Abstract
Rationale & Objective
Poor sleep quality and insomnia are pervasive among patients with advanced chronic kidney disease (CKD); however, these health issues have not been systematically evaluated.
Study Design
Systematic review and meta-analysis.
Setting & Study Populations
Adult patients with CKD not receiving kidney replacement therapy (KRT), as well as adults receiving KRT, including hemodialysis, peritoneal dialysis, and kidney transplantation.
Selection Criteria for Studies
A systematic literature search using PubMed, Embase, and PsycNET, was conducted for articles published between January 1, 1990, and September 28, 2018.
Data Extraction
Data on the prevalences of poor sleep quality and insomnia in patients with CKD, including those receiving and not receiving KRT, were extracted.
Analytical Approach
Pooled prevalences were estimated using a random-effects meta-analysis and were stratified according to age, CKD stage, World Health Organization region, risk of bias, Pittsburgh Sleep Quality Index score, and the different criteria for insomnia that were used at diagnosis.
Results
Of 3,708 articles, 93 were selected, and significant methodological heterogeneity was present. The pooled prevalences of poor sleep quality for CKD without KRT, hemodialysis, peritoneal dialysis, and kidney transplantation were 59% (95% CI, 44%-73%), 68% (95% CI, 64%-73%), 67% (95% CI, 44%-86%), and 46% (95% CI, 34%-59%), respectively. The corresponding prevalences of insomnia were 48% (95% CI, 30%-67%), 46% (95% CI, 39%-54%), 61% (95% CI, 41%-79%), and 26% (95% CI, 9%-49%), respectively. Insomnia was significantly more prevalent among patients aged 51-60 years and those aged >60 years than among those aged <50 years. The prevalence of insomnia in the European region was the lowest of all World Health Organization regions.
Limitations
High interstudy heterogeneity.
Conclusions
Approximately half of the patients with advanced CKD had poor sleep quality or insomnia, and the prevalence was even higher among those who received KRT. Kidney transplantation may reduce the burden of poor sleep quality and insomnia.
Index Words: Chronic kidney disease, hemodialysis, insomnia, kidney transplantation, peritoneal dialysis, poor sleep quality
Plain-Language Summary.
Sleep disorders, such as insomnia and poor sleep quality, are common among patients with chronic kidney disease (CKD) and are often associated with health problems. By systematically reviewing the literature, the present study provides quantitative evidence showing that half of the patients with CKD have trouble falling asleep or staying asleep. Patients with CKD aged >50 years are particularly vulnerable to insomnia. The current study also found that patients with kidney failure who had undergone kidney transplantation were associated with better sleep than those with hemodialysis or peritoneal dialysis. These findings call for attention to sleep problems in patients with CKD and identify a critical, unmet need for designing effective interventions to improve sleep in this vulnerable population.
Sleeplessness is an emerging global epidemic and has been closely associated with noncommunicable diseases such as type 2 diabetes, chronic kidney disease (CKD), cognitive dysfunction, and neuropsychiatric disorders.1, 2, 3 When indirect costs such as reduced job productivity and increased use of health care services are accounted for, the annual costs related to sleeplessness and insomnia range from US$30-$107 billion.4,5
Patients with CKD are vulnerable to sleeping disorders, particularly in the elderly, leading to chronic fatigue and reduced quality of life.6, 7, 8 An estimated 50%-75% of patients with kidney failure and approximately 8%-36% of patients with earlier stages of CKD have reported insomnia symptoms.9, 10, 11 Poor sleep and insomnia may accelerate CKD progression and increase mortality in patients on maintenance dialysis.12, 13, 14, 15 Additionally, patients with a more advanced stage of CKD, particularly kidney failure requiring kidney replacement therapy (KRT), encounter relatively more sleeping problems because of uremia-related metabolic abnormalities, such as refractory pruritus or restless leg syndrome.9,15, 16, 17, 18, 19 There have been several patient-centered studies emphasizing the need for approaches to address sleep disorders in patients requiring KRT.20, 21, 22 Medical therapies for sleep dysfunction, such as hypnotics or antipsychotics, may further magnify complications of CKD by increasing the risk of accidental events, particularly among older adults.23,24
Although many studies have reported the prevalences of insomnia and poor sleep among patients with CKD, relatively less attention has been paid to quantitative syntheses related to studies on insomnia or poor sleep prevalences on a global scale.6,9 We hypothesized that the prevalences of poor sleep quality and insomnia would be higher in patients with CKD than in those without CKD. However, at the time of writing, to our knowledge, no systematic review in the literature had evaluated poor sleep quality and insomnia prevalences across all stages and severity levels of CKD, including early CKD, CKD not treated by KRT, hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation. To fill this knowledge gap, this systematic review and meta-analysis synthesized existing findings on the prevalence estimates for poor sleep quality and insomnia across the different CKD stages. We explored variations in prevalence estimates by investigating the effects that different CKD stages, diagnostic criteria, demographics, and methodological characteristics have on such estimates.
Methods
Search Strategy and Selection Criteria
The systematic search and review processes were conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses Statement and the Meta-Analysis of Observational Studies in Epidemiology criteria.25,26 We searched the PubMed or MEDLINE, Embase, and APA PsycNET databases for the period from January 1, 1990, to September 28, 2018, for epidemiologic studies that investigated the prevalences of sleep disorders in patients (1) with CKD, (2) on HD, (4) on PD, and (5) who received kidney transplantation, using Medical Subject Headings terms and other specific terms related to our key research concepts. Table S1 details the search strategies.
Studies were included if they (1) were observational studies or randomized controlled trials; (2) investigated patients with CKD, with or without a comparison group; and (3) evaluated or provided relevant, quantitative data on the prevalence of poor sleep quality or insomnia. There was no language restriction for the included studies. Google Translate was used for translating studies using languages other than English.
After removing duplicates, we excluded studies that lacked original data, including reviews, editorials, letters, case reports, or commentaries. Studies involving study populations without CKD (except for the control group), those with fewer than 10 patients, and those without reported endpoints of interest were also excluded. Conference abstracts were also excluded. We assessed the remaining articles by reading the full text of each article and excluded studies without measured point prevalences and appropriate definitions of insomnia (see under definitions). We identified the duplicate populations and excluded them. We excluded randomized controlled trials that did not show baseline sleep data on the selection of the participants because the data may have been altered after the intervention, which could, in turn, bias findings on the prevalences of poor sleep quality and insomnia. We also excluded studies in which insomnia was diagnosed before the patients had CKD. Studies that included children and used poor sleep assessments other than the Pittsburgh Sleep Quality Index (PSQI) were also excluded. The remaining studies were assessed for risk of bias. Studies with a total score > 8 were categorized as having a very high risk of bias and were excluded. We also excluded poor sleep-quality studies with population sizes < 100. This study followed the protocol registered in PROSPERO (www.crd.york.ac.uk/PROSPERO; protocol number, CRD42020133673).
Definition of Poor Sleep Quality and Insomnia
Poor sleep quality was defined by a PSQI score ≥ 5.27 Furthermore, owing to the significant variation in studies’ insomnia definitions, studies were included only if the following conditions were met: (1) patients were determined to have insomnia by presenting at least 1 of 4 insomnia complaints listed in the primary criteria and at least 1 of 4 insomnia complaints listed in the secondary criteria in Table S2 (modified from the insomnia inclusion criteria provided by the American Academy of Sleep Medicine Working Group Report from 2004, as shown in Table S3); (2) patients were diagnosed as having insomnia disorder according to the criteria of either the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision, or the International Classification of Sleep Disorders, version 2; (3) patients were assessed according to the Athens Insomnia Scale and Insomnia Severity Index, with cutoff scores of ≥6 and >13, respectively; and (4) patients had diagnoses of insomnia in their medical records.28, 29, 30, 31, 32
Data Abstraction
Two authors (L-HT and C-CK) independently evaluated all references by titles and abstracts and retrieved the full texts of any article that seemed relevant. We contacted the authors whose full-text articles were not accessible. Information such as study characteristics and prevalence estimates of poor sleep quality and insomnia were systematically extracted (Tables S4 and S5). If >1 study reported the same population, the study with the largest sample size and most informative data was selected. Reviewers resolved disagreements on study inclusion through consensus. In studies that did not directly report the prevalence estimates, we calculated the prevalence estimates from the proportion of patients with poor sleep quality or insomnia. A study quality assessment form, which was modified on the basis of a published risk-of-bias tool for prevalence studies, was used to evaluate the quality of the included studies.33 According to the specific justification of individual quality items, each of the 5 quality items had a range of scores between 0-2 (the smaller the better). All scores in each item were summated to calculate an overall score, categorized into low, moderate, and high risks of bias (Table S6; Fig S1). We excluded all studies with a very high risk of bias (total score > 8) from the analysis.
Data Analysis
All analyses were performed using the Metafor package for R (R Foundation for Statistical Computing). To manage the confidence intervals (CIs) outside the 0-1 range and to address variance instability, we applied the Freeman-Tukey (double arcsine) transformation (using the escalc function with the measure argument “PFT”) to calculate the pooled prevalence estimates from the raw data or the prevalence estimates provided in the indicated studies. The double arcsine–transformed prevalence is equal to , where N and n represent the population size and the number of people with the indicated condition (eg, insomnia), respectively; the prevalence estimates in the results have been back-transformed. The meta-analysis was conducted using a random-effects model (by using the “rma” function with the method argument “restricted maximum-likelihood estimator”) when the prevalence estimates and variances of poor sleep quality and insomnia (with 95% CIs) were pooled.34 Results obtained using the fixed-effects models (using the “rma” function with the method argument “fixed-effects model”) have also been provided (Table S7).
Subgroup analyses were performed on the prevalence estimates of poor sleep quality and insomnia to explore potential sources of heterogeneity. We analyzed the following covariates: (1) mean age; (2) CKD stage; (3) World Health Organization (WHO) region; (4) risk of bias; and (5) PSQI cutoff score or the definition used to define insomnia. We further constructed univariate meta-regression plots to represent the correlations between pooled estimates and mean age, and we plotted each study with circles using inverse-variance weights to represent the sample size.
The heterogeneity of prevalence estimates was assessed using the I2 statistic and the Cochran Q statistic:
where is the degree of freedom.
where are the weight and prevalence of each study, respectively.
The proportion of heterogeneity explained by a priori covaries was calculated as , where represents the between-studies component of variance in the null model and represents the between-studies component of variance in the model, including covariates.35 Publication bias was determined through a visual inspection of the funnel plot (using the “funnel” function) with a set of significance thresholds, including P > 0.10, 0.05 < P < 0.10, 0.01 < P < 0.05, and P < 0.01, for double arcsine–transformed prevalences and by testing for asymmetry using Egger’s tests (by applying the “regtest” function). All analyses were completed using R version 3.6.0 (R Foundation for Statistical Computing). The data collection form and analytic codes are available on request.
Results
Overview of Enrolled Studies
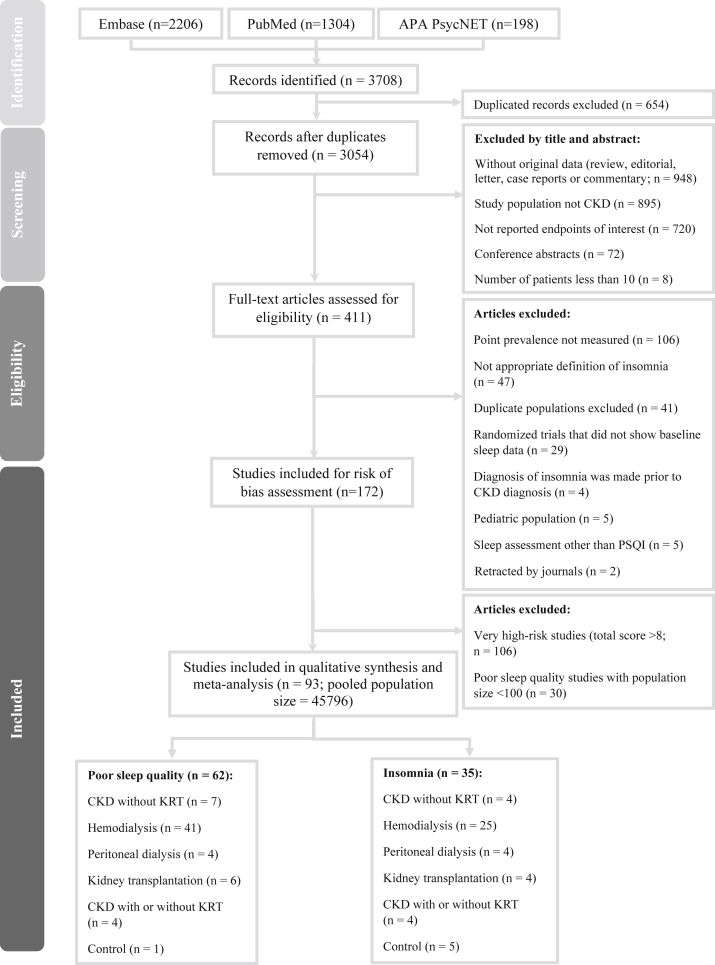
We identified 3,708 studies in the secondary databases, and 411 studies were eligible for full-text screening (Fig 1). We identified 93 articles that met the inclusion criteria and provided prevalence data for either insomnia or poor sleep quality from the eligible articles. Of the 45,796 participants from the 93 included studies, 32,949 patients were diagnosed with CKD and the rest were controls who did not have CKD (Fig 1). Four studies included data on both poor sleep quality and insomnia.36, 37, 38, 39
Figure 1.
Flowchart of the selection process of the included studies. Abbreviations: APA, American Psychiatric Association; CKD, chronic kidney disease; KRT, kidney replacement therapy; PSQI, Pittsburgh Sleep Quality Index.
Studies on Poor Sleep Quality
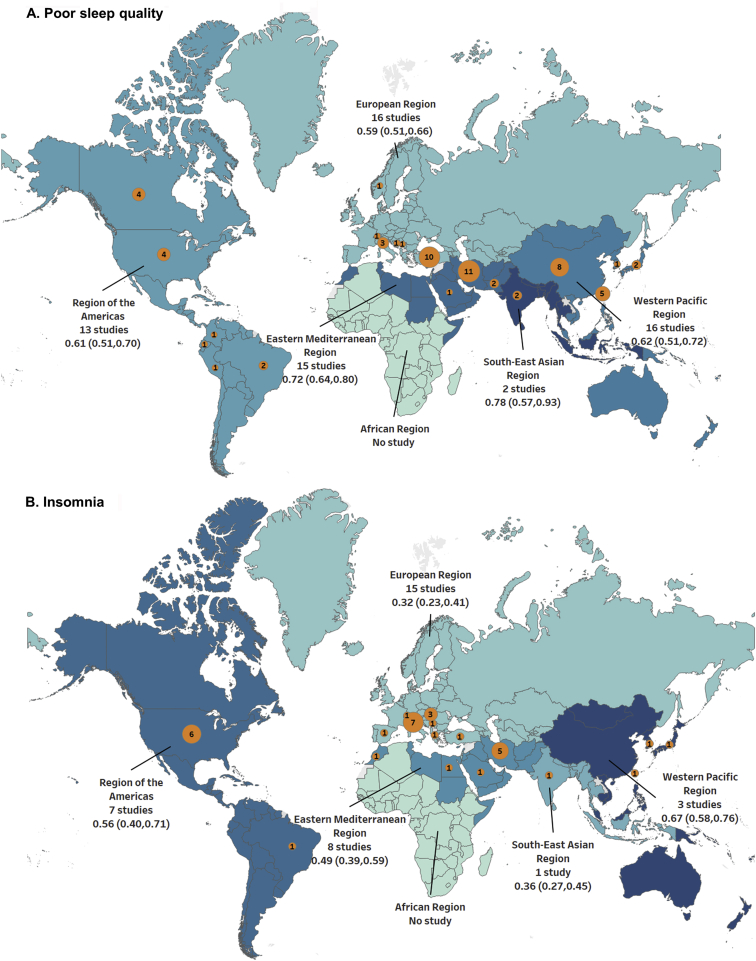
In 62 studies, the prevalence of poor sleep quality was estimated, and most of the studies focused primarily on patients on HD (41 studies; n = 8,747 patients) followed by patients with CKD without KRT (7 studies; n = 2,038), kidney transplantation (6 studies; n = 1,756), and PD (4 studies; n = 8,638; Table S4). The mean age of all enrolled patients with poor sleep quality was 55.6 years. Almost all studies in the group with poor sleep quality were cross-sectional and used the consecutive sampling method. In addition, all studies in the group with poor sleep quality collected data through a patient-administered questionnaire (Table S4). Fig 2A depicts the wide geographical distribution of study populations across the 5 continents, according to WHO regions. For instance, 15 studies were conducted in the eastern Mediterranean region, whereas no study was conducted in the African region (Fig 2A).
Figure 2.
Geographic distribution showing the pooled prevalences of (A) poor sleep. quality and (B) insomnia across WHO regions. The numbers in the orange circles indicate the number of studies from respective countries. The size of the orange circles is proportional to the number of studies. Abbreviation: WHO, World Health Organization.
Studies on Insomnia
Thirty-five studies that evaluated the prevalence of insomnia were conducted, primarily with patients who required HD (25 studies; n = 13,456), followed by patients with CKD not receiving KRT (4 studies; n = 2,006), patients who required kidney transplantation (4 studies; n = 1,178), and patients who required PD (4 studies; n = 372). The mean age of all patients in the insomnia group was 56.7 years. Nearly all studies (94.3%) had a cross-sectional design. Twenty-seven (77.1%) studies used convenience sampling, and 8 (22.9%) studies used random sampling (Table S5). Most studies collected data through a patient-administered questionnaire (25 studies) or interview-based assessment (9 studies; Table S5). A wide diversity in the study populations’ geographical locations was observed, and 42.9% of studies were conducted in the European region (Fig 2B).
Risk Assessment of Bias
Summaries of the risks of bias of the selected studies for insomnia and poor sleep quality are presented in Fig S1. Of the 62 included studies on poor sleep quality, 49 (79.0%) studies were judged as having a moderate risk and 13 (20.1%) studies were deemed to have a high risk. Among the 35 included studies on insomnia, only 7 (20.0 %) studies were considered to have a low risk, whereas 14 (40.0%) studies had a moderate risk and 14 (40.0%) studies had a high risk (Fig S1).
Meta-analysis
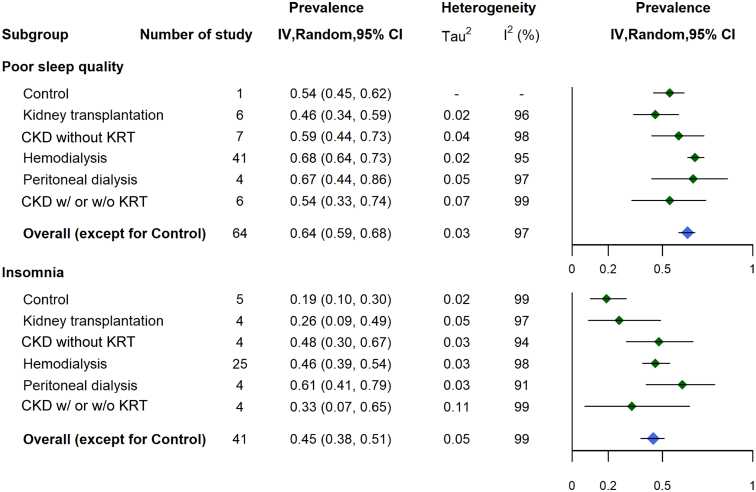
The overall pooled prevalence of poor sleep quality among patients with CKD was 64% (95% CI, 59%-68%), with a high degree of heterogeneity (I2 = 97%). The overall pooled prevalence of insomnia was 45% (95% CI, 38%-51%), and the degree of heterogeneity was also high (I2 = 99%). Prevalence estimates of poor sleep quality ranged from 21%-98%, whereas those of insomnia ranged from 6%-85%. The pooled prevalences of poor sleep quality were 54% (95% CI, 45%-62%) in the control group, 46% (95% CI, 34%-59%) in the kidney transplantation–treated group, 59% (95% CI, 44%-73%) in the CKD without KRT group, 68% (95% CI, 64%-73%) in the HD group, and 67% (95% CI, 44%-86%) in the PD group (Fig 3; Fig S2). The corresponding pooled prevalences of insomnia were 19% (95% CI, 10%-30%), 26% (95% CI 9%-49%), 48% (95% CI, 30%-67%), 46% (95% CI 39%-54%), and 61% (95% CI 41%-79%) in the control, kidney transplantation, CKD without KRT, HD, and PD groups, respectively (Fig 3).
Figure 3.
Summary pooled prevalence of poor sleep quality and insomnia according to the whole spectrum of CKD. Abbreviations: CI, confidence interval; CKD, chronic kidney disease; IV, inverse variance; KRT, kidney replacement therapy.
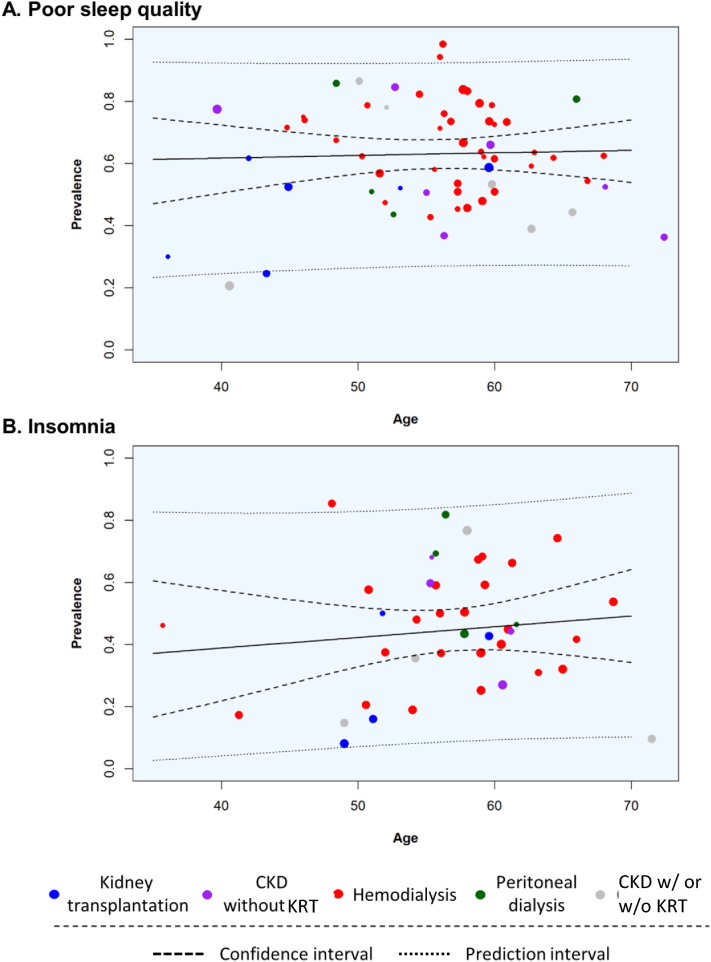
In the subgroup analysis and univariate random-effects meta-regression for poor sleep quality and insomnia, the control groups had the lowest prevalences than other groups. We also observed an increasing prevalence trend across the CKD stage spectrum (Tables 1 and 2; Fig 3). Furthermore, the group with kidney transplantation had a significantly lower prevalence of poor sleep quality than the group with PD (P < 0.01); for insomnia, a significantly lower prevalence was observed in the group with kidney transplantation than the group with HD (P = 0.02; Tables 1 and 2). The age category was not associated with the prevalence of poor sleep quality; however, patients aged 51-60 years and those aged >60 years had significantly higher prevalences of insomnia than those belonging to other age groups (Tables 1 and 2; Fig 4). The prevalence of poor sleep quality declined with age among patients receiving HD; nevertheless, the trends of insomnia prevalence with age were similar in patients with CKD and HD (Fig S3). The prevalences of poor sleep quality in patients with automated peritoneal dialysis (APD) and continuous ambulatory peritoneal dialysis (CAPD) were 62% and 67%, respectively (Fig S4). For insomnia, the prevalences were 83% and 81% for patients with APD and CAPD, respectively (Fig S4). There was no significant difference between these 2 subgroups. Risks of bias, definitions of insomnia, and PSQI cutoff scores did not alter the prevalence estimates of insomnia or poor sleep quality (Tables 1 and 2; Fig 4). In applying a univariate random-effects meta-regression for insomnia, we excluded the study by Elder et al15 from a WHO region analysis because it involved multiple WHO regions. The prevalence estimate of insomnia in the European region was significantly lower than that in other WHO regions, except in South-East Asia. The eastern Mediterranean region had a significantly higher pooled prevalence for poor sleep quality than the European region (Table 2). A visual inspection of the funnel plot showed potential publication bias in the group with insomnia but no evidence of publication bias in the group with poor sleep quality (Fig S5).
Table 1.
Univariate Random-effects Meta-regression for the Prevalence of Poor Sleep Quality According to Age Categories, CKD Stage, WHO Regions, Risk of Bias, and Diagnostic Criteria of Poor Sleep Quality
| Variables | No. of Studies | Beta Estimates (95% CI) | Prevalence (95% CI) | P Value | I2, % |
|---|---|---|---|---|---|
| Age, y | |||||
| Per 1-y increase | 62 | 0.001 (−0.01 to 0.01) | 0.63 (0.58 to 0.67) | 0.72 | 96.99 |
| <50 | 12 | Ref | 0.58 (0.48 to 0.68) | Ref | 96.89 |
| 51-60 | 39 | 0.08 (−0.04 to 0.20) | 0.66 (0.60 to 0.71) | 0.20 | |
| >60 | 11 | −0.01 (−0.16 to 0.14) | 0.57 (0.46 to 0.68) | 0.92 | |
| CKD stage | 96.48 | ||||
| Control | 1 | 0.07 (−0.31 to 0.45) | 0.54 (0.20 to 0.85) | 0.71 | |
| Kidney transplantation | 6 | Ref | 0.46 (0.32 to 0.61) | Ref | |
| CKD without KRT | 7 | 0.12 (−0.07 to 0.32) | 0.59 (0.45 to 0.71) | 0.22 | |
| Hemodialysis | 41 | 0.22 (0.07 to 0.38) | 0.68 (0.63 to 0.73) | 0.005 | |
| Peritoneal dialysis | 4 | 0.21 (−0.02 to 0.43) | 0.67 (0.49 to 0.82) | 0.08 | |
| CKD with or without KRT | 6 | 0.07 (−0.13 to 0.28) | 0.54 (0.40 to 0.68) | 0.47 | |
| WHO regions | 96.93 | ||||
| European | 16 | Ref | 0.59 (0.50 to 0.68) | Ref | |
| Americas | 13 | 0.02 (−0.12 to 0.16) | 0.61 (0.51 to 0.71) | 0.77 | |
| Western Pacific | 16 | 0.03 (−0.10 to 0.16) | 0.62 (0.53 to 0.70) | 0.67 | |
| Eastern Mediterranean | 15 | 0.14 (0.01 to 0.27) | 0.72 (0.63 to 0.80) | 0.04 | |
| South-East Asia Region | 2 | 0.20 (−0.07 to 0.48) | 0.78 (0.54 to 0.95) | 0.14 | |
| Risk of bias | 96.97 | ||||
| Low | - | - | - | - | |
| Moderate | 49 | Ref | 0.66 (0.61 to 0.71) | Ref | |
| High | 13 | −0.10 (−0.21 to 0.01) | 0.56 (0.46 to 0.66) | 0.09 | |
| PSQI cutoff scores | 96.95 | ||||
| ≥5 | 59 | Ref | 0.72 (0.55 to 0.87) | Ref | |
| >6 | 6 | −0.08 (−0.24 to 0.08) | 0.64 (0.60 to 0.69) | 0.31 |
Abbreviations: CKD, chronic kidney disease; KRT, kidney replacement therapy; PSQI, Pittsburgh Sleep Quality Index; Ref, reference; WHO, World Health Organization.
Table 2.
Univariate Random-effects Meta-regression for the Prevalence of Insomnia According to Age Categories, CKD Stage, WHO Regions, Risk of Bias, and Diagnostic Criteria of Insomnia
| Variables | No. of Studies | Beta Estimates (95% CI) | Prevalence (95% CI) | P Value | I2, % |
|---|---|---|---|---|---|
| Age,Y | |||||
| Per 1-y increase | 45 | 0.01 (0.00 to 0.02) | 0.42 (0.36 to 0.49) | 0.21 | 99.11 |
| <50 | 8 | Ref | 0.24 (0.12 to 0.38) | Ref | 98.64 |
| 51-60 | 24 | 0.26 (0.09 to 0.43) | 0.49 (0.40 to 0.57) | 0.003 | |
| >60 | 13 | 0.19 (0.00 to 0.37) | 0.41 (0.30 to 0.53) | 0.05 | |
| CKD stage | 98.71 | ||||
| Control | 5 | −0.10 (−0.36 to 0.17) | 0.18 (0.07 to 0.34) | 0.49 | |
| Kidney transplantation | 4 | Ref | 0.26 (0.11 to 0.46) | Ref | |
| CKD without KRT | 4 | 0.23 (−0.06 to 0.52) | 0.49 (0.29 to 0.69) | 0.12 | |
| Hemodialysis | 25 | 0.21 (−0.01 to 0.43) | 0.46 (0.39 to 0.55) | 0.06 | |
| Peritoneal dialysis | 4 | 0.35 (0.06 to 0.64) | 0.61 (0.40 to 0.80) | 0.02 | |
| CKD with or without KRT | 4 | 0.07 (−0.21 to 0.35) | 0.33 (0.16 to 0.52) | 0.63 | |
| WHO regionsa | 97.67 | ||||
| European | 15 | Ref | 0.32 (0.23 to 0.41) | Ref | |
| Americas | 7 | 0.24 (0.08 to 0.40) | 0.56 (0.42 to 0.69) | 0.003 | |
| Western Pacific | 3 | 0.36 (0.13 to 0.59) | 0.67 (0.47 to 0.85) | 0.002 | |
| Eastern Mediterranean | 8 | 0.17 (0.02 to 0.33) | 0.49 (0.36 to 0.62) | 0.03 | |
| South-East Asia Region | 1 | 0.04 (−0.33 to 0.41) | 0.36 (0.07 to 0.71) | 0.83 | |
| Risk of bias | 98.47 | ||||
| Low | 7 | Ref | 0.50 (0.35 to 0.65) | Ref | |
| Moderate | 13 | −0.15 (−0.34 to 0.03) | 0.35 (0.25 to 0.46) | 0.10 | |
| High | 15 | −0.01 (−0.19 to 0.17) | 0.49 (0.39 to 0.59) | 0.93 | |
| Diagnostic criteria of insomnia | 98.66 | ||||
| Complaint-basedb | 16 | Ref | 0.49 (0.38 to 0.59) | Ref | |
| Diagnostic tool–basedc | 19 | −0.09 (−0.23 to 0.05) | 0.40 (0.31 to 0.49) | 0.21 |
Abbreviations: CKD, chronic kidney disease; KRT, kidney replacement therapy; Ref, reference; WHO, World Health Organization.
In our analysis of WHO regions, we excluded the study by Elder et al,15 which comprised data from 3 WHO regions.
Primary criteria (difficulty initiating sleep, difficulty maintaining sleep, early morning awakening, and nonrestorative sleep), together with at least 1 of the secondary criteria listed in Table S2.
Included the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision; International Classification of Sleep Disorders, version 2; Athens Insomnia Scale; and Insomnia Severity Index.
Figure 4.
Random-effects meta-regression plots showing the relationships between age and the prevalences of (A) poor sleep quality and (B) insomnia, according to different CKD spectrums and types of kidney replacement therapy. Abbreviations: CKD, chronic kidney disease; KRT, kidney replacement therapy.
Discussion
The results of this systematic review suggest that poor sleep quality and insomnia result in considerable health care burdens, with prevalences of 64% and 45%, respectively, in patients with CKD, which are much higher than the prevalences in the control populations without CKD in this systematic review (54% and 18%, respectively) or estimates derived from general adult populations in the literature (10%-48% for poor sleep quality and 10%-15% for insomnia).40, 41, 42, 43 Kidney transplantation significantly reduced the prevalence of insomnia among patients receiving HD or PD. Non-European patients with CKD and patients aged >50 years tended to have higher prevalences of insomnia. Nonetheless, the meta-analysis findings must be carefully interpreted due to high heterogeneity, which reflected significant variation among the included studies in the definitions of outcomes, study designs, and study populations.
Insomnia and poor sleep quality often co-occur with cardiovascular disease, psychiatric illnesses, and impaired social and physical functioning, all of which frequently complicate the course of CKD.44, 45, 46, 47 Given the mutually dependent and reinforcing interactions of kidney-heart-brain crosstalk, the prevalences of insomnia and poor sleep quality have unsurprisingly increased substantially worldwide among patients with CKD.48, 49, 50 The gradient increase in the magnitude of prevalence of sleep disturbances across stages and severity levels of CKD and the ability to reduce the prevalence of these problems among patients with kidney failure treated with kidney transplantation further supports the hypothesis that CKD is an independent risk factor for insomnia and poor sleep quality.51,52 The progression of CKD likely creates a vicious cycle of sleep disorders, CKD, and CKD-related complications. However, due to insufficient robust evidence on the relationships between insomnia and poor sleep quality and CKD, the latest KDIGO guidelines for CKD care did not emphasize the problem or recommend specific treatments for insomnia and poor sleep quality.53
Only a few clinical trials have evaluated the effects of cognitive behavioral therapy for insomnia on patients undergoing PD or HD.54, 55, 56 However, the sample sizes of these trials were small, and they did not evaluate health outcomes, such as mortality or cardiovascular events. Large, prospective studies are warranted to assess whether cognitive behavioral therapy for insomnia can modify the course of CKD and to further extend the breadth of evidence to dialysis quality and improvement among patients with kidney failure. Similarly, insufficient evidence was found to support the effectiveness of pharmacological therapies for insomnia in patients with CKD in terms of drug selection, dosing, and duration.6 Some studies have reported that melatonin use at bedtime improved sleep problems in HD patients; however, the long-term efficacy of melatonin was not observed.57, 58, 59 It would be an urgent priority to pursue an effective, pragmatic intervention to alleviate the potential long-term, adverse impacts of insomnia on CKD, such as cardiovascular events and mortality, in this vulnerable population.60
Insomnia and poor sleep quality have been linked to increased risks of mortality among patients with CKD, particularly when hypnotic therapy is used for a prolonged period.15,61, 62, 63, 64 However, the causality remains debated, because sleep disturbances may only be a downstream marker of major underlying, confounding factors, such as chronic inflammation, a heavy burden of comorbid conditions, or inadequate dialysis.65 For example, HD timing may affect sleep quality; several studies have reported that evening dialysis is associated with better sleep quality than is daytime dialysis.66,67
The considerable heterogeneity in the present meta-analysis may be because of the poor methodological quality of most studies, which poses a serious threat to validity, especially when comparisons of the burden of sleep disturbances are being made with regard to dialysis modalities, geographical regions, and definitions of outcome diagnoses. For example, a high pooled prevalence of sleep disturbance was calculated among patients who received HD or PD; however, few studies have evaluated (with high interstudy heterogeneity) the prevalences and incidences of insomnia and poor sleep quality in patients who receive PD, which renders valid comparisons between HD and PD impossible.68, 69, 70, 71
The pathogenic mechanism underlying sleep disturbances in CKD remains unclear. Although chronic inflammation because of uremia has been implicated in the development of sleep disturbances, the associations between inflammatory markers, such as C-reactive protein, tumor necrosis factor α, interleukin 6, and interleukin 18, and insomnia and poor sleep quality are not universally present among patients with CKD who require dialysis.72, 73, 74, 75 Other possible mechanisms, such as elevated blood orexin and the depletion of melatonin, may induce sleep disturbances by altering patients’ circadian rhythms.57,76 Common symptoms of CKD, and especially kidney failure treated by KRT, such as refractory pruritus, restless leg syndrome, depression, and anxiety, may also contribute to the development of insomnia and poor sleep quality.6,16, 17, 18,77
This study has several limitations. First, the pooled prevalence estimates must be interpreted with caution because of high interstudy heterogeneity. Moreover, the observed publication bias regarding insomnia studies compromised the validity of the prevalence estimates. Second, of the selected patients with kidney failure treated by dialysis, 21,422 (approximately 95.5%) received HD, whereas only 1,010 (approximately 4.5%) received PD. Such an imbalance prevented the comparison of the pooled prevalences between HD and PD. Third, only a few studies provided individual participant data, which rendered thorough investigations of the sources of heterogeneity and biases using aggregated data impossible. Fourth, this study may have missed some relevant work because we did not include unpublished literature, such as conference abstracts or research theses, and we did not update the literature search to the present time. However, publication bias was not evident in our study. Fifth, we did not include studies measuring poor sleep quality using a tool other than the PSQI, such as the Kidney Disease and Quality of Life, Choices for Healthy Outcomes in Caring for ESRD Health Experience Questionnaire, or Medical Outcomes Study Instruments. Nonetheless, as we discovered in the initial phase of our study, regardless of the diagnostic criteria adopted, the pooled prevalences were 50%, 61%, 63%, and 48% for patients with CKD without KRT, HD, PD, and kidney transplantation.
In summary, insomnia and poor sleep quality are pervasive in patients with CKD, and kidney transplantation treatment may solve those problems. The actual prognostic benefits resulting from the improvement of sleep hygiene in patients with CKD warrant further investigation. More research, such as intervention trials, is also required to assess the effectiveness and potentially harmful consequences of the current treatments for sleep disturbances associated with CKD.
Article Information
Authors’ Full Names and Academic Degrees
Lek-Hong Tan, MD, MPH, Pei-Shan Chen, MS, Hsiu-Yin Chiang, PhD, MS, Emily King, MPH, Hung-Chieh Yeh, MD, Ya-Luan Hsiao, MD, MPH, David Ray Chang, MD, Sheng-Hsuan Chen, MS, Min-Yen Wu, MS, and Chin-Chi Kuo, MD, PhD.
Authors’ Contributions
Study design: L-HT, H-YC, EK, C-CK; data analysis: P-SC, S-HC, M-YW; hypothesis review and clinical insights: H-CY, DRC, EK; data interpretation: all authors; mentorship/supervision: L-HT, C-CK. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved.
Support
This study was supported by the Ministry of Science and Technology of Taiwan (grants: DMR-HHC-110-5, MOST-108-2314-B-039-038-MY3, MOST-110-2314-B-039-013, and MOST-110-2321-B-468-001-).
Financial Disclosure
The authors declare that they have no relevant financial interests.
Acknowledgements
We appreciate the data exploration, statistical analysis, manuscript preparation, and support of the iHi Clinical Research Platform from the Big Data Center of China Medical University Hospital. We would like to thank the Health and Welfare Data Science Center, Ministry of Health Welfare, and Health Data Science Center, China Medical University Hospital for providing administrative, technical, and funding support.
Peer Review
Received July 14, 2021. Evaluated by 3 external peer reviewers, with direct editorial input from an Associate Editor and the Editor-in-Chief. Accepted in revised form February 6, 2022.
Footnotes
Complete author and article information provided before references.
Figure S1: Risk assessment of bias for included studies.
Figure S2: Forest plots of the prevalence of insomnia and poor sleep, by chronic kidney disease (CKD) stage.
Figure S3: Random-effects meta-regression plots showing relationships between age and prevalences of (A) poor sleep quality and (B) insomnia in patients with hemodialysis.
Figure S4: Forest plots of the prevalences of insomnia and poor sleep by different peritoneal dialysis types.
Figure S5: Funnel plot of studies evaluating the prevalences of (A) poor sleep quality and (B) insomnia in patients with chronic kidney disease (CKD).
Table S1: Detailed search strategies for PubMed or MEDLINE, Embase, and APA PsycNET from January 1990 to September 2018.
Table S2: Inclusion criteria of insomnia disorders assimilated from the American Academy of Sleep Medicine (AASM) inclusion criteria.
Table S3: Fourteen American Academy of Sleep Medicine (AASM) inclusion criteria of insomnia before assimilation.
Table S4: Studies evaluating the prevalence of poor sleep among patients with chronic kidney disease (CKD).
Table S5: Studies evaluating the prevalence of insomnia among patients with chronic kidney disease (CKD).
Table S6: Risk assessment of bias for included studies.
Table S7: Pooled prevalence of poor sleep quality and insomnia calculated by random and fixed effects.
Supplementary Material
Fig S1-S5, Table S1-S7.
References
- 1.Lyon L. Is an epidemic of sleeplessness increasing the incidence of Alzheimer’s disease? Brain. 2019;142(6):e30. doi: 10.1093/brain/awz087. [DOI] [PubMed] [Google Scholar]
- 2.Morin C.M., Benca R. Chronic insomnia. Lancet. 2012;379(9821):1129–1141. doi: 10.1016/S0140-6736(11)60750-2. [DOI] [PubMed] [Google Scholar]
- 3.Stranges S., Tigbe W., Gómez-Olivé F.X., Thorogood M., Kandala N.B. Sleep problems: an emerging global epidemic? Findings from the INDEPTH WHO-SAGE study among more than 40,000 older adults from 8 countries across Africa and Asia. Sleep. 2012;35(8):1173–1181. doi: 10.5665/sleep.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walsh J.K., Engelhardt C.L. The direct economic costs of insomnia in the United States for 1995. Sleep. 1999;22(suppl 2):S386–S393. [PubMed] [Google Scholar]
- 5.Stoller M.K. Economic effects of insomnia. Clin Ther. 1994;16(5):873–897. discussion 854. [PubMed] [Google Scholar]
- 6.Lindner A.V., Novak M., Bohra M., Mucsi I. Insomnia in patients with chronic kidney disease. Semin Nephrol. 2015;35(4):359–372. doi: 10.1016/j.semnephrol.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 7.Jhamb M., Liang K., Yabes J., et al. Prevalence and correlates of fatigue in chronic kidney disease and end-stage renal disease: are sleep disorders a key to understanding fatigue? Am J Nephrol. 2013;38(6):489–495. doi: 10.1159/000356939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Iliescu E.A., Coo H., McMurray M.H., et al. Quality of sleep and health-related quality of life in haemodialysis patients. Nephrol Dial Transplant. 2003;18(1):126–132. doi: 10.1093/ndt/18.1.126. [DOI] [PubMed] [Google Scholar]
- 9.Maung S.C., El Sara A.E., Chapman C., Cohen D., Cukor D. Sleep disorders and chronic kidney disease. World J Nephrol. 2016;5(3):224–232. doi: 10.5527/wjn.v5.i3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tu C.Y., Chou Y.H., Lin Y.H., Huang W.L. Sleep and emotional disturbance in patients with non-dialysis chronic kidney disease. J Formos Med Assoc. 2019;118(6):986–994. doi: 10.1016/j.jfma.2018.10.016. [DOI] [PubMed] [Google Scholar]
- 11.Ogna A., Forni Ogna V., Haba Rubio J., et al. Sleep characteristics in early stages of chronic kidney disease in the HypnoLaus cohort. Sleep. 2016;39(4):945–953. doi: 10.5665/sleep.5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brekke F.B., Waldum B., Amro A., et al. Self-perceived quality of sleep and mortality in Norwegian dialysis patients. Hemodial Int. 2014;18(1):87–94. doi: 10.1111/hdi.12066. [DOI] [PubMed] [Google Scholar]
- 13.Unruh M.L., Buysse D.J., Dew M.A., et al. Sleep quality and its correlates in the first year of dialysis. Clin J Am Soc Nephrol. 2006;1(4):802–810. doi: 10.2215/CJN.00710206. [DOI] [PubMed] [Google Scholar]
- 14.Lu J.L., Freire A.X., Molnar M.Z., Kalantar-Zadeh K., Kovesdy C.P. Association of chronic insomnia with mortality and adverse renal outcomes. Mayo Clin Proc. 2018;93(11):1563–1570. doi: 10.1016/j.mayocp.2018.05.032. [DOI] [PubMed] [Google Scholar]
- 15.Elder S.J., Pisoni R.L., Akizawa T., et al. Sleep quality predicts quality of life and mortality risk in haemodialysis patients: results from the Dialysis Outcomes and Practice Patterns Study (DOPPS) Nephrol Dial Transplant. 2008;23(3):998–1004. doi: 10.1093/ndt/gfm630. [DOI] [PubMed] [Google Scholar]
- 16.Novak M., Shapiro C.M., Mendelssohn D., Mucsi I. Diagnosis and management of insomnia in dialysis patients. Semin Dial. 2006;19(1):25–31. doi: 10.1111/j.1525-139X.2006.00116.x. [DOI] [PubMed] [Google Scholar]
- 17.Virga G., Mastrosimone S., Amici G., Munaretto G., Gastaldon F., Bonadonna A. Symptoms in hemodialysis patients and their relationship with biochemical and demographic parameters. Int J Artif Organs. 1998;21(12):788–793. [PubMed] [Google Scholar]
- 18.Loewen A., Siemens A., Hanly P. Sleep disruption in patients with sleep apnea and end-stage renal disease. J Clin Sleep Med. 2009;5(4):324–329. [PMC free article] [PubMed] [Google Scholar]
- 19.Koch B.C., Nagtegaal J.E., Hagen E.C., et al. Subjective sleep efficiency of hemodialysis patients. Clin Nephrol. 2008;70(5):411–416. doi: 10.5414/cnp70411. [DOI] [PubMed] [Google Scholar]
- 20.Abassi M.R., Safavi A., Haghverdi M., Saedi B. Sleep disorders in ESRD patients undergoing hemodialysis. Acta Med Iran. 2016;54(3):176–184. [PubMed] [Google Scholar]
- 21.Hamzi M.A., Hassani K., Asseraji M., El Kabbaj D. Insomnia in hemodialysis patients: a multicenter study from morocco. Saudi J Kidney Dis Transpl. 2017;28(5):1112–1118. doi: 10.4103/1319-2442.215152. [DOI] [PubMed] [Google Scholar]
- 22.Sabry A.A., Abo-Zenah H., Wafa E., et al. Sleep disorders in hemodialysis patients. Saudi J Kidney Dis Transpl. 2010;21(2):300–305. [PubMed] [Google Scholar]
- 23.Ponticelli C., Sala G., Glassock R.J. Drug management in the elderly adult with chronic kidney disease: a review for the primary care physician. Mayo Clin Proc. 2015;90(5):633–645. doi: 10.1016/j.mayocp.2015.01.016. [DOI] [PubMed] [Google Scholar]
- 24.Goto N.A., Hamaker M.E., Willems H.C., Verhaar M.C., Emmelot-Vonk M.H. Accidental falling in community-dwelling elderly with chronic kidney disease. Int Urol Nephrol. 2019;51(1):119–127. doi: 10.1007/s11255-018-1992-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stroup D.F., Berlin J.A., Morton S.C., et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D.G., PRISMA Group Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269. doi: 10.7326/0003-4819-151-4-200908180-00135. W64. [DOI] [PubMed] [Google Scholar]
- 27.Buysse D.J., Reynolds C.F., Monk T.H., Berman S.R., Kupfer D.J. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 28.Edinger J.D., Bonnet M.H., Bootzin R.R., et al. Derivation of research diagnostic criteria for insomnia: report of an American Academy of Sleep Medicine Work Group. Sleep. 2004;27(8):1567–1596. doi: 10.1093/sleep/27.8.1567. [DOI] [PubMed] [Google Scholar]
- 29.American Psychiatric Association . 4th ed. American Psychiatric Association; 2000. Diagnostic and Statistical Manual of Mental Disorders. [Google Scholar]
- 30.American Sleep Disorders Association . American Sleep Disorders Association; 1997. International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manuals. [Google Scholar]
- 31.Soldatos C.R., Dikeos D.G., Paparrigopoulos T.J. Athens Insomnia Scale: validation of an instrument based on ICD-10 criteria. J Psychosom Res. 2000;48(6):555–560. doi: 10.1016/s0022-3999(00)00095-7. [DOI] [PubMed] [Google Scholar]
- 32.Bastien C.H., Vallières A., Morin C.M. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi: 10.1016/s1389-9457(00)00065-4. [DOI] [PubMed] [Google Scholar]
- 33.Hoy D., Brooks P., Woolf A., et al. Assessing risk of bias in prevalence studies: modification of an existing tool and evidence of interrater agreement. J Clin Epidemiol. 2012;65(9):934–939. doi: 10.1016/j.jclinepi.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Barendregt J.J., Doi S.A., Lee Y.Y., Norman R.E., Vos T. Meta-analysis of prevalence. J Epidemiol Community Health. 2013;67(11):974–978. doi: 10.1136/jech-2013-203104. [DOI] [PubMed] [Google Scholar]
- 35.Veroniki A.A., Jackson D., Viechtbauer W., et al. Methods to estimate the between-study variance and its uncertainty in meta-analysis. Res Synth Methods. 2016;7(1):55–79. doi: 10.1002/jrsm.1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Burkhalter H., Brunner D.P., Wirz-Justice A., et al. Self-reported sleep disturbances in renal transplant recipients. BMC Nephrol. 2013;14:220. doi: 10.1186/1471-2369-14-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chavoshi F., Einollahi B., Sadeghniat Haghighi K., Saraei M., Izadianmehr N. Prevalence and sleep related disorders of restless leg syndrome in hemodialysis patients. Nephrourol Mon. 2015;7(2) doi: 10.5812/numonthly.24611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Razeghi E., Sahraian M.A., Heidari R., Bagherzadeh M. Association of inflammatory biomarkers with sleep disorders in hemodialysis patients. Acta Neurol Belg. 2012;112(1):45–49. doi: 10.1007/s13760-012-0003-7. [DOI] [PubMed] [Google Scholar]
- 39.Yazdi Z., Sadeghniiat-Haghighi K., Kazemifar A.M., Kordi A., Naghipour S. Restless leg syndrome in hemodialysis patients: a disorder that should be noticed. Saudi J Kidney Dis Transpl. 2015;26(3):625–650. doi: 10.4103/1319-2442.157431. [DOI] [PubMed] [Google Scholar]
- 40.Insomnia: assessment and management in primary care. National Heart, Lung, and Blood Institute Working Group on Insomnia. Am Fam Physician. 1999;59(11):3029–3038. [PubMed] [Google Scholar]
- 41.Schutte-Rodin S., Broch L., Buysse D., Dorsey C., Sateia M. Clinical guideline for the evaluation and management of chronic insomnia in adults. J Clin Sleep Med. 2008;4(5):487–504. [PMC free article] [PubMed] [Google Scholar]
- 42.Ohayon M.M., Smirne S. Prevalence and consequences of insomnia disorders in the general population of Italy. Sleep Med. 2002;3(2):115–120. doi: 10.1016/s1389-9457(01)00158-7. [DOI] [PubMed] [Google Scholar]
- 43.Wong W.S., Fielding R. Prevalence of insomnia among Chinese adults in Hong Kong: a population-based study. J Sleep Res. 2011;20(1 Pt 1):117–126. doi: 10.1111/j.1365-2869.2010.00822.x. [DOI] [PubMed] [Google Scholar]
- 44.Larsson S.C., Markus H.S. Genetic liability to insomnia and cardiovascular disease risk. Circulation. 2019;140(9):796–798. doi: 10.1161/CIRCULATIONAHA.119.041830. [DOI] [PubMed] [Google Scholar]
- 45.Freeman D., Sheaves B., Waite F., Harvey A.G., Harrison P.J. Sleep disturbance and psychiatric disorders. Lancet Psychiatry. 2020;7(7):628–637. doi: 10.1016/S2215-0366(20)30136-X. [DOI] [PubMed] [Google Scholar]
- 46.Wade A.G. The societal costs of insomnia. Neuropsychiatr Dis Treat. 2010;7:1–18. doi: 10.2147/NDT.S15123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Murtagh F.E., Addington-Hall J., Higginson I.J. The prevalence of symptoms in end-stage renal disease: a systematic review. Adv Chronic Kidney Dis. 2007;14(1):82–99. doi: 10.1053/j.ackd.2006.10.001. [DOI] [PubMed] [Google Scholar]
- 48.Rangaswami J., Bhalla V., Blair J.E.A., et al. Cardiorenal syndrome: classification, pathophysiology, diagnosis, and treatment strategies: a scientific statement from the American Heart Association. Circulation. 2019;139(16):e840–e878. doi: 10.1161/CIR.0000000000000664. [DOI] [PubMed] [Google Scholar]
- 49.Lu R., Kiernan M.C., Murray A., Rosner M.H., Ronco C. Kidney-brain crosstalk in the acute and chronic setting. Nat Rev Nephrol. 2015;11(12):707–719. doi: 10.1038/nrneph.2015.131. [DOI] [PubMed] [Google Scholar]
- 50.Tanaka S., Okusa M.D. Crosstalk between the nervous system and the kidney. Kidney Int. 2020;97(3):466–476. doi: 10.1016/j.kint.2019.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Russcher M., Nagtegaal J.E., Nurmohamed S.A., et al. The effects of kidney transplantation on sleep, melatonin, circadian rhythm and quality of life in kidney transplant recipients and living donors. Nephron. 2015;129(1):6–15. doi: 10.1159/000369308. [DOI] [PubMed] [Google Scholar]
- 52.Novak M., Molnar M.Z., Ambrus C., et al. Chronic insomnia in kidney transplant recipients. Am J Kidney Dis. 2006;47(4):655–665. doi: 10.1053/j.ajkd.2005.12.035. [DOI] [PubMed] [Google Scholar]
- 53.KDIGO Working Group KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 54.Chen H.Y., Chiang C.K., Wang H.H., et al. Cognitive-behavioral therapy for sleep disturbance in patients undergoing peritoneal dialysis: a pilot randomized controlled trial. Am J Kidney Dis. 2008;52(2):314–323. doi: 10.1053/j.ajkd.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 55.Chen H.Y., Cheng I.C., Pan Y.J., et al. Cognitive-behavioral therapy for sleep disturbance decreases inflammatory cytokines and oxidative stress in hemodialysis patients. Kidney Int. 2011;80(4):415–422. doi: 10.1038/ki.2011.151. [DOI] [PubMed] [Google Scholar]
- 56.Hou Y., Hu P., Liang Y., Mo Z. Effects of cognitive behavioral therapy on insomnia of maintenance hemodialysis patients. Cell Biochem Biophys. 2014;69(3):531–537. doi: 10.1007/s12013-014-9828-4. [DOI] [PubMed] [Google Scholar]
- 57.Edalat-Nejad M., Haqhverdi F., Hossein-Tabar T., Ahmadian M. Melatonin improves sleep quality in hemodialysis patients. Indian J Nephrol. 2013;23(4):264–269. doi: 10.4103/0971-4065.114488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koch B.C., Nagtegaal J.E., Hagen E.C., et al. The effects of melatonin on sleep-wake rhythm of daytime haemodialysis patients: a randomized, placebo-controlled, cross-over study (EMSCAP study) Br J Clin Pharmacol. 2009;67(1):68–75. doi: 10.1111/j.1365-2125.2008.03320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Russcher M., Koch B.C., Nagtegaal J.E., et al. Long-term effects of melatonin on quality of life and sleep in haemodialysis patients (Melody study): a randomized controlled trial. Br J Clin Pharmacol. 2013;76(5):668–679. doi: 10.1111/bcp.12093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rouleau C.R., Toivonen K., Aggarwal S., Arena R., Campbell T.S. The association between insomnia symptoms and cardiovascular risk factors in patients who complete outpatient cardiac rehabilitation. Sleep Med. 2017;32:201–207. doi: 10.1016/j.sleep.2017.01.005. [DOI] [PubMed] [Google Scholar]
- 61.Sofi F., Cesari F., Casini A., Macchi C., Abbate R., Gensini G.F. Insomnia and risk of cardiovascular disease: a meta-analysis. Eur J Prev Cardiol. 2014;21(1):57–64. doi: 10.1177/2047487312460020. [DOI] [PubMed] [Google Scholar]
- 62.de Zambotti M., Goldstone A., Colrain I.M., Baker F.C. Insomnia disorder in adolescence: diagnosis, impact, and treatment. Sleep Med Rev. 2018;39:12–24. doi: 10.1016/j.smrv.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Amaral M.O., de Almeida Garrido A.J., de Figueiredo Pereira C., Master N.V., de Rosário Delgado Nunes C., Sakellarides C.T. Quality of life, sleepiness and depressive symptoms in adolescents with insomnia: a cross-sectional study. Aten Primaria. 2017;49(1):35–41. doi: 10.1016/j.aprim.2016.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lovato N., Lack L. Insomnia and mortality: a meta-analysis. Sleep Med Rev. 2019;43:71–83. doi: 10.1016/j.smrv.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 65.Perl J., Unruh M.L., Chan C.T. Sleep disorders in end-stage renal disease: “markers of inadequate dialysis”? Kidney Int. 2006;70(10):1687–1693. doi: 10.1038/sj.ki.5001791. [DOI] [PubMed] [Google Scholar]
- 66.Barmar B., Dang Q., Isquith D., Buysse D., Unruh M. Comparison of sleep/wake behavior in CKD stages 4 to 5 and hemodialysis populations using wrist actigraphy. Am J Kidney Dis. 2009;53(4):665–672. doi: 10.1053/j.ajkd.2008.10.045. [DOI] [PubMed] [Google Scholar]
- 67.Hsu C.Y., Lee C.T., Lee Y.J., et al. Better sleep quality and less daytime symptoms in patients on evening hemodialysis: a questionnaire-based study. Artif Organs. 2008;32(9):711–716. doi: 10.1111/j.1525-1594.2008.00593.x. [DOI] [PubMed] [Google Scholar]
- 68.Hui D.S.C., Wong T.Y.H., Ko F.W.S., et al. Prevalence of sleep disturbances in Chinese patients with end-stage renal failure on continuous ambulatory peritoneal dialysis. Am J Kidney Dis. 2000;36(4):783–788. doi: 10.1053/ajkd.2000.17664. [DOI] [PubMed] [Google Scholar]
- 69.Huyge L., Locking-Cusolito H. Incidence of sleep pattern disturbance in a peritoneal dialysis sample. Adv Perit Dial. 2000;16:156–162. [PubMed] [Google Scholar]
- 70.Losso R.L.M., Minhoto G.R., Riella M.C. Sleep disorders in patients with end-stage renal disease undergoing dialysis: comparison between hemodialysis, continuous ambulatory peritoneal dialysis and automated peritoneal dialysis. Int Urol Nephrol. 2015;47(2):369–375. doi: 10.1007/s11255-014-0860-5. [DOI] [PubMed] [Google Scholar]
- 71.Yngman-Uhlin P., Edéll-Gustafsson U. Self-reported subjective sleep quality and fatigue in patients with peritoneal dialysis treatment at home. Int J Nurs Pract. 2006;12(3):143–152. doi: 10.1111/j.1440-172X.2006.00566.x. [DOI] [PubMed] [Google Scholar]
- 72.Chiu Y.L., Chuang Y.F., Fang K.C., et al. Higher systemic inflammation is associated with poorer sleep quality in stable haemodialysis patients. Nephrol Dial Transplant. 2009;24(1):247–251. doi: 10.1093/ndt/gfn439. [DOI] [PubMed] [Google Scholar]
- 73.Erten Y., Kokturk O., Yuksel A., et al. Relationship between sleep complaints and proinflammatory cytokines in haemodialysis patients. Nephrology (Carlton) 2005;10(4):330–335. doi: 10.1111/j.1440-1797.2005.00418.x. [DOI] [PubMed] [Google Scholar]
- 74.Yang J.Y., Huang J.W., Chiang C.K., et al. Higher plasma interleukin-18 levels associated with poor quality of sleep in peritoneal dialysis patients. Nephrol Dial Transplant. 2007;22(12):3606–3609. doi: 10.1093/ndt/gfm231. [DOI] [PubMed] [Google Scholar]
- 75.Fornadi K., Lindner A., Czira M.E., et al. Lack of association between objectively assessed sleep disorders and inflammatory markers among kidney transplant recipients. Int Urol Nephrol. 2012;44(2):607–617. doi: 10.1007/s11255-011-0095-7. [DOI] [PubMed] [Google Scholar]
- 76.Rayner H.C. Orexin as a possible cause of insomnia in dialysis patients. Am J Kidney Dis. 2003;41(6):1335–1336. doi: 10.1016/s0272-6386(03)00516-x. author reply 1336. [DOI] [PubMed] [Google Scholar]
- 77.Hanly P.J., Pierratos A. Improvement of sleep apnea in patients with chronic renal failure who undergo nocturnal hemodialysis. N Engl J Med. 2001;344(2):102–107. doi: 10.1056/NEJM200101113440204. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1-S5, Table S1-S7.