Abstract
Although cardiac troponin is a highly specific biomarker for myocardial cell injury, it is important to recognize the pitfalls of this test in the diagnosis and management of immune checkpoint inhibitor (ICI) myocarditis. We describe the challenging case of an 81‐year‐old woman with persistently high troponin after undergoing immunotherapy with ipilimumab and nivolumab, and histological evidence of amyloid deposition in the myocardium. The patient received immunosuppressive treatments based on the magnitude of troponin changes because myocarditis was clinically suspected. However, histological examination revealed the deposition of transthyretin amyloid fibrils with only minimal T‐lymphocyte infiltration and no myocyte necrosis, suggesting transthyretin cardiac amyloidosis rather than ICI myocarditis. This case highlights the importance of assessing other causes of persistently high troponin, and the necessity of incorporating comprehensive histological and immunohistochemical examinations of the endomyocardial biopsy, especially when cardiovascular magnetic resonance imaging is inconclusive.
Keywords: Amyloid, Cardiovascular magnetic resonance imaging, Endomyocardial biopsy, Immune checkpoint inhibitor, Immunosuppressive therapy, Myocarditis, Pathophysiology
Introduction
Immune checkpoint inhibitor (ICI) myocarditis is a rare but serious side effect with an estimated incidence of 0.06% to over 1%. Commonly used diagnostic testing for ICI myocarditis includes ECGs, echocardiography, biomarkers, and cardiovascular magnetic resonance. However, ICI myocarditis has a variable clinical presentation and, until recently, had no widely accepted definition. Its diagnosis relies on clinical suspicion, and if there is a high level of suspicion for myocarditis, treatment will then be considered.
Case Report
An 81‐year‐old woman with a history of advanced melanoma, hypertension, and dyslipidaemia presented with high‐grade fever, whole‐body rash, and altered consciousness. Following primary tumour resection and lymph node dissection, recurrent, distant metastases occurred after nine cycles of pembrolizumab. Subsequently, she received her first round of combination immunotherapy with ipilimumab and nivolumab 11 days prior to her current presentation (Figure 1 ). Her blood pressure was 74/46 mmHg; heart rate, 106 b.p.m.; respiratory rate, 25 breaths/min; oxygen saturation, 92% at 3 L/min via nasal cannula; temperature, 38.5°C. Her initial laboratory test results were as follows: leukocyte count, 17 960/L (normal range; 3300–8600/L), creatine kinase (CK), 407 U/L (normal range; 59–248 U/L), CK‐MB, 6.2 U/L (normal range; <12 U/L), troponin I, 55.0 pg/mL (normal range; <34.2 pg/mL), and C‐reactive protein, 26.2 mg/dL (normal range; <0.14 mg/dL). There were no new electrocardiographic or echocardiographic abnormalities, leading to the preliminary diagnosis of serum sickness/systemic inflammatory response syndrome. The patient was provided best supportive care and a 1.5 mg/kg equivalent dose of prednisolone. One week later, electrocardiography (ECG) demonstrated diffuse T‐wave inversions in the precordial leads and the cardiac troponin level increased to 1053 pg/mL.
Figure 1.
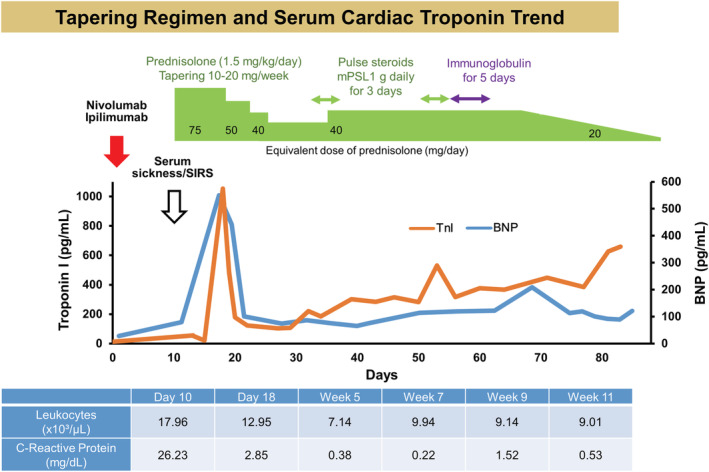
Tapering regimen and serum cardiac troponin. Timeline of the cardiac troponin and BNP levels, and treatment approach. BNP, brain natriuretic peptide.
Cine cardiovascular magnetic resonance (CMR) demonstrated normal left ventricular (LV) ejection fraction of 59%, normal LV mass index of 46.1 g/m2, and pericardial effusion. There was patchy mid‐wall late gadolinium enhancement (LGE) on LGE‐CMR without increased myocardial native T1, T2, and extracellular volume fraction (ECV) on multi‐parametric mapping or high signal intensity on T2‐weighted short‐TI inversion recovery (STIR), suggesting the low likelihood of ICI myocarditis and acute myocardial infarction (Figure 2 ; also see Supporting Information, Movie S1 ). The troponin level rapidly decreased from 1053 to 104 pg/mL by 10 days despite the subsequent prednisolone taper. Cardiac troponin levels also gradually increased after tapering. As ICI myocarditis was unable to be excluded, the patient was re‐treated with methylprednisolone at 1 g daily for 3 days, followed by prednisolone at 1 mg/kg/day. Two weeks later, secondary methylprednisolone pulse therapy and a 5 day course of intravenous immunoglobulin were initiated. In order to determine the aetiology and guide therapy, endomyocardial biopsy samples were obtained from the mid interventricular septum. There were small amounts of amyloid deposits on both direct fast scarlet and TTR staining, indicating ATTR cardiac amyloidosis. Immunohistochemistry depicted only minimal T‐lymphocyte infiltration with myocyte degeneration but not necrosis (Figure 3 ). Furthermore, PD‐L1 staining was negative. Considering the long‐term risks of steroids, the relatively low degree of persistently high cardiac troponin and, most importantly, the patient's overall clinical stability, steroids were gradually tapered and eventually discontinued. At the 2 month follow‐up, neither increased T1, T2 values nor significant differences in LV function, volumes, or mass were noted on CMR (Supporting Information, Movie S2 ). There was no further evolution of diffuse T‐wave inversions on follow‐up ECG. No cardioprotective drugs were administered during the course of hospitalization because of low blood pressure. Five months later, the patient had no clinical events indicative of heart failure. There was no evidence of recurrent melanoma.
Figure 2.
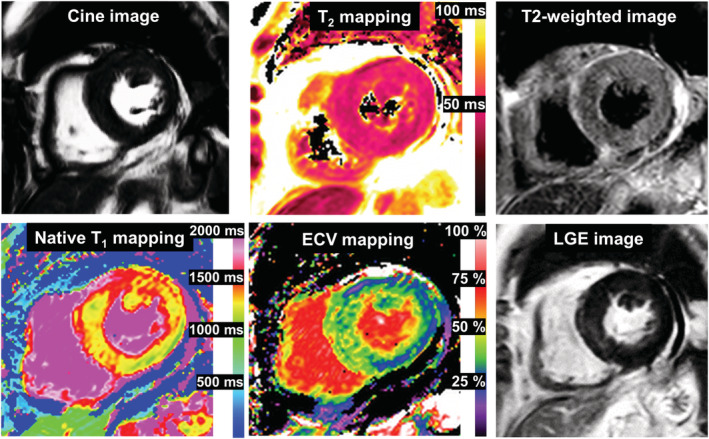
Baseline cardiovascular magnetic resonance. There was patchy mid‐wall LGE and mildly increased ECV of 36% in the basal septum; no increased global myocardial native T1, T2 values (1334 and 51 ms, respectively) or signal intensities on T2‐weighted images were observed. These findings were inconsistent with the 2018 Lake Louise criteria for a diagnosis of acute myocarditis. (Normal values at our institution: native T1:1314 ± 29 ms, T2:46 ± 5 ms, ECV: 26 ± 5%). ECV, extracellular volume fraction; LGE, late gadolinium enhancement.
Figure 3.
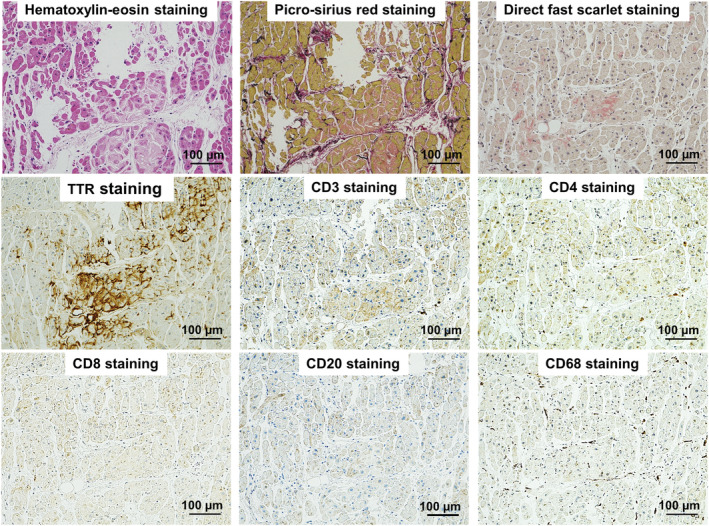
Pathological findings. Haematoxylin–eosin and picrosirius red‐stained samples showed mild extracellular expansion and minimal lymphocytic infiltrates with mild infiltrative interstitial fibrosis. Anti‐transthyretin immunohistochemistry was positive for transthyretin. There was only a minimal increase in CD3+ T‐lymphocytes, with a predominance of CD4+ T‐lymphocytes, and myocardial CD68+ macrophages, which did not fulfil the pathological diagnostic criteria of myocarditis.
Discussion
Immune checkpoint inhibitor myocarditis is a severe complication of CTLA‐4, PD‐1, and PD‐L1 antibodies used for the treatment of multiple cancer types. Its diagnosis relies on clinical suspicion, and if there is a high level of suspicion for myocarditis, treatment will then be considered. The treatment of ICI myocarditis has largely been based on the use of glucocorticoids. 1 Some centres stratify steroid management based on the magnitude of troponin changes. However, caution is required when relying on this biomarker to guide management. Although troponin biomarkers are highly specific for myocardial cell injury, there are no specific laboratory tests for myocarditis per se. Many clinical factors are involved in increased troponin, and other common causes should be considered. ECG is a widely available and inexpensive test that should be performed immediately upon presentation. In this clinical scenario, the patient did not exhibit further evolution of electrocardiographic changes indicative of myocarditis or myocardial ischaemia despite persistently high cardiac troponin. These clues further underscore the clinical importance of serial ECG for the differential diagnosis of high troponin. CMR is increasingly employed to address high troponin levels due to its ability to characterize tissue noninvasively. Although CMR is not the primary imaging modality for evaluating pericardial effusion, it provides information on the location and exact volume of fluid. Phase‐sensitive inversion‐recovery LGE imaging is able to detect even minute amounts of fluid not visible by echocardiography. 2 Importantly, depending on the LGE pattern and distribution, LGE CMR can differentiate ischaemic heart disease from nonischaemic injury. 3 In this patient, endomyocardial biopsy revealed small amounts of cardiac amyloid. Thus, this small amount of cardiac amyloid was likely beyond the spatial resolution of CMR, which is typically around 1.2 × 1.8 mm. This case study demonstrated the importance of assessing other causes of persistently high troponin and the necessity of incorporating comprehensive histological and immunohistochemical examinations of the endomyocardial biopsy if CMR is inconclusive.
Furthermore, this case highlights the diagnostic and therapeutic challenges associated with ICI myocarditis, especially when accompanied by amyloid deposits in the myocardium. Currently, it is estimated that 25% of all individuals older than 80 years have cardiac TTR amyloidosis. 4 Of note, in a recent study by Siegismund et al., 48% of patients with cardiac amyloidosis exhibited lymphocytic myocardial inflammation regardless of the extent of amyloid deposition. 5 However, we have a limited understanding of the clinical ramifications of myocardium amyloid deposits which may occur in response to ICI therapy. The misfolded forms of TTR are directly toxic to the neighbouring cardiomyocytes; thus, these cells may be more susceptible to the development of an inflammatory process, such as myocarditis, when subjected to an unrestrained immune response. Moreover, the inflammation may represent a predictor/marker of active cardiac amyloidosis. These novel findings raise important questions about the clinical management of patients receiving ICI therapy. If inflammation is induced by an unrestrained immune response, immune modulatory treatments may be effective. Conversely, if inflammation is a predictor/marker of the disease progression of cardiac amyloid, immune‐modulating strategies are unlikely to work. Indeed, persistently high cardiac troponin refractory to high‐dose glucocorticoids may suggest active cardiac amyloid, as in our patient. This was also confirmed by the stable biventricular systolic function and lack of increase in myocardial native T1 on follow‐up CMR. As cardiac amyloidosis is associated with an unfavourable prognosis, 6 the addition of pharmacological treatments for ATTR cardiac amyloidosis should be tailored. Further studies are warranted to determine whether treatment for amyloidosis and immunosuppression should be co‐administered as a clinical mechanism to improve cardiac outcomes in cancer survivors.
Conflict of interest
None declared.
Funding
This study was supported by JSPS KAKENHI (19K08579).
Supporting information
Movie S1. Baseline Short‐axis Cine CMR.
Movie S2. Follow‐up Short‐axis Cine CMR.
Acknowledgement
We thank Susan M. Miller, MD, MPH, at Houston Methodist Hospital for reviewing the manuscript and her editorial assistance.
Ida, M. , Nakamori, S. , Yamamoto, S. , Watanabe, S. , Imanaka‐Yoshida, K. , Ishida, M. , Sakuma, H. , Yamanaka, K. , and Dohi, K. (2022) Subtle‐but‐smouldering myocardial injury after immune checkpoint inhibitor treatment accompanied by amyloid deposits. ESC Heart Failure, 9: 2027–2031. 10.1002/ehf2.13915.
References
- 1. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, Sullivan RJ, Damrongwatanasuk R, Chen CL, Gupta D, Kirchberger MC, Awadalla M, Hassan MZO, Moslehi JJ, Shah SP, Ganatra S, Thavendiranathan P, Lawrence DP, Groarke JD, Neilan TG. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol. 2018; 71: 1755–1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bogaert J, Francone M. Cardiovascular magnetic resonance in pericardial diseases. J Cardiovasc Magn Reson. 2009; 11: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McCrohon JA, Moon JC, Prasad SK, McKenna WJ, Lorenz CH, Coats AJ, Pennell DJ. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium‐enhanced cardiovascular magnetic resonance. Circulation. 2003; 108: 54–59. [DOI] [PubMed] [Google Scholar]
- 4. Ruberg FL, Berk JL. Transthyretin (TTR) cardiac amyloidosis. Circulation. 2012; 126: 1286–1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Siegismund CS, Escher F, Lassner D, Kühl U, Gross U, Fruhwald F, Wenzel P, Münzel T, Frey N, Linke RP, Schultheiss HP. Intramyocardial inflammation predicts adverse outcome in patients with cardiac AL amyloidosis. Eur J Heart Fail. 2018; 20: 751–757. [DOI] [PubMed] [Google Scholar]
- 6. Grogan M, Scott CG, Kyle RA, Zeldenrust SR, Gertz MA, Lin G, Klarich KW, Miller WL, Maleszewski JJ, Dispenzieri A. Natural history of wild‐type transthyretin cardiac amyloidosis and risk stratification using a novel staging system. J Am Coll Cardiol. 2016; 68: 1014–1020. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Movie S1. Baseline Short‐axis Cine CMR.
Movie S2. Follow‐up Short‐axis Cine CMR.


