Abstract
The present study aimed to evaluate the impact of feeding peanut meal and linseed meal (LSM) with or without enzyme mixture on growth, plasma metabolites, muscle amino acid (AA) profile, nutrient digestibility, and expression of nutrient absorption-related genes in broilers. A total of 560 one-day-old Cobb-500 male broiler chicks were distributed into eight experimental treatments (7 replications of 10 chicks each) as follows: This study was designed by using 560 one-day-old Cobb-500 male broiler chicks were distributed into eight experimental groups (7 replications of 10 chicks each) to evaluate the differences in body weight, body weight gain, feed intake, feed conversion rate, carcass parts, blood biochemical and mRNA expression genes. Group 1 (C) control fed the basal diet without supplements, Group 2 (C + E) is control group fed on 350 g/ton enzyme mixture, Group 3 (C + PNM100) is control group fed 100 kg/ton peanut meal, Group 4 (C + E + PNM100) is a control group fed on 350 g/ton enzyme mixture and 100 kg/ton peanut meal, Group 5 (C + LSM100) is a control group fed on 100 kg/ton linseed meal, Group 6 (C + E + LSM100) is a control group fed on 350 g/ton enzyme mixture and 100 kg/ton linseed meal, Group 7 (C + PNM50 + LSM50) is control group fed on 50 kg/ton peanut meal and 50 kg/ton linseed meal. Group 8 (C + E + PNM50 + LSM50) is the control group fed on 50 kg/ton peanut meal and 50 kg/ton linseed meal. Each gram of the enzyme mixture contains 11,000 U Xylanase, 6000 U Cellulase, 700 U β-Mannanase, 1500 U Phytase, 5 mg α-Amylase, and 2 mg Protease. No differences in Bodyweight, Bodyweight gain, Feed intake, and carcass parts were noticed among experimental groups, while abdominal fat (%) and FCR were reduced (P < 0.05) in PNM50 + LSM50 + E and LSM100 groups. Plasma metabolites were not altered except total cholesterol, triglyceride, and LDL, reduced (P < 0.01) in treated birds. Dietary inclusion of 100 kg PNM or LSM reduced (P < 0.05) methionine concentration in muscle, while all remaining AA and ammonia concentrations were unaffected. Hepatic MDA contents were reduced (P < 0.001) in treated groups. Nutrient digestibility was not altered among groups except for protein digestibility, which was elevated (P < 0.05) in PNM50 + LSM50 + E, E, and PNM100 + E groups. The highest mRNA expressions of PepT1, APN, SGLT1, HMGCR, GHr, and IGF-1 genes were noticed in PNM50 + LSM50 + E. Conclusively, PNM and LSM can efficiently substitute corn and soybean meal in broiler diets, particularly when fortified with exogenous enzymes, without negative impacts on broiler performance.
Keywords: Peanut meal, Linseed meal, Growth performance, Muscle AAs profile, Gene expression, Broilers
1. Introduction
The limited amounts and continuous rising prices of high-quality feed ingredients are real challenges facing poultry producers. This enormous challenge remains the most significant determinant of profit margins in poultry production (Ibrahim et al., 2020). Soybean meal and corn are the most expensive ingredients in traditional poultry rations. Nevertheless, must be many African, Asian, and European countries. External fluctuation influences their prices (Abdel-Moneim, Sabic, et al., 2020). Therefore, constant evaluation of alternative and locally available feedstuffs is required to minimize feed costs.
Peanut is one of the legumes plants cultivated in tropical and subtropical regions mainly used as an oilseed, and its meal can be used as a protein source in animal diets (Sarbaz et al., 2018). Peanut meal (PNM) is considered fibrous since it contains about 10% crude fibre and multiple skins and shell residues (Davis and Dean, 2016). Due to the comprehensive extraction procedures, the residual oil content is highly variable, ranging from 3% or higher for a solvent-extracted meal to 10% in mechanically extracted cakes. More than 90% of the peanut oil's fatty acids (FAs) are unsaturated FAs, mainly linoleic and oleic (Bera et al., 2019). However, several disadvantages of incorporating PNM in broiler diets include a lack of lysine, threonine, and methionine (Piva et al., 1995, Zhang and Parsons, 1996) the risk of aflatoxin contamination (Kana et al., 2013). Mineral deficiency is another limiting factor for using PNM in broiler diets. However, mineral availability has been found to improve with the addition of phytase enzyme, increasing metabolizable energy. It's worth noting that PNM cannot be used as a single protein source in broiler diets, but when combined with other protein sources, it can be used efficiently at levels up to 15% (Costa et al., 2001, Diaw et al., 2010, Ghadge et al., 2009). Therefore, by balancing the diet and detoxifying peanut meal, the pre-existing restrictions can be removed (Driver et al., 2006).
Linseed is an oily crop that contains a considerable level of protein (25–35%) and a high source of ω-3 polyunsaturated fatty acids (PUFA) and produces linseed meal (LSM) as a by-product after oil extraction. However, several factors limit the incorporation of LSM in poultry diets in high proportions, including low content of lysine and methionine compared to other oilseeds, relatively high fibre content, and anti-nutritional factors (Iji et al., 2017). The presence of anti-nutritional factors, such as, e.g., cyanogenic glycosides, phytic acid, mucilage, anti-pyridoxine factor, allergens, and goitrogens, in PNM and LSM can bind to water and increase digesta viscosity. The high viscosity of digesta leads to the formation of a biofilm on the intestinal epithelium. It increases the fermentation processes and the proliferation of pathogenic microbes, elevating the risk of diseases and decreasing the productive performance of birds (Pirmohammadi et al., 2019, Rodriguez et al., 2001, Schöne et al., 1996). To the best of our knowledge, minimal studies have investigated the nutritional patterns of feeding PNM and LSM in the amino acid profile of broiler meat. It is worth noting that dietary protein is the primary source providing the essential amino acids required for muscle growth, tissue repair, and maintaining vital physiological functions (Toomer, Livingston, et al., 2020). Plant or animal protein sources can be used to formulate the diets; however, plant-based feedstuffs are typically included in broiler diets because of their availability and low costs (Babatunde et al., 2021).
Fortunately, recent studies have reported that adding exogenous enzymes to the diets containing linseed or peanut meal improved birds' growth performance, nutrient digestibility, and gut health (Driver et al., 2006, Pirmohammadi et al., 2019). However, limited studies have investigated the dietary incorporation impacts of PNM and LSM with exogenous enzymes on broiler performance and nutrient digestibility. Therefore, and for the first time, this study was conducted to evaluate the effects of feeding PNM and LSM with or without enzyme mixture on muscle amino acids profile and expression of nutrients absorption related genes in broilers.
2. Material and methods
2.1. Et hical statement
The study was approved by the Ethics Committee of Local Experimental Animals Care Committee and conducted following Kafrelsheikh University, Egypt (Number 4/2016 EC) guidelines. All precautions were followed to decrease suffering during the entire experimental period.
2.2. Birds and experimental design
Five hundred sixty one-day-old male Cobb-500 broiler chicks (average initial weight of 41 ± 0.6 g) were allocated into eight experimental treatments (7 replications of 10 chicks each). Birds were housed in floor pens littered with wood shavings and supplied with a chain feeder and automatic nipple cup drinker systems. All birds were reared under the same uniform management conditions. The experimental diets were fed on three phases (starter, 0–10 d; grower, 11–24 d; and finisher, 25–35d), isocaloric and isonitrogenous and formulated to meet or exceed the nutrient requirements of Cobb-500 following the guidelines of the strain (Table 1). The eight experimental diets were as follows: Group 1 (C) control fed the basal diet without supplements, Group 2 (C + E) is control group fed on 350 g/ton enzyme mixture, Group 3 (C + PNM100) is control group fed 100 kg/ton peanut meal, Group 4 (C + E + PNM100) is a control group fed on 350 g/ton enzyme mixture and 100 kg/ton peanut meal, Group 5 (C + LSM100) is a control group fed on 100 kg/ton linseed meal, Group 6 (C + E + LSM100) is a control group fed on 350 g/ton enzyme mixture and 100 kg/ton linseed meal, Group 7 (C + PNM50 + LSM50) is control group fed on 50 kg/ton peanut meal and 50 kg/ton linseed meal and Group 8 (C + E + PNM50 + LSM50). Feed and drinking water were offered ad libitum. Each gram of the enzyme mixture (Natuzyme, Bioproton Europe Oy, Finland) contains 11,000 U Xylanase, 6000 U Cellulase, and 700 U β-Mannanase (Trichoderma longibrachiatum), 1500 U Phytase (Escherichia coli), 5 mg α-Amylase (Bacillus subtilis), and 2 mg Protease (Aspergillus niger).
Table 1.
Composition of the experimental starter, grower, and finisher diets.
| Ingredient, g/kg |
Starter, (1–10 days) |
Grower, (11–25 days) |
Finisher, (26–35 days) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | PNM100 | LSM100 | PNM50 + LSM50 | Control | PNM100 | LSM100 | PNM50 + LSM50 | Control | PNM100 | LSM100 | PNM50 + LSM50 | |
| Yellow corn | 549.0 | 483.5 | 485.5 | 482.5 | 570.0 | 531.0 | 529.0 | 530.5 | 616.5 | 579.0 | 575.0 | 575.5 |
| Soybean meal, 46% | 360 | 332 | 360 | 348 | 358 | 280 | 312 | 296 | 305 | 226 | 259 | 244 |
| Corn gluten meal, 62% | 33 | – | – | – | 0 | – | – | – | 0 | – | – | – |
| PCM, 23% | – | 100 | – | 50 | – | 100 | – | 50 | – | 100 | – | 50 |
| LCM, 36% | – | – | 100 | 50 | – | – | 100 | 50 | – | – | 100 | 50 |
| Soya oil | 17.0 | 41.5 | 13.0 | 27.7 | 35.0 | 49.0 | 21.0 | 35.0 | 43.0 | 57.0 | 29.0 | 43.0 |
| Premix* | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| NaCl | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| NaCo3 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| Monocalcium phosphate | 15.8 | 16.2 | 16.0 | 16.0 | 14.0 | 14.4 | 14.0 | 14.0 | 12.8 | 12.8 | 13.0 | 13.0 |
| K2Co3 | 15.5 | 15.0 | 15.0 | 15.0 | 14.0 | 13.5 | 14.0 | 13.5 | 13.0 | 12.7 | 13.0 | 12.8 |
| L-Lysine HCl, 98% | 1.3 | 2.5 | 1.5 | 1.8 | 0.1 | 2.3 | 1.4 | 2.1 | 0.7 | 2.9 | 1.9 | 2.4 |
| Dl Methionine, 99% | 1.4 | 2.3 | 2.0 | 2.0 | 1.8 | 2.8 | 1.6 | 1.9 | 2.5 | 2.4 | 2.1 | 2.2 |
| Chemical analysis | ||||||||||||
| AME kcal/kg | 3000 | 3000 | 3000 | 3000 | 3100 | 3100 | 3100 | 3100 | 3200 | 3200 | 3200 | 3200 |
| Crude protein, % | 23 | 23 | 23 | 23 | 21 | 21 | 21 | 21 | 19 | 19 | 19 | 19 |
| Crude fat, % | 4.2 | 7.2 | 5.6 | 6.4 | 6.0 | 8.0 | 6.5 | 7.3 | 6.9 | 9.0 | 7.5 | 8.25 |
| Crude fiber, % | 3.2 | 3.6 | 3.0 | 3.0 | 2.4 | 3.0 | 3.0 | 3.0 | 2.4 | 3.0 | 2.8 | 2.9 |
| Digestible Lys, % | 1.44 | 1.44 | 1.44 | 1.44 | 1.29 | 1.29 | 1.29 | 1.29 | 1.19 | 1.19 | 1.19 | 1.19 |
| Digestible Met, % | 0.56 | 0.56 | 0.56 | 0.56 | 0.55 | 0.55 | 0.55 | 0.55 | 0.53 | 0.53 | 0.53 | 0.53 |
| Digestible Met + Cys, % | 0.94 | 0.94 | 0.94 | 0.94 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 | 0.90 |
| Digestible Thr, % | 0.94 | 0.94 | 0.94 | 0.9 | 0.87 | 0.8 | 0.8 | 0.8 | 0.79 | 0.8 | 0.8 | 0.8 |
| Ca, % | 0.97 | 0.97 | 0.97 | 0.97 | 0.87 | 0.87 | 0.87 | 0.87 | 0.81 | 0.81 | 0.81 | 0.81 |
| Available P, % | 0.48 | 0.48 | 0.48 | 0.48 | 0.43 | 0.43 | 0.43 | 0.43 | 0.40 | 0.40 | 0.40 | 0.40 |
| Cl, % | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Na, % | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 | 0.16 |
* Hero mix® (Hero pharm, Cairo, Egypt). Composition (per 3 kg): Vitamin A 12,000,000 IU, vitamin D3 2,500,000 IU, vitamin E 10,000 mg, vitamin K3 2000 mg, vitamin B1 1000 mg, vitamin B2 5000 mg, vitamin B6 1500 mg, vitamin B12 10 mg, niacin 30,000 mg, biotin 50 mg, folic acid 1000 mg, pantothenic acid 10,000 mg, manganese 60,000 mg, zinc 50,000 mg, iron 30,000 mg, copper 4000 mg, iodine 300 mg, selenium 100 mg, and cobalt 100 mg. There no any feed additive added to the diets. Peanut cake meal (PCM); Linseed cake meal (LCM).
2.3. Nutrients composition of peanut and linseed meals
The chemical analysis of PNM and LSM (Table 2) was conducted at the feed analysis laboratory of Kafrelsheikh University, Egypt, following the AOAC International procedures (AOAC, 2003). The metabolizable energy of PCM and LCM has calculated the following equation: Metabolizable energy = 26.7 × dry matter (%) + 77 × ether extract (%) – 51.22 × crude fibre (%) (Saleh, El-Awady, et al., 2021).
Table 2.
Chemical analysis of peanut meal and linseed meal as dry matter (%) basis.
| Nutrients | Peanut meal | Linseed meal |
|---|---|---|
| Moisture, % | 7.30 | 8.70 |
| Metabolizable energy, kcal/kg | 3670 | 1850 |
| Crude protein, % | 23.00 | 36.00 |
| Crude fiber, % | 11.40 | 8.50 |
| Crude fat, % | 21.00 | 8.70 |
| Lysine, % | 0.30 | 0.40 |
| Methionine, % | 0.20 | 0.15 |
| Calcium, % | 0.10 | 0.20 |
| Phosphorus, % | 0.10 | 0.10 |
| Aflatoxin, mg/kg | 7.30 | 3.10 |
| Ochratoxin, mg/kg | 2.00 | 1.70 |
2.4. Growth performance, carcass parts, and sampling
At 35 d, all birds were individually weighed, and final body weight (BW), weight gain (WG), and feed intake (FI) were recorded on a replication basis. The feed conversion ratio was calculated as .
Seven birds per group (1 bird/replicate; 56 birds total) were randomly chosen, slaughtered, and dissected to evaluate carcass parts. Fresh weights of the hot carcass, breast and thigh muscles, heart, gizzard, spleen, liver, and abdominal fat were recorded and expressed as relative weights to live body weight (Abdel-Moneim et al., 2020b, Abdel-Moneim et al., 2020c).
Blood samples were gathered into heparinized test tubes for biochemical analysis at slaughtering. Samples of the liver, superficial pectoral muscle, and ileum were collected appropriately for further biochemical and real-time PCR analyses (Saleh, Shukry, et al., 2021).
2.5. Plasma biochemical analysis
To separate plasma, blood samples were centrifuged at 4 °C for 20 min (3000g), and collected samples were stored at −20 °C pending analysis. Using spectrophotometric analysis and following the manufacturer instructions, triglycerides, total cholesterol, high- and low-density lipoprotein (HDL and LDL), glucose, albumin, total protein, creatinine, uric acid, alanine, and aspartate transaminases (ALT and AST) were calorimetrically measured using commercial kits (Diamond Diagnostics, Egypt).
2.6. Muscle amino acids profiles and hepatic MDA
Collected muscle samples were used to analyze the amino acid using gas-liquid chromatography (GLC) following the procedure described by Ahmed Ali Saleh (2013). In brief, after homogenizing 10 g of tissue in 40 mL of 0.1 N HCl for 45 s at 4 °C, the sample was centrifuged at 15,000 g for one hour minutes at 4 °C Celsius. Filtration and analysis of the supernatants were carried out (GC-4 CM-PFE, Shimadzu gas chromatograph, Tokyo, Japan) outfitted with a flame ionization detector (FID).
Concentrations of malondialdehyde (MDA) in hepatic tissues were also measured using a commercial test kit(Bio-Diagnostic kit, Giza, Egypt) according to Ohkawa et al. (1979). A 100 μl aliquot of tissue homogenate was combined with thiobarbituric acid reagent, incubated at 95 °C for 30 min, then centrifuged for 3 min at 10,000 r.p.m. At an absorbance level of 534 nm, the clear pink supernatant (thiobarbituric acid reactive products) was detected.
2.7. Nutrients digestibility
Four days before slaughtering, seven chicks per group were weighed, allocated individually in metabolic cages with free access to water and feed, and kept for 24 h adaptation period. Then, for three consecutive days, faeces samples were gathered. The final body weight of birds was recorded to ensure maintaining their weight. Diets and dried excreta were analyzed for crude fiber (#978.10), ether extract (#920.29), and crude protein (#954.01) following the (AOAC, 2003). According to Jacobsen et al. (1960), Faecal nitrogen was estimated using trichloroacetic acid.
2.8. Gene expression
RT-PCR was used to determine the mRNA expression of duodenal and hepatic genes. In brief, total RNA extraction was conducted using TRIzol reagent from roughly 50 mg of tissue (Invitrogen, Life Technologies, Carlsbad, CA, USA). The quantification was made using Nanodrop for a cDNA synthesis package; RNA samples with an A260/A280 ratio of 1.8 or greater were used to synthesize DNA. They used a cDNA synthesis package (Fermentas, Waltham, MA, United States). The SYBR Green master mix was used with the primers to replicate cDNA given in Table 8. GAPDH is used as a housekeeping gene using 2 − ΔΔT methods (Livak and Schmittgen, 2001). The primers used are listed in Table 8.
Table 8.
Primers sequences and target genes for SYBR green RT-PCR.
| Gene | Forward | Reverse | Accession number |
|---|---|---|---|
| PepT1 | CCCCTGAGGAGGATCACTGTTGGCAGTT | CAAAAGAGCAGCAGCAACGA | NM_204365 |
| APN | AATACGCGCTCGAGAAAACC | AGCGGGTACGCCGTGTT | NM_204861 |
| SGLT1 | GCCATGGCCAGGGCTTA | CAATAACCTGATCTGTGCACCAGTA | XM_415247 |
| GHr | GATCCACCACCAACAGCAGA | TGTCGCTTGCACTGAAGTCT | AH007350.1 |
| IGF-I | GGTGCTGAGCTGGTTGATGC | CGTACAGAGCGTGCAGATTTAGGT | JN942578 |
| SOD1 | ATTACCGGCTTGTCTGATGG | CCTCCCTTTGCAGTCACATT | NM205064.1 |
| CAT | ACTGCAAGGCGAAAGTGTTT | GGCTATGGATGAAGGATGGA | NM001031215.1 |
| HMGCR | TGTTGTAAGGCTGCCCTCTG | TAGGCGGGCAAACCTACTTG | NM_204485.2 |
| FAS | GGTCAGTGCTGCACGAAAT | CATCTCATACACTCGTCCAAATC | NM_001199487.1 |
| GAPDH | CAGGCTTATCCCGTTTAGCA | AGTCGGAGTCAACGGATTTG | NM_204305 |
PepT1, peptide transporter 1; APN, aminopeptidase N; SGLT1, Na + -dependent glucose transporter1; IGF-I, insulin-like growth factor 1; GHr, Growth hormone receptor; SOD1, superoxide dismutase 1; CAT, catalase; HMGCR, 3-hydroxy-3-methylglutaryl-coenzyme A reductase; FAS, fatty acid synthase; GAPDH, glyceraldehyde-3-phosphate dehydrogenase.
2.9. Statistical analysis
The collected data were analyzed following the completely randomized design using SPSS statistical software version 26 (IBM SPSS stats for Windows Armonk, NY: IBM Corp). Tukey's multiple comparison test distinguished the significant differences among means at P < 0.05.
3. Results
3.1. Growth performance and carcass parts
The effects of dietary incorporation of PNM and LSM with or without enzyme mixture on growth performance of broiler chickens at 35 d of age are presented in Table 3. No significant differences were observed among experimental groups in the BW, WG, and FI. FCR was reduced (P < 0.05) in PNM50 + LSM50 + E and LSM100 groups compared to the control group. The PNM50 + LSM50 + E group recorded the highest BW and WG and the lowest FCR.
Table 3.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on growth performance of broilers at 35 days of age.
| Item |
Experimental diets1 |
SEM | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | PNM100 | PNM100 + E | LSM100 | LSM100 + E | PNM50 + LSM50 | PNM50 + LSM50 + E | |||
| Final body weight, g | 2016.71ab | 2067.29ab | 1966.14b | 1995.86b | 1989.86b | 2016.71ab | 1980.71b | 2160.43a | 33.500 | 0.0039 |
| Body weight gain, g/period | 1974.33ab | 2024.94ab | 1923.76b | 1953.53b | 1947.54b | 1974.33ab | 1938.34b | 2118.10a | 33.505 | 0.0039 |
| Feed intak, g/period | 3346.07 | 3222.40 | 3224.14 | 3255.43 | 3242.14 | 3262.00 | 3213.14 | 3220.93 | 39.049 | 0.3079 |
| FCR, g feed/g gain | 1.66a | 1.56ab | 1.65ab | 1.63ab | 1.503b | 1.62ab | 1.62ab | 1.49b | 0.035 | 0.0230 |
a-b Means in each row with different superscripts are significantly different at P < 0.05. SEM; standard error of mean. 1C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
Feeding on PNM and LSM with or without enzyme mixture did not affect the relative weights of carcass, breast and thigh muscles, liver, spleen gizzard, and heart among experimental groups (Table 4). The relative weight of abdominal fat was decreased (P < 0.01) in LSM100 (0.553) and PNM50 + LSM50 + E (0.642) groups compared to the control group.
Table 4.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on carcass parts of broilers at 35 days of age.
| Item |
Experimental Diets1 |
SEM | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | PNM100 | PNM100 + E | LSM100 | LSM100 + E | PNM50 + LSM50 | PNM50 + LSM50 + E | |||
| Carcass, % | 61.51 | 61.91 | 61.95 | 62.75 | 63.07 | 63.39 | 62.43 | 63.34 | 0.942 | 0.7797 |
| Breast muscle, % | 23.72 | 23.22 | 23.45 | 23.54 | 23.93 | 23.34 | 23.22 | 23.39 | 0.825 | 0.9986 |
| Thigh muscle, % | 16.89 | 16.85 | 16.67 | 16.80 | 16.16 | 16.13 | 16.48 | 16.18 | 0.595 | 0.9052 |
| Gizzard, % | 1.53 | 1.45 | 1.61 | 1.53 | 1.60 | 1.63 | 1.58 | 1.63 | 0.064 | 0.4585 |
| Heart, % | 0.535 | 0.591 | 0.583 | 0.588 | 0.508 | 0.525 | 0.503 | 0.502 | 0.042 | 0.5199 |
| Spleen, % | 0.163 | 0.185 | 0.205 | 0.175 | 0.182 | 0.190 | 0.180 | 0.197 | 0.016 | 0.7081 |
| Liver, % | 2.382 | 2.535 | 2.867 | 2.615 | 2.768 | 2.327 | 2.455 | 2.395 | 0.166 | 0.2487 |
| Abdominal fat, % | 1.027a | 0.683ab | 0.828ab | 0.670ab | 0.553b | 0.697ab | 0.702ab | 0.642b | 0.941 | 0.0096 |
a-b Means in each row with different superscripts are significantly different at P < 0.05. SEM; standard error of mean. 1C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
3.2. Plasma biochemical analysis
As presented in Table 5, dietary supplementation of PNM and LSM with or without enzyme mixture did not alter plasma concentrations of HDL, total protein, creatinine, globulin, albumin, AST, ALT, uric acid, and glucose. However, total cholesterol, triglyceride, and LDL were reduced (P < 0.01) in all treated birds compared to the control group except for total cholesterol and LDL in the E group.
Table 5.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on blood biochemical analysis of broilers.
| Item |
Experimental Diets1 |
SEM | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | PNM100 | PNM100 + E | LSM100 | LSM100 + E | PNM50 + LSM50 | PNM50 + LSM50 + E | |||
| Lipid profile, mg/dl | ||||||||||
| Total cholesterol | 156.75a | 141.25ab | 125.50b | 127.50b | 130.25b | 133.25b | 133.25b | 125.50b | 3.678 | 0.0001 |
| Triglycerides | 26.00a | 15.75b | 18.00b | 17.75b | 13.75b | 14.50b | 13.50b | 14.75b | 1.287 | 0.0001 |
| LDL-cholesterol | 91.00a | 67.00ab | 48.25b | 48.25b | 51.50b | 66.00b | 51.00b | 47.00b | 7.180 | 0.0025 |
| HDL-cholesterol | 62.75 | 71.25 | 74.25 | 76.25 | 75.75 | 64.25 | 79.25 | 75.50 | 5.101 | 0.2688 |
| Protein fractions, g/dl | ||||||||||
| Total protein | 3.03 | 3.25 | 3.28 | 3.30 | 3.38 | 3.15 | 3.43 | 3.48 | 0.575 | 0.9995 |
| Globulin | 1.95 | 2.15 | 1.75 | 2.10 | 2.03 | 1.83 | 1.83 | 2.45 | 0.246 | 0.5577 |
| Albumin | 0.58 | 0.66 | 0.72 | 0.96 | 0.76 | 0.68 | 0.80 | 0.51 | 0.125 | 0.3333 |
| Hepatic and renal function biomarkers, mg/dl | ||||||||||
| Creatinine | 0.35 | 0.34 | 0.35 | 0.36 | 0.37 | 0.34 | 0.35 | 0.37 | 0.025 | 0.9786 |
| ALT | 3.25 | 3.18 | 3.33 | 3.28 | 3.38 | 3.33 | 3.23 | 3.05 | 0.309 | 0.9970 |
| AST | 284.00 | 284.50 | 271.25 | 284.00 | 264.75 | 284.75 | 275.75 | 284.25 | 23.931 | 0.9976 |
| Uric acid | 2.98 | 2.80 | 2.93 | 2.68 | 2.93 | 2.80 | 2.60 | 2.78 | 0.630 | 0.9999 |
| Glucose, mg/dl | 119.50 | 117.75 | 118.00 | 125.75 | 119.50 | 118.75 | 122.25 | 123.50 | 10.640 | 0.9992 |
a-b Means in each row with different superscripts are significantly different at P < 0.05. SEM; standard error of mean. 1C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
3.3. Muscle amino acids profiles and hepatic MDA
Respective to the effects of the feeding PNM and LSM with or without enzyme mixture on amino acids profile of broilers' superficial pectoral muscle is presented in Table 6. Dietary inclusion of 100 kg of PNM or LSM reduced (P < 0.05) methionine concentration, 4.867 and 4.833, respectively in muscle compared to the control group. Hepatic MDA contents were significantly reduced (P < 0.001) in all treatment groups compared to the control one (Table 7). Concentrations of all remaining essential and non-essential amino acids and ammonia were not significantly affected by all dietary supplements.
Table 6.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on amino acids (AA) profile and ammonia of superficial pectoral muscle in broilers.
| Item |
Experimental Diets1 |
SEM | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | PNM100 | PNM100 + E | LSM100 | LSM100 + E | PNM50 + LSM50 | PNM50 + LSM50 + E | |||
| Essential AA, mg/100 g protein | ||||||||||
| Lys | 10.200 | 10.000 | 10.767 | 11.200 | 10.133 | 10.500 | 10.833 | 10.300 | 0.262 | 0.0641 |
| Ile | 10.267 | 10.500 | 11.167 | 10.967 | 10.200 | 9.867 | 11.467 | 11.467 | 0.420 | 0.1000 |
| Leu | 2.700 | 2.667 | 2.700 | 2.633 | 2.533 | 2.300 | 2.600 | 2.800 | 0.189 | 0.7255 |
| Val | 2.067 | 1.967 | 1.800 | 1.733 | 1.533 | 1.967 | 1.867 | 1.767 | 0.159 | 0.3997 |
| Thr | 2.033 | 2.533 | 2.367 | 2.233 | 2.700 | 2.500 | 2.533 | 2.833 | 0.243 | 0.4147 |
| Phe | 0.767 | 0.800 | 0.867 | 0.900 | 0.767 | 0.833 | 0.867 | 1.033 | 0.079 | 0.3497 |
| Met | 5.567a | 5.267ab | 4.867b | 4.933ab | 4.833b | 4.967ab | 5.300ab | 5.200ab | 0.132 | 0.0129 |
| Cys | 8.333 | 7.533 | 8.367 | 8.567 | 8.100 | 8.733 | 7.600 | 8.267 | 0.325 | 0.1719 |
| His | 6.433 | 6.233 | 6.000 | 6.433 | 6.433 | 6.433 | 6.267 | 6.300 | 0.191 | 0.7167 |
| Non-essential AA, mg/100 g protein | ||||||||||
| Arg | 8.933 | 8.500 | 8.833 | 8.567 | 7.900 | 8.333 | 8.000 | 8.333 | 0.395 | 0.5669 |
| Glu | 12.067 | 13.200 | 12.300 | 11.700 | 16.100 | 13.833 | 12.167 | 13.433 | 1.002 | 0.1154 |
| Ser | 0.733ab | 0.833ab | 0.700b | 0.667b | 1.400a | 0.967ab | 1.233a | 0.833ab | 0.158 | 0.0408 |
| Asp | 8.267 | 8.033 | 7.700 | 7.067 | 8.767 | 8.467 | 8.367 | 7.700 | 0.394 | 0.1388 |
| Gly | 4.200 | 4.500 | 4.600 | 4.767 | 4.333 | 4.167 | 3.900 | 4.467 | 0.195 | 0.1213 |
| Tyr | 4.533ab | 4.167ab | 4.233ab | 4.167ab | 4.033ab | 4.900ab | 3.667b | 3.667ab | 0.225 | 0.0449 |
| Ala | 8.433 | 9.733 | 8.700 | 9.200 | 6.700 | 7.133 | 10.033 | 7.733 | 0.829 | 0.1043 |
| Ammonia, mg/ 100 g protein | 3.700ab | 3.600ab | 3.900ab | 4.267a | 3.400ab | 3.367ab | 3.300b | 3.900ab | 0.192 | 0.0337 |
a-b Means in each row with different superscripts are significantly different at P < 0.05. SEM; standard error of mean. 1C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
Table 7.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on nutrients digestibility and hepatic MDA content of broilers.
| Item |
Experimental Diets1 |
SEM | P-Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C | E | PNM100 | PNM100 + E | LSM100 | LSM100 + E | PNM50 + LSM50 | PNM50 + LSM50 + E | |||
| Liver MDA, g/100 g bw | 13.047a | 10.007bc | 6.433d | 5.093d | 10.443b | 9.940bc | 7.463 cd | 7.660 cd | 0.833 | 0.0001 |
| Crude protein digestibility, % | 62.667c | 65.667a | 63.667abc | 65.667ab | 63.000bc | 65.000abc | 64.667abc | 66.000a | 0.726 | 0.0348 |
| Crude fiber digestibility, % | 23.000 | 24.333 | 24.000 | 23.667 | 23,667 | 24.667 | 24.333 | 23.333 | 1.000 | 0.9362 |
| Crude fat digestibility, % | 17.000 | 19.000 | 17.000 | 21.333 | 20.000 | 19.667 | 21.000 | 19.000 | 1.149 | 0.1203 |
a-d Means in each row with different superscripts are significantly different at P < 0.05. SEM, standard error of mean; MDA, malondialdehyde. 1C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
3.4. Nutrients digestibility
The effect of feeding PNM and LSM with or without enzyme mixture on the nutrient digestibility of broilers is summarized in Table 7. Digestibility coefficients of crude protein, fiber and fat were not significantly differ among experimental groups except for crude protein digestibility in PNM50 + LSM50 + E (66%), E (65.667%) and PNM100 + E (65.667%) groups, which was elevated (P < 0.05) compared to the control group.
3.5. Gene expression
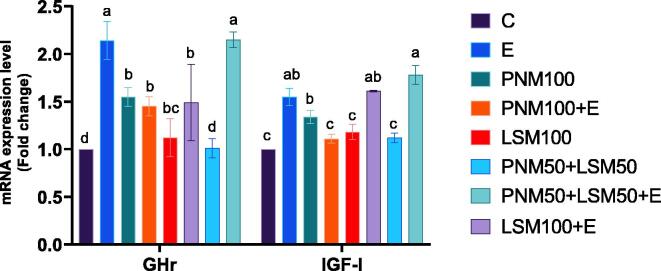
The mRNA expressions of PepT1, APN, SGLT1, and IGF-1 were significantly (P < 0.05) upregulated in E, LSM100 + E, and PNM50 + LSM50 + E groups compared to the remaining groups (Fig. 1, Fig. 2). The mRNA expression GHr was upregulated (P < 0.05) in all treated groups except the PNM50 + LSM50 group compared to the control (Fig. 2). HMGCR mRNA expression was elevated (P < 0.05) in all treated groups except for E and PNM50 + E groups (Fig. 3). There are no significant changes in SOD1, CAT, and FAS expression among the experimental groups (Fig. 3, Fig. 4). The highest mRNA expression of the genes mentioned above was recorded in the PNM50 + LSM50 + E group.
Fig. 1.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on mRNA expression of PepT1, APN and SGLT1. a-d Means with different superscripts are significantly different at P < 0.05. C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
Fig. 2.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on mRNA expression of GHr and IGF-I. a-d Means with different superscripts are significantly different at P < 0.05. C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
Fig. 3.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on mRNA expression of FAS and HMGCR. a-f Means with different superscripts are significantly different at P < 0.05. C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
Fig. 4.
Effect of feeding peanut meal (PNM) and linseed meal (LSM) with or without enzyme mixture on mRNA expression of SOD1, and, CAT. C = control, E = C + 350 g/ton enzyme mixture, PNM100 = C + 100 kg/ton PNM, PNM100 + E = C + E + PNM100, LSM100 = C + 100 kg/ton LSM, LSM100 + E = C + E + LSM100, PNM50 + LSM50 = C + 50 kg/ton PMN and 50 kg/ton LSM, PNM50 + LSM50 + E = C + E + PNM50 + LSM50.
4. Discussion
The present study revealed non-significant changes in growth performance indices and carcass traits when broilers fed PNM and LSM with or without enzyme mixture. The effects of feeding PNM and LSM on the growth of broiler chickens are diversified among studies. The variations among the results of earlier studies may depend on dietary inclusion level, chemical structure and percent of oil residue, and the concentrations of the anti-nutritional factors in both meals (Abdel-Moneim et al., 2020b, Mridula et al., 2011, Toomer et al., 2020a). In line with our findings, Mridula et al. (2011) reported that the growth performance of broiler chicks was not affected by dietary incorporation of 10% LSM while omega-3 fatty acid content in the meat yield was improved. Moreover, Meherunnisa et al. (2017) found that feeding 15% water-treated linseed meal can be used in broiler diets without any adverse effects. Contrarily, Toomer et al. (2019) demonstrated that broilers fed a high oleic-PNM diet recorded lower BW than the control group. In the present study, the highest BW and WG and the lowest FCR were recorded in the PNM50 + LSM50 + E group. This indicates that using various plant protein sources in poultry diets and exogenous enzymes efficiently enhances nutrient digestibility and improve utilization. This improvement might be attributed to the synergistic effect between different protein sources, which provide the essential amino acids required by the chicken growth without shortages or excesses (Pesti et al., 2003). Confirming this hypothesis, Driver et al. (2006) revealed that supplementation of an exogenous enzyme (phytase) to broilers' diet based on PNM increased their metabolizable energy. Musharaf and Latshaw (1991) showed that formulating a starter broiler diet (23% crude protein) composed of different protein sources, such as fish, meat, soybean, PNM, and sesame meals, resulted in an improvement in growth rate and FCR.
Furthermore, dietary inclusion of 0.10% threonine to PNM based-diet improved WG and FCR of broilers compared to the control (Costa et al., 2001). The authors also documented that the source of protein in the broiler diet did not significantly affect the carcass part's weights. In the same line, Pekel et al. (2009) reported significant changes in carcass yield and weights of carcass and breast in broilers fed flaxseed-based diets. Nevertheless, Toomer et al., 2020a, Toomer et al., 2019 found that carcass parts weights were decreased by providing on high oil PNM compared to the other treatments. However, Sarbaz et al. (2018) noticed higher carcass weight and lower abdominal fat pad when birds were fed 50 g/kg peanut pod. The reduction in abdominal fat deposition might be attributed to the increasing omega-3 fatty acids in PNM and LSM and the balance of amino acids, especially lysine, in the broiler diet (Ajuyah et al., 1991).
Inclusion of PNM and LSM with or without enzyme mixture in broiler diets did not affect hepatic and renal function biomarkers in the plasma. The lack of differences among hepatic and renal function biomarkers indicates the absence of any adverse health effects of dietary supplements at tested levels on treated birds (Abd El-Moneim and Sabic, 2019). Nevertheless, the hypocholesterolemic impacts of these supplements were well-presented as plasma levels of total cholesterol, triglyceride, and LDL were reduced in treated birds. These results align with Toomer, Livingston, et al. (2020), who demonstrated that feeding on diets fortified with high-oleic PNM recorded the lowest total cholesterol content compared to the remaining groups. Similarly, fat and cholesterol contents of meat were reduced when 100 g/kg LSM for at least three weeks (Kumar et al., 2019).
Additionally, dietary inclusion of PNM at 5.3% and 10.6% reduced egg yolk concentration of cholesterol (Lu et al., 2013). The reduction in cholesterol and triglycerides levels could be attributed to the high contents of unsaturated fatty acids of oil residues in PNM and LSM (Bayrak et al., 2010, Bera et al., 2019), which contribute to reducing plasma cholesterol levels and increasing LDL turnover in the liver by increasing hepatic LDL receptor number (Fernandez and West, 2005). Another potential reason for the hypocholesterolemic effect of PNM and LSM is their high phytosterols content (Awad et al., 2000, Han et al., 2015), which have a similar chemical structure to animal cholesterol; leading to decreasing blood cholesterol (Abdel-Moneim, Selim, et al., 2020). Furthermore, previous studies showed that the Linseeds might produce viscous digestion, obstruct bile acid recycling and impair fat digestion, resulting in lower serum cholesterol and triglyceride levels (Kristensen et al., 2013). Moreover, soluble fibre intake can lower total and LDL cholesterol and triglycerides by increasing bile acid losses in the faeces (Maki et al., 1999).
In the present study, feeding 100 kg/ton of PNM or LSM reduced methionine concentration in muscle, while all remaining essential and non-essential amino acids and ammonia were not significantly affected by the dietary supplements. This might be due to the low methionine concentration in PNM and LSM. Although the amino acids profile of PNM and LSM is deficient in threonine, lysine, and methionine (Batal et al., 2005, Iji et al., 2017), rebalancing broiler diets with synthetic amino acids will become PNM and LSM comparable to conventional protein sources such as soybean meal. The findings of Toomer, Livingston, et al. (2020) are agreed with ours, as the authors reported that all amino acids profiles and ammonia content in breast muscle of birds fed high-oleic PNM were adequate and similar to the control except the concentrations of methionine and cysteine, which were reduced in treated birds. In the present study, the combination between 50 kg/ton of PNM and LSM, particularly with enzymes addition, overcomes the deficiencies in these amino acids. This improvement might be attributed to the aan dequate amount of PNM and LSM producing a more balanced diet by providing the essential amino acids required by the chicken growth without shortages or excesses.
To date, minimal studies examined the effect of PNM, LSM, or their combination with or without enzyme mixture on nutrient digestibility of broilers (Toomer et al., 2020a, Toomer et al., 2020b). Feeding on PNM and LSM with or without enzyme mixture did not affect the digestibility coefficients of crude fibre and crude fat in broiler chickens. Protein digestibility was improved only in PNM50 + LSM50 + E, E, and PNM100 + E groups. In a study by Rodriguez et al. (2001), the authors reported that dietary inclusion of LSM negatively affected amino acid digestibility. Toomer, Sanders, et al. (2020) said that apparent fat and protein digestibility did not alter by dietary incorporation of 10% high-oleic PNM. The authors revealed that treated birds recorded the highest apparent metabolizable energy, suggesting improved nutrient uptake. This could explain the elevation in protein digestibility in PNM50 + LSM50 + E, E, and PNM100 + E groups. The improvement in nutrient uptake could be attributed to the presence of phytochemicals (Abdel‐Moneim et al., 2020) and polyunsaturated fatty acids (Fernandez and West, 2005) in PNM and LSM, or the effect of enzyme mixture as a type of meal processing. Jahanian and Rasouli (2016) documented that applying some processing on the crude meals enhances the nutrient digestibility in treated birds.
Hepatic MDA contents were reduced in all treated groups compared to the control one in the current study. The reduction in lipid peroxidation in the liver could be attributed to the polyunsaturated fatty acids contents in oil residues in PNM and LSM. Polyunsaturated fatty acids can act as prooxidants and indirect antioxidants in vascular endothelial cells and reduce the production of reactive oxygen species and scavenge superoxide radicals leading to diminishing inflammation and cardiovascular diseases (Richard et al., 2008).
Uptake of nutrients, e.g., short peptides, glucose, galactose, fatty acids …etc., is a vital biological phenomenon responsible for growth and maintenance and regulated by several genes, including PepT1, APN, and SGLT1. These genes are predominantly expressed in the intestinal epithelial cells membrane and have tremendous nutritional importance (Aito‐Inoue et al., 2007). PepT1 and APN are responsible for absorbing short chain peptides and amino acids through the intestinal epithelial membrane and their transportation from the intestine lumen into the bloodstream. These genes play profound roles during absorptive and post-absorptive states, in the whole metabolism of nitrogen in the body (Christensen, 1990). SGLT1 is responsible for absorbing and transporting galactose and glucose across the intestinal epithelial brush-border membrane, where it is mainly located (Yamazaki et al., 2018). The abundance of these nutrient transporters in the gastrointestinal tract is a vital determinant of protein and carbohydrate absorption efficiency. In the present study, duodenal PepT1, APN, and SGLT1 were upregulated in groups supplemented with PNM and LSM with an exogenous enzyme mixture. The enzyme mixture used in this study contained xylanase, cellulase, β-mannanase, phytase, α-amylase, and protease, which are mainly responsible for carbohydrate and protein digestion. Zuo et al. (2015) reported that dietary supplementation of exogenous protease improved the efficiency of peptide and amino acids absorption and increased the mRNA expression of duodenal PepT1.
Both metabolites affect the growth and several functions in the body. GHr gene is responsible for the translation of growth hormone receptor protein which binds to growth hormone triggering a signalling process that stimulates the division and growth of cells. Primarily by hepatic cells, this signalling also leads to the production of IGF-1 (Ketelslegers et al., 1995). IGF-1 is used as a marker to evaluate the nutritional status, and its mRNA expression is regulated quantity and quality of dietary proteins (Miura et al., 1992). In the present study, GHr and IGF-1 were upregulated in birds treated with dietary supplements and exogenous enzymes. As mentioned earlier, the highest mRNA expression of the genes was recorded in the PNM50 + LSM50 + E group. This indicates the importance of the enzyme mixture to enhance the nutritional potential of PNM and LSM by elevating the available nutrients in the intestine lumen for absorption, which enhances the expression of nutrients transportation- and growth- related genes leading to improve growth and health status of treated birds.
5. Conclusion
Feeding broilers with diets incorporated with 100 kg/ton PNM, LSM, or combination in equal amounts with or without enzyme mixture as substitutes for corn and soybean meal did not affect their growth performance and health status parameters. Hypocholesterolemic impacts of these supplements were noticed. Dietary incorporation of PNM or LSM did not affect the nutrient digestibility and amino acids profile of superficial pectoral muscle. Nutrient transportation-related genes were upregulated in the duodenum of treated birds. The finding of this study suggests the use of PNM and LSM in broiler diets, particularly their combination (50 kg/ton each) with enzyme mixture, as an efficient tool to reduce feed costs without affecting the productive performance of broiler chickens.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgements
The authors wish to acknowledge the helpful suggestions of members of the department of poultry production, faculty of agriculture, Kafrelsheikh University, Egypt as well as, Taif University Researchers Supporting Project number (TURSP-2020/105), Taif University, Taif, Saudi Arabia.
Data availability statement
All data sets collected and analyzed during the current study are available from the corresponding author on reasonable request.
Funding
This study was supported by Taif University Researchers Supporting Project number (TURSP-2020/105), Taif University, Taif, Saudi Arabia.
Footnotes
Peer review under responsibility of King Saud University.
References
- Abd El-Moneim A.E., Sabic E.M. Beneficial effect of feeding olive pulp and Aspergillus awamori on productive performance, egg quality, serum/yolk cholesterol and oxidative status in laying Japanese quails. J. Animal Feed Sci. 2019;28(1):52–61. doi: 10.22358/jafs/105537/2019. [DOI] [Google Scholar]
- Abdel-Moneim A.-M.E., Sabic E.M., Abu-Taleb A.M., Ibrahim N.S. Growth performance, hemato-biochemical indices, thyroid activity, antioxidant status, and immune response of growing Japanese quail fed diet with full-fat canola seeds. Trop. Anim. Health Prod. 2020;52(4):1853–1862. doi: 10.1007/s11250-020-02200-1. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.-M.-E., Selim D.A., Basuony H.A., Sabic E.M., Saleh A.A., Ebeid T.A. Effect of dietary supplementation of Bacillus subtilis spores on growth performance, oxidative status and digestive enzyme activities in Japanese quail birds. Trop. Anim. Health Prod. 2020;52(2):671–680. doi: 10.1007/s11250-019-02055-1. [DOI] [PubMed] [Google Scholar]
- Abdel-Moneim A.E., Elbaz A.M., Khidr R.E., Badri F.B. Effect of in ovo inoculation of Bifidobacterium spp. on growth performance, thyroid activity, ileum histomorphometry and microbial enumeration of broilers. Probiotics Antimicrob. Proteins. 2020;12:873–882. doi: 10.1007/s12602-019-09613-x. [DOI] [PubMed] [Google Scholar]
- Abdel‐Moneim A.-M., Shehata A.M., Alzahrani S.O., Shafi M.E., Mesalam N.M., Taha A.E., Swelum A.A., Arif M., Fayyaz M., Abd El‐Hack M.E. The role of polyphenols in poultry nutrition. J. Animal Physiol. Animal Nutrition. 2020;104(6):1851–1866. doi: 10.1111/jpn.13455. [DOI] [PubMed] [Google Scholar]
- Aito-Inoue M., Lackeyram D., Fan M.Z., Sato K., Mine Y. Transport of a tripeptide, Gly-Pro-Hyp, across the porcine intestinal brush-border membrane. J. Peptide Sc.: Off. Publ. Europ. Peptide Soc. 2007;13(7):468–474. doi: 10.1002/psc.870. [DOI] [PubMed] [Google Scholar]
- Ajuyah A., Lee K., Hardin R., Sim J. Changes in the yield and in the fatty acid composition of whole carcass and selected meat portions of broiler chickens fed full-fat oil seeds. Poult. Sci. 1991;70(11):2304–2314. [Google Scholar]
- AOAC, 2003. Official methods of analysis of AOAC, 17th ed. AOAC, Gaithersburg, MD, USA.
- Awad A.B., Chan K.C., Downie A.C., Fink C.S. Peanuts as a source of β-sitosterol, a sterol with anticancer properties. Nutr. Cancer. 2000;36(2):238–241. doi: 10.1207/S15327914NC3602_14. [DOI] [PubMed] [Google Scholar]
- Babatunde O., Park C., Adeola O. Nutritional Potentials of Atypical Feed Ingredients for Broiler Chickens and Pigs. Animals. 2021;11:1196. doi: 10.3390/ani11051196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batal A., Dale N., Café M. Nutrient composition of peanut meal. J. Appl. Poult. Res. 2005;14(2):254–257. [Google Scholar]
- Bayrak A., Kiralan M., Ipek A., Arslan N., Cosge B., Khawar K. Fatty acid compositions of linseed (Linum usitatissimum L.) genotypes of different origin cultivated in Turkey. Biotechnol. Biotechnol. Equip. 2010;24(2):1836–1842. [Google Scholar]
- Bera S.K., Kamdar J.H., Kasundra S.V., Patel S.V., Jasani M.D., Maurya A.K., Dash P., Chandrashekar A.B., Rani K., Manivannan N., Janila P., Pandey M.K., Vasanthi R.P., Dobariya K.L., Radhakrishnan T., Varshney R.K., Prasad M. Steady expression of high oleic acid in peanut bred by marker-assisted backcrossing for fatty acid desaturase mutant alleles and its effect on seed germination along with other seedling traits. PLoS ONE. 2019;14(12):e0226252. doi: 10.1371/journal.pone.0226252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen H.N. Role of amino acid transport and countertransport in nutrition and metabolism. Physiol. Rev. 1990;70(1):43–77. doi: 10.1152/physrev.1990.70.1.43. [DOI] [PubMed] [Google Scholar]
- Costa E., Miller B., Pesti G., Bakalli R., Ewing H. Studies on feeding peanut meal as a protein source for broiler chickens. Poult. Sci. 2001;80(3):306–313. doi: 10.1093/ps/80.3.306. [DOI] [PubMed] [Google Scholar]
- Davis, J.P., Dean, L.L., 2016. Peanut composition, flavor and nutrition. In: Peanuts. Elsevier, pp. 289-345.
- Diaw M.T., Dieng A., Mergeai G., Dotreppe O., Youssouf I., Hornick J.-L. Effect of groundnut cake substitution by glandless cottonseed kernels on broilers production: animal performance, nutrient digestibility, carcass characteristics and fatty acid composition of muscle and fat. Int. J. Poultry Sci. 2010;9(5):473–481. [Google Scholar]
- Driver J., Atencio A., Edwards H., Jr, Pesti G. Improvements in nitrogen-corrected apparent metabolizable energy of peanut meal in response to phytase supplementation. Poult. Sci. 2006;85(1):96–99. doi: 10.1093/ps/85.1.96. [DOI] [PubMed] [Google Scholar]
- Fernandez M.L., West K.L. Mechanisms by which dietary fatty acids modulate plasma lipids. J. Nutrit. 2005;135(9):2075–2078. doi: 10.1093/jn/135.9.2075. [DOI] [PubMed] [Google Scholar]
- Ghadge V., Upase B., Patil P. Effect of replacing groundnut cake by soybean meal on performance of broilers. Veterinary World. 2009;2(5):183. [Google Scholar]
- Han, H., Yan, P., Chen, L., Luo, C., Gao, H., Deng, Q., Liu, L., 2015. Flaxseed oil containing α-linolenic acid ester of plant sterol improved atherosclerosis in apoE deficient mice. Oxidative Med. Cell. Longevity. [DOI] [PMC free article] [PubMed]
- Ibrahim N., Sabic E., Abu-Taleb A., Abdel-Moneim A. Effect of dietary supplementation of full-fat canola seeds on productive performance, blood metabolites and antioxidant status of laying Japanese quails. Braz. J. Poultry Sci. 2020;22(1):1–10. [Google Scholar]
- Iji P., Toghyani M., Ahiwe E., Omede A. Alternative sources of protein for poultry nutrition. Achieving Sustain. Prod. Poultry Meat. 2017;2:237–269. [Google Scholar]
- Jacobsen, D, Gertovey, S. Nielson, H Digestibility trials with poultry. 322 Bertning fra forsg slabooratoriel udgbet of statens. In Husdyrbugsudvaly–Kobengaven; Københavns Universitet: Copenhagen, Denmark. 1960 [Google Scholar]
- Jahanian R., Rasouli E. Effect of extrusion processing of soybean meal on ileal amino acid digestibility and growth performance of broiler chicks. Poult. Sci. 2016;95(12):2871–2878. doi: 10.3382/ps/pew178. [DOI] [PubMed] [Google Scholar]
- Kana J.R., Gnonlonfin B.G.J., Harvey J., Wainaina J., Wanjuki I., Skilton R.A., Teguia A. Assessment of aflatoxin contamination of maize, peanut meal and poultry feed mixtures from different agroecological zones in Cameroon. Toxins. 2013;5(5):884–894. doi: 10.3390/toxins5050884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ketelslegers, J.-M, Maiter, D. Maes, M. Underwood, L.E. Thissen, J. Nutritional regulation of insulin-like growth factor-I. Metabolism. 1995;44:50–57. doi: 10.1016/0026-0495(95)90221-x. [DOI] [PubMed] [Google Scholar]
- Kristensen M., Knudsen K., Jørgensen H., Oomah D., Bügel S., Toubro S., Tetens I., Astrup A. Linseed dietary fibers reduce apparent digestibility of energy and fat and weight gain in growing rats. Nutrients. 2013;5(8):3287–3298. doi: 10.3390/nu5083287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar F., Tyagi P.K., Mir N.A., Tyagi P.K., Dev K., Bera I., Biswas A.K., Sharma D., Mandal A.B., Deo C. Role of flaxseed meal feeding for different durations in the lipid deposition and meat quality in broiler chickens. J. Am. Oil. Chem. Soc. 2019;96(3):261–271. [Google Scholar]
- Livak, K.J., Schmittgen, T.D Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. method. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lu J., Wang K., Tong H., Shi S., Wang Q. Effects of graded replacement of soybean meal by peanut meal on performance, egg quality, egg fatty acid composition and cholesterol content in laying hens. Archiv fur Geflugelkunde. 2013;77(1):43–50. [Google Scholar]
- Maki K.C., Davidson M.H., Malik K.C., Albrecht H.H., O’Mullane J., Daggy B.P. Cholesterol lowering with high-viscosity hydroxypropylmethylcellulose. Am. J. Cardiol. 1999;84(10):1198–1203. doi: 10.1016/s0002-9149(99)00534-2. [DOI] [PubMed] [Google Scholar]
- Meherunnisa L., Imdad H., Abdullah S., Gul H., Farzana M. Effect of Linseed Meal on Broiler Perform Acne and Fat Content. Dairy Vet. Sci. J. 2017;4(1):555628. [Google Scholar]
- Miura Y., Kato H., Noguchi T. Effect of dietary proteins on insulin-like growth factor-1 (IGF-1) messenger ribonucleic acid content in rat liver. Br. J. Nutr. 1992;67(2):257–265. doi: 10.1079/bjn19920029. [DOI] [PubMed] [Google Scholar]
- Mridula D., Kaur D., Nagra S., Barnwal P., Gurumayum S., Singh K. Growth performance, carcass traits and meat quality in broilers, fed flaxseed meal. Asian-Australasian J. Animal Sci. 2011;24(12):1729–1735. [Google Scholar]
- Musharaf N., Latshaw D. Subsititutan of GNM and CSM for soybean meal in broiler starter diet. Sudan. J. Vet. Sci. Animal Husb. 1991;30:6–12. [Google Scholar]
- Ohkawa H., Ohishi N., Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal. Biochem. 1979;95(2):351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- Pekel A., Patterson P., Hulet R., Acar N., Cravener T., Dowler D., Hunter J. Dietary camelina meal versus flaxseed with and without supplemental copper for broiler chickens: Live performance and processing yield. Poult. Sci. 2009;88(11):2392–2398. doi: 10.3382/ps.2009-00051. [DOI] [PubMed] [Google Scholar]
- Pesti G., Bakalli R., Driver J., Sterling K., Hall L., Bell E. Comparison of peanut meal and soybean meal as protein supplements for laying hens. Poult. Sci. 2003;82(8):1274–1280. doi: 10.1093/ps/82.8.1274. [DOI] [PubMed] [Google Scholar]
- Pirmohammadi A., Khalaji S., Yari M. Effects of Linseed Expansion on its Dietary Molecular Structures, and on Broiler Chicks Digestive Enzymes Activity, Serum Metabolites, and Ileal Morphology. J. Appl. Poult. Res. 2019;28(4):997–1012. [Google Scholar]
- Piva G., Galvano F., Pietri A., Piva A. Detoxification methods of aflatoxins. A review. Nutrit. Res. 1995;15(5):767–776. [Google Scholar]
- Richard D., Kefi K., Barbe U., Bausero P., Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol. Res. 2008;57(6):451–455. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- Rodriguez M., Alzueta C., Rebole A., Ortiz L., Centeno C., Trevino J. Effect of inclusion level of linseed on the nutrient utilisation of diets for growing broiler chickens. Br. Poult. Sci. 2001;42(3):368–375. doi: 10.1080/00071660120055359. [DOI] [PubMed] [Google Scholar]
- Saleh A.A. Effects of fish oil on the production performances, polyunsaturated fattyacids and cholesterol levels of yolk in hens. Emirates J. Food Agric. 2013:605–612. [Google Scholar]
- Saleh A.A., El-Awady A., Amber K., Eid Y.Z., Alzawqari M.H., Selim S., Soliman M.M., Shukry M. Effects of Sunflower Meal Supplementation as a Complementary Protein Source in the Laying Hen’s Diet on Productive Performance, Egg Quality, and Nutrient Digestibility. Sustainability. 2021;13(6):3557. [Google Scholar]
- Saleh A.A., Shukry M., Farrag F., Soliman M.M., Abdel-Moneim A.-M.-E. Effect of feeding wet feed or wet feed fermented by Bacillus licheniformis on growth performance, histopathology and growth and lipid metabolism marker genes in broiler chickens. Animals. 2021;11(1):83. doi: 10.3390/ani11010083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarbaz E., Navidshad B., Aghjegheshlagh F.M. The effect of peanut pod on performance, small intestine pH and ileum bacteria population in broiler chickens. South African J. Animal Sci. 2018;48(3):435–444. [Google Scholar]
- Schöne F., Kirchheim U., Ochrimenko W., Rudolph B., Peglow K., Lüdke H. Evaluation of ground linseed with pigs-crude nutrient digestibility and excretion of fibre respectively non starch polysaccharides, growth and blood serum concentration of thiocyanate (SCN) and thyroid hormones. Wirtschaftseigene Futter. 1996 [Google Scholar]
- Toomer O.T., Livingston M., Wall B., Sanders E., Vu T., Malheiros R.D., Livingston K.A., Carvalho L.V., Ferket P.R., Dean L.L. Feeding high-oleic peanuts to meat-type broiler chickens enhances the fatty acid profile of the meat produced. Poult. Sci. 2020;99(4):2236–2245. doi: 10.1016/j.psj.2019.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toomer O.T., Livingston M.L., Wall B., Sanders E., Vu T.C., Malheiros R.D., Livingston K.A., Carvalho L.V., Ferket P.R. Meat quality and sensory attributes of meat produced from broiler chickens fed a high oleic peanut diet. Poult. Sci. 2019;98(10):5188–5197. doi: 10.3382/ps/pez258. [DOI] [PubMed] [Google Scholar]
- Toomer O.T., Sanders E., Vu T.C., Malheiros R.D., Redhead A.K., Livingston M.L., Ferket P.R. The effects of high-oleic peanuts as an alternative feed ingredient on broiler performance, ileal digestibility, apparent metabolizable energy, and histology of the intestine. Trans. Anim. Sci. 2020;4(3):txaa137. doi: 10.1093/tas/txaa137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki Y., Harada S., Tokuyama S. Sodium–glucose transporter as a novel therapeutic target in disease. Eur. J. Pharmacol. 2018;822:25–31. doi: 10.1016/j.ejphar.2018.01.003. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Parsons C.M. Effects of overprocessing on the nutritional quality of peanut meal. Poult. Sci. 1996;75(4):514–518. doi: 10.3382/ps.0750514. [DOI] [PubMed] [Google Scholar]
- Zuo J., Ling B., Long L., Li T., Lahaye L., Yang C., Feng D. Effect of dietary supplementation with protease on growth performance, nutrient digestibility, intestinal morphology, digestive enzymes and gene expression of weaned piglets. Animal Nutrition. 2015;1(4):276–282. doi: 10.1016/j.aninu.2015.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data sets collected and analyzed during the current study are available from the corresponding author on reasonable request.