Abstract
Aims
The HINODE study aimed to analyse rates of mortality, appropriately treated ventricular arrhythmias (VA), and heart failure in Japanese patients and compared with those in Western patients.
Methods and results
After treatment decisions following contemporary practice in Japan, patients were prospectively enrolled into four cohorts: (i) internal cardioverter‐defibrillator (ICD), (ii) cardiac resynchronization therapy (CRT) defibrillator (CRT‐D), (iii) standard medical therapy (‘non‐device’: ND), or (iv) pacing (indicated for CRT; received pacemaker or CRT pacing). Cohorts 1–3 required a left ventricular ejection fraction ≤35%, a history of heart failure, and a need for primary prevention of sudden cardiac death based on two to five previously identified risk factors. Endpoint outcomes were adjudicated by the independent committees. ICD and CRT‐D cohorts, considered as high‐voltage (HV) cohorts, were pooled for Kaplan–Meier analysis and propensity‐matched to Multicenter Automatic Defibrillator Implantation Trial‐Reduce Inappropriate Therapy (MADIT‐RIT) arm B and C patients. The study enrolled 354 patients followed for 19.6 ± 6.5 months, with a minimum of 12 months. Propensity‐matched HV cohorts showed comparable VA (P = 0.61) and mortality rates (P = 0.29) for HINODE and MADIT‐RIT. The ND cohort presented a high crossover rate to ICD therapy (6.1%, n = 7/115), and the CRT‐D cohort showed elevated mortality rates. The pacing cohort revealed that patients implanted with pacemakers had higher mortality (26.0%) than those with CRT‐Pacing (8.4%, P = 0.05).
Conclusions
The mortality and VA event rates of landmark trials are applicable to patients with primary prevention in Japan. Patients who did not receive guideline‐indicated CRT devices had poor outcomes.
Keywords: Defibrillator therapy; Ventricular arrhythmia; Sudden cardiac death; Japan, primary prevention; Electrophysiologic studies
Introduction
Despite increase in the incidence of chronic heart failure and sudden cardiac death (SCD), 1 , 2 , 3 , 4 the current use of implantable cardioverter‐defibrillator (ICD) and cardiac resynchronization therapy (CRT) with defibrillator (CRT‐D) in Japan is much lower than in Western countries. 3 , 5 , 6 , 7 The present Japanese guidelines, 8 especially for primary prevention, do not fully implement findings from landmark trials on ICD and CRT‐D. 9 , 10 , 11 , 12 , 13 The benefit of ICD therapy for patients from Japan is widely questioned as not very cost‐effective in Japan. 5 , 8 , 14 For a class I ICD indication, primary prevention patients need a history of non‐sustained ventricular tachycardia (NSVT) and/or an electrophysiologic study with an inducible sustained ventricular arrhythmia (VA). 15 If there is no history of NSVT or positive EPS testing, there is only a class IIa recommendation (II: Conflicting evidence and/or a divergence of opinion about the usefulness/efficacy of the given procedure or treatment; IIa Weight of evidence/opinion is in favour of usefulness/efficacy) by the Japanese Circulation Society (JCS). Only patients with an ICD indication can be considered for CRT‐D per JCS guideline. CRT‐D class I indication is limited to patients in sinus rhythm with LBBB: NYHA class III/IV and QRS ≥ 120 ms or NYHA class II and QRS ≥ 150 ms. Without any VA history (NSVT or EPS) there is no expected reimbursement for CRT‐D therapy. Specifically, the actual rate of ventricular arrhythmias (VAs) in ICD and CRT patients, the diminished value of electrophysiologic testing for risk stratification, and the use of CRT therapy for mildly symptomatic patients in New York Heart Association class I and II have not been well‐established in Japan. While CRT‐D has been proven to be a highly effective treatment for some patients with heart failure, 16 it remains unknown whether study data from the USA and Europe are fully applicable in Japan considering genetic and cultural differences. Kawashiro and colleagues (2015) stated that CRT‐D therapy is very limited in Japan suggesting that ‘it will be necessary to formulate evidence unique to Japan on the extent to which cutting‐edge non‐pharmacological treatments for arrhythmias improve the prognosis of patients’. 8 Notably, the occurrence of HF related mortality in non‐ischaemic patients might be underestimated and having an ICD indication prior to CRT‐D therapy may lead to delayed treatment. 17
The Heart Failure Indication and Sudden Cardiac Death Prevention Trial Japan (HINODE) study was designed to prospectively assess the rates of mortality, appropriately treated VA, and heart failure for comparison with references from historical landmark trials like MADIT‐RIT in Japanese patients who meet the European Society of Cardiology (ESC) guidelines for the SCD primary prevention or CRT treatment of heart failure across four clinical therapy cohorts: ICD, CRT‐D, pacing (PA), and non‐device (ND). 18 In addition to reduced left ventricular ejection fraction (LVEF) and heart failure, subjects were required to have two to five previously identified risk factors for sudden death. 19 , 20 The HINODE study aimed to identify current treatment practices for ICD and CRT‐D in Japan for comparison with reference data from Multicenter Automatic Defibrillator Implantation Trial Reducing Inappropriate Therapy (MADIT‐RIT). 21 This novel comparison is critical to evaluating current assumptions that patient outcomes in Japan are distinct from those observed in western studies.
Methods
Study design
Details of the study design, including inclusion and exclusion criteria, device programming, and event adjudication, have been published previously. 18 The protocol was approved by the ethics committees of the participating centres, and written informed consent was obtained from each enrolled subject. The investigation conformed to the principles outlined in the Declaration of Helsinki.
The study followed patients in four treatment cohorts to determine the rates of life‐threatening VAs, all‐cause mortality and occurrence of serious heart failure events. Equivalence in event rates between Japanese and western patients is essential evidence for acceptance of ESC like guidelines following clinical trial results. After the treatment plan was decided, based on current JCS guidelines, patients indicated for primary prevention according to the ESC guidelines were prospectively enrolled into one of four treatment cohorts (ICD, CRT‐D, PA, or ND). Stable OMT was required prior to any device implant by JCS guidelines. Information on drug groups was collected at enrolment and closeout as well in relation to adverse events, re‐implants and new device implants. Enrolment was limited to subjects with two to five of the following previously identified risk factors for SCD: (i) LVEF ≤35%, (ii) New York Heart Association (NYHA) functional class III or IV, (iii) left bundle branch block (LBBB) with QRS ≥ 130 ms or any QRS morphology ≥150 ms; (iv) renal dysfunction, defined as chronic blood urea nitrogen (BUN) > 26 mg/dL or ≥9.28 mmol/L; (v) diabetes mellitus type I and II; (vi) chronic atrial fibrillation; (vii) prior myocardial infarction (MI); (viii) age >70 years; or (ix) smoking currently or during the last 5 years. Notably, all patients had LVEF ≤35%. The criteria for cohort enrolment are as follows:
ICD Device Therapy Cohort: NYHA Class II‐III, ischaemic heart disease or dilated cardiomyopathy, optimal medical therapy (OMT), and LVEF ≤35%.
CRT‐D Device Therapy Cohort: NYHA class I‐IV despite OMT, LVEF ≤35%, sinus rhythm + QRS > 130 ms and LBBB, or QRS > 150 ms and non‐LBBB; alternatively, AF rhythm, NYHA class III, QRS ≥ 130 ms.
Non‐Device (ND) Therapy Cohort: fulfilment of either the ICD or CRT‐D cohort criteria
Pacing (PA) Cohort with standard right ventricular pacemaker (PM) or CRT‐Pacing (CRT‐P) Therapy: NYHA class I‐IV despite OMT, LVEF ≤50%
ICD device programming required a high cut‐off rate or delayed therapy and followed the principles of MADIT‐RIT. Patients' medical therapy was at the investigator's discretion except for ICD indicated patients OMT was required. Patients were followed up for a minimum of 12 months. All patients using a device were required to be connected to a home monitoring system.
The primary endpoint for the ICD and CRT‐D cohorts was the first appropriately treated VA along with possible life‐threatening symptoms, while the primary endpoint for the PA and ND cohorts was all‐cause mortality. Events were adjudicated by independent committees following the MADIT‐RIT and MADIT‐CRT criteria for the classification of endpoint VA events.
Statistical considerations
The appropriate anti‐tachycardia pacing or shock treatment for VA was 5% in ICD and 3% in CRT‐D patients at 12 months based on the results from MADIT‐RIT. This data was used for sample size calculation. 22 Meanwhile, the sample sizes for the PA and ND cohorts were calculated using a 10% estimated all‐cause mortality at 12 months based on the CARE‐HF and MADIT‐II study results. 23 The Kaplan–Meier estimates for freedom from all‐cause mortality and first VA were determined at the 12‐ and 24‐month follow‐up for all cohorts. Estimates were further compared by subgroups within each cohort, as well as to MADIT‐RIT outcomes using log‐rank tests (Table 3 ).
Table 3.
Subgroups for patient outcomes of ventricular arrhythmia and mortality rate
| Cohort | Subgroup | Ventricular arrhythmia associated symptoms free rate from 0–24 months b | All‐cause mortality free rate from 0–24 months b | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Subjects at risk | Number of failures | VA‐free rate | P‐value | Hazard ratio (95% CI) | Number of failures | Survival rate | P‐value | Hazard ratio (95% CI) | ||
| Full ICD cohort | All | 102 | 12 | 86.3% | 11 | 83.5% | ||||
| Without CRT‐D indication a | 92 | 8 | 90.7% | 0.002 | 5.5 (1.6, 18.3) | 8 | 88.0% | 0.033 | 3.8 (1.0, 14.5) | |
| With CRT‐D indication a | 10 | 4 | 40.0% | 3 | 45.7% | |||||
| QRS ≥ 130 ms | 29 | 5 | 77.9% | 0.283 | 1.9 (0.6, 5.8) | 4 | 77.5% | 0.644 | 1.3 (0.4, 4.6) | |
| QRS < 130 ms | 73 | 7 | 89.6% | 7 | 86.7% | |||||
| ICD COHORT Without CRT‐D indication a | Age ≤70 years | 49 | 6 | 86.9% | 0.213 | 0.4 (0.1, 1.9) | 2 | 95.7% | 0.086 | 3.7 (0.8 18.4) |
| Age >70 years | 43 | 2 | 95.3% | 6 | 78.7% | |||||
| Full CRT‐D cohort | All | 69 | 4 | 94.0% | 7 | 89.6% | ||||
| QRS ≥ 130 ms | 54 | 3 | 94.4% | 0.645 | 0.6 (0.1, 5.7) | 3 | 94.1% | 0.004 | 0.1 (0.0, 0.7) | |
| QRS < 130 ms | 13 | 1 | 92.3% | 4 | 69.2% | |||||
| With LBBB | 42 | 1 | 97.6% | 0.085 | 0.2 (0.0, 1.7) | 2 | 95.1% | 0.052 | 0.2 (0.0, 1.2) | |
| Others | 25 | 3 | 87.4% | 5 | 79.8% | |||||
| CRT‐D cohort with QRS ≥ 130 | Age ≤70 years | 28 | 2 | 92.9% | 0.601 | 0.5 (0.0, 5.9) | 0 | 100.0% | 0.071 | NA d |
| Age >70 years | 26 | 1 | 96.0% | 3 | 88.0% | |||||
| High‐voltage ICD + CRT‐D | All | 171 | 16 | 89.4% | 18 | 85.8% | ||||
| ICD therapy | QRS < 130 ms | 73 | 7 | 89.6% | 0.979 | 1.0 (0.1, 8.4) | 7 | 86.7% | 0.013 | 0.2 (0.1, 0.8) |
| CRT‐D therapy | QRS < 130 ms | 13 | 1 | 92.3% | 4 | 69.2% | ||||
| High‐voltage ICD + CRT‐D | Female | 37 | 5 | 85.1% | 0.387 | 0.6 (0.2, 1.8) | 5 | 82.5% | 0.623 | 0.77 (0.3, 2.2) |
| Male | 134 | 11 | 90.7% | 13 | 87.2% | |||||
| High‐voltage ICD (ESC class I excluded) + CRT‐D | Age ≤70 | 77 | 8 | 89.1% | 0.179 | 0.4 (0.1, 1.6) | 2 | 97.2% | 0.019 c | 5.2 (1.1, 24.0) |
| Age >70 | 69 | 3 | 95.4% | 9 | 82.9% | |||||
CRT‐D indication refers to subjects with the third risk factor: Left bundle branch block (LBBB) with QRS > 130 ms or QRS > 150 ms.
Rates calculated using Kaplan–Meier estimation with P‐values from log‐rank test.
Most significant split in age.
Hazard ratio not available due to non‐proportional hazards model violation.
CRT‐D, cardiac resynchronization therapy defibrillator; ICD, internal cardioverter defibrillator; VA, ventricular arrhythmia.
An exploratory propensity score (PS) match analysis was performed per protocol to compare event rates for the high‐voltage (HV) cohorts, ICD and CRT‐D, to those in MADIT‐RIT. To parallel the MADIT‐RIT exclusion criteria in the HV cohort, ICD patients with CRT‐D indication (LVEF >35% and LBBB with QRS > 130 ms or QRS > 150 ms) were excluded, along with patients with chronic AF. The remaining patients were matched 1:1 to the MADIT‐RIT arm B and C patients (novel ICD programming) on select baseline characteristics that match the primary prevention indication. A reduction in the standardized mean difference (<0.20), post‐PS match, and model fit were desired (Supporting Information, Table S2 ). Baseline characteristics included those with high relevance to the endpoint that were present in both studies. QRS width was excluded due to its unavailability for the MADIT‐RIT ICD cohort, while BMI was excluded due to the burden on model fit. Event rates were re‐estimated in the matched HINODE and MADIT‐RIT cohorts and were compared using a log‐rank test stratified on the quintiles of the PS. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, USA).
Results
Between June 2017 and June 2019, 354 patients from 34 hospitals were enrolled in the HINODE trial. Due to the extended enrolment period, the mean follow‐up time both overall (19.6 ± 6.5 months) and in three cohorts (ICD: 20.5 ± 6.2 months, CRT‐D: 19.7 ± 7.1 months, and ND: 17.8 ± 5.5 months) exceeded the anticipated period and compensated for the low enrolment rate. The follow‐up time for the PA cohort was 20.9 ± 7.3 months for 68 patients (1422.6 patient‐months), in contrast with the expected 137 patients (1644 patient‐months, Supporting Information, Table S1 ). No patients were lost to follow‐up.
The four cohorts differed in terms of patient characteristics and disease history. Notably, CRT‐D included patients across the ICD, ND, and PA cohorts. ICD and CRT‐D patients had a median of four out of nine risk factors, while ND and PA patients had a median of three risk factors (Table 1 ). More than 10% of patients who underwent ICD or CRT‐D therapy had a history of inducible VA (Table 1 ). The ICD cohort enrolled 68.6% DDDR (Resonate™ ICD EL D433, Dynagen™ D152 and Perciva™ DF4 D413), 15.7% S‐ICD (Emblem MRI™ A219) and 15.7% VR devices (Resonate™ ICD D432, Perciva™ DF4 D412), the CRT‐D cohort contained 100% Resonate™ X4 G447, G437 devices and the Pacing cohort included 22 pacemakers (Accolade™ [77.3% MRI DR (L331, L311) 18.2% MRI VR L310, and 4.5% DR L301]) and 46 CRT‐P (100% Valitude™ X4 MRI, U128, U125).
Table 1.
Patient characteristics in the four treatment cohorts
| Characteristic | Measurement | ICD cohort (N = 102) | CRT‐D cohort (N = 69) | Pacing cohort (N = 68) | Non‐device cohort (N = 115) |
|---|---|---|---|---|---|
| Risk factors at enrolment | |||||
| 1. LVEF ≤ 35% | N (%) | 102 (100.0%) | 69 (100.0%) | 37 (54.4%) | 114 (99.1%) |
| 2. NYHA Class III or IV | N (%) | 20 (19.6%) | 38 (55.1%) | 20 (29.4%) | 21 (18.3%) |
| 3. Left Bundle Branch Block (LBBB) with QRS > 130 ms or QRS > 150 ms | N (%) | 10 (9.8%) | 64 (92.8%) | 47 (69.1%) | 29 (25.2%) |
| 4. Renal dysfunction | N (%) | 25 (24.5%) | 11 (15.9%) | 17 (25.0%) | 32 (27.8%) |
| 5. Diabetes Type I and II | N (%) | 44 (43.1%) | 25 (36.2%) | 19 (27.9%) | 44 (38.3%) |
| 6. Chronic atrial fibrillation | N (%) | 22 (21.6%) | 6 (8.7%) | 9 (13.2%) | 24 (20.9%) |
| 7. Prior MI | N (%) | 48 (47.1%) | 11 (15.9%) | 11 (16.2%) | 39 (33.9%) |
| 8. Age >70 years | N (%) | 50 (49.0%) | 32 (46.4%) | 46 (67.6%) | 63 (54.8%) |
| 9. Smoking history (last 5 years) | N (%) | 32 (31.4%) | 14 (20.3%) | 10 (14.7%) | 31 (27.0%) |
| Risk factors per cohort | Median (Range) | 4.0 (2.0–5.0) | 4.0 (2.0–5.0) | 3.0 (1.0–5.0) | 3.0 (2.0–5.0) |
| Age at time of consent (years) |
Mean ± SD Median (Range) |
68.2 ± 10.4 70.0 (35.0–85.0) |
66.4 ± 13.4 69.0 (23.0–88.0) |
75.0 ± 10.9 76.5 (41.0–95.0) |
68.8 ± 11.8 70.0 (33.0–92.0) |
| Gender/female | (%) | 14.7% | 31.9% | 35.3% | 21.7% |
| BMI (kg/m2) | Mean ± SD | 23.2 ± 3.8 | 22.2 ± 4.1 | 22.3 ± 3.3 | 23.7 ± 4.0 |
| Systolic blood pressure (mmHg) | Mean ± SD | 108.2 ± 14.7 | 104.8 ± 17.2 | 113.9 ± 17.4 | 112.6 ± 17.0 |
| Diastolic blood pressure (mmHg) | Mean ± SD | 63.7 ± 10.9 | 63.5 ± 14.1 | 64.8 ± 11.2 | 66.3 ± 12.0 |
| NYHA class | I [N (%)] | 0/102 (0.0%) | 3/68 (4.4%) | 10/65 (15.4%) | 4/115 (3.5%) |
| II [N (%)] | 82/102 (80.4%) | 28/68 (41.2%) | 36/65 (55.4%) | 92/115 (80.0%) | |
| III [N (%)] | 20/102 (19.6%) | 34/68 (50.0%) | 18/65 (27.7%) | 18/115 (15.7%) | |
| IV [N (%)] | 0/102 (0.0%) | 3/68 (4.4%) | 1/65 (1.5%) | 1/115 (0.9%) | |
| LVEF (%) a |
Mean ± SD Median (Range) |
27.1 ± 6.0 29.0 (4.0–35.0) |
24.3 ± 6.1 24.0 (3.0–35.0) |
35.8 ± 9.6 34.0 (12.0–51.0) |
26.0 ± 5.9 27.0 (10.0–35.0) |
| LVEF category a ≤25% | (%) | 38.2% | 60.9% | 10.3% | 44.4% |
| Resting heart rate (b.p.m.) | Mean ± SD | 68.9 ± 14.6 | 71.3 ± 15.9 | 63.0 ± 16.4 | 74.0 ± 15.1 |
| QRS width (ms) |
Mean ± SD Median (Range) |
120.2 ± 23.1 110.0 (91.0–223.0) |
152.2 ± 25.0 150.0 (101.0–218.0) |
153.8 ± 29.3 155.0 (84.0–225.0) |
120.5 ± 21.8 116.0 (82.0–190.0) |
| QRS width ≥120 ms | (%) | 38.2% | 91.0% | 86.8% | 44.3% |
| QRS width ≥150 ms | (%) | 13.7% | 58.2% | 58.8%) | 13.9% |
| PR interval (ms) | Mean ± SD | 193.2 ± 35.0 | 200.6 ± 46.7 | 194.0 ± 51.6 | 183.8 ± 28.1 |
| QT interval (ms) | Mean ± SD | 438.4 ± 47.6 | 457.1 ± 58.9 | 492.0 ± 74.3 | 426.7 ± 45.3 |
| QRS morphology | Normal (%) | 47.5% | 9.0% | 14.9% | 53.0% |
| RBBB (%) | 13.9% | 10.4% | 11.9% | 10.4% | |
| LBBB (%) | 8.9% | 62.7% | 50.7% | 18.3% | |
| Other (%) | 29.7% | 17.9% | 22.4% | 18.3% | |
| Ischaemic cardiomyopathy | Yes (%) | 52.9% | 26.1% | 29.4% | 42.6% |
| Hypertension | Yes (%) | 50.0% | 39.1% | 50.0% | 52.2% |
| Rhythm (pre‐implant per core lab) | Sinus rhythm (%) | 75.2% | 86.8% | 47.1% | 76.6% |
| Atrial fibrillation history | Yes (%) | 34.3% | 17.4% | 25.0% | 29.6% |
| Type of atrial fibrillation | Paroxysmal (%) | 37.1% | 66.7% | 41.2% | 26.5% |
| Chronic (%) | 40.0% | 33.3% | 35.3% | 44.1% | |
| Persistent (%) | 22.9% | 0.0% | 17.6% | 29.4% | |
| Unknown (%) | 0.0% | 0.0% | 5.9% | 0.0% | |
| Previous hospitalization for heart failure | Yes (%) | 61.8% | 71.0% | 52.9% | 58.3% |
| History of non‐sustained spontaneous ventricular arrhythmias >20–30s | Yes (%) | 16.7% | 11.6% | 2.9% | 4.3% |
| History of inducible ventricular arrhythmias during last 12 months | Yes (%) | 10.8% | 10.1% | 2.9% | 5.2% |
| Previous ablation | Yes (%) | 15.7% | 14.5% | 14.7% | 14.8% |
| Previously implanted medical devices | Yes [N (%)] | 6 (5.9%) | 6 (8.7%) | 21 (30.9%) | 15 (13.0%) |
| Type of previously implanted device | Pacemaker [N (%)] | 0/6 (0.0%) | 0/5 (0.0%) | 19/21 (90.5%) | 0/15 (0.0%) |
| CRT‐P [N (%)] | 0/6 (0.0%) | 0/5 (0.0%) | 2/21 (9.5%) | 0/15 (0.0%) | |
| Stent [N (%)] | 5/6 (83.3%) | 3/5 (60.0%) | 0/21 (0.0%) | 15/15 (100.0%) | |
| Other devices [N (%)] | 1/6 (16.7%) | 2/5 (40.0%) | 0/21 (0.0%) | 0/15 (0.0%) | |
| Medication use | |||||
| Angiotensin converting enzyme | (%) | 46.1% | 47.8% | 32.4% | 47.8% |
| ARB | (%) | 31.4% | 20.3% | 27.9% | 31.3% |
| Antiarrhythmic | (%) | 36.3% | 33.3% | 19.1% | 21.7% |
| Anticoagulant | (%) | 52.0% | 37.7% | 41.2% | 47.0% |
| Antiplatelet | (%) | 52.0% | 34.8% | 47.1% | 43.5% |
| Aldosterone‐antagonist | (%) | 40.2% | 55.1% | 30.9% | 38.3% |
| Beta‐blocker | (%) | 94.1% | 79.7% | 54.4% | 89.6% |
| Digitalis | (%) | 3.9% | 10.1% | 2.9% | 10.4% |
| Diuretics | (%) | 76.5% | 75.4% | 61.8% | 81.7% |
| Statins | (%) | 57.8% | 39.1% | 42.6% | 56.5% |
| Calcium antagonists | (%) | 14.7% | 8.7% | 25.0% | 10.4% |
If echocardiography or equivalent method assessed within 12 months but not before 3 months, exact LVEF % not available.
ACE, angiotensin‐converting enzyme; ARB, angiotensin‐receptor blocker; BMI, body mass index; CRT‐P, cardiac resynchronization therapy pacemaker; CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; LVEF, left ventricular ejection fraction; LBBB, left bundle branch block; LVEDV, left ventricular end‐diastolic volume; LVESV, left ventricular end‐systolic volume; NYHA, New York Heart Association; PM, pacemaker; RBBB, right bundle branch block (RBBB); SD, standard deviation.
The Ventricular Event Adjudication Committee assessed a total of 104 events, including first and subsequent treatments, as well as untreated ventricular events. Of 104 events, 72 (69.2%) were classified as life‐threatening and appropriately treated. Of 171 HV patients, 15 (8.8%) received appropriate antitachycardia pacing (ATP) or shock therapy within the trial. This resulted in 12‐ and 24‐month estimated event rates of 7.9% and 13.3%, respectively, for the ICD cohort and 3.0% and 4.6%, respectively, for the CRT‐D cohort. Unmatched and propensity‐matched MADIT‐RIT subjects with ICD and CRT‐D from the arms B and C had similar event rates, with no significant difference based on the Kaplan–Meier analysis at 12 and 24 months (Table 2 ). Other episodes (n = 32/104) did not qualify for endpoint events due to the rate, duration, or absence of symptoms during the arrhythmia. There were four instances of inappropriate ICD therapy (ATP or shock), resulting in an inappropriate therapy rate of 1.8% at 1 year (CRT‐D, n = 1; ICD, n = 3). For comparison, the inappropriate therapy rate for the MADIT‐RIT arms B and C with ICD and CRT‐D devices at 1 year was approximately 5%. 22
Table 2.
Endpoint results for HINODE ICD and CRT‐D treatment cohorts compared with matched and unmatched MADIT‐RIT results
| Cohort | ICD cohorts | CRT‐D cohorts | ICD and CRT‐D cohorts | ICD and CRT‐D MADIT‐RIT matched 1:1 to HINODE cohorts | ||||
|---|---|---|---|---|---|---|---|---|
| HINODE | MADIT‐RIT | HINODE | MADIT‐RIT | HINODE | MADIT‐RIT | HINODE | MADIT‐RIT | |
| Number of patients | 102 | 484 | 69 | 501 | 171 | 985 | 134 | 134 |
| Appropriately treated VA‐free at 12 m | 92.1% | 94.3% | 97.0% | 96.1% | 94.0%) | 95.2% | 94.7%* | 96.8%* |
| at 24 m | 86.3% | 87.7% | 95.4% | 91.8% | 90.0% | 89.8% | 92.8%* | 94.5%* |
| Mortality‐free at 12 m | 97.1% | 96.7% | 92.8% | 98.7% | 95.3% | 97.7% | 96.3%* | 96.9%* |
| at 24 m | 83.5% | 92.8% | 89.6%*** | 95.3%*** | 85.8%** | 94.1%** | 88.6%* | 92.5%* |
| Total study mortality, % (N) | 13.7% (14) | 4.8% (23) | 13.0% (9) | 2.8% (14) | 13.5% (23) | 3.8% (37) | 11.9% (16) | 4.5% (6) |
| Mortality: Non‐cardiac or unknown % | 42.9% | 52.2% | 22.2% | 35.7% | 34.8% | 45.9% | 37.6% | 0.0% |
| Mortality: Cardiac % | 57.4% | 47.8% | 77.8% | 64.3% | 65.2% | 54.1% | 62.5% | 100.0% |
| Out of cardiac: Arrhythmic % | 12.5% | 27.3% | 14.3% | 33.3% | 13.3% | 30.0% | 10.0% | 50.0% |
| Out of cardiac: Pump failure % | 75.0% | 63.6% | 85.7% | 44.4% | 80.0% | 55.0% | 90.0% | 50.0% |
| Out of cardiac: unknown % | 12.5% | 9.1% | 0.0% | 22.2% | 6.7% | 15.0% | 0.0% | 0.0% |
All P‐values for matched groups >0.05; P‐value from stratified log‐rank test.
Kaplan–Meier, P‐value, 0.002; P‐value from log‐rank test.
Kaplan–Meier, P‐value, 0.005; P‐value from log‐rank test.
CRT‐D, cardiac resynchronization therapy defibrillator; ICD, implantable cardioverter‐defibrillator; VA, ventricular arrhythmia.
High‐voltage cohort
The risk of mortality tended to be higher in the ICD and CRT‐D cohorts than in the MADIT‐RIT unmatched cohort and was significantly different at 24 months for CRT‐D (P = 0.002) and HV (P = 0.005, Table 2 ). PS matching resulted in more comparable cohorts, with 134 subjects for each study (Table 2 ), improving the balance in the proportion of subjects by device type, age, sex, ischaemic cardiomyopathy, hypertension, and prior MI (Supporting Information, Table S2 ). Stratified log‐rank comparisons of mortality and VA‐free rates showed no difference in the PS‐matched data (Figure 1 ).
Figure 1.
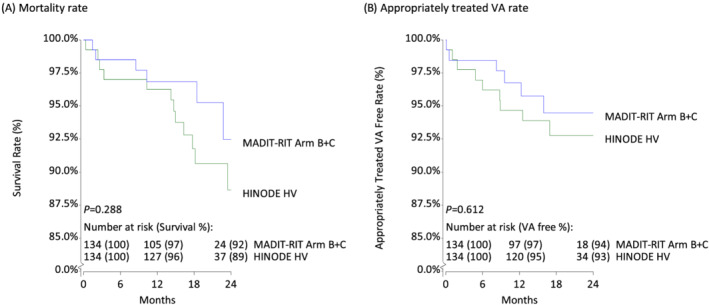
High‐voltage (HV) cohort of HINODE matched 1:1 with MADIT‐RIT cohort. Survival curves for mortality (A) and appropriately treated VA (B) comparing HV HINODE patients to 1:1 matched data from MADIT‐RIT demonstrated similar rates. Stratified log‐rank tests showed no difference in event occurrence between HINODE and MADIT‐RIT patients. However, it is notable that event‐free rates in HINODE are slightly lower than those in MADIT‐RIT.
The HV cohort was stratified using a cut‐off age of 70 years and was limited to subjects treated according to guidelines to assess the impact of age. There was no significant association between age and VA‐associated symptom‐free rates from 0–24 months (P = 0.179). However, a significant relationship was found between survival rates. Subjects aged 70 years or less had an estimated 97.2% survival at 24 months, whereas subjects older than 70 years had an estimated 82.9% survival (P = 0.019, Table 3 ). A similar analysis by gender revealed no significant differences for VA‐free rate (P = 0.387) and survival (P = 0.623). Although HINODE patients are older than those in MADIT‐RIT, the age of 70 remains a discriminator for mortality risk. 24
In additional analysis the predictive value of electrophysiology studies (EPS) was assessed for all HV patients by comparing patients with a positive test and an inducible VA to patients who did not undergo EPS or obtained a negative test. No predictive association between positive findings and mortality or VA was found at 12 or 24 months (Table 4 ), which is consistent with current ESC guidelines.
Table 4.
Patient endpoint outcomes and history of inducible VA within 12 months prior to enrolment
| Endpoint | Cohort and time in months | Event‐free rate a (N at risk) | Log‐rank P‐value | |
|---|---|---|---|---|
| No EPS or negative EPS | Patients with inducible VA during EPS | |||
| All‐cause mortality‐free rate | Combined ICD/CRT‐D 0–12 | 94.4% (N = 144) | 100.0% (N = 18) | 0.311 |
| 0–24 | 83.4% | 100.0% | 0.140 | |
| CRT‐D only 0–12 | 91.5% (N = 59) | 100.0% (N = 7) | 0.433 | |
| 0–24 | 87.7% | 100.0% | 0.341 | |
| ICD only 0–12 | 96.5% (N = 85) | 100.0% (N = 11) | 0.531 | |
| 0–24 | 80.3% | 100.0% | 0.231 | |
| Ventricular arrhythmia‐associated symptom‐free rate | Combined ICD/CRT‐D 0–12 | 93.7% (N = 144) | 94.4% (N = 18) | 0.915 |
| 0–24 | 88.8% | 94.4% | 0.576 | |
| CRT‐D only 0–12 | 94.8% (N = 59) | 100.0% (N = 7) | 0.542 | |
| 0–24 | 92.9% | 100% | 0.476 | |
| ICD only 0–12 | 92.9% (N = 85) | 90.9% (N = 11) | 0.786 | |
| 0–24 | 86.0% | 90.9% | 0.842 | |
Kaplan–Meier calculation of event‐free rate.
CRT‐D, cardiac resynchronization therapy defibrillator; EPS, electrophysiology studies; ICD, internal cardioverter defibrillator; VA, ventricular arrhythmia.
Internal cardioverter‐defibrillator treatment cohort
Exploratory subgroup analysis showed that the ICD cohort enrolled 9.8% (n = 10/102) of patients with CRT‐D indication (risk factor: LBBB with QRS > 130 ms or QRS > 150 ms) and 28.4% (n = 29/102) with QRS ≥ 130 ms. Sub‐group analysis on ESC guideline indicated CRT‐D patients showed a significantly greater VA symptom probability (P = 0.002) and mortality (P = 0.033) at 24 months compared with the rest of the ICD cohort (Table 3 ). Further, patients with QRS ≥ 130 ms had a VA‐free rate of 77.9% and a survival rate of 77.5% compared with a VA‐free rate of 89.6% and a survival rate of 86.7% for patients with QRS < 130 ms (VA‐free P = 0.283, Survival P = 0.644, Table 3 ). For ICD sub‐group patients without CRT‐D indication, no significant differences in endpoints were found by the assumed risk factor cut‐off age of 70 years. Though not significant, older patients (> 70 years) tended to have lower VA rates(P = 0.213), and higher mortality rates (P = 0.086, Table 3 ). Of the 102 patients in the ICD cohort, 16 received S‐ICD and the S‐ICD subgroup had one death.
Subgroup analysis
Cardiac resynchronization therapy defibrillator treatment cohort
The CRT‐D cohort included 42 patients with LBBB and 25 patients with non‐LBBB. Patients with LBBB tended to have lower VA rates (P = 0.085) and lower mortality (P = 0.052, Table 3 ) than those without LBBB. CRT‐D‐treated patients with a QRS < 130 ms presented a similar 24‐month VA rate (P = 0.645) but a significantly higher mortality rate compared to those with longer QRS width (30.8% vs. 5.9%; P = 0.004; Table 3 ). HINODE patients with LBBB (n = 42/67), compared with those with non‐LBBB (n = 25/67), presented a wider QRS (159.4 ± 23.8 vs. 140.1 ± 22.6; P = 0.002) and tended to have less renal dysfunction (11.9% vs. 24.0%; P = 0.200).
Non‐device cohort
The estimated mortality of the ND cohort was 4.4% at 12 months and 9.0% at 24 months. The ND cohort enrolled 25.2% (n = 29/115) patients with CRT‐D indication (with LBBB, QRS > 130 ms or QRS > 150 ms, Table 5 ) and 74.8% with ICD indication (without LBBB, QRS > 130 ms or QRS > 150 ms). Further exploratory analyses suggested that patients with CRT‐D had lower estimated survival at 24 months (87.9%) compared with patients with primary prevention ICD indication (91.4%), although the difference was not significant (P = 0.352). ND patients presented a high crossover rate (n = 9/115) to ICD (3) and CRT therapy (6).
Table 5.
All‐cause mortality in non‐device and pacing cohorts with select subgroups
| Mortality statistic | Non‐device cohort and subgroups | Pacing cohort and subgroup | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| All | CRT‐D indicated a | ICD indicated | QRS width ≥130 ms | QRS width <130 ms | No prior MI or ischaemic | Prior MI or ischaemic | All | CRT‐P therapy | PM therapy | |
| P‐value b at 24 months | 0.352 | 0.693 | 0.532 | 0.05 | ||||||
| Subjects at risk | 115 | 29 | 86 | 38 | 77 | 66 | 49 | 68 | 46 | 22 |
| Survival rate at: | ||||||||||
| 12 months b | 95.6% | 93.1% | 96.5% | 94.7% | 96.1% | 97.0% | 93.7% | 94.1% | 95.6% | 90.9% |
| 24 months b | 91.0% | 87.9% | 91.4% | 88.8% | 93.6% | 89.5% | 90.5% | 86.2% | 91.6% | 74.0% |
| Total mortality % (N) | 6.1% (7) | 10.3% (3) | 4.7% (4) | 13.2% (9) | 8.7% (4) | 22.7% (5) | ||||
| Non‐cardiac/unknown (N) | 4 | 0 | 4 | 6 | 2 | 4 | ||||
| Cardiac (N) | 3 | 3 | 0 | 3 | 2 | 1 | ||||
CRT‐D indication refers to subjects with the third risk factor: Left bundle branch block (LBBB) with QRS > 130 ms or QRS > 150 ms.
Rates calculated using Kaplan Meier estimation with P‐values from log‐rank test on the event‐free rates.
CRT‐P, cardiac resynchronization therapy pacemaker; PM, pacemaker; CRT‐D, cardiac resynchronization therapy defibrillator; ICD, internal cardioverter defibrillator; MI, myocardial infarction.
Pacing cohort
The PA cohort enrolled 68 patients with ESC guideline indications for CRT (40 for CRT‐P and 28 for CRT‐D). Of these, 22 received standard PM therapy, and 46 received CRT‐P therapy. A total of 54.4% (n = 37/68) of the patients had an LVEF ≤35%. Most patients receiving a PM did not meet JCS criteria for CRT due to having an LVEF ≥35% (21/22). This included patients with NYHA class I (n = 8), class II/III (n = 11), LBBB (n = 8), QRS ≥ 130 ms (n = 14), and AV‐block (n = 15). The final PM subject had an unknown JCS criteria violation. The estimated survival rate in the pacing cohort was at 94.1% at 12 months and 86.2% at 24 months.
An additional comparison of patients who received PM and CRT‐P revealed that patients who received a CRT‐P device had a higher probability of 24‐month survival (91.6% vs. 74.0%, P = 0.05).
Four deaths out of 46 CRT‐P patients were observed: two among CRT‐P subjects with ischaemic cardiomyopathy (N = 14) (plus LBBB) and two among those with non‐ischaemic cardiomyopathy (N = 32) (no LBBB). All four subjects had LVEF ≥30%.
In the pacing cohort, 21 subjects (30.9%) had a previous device. Two CRT‐P subjects had previously been implanted with CRT‐P device and 14 had a previously implanted PM (n = 16/46). Also, five PM subjects had a previously implanted PM device (n = 5/22). There were three deaths among patients with a previous device (n = 21) and six deaths among those without (n = 47) leading to a probability of survival through 24 months of 81.8% and 87.8% respectively (P = 0.509).
Discussion
HINODE is a large single ethnicity population study of patients in four different therapy cohorts. One important implication of this study is that for ICD and sudden death, findings are neither geocentric nor ethnocentric.
ICD and CRT‐D patients with a high risk of SCD have not previously been prospectively studied in Japan. The ASIAN HF study closed a large knowledge gap on Asian patients with heart failure by enrolling patients across many countries, including 540 in Japan. 25 That registry revealed a large pool of patients indicated for ICD or CRT‐D primary prevention according to the ESC guidelines (with two to five risk factors) that did not receive the appropriate therapy. The study concluded that variation in phenotypes and ethnic differences might need to be considered when allocating public health resources for heart failure management. 25 Because the benefits of ICD and CRT‐D therapy are not yet well‐established in Japan, the validation of the applicability of VA, SCD, and heart failure rates was an important follow‐up to ASIAN HF. HINODE demonstrates that results from previous landmark trials conducted outside of Japan (e.g. MADIT‐RIT 21 ) on VA and mortality in ICD and CRT‐D are likely generalizable to Japanese primary prevention patients. Because heart failure prevalence is expected to increase in many industrialized countries, including Japan, 2 these findings have direct implications for clinical practice decisions. The present study fills a critical gap by providing data on the effectiveness of ICD and CRT‐D in the primary prevention of heart failure and by comparing patients with HV devices (ICD and CRT‐D) to those with similar indications that did not receive the appropriate treatment.
The MADIT‐RIT trial was a randomized controlled trial examining algorithm effectiveness in reducing the rate of inappropriate ICD therapy in 1500 subjects with dual chamber ICDs implanted mainly from the USA and Europe. 26 MADIT‐RIT reported rates of appropriate and inappropriate therapy, VAs, and mortality. The HINODE ICD cohort included 67.7% dual chamber, 15.7% single chamber, and 15.7% S‐ICD devices. For S‐ICDs no inappropriate treatments were found in HINODE, which followed the UNTOUCHED study programming 27 (required per protocol). A limitation to the VA rate of the HINODE ICD cohort might be the mix of device types. Though it is notable that UNTOUCHED had similar rates of both inappropriate and appropriate therapies, particularly for newer S‐ICD models, thus it is unclear if there is any difference in VA detection and treatment between single and dual chamber ICD. The rate of inappropriate therapy in HINODE is very low. Thus, it is a valuable comparison between Japan and Western countries. Patients with ICD and CRT‐D in HINODE followed MADIT‐RIT programming arm B/C to allow direct comparison of event rates. Based on the comparison of patient characteristics, we assumed that the HINODE ICD and CRT‐D treatment cohorts likely had more advanced disease at diagnosis and were treated at a later stage than patients in MADIT‐RIT (Supporting Information, Table S2 ). Additionally, treatments did not always follow guidelines, which may have contributed to the increased risk of VA and mortality.
Post‐hoc analysis of CRT‐D patient sub‐groups revealed trends toward lower VA rates (P = 0.085) and mortality rates (P = 0.052, Table 3 ) in LBBB patients. Similarly, MADIT‐CRT reported that non‐LBBB patients with a PR‐interval >230 ms had increased VA and mortality rates. 28 MADIT‐CRT also showed a higher risk of VA due to delayed remodelling and a low CRT response. 29 A similar relationship was hypothesized in MADIT‐RIT, where a lower risk of VA was observed in patients receiving CRT compared with ICD therapy alone after the first 6 months of CRT therapy in which remodelling predominantly occurs. The present results of lower VA and mortality rates for LBBB patients align with earlier studies and support the importance of remodelling in CRT therapy for patients with LBBB and wide QRS complex.
The relative non‐cardiac or unknown cause death rates were similar between the MADIT‐RIT and HINODE HV cohorts (Table 2 ). The higher absolute mortality in HINODE (ICD: 13.7%, CRT‐D: 13.0%) compared with the MADIT‐RIT arm B and C (ICD: 4.8%, CRT‐D: 2.8%) suggests that HINODE may have had a higher risk population. The rate of ICD penetration is still low in Japan, possibly due to socio‐cultural and guideline and/or reimbursement factors. This is reflected by enrolment across cohorts in HINODE, where ND cohort had the largest enrolment. Additionally, in both studies, a large proportion of deaths were attributed to cardiac causes (over half of which were pump failure; Table 2 ). The ICD cohort included 29/102 patients with QRS ≥ 130 ms and LVEF ≤35%. The restrictive use of CRT‐D therapy due to the necessity of ICD indication, which depends again on positive EPS or NSVT, may be negatively impacting effectiveness of ICD therapy. Patients with CRT‐D or CRT‐P indication who did not receive appropriate therapies drove mortality and device upgrade rates across cohorts, suggesting that they may have benefited from earlier CRT device implant.
Current ESC and US primary prevention guidelines do not recommend the use of EPS to induce arrhythmias during ICD or CRT‐D implantation due to low sensitivity and specificity for predicting subsequent VA events. Results from the HINODE trial support this recommendation because electrophysiologic testing did not predict outcomes.
It is important to note that a subgroup of 29 ND patients with CRT indication did not receive ESC guideline‐indicated CRT devices and subsequently had worse outcomes. This highlights the importance of guideline‐directed CRT implantation in eligible patients despite NSVT and positive EPS. Beyond Japan, these findings may also be relevant to healthcare systems where limited access to CRT devices may force clinicians to triage implant in some patients.
Additionally, we analysed medication between five subgroups (ICD cohort: CRTD indicated class 1/not CRT‐D indicated; QRS >/< 130 ms; Non‐Device cohort: CRT‐D indicated/ICD indicated; Pacing cohort: PM versus CRT‐P). PM patients received less Beta blocker (18.2%) compared with CRT‐P treated patients (71.7%, P < .001). There were no differences in the remaining groups.
According to the HINODE protocol, patients in this study had to have two to five risk factors associated with increased incidence of non‐sustained ventricular tachycardia (NSVT) or life‐threatening VA. Notably, patients in the ND cohort had fewer risk factors than those in the device‐treated cohorts. While this suggests that they should have had a lower risk of VA than other patients, high rates of VA and mortality were still observed, which may have been prevented by the use of device implants. 19 This suggests that regardless of risk factors, all patients with indications for primary prevention with an ICD may benefit from timely and appropriate use of these devices.
The low rate of inappropriate therapy in the HV cohort (<2% at 1 year) supports the programming of high cut‐off rates or delayed therapy, as shown in MADIT‐RIT and UNTOUCHED. 27
This study showed favourable outcomes in a small number of implanted S‐ICD devices. S‐ICD is a novel technology that does not require an intracardiac electrode, thus avoiding complications related to transvenous leads. Given its favourable risk profile, it is a reasonable option for patients with primary prevention. Future studies may further examine S‐ICD outcomes in primary prevention patients.
This study has some limitations. The sample size in this multi‐cohort study was smaller than initially planned. However, no patients were lost to follow‐up, and the study period was extended by 6 months. The increased mean follow‐up time and total patient months in the trial allowed Kaplan–Meier analysis up to 24 months. Overall, the study design and low attrition rate facilitated a better estimate of event rates than a study with a larger sample size but shorter follow‐up. The discrepancies between JCS and ESC guidelines and individual clinician judgement on therapy could have resulted in residual confounding despite propensity matching of the HV cohort. Due to a complex bias between CIDE and Non‐device patients a direct comparison was not possible. OMT was required for all patients by the JCS guideline and although we tested for discrepancies between sub‐groups, we cannot exclude the possibility that medication had an impact on differences between subgroups. Patient subgroups were identified for all four cohorts to better understand the cohort outcome, however not all were pre‐defined. Additionally, this was an intention to treat study with therapy treatment conditions not directly manipulated or randomly assigned. Thus, the conclusions are not as robust as in a randomized controlled trial.
Future analysis of the data from HINODE will focus on patients in the ND cohort with an indication for ICD, as well as the risk of heart failure across all cohorts. Future studies should address the need for large‐scale randomized studies to directly assess the effectiveness of new therapies in the Japanese population.
In conclusion, mortality and VA event rates in primary prevention patients in Japan are similar to those in matched MADIT‐RIT patients, indicating that the results of landmark trials are applicable to Japan. This study found that regardless of cohort or indicated treatment patients that did not receive ESC guideline indicated care had worse outcomes than those reported in MADIT‐RIT. The novel comparison facilitated by this study design allows direct comparison to western clinical data, and the present data suggest that Japanese patients may have better outcomes if they are treated in accordance with the western Guidelines.
Conflict of interest
Ando K. received lecture fees from Japan Lifeline Co., Ltd., Terumo Co., Ltd., Bristol‐Myers Squibb Co., Ltd., Medtronic Japan Co. Ltd., Biotronik Japan, and Bayer Co., Ltd., and consulting honoraria from Boston Scientific. Aonuma K. received speaker honoraria from Abbott Japan, Boehringer‐Ingelheim, and Daiichi‐Sankyo Co., Ltd., consulting honoraria from Boston Scientific, and belonged to the endowment department of Abbott Japan. Azlan H. received consulting fees from the ventricular event committee in Hinode. Chan Y. S. received consulting fees for the ventricular event committee in Hinode. Ikeda T. received scholarship funds or donations scholarship funds from Medtronic Japan Co., Ltd., Japan Lifeline Co., Ltd., Daiichi Sankyo Co., Ltd., honoraria for lectures from Bayer Co., Ltd., Ono Pharmaceutical Co., Ltd., and Bristol‐Myers Squibb Co., Ltd., and consulting honoraria from Boston Scientific. Kutyifa V. received research grants from Boston Scientific, ZOLL, Biotronik, Spire Inc., and consultant fees from Biotronik, and ZOLL. Mitsuhashi T. received lecture fees from Medtronic Japan Co., Ltd. and Abbott Japan, and consulting honoraria from Boston Scientific. Murohara T. received consulting honoraria from Boston Scientific. Nishii N. belonged to the endowed department by Medtronic Japan Co., Ltd. and received lecture fees from Medtronic Japan Co., Ltd., Cook Japan, and Boston Scientific Japan, and consulting honoraria from Boston Scientific. Nogami A. received honoraria from Johnson & Johnson, Boehringer‐Ingelheim, Daiichi‐Sankyo Co., Ltd., and Abbott Japan, an endowment from Medtronic Japan Co., Ltd. and DVx Co., Ltd., and consulting honoraria from Boston Scientific. Sakata Y. received a scholarship fund and consulting honoraria from Boston Scientific Japan. Shimizu W. received scholarship funds from Abbott Japan Co., Ltd., Japan Lifeline Co., Ltd., Boehringer‐Ingelheim, and Daiichi Sankyo Co., Ltd. and remuneration from Boehringer‐Ingelheim, and Daiichi Sankyo Co., Ltd., Ono Pharmaceutical Co., Ltd., Bayer Co., Ltd., and Bristol‐Myers Squibb Co., Ltd., and a consulting honoraria from Boston Scientific. Simon T., Beaudoint C., and Kayser T. were employees of Boston Scientific. Tachapong N. is a member of the advisory board of Boston Scientific and received consulting fees for the ventricular event committee in HINODE, lecture honoraria, and travel support from the company, in addition to lecture fees from Medtronic. Asai T., Inamura Y., Inoue K., and Kusano K.: none declared.
Funding
This work was supported and funded by Boston Scientific.
Supporting information
Table S1. Study characteristics, follow‐up availability, and endpoint data compliance.
Table S2. Baseline characteristics for HINODE high‐voltage cohort compared to matched and unmatched MADIT‐RIT cohorts.
Table S3. Ventricular arrhythmia classification in the HV cohort.
Table S4. Medication at enrolment in the pacing cohort by treatment device subgroup.
Table S5. JCS guideline overview on CRT indication for patient in sinus rhythm.
Table S6. JCS guideline overview on CRT indication for patient in AF and/or high frequency pacing needs.
Table S7. JCS guideline overview on conditions for ICD indication effecting ICD and CRT‐D therapy.
Acknowledgements
The authors would like to sincerely thank the HINODE enrolling sites, physicians, and study coordinators. We would like to thank all members of the adjudication committees who reviewed endpoint events and electrocardiograms. Special thanks to the Boston Scientific technical support team and the ICON study team, who supported all trial‐related preparations and monitoring. We would like to also thank JD Raybuck (Boston Scientific SciComm) for assistance in editing this manuscript. Additionally, we would like to thank Hiroshi Takagi, Kai Lee Yew, and Shuichi Matsumoto from the Boston Scientific clinical team, who were essential for project progress and conduct.
Aonuma, K. , Ando, K. , Kusano, K. , Asai, T. , Inoue, K. , Inamura, Y. , Ikeda, T. , Mitsuhashi, T. , Murohara, T. , Nishii, N. , Nogami, A. , Shimizu, W. , Beaudoint, C. , Simon, T. , Kayser, T. , Azlan, H. , Tachapong, N. , Chan, J. Y.‐S. , Kutyifa, V. , Sakata, Y. , and For the HINODE Investigators (2022) Primary results from the Japanese Heart Failure and Sudden Cardiac Death Prevention Trial (HINODE). ESC Heart Failure, 9: 1584–1596. 10.1002/ehf2.13901.
References
- 1. Shiba N, Shimokawa H. Chronic heart failure in Japan: implications of the CHART studies. Vasc Health Risk Manag 2008; 4: 103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Tsutsui H, Isobe M, Ito H, Ito H, Okumura K, Ono M, Kitakaze M, Kinugawa K, Kihara Y, Goto Y, Komuro I, Saiki Y, Saito Y, Sakata Y, Sato N, Sawa Y, Shiose A, Shimizu W, Shimokawa H, Seino Y, Node K, Higo T, Hirayama A, Makaya M, Masuyama T, Murohara T, Momomura SI, Yano M, Yamazaki K, Yamamoto K, Yoshikawa T, Yoshimura M, Akiyama M, Anzai T, Ishihara S, Inomata T, Imamura T, Iwasaki YK, Ohtani T, Onishi K, Kasai T, Kato M, Kawai M, Kinugasa Y, Kinugawa S, Kuratani T, Kobayashi S, Sakata Y, Tanaka A, Toda K, Noda T, Nochioka K, Hatano M, Hidaka T, Fujino T, Makita S, Yamaguchi O, Ikeda U, Kimura T, Kohsaka S, Kosuge M, Yamagishi M, Yamashina A, on behalf of the Japanese Circulation Society and the Japanese Heart Failure Society Joint Working Group . JCS 2017/JHFS 2017 guideline on diagnosis and treatment of acute and chronic heart failure—digest version. Circ J Sep 25 2019; 83: 2084–2184. [DOI] [PubMed] [Google Scholar]
- 3. FDMA . White Paper on Firefighting. In: Agency FaDM, ed. Japan; 2015. https://www.fdma.go.jp/publication/hakusho/h27/ Accessed December 17, 2021.
- 4. Shiba N, Shimokawa H. Prospective care of heart failure in Japan: lessons from CHART studies. EPMA J 2011; 2: 425–438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shiga T, Hagiwara N, Ogawa H, Takagi A, Nagashima M, Yamauchi T, Tsurumi Y, Koyanagi R, Kasanuki H, for the Heart Institute of Japan Acute Myocardial Infarction‐II (HIJAMI‐II) Investigators . Sudden cardiac death and left ventricular ejection fraction during long‐term follow‐up after acute myocardial infarction in the primary percutaneous coronary intervention era: results from the HIJAMI‐II registry. Heart 2009; 95: 216–220. [DOI] [PubMed] [Google Scholar]
- 6. Raatikainen MJ, Arnar DO, Merkely B, Camm AJ, Hindricks G. Access to and clinical use of cardiac implantable electronic devices and interventional electrophysiological procedures in the European Society of Cardiology Countries: 2016 Report from the European Heart Rhythm Association. Europace 2016; 18: iii1–iii79. [DOI] [PubMed] [Google Scholar]
- 7. JADIA . ICD and CRT‐D implants by prefecture annual repots. Japan Arrythmia Device Industry Association; 2021. https://www.jadia.or.jp/medical/crt‐d.html Accessed December 17, 2021.
- 8. Nogami A, Kurita T, Abe H, Ando K, Ishikawa T, Imai K, Usui A, Okishige K, Kusano K, Kumagai K, Goya M. JCS/JHRS 2019 Guideline on Non‐Pharmacotherapy of Cardiac Arrhythmias. Circ J Jun 1 2021; 85: 1104–1244. [DOI] [PubMed] [Google Scholar]
- 9. Bardy GH, Lee KL, Mark DB, Poole JE, Packer DL, Boineau R, Domanski M, Troutman C, Anderson J, Johnson G, McNulty SE, Clapp‐Channing N, Davidson‐Ray LD, Fraulo ES, Fishbein DP, Luceri RM, Ip JH. Amiodarone or an implantable cardioverter‐defibrillator for congestive heart failure. N Engl J Med Jan 20 2005; 352: 225–237. [DOI] [PubMed] [Google Scholar]
- 10. Goldenberg I, Gillespie J, Moss AJ, Hall WJ, Klein H, McNitt S, Brown MW, Cygankiewicz I, Zareba W, and the Executive Committee of the Multicenter Automatic Defibrillator Implantation Trial II . Long‐term benefit of primary prevention with an implantable cardioverter‐defibrillator: an extended 8‐year follow‐up study of the Multicenter Automatic Defibrillator Implantation Trial II. Circulation Sep 28 2010; 122: 1265–1271. [DOI] [PubMed] [Google Scholar]
- 11. Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NAM III, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac‐resynchronization therapy for the prevention of heart‐failure events. N Engl J Med Oct 1 2009; 361: 1329–1338. [DOI] [PubMed] [Google Scholar]
- 12. Moss AJ, Zareba W, Hall WJ, Klein H, Wilber DJ, Cannom DS, Daubert JP, Higgins SL, Brown MW, Andrews ML, Multicenter Automatic Defibrillator Implantation Trial II Investigators . Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med Mar 21 2002; 346: 877–883. [DOI] [PubMed] [Google Scholar]
- 13. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, González‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, ESC Scientific Document Group . 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J Jul 14 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 14. Tanno K, Miyoshi F, Watanabe N, Minoura Y, Kawamura M, Ryu S, Asano T, Kobayashi Y, Katagiri T, MADIT II. The Multicenter Automtic Defibrillator Implantation Trial . Are the MADIT II criteria for ICD implantation appropriate for Japanese patients? Circ J 2005; 69: 19–22. [DOI] [PubMed] [Google Scholar]
- 15. Satake H, Fukuda K, Sakata Y, Miyata S, Nakano M, Kondo M, Hasebe Y, Segawa M, Shimokawa H, on behalf of the CHART‐2 Investigators . Current status of primary prevention of sudden cardiac death with implantable cardioverter defibrillator in patients with chronic heart failure—a report from the CHART‐2 study. Circ J 2015; 79: 381–390. [DOI] [PubMed] [Google Scholar]
- 16. Goldenberg I, Kutyifa V, Klein HU, Cannom DS, Brown MW, Dan A, Daubert JP, Estes NAM III, Foster E, Greenberg H, Kautzner J, Klempfner R, Kuniss M, Merkely B, Pfeffer MA, Quesada A, Viskin S, McNitt S, Polonsky B, Ghanem A, Solomon SD, Wilber D, Zareba W, Moss AJ. Survival with cardiac‐resynchronization therapy in mild heart failure. N Engl J Med May 1 2014; 370: 1694–1701. [DOI] [PubMed] [Google Scholar]
- 17. Kawashiro N, Kasanuki H, Ogawa H, Matsuda N, Hagiwara N, The Heart Institute of Japan ‐ Depa . Clinical characteristics and outcome of hospitalized patients with congestive heart failure: results of the HIJC‐HF registry. Circ J 2008; 72: 2015–2020. [DOI] [PubMed] [Google Scholar]
- 18. Yamasaki HAK, Ikeda T, Mitsuhashi T, Murohara T, Nishii N, Nogami A, Sakata Y, Shimizu W, Simon T, Beaudoint C. Rationale and design of the HINODE study: heart failure indication and sudden cardiac death prevention trial Japan. J Arrhythm 2021; 37: 1031–1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldenberg I, Vyas AK, Hall WJ, Moss AJ, Wang H, He H, Zareba W, McNitt S, Andrews ML, MADIT‐II Investigators . Risk stratification for primary implantation of a cardioverter‐defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol Jan 22 2008; 51: 288–296. [DOI] [PubMed] [Google Scholar]
- 20. Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ, MADIT‐CRT Investigators . Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy (MADIT‐CRT). Circulation Mar 15 2011; 123: 1061–1072. [DOI] [PubMed] [Google Scholar]
- 21. Moss AJ, Schuger C, Beck CA, Brown MW, Cannom DS, Daubert JP, Estes NAM III, Greenberg H, Hall WJ, Huang DT, Kautzner J, Klein H, McNitt S, Olshansky B, Shoda M, Wilber D, Zareba W. Reduction in inappropriate therapy and mortality through ICD programming. N Engl J Med Dec 13 2012; 367: 2275–2283. [DOI] [PubMed] [Google Scholar]
- 22. Kutyifa V, Daubert JP, Schuger C, Goldenberg I, Klein H, Aktas MK, McNitt S, Stockburger M, Merkely B, Zareba W, Moss AJ. Novel ICD programming and inappropriate ICD therapy in CRT‐D versus ICD patients: A MADIT‐RIT sub‐study. Circ Arrhythm Electrophysiol 2016; 9: e001965. [DOI] [PubMed] [Google Scholar]
- 23. Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med Apr 14 2005; 352: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 24. Pellegrini CN, Lee K, Olgin JE, Turakhia MP, Tseng ZH, Lee R, Badhwar N, Lee B, Varosy PD. Impact of advanced age on survival in patients with implantable cardioverter defibrillators. Europace 2008; 10: 1296–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kubota Y, Tay WT, Asai K, Murai K, Nakajima I, Hagiwara N, Ikeda T, Kurita T, Teng THK, Anand I, Lam CSP, Shimizu W, on behalf of the ASIA‐HF Study investigators . Chronic obstructive pulmonary disease and beta‐blocker treatment in Asian patients with heart failure. ESC Heart Fail 2018; 5: 297–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schuger C, Daubert JP, Brown MW, Cannom D, Estes NAM III, Hall WJ, Kayser T, Klein H, Olshansky B, Power KA, Wilber D, Zareba W, Moss AJ. Multicenter automatic defibrillator implantation trial: reduce inappropriate therapy (MADIT‐RIT): background, rationale, and clinical protocol. Ann Noninvasive Electrocardiol 2012; 17: 176–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Gold MR, Lambiase PD, El‐Chami MF, Knops RE, Aasbo JD, Bongiorni MG, Russo AM, Deharo JC, Burke MC, Dinerman J, Barr CS. Primary results from the understanding outcomes with the S‐ICD in primary prevention patients with low ejection fraction (UNTOUCHED) trial. Circulation Jan 5 2021; 143: 7–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kutyifa V, Stockburger M, Daubert JP, Holmqvist F, Olshansky B, Schuger C, Klein H, Goldenberg I, Brenyo A, McNitt S, Merkely B, Zareba W, Moss AJ. PR interval identifies clinical response in patients with non‐left bundle branch block: a multicenter automatic defibrillator implantation trial‐cardiac resynchronization therapy substudy. Circ Arrhythm Electrophysiol 2014; 7: 645–651. [DOI] [PubMed] [Google Scholar]
- 29. Barsheshet A, Wang PJ, Moss AJ, Solomon SD, al‐Ahmad A, McNitt S, Foster E, Huang DT, Klein HU, Zareba W, Eldar M, Goldenberg I. Reverse remodeling and the risk of ventricular tachyarrhythmias in the MADIT‐CRT (Multicenter Automatic Defibrillator Implantation Trial‐Cardiac Resynchronization Therapy). J Am Coll Cardiol Jun 14 2011; 57: 2416–2423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Study characteristics, follow‐up availability, and endpoint data compliance.
Table S2. Baseline characteristics for HINODE high‐voltage cohort compared to matched and unmatched MADIT‐RIT cohorts.
Table S3. Ventricular arrhythmia classification in the HV cohort.
Table S4. Medication at enrolment in the pacing cohort by treatment device subgroup.
Table S5. JCS guideline overview on CRT indication for patient in sinus rhythm.
Table S6. JCS guideline overview on CRT indication for patient in AF and/or high frequency pacing needs.
Table S7. JCS guideline overview on conditions for ICD indication effecting ICD and CRT‐D therapy.


