Abstract
Acetylsalicylic acid (Aspirin) has been used for a long time as an antipyretic and analgesic. Nevertheless, aspirin use is associated with severe morbidity and death because of its detrimental impacts on several organs, including the liver, kidneys, and stomach. The current investigation sought to ascertain the influence of thymol in mitigating aspirin-mediated gastric and hepato-renal injury. This was done by 1) evaluating gastric juice volume and pH as well as pepsin and prostaglandin E2 level; 2) measuring serum biochemical parameters and proinflammatory cytokines; 3) determining tissue oxidant/antioxidant status, and 4) identifying a link with gastric, hepatic and renal histopathological changes. Forty-eight rats were segregated to six groups: normal control, Th100, Th200, ASA, Th100 + ASA, Th200 + ASA. Daily administration of aspirin (ASA, 150 mg/kg body weight) for 3 successive days induced a significant increase in gastric juice volume, pepsin activity, serum transaminases, alkaline phosphatase, urea, creatinine, tumor necrosis factor-α, myeloperoxidase, and tissue malondialdehyde levels. In contrast, a significant reduction in gastric pH, prostaglandin E2, tissue non-enzymatic antioxidant (glutathione), and enzymatic antioxidant (superoxide dismutase, glutathione peroxidase, and catalase) levels. These biochemical changes were accompanied by histological modifications that included changes to the normal gastric, hepatic, and renal architectures. Pretreatment and simultaneous oral treatment with thymol (100 or 200 mg/kg body weight) plus ASA significantly improved all biochemical and histological changes in a dose-dependent way. Thymol's antiinflammatory and antioxidative properties may contribute to its protective action. As a result, thymol may represent a promising medication for preventing aspirin-induced gastric, liver, and renal damage.
Keywords: Aspirin, Oxidative stress, Prostaglandin E2, Rats, Side effects, Thymol
1. Introduction
Acetylsalicylic acid (aspirin; ASA) is a nonsteroidal anti-inflammatory medication (NSAIDS) that lowers inflammatory symptoms and has a wide range of pharmacological effects including antipyretic, analgesic, and antiplatelet properties (Cadavid 2017). ASA exerts its therapeutic (analgesic and anti-inflammatory) and adverse (gastrointestinal ulcers) effects by inhibiting cyclooxygenase (COX), which leads to the inhibition of prostaglandin (PG) synthesis, which normally protects the stomach mucosa from damage, maintains kidney function, and aggregates platelets when needed (Vane and Botting 2003). Nevertheless, aspirin use is associated with severe morbidity and death because of its detrimental impacts on several organs, including the liver, stomach, and kidneys. Prolonged ASA use is linked to the development of gastrointestinal (GI) ulcers (the most popular side effect), hepatotoxicity, nephrotoxicity, and even renal cell carcinoma (Merchant and Modi, 2004, Vyas et al., 2016).
Herbal medicines are playing an increasing role in healthcare around the world, particularly in underdeveloped countries. This is primarily the result of the widespread perception that herbal medications have no negative effects and are inexpensive and readily available. Natural compounds have been utilized to reduce pharmacological adverse effects (Edrees et al., 2018). Thymol, 2-isopropyl-5-methyl phenol, is a colorless crystalline monoterpene phenol and is one of the main ingredients of thyme (Thymus vulgaris L.) and essential oils (Kowalczyk et al., 2020). Thymol exhibits a gastroprotective effect in acute and chronic ulcer models by increasing mucus and prostaglandin production (Ribeiro et al., 2016). Through anti-inflammatory, anti-apoptotic, and anti-oxidative mechanisms, thymol has the potential to ameliorate stomach mucosal damage caused by indomethacin (Koc et al., 2020). It also has anti-inflammatory (through decreasing cytokines and chemokines recruitment) and antioxidant properties (through scavenging of free radicals, augmenting the non-enzymatic and enzymatic antioxidants, and chelation of metal ions) (Nagoor Meeran et al., 2017), antimicrobial (through disruption of the bacterial plasma membrane's lipid portion, resulting in intracellular material leakage) (Trombetta et al., 2005), anticarcinogenic (via apoptosis induction, anti-proliferation, angiogenesis and migration inhibition by modulation of carcinogen metabolising enzyme activity) (Salehi et al., 2018), antispasmodic (via opposing Ca2+ activation and the ATP-dependent activity of the muscle) (Tamura and Iwamoto 2004), growth enhancer (via increasing antioxidant and digestive enzyme activity, as well as an improved immunological response, which may have a positive impact on health and performance) (Hashemipour et al., 2013), and immunomodulatory (via increasing the expression CD4, CD8, and Th1 cytokines through increased IFN- γ expression and increased interleukin-12 release (Chauhan et al., 2010). Furthermore, it exhibits hepatoprotective (Jafari et al., 2018), nephroprotective (Jamshidi and Taheri 2021), and cardioprotective properties (El-Marasy et al., 2020).
Based on this information, we evaluated the influence of thymol in mitigating aspirin-mediated gastric and hepato-renal injury. This was done by 1) evaluating gastric juice volume and pH as well as pepsin and prostaglandin E2 level; 2) measuring serum biochemical parameters and proinflammatory cytokines; 3) determining tissue oxidant/antioxidant status, and 4) identifying a link with gastric, hepatic and renal histopathological changes.
2. Material and methods
2.1. Acetylsalicylic acid
Acetylsalicylic acid (Ezacard®, 75 mg tablets) was obtained from Multipharma Company, Egypt. Thymol (>99.5%), as a white crystalline powder was provided by Sigma-Aldrich (St. Louis, MO, USA).
2.2. Animals and investigational protocol
The experiments were carried out using 48 mature male albino rats, 270–280 g, provided by the animal house. The animals were maintained in a conventional lab environment. They were given unlimited water and fed barley and milk. Before beginning the experiments, the animals were acclimatized for one week.
The experimental animals were weighed and haphazardly segregated into six equal groups.
Group I (control group): each rat was orally administered 0.5 mL of 0.2% Tween 80 solution for 10 successive days.
Group II (Th 100): animals were orally given thymol (100 mg/kg BW) (Koc et al., 2020) dissolved in 0.2% Tween 80 solution once daily for 10 days.
Group III (Th 200): animals were orally given thymol (200 mg/kg BW) (Koc et al., 2020) dissolved in 0.2% Tween 80 solution once daily for 10 successive days.
Group IV (ASA): rats were orally administered 0.2% Tween 80 solution for 7 days, then on the 8th day, they were orally administered acetylsalicylic acid (150 mg/kg BW) once daily for 3 successive days (Adefisayo et al., 2017). The rats were fasted before the ASA administration (Shah and Patel 2012).
Group V (Th100 + ASA) and Group VI (Th200 + ASA): rats were orally administered thymol (100 and 200 mg/kg BW), respectively, for 7 successive days, then on the 8th day, they were concurrently administered thymol and ASA for 3 consequent days.
2.3. Sampling
The rats were fasted overnight at the end of the study (10 days). They were weighed and sedated via intramuscular injection with a xylazine (5 mg/kg) and ketamine HCl (50 mg/kg) mixture (El‐Sheikh et al., 2021a, El-Sheikh et al., 2021b). A fine sterilized glass capillary tube was used to puncture the retroorbital venous plexus and collect blood samples. Before preparing serum, blood was collected into a sterile centrifuge tube without EDTA and left to coagulate at room temperature for 10 min. The resulting serum was maintained at −20 °C for the measurement of liver and kidney damage indicators and proinflammatory cytokines. The animals were then decapitated and their stomachs, livers, and kidneys were rapidly harvested for further examination. The liver, stomach, and one kidney were maintained at −20 °C until tissue homogenates were processed for screening of gastric, hepatic, and renal oxidant/antioxidant biomarkers as well as gastric prostaglandin E2 level. For the histological study, other sections of the liver and stomach, and the second kidney, were immersed in 10% neutral buffered formalin.
2.4. Measurement of gastric juice volume, pH, and pepsin activity
After euthanization, the stomach was immediately dissected by opening along - the greater curvature and the gastric content was emptied into a glass tube. To separate the aqueous phase, the stomach content was centrifuged at 3000 rpm for 10 min. A graduated cylinder was used to determine the amount of centrifuged gastric juice. Gastric pH was assessed by a pH meter (Adefisayo et al., 2017), whereas the technique of Rafsanjani and Vahedian (2004) was used to determine pepsin activity in the gastric secretion.
2.5. Evaluation of some biochemical parameters and proinflammatory cytokines
Renal injury biomarkers (creatinine and urea) and hepatic injury biomarkers [alkaline phosphatase (ALP), aspartate aminotransferase (AST), and alanine aminotransferase (ALT) concentrations were evaluated according to instructions of the kits (Randox diagnostic; London, UK), with Cat.No. for creatinine (CR2336), urea (UR446), ALP (AP9764), AST (AS3804), and ALT (AL146). Serum Tumor necrosis factor-α (TNF-α) and myeloperoxidase (MPO) concentrations were evaluated following the procedure of a specific rat ELISA kit (MyBioSource; California, USA.), with Cat.No. for TNF-α (MBS2507393) and MPO (MBS704859).
2.6. Evaluation of oxidant/antioxidant status and gastric prostaglandin E2 (PGE2) activity
One gram of the stomach, liver, and kidney tissue was homogenized to prepare a clear supernatant for the colorimetric determination of glutathione (GSH), malondialdehyde (MDA), superoxide dismutase (SOD), and glutathione peroxidase (GPx) levels using kits (Biodiagnostic Co., Cairo, Egypt.), with Cat. No for GSH (GR 25 11) (Beutler 1963), MDA. (MD 25 29) (Ohkawa et al., 1979), SOD (SD 25 21) (Nishikimi et al., 1972), and GPx (GP 2524) (Paglia and Valentine 1967). PGE2 level was measured in the stomach tissue homogenate using a rat ELISA kit (MyBioSource; California, USA, Cat.NO. MBS262150).
2.7. Histopathological investigations
At the completion of the investigational period, decapitation was used to sacrifice the rats. Representative stomach, liver, and kidney specimens from all of the animals were obtained following standardized necropsy procedures (Ruehl-Fehlert et al., 2003, Morawietz et al., 2004). To avoid folds in the gastric mucosa, the ingesta was removed, and the mucosae were cleaned carefully with normal saline solution, spread out, and fixed with pins. Next, all specimens were fixed in 10% neutral buffered formalin for 24 h, washed with distilled water, dehydrated in ascending grades of ethyl alcohol, cleared in UltraClear™ clearing agent, impregnated, and embedded in paraffin wax. After preparing 5 µm-thick sections, the slides were stained with hematoxylin and eosin, mounted with dibutyl phthalate polystyrene xylene (Suvarna et al., 2018), and examined by light microscopy. For lesion scoring, five randomly selected microscopic fields (40 objectives for the liver and kidneys and 10 objectives for the stomach; 40 images per organ per group) were imaged using an AmScope digital camera. Next, multiparametric quantitative lesion scoring was carried out on the images. For the hepatic lesions, the percentage of hepatocytes showing vacuolar and hydropic degeneration, swelling, pyknosis, and single-cell necrosis relative to the total numbers of hepatocytes/images, were counted instinctively. For the renal lesions, the percentages of renal tubules that showed vacuolar and hydropic degeneration, swelling, cytoplasmic acidophilia, pyknosis, single-cell necrosis, luminal debris, and or cast formation relative to the total numbers of tubules/images, were assessed subjectively. The other hepatic and renal and gastric lesions (necrosis, desquamation, erosions, ulcers, congestion, hemorrhage, and leukocytic infiltrations) were determined by calculating the % of lesion frequencies per image. Finally, the results were presented as the mean ± SE.
2.8. Statistical investigation
The data were shown as the mean SE for each group. For pairwise comparisons, a one-way analysis of variance was used followed by Duncan's multiple range post hoc test to examine the difference between groups. At p < 0.05, variations were considered statistically significant (Bewick et al., 2004).
3. Results
3.1. 3.1. Effects of pretreatment and concurrent administration of thymol on gastric juice volume, pH, and pepsin activity, along with gastric prostaglandin E2 activity of acetylsalicylic acid-treated rats
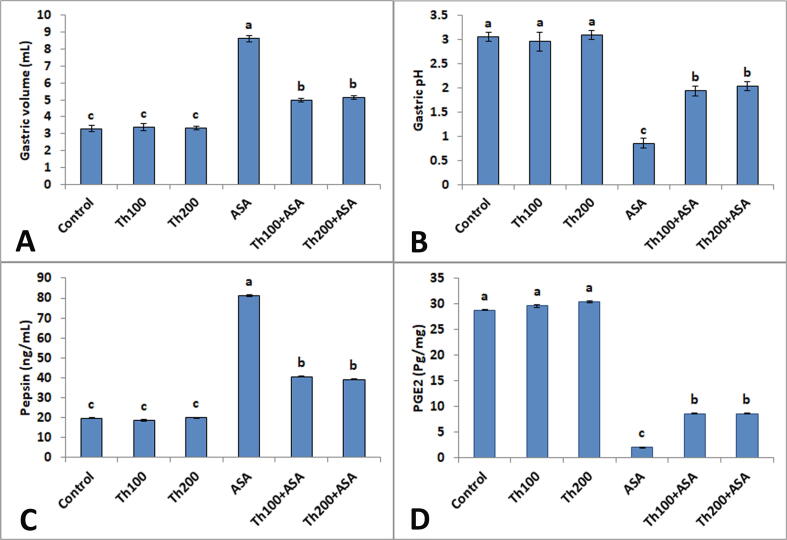
Daily oral administration of ASA (150 mg/kg BW) to adult male rats for 3 successive days induced a considerable (p < 0.05) rise in gastric juice volume (8.62 ± 0.2 mL) and pepsin activity (81.36 ± 0.5) as well as a considerable (p < 0.05) decline in gastric pH (0.86 ± 0.1) and PGE2 level (1.96 ± 0.1 pg/mg) compared with gastric juice volume (3.30 ± 0.2), pepsin activity (19.66 ± 0.2 ng/mL), gastric pH (3.06 ± 0.1) and PGE2 level (28.79 ± 0.10 pg/mg) in the control animals. While, pretreatment and simultaneous administration of thymol (100 or 200 mg/kg BW) with ASA produced a considerable (p < 0.05) decline in volume of gastric juice (4.96 ± 0.1 mL and 5.14 ± 0.1 mL) and pepsin activity (40.68 ± 0.3 and 39.26 ± 0.2 ng/mL) and a marked (p < 0.05) elevation in gastric pH (1.94 ± 0.1 and 2.04 ± 0.1) and PGE2 level (8.60 ± 0.1 and 8.52 ± 0.1 pg/mg) compared with the gastric juice volume (8.62 ± 0.2 mL), pepsin activity (81.36 ± 0.5 ng/mL), gastric pH (0.86 ± 0.1), and PGE2 level (1.96 ± 0.1 pg/mg) of ASA-treated rats (Fig. 1).
Fig. 1.
Effects of pre and concurrent administration of thymol on gastric juice volume (A), pH (B) and pepsin activity (C) as well as gastric prostaglandin E2 level (D) of Acetylsalicylic acid-treated rats. Values are represented as the mean ± SE (n = 5). Different letters (a, b, c) on the bars indicate statistically significant differences at p < 0.05.
3.2. Influences of pretreatment and concurrent administration of thymol (100 or 200 mg/kg BW) on some serum biochemical parameters of acetylsalicylic acid-treated rats
ASA-treated rats exhibited a considerable (p < 0.05) elevation in the level of serum ALT (66.40 ± 0.22 U/L), AST (95.58 ± 1.1U/L), ALP (104.60 ± 1.61 IU/L), urea (83.20 ± 1.1 mg/dL), and creatinine (2.67 ± 0.04 mg/dL) compared with ALT (18.74 ± 0.16 U/L), AST (24.06 ± 0.31 U/L), ALP (33.46 ± 0.12 IU/L), urea (21.06 ± 0.3 mg/dL), and creatinine (0.86 ± 0.01 mg/dL) in the control rats. However, pretreatment and concurrent administration of thymol (100 or 200 mg/kg BW) with ASA produced a substantial (p < 0.05) decline in the levels of serum ALT (41.11 ± 0.33 and 36.54 ± 1.17 U/L), AST (42.24 ± 0.20 and 35.94 ± 0.14 U/L), ALP (48.46 ± 0.31 IU/L and 42.72 ± 0.41 IU/L), urea (40 ± 0.5 and 42.28 ± 0.3 mg/dL) and creatinine (1.78 ± 0.01 and 1.62 ± 0.01 mg/dL) compared with ALT (66.40 ± 0.22 U/L), AST (95.58 ± 1.1 U/L), ALP (104.60 ± 1.61 IU/L), urea (83.20 ± 1.1 mg/dL) and creatinine (2.67 ± 0.04 mg/dL) in ASA-treated rats.
Pretreatment and concurrent treatment with thymol exhibited a dose-dependent reversal in the effects against aspirin-induced hepatic and renal dysfunction. Thus, thymol treatment significantly reduced aspirin-induced hepatic and renal dysfunction. Thymol at a dose of 200 mg /kg BW yielded better protection compared with thymol at a dose of 100 mg/kg BW, although both doses failed to maintain normal hepatic and renal function (Table 1).
Table 1.
Effects of oral administration Effects of pre and concurrent oral administration of thymol (100 or 200 mg/kg BW) on serum level of ALT, AST, ALP, creatinine, urea, TNFα and MPO of Acetylsalicylic acid-treated rats.
| Group |
Parameters |
||||||
|---|---|---|---|---|---|---|---|
| ALT (U/L) | AST (U/L) | ALP (IU/L) | Creatinine (mg/dl) | Urea(mg/dl) | TNFα (pg/ml) | MPO (ng/ml) | |
| Control | 18.74 ± 0.16d | 24.1 ± 0.31d | 33.46 ± 0.1d | 0.86 ± 0.01c | 21.06 ± 0.3d | 43.2 ± 0.6c | 9.07 ± 0.1c |
| Th100 | 18.66 ± 0.44d | 23.66 ± 0.1d | 33.8 ± 0.1d | 0.85 ± 0.01c | 21.80 ± 0.5d | 42.6 ± 0.6c | 9.22 ± 0.1c |
| Th200 | 18.61 ± 0.42d | 24.1 ± 0.13d | 33.8 ± 0.1d | 0.87 ± 0.01c | 20.9 ± 0.4d | 42.5 ± 0.8c | 9.55 ± 0.1c |
| ASA | 66.40 ± 0.22a | 95.58 ± 1.1a | 104.6 ± 1.6 a | 2.67 ± 0.04 a | 83.20 ± 1.1a | 176.4 ± 1.4a | 31.2 ± 0.2a |
| Th100 + ASA | 41.11 ± 0.33b | 42.24 ± 0.2b | 48.46 ± 0.3b | 1.78 ± 0.01b | 46.40 ± 0.5b | 56.6 ± 0.6b | 15.5 ± 0.1b |
| Th200 + ASA | 36.54 ± 1.2c | 35.94 ± 0.1c | 42.7 ± 0.4c | 1.62 ± 0.01b | 42.28 ± 0.3c | 55.4 ± 0.7b | 15.8 ± 0.1b |
Values are represented as the mean ± SE (n = 5). Mean values for the same parameter carrying different superscripts (a, b, c) are significantly different at p < 0.05. ALT: alanine aminotransferase; AST: aspartate aminotransferase; ALP: alkaline phosphatase; TNFα: tumor necrosis factor-alpha; MPO: myeloperoxidase.
3.3. Influences of pretreatment and concurrent administration of thymol (100 or 200 mg/kg BW) on inflammatory and oxidative stress biomarkers in acetylsalicylic acid-treated rats
Daily oral administration of ASA (150 mg/kg BW) to adult male rats for 3 successive days induced a considerable (p < 0.05) raise in serum TNF-α concentration (176.40 ± 1.36 pg/mL) and MPO activity (31.24 ± 0.2 ng/mL) compared with the serum TNF-α concentration (43.22 ± 0.34 pg/mL) and MPO activity (9.07 ± 0.1 ng/mL) of control animals. While, pretreatment and concomitant administration of thymol (100 or 200 mg/kg BW) with ASA encouraged a substantial (p < 0.05) decrease in TNF-α concentration (56.60 ± 2.65 and 55.40 ± 1.74 pg/mL) and MPO activity (15.46 ± 0.1 and 15.82 ± 0.1 ng/mL) compared with TNF-α concentration (176.40 ± 1.3 pg/mL) and MPO activity (31.24 ± 0.2 ng/mL) of ASA-treated rats.
Compared with control rats, ASA-treated rats exhibited a noticeable (p < 0.05) decline in GSH concentration, SOD, and GPx activities, besides a noticeable (p < 0.05) rise in MDA concentration in hepatic, renal, and gastric tissues. Pretreatment and concurrent administration of thymol with ASA caused a marked increase in GSH concentration, SOD, and GPx activities and a marked (p < 0.05) decline in MDA levels in hepatic, renal, and gastric tissues compared with that of ASA-treated rats (Table 2).
Table 2.
Effects of pre and concurrent oral administration of thymol (100 or 200 mg/kg BW) on gastric, hepatic and renal oxidant/antioxidant status of Acetylsalicylic acid-treated rats.
| Groups |
Parameters |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
GSH (mmol/g tissue) |
GPx (U/g tissue) |
SOD (U/g tissue) |
MDA(nmol/g tissue) |
|||||||||
| Stomach | Liver | Kidney | Stomach | Liver | Kidney | Stomach | Liver | Kidney | Stomach | Liver | Kidney | |
| Control | 156±1.3a | 158.8±1.1a | 152.2 ± 1.4a | 6.77 ± 0.1a | 3.56 ±0.02a | 6.15 ± 0.46a | 171.60 ± 1.07a | 125±1.81a | 213.6 ± 1.02a | 0.30 ± 0.02c | 0.55 ± 0.01b | 0.53 ± 0.01b |
| Th100 | 155 ± 1.7a | 153.6 ± 1.9a | 151.8 ± 1.4a | 6.86 ± 0.1a | 3.32 ± 0.01a | 8.26 ± 0.12a | 170.2 ± 1.01a | 125 ± 1.31a | 212.2 ± 1.15a | 0.31 ± 0.02c | 0.56 ± 0.01b | 0.51 ± 0.01b |
| Th200 | 157 ± 1a | 154.6 ± 1.4a | 155.4 ± 1.46a | 6.98 ± 0.1a | 3.42 ± 0.01a | 8.34 ± 0.1a | 172 ± 1.14a | 125.60 ± 1.02a | 213.6 ± 1.02a | 13.30 ± 0.1a | 0.55 ± 0.01b | 0.50 ± 0.01b |
| ASA | 11.73 ± 0.2d | 24.2 ± 0.9d | 14.1 ± 0.6d | 0.34 ± 0.1d | 0.26 ± 0.01c | 2.15 ± 0.02c | 33 ± 0.1d | 34.40 ± 0.50d | 22.60 ± 0.8d | 1.68 ± 0.1b | 2.84 ± 0.01a | 21.90 ± 1.08a |
| Th100 + ASA | 66.48 ± 1.4c | 105.6 ± 1.4c | 95.4 ± 1.4c | 2.9± 0.1c |
3.56 ±0.02a | 4.26 ± 0.03b | 116 ± 1.1c | 84.60 ± 1.01c | 106.2 ± 1.35b | 1.96 ± 0.1b | 0.55 ± 0.01b | 0.51 ± 0.01b |
| Th200 + ASA | 95.18 ± 1.5b | 135 ± 1.4b | 118.6 ± 1.2b | 4.86 ± 0.1b | 3.32 ± 0.01a | 5.73 ± 0.02b | 135.8 ± 1.2b | 96 ± 1.02b | 130.40 ± 1.63c | 0.30 ± 0.02c | 0.65 ± 0.01b | 0.36 ± 0.01b |
Values are represented as the mean ± SE (n = 5). Mean values for the same parameter carrying different superscripts (a, b, c) are significantly different at p < 0.05. GSH: reduced glutathione; GPx: glutathione peroxidase; MDA: Malodialdehyde.
3.4. Histopathological findings
3.4.1. Stomach
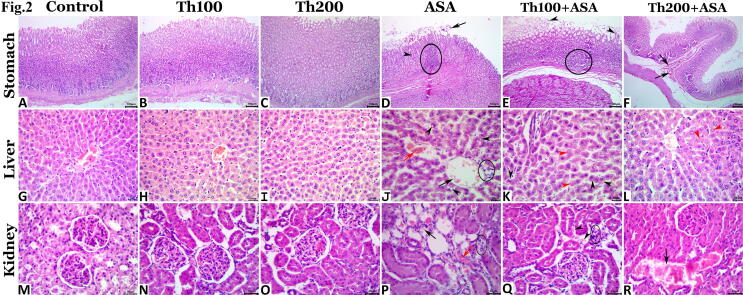
Light microscopic inspection of gastric tissue specimens exhibited normal histological architectures in the control, Th100-, and Th200-treated rats (Fig. 2A, B, and C), whereas the gastric mucosae of the aspirin-treated rats exhibited various morphological alterations including necrosis of the epithelial cells above the gastric pits and the upper parts of the gastric glands associated with widespread desquamation of these necrotic cells that sometimes extended to erosions of the surface epithelial lining. Minute hemorrhage, inflammatory cell infiltrate in the lamina propria and submucosa with vascular congestion were evident in most tissue specimens (Fig. 2 D). Treatment with thymol resulted in a dose-dependent restoration of the effects of the aspirin-induced gastric injury. Thus, thymol treatment considerably lowered the severity and frequency of aspirin-mediated lesions, and the 200 mg/kg BW dose exerted better protective effects compared with the 100 mg/kg BW dose, however, both doses failed to maintain the normal gastric histology. Minute hemorrhage, mild vascular congestion, focal inflammatory cell infiltrate, and epithelial desquamation were still noticed in the gastric mucosae of the Th100 + ASA- and Th200 + ASA-administered rats (Fig. 2 E and F). Lesion scoring for the gastric lesions is presented in Table 3.
Fig. 2.
A-F; Representative photomicrographs of the hematoxylin and eosin-stained gastric tissue sections showing normal histological pictures in the control (A), Th100-treated (B), and Th200-treated (C) rats. The aspirin-treated gastric tissue showing desquamated necrotic epithelial cells (arrow), minute hemorrhage (arrowhead) and inflammatory cell infiltrate in the lamina propria and submucosa (ellipse) (D). The Th100 + ASA-treated gastric tissue showing necrotic surface epithelium (arrow) and inflammatory cell infiltrate in the lamina propria and submucosa (ellipse) (E). The Th200 + ASA-treated gastric tissue showing submucosal vascular congestion (arrows) (F).Fig. 2. G-L; Representative photomicrographs of the hematoxylin and eosin-stained hepatic tissue sections showing normal histological pictures in the control (G), Th100-treated (H), and Th200-treated (I) rats. The aspirin-treated hepatic tissue showing congestion of the central vein (black arrow), sinusoidal dilatation (red arrow), vacuolar degeneration (black arrowheads), and very few numbers of leukocyte (ellipse) (J). The Th100 + ASA-treated hepatic tissue showing vacuolar degeneration (black arrowheads) and single-cell necrosis (red arrowheads) (K). The Th200 + ASA-treated hepatic tissue showing single-cell necrosis (red arrowheads) (L).Fig. 2. M−R; Representative photomicrographs of the hematoxylin and eosin-stained renal tissue sections showing normal histological pictures in the control (M), Th100-treated (N), and Th200-treated (O) rats. The aspirin-treated renal tissue showing vascular congestion (red arrow), RBCs casts (black arrow), and interstitial scanty leukocytic infiltration (ellipse) (P). The Th100 + ASA-treated hepatic tissue showing minute hemorrhages (arrowheads) with very few numbers of interstitial leukocytes (ellipse) (Q). The Th200 + ASA-treated renal tissue showing vascular congestion (arrow) (R).
Table 3.
Lesion scoring in the gastric, hepatic, and renal tissues of all groups.
| Organ | lesion | Control | Th100 | Th200 | ASA | Th100 + ASA | Th200 + ASA |
|---|---|---|---|---|---|---|---|
| Stomach | Necrosis of the surface epithelium | 0 ± 0d | 0 ± 0 d | 0 ± 0 d | 100 ± 0.0 a | 53 ± 2b | 25 ± 0.0c |
| Epithelial desquamation | 0 ± 0d | 0 ± 0 d | 0 ± 0 d | 100 ± 0.0 a | 26.60 ± 1.02b | 23 ± 0.94c | |
| Erosion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 25 ± 0.0 a | 0 ± 0b | 0 ± 0b | |
| Ulcer formation | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | |
| Hemorrhage | 0 ± 0b | 0 ± 0b | 0 ± 0b | 25 ± 0.0a | 0 ± 0b | 0 ± 0b | |
| Congestion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 100 ± 0.0a | 0 ± 0b | 0 ± 0b | |
| Leukocytic infiltration | 0 ± 0b | 0 ± 0b | 0 ± 0b | 25 ± 0.0 a | 0 ± 0b | 0 ± 0b | |
| Liver | Vacuolar degeneration | 0 ± 0c | 0 ± 0c | 0 ± 0c | 37.12 ± 1.28a | 2.94 ± 0.08b | 1.34 ± 0.06bc |
| Hydropic degeneration | 0 ± 0d | 0 ± 0 d | 0 ± 0 d | 6.80 ± 0.54a | 4.94 ± 0.3.5b | 1.16 ± 0.03c | |
| Pyknosis | 0 ± 0c | 0 ± 0c | 0 ± 0c | 3.53 ± 0.05a | 1.58 ± 0.03b | 0.96 ± 0.02b | |
| Single-cell necrosis | 0 ± 0c | 0 ± 0c | 0 ± 0c | 2.05 ± 0.10a | 1.39 ± 0.02b | 1.17 ± 0.02b | |
| Cholestasis | 0 ± 0b | 0 ± 0b | 0 ± 0b | 28 ± 1.37 a | 0 ± 0b | 0 ± 0b | |
| Central vein congestion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 92.66 ± 2.27a | 0 ± 0b | 0 ± 0b | |
| Portal congestion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 60.60 ± 3.21a | 0 ± 0b | 0 ± 0b | |
| Sinusoidal dilatation | 0 ± 0b | 0 ± 0b | 0 ± 0b | 92.40 ± 3.31a | 0 ± 0b | 0 ± 0b | |
| Leukocytic infiltration | 0 ± 0b | 0 ± 0b | 0 ± 0b | 49.20 ± 2.13a | 0 ± 0b | 0 ± 0b | |
| Kidney | Vacuolar degeneration | 0 ± 0c | 0 ± 0c | 0 ± 0c | 9.78 ± 1.23a | 5.44 ± 0.36b | 0 ± 0c |
| Hydropic degeneration | 0 ± 0c | 0 ± 0c | 0 ± 0c | 5.61 ± 0.43a | 1.59 ± 0.02b | 0 ± 0c | |
| Pyknosis | 0 ± 0c | 0 ± 0c | 0 ± 0c | 4.44 ± 0.31 a | 1.93 ± 0.01b | 0 ± 0c | |
| Single-cell necrosis | 0 ± 0c | 0 ± 0c | 0 ± 0c | 4.77 ± 0.10 a | 1.17 ± 00.1b | 0 ± 0c | |
| RBC cast | 0 ± 0b | 0 ± 0b | 0 ± 0b | 28 ± 1.37 a | 0 ± 0b | 0 ± 0b | |
| Non-cellular cast | 0 ± 0b | 0 ± 0b | 0 ± 0b | 21.55 ± 0.54a | 0 ± 0b | 0 ± 0b | |
| Interstitial congestion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 5.11 ± 0.33a | 0 ± 0b | 0 ± 0b | |
| Glomerular congestion | 0 ± 0b | 0 ± 0b | 0 ± 0b | 2.37 ± 0.02a | 0 ± 0b | 0 ± 0b | |
| Hemorrhage | 0 ± 0b | 0 ± 0b | 0 ± 0b | 1.68 ± 0.01a | 0 ± 0b | 0 ± 0b | |
| Leukocytic infiltration | 0 ± 0b | 0 ± 0b | 0 ± 0b | 1.69 ± 0.01a | 0 ± 0b | 0 ± 0b |
Values are represented as the mean ± SE (n = 5). Mean values for the same parameter carrying different superscripts (a, b, c) are significantly different at p < 0.05.
3.4.2. Liver
Normal histological images were observed in the hepatic tissue sections prepared from the control, Th100-, and Th200-administered rats (Fig. 2G, H, and I), whereas mild degenerative and circulatory alterations were observed in the aspirin-treated specimens. The basic alterations were cytoplasmic vacuolation of the hepatocytes with vacuolar or hydropic degeneration accompanied by varying degrees of vascular congestion and sinusoidal dilatation (Fig. 2J). Some sections exhibited nuclear pyknosis and single-cell necrosis with negligible leukocytic infiltration. No histological alterations were noticed in the biliary system; however, a few sections showed cholestatic changes. It was evident that the thymol hepatoprotective effects against aspirin-induced hepatopathy were dose-dependent, with the higher the dose (200 mg/kg BW) yielding better results. The hepatic tissue sections of the rats treated with Th100 + ASA still showed a few histological alterations including vacuolar degeneration, single-cell necrosis, and sinusoidal dilatation (Fig. 2K), whereas those treated with Th200 + ASA appeared almost normal, except for a few pyknotic hepatocytes which were sometimes accompanied with single-cell necrosis (Fig. 2L). Lesion scoring for the hepatic lesions is presented in Table 3.
3.4.3. Kidneys
Light microscopic inspection of renal tissue specimens exhibited normal histological architectures in the control, Th100-, and Th200-treated rats (Fig. 2M, N, and O). The renal tissues of the ASA-administered rats showed very mild degenerative and circulatory changes. The basic change was interstitial congestion, sometimes associated with minute hemorrhages with the existence of extravasated erythrocytes in the lumen of the renal tubules (Fig. 2P). Interstitial inflammatory infiltration was negligible in most specimens, whereas debris and cast luminal formation was a consistent finding. Few specimens showed tubular vacuolations with or without nuclear pyknosis. Thymol treatment ameliorated the aspirin-induced nephropathic changes, particularly at a dose of 200 mg/kg BW. The renal tissue sections of the Th100 + ASA-treated rats showed minute hemorrhages with very few numbers of interstitial leukocytes (Fig. 2Q), whereas the kidneys of the Th200 + ASA-treated rats appeared almost normal, although few sections manifested by vascular congestion (Fig. 2R). Lesion scoring for the renal lesions is summarized in Table 3.
4. Discussion
Aspirin is one of the world's most extensively used pharmaceutical products. It is extensively used as an anti-inflammatory and antipyretic agent for the management of joint and soft tissue inflammation, Kawasaki and acute rheumatic fever, and also prophylaxis of cardiovascular thrombotic diseases (Bouzenna et al., 2016). Aspirin at higher doses or repeated use over a long period may cause oxidative stress, which can lead to GI, hepatic, renal, and splenic problems (Bhattacharyya et al., 2014, Bouzenna et al., 2016, Mahmoud and Abd El-Ghffar, 2019). More emphasis has been placed on the preventive effects of natural herbal antioxidants against drug-induced side effects. Thymol exhibits many pharmacological properties, including gastroprotective, neuroprotective, hepatoprotective, and nephroprotective effects. Thymol's health benefits are achieved through the preservation of natural antioxidant defense networks and the suppression of inflammatory mediators such as cytokines and enzymes. Therefore, we evaluated the influence of thymol in mitigating aspirin-mediated gastric and hepato-renal injury.
The results showed that daily oral administration of ASA (150 mg/kg BW) to adult male rats for 3 successive days induced a significant increase in the volume of gastric juice, pepsin activity, and a decrease in gastric pH and prostaglandin E2 activity compared with the control group. Prostaglandins normally safeguard the GI mucosa by sustaining blood flow and boosting mucous and bicarbonate secretion. NSAIDs, such as aspirin, inhibit PG activity in the gastric mucosa, resulting in an elevation in stomach acid secretion and a reduction in mucus, bicarbonate secretion, and mucosal blood flow (Wallace 2008). A decrease in gastric pH indicates a decrease in H+ concentration in the gastric juice, which has been associated with the pathophysiology of ulcers and GI injury in lab animals (Adefisayo et al., 2017). The reduction of gastric pH leads to an increase in the activity of pepsin, which is considered to be the most damaging, aggressive enzyme of the gastrointestinal tract.
Previous studies have linked the inhibitory action of ASA on PG synthesis with free radical generation as an essential biochemical processe in the pathophysiology of gastric damage (Naito et al., 2001, Wang et al., 2011, Adefisayo et al., 2017). Our outcomes revealed that daily oral administration of ASA (150 mg/kg BW) to adult male rats for 3 successive days resulted in a significant (p < 0.05) decline in GSH concentration, SOD, and GPx activities, in addition to a significant (p < 0.05) elevation of MDA levels in gastric tissues compared with that of the control rats. In addition, the gastric mucosae of aspirin-treated rats showed various morphological alterations, including necrosis of the epithelial cells above the gastric pits and the upper parts of the gastric glands associated with widespread desquamation of necrotic cells that sometimes extended to erosion of the surface epithelial lining. In previous studies, a significant elevation in the volume of gastric juice and pepsin activity, a decrease in gastric pH and prostaglandin activity, as well as alteration of the oxidant/antioxidant status of gastric tissue, were demonstrated in aspirin-treated rats (Bouzenna et al., 2016, Adefisayo et al., 2017, Mohamed et al., 2019).
However, pretreatment and simultaneous administration of thymol (100 or 200 mg/kg BW) with ASA caused a significant increase in gastric pH and PGE2 activity and a decrease in the pepsin activity and volume of gastric juice compared with the ASA only-treated group. This improvement in the prostaglandin level is responsible for an increase in the protective factors to maintain te integrity of gastric mucosal. The increase in PG activity may lead to decreased gastric acid secretion, increased gastric pH, and subsequently, decreased pepsin activity. Furthermore, an increase in PG level in rats from the Th100 + ASA and Th200 + ASA groups may lead to enhancement of the gastric oxidant/antioxidant status, which is manifested by a significant elevation in GSH concentration, SOD, and GPx activities, besides a substantial decline in MDA level in gastric tissues compared with that of ASA only-treated rats. Furthermore, treatment with thymol resulted in dose-dependent rescue effects against aspirin-induced gastric injury. Thus, thymol treatment significantly reduced the severity and frequency of aspirin-mediated lesions. The 200 mg/kg BW dose yielded better protective effects compared with the 100 mg/kg BW dose.
Thymol is a phenolic compound with a strong capacity for scavenging oxidative radicals by giving protons from its phenolic hydroxyl groups (Michiels et al., 2010). According to an earlier study, thymol exhibited gastroprotective activities in acute and chronic ulcer models through various mechanisms, including an increase in PG levels, mucus secretion, and ATP-sensitive K+ channel activity (Ribeiro et al., 2016). Furthermore, lower doses of thymol (50, 100, and 200 mg/kg BW) exhibited gastroprotective activities in an acute ulcer model (25 mg/kg indomethacin) through various mechanisms, including an increase in PG activity as well as reduced inflammation, oxidant/antioxidant imbalance, and apoptosis (Koc et al., 2020).
Our findings show that ASA-treated rats exhibit hepatic and renal dysfunction as evidenced by a marked increase in serum AST, ALT, ALP (diagnostic markers of liver function), creatinine, and urea (diagnostic marker of renal function) levels compared with control rats. The biochemical alterations were confirmed by hepatic and renal histopathological findings in ASA-treated rats. A significant increase of serum ALT, AST, ALP, urea, and creatinine levels were observed in ASA-treated rats (Bouzenna et al., 2016, Asif and Malik, 2017). Other NSAIDs induce hepatic and renal dysfunction (Mostafa et al., 2020, Elshopakey and Elazab, 2021). Inhibition of prostaglandin synthesis by NSAIDs causes adverse renal effects ranging from electrolyte retention and decreased glomerular filtration to nephritic syndrome and chronic renal failure (Harirforoosh and Jamali 2009).
Inflammation and oxidative stress are involved in the pathophysiology of nephrotoxicity and hepatotoxicity induced by many drugs (Edrees et al., 2018, El‐Sheikh et al., 2021a, El-Sheikh et al., 2021b). Our results indicate that ASA treatment-induced inflammation and oxidant/antioxidant imbalance, which was manifested by a significant elevation of serum TNF-α (pro-inflammatory cytokine), MPO (A vital mechanistic link between inflammation and oxidation) (Mostafa et al., 2018), MDA (lipid peroxidation biomarker) concentration as well as a significant reduction in GSH levels (non-enzymatic antioxidant), SOD and GPx activities (enzymatic antioxidant). In the same context, aspirin and other NSAIDs induced inflammation and an oxidant/antioxidant imbalance (Koc et al., 2020, Mostafa et al., 2020).
Compared with ASA- only-treated rats, pretreatment and simultaneous thymol treatment with ASA significantly lowered serum ALT, ALP, AST, creatinine, and urea levels. The effect on hepatic and renal function was confirmed by hepatorenal histological improvement in Th100 + ASA- and Th200 + ASA-treated rats compared with that of ASA only-treated rats. It was evident that the thymol hepatoprotective and nephroprotective effects against aspirin-induced hepatopathic and nephropathic changes were dose-dependent. Thus, the higher the dose (200 mg/kg BW), the greater the effect. The biochemical and histological enhancements may be attributed to the antioxidant and anti-inflammatory activities which are manifested by a substantial reduction in serum TNF-α and MPO concentration, hepatorenal MDA concentration, along with a significant reduction in hepatorenal GSH concentration, SOD, and GPx activities in Th100 + ASA and Th200 + ASA-treated rats compared to ASA only-treated rats. The notable influences of thymol are primarily associated with its anti-inflammatory (through decreasing cytokines and chemokines recruitment) and antioxidant properties (through scavenging of free radicals, augmenting the non-enzymatic and enzymatic antioxidants, and chelation of metal ions) (Nagoor Meeran et al., 2017). Thymol inhibited myeloperoxidase activity and reduced the formation of ROS in human neutrophils (Pérez-Rosés et al., 2016). Our findings are consistent with those of Aboelwafa and Yousef (2015) who demonstrated that thymol significantly reduces oxidative stress injury caused by hydrocortisone in hepatic tissues (Aboelwafa and Yousef 2015). Additionally, the antioxidant effects of thymol significantly protected rats from nano titanium dioxide-induced hepatotoxicity (Jafari et al., 2018).
Thymol treatment markedly reduced liver injury induced by indomethacin administration, and this effect is associated with its antioxidant or anti-inflammatory action, as well as the induction of PGE2 (Geyikoglu et al., 2019). Thymol may be useful in preventing cisplatin-induced nephrotoxicity because of its antioxidant, anti-inflammatory, and anti-apoptotic properties (El-Sayed et al., 2015). Through its antioxidant properties, thymol can prevent mercury chloride-induced kidney damage at various dosages (Jamshidi and Taheri 2021).
5. Conclusion
In conclusion, our study found that administering thymol orally reduces aspirin-induced gastric, hepatic, and renal damage. Thymol's antiinflammatory and antioxidative properties may contribute to its protective action. As a result, thymol may represent a promising medication for preventing aspirin-induced gastric, liver, and renal damage.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Aboelwafa H.R., Yousef H.N. The ameliorative effect of thymol against hydrocortisone-induced hepatic oxidative stress injury in adult male rats. Biochem. Cell Biol. 2015;93(4):282–289. doi: 10.1139/bcb-2014-0154. [DOI] [PubMed] [Google Scholar]
- Adefisayo M.A., Akomolafe R.O., Akinsomisoye S.O., Alabi Q.K., Ogundipe O.L., Omole J.G., Olamilosoye K.P. Gastro-protective effect of methanol extract of vernonia amygdalina (del.) leaf on aspirin-induced gastric ulcer in wistar rats. Toxicol. Rep. 2017;4:625–633. doi: 10.1016/j.toxrep.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asif, S., Malik, L., 2017. Protective effects of nigella sativaon acetylsalicylic acid-induced nephrotoxicity in albino rats. J. Coll. Physicians Surg. Pak. [PubMed]
- Beutler, E., 1963. Improved method for the determination of blood glutathione. J. lab. clin. Med. [PubMed]
- Bewick V., Cheek L., Ball J. Statistics review 9: one-way analysis of variance. Critical Care (London, England) 2004 doi: 10.1186/cc2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S., Ghosh S., Sil P.C., Gnoni G.V. Amelioration of aspirin induced oxidative impairment and apoptotic cell death by a novel antioxidant protein molecule isolated from the herb phyllanthus niruri. PLoS ONE. 2014;9(2):e89026. doi: 10.1371/journal.pone.0089026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouzenna H., Dhibi S., Samout N., Rjeibi I., Talarmin H., Elfeki A., Hfaiedh N. The protective effect of citrus limon essential oil on hepatotoxicity and nephrotoxicity induced by aspirin in rats. Biomed. Pharmacother. 2016;83:1327–1334. doi: 10.1016/j.biopha.2016.08.037. [DOI] [PubMed] [Google Scholar]
- Cadavid A.P. Aspirin: the mechanism of action revisited in the context of pregnancy complications. Front. Immunol. 2017 doi: 10.3389/fimmu.2017.00261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan P.S., Satti N.K., Suri K.A., Amina M., Bani S. Stimulatory effects of cuminum cyminum and flavonoid glycoside on cyclosporine-a and restraint stress induced immune-suppression in swiss albino mice. Chem. –Biol. Interact. 2010;185(1):66–72. doi: 10.1016/j.cbi.2010.02.016. [DOI] [PubMed] [Google Scholar]
- Edrees N.E., Galal A.A.A., Abdel Monaem A.R., Beheiry R.R., Metwally M.M.M. Curcumin alleviates colistin-induced nephrotoxicity and neurotoxicity in rats via attenuation of oxidative stress, inflammation and apoptosis. Chem. –Biol. Interact. 2018;294:56–64. doi: 10.1016/j.cbi.2018.08.012. [DOI] [PubMed] [Google Scholar]
- El-Marasy S.A., El Awdan S.A., Hassan A., Abdallah H.M.I. Cardioprotective effect of thymol against adrenaline-induced myocardial injury in rats. Heliyon. 2020;6(7):e04431. doi: 10.1016/j.heliyon.2020.e04431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sayed, E., Abd‐Allah, A., Mansour, A., EL‐Arabey, A., 2015. Thymol and carvacrol prevent cisplatin‐induced nephrotoxicity by abrogation of oxidative stress, inflammation, and apoptosis in rats. J. Biochem. Mol. Toxicol. [DOI] [PubMed]
- El‐Sheikh S.M.A., Eleiwa N.Z., Khairy G.M., Abd El‐Aziz R.M., Metwally M.M.M., Galal A.A.A. Comparative effect of administration and discontinuation of sildenafil and/or clomipramine on the hepatic, cardiac and testicular tissues of male rats. Andrologia. 2021;53(4) doi: 10.1111/and.13983. [DOI] [PubMed] [Google Scholar]
- El-Sheikh S.M.A., Khairy M.H., Osama E., Metwally M.M.M., Galal A.A.A. Nanotechnology improves the therapeutic efficacy of gemcitabine against a human hepatocellular carcinoma cell line and minimizes its in vivo side effects. Naunyn-Schmiedeberg's Arch. Pharmacol. 2021;394(4):631–643. doi: 10.1007/s00210-020-02004-y. [DOI] [PubMed] [Google Scholar]
- Elshopakey G.E., Elazab S.T. Cinnamon aqueous extract attenuates diclofenac sodium and oxytetracycline mediated hepato-renal toxicity and modulates oxidative stress, cell apoptosis, and inflammation in male albino rats. Vet. Sci. 2021;8(1):9. doi: 10.3390/vetsci8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyikoglu, F., Yilmaz, E.G., Erol, H.S., Koc, K., Cerig, S., Ozek, N.S., Aysin, F., 2019. Hepatoprotective role of thymol in drug-induced gastric ulcer model. Ann. Hepatol. [DOI] [PubMed]
- Harirforoosh S., Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin. Drug Saf. 2009;8(6):669–681. doi: 10.1517/14740330903311023. [DOI] [PubMed] [Google Scholar]
- Hashemipour H., Kermanshahi H., Golian A., Veldkamp T. Effect of thymol and carvacrol feed supplementation on performance, antioxidant enzyme activities, fatty acid composition, digestive enzyme activities, and immune response in broiler chickens. Poultry Sci. 2013;92(8):2059–2069. doi: 10.3382/ps.2012-02685. [DOI] [PubMed] [Google Scholar]
- Jafari A., Rasmi Y., Hajaghazadeh M., Karimipour M. Hepatoprotective effect of thymol against subchronic toxicity of titanium dioxide nanoparticles: Biochemical and histological evidences. Environ. Toxicol. Pharmacol. 2018;58:29–36. doi: 10.1016/j.etap.2017.12.010. [DOI] [PubMed] [Google Scholar]
- Jamshidi H.R., Taheri F. The effect of thymol on renal toxicity induced by mercury chloride in rats. Int. J. Med. Lab. 2021 doi: 10.18502/ijml.v8i3.7335. [DOI] [Google Scholar]
- Koc K., Cerig S., Ucar S., Colak S., Bakir M., Erol H.S., Yildirim S., Hosseinigouzdagani M., Simsek Ozek N., Aysin F., Fehim Kocpinar E., Budak H., Geyikoglu F. Gastroprotective effects of oleuropein and thymol on indomethacin-induced gastric ulcer in sprague-dawley rats. Drug Chem. Toxicol. 2020;43(5):441–453. doi: 10.1080/01480545.2018.1530261. [DOI] [PubMed] [Google Scholar]
- Kowalczyk A., Przychodna M., Sopata S., Bodalska A., Fecka I. Thymol and thyme essential oil—new insights into selected therapeutic applications. Molecules. 2020;25(18):4125. doi: 10.3390/molecules25184125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmoud Y.I., Abd El-Ghffar E.A. Spirulina ameliorates aspirin-induced gastric ulcer in albino mice by alleviating oxidative stress and inflammation. Biomed. Pharmacother. 2019;109:314–321. doi: 10.1016/j.biopha.2018.10.118. [DOI] [PubMed] [Google Scholar]
- Merchant, M.A., Modi, D.N., 2004. Acute and chronic effects of aspirin on hematological parameters and hepatic ferritin expression in mice. Indian J. Pharmacol.
- Michiels J., Missotten J., Van Hoorick A.n., Ovyn A., Fremaut D., De Smet S., Dierick N. Effects of dose and formulation of carvacrol and thymol on bacteria and some functional traits of the gut in piglets after weaning. Arch. Anim. Nutr. 2010;64(2):136–154. doi: 10.1080/17450390903499915. [DOI] [PubMed] [Google Scholar]
- Mohamed W.A., Abd-Elhakim Y.M., Ismail S.A.A. Involvement of the anti-inflammatory, anti-apoptotic, and anti-secretory activity of bee venom in its therapeutic effects on acetylsalicylic acid-induced gastric ulceration in rats. Toxicology. 2019;419:11–23. doi: 10.1016/j.tox.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Morawietz G., Ruehl-Fehlert C., Kittel B., Bube A., Keane K., Halm S., Heuser A., Hellmann J. Revised guides for organ sampling and trimming in rats and mice – part 3: A joint publication of the rita1)and nacad2)groups. Exp. Toxicol. Pathol. 2004;55(6):433–449. doi: 10.1078/0940-2993-00350. [DOI] [PubMed] [Google Scholar]
- Mostafa R.E., El-Marasy S.A., Abdel Jaleel G.A., Bakeer R.M. Protective effect of royal jelly against diclofenac-induced hepato-renal damage and gastrointestinal ulcerations in rats. Heliyon. 2020;6(2):e03330. doi: 10.1016/j.heliyon.2020.e03330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mostafa R.E., Saleh D.O., Mansour D.F. Cisplatin-induced nephrotoxicity in rats: modulatory role of simvastatin and rosuvastatin against apoptosis and inflammation. JAPS. 2018 [Google Scholar]
- Nagoor Meeran M.F., Javed H., Al Taee H., Azimullah S., Ojha S.K. Pharmacological properties and molecular mechanisms of thymol: prospects for its therapeutic potential and pharmaceutical development. Front. Pharmacol. 2017 doi: 10.3389/fphar.2017.00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naito Y., Yoshikawa T., Yagi N., Matsuyama K., Yoshida N., Seto K., Yoneta T. Effects of polaprezinc on lipid peroxidation, neutrophil accumulation, and tnf-α expression in rats with aspirin-induced gastric mucosal injury. Dig. Dis. Sci. 2001 doi: 10.1023/a:1010716804594. [DOI] [PubMed] [Google Scholar]
- Nishikimi M., Appaji Rao N., Yagi K. The occurrence of superoxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem. Biophys. Res. Commun. 1972;46(2):849–854. doi: 10.1016/s0006-291x(72)80218-3. [DOI] [PubMed] [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Analytical. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Paglia D.E., Valentine W.N. Studies on the quantitative and qualitative characterization of erythrocyte glutathione peroxidase. J. Lab. Clin. Med. 1967 [PubMed] [Google Scholar]
- Pérez-Rosés R., Risco E., Vila R., Peñalver P., Cañigueral S. Biological and nonbiological antioxidant activity of some essential oils. J. Agric. Food. Chem. 2016;64(23):4716–4724. doi: 10.1021/acs.jafc.6b00986. [DOI] [PubMed] [Google Scholar]
- Rafsanjani F.N., Vahedian J. The effect of insulin-dependent diabetes mellitus on basal and distention-induced acid and pepsin secretion in rat. Diabetes Res. Clin. Pract. 2004 doi: 10.1016/j.diabres.2004.02.017. [DOI] [PubMed] [Google Scholar]
- Ribeiro A.R.S., Diniz P.B.F., Pinheiro M.S., Albuquerque-Júnior R.L.C., Thomazzi S.M. Gastroprotective effects of thymol on acute and chronic ulcers in rats: The role of prostaglandins, atp-sensitive k+ channels, and gastric mucus secretion. Chem. –Biol. Interact. 2016;244:121–128. doi: 10.1016/j.cbi.2015.12.004. [DOI] [PubMed] [Google Scholar]
- Ruehl-Fehlert C., Kittel B., Morawietz G., Deslex P., Keenan C., Mahrt C.R., Nolte T., Robinson M., Stuart B.P., Deschl U. Revised guides for organ sampling and trimming in rats and mice–part 1. Exp. Toxicol. Pathol. 2003;55(2-3):91–106. [PubMed] [Google Scholar]
- Salehi B., Mishra A.P., Shukla I., Sharifi-Rad M., Contreras M.D.M., Segura-Carretero A., Fathi H., Nasrabadi N.N., Kobarfard F., Sharifi-Rad J. Thymol, thyme, and other plant sources: Health and potential uses. Phytother. Res. 2018;32(9):1688–1706. doi: 10.1002/ptr.6109. [DOI] [PubMed] [Google Scholar]
- Shah J.S., Patel J.R. Anti-ulcer activity of lucer against experimentally induced gastric ulcers in rats. Ayu. 2012;33(2):314. doi: 10.4103/0974-8520.105260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna S.K., Layton C., Bancroft J.D. Oxford; Churchill Livingstone Elsevier: 2018. Bancroft’s Theory and Practice of Histological Techniques. [Google Scholar]
- Tamura T., Iwamoto H. Thymol: A classical small-molecule compound that has a dual effect (potentiating and inhibitory) on myosin. Biochem. Biophys. Res. Commun. 2004;318(3):786–791. doi: 10.1016/j.bbrc.2004.04.085. [DOI] [PubMed] [Google Scholar]
- Trombetta D., Castelli F., Sarpietro M.G., Venuti V., Cristani M., Daniele C., Saija A., Mazzanti G., Bisignano G. Mechanisms of antibacterial action of three monoterpenes. Antimicrob. Agents Chemother. 2005;49(6):2474–2478. doi: 10.1128/AAC.49.6.2474-2478.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vane J.R., Botting R.M. The mechanism of action of aspirin. Thromb. Res. 2003;110(5-6):255–258. doi: 10.1016/s0049-3848(03)00379-7. [DOI] [PubMed] [Google Scholar]
- Vyas A., Ram H., Purohit A., Jatwa R. Adverse effects of subchronic dose of aspirin on reproductive profile of male rats. J. Pharm. 2016;2016:1–9. doi: 10.1155/2016/6585430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace J.L. Prostaglandins, nsaids, and gastric mucosal protection: why doesn't the stomach digest itself? Physiol. Rev. 2008;88(4):1547–1565. doi: 10.1152/physrev.00004.2008. [DOI] [PubMed] [Google Scholar]
- Wang, Z., Hasegawa, J., Wang, X., Matsuda, A., Tokuda, T., Miura, N., Watanabe, T., 2011. Protective effects of ginger against aspirin-induced gastric ulcers in rats. Yonago Acta Medica. [PMC free article] [PubMed]