Highlights
-
•
Glucocorticoid receptor expression was higher in the sarcomatous than the carcinomatous components.
-
•
Estrogen and progesterone receptor expression were both higher in the carcinomatous components.
-
•
Androgen receptor expression was low overall in Mullerian carcinosarcomas.
Keywords: Carcinosarcoma, Glucocorticoid receptor, Estrogen receptor, Progesterone receptor
Abstract
Glucocorticoid receptor can be associated with poor prognosis among a variety of solid tumors in the absence of other nuclear hormone receptors. Our objective was to characterize differences in glucocorticoid receptor (GR), estrogen receptor (ER), progesterone receptor (PR), and androgen receptor expression in the sarcomatous versus carcinomatous components of ovarian and uterine carcinosarcomas. Eighteen patients diagnosed with Mullerian carcinosarcoma between May 2009 and August 2014 were included. Nuclear receptor expression was evaluated by immunohistochemistry using whole tissue specimens. Receptor expression was quantified using the H-score. Mean H-scores were compared between the sarcomatous and carcinomatous components of tumors using Wilcoxon signed-rank tests. We found that GR expression was significantly higher in the sarcomatous components than in the carcinomatous components of the cancers (mean H score 144.4 vs 38.9, p = 0.002). Conversely, ER (3.1 vs 63.1, p = 0.002) and PR (1.7 vs 47.2, p < 0.0001) expression were significantly decreased in the sarcomatous component compared to the carcinomatous component. Androgen receptor expression was low overall (0 versus 2.8, p = 0.04). We hypothesize that GR-high, ER/PR-low expression is associated with epithelial to mesenchymal transition in the sarcomatous cells and may serve as a potential therapeutic target.
1. Introduction
Carcinosarcomas are rare and aggressive gynecologic malignancies defined by a biphasic tumor histology including both carcinomatous and sarcomatous components (Berton-Rigaud et al., 2014). Mullerian carcinosarcomas have been reported to account for 5% or less of all ovarian and endometrial malignancies, but the survival outcomes are worse than for high grade serous and endometrioid tumors of the ovary and endometrium, respectively (Berton-Rigaud et al., 2014). Primary tumors with a greater proportion of sarcomatous histology – sometimes referred to as sarcoma-dominant – behave more aggressively than carcinoma-dominant tumors and carry a worse prognosis (Matsuo et al., 2018). While carcinosarcomas were initially classified and treated as sarcomas, a growing body of evidence suggests that carcinosarcomas arise from a precursor epithelial cell with transformation into sarcomatous components (McCluggage, 2002). Accordingly, gynecologic carcinosarcomas are managed as aggressive carcinomas rather than sarcomas (Berton-Rigaud et al., 2014). The carcinoma and sarcomatous components share common somatic mutations (Zhao et al., 2016, Cherniack et al., 2017). However, there is also intercomponent heterogeneity, e.g., in epithelial to mesenchymal transition (EMT) related genes, with greater heterogeneity noted in more advanced or aggressive cases (Liu et al., 2018). Examples of relevant genes include epithelial markers such as E-cadherin, mesenchymal markers such as vimentin, and transcription factors such as Slug and Snail. Understanding the differences between the epithelial and mesenchymal components may help us to understand the lineage-specific biology of these difficult-to-treat cancers and target treatments more effectively.
Glucocorticoid receptor (GR) signaling is increasingly recognized as an important mediator of solid tumor biology and is expressed to varying degrees in many carcinomas (Block, 2017). Its impact on tumor behavior is extremely context dependent. For example, high GR expression in estrogen receptor (ER)-positive breast cancer is associated with cell differentiation, decreased proliferation, and better clinical outcomes (West et al., 2016), whereas in ER-negative breast cancer high GR expression is correlated with EMT-associated gene expression, de-differentiation, and a poor prognosis (Pan et al., 2011). In preclinical studies of castrate-sensitive prostate cancer, GR activation appears to slow disease progression, but when prostate cancer becomes castrate-resistant, GR signaling drives more aggressive disease (Kach et al., 2015). Similarly, recent data suggest that GR signaling becomes increasingly important in driving metastases as breast cancer becomes more advanced (Obradović et al., 2019). Increased GR expression correlates with poor outcomes in both endometrial cancer (Tangen et al., 2017) and ovarian cancer (Veneris et al., 2017). Information about the other hormonal receptors was not available for the study in ovarian cancer patients, but among the endometrial cohort, this poor prognosis was somewhat ameliorated in the presence of positive estrogen, progesterone, or androgen receptor expression. Based on the observed association of EMT with GR expression in other estrogen and progesterone receptor (PR) negative cancers, as well as prior research demonstrating GR expression in sarcomas (Block, 2017), we hypothesized that tumor cells in the sarcomatous regions of carcinosarcoma would have higher GR expression and lower ER/PR expression, while the carcinoma cells would express relatively decreased GR and higher ER and PR expression.
2. Methods
2.1. Patient specimens
Archival primary Mullerian carcinosarcoma specimens were retrieved from patients diagnosed at The University of Chicago between May 2009 and August 2014 using the in-house ovarian cancer database (Institutional Review Board [IRB] 13372B) (Sawada et al., 2007) and a retrospective review of endometrial specimens under a separate protocol (IRB 18–1837). All specimens were evaluated by a gynecologic pathologist (RL) to confirm the diagnosis using H&E staining.
2.2. Immunohistochemistry (IHC)
Four-micron sections were cut from formalin-fixed paraffin-embedded (FFPE) whole tissue sections. Standard techniques were used to stain tissue sections for estrogen receptor (Leica Biosystems, mouse IgG1 clone 6F11, 1:60 dilution), progesterone receptor (Leica Biosystems, mouse IgG1 clone 16, 1:300 dilution), androgen receptor (Leica Biosystems, mouse IgG clone AR441, 1:250 dilution), and GR (Leica Microsystems, rabbit IgG Cat#3660, dilution 1:1500). One pathologist (RL) reviewed the IHC slides. Receptor expression was quantified using the H-score, as previously described (Block, 2017). H-score is a commonly used expression assessment tool which takes into account both staining intensity and staining frequency throughout the tumor. Staining intensity was interpreted as none (0), weak (1 + ), moderate (2 + ), and strong (3 + ). Percentage of component staining was scored as a continuous variable from 0 to 100%. H-score was calculated by multiplying staining intensity by percentage of cells with staining (range: 0–300).
2.3. Statistical analysis
H-scores for the sarcomatous and carcinomatous components of the specimens were compared using a Wilcoxon signed-rank test. Statistical significance was defined as p < 0.05.
3. Results
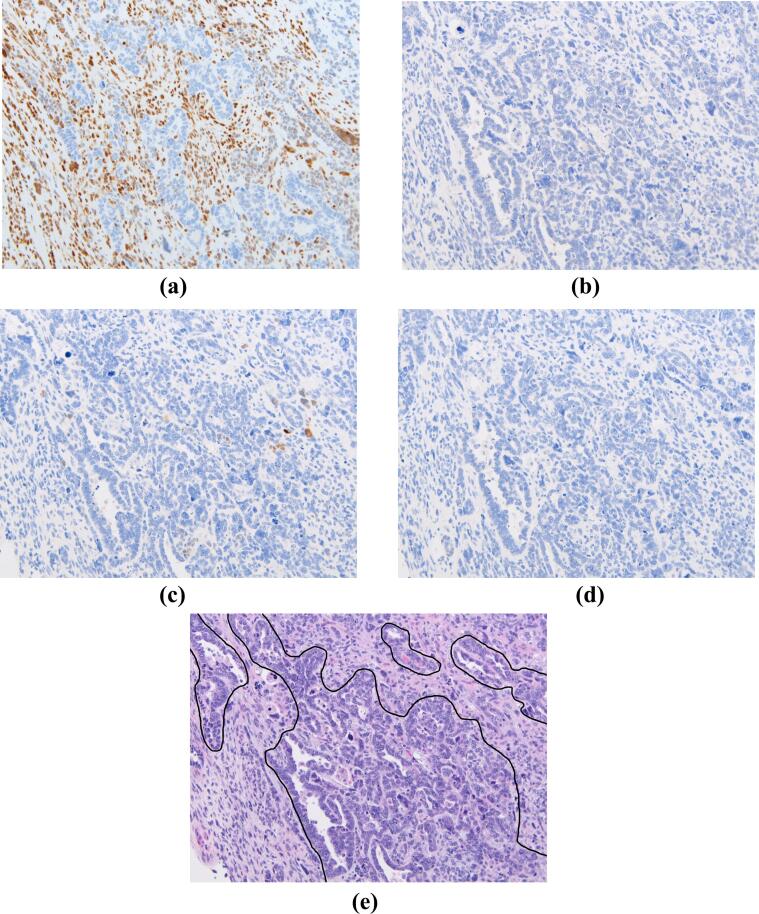
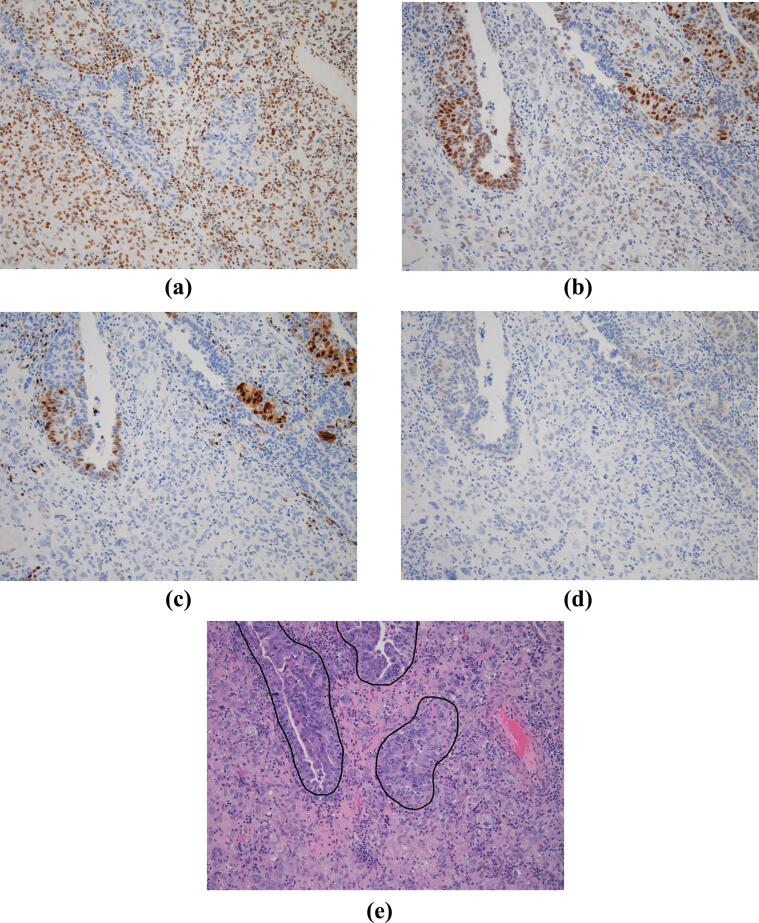
Eight endometrial carcinosarcomas and ten ovarian carcinosarcomas were identified. An example of GR, ER, PR, and androgen receptor tissue staining is shown in Fig. 1, Fig. 2. Table 1 summarizes the differences in nuclear receptor expression between the sarcomatous and carcinomatous components of tumors. Overall, mean GR H-score was significantly increased in the sarcomatous component when compared to the carcinomatous component of tumors (144.4 vs 38.9, Wilcoxon test, p = 0.002). A summary of differences in receptor expression by tumor type is also listed in Table 1.
Fig. 1.
Human tissue from an ovarian carcinosarcoma, with staining for a) glucocorticoid receptor; b) estrogen receptor; c) progesterone receptor; d) androgen receptor. H&E staining with the carcinomatous component outlined is shown in panel e). All magnifications are 20X.
Fig. 2.
Human tissue from an endometrial carcinosarcoma, with staining for a) glucocorticoid receptor; b) estrogen receptor; c) progesterone receptor; d) androgen receptor. H&E staining with the carcinomatous component outlined is shown in panel e). All magnifications are 20X.
Table 1.
Differential Nuclear Receptor Expression in Carcinomatous versus Sarcomatous Components of Gynecologic Carcinosarcomas.
| GR H-score | ER H-score | PR H-score | AR H-score | ||
|---|---|---|---|---|---|
| Overall (n = 18) | Sarcomatous - mean (range) |
144.4 (0–300) |
3.1 (0–50) |
1.7 (0–20) |
0 (0–0) |
| Carcinomatous - mean (range) |
38.9 (0–160) |
63.1 (0–300) |
47.2 (0–210) |
2.8 (0–20) |
|
| Mean of differences (range) |
105.6 (-70–300) |
−60 (-270–5) |
−45.6 (-210–0) |
−2.8 (-20–0) |
|
| 95% Confidence Interval | 47.3–163.8 | −107.4 to −12.6 | −74.7 to −16.5 | −5.6 to 0.1 | |
| P-value | 0.002 | 0.002 | <0.0001 | 0.04 | |
| Ovarian (n = 10) | Sarcomatous - mean (range) |
185 (10–300) |
0.5 (0–50) |
3 (0–20) |
0 (0–0) |
| Carcinomatous - mean (range) |
68 (10–160) |
96.5 (0–300) |
63 (0–210) |
4 (0–20) |
|
| Mean of differences (range) |
117 (-70–290) |
−91.5 (-270–0) |
−60 (-210–0) |
−4 (-20–0) |
|
| 95% Confidence Interval | 29.5 to 204.5 | −173.9 to −9.1 | −111.9 to −8.1 | −9.0 to 1.0 | |
| P-value | 0.01 | 0.008 | 0.007 | 0.08 | |
| Uterine (n = 8) | Sarcomatous - mean (range) |
93.8 (0–300) |
0.6 (0–5) |
0 (0–0) |
0 (0–0) |
| Carcinomatous - mean (range) |
2.5 (0–20) |
21.3 (0–120) |
27.5 (0–90) |
1.3 (0–10) |
|
| Mean of differences (range) |
91.3 (0–300) |
−20.6 (-40–5) |
−27.5 (-90–0) |
−1.3 (-10–0) |
|
| 95% Confidence Interval | −6.5 to 189 | −56.2 to 15.0 | −52.3 to −2.7 | −4.2 to 1.7 | |
| P-value | 0.005 | 0.11 | 0.005 | 0.38 |
Legend:
GR = glucocorticoid receptor; ER = estrogen receptor; PR = progesterone receptor; AR = androgen receptor.
p-value: computed using t-tests.
H-score was calculated by multiplying staining intensity (0 to 3 + ) by percentage of cells (0 to 100%) with staining.
Expression of the other steroid hormone receptors also differed between the sarcomatous and carcinomatous components, with estrogen, progesterone, and androgen receptors all higher in the carcinomatous components relative to the sarcomatous components. The mean estrogen receptor H-score was 3.1 in the sarcomatous components versus 63.1 in the carcinomatous components (Wilcoxon test, p = 0.002). Mean progesterone receptor H-score was 1.7 in the sarcomatous components, versus 47.2 in the carcinomatous components (Wilcoxon test, p < 0.001). Androgen receptor expression was low overall and not expressed in the sarcomatous component of any tumor, resulting in a mean androgen receptor H-score of 0 in the sarcomatous components and 2.8 in the carcinomatous components (Wilcoxon test, p = 0.04).
4. Discussion
The present study is the first to assess GR expression in carcinosarcomas of Mullerian origin. Carcinosarcomas are one model for epithelial-mesenchymal transition (Zhao et al., 2016), with increased expression of EMT-associated genes in the sarcomatous component of carcinosarcomas relative to the carcinomatous component. Although most likely derived from a single progenitor cell, the two compartments do have some important differences. It is also worth noting that the trend for expression differences of each of the receptors in the carcinoma versus sarcoma components were similar in the uterine and ovarian tumors; e.g., glucocorticoid receptor expression was higher in the sarcoma component, and estrogen, progesterone, and androgen receptors were higher in the carcinoma component. Our results add to that growing body of literature by highlighting differences in glucocorticoid receptor expression, which has not been previously explored, and which could have both mechanistic and therapeutic implications.
Recent molecular evaluations have highlighted the role that EMT plays in the evolution from carcinomatous to sarcomatous features. One recent study performed whole exome sequencing on 41 Mullerian carcinosarcomas (Zhao et al., 2016). Multiregion whole-exome sequencing was performed in six of these tumors in order to investigate their origins. In these tumors, the sarcomatous and carcinomatous components were analyzed separately, confirming a common, epithelial precursor lesion. Along with identification of many common mutations among epithelial ovarian and endometrial carcinomas, these sequencing data also demonstrated a significant number of alterations among histone genes, including several that were previously unrecognized. The authors hypothesize that these changes may contribute to some of the differential expression in the carcinomatous and sarcomatous components, and to the EMT that is seen in these tumors (Zhao et al., 2016). In laboratory data from triple-negative breast cancer, GR expression and activity also appear to drive EMT-associated gene expression (Pan et al., 2011). In this preclinical study, ER-negative breast cancer cell lines were treated with dexamethasone and 187 glucocorticoid receptor direct target genes were identified. Using gene enrichment pathway analysis, functional pathways associated with glucocorticoid receptor activation were identified. One of the top 10 pathways associated with activation was that associated with regulation of EMT. Extrapolating from those data, we speculate that GR may similarly act as a transcriptional driver of EMT in the transformation of carcinomatous to sarcomatous cells in these interesting tumors.
Whereas glucocorticoid receptor expression was upregulated, estrogen receptor and progesterone receptor were relatively decreased in the sarcomatous component of carcinosarcomas. We hypothesize that one of the key events leading to aggressive disease in hormone-driven cancer may be the upregulation of glucocorticoid receptor. However, it remains unclear whether it is glucocorticoid upregulation on its own, or glucocorticoid receptor upregulation in the context of downregulation of other hormonal receptor expression. In addition to the potential prognostic and mechanistic implications, GR expression may also represent a potential therapeutic target (Kach et al., 2015) for patients with carcinosarcomas. Given the relative chemotherapy-resistance of carcinosarcomas, identifying new treatment options for this subset of patients is critical. Studies testing GR antagonists as chemotherapy-sensitizing agents have been developed. Preliminary data from a Phase II study of relacorilant, a selective GR modulator, demonstrated an improvement in median progression-free survival for patients with platinum-resistant ovarian cancer who received the combination of intermittent relacorilant and nab-paclitaxel over nab-paclitaxel alone (5.6 months versus 3.8 months, p < 0.05) (Colombo et al., 2021). Preclinical data suggest that the combination of paclitaxel and a glucocorticoid receptor antagonist may also be worthy of investigation in pancreatic cancer and breast cancer patients (Greenstein and Hunt, 2021, Skor et al., 2013). Future studies should elucidate the mechanistic role that high GR expression and activity may play in the aggressive nature of these gynecologic cancers, with the hope that new treatment strategies can be tested.
Funding
GFF, SDC, SJW, and EL acknowledge support from the Mayo Clinic SPORE in Ovarian Cancer Developmental Research Program, Pilot Study Award (5P50CA136393-09); SDC and GFF received funding from NIH R21CA223426-02. KCK, MS, JTV, RRL, EL, GFF acknowledge support from the University of Chicago Comprehensive Cancer Center Core Immunohistochemistry Facility, grant #5P30CA014599-46.
CRediT authorship contribution statement
Katherine C. Kurnit: Writing – original draft, Writing – review & editing, Data curation, Validation, Visualization. Meghan Steiner: Writing – original draft, Visualization. Ricardo R. Lastra: Investigation, Writing – review & editing, Visualization. S. John Weroha: Writing – review & editing, Visualization. John Cursio: Formal analysis, Writing – review & editing. Ernst Lengyel: Writing – review & editing, Resources. Gini F. Fleming: Investigation, Conceptualization, Methodology, Writing – review & editing, Resources, Supervision. Suzanne D. Conzen: Investigation, Conceptualization, Methodology, Writing – review & editing, Resources, Supervision.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
The authors would like to acknowledge Dr. Jennifer Veneris for her contributions to this work.
Ernst Lengyel receives research funding from AbbVie and Arsenal Bio to perform translational ovarian cancer research that is completely unrelated to this study. Suzanne Conzen is an inventor on patents licensed by The University of Chicago to Corcept Therapeutics regarding a method to use glucocorticoid receptor modulators as therapies for triple-negative breast cancer and prostate cancer that is not directly relevant to Mullerian carcinosarcomas. Gini Fleming has served on an advisory board to GSK on a topic not directly related to this study.
References
- Berton-Rigaud D., Devouassoux-Shisheboran M., Ledermann J.A., Leitao M.M., Powell M.A., Poveda A., Beale P., Glasspool R.M., Creutzberg C.L., Harter P., Kim J.-W., Reed N.S., Ray-Coquard I. Gynecologic Cancer InterGroup (GCIG) consensus review for uterine and ovarian carcinosarcoma. Int. J. Gynecol. Cancer. 2014;24(Supp 3):S55–S60. doi: 10.1097/IGC.0000000000000228. [DOI] [PubMed] [Google Scholar]
- Matsuo K., Takazawa Y., Ross M.S., Elishaev E., Yunokawa M., Sheridan T.B., Bush S.H., Klobocista M.M., Blake E.A., Takano T., Baba T., Satoh S., Shida M., Ikeda Y., Adachi S., Yokoyama T., Takekuma M., Yanai S., Takeuchi S., Nishimura M., Iwasaki K., Johnson M.S., Yoshida M., Hakam A., Machida H., Mhawech-Fauceglia P., Ueda Y., Yoshino K., Kajiwara H., Hasegawa K., Yasuda M., Miyake T.M., Moriya T., Yuba Y., Morgan T., Fukagawa T., Pejovic T., Nagano T., Sasaki T., Richmond A.M., Post M.D., Shahzad M.M.K., Im D.D., Yoshida H., Enomoto T., Omatsu K., Ueland F.R., Kelley J.L., Karabakhtsian R.G., Roman L.D. Proposal for a Risk-Based Categorization of Uterine Carcinosarcoma. Ann. Surg. Oncol. 2018;25(12):3676–3684. doi: 10.1245/s10434-018-6695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCluggage W.G. Malignant biphasic uterine tumours: carcinosarcomas or metaplastic carcinomas? J. Clin. Pathol. 2002;55(5):321–325. doi: 10.1136/jcp.55.5.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao S., Bellone S., Lopez S., Thakral D., Schwab C., English D.P., Black J., Cocco E., Choi J., Zammataro L., Predolini F., Bonazzoli E., Bi M., Buza N., Hui P., Wong S., Abu-Khalaf M., Ravaggi A., Bignotti E., Bandiera E., Romani C., Todeschini P., Tassi R., Zanotti L., Odicino F., Pecorelli S., Donzelli C., Ardighieri L., Facchetti F., Falchetti M., Silasi D.-A., Ratner E., Azodi M., Schwartz P.E., Mane S., Angioli R., Terranova C., Quick C.M., Edraki B., Bilgüvar K., Lee M., Choi M., Stiegler A.L., Boggon T.J., Schlessinger J., Lifton R.P., Santin A.D. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc. Natl. Acad. Sci. U.S.A. 2016;113(43):12238–12243. doi: 10.1073/pnas.1614120113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherniack A.D., et al. Integrated molecular characterization of uterine Carcinosarcoma. Cancer Cell. 2017;31(3):411–423. doi: 10.1016/j.ccell.2017.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Weber Z., San Lucas F.A., Deshpande A., Jakubek Y.A., Sulaiman R., Fagerness M., Flier N., Sulaiman J., Davis C.M., Fowler J., Starks D., Rojas-Espaillat L., Lazar A.J., Davies G.E., Ehli E.A., Scheet P. Assessing inter-component heterogeneity of biphasic uterine carcinosarcomas. Gynecol. Oncol. 2018;151(2):243–249. doi: 10.1016/j.ygyno.2018.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block T.S., et al. Glucocorticoid receptor expression in 20 solid tumor types using immunohistochemistry assay. CancerManag Res. 2017;9:65–72. doi: 10.2147/CMAR.S124475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West D.C., Pan D., Tonsing-Carter E.Y., Hernandez K.M., Pierce C.F., Styke S.C., Bowie K.R., Garcia T.I., Kocherginsky M., Conzen S.D. GR and ER Coactivation Alters the Expression of Differentiation Genes and Associates with Improved ER+ Breast Cancer Outcome. Mol. Cancer Res. 2016;14(8):707–719. doi: 10.1158/1541-7786.MCR-15-0433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan D., Kocherginsky M., Conzen S.D. Activation of the glucocorticoid receptor is associated with poor prognosis in estrogen receptor-negative breast cancer. Cancer Res. 2011;71(20):6360–6370. doi: 10.1158/0008-5472.CAN-11-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kach J., Conzen S.D., Szmulewitz R.Z. Targeting the glucocorticoid receptor in breast and prostate cancers. Sci. Transl. Med. 2015;7(305):p. 305ps19. doi: 10.1126/scitranslmed.aac7531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obradović M.M.S., Hamelin B., Manevski N., Couto J.P., Sethi A., Coissieux M.-M., Münst S., Okamoto R., Kohler H., Schmidt A., Bentires-Alj M. Glucocorticoids promote breast cancer metastasis. Nature. 2019;567(7749):540–544. doi: 10.1038/s41586-019-1019-4. [DOI] [PubMed] [Google Scholar]
- Tangen I.L., Veneris J.T., Halle M.K., Werner H.M., Trovik J., Akslen L.A., Salvesen H.B., Conzen S.D., Fleming G.F., Krakstad C. Expression of glucocorticoid receptor is associated with aggressive primary endometrial cancer and increases from primary to metastatic lesions. Gynecol. Oncol. 2017;147(3):672–677. doi: 10.1016/j.ygyno.2017.09.013. [DOI] [PubMed] [Google Scholar]
- Veneris J.T., Darcy K.M., Mhawech-Fauceglia P., Tian C., Lengyel E., Lastra R.R., Pejovic T., Conzen S.D., Fleming G.F. High glucocorticoid receptor expression predicts short progression-free survival in ovarian cancer. Gynecol. Oncol. 2017;146(1):153–160. doi: 10.1016/j.ygyno.2017.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawada K., Radjabi A.R., Shinomiya N., Kistner E., Kenny H., Becker A.R., Turkyilmaz M.A., Salgia R., Yamada S.D., Vande Woude G.F., Tretiakova M.S., Lengyel E. c-Met overexpression is a prognostic factor in ovarian cancer and an effective target for inhibition of peritoneal dissemination and invasion. Cancer Res. 2007;67(4):1670–1679. doi: 10.1158/0008-5472.CAN-06-1147. [DOI] [PubMed] [Google Scholar]
- Colombo N., Nguyen D.D., Fleming G.F., Grisham R.N., Lorusso D., Van Gorp T., Oaknin A., Pashova H.I., Grauer A. 721O Relacorilant, a selective glucocorticoid receptor modulator, in combination with nab-paclitaxel improves progression-free survival in patients with recurrent platinum-resistant ovarian cancer: A 3-arm, randomized, open-label, phase II study. Ann. Oncol. 2021;32:S725. doi: 10.1016/j.annonc.2021.08.1164. [DOI] [Google Scholar]
- Greenstein A.E., Hunt H.J. Glucocorticoid receptor antagonism promotes apoptosis in solid tumor cells. Oncotarget. 2021;12(13):1243–1255. doi: 10.18632/oncotarget.27989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skor M.N., Wonder E.L., Kocherginsky M., Goyal A., Hall B.A., Cai Y.i., Conzen S.D. Glucocorticoid receptor antagonism as a novel therapy for triple-negative breast cancer. Clin. Cancer Res. 2013;19(22):6163–6172. doi: 10.1158/1078-0432.CCR-12-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]