Abstract
Aims
Left ventricular assist devices (LVADs) have reduced the mortality of patients with advanced heart failure both as bridge‐to‐transplant and as destination therapy. However, LVADs are associated with various complications, including bleedings, which affect the prognosis. The aim of the study was to explore the prevalence, management, and outcomes of haemorrhagic adverse events in LVAD recipients.
Methods and results
We conducted a retrospective, single‐centre, cohort study including all patients who received an LVAD from January 2008 to December 2019 in our tertiary centre (Rangueil University Hospital, Toulouse, France). Bleeding events, death, and heart transplantation were collected from electronic medical files. Eighty‐eight patients were included, and 43 (49%) presented at least one bleeding event. Gastrointestinal (GI) bleeding was the most frequent (n = 21, 24%), followed by epistaxis (n = 12, 14%) and intracranial haemorrhage (n = 9, 10%). Bleeding events were associated with increased mortality [hazard ratio (HR) 3.8, 95% confidence interval (CI) 1.5–9.3, P < 0.01], particularly in case of intracranial haemorrhage (HR 14.6, 95% CI 4.2–51.1, P < 0.0001). GI bleedings were associated with a trend towards increased mortality (HR 3.0, 95% CI 0.9–9.3, P = 0.05). Each bleeding episode multiplied the risk of death by 1.8 (95% CI 1.2–2.7, P < 0.01). Finally, only early bleedings (<9 months post‐implantation) had an impact on mortality (HR 4.2, 95% CI 1.6–11.1, P < 0.01). Therapeutic management was mainly based on temporary interruption of anticoagulation and permanent interruption of antiplatelet therapy. Invasive management was rarely performed.
Conclusions
Haemorrhagic events in LVAD recipients are frequent and associated with increased mortality. GI bleedings are the most frequent, and intracranial haemorrhages the most associated with mortality. Management remains empirical requiring more research.
Keywords: Left ventricular assist device, Bleeding, Heart failure, Mortality
Introduction
Left ventricular assist devices (LVADs) have been in development since 1964, leading to the first successful human implantation by Dr De Bakey in 1966. 1 The first generation of LVAD, pneumatically powered and with a pulsatile flow, demonstrated improvement in survival compared with the medical treatment, first as a bridge‐to‐transplantation and then as a destination therapy. 2 , 3 Despite favourable outcomes, these devices are associated with complications such as infections, strokes, bleedings, or device failure that may lead to reinterventions or even death. The second generation of LVAD [HeartMate II (Thoratec Corp, Pleasanton, CA, USA) and the Jarvik 2000 (Jarvik Heart, Inc, New York, NY, USA)], based on axial pumps with continuous flow, have demonstrated a decrease in the number of strokes and pump failure, 4 , 5 , 6 but an increase in gastrointestinal (GI) bleeding. 7 To improve the haemocompatibility of the pump, a third generation of LVAD has been developed with the HeartMate 3 (Thoratec Corp, Pleasanton, CA, USA) and the HeartWare HVAD (Medtronic, Mounds View, MN, USA), which are continuous flow and centrifugal pumps. 8 , 9 The MOMENTUM 3 study has shown the superiority of the HeartMate III compared with the HeartMate II with less thrombosis, pump replacement, disabling stroke, but also less major bleeding and GI bleeding. 10
Despite all the improvements with the last generations of LVAD, bleeding remains frequent and a source of morbidity. In this study, we aim to explore the prevalence, management and outcomes of haemorrhagic events in LVAD recipients in our tertiary centre.
Methods
Study design and population
We conducted a retrospective, single‐centre, cohort study including all patients who underwent LVAD implantation from January 2008 to December 2019 at Rangueil University Hospital (Toulouse, France), a tertiary cardiac centre with regional expertise in cardiac transplantation and mechanical assist device implantation. Patients with premature death, defined as death occurring within 30 days post‐implantation, were excluded.
The study was consistent with the principles set out in the Helsinki Declaration. After evaluation and validation by the data protection officer and in accordance with the General Data Protection Regulation, this study has met all the criteria and is registered in the retrospective study register of the Toulouse University Hospital (number's register: RnIPH 2021‐87) and covered by the MR‐004 (CNIL number: 2206723 v 0).
Follow‐up and endpoints
Follow‐up data were collected up to July 2021 or the occurrence of heart transplantation or death.
The primary endpoint was the occurrence of any haemorrhagic event, including GI bleeding, intracranial haemorrhage, epistaxis, or any other bleeding leading to medical consultation or hospitalization. Only events occurring more than 30 days after LVAD implantation were considered to avoid surgery‐associated events. GI bleeding was defined as the occurrence of at least one of the following events: haematemesis, melaena, haematochezia or drop of haemoglobin > 2 g/dL requiring blood transfusion, and digestive explorations without extra‐digestive aetiology found. GI bleeding was classified as ‘upper’ tract bleeding if the origin was located above the suspensory muscle of the duodenum; otherwise, it was classified as ‘lower’ tract bleeding. Intracranial haemorrhage was defined as the occurrence of one of the following events: intracerebral haemorrhage, subarachnoid haemorrhage, subdural haematoma, or epidural haematoma.
Collected data included demographic data such as age, gender, comorbidities, and type of cardiomyopathy; type of device and device strategy; biological values such as haemoglobin level, platelet count, and international normalized ratio (INR) before implantation and at the time of the event; long‐term treatment and during event; and number of packed red blood cell transfused during event as well as the various diagnostic and therapeutic explorations carried out during event.
Statistical analysis
Continuous variables are presented as median [interquartile range] and categorical variables as counts (percentage). Continuous variable differences were tested with Wilcoxon signed rank tests and categorical variables with χ 2 tests or Fisher's exact tests according to their respective conditions of applicability.
Survival analyses consisted of multistate models considering the competitive risks represented by two absorbing states: death and heart transplant. Bleeding events were used as an explanatory variable for death using time‐dependent Cox regressions; applicability was verified with Schoenfeld tests. Otherwise, a log rank test was used. In both cases, we applied a robust estimation of the coefficients in order to avoid an artificial inflation of the number of patients when more than one event occurred in the same patient. Graphical representation relied on time‐dependent Cox curves. To assess the effect of the precocity of the bleeding on mortality, early bleeding was a posteriori defined as occurring prior to the median onset of bleeding, that is, prior to the ninth month post‐implantation of the LVAD, and late bleeding as occurring after this timepoint.
All tests were two‐sided, and P values < 0.05 were considered statistically significant. Missing data were systematically reported as they were removed from the statistical analysis.
All statistical analyses were performed with R Version 4.1.0 (R Foundation for Statistical Computing, Vienna, Austria). Survival analysis relied on the survival and mstate packages.
Results
Patient characteristics
A total of 102 patients received LVADs during the study period. Fourteen (14%) were excluded from analysis due to short survival times (<30 days) after implantation. Finally, 88 patients were included in the study (Figure 1 ). The median age was 58 [53–65] years, 78 (88%) patients were male, and 64 (73%) had ischaemic cardiomyopathy (ICM). At implantation, median left ventricular ejection fraction was 20 [15–25] %, and TAPSE was 17 [15–19] mm. The main comorbidities were hypertension, smoking, and chronic kidney disease with 41%, 77%, and 31% of the population, respectively. Sixty‐one (69%) patients were implanted with a HeartMate II and 53 (60%) patients as a bridge‐to‐transplant strategy. Twenty‐one (24%) patients received inotropes, and 16 (18%) had a temporary circulatory support including 9 (10%) patients under extracorporeal membrane oxygenation. Most patients were INTERMACS ≥ 4 (n = 53, 61%). The median duration of follow‐up was 2.4 [1.1–3.2] years (Table 1 ).
Figure 1.
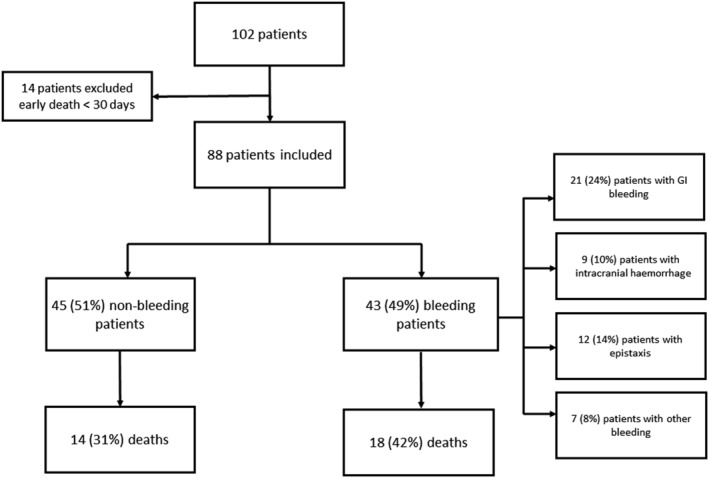
Study flowchart.
Table 1.
Population characteristics
| Patients characteristics | Total population | Non‐bleeding patients | Bleeding patients | P value |
|---|---|---|---|---|
| Population | N = 88 | N = 45 | N = 43 | |
| Age at LVAD implant (year) | 58 (53–65) | 58 (53–64) | 58 (54–66) | 0.6 |
| Male | 78 (88%) | 40 (89%) | 38 (88%) | 1 |
| BMI (kg/m2) |
25.4 (23.6–29) n = 87 |
25 (23–28.5) | 25.5 (24.5–29.2) | 0.1 |
| Heart failure aetiology | 0.03 | |||
| ICM | 64 (73%) | 38 (85%) | 26 (60%) | |
| DCM | 21 (24%) | 6 (13%) | 15 (35%) | |
| Other | 3 (3%) | 1 (2%) | 2 (5%) | |
| Comorbidities | ||||
| Obesity |
17 (20%) n = 87 |
8 (18%) | 9 (21%) | 0.9 |
| HTN | 36 (41%) | 15 (33%) | 21 (49%) | 0.2 |
| Diabetes | 21 (24%) | 8 (18%) | 13 (30%) | 0.3 |
| Smoking | 68 (77%) | 32 (71%) | 36 (84%) | 0.02 |
| Active | 18 (21%) | 13 (29%) | 5 (12%) | |
| CKD | 27 (31%) | 8 (18%) | 19 (44%) | 0.01 |
| ICD | 56 (64%) | 26 (58%) | 30 (79%) | 0.3 |
| Echocardiography at implantation | ||||
| LVEF |
20 (15–25) n = 87 |
20 (15–24) | 19 (15–25) | 1 |
| TAPSE |
17 (15–19) n = 80 |
18 (15–20) | 15 (13–17) | 0.006 |
| TDI S′ |
10 (8–11) n = 75 |
10 (8–11) | 10 (8–11) | 0.3 |
| RV dysfunction a | 27 (41.5%) | 14 (41.2%) | 13 (41.9%) | 1 |
| Valvular heart diseases | ||||
| AR (≥grade 2) | 2 (2%) | 1 (2%) | 1 (2%) | 1 |
| MR (>grade 2) |
17 (19%) n = 87 |
8 (18%) | 9 (21%) | 0.9 |
| Medications pre‐implantation, n (%) | ||||
| Betablockers | 35 (39.8%) | 14 (31.1%) | 21 (50%) | 0.15 |
| ACE inhibitors or ARB | 35 (39.8%) | 16 (35.6%) | 19 (44.2%) | 0.54 |
| Sacubitril/valsartan | 7 (8%) | 2 (4.4%) | 5 (11.6%) | 0.26 |
| Aldosteron antagonist | 43 (48.9%) | 20 (44.4%) | 23 (53.5%) | 0.53 |
| Loop Diuretics | 75 (85.2%) | 39 (86.7%) | 36 (83.7%) | 0.93 |
| Statins | 61 (73.5%) | 33 (78.6%) | 28 (68.3%) | 0.42 |
| APT | 48 (56.5%) | 28 (63.6%) | 20 (48.8%) | 0.25 |
| Characteristics at implantation | ||||
| INTERMACS | 0.5 | |||
| 1 | 16 (18%) | 10 (22%) | 6 (14%) | |
| 2 | 3 (3%) | 1 (2%) | 2 (5%) | |
| 3 | 16 (18%) | 10 (22%) | 6 (14%) | |
| ≥4 | 53 (61%) | 24 (54%) | 29 (67%) | |
| Type of device | 0.9 | |||
| HM 2 | 61 (69%) | 32 (71%) | 29 (67%) | |
| HM 3 | 27 (31%) | 13 (29%) | 14 (33%) | |
| Device strategy | 0.7 | |||
| Destination therapy | 27 (31%) | 12 (27%) | 15 (35%) | |
| Bride to transplant | 53 (60%) | 29 (64%) | 24 (56%) | |
| Bridge to candidacy | 8 (9%) | 4 (9%) | 4 (9%) | |
| Temporary circulatory support | 16 (18%) | 0.5 | ||
| ECMO | 9 (10%) | 5 (11%) | 4 (9%) | |
| Impella | 7 (8%) | 5 (11%) | 2 (5%) | |
| Inotropes |
21 (24%) n = 8 |
12 (27%) | 9 (21%) | 0.7 |
| Time to diagnosis | <0.05 | |||
| <3 months | 22 (25%) | 16 (36%) | 6 (14%) | |
| 3 months to 1 year | 7 (8%) | 5 (11%) | 2 (5%) | |
| 1–5 years | 14 (16%) | 6 (13%) | 8 (18%) | |
| >5 years | 45 (51%) | 18 (40%) | 27 (63%) | |
| Medications at hospital discharge, n (%) | ||||
| Betablockers | 53 (63.9%) | 25 (59.5%) | 28 (68.3%) | 0.55 |
| ACE inhibitors or ARB | 43 (51.8%) | 20 (47.6%) | 23 (56.1%) | 0.58 |
| Sacubitril/valsartan | 0 | 0 | 0 | — |
| Aldosteron antagonist | 36 (43.4%) | 16 (38.1%) | 20 (48.8%) | 0.45 |
| Statins | 61 (73.5%) | 33 (78.6%) | 28 (68.3%) | 0.42 |
| APT | 80 (96.4%) | 41 (97.6%) | 39 (95.1%) | 0.62 |
| Proton‐pump inhibitors | 70 (84.3%) | 34 (81%) | 36 (87.8%) | 0.58 |
| Events | ||||
| Mortality (all cause) | 32 (36%) | 14 (31%) | 18 (42%) | 0.4 |
| Patients with ≥1 bleeding | 43 (49%) | 43 | ||
| Total events | 87 | 87 | ||
| GI bleeding | 56 (64%) | 56 | ||
| Intracranial haemorrhage | 10 (12%) | 10 | ||
| Epistaxis | 13 (15%) | 13 | ||
| Other | 8 (9%) | 8 | ||
| Transplant | 28 (32%) | 19 (42%) | 9 (21%) | 0.06 |
| Follow‐up | ||||
| Follow‐up (year) | 2.4 (1.1–3.2) | 2.1 (1–2.8) | 2.7 (1.3–4.3) | 0.07 |
APT, antiplatelet therapy; AR, aortic regurgitation; BMI, body mass index; CKD, chronic kidney disease; DCM, dilated cardiomyopathy; ECMO, extracorporeal membrane oxygenation; GI: gastrointestinal; HM, HeartMate; HTN, hypertension; ICD, implantable cardioverter defibrillator; ICM, ischaemic cardiomyopathy; INTERMACS, Interagency Registry for Mechanically Assisted Circulatory Support classification; LVAD, left ventricular assist device; LVEF, left ventricular ejection fraction; MR, mitral regurgitation; TAPSE, tricuspid annular plane systolic excursion; TDI S′, tricuspid annular systolic velocity.
RV dysfunction was defined by the association of TAPSE < 14 mm and TDI S′ < 8 cm/s.
Bleeding events
Of the 88 patients included in the study, 43 (49%) had at least one bleeding event with a total of 87 events during follow‐up, the median number of events by patients was 1 [1–2.5]. The median time to first bleed was 9 [3–21] months. Fifty‐six (64%) GI bleedings, 10 (12%) intracranial haemorrhages, 13 (15%) epistaxis, and 8 (9%) other bleedings were recorded (Table 2 ). There was no significant difference between groups with and without bleeding for age, gender, body mass index, hypertension, diabetes, type of device, and assistance strategy. Among patients who bled, there were more smokers and more chronic kidney disease. The aetiology of cardiomyopathy was different with more dilated cardiomyopathy and less ICM in the bleeding group. TAPSE was also lower in the bleeding group (Table 1 ). There was no difference in bleeding events between HeartMate 2 and HeartMate 3 recipients in a subgroup analysis [hazard ratio (HR) 1.3, 95% confidence interval (CI) 0.7–2.5, P = 0.4] (Supporting Information, Table S2 ).
Table 2.
Bleeding characteristics
| Events (N = 87) | |
|---|---|
| Patients with ≥1 bleeding episode | 43 (49%) |
| Patients with 2 different types of bleeding | 7 (8%) |
| Time to first bleeding (months) | 9 (3–21) |
| Total number of episodes | 87 |
| GI bleeding | 56 (64%) |
| Intracranial haemorrhage | 10 (12%) |
| Epistaxis | 13 (15%) |
| Other | 8 (9%) |
| Patients with GI bleeding | 21 (24%) |
| 1 episode | 5 |
| 2 episodes | 7 |
| 3 episodes | 4 |
| 4 episodes | 2 |
| 5 episodes | 1 |
| 6 episodes | 2 |
| Patients with intracranial haemorrhage | 9 (10%) |
| 1 episode | 8 |
| 2 episodes | 1 |
| Patients with epistaxis | 12 (14%) |
| 1 episode | 11 |
| 2 episodes | 1 |
| Patients with other bleeding | 7 (8%) |
Gastrointestinal bleeding
Gastrointestinal bleeding occurred in 21 (24%) patients with a total of 56 events during the follow‐up. Among patients who had GI bleeding, 16 (76%) had recurrent episodes (Table 2 ). The median time to first GI bleeding after LVAD implantation was 2.4 [1.2–3.5] years. The most frequent symptom at admission was melaena (n = 22, 39%) followed by haematochezia (n = 13, 23%). At admission, the median haemoglobin level was 7.3 [6.4–8.8] g/dL and INR was 2.9 [2.4–3.6]. Thirty‐four (61%) patients were on dual antithrombotic therapy [vitamin K antagonists (VKA) and aspirin], and 48 (86%) were receiving a proton‐pump inhibitor.
Among all the events, diagnostic procedures were performed in 45 (80%) cases with an esophagogastroduodenoscopy alone in 13 (23%) cases. A total of 73 procedures were performed, which allowed determining the bleeding source in 28 (38%) explorations. The bleeding origin was most often unknown (n = 30, 54%). However, when the origin was identified, it was mainly located in the lower GI tract and more particularly in the colon (n = 10, 18%), followed by a gastric origin (n = 8, 14%). Ulcerative lesions were the most common (n = 8, 14%).
Patients required transfusion in 46 (82%) GI bleedings with a median of 4 [2–4] units of PRBC administered. Therapeutic endoscopic management was performed in 11 (20%) cases. Antiplatelet therapy (APT) was definitively discontinued in 21 (55%) cases (Tables 3 and S1 ).
Table 3.
Presentation, diagnosis, location, aetiology, and management of GI bleeding
| Events (N = 56) | ||
|---|---|---|
| Time to first bleed (years) | 2.4 (1.2–3.5) | |
| Presenting symptoms | ||
| Haematemesis | 1 (2%) | |
| Melaena | 22 (39%) | |
| Haematochezia | 13 (23%) | |
| Haematemesis + haematochezia | 1 (2%) | |
| Melaena + haematochezia | 7 (13%) | |
| Symptomatic anaemia | 12 (21%) | |
| Biology | ||
| INR | 2.9 (2.4–3.6) | |
| Hb (g/dL) | 7.3 (6.4–8.8) | |
| Platelets (G/L) | 231 (190–269) | |
| Treatment | ||
| Antithrombotic treatment | ||
| VKA + APT | 34 (61%) | |
| VKA alone | 17 (30%) | |
| VKA + heparin | 1 (2%) | |
| VKA + APT + heparin | 1 (2%) | |
| APT + heparin | 3 (5%) | |
| PPI | 48 (86%) | |
| Procedures performed a | Number (% diagnoses) | |
| Total number | 73 | 28 (38%) |
| EGD | 32 (57%) | 10 (31%) |
| Colonoscopy | 19 (34%) | 10 (53%) |
| VCE | 15 (27%) | 5 (33%) |
| Push enteroscopy | 3 (5%) | 1 (33%) |
| Technetium‐labelled RBC scintigraphy | 4 (7%) | 2 (50%) |
| 1 procedure performed | 25 (45%) | |
| 2 procedures performed | 15 (27%) | |
| ≥3 procedure performed | 5 (9%) | |
| No procedure performed | 11 (19%) | |
| Location of bleeding a | ||
| Upper GI bleeding | 12 (21%) | |
| Oesophagus | 1 | |
| Stomach | 8 | |
| Duodenum | 3 | |
| Lower GI bleeding | 17 (30%) | |
| Small bowel | 5 | |
| Colon | 10 | |
| Rectum | 2 | |
| Unknown | 30 (54%) | |
| Type of lesions a | ||
| Ulcer/erosions | 8 (14%) | |
| Bleeding polyps | 5 (9%) | |
| GIAD | 6 (11%) | |
| Diverticula | 2 (4%) | |
| Haemorrhoids | 2 (4%) | |
| Tumour | 1 (2%) | |
| Unknown | 33 (59%) | |
| Therapeutic management | ||
| Invasive management | 11 (20%) | |
| Endoclips/sclerosis | 2 | |
| Argon plasma coagulation | 4 | |
| Polypectomy | 4 | |
| Epinephrine injection | 1 | |
| Non‐invasive management | ||
| Blood transfusion | 46 (82%) | |
| Units of PRBC | 4 (2–4) | |
| Heparin switch during hospitalization | 25 (45%) | |
| APT interruption | 21 (55%) | |
| Reduced INR range | 10 (18%) | |
| PPI increase/initiation | 14 (25%) | |
| Iron supplementation | 17 (30%) | |
| Willfactin | 4 (7%) | |
| Octreotide | 6 (11%) |
APT, antiplatelet therapy; EGD, esophagogastroduodenoscopy; GI, gastrointestinal; GIAD, gastrointestinal angiodysplasia; Hb, haemoglobin, g/dL; INR, international normalized ratio; PPI: proton‐pump inhibitors; PRBC: packed red blood cells; RBC, red blood cells; VCE, video capsule endoscopy; VKA, vitamin K antagonists.
Categories are not mutually exclusive. Unknown category includes the events without procedure performed and the ones with non‐contributory procedures. APT interruption only concerns patients on APT during the event (n = 38).
Intracranial haemorrhage
During follow‐up, 9 (10%) patients had intracranial haemorrhage with a total of 10 events (Table 2 ). The median time from LVAD implantation to first intracranial haemorrhage was 1.7 [0.7–2.7] year. Median INR was 2 [1.8–2.5]. Most patients were on dual antithrombotic therapy: four (40%) on VKA and APT and four (40%) on heparin and APT. Patients mainly presented with an intracerebral haemorrhage associated with subarachnoid haemorrhage (n = 4, 40%). Regarding the aetiology, four (40%) events occurred spontaneously, three (30%) occurred as a consequence of a fall, one (10%) involved an arteriovenous malformation, one (10%) consisted in a mycotic aneurysm rupture, and one (10%) consisted in the haemorrhagic transformation of an ischaemic stroke (Table 4 ). In the acute phase, VKA were discontinued in six (100%) cases, APT in four (40%), and a switch for heparin was performed in seven (70%) cases. There was only one invasive management with external ventricular drain for one (10%) patient. INR target at discharge was reduced in three (60%) patients. Intracranial haemorrhage led to death in five (50%) patients during hospitalization.
Table 4.
Presentation, diagnosis, and management of intracranial haemorrhage
| Events (N = 10) | |
|---|---|
| Time to first bleed (year) | 1.7 (0.7–2.7) |
| Presenting symptoms a | |
| Impaired consciousness | 7 (70%) |
| Neurological deficit | 7 (70%) |
| Syncope | 1 (10%) |
| Seizure | 1 (10%) |
| Biology | |
| INR | 2 (1.8–2.5) |
| Hb (g/dL) | 10.6 (9.7–12.7) |
| Platelets (G/L) | 193 (166–238) |
| Treatment | |
| Antithrombotic treatment | |
| VKA + APT | 4 (40%) |
| VKA + APT + heparin | 2 (20%) |
| APT + heparin | 4 (40%) |
| Procedures performed | |
| Cranial CT scan | 10 (100%) |
| Radiological diagnosis | |
| Intracerebral haemorrhage | 3 (30%) |
| Subarachnoid haemorrhage | 2 (20%) |
| Subdural haematoma | 1 (10%) |
| Intracerebral haemorrhage + SAH | 4 (40%) |
| Therapeutic management | |
| Invasive management | 1 (10%) |
| EVD placement | 1 |
| Non‐invasive management | |
| VKA interruption | 6 (100%) |
| APT interruption | 4 (40%) |
| Heparin switch | 7 (70%) |
| Interruption of any anticoagulation | 2 (20%) |
| Vitamin K | 2 (20%) |
| Reduced INR range at discharge | 3 (60%) |
| Death during the event | 5 (50%) |
APT, antiplatelet therapy; CT, computed tomography; EVD, external ventricular drain; Hb, haemoglobin, g/dL; INR, international normalized ratio; SAH, subarachnoid haemorrhage; VKA, vitamin K antagonists.
Categories are not mutually exclusive. VKA interruption only concerns patients on VKA during the event (n = 6).
Epistaxis
Epistaxis occurred in 12 (14%) patients during the follow‐up with a total of 13 events (Table 2 ). Median time from LVAD implantation to first epistaxis was 6.2 [1.6–11.8] months. On admission, median INR was 2.7 [2.2–3.3], and median haemoglobin level was 11.2 [8.4–13.3] g/dL. Most patients were on dual antithrombotic therapy with VKA and APT (n = 6, 46%). One patient presented with haemorrhagic shock. Compared with Heartmate II device, Heartmate 3 was associated with a significant increased risk of epistaxis (HR 7.3, 95% CI 2.0–26.7, P < 0.01) (Table S2 ). Nasofibroscopy was performed in six (46%) cases with evidence of Kiesselbach's plexus ectasia in four (31%) patients. As therapeutical management, four (31%) patients received a blood transfusion with a median of 3 [2–6] units of PRBC. APT was definitively discontinued in five (71%) patients. Specific otorhinolaryngologic care was performed with topical medications for eight (61%) patients, nasal packing for seven (54%) patients, and silver nitrate chemical cautery for four (31%) patients (Table 5 ).
Table 5.
Presentation, diagnosis, and management of epistaxis
| Events (N = 13) | |
|---|---|
| Time to first bleed (months) | 6.2 (1.6–11.8) |
| Procedures performed | |
| Nasofibroscopy | 6 (46%) |
| Diagnosis | 4/6 (66%) |
| Kiesselbach's plexus ectasia | 4/4 |
| Biology | |
| INR | 2.7 (2.2–3.3) |
| Hb (g/dL) | 11.2 (8.4–13.3) |
| Platelets (G/L) | 213 (200–260) |
| Treatment | |
| Antithrombotic treatment | |
| VKA + APT | 6 (46%) |
| VKA + APT + heparin | 1 (8%) |
| VKA alone | 3 (23%) |
| Unknown | 3 (23%) |
| PPI | 6 (46%) |
| Therapeutic management | |
| Non‐invasive management | |
| Blood transfusion | 4 (31%) |
| Units of PRBC | 3 (2–6) |
| Reduced INR range | 1 (8%) |
| APT interruption | 5 (71%) |
| Octreotide | 1 (8%) |
| Invasive management | |
| Nasal packing | 7 (54%) |
| Double‐balloon catheter | 1 (8%) |
| Topical medications | 8 (61%) |
| Endoscopic cauterization | 4 (31%) |
APT, antiplatelet therapy; Hb, haemoglobin, g/dL; INR, international normalized ratio; PPI: proton‐pump inhibitors; PRBC, packed red blood cells; VKA, vitamin K antagonists.
APT interruption only concerns patients on APT during the event (n = 7).
Other bleeding
During follow‐up, seven (8%) patients had a total of eight bleeding events, consisting in two haematurias, two muscle haematomas, one haemoptysis, one retroperitoneal haematoma, one bleeding around the cable, and one gingivorrhagia. Most patients were on dual antithrombotic therapy with VKA and APT (n = 5, 63%). On admission, median haemoglobin level was 9.8 [8.5–11.7] g/dL, and median INR was 2 [1.5–4]. Five (63%) patients needed a blood transfusion with a median of 5.5 [7–7.7] units of PRBC. VKAs were discontinued in four (50%) patients with a switch for heparin. Invasive management was performed four (50%) times.
Outcomes
The prevalence of all‐cause mortality was 36% (n = 32) with respectively 14 (31%) and 18 (42%) for non‐bleeding and bleeding patients during a median follow‐up of 2.4 [1.1–3.2] years. Bleeding events multiplied the risk of death by 3.8 (95% CI 1.5–9.3, P < 0.01) (Figure 2 ). According to the type of bleeding, only intracranial haemorrhage significantly increased mortality (HR 14.6, 95% CI 4.2–51.1, P < 0.0001). There was a trend for GI bleeding to increase the risk of death (HR 3.0, 95% CI 0.9–9.3, P = 0.05) (Figure 3 ). In case of recurrent bleedings, only the first and third ones had an impact on mortality (HR 3.4, 95% CI 1.2–9.4, P < 0.05 and HR 6.3, 95% CI 1.8–21.8, P < 0.01, respectively). Each bleeding episode multiplied the risk of death by 1.8 (95% CI 1.2–2.7, P < 0.01). Finally, only early bleedings (<9 months after LVAD implantation) had an impact on mortality (HR 4.2, 95% CI 1.6–11.1, P < 0.01) (Figure 4 ). In the subgroup analysis comparing mortality between HeartMate 2 and Heartmate 3 recipients, there was a trend for HeartMate 3 to decrease mortality (HR 0.4, 95% CI 0.1–1.2, P = 0.09) ( Table S2 ).
Figure 2.
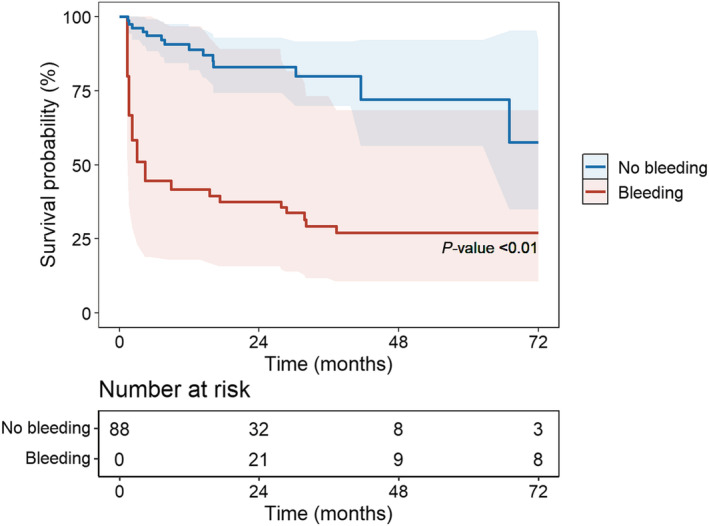
Time‐dependent Cox survival curves according to bleeding event.
Figure 3.
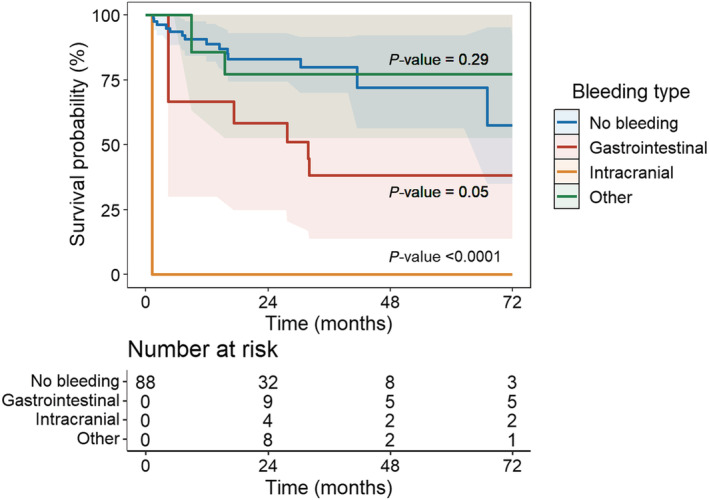
Time‐dependent Cox survival curves according to the type of bleeding. No bleeding is the reference for statistic tests.
Figure 4.
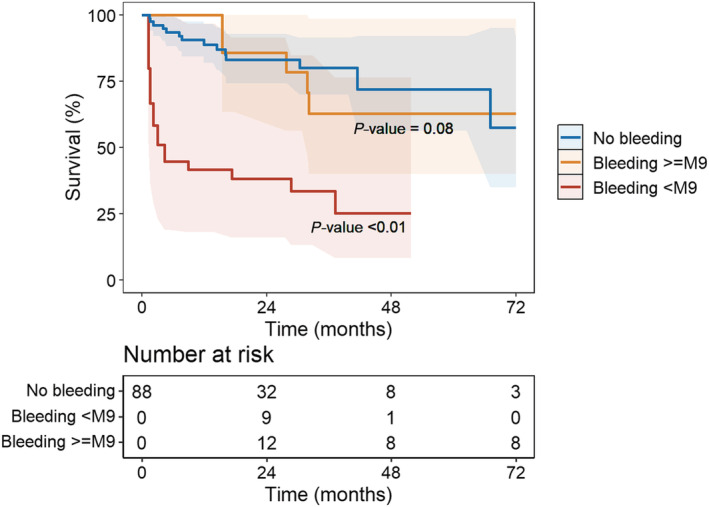
Time‐dependent Cox survival curves according to the time of bleeding (early <9 months) vs late.
Discussion
In this retrospective, single‐centre study, we found the following results: (i) haemorrhagic events are frequent with almost half of patients (49%) presenting at least one bleeding event during a median follow‐up of 2.4 [1.1–3.2] years, (ii) bleeding events are associated with an increased risk of mortality (HR 3.8), (iii) each bleeding episode multiplied the risk of death by 1.8, and (iv) intracranial haemorrhage and early bleeding (<9 months) have a significant impact on mortality (HR 14.6 and 4.2, respectively).
From 2008 to 2019, patients who received an LVAD in our centre were mainly men suffering from ICM, in bridge‐to‐transplant and INTERMACS ≥ 4. These patients differ from the patients of the American INTERMACS registry 11 where most of them were implanted because of non‐ischaemic cardiomyopathy, in precarious condition (INTERMACS < 4) and in destination therapy. Our sex ratio with large predominance of male recipients (88%) is in accordance with previous report of LVAD registry in France (576 on 671 patients (85.8%) in the ASSIST‐ICD registry. 12 In our study and with a median follow‐up of 2.4 years, about a third of patients were transplanted, another third died, and finally, 30% were still under assistance at the end of the study. While more than 60% of patients were implanted in bridge‐to‐transplant, only 32% were transplanted during follow‐up. There is obviously a donor shortage, but the lack of prioritization in France of patients on LVAD on the waiting list until 2020 also contributes to it.
We demonstrated that bleedings were frequent with about half patients presenting a haemorrhagic event during follow‐up. GI bleeding was the leading cause of bleeding (24%) while intracranial haemorrhage, although associated with a poor prognosis, was less frequent (10%), as previously reported. 8 , 10 , 11 , 13 , 14 As expected, there was an excess of mortality in case of bleeding (HR 3.8). Intracranial haemorrhages were associated with a major and significantly increased risk of mortality (HR 14.6), which is already reported. 15 , 16 It also appears that half of patients died at the acute phase of bleeding. In our study, most patients did not have VKA overdose at admission as the median INR was 2 [1.8–2.5]. Management is complex because of thrombotic risk, empirical, and almost exclusively medical based on a multidisciplinary approach. It was mainly based on temporary interruption of curative anticoagulation and antiplatelet aggregation with possible switch for heparin or even reversion of anticoagulation. In survivors, the target INR range was lowered in 60% of cases and the APT was stopped in only 40% of cases.
In case of GI bleeding, there was a trend for an increased risk of death (HR 3.0, P = 0.05), probably because of lack of statistical power in our study. However, data in the literature remain discordant with studies showing an excess of mortality 14 , 17 and others not. 7 , 18 , 19 Most patients with a first episode had recurrences and more than 40% had more than three episodes leading to many rehospitalization and procedures. In our study, among the 56 events, explorations were carried out in more than 80%, with a total of 73 exams. The diagnostic yield remains unsatisfactory as only 38% of these explorations lead to a diagnosis. Eventually, on the 56 episodes, a diagnosis was retained in 46% with evidence of a lesion in 58% of explored patients. This raises the question of the best diagnostic strategy and the prioritization of explorations in order to improve the efficiency of our strategy. Some teams have already developed a diagnostic algorithm adapted to the initial presentation of GI bleeding. 20 Their results seem to be encouraging but have to be validated on a larger scale. 21 Interestingly, 86% of patients were taking proton‐pump inhibitor during GI bleeding, raising the question of the value of such systematic treatment in LVAD recipients. Invasive management was rarely performed (20%) because the bleeding source remained largely unknown and if a lesion is found, invasive management is not always possible. New therapeutic approaches are emerging mainly in secondary prevention such as octreotide, a molecule well‐known by gastroenterologists, with satisfactory results on recurrent GI bleeding. 22 , 23 , 24 Prospective, randomized, and larger studies are underway to confirm these results (NCT01707225). Thalidomide and danazol also seem to decrease recurrent bleeding, 25 , 26 , 27 , 28 to be confirmed by prospective and larger studies. Recently, a pilot study reported a significant reduction of blood transfusions, bleeding‐related hospitalizations, and endoscopy procedures in LVAD's patients with refractory GI angiodysplasia related bleeding after intravenous infusion of a humanized monoclonal antibody against VEGF (bevacizumab Avastin). 29 In primary prevention, there seems to be a protective role for ACE inhibitors and ARBs, 30 , 31 that we however did not find in our study, probably due to lack of power. Eventually, Omega‐3 fatty acid administration would also tend to reduce the occurrence of bleeding. 32
Our study has several limitations. First, because of its design, this is a single‐centre study with a small number of patients, therefore leading to a lack of power. The retrospective approach is also the cause of significant missing data during the collection process by examining electronic files. As it is a single‐centre study, the external validity is limited with results that cannot always be extrapolated to the entire target population. Our study also has the limitation of observational protocols and consequently can only generate hypotheses and lines of research. Finally, it should be noticed that our population initially and mainly benefited from a HeartMate 2 implantation, then from the end of 2017, HeartMate 3 were implanted, which may have further limited the number of events as bleeding is less common with HeartMate 3 devices. 33 The size of our population and the number of events did not allow subgroup analysis. It is necessary for future studies to establish clear protocols and to standardize inclusion criteria but also the definition of haemorrhagic events, in particular for GI bleeding.
Conclusion
Haemorrhagic events in LVAD recipients remain a frequent complication that occurs in nearly half of patients. In addition, it is associated with a significant increase in the risk of death. GI bleeding ranks first in the bleeding complication with many recurrent episodes. Although intracranial haemorrhage is less frequent, it leads to a worst prognosis. Management remains complex and empirical.
Conflict of interest
None declared.
Funding
This research received no specific grant from public, commercial, or non‐profit funding agencies.
Supporting information
Table S1. Details of the procedures performed during GI bleeding
Legend: EGD: Esophagogastroduodenoscopy; VCE: video capsule endoscopy; RBC: red blood cells.
Table S2. Subgroup analysis according to the type of LVAD (Heartmate 2 vs Heartmate 3)
Legend: HR: hazard ratio; CI: confidence interval. Subgroup analysis comparing mortality and bleeding occurrence between HeartMate 2 and HeartMate 3. HeartMate 2 is the reference.
Acknowledgements
We would like to thanks our VAD coordinator Sophie Bernard for her continuous assistance to LVAD recipients and medical team managing these patients for several years.
Pourtau, L. , Beneyto, M. , Porterie, J. , Roncalli, J. , Massot, M. , Biendel, C. , Fournier, P. , Itier, R. , Galinier, M. , Lairez, O. , and Delmas, C. (2022) Prevalence, management, and outcomes of haemorrhagic events in left ventricular assist device recipients. ESC Heart Failure, 9: 1931–1941. 10.1002/ehf2.13899.
References
- 1. DeBakey ME. Left ventricular bypass pump for cardiac assistance. Am J Cardiol. 1971; 27: 3–11. [DOI] [PubMed] [Google Scholar]
- 2. Goldstein DJ, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998; 12: 1522–1533. [DOI] [PubMed] [Google Scholar]
- 3. Rose EA, Stevenson LW, Tierney AR. Long‐term use of a left ventricular assist device for end‐stage heart failure. N Engl J Med. 2001; 9: 1435–1443. [DOI] [PubMed] [Google Scholar]
- 4. Miller LW, Pagani FD, Russell SD, John R, Boyle AJ, Aaronson KD, Conte JV, Naka Y, Mancini D, Delgado RM, MacGillivray TE, Farrar DJ, Frazier OH. Use of a continuous‐flow device in patients awaiting heart transplantation. N Engl J Med. 2007; 357: 885–896. [DOI] [PubMed] [Google Scholar]
- 5. Slaughter MS, Rogers JG, Milano CA, Russell SD, Conte JV, Feldman D, Sun B, Tatooles AJ, Delgado RM III, Long JW, Wozniak TC, Ghumman W, Farrar DJ, Frazier OH. Advanced heart failure treated with continuous‐flow left ventricular assist device. N Engl J Med. 2009; 361: 2241–2251. [DOI] [PubMed] [Google Scholar]
- 6. Pagani FD, Miller LW, Russell SD, Aaronson KD, John R, Boyle AJ, Conte JV, Bogaev RC, MacGillivray T, Naka Y, Mancini D, Massey HT, Chen L, Klodell CT, Aranda JM, Moazami N, Ewald GA, Farrar DJ, Frazier OH, HeartMate II Investigators . Extended mechanical circulatory support with a continuous‐flow rotary left ventricular assist device. J Am Coll Cardiol. 2009; 54: 312–321. [DOI] [PubMed] [Google Scholar]
- 7. Crow S, John R, Boyle A, Shumway S, Liao K, Colvin‐Adams M, Toninato C, Missov E, Pritzker M, Martin C, Garry D, Thomas W, Joyce L. Gastrointestinal bleeding rates in recipients of nonpulsatile and pulsatile left ventricular assist devices. J Thorac Cardiovasc Surg. 2009; 137: 208–215. [DOI] [PubMed] [Google Scholar]
- 8. Aaronson KD, Slaughter MS, Miller LW, McGee EC, Cotts WG, Acker MA, Jessup ML, Gregoric ID, Loyalka P, Frazier OH, Jeevanandam V, Anderson AS, Kormos RL, Teuteberg JJ, Levy WC, Naftel DC, Bittman RM, Pagani FD, Hathaway DR, Boyce SW, HeartWare Ventricular Assist Device (HVAD) Bridge to Transplant ADVANCE Trial Investigators . Use of an intrapericardial, continuous‐flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012; 125: 3191–3200. [DOI] [PubMed] [Google Scholar]
- 9. Netuka I, Sood P, Pya Y, Zimpfer D, Krabatsch T, Garbade J, Rao V, Morshuis M, Marasco S, Beyersdorf F, Damme L, Schmitto JD. Fully magnetically levitated left ventricular assist system for treating advanced HF. J Am Coll Cardiol. 2015; 66: 2579–2589. [DOI] [PubMed] [Google Scholar]
- 10. Mehra MR, Uriel N, Naka Y, Cleveland JC, Yuzefpolskaya M, Salerno CT, Walsh MN, Milano CA, Patel CB, Hutchins SW, Ransom J. A fully magnetically levitated left ventricular assist device—final report. N Engl J Med. 2019; 380: 1618–1627. [DOI] [PubMed] [Google Scholar]
- 11. Molina EJ, Shah P, Kiernan MS, Cornwell WK, Copeland H, Takeda K, Fernandez FG, Badhwar V, Habib RH, Jacobs JP, Koehl D. The society of thoracic surgeons intermacs 2020 annual report. Ann Thorac Surg. 2021; 111: 778–792. [DOI] [PubMed] [Google Scholar]
- 12. Anselmi A, Galand V, Vincentelli A, Boule S, Dambrin C, Delmas C, Barandon L, Pernot M, Kindo M, Tam HM, Gaudard P, Rouviere P, Senage T, Michel M, Boignard A, Chavanon O, Verdonk C, Para M, Gariboldi V, Pelce E, Pozzi M, Obadia JF, Anselme F, Litzler PY, Babatasi G, Belin A, Garnier F, Bielefeld M, Guihaire J, Kloeckner M, Radu C, Lellouche N, Bourguignon T, Genet T, D'Ostrevy N, Duband B, Jouan J, Bories MC, Vanhuyse F, Blangy H, Colas F, Verhoye JP, Martins R, Flecher E. Current results of left ventricular assist device therapy in France: the ASSIST‐ICD registry. Eur J Cardiothorac Surg. 2020; 58: 112–120. [DOI] [PubMed] [Google Scholar]
- 13. Boyle AJ, Jorde UP, Sun B, Park SJ, Milano CA, Frazier OH, Sundareswaran KS, Farrar DJ, Russell SD, HeartMate II Clinical Investigators . Pre‐operative risk factors of bleeding and stroke during left ventricular assist device support. J Am Coll Cardiol. 2014; 63: 880–888. [DOI] [PubMed] [Google Scholar]
- 14. Malik S, Malik SA, Ulmer LL, Jha LK, Strupp MS, Raichlin E, Lyden ER, Hewlett AT. Gastrointestinal bleeding with left ventricular assist devices (LVAD): locating the leak and identifying outcomes. J Clin Gastroenterol. 2019; 53: e202–e207. [DOI] [PubMed] [Google Scholar]
- 15. Wilson TJ, Stetler WR, Al‐Holou WN, Sullivan SE, Fletcher JJ. Management of intracranial hemorrhage in patients with left ventricular assist devices: clinical article. JNS. 2013; 118: 1063–1068. [DOI] [PubMed] [Google Scholar]
- 16. Ramey WL, Basken RL, Walter CM, Khalpey Z, Lemole GM, Dumont TM. Intracranial hemorrhage in patients with durable mechanical circulatory support devices: institutional review and proposed treatment algorithm. World Neurosurg. 2017; 108: 826–835. [DOI] [PubMed] [Google Scholar]
- 17. Thohan V, Shi Y, Rappelt M, Yousefzai R, Sulemanjee NZ, Hastings TE, Cheema OM, Downey F, Crouch JD. The association between novel clinical factors and gastrointestinal bleeding among patients supported with continuous‐flow left ventricular assist device therapy. J Card Surg. 2019; 34: 453–462. [DOI] [PubMed] [Google Scholar]
- 18. Joy PS, Kumar G, Guddati AK, Bhama JK, Cadaret LM. Risk factors and outcomes of gastrointestinal bleeding in left ventricular assist device recipients. Am J Cardiol. 2016; 117: 240–244. [DOI] [PubMed] [Google Scholar]
- 19. Morgan JA, Paone G, Nemeh HW, Henry SE, Patel R, Vavra J, Williams CT, Lanfear DE, Tita C, Brewer RJ. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012; 31: 715–718. [DOI] [PubMed] [Google Scholar]
- 20. Axelrad JE, Pinsino A, Trinh PN, Thanataveerat A, Brooks C, Demmer RT, Effner L, Parkis G, Cagliostro B, Han J, Garan AR, Topkara V, Takeda K, Takayama H, Naka Y, Ramirez I, Garcia‐Carrasquillo R, Colombo PC, Gonda T, Yuzefpolskaya M. Limited usefulness of endoscopic evaluation in patients with continuous‐flow left ventricular assist devices and gastrointestinal bleeding. J Heart Lung Transplant. 2018; 37: 723–732. [DOI] [PubMed] [Google Scholar]
- 21. Axelrad JE, Faye AS, Pinsino A, Thanataveerat A, Cagliostro B, Pineda MFT, Ross K, te‐Frey RT, Effner L, Garan AR, Topkara VK, Takayama H, Takeda K, Naka Y, Ramirez I, Garcia‐Carrasquillo R, Colombo PC, Gonda T, Yuzefpolskaya M. Endoscopic algorithm for management of gastrointestinal bleeding in patients with continuous flow LVADs: a prospective validation study. J Card Fail. 2020; 26: 324–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Shah KB, Gunda S, Emani S, Kanwar MK, Uriel N, Colombo PC, Uber PA, Sears ML, Chuang J, Farrar DJ, Brophy DF, Smallfield GB. Multicenter evaluation of octreotide as secondary prophylaxis in patients with left ventricular assist devices and gastrointestinal bleeding. Circ Heart Fail. 2017; 10: e004500. [DOI] [PubMed] [Google Scholar]
- 23. Juricek C, Imamura T, Nguyen A, Chung B, Rodgers D, Sarswat N, Kim G, Raikhelkar J, Ota T, Song T, Burkhoff D, Sayer G, Jeevanandam V, Uriel N. Long‐acting octreotide reduces the recurrence of gastrointestinal bleeding in patients with a continuous‐flow left ventricular assist device. J Card Fail. 2018; 24: 249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dang G, Grayburn R, Lamb G, Umpierrez De Reguero A, Gaglianello N. Octreotide for the management of gastrointestinal bleeding in a patient with a HeartWare left ventricular assist device. Case Rep Cardiol. 2014; 2014: 1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schettle S, Bawardy BA, Asleh R, Sherazi S, Rajan E, Stulak J, Pereira N. Danazol treatment of gastrointestinal bleeding in left ventricular assist device‐supported patients. J Heart Lung Transplant. 2018; 37: 1035–1037. [DOI] [PubMed] [Google Scholar]
- 26. Seng BJJ, Teo LLY, Chan LL, Sim DKL, Kerk KL, Soon JL, Tan TE, Sivathasan C, Lim CP. Novel use of low‐dose thalidomide in refractory gastrointestinal bleeding in left ventricular assist device patients. Int J Artif Organs. 2017; 40: 636–640. [DOI] [PubMed] [Google Scholar]
- 27. Chan LL, Lim CP, Lim CH, Tan TE, Sim D, Sivathasan C. Novel use of thalidomide in recurrent gastrointestinal tract bleeding in patients with left ventricular assist devices: a case series. Heart, Lung and Circulation. 2017; 26: 1101–1104. [DOI] [PubMed] [Google Scholar]
- 28. Namdaran P, Zikos TA, Pan JY, Banerjee D. Thalidomide use reduces risk of refractory gastrointestinal bleeding in patients with continuous flow left ventricular assist devices. ASAIO J. 2020; 66: 645–651. [DOI] [PubMed] [Google Scholar]
- 29. Asleh R, Albitar HAH, Schettle SD, Kushwaha SS, Pereira NL, Behfar A, Stulak JM, Rodeheffer RJ, Iyer VN. Intravenous bevacizumab as a novel treatment for refractory left ventricular assist device‐related gastrointestinal bleeding. J Heart Lung Transplant. 2020; 39: 492–495. [DOI] [PubMed] [Google Scholar]
- 30. Kittipibul V, Vutthikraivit W, Kewcharoen J, Rattanawong P, Tantrachoti P, Putthapiban P, Nair N. Angiotensin II antagonists and gastrointestinal bleeding in left ventricular assist devices: a systematic review and meta‐analysis. Int J Artif Organs. 2021; 44: 215–220. [DOI] [PubMed] [Google Scholar]
- 31. Converse MP, Sobhanian M, Taber DJ, Houston BA, Meadows HB, Uber WE. Effect of angiotensin II inhibitors on gastrointestinal bleeding in patients with left ventricular assist devices. J Am Coll Cardiol. 2019; 73: 1769–1778. [DOI] [PubMed] [Google Scholar]
- 32. Imamura T, Nguyen A, Rodgers D, Kim G, Raikhelkar J, Sarswat N, Kalantari S, Smith B, Chung B, Narang N, Juricek C, Burkhoff D, Song T, Ota T, Jeevanandam V, Sayer G, Uriel N. Omega‐3 therapy is associated with reduced gastrointestinal bleeding in patients with continuous‐flow left ventricular assist device. Circ Heart Failure. 2018; 11: e000030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Goldstein DJ, Naka Y, Horstmanshof D, Ravichandran AK, Schroder J, Ransom J, Itoh A, Uriel N, Cleveland JC Jr, Raval NY, Cogswell R, Suarez EE, Lowes BD, Kim G, Bonde P, Sheikh FH, Sood P, Farrar DJ, Mehra MR. Association of clinical outcomes with left ventricular assist device use by bridge to transplant or destination therapy intent: the multicenter study of MagLev Technology in Patients Undergoing Mechanical Circulatory Support Therapy With HeartMate 3 (MOMENTUM 3) randomized clinical trial. JAMA Cardiol. 2020; 5: 411–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Details of the procedures performed during GI bleeding
Legend: EGD: Esophagogastroduodenoscopy; VCE: video capsule endoscopy; RBC: red blood cells.
Table S2. Subgroup analysis according to the type of LVAD (Heartmate 2 vs Heartmate 3)
Legend: HR: hazard ratio; CI: confidence interval. Subgroup analysis comparing mortality and bleeding occurrence between HeartMate 2 and HeartMate 3. HeartMate 2 is the reference.


