Abstract
Aims
The study aimed to investigate the prevalence, phenotypic characteristics, and predictors of atrial fibrillation (AF) in patients presenting with cardiac amyloidosis (CA) of light‐chain (AL) or transthyretin (ATTR) type.
Methods and results
Clinical, biochemical, and echocardiographic data of patients presenting with CA between 2005 and 2020 were retrospectively collected. CA staging was based on established biomarker systems. Binomial logistic regression was run to analyse the effects of clinical variables on the likelihood of AF. The study included 133 patients [53% AL, 41% wild‐type (wt) ATTR‐CA, & 6% hereditary ATTR‐CA]. Mean age was 71 years, and 80% were male patients. AF was diagnosed in 64 (48%) patients (28% in AL‐CA, 80% in wtATTR, 13% in hATTR, P < 0.001). Patients with AF were older (74 vs. 69 years, P < 0.001), more likely to have wtATTR‐CA (67 vs. 16%, P < 0.001), exhibited more often New York Heart Association ≥ III symptoms (66 vs. 45%, P = 0.02) and carried a higher burden of comorbidities. AF patients had lower left ventricular ejection fraction (47 vs. 53%, P < 0.005), higher left atrial volume index (54 vs. 46 mL/m2, P = 0.007), higher pulmonary artery pressure (42 vs. 31 mmHg, P = 0.008), and worse tricuspid annular plane systolic excursion values (17 vs. 20 mm, P = 0.01). Mitral regurgitation ≥ Grade 2 was more frequent in AF (56 vs. 25%, P < 0.001). Higher ATTR‐CA stage was associated with higher AF prevalence (47% vs. 74% vs. 94%, P < 0.001, for Stages I, II, & III, respectively). Higher AL‐CA stage was associated with lower AF prevalence (0% vs. 40% vs. 31% vs. 18%, P < 0.001, for Stages I, II, IIIa, & IIIb, respectively). Three independent predictors for AF were identified in a multivariate logistic regression model with 81.5% classification accuracy: AL type [odds ratio (OR) 0.1, confidence interval (CI) 0.01–0.29, P = 0.001], estimated glomerular filtration rate (OR 0.9, CI 0.93–0.99, P = 0.03), and body mass index (OR 1.3, CI 1.07–1.66, P = 0.01). ATTR amyloidosis was associated with a 10‐fold higher risk of AF. During 1 year follow‐up, only one episode of ischaemic stroke was reported.
Conclusions
Atrial fibrillation affects nearly half of all patients with CA. Patients presenting with AF have more severe symptoms and higher burden of comorbidities. ATTR type of amyloidosis is the strongest predictor of AF. Prospective screening for occult AF may be considered in ATTR‐CA.
Keywords: Atrial fibrillation, Amyloidosis, Transthyretin, Light‐chain, Cardiomyopathy
Introduction
Cardiac amyloidosis (CA) has emerged as a previously underdiagnosed cardiomyopathy with hypertrophic and/or restrictive phenotype leading to progressive heart failure with high mortality rates. Two major types of amyloidosis affect the heart, transthyretin (ATTR), and light‐chain (AL) amyloidosis, each with distinct clinical features. While the hereditary form of ATTR amyloidosis (hATTR) and AL amyloidosis are universally recognized as rare diseases, wild‐type ATTR (wtATTR) amyloidosis has been repeatedly shown to affect a significant proportion of heart failure patients with increasing prevalence in older age groups. It is now known that up to 14% of patients with heart failure and preserved ejection function 1 , 2 , 3 and up to 16% of patients with severe aortic stenosis undergoing transcatheter aortic valve replacement 4 , 5 , 6 suffer from amyloid cardiomyopathy. Approximately 10% of patients with carpal tunnel syndrome have tenosynovial ATTR deposits often accompanied by occult amyloid cardiomyopathy. 7
Beyond left ventricular systolic and diastolic dysfunction, extracellular amyloid infiltration leads to further cardiac abnormalities including atrial dysfunction, valve stenosis or regurgitation, conduction abnormalities, tachyarrhythmias, and pericardial effusion. It has been shown that atrial fibrillation (AF) is one of the most common arrhythmias in CA. 8 , 9 , 10 , 11 To date, the association of AF with prognosis and other adverse clinical events has not been prospectively investigated in CA. However, retrospective studies reported high rates of intracardiac thrombus formation, arrhythmic, and thrombotic complications linked to external cardioversion and high rates of thromboembolic events in patients with concomitant CA and AF. 12 , 13 , 14 , 15 , 16 This implicates that screening for AF may be beneficial in these patients and that oral anticoagulation may be considered even in the absence of high thromboembolic risk according to traditional risk scores as in hypertrophic cardiomyopathy. The current study aims to investigate the prevalence, phenotypic characteristics, and predictors of AF in a contemporary cohort of treatment‐naïve patients presenting with AL or ATTR amyloidosis.
Methods
Design and material
This is a retrospective study including patients diagnosed with AL or ATTR‐CA at a tertiary care academic centre between May 2005 and June 2020. Patients with full medical reports and confirmed cardiac involvement were included in this analysis. Diagnosis of ATTR‐CA was made by endomyocardial biopsy or non‐invasive work‐up with bone scintigraphy and laboratory testing for plasma cell disease (serum‐free light‐chain assay, serum, and urine immunofixation) in compliance with current recommendations. The bone scintigraphy protocol included planar whole‐body acquisition followed by single‐photon emission computed tomography and low‐dose, non‐contrast computed tomography scan of the chest for the purposes of attenuation correction and anatomic localization 3 h after intravenous injection of approximately 700 Mbq of 99mTc‐labelled 3,3‐diphosphono‐1,2‐propanodicarboxylic acid (99mTc‐DPD). Cardiac uptake of Perugini Grade 2 or 3 was considered diagnostic of ATTR‐CA in the absence of indices of AL amyloidosis. Diagnosis of AL‐CA was based on tissue biopsy, this being endomyocardial biopsy in the majority of cases or biopsy of a surrogate site (subcutaneous fat or mucosal tissue) in combination with the presence of typical signs of CA by echocardiography or cardiac magnetic resonance imaging including late gadolinium enhancement studies and T1 mapping. Whole‐exome sequencing of the transthyretin gene was performed in all patients with ATTR amyloidosis to define disease genotype. Clinical and echocardiographic data were collected prospectively in an electronic database dedicated to clinical surveys. Data regarding past medical history were retrieved from the individual health records. The study received institutional review board approval (20‐9446‐BO).
Classification and staging
Patients were assigned to the AF group based on written reports with documented AF episodes, if they had previously received treatment for AF or if they were firstly diagnosed with AF at their initial presentation for CA. Symptom severity, arrhythmic burden, and thromboembolic risk were assessed according to the 2020 ESC Guidelines for AF. Staging for ATTR‐CA was based on the scoring system proposed by Gillmore et al. In this scoring system, Stage I is defined as N‐terminal pro‐B‐type natriuretic peptide (NT‐proBNP) ≤ 3000 ng/L and estimated glomerular filtration rate (eGFR) ≥ 45 mL/min, and Stage III is defined as NT‐proBNP > 3000 ng/L and eGFR < 45 mL/min while all other patients are assigned to Stage II. 17 Staging for AL amyloidosis was based on the modified Mayo 2004 criteria using cardiac troponin (cTn) and NT‐proBNP values with the following thresholds: cTnT > 0.035 μg/L or cTnI > 0.1 μg/L or high sensitivity TnT > 54 pg/mL and NT‐proBNP > 332 ng/L with a higher threshold of NT‐proBNP > 8500 ng/L to further define Stage IIIa and IIIb. Accordingly, patients were assigned to the following stages: Stage I: both biomarkers below threshold, Stage II: 1 biomarker above threshold, Stage IIIa: both biomarkers above threshold and NT‐proBNP < 8500 ng/L, and Stage IIIb: both biomarkers above threshold and additionally NT‐proBNP > 8500 ng/L. 18
Echocardiography
Transthoracic echocardiography studies were performed at diagnosis and included a comprehensive assessment by two‐dimensional measurements, colour flow, tissue, pulsed wave and continuous wave Doppler measurements for chamber quantification, estimation of systolic and diastolic function, and quantification of valve lesions according to current international guidelines. Native aortic valve stenosis was assessed based on the continuity equation. The severity of native mitral regurgitation was estimated by multiparametric assessment including vena contracta width and/or the proximal iso‐velocity surface area method. Measurements of left ventricular muscle mass and left atrial volume were indexed for body surface area. All images were prospectively stored and were available for retrospective analysis. Longitudinal strain was calculated using speckle tracking from the apical four‐chamber, three‐chamber, and two‐chamber views. Strain values from all segments were averaged to obtain the global longitudinal strain value.
Statistical analysis
Continuous variables are summarized as means (standard deviations) unless otherwise indicated, and categorical variables as counts (percentages). Continuous data were evaluated for normality of distribution with the Shapiro–Wilk test. The two‐sided t‐test was used for comparison of continuous, normally distributed data, otherwise the non‐parametric Mann–Whitney U‐test. The χ 2 test was used for testing the association between categorical variables. The Cochran–Armitage test of trend was run to determine whether a linear trend exists between the stage of CA and the proportion of patients who were concurrently diagnosed with AF. Binomial logistic regression was run to analyse the effects of clinical variables on the likelihood of AF. Variables were selected based on clinical relevance and were introduced in the model with the one‐step enter method. Linearity of the continuous variables with respect to the logit of the dependent variable was assessed via the Box–Tidwell procedure. The level of significance was set at 0.05. All analyses were performed using SPSS (IBM Corp., SPSS Statistics, Version 23.0. Armonk, NY).
Results
During the study period, 133 patients were diagnosed with CA and were included in the current analysis. The vast majority of patients (n = 101, 76%) were diagnosed after 2017 following the establishment of an interdisciplinary amyloidosis programme at our institution. Mean age was 71 years, and 80% were male patients. AL‐CA was diagnosed in 53%, wtATTR‐CA in 41%, and hATTR‐CA in 6% of patients. More than half of all patients suffered from severe HF symptoms New York Heart Association class III or IV and exhibited a significant burden of comorbidities, as shown in Table 1 . AF was diagnosed in 64 patients with CA (48%) either at presentation or prior to the diagnosis of CA. In Tables 1 and 2 , clinical, biochemical, and echocardiographic characteristics of patients with and without AF are shown. Patients with AF were older (74 vs. 69 years, P < 0.001) and more frequently diagnosed with wtATTR‐CA than patients in sinus rhythm (67 vs. 16%, P < 0.001). AF was diagnosed in 28% of patients with AL‐CA and 71% of those with ATTR‐CA.
Table 1.
Clinical characteristics of the study patients
| Variable | Overall (n = 133) | AF (n = 64) | SR (n = 69) | P value |
|---|---|---|---|---|
| Age | 70.6 ± 11.6 | 74.3 ± 8.8 | 68.8 ± 10.8 | <0.001 |
| Male | 106 (80) | 54 (84) | 52 (75) | 0.20 |
| CA type | ||||
| AL | 71 (53) | 20 (31) | 51 (74) | <0.001 |
| Hereditary ATTR | 8 (6) | 1 (2) | 7 (10) | |
| Wild‐type ATTR | 54 (41) | 43 (67) | 11 (16) | |
| Body mass index (kg/m2) | 25.6 ± 5.6 | 25.2 ± 3.4 | 26.1 ± 6.9 | 0.45 |
| NYHA class | ||||
| I/II | 60 (45) | 22 (34) | 38 (55) | 0.02 |
| III/IV | 73 (55) | 42 (66) | 31 (45) | |
| Comorbidities | ||||
| Arterial hypertension | 60 (45) | 33 (52) | 27 (39) | 0.15 |
| Chronic kidney disease | 55 (41) | 33 (52) | 22 (32) | 0.02 |
| Diabetes | 22 (16.5) | 15 (23) | 7 (10) | 0.04 |
| COPD | 5 (4) | 2 (3) | 3 (4) | 0.70 |
| Coronary artery disease | 40 (30) | 28 (44) | 12 (17) | 0.001 |
| Stroke | 11 (8) | 4 (6) | 7 (10) | 0.42 |
| Dyslipidaemia | 33 (25) | 21 (33) | 12 (17) | 0.04 |
| Anaemia | 64 (48) | 32 (50) | 32 (46) | 0.68 |
| Pacemaker | 13 (10) | 9 (14) | 4 (6) | 0.11 |
| Laboratory analysis | ||||
| NT‐proBNP (pg/mL) a | 4527 (8712) | 6141 (9890) | 3003 (10 925) | 0.08 |
| Haemoglobin (g/dL) | 12.4 ± 2.1 | 11.9 ± 2.3 | 12.1 ± 1.9 | 0.45 |
| eGFR (mL/min/1.73 m2) | 55 ± 23.7 | 47.3 ± 22.2 | 58.3 ± 27.1 | 0.41 |
| Creatinine (mg/dL) a | 1.6 (1.4) | 1.7 (0.9) | 1.5 (0.9) | 0.39 |
| Leucocyte count | 7.8 ± 3.2 | 7.7 ± 2.2 | 8.6 ± 5.0 | 0.90 |
| C‐reactive protein (mg/dL) a | 0.1 (1.3) | 0.1 (1.5) | 0.1 (1.6) | 0.20 |
AF, atrial fibrillation; AL, light‐chain amyloidosis; ATTR, transthyretin amyloidosis; CA, cardiac amyloidosis; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; NT‐proBNP, N‐terminal pro‐B‐type natriuretic peptide; NYHA, New York Heart Association; SR, sinus rhythm.
Values in brackets represent median (interquartile range).
Table 2.
Echocardiographic findings
| Variable | Overall (n = 133) | AF (n = 64) | SR (n = 69) | P value |
|---|---|---|---|---|
| LVEF (%) | 49 ± 12 | 47 ± 12 | 53 ± 9 | 0.005 |
| GLS (%) | −10.7 ± 4.1 | 10 ± 3.8 | −11 ± 4.3 | 0.44 |
| IVSd (mm) | 16 ± 5 | 17 ± 5 | 16 ± 5 | 0.36 |
| PWd (mm) | 15 ± 6 | 14 ± 4 | 14 ± 4 | 0.14 |
| LVEDD (mm) | 47 ± 8 | 47 ± 9 | 46 ± 8 | 0.30 |
| LVMMI (g/m2) | 168 ± 48 | 167 ± 47 | 162 ± 41 | 0.15 |
| LAVI (mL/m2) | 50 ± 20 | 54 ± 18 | 46 ± 20 | 0.007 |
| e' (cm/s) | 6.5 ± 2.5 | 6.1 ± 2.0 | 6.5 ± 2.7 | 0.09 |
| E/e' | 15 ± 7 | 17 ± 8 | 15 ± 7 | 0.13 |
| TRV (m/s) | 2.7 ± 0.5 | 2.8 ± 0.5 | 2.6 ± 0.6 | 0.07 |
| Systolic PAP (mmHg) a | 40 ± 15 | 42 ± 10 | 31 ± 20 | 0.008 |
| TAPSE (mm) | 18 ± 7 | 17 ± 6 | 20 ± 7 | 0.01 |
| RV free wall thickness (mm) | 7.3 ± 2.7 | 6.9 ± 2.3 | 7.5 ± 2.9 | 0.92 |
| Pericardial effusion | 23 (17) | 12 (19) | 11 (16) | 0.90 |
| Mitral regurgitation ≥ Grade 2 | 53 (40) | 36 (56) | 17 (25) | <0.001 |
| Aortic stenosis ≥ Grade 2 | 2 (1.5) | 1 (2) | 1 (1) | 1.00 |
AF, atrial fibrillation; e', peak early diastolic velocity of mitral valve annulus; E/e', peak early mitral inflow velocity to peak early diastolic velocity of mitral valve annulus; GLS, global longitudinal strain; IVSd, end‐diastolic interventricular septal diameter; LAVI, left atrial volume index; LVEDD, left ventricular end‐diastolic diameter; LVEF, left ventricular ejection fraction; LVMMI, left ventricular muscle mass index; PAP, pulmonary artery pressure; PWd, end‐diastolic posterior wall diameter; RV, right ventricular; SR, sinus rhythm; TAPSE, tricuspid annular plane systolic excursion; TRV, tricuspid regurgitation velocity.
Values in brackets represent median (interquartile range).
Overall, the echocardiographic phenotype of the study cohort was consistent with the cardinal abnormalities of CA with biventricular hypertrophy, preserved, or mildly reduced left ventricular ejection fraction (LVEF), and elevated LV filling pressures. AF patients had lower LVEF (47 vs. 53%, P < 0.005) and higher left atrial volume index (54 vs. 46 mL/m2, P = 0.007), higher systolic pulmonary artery pressure (42 vs. 31 mmHg, P = 0.008) and worse RV function as indicated by the tricuspid annular plane systolic excursion values (17 vs. 20 mm, P = 0.01). Mitral regurgitation of at least moderate severity was also more frequent in the AF group (56 vs. 25%, P < 0.001). Aortic stenosis was overall rarely observed in this cohort with no observed differences across the groups with AF and sinus rhythm.
For Stages I, II, and III of ATTR‐CA, the proportion of patients with AF was 47%, 74%, and 94%, respectively. The Cochran–Armitage test of trend showed a statistically significant linear trend (P < 0.001) with higher stage of ATTR‐CA being associated with a higher proportion of patients with AF (Table 3 ). For Stages I, II, IIIa, and IIIb of AL‐CA, the proportion of patients with AF was 0%, 40%, 31%, and 18%, respectively. The Cochran–Armitage test of trend showed a lower proportion of patients with AF in higher stages of AL‐CA (P < 0.001) (Table 3 and Figure 1 ).
Table 3.
Linear trend between AF and stage of CA
| AF*ATTR‐CA | Stage I, n = 19 | Stage II, n = 27 | Stage III, n = 16 | P value | |
|---|---|---|---|---|---|
| 9 (47) | 20 (74) | 15 (94) | <0.001 | ||
| AF*AL‐CA | Stage I, n = 3 | Stage II, n = 20 | Stage IIIa, n = 26 | Stage IIIb, n = 22 | P value |
|---|---|---|---|---|---|
| 0 (0) | 8 (40) | 8 (31) | 4 (18) | <0.00 |
AF, atrial fibrillation; ATTR‐CA, transthyretin cardiac amyloidosis; AL‐CA, light‐chain cardiac amyloidosis.
Figure 1.
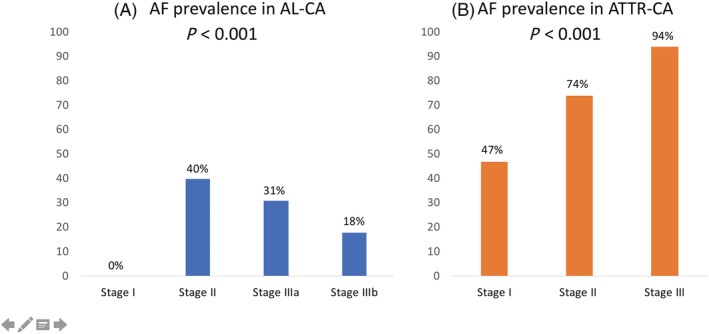
Prevalence of atrial fibrillation (AF) in different stages of (A) light‐chain cardiac amyloidosis (AL‐CA) and (B) transthyretin cardiac amyloidosis (ATTR‐CA). Cochran–Armitage test of trend revealed a linear trend for both AL‐CA and ATTR‐CA (P < 0.001).
Binomial logistic regression was performed to assess the effects of clinical variables on the likelihood of AF. These included age, body mass index (BMI), eGFR, amyloidosis type, gender, coronary artery disease, LVEF, left atrial volume index, mitral valve annulus velocity in tissue doppler imaging (e'), ratio of mitral inflow velocity of early ventricular filling by pulsed wave Doppler to mitral valve annular velocity in tissue Doppler imaging E/e', tricuspid annular plane systolic excursion, tricuspid regurgitation velocity, and NT‐proBNP. Based on the Box–Tidwell procedure, all continuous independent variables were found to be linearly related to the logit of the dependent variable. There was one standardized residual with a value of 2.86 standard deviations, which was kept in the analysis. The logistic regression model was statistically significant (x2(12) = 49.32, P < .001, Hosmer and Lemeshow goodness of fit test P = 0.768) with an overall classification accuracy of 81.5%. Of the predictor variables entered in Model 3 were found statistically significant: type of amyloidosis, eGFR, and BMI as shown in Table 4 . Patients with ATTR‐CA had 10 times higher odds for AF. Decreasing eGFR was associated with an increased likelihood of AF, and increasing BMI was associated with higher likelihood of AF (Table 4 ).
Table 4.
Binomial logistic regression predicting the likelihood of AF in CA
| Variable | Odds ratio | 95% CI | P value |
|---|---|---|---|
| AL type | 0.1 | 0.01, 0.29 | 0.001 |
| eGFR | 0.9 | 0.93, 0.99 | 0.03 |
| Body mass index | 1.3 | 1.07, 1.61 | 0.01 |
AF, atrial fibrillation; AL, light‐chain amyloidosis; CA, cardiac amyloidosis; eGFR, estimated glomerular filtration rate.
Further phenotypic analysis of the group of patients with AF according to the subtype of CA is shown in Table 5 . Patients with ATTR‐CA and AF were older (79 vs. 67 years, P < 0.001) and had higher thromboembolic risk according to the CHA2DS2‐VASc score (4.4 vs. 2.3, P < 0.001). Symptom severity and arrhythmic burden did not differ among the two amyloidosis types. Overall, 63% of patients with AF and CA had permanent or persistent AF. Patients with ATTR‐CA and AF received more frequent anticoagulation than patients with AL (86 vs. 60%, P = 0.03) and more frequently direct oral anticoagulants than the AL patients (68 vs. 30%, P = 0.004). There were no differences in other pharmacologic agents implemented for rhythm or rate control but interestingly 65% of all AF and CA patients received heart rate or rhythm modulating agents (59% beta‐blocker or calcium channel blocker, 6% digitalis, & 8% amiodaron). Regarding non‐pharmacologic antiarrhythmic treatment, 23% of AF patients had previously undergone external cardioversion and 13% at least once catheter ablation, with no differences in rates of interventional treatment across the two major types of CA. History of extracranial bleeding was reported in 16% of patients and ischaemic stroke or transient ischaemic attack in 6% of patients with no differences across the two major types of CA. No intracranial bleedings were reported in this cohort. During a 12 month follow‐up, only one episode of ischaemic stroke was reported in a patient with ATTR‐CA and AF who was on vitamin K oral anticoagulation.
Table 5.
Characteristics and treatment modalities of atrial fibrillation in patients with cardiac amyloidosis
| Variable | Overall (n = 64) | AL‐CA (n = 20) | ATTR‐CA (n = 44) | P value |
|---|---|---|---|---|
| Age | 75 ± 9 | 67 ± 9 | 79 ± 5 | <0.001 |
| Male | 54 (84) | 13 (20) | 41 (64) | 0.004 |
| CHA2DS2‐VASc score | 3.7 (1.5) | 2.3 (1.0) | 4.4 (1.2) | <0.001 |
| EHRA symptom severity scale | ||||
| I | 20 (31) | 4 (20) | 16 (36) | 0.41 |
| IIa/b | 32 (50) | 12 (60) | 20 (46) | |
| III | 12 (19) | 4 (20) | 8 (18) | |
| IV | 0 (0) | 0 (0) | 0 (0) | |
| AF burden | ||||
| Permanent | 24 (38) | 5 (25) | 19 (43) | 0.14 |
| Persistent | 16 (25) | 4 (20) | 12 (27) | |
| Paroxysmal | 24 (38) | 11 (55) | 13 (54) | |
| Pharmacologic treatments | ||||
| Anticoagulation | 50 (78) | 12 (60) | 38 (86) | 0.03 |
| Vitamin K antagonist | 14 (22) | 6 (43) | 8 (57) | 0.29 |
| Direct oral anticoagulant | 36 (56) | 6 (30) | 30 (68) | 0.004 |
| Digitalis glycosides | 4 (6) | 2 (10) | 2 (5) | 0.37 |
| Beta‐blocker/Calcium channel blocker | 38 (59) | 10 (50) | 28 (64) | 0.41 |
| Amiodaron | 5 (8) | 1 (5) | 4 (9) | 0.50 |
| Non‐pharmacologic treatments | ||||
| Pacemaker | 9 (14) | 2 (10) | 7 (16) | 0.53 |
| Current cardioversion | 15 (23) | 3 (15) | 12 (27) | 0.28 |
| Catheter ablation | 8 (13) | 1 (5) | 7 (16) | 0.22 |
| Adverse clinical events | ||||
| Bleeding | 10 (16) | 4 (20) | 6 (14) | 0.71 |
| Stroke/TIA | 4 (6) | 2 (10) | 2 (5) | 0.37 |
AL, light‐chain amyloidosis; ATTR, transthyretin amyloidosis; CA, cardiac amyloidosis; EHRA, European Heart Rhythm Association; TIA, transient ischaemic attack.
Discussion
Our analysis demonstrates that nearly half of patients with AL‐CA or ATTR‐CA have concomitant AF at presentation. AF prevalence in wtATTR‐CA was 80% in our cohort, which is nearly three‐fold higher than that of AL‐CA patients and the second highest rate reported in patients with wtATTR‐CA in the literature to date (40–88%). 8 , 9 , 10 , 15 , 19 , 20 Overall, AF prevalence was much higher than the age‐adjusted prevalence in the general population, 21 and the 13–27% reported rate in major HF trials. 22 Thus, it has to be assumed that AF and CA have a causal relationship and are not solely comorbid conditions. This is also supported by previously published histopathologic and imaging findings of a quasi‐ubiquitous amyloid infiltration of the interstitial space of both atrial and ventricular myocardium with a potentially direct impact on the electrical properties of the atrial myocardium and conduction system. 23 , 24
Patients with AF had more severe HF symptoms, more comorbidities, and more severe echocardiographic abnormalities including worse left and right ventricular function, higher pulmonary artery pressures, and higher rates of mitral regurgitation. It was not part of the study protocol to evaluate the net impact of AF on survival and clinical outcomes and if different treatment modalities of AF, for example, rate vs. rhythm control would be beneficial in terms of improved prognosis. However, previous retrospective cohorts already demonstrated that AF did not have an impact on the long‐term survival of CA patients. 8 , 9 , 10 , 20 Prospective data are still needed to sufficiently address any association of AF with clinical events and the potential to modify any incremental risk associated with AF in amyloidosis.
Atrial fibrillation demonstrated a linear relationship with the stage of ATTR‐CA with increasing prevalence from Stages I to III. Patients in Stage III ATTR‐CA had 96% prevalence of AF at diagnosis. This finding also supports the causal association of AF with amyloid deposits beyond other traditional risk factors for AF that often coexist in patients of older age. In contrary, patients with AL‐CA had lower AF prevalence with increasing disease stage. This is an interesting finding that suggests investigation for AF even at early disease stages in AL‐CA. AF has been investigated in AL‐CA by two groups so far without previous analysis of its association with the disease stage. 8 , 9 Although this relationship is not anticipated, it must be taken into account that biomarker‐based disease staging in AL (based on NT‐proBNP and troponin values) does not reflect hemodynamic alterations that are also involved in the pathogenesis of AF. Amyloidogenic light chains modulate p38 mitogen‐activated protein kinase, which can directly promote NT‐proBNP expression. As such, for the same range of haemodynamic abnormalities plasma levels of NT‐proBNP are possibly higher in AL‐CA than in ATTR‐CA. Another explanation of this negative correlation of AF and stage of AL‐CA could be the rapidly evolving interstitial amyloid infiltration in AL‐CA (frequently a few months) as opposed to the slower development of structural changes in ATTR patients with chronic progressive atrial dilatation and elevated filling pressures presumably evolving over several years.
Transthyretin type of amyloidosis was shown to be a strong predictor of AF associated with 10‐fold higher risk for AF. BMI and eGFR were also independent predictors of AF, a finding that was previously reported from other groups. 8 These results justify systematic screening for occult AF in patients with CA, especially in those with ATTR and higher disease stage, that should be integrated in the standardized follow‐up assessment of these patients.
Among patients with AF and CA, we observed that ATTR‐CA patients were older, more frequently male, had higher CHA2DS2‐VASc scores, and received more frequently anticoagulants compared with AL patients. Overall, nearly 60% of AF patients received beta‐blockers or calcium channel blockers, an issue requiring attention in the context of a disease known to negatively affect the conduction system and chronotropy and to be associated with hypotension and intolerance of pharmacological blood pressure reductions. Furthermore, 23% of AF patients reported at least one cardioversion, 13% had at least one catheter ablation for AF prior to diagnosis, and 14% had implanted pacemaker. In patients with CA, recurrence rates after external cardioversion have been reported to be high after 1 year, ranging from 48% to 70%. 9 , 20 A retrospective study in 119 patients with AF and ATTR‐CA who underwent cardioversion reported that maintenance of sinus rhythm after 1 year was associated with the disease stage (74%, 33%, and 18% for Stages I, II, & III after 1 year, respectively). 20 Similarly, a small study (n = 24) showed high recurrence rates of AF after catheter ablation (58% in 39 months) with Stage III disease being associated with 90% recurrence rate. Overall, scarce data exist regarding the prognostic value of cardioversion and ablation procedures in CA. With the exception of more anticoagulated patients in the ATTR group, there were no differences regarding pharmacologic and non‐pharmacologic treatments across the two subtypes of CA in our study. A further analysis of the timeline and effectiveness of the various treatments of AF before presentation was not possible at this point due to the fact that our clinic serves as a referral centre and many patients were previously treated in remote facilities (missing data and source of recall bias).
We demonstrated that CHA2DS2‐VASc score in patients with concomitant CA and AF was high with nearly double scores in ATTR‐CA compared with AL‐CA, which is an anticipated finding in light of the higher age and higher comorbidities of patients with ATTR‐CA. However, there is ambiguity regarding the predictability of thrombotic risk with the CHA2DS2‐VASc score in CA due to growing evidence of an inherent thrombogenicity beyond traditional risk factors. This is substantiated by retrospective observational studies reporting high rates of intracardiac thrombosis in CA detected either post‐mortem or during transesophageal echocardiography with a high proportion of these patients (20%) being in sinus rhythm. 25 , 26 , 27 A recent study reported 38% rates of systemic thromboembolism [peripheral embolism, ischaemic stroke, or transient ischemic attack (TIA)] in 68 patients with wtATTR‐CA and concomitant AF at a median follow‐up of 3.6 years despite oral anticoagulation in 96% of patients. 15 Current recommendations for CA consider initiation of anticoagulation in patients with AF and CA even with low CHA2DS2‐VASc score, as it is also the case for hypertrophic cardiomyopathy. 28 Whether this strategy will reduce thromboembolic complications remains to be evaluated in future studies. In our study, prior ischaemic strokes or transient ischaemic attacks (TIA) were reported in 6% of all CA patients. At 12 month follow‐up, only one patient with known AF and ATTR‐CA out of 133 patients experienced an ischemic stroke while on treatment with oral vitamin K antagonist. This is in accordance with the results of a previous study reporting no thromboembolic events in 38 patients with AF and CA at 2.5 years of follow‐up. 8 All patients in that study received anticoagulation with warfarin. It is, however, still possible that the incidence of stroke would rise with longer follow‐up of the study. Despite lack of robust data regarding the prognostic impact of AF in CA, the overall high arrhythmic burden in these patients including conduction disease and ventricular tachyarrhythmias justifies upfront screening with Holter monitoring or even implantable loop recorders.
The main limitation of the current study is its retrospective design. Data retrieval from digital reports hampers non‐biased analysis of important clinical questions including arrhythmic burden, effectiveness of AF treatment modalities, and the temporal association of AF with CA. Standardized evaluation and follow‐up of patients with CA was established in 2018 at our site and ad hoc Holter assessments were not performed in all patients before this period. Therefore, occult AF may have gone undetected. The strength of the study is the comprehensive phenotypic characterization of a large contemporary patient cohort with both major types of CA equally represented.
Our study highlights the high incidence of AF in CA and the importance of systematic screening for AF especially in patients with ATTR‐CA of higher disease stage. Patients presenting with AF have more severe symptoms, more depressed cardiac function, and a higher burden of comorbidities. The incidence of ischaemic stroke or TIA was low at 12 months after diagnosis in this cohort with 78% anticoagulation rate. The optimal treatment strategy of AF in CA remains an issue of high clinical relevance that needs to be addressed by prospective studies with randomized study design.
Conflict of interest
None declared.
Funding
This work was supported by the Universitätsmedizin Essen Clinician Scientist Acedemy (UMEA) and the German Research Foundation (DFG, Deutsche Forschungs‐Gemeinschaft) (FU356/12‐1 to M.P.) and the DFG (LU2139/2‐1 to P.L. and RA969/12‐1 to T.R.).
Acknowledgements
We would like to thank Mrs Lucie Hiepen for her contribution to data collection. Open Access funding enabled and organized by Projekt DEAL.
Papathanasiou, M. , Jakstaite, A.‐M. , Oubari, S. , Siebermair, J. , Wakili, R. , Hoffmann, J. , Carpinteiro, A. , Hagenacker, T. , Thimm, A. , Rischpler, C. , Kessler, L. , Rassaf, T. , and Luedike, P. (2022) Clinical features and predictors of atrial fibrillation in patients with light‐chain or transthyretin cardiac amyloidosis. ESC Heart Failure, 9: 1740–1748. 10.1002/ehf2.13851.
References
- 1. González‐López E, Gallego‐Delgado M, Guzzo‐Merello G, de Haro‐del Moral FJ, Cobo‐Marcos M, Robles C, Bornstein B, Salas C, Lara‐Pezzi E, Alonso‐Pulpon L, Garcia‐Pavia P. Wild‐type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J 2015; 36: 2585–2594. [DOI] [PubMed] [Google Scholar]
- 2. Mohammed SF, Mirzoyev SA, Edwards WD, Dogan A, Grogan DR, Dunlay SM, Roger VL, Gertz MA, Dispenzieri A, Zeldenrust SR, Redfield MM. Left ventricular amyloid deposition in patients with heart failure and preserved ejection fraction. JACC Hear Fail 2014; 2: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hahn VS, Yanek LR, Vaishnav J, Ying W, Vaidya D, Lee YZJ, Riley SJ, Subramanya V, Brown EE, Hopkins CD, Ononogbu S, Perzel Mandell K, Halushka MK, Steenbergen C Jr, Rosenberg AZ, Tedford RJ, Judge DP, Shah SJ, Russell SD, Kass DA, Sharma K. Endomyocardial biopsy characterization of heart failure with preserved ejection fraction and prevalence of cardiac amyloidosis. JACC Hear Fail 2020; 8: 712–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Treibel TA, Fontana M, Gilbertson JA, Castelletti S, White SK, Scully PR, Roberts N, Hutt DF, Rowczenio DM, Whelan CJ, Ashworth MA, Gillmore JD, Hawkins PN, Moon JC. Occult transthyretin cardiac amyloid in severe calcific aortic stenosis. Circ Cardiovasc Imaging 2016; 9: 1–10. [DOI] [PubMed] [Google Scholar]
- 5. Castano A, Narotsky DL, Hamid N, Khalique OK, Morgenstern R, DeLuca A, Rubin J, Chiuzan C, Nazif T, Vahl T, George I. Unveiling transthyretin cardiac amyloidosis and its predictors among elderly patients with severe aortic stenosis undergoing transcatheter aortic valve replacement. Eur Heart J 2017; 38: 2879–2887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scully PR, Treibel TA, Fontana M, Lloyd G, Mullen M, Pugliese F, Hartman N, Hawkins PN, Menezes LJ, Moon JC. Prevalence of cardiac amyloidosis in patients referred for transcatheter aortic valve replacement. J Am Coll Cardiol 2018; 71: 463–464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, Shapiro D, Evans PJ, Maschke S, Kilpatrick SE, Tan CD, Rodriguez ER, Monteiro C, Tang WHW, Kelly JW, Seitz WH Jr, Hanna M. Tenosynovial and cardiac amyloidosis in patients undergoing carpal tunnel release. J Am Coll Cardiol 2018; 72: 2040–2050. [DOI] [PubMed] [Google Scholar]
- 8. Longhi S, Quarta CC, Milandri A, Lorenzini M, Gagliardi C, Manuzzi L, Bacchi‐Reggiani ML, Leone O, Ferlini A, Russo A, Gallelli I, Rapezzi C. Atrial fibrillation in amyloidotic cardiomyopathy: prevalence, incidence, risk factors and prognostic role. Amyloid 2015; 22: 147–155. [DOI] [PubMed] [Google Scholar]
- 9. Sanchis K, Cariou E, Colombat M, Ribes D, Huart A, Cintas P, Fournier P, Rollin A, Carrié D, Galinier M, Maury P, Duparc A, Lairez O, On behalf of the Toulouse Amyloidosis Research Network collaborators . Atrial fibrillation and subtype of atrial fibrillation in cardiac amyloidosis: clinical and echocardiographic features, impact on mortality. Amyloid 2019; 26: 128–138. [DOI] [PubMed] [Google Scholar]
- 10. Mints YY, Doros G, Berk JL, Connors LH, Ruberg FL. Features of atrial fibrillation in wild‐type transthyretin cardiac amyloidosis: a systematic review and clinical experience. ESC Hear Fail 2018; 5: 772–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Giancaterino S, Urey MA, Darden D, Hsu JC. Management of arrhythmias in cardiac amyloidosis. JACC Clin Electrophysiol 2020; 6: 351–361. [DOI] [PubMed] [Google Scholar]
- 12. Feng DL, Edwards WD, Oh JK, Chandrasekaran K, Grogan M, Martinez MW, Syed II, Hughes DA, Lust JA, Jaffe AS, Gertz MA, Klarich KW. Intracardiac thrombosis and embolism in patients with cardiac amyloidosis. Circulation 2007; 116: 2420–2426. [DOI] [PubMed] [Google Scholar]
- 13. Feng DL, Syed IS, Martinez M, Oh JK, Jaffe AS, Grogan M, Edwards WD, Gertz MA, Klarich KW. Intracardiac thrombosis and anticoagulation therapy in cardiac amyloidosis. Circulation 2009; 119: 2490–2497. [DOI] [PubMed] [Google Scholar]
- 14. Cappelli F, Tini G, Russo D, Emdin M, del Franco A, Vergaro G, di Bella G, Mazzeo A, Canepa M, Volpe M, Perfetto F, Autore C, di Mario C, Rapezzi C, Musumeci MB. Arterial thrombo‐embolic events in cardiac amyloidosis: a look beyond atrial fibrillation. Amyloid 2021; 28: 12–18. [DOI] [PubMed] [Google Scholar]
- 15. Bukhari S, Barakat AF, Eisele YS, Nieves R, Jain S, Saba S, Follansbee WP, Brownell A, Soman P. Prevalence of atrial fibrillation and thromboembolic risk in wild‐type transthyretin amyloid cardiomyopathy. Circulation 2021; 143: 1335–1337. [DOI] [PubMed] [Google Scholar]
- 16. Ballantyne B, Manian U, Sheyin O, Davey R, De S. Stroke risk and atrial mechanical dysfunction in cardiac amyloidosis. ESC Hear Fail 2020; 7: 705–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gillmore JD, Damy T, Fontana M, Hutchinson M, Lachmann HJ, Martinez‐Naharro A, Quarta CC, Rezk T, Whelan CJ, Gonzalez‐Lopez E, Lane T, Gilbertson JA, Rowczenio D, Petrie A, Hawkins PN. A new staging system for cardiac transthyretin amyloidosis. Eur Heart J 2018; 39: 2799–2806. [DOI] [PubMed] [Google Scholar]
- 18. Wechalekar AD, Schonland SO, Kastritis E, Gillmore JD, Dimopoulos MA, Lane T, Foli A, Foard D, Milani P, Rannigan L, Hegenbart U, Hawkins PN, Merlini G, Palladini G. A European collaborative study of treatment outcomes in 346 patients with cardiac stage III AL amyloidosis. Blood 2013; 121: 3420–3427. [DOI] [PubMed] [Google Scholar]
- 19. Pinney JH, Whelan CJ, Petrie A, Dungu J, Banypersad SM, Sattianayagam P, Wechalekar A, Gibbs SD, Venner CP, Wassef N, McCarthy C, Gilbertson JA, Rowczenio D, Hawkins PN, Gillmore JD, Lachmann HJ. Senile systemic amyloidosis: clinical features at presentation and outcome. J Am Heart Assoc 2013; 2: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Donnellan E, Wazni O, Kanj M, Elshazly MB, Hussein A, Baranowski B, Hanna M, Patel D, Trulock K, Martyn M, Menon V, Saliba W, Jaber WA. Atrial fibrillation ablation in patients with transthyretin cardiac amyloidosis. Europace 2020; 22: 259–264. [DOI] [PubMed] [Google Scholar]
- 21. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, la Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Thomas GN, Valgimigli M, van Gelder IC, van Putte BP, Watkins CL, ESC Scientific Document Group , Kirchhof P, Kühne M, Aboyans V, Ahlsson A, Balsam P, Bauersachs J, Benussi S, Brandes A, Braunschweig F, Camm AJ, Capodanno D, Casadei B, Conen D, Crijns HJGM, Delgado V, Dobrev D, Drexel H, Eckardt L, Fitzsimons D, Folliguet T, Gale CP, Gorenek B, Haeusler KG, Heidbuchel H, Iung B, Katus HA, Kotecha D, Landmesser U, Leclercq C, Lewis BS, Mascherbauer J, Merino JL, Merkely B, Mont L, Mueller C, Nagy KV, Oldgren J, Pavlović N, Pedretti RFE, Petersen SE, Piccini JP, Popescu BA, Pürerfellner H, Richter DJ, Roffi M, Rubboli A, Scherr D, Schnabel RB, Simpson IA, Shlyakhto E, Sinner MF, Steffel J, Sousa‐Uva M, Suwalski P, Svetlosak M, Touyz RM, Dagres N, Arbelo E, Bax JJ, Blomström‐Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, Fauchier L, Filippatos G, Kalman JM, la Meir M, Lane DA, Lebeau JP, Lettino M, Lip GYH, Pinto FJ, Neil Thomas G, Valgimigli M, van Gelder IC, Watkins CL, Delassi T, Sisakian HS, Scherr D, Chasnoits A, Pauw MD, Smajić E, Shalganov T, Avraamides P, Kautzner J, Gerdes C, Alaziz AA, Kampus P, Raatikainen P, Boveda S, Papiashvili G, Eckardt L, Vassilikos V, Csanádi Z, Arnar DO, Galvin J, Barsheshet A, Caldarola P, Rakisheva A, Bytyçi I, Kerimkulova A, Kalejs O, Njeim M, Puodziukynas A, Groben L, Sammut MA, Grosu A, Boskovic A, Moustaghfir A, Groot N, Poposka L, Anfinsen OG, Mitkowski PP, Cavaco DM, Siliste C, Mikhaylov EN, Bertelli L, Kojic D, Hatala R, Fras Z, Arribas F, Juhlin T, Sticherling C, Abid L, Atar I, Sychov O, Bates MGD, Zakirov NU. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio‐Thoracic Surgery (EACTS). Eur Heart J 2021; 42: 373–498. [DOI] [PubMed] [Google Scholar]
- 22. Staerk L, Sherer JA, Ko D, Benjamin EJ, Helm RH. Atrial fibrillation: epidemiology, pathophysiology, clinical outcomes. Circ Res 2017; 120: 1501–1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Leone O, Boriani G, Chiappini B, Pacini D, Cenacchi G, Martin Suarez S, Rapezzi C, Bacchi Reggiani ML, Marinelli G. Amyloid deposition as a cause of atrial remodelling in persistent valvular atrial fibrillation. Eur Heart J 2004; 25: 1237–1241. [DOI] [PubMed] [Google Scholar]
- 24. Röcken C, Peters B, Juenemann G, Saeger W, Klein HU, Huth C, Roessner A, Goette A. Atrial amyloidosis: an arrhythmogenic substrate for persistent atrial fibrillation. Circulation 2002; 106: 2091–2097. [DOI] [PubMed] [Google Scholar]
- 25. Henein MY, Lindqvist P. Response: Atrial impairment in transthyretin cardiac amyloidosis: an early marker of cardiac involvement and a prognostic factor. Amyloid 2018; 25: 136. [DOI] [PubMed] [Google Scholar]
- 26. Russo D, Rosario L, Arcari L, Autore C, Musumeci MB. Predicting the unpredictable: how to score the risk of stroke in cardiac amyloidosis? J Am Coll Cardiol 2019; 73: 2910–2911. [DOI] [PubMed] [Google Scholar]
- 27. El‐Am EA, Dispenzieri A, Grogan M, Nkomo VT. Reply: cardiac amyloidosis, atrial arrhythmias, thrombus, and stroke: a vexing problem. J Am Coll Cardiol 2019; 73: 2911–2913. [DOI] [PubMed] [Google Scholar]
- 28. Garcia‐Pavia P, Rapezzi C, Adler Y, Arad M, Basso C, Brucato A, Burazor I, Caforio ALP, Damy T, Eriksson U, Fontana M, Gillmore JD, Gonzalez‐Lopez E, Grogan M, Heymans S, Imazio M, Kindermann I, Kristen AV, Maurer MS, Merlini G, Pantazis A, Pankuweit S, Rigopoulos AG, Linhart A. Diagnosis and treatment of cardiac amyloidosis: a position statement of the ESC working group on myocardial and pericardial diseases. Eur Heart J 2021; 42: 1554–1568. [DOI] [PMC free article] [PubMed] [Google Scholar]


