Abstract
Climate change has been implicated in an increased number of distributional shifts of marine species during the last century. Nonetheless, it is unclear whether earlier climatic fluctuations had similar impacts. We use ancient DNA to investigate the long-term spawning distribution of the Northeast Arctic cod (skrei) which performs yearly migrations from the Barents Sea towards spawning grounds along the Norwegian coast. The distribution of these spawning grounds has shifted northwards during the last century, which is thought to be associated with food availability and warming temperatures. We genetically identify skrei specimens from Ruskeneset in west Norway, an archaeological site located south of their current spawning range. Remarkably, 14C analyses date these specimens to the late Holocene, when temperatures were warmer than present-day conditions. Our results either suggest that temperature is not the only driver influencing the spawning distribution of Atlantic cod, or could be indicative of uncertainty in palaeoclimate reconstructions in this region. Regardless, our findings highlight the utility of aDNA to reconstruct the historical distribution of economically important fish populations and reveal the complexity of long-term ecological interactions in the marine environment.
Keywords: warm period, historical distribution, spawning distribution, feeding grounds, re-distribution, ecological interactions
1. Introduction
Significant poleward shifts in the distribution of marine species have been observed during the last century and have been associated with global warming [1]. The description of species distributions under a changing climate may yield fundamental insights into ecosystem dynamics and responses to future climate change. Nonetheless, we still have a poor understanding of the historical distribution of marine species during the late Holocene.
Atlantic cod, an economically important and highly exploited fish species in the North Atlantic Ocean, comprises various stocks with different life-history characteristics. Along the Norwegian coast, two distinct ecological ecotypes of Atlantic cod have been identified. The ‘stationary’ ecotype (Norwegian coastal cod, NCC) spawns along the Norwegian coast and has limited migration between spawning and feeding areas [2,3]. By contrast, the ‘migratory’ ecotype (Northeast Arctic cod, NEAC), also known as ‘skrei’ (from the old Norse ‘the wanderer’), migrates every year during winter–spring (March to beginning of May) from colder feeding grounds (down to −1.5° C) in the Barents Sea towards warmer (up to 8° C) spawning areas along the Norwegian coast like Finnmark, Troms, Lofoten and Møre (figure 1a) [4,5]. In particular, the Lofoten archipelago has been the major spawning ground of skrei since at least medieval times, when relevant historical records first appeared [6]. Right after spawning, skrei eggs, larvae and juveniles will drift ca 600–1200 km towards the northeast of the Barents Sea, following the Norwegian Coastal Current (NwCC) and the Norwegian Atlantic Current (NwAC; figure 1a) [3,7].
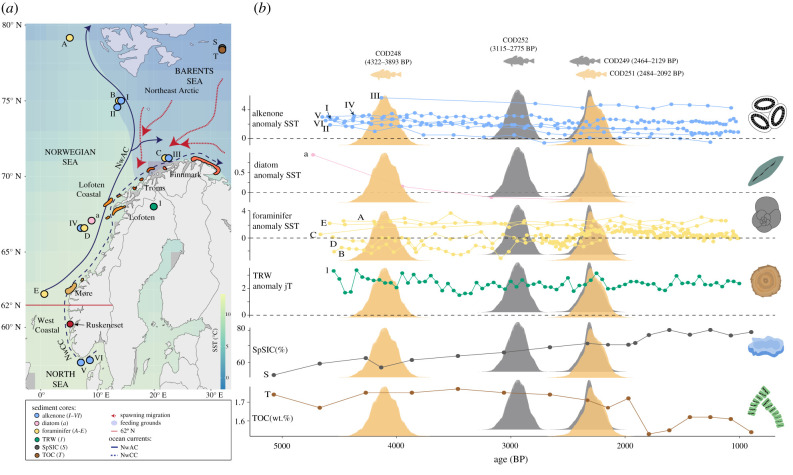
Figure 1.
(a) Distribution of spawning sites for the migratory skrei ecotype from top to bottom: Finnmark (orange), Troms (dark orange), Lofoten (light orange) and Møre (light brown). Spawning map and details are adapted from Sundby and Nakken [4]. Blue arrows indicate the pathway of the NwAC and the NwCC. Red arrows indicate the spawning migration of skrei from feeding grounds. No skrei is currently observed below 62° N [3]. The background colour indicates average Norwegian and Barents Sea sea-surface temperature (SST) during January to December 2021. The distribution of the sediment core locations used in the study are highlighted per colour according to each proxy: alkenone (in blue: I, II, III, IV, V and VI), diatom (in pink: a), foraminifer (in yellow: A,B,C,D and E), tree-ring (TRW, in green: 1), spring sea-ice composition (SpSIC, in grey: S) and total organic carbon (TOC, in brown: T). (b) Historical climate reconstructions presented as an individual line for each sediment core for each proxy (electronic supplementary material, figure S2 for individual locations) with a dotted reference at 0° C in all temperature graphs. SST and July temperatures (jT) anomalies were calculated with respect to the long-term 1981–2010 average for their specific location (see electronic supplementary material, methods for details on long-term means). 14C dating range (orange for skrei and grey for stationary ecotype) are shown for each ancient Atlantic cod (see electronic supplementary material, figure S1 and table S1 for details). Specimen COD253 was not dated due to insufficient bone material. Fish illustrations were drawn by Geir Holm. Tree-ring, diatom, foraminifer, alkenone, sea ice and TOC illustrations were drawn by Lourdes Martínez-García.
Recent observations have shown a pronounced northward re-distribution of skrei [3,5]. The causes for this shift have been debated [2,4,8], although a northward movement of prey in the Barents Sea, directly influenced by an increase in sea temperatures, has been implicated [4]. Displacement of skrei feeding grounds lengthens migration distances to southern spawning locations (i.e. Møre) and could potentially influence the spawning latitude of skrei [5]. Nonetheless, it is unclear whether historical climate fluctuations along with ecological interactions (i.e. prey–predator interaction) have similarly influenced such distributions over longer temporal scales. Archaeological bone assemblages of skrei and the stationary ecotype are morphologically similar and difficult to distinguish with certainty [6]. Yet, the two ecotypes are genetically different, with significant population differentiation in several chromosomal inversions [6,9]. Genome-wide scans of such chromosomal regions allow the identification of individual skrei ecotypes with high confidence [6], even when using low-coverage sequence data from poorly preserved archaeological specimens [10].
We used ancient DNA (aDNA) to study the long-term spawning distribution of the stationary and migratory Atlantic cod ecotypes. We obtained genome-wide data of five archaeological specimens (ca 4322–2092 cal. BP) from Ruskeneset, west Norway (figure 1a and electronic supplementary material, table S1). Given the low latitudes of Ruskeneset, overall warmer climatic conditions and low sea-ice conditions in the Barents Sea during the late Holocene (within the past ca 5000 years, figure 1b and electronic supplementary material, figure S2) [11], and the contemporary temperature-related shifts in distribution, we expected that our ancient specimens would comprise stationary ecotypes.
2. Material and methods
(a) . Sample collection and age calibration
Ancient samples (n = 6) retrieved in 1914–1916 at the archaeological site Ruskeneset in the municipality of Bergen, west Norway (60.23° N – 5.15° E) [12] were used to extract DNA. The zooarchaeological assemblage (bones) from Ruskeneset are in the osteological collections at the Natural History Department, the University Museum, University of Bergen.
Ruskeneset is a rock-shelter area in the western coast of Norway which preserves evidence of human activities (e.g. bones, shells and archaeological elements) dating back to the late Neolithic and Bronze Age [13]. During the Bronze Age, the shelter would have been nearly inaccessible from land due to steep cliffs on both east and west, with easier access from the seaside by boat (N. Anfinset pers. comm.). Moreover, the fishing and hunting gear findings (e.g. harpoons, hooks, arrowheads and daggers) indicate that this was a hunting and fishing station rather than a permanent coastal settlement [13]. The site is located close to tidal current channels and is at a lower latitude than the current spawning grounds of skrei [3,4]. Four specimens were dated using 14C content (figure 1b; electronic supplementary material, figure S1 and table S1). Age calibration of the samples was calculated in OxCal v. 4.4.4 [14] using the Marine20 calibration curve [15]. We used slightly different ΔR values for the stationary (−164 ± 29) and skrei (−144 ± 46) ecotypes to account for differences in the marine reservoir effect given that these ecotypes feed either around the coast of Norway or in the Barents Sea (figure 1b; electronic supplementary material, figure S1) [15,16].
(b) . DNA extraction and library amplification
All ancient samples were processed in the aDNA laboratory at the University of Oslo under rigorous conditions [17,18]. DNA extraction and library preparation were according to Ferrari et al. [19]. Ancient read data for five specimens were processed using PALEOMIX 2.13 [20]. Sequencing reads were trimmed, filtered and collapsed using AdapterRemoval v. 2.1.7 [21], and aligned to the Atlantic cod gadMor2 nuclear genome [22,23] using BWA backtrack v. 0.7.12 [24] with a minimum quality score of 25. DNA postmortem damage was assessed using MapDamage v. 2.0.9 [25] and the resulting BAM files were indexed with samtools v. 1.9 [26]. Additional details of the laboratory protocols are provided in the electronic supplementary material.
(c) . Genomic statistical analyses
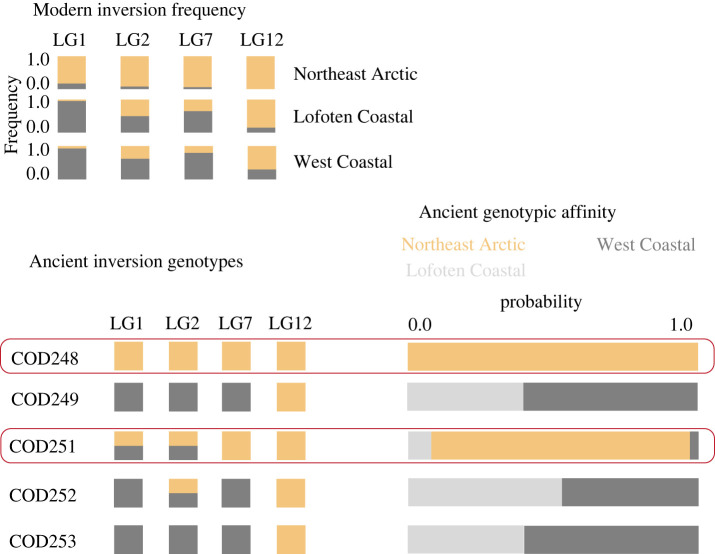
Four different chromosomal inversions associated with migratory behaviour and temperature clines were investigated (LG1, LG2, LG7 and LG12) to determine the probability of the ancient Atlantic cod specimens to be skrei [9,27–30]. These chromosomal inversions differ in their affinity towards a particular geographic area as previously described in Star et al. [6]. The BAMscorer pipeline [10] was used to assign inversion haplotypes. First, the Atlantic cod reference SNP database from Ferrari et al. [10] was used to associate divergent SNPs to different haplotypes. This reference SNP database includes 276 Atlantic cod individuals from three geographical locations (western Atlantic, eastern Atlantic and Baltic Sea) [31,32] across the species' range. Second, five ancient Atlantic cod specimens were compared to the reference dataset with score_bams. Ancient specimens were identified as skrei or stationary Lofoten Coastal or stationary (Norwegian) West Coastal individuals using the population specific chromosomal inversion frequencies obtained from Star et al. [6] and Johansen et al. [33].
(d) . Reference palaeoclimate datasets
To describe the climate as reflected during the late Holocene, particularly during the period of the Atlantic cod ancient samples (ca 4322–2092 cal. BP), a range of previously published marine and terrestrial palaeoreconstructions were compiled using temperature, spring sea-ice conditions (SpSIC) and total organic carbon (TOC) reconstructions along the Norwegian coast, Scandinavia and northern Barents Sea (electronic supplementary material, table S2). The localities of these datasets overlap with the spatial distribution of spawning and feeding areas of skrei (figure 1a).
Marine palaeoreconstructions are established from reference sea-surface temperature (SST) datasets based on three different proxies: alkenone (UK’37) [34–36], planktic foraminifer [34,37,38] and diatom assemblages [39]. Reference SpSIC dataset is based on the seasonal sea-ice biomarker IP25 [40], while TOC is based on the open water phytoplankton biomarkers brassicasterol and HBI III [40]. For further comparisons, SpSIC previously reported in Pieńkowski et al. [41] was included. This dataset includes recent observations of persisting levels of seasonal sea-ice during the Holocene Thermal Maximum (6000–10 000 cal. BP; electronic supplementary material, figure S2). The terrestrial palaeoreconstruction is established from a reference July temperature (jT) dataset based on tree-ring width (TRW) data [42]. TRW was selected because tree growth is a reliable and sensitive proxy for climatic conditions (e.g. temperatures, precipitation and drought) [43]. All temperatures are presented as an individual line for each sediment core for each proxy (figure 1b) and as individual graphs (electronic supplementary material, figure S2) to avoid introducing uncertainty between proxies. Full details of climate datasets are provided in the electronic supplementary material and electronic supplementary material, table S2.
3. Results
We successfully extracted aDNA from five out of six Atlantic cod specimens and radiocarbon dated four specimens (electronic supplementary material, table S1). Sequencing reads showed the patterns of DNA fragmentation and deamination rates that are associated with authentic aDNA (electronic supplementary material, figure S3). Our sequencing results yield approximately 59 million paired reads, with between 1% and 7% endogenous DNA and approximately 74 000 to approximately 1 million aligned reads for five specimens (electronic supplementary material, table S1). This is sufficient coverage to unequivocally determine the genotype of the four major chromosomal inversions of Atlantic cod (LG1, LG2, LG7 and LG12; electronic supplementary material, table S3). Two out of five specimens (40%) were identified as skrei with a near 100% probability (figure 2; electronic supplementary material, tables S3 and S4). The specimens were dated to three different periods approximately 4300, approximately 3100 and approximately 2400 cal. BP which is consistent with previous dates obtained for Ruskeneset [13,44]. These specimens represent the oldest genetically identified southern skrei to date. Although our sample size remains limited, our findings suggest a presence of skrei at Ruskeneset between ca. 4322 and 2092 cal. BP at overall warmer temperatures than present-day conditions (figure 1b; electronic supplementary material, figure S2).
Figure 2.
Modern inversion frequencies for LG1, LG2, LG7 and LG12 in Northeast Arctic, Lofoten Coastal and (Norwegian) West Coastal populations, and individual ancient inversion status. Binomial probability calculations identify two Atlantic cod specimens as skrei following Star et al. [6] (see electronic supplementary material, tables S3 and S4 for complete assignment probabilities).
4. Discussion
Several reasons may explain the historical skrei presence at lower and warmer latitudes than today. First, skrei could have been obtained from northern latitudes and transported to Ruskeneset. Such transport would indicate the mobility of human settlements during the late Neolithic and/or Bronze Age from northern to southern Norway. Nonetheless, bone material and artefact evidence indicate that Ruskeneset was a hunting site associated with local marine exploitation [13]. Transport of skrei from northern Norway to this location therefore seems improbable.
Second, it is possible that the association of these inversion haplotypes with the skrei ecotype is of a recent evolutionary origin, and that the adaptive association we use to distinguish each ecotype has not remained stable across time. Nevertheless, the evolutionary origin of these inversions is dated to 0.4 and 1.66 million years ago, and they have been selectively maintained within the Atlantic cod populations ever since [9,30]. It would seem unlikely that the association of such evolutionary ancient genetic variants with this distinct behaviour evolved as recently as the last millennia.
Third, although no skrei is currently observed below 62° N (figure 1a), they were sporadically observed below 62° N during the start of the twentieth century [8]. The impact of fishing has been hypothesized for the current absence of such southern spawners—through the removal of larger individuals with greater capacity for migration—however, larger fish are not associated with increased migration distance [2]. The reasons for these observations, therefore, remain unknown. The archaeological southern latitude of skrei could reflect such sporadic spawning events, possibly during short cold spells—representing annual inter-variability—experienced within the date range of each individual and the temperature variability resolution of the palaeoclimate reconstructions (figure 1b; electronic supplementary material, figure S2). Moreover, the complexity of marine reservoir correction on tissues from animals feeding at latitudes higher than 50° N adds uncertainty to the precision of radiocarbon dates. Regardless, the probability of observing such sporadic southern spawning events would appear low, given that only a few specimens were sampled over a ca 4000 years period of natural history. Our results, therefore, tentatively suggest more frequent southern spawning of skrei during the late Holocene.
Finally, there may be uncertainty around the climatic reconstructions in the Barents Sea. A recent observation has identified persisting levels of seasonal sea-ice during the entire Holocene Thermal Maximum (6000–10 000 cal. BP) in this region [41] (electronic supplementary material, figure S2). Consequently, as the climate in the Barents Sea further cooled during the late Holocene (ca 5900 cal. BP to present) [11], this region may have had reduced primary productivity and more significant ice cover than currently estimated [40]. Such a scenario could have resulted in more southern located feeding grounds and decreasing migration distance towards lower latitude spawning areas. Our observations would agree with such more extensive presence of sea-ice than currently assumed during the late Holocene in the Barents Sea.
Taken together, we here identify the oldest known migratory ecotype in an archaeological Atlantic cod fishbone assemblage. Although the reasons for their southern distribution during the late Holocene remain unclear, our results highlight the utility of aDNA to reconstruct the historical distribution of economically important fish populations. Our findings indicate that the response of marine species to present-day and future climate change may be more complex than currently anticipated.
Acknowledgements
We thank Angélica Cuevas for advice during analyses and discussion. We thank Birgitte Skar and Erlend Jørgensen for providing advice regarding Neolithic and Bronze Age trade patterns. We thank Nils Anfinset for providing Ruskeneset excavation details. We also thank Tor Eldevik, Bjørg Risebrobakken and Hans Sejrup for providing information about palaeoreconstructions and for literature advice. Finally, we thank M. Skage, S. Kollias and A. Tooming-Klunderud at the Norwegian Sequencing Centre for sequencing and processing of samples. The NOAA Extended Reconstructed Sea Surface Temperature SST V5 data (https://psl.noaa.gov/data/gridded/data.noaa.ersst.v5.html) and the Global Historical Climatology and the Climate Anomaly Monitoring System Gridded 2 m Temperature (Land) (https://psl.noaa.gov/data/gridded/data.ghcncams.html) were provided by the NOAA/OAR/ESRL PSL, Boulder, Colorado, USA, from their website (https://psl.noaa.gov/). Analyses were performed on resources provided by UNINETT Sigma2 – the National Infrastructure for High Performance Computing and Data Storage in Norway.
Contributor Information
Lourdes Martínez-García, Email: l.m.garcia@ibv.uio.no.
Bastiaan Star, Email: bastiaan.star@ibv.uio.no.
Ethics
No modern specimens were sampled for this study. Sampling of the zooarchaeological assemblage (bones) from Ruskeneset was approved by the University Museum, Department of Culture, Bergen with reference no. 2018/13857.
Data accessibility
Modern reference raw sequence data have been released earlier under ENA accession nos. PRJEB29231 and PRJEB41431. The raw reads for the ancient specimens for this study are released under ENA accession no. PRJEB49220.
The data are provided in the electronic supplementary material [45].
Authors' contributions
L.M.-G.: conceptualization, data curation, formal analysis, visualization, writing—original draft and writing—review and editing; G.F.: data curation, methodology and writing—review and editing; A.K.H.: resources and writing—review and editing; K.S.J.: funding acquisition and writing—review and editing; S.J.: funding acquisition and writing—review and editing; J.H.B.: conceptualization, formal analysis, funding acquisition, resources, supervision, visualization and writing—review and editing; B.S.: conceptualization, formal analysis, funding acquisition, project administration, supervision, visualization, writing—original draft and writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
This work was supported by Research Council of Norway projects ‘Catching the Past’ (grant no. 262777) and the European Union's Horizon 2020 Research and Innovation Programme under the Marie Sklodowska-Curie grant agreement no. 813383. The European Research Agency is not responsible for any use that may be made of the information this work contains.
References
- 1.Hastings RA, Rutterford LA, Freer JJ, Collins RA, Simpson SD, Genner MJ. 2020. Climate change drives poleward increases and equatorward declines in marine species. Curr. Biol. 30, 1572-1577. ( 10.1016/j.cub.2020.02.043) [DOI] [PubMed] [Google Scholar]
- 2.Langangen Ø, et al. 2019. Ticket to spawn: combining economic and genetic data to evaluate the effect of climate and demographic structure on spawning distribution in Atlantic cod. Glob. Change Biol. 25, 134-143. ( 10.1111/gcb.14474) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jorde PE, Huserbråten MBO, Seliussen BB, Myksvoll MS, Vikebø FB, Dahle G, Aglen A, Johansen T. 2021. The making of a genetic cline: introgression of oceanic genes into coastal cod populations in the Northeast Atlantic. Can. J. Fish. Aquat. Sci. 78, 958-968. ( 10.1139/cjfas-2020-0380) [DOI] [Google Scholar]
- 4.Sundby S, Nakken O. 2008. Spatial shifts in spawning habitats of Arcto-Norwegian cod related to multidecadal climate oscillations and climate change. ICES J. Mar. Sci. 65, 953-962. ( 10.1093/icesjms/fsn085) [DOI] [Google Scholar]
- 5.Sandø A, Johansen G, Aglen A, Stiansen JE, Renner A. 2020. Climate change and new potential spawning sites for Northeast Arctic cod. Front. Mar. Sci. 7, 28. ( 10.3389/fmars.2020.00028) [DOI] [Google Scholar]
- 6.Star B, et al. 2017. Ancient DNA reveals the Arctic origin of Viking Age cod from Haithabu, Germany. Proc. Natl Acad. Sci. USA 114, 9152-9157. ( 10.1073/pnas.1710186114) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ottersen G, Bogstad B, Yaragina NA, Stige LC, Vikebø FB, Dalpadado P. 2014. A review of early life history dynamics of Barents Sea cod (Gadus morhua). ICES J. Mar. Sci. 71, 2064-2087. ( 10.1093/icesjms/fsu037) [DOI] [Google Scholar]
- 8.Jørgensen C, Dunlop ES, Opdal AF, Fiksen Ø. 2008. The evolution of spawning migrations: state dependence and fishing-induced changes. Ecology 89, 3436-3448. ( 10.1890/07-1469.1) [DOI] [PubMed] [Google Scholar]
- 9.Matschiner M, et al. 2022. Supergene origin and maintenance in Atlantic cod. Nat. Ecol. Evol. 6, 469-481. ( 10.1038/s41559-022-01661-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ferrari G, Atmore LM, Jentoft S, Jakobsen KS, Makowiecki D, Barrett JH, Star B. 2021. An accurate assignment test for extremely low-coverage whole-genome sequence data. Mol. Ecol. Res. 1, 15. ( 10.1101/2021.06.04.447098) [DOI] [PubMed] [Google Scholar]
- 11.Wanner H, et al. 2008. Mid- to Late Holocene climate change: an overview. Quat. Sci. Rev. 27, 1791-1828. ( 10.1016/j.quascirev.2008.06.013) [DOI] [Google Scholar]
- 12.Brinkmann A, Shetelig H. 1920. III ruskenesset: en Stenalders Jaktplass (III Ruskenesset: a Stone Age Hunter Site). Christiania, Norway: A.W. Brøggers Boktrykkeri A/S. [Google Scholar]
- 13.Melheim AL. 2012. Recycling ideas: bronze age metal production in southern Norway. PhD thesis, University of Oslo, Oslo, Norway. [Google Scholar]
- 14.Ramsey CB. 2009. Bayesian analysis of radiocarbon dates. Radiocarbon 51, 337-360. ( 10.1017/S0033822200033865) [DOI] [Google Scholar]
- 15.Heaton TJ, et al. 2020. Marine20—the marine radiocarbon age calibration curve (0–55,000 cal BP). Radiocarbon 62, 779-820. ( 10.1017/RDC.2020.68) [DOI] [Google Scholar]
- 16.Reimer PJ, Reimer RW. 2001. A marine reservoir correction database and on-line interface. Radiocarbon 43, 461-463. ( 10.1017/S0033822200038339) [DOI] [Google Scholar]
- 17.Cooper A, Poinar HN. 2000. Ancient DNA: do it right or not at all. Science 289, 1139. ( 10.1126/science.289.5482.1139b) [DOI] [PubMed] [Google Scholar]
- 18.Gilbert MTP, Bandelt HJ, Hofreiter M, Barnes I. 2005. Assessing ancient DNA studies. Trends Ecol. Evol. 20, 541-544. ( 10.1016/j.tree.2005.07.005) [DOI] [PubMed] [Google Scholar]
- 19.Ferrari G, et al. 2021. The preservation of ancient DNA in archaeological fish bone. J. Archaeol. Sci. 126, 105317. ( 10.1016/j.jas.2020.105317) [DOI] [Google Scholar]
- 20.Schubert M, et al. 2014. Characterization of ancient and modern genomes by SNP detection and phylogenomic and metagenomic analysis using PALEOMIX. Nat. Protoc. 9, 1056. ( 10.1038/nprot.2014.063) [DOI] [PubMed] [Google Scholar]
- 21.Lindgreen S. 2012. AdapterRemoval: easy cleaning of next-generation sequencing reads. BMC Res. Notes 5, 337. ( 10.1186/1756-0500-5-337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Star B, et al. 2011. The genome sequence of Atlantic cod reveals a unique immune system. Nature 477, 207-210. ( 10.1038/nature10342) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tørresen OK, et al. 2017. An improved genome assembly uncovers prolific tandem repeats in Atlantic cod. BMC Genomics 18, 1-23. ( 10.1186/s12864-016-3448-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754-1760. ( 10.1093/bioinformatics/btp324) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jónsson H, Ginolhac A, Schubert M, Johnson PL, Orlando L. 2013. mapDamage2. 0: fast approximate Bayesian estimates of ancient DNA damage parameters. Bioinformatics 29, 1682-1684. ( 10.1093/bioinformatics/btt193) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li H, Handsaker B, Wysoker A, Fennell T, Ruan J, Homer N, Marth G, Abecasis G, Durbin R. 2009. The sequence alignment/map format and SAMtools. Bioinformatics 25, 2078-2079. ( 10.1093/bioinformatics/btp352) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Barney BT, Munkholm C, Walt DR, Palumbi SR. 2017. Highly localized divergence within supergenes in Atlantic cod (Gadus morhua) within the Gulf of Maine. BMC Genomics 18, 1-14. ( 10.1186/s12864-017-3660-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodeland M, et al. 2016. ‘Islands of divergence’ in the Atlantic cod genome represent polymorphic chromosomal rearrangements. Genome Biol. Evol. 8, 1012-1022. ( 10.1093/gbe/evw057) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berg PR, Star B, Pampoulie C, Sodeland M, Barth JM, Knutsen H, Jakobsen KS, Jentoft S. 2016. Three chromosomal rearrangements promote genomic divergence between migratory and stationary ecotypes of Atlantic cod. Sci. Rep. 6, 1-12. ( 10.1038/s41598-016-0001-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Berg PR, Star B, Pampoulie C, Bradbury IR, Bentzen P, Hutchings JA, Jentoft S, Jakobsen KS. 2017. Trans-oceanic genomic divergence of Atlantic cod ecotypes is associated with large inversions. Heredity 119, 418-428. ( 10.1038/hdy.2017.54) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barth JMI, et al. 2019. Disentangling structural genomic and behavioural barriers in a sea of connectivity. Mol. Ecol. 28, 1394-1411. ( 10.1111/mec.15010) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinsky ML, et al. 2021. Genomic stability through time despite decades of exploitation in cod on both sides of the Atlantic. Proc. Natl Acad. Sci. USA 118, e2025453118. ( 10.1073/pnas.2025453118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Johansen T, Besnier F, Quintela M, Jorde PE, Glover KA, Westgaard JI, Dahle G, Lien S, Kent MP. 2020. Genomic analysis reveals neutral and adaptive patterns that challenge the current management regime for East Atlantic cod Gadus morhua L. Evol. Appl. 13, 2673-2688. ( 10.1111/eva.13070) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Eldevik T, et al. 2014. A brief history of climate—the northern seas from the Last Glacial Maximum to global warming. Quat. Sci. Rev. 106, 225-246. ( 10.1016/j.quascirev.2014.06.028) [DOI] [Google Scholar]
- 35.Emeis KC, Struck U, Blanz T, Kohly A, Voβ M. 2003. Salinity changes in the central Baltic Sea (NW Europe) over the last 10 000 years. The Holocene 13, 411-421. ( 10.1191/0959683603hl634rp) [DOI] [Google Scholar]
- 36.Rigual-Hernandez AS, et al. 2017. Svalbard ice-sheet decay after the Last Glacial Maximum: new insights from micropalaeontological and organic biomarker paleoceanographical reconstructions. Palaeogeogr. Palaeoclimatol. Palaeoecol. 465, 225-236. ( 10.1016/j.palaeo.2016.10.034) [DOI] [Google Scholar]
- 37.Werner K, Müller J, Husum K, Spielhagen RF, Kandiano ES, Polyak L. 2016. Holocene sea subsurface and surface water masses in the Fram Strait—comparisons of temperature and sea-ice reconstructions. Quat. Sci. Rev. 147, 194-209. ( 10.1016/j.quascirev.2015.09.007) [DOI] [Google Scholar]
- 38.Risebrobakken B, Dokken T, Smedsrud LH, Andersson C, Jansen E, Moros M, Ivanova EV. 2011. Early Holocene temperature variability in the Nordic Seas: the role of oceanic heat advection versus changes in orbital forcing. Paleoceanography 26, PA4206. ( 10.1029/2011PA002117) [DOI] [Google Scholar]
- 39.Koç N, Jansen E, Haflidason H. 1993. Paleoceanographic reconstructions of surface ocean conditions in the Greenland, Iceland and Norwegian seas through the last 14 ka based on diatoms. Quat. Sci. Rev. 12, 115-140. ( 10.1016/0277-3791(93)90012-B) [DOI] [Google Scholar]
- 40.Berben SM, Husum K, Navarro-Rodriguez A, Belt ST, Aagaard-Sørensen S. 2017. Semi-quantitative reconstruction of early to late Holocene spring and summer sea ice conditions in the northern Barents Sea. J. Q. Sci. 32, 587-603. ( 10.1002/jqs.2953) [DOI] [Google Scholar]
- 41.Pieńkowski AJ, et al. 2021. Seasonal sea ice persisted through the Holocene thermal maximum at 80°N. Commun. Earth Environ. 2, 124. ( 10.1038/s43247-021-00191-x) [DOI] [Google Scholar]
- 42.Helama S, Seppä H, Bjune AE, Birks HJB. 2012. Fusing pollen-stratigraphic and dendroclimatic proxy data to reconstruct summer temperature variability during the past 7.5 ka in subarctic Fennoscandia. J. Paleolimnol. 48, 275-286. ( 10.1007/s10933-012-9598-1) [DOI] [Google Scholar]
- 43.Linderholm HW, Chen D. 2005. Central Scandinavian winter precipitation variability during the past five centuries reconstructed from Pinus sylvestris tree rings. Boreas 34, 43-52. ( 10.1111/j.1502-3885.2005.tb01003.x) [DOI] [Google Scholar]
- 44.Hjelle KL, Hufthammer AK, Bergsvik KA. 2006. Hesitant hunters: a review of the introduction of agriculture in western Norway. Environ. Archaeol. 11, 147-170. ( 10.1179/174963106(123188) [DOI] [Google Scholar]
- 45.Martínez-García L, Ferrari G, Hufthammer AK, Jakobsen KS, Jentoft S, Barrett JH, Star B. 2022. Ancient DNA reveals a southern presence of the Northeast Arctic cod during the Holocene. FigShare. ( 10.6084/m9.figshare.c.5942011) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Martínez-García L, Ferrari G, Hufthammer AK, Jakobsen KS, Jentoft S, Barrett JH, Star B. 2022. Ancient DNA reveals a southern presence of the Northeast Arctic cod during the Holocene. FigShare. ( 10.6084/m9.figshare.c.5942011) [DOI] [PMC free article] [PubMed]
Data Availability Statement
Modern reference raw sequence data have been released earlier under ENA accession nos. PRJEB29231 and PRJEB41431. The raw reads for the ancient specimens for this study are released under ENA accession no. PRJEB49220.
The data are provided in the electronic supplementary material [45].