Abstract
Aims
Chronic pressure overload and right ventricular (RV) dysfunction can lead to RV–pulmonary artery (PA) uncoupling in patients with heart failure. The evolution and prognostic values of RV–PA coupling assessed by echocardiography in patients undergoing cardiac resynchronization therapy (CRT) have not been thoroughly investigated. The aim of this study was to evaluate the evolution and prognostic value of tricuspid annular plane systolic excursion (TAPSE)/pulmonary artery systolic pressure (PASP) ratio in CRT recipients.
Methods and results
The RV–PA coupling was measured non‐invasively with echocardiography using the TAPSE/PASP ratio at baseline and 6 month follow‐up in CRT recipients. The cut‐off value for TAPSE/PASP uncoupling was derived from spline curve analysis (i.e. <0.45 mm/mmHg). The primary endpoint was all‐cause mortality. A total of 807 patients (age 66 ± 11 years, 76% men) were analysed. During a median follow‐up of 97 (54–143) months, 483 (60%) patients died. Survival rates at 3 and 5 year follow‐up were significantly lower for patients with a TAPSE/PASP ratio <0.45 mm/mmHg (76% and 58%, respectively), compared with those with a TAPSE/PASP ratio ≥0.45 mm/mmHg (91% and 82%, respectively) (P < 0.001). On multivariable analysis, TAPSE/PASP ratio <0.45 mm/mmHg (hazard ratio 1.437; 95% confidence interval: 1.145–1.805; P = 0.002) was independently associated with all‐cause mortality, whereas TAPSE <17 mm (hazard ratio 1.237; 95% confidence interval: 0.990–1.546; P = 0.061) was not. In addition, no improvement of the TAPSE/PASP ratio after CRT implantation was independently associated with worse survival.
Conclusions
The TAPSE/PASP ratio at baseline is independently associated with long‐term outcomes in CRT recipients. The baseline TAPSE/PASP ratio has incremental value over TAPSE, which does not take account of RV afterload. A lack of improvement in the TAPSE/PASP ratio after CRT implantation is associated with worse survival.
Keywords: Right ventricular–pulmonary artery coupling, Heart failure, Cardiac resynchronization therapy, Mortality
Introduction
Cardiac resynchronization therapy (CRT) is an effective treatment for selected patients with heart failure (HF), who are already receiving optimal guideline‐directed medical therapy. 1 Both right ventricular (RV) dysfunction and pulmonary hypertension are major determinants of outcomes in patients with HF and reduced left ventricular ejection fraction (LVEF), including those eligible for CRT. 2 , 3 , 4 , 5 Increased RV afterload is frequently associated with HF as a result of LV diastolic dysfunction and secondary mitral regurgitation, which leads to retrograde transmission of elevated left‐sided filling pressures, pulmonary hypertension, and pulmonary vascular remodelling. 6 Because the RV adapts less well to pressure overload than to volume overload, an increase in RV afterload may lead to RV dilatation and dysfunction. Despite the fact that an intimate relationship exists between the pulmonary circulation and RV function, most CRT studies have characterized these parameters as separate entities.
The RV to pulmonary artery (PA) coupling refers to the degree of functional matching between RV contractility and the afterload imposed by the pulmonary vascular bed. The gold standard for evaluating RV–PA coupling is the RV end‐systolic elastance (Ees) to pulmonary arterial elastance (Es) ratio, derived from invasively‐measured pressure–volume loops. 7 Catheter‐based measurement of this ratio though is impractical for routine clinical use. RV–PA coupling can be reliably estimated non‐invasively by using the echocardiographically calculated ratio of the tricuspid annular plane systolic excursion (TAPSE) to pulmonary artery systolic pressure (PASP). 8 The prognostic significance of the pre‐implant TAPSE/PASP ratio, as well its impact on the evolution of non‐invasively measured RV‐PA coupling after CRT, has not been thoroughly investigated. The aim of the current study was therefore to (i) establish the prevalence and prognostic value of echocardiographically estimated RV–PA uncoupling by using the TAPSE/PASP ratio before CRT implantation and (ii) characterize the evolution of the TAPSE/PASP ratio following CRT, as well as its prognostic impact, in a large cohort of CRT recipients.
Methods
Patient population and clinical data collection
Patients with HF undergoing CRT implantation in a single tertiary care center (Leiden University Medical Center, the Netherlands) were included from an ongoing registry. CRT implantation was performed in accordance with the European Society of Cardiology guidelines on HF. 1 All patients underwent complete clinical and echocardiographic evaluation before CRT implantation, and patient information was prospectively collected in the departmental cardiology information system (EPD‐vision; Leiden University Medical Center, Leiden, the Netherlands). Clinical data included demographic characteristics, cardiovascular risk factors, comorbidities, and functional status (New York Heart Association (NYHA) functional class). An ischaemic aetiology of HF was diagnosed by the presence of significant coronary artery disease on invasive coronary angiography. Quality of life was evaluated with the Minnesota Living with Heart Failure Questionnaire, and if feasible, a 6 min walk test was performed. Renal function was quantified by estimating the glomerular filtration rate with the Modification of Diet in Renal Disease Study equation. The study complied with the Declaration of Helsinki and was approved by the Institutional Review Board. Due to the retrospective study design, the Medical Ethical Committee waived the need for written informed consent.
Echocardiographic data acquisition and analysis
All patients underwent transthoracic echocardiography before CRT implantation in the left lateral decubitus position with commercially available ultrasound equipment (Vivid 7 and E9, GE‐Vingmed, Horten, Norway). The same protocol was repeated at 6 months follow‐up, and electrocardiogram‐triggered echocardiographic data were stored digitally in a cine‐loop format for offline analysis using EchoPAC version 203 (GE Medical Systems, Horten, Norway). To account for interbeat variability, echocardiographic measurements in patients with atrial fibrillation were calculated and averaged over five cardiac cycles. 9 LV volumes, LVEF, and left atrial volumes were measured using the Simpson's biplane method. 9 RV end‐systolic and end‐diastolic areas were traced in a focused RV apical view. 9 TAPSE was measured on M‐mode recordings of the lateral tricuspid annulus in an RV‐focused view. 9 PASP was derived from the peak velocity of the tricuspid regurgitant jet according to the Bernoulli equation, subsequently adding the right atrial pressure (estimated by the inspiratory collapse and diameter of the inferior vena cava). 9 RV–PA coupling was non‐invasively assessed using the ratio between TAPSE and PASP, which is a surrogate for the ratio between RV Ees and pulmonary Ea, when derived from invasively measured pressure–volume loops. 7 The TAPSE/PASP ratio has been shown to be the only reliable echocardiographic index that is independently associated with Ees/Ea (i.e. the gold standard for quantifying RV–PA coupling). 8 The severity of mitral and tricuspid regurgitation was graded using a multiparametric approach, as recommended by current guidelines. 10
Cardiac resynchronization therapy implantation
Cardiac resynchronization therapy implantation was performed according to a standard approach, that is, insertion of the right atrial and ventricular leads via the subclavian or cephalic veins. Before insertion of the LV lead, coronary sinus venography was performed. The LV pacing lead was then introduced into the coronary sinus through an 8 Fr guiding catheter, and positioned in a posterior or posterolateral vein, if possible. Defibrillator functionality was included in most (96%) of the implanted devices. CRT recipients were followed up at regular intervals at the HF outpatient clinic, at which time the device was interrogated. Atrioventricular and interventricular delays were empirically set at 120–140 ms and 0 ms, respectively. CRT optimization was performed during follow‐up visits at the discretion of the treating physician.
Clinical endpoints
Patients were followed up for the occurrence of all‐cause mortality. Data on mortality were obtained from the departmental cardiology information system (EPD Vision, Leiden University Medical Center, Leiden, the Netherlands), which is linked to the governmental death registry database. Follow‐up data were complete for all patients.
Statistical analysis
Continuous data are presented as mean ± standard deviation when normally distributed and as median and interquartile range when not normally distributed. Categorical data are presented as frequencies and percentages. Continuous variables were compared using the independent samples Student's t test when normally distributed, whereas the Mann–Whitney U test was used to compare continuous variables that did not follow a normal distribution. Categorical variables were compared using the Pearson χ 2 test. A spline curve analysis was performed to assess the hazard ratio (HR) for all‐cause mortality across a range of TAPSE/PASP values. A threshold of 0.45 mm/mmHg was used to dichotomize the study population, based on mortality excess (i.e. where the predicted HR was ≥1) (Figure 1 ). Event‐free survival curves were generated using the Kaplan–Meier method, and differences between groups were analysed using the log‐rank test. To assess the association of TAPSE/PASP ratio at baseline and follow‐up with all‐cause mortality, univariable and multivariable Cox proportional hazard models were constructed. Variables that were statistically significant (P < 0.05) in the univariable analysis were included in the multivariable model. HRs with 95% confidence intervals (CIs) were calculated. To investigate the incremental value of TAPSE/PASP ratio over clinical and conventional echocardiographic parameters to predict outcome, a likelihood ratio test was performed. The change in global χ 2 values was calculated and reported. A two‐sided P value <0.05 was considered statistically significant. Statistical analysis was performed using SPSS for Windows, version 25.0 (IBM, Armonk, New York, USA) and R version 4.0.1 (R Foundation for Statistical Computing, Vienna, Austria).
Figure 1.
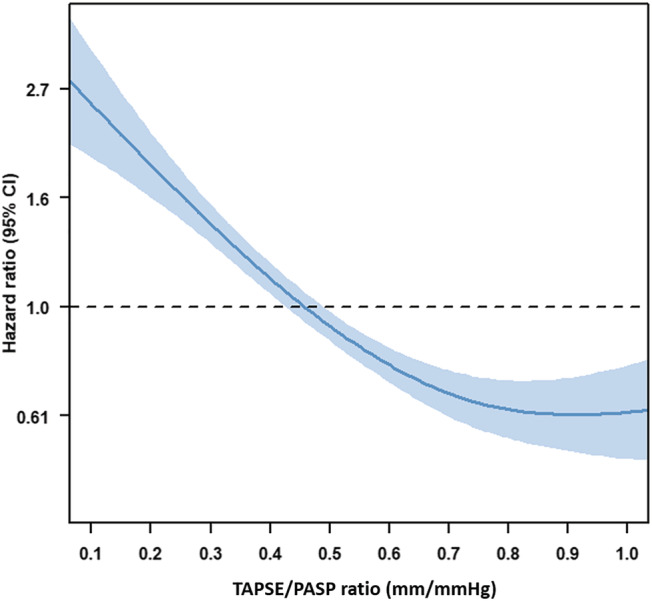
Association between TAPSE/PASP ratio and the risk of all‐cause mortality among cardiac resynchronization therapy (CRT) recipients. The curve represents the hazard ratio change for all‐cause mortality with overlaid 95% confidence intervals (blue) across TAPSE/PASP ratio as a continuous variable before CRT implantation. PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Results
Clinical and echocardiographic characteristics at baseline
A total of 807 patients (age 66 ± 11 years; 76% men) were included (Figure S1 ). Baseline clinical characteristics are shown in Table 1 . An ischaemic aetiology of HF was present in 471 (58%) patients and 522 (65%) patients had NYHA functional Class III–IV before CRT implantation. Baseline echocardiographic data are shown in Table 2 . The mean pre‐implantation LVEF was 28 ± 8%, while the mean TAPSE and PASP pre‐CRT were 16 ± 5 mm and 35 ± 14 mmHg, respectively.
Table 1.
Baseline clinical characteristics
| Overall population (n = 807) | TAPSE/PASP ratio <0.45 mm/mmHg (n = 360) | TAPSE/PASP ratio ≥0.45 mm/mmHg (n = 447) | P value | |
|---|---|---|---|---|
| Age, years | 65.5 (±10.5) | 65.7 (±10.9) | 65.4 (±10.2) | 0.618 |
| Male sex (%) | 613 (76.0%) | 281 (78.1%) | 332 (74.3%) | 0.211 |
| Arterial hypertension (%) | 377 (46.9%) | 163 (45.8%) | 214 (47.9%) | 0.556 |
| Diabetes mellitus (%) | 165 (20.4%) | 87 (24.2%) | 78 (17.4%) | 0.019 |
| Dyslipidaemia (%) | 346 (43.1%) | 146 (41.0%) | 200 (44.8%) | 0.276 |
| Current smoker (%) | 116 (14.4%) | 43 (11.9%) | 73 (16.3%) | 0.177 |
| BMI, kg/m2 | 26.4 (±4.3) | 25.9 (±4.1) | 26.8 (±4.3) | 0.002 |
| Ischaemic aetiology (%) | 471 (58.4%) | 223 (61.9%) | 248 (55.5%) | 0.064 |
| QoL score | 31.2 (±19.2) | 34.4 (±19.3) | 28.6 (±18.7) | <0.001 |
| 6MWT, m | 335.9 (±119.0) | 310.1 (±116.4) | 356.7 (±117.2) | <0.001 |
| NYHA III–IV (%) | 522 (65.0%) | 261 (73.5%) | 261 (59.9%) | <0.001 |
| Sinus rhythm (%) | 559 (69.3%) | 219 (60.8%) | 340 (76.1%) | <0.001 |
| QRS duration, ms | 153.0 (±35.2) | 154.2 (±36.5) | 152.0 (±34.0) | 0.396 |
| Beta‐blocker (%) | 602 (74.6%) | 257 (71.4%) | 345(77.2%) | 0.060 |
| ACE‐i/ARB (%) | 709 (87.9%) | 307 (85.3%) | 402 (89.9%) | 0.044 |
| MRA (%) | 362 (44.9%) | 176 (48.9%) | 186 (41.6%) | 0.039 |
| Diuretics (%) | 641 (79.4%) | 310 (86.1%) | 331 (74.0%) | <0.001 |
| Statin (%) | 514 (63.7%) | 216 (60.0%) | 298 (66.7%) | 0.050 |
| eGFR, mL/min/1.73 m2 | 67.2 (±23.5) | 63.3 (±23.9) | 70.4 (±22.8) | <0.001 |
| Haemoglobin, g/dL | 13.4 (±1.6) | 13.2 (±1.8) | 13.5 (±1.6) | 0.001 |
ACE‐i, angiotensin‐converting enzyme inhibitor; ARB, angiotensin receptor blocker; BMI, body mass index; eGFR, estimated glomerular filtration rate; MRA, mineralocorticoid receptor antagonist; MWT, minute walking test; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; QoL, quality of life; TAPSE, tricuspid annular plane systolic excursion.
Table 2.
Baseline echocardiographic characteristics
| Overall population (n = 807) | TAPSE/PASP ratio <0.45 mm/mmHg (n = 360) | TAPSE/PASP ratio ≥0.45 mm/mmHg (n = 447) | P value | |
|---|---|---|---|---|
| LVEDV, mL | 187 (146–243) | 196 (150–245) | 183 (144–243) | 0.086 |
| LVESV, mL | 137 (99–178) | 144 (105–187) | 129 (96–172) | 0.004 |
| LVEF, % | 27.8 (±8.3) | 26.1 (±8.0) | 29.2 (±8.3) | <0.001 |
| LAVi, mL/m2 | 44.2 (±20.2) | 49.8 (±20.2) | 39.7 (±19.1) | <0.001 |
| Moderate to severe MR (%) | 314 (42.7%) | 176 (56.6%) | 138 (32.5%) | <0.001 |
| RVEDA, cm2 | 22.5 (±7.1) | 24.5 (±7.9) | 20.9 (±5.8) | <0.001 |
| RVESA, cm2 | 14.6 (±6.3) | 17.0 (±7.0) | 12.7 (±4.9) | <0.001 |
| RVFAC, % | 36.6 (±12.9) | 31.8 (±12.4) | 40.6 (±11.9) | <0.001 |
| RA area, cm2 | 18.5 (14.6–23.9) | 20.7 (16.7–25.9) | 16.8 (13.6–21.7) | <0.001 |
| TAPSE, mm | 16.1 (±4.8) | 13 (±4.0) | 18 (±4.0) | <0.001 |
| TR velocity, m/s | 2.58 (±0.58) | 2.93 (±0.52) | 2.29 (±0.45) | <0.001 |
| PASP, mmHg | 35 (± 14) | 44 (±14) | 27 (± 8) | <0.001 |
| Moderate to severe TR (%) | 212 (28.0%) | 154 (45.8%) | 58 (13.8%) | <0.001 |
EDA, end‐diastolic area; EDV, end‐diastolic volume; EF, ejection fraction; ESA, end‐systolic area; ESV, end‐systolic volume; FAC, fractional area change; LAVi, left atrial volume index; LV, left ventricular; MR, mitral regurgitation; PASP, pulmonary artery systolic pressure; RA, right atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
There were 360 (45%) patients with a TAPSE/PASP ratio <0.45 mm/mmHg. Patients with a TAPSE/PASP ratio <0.45 mm/mmHg more frequently had diabetes mellitus, a lower body mass index, more impaired renal function, worse quality of life score, lower performance on 6 min walk test and were more likely to have NYHA functional Class III–IV (Table 1 ). CRT recipients with a TAPSE/PASP ratio <0.45 mm/mmHg had larger LV end‐systolic volumes, lower LVEF, larger left atrial volumes, larger RV dimensions, and more severe mitral and tricuspid regurgitation at baseline (Table 2 ).
Baseline tricuspid annular plane systolic excursion/pulmonary artery systolic pressure ratio and all‐cause mortality
During a median follow‐up of 97 (54–143) months, 483 (60%) patients died. Individuals with a more impaired TAPSE/PASP ratio had significantly worse survival rates when compared with patients with a more preserved TAPSE/PASP ratio. Survival rates at 1, 3, and 5 years were 91%, 76%, and 58%, respectively, in patients with a TAPSE/PASP ratio <0.45 mm/mmHg vs. 98%, 91%, 82%, respectively, in those with TAPSE/PASP ratio ≥0.45 mm/mmHg (P < 0.001) (Figure 2 ). To further investigate the association between TAPSE/PASP ratio and all‐cause mortality, univariable and multivariable Cox proportional hazards models were performed (Table 3 ). On multivariable analysis, TAPSE/PASP ratio, analysed as a continuous variable (HR 0.602; 95% CI 0.394–0.919; P = 0.019), as well as a categorical variable (i.e. TAPSE/PASP ratio <0.45 mm/mmHg) (HR 1.437; 95% CI 1.145–1.805; P = 0.002), was independently associated with all‐cause mortality. Interestingly, TAPSE/PASP ratio and LV end‐systolic volume were the only two echocardiographic variables that remained significantly associated with all‐cause mortality on multivariable analysis. TAPSE <17 mm (instead of TAPSE/PASP <0.45 mm/mmHg) did not remain independently associated with all‐cause mortality (HR 1.237; 95% CI 0.990–1.546; P = 0.061) on multivariable analysis. The addition of TAPSE/PASP ratio <0.45 mm/mmHg to a baseline model (including all covariables used in the multivariable Cox regression analysis) yielded a significantly greater incremental prognostic value, compared with the addition of TAPSE (using the guideline‐recommended cut‐off value of <17 mm 9 ) (Figure 3 ).
Figure 2.
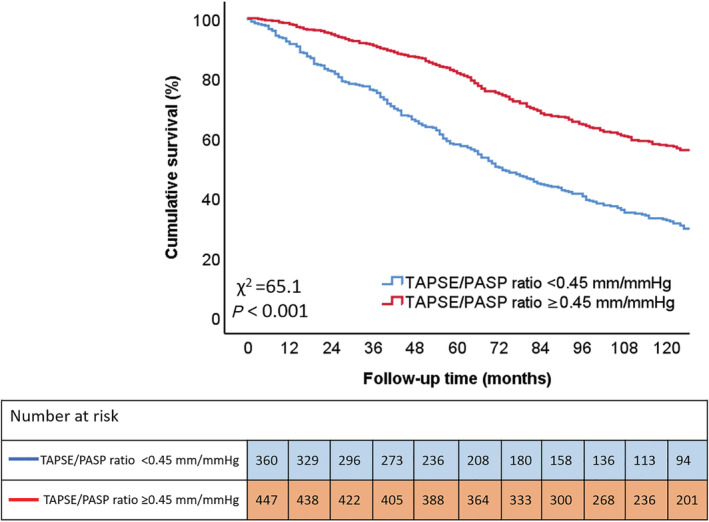
Kaplan–Meier curve for all‐cause mortality, according to TAPSE/PASP ratio. The Kaplan–Meier survival curve demonstrates lower survival rates for patients with a TAPSE/PASP ratio <0.45 mm/mmHg (blue), compared with patients with TAPSE/PASP ratio ≥0.45 mm/mmHg (red). PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion.
Table 3.
Univariable and multivariable Cox regression analysis to assess the association between TAPSE/PASP ratio and all‐cause mortality
| Univariable analysis | Multivariable analysis a | Multivariable analysis b | ||||
|---|---|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | 1.044 (1.034–1.055) | <0.001 | 1.021 (1.007–1.035) | 0.004 | 1.021 (1.007–1.036) | 0.003 |
| Male sex | 1.305 (1.048–1.624) | 0.017 | 1.071 (0.807–1.421) | 0.633 | 1.055 (0.794–1.401) | 0.712 |
| Arterial hypertension | 1.102 (0.921–1.318) | 0.291 | ||||
| Diabetes mellitus | 1.680 (1.365–2.067) | <0.001 | 1.539 (1.199–1.976) | 0.001 | 1.566 (1.219–2.011) | <0.001 |
| Dyslipidaemia | 1.294 (1.082–1.549) | 0.005 | 1.105 (0.891–1.371) | 0.364 | 1.125 (0.906–1.396) | 0.288 |
| Smoking | 0.958 (0.847–1.084) | 0.497 | ||||
| Body mass index, kg/m2 | 0.982 (0.961–1.003) | 0.095 | ||||
| Ischaemic aetiology of heart failure | 1.587 (1.315–1.916) | <0.001 | 1.378 (1.090–1.743) | 0.007 | 1.339 (1.057–1.697) | 0.016 |
| NYHA III–IV | 1.779 (1.449–2.186) | <0.001 | 1.381 (1.095–1.743) | 0.006 | 1.346 (1.067–1.699) | 0.012 |
| Sinus rhythm | 0.625 (0.229–1.710) | 0.361 | ||||
| QRS duration before implantation, ms | 1.001 (0.999–1.004) | 0.333 | ||||
| Haemoglobin, g/dL | 0.790 (0.724–0.862) | <0.001 | 0.992 (0.890–1.105) | 0.878 | 0.992 (0.890–1.106) | 0.881 |
| eGFR, mL/min/1.73 m2 | 0.981 (0.977–0.984) | <0.001 | 0.983 (0.978–0.988) | <0.001 | 0.984 (0.979–0.989) | <0.001 |
| LVESV, mL | 1.003 (1.001–1.004) | <0.001 | 1.003 (1.000–1.005) | 0.018 | 1.003 (1.000–1.005) | 0.018 |
| LVEF, % | 0.985 (0.974–0.995) | 0.005 | 1.001 (0.984–1.017) | 0.944 | 1.002 (0.985–1.018) | 0.856 |
| LAVi, mL/m2 | 1.013 (1.010–1.017) | <0.001 | 1.005 (1.000–1.010) | 0.053 | 1.005 (1.000–1.010) | 0.063 |
| Moderate to severe MR | 1.462 (1.211–1.765) | <0.001 | 1.043 (0.840–1.295) | 0.704 | 1.027 (0.826–1.276) | 0.812 |
| RVEDA, cm2 | 1.026 (1.014–1.038) | <0.001 | 1.016 (0.998–1.035) | 0.086 | 1.015 (0.997–1.033) | 0.111 |
| RA area, cm2 | 1.022 (1.014–1.030) | <0.001 | 1.008 (0.995–1.022) | 0.234 | 1.007 (0.994–1.021) | 0.290 |
| PASP, mmHg | 1.024 (1.018–1.030) | <0.001 | ||||
| TAPSE, mm | 0.950 (0.932–0.968) | <0.001 | ||||
| TAPSE <17 mm | 1.457 (1.206–1.761) | <0.001 | ||||
| TAPSE/PASP ratio, mm/mmHg (continuous) | 0.299 (0.205–0.434) | <0.001 | 0.602 (0.394–0.919) | 0.019 | ||
| TAPSE/PASP ratio, mm/mmHg (cut‐off <0.45) | 2.060 (1.721–2.465) | <0.001 | 1.437 (1.145–1.805) | 0.002 | ||
CI, confidence interval; EDA, end‐diastolic area; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESV, end‐systolic volume; HR, hazard ratio; LAVi, left atrial volume index; LV, left ventricular; MR, mitral regurgitation; NYHA, New York Heart Association; PASP, pulmonary artery systolic pressure; RA, right atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion.
Multivariable analysis, using TAPSE/PASP ratio as a continuous variable.
Multivariable analysis, using TAPSE/PASP ratio as a categorical variable (i.e. TAPSE/PASP ratio <0.45 mm/mmHg compared to ≥0.45 mm/mmHg).
Figure 3.
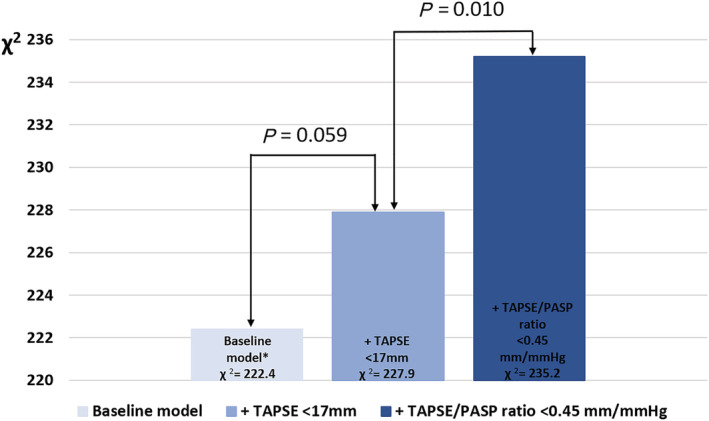
Likelihood ratio test for the incremental prognostic value of TAPSE/PASP ratio. The addition of TAPSE/PASP ratio <0.45 mm/mmHg to a baseline model shows a greater increase in the chi‐square value, compared to the addition of TAPSE <17 mm. PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion. *The baseline model includes age, sex, diabetes mellitus, dyslipidaemia, ischaemic aetiology for heart failure, New York Heart Association functional Class III–IV, haemoglobin, estimated glomerular filtration rate, left ventricular end‐systolic volume, left ventricular ejection fraction, left atrial volume index, significant (i.e. ≥moderate) mitral regurgitation, right ventricular end‐diastolic area, and right atrial area.
Evolution of tricuspid annular plane systolic excursion/pulmonary artery systolic pressure ratio following cardiac resynchronization therapy and prognostic implications
The TAPSE/PASP ratio was measurable in 663/807 (82%) patients at 6 month follow‐up. Of the 299 individuals with impaired baseline TAPSE/PASP ratio (TAPSE/PASP <0.45 mm/mmHg) who were re‐evaluated at 6 months after CRT implantation, 110 (37%) showed significant improvement in this parameter (mean TAPSE/PASP ratio increased to ≥0.45 mm/mmHg). Among 364 individuals with a preserved baseline TAPSE/PASP ratio (TAPSE/PASP ≥0.45 mm/mmHg), 81 (22%) worsened, with a follow‐up TAPSE/PASP ratio which had decreased to <0.45 mm/mmHg. CRT recipients with an impaired TAPSE/PASP ratio, which had improved to ≥0.45 mm/mmHg at 6 months' follow‐up, displayed significantly higher survival rates than those whose 6 month TAPSE/PASP ratio remained <0.45 mm/mmHg (P < 0.001) (Figure 4 ). On multivariable analysis, baseline CRT TAPSE/PASP ratio ≥0.45 mm/mmHg, which worsened to TAPSE/PASP ratio <0.45 mm/mmHg at 6 months (HR 1.853; 95% CI 1.274–2.696; P = 0.001), and baseline CRT TAPSE/PASP ratio <0.45 mm/mmHg, which remained <0.45 mm/mmHg at 6 months (HR 1.836; 95% CI 1.340–2.515; P < 0.001), were independently associated with higher all‐cause mortality (Table 4 ).
Figure 4.
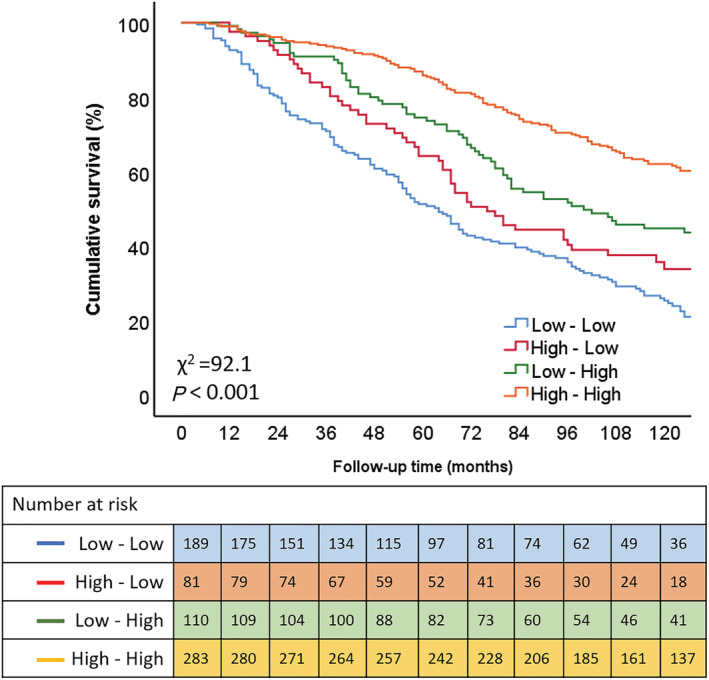
Kaplan–Meier curve for all‐cause mortality according to different groups of TAPSE/PASP ratio evolution [based on the response following cardiac resynchronization therapy (CRT) implantation]. PASP, pulmonary artery systolic pressure; TAPSE, tricuspid annular plane systolic excursion. Low–low indicates individuals with pre‐CRT TAPSE/PASP ratio <0.45 mm/mmHg and 6 month TAPSE/PASP ratio <0.45 mmHg; high–low indicates a pre‐CRT TAPSE/PASP ratio ≥0.45 mm/mmHg and 6 month TAPSE/PASP ratio <0.45 mm/mmHg; low–high indicates a pre‐CRT TAPSE/PASP ratio <0.45 mm/mmHg and a 6 month TAPSE/PASP ratio ≥0.45 mm/mmHg; high–high indicates a TAPSE/PASP ratio ≥0.45 mm/mmHg and a 6 month TAPSE/PASP ratio ≥0.45 mm/mmHg.
Table 4.
Univariable and multivariable Cox regression analysis to assess the association of TAPSE/PASP ratio evolution (based on the response following CRT implantation) and all‐cause mortality
| Univariable analysis | Multivariable analysis | |||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age, years | 1.043 (1.031–1.055) | <0.001 | 1.031 (1.017–1.047) | <0.001 |
| Male sex | 1.404 (1.099–1.793) | 0.007 | 1.156 (0.839–1.592) | 0.375 |
| Arterial hypertension | 1.059 (0.869–1.292) | 0.568 | ||
| Diabetes mellitus | 1.652 (1.307–2.088) | <0.001 | 1.397 (1.049–1.861) | 0.022 |
| Dyslipidaemia | 1.244 (1.019–1.518) | 0.032 | 1.060 (0.833–1.349) | 0.635 |
| Smoking | 0.994 (0.867–1.139) | 0.929 | ||
| Body mass index, kg/m2 | 0.986 (0.963–1.010) | 0.254 | ||
| Ischaemic aetiology | 1.504 (1.225–1.848) | <0.001 | 0.818 (0.633–1.058) | 0.126 |
| NYHA III–IV | 1.834 (1.463–2.300) | <0.001 | 1.305 (1.007–1.691) | 0.044 |
| Sinus rhythm | 1.015 (0.883–1.167) | 0.838 | ||
| QRS duration before implantation, ms | 1.002 (0.999–1.005) | 0.125 | ||
| Haemoglobin, g/dL | 0.799 (0.722–0.883) | <0.001 | 0.969 (0.861–1.092) | 0.609 |
| eGFR, mL/min/1.73 m2 | 0.978 (0.973–0.983) | <0.001 | 0.986 (0.980–0.992) | <0.001 |
| LVESV, mL | 1.003 (1.001–1.004) | <0.001 | 1.002 (1.000–1.005) | 0.085 |
| LVEF, % | 0.985 (0.973–0.997) | 0.011 | 1.001 (0.982–1.019) | 0.953 |
| LAVi, mL/m2 | 1.014 (1.010–1.018) | <0.001 | 1.004 (0.999–1.010) | 0.131 |
| Moderate to severe MR | 1.399 (1.134–1.725) | 0.002 | 1.004 (0.787–1.280) | 0.975 |
| RVEDA, cm2 | 1.036 (1.022–1.051) | <0.001 | 1.007 (0.986–1.028) | 0.517 |
| RA area, cm2 | 1.022 (1.013–1.030) | <0.001 | 1.007 (0.992–1.022) | 0.352 |
| TAPSE/PASP ratio groups | ||||
| High–high | Reference | Reference | ||
| Low–high | 1.540 (1.139–2.082) | 0.005 | 1.318 (0.930–1.868) | 0.121 |
| High–low | 2.177 (1.583–2.992) | <0.001 | 1.853 (1.274–2.696) | 0.001 |
| Low–low | 3.000 (2.363–3.809) | <0.001 | 1.836 (1.340–2.515) | <0.001 |
CI, confidence interval; EDA, end‐diastolic area; EF, ejection fraction; eGFR, estimated glomerular filtration rate; ESV, end‐systolic volume; LAVi, left atrial volume index; LV, left ventricular; MR, mitral regurgitation; NYHA, New York Heart Association; OR, odds ratio; PASP, pulmonary artery systolic pressure; RA, right atrial; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion.
Low–low indicates individuals with pre‐CRT TAPSE/PASP ratio <0.45 mm/mmHg and 6 month TAPSE/PASP ratio <0.45 mmHg; high–low indicates a pre‐CRT TAPSE/PASP ratio ≥0.45 mm/mmHg and 6 month TAPSE/PASP ratio <0.45 mm/mmHg; low–high indicates a pre‐CRT TAPSE/PASP ratio <0.45 mm/mmHg and a 6 month TAPSE/PASP ratio ≥0.45 mm/mmHg; high–high indicates a TAPSE/PASP ratio ≥0.45 mm/mmHg and a 6 month TAPSE/PASP ratio ≥0.45 mm/mmHg.
Discussion
The main findings of the current study can be summarized as follows: (i) TAPSE/PASP ratio at baseline is independently associated with long‐term survival in CRT recipients, (ii) baseline TAPSE/PASP ratio has incremental prognostic value over TAPSE, and (iii) no improvement in TAPSE/PASP ratio after CRT implantation is associated with worse survival.
Rationale for assessment of right ventricular–pulmonary artery coupling in cardiac resynchronization therapy recipients
Pulmonary hypertension is frequently observed in patients with HF and reduced LVEF, including those eligible for CRT. 6 It is most often the consequence of LV diastolic dysfunction and secondary mitral regurgitation, which leads to retrograde transmission of elevated left‐sided filling pressures to the pulmonary vascular bed. 6 , 11 RV adaptation to increased afterload is initially characterized by a hypertrophic response, which maintains RV contractility and RV‐PA coupling. However, the thin‐walled RV cannot cope effectively with a persistent increase in afterload and pulmonary hypertension may eventually result in increased RV wall tension, leading to RV dilatation, reduced RV contractility, and RV‐PA uncoupling. 12 Both RV systolic dysfunction and pulmonary hypertension are important determinants of outcomes in CRT recipients, 2 , 13 , 14 , 15 although most studies have focused on RV function and the pulmonary vasculature as separate variables, overlooking the basic concept that the RV is highly sensitive to the imposed pressure load. A more integrative assessment of the RV function/RV afterload relation may be more predictive of outcomes than each of the two variables in isolation, but has never been adequately studied in CRT recipients. 16 , 17
Non‐invasive estimation of right ventricular–pulmonary artery coupling
The gold standard for evaluating RV–PA coupling is the invasive measurement of the ratio of RV Ees to pulmonary Ea. Ees describes the relation between end‐systolic ventricular pressure and end‐systolic ventricular volume, thereby representing ventricular performance, whereas Ea is calculated by the ratio between end‐systolic pressure and stroke volume, reflecting vascular load (including peripheral resistance, vascular compliance, and systolic/diastolic time intervals). 7 , 18 In patients with HF and reduced LVEF, Ees is often reduced and Ea increased, indicating RV‐PA uncoupling. Invasive measurements of RV‐PA coupling are impractical for routine clinical use, but can be reliably estimated by the echocardiographic ratio of TAPSE and PASP. 8 , 17 This non‐invasive index of RV–PA coupling has demonstrated prognostic value in various cardiovascular diseases. 19 , 20 , 21 , 22
Prognostic implications of tricuspid annular plane systolic excursion/pulmonary artery systolic pressure ratio in cardiac resynchronization therapy recipients
Guazzi et al. demonstrated that the TAPSE/PASP ratio is a strong and independent predictive variable in patients with HF and preserved as well as reduced LVEF, and also has incremental prognostic value over each parameter measured separately. 16 The interplay of RV function and afterload was elegantly demonstrated by Ghio et al., who found that impaired RVEF or pulmonary hypertension in isolation did not impact on survival. In contrast, in those patients with impaired RV function and pulmonary hypertension, survival was impacted negatively. 5 Similar findings were described in 293 patients with HF (both preserved and reduced LVEF), using TAPSE and PASP measured non‐invasively with echocardiography. 12 , 16 The abovementioned studies underpin the use of RV‐PA coupling parameters in HF patients.
Although the TAPSE/PASP ratio has been shown to predict outcomes in various cardiovascular conditions, 19 , 20 , 21 , 22 it has never been thoroughly evaluated in patients with HF and reduced LVEF undergoing CRT implantation. In a study of 54 CRT recipients, Deaconu et al. demonstrated that the TAPSE/PASP ratio was linked to both CRT response (defined as ≥15% reduction in LV end‐systolic volume) as well as cardiovascular events. 23 Using a different definition (≥5% improvement in LVEF), Bragança et al. also found the TAPSE/PASP ratio to be associated with CRT response. 24 The current study expands on these results by demonstrating a strong, independent link between the TAPSE/PASP ratio and long‐term outcome in a large, unselected cohort of CRT recipients. The TAPSE/PASP ratio also showed incremental prognostic value over TAPSE alone, which does not take account of RV afterload. In our population, CRT showed the unique ability of being able to improve RV–PA coupling in a significant proportion (37%) of patients with mismatched TAPSE and PASP pre‐implant. In a proof‐of‐concept study, Martens et al. linked improved RV–PA coupling in CRT recipients during exercise to beneficial LV remodelling and a reduction mitral regurgitation during exercise. 18
Clinical implications
Our findings demonstrate that baseline RV–PA uncoupling is associated with worse long‐term survival in CRT recipients. Calculating the TAPSE/PASP ratio adds little time to an echocardiographic examination and may improve risk stratification, thereby identifying patients who are at higher risk of adverse events and may benefit from closer follow‐up after CRT implantation.
Assessment of RV–PA coupling at follow‐up may help to elucidate reasons for lack of response to CRT. Targeting a normalization in RV–PA coupling in CRT patients is an attractive strategy, but will require prospective evaluation.
Study limitations
This was a retrospective, single‐centre study. RV–PA coupling was measured only at rest, and we were unable to evaluate coupling reserve under different loading conditions. No invasively measured Ees/Ea data were available, and therefore, the non‐invasive TAPSE/PASP ratio could not be validated in our cohort. All‐cause mortality was chosen as the primary endpoint, and no distinction could be made between cardiac and non‐cardiac mortality.
Conclusions
The TAPSE/PASP ratio, measured non‐invasively with echocardiography, is independently associated with long‐term outcomes in CRT recipients. Baseline TAPSE/PASP ratio has incremental value over TAPSE, which does not take account of RV afterload, and may therefore improve risk stratification of patients receiving CRT. A lack of significant improvement in the TAPSE/PASP ratio after CRT implantation was associated with worse survival.
Conflict of interest
The Department of Cardiology, Heart Lung Center, Leiden University Medical Center received research grants from Abbott Vascular, Bayer, Biotronik, Bioventrix, Boston Scientific, Edwards Lifesciences, GE Healthcare, Ionis and Medtronic. J. J. B. received speaker fees from Abbott Vascular. NAM received speaker fees from Abbott Vascular and GE Healthcare. V. D. received speaker fees from Abbott Vascular, Edwards Lifesciences, GE Healthcare, Medtronic, MSD and Novartis. The remaining authors have nothing to disclose.
Funding
J.S. received funding from the European Society of Cardiology (ESC Training Grant App000064741).
Supporting information
Figure S1 – Flow Chart.
CRT = cardiac resynchronization therapy; LUMC = Leiden University Medical Center; PA = pulmonary artery; PASP = pulmonary artery systolic pressure; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion.
Stassen, J. , Galloo, X. , Hirasawa, K. , Chimed, S. , Marsan, N. A. , Delgado, V. , van der Bijl, P. , and Bax, J. J. (2022) Right ventricular–pulmonary artery coupling in cardiac resynchronization therapy: evolution and prognosis. ESC Heart Failure, 9: 1597–1607. 10.1002/ehf2.13857.
References
- 1. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Böhm M, Burri H, Butler J, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, de Boer RA, Christian Schulze P, Abdelhamid M, Aboyans V, Adamopoulos S, Anker SD, Arbelo E, Asteggiano R, Bauersachs J, Bayes‐Genis A, Borger MA, Budts W, Cikes M, Damman K, Delgado V, Dendale P, Dilaveris P, Drexel H, Ezekowitz J, Falk V, Fauchier L, Filippatos G, Fraser A, Frey N, Gale CP, Gustafsson F, Harris J, Iung B, Janssens S, Jessup M, Konradi A, Kotecha D, Lambrinou E, Lancellotti P, Landmesser U, Leclercq C, Lewis BS, Leyva F, Linhart A, Løchen M‐L, Lund LH, Mancini D, Masip J, Milicic D, Mueller C, Nef H, Nielsen J‐C, Neubeck L, Noutsias M, Petersen SE, Sonia Petronio A, Ponikowski P, Prescott E, Rakisheva A, Richter DJ, Schlyakhto E, Seferovic P, Senni M, Sitges M, Sousa‐Uva M, Tocchetti CG, Touyz RM, Tschoepe C, Waltenberger J, Adamo M, Baumbach A, Böhm M, Burri H, Čelutkienė J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gardner RS, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Piepoli MF, Price S, Rosano GMC, Ruschitzka F, Skibelund AK. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 42: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 2. Leong DP, Höke U, Delgado V, Auger D, Witkowski T, Thijssen J, van Erven L, Bax JJ, Schalij MJ, Marsan NA. Right ventricular function and survival following cardiac resynchronisation therapy. Heart 2013; 99: 722–728. [DOI] [PubMed] [Google Scholar]
- 3. Patel D, Trulock K, Kumar A, Kiehl E, Toro S, Moennich LA, Gorodeski E, Hussein A, Cantillon D, Tarakji KG, Niebauer M, Wazni O, Varma N, Wilkoff B, Rickard JW. Baseline right ventricular dysfunction predicts worse outcomes in patients undergoing cardiac resynchronization therapy implantation. J Card Fail 2020; 26: 227–232. [DOI] [PubMed] [Google Scholar]
- 4. Dupont M, Mullens W, Skouri HN, Abrahams Z, Wu Y, Taylor DO, Starling RC, Tang WHW. Prognostic role of pulmonary arterial capacitance in advanced heart failure. Circ Heart Fail 2012; 5: 778–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ghio S, Gavazzi A, Campana C, Inserra C, Klersy C, Sebastiani R, Arbustini E, Recusani F, Tavazzi L. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001; 37: 183–188. [DOI] [PubMed] [Google Scholar]
- 6. Guazzi M, Borlaug BA. Pulmonary hypertension due to left heart disease. Circulation 2012; 126: 975–990. [DOI] [PubMed] [Google Scholar]
- 7. Sanz J, Sánchez‐Quintana D, Bossone E, Bogaard HJ, Naeije R. Anatomy, function, and dysfunction of the right ventricle: JACC state‐of‐the‐art review. J Am Coll Cardiol 2019; 73: 1463–1482. [DOI] [PubMed] [Google Scholar]
- 8. Tello K, Wan J, Dalmer A, Vanderpool R, Ghofrani HA, Naeije R, Roller F, Mohajerani E, Seeger W, Herberg U, Sommer N, Gall H, Richter MJ. Validation of the tricuspid annular plane systolic excursion/systolic pulmonary artery pressure ratio for the assessment of right ventricular‐arterial coupling in severe pulmonary hypertension. Circ Cardiovasc Imaging 2019; 12: e009047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015; 1: 1–39. [DOI] [PubMed] [Google Scholar]
- 10. Zoghbi WA, Adams D, Bonow RO, Enriquez‐Sarano M, Foster E, Grayburn PA, Hahn RT, Han Y, Hung J, Lang RM, Little SH, Shah DJ, Shernan S, Thavendiranathan P, Thomas JD, Weissman NJ. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017; 30: 303–371. [DOI] [PubMed] [Google Scholar]
- 11. Miller WL, Mahoney DW, Michelena HI, Pislaru SV, Topilsky Y, Enriquez‐Sarano M. Contribution of ventricular diastolic dysfunction to pulmonary hypertension complicating chronic systolic heart failure. JACC Cardiovasc Imaging 2011; 4: 946–954. [DOI] [PubMed] [Google Scholar]
- 12. Guazzi M, Naeije R. Pulmonary hypertension in heart failure: pathophysiology, pathobiology, and emerging clinical perspectives. J Am Coll Cardiol 2017; 69: 1718–1734. [DOI] [PubMed] [Google Scholar]
- 13. Damy T, Ghio S, Rigby AS, Hittinger L, Jacobs S, Leyva F, Delgado JF, Daubert JC, Gras D, Tavazzi L, Cleland JGF. Interplay between right ventricular function and cardiac resynchronization therapy: an analysis of the CARE‐HF trial (Cardiac Resynchronization‐Heart Failure). J Am Coll Cardiol 2013; 61: 2153–2160. [DOI] [PubMed] [Google Scholar]
- 14. Chatterjee NA, Upadhyay GA, Singal G, Parks KA, Dec GW, Singh JP, Lewis GD. Pre‐capillary pulmonary hypertension and right ventricular dilation predict clinical outcome in cardiac resynchronization therapy. JACC Heart Fail 2014; 2: 230–237. [DOI] [PubMed] [Google Scholar]
- 15. Stern J, Heist EK, Murray L, Alabiad C, Chung J, Picard MH, Semigran MJ, Ruskin JN, Singh JP. Elevated estimated pulmonary artery systolic pressure is associated with an adverse clinical outcome in patients receiving cardiac resynchronization therapy. Pacing Clin Electrophysiol 2007; 30: 603–607. [DOI] [PubMed] [Google Scholar]
- 16. Guazzi M, Bandera F, Pelissero G, Castelvecchio S, Menicanti L, Ghio S, Temporelli PL, Arena R. Tricuspid annular plane systolic excursion and pulmonary arterial systolic pressure relationship in heart failure: an index of right ventricular contractile function and prognosis. Am J Physiol Heart Circ Physiol 2013; 305: H1373–H1381. [DOI] [PubMed] [Google Scholar]
- 17. Guazzi M, Naeije R, Arena R, Corrà U, Ghio S, Forfia P, Rossi A, Cahalin LP, Bandera F, Temporelli P. Echocardiography of right ventriculoarterial coupling combined with cardiopulmonary exercise testing to predict outcome in heart failure. Chest 2015; 148: 226–234. [DOI] [PubMed] [Google Scholar]
- 18. Martens P, Verbrugge FH, Bertrand PB, Verhaert D, Vandervoort P, Dupont M, Tang WHW, Janssens S, Mullens W. Effect of cardiac resynchronization therapy on exercise‐induced pulmonary hypertension and right ventricular‐arterial coupling. Circ Cardiovasc Imaging 2018; 11: e007813. [DOI] [PubMed] [Google Scholar]
- 19. Guazzi M, Dixon D, Labate V, Beussink‐Nelson L, Bandera F, Cuttica MJ, Shah SJ. RV contractile function and its coupling to pulmonary circulation in heart failure with preserved ejection fraction: stratification of clinical phenotypes and outcomes. JACC Cardiovasc Imaging 2017; 10: 1211–1221. [DOI] [PubMed] [Google Scholar]
- 20. Trejo‐Velasco B, Estevez‐Loureiro R, Carrasco‐Chinchilla F, Fernández‐Vázquez F, Arzamendi D, Pan M, Pascual I, Nombela‐Franco L, Amat‐Santos IJ, Freixa X, Hernández‐Antolín RA, Trillo‐Nouche R, Andraka Ikazuriaga L, López‐Mínguez JR, Sanmiguel Cervera D, Sanchis J, Diez‐Gil JL, Ruiz‐Quevedo V, Urbano‐Carrillo C, Becerra‐Muñoz VM, Benito‐González T, Li CH, Mesa D, Avanzas P, Armijo G, Serrador‐Frutos AM, Sanchis L, Lobán CFG, Cid‐Álvarez B, Hernández‐García JM, Garrote‐Coloma C, Fernández‐Peregrina E, Romero M, León Arguero V, Cruz‐González I. Prognostic role of TAPSE to PASP ratio in patients undergoing MitraClip procedure. J Clin Med 2021; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sultan I, Cardounel A, Abdelkarim I, Kilic A, Althouse AD, Sharbaugh MS, Gupta A, Xu J, Fukui M, Simon MA, Schindler JT, Lee JS, Gleason TG, Cavalcante JL. Right ventricle to pulmonary artery coupling in patients undergoing transcatheter aortic valve implantation. Heart 2019; 105: 117–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tello K, Axmann J, Ghofrani HA, Naeije R, Narcin N, Rieth A, Seeger W, Gall H, Richter MJ. Relevance of the TAPSE/PASP ratio in pulmonary arterial hypertension. Int J Cardiol 2018; 266: 229–235. [DOI] [PubMed] [Google Scholar]
- 23. Deaconu S, Deaconu A, Scarlatescu A, Petre I, Onciul S, Vijiac A, Onut R, Zamfir D, Marascu G, Iorgulescu C, Radu DA, Bogdan S, Vatasescu R, Dorobantu M. Right ventricular‐arterial coupling—a new perspective for right ventricle evaluation in heart failure patients undergoing cardiac resynchronization therapy. Echocardiography 2021; 38: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 24. Bragança B, Trêpa M, Santos R, Silveira I, Fontes‐Oliveira M, Sousa MJ, Reis H, Torres S, Santos M. Echocardiographic assessment of right Ventriculo‐arterial coupling: Clinical correlates and prognostic impact in heart failure patients undergoing cardiac resynchronization therapy. J Cardiovasc Imaging 2020; 28: 109–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 – Flow Chart.
CRT = cardiac resynchronization therapy; LUMC = Leiden University Medical Center; PA = pulmonary artery; PASP = pulmonary artery systolic pressure; RV = right ventricular; TAPSE = tricuspid annular plane systolic excursion.


