Abstract
Aim
Cancer treatments are associated with cardiotoxic events that predispose to cardiac pathology and compromise the survival of patients, making necessary the identification of new molecular biomarkers to detect cardiotoxicity. This scoping review aims to identify the available evidence on novel molecular biomarkers associated with cardiotoxicity in the adult population undergoing cancer therapy.
Methods and results
The databases Medline, Web of Science, Scopus, and Embase were screened for the identification of published studies until 23 August 2020, searching for novel molecular biomarkers reported in cancer therapy‐related cardiac dysfunction in adult patients. A total of 42 studies that met the eligibility criteria were included. Fourteen studies reported 44 new protein biomarkers, 18 studies reported 57 new single nucleotide polymorphism biomarkers, and 11 studies reported 171 new gene expression profiles associated with cardiotoxicity. Data were extracted for 272 novel molecular biomarkers reported and evaluated in 7084 cancer patients, of which only 13 were identified in more than one study (MPO, sST2, GDF‐15, TGF‐B1, rs1056892, rs1883112, rs4673, rs13058338, rs1695, miR‐1, miR‐25‐3p, miR‐34a‐5p, and miR‐423‐5p), showing values for area under the curve > 0.73 (range 0.74–0.85), odds ratio 0.26–7.17, and hazard ratio 1.28–1.80.
Conclusions
Multiple studies presented a significant number of novel molecular biomarkers as promising predictors for risk assessment of cardiac dysfunction related to cancer therapy, but the characteristics of the studies carried out and the determinations applied do not allow suggesting the clinical use of these molecular biomarkers in the assessment of cancer therapy‐induced cardiotoxicity.
Keywords: Cardiotoxicity, Molecular biomarkers, LVEF, CTRCD, Cancer therapy, Cardio‐oncology/onco‐cardiology
Introduction
The relationship between cancer and cardiovascular diseases (CVDs) is studied in the field of cardio‐oncology/onco‐cardiology. 1 , 2 This relationship includes the cardiotoxicity associated to cancer therapies. 3 Cancer therapy‐related cardiac dysfunction (CTRCD) is recognized as one of the main causes of mortality among breast, prostate, and bladder cancer survivors, even surpassing the mortality related to recurrence of the baseline malignancy, 3 , 4 , 5 and impact the prognosis of patients treated in the short and long term. 6 The cardiotoxic events include myocardial dysfunction, heart failure (HF), coronary artery disease, valvular disease, arrhythmias, pericardial disease, hypertension, and thrombolytic events, among others. 7 , 8 The cardiac imaging societies define cardiotoxicity as a serial decline of left ventricular ejection fraction (LVEF) despite symptoms, 9 and despite existence of other cardiac imaging parameters, such as global longitudinal strain (GLS), which could be used to predict CTRCD. 10 In addition, the LVEF as a diagnostic tool for early cardiomyopathy has poor sensitivity and variable reproducibility, 11 highlighting the need to identify novel cardiotoxicity biomarkers. The molecular biomarkers (as protein, DNA, or RNA), among other characteristics, must detect cardiac lesions in earlier stages of the disease, monitoring the therapeutic benefit of cancer therapy with the risk of cardiotoxicity and identifying high‐risk patients who required closer cardiac surveillance or the establishment of cardioprotective strategies. 12 , 13
The molecular biomarkers currently accepted in the clinic are cardiac troponins and natriuretic peptides (BNPs) and their use for cardiovascular toxicity diagnosis, during and after cancer therapies, have been recently discussed. For example, Pudil et al., 2020, reviewed the role of cardiac biomarkers to monitor cardiac safety during surveillance of cancer patients receiving radiotherapy, chemotherapy, or targeted therapies. 14 Cardiac troponins are the gold standard biomarkers for the detection of cardiac injury and cardiomyocyte necrosis, and the most extensively used biomarkers to detect cardiac toxicity. BNP and N‐terminal pro‐B type natriuretic peptides (NT‐proBNPs) are biomarkers of long‐term cardiovascular dysfunction in asymptomatic patients. 15 , 16 However, these biomarkers are not useful in all cases, generating contradictory results. 17 , 18 Therefore, the search for new biomarkers is necessary. For instance, microRNAs (miRNAs) that mediate cardiac hypertrophy and fibrosis may represent important biomarkers of HF, 19 as well as single nucleotide polymorphisms (SNPs) that are associated with patients at increased risk of cardiotoxicity due to cancer treatment. 20
Several studies have reported new molecular biomarkers of cardiac dysfunction, but there is no clarity on its potential as biomedical tools to recognize cardiotoxicity risk. Therefore, we conducted a scoping review 21 to identify the emerging evidence on novel molecular biomarkers of cancer therapy‐induced cardiotoxicity in adult population, as well as to identify existing gaps in scientific knowledge on this topic.
Material and methods
Our protocol was developed and written according to the scoping review methodological framework proposed by Arksey and O'Malley. 22
Literature searches were performed in the following information sources: Medline (by PubMed), Web of Science (WoS), Scopus, and Embase. An initial search was performed by two authors (A. L. R.‐C. and I. C.‐E.) on all included databases using keywords and index terms, followed by an analysis of the text contained in the title and abstracts, and the index terms used to describe the study (Supporting Information, Table S1 ). In addition to electronic databases, searching bibliographies and reference lists from relevant publications were checked for potentially relevant studies. All searches were carried out until 23 August 2020.
All identified citations were collated, and duplicates were removed. Abstracts and full‐text reviews were screened and selected by two reviewers (A. L. R.‐C. and I. C.‐E.) based on the inclusion–exclusion criteria (Supporting Information, Table S2 ). Inter‐reviewer disagreement was handled by a third reviewer (M. T.‐F.). The scoping review was guided following the Preferred Reporting Items for Systematic Reviews and Meta‐Analyses extension for Scoping Reviews (PRISMA‐ScR). 23
For all included studies, the following data collection item was recorded: study characteristics, doi, author, year of publication, country, study designs, population characteristics, cardiotoxicity definition, and biological sample (Supporting Information, Table S3 , Sheets A–E). The collection information was performed by I. C.‐E. and cross‐checked by A. L. R.‐C.
Results
Study selection and characteristics
A total of 3484 publications from 2012 to 2020 were identified (Figure 1 ). Duplicated articles and those that did not meet the inclusion criteria during the initial screening of titles and abstracts and full‐text analysis were excluded. Additionally, nine articles were incorporated by hand searching by checking the reference lists of relevant studies. Finally, 42 studies were examined in this scoping review (Supporting Information, Table S3 , Sheet E).
Figure 1.
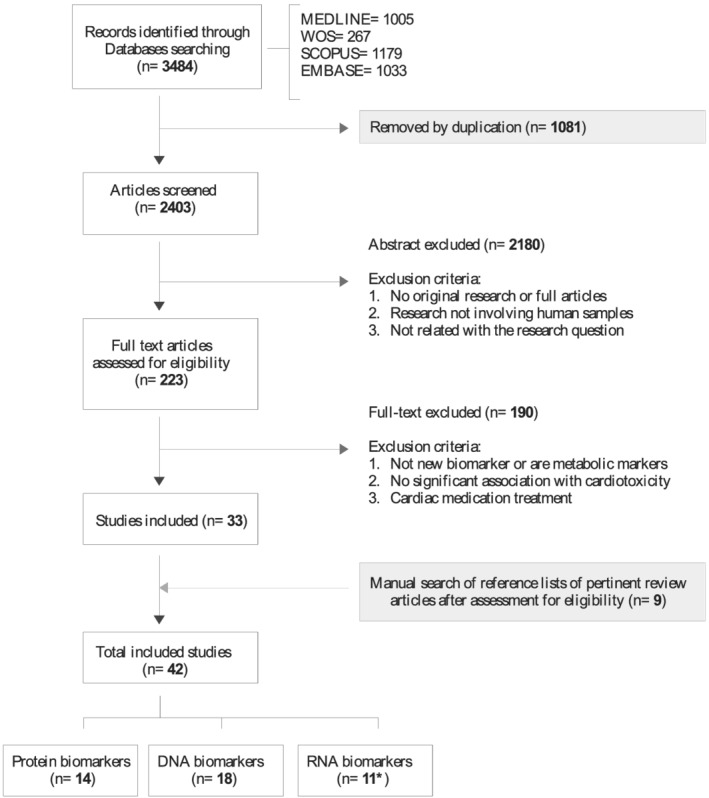
PRISMA flow diagram of the selection process and exclusion criteria. *The article Frères et al., 2018, reported protein and RNA biomarkers and it is repeated in the n of these biomarker types, but not in the total included studies.
The study design was correctly defined in 22 studies (cohort n = 12, case–control n = 7, cross‐sectional n = 2, and randomized n = 1) and only 40.4% of the studies were prospective (Table 1 ). The studies were conducted in North America (n = 17), Europe (n = 16), Asia (n = 8), and South America (n = 1). The most common type of cancer was breast cancer (n = 30), followed by haematological malignancies (leukaemia, lymphomas, or multiple myeloma) (n = 5), lung cancer (n = 1), and colorectal cancer (n = 1). For the remaining five studies, multiple cancer types were examined. Cancer therapies were chemotherapy (n = 22), radiotherapy (n = 4), or target therapy (n = 7), and nine articles reported the use of combined therapies. The definition of cardiotoxicity varied across studies, but 66.7% used the reduction in LVEF defined by the CTCAE. 24
Table 1.
Study characteristics summary
| Variable | Treated (n) | Cardiotoxicity (n) | Included studies | |
|---|---|---|---|---|
| Patients | 6766 | 1166 | 42 | |
| Type of cancer | Breast | 4532 | 7 71 | 30 |
| Haematological | 801 | 149 | 5 | |
| Colorectal | 198 | 98 | 1 | |
| Lung | 63 | 11 | 1 | |
| Various types | 1172 | 37 | 5 | |
| Cancer treatment | Chemotherapy | 4667 | 571 | 24 |
| Target therapy | 1155 | 219 | 7 | |
| Radiotherapy | 253 | 64 | 4 | |
| Combined | 691 | 31 2 | 7 | |
| Continent | North America | 2606 | 305 | 17 |
| Europe | 2351 | 584 | 16 | |
| Asia | 1753 | 267 | 8 | |
| South America | 56 | 10 | 1 | |
| Study design | Cohort | 1538 | 661 | 12 |
| Case–control | 467 | 165 | 7 | |
| Cross‐sectional | 338 | 191 | 2 | |
| Randomized | 450 | 87 | 1 | |
| Others | 3973 | 62 | 20 | |
| Cardiotoxicity | LVEF decline | 4251 | 795 | 28 |
| Clinical symptoms | 2080 | 220 | 10 | |
| Others | 526 | 151 | 4 | |
| Markers | Protein | 1002 | 338 | 14 |
| DNA | 4920 | 680 | 18 | |
| RNA | 899 | 148 | 11 | |
LVEF, left ventricular ejection fraction; n, number.
In the haematological type of cancer, leukaemia, lymphomas, or multiple myeloma were included. North America included USA and Canada; Europe included the Netherlands, France, Belgium, Spain, Greece, Romania, Italia, France, Norway, Turkey, and Finland; Asia included China and Japan; and South America included Brazil.
Finally, 14, 18, and 11 studies reported novel proteins, DNA, and RNA molecular biomarkers, respectively, associated with cardiotoxicity (Figure 1 and Table 1 ).
Patients characteristics
The analysed population consisted of 7084 cancer patients (age range from 43 to 65.7, median 50 years) with 70.9% (n = 5018) of females. A total of 6766 patients were treated with cancer therapy and 1166 patients reported cardiotoxicity.
Novel protein biomarkers associated with cardiotoxicity
Fourteen studies evaluated the relationship between levels of 44 circulating proteins and cardiotoxicity induced by cancer therapy (Table 2 and Supporting Information, Table S3 , Sheet F). 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 , 33 , 34 , 35 , 36 , 37 , 38 Only transforming growth factor‐beta 1 (TGF‐β1), interleukin‐1 suppression of tumorigenicity 2 (ST‐2), myeloperoxidase (MPO), phosphatidylinositol glycan anchor biosynthesis class F (PIGF), and growth differentiation factor 15 (GDF‐15) were repeatedly evaluated in eight studies. 25 , 26 , 27 , 28 , 29 , 30 , 31 , 32 All these proteins have been associated with cardiac dysfunction; for example, the soluble form of ST‐2 is secreted by cardiac cells in response to myocardial stress and fibrosis. 39 The determination of proteins in plasma was done by immunoassays (n = 11) and high‐throughput (n = 3) techniques (Table 2 ).
Table 2.
Proteins associated with cancer therapy‐induced cardiotoxicity
| Study ref. | Sample size | Events | Type of cancer | Therapy | Biomarker | Cardiotoxicity association | Detection method | AUC | Timing of biomarker assessment |
|---|---|---|---|---|---|---|---|---|---|
| 25 | 66 | 20 | Breast | Radiotherapy | TGF‐β1 | Associated with a ≥15% decrease in TAPSE, OR = 0.85 (0.75–0.96), P < 0.05 | ELISA | After completion of radiotherapy | |
| 26 | 66 | 20 | Breast | Radiotherapy | TGF‐β1 | Associated with worsening in GLS, P = 0.013 | ELISA | 3 years after radiotherapy | |
| 27 | 84 | 42 | Breast | Doxorubicin + trastuzumab | ST‐2 | Associated with LVEF decline, P = 0.008 | ELISA | AUC = 0.74 | 6 months after therapy |
| 28 | 78 | 36 | Breast | Doxorubicin + trastuzumab | MPO | Interval changes associated with LVEF decline >10%, HR = 1.34 (1.00–1.80), P = 0.048 | CLIA | 0 | |
| 29 | 78 | 23 | Breast | Doxorubicin + taxanes + trastuzumab | GDF‐15 | Associated with risk cardiotoxicity, HR = 1.80 (1.20–2.69), P = 0.007 | CLIA | 0 | |
| PIGF | Associated with risk cardiotoxicity, HR = 3.77 (1.43–9.89), P = 0.04 | ||||||||
| MPO | Associated with risk cardiotoxicity, HR = 1.37 (1.11–1.69), P = 0.02 | ||||||||
| 30 | 53 | 18 | Breast | Doxorubicin + trastuzumab | MPO | Associated with CTRCD risk, HR = 1.28 (1.04–1.57), P = 0.019 | ELISA | 3.7 years after therapy | |
| 31 | 61 | 14 | Breast | Radiotherapy | ST2 | Associated with asymptomatic decline LVEF (P = 0.006) | ELISA | 3 years after radiotherapy | |
| 32 | 45 | 1 | Breast | Anthracyclines | ST2 | Patients with CHF presented values for sST2 higher than average | ELISA | After completion of chemotherapy | |
| 33 | 42 | 19 | Breast | Doxorubicin + trastuzumab | IgE | Associated with lower risk of CTRCD, OR = 0.52 (0.31–0.90), P = 0.018 | Mass spectrometry | AUC = 0.73 | 0 |
| 34 | 129 | 31 | Haematological | Anthracyclines | GPBB | Higher levels in patients with cardiotoxicity | Double‐ELISA | AUC = 0.814 | 0 |
| Myoglobin | Higher levels in patients with cardiotoxicity | AUC = 0.810 | |||||||
| 35 | 27 | 10 | Breast | Doxorubicin | CCL23 | Higher levels in patients with LVEF decline >10% | Magnetic bead‐based multiplex immunoassay | Before each DOX cycle |
AUC, area under the curve; CCL23, C–C motif chemokine 23; CHF, congestive heart failure; CLIA, chemiluminescent immunoassay; DOX, doxorubicin; ELISA, enzyme‐linked immunosorbent assay; GDF‐15, growth differentiation factor 15; GPBB, platelet glycoprotein Ib beta chain; HR, hazard ratio; IgE, immunoglobulin E; LVEF, left ventricular ejection fraction; MPO, myeloperoxidase; OR, odds ratio; PIGF, phosphatidylinositol glycan biosynthesis class F; ST‐2, suppression of tumorigenicity 2; TGF‐β1, transforming growth factor beta‐1 protein.
Sample size column is referred to treated patients. Haematological cancer includes leukaemia, lymphomas, or multiple myeloma.
A protein profile performed in breast cancer patients showed that ≥15% tricuspid annular plane systolic excursion (TAPSE) decline was associated with a significant decrease in TGF‐β1 after adjuvant radiotherapy (P < 0.001). 25 Also, a decrease in TGF‐β1 levels before and after 3 years of follow‐up were independent risk factors for worsening LV systolic dysfunction (P = 0.013). 26 Three studies evaluated the association of soluble ST‐2 (sST‐2) with cardiotoxicity. 27 , 31 , 32 Patients under anthracycline‐containing chemotherapy presented increased sST‐2 levels after treatment and were positively correlated with elevation of the cardiac‐specific isoenzyme troponin T (TnT) (P < 0.001). 32 In addition, levels of sST‐2 after 6 months of chemotherapy were negatively correlated with LVEF (P < 0.05) and were considered an independent predictor of LVEF change [area under the curve (AUC) of 0.74] with a sensitivity and specificity of 73 and 79%, respectively. 27 Finally, increased levels of sST‐2 in chemo‐naïve breast cancer patients treated with radiotherapy during 3 years were associated with a worse GLS (P = 0.034) and LVEF (P = 0.006). However, baseline patients' characteristics and cardiac doses were also associated with impairment in GLS. 31
The level of MPO, GDF‐15, and PIGF proteins increases after anthracyclines and trastuzumab therapy, but only changes of MPO were related to subsequent cardiotoxicity [hazard ratio (HR) = 1.34 (1.00–1.80), P = 0.048]. The combination of MPO and troponin I (TnI) was able to improve the risk prediction of cardiotoxicity. 28 A second study confirmed that MPO, GDF‐15, and PIGF biomarkers increase over 3 months and were associated with the prediction of subsequent cardiotoxicity against doxorubicin and trastuzumab therapy. 29 A prospective cohort study of breast cancer patients treated with anthracyclines and trastuzumab demonstrated that higher baseline MPO levels (median 325 pmol/L) before treatment were associated with an increased risk of cardiotoxicity for over an extended period of 3.7 years [HR: 1.28 (1.04–1.57), P = 0.019]. 30
Novel DNA biomarkers associated with cardiotoxicity
Eighteen new studies describe the significant association of 57 polymorphic variations with cardiac toxicity induced by cancer therapy 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 (Table 3 and Supporting Information, Table S3 , Sheet G). These DNA polymorphisms are presented in genes associated with drug metabolism and detoxification. Thus, these can be important in establishing a genetic predisposition or protection for cardiotoxicity. The SNPs were classified as intronic (22 SNPs), intergenic (14 SNPs), UTRs (3 SNPs), splice acceptor variants (1 SNP), non‐coding genes (2 SNPs), and missense/synonymous coding genes (15 SNPs). Of the total SNP evaluated, only rs1883112 (NCF4), rs4673 (CYBA), rs13058338 (RAC2), rs1695 (GSTP1), and rs1056892 (CBR3) were presented in more than one study. 41 , 42 , 43 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 Genotyping was performed by PCR‐RFLP (n = 1), direct sequencing PCR (n = 1), Taqman (n = 5), pyrosequencing (n = 2), array/bead‐based (n = 5), or mass spectrometry‐based (n = 4) (Table 3 ).
Table 3.
Genetic polymorphisms associated with cancer therapy‐induced cardiotoxicity
| Study ref. | Sample size | Events | Type of cancer | Therapy | Gene | Amino acid change | SNP rsID | Cardiotoxicity association | Detection method | HWE |
|---|---|---|---|---|---|---|---|---|---|---|
| 41 | 48 | 7 | Various | Anthracyclines | NCF4 | NR | rs1883112 | A allele associated with cardiac interstitial fibrosis, OR = 5.11 (95% CI: 1.59–16.43, P = 0.018) | qPCR | Yes |
| CYBA | NR | rs4673 | C/C genotype associated with patched myocardial necrosis, OR = 0.112 (95% CI: 0.20–0.63, P = 0.039) | Yes | ||||||
| 42 | 61 | 5 | Breast | Trastuzumab | HER2 | Ile/Val | NR | SNP is significantly associated with asymptomatic LVEF decline ≥20%, P = 0.0058 | PCR‐RFLP | Yes |
| 43 | 70 | 25 | Various | Anthracyclines + trastuzumab | CBR3 | V244M | rs1056892 | AA genotype associated with high LVEF decrease, P = 0.039 | qPCR | Yes |
| GSTP1 | I105V | rs1695 | G (I105V) allele had lower fractional shorting, P = 0.024 | Yes | ||||||
| 50 | 106 | 17 | Haematological | R‐CHOP | ABCB1 | NR | rs2229109 | AG heterozygote variant associated with Grade 2–4 cardiac toxicity, OR 1.89 (95% CI: 1.15–3.12, P = 0.010) | SNP minisequencing | Yes |
| NCF4 | NR | rs1883112 | AG/GG genotypes independent predictor of Grade 2–4 cardiac toxicity, OR = 0.37 (95% CI: 0.16–0.87, P = 0.023) | Yes | ||||||
| CYBA | NR | rs4673 | TT homozygote variant associated with Grade 2–4 cardiac toxicity, OR = 1.86 (95% CI: 1.15–2.99, P = 0.010) | Yes | ||||||
| RAC2 | NR | rs13058338 | AA homozygote variant associated with Grade 2–4 cardiac toxicity, OR = 1.84 (95% CI: 1.10–3.10, P = 0.019) | Yes | ||||||
| GSTP1 | NR | rs1695 | GG homozygote variant associated with Grade 2–4 cardiac toxicity, OR = 1.83 (95% CI: 1.12–3.01, P = 0.015) | Yes | ||||||
| 51 | 1119 | 17 | Breast | Anthracyclines | CBR3 | NR | rs1056892 | SNP associated with LVEF decline (P = 0.004) | Axiom array | NR |
| NCF4 | NR | rs1883112 | SNP associated in patients with CHF, OR = 0.35 (95% CI: 0.15–0.78, P = 0.011) | NR | ||||||
| ABCB1 | NR | rs2235047 | SNP associated with LVEF decline (P = 0.018) | NR | ||||||
| 52 | 450 | 87 | Haematological | Doxorubicin | NCF4 | NR | rs1883112 | AA genotype associated with CHF, OR = 2.5 (95% CI: 1.3–5.0, P = 0.031) | Pyrosequencing | NR |
| CYBA | NR | rs4673 | T allele acute ACT, OR = 2.0 (95% CI: 1.0–3.9, P = 0.01) | No | ||||||
| RAC2 | NR | rs13058338 | 7508T<A acute ACT, OR = 2.6 (95% CI: 1.3–5.1, P = 0.005) | NR | ||||||
| ABCC1 | G671V | rs45511401 | T allele with acute ACT, OR = 3.6 (95% CI: 1.6–8.4, P = 0.005) | NR | ||||||
| 53 | 877 | 153 | Breast | Epirubicin | ABCC1 | NR | rs246221 | TT genotype was associated with LVEF decline, OR 1.59 (95% CI: 1.07–2.35, P = 0.021) | Sequenom MassArray | NR |
| 54 | 166 | 19 | Breast | Doxorubicin | CBR3 | NR | rs1056892 | SNP associated with increased risk of EF < 55%, OR = 2.50 (95% CI: 1.22–5.11, P = 0.012) | qPCR | Yes |
| ABCB1 | NR | rs1045642 | The variant allele 3435C>T had an additive protective effect for EF decline, OR = 0.48 (95% CI: 0.23–1.00, P = 0.049) | Yes | ||||||
| NR | rs1045642 | The variant allele 3435C > T had an additive protective effect for EF decline, OR 0.48 (95% CI: 0.23–1.00, P = 0.049) | Yes | |||||||
| 55 | 78 | 18 | Breast | Trastuzumab | HER2 | NR | rs1136201 | AG genotype associated with cardiotoxicity risk, OR = 7.17 (95% CI: 1.82–28.29, P = 0.005) | qPCR | NR |
| 56 | 140 | 29 | Breast | Trastuzumab | HER2 | P1170A | rs1058808 | CC genotype Pro1170Ala associated with cardiomyopathy, OR = 2.60 (95% CI: 1.02–6.68, P = 0.046) | qPCR | Yes |
| 57 | 132 | 13 | Breast | Trastuzumab | HER2 | I655V | rs1801201 | Val carrier genotype associated with asymptomatic LVEF < 50%, OR = 3.83 (95% CI: 1.11–13.18, P = 0.025) | PCR | Yes |
| 45 | 427 | 18 | Breast | Anthracyclines | UGT2B7 | NR | rs7668258 | Low occurrence of cardiotoxicity, OR = 0.259 (95% CI: 0.103–0.651, P = 0.004) | Pyrosequencing | NR |
ABCB1, ATP binding cassette subfamily B member 1; ABCC1, ATP binding cassette subfamily C member 1; CBR3, carbonyl reductase 3; CHF, congestive heart failure; CI, confidence interval; CYBA, cytochrome B‐245 alpha chain; EF, ejection fraction; GSTP1, glutathione S‐transferase Pi 1; HER2, Erb‐B2 receptor tyrosine kinase 2; HWE, Hardy–Weinberg equilibrium; LVEF, left ventricular ejection fraction; NCF4, neutrophil cytosolic factor 4; NR, not reported; OR, odds ratio; RAC2, Rac family small GTPase 2; SNP, single nucleotide polymorphism; UGT2B7, UDP glucuronosyl transferase family 2 member B7.
Sample size column is referred to treated patients. Haematological cancer includes leukaemia, lymphomas, or multiple myeloma.
The SNP rs1883112 conferred a protective role against cardiac toxicity for R‐CHOP therapy [odds ratio (OR) = 0.37 (95% confidence interval, CI: 0.16–0.87), P = 0.023]. 50 In concordance with these results, a genome‐wide association study showed that a lower frequency of the rs1883112 A allele was related to congestive heart failure (CHF) [OR = 0.35, 0.15–0.78), P = 0.011] after chemotherapy. 51 This was in contradiction to two other studies where the presence of this variant correlated with cardiac interstitial fibrosis [OR = 5.11 (95% CI: 1.59–16.43), P = 0.018] and CHF (P = 0.031). 41 , 52 The rs4673 242‐T allele was associated with protection against patched myocardial necrosis due to anthracyclines [OR = 0.11 (95% CI: 0.20–0.63), P = 0.039], 41 but in contrast, other two studies associated the rs4673 SNP with chronic HF in lymphoma patients treated with R‐CHOP or doxorubicin. 50 , 52 Two articles reported that the rs13058338 polymorphism could be associated with CHF induced by anthracycline cancer therapy. 50 , 52 The SNP rs1695 in R‐CHOP was related to Grade 2–4 cardiotoxicity in R‐CHOP treatment, and carriers of the G‐allele (I105V) had significantly lower fractional shortening (FS) after anthracycline therapy (P = 0.024). 43 , 50
Lastly, patients that carried the rs10556892 polymorphism presented significant deterioration in cardiac functions after anthracycline treatment confirmed by a second study where this SNP was associated with risk of EF < 55% [OR = 2.50 (95% CI: 1.22–5.11), P = 0.012]. 43 , 54 A genome‐wide study of 1191 breast cancer participants observed an association between the rs10556892 SNP and the maximum LVEF decline (P = 0.004). 51
Nine articles reported several SNP mutations on genes ABCC1 (rs45511401 and rs246221), HER2 (rs1801201, rs1136201, and rs1058808), and ABCB1 (rs2229109, rs1045642, and rs2235047). 42 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 In the ABCC1 gene, lymphoma patients carrying the rs45511401 polymorphism were predisposed to CHF by anthracycline therapy [OR = 3.6 (95% CI: 1.6–8.4), P = 0.005]. Due to the low number of CHF patients, these results could not be confirmed in a second cohort study. 52 , 53 The second SNP on the ABCC1 gene, rs246221, was associated with LVEF decline >10% (P < 0.001) in patients carrying the T‐allele. 53 From the articles that evaluated the influence of polymorphisms on the HER2 gene, the Ile655Val genotype (rs1801201 and rs1136201) was associated with trastuzumab cardiotoxicity by LVEF decline ≥20% (P = 0.0058) or LVEF < 50% (P = 0.025). 42 , 55 , 57 On the other hand, the Pro1170Ala HER2 genotype (rs1058808) was frequently found in patients with cardiomyopathy due to trastuzumab [OR = 2.60 (95% CI: 1.02–6.68), P = 0.046]. 56 Three SNPs on ABCB1 gene were associated with cancer therapy‐induced toxicity. Assuming additive genetic effects, the variant rs1045642 presented a protective role [OR = 0.48 (95% CI: 0.23–1.00), P = 0.049] while the SNPs rs2235047 and rs2229109 were associated with LVEF decline. 50 , 51 , 54
Novel RNA biomarkers associated with cardiotoxicity
A total of 11 studies explored the relationship between drug‐induced cardiotoxicity and 171 RNA expression markers (Table 4 and Supporting Information, Table S3 , Sheet H). 32 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 Four studies identified differential expression profile in 96 genes and seven determined the potential of 75 circulating microRNAs. The miR‐1, miR‐25‐3p, miR‐34a‐5p, miR‐423‐5p, and BANK1 transcripts were found in two independent studies. 32 , 58 , 59 , 60 , 62 , 66 The gene expression screening was performed using high‐throughput sequencing (n = 5) or RT‐qPCR over RNAs previously selected by literature (n = 6) (Table 4 ).
Table 4.
RNA biomarkers associated with cancer therapy‐induced cardiotoxicity
| Study ref. | Sample size | Events | Type of cancer | Therapy | Biomarker | Cardiotoxicity association | Detection method | AUC/HR | Timing of biomarker assessment |
|---|---|---|---|---|---|---|---|---|---|
| 32 | 45 | 1 | Breast | Anthracyclines | miR‐423‐5p | Associated with LVEF (P = 0.045) and CHF | RT‐qPCR | After completion of chemotherapy | |
| miR‐34a‐5p | Up‐regulated | ||||||||
| 58 | 15 | 5 | Breast | Doxorubicin | BANK1 | Down‐regulated (P ≤ 0.05) in patients with low ejection fractions | GeneChip Array | After completion of DOX chemotherapy | |
| 59 | 56 | 10 | Breast | Doxorubicin | miR‐1 | Up‐regulation associated with LVEF (P < 0.0001) | RT‐qPCR | AUC = 0.851 | 3 months after DOX chemotherapy |
| miR‐423‐5p | Up‐regulated in patients with cardiotoxicity | ||||||||
| 60 | 30 | 10 | Breast | Doxorubicin | miR‐1 | Down‐regulated (P = 0.003) in patients with abnormal LVEF after 2 cycles of chemotherapy | RT‐qPCR | After 1 cycle of DOX chemotherapy | |
| miR‐25‐3p | Up‐regulated (P = 0.017) in patients with abnormal LVEF | ||||||||
| 61 | 63 | 11 | Lung | Radiotherapy | miR‐25‐3p | Up‐regulated | miRNA PCR array | HR = 0.826 | 1 week prior to initiation of radiotherapy |
| miR‐34a‐5p | Up‐regulated | HR = 0.722 | |||||||
| miR‐223‐3p | Up‐regulated | HR = 0.968 | |||||||
| 62 | 33 | 8 | Breast | Doxorubicin | BANK1 | Down‐regulated | BeadChip Array | After completion of chemotherapy | |
| 63 | 363 | 19 | Breast | Anthracyclines | miR‐17‐5p | Prediction cardiotoxicity risk, OR = 0.000 (95% CI: 0.000–0.000, P = 0.014) | RT‐qPCR | AUC = 0.702 | Before chemotherapy |
| miR‐20a | Prediction cardiotoxicity risk, OR = 0.005 (95% CI: 0.000–0.484, P = 0.024) | AUC = 0.754 | |||||||
| 64 | 198 | 98 | Colorectal | Bevacizumab | miR‐1254 | Up‐regulated | miRNA array | Within 24 h of the onset of symptoms |
AUC, area under the curve; CHF, congestive heart failure; CI, confidence interval; DOX, doxorubicin; HR, hazard ratio; LVEF, left ventricular ejection fraction; OR, odds ratio; RT‐qPCR, quantitative reverse transcription PCR.
Sample size column is referred to treated patients.
The miR‐1 showed down‐regulation in breast cancer patients who developed LVEF decline after the completion of doxorubicin‐based chemotherapy but, in a second study, was associated with decreased LVEF (P < 0.0001) and prediction of cardiotoxicity (AUC = 0.851). 59 , 60 The miR‐25‐3p was up‐regulated in lung and breast cancer patients treated with radiotherapy or doxorubicin chemotherapy who developed cardiotoxicity. 60 , 66 Up‐regulation of miR‐34a‐5p in treated‐naïve breast cancer patients immediately after anthracycline was identified as a possible risk factor for cardiotoxicity development. 32 The last miRNA, the miR‐423‐5p, was up‐regulated during chemotherapy treatment and was associated with decreased LVEF (P = 0.045). 32 , 59 A genomic profile in patients who presented abnormal LVEF by doxorubicin treatment showed a significant down‐regulation of BANK1 gene. 58 , 62
Other novel molecular biomarkers of interest
Some novel biomarkers such as miR‐223‐3p, the protein IgE, and the rs1045642 (ABCB1) and rs7668258 (UGT2B7) polymorphisms were associated with a protective role or lower occurrence of cardiotoxicity due to cancer therapy. 33 , 45 , 54 , 66 Noteworthy, the combined use of MPO and TnI was able to provide additive value in predicting subsequent cardiotoxicity, 28 and the measure of proteins BNP, myoglobin, and GPBB (a glycogen phosphorylase released into circulation after myocardial injuries) together in patients with anthracycline risk cardiotoxicity exhibited an AUC of 0.930 with a sensitivity of 90.3% and specificity of 85.7%. 34 Further on, the combination of miR‐17‐5p and miR‐20a expressions displayed a predictive benefit for low cardiotoxicity risk (AUC = 0.842). 63 High baseline concentration of chemokine CCL23 plasma levels before early doses of doxorubicin treatment could predict subsequent LVEF decline in breast cancer patients (P < 0.05). 35 Lastly, the expression profile of circulating miRNAs in two independent cohorts of colorectal cancer showed that only up‐regulation of miR‐1254 could be considered as a putative biomarker for bevacizumab‐induced cardiotoxicity (Tables 2, 3, 4). 64
Discussion
Summary of findings
To the best of our knowledge, this is the first and only scoping review focused on novel molecular biomarkers associated with cancer therapy‐induced cardiotoxicity in adulthood. We found a total of 42 articles, examining 44 proteins, 57 SNPs, and 171 differential gene expression profiles. Within these studies, two approaches are used for the identification of new biomarkers. The first approach utilizes high‐throughput analysis to identify new biomarkers among the total of molecules studied. 33 , 35 , 37 , 40 , 43 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 58 , 62 , 64 , 67 The second approach, used in the most of articles, is a directed selection based on a previous literature search. Further, in an attempt to provide a biological context of these novel molecular biomarkers with cancer therapy‐induced cardiotoxicity, three articles performed a network analysis where they identified an over‐representation of biological processes involved in apoptosis, immune response, drug/response transport, collagen metabolism, and activation of matrix metalloproteinases. 37 , 58 , 62 However, the lack of complete knowledge on the molecular mechanisms of drug action constitutes one of the major problems to improve the biomarker discovery and its role on cardiotoxicity. 68 From the total 272 novel molecular biomarkers reported, only 13 were assessed by two or more studies (MPO, sST2, GDF‐15, TGF‐B1, rs1056892, rs1883112, rs4673, rs13058338, rs1695, miR‐1, miR‐25‐3p, miR‐34a‐5p, and miR‐423‐5p). Furthermore, contradictory results were reported when the role of the same molecular biomarker of cardiotoxicity was examined in the different studies. For example, the HER2 Ile655Val polymorphism was significantly associated with cardiotoxicity, but a genome‐wide study failed to observe any association between HER2 gene and LVEF decline in breast cancer patients treated with trastuzumab. 42 , 51 , 55 , 57 Another approximation used by some authors is attempting to study the prediction role of these novel molecular biomarkers in combination with previously recognized cardiac biomarkers, such as TnI or BNP, to evaluate cardiotoxicity risk due to cancer therapy. The authors recognize a superior performance when two molecular biomarkers were used together, suggesting the usefulness of this strategy to identify subgroups of patients with considerable risk to suffer cardiotoxicity. 28 , 34 , 63
Regarding the types of therapy studied, a lack is observed in the identification of molecular biomarkers for the new types of therapy, as immunotherapy. For instance, reviews highlight the cardiotoxic effects of CAR‐T cell treatment such as tachycardia, hypotension, hypertension, LVEF decrease, and cardiogenic shock, 69 , 70 , 71 but associated molecular biomarkers have not been investigated. There is also a lack of information regarding the types of molecular biomarker identified. For example, studies evaluating the role of epigenetic biomarkers with cardiotoxicity in patients were not reported, despite that the recent findings demonstrate that decreased DNA methylation and increased acetylation are associated to doxorubicin induced in in vivo and in vitro models. 72 These gaps in knowledge added to the fact that the molecular factors related to cardiotoxicity derived from cancer treatment are not reported in all types of cancer, which opens a new field for the discovery of new molecular biomarkers.
On the other hand, the majority of the studies focused on the identification of novel molecular biomarkers before or immediately after treatment to predict early changes in cardiac parameters to identify patients at high risk of cardiotoxicity, but cancer therapies may also induce cardiovascular late effects. 73 , 74 For instance, study conducted in long‐term survivors of paediatric cancer showed that they had more than nine‐fold increased rates of suffering CHF, CVD, or stroke compared with general population after chemotherapy and radiation therapy. 75 Therefore, long follow‐up studies for these molecular biomarkers are suggested to understand late cardiac effects due to cancer therapy. In this sense, the absence of a uniform definition for cardiotoxicity among trials could significantly influence the validity of the results. 29 Even more, establishing a consensus in the definition of cardiotoxicity is an urgent need. Some interesting studies found in the systematic search were left out of the selection due to the definition of cardiotoxicity used. A recent study has made an attempt to uniform these diagnostic criteria suggesting a division of cardiotoxicity into four exclusive degrees groups according to their myocardial injury/dysfunction that could serve as a guide for future studies in cardio‐oncology field. 76
Regardless of the above and despite advances in the identification of new molecular biomarkers, further research is needed to outline whether all these novel molecular biomarkers could provide additional value in cardiotoxicity management and risk evaluation over already established cardiac biomarkers and cardiac imaging techniques.
Limitation of included studies
This scoping review suggests that knowledge related to novel molecular biomarkers in cardiotoxicity is in an early stage of understanding. A common limitation in most of the studies is the small sample size and reduced number of individuals experiencing cardiotoxicity events, which reduce the statistical power and limits the association between molecular biomarker changes and cardiotoxicity. Further, due to the novelty of these molecular biomarkers, the authors did not provide the potential implications of additional risk factors such as underlying CVDs, age, cumulative dose, or concomitant administration of multiple treatment modalities, which could have been contributing to the cardiotoxicity outcomes.
There is also a selection bias due to single‐institution studies, cancer types, and population characteristics. International collaboration research and additional replication studies could help to validate and add consistency to these novel molecular biomarkers on cardiotoxicity towards their clinical use. Furthermore, longer follow‐up trials and larger prospective clinical studies are required to establish molecular biomarker associations as it takes years for some cardiotoxic regimens to manifest symptoms.
Finally, the exclusion of patients with underlying CVDs and the lack of control groups, among others, hinder the application of novel molecular biomarkers in clinical practices.
Strengths and limitations of scoping review
The purpose of this review was to assess the amount and the preliminary nature of published evidence for novel molecular biomarkers associated with cancer therapy‐induced cardiotoxicity in the adult population. This scoping review used precise methods to ensure a broad search of the literature, including four electronic databases and a reference list of relevant publications. The screening and data characterization forms were pre‐tested by at least two reviewers and revised as needed before implementation. Each citation and article was reviewed by two independent reviewers who met to resolve conflicts. A bias towards English‐language terms could have arisen on missing documents of interest in our scoping review. Our literature search included different terms used to describe cardiotoxicity, but other words may also exist. Only 22 articles reported the study design, and because of the heterogeneity, we could not infer the level of evidence for the literature search.
Conclusions
There is currently a limited amount of research on novel molecular biomarkers to predict cardiac toxicity induced by cancer therapies. This scoping review showed that the investigation of new protein, DNA, and RNA biomarkers in cardio‐oncology is still at early stages and highlighted several limitations to confirm their added value in cardiotoxicity. Even though, molecular biomarker research is a promising area that would help to establish new strategies for CVD prevention and diagnosis for patients undergoing cancer therapy.
Conflict of interest
None declared.
Funding
This work was supported by the Chilean ANID/Fondecyt‐Postdoctorado No. 3180486 (A.L.R.‐C), and Grants ANID/Doctorado Nacional No. 21150650 (I.C.‐E.) and supported by the Universidad de La Frontera, project DI22‐0014 (A.L.R.‐C and I.C.‐E.).
Supporting information
Table S1. Search strategy.
Table S2. Eligibility criteria for the clinical research question.
Table S3. Data collection of included studies.
Acknowledgements
The role as research assistant of Ignacio Aravena Fica, an undergraduate student at the Universidad de La Frontera, is appreciated.
Cartas‐Espinel, I. , Telechea‐Fernández, M. , Manterola Delgado, C. , Ávila Barrera, A. , Saavedra Cuevas, N. , and Riffo‐Campos, A. L. (2022) Novel molecular biomarkers of cancer therapy‐induced cardiotoxicity in adult population: a scoping review. ESC Heart Failure, 9: 1651–1665. 10.1002/ehf2.13735.
References
- 1. Han X, Zhou Y, Liu W. Precision cardio‐oncology: understanding the cardiotoxicity of cancer therapy. NPJ Precis Oncologia 2017; 1: 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brown S. Preventive cardio‐oncology: the time has come. Front Cardiovasc Med 2020; 6: 187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cautela J, Lalevée N, Ammar C, Ederhy S, Peyrol M, Debourdeau P, Serin D, le Dolley Y, Michel N, Orabona M, Barraud J, Laine M, Bonello L, Paganelli F, Barlési F, Thuny F. Management and research in cancer treatment‐related cardiovascular toxicity: challenges and perspectives. Int J Cardiol 2016; 224: 366–375. [DOI] [PubMed] [Google Scholar]
- 4. Sturgeon KM, Deng L, Bluethmann SM, Zhou S, Trifiletti DM, Jiang C, Kelly SP, Zaorsky NG. A population‐based study of cardiovascular disease mortality risk in US cancer patients. Eur Heart J 2019; 40: 3889–3897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Alizadehasl A, Amin A, Maleki M, Noohi F, Ghavamzadeh A, Farrashi M. Cardio‐oncology discipline: focus on the necessities in developing countries. ESC Hear Fail 2020; 7: 2175–2183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tajiri K, Aonuma K, Sekine I. Cardio‐oncology: A multidisciplinary approach for detection, prevention and management of cardiac dysfunction in cancer patients. Vol. 47, Jpn J Clin Oncol 2017. p. 678–682. [DOI] [PubMed] [Google Scholar]
- 7. Michel L, Rassaf T, Totzeck M. Biomarkers for the detection of apparent and subclinical cancer therapy‐related cardiotoxicity. J Thorac Dis 2018; 10: S4282–S4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Manrique CR, Park M, Tiwari N, Plana JC, Garcia MJ. Diagnostic strategies for early recognition of cancer therapeutics‐related cardiac dysfunction. Clin Med Insights Cardio 2017; 11: 1179546817697983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer‐Crosbie M, Ganame J, Sebag IA, Agler DA, Badano LP, Banchs J, Cardinale D, Carver J, Cerqueira M, DeCara JM, Edvardsen T, Flamm SD, Force T, Griffin BP, Jerusalem G, Liu JE, Magalhães A, Marwick T, Sanchez LY, Sicari R, Villarraga HR, Lancellotti P. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2014; 27: 911–939. [DOI] [PubMed] [Google Scholar]
- 10. Araujo‐Gutierrez R, Chitturi KR, Xu J, Wang Y, Kinder E, Senapati A, Chebrolu LB, Kassi M, Trachtenberg BH. Baseline global longitudinal strain predictive of anthracycline‐induced cardiotoxicity. Cardio‐Oncology 2021; 7: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground? J Clin Oncol 2008; 26: 1201–1203. [DOI] [PubMed] [Google Scholar]
- 12. Yu AF, Ky B. Roadmap for biomarkers of cancer therapy cardiotoxicity. Heart 2016; 102: 425–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Alexandre J, Cautela J, Ederhy S, Damaj GL, Salem JE, Barlesi F, Farnault L, Charbonnier A, Mirabel M, Champiat S, Cohen‐Solal A, Cohen A, Dolladille C, Thuny F. Cardiovascular toxicity related to cancer treatment: a pragmatic approach to the American and European cardio‐oncology guidelines. J Am Heart Assoc 2020; 9: e018403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pudil R, Mueller C, Čelutkienė J, Henriksen PA, Lenihan D, Dent S, Barac A, Stanway S, Moslehi J, Suter TM, Ky B, Štěrba M, Cardinale D, Cohen‐Solal A, Tocchetti CG, Farmakis D, Bergler‐Klein J, Anker MS, von Haehling S, Belenkov Y, Iakobishvili Z, Maack C, Ciardiello F, Ruschitzka F, Coats AJS, Seferovic P, Lainscak M, Piepoli MF, Chioncel O, Bax J, Hulot JS, Skouri H, Hägler‐Laube ES, Asteggiano R, Fernandez TL, Boer RA, Lyon AR. Role of serum biomarkers in cancer patients receiving cardiotoxic cancer therapies: a position statement from the Cardio‐Oncology Study Group of the Heart Failure Association and the Cardio‐Oncology Council of the European Society of Cardiology. Eur J Heart Fail 2020; 22: 1966–1983. [DOI] [PubMed] [Google Scholar]
- 15. Ananthan K, Lyon AR. The role of biomarkers in cardio‐oncology. J Cardiovasc Transl Res 2020; 13: 431–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hinrichs L, Mrotzek SM, Mincu RI, Pohl J, Röll A, Michel L, Mahabadi AA, Al‐Rashid F, Totzeck M, Rassaf T. Troponins and natriuretic peptides in cardio‐oncology patients—data from the ECoR registry. Front Pharmacol 2020; 11: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tan LL, Lyon AR. Role of biomarkers in prediction of cardiotoxicity during cancer treatment. Curr Treat Options Cardiovasc Med 2018; 20: 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bracun V, Aboumsallem JP, van der Meer P, de Boer RA. Cardiac biomarkers in patients with cancer: considerations, clinical implications, and future avenues. Curr Oncol Rep 2020; 22: 67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Colpaert RMW, Calore M. MicroRNAs in cardiac diseases. Cell 2019; 8: 737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Garcia‐Pavia P, Kim Y, Restrepo‐Cordoba MA, Lunde IG, Wakimoto H, Smith AM, Toepfer CN, Getz K, Gorham J, Patel P, Ito K, Willcox JA, Arany Z, Li J, Owens AT, Govind R, Nuñez B, Mazaika E, Bayes‐Genis A, Walsh R, Finkelman B, Lupon J, Whiffin N, Serrano I, Midwinter W, Wilk A, Bardaji A, Ingold N, Buchan R, Tayal U, Pascual‐Figal DA, de Marvao A, Ahmad M, Garcia‐Pinilla JM, Pantazis A, Dominguez F, John Baksi A, O'Regan DP, Rosen SD, Prasad SK, Lara‐Pezzi E, Provencio M, Lyon AR, Alonso‐Pulpon L, Cook SA, DePalma SR, Barton PJR, Aplenc R, Seidman JG, Ky B, Ware JS, Seidman CE. Genetic variants associated with cancer therapy‐induced cardiomyopathy. Circulation 2019; 140: 31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Munn Z, Peters M, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol 2018; 18: 143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol Theory Pract 2005; 8: 19–32. [Google Scholar]
- 23. Tricco AC, Lillie E, Zarin W, O'Brien KK, Colquhoun H, Levac D, Moher D, Peters MDJ, Horsley T, Weeks L, Hempel S, Akl EA, Chang C, McGowan J, Stewart L, Hartling L, Aldcroft A, Wilson MG, Garritty C, Lewin S, Godfrey CM, Macdonald MT, Langlois EV, Soares‐Weiser K, Moriarty J, Clifford T, Tunçalp Ö, Straus SE. PRISMA extension for scoping reviews (PRISMA‐ScR): checklist and explanation. Ann Intern Med 2018; 169: 467–473. [DOI] [PubMed] [Google Scholar]
- 24. Hossain A, Chen A, Ivy P, Lenihan DJ, Kaltman J, Taddei‐Peters W, Remick SC. The importance of clinical grading of heart failure and other cardiac toxicities during chemotherapy: updating the common terminology criteria for clinical trial reporting. Heart Fail Clin 2011; 7: 373–384. [DOI] [PubMed] [Google Scholar]
- 25. Aula H, Skyttä T, Tuohinen S, Luukkaala T, Hämäläinen M, Virtanen V, Raatikainen P, Moilanen E, Kellokumpu‐Lehtinen PL. Decreases in TGF‐β1 and PDGF levels are associated with echocardiographic changes during adjuvant radiotherapy for breast cancer. Radiat Oncol 2018; 13: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Aula H, Skyttä T, Tuohinen S, Luukkaala T, Hämäläinen M, Virtanen V, Raatikainen P, Moilanen E, Kellokumpu‐Lehtinen PL. Transforming growth factor beta 1 levels predict echocardiographic changes at three years after adjuvant radiotherapy for breast cancer. Radiat Oncol 2019; 14: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Huang G, Zhai J, Huang X, Zheng D. Predictive value of soluble ST‐2 for changes of cardiac function and structure in breast cancer patients receiving chemotherapy. Med (United States) 2018; 97: e12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ky B, Putt M, Sawaya H, French B, Januzzi JL, Sebag IA, Plana JC, Cohen V, Banchs J, Carver JR, Wiegers SE, Martin RP, Picard MH, Gerszten RE, Halpern EF, Passeri J, Kuter I, Scherrer‐Crosbie M. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol 2014; 63: 809–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Putt M, Hahn VS, Januzzi JL, Sawaya H, Sebag IA, Plana JC, Picard MH, Carver JR, Halpern EF, Kuter I, Passeri J, Cohen V, Banchs J, Martin RP, Gerszten RE, Scherrer‐Crosbie M, Ky B. Longitudinal changes in multiple biomarkers are associated with cardiotoxicity in breast cancer patients treated with doxorubicin, taxanes, and trastuzumab. Clin Chem 2015; 16: 1164–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Demissei BG, Hubbard RA, Zhang L, Smith AM, Sheline K, McDonald C, Narayan V, Domchek SM, DeMichele A, Shah P, Clark AS, Fox K, Matro J, Bradbury AR, Knollman H, Getz KD, Armenian SH, Januzzi JL, Tang WHW, Liu P, Ky B. Changes in cardiovascular biomarkers with breast cancer therapy and associations with cardiac dysfunction. J Am Heart Assoc 2020; 9: e014708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aula H, Skyttä T, Tuohinen S, Luukkaala T, Hämäläinen M, Virtanen V, Raatikainen P, Moilanen E, Kellokumpu‐Lehtinen PL. ST2 levels increased and were associated with changes in left ventricular systolic function during a three‐year follow‐up after adjuvant radiotherapy for breast cancer. The Breast 2020; 49: 183–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Frères P, Bouznad N, Servais L, Josse C, Wenric S, Poncin A, Thiry J, Moonen M, Oury C, Lancellotti P, Bours V, Jerusalem G. Variations of circulating cardiac biomarkers during and after anthracycline‐containing chemotherapy in breast cancer patients. BMC Cancer 2018; 18: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Beer LA, Kossenkov AV, Liu Q, Luning Prak E, Domchek S, Speicher DW, Ky B. Baseline immunoglobulin E levels as a marker of doxorubicin and trastuzumab‐associated cardiac dysfunction. Circ Res 2016; 119: 1135–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Di JY, Zhang ZX, Xin SJ. Glycogen phosphorylase isoenzyme bb, myoglobin and BNP in ANT‐induced cardiotoxicity. Open Life Sci 2018; 13: 561–568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yu LR, Cao Z, Makhoul I, Daniels JR, Klimberg S, Wei JY, Bai JPF, Li J, Lathrop JT, Beger RD, Todorova VK. Immune response proteins as predictive biomarkers of doxorubicin‐induced cardiotoxicity in breast cancer patients. Exp Biol Med 2018; 243: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kouloubinis A, Sofroniadou S, Panoulas VF, Makaritsis K, Revela I, Karavolias G, Voudris V, Adamopoulos S. The role of TNF‐α, Fas/Fas ligand system and NT‐proBNP in the early detection of asymptomatic left ventricular dysfunction in cancer patients treated with anthracyclines. IJC Heart Vasc 2015; 6: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tromp J, Boerman LM, Sama IE, Maass SWMC, Maduro JH, Hummel YM, Berger MY, Bock GH, Gietema JA, Berendsen AJ, Meer P. Long‐term survivors of early breast cancer treated with chemotherapy are characterized by a pro‐inflammatory biomarker profile compared to matched controls. Eur J Heart Fail 2020; 22: 1239–1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Geisberg CA, Abdallah WM, da Silva M, Silverstein C, Smith HM, Abramson V, Mayer I, Means‐Powell J, Freehardt D, White B, Lenihan D, Sawyer DB. Circulating neuregulin during the transition from Stage A to Stage B/C heart failure—a breast cancer cohort. J Card Fail 2013; 19: 10–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Vianello E, Dozio E, Tacchini L, Frati L, Corsi Romanelli MM. ST2/IL‐33 signaling in cardiac fibrosis. Int J Biochem Cell Biol 2019; 116: 105619. [DOI] [PubMed] [Google Scholar]
- 40. Lubieniecka JM, Graham J, Heffner D, Mottus R, Reid R, Hogge D, Grigliatti TA, Riggs WK. A discovery study of daunorubicin induced cardiotoxicity in a sample of acute myeloid leukemia patients prioritizes P450 oxidoreductase polymorphisms as a potential risk factor. Front Genet 2013; 4: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cascales A, Pastor‐Quirante F, Sánchez‐Vega B, Luengo‐Gil G, Corral J, Ortuño‐Pacheco G, Vicente V, Peña FA. Association of anthracycline‐related cardiac histological lesions with NADPH oxidase functional polymorphisms. Oncologist 2013; 18: 446–453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Beauclair S, Formento P, Fischel JL, Lescaut W, Largillier R, Chamorey E, Hofman P, Ferrero JM, Pagès G, Milano G. Role of the HER2 [Ile655Val] genetic polymorphism in tumorogenesis and in the risk of trastuzumab‐related cardiotoxicity. Ann Oncol 2007; 18: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 43. Salanci BV, Aksoy H, Kiratli ÖP, Tülümen E, Güler N, Öksüzoglu B, Tokgözoğlu L, Erbaş B, Alikaşifoğlu M. The relationship between changes in functional cardiac parameters following anthracycline therapy and carbonyl reductase 3 and glutathione s transferase pi polymorphisms. J Chemother 2012; 24: 285–291. [DOI] [PubMed] [Google Scholar]
- 44. Christidi E, Huang H, Shafaattalab S, Maillet A, Lin E, Huang K, Laksman Z, Davis MK, Tibbits GF, Brunham LR. Variation in RARG increases susceptibility to doxorubicin‐induced cardiotoxicity in patient specific induced pluripotent stem cell‐derived cardiomyocytes. Sci Rep 2020; 10: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li H, Hu B, Guo Z, Jiang X, Su X, Zhang X. Correlation of UGT2B7 polymorphism with cardiotoxicity in breast cancer patients undergoing epirubicin/cyclophosphamide‐docetaxel adjuvant chemotherapy. Yonsei Med J 2019; 60: 30–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Ruiz‐Pinto S, Pita G, Martín M, Alonso‐Gordoa T, Barnes DR, Alonso MR, Herraez B, García‐Miguel P, Alonso J, Pérez‐Martínez A, Cartón AJ, Gutiérrez‐Larraya F, García‐Sáenz JA, Benítez J, Easton DF, Patiño‐García A, González‐Neira A. Exome array analysis identifies ETFB as a novel susceptibility gene for anthracycline‐induced cardiotoxicity in cancer patients. Breast Cancer Res Treat 2018; 167: 249–256. [DOI] [PubMed] [Google Scholar]
- 47. Wells QS, Veatch OJ, Fessel JP, Joon AY, Levinson RT, Mosley JD, Held EP, Lindsay CS, Shaffer CM, Weeke PE, Glazer AM, Bersell KR, van Driest SL, Karnes JH, Blair MA, Lagrone LW, Su YR, Bowton EA, Feng Z, Ky B, Lenihan DJ, Fisch MJ, Denny JC, Roden DM. Genome‐wide association and pathway analysis of left ventricular function after anthracycline exposure in adults Quinn. Pharmacogenet Genomics 2017; 27: 247–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Todorova VK, Makhoul I, Dhakal I, Wei J, Stone A, Carter W, Owen A, Klimberg VS. Polymorphic variations associated with doxorubicin‐induced cardiotoxicity in breast cancer patients. Oncol Res 2017; 25: 1223–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Nakano MH, Udagawa C, Shimo A, Kojima Y, Yoshie R, Zaha H, Abe N, Motonari T, Unesoko M, Tamura K, Shimoi T, Yoshida M, Yoshida T, Sakamoto H, Kato K, Mushiroda T, Tsugawa K, Zembutsu H. A genome‐wide association study identifies five novel genetic markers for trastuzumab‐induced cardiotoxicity in Japanese population. Biol Pharm Bull 2019; 42: 2045–2053. [DOI] [PubMed] [Google Scholar]
- 50. Rossi D, Rasi S, Franceschetti S, Capello D, Castelli A, de Paoli L, Ramponi A, Chiappella A, Pogliani EM, Vitolo U, Kwee I, Bertoni F, Conconi A, Gaidano G. Analysis of the host pharmacogenetic background for prediction of outcome and toxicity in diffuse large B‐cell lymphoma treated with R‐CHOP21. Leukemia 2009; 23: 1118–1126. [DOI] [PubMed] [Google Scholar]
- 51. Serie DJ, Crook JE, Necela BM, Dockter TJ, Wang X, Asmann YW, Fairweather D, Bruno KA, Colon‐Otero G, Perez EA, Thompson EA, Norton N. Genome‐wide association study of cardiotoxicity in NCCTG N9831 (Alliance) Adjuvant Trastuzumab Trial. Pharmacogenet Genomics 2017; 27: 378–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wojnowski L, Kulle B, Schirmer M, Schlüter G, Schmidt A, Rosenberger A, Vonhof S, Bickeböller H, Toliat MR, Suk EK, Tzvetkov M, Kruger A, Seifert S, Kloess M, Hahn H, Loeffler M, Nürnberg P, Pfreundschuh M, Trümper L, Brockmöller J̈, Hasenfuss G. NAD(P)H oxidase and multidrug resistance protein genetic polymorphisms are associated with doxorubicin‐induced cardiotoxicity. Circulation 2005; 112: 3754–3762. [DOI] [PubMed] [Google Scholar]
- 53. Vulsteke C, Pfeil AM, Maggen C, Schwenkglenks M, Pettengell R, Szucs TD, Lambrechts D, Dieudonné AS, Hatse S, Neven P, Paridaens R, Wildiers H. Clinical and genetic risk factors for epirubicin‐induced cardiac toxicity in early breast cancer patients. Breast Cancer Res Treat 2015; 152: 67–76. [DOI] [PubMed] [Google Scholar]
- 54. Hertz DL, Caram MV, Kidwell KM, Thibert JN, Gersch C, Seewald NJ, Smerage J, Rubenfire M, Henry NL, Cooney KA, Leja M, Griggs JJ, Rae JM. Evidence for association of SNPs in ABCB1 and CBR3, but not RAC2, NCF4, SLC28A3 or TOP2B, with chronic cardiotoxicity in a cohort of breast cancer patients treated with anthracyclines. Pharmacogenomics 2016; 17: 231–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gómez Peña C, Dávila‐Fajardo CL, Martínez‐González LJ, Carmona‐Sáez P, Soto Pino MJ, Sánchez Ramos J, Moreno Escobar E, Blancas I, Fernández JJ, Fernández D, Correa C, Cabeza Barrera J. Influence of the HER2 Ile655Val polymorphism on trastuzumab‐induced cardiotoxicity in HER2‐positive breast cancer patients: a meta‐analysis. Pharmacogenet Genomics 2015; 25: 388–393. [DOI] [PubMed] [Google Scholar]
- 56. Stanton SE, Ward MM, Christos P, Sanford R, Lam C, Cobham MV, Donovan D, Scheff RJ, Cigler T, Moore A, Vahdat LT, Lane ME, Chuang E. Pro1170 Ala polymorphism in HER2‐neu is associated with risk of trastuzumab cardiotoxicity. BMC Cancer 2015; 15: 267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Roca L, Diéras V, Roché H, Lappartient E, Kerbrat P, Cany L, Chieze S, Canon JL, Spielmann M, Penault‐Llorca F, Martin AL, Mesleard C, Lemonnier J, de Cremoux P. Correlation of HER2, FCGR2A, and FCGR3A gene polymorphisms with trastuzumab related cardiac toxicity and efficacy in a subgroup of patients from UNICANCER‐PACS04 trial. Breast Cancer Res Treat 2013; 139: 789–800. [DOI] [PubMed] [Google Scholar]
- 58. McCaffrey TA, Tziros C, Lewis J, Katz R, Siegel R, Weglicki W, Kramer J, Mak IT, Toma I, Chen L, Benas E, Lowitt A, Rao S, Witkin L, Lian Y, Lai Y, Yang Z, Fu SW. Genomic profiling reveals the potential role of TCL1A and MDR1 deficiency in chemotherapy‐induced cardiotoxicity. Int J Biol Sci 2013; 9: 350–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Rigaud VOC, Ferreira LRP, Ayub‐Ferreira SM, Ávila MS, Brandão SMG, Cruz FD, Santos MHH, Cruz CBBV, Alves MSL, Issa VS, Guimarães GV, Cunha‐Neto E, Bocchi EA. Circulating miR‐1 as a potential biomarker of doxorubicininduced cardiotoxicity in breast cancer patients. Oncotarget 2017; 8: 6994–7002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Todorova VK, Makhoul I, Wei J, Klimberg VS. Circulating miRNA profiles of doxorubicin‐induced cardiotoxicity in breast cancer patients. Ann Clin Lab Sci 2017; 47: 115–119. [PubMed] [Google Scholar]
- 61. Zhang S, Wang Y, Wang Y, Peng J, Yuan C, Zhou L, Xu S, Lin Y, Du Y, Yang F, Zhang J, Dai H, Yin W, Lu J. Serum miR‐222‐3p as a double‐edged sword in predicting efficacy and trastuzumab‐induced cardiotoxicity for HER2‐positive breast cancer patients receiving neoadjuvant target therapy. Front Oncol 2020; 10: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Todorova VK, Makhoul I, Siegel ER, Wei J, Stone A, Carter W, Beggs ML, Owen A, Klimberg VS. Biomarkers for presymptomatic doxorubicin‐induced cardiotoxicity in breast cancer patients. PLoS ONE 2016; 11: 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63. Qin X, Chang F, Wang Z, Jiang W. Correlation of circulating pro‐angiogenic miRNAs with cardiotoxicity induced by epirubicin/cyclophosphamide followed by docetaxel in patients with breast cancer. Cancer Biomark 2018; 23: 473–484. [DOI] [PubMed] [Google Scholar]
- 64. Zhao Z, He J, Zhang J, Liu M, Yang S, Li N, Li X. Dysregulated miR1254 and miR579 for cardiotoxicity in patients treated with bevacizumab in colorectal cancer. Tumor Biol 2014; 35: 5227–5235. [DOI] [PubMed] [Google Scholar]
- 65. Pop‐Moldovan AL, Trofenciuc NM, Dărăbanţiu DA, Precup C, Branea H, Christodorescu R, Puşchiţă M. Customized laboratory TLR4 and TLR2 detection method from peripheral human blood for early detection of doxorubicin‐induced cardiotoxicity. Cancer Gene Ther 2017; 24: 203–207. [DOI] [PubMed] [Google Scholar]
- 66. Hawkins PG, Sun Y, Dess RT, Jackson WC, Sun G, Bi N, Tewari M, Hayman JA, Kalemkerian GP, Gadgeel SM, Lawrence TS, Haken RKT, Matuszak MM, Kong FM, Schipper MJ, Jolly S. Circulating microRNAs as biomarkers of radiation‐induced cardiac toxicity in non‐small‐cell lung cancer. J Cancer Res Clin Oncol 2019; 145: 1635–1643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Peng J, Wang Z, Li Y, Lv D, Zhao X, Gao J, Teng H. Identification of differential gene expression related to epirubicin‐induced cardiomyopathy in breast cancer patients. Hum Exp Toxicol 2020; 39: 393–401. [DOI] [PubMed] [Google Scholar]
- 68. Braumann S, Baldus S, Pfister R. Molecular mechanisms underlying cardiotoxicity of novel cancer therapeutics. J Thorac Dis 2018; 10: S4335–S4343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Ganatra S, Carver JR, Hayek SS, Ky B, Leja MJ, Lenihan DJ, Lenneman C, Mousavi N, Park JH, Perales MA, Ryan TD, Scherrer‐Crosbie M, Steingart RM, Yang EH, Zaha V, Barac A, Liu JE. Chimeric antigen receptor T‐cell therapy for cancer and heart: JACC council perspectives. J Am Coll Cardiol 2019; 74: 3153–3163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Ghosh AK, Chen DH, Guha A, Mackenzie S, Walker JM, Roddie C. CAR T cell therapy‐related cardiovascular outcomes and management. JACC CardioOncology 2020; 2: 97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol 2020; 17: 474–502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Kumari H, Huang W‐H, Chan MWY. Review on the role of epigenetic modifications in doxorubicin‐induced cardiotoxicity. Front Cardiovasc Med 2020; 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Aleman BMP, Moser EC, Nuver J, Suter TM, Maraldo MV, Specht L, Vrieling C, Darby SC. Cardiovascular disease after cancer therapy. Eur J Cancer, Suppl 2014; 12: 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Okwuosa TM, Anzevino S, Rao R. Cardiovascular disease in cancer survivors. Postgrad Med J 2017; 93: 82–90. [DOI] [PubMed] [Google Scholar]
- 75. Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan‐Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med 2006; 355: 1572–1582. https://www.nejm.org/doi/full/10.1056/NEJMsa060185 [DOI] [PubMed] [Google Scholar]
- 76. López‐Sendón J, Álvarez‐Ortega C, Zamora Auñon P, Buño Soto A, Lyon AR, Farmakis D, Cardinale D, Canales Albendea M, Feliu Batlle J, Rodríguez Rodríguez I, Rodríguez Fraga O, Albaladejo A, Mediavilla G, González‐Juanatey JR, Martínez Monzonis A, Gómez Prieto P, González‐Costello J, Serrano Antolín JM, Cadenas Chamorro R, López Fernández T. Classification, prevalence, and outcomes of anticancer therapy‐induced cardiotoxicity: the CARDIOTOX registry. Eur Heart J 2020; 41: 1720–1729. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Search strategy.
Table S2. Eligibility criteria for the clinical research question.
Table S3. Data collection of included studies.


