Abstract
Aims
Ischaemic coronary artery disease (CAD) remains the leading cause of mortality globally due to sudden death and heart failure (HF). Invasive coronary angiography (CAG) is the gold standard for evaluating the presence and severity of CAD. Our objective was to assess temporal trends in CAG utilization, patient characteristics, and prognosis in HF patients undergoing CAG at a national level.
Methods and results
We used data from the Swedish Coronary Angiography and Angioplasty Registry. Data on all patients undergoing CAG for HF indication in Sweden between 2000 and 2018 were collected and analysed. Long‐term survival was estimated with multivariable Cox proportional hazards regression adjusted for differences in patient characteristics. In total, 22 457 patients (73% men) with mean age 64.2 ± 11.3 years were included in the study. The patients were increasingly older with more comorbidities over time. The number of CAG specifically for HF indication increased by 5.5% per calendar year (P < 0.001). No such increase was seen for indications angina pectoris and ST‐elevation myocardial infarction. A normal CAG or non‐obstructive CAD was reported in 63.2% (HF‐NCAD), and 36.8% had >50% diameter stenosis in one or more coronary arteries (HF‐CAD). The median follow‐up time was 3.6 years in HF‐CAD and 5 years in HF‐NCAD. Age and sex‐adjusted survival improved linearly by 1.3% per calendar year in all patients. Compared with HF‐NCAD, long‐term mortality was higher in HF‐CAD patients. The risk of death increased with the increasing severity of CAD. Compared with HF‐NCAD, the risk estimate in patients with a single‐vessel disease was higher [hazard ratio (HR) 1.3; 95% confidence interval (CI) 1.20–1.41; P < 0.001], a multivessel disease without the involvement of left main coronary artery (HR 1.72; 95% CI 1.58–1.88; P < 0.001), and with left main disease (HR 2.02; 95% CI 1.88–2.18; P < 0.001). The number of HF patients undergoing revascularization with percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG) increased by 7.5% (P < 0.001) per calendar year. The majority (53.4%) of HF‐CAD patients were treated medically, while a minority (46.6%) were referred for revascularization with PCI or CABG. Compared with patients treated with PCI, the proportion of patients treated medically or with CABG decreased substantially (P < 0.001).
Conclusions
Over 18 years, the number of patients with HF undergoing CAG has increased substantially. Expanded utilization of CAG increased the number of HF patients treated with percutaneous coronary intervention and coronary artery bypass surgery. Long‐term survival improved in all HF patients despite a steady increase of elderly patients with comorbidities.
Keywords: Coronary angiography, Coronary artery disease, Heart failure, Long‐term survival
Introduction
Ischaemic coronary artery disease (CAD) remains the leading cause of mortality globally due to sudden death and heart failure (HF). 1 , 2 Despite improved pharmacological and device‐based therapy, patients with HF due to CAD (HF‐CAD) have a worse prognosis than HF patients without CAD (HF‐NCAD). 3 , 4 , 5 Invasive coronary angiography (CAG) is the ‘gold standard’ for assessing the presence and severity of CAD. 6 During the last two decades, there have been variable recommendations regarding the utilization of CAG for the diagnosis of HF. Indeed, in the first European Society of Cardiology guidelines from 1995 to 2001, CAG was required to exclude CAD when a diagnosis of idiopathic cardiomyopathy is considered. 7 , 8 The recommendation about CAG shifted to a more restrictive use of CAG since 2005. 9 , 10
One clear indication for CAG in HF is to ascertain the aetiology of HF and consequently the eligibility for revascularization because revascularization in HF‐CAD patients provides superior clinical outcomes compared with medical therapy alone. The current guidelines recommend coronary revascularization on top of optimal medical treatment in patients with reduced systolic left ventricular ejection fraction and CAD. 11 The evidence from randomized controlled trials regarding revascularization is limited only to coronary artery bypass grafting (CABG), which has shown superior long‐term outcomes compared with medical treatment. 12
Our aim was to evaluate the temporal trends in patient characteristics, utilization of CAG, and outcomes in HF patients undergoing CAG over 18 years. We used data from the Swedish Coronary Angiography and Angioplasty Registry (SCAAR), which covers the entire Swedish population.
Methods
Database and study population
Established in 1992, the SCAAR registry provides a web‐based platform dedicated to data collection from all CAG, and percutaneous coronary interventions (PCI) performed at all coronary catheterization laboratories (n = 31) in Sweden (https://www.ucr.uu.se/swedeheart/). Each catheterization procedure is described with ~50 angiographic and 200 PCI demographic and procedure‐related variables. The registry is sponsored by the Swedish Health Authorities and provides almost complete procedure coverage in Sweden. The SCAAR database is continuously merged with The Swedish Tax Agency by the Swedish National Board of Health and Welfare to obtain information about the patients' vital status. More detailed information about SCAAR's organization has been published elsewhere. 13 , 14 , 15 The study design was complied with the Declaration of Helsinki, and the study approval was obtained from the Institutional Review Board at the University of Gothenburg (Dnr. 759‐13, date of approval 2014‐05‐06). We included all adults (age ≥ 18) who underwent CAG in whom the primary indication was HF between 1 January 2000 and 31 March 2018. Only Swedish residents with a unique personal identification number were included. The patients who underwent CAG due to any other indications than explicitly HF were excluded. To compare the temporal trends in CAG utilization during the same period for relevant indications other than HF, we compared the HF CAG data with ST‐elevation myocardial infarction and stable angina. Patients with ST‐elevation myocardial infarction and stable angina were not included in any analyses other than descriptive frequencies per calendar year.
Definitions and outcomes
In this study, HF was defined as a clinical diagnosis determined by physicians who referred patients to CAG and the International Classification of Diseases codes. HF‐CAD was defined as HF and the presence of lumen narrowing ≥ 50% in one or more coronary arteries. HF‐NCAD was defined as HF with normal or non‐obstructive lumen narrowing. Patients were considered to have diabetes, hypertension, hyperlipidaemia, previous myocardial infarction, or previous stroke according to the International Classification of Diseases codes. 13 Standardized definitions were used for procedure‐related information. The outcome was all‐cause mortality. Vital status and date of death were obtained from the Swedish National Population Registry until 15 March 2018. Because the use of unique identification numbers is mandatory in Sweden, the death registry in Sweden has virtually complete follow‐up within 30 days.
Statistical analysis
Missing data were imputed using the multiple imputation chain‐equation method 16 with five data sets. Calendar year, an indicator of missingness, and an event indicator were included as regular variables. Continuous variables were imputed by ordinary least‐squares multiple regression, binary variables by logistic regression, and categorical variables by multinomial logistic regression. The imputation procedure and subsequent analyses were done according to Rubin's protocol 17 under the assumption that missing data are missing at random.
Continuous variables were presented as a mean and standard deviation, and categorical variables as frequencies. The normal distribution of variables was assessed by inspecting the distribution of values on histograms and the Shapiro–Wilk test. Between‐group differences of continuous variables were tested by linear regression. Differences in categorical variables were tested by logistic regression. All‐cause mortality was based on Kaplan–Meier estimates in time‐to‐first‐event analyses. Unadjusted differences in survival were compared with the log‐rank test. Between‐group differences in time to all‐cause mortality were assessed using a Cox proportional hazards model adjusted for variables presented in Table 1 and calendar year. Patients reported multiple times in the registry were included at the time of first CAG. The proportionality of hazards assumption was evaluated by including treatment–time interaction in the Cox proportional hazards models. Treating hospitals were included in the model as a random effect variable to account for clustering of patients. Effect modification between HF and clinically relevant patient subgroups was evaluated by having interaction terms in the regression. Goodness‐of‐fit was assessed with the Groennesby and Borgan test for the Cox proportional hazards models. Multicollinearity between the variables was evaluated by calculating the variance inflation factor.
Table 1.
Patient characteristics stratified by time period
| 2000–2004 | 2005–2008 | 2009–2011 | 2012–2014 | 2015–2018 | P value a | |
|---|---|---|---|---|---|---|
| n = 2950 | n = 4806 | n = 4167 | n = 4899 | n = 6381 | ||
| Age [mean (SD)] | 59 (11) | 62 (11) | 63 (11) | 64 (11) | 65 (11) | <0.001 |
| Age category (%) | ||||||
| <59 | 46.3 | 37.7 | 32.7 | 29.4 | 26.4 | <0.001 |
| 60–69 | 33.3 | 34.8 | 36.1 | 34.2 | 30.6 | |
| 70–79 | 18.1 | 24.3 | 26.3 | 29.7 | 34.6 | |
| ≥80 | 2.3 | 3.3 | 4.9 | 7.1 | 8.4 | |
| Male sex (%) | 74 | 74 | 74 | 73 | 72 | 0.317 |
| BMI, mean (SD) | NA | 27.6 (5.4) | 28.1 (7.2) | 28 (6.7) | 28.1 (5.9) | 0.007 |
| Severity of CAD (%) | <0.001 | |||||
| Normal/non‐obstructive | 65.7 | 63.1 | 59.0 | 60.7 | 61.1 | |
| SVD | 13.1 | 13.9 | 15.9 | 14.5 | 14.6 | |
| MVD | 7.9 | 8.5 | 9.4 | 9.4 | 9.7 | |
| LM | 13.2 | 14.4 | 15.7 | 15.3 | 14.7 | |
| Diabetes, (%) | 20.6 | 19.1 | 23.0 | 23.6 | 22.5 | <0.001 |
| Hypertension (%) | 39.5 | 44.6 | 51.1 | 60.4 | 65.0 | <0.001 |
| Previous MI (%) | 12.3 | 13.6 | 15.1 | 14.7 | 13.2 | <0.001 |
| Previous PCI (%) | 4.2 | 5.3 | 7.2 | 8.0 | 9.7 | <0.001 |
| Previous CABG (%) | 4.9 | 5.6 | 6.2 | 7.2 | 5.5 | |
| eGFR (mL/min/1.73 m2) | ||||||
| ≥60 | 99.9. | 84.6 | 83.0 | 79.7 | 77.8 | <0.001 |
| <60 | 0.1 | 15.4 | 17.0 | 20.3 | 22.2 | |
| Smoking (%) | <0.001 | |||||
| Never smoker | 36.3 | 45.3 | 42.0 | 40.1 | 41.9 | |
| Past smoker | 45.5 | 38.5 | 39.6 | 42.9 | 42.5 | |
| Current smoker | 18.2 | 16.2 | 18.4 | 17.0 | 15.6 | |
| Smokeless tobacco (%) | <0.001 | |||||
| Never user | NA | NA | 87.9 | 85.0 | 84.5 | |
| Past user | NA | NA | 4.9 | 6.2 | 5.9 | |
| Current user | NA | NA | 7.2 | 8.8 | 9.6 | |
| ASA (%) | 2.9 | 82.0 | 83.0 | 82.9 | 76.8 | <0.001 |
| Statins (%) | 48.7 | 38.9 | 40.3 | 42.6 | 43.2 | <0.001 |
| Amount of contrast (mL) | 80 (60) | 87 (54) | 75 (48) | 78 (55) | 78 (56) | <0.001 |
| Radiation time (s) | ||||||
| Mean (SD) | 350 (393) | 317 (363) | 315 (356) | 383 (516) | 425 (581) | <0.001 |
ASA, acetylsalicylic acid; BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary disease; eGFR, estimated glomerular filtration rate (Cockcroft–Gault); LM, disease in left main artery; MVD, multivessel disease (stenosis > 50% in more than one vessel without left main disease); MI, myocardial infarction; NA, not available; SVD, single‐vessel diseases (stenosis > 50% in one coronary artery).
Compared with period 2000–2004.
We evaluated the relative importance of all variables from Table 1 for long‐term survival. Relative variable importance was calculated using a random forest method as implemented in the R package party. 18 The random forest is a highly efficient machine‐learning method that handles a large number of observations and predictors and allows for complex modelling of interactions and non‐linear functions.
All statistical analyses were performed using Stata software (Version 16.1, StataCorp, College Station, Texas, USA) and R (Version 4.1.0, R Core Team, 2020). Figures were produced using the R package ggplot2. All reported P values are two‐sided and are not adjusted for multiple testing.
Results
Patient characteristics
In total, 22 457 patients, 16 393 (73%) men and 6064 (27%) women, were included in the study. The mean age was 64.2 ± 11.3 years, and one‐third were <60 years old. We found that patient characteristics changed substantially over the 18‐year study period (Table 1 ). Patients who underwent CAG during 2015–2018 were older; had higher body mass index; were more likely to have diabetes, hypertension, renal failure, previous myocardial infarction, and previous revascularization (PCI/CABG); and on statin treatment but were less likely to be smokers and to receive treatment with acetylsalicylic acid than patients investigated between 2000 and 2004. We found that the severity of CAD increased over the study period, with more patients having MVD and significant lesions in the left main coronary artery. Sex distribution did not change over the study period, with ~70% being male. Between 2000 and 2018, the number of catheterization laboratories in Sweden increased from 22 to 31. Demographic data of HF patients with (HF‐CAD) or without CAD (HF‐NCAD) are presented in Table 2 . HF‐CAD patients were older and were more likely to be male, smokers, have diabetes, hypertension, previous myocardial infarction, and were more likely to be treated previously with PCI (P < 0.01). Similarly, patients with more advanced CAD (i.e. MVD and LM) were more likely to have traditional risk factors including diabetes, hypertension, previous myocardial infarction, and prior PCI than patients with less severe CAD (P < 0.01, Table 2 ).
Table 2.
Patient characteristics stratified by coronary angiography findings
| HF‐NCAD | HF‐CAD | SVD | MVD | LM | P value a | |
|---|---|---|---|---|---|---|
| n = 13 838 | n = 8619 | n = 3252 | n = 2052 | n = 3315 | ||
| Age, year [mean (SD)] | 61 (11) | 67 (10) | 66 (9) | 67 (9) | 68 (9) | <0.001 |
| Age category (%) | <0.001 | |||||
| <59 years | 41.2 | 19.4 | 22.2 | 19.8 | 16.4 | |
| 60–69 years | 32.9 | 34.5 | 34.7 | 34.6 | 34.2 | |
| 70–79 years | 22.3 | 37.0 | 35.3 | 37.3 | 38.5 | |
| ≥80 years | 3.6 | 9.1 | 7.8 | 8.2 | 10.9 | |
| Male sex (%) | 68.9 | 80.4. | 77.0 | 81.4 | 83.2 | <0.001 |
| Diabetes (%) | 14.8 | 32.0 | 25.2 | 33.3 | 38 | <0001 |
| BMI, kg/m2 (SD) | 28.1 (5.9) | 27.8 (6.9) | 27.9 (8.2) | 27.9 (6.8) | 27.6 (5.2) | 0.005 |
| Hypertension (%) | 50.1 | 64.9 | 61.5 | 65.5 | 67.9 | <0.001 |
| Missing | 1.0 | 1.4 | 1.4 | 1.3 | 1.4 | |
| Previous MI (%) | 0 | 35.3 | 26.4 | 37.7 | 42.7 | <0.001 |
| Missing | 1.9 | 3.8 | 3.8 | 3.6 | 3.6 | |
| Previous PCI | 1.6 | 16.9 | 16.4 | 19.9 | 15.4 | <0001 |
| Previous CABG | 0.6 | 14.7 | 4.0 | 10.7 | 27.6 | <0001 |
| eGFR (mL/min/1.73 m2) | <0001 | |||||
| ≥60 mL/min (%) | 87.5 | 76.3 | 79.6 | 76.5 | 72.8 | |
| <60 mL/min (%) | 12.5 | 23.7 | 20.4 | 23.5 | 27.2 | |
| Smoking (%) | ||||||
| Never smoker | 44.6 | 33.0 | 34.1 | 31.9 | 32.5 | |
| Past smoker | 36.6 | 43.5 | 42.7 | 43.3 | 44.6 | <0.001 |
| Current smoker | 14.4 | 18.0 | 18.2 | 19.0 | 17.2 | |
| Missing | 4.4 | 5.4 | 5.0 | 5.8 | 5.7 | |
| Smokeless tobacco (%) | <0.001 | |||||
| Never user | 71.9 | 70.4 | 71.8 | 70.4 | 69.0 | |
| Past user | 5.7 | 6.0 | 6.1 | 5.5 | 6.1 | |
| Current user | 9.7 | 7.5 | 8.4 | 7.2 | 6.7 | |
| Missing | 12.7 | 16.2 | 13.7 | 16.9 | 18.2 | |
| ASA (%) | 15.7 | 64.2 | 65.6 | 67.2 | 59.5 | <0001 |
| Statins (%) | 29.7 | 58.7 | 51.7 | 60.8 | 64.3 | <0001 |
| Missing | 1.4 | 1.7 | 1.5 | 1.6 | 2.0 | |
| Amount of contrast [mL (SD)] | 62 (31) | 108 (70) | 101 (62) | 120 (80) | 106 (66) | <0.001 |
| Radiation time [s (SD)] | 242 (232) | 570 (671) | 518 (658) | 673 (773) | 557 (607) | <0.001 |
ASA, acetylsalicylic acid; CABG, coronary artery bypass grafting; eGFR, estimated glomerular filtration rate (Cockcroft–Gault); HF‐CAD, heart failure with coronary artery disease (i.e. SVD + MVD + LM); HF‐NCAD, heart failure with normal/non‐obstructive coronary artery disease; LM, stenosis > 50% in left main coronary artery; MI, myocardial infarction, MVD, multivessel disease (stenosis > 50% in more than one coronary artery without left main disease); Normal/non‐obstructive, no stenosis > 50% in coronary arteries; PCI, percutaneous coronary intervention; SVD, single‐vessel diseases (stenosis > 50% in one coronary artery).
Compared with HF‐NCAD.
During the study period, there was a steady (linear) annual increase in the number of coronary angiographies for HF (Figure 1A ), by 5.5% (P < 0.001), while no such increase was seen for the indications of angina pectoris or ST‐elevation myocardial infarction (Figure 1CB–1 ). Similarly, the number of HF patients undergoing revascularization with PCI or CABG increased by 7.5% (P < 0.001) per calendar year (Figure 1D ). The majority (53.4%) of HF‐CAD patients were treated medically (including device therapy according to the guidelines), while a minority (46.6%) were referred for revascularization with PCI or CABG. Compared with patients treated with PCI, the proportion of patients treated medically or with CABG decreased substantially (P < 0.001; Figure 1D ).
Figure 1.
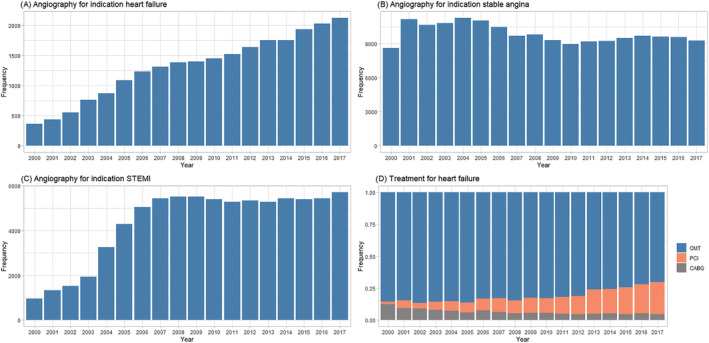
The number of coronary angiographies performed annually in Sweden between 2000 and 2018 for indications: (A) heart failure; (B) stable angina; (C) ST‐elevation myocardial infarction (STEMI). The rise in primary PCI until 2006 was due to the increasing number of catheterization laboratories in Sweden during the same period when PCI became the preferred reperfusion method for STEMI. (D) Initial treatment for heart failure after angiography. CABG, coronary artery bypass grafting; OMT, optimal medical treatment including device therapy; PCI, percutaneous coronary intervention.
Long‐term survival
During the study period, age and sex‐adjusted survival in HF patients undergoing angiography increased by 1.3% (P < 0.001) per the calendar year, both in patients with and without CAD. Median follow‐up time was 3.6 years (range 1 day to 18 years) in HF‐CAD and 5 years (range 1 day to 18 years) in HF‐NCAD patients. The median survival time in HF with CAD was 7.8 [95% confidence interval (CI) 7.46–8.1] and 16.8 (95% CI 7.46–8.1) in patients with HF‐NCAD. One‐year mortality was 10.9% in HF‐CAD vs. 3.6% in HF‐NCAD, while 5‐year mortality was 15.3% in HF‐NCAD and 35.0% in HF‐CAD (Figure 2 ). The probability of survival in patients with HF and advanced CAD (MVD and LM) was lower than in those with HF and single‐vessel disease or normal/non‐obstructive CAD (Figure 3 ). Compared with HF patients without CAD, adjusted long‐term mortality increased with augmented severity of CAD [hazard ratio (HR) 1.30; 95% CI 1.20–1.41; P < 0.001] in HF patients with single‐vessel disease, in MVD (HR 1.72; 95% CI 1.58–1.88; P < 0.001) and in MVD with left main CAD (HR 2.02; 95% CI 1.88–2.18; P < 0.001).
Figure 2.
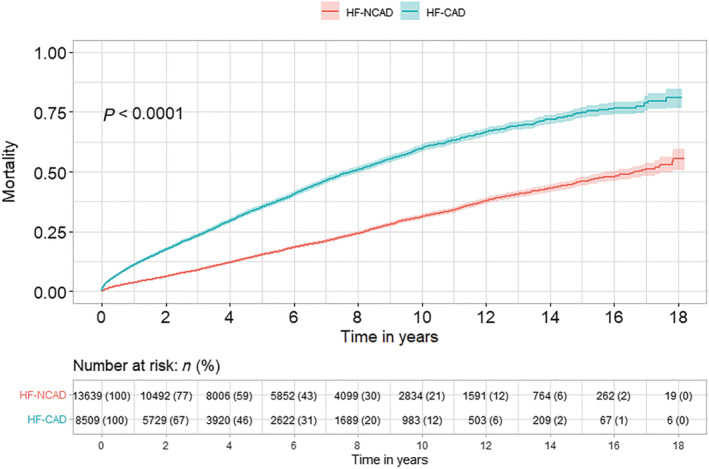
Kaplan–Meier estimates of mortality stratified by the presence (HF‐CAD) or absence of coronary artery disease (HF‐NCAD) in patients with heart failure.
Figure 3.
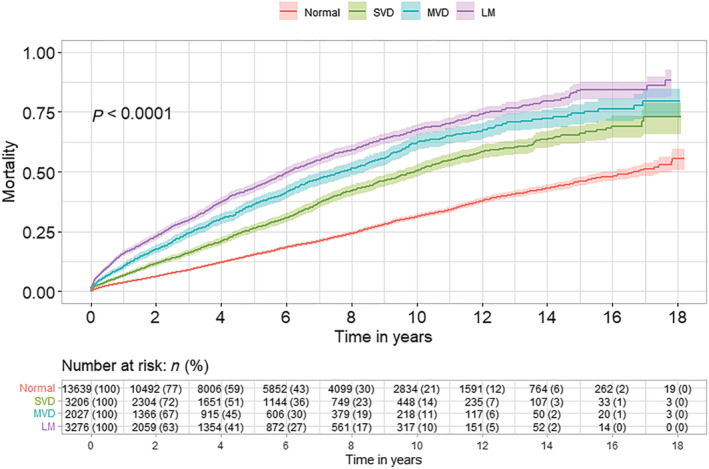
Kaplan–Meier estimates of mortality stratified by the presence and severity of coronary artery disease in patients with HF. Normal (normal/non‐obstructive CAD); SVD [single‐vessel diseases (stenosis > 50% in one coronary artery)]; MVD [multivessel disease (stenosis > 50% in more than one coronary artery without left main disease)]; LM [disease in a left main coronary artery (stenosis > 50% in left main coronary artery)].
Subgroup analyses
We found effect modification between HF‐CAD/HF‐NCAD, renal function, and time period. HF‐NCAD patients with creatinine clearance ≥ 60 mL/min had a better prognosis than patients with HF‐CAD (Table 3 ). The risk of death was higher in HF patients with CAD during 2015–2018 compared with the reference period (Figure 4 ). We found no interaction between age, sex, diabetes, and type of HF (Figure 4 ).
Table 3.
Cox proportional hazard regression
| HR | 95% CI | P value | |
|---|---|---|---|
| Severity of CAD | <0.000 | ||
| Normal/non‐obstructive | 1 (reference) | ||
| SVD | 1.36 | 1.26–1.47 | |
| MVD | 1.75 | 1.61–1.90 | |
| LM | 2.06 | 1.91–2.22 | |
| Age | |||
| <59 | 1 (reference) | ||
| 60–69 | 1.80 | 1.68–1.93 | <0.000 |
| 70–79 | 2.84 | 2.64–301 | <0.000 |
| ≥80 | 4.25 | 3.82–4.74 | <0.000 |
| Female sex | 0.86 | 0.81–0.91 | <0.000 |
| Diabetes | 1.38 | 1.30–1.46 | <0.000 |
| Hypertension | 1.03 | 0.97–1.08 | 0.352 |
| Hyperlipidaemia | 0.99 | 0.93–1.05 | 0.789 |
| History of smoking | |||
| Never smoker | 1 (reference) | ||
| Previous | 1.04 | 0.99–1.10 | 0.894 |
| Current | 1.38 | 1.24–1.43 | <0.000 |
| Previous MI | 1.15 | 1.07–1.25 | <0.000 |
| Previous PCI | 1.00 | 0.92–1.09 | 0.904 |
| Previous CABG | 1.16 | 1.07–1.28 | 0.001 |
| eGFR (mL/min/1.73 m2) | 0.99 | 0.99–0.99 | <0.000 |
| <60 | 1 (reference) | ||
| ≥60 | 1.66 | 1.07–1.28 | 0.001 |
| BMI | |||
| <18 | 1.28 | 1.03–1.59 | 0.028 |
| 18–25 | 1 (reference) | ||
| 26–30 | 0.93 | 0.88–0.98 | 0.019 |
| >30 | 0.96 | 0.91–1.03 | 0.364 |
| Time period | |||
| 2000–2004 | 1 (reference) | ||
| 2005–2008 | 0.85 | 0.79–0.92 | <0.000 |
| 2009–2011 | 0.88 | 0.81–0.95 | 0.002 |
| 2012–2014 | 0.88 | 0.81–0.96 | 0.006 |
| 2015–2018 | 0.73 | 0.66–0.83 | <0.000 |
BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; CI, confidence interval; eGFR, estimated glomerular filtration rate (Cockcroft–Gault); HF, heart failure; HR, hazard ratio, LM, disease in left main artery; MI, myocardial infarction; MVD, multivessel disease (stenosis > 50% in more than one vessel without left main disease); Normal/non‐obstructive, no stenosis > 50% in coronary arteries; PCI, percutaneous coronary intervention; SVD, single‐vessel diseases (stenosis > 50% in one of the coronary arteries).
Figure 4.
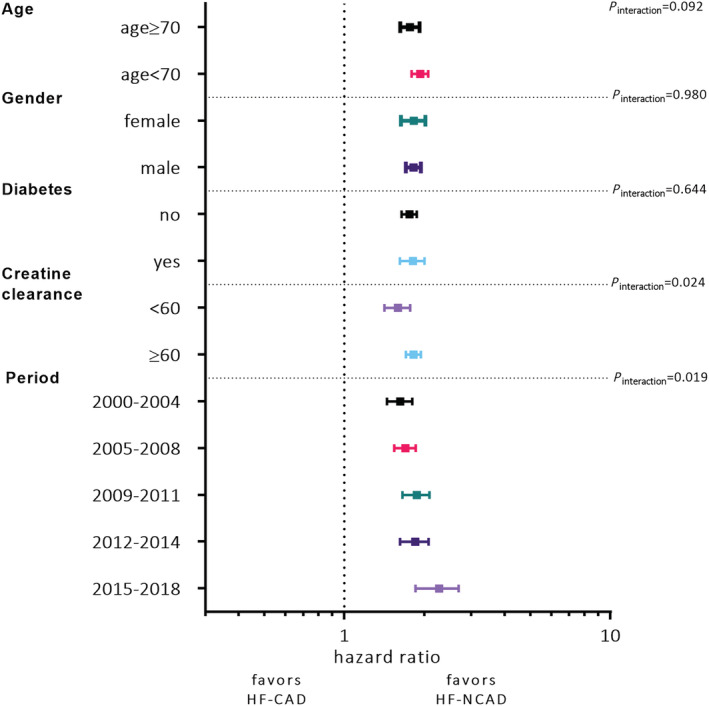
Forest plot depicting the interaction between the type of heart failure (HF‐CAD vs. HF‐NCAD) and age, sex, diabetes, renal function, and time period.
The relative importance of individual predictors of survival
The variable with the highest predictive value was the time period of CAG followed by age, the severity of CAD, and renal function. The combined variable importance of these four variables was ~90% for all‐cause mortality (Figure 5 ).
Figure 5.
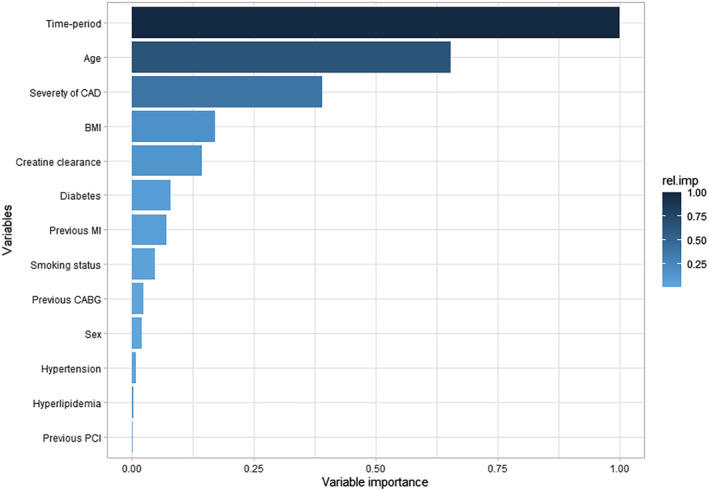
Variables' relative importance for predicting long‐term mortality. BMI, body mass index; CABG, coronary artery bypass grafting; CAD, coronary artery disease; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Discussion
In this nationwide study based on 22 457 patients referred for CAG due to HF between 2000 and 2018, the most important findings are as follows: (i) the number of CAG performed annually increased five times; (ii) survival increased considerably in all patients with HF but more in HF‐NCAD than HF‐CAD; (iii) during the study period, patients undergoing CAG for HF were increasingly older and had more comorbidities and more severe CAD; and (iv) the proportion of PCI‐treated patients increased substantially while the proportion of patients only treated pharmacologically or with CABG decreased. To our knowledge, this is the first prospective study reporting temporal trends in patient characteristics, treatment strategies, and long‐term survival in consecutive patients undergoing CAG due to HF based on nationwide data.
Coronary artery disease is the most common cause of HF. Accurate and timely identification of the cause of compromised cardiac function is a fundamental goal of the diagnostic process in HF. 19 Information from CAG is essential to distinguish left ventricular dysfunction primarily due to CAD from HF caused by other reasons because the treatment options are very different. When available, decision‐making rests heavily on CAG information, which links CAG with short‐term and long‐term prognosis. 20 , 21 , 22 The latest 2021 European Society of Cardiology guidelines for diagnosis and treatment of acute and chronic HF recommends CAG as a part of the diagnostic workup in HF patients with angina pectoris despite pharmacological therapy, history of ventricular arrhythmias, or aborted cardiac death (Class I, level of evidence B). 23 Guidelines from the European Society of Cardiology and the American College of Cardiology recommend CAG when ischaemia is considered contributing to HF or in patients with an intermediate‐to‐high pre‐test probability of CAD and positive non‐invasive stress test (Class IIa, level of evidence C). 24 , 25 Because of the absence of solid evidence for benefit and, consequently, varying recommendation levels during the last decades, only a minority of eligible HF patients undergo CAG. 26 , 27 However, our data show a constant and substantial growth in HF patients undergoing CAG in Sweden. This development is likely due to the increased number of catheterization laboratories generating improved hospital access to procedures, decreasing costs, decreased risk of complications, higher adherence to guidelines, and a better understanding of the importance of myocardial ischaemia in the pathophysiology of HF, 28 among other factors. Nonetheless, under the assumption that CAG is not performed in ~50% of patients with HF (unpublished data from SCAAR and Swedish Heart Failure Registry), we estimate that there are ~30 000 HF patients at present in Sweden in whom advanced CAD is not diagnosed but who may benefit from revascularization. This estimation is based on the known prevalence of HF‐CAD in Sweden 29 and the frequency of MVD or left main disease in HF‐CAD from the present study.
Information about the location and severity of CAD is pivotal for subsequent clinical decisions, including the choice of revascularization method. However, whether HF patients with complex CAD (i.e. MVD and LM) should be revascularized with PCI rather than with CABG is controversial. To date, no randomized clinical trial has demonstrated the superiority of PCI over medical treatment in HF‐CAD. On the other hand, the STICH study established that CABG is superior to medical therapy in patients with HF‐CAD. 12 Two large observational studies from Canada 30 and Sweden 15 recently confirmed the results from the STICH trial. According to the latest European Society of Cardiology guidelines on myocardial revascularization, treatment with both PCI and CABG are recommended for patients with severe left ventricular dysfunction and CAD suitable for intervention. CABG is recommended as the first choice in patients with MVD and acceptable surgical risk. 11
While prognosis improved steadily for each calendar year by 1.3% in all patients with HF during the last two decades, this development has been more pronounced in HF‐NCAD than in HF‐CAD. 5 Our study is consistent with these findings because we report that improvement in survival was attenuated during the last 5 years in HF‐CAD. The demographics of HF patient undergoing CAG in Sweden changed substantially in the direction of an older population with a higher comorbidity burden and more severe CAD. Change in patient characteristics may at least partly explain the discrepancy in outcome trends between HF‐NCAD and HF‐CAD. Another explanation may be the increased prevalence of HF with preserved ejection fraction, which associates less with CAD and has a better prognosis than HF with reduced ejection fraction. 31 Our observation that CAD and severity of CAD were critical prognostic factors for all‐cause mortality is supported by previous studies. 3 , 32 , 33 Studies using computed tomography angiography have also reported that the presence and extent of CAD increase the risk of death and adverse cardiac events. 34 , 35 However, none of these studies have specifically examined patients with HF. At present, ~80% of HF‐CAD undergoing CAG in Sweden (including patients with MVD and LM) are revascularized with PCI. The most likely explanation for PCI predominance is the growing number of HF patients with a high risk of CABG‐related complications paralleled with better access to catheterization laboratory facilities, improved PCI operators' skills, advancement in PCI technology, and patient's preference for PCI. On the other hand, the predominant and accelerated utilization of PCI for revascularisation in HF‐CAD could explain the attenuated survival benefit during the latest years.
Study limitations
There are several limitations to this study that need to be addressed. Patients with HF referred to CAG in this study may not be representative of the general HF population. We acknowledge that the observational design of our study carries an inherent risk for residual confounding. However, we have used appropriate statistical adjustment to reduce bias in risk estimates. Events in the SCAAR registry are not adjudicated. However, regular external monitoring and data validation are performed and have ascertained high data accuracy. 14 Our data did not allow differentiation between cardiac and non‐cardiac death. Nevertheless, all‐cause death is an important clinical outcome and is not subject to complex adjudication issues. Administrative databases reliably capture vital status in Sweden, and nearly 100% of all deaths are registered within the first month. We did not have data about left ventricular function or medications before or after the CAG. We could not discern new‐onset HF from chronic HF nor between HF with reduced ejection fraction from HF with preserved ejection fraction. Invasive CAG quantifies CAD based on the measurement of the vessel lumen does not provide information about the vessel wall. We were not able to compare patients with normal coronary angiograms with those having non‐obstructive CAD.
Conclusions
The number of patients with HF undergoing CAG has increased substantially over the last two decades in Sweden. Expanded utilization of CAG increases the number of revascularized HF‐CAD patients. Long‐term survival increased considerably despite higher age and increased comorbidity burden.
Conflict of interest
Dr Ljungman has received honoraria for presentations, educational events from Novartis, Astra Zeneca, and Pfizer; Dr Alfredsson has received honoraria for lectures from Boehringer‐Ingelheim and advisory board from Astra Zeneca; Dr Braun has received honoraria for lectures, presentations, educational events, or advisory boards from Novartis, Astra Zeneca, Bayer, Boehringer‐Ingelheim, Orion Pharma, and Pharmacosmos; Dr Fröberg reports grants from Sanofi Pasteur, outside the submitted work. All other authors have reported that they have no relationships relevant to the contents of this paper to disclose.
Funding
This work was supported by the Swedish Heart Lung Foundation, the Swedish Research Council, and Swedish federal government under the ALF agreement. This work was also supported by Sahlgrenska Akademin and Västra Götalandsregionen.
Bollano, E. , Redfors, B. , Rawshani, A. , Venetsanos, D. , Völz, S. , Angerås, O. , Ljungman, C. , Alfredsson, J. , Jernberg, T. , Råmunddal, T. , Petursson, P. , Smith, J. G. , Braun, O. , Hagström, H. , Fröbert, O. , Erlinge, D. , and Omerovic, E. (2022) Temporal trends in characteristics and outcome of heart failure patients with and without significant coronary artery disease. ESC Heart Failure, 9: 1812–1822. 10.1002/ehf2.13875.
References
- 1. Priori SG, Blomstrom‐Lundqvist C, Mazzanti A, Blom N, Borggrefe M, Camm J, Elliott PM, Fitzsimons D, Hatala R, Hindricks G, Kirchhof P, Kjeldsen K, Kuck KH, Hernandez‐Madrid A, Nikolaou N, Norekval TM, Spaulding C, Van Veldhuisen DJ, Group ESCSD . 2015 ESC guidelines for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death: the task force for the management of patients with ventricular arrhythmias and the prevention of sudden cardiac death of the European Society of Cardiology (ESC). Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC). Eur Heart J 2015; 36: 2793–2867. [DOI] [PubMed] [Google Scholar]
- 2. Patel MR, Calhoon JH, Dehmer GJ, Grantham JA, Maddox TM, Maron DJ, Smith PK. ACC/AATS/AHA/ASE/ASNC/SCAI/SCCT/STS 2017 appropriate use criteria for coronary revascularization in patients with stable ischemic heart disease: a report of the American College of Cardiology Appropriate Use Criteria Task Force, American Association for Thoracic Surgery, American Heart Association, American Society of Echocardiography, American Society of Nuclear Cardiology, Society for Cardiovascular Angiography and Interventions, Society of Cardiovascular Computed Tomography, and Society of Thoracic Surgeons. J Am Coll Cardiol 2017; 69: 2212–2241. [DOI] [PubMed] [Google Scholar]
- 3. Lee DS, Gona P, Vasan RS, Larson MG, Benjamin EJ, Wang TJ, Tu JV, Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: insights from the Framingham heart study of the National Heart, Lung, and Blood Institute. Circulation 2009; 119: 3070–3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vedin O, Lam CSP, Koh AS, Benson L, Teng THK, Tay WT, Braun OO, Savarese G, Dahlstrom U, Lund LH. Significance of ischemic heart disease in patients with heart failure and preserved, midrange, and reduced ejection fraction: a nationwide cohort study. Circulation Heart Fail 2017; 10: e003875. [DOI] [PubMed] [Google Scholar]
- 5. Silverdal J, Sjoland H, Bollano E, Pivodic A, Dahlstrom U, Fu M. Prognostic impact over time of ischaemic heart disease vs. non‐ischaemic heart disease in heart failure. ESC Heart Fail 2020; 7: 265–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Levine GN, Bates ER, Bittl JA, Brindis RG, Fihn SD, Fleisher LA, Granger CB, Lange RA, Mack MJ, Mauri L, Mehran R, Mukherjee D, Newby LK, O'Gara PT, Sabatine MS, Smith PK, Smith SC Jr. 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA Guideline for Coronary Artery Bypass Graft Surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS Guideline for the Diagnosis and Management of Patients With Stable Ischemic Heart Disease, 2013 ACCF/AHA Guideline for the Management of ST‐Elevation Myocardial Infarction, 2014 AHA/ACC Guideline for the Management of Patients With Non‐ST‐Elevation Acute Coronary Syndromes, and 2014 ACC/AHA Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery. Circulation 2016; 134: e123–e155. [DOI] [PubMed] [Google Scholar]
- 7. Guidelines for the diagnosis of heart failure . The task force on heart failure of the European Society of Cardiology. Eur Heart J 1995; 16: 741–751. [PubMed] [Google Scholar]
- 8. Remme WJ, Swedberg K, Task Force for the D and Treatment of Chronic Heart Failure ESoC . Guidelines for the diagnosis and treatment of chronic heart failure. Eur Heart J 2001; 22: 1527–1560. [DOI] [PubMed] [Google Scholar]
- 9. Swedberg K, Cleland J, Dargie H, Drexler H, Follath F, Komajda M, Tavazzi L, Smiseth OA, Gavazzi A, Haverich A, Hoes A, Jaarsma T, Korewicki J, Levy S, Linde C, Lopez‐Sendon JL, Nieminen MS, Pierard L, Remme WJ, Task Force for the D and Treatment of Chronic Heart Failure of the European Society of C . Guidelines for the diagnosis and treatment of chronic heart failure: executive summary (update 2005): the task force for the diagnosis and treatment of chronic heart failure of the European Society of Cardiology. Eur Heart J; 2005: 1115–1140. [DOI] [PubMed] [Google Scholar]
- 10. Dickstein K, Cohen‐Solal A, Filippatos G, McMurray JJ, Ponikowski P, Poole‐Wilson PA, Stromberg A, van Veldhuisen DJ, Atar D, Hoes AW, Keren A, Mebazaa A, Nieminen M, Priori SG, Swedberg K, Guidelines ESCCfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: the task force for the diagnosis and treatment of acute and chronic heart failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM). Eur Heart J 2008; 29: 2388–2442. [DOI] [PubMed] [Google Scholar]
- 11. Neumann FJ, Sousa‐Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Juni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbing D, Stefanini GG, Windecker S, Yadav R, Zembala MO, Group ESCSD . ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2018; 2019: 87–165. [DOI] [PubMed] [Google Scholar]
- 12. Velazquez EJ, Lee KL, Jones RH, Al‐Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, Oh JK, She L, Moore VL, Desvigne‐Nickens P, Sopko G, Rouleau JL, Investigators S. Coronary‐artery bypass surgery in patients with ischemic cardiomyopathy. N Engl J Med 2016; 374: 1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frobert O, Lagerqvist B, Olivecrona GK, Omerovic E, Gudnason T, Maeng M, Aasa M, Angeras O, Calais F, Danielewicz M, Erlinge D, Hellsten L, Jensen U, Johansson AC, Karegren A, Nilsson J, Robertson L, Sandhall L, Sjogren I, Ostlund O, Harnek J, James SK, Trial T. Thrombus aspiration during ST‐segment elevation myocardial infarction. N Engl J Med 2013; 369: 1587–1597. [DOI] [PubMed] [Google Scholar]
- 14. Jernberg T, Attebring MF, Hambraeus K, Ivert T, James S, Jeppsson A, Lagerqvist B, Lindahl B, Stenestrand U, Wallentin L. The Swedish web‐system for enhancement and development of evidence‐based care in heart disease evaluated according to recommended therapies (SWEDEHEART). Heart (British Cardiac Society) 2010; 96: 1617–1621. [DOI] [PubMed] [Google Scholar]
- 15. Volz S, Redfors B, Angeras O, Ioanes D, Odenstedt J, Koul S, Valeljung I, Dworeck C, Hofmann R, Hansson E, Venetsanos D, Ulvenstam A, Jernberg T, Ramunddal T, Petursson P, Frobert O, Erlinge D, Jeppsson A, Omerovic E. Long‐term mortality in patients with ischaemic heart failure revascularized with coronary artery bypass grafting or percutaneous coronary intervention: insights from the Swedish coronary angiography and angioplasty registry (SCAAR). Eur Heart J 2021; 42: 2657–2664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. van Buuren S. Multiple imputation of discrete and continuous data by fully conditional specification. Stat Methods Med Res 2007; 16: 219–242. [DOI] [PubMed] [Google Scholar]
- 17. Rubin DB. Inference and missing data. Biometrika 1976; 63: 581–590. [Google Scholar]
- 18. Strobl C, Boulesteix AL, Kneib T, Augustin T, Zeileis A. Conditional variable importance for random forests. BMC Bioinform 2008; 9: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Roger VL. Epidemiology of heart failure. Circ Res 2013; 113: 646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Flaherty JD, Bax JJ, De Luca L, Rossi JS, Davidson CJ, Filippatos G, Liu PP, Konstam MA, Greenberg B, Mehra MR, Breithardt G, Pang PS, Young JB, Fonarow GC, Bonow RO, Gheorghiade M, Acute Heart Failure Syndromes International Working G . Acute heart failure syndromes in patients with coronary artery disease early assessment and treatment. J Am Coll Cardiol 2009; 53: 254–263. [DOI] [PubMed] [Google Scholar]
- 21. Jourdain P, Jondeau G, Funck F, Gueffet P, Le Helloco A, Donal E, Aupetit JF, Aumont MC, Galinier M, Eicher JC, Cohen‐Solal A, Juilliere Y. Plasma brain natriuretic peptide‐guided therapy to improve outcome in heart failure: the STARS‐BNP multicenter study. J Am Coll Cardiol 2007; 49: 1733–1739. [DOI] [PubMed] [Google Scholar]
- 22. Taylor AJ, Bindeman J, Feuerstein I, Le T, Bauer K, Byrd C, Wu H, O'Malley PG. Community‐based provision of statin and aspirin after the detection of coronary artery calcium within a community‐based screening cohort. J Am Coll Cardiol 2008; 51: 1337–1341. [DOI] [PubMed] [Google Scholar]
- 23. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, Burri H, Butler J, Celutkiene J, Chioncel O, Cleland JGF, Coats AJS, Crespo‐Leiro MG, Farmakis D, Gilard M, Heymans S, Hoes AW, Jaarsma T, Jankowska EA, Lainscak M, Lam CSP, Lyon AR, McMurray JJV, Mebazaa A, Mindham R, Muneretto C, Francesco Piepoli M, Price S, Rosano GMC, Ruschitzka F, Kathrine Skibelund A, Group ESCSD . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021; 2021: 3599–3726. [DOI] [PubMed] [Google Scholar]
- 24. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 2017; 70: 776–803. [DOI] [PubMed] [Google Scholar]
- 25. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, Falk V, Gonzalez‐Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GMC, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P, Group ESCSD . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 2016: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 26. Ferreira JP, Rossignol P, Demissei B, Sharma A, Girerd N, Anker SD, Cleland JG, Dickstein K, Filippatos G, Hillege HL, Lang CC, Metra M, Ng LL, Ponikowski P, Samani NJ, van Veldhuisen DJ, Zwinderman AH, Voors A, Zannad F. Coronary angiography in worsening heart failure: determinants, findings and prognostic implications. Heart 2018; 104: 606–613. [DOI] [PubMed] [Google Scholar]
- 27. Doshi D, Ben‐Yehuda O, Bonafede M, Josephy N, Karmpaliotis D, Parikh MA, Moses JW, Stone GW, Leon MB, Schwartz A, Kirtane AJ. Underutilization of coronary artery disease testing among patients hospitalized with new‐onset heart failure. J Am Coll Cardiol 2016; 68: 450–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ryan MJ, Perera D. Identifying and managing hibernating myocardium: what's new and what remains unknown? Curr Heart Fail Rep 2018; 15: 214–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Zarrinkoub R, Wettermark B, Wandell P, Mejhert M, Szulkin R, Ljunggren G, Kahan T. The epidemiology of heart failure, based on data for 2.1 million inhabitants in Sweden. Eur J Heart Fail 2013; 15: 995–1002. [DOI] [PubMed] [Google Scholar]
- 30. Sun LY, Gaudino M, Chen RJ, Bader Eddeen A, Ruel M. Long‐term outcomes in patients with severely reduced left ventricular ejection fraction undergoing percutaneous coronary intervention vs coronary artery bypass grafting. JAMA Cardiol 2020; 5: 631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Steinberg BA, Zhao X, Heidenreich PA, Peterson ED, Bhatt DL, Cannon CP, Hernandez AF, Fonarow GC, Get With the Guidelines Scientific Advisory C and Investigators . Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: prevalence, therapies, and outcomes. Circulation 2012; 126: 65–75. [DOI] [PubMed] [Google Scholar]
- 32. Harris PJ, Behar VS, Conley MJ, Harrell FE Jr, Lee KL, Peter RH, Kong Y, Rosati RA. The prognostic significance of 50% coronary stenosis in medically treated patients with coronary artery disease. Circulation 1980; 62: 240–248. [DOI] [PubMed] [Google Scholar]
- 33. Arbab‐Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol 2016; 68: 2467–2478. [DOI] [PubMed] [Google Scholar]
- 34. Lin FY, Shaw LJ, Dunning AM, Labounty TM, Choi JH, Weinsaft JW, Koduru S, Gomez MJ, Delago AJ, Callister TQ, Berman DS, Min JK. Mortality risk in symptomatic patients with nonobstructive coronary artery disease: a prospective 2‐center study of 2,583 patients undergoing 64‐detector row coronary computed tomographic angiography. J Am Coll Cardiol 2011; 58: 510–519. [DOI] [PubMed] [Google Scholar]
- 35. Ostrom MP, Gopal A, Ahmadi N, Nasir K, Yang E, Kakadiaris I, Flores F, Mao SS, Budoff MJ. Mortality incidence and the severity of coronary atherosclerosis assessed by computed tomography angiography. J Am Coll Cardiol 2008; 52: 1335–1343. [DOI] [PubMed] [Google Scholar]


