Abstract
Aims
The purpose of this study was to compare the impact of catheter ablation on cardiac structural reverse remodelling and atrial (AFMR) and ventricular (VFMR) functional mitral regurgitation (MR), and the long‐term prognosis of patients with AFMR and VFMR.
Methods and results
The retrospective study included persistent AF patients who had AFMR (n = 136, left atrial (LA) volume index >30 mL/m2 and left ventricular (LV) ejection fraction ≥40%) or VFMR (n = 31, LV ejection fraction <40% or LV regional asynergy) and had undergone the initial AF ablation from April 2015 to December 2019. Baseline and 6 month follow‐up echocardiography were performed to assess MR, LA, and LV sizes. MR improvement after ablation was comparable in the AFMR (64%) and VFMR groups (52%, P = 0.20). Patients with AFMR improvement showed a greater decrease in left atrial volume after ablation than those without (amount of change: −11.4 ± 15.1 vs. −2.3 ± 21.1 mL/m2, P = 0.01). Patients with VFMR improvement showed a greater increase in LV ejection fraction than those without (amount of change: 28.5 ± 13.6% vs. 9.0 ± 14.8%, P = 0.001). The composite endpoint of all‐cause death and heart failure hospitalization during the 2 year follow‐up period was more frequently observed in the VFMR than in the AFMR group (22.6% vs. 3.7%, P < 0.0001). Patients with MR improvement after catheter ablation less frequently demonstrated the composite endpoint than those without (1.9% vs. 15.6%, P < 0.0001).
Conclusions
Atrial functional mitral regurgitation and VFMR improvement after ablation were associated with atrial and ventricular reverse remodelling, respectively. It is possible that long‐term prognosis is better in patients with AFMR than with VFMR, and in those with MR improvement than in those without.
Keywords: Functional mitral regurgitation, Atrial fibrillation, Ablation, reverse remodelling
Introduction
Atrial fibrillation (AF) and heart failure represent a vicious twin. Heart failure can develop as a result of AF persistence. In contrast, AF often follows heart failure as a result of multiple factors that cause myocardial remodelling, including increased wall stress and neurohormonal abnormity. 1 , 2 Functional mitral regurgitation (MR) is known to exacerbate this vicious relationship. AF patients with MR are hospitalized for heart failure more frequently than those without. 3 , 4 Traditional functional MR ‐ which has recently been termed ‘ventricular functional MR (VFMR)’—is associated with mitral leaflet tethering and/or decreased closing forces in patients with a dilated left ventricle. Recently, atrial functional MR (AFMR), which is caused by a lack of leaflet coaptation due to left atrial (LA) and mitral annular dilatation, has been recognized as an important cause of heart failure following AF. 3 , 5
Catheter ablation is widely performed as a therapeutic option for AF. Several observational studies have reported AFMR improvement in line with atrial reverse remodelling after maintaining sinus rhythm after catheter ablation for AF. 5 , 6 These studies included patients with relatively preserved ventricular function, and clearly illustrated the impact of catheter ablation on AFMR. In the clinical setting, functional MR in AF patients consists of both AFMR and VFMR, because myocardial remodelling develops not only at the atrium but also at the ventricle. However, little is known about the influence of catheter ablation on these different types of functional MR.
Here, we compared the impact of catheter ablation on cardiac structural reverse remodelling and MR severity, as well as long‐term prognosis in patients with AFMR and VFMR.
Methods
Study design
This single centre retrospective study included patients with symptomatic persistent AF who had a significant AFMR or VFMR graded as mild or more severe and who underwent index AF ablation at Kansai Rosai Hospital from April 2015 to December 2019. AFMR was defined as functional MR with LA enlargement (LA volume index >30 mL/m2 which is the upper limit of the 95% normal range of healthy Japanese subjects) and preserved left ventricular (LV) contraction (LV ejection fraction ≥40%) without LV regional asynergy as reported in prior studies. 4 , 7 VFMR consisted of functional MR with impaired LV contraction, defined as LV ejection fraction <40% or LV regional asynergy. Patients with any evidence of primary leaflet involvement, such as from prior endocarditis, rheumatic valve disease, congenital anomaly, or significant mitral annular calcification, were excluded. Other exclusion criteria were as follows: sinus rhythm at the time of baseline echocardiography; MR of an unknown cause; age <20 years; prior cardiac surgery; prior catheter ablation in the left atrium; and lack of appropriate echocardiography, including lost follow‐up echocardiography and poor images of obtained echocardiography.
This study complied with the Declaration of Helsinki. Written informed consent for ablation and participation in the study was obtained from all patients. The protocol was approved by our institutional review board.
Echocardiography
Transthoracic echocardiography was performed before and 6 months after AF ablation using an Epic7Q (Phillips, Andover, MA, USA), Vivid E9 (GE Healthcare, Boston, MA, USA), Artida or Aplio XL (Canon Medical Systems, Ohtawara, Tochigi, Japan). All measurements in AF were conducted in a single beat after serial beats with average R‐R interval. Echocardiograms were then analysed offline using digital analysis software (KinetDx, Siemens, Mountain View, CA, USA) by a single research echocardiographer who was blinded to patient outcomes. LA diameter at end systole, and LV systolic and diastolic diameters were measured in the parasternal long‐axis view. LA volume at end systole was measured in the apical 2‐chamber and 4‐chamber views using biplane disk summation method. Anterior–posterior and medial–lateral mitral annular dimensions were measured in apical long‐axis and apical 4‐chamber views. MR colour jet area was measured in the apical 4‐chamber, apical 2‐chamber, and apical long‐axis views. Colour Doppler scale, and therefore Nyquist limit, were determined by the clinical ultrasonographer and in general were set to 50 to 70 cm/s. The ratio of MR colour jet area to LA area was then calculated using the largest measured values among three views. The severity of MR was classified into none, trivial, mild, moderate, and severe, using multiple parameters including the area of the regurgitant jet and vena contracta width. 8 Leaflet motion was characterized as normal, excessive, or restrictive. MR improvement was defined as a decrease in MR degree at 6 months' follow‐up echocardiography by 1 or more of that at baseline. The echocardiographic data measurement was performed retrospectively by a research echocardiographer who has 20 years of experience as a clinical sonographer. In addition, she has been involved in the field of clinical research for 12 years. She is certified as a cardiac sonographer specialist by the Japan Society of Ultrasonics in Medicine.
Catheter ablation
Electrophysiological studies and catheter ablation were performed under intravenous sedation with dexmedetomidine. A 6‐Fr decapolar electrode was inserted into the coronary sinus, and a second 6‐Fr decapolar electrode was placed in the right atrium. Following transseptal puncture at the fossa ovalis, two long sheaths were introduced into the left atrium using a single transseptal puncture technique. A 20‐pole circular catheter was placed at one of four pulmonary veins via a long sheath. Mapping and ablation were then performed under guidance with an electroanatomical mapping system (Carto3; Biosense Webster, Diamond Bar, CA, USA). Circumferential ablation around both ipsilateral pulmonary veins was performed using a point‐by‐point technique.
An open‐irrigated ablation catheter with a 3.5‐mm tip (Thermocool SmartTouch®; Biosense Webster) via an Agilis® or SL0® sheath (St. Jude Medical, St. Paul, MN, USA) was used. Radiofrequency energy was applied for 30 s at each site using a maximum temperature of 42°C, maximum power of 35 W, and flow rate of 17 mL/min. At the posterior wall near the oesophagus, the duration of radiofrequency energy delivery was limited to 15 s. Operators attempted to maintain contact force values between 5 and 20 g to ensure an appropriate degree of contact between the catheter and myocardium. Pulmonary vein isolation was considered complete when the circular catheters no longer recorded any pulmonary vein potentials. Electrical cardioversion was performed in cases where AF persisted at the end of the pulmonary vein isolation procedure.
Following pulmonary vein isolation, LA voltage mapping during 100 b.p.m. paced rhythm at the right atrium was performed using a 20‐pole multielectrode mapping catheter (Pentaray®, or Lasso NAV®, Biosense Webster) as previously reported. 9 Low‐voltage areas were defined as areas with bipolar peak‐to‐peak voltage <0.50 mV covering >5 cm2 of LA surface.
Patients were followed every 4–8 weeks at the dedicated arrhythmia clinic of our institution for a minimum of 2 years. Routine ECGs were obtained at each outpatient visit, and 24 h ambulatory Holter monitoring was performed at 6 months post‐ablation. When patients experienced symptoms suggestive of an arrhythmia, a surface ECG, ambulatory ECG, and/or cardiac event recording were also obtained. Either of the following events after the initial 3 months from the ablation (blanking period) was considered to indicate AF recurrence: (i) atrial tachyarrhythmia recorded on a routine or symptom‐triggered ECG during an outpatient visit or (ii) atrial tachyarrhythmia of at least a 30 s duration on ambulatory ECG monitoring. No antiarrhythmic drugs were prescribed after the ablation procedure unless recurrent AF was observed.
Statistics
Continuous data are expressed as the mean ± standard deviation or median (interquartile range). Categorical data are presented as absolute values and percentages. Tests for significance were conducted using the unpaired t‐test or a nonparametric test (Mann–Whitney U‐test) for continuous variables and the χ 2 test or Fisher's exact test for categorical variables. Univariate and multivariate logistic regression analyses was used to determine the clinical factors that were associated with MR improvement after catheter ablation. Variables with a P value ≤0.05 in the univariate models were included in the multivariate analysis. Long‐term clinical event rates were calculated using the Kaplan–Meier method. Comparison of survival curves between the groups was performed using the two‐sided Mantel–Haenszel (log‐rank) test. All analyses were performed using commercial software (SPSS® version 22.0 software, SPSS, Inc., Chicago IL, USA).
Results
Patients
During the study period, a total of 513 persistent AF patients underwent catheter ablation at Kansai Rosai Hospital. After excluding patients with no appropriate echocardiography data (n = 61), no or trivial MR grades (n = 274), and primary or unknown‐cause MR (n = 3), 167 patients with a significant functional MR were enrolled in the study. Study patients were classified into AFMR (n = 136) and VFMR (n = 31) groups.
Comparison of baseline data between atrial and ventricular functional mitral regurgitation
Baseline data between the AFMR and VFMR groups are compared in Table 1 . History of symptomatic heart failure was more common in the VFMR group than in the AFMR group. Heart rate before ablation was significantly higher in the VFMR group than that in the AFMR group. Patients in the AFMR group tended to be older, more likely to have long‐standing persistent AF and concomitant hypertension, and less likely to have history of stroke.
Table 1.
Baseline characteristics
| AFMR (n = 136) | VFMR (n = 31) | P | |
|---|---|---|---|
| Age, years | 70.0 ± 7.9 | 66.3 ± 10.2 | 0.063 |
| Male, n (%) | 82 (60) | 23 (74) | 0.15 |
| Body mass index, kg/m2 | 23.1 ± 3.6 | 22.8 ± 2.5 | 0.57 |
| Systolic blood pressure, mmHg | 124 ± 15 | 113 ± 14 | <0.0001 |
| AF history | |||
| Duration | 11 (7, 16) | 7 (4, 12) | 0.009 |
| Long‐standing persistent AF a , n (%) | 27 (20) | 2 (7) | 0.057 |
| Coronary artery disease, n (%) | 17 (13) | 3 (10) | 0.47 |
| Hypertension, n (%) | 74 (54) | 11 (36) | 0.057 |
| Diabetes mellitus, % | 19 (14) | 6 (19) | 0.45 |
| History of symptomatic heart failure, n (%) | 45 (33) | 23 (74) | <0.0001 |
| History of stroke, n (%) | 11 (8) | 6 (19) | 0.061 |
| CHA2DS2 VASc score | 2.7 ± 1.4 | 2.8 ± 1.7 | 0.68 |
| Heart rate, beats per minute | 89 ± 22 | 105 ± 30 | 0.004 |
| Haemoglobin, g/dL | 13.9 ± 1.5 | 14.4 ± 1.7 | 0.21 |
| eGFR, mL/min/1.73 m2 | 60.0 ± 16.5 | 59.2 ± 21.3 | 0.84 |
| NT‐pro BNP, pg/mL | 895 (664, 1577) | 1509 (850, 3166) | 0.12 |
| Medications | |||
| ACE inhibitor/ARB | 53 (39) | 26 (84) | <0.0001 |
| Beta‐blocker | 50 (37) | 23 (74) | <0.0001 |
| MRA | 14 (10) | 15 (48) | <0.0001 |
| Echocardiography | |||
| LA volume, mL | 82.8 ± 28.4 | 102.4 ± 33.5 | 0.004 |
| LA volume index, mL/m2 | 50.9 ± 18.7 | 63.0 ± 23.2 | 0.010 |
| Anterior–posterior mitral annular diameter, mm | 34.8 ± 3.6 | 36.7 ± 5.5 | 0.067 |
| Medical‐lateral mitral annular diameter, mm | 36.5 ± 3.5 | 39.6 ± 4.4 | <0.0001 |
| Mitral valve leaflet motion | <0.0001 | ||
| Normal, n (%) | 132 (97) | 20 (65) | |
| Restricted, n (%) | 4 (3) | 11 (36) | |
| Mitral valve tethering height, mm | 4.1 ± 1.8 | 7.0 ± 3.2 | <0.0001 |
| Tricuspid valve regurgitation | |||
| Severity grade, n (%) | 0.20 | ||
| None or trivial | 18 (13) | 2 (7) | |
| Mild | 77 (57) | 22 (71) | |
| Moderate | 38 (28) | 5 (16) | |
| Severe | 3 (2) | 2 (7) | |
| Pressure gradient b | 25.4 ± 6.8 | 25.1 ± 6.4 | 0.83 |
| LV ejection fraction, % | 59.8 ± 8.3 | 29.2 ± 6.6 | <0.0001 |
| LV diastolic diameter, mm | 46.7 ± 6.1 | 55.8 ± 8.4 | <0.0001 |
| LV diastolic diameter index, mm/m2 | 28.6 ± 4.3 | 34.1 ± 5.6 | <0.0001 |
| LV systolic diameter, mm | 31.7 ± 5.3 | 48.8 ± 7.0 | <0.0001 |
| LV systolic diameter index, mm/m2 | 19.4 ± 3.5 | 29.8 ± 4.9 | <0.0001 |
| LV mass, g | 178.6 ± 55.2 | 241.9 ± 80.5 | <0.0001 |
| LV mass index, g/m2 | 108.4 ± 31.8 | 146.5 ± 42.7 | <0.0001 |
| E, cm/s | 0.89 ± 0.20 | 0.93 ± 0.31 | 0.52 |
| E/e′ | 11.2 ± 5.6 | 15.3 ± 8.7 | <0.002 |
ACE inhibitor, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; AFMR, atrial functional mitral regurgitation; ARB, angiotensin II receptor blocker; E, transmitral diastolic early flow peak velocity; e′, tissue Doppler diastolic early myocardial velocity from septal mitral annulus; eGFR, estimated glomerular filtration rate; LA, left atrial; LV, left ventricular; MRA, mineral corticoid receptor antagonist; NT‐pro BNP; N‐terminal pro‐brain natriuretic peptide; VFMR, ventricular functional mitral regurgitation.
Long‐standing persistent atrial fibrillation lasting >1 year.
Pressure gradient of tricuspid valve regurgitation was obtained from 113 patients in the AFMR group and 28 in the VFMR group.
Mitral regurgitation was more severe in the VFMR group than in the AFMR group in terms of severity grade, regurgitation jet area, and regurgitation jet contracta width (Figure 1CA–1 ). Patients in VFMR group demonstrated larger LA and mitral annular sizes and were more likely to have restricted mitral valve leaflet motion and a larger mitral valve tethering height than those in the AFMR group (Table 1 ). LV remodelling was more advanced in the VFMR group than in the AFMR group, as represented by lower LV ejection fraction, larger LV size, and larger LV mass. In addition, LV filling pressure surrogated by E/e′ was also higher in the VFMR group than in the AFMR group.
Figure 1.
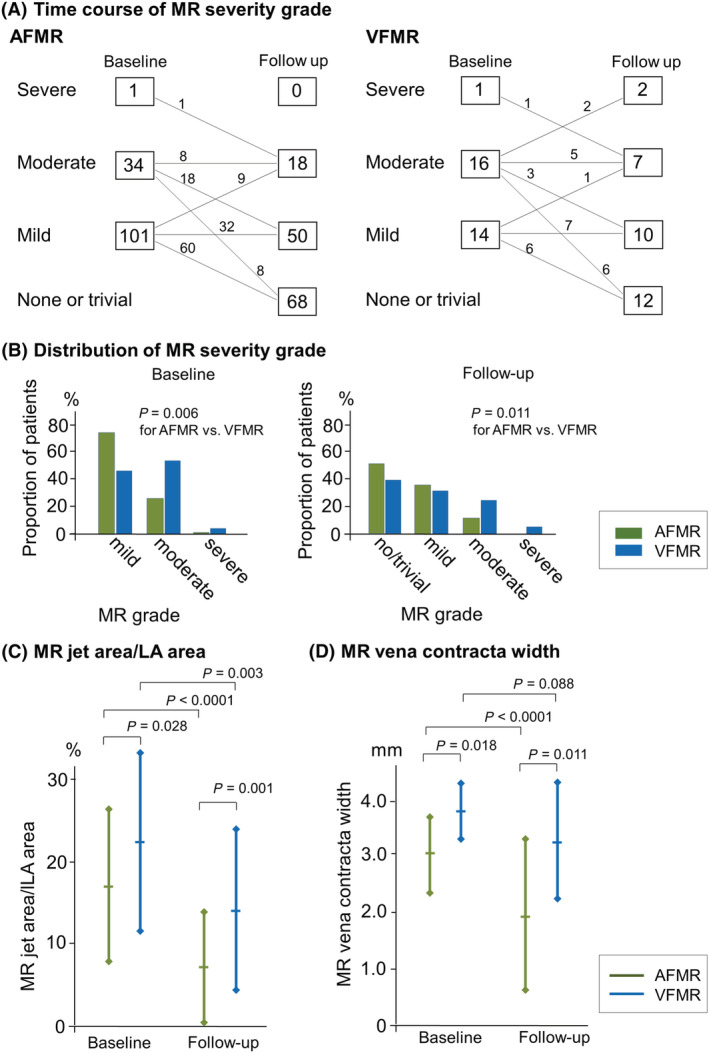
MR severity at baseline and follow‐up echocardiography. Time course of MR severity grade (A), patient distributions at each severity grade (B), and MR jet area/LA area (C), and MR vena contracta width (D). MR severity was less in AFMR than in VFMR at both baseline and 6 month follow‐up echocardiography. MR improvement was observed, irrespective of AFMR and VFMR after catheter ablation. AFMR, atrial functional mitral regurgitation; LA, left atrial; MR, mitral regurgitation; VFMR, ventricular functional mitral regurgitation.
Echocardiographic and clinical parameters associated with mitral regurgitation improvement
MR improvement was comparable in the AFMR (87 [64%]) and VFMR groups (16 [52%], P = 0.20, Figure 1 A ). MR jet area and vena contracta width also decreased or tended to decrease after ablation (Figure 1 C and 1 D ). VFMR demonstrated more severe MR than AFMR even after catheter ablation (Figure 1 B ).
Changes in echocardiographic parameters after catheter ablation are shown in Table 2 . In the AFMR group, LA volume decrease was observed only in patients with MR improvement. Decrease in mitral annular diameters was observed in with and without MR improvement, and the amount of mitral annular diameter decrease was more in patients with MR improvement than those without. A tendency toward an increase in LV ejection fraction was more common in patients with AFMR improvement than those without. Tricuspid valve regurgitation was significantly improved in patients with MR improvement compared with those without. Among patients with sinus rhythm at follow‐up echocardiography, the ratio of the early to late transmitral flow velocities (E/A) was lower in patients with MR improvement (n = 80, E/A = 1.02 ± 0.39 cm/s) than those without (n = 40, E/A = 1.50 ± 0.77 cm/s, P < 0.0001).
Table 2.
Comparison of echocardiographic parameters with and without MR improvement
| AFMR improvement | VFMR improvement | |||||
|---|---|---|---|---|---|---|
| With (n = 87) | Without (n = 49) | P | With (n = 16) | Without (n = 15) | P | |
| LA volume index | ||||||
| Baseline, mL/m2 | 48.8 ± 15.3 | 54.6 ± 23.3 | 0.081 | 56.9 ± 20.6 | 69.5 ± 24.6 | 0.14 |
| Change a , mm/m2 | −11.4 ± 15.1 b | −2.3 ± 21.1 | 0.010 | −12.3 ± 16.7 b | −17.5 ± 16.9 b | 0.39 |
| Change ratio, % | −17.8 ± 35.3 | 1.9 ± 46.2 | 0.012 | −19.1 ± 26.4 | −24.4 ± 26.4 | 0.58 |
| Anterior–posterior mitral annular diameter | ||||||
| Baseline, mm | 34.7 ± 3.7 | 34.9 ± 3.7 | 0.81 | 35.4 ± 4.0 | 38.2 ± 6.5 | 0.17 |
| Change, mm | −3.0 ± 3.4 b | −1.7 ± 3.6 b | 0.042 | −2.4 ± 1.9 b | −2.8 ± 5.2 | 0.79 |
| Change ratio, % | −8.3 ± 9.3 | −4.3 ± 10.0 | 0.026 | −6.7 ± 5.1 | −6.3 ± 13.4 | 0.91 |
| Medial‐lateral mitral annular diameter | ||||||
| Baseline, mm | 36.5 ± 3.5 | 36.5 ± 3.6 | 0.97 | 37.8 ± 4.1 | 41.5 ± 4.0 | 0.017 |
| Change, mm | −3.4 ± 4.1 b | −1.2 ± 3.1 b | 0.001 | −3.5 ± 3.4 b | −3.8 ± 3.5 | 0.84 |
| Change ratio, % | −9.8 ± 12.5 | −3.0 ± 8.6 | 0.001 | −8.9 ± 7.7 | −8.7 ± 7.8 | 0.95 |
| Mitral valve tethering height | ||||||
| Baseline, mm | 4.2 ± 1.8 | 4.2 ± 1.6 | 0.79 | 5.9 ± 2.6 | 8.3 ± 3.3 | 0.032 |
| Change, mm | −0.6 ± 1.5 b | −0.6 ± 1.4 b | 0.96 | −2.1 ± 2.4 b | −0.9 ± 1.3 | 0.095 |
| Change ratio, % | −4.4 ± 31.0 | −7.0 ± 32.7 | 0.66 | −28.3 ± 30.0 | −10.4 ± 17.8 | 0.053 |
| Mitral valve regurgitation | ||||||
| Baseline severity grade, n (%) | 0.15 | 0.47 | ||||
| Mild | 60 (69) | 41 (84) | 6 (38) | 8 (53) | ||
| Moderate | 26 (30) | 8 (16) | 9 (56) | 7 (47) | ||
| Severe | 1 (1) | 0 (0) | 1 (6) | 0 (0) | ||
| Change in grades, grade | −1.1 ± 0.3 b | 0.2 ± 0.4 | 0.001 | −1.4 ± 0.5 b | 0.2 ± 0.4 | 0.001 |
| Tricuspid valve regurgitation | ||||||
| Baseline severity grade, n (%) | 0.84 | 0.29 | ||||
| None or trivial | 12 (14) | 6 (12) | 0 (0) | 2 (13) | ||
| Mild | 47 (54) | 30 (61) | 11 (69) | 11 (73) | ||
| Moderate | 26 (30) | 12 (25) | 4 (25) | 1 (7) | ||
| Severe | 2 (3) | 1 (2) | 1 (6) | 1 (7) | ||
| Change in grades, grade | −0.6 ± 0.7 b | −0.2 ± 0.7 | 0.006 | −0.9 ± 0.8 b | −0.2 ± 0.9 | 0.032 |
| Pressure gradient of tricuspid regurgitation | ||||||
| Baseline, mmHg | 25.5 ± 6.8 | 25.4 ± 6.8 | 0.65 | 25.1 ± 6.2 | 25.2 ± 7.0 | 0.99 |
| Change, mmHg | −2.5 ± 7.6 b | 0.5 ± 7.1 | 0.086 | −4.5 ± 4.0 b | 2.1 ± 11.9 | 0.18 |
| Change ratio, % | −5.6 ± 26.1 | 4.2 ± 26.1 | 0.12 | −16.7 ± 14.1 | 8.7 ± 40.8 | 0.14 |
| LV ejection fraction, % | ||||||
| Baseline, % | 60.0 ± 8.3 | 59.4 ± 8.5 | 0.72 | 29.9 ± 6.7 | 28.5 ± 6.7 | 0.55 |
| Change, % | 6.0 ± 9.2 b | 3.5 ± 7.2 b | 0.079 | 28.5 ± 13.6 b | 9.0 ± 14.8 b | 0.001 |
| Change ratio, % | 11.9 ± 17.9 | 6.4 ± 13.2 | 0.064 | 102.6 ± 61.1 | 38.8 ± 63.8 | 0.008 |
| LV diastolic diameter | ||||||
| Baseline, mm | 46.4 ± 6.4 | 47.2 ± 5.6 | 0.43 | 50.1 ± 4.0 | 61.3 ± 8.6 | <0.0001 |
| Change, mm | −0.4 ± 5.5 | 0.5 ± 4.5 | 0.33 | −2.4 ± 4.6 | −1.6 ± 3.9 | 0.19 |
| Change ratio, % | 0.5 ± 10.8 | 1.6 ± 9.5 | 0.39 | −4.3 ± 8.7 | −2.9 ± 7.1 | 0.16 |
| LV systolic diameter | ||||||
| Baseline, mm/m2 | 31.3 ± 5.0 | 32.4 ± 5.9 | 0.30 | 45.0 ± 4.6 | 52.8 ± 7.0 | 0.001 |
| Change, mm/m2 | −5.4 ± 13.3 b | −2.3 ± 12.3 | 0.18 | −22.2 ± 11.8 b | −9.3 ± 11.9 b | 0.005 |
| Change ratio, % | −13.4 ± 43.5 | −3.8 ± 42.2 | 0.21 | −49.6 ± 25.0 | −19.4 ± 24.9 | 0.002 |
| E/e′ | ||||||
| Baseline | 10.8 ± 4.9 | 12.0 ± 6.9 | 0.27 | 15.4 ± 7.8 | 15.2 ± 10.0 | 0.95 |
| Change | −0.8 ± 4.7 | 1.4 ± 8.2 | 0.094 | −2.0 ± 4.1 | −0.7 ± 5.1 | 0.49 |
| Change ratio, % | −1.8 ± 31.2 | 21.8 ± 69.5 | 0.036 | −6.9 ± 28.5 b | 1.4 ± 44.0 | 0.58 |
AFMR, atrial functional mitral regurgitation; E, transmitral diastolic early flow peak velocity; LA, left atrial; LV, left ventricular; VFMR, ventricular functional mitral regurgitation; e′, tissue Doppler diastolic early myocardial velocity from septal mitral annulus.
Change, difference in each parameter between that at baseline and 6 months after catheter ablation.
P < 0.05 for the difference of each parameter between baseline and 6 months after catheter ablation.
In the VFMR group, LA volume was comparably decreased in patients with and without MR improvement. There was no difference in the amount of changes in mitral annular size between patients with and without VFMR improvement. Although LV ejection fraction increased in both with and without MR improvement, patients with MR improvement demonstrated a greater increase in LV ejection fraction, a greater decrease in LV systolic diameter, and a tendency toward a reduction in mitral valve tethering height than those without MR improvement. Greater improvement in tricuspid valve regurgitation was also observed in patients with MR improvement than those without. Among patients with sinus rhythm at follow‐up echocardiography, the E/A ratio was not statistically different between patients with MR improvement (n = 16, E/A = 0.96 ± 0.31 cm/s) and without (n = 11, E/A = 1.6 ± 1.33 cm/s, P = 0.15).
Clinical and ablation data with and without MR improvement are compared in Table 3 . In the AFMR group, smaller proportions of patients with MR improvement had LA low‐voltage areas, underwent posterior mitral isthmus linear ablation, and experienced AF recurrence than those without MR improvement. On the other hand, VFMR improvement was associated with a less frequent history of heart failure. Incidence of low‐voltage areas was comparable between those with and without VFMR improvement.
Table 3.
Comparison of clinical and ablation parameters with and without MR improvement after ablation
| AFMR improvement | VFMR improvement | |||||
|---|---|---|---|---|---|---|
| With (n = 87) | Without (n = 49) | P | With (n = 16) | Without (n = 15) | P | |
| Age, years | 70.0 ± 8.4 | 70.0 ± 7.0 | 0.99 | 67.6 ± 8.7 | 64.9 ± 11.7 | 0.48 |
| Male, n (%) | 52 (60) | 30 (61) | 1.00 | 10 (63) | 13 (87) | 0.13 |
| Body mass index, kg/m2 | 23.5 ± 3.8 | 22.5 ± 3.3 | 0.12 | 22.6 ± 2.4 | 22.9 ± 2.6 | 0.75 |
| Systolic blood pressure, mmHg | 124 ± 15 | 125 ± 16 | 0.62 | 117 ± 15 | 110 ± 13 | 0.18 |
| AF history | ||||||
| Duration | 11 (7, 15) | 11 (8, 20) | 0.12 | 7 (4, 11) | 7 (4, 12) | 0.54 |
| Long‐standing persistent AF a , n (%) | 18 (21) | 9 (18) | 0.74 | 0 (0) | 2 (13) | 0.23 |
| Coronary artery disease, n (%) | 9 (10) | 8 (16) | 0.31 | 1 (6) | 2 (13) | 0.48 |
| History of symptomatic heart failure, n (%) | 26 (30) | 19 (39) | 0.29 | 9 (56) | 14 (93) | 0.037 |
| CHA2DS2 VASc score | 2.7 ± 1.4 | 2.6 ± 1.4 | 0.63 | 3.1 ± 1.6 | 2.5 ± 1.7 | 0.29 |
| Heart rate, b.p.m. | 89 ± 22 | 85 ± 22 | 0.34 | 112 ± 34 | 98 ± 23 | 0.18 |
| NT‐pro BNP, pg/mL | 895 (684, 1444) | 894 (519, 1629) | 0.21 | 1202 (757, 2743) | 2354 (1062, 3279) | 0.25 |
| Medications | ||||||
| ACE inhibitor/ARB | 35 (40) | 18 (37) | 0.72 | 13 (81) | 13 (87) | 1.00 |
| Beta‐blocker | 30 (35) | 20 (41) | 0.47 | 12 (75) | 11 (73) | 1.00 |
| MRA | 6 (7) | 8 (16) | 0.14 | 9 (56) | 6 (40) | 0.48 |
| Left atrial low‐voltage areas | ||||||
| ≥5 cm2 | 22 (26) | 22 (45) | 0.021 | 4 (25) | 2 (14) | 0.40 |
| ≥20 cm2 | 3 (4) | 11 (22) | 0.001 | 1 (6) | 2 (14) | 0.45 |
| Ablation procedure | ||||||
| Delivered radiofrequency energy, KJ | 45.9 ± 17.7 | 51.6 ± 20.6 | 0.14 | 44.7 ± 16.6 | 57.4 ± 25.7 | 0.17 |
| Left atrial roof linear ablation, % | 11 (13) | 6 (12) | 0.96 | 0 (0) | 2 (13) | 0.23 |
| Left atrial bottom linear ablation, % | 2 (2) | 2 (4) | 0.46 | 0 (0) | 1 (7) | 0.48 |
| Posterior mitral isthmus linear ablation, % | 0 (0) | 3 (6) | 0.045 | 0 (0) | 0 (0) | 1.0 |
| Left atrial septal linear ablation, % | 6 (7) | 2 (4) | 0.40 | 0 (0) | 0 (0) | 1.0 |
| Isolation of superior vena cava | 2 (2) | 0 (0) | 0.54 | 0 (0) | 1 (7) | 0.48 |
| Cavo‐tricuspid isthmus ablation, % | 18 (21) | 13 (27) | 0.44 | 3 (19) | 3 (20) | 0.64 |
| Low‐voltage area ablation, % | 13 (15) | 5 (10) | 0.43 | 2 (13) | 1 (7) | 0.53 |
| AF recurrence | ||||||
| Any recurrence, % | 24 (28) | 24 (49) | 0.012 | 2 (13) | 6 (40) | 0.090 |
| Recurrence prior to 6 m follow‐up echocardiography, % | 11 (13) | 14 (29) | 0.021 | 1 (6) | 3 (20) | 0.28 |
| AF rhythm at 6 m follow‐up echocardiography, % | 5 (6) | 8 (16) | 0.044 | 0 (0) | 2 (13) | 0.23 |
ACE inhibitor, angiotensin converting enzyme inhibitor; AF, atrial fibrillation; ARB, angiotensin II receptor blocker; MR, mitral regurgitation; MRA, mineral corticoid receptor antagonist; NT‐pro BNP; N‐terminal pro‐brain natriuretic peptide.
Δ, difference in parameter between baseline and 6 month follow‐up.
Multivariate analysis incorporating factors with a P value <0.10 in Tables 2 and 3 showed that independent predictors of MR improvement were the absence of extensive low‐voltage areas (≥20 cm2) for AFMR improvement and smaller baseline LV diastolic diameter for VFMR (Supporting Information, Table S1 ).
Impact of left atrial fibrosis on left atrial reverse remodelling and mitral regurgitation improvement after sinus rhythm maintenance
Figure 2 compares LA reverse remodelling and MR improvement after catheter ablation between cases with (n = 10) and without extensive LA low‐voltage areas ≥20 cm2 (n = 100) among AFMR group patients without AF recurrence. Patients with extensive low‐voltage areas had a greater LA volume at baseline and 6 months after sinus rhythm recovery by catheter ablation. In addition, a smaller LA volume change ratio was observed in patients with extensive low‐voltage areas than those without. Reduction of MR jet area/LA area was also smaller in patients with extensive low‐voltage areas than those without.
Figure 2.

Influence of LA extensive low‐voltage areas after sinus rhythm maintenance on AFMR and LA reverse remodelling. Patients who remained in sinus rhythm after catheter ablation in the AFMR group were classified by the presence (n = 10) or absence (n = 100) of LA extensive low‐voltage areas (≥20 cm2). MR jet area/LA area (A) and LA volume index (B) were compared. Patients with extensive low‐voltage areas had less MR improvement and LA reverse remodelling. AFMR, atrial functional mitral regurgitation; LA, left atrial; MR, mitral regurgitation.
Long‐term prognosis
During 2 year follow‐up, four patients died from a cardiovascular cause (heart failure in all four patients), namely, two patients in the VFMR group without MR improvement, one in the VFMR group with MR improvement, and one in the AFMR group without MR improvement. Non‐cardiovascular death was observed in one patient (tongue cancer in the VFMR group without MR improvement). No patient underwent cardiac surgery for MR. The composite endpoint of all‐cause death and heart failure hospitalization was more frequent in the VFMR group than in the AFMR group (22.6% vs. 3.7%, P < 0.0001, Figure 3 A ). In addition, the composite endpoint was more frequent in patients without MR improvement after catheter ablation than those with MR improvement (15.6% vs. 1.9%, P < 0.0001), and even more frequent in patients without MR improvement in both the AFMR and VFMR groups (Figure 3 B ).
Figure 3.

Comparison of long‐term outcomes. Event‐free rates of all‐cause death and heart failure hospitalization (A), heart failure requiring medical intervention (B), and AF recurrence (C) during the 2 year follow‐up period. The composite endpoint of all‐cause death and heart failure hospitalization, and heart failure requiring medical intervention were more frequent in the VFMR than in the AFMR group, and in patients without MR improvement than those with improvement. AF recurrence rates were comparable between the AFMR and VFMR groups. On the other hand, patients with AFMR and VFMR showed less frequent or the tendency toward less frequent AF recurrence. AF atrial fibrillation; AFMR, atrial functional mitral regurgitation; MR, mitral regurgitation; VFMR, ventricular functional mitral regurgitation.
Exacerbation of heart failure requiring medical intervention was also more frequent in the VFMR group than in the AFMR group (35.5% vs. 11.8%, P = 0.001; Figure 3 B ) and in patients without MR improvement than those with improvement (31.3% vs. 6.8%, P < 0.0001), irrespective of AFMR or VFMR group (Figure 3 B ).
Atrial fibrillation recurrence was comparable between the AFMR and VFMR groups. On the other hand, patients with MR improvement had or tended to have fewer AF recurrences than those without MR improvement.
Discussion
This observational study was conducted in patients with persistent AF who had significant AFMR or VFMR and who underwent the index AF ablation. MR improvement in the groups was comparable, at approximately half of the AFMR (64%) and VFMR groups (52%). MR improvement was associated with structural reverse remodelling after catheter ablation: reduction in LA volume and mitral annular sizes for AFMR improvement, and increase in LV ejection fraction for VFMR. Predictors of AFMR and VFMR improvement were the absence of extensive LA fibrosis and small LV diastolic diameter at baseline, respectively. Long‐term heart failure prognosis was better in patients with AFMR than those with VFMR, and in patients with MR improvement than those without MR improvement.
Atrial functional mitral regurgitation improvement
The AFMR group showed an enlarged left atrium, with an LA volume of 82.8 ± 28.4 mL and LA volume index of 50.9 ± 18.7 mL/m2, which are higher than those of healthy Japanese subjects: 37 ± 8 mL and 22 ± 4 mL/m2, respectively. 7 In addition, most patients in the AFMR group had normal mitral valve leaflet motion without restriction or prolapse. We therefore presume that the structural mechanism of MR in the AFMR group was a lack of leaflet coaptation due to dilatation of the left atrium and mitral annulus. This theory is supported by our finding that patients with AFMR improvement had a greater reduction in LA and mitral annular sizes than those without this improvement. Similar observations have been reported previously, including in patients who underwent catheter ablation. 5 , 6
Of note, the degree of LA volume reduction and MR improvement after sinus rhythm maintenance was significantly less in patients with extensive LA low‐voltage areas. Low‐voltage areas are thought to represent fibrosis of myocardial tissue. 10 On this basis, patients with extensive low‐voltage areas would have advanced and broad LA myocardial fibrotic degeneration which would lead to irreversible LA remodelling even after sinus rhythm maintenance by catheter ablation.
Future clinical studies investigating the impact of catheter ablation on atrial and ventricular three‐dimensional shape observed using echocardiography would improve our understanding of the mechanisms of functional MR in AF patients.
Ventricular functional mitral regurgitation improvement
Baseline echocardiography in the VFMR group demonstrated dilated LA and LV sizes and frequent restriction of mitral leaflet motion compared with the AFMR group. Functional MR in the VFMR group is therefore likely attributable to a lack of leaflet coaptation due to the combination of mitral valvular tethering and mitral annular dilation.
Patients with improved VFMR showed an increase in LV ejection fraction. In contrast, change in LA size was comparable between patients with and without VFMR improvement. These results suggest that catheter ablation improves VFMR by ventricular rather than atrial reverse remodelling. One of the main aetiologies of reduced LV ejection fraction in the VFMR group is likely tachycardia‐induced cardiomyopathy, in which ventricular reverse remodelling is common after maintenance of appropriate cardiac rhythm. 11 In contrast, cases of primary ventricular cardiomyopathy, such as dilated cardiomyopathy and ischaemic cardiomyopathy, cannot expect significant reverse remodelling after sinus rhythm maintenance, as is expected in tachycardia‐induced cardiomyopathy.
No enlargement of LV diastolic diameter at baseline was independently associated with VFMR improvement. The absence of LV enlargement suggests relatively mild LV remodelling and may indicate that LV remodelling is still in the reversible stage.
Over a half of patients with VFMR demonstrated MR improvement after ablation in this study. A high rate of VFMR improvement after ablation would suggest that majority of patients with VFMR had tachycardia‐induced cardiomyopathy with relatively mild LV remodelling which is represented by short duration of AF persistence (7 [4,12] months) and small LV diastolic diameter at baseline (55.8 ± 8.4 mm) for reduced LV ejection fraction (29.2 ± 6.6%).
Better heart failure prognosis in patients with atrial functional mitral regurgitation than those with ventricular functional mitral regurgitation
Prior studies reported better heart failure prognosis in medically‐treated patients with AFMR than those with VFMR. 4 , 12 In addition, catheter ablation possibly improved heart failure prognosis to a greater extent in patients with AFMR than those with VFMR. Patients with persistent AF likely have atrial remodelling caused by AF persistence itself, the so‐called ‘AF begets AF’ phenomenon. 13 Elimination of AF by catheter ablation might break this vicious circle, and atrial reverse remodelling would then be particularly expected in the AFMR group. On the other hand, patients with VFMR have remodelling of both the atrium and ventricle. The majority of patients have ventricular cardiomyopathy leading to both ventricular and atrial remodelling, resulting in both AF and functional MR. In such cases, ventricular remodelling would continue even after the elimination of AF by catheter ablation. Exceptionally, when ventricular remodelling is due to tachycardia‐induced cardiomyopathy, catheter ablation of AF could improve ventricular remodelling and thereby improve the prognosis. 14
Mitral regurgitation improvement and heart failure prognosis
The better clinical outcome seen in patients with MR improvement than those without may be explained by the direct benefit of MR reduction, such as improved cardiac performance and suppression of further LV remodelling. The beneficial impact of MR reduction on long‐term prognosis was supported by the results of the randomized controlled COAPT trial, which reported a better heart failure prognosis in patients undergoing percutaneous mitral valve repair than those without. 15
In addition, given that MR improvement in the present study was observed together with other structural reverse remodelling, such as LA and LV volume reduction, it is possible that MR improvement is a result of cardiac reverse remodelling after catheter ablation, and cardiac reverse remodelling itself may contribute to the improved heart failure prognosis.
Improvement of heart failure outcomes by catheter ablation reported in the CASTLE AF trial is likely explained by the multiple benefits of catheter ablation on haemodynamics in heart failure patients. 16 Beneficial effects of sinus rhythm maintenance include the recovery of atrial mechanical function, optimized frequency of ventricular contraction, and possibly also improvement in functional MR.
Catheter ablation of persistent atrial fibrillation associated with functional mitral regurgitation
Observation of this study indicated that a part of the functional MR in patients with persistent AF could be improved by catheter ablation. Improvements in the AFMR and VFMR were accompanied by atrial and ventricular reverse remodelling, respectively. The absence of extensive LA low‐voltage areas was an independent predictor of an AFMR improvement and could be estimated by the previously published clinical risk score. 17 A VFMR improvement was expected in patients with a small LV size in spite of a reduced LV ejection fraction. This information would be useful in selecting the patients in whom functional MR would improve after catheter ablation.
Limitations
Several limitations of this study warrant mention. First, this is a non‐randomized observational study. Prognosis in each group was likely influenced by multiple confounding factors. Second, patient categorization of AFMR and VFMR was based on the presence or absence of LA enlargement and LV contraction disorder, rather than the aetiology of functional MR. Therefore, patient groups and MR aetiology do not necessarily have a one‐to‐one correspondence. Third, this study was limited to patients with prespecified follow‐up data, possibly resulting in a degree of selection bias. Fourth, heart failure hospitalizations would range from treatment for life‐threatening conditions to therapeutic optimization in patients with a stable condition, and the clinical impact would be quite different. Fifth, AF recurrence after discharge was quantified on the basis of intermittent short‐time ECGs and the patients' symptom‐triggered ECGs, giving rise to the possibility that asymptomatic episodes of AF might have been missed. Sixth, this study did not collect data on atrial mechanical function such as booster pump function and reservoir function which could affect post‐procedural reverse remodelling and heart failure prognosis. Seventh, the effects of the medications on cardiac reverse remodelling during the follow‐up period were difficult to determine because the medical therapy protocol was not consistent. Eighth, the comparisons of the outcomes between the AFMR and VFMR groups were possibly influenced by selection bias arising from the different baseline MR severity grades between the groups. Finally, the study was retrospectively conducted using a small sample size at a single centre. Accordingly, the results were possibly influenced by many confounding factors, and the statistical accuracy was limited. Therefore, the study results are not conclusive. Multicentre prospective trials including a larger number of patients are warranted.
Conclusion
In patients with persistent AF who underwent index AF ablation, approximately one‐half showed an improvement in functional MR after catheter ablation, irrespective of AFMR or VFMR. AFMR was associated with atrial reverse remodelling, whereas VFMR was associated with ventricular reverse remodelling. Although better long‐term prognosis was suggested in patients with AFMR and those with MR improvement after catheter ablation, further prospective studies are needed to verify the results of this study.
Conflict of interest
None declared.
Funding
Our institute received research fund from Japan Lifeline.
Supporting information
Table S1. Predictors associated with MR improvement.
Masuda, M. , Sekiya, K. , Asai, M. , Iida, O. , Okamoto, S. , Ishihara, T. , Nanto, K. , Kanda, T. , Tsujimura, T. , Matsuda, Y. , Hata, Y. , Uematsu, H. , Toyoshima, T. , Higashino, N. , and Mano, T. (2022) Influence of catheter ablation for atrial fibrillation on atrial and ventricular functional mitral regurgitation. ESC Heart Failure, 9: 1901–1913. 10.1002/ehf2.13896.
References
- 1. Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D'Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003; 107: 2920–2925. [DOI] [PubMed] [Google Scholar]
- 2. Tsuji A, Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, Hata Y, Mano T. Impact of the temporal relationship between atrial fibrillation and heart failure on prognosis after ablation. Circ J. 2020; 84: 1467–1474. [DOI] [PubMed] [Google Scholar]
- 3. Saito C, Minami Y, Arai K, Haruki S, Yagishita Y, Jujo K, Ashihara K, Hagiwara N. Prevalence, clinical characteristics, and outcome of atrial functional mitral regurgitation in hospitalized heart failure patients with atrial fibrillation. J Cardiol. 2018; 72: 292–299. [DOI] [PubMed] [Google Scholar]
- 4. Dziadzko V, Dziadzko M, Medina‐Inojosa JR, Benfari G, Michelena HI, Crestanello JA, Maalouf J, Thapa P, Enriquez‐Sarano M. Causes and mechanisms of isolated mitral regurgitation in the community: clinical context and outcome. Eur Heart J. 2019; 40: 2194–2202. [DOI] [PubMed] [Google Scholar]
- 5. Gertz ZM, Raina A, Saghy L, Zado ES, Callans DJ, Marchlinski FE, Keane MG, Silvestry FE. Evidence of atrial functional mitral regurgitation due to atrial fibrillation: reversal with arrhythmia control. J Am Coll Cardiol. 2011; 58: 1474–1481. [DOI] [PubMed] [Google Scholar]
- 6. Nishino S, Watanabe N, Ashikaga K, Morihisa K, Kuriyama N, Asada Y, Shibata Y. Reverse remodeling of the mitral valve complex after radiofrequency catheter ablation for atrial fibrillation: a serial 3‐dimensional echocardiographic study. Circ Cardiovasc Imaging. 2019; 12: e009317. [DOI] [PubMed] [Google Scholar]
- 7. Yamaguchi K, Tanabe K, Tani T, Yagi T, Fujii Y, Konda T, Kawai J, Sumida T, Morioka S, Kihara Y. Left atrial volume in normal Japanese adults. Circ J. 2006; 70: 285–288. [DOI] [PubMed] [Google Scholar]
- 8. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography Developed in Collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr. 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 9. Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Matsuda Y, Okuno S, Hata Y, Mano T. Additional low‐voltage‐area ablation in patients with paroxysmal atrial fibrillation: results of the randomized controlled VOLCANO trial. J Am Heart Assoc. 2020; 9: e015927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malcolme‐Lawes LC, Juli C, Karim R, Bai W, Quest R, Lim PB, Jamil‐Copley S, Kojodjojo P, Ariff B, Davies DW, Rueckert D, Francis DP, Hunter R, Jones D, Boubertakh R, Petersen SE, Schilling R, Kanagaratnam P, Peters NS. Automated analysis of atrial late gadolinium enhancement imaging that correlates with endocardial voltage and clinical outcomes: a 2‐center study. Heart Rhythm. 2013; 10: 1184–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Huizar JF, Ellenbogen KA, Tan AY, Kaszala K. Arrhythmia‐induced cardiomyopathy: JACC state‐of‐the‐art review. J Am Coll Cardiol. 2019; 73: 2328–2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim K, Kitai T, Kaji S, Pak M, Toyota T, Sasaki Y, Ehara N, Kobori A, Kinoshita M, Furukawa Y. Outcomes and predictors of cardiac events in medically treated patients with atrial functional mitral regurgitation. Int J Cardiol. 2020; 316: 195–202. [DOI] [PubMed] [Google Scholar]
- 13. Ausma J, van der Velden HM, Lenders MH, van Ankeren EP, Jongsma HJ, Ramaekers FC, Borgers M, Allessie MA. Reverse structural and gap‐junctional remodeling after prolonged atrial fibrillation in the goat. Circulation. 2003; 107: 2051–2058. [DOI] [PubMed] [Google Scholar]
- 14. Ullah W, Ling LH, Prabhu S, Lee G, Kistler P, Finlay MC, Earley MJ, Sporton S, Bashir Y, Betts TR, Rajappan K, Thomas G, Duncan E, Staniforth A, Mann I, Chow A, Lambiase P, Schilling RJ, Hunter RJ. Catheter ablation of atrial fibrillation in patients with heart failure: impact of maintaining sinus rhythm on heart failure status and long‐term rates of stroke and death. Europace. 2016; 18: 679–686. [DOI] [PubMed] [Google Scholar]
- 15. Stone GW, Lindenfeld J, Abraham WT, Kar S, Lim DS, Mishell JM, Whisenant B, Grayburn PA, Rinaldi M, Kapadia SR, Rajagopal V, Sarembock IJ, Brieke A, Marx SO, Cohen DJ, Weissman NJ, Mack MJ, Investigators COAPT. Transcatheter mitral‐valve repair in patients with heart failure. N Engl J Med. 2018; 379: 2307–2318. [DOI] [PubMed] [Google Scholar]
- 16. Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, Schunkert H, Christ H, Vogt J, Bänsch D, CASTLE‐AF Investigators . Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018; 378: 417–427. [DOI] [PubMed] [Google Scholar]
- 17. Matsuda Y, Masuda M, Asai M, Iida O, Okamoto S, Ishihara T, Nanto K, Kanda T, Tsujimura T, Hata Y, Uematsu H, Mano T. A new clinical risk score for predicting the prevalence of low‐voltage areas in patients undergoing atrial fibrillation ablation. J Cardiovasc Electrophysiol. 2020; 31: 3150–3158. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Predictors associated with MR improvement.


