Abstract
Predators can strongly influence prey populations through both consumptive and non-consumptive effects. Nevertheless, most studies have focused on the consumptive effects in driving evolutionary changes. By integrating experimental evolution and resurrection ecology, we tested the roles of non-consumptive and consumptive effects in driving evolution in a Daphnia magna population that experienced strong changes in fish predation pressure. All resurrected genotypes were pooled, inoculated in outdoor mesocosms, and exposed to free-fish or caged-fish treatments. Non-consumptive effects induced rapid, repeatable changes in the clonal composition and associated genotypic trait changes that were similar in magnitude and direction to those imposed by killing. Both non-consumptive and consumptive effects caused a shift towards a dominance of the high-fish period clones that can perform better under fish predation, and this may be explained by the higher intrinsic growth rate of the high-fish period clones under predation risk. The genotypic trait changes (e.g. reduced body sizes, earlier maturation, more and smaller offspring) of the Daphnia in the mesocosm experiments were in the same direction as the adaptive trait shifts observed in situ through resurrection ecology. Our results demonstrate that non-consumptive effects can induce rapid adaptive evolution and may represent an overlooked driver of eco-evolutionary dynamics.
Keywords: eco-evolutionary dynamics, experimental evolution, non-consumptive effects, predator–prey interactions, micro-evolution, resurrection ecology
1. Introduction
Predators play a pivotal role in population, community and ecosystem ecology [1]. Predators can affect prey in two ways: through consumptive and non-consumptive effects [2–4]. Direct killing is well known as a strong selective agent that may cause rapid adaptive evolution in prey populations [5–7]. Non-consumptive effects in turn are well known to plastically induce changes in prey life history, behaviour, morphology and physiology [8–10], and may even cause mortality [11–13]. Nevertheless, non-consumptive effects have been understudied as a selective agent and the potential role of non-consumptive effects in driving rapid evolution remains untested. Quantifying and contrasting both types of predator effects that co-occur in nature is crucial because they may be of the same magnitude [1], yet may differ in direction [12]. For instance, if the development of costly defences induced by non-consumptive effects results in a lower fitness of the prey in the absence of actual killing [14], one may expect that there would be selection against individuals that are very sensitive to predator kairomones in conditions where the predator does not impose a real threat.
Two powerful ways to study rapid evolution are experimental evolution [15], where a selective factor is directly imposed on experimental populations, and resurrection ecology [16], where subpopulations separated in time of a natural population that underwent known changes in a selective factor are resurrected to reconstruct evolution as it occurred in nature [17,18]. Both approaches have been widely used, and by integrating both approaches, one can obtain a powerful test of hypotheses in a controlled and replicated way on whether specific selective factor(s) can drive evolution along the trajectory as observed in nature. When using predators as a selective factor, experimental designs including treatments with a caged-fish or free-fish predator would allow disentangling the non-consumptive and consumptive effects in driving rapid evolution.
Here, we carried out experimental evolution trials under replicated, semi-natural outdoor conditions to test, and compared the roles of non-consumptive and consumptive effects in driving rapid evolution in the water flea Daphnia. We capitalized on a resurrection study of a natural population of Daphnia magna in which evolution was quantified across three historical fish-stocking periods with distinct selection pressures: no fish predation pressure (pre-fish), high fish predation pressure (high-fish) and reduced fish predation pressure (reduced-fish) [6,19]. We then carried out experimental evolution trials with the three subpopulations matching these historical fish-stocking periods under three mesocosm treatments: no fish present (no-fish), a caged fish (caged-fish, where fine gauze prevented direct predation) and a free fish (free-fish). The experimental mesocosm populations were composed of an equal mixture of six clones from each of the three resurrected subpopulations matching the historical fish-stocking periods that were studied earlier [6,19]. After six weeks of selection, we quantified shifts in clonal composition of each experimental Daphnia population, and found that not only direct killing but also non-consumptive effects itself favoured the D. magna clones from the high-fish period that can better deal with fish predation. By integrating the shifts in clonal composition with the previously determined genotypic trait values for 14 life-history, morphology and behaviour traits [6], we reconstructed the evolutionary shifts of the multivariate genotypic trait values for the experimentally selected populations and compared these with the resurrected natural population. This approach enabled us to directly compare the outcome of the experimental evolution trials with realized evolutionary trajectories in nature. We found that the genotypic trait changes in the experimental mesocosm populations were similar under non-consumptive effects and under direct killing, and moreover in the same direction as those in the natural population under fish predation pressure. These results show that non-consumptive effects may induce rapid adaptive evolution of anti-predator traits in prey populations, and thus may be an overlooked driver of eco-evolutionary dynamics between predators and their prey.
2. Material and methods
(a) . Daphnia population and laboratory culture
Clones of D. magna were hatched from dormant eggs retrieved from layered sediments of a natural pond in Oud-Heverlee, Belgium (50°50′22.16″ N, 4°39′18.16″ E). This pond has a well-documented historical record of fish abundance over 30 years: no fish stocking in the first years after digging from 1970 to 1972 (‘pre-fish' period); high fish stocking (greater than 250 kg ha−1) from 1976 to 1979 (‘high-fish' period); and reduced fish stocking from 1988 to 1990 (‘reduced-fish' period) [19]. Resting eggs were hatched from three depths of a sediment core, corresponding to these three historical fish stocking periods [19]. The low genetic differentiation in neutral microsatellite markers among clones of the three periods indicates that they are one continuous population [19]. For the current study, we used six clones of each resurrected subpopulation matching the historical fish-stocking periods (total of 18 clones), a subset of the clones used in a previous study [6]. Clonal lineages were established and kept in the laboratory prior to the experiment. The probability of mutations impacting the genotypic trait values of individual clones is low even over a period of 15 years due to the low annual turnover in individuals [20].
To prepare the experiment, we started five independent maternal lines of each clone under standardized conditions (bio-filtered tap water, 20 ± 1°C, 14 light : 10 dark, daily feeding with 1 × 105 cells ml−1 of the green alga Scenedesmus obliquus, refreshment of the culture medium every other day). The animals were cultured under those standard conditions for two generations in the laboratory to minimize the interference from maternal effects prior to the mesocosm experiment. For the mesocosm experiment, we worked with juveniles from the second brood.
(b) . Mesocosm experiment
To quantify and compare the non-consumptive and consumptive effects, we exposed experimental mesocosm populations for six weeks to one of three predator selection regimes in outdoor mesocosms: no-fish treatment, caged-fish treatment and free-fish treatment. In the caged-fish treatment, the fish predators could not consume the prey but could impose non-consumptive effects through chemical cues (i.e. fish kairomones). In the free-fish treatment, both non-consumptive and consumptive effects could occur. Experimental mesocosm populations containing six clones from each of the three historical fish-stocking periods were inoculated in the mesocosms. This allowed to test for rapid evolution by quantifying shifts in the frequencies of the clones from the three historical fish-stocking periods. Each mesocosm was started with an experimental population of 144 juveniles where each clone (i.e. eight juveniles per clone) was equally represented. Juveniles from the second brood that were 24–48 h old were used for inoculation. Each treatment combination was replicated in five mesocosms resulting in total of 15 mesocosms (3 mesocosm predator treatments × 5 replicates).
Mesocosms were cylindrical polyethylene 210 l containers placed on an open grass field at the outdoor experimental area of the Division of Ecology, Evolution and Conservation Biology in Heverlee, Belgium. All mesocosms contained a fish cage made of plastic netting (mesh size 5.5 mm) that occupied the upper third of the mesocosm volume, leaving a refuge at the bottom and along the sides of the mesocosm which could be used by Daphnia to avoid fish predation through diel vertical and horizontal migration [20]. All mesocosms were covered on top with netting (mesh size 1.2 mm) to prevent insects entering. For the caged-fish treatment, the fish was kept in the upper third of the fish cage using a gauze (mesh size 0.15 mm) that allowed kairomones to diffuse but that prevented fish from killing Daphnia. The gauze was placed inside the plastic netting and the Daphnia could not move through the gauze, hence could not be directly eaten by the fish. The gauze used in the caged-fish treatment did not affect the water temperature compared to the other two treatments (electronic supplementary material, figure S1). For the free-fish treatment, there were no gauze that the fish could swim freely inside the plastic netting.
On 27 June 2016 (day 1), each mesocosm was filled with 170 l of tap water and 20 l of twice filtered (64 µm mesh size) pond water (mixture from three ponds). On 1 July (day 5), a 50 ml inoculum (100 × 106 cells ml−1) of S. obliquus algae was added to each mesocosm. The addition of pond water and the Scenedesmus inoculum aimed at stimulating the phytoplankton growth. After two weeks, the mesocosms were randomly assigned to one of the three predation treatments (no-fish, caged-fish, free-fish). On that day (14 July, day 18), each mesocosm received 144 juvenile Daphnia equally representing the mixed experimental population (see above for details). Four weeks after Daphnia inoculation (12 August, day 46), approximately two parthenogenetic Daphnia generations at 20°C, one three-spined stickleback (Gasterosteus aculeatus, body length 4.7 ± 0.4 cm) was added to each cage of the free-fish and caged-fish mesocosms. During the experiment, the fish from the caged-fish treatment were fed frozen Daphnia for two hours outside the mesocosm every 2 days. Caged fish were not allowed to eat in the mesocosms to avoid the release of Daphnia alarm cues in the mesocosms. All fish (from both caged-fish and free-fish treatments) were randomly redistributed once a week within each treatment group to eliminate any possible biases that might arise due to differential activity among individual fishes.
Nutrients were supplied to the mesocosms to support algal growth as food resource for Daphnia. On 7 July (day 11) an initial dose of 1.942 g NaNO3 and 0.774 g KH2PO4 was added to each mesocosm. This corresponds to 1600 µg l−1 nitrogen and 100 µg l−1 phosphorus which are medium nutrient levels [21,22]. Afterwards, a maintenance dose of 50% of the original dosage of N and p was supplied once a week throughout the experiment. It has been shown that any nutrient input by fish excretion in the mesocosms would be overwhelmed by this external nutrient supply [22].
(c) . Mesocosm sampling
Sampling was done by taking a 4 l water sample after gently mixing the water in the mesocosm and filtering it over a plankton gauze (mesh size 64 µm). To determine the clonal composition in the experimental mesocosm populations, 24 individuals were randomly picked out per mesocosm, immediately preserved in absolute ethanol and stored at 4°C. The remaining Daphnia in the samples were preserved in 4% formaldehyde for quantifying density and body size. For some mesocosms (especially those of the free-fish treatment), more volume of water was sampled to get enough individuals. Daphnia were sampled every two weeks to monitor the Daphnia population densities and after eight weeks of selection the experiment was terminated (7 October, day 102). As temperatures suddenly dropped below 15°C on 29 September (electronic supplementary material, figure S1), thereby strongly affecting the Daphnia population densities, Daphnia samples after six weeks of selection (25 September, day 90) were used for further analyses.
(d) . Body size and density of Daphnia
Average Daphnia body size per sample was quantified both based on the first 10 adults and on the first 10 random individuals from each sample. The number of adult and juvenile D. magna individuals was quantified by counting a minimum of 300 individuals from each sample using a stereomicroscope (Olympus ZS X-12). The counts were extrapolated to densities in the mesocosms (number of individuals per litre) [20]. Daphnia adults (all female) and juveniles were differentiated based on the length of the first abdominal process, which is clearly elongated in adults compared to immature females [23].
(e) . Shifts in clonal composition
We quantified the clonal composition in each experimental mesocosm population after six weeks of selection to assess the shifts of clonal composition in response to fish predation. We genotyped 24 randomly selected individuals from each mesocosm following [24,25]. The details of the genotyping procedures are in the electronic supplementary material, methods. All individuals per mesocosm sample were attributed to one of the experimental clones based on their microsatellite signature and clones were further classified into each of the three historical fish-stocking periods.
(f) . Statistical analyses
To test the effects of the mesocosm predator treatment on the frequencies of clones from the different historical fish-stocking periods in the experimental mesocosm populations we used a general linear mixed model (GLMM) with the predator treatment, historical fish-stocking period and their interaction as fixed effects. To take into account that each set of three clonal frequencies (one frequency per historical fish-stocking period) belonged to the same mecosom, we added mesocosm nested within the predator treatment as random effect. A significant mesocosm predator treatment × historical fish-stocking period interaction would indicate that any specific shifts in the proportional representation of clones from different historical fish-stocking periods depended on the specific predator treatment. The relative frequencies of the clones from the different historical fish-stocking periods were compared within each treatment using linear contrasts with false discovery rate corrected p-values. For the caged-fish treatment, we identified one mesocosm as an outlier for the frequency of high-fish period clones based on the interquartile range method [26,27] (see electronic supplementary material, figure S2), and we also ran the analyses without this outlier mesocosm. As a measure of effect size for the interaction term in the analyses with and without the outlier, we used partial η2-values [28]. In addition, as we expected the high-fish period clones to better cope with predation, we also specifically compared the relative frequencies of these clones among the three treatments with a one-way ANOVA. To compare the clonal composition of the experimental mesocosm populations among the three mesocosm treatments after six weeks of selection to the start population in the mesocosms, we used a GLMM that included historical fish-stocking period, experimental stage (start versus after six weeks) and their interactions. Densities were log-transformed to meet the assumptions of normality and homogeneity of variance. All tests were conducted using the packages lme4, car, effects, lsmeans and multcomp in R v. 3.4.1. p-values < 0.05 were considered significant.
To reconstruct the evolutionary shifts in the multivariate genotypic trait values under fish predation in the mesocosm experiment and compare these shifts to evolution in the natural population, we combined the information on the final clonal composition under each of the three mesocosm predator treatments (no-fish, caged-fish, free-fish) and the previously determined genotypic trait values of each of the 18 clones [6]. For the latter, we used the following 14 life-history, behavioural and morphological traits known to be important to deal with consumptive fish predation in this natural population: size of neonates, size at maturity, early and late offspring size, somatic growth rate, spine length neonates, spine length at maturity, age at maturity, early and late fecundity, intrinsic growth rate, phototactic behaviour, horizontal migration index and alertness (see details in electronic supplementary material, Supporting Information). We first performed a discriminant function analysis on the genotypic trait values of all 18 clones for these 14 traits measured under control (absence of fish cues) conditions to extract two roots. Then, we combined the genotypic values for each clone and the clonal composition of each resurrected subpopulation and mesocosm experimental population to calculate the bivariate means (for the first two roots) of each (sub)population using the equations of both discriminant functions. We did so for the experimental mesocosm populations at the start and at the end of the experiment as well as for the resurrected natural subpopulations. Using the same equations of both discriminant functions, we also calculated the bivariate means for the trait values measured in the presence of fish cues. We plotted the resulting bivariate means for each (sub)population to visualize the (sub)population shifts in the phenotypic space determined by the first two roots. The differences among the natural subpopulations were compared using MANOVA with the bivariate means as response variables. The bivariate means for each experimental mesocosm population after six weeks of selection were compared to the start condition (bivariate mean: 0, 0) using separate one-sample t-tests.
3. Results
(a) . Predator-induced shifts in prey body size and population densities in experimental mesocosms
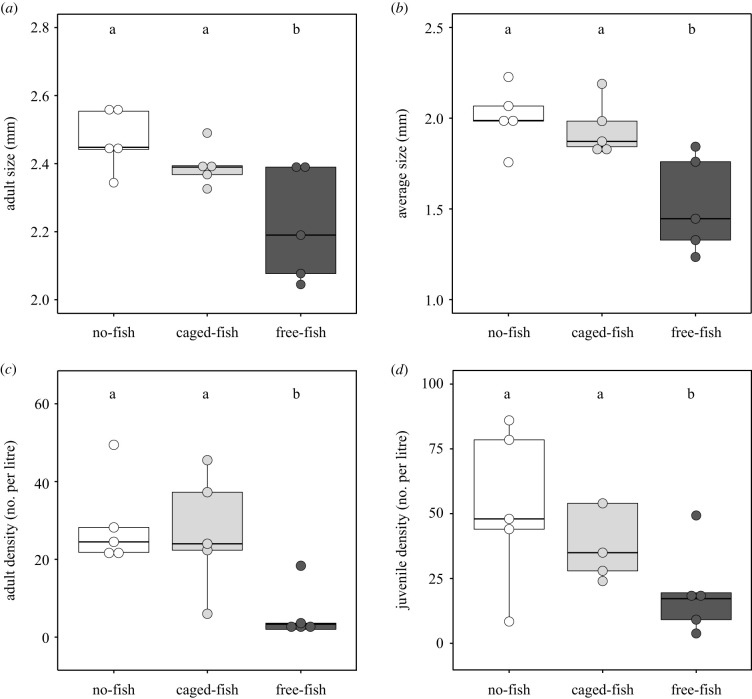
The predator treatments changed the body size of D. magna in the mesocosms after six weeks (adult size: , p < 0.0001, average size: , p < 0.0001; figure 1a,b). Daphnia in the free-fish treatment had a smaller body size compared to the caged-fish and the no-fish treatments (figure 1a,b). The predator treatments also changed the density of adult Daphnia (, p < 0.0001, figure 1c). Specifically, there were fewer adult Daphnia in the free-fish treatment compared to the caged-fish and the no-fish treatments (figure 1c). The overall pattern was similar for juvenile densities, but not significant (, p = 0.066, figure 1d).
Figure 1.
Size and density of Daphnia magna at the end of the six weeks selection experiment for the three mesocosm fish predation treatments. (a) Adult size; (b) average size; (c) adult density; (d) juvenile density. Letters above the boxplots represent significantly different means based on Tukey post hoc comparisons.
(b) . Predator-induced shifts in prey clonal composition
The final clonal composition in the experimental prey populations after six weeks of selection differed among the three mesocosm predator treatments (mesocosm predator treatment × period, , p = 0.019, partial η2-value = 0.24; figure 2). The differences in clonal composition were more pronounced if we removed one outlier mesocosm (mesocosm predator treatment × period, , p < 0.0001, partial η2-value = 0.47; electronic supplementary material, figure S3). Focusing on the clones from the historical high-fish stocking period showed that their relative frequency differed among the three mesocosm predator treatments (, p = 0.015, partial η2-value = 0.41; figure 2). The relative frequencies of the high-fish period clones were higher in the caged-fish (p = 0.04) and in the free-fish (p = 0.03) treatments compared to the no-fish treatment (figure 2). Without the outlier mesocosm, the relative frequencies of the high-fish period clones also differed among the three mesocosm predator treatments (, p < 0.0001, partial η2-value = 0.72; electronic supplementary material, figure S3), and the higher relative frequency of the high-fish period clones in the caged-fish (p < 0.001) and in the free-fish (p = 0.002) treatments compared to the no-fish treatment was more pronounced (electronic supplementary material, figure S3). The clonal composition in the no-fish treatment did not significantly change compared to the initial composition (experimental stage × period: , p = 0.11; figure 2), while the caged-fish and free-fish treatments deviated from the initial composition (experimental stage × period: both p < 0.0001; figure 2). Additional analyses showed that the high-fish period clones had higher relative frequencies compared with the pre-fish and reduced-fish period clones in both the free-fish and caged-fish treatments (both p < 0.0001; figure 2).
Figure 2.
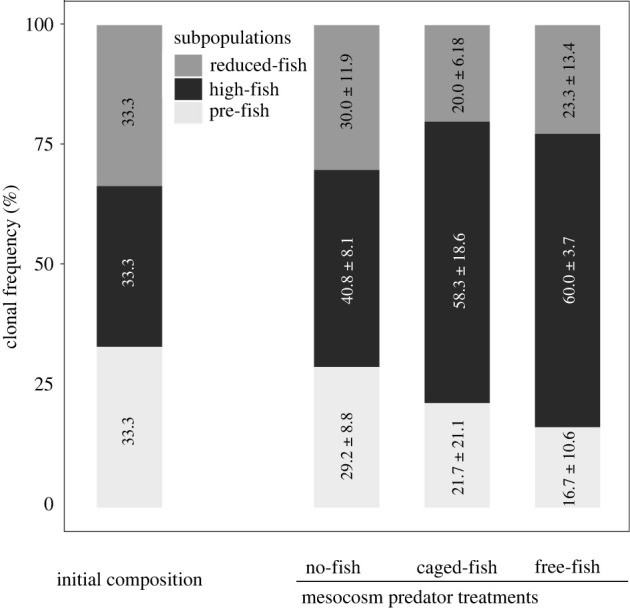
Clonal frequencies of Daphnia magna from the different historical fish-stocking periods in the experimental mesocosm populations at the start and at the end of the experimental evolution trial. Mean (±1 s.e.) clonal frequencies of each resurrected subpopulation are given based on five mesocosms per predator treatment.
(c) . Reconstruction of evolutionary shifts in genotypic trait values
Combining the clonal composition with the 14 genotypic trait values in the absence of fish cues of each clone, we reconstructed the average genotypic trait value of each resurrected subpopulation (pre-fish, high-fish, reduced-fish) consisting of six clones and of each experimental mesocosm population (no-fish, caged-fish, free-fish) at the start of the selection experiment and after six weeks of selection. In the phenotypic space determined by the first two roots of the discriminant function analysis, the bivariate means (each time based on six clones) of the natural subpopulations associated with each historical fish-stocking period strongly differed from each other (Pillai's MANOVA F4,30 = 61.536, p < 0.0001; open symbols in figure 3).
Figure 3.
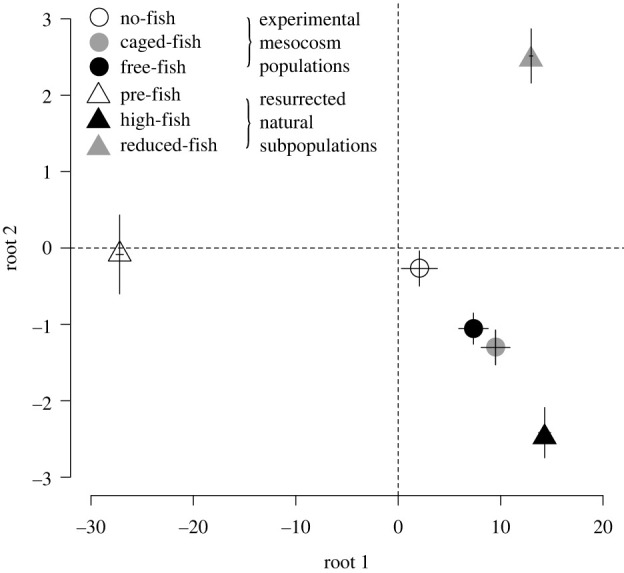
Multivariate genotypic trait values of the experimental mesocosm populations (no-fish, caged-fish and free-fish treatments) after six weeks of selection and of the three resurrected natural Daphnia magna subpopulations reflecting the population of a single pond (Oud-Heverlee Zuid) during three different historical fish-stocking periods (pre-fish, high-fish, reduced-fish periods) as reconstructed by resurrection ecology [6]. Population means are given with 1 s.e. Axes are the first two roots from a linear discriminant analysis based on 14 life-history, morphology and behaviour traits for the 18 clones used in this experiment. The traits most strongly associated with root 1 were somatic growth rate (+44.17), spine length at maturity (+38.54), size at maturity (−27.03), age at maturity (+24.35) and size of neonates (+18.64). For root 2, these were somatic growth rate (−28.65), size at maturity (+14.90), age at maturity (−19.54) and size of neonates (−12.98) (see details in electronic supplementary material, table S1). The [0,0] coordinate (intersection of hatched lines) represents the genotypic trait values for the 14 traits of the population that was inoculated in all mesocosms at the start of the selection experiment. This starting population consisted of an equal density of all 18 clones.
After six weeks of selection in the outdoor mesocosms, the bivariate mean of the no-fish experimental mesocosm population remained in the centre of the phenotypic space and did not significantly differ from the start population (one sample t-tests: p > 0.27 for both roots; figure 3). However, the bivariate means of the caged-fish and free-fish experimental mesocosm populations strongly deviated from the start situation (one sample t-tests: p < 0.05 for both roots) and shifted toward the bivariate mean of the resurrected high-fish subpopulation (figure 3). The bivariate means of the caged-fish and free-fish experimental populations did not differ from each other (p > 0.96 for both roots). When using the trait values measured in the presence of fish cues, the bivariate means for the first two roots showed very similar results (electronic supplementary material, figure S4) to those when using the trait values in the absence of fish cues.
4. Discussion
Combining experimental evolution and resurrection ecology, we showed that non-consumptive effects can induce rapid evolution in a period of six weeks (corresponding to approximately three to four generations of parthenogenetic reproduction) in prey populations as detected by shifts in clonal composition and associated genotypic trait values. Moreover, these shifts were similar to the previously documented evolutionary trajectories in a resurrected natural prey population and in line with predictions on adaptive responses of prey under predation risk (see the predictions in electronic supplementary material). As we worked with mixed populations that contained clones that were already adapted to fish predation, these trajectories may differ to what would happen in natural populations. However, there is large standing genetic variation in this natural Daphnia population, including the presence of clones adapted to fish predation in the pre-fish period (electronic supplementary material, figure S2 in [6]), that may allow rapid adaptation as confirmed recently by whole-genome sequencing [29]. Shifts induced by non-consumptive effects in clonal composition and genotypic trait values were largely parallel and of the same magnitude to those imposed by free predators that also affected prey by direct killing. In analysing the changes in clonal frequencies and associated shifts in genotypic trait values, the free-fish and caged-fish treatments showed very similar responses. Hence, non-consumptive effects of a predator induced a similar evolutionary response as direct killing by the predator. This makes a nice illustration of soft selection, i.e. the replacement by different genotypes without an effect on densities [30]. Our observation that non-consumptive effects alone causes evolution in prey populations extends earlier reports that non-consumptive effects can have important effects on prey population dynamics [1,9,31] and can change the species composition in prey communities [22]. Non-consumptive effects may affect population dynamics by long-term effects on fecundity and lifespan [32,33] and by directly imposing mortality [11–13]. Direct killing in our experiment did affect prey densities and the phenotypic trait distribution of body sizes. Indeed, at the end of the selection experiment, body size and population densities contrasted sharply between the free-fish and the other two treatments. The observed shift towards smaller body sizes in the presence of fish is in line with expectations when predators tend to result in a population dominated by younger individuals or prey in a size-selective manner [34]. These differences in phenotypic trait values hide that the evolutionary trajectory in free and caged fish is similar, as revealed by the results on clonal composition and associated genotypic trait values.
Strikingly, evolution in the presence of non-consumptive effects lead to the same evolutionary trajectory as in the presence of a free predator, leading to a population that is genetically adapted to better cope with the predator. Indeed, after six weeks of selection, both in the mesocosms with the caged fish and with the free fish, the mesocosm populations were dominated by the high-fish period clones. Stoks et al. [6] in their resurrection ecology analysis studying 14 different life-history and behavioural traits showed that the high-fish period subpopulation is genetically adapted to cope with fish predation, both in terms of mean trait values as well as the phenotypic plasticity responses in the presence of fish kairomones. The shift towards a dominance of high-fish clones reflects a shift to smaller animals that mature faster, produce more and smaller offspring, show stronger vertical migration, and generally show stronger phenotypic plasticity responses in response to fish kairomones [6] compared to the original inoculum consisting of an equal number of clones from all three subpopulations. Note that the genotypic trait values of the clones studies here were determined under laboratory conditions in a previous study [6], whereas in the current experiment we exposed clones to fish predators under outdoor mesocosm conditions. The reconstruction of changes in genotypic trait values is therefore assuming standardized conditions. Yet the observation that free fish (and caged fish) induced a shift in population frequencies that were in line with what was observed in nature (i.e. a shift to a dominance of clones from the high-fish period) suggests that this reconstruction in the laboratory captures key aspects of reality. This is reinforced by the observation that the clones from the high-fish predation period are characterized by traits that make them better adapted to coexist with fish [16]. The evolutionary responses we observe are generally in line with other studies reporting predator-induced evolution in prey populations [5,12] and with theoretically predicted responses to size-selective fish predation [35]. However, these responses have been typically attributed to the direct killing effect, whereas our results show that they can also result from responses to non-consumptive effects alone. The only other study that directly tested for the effects of selection imposed by non-consumptive effects showed that predator cues of dragonfly larvae selected for more active damselfly larvae that had a greater propensity to evade predators [12]. However, this study only focused on short-term selection and did not look at evolution across generations.
The question arises why non-consumptive effects of predators can induce a similar evolutionary response as direct killing. To the extent that predator-induced defences are costly [14], one would expect that non-consumptive effects alone, while inducing predator-induced responses through phenotypic plasticity, would not result in evolutionary shifts in the direction of stronger defences, as the animals would incur the costs but not the benefits of these responses. However, in the case of positively size-selective, visual predators such as fish, predator-induced defences involve the production of more but smaller offspring [36], and this might lead to a higher fitness if environmental conditions are such that a fast population growth rate is a good measure of fitness, which is often the case when the population suffers losses from, for instance, predation. Non-consumptive effects alone can then lead to the selection of genotypes that show strong predator defences, at least under conditions that promote high population growth rates (i.e. no strong intra- or interspecific competition). Previous work showed that the high-fish period clones have a higher intrinsic growth rate in the presence of fish kairomones than no-fish and reduced-fish period clones [6], which is adaptive under consumptive predation as predicted by theory [35]. Possibly, this plastic response was further enlarged by transgenerational effects. Indeed, parental exposure to predator cues has been shown to result in an earlier age at maturation in the offspring generation in Daphnia [37]. The higher intrinsic growth rate of the high-fish period clones may also reflect more active foraging in the presence of fish cues when prey are experiencing constant high predation risk, as predicted by the risk allocation hypothesis [38].
The higher intrinsic growth rate of the high-fish period clones under predation risk may explain why more high-fish period clones were present after six weeks of selection in the experimental populations with the caged fish predators. Indeed, differences in intrinsic growth rate have been shown to underlie clonal shifts in experimental Daphnia populations [39]. In support of this, when the pre-fish and high-fish period clones were separately exposed to the same mesocosm predator treatments, only the pre-fish clones suffered reductions in population density in the caged-fish treatment while the high-fish clones did not (electronic supplementary material, figure S5c). Note this does not conflict with the observation that the final densities of the experimental mesocosm populations did not differ between the no-fish and caged-fish treatments, as any reductions in the density of some clonal groups may have been countered by competitive release of other clonal groups (cf. soft selection [30]).
Our results support the intriguing hypothesis that non-consumptive effects, separate from direct predation, might contribute to predator-mediated evolution in natural populations. This effect might be widespread, as many predators induce evolution towards earlier maturation and the production of more offspring in their prey populations [35,40]. Evolution induced by non-consumptive effects might be important to increase the rate of evolution to predation. If non-consumptive effects alone can promote the dominance of better-defended genotypes, then evolutionary adaptation to predation can take a head start in settings in which predators are too low in number or prey are at such high densities that the individual risk of being consumed is low. In settings where the predators need to show a numerical response before they exert a strong impact through killing, evolution induced by non-consumptive effects might be very important in fostering a faster adaptation of prey to predators. Similarly, adaptation to predation would be sustained under conditions in which predation rates are temporarily strongly reduced, such as in temporarily turbid waters in which visually hunting predators cannot hunt effectively.
Our results under controlled and replicated conditions suggest that, at least under the conditions mimicked in our mesocosm experiment, non-consumptive effects can induce rapid evolution that is very similar in amplitude and direction to the realized evolutionary trajectories in nature. In the phenotypic space determined by the 14 traits in this natural population [6], the evolutionary shifts induced by non-consumptive and consumptive effects were both in the direction of the genotypic trait values of the natural high-fish predation subpopulation. Moreover, the shifts of the genotypic trait values induced by non-consumptive and consumptive effects were of similar magnitude. This match between evolution mediated by non-consumptive and consumptive effects will likely depend on environmental conditions and on the type of predators. Our results suggest that the parallel trajectories might depend on the degree to which environmental conditions favour fast population growth. In more competitive environments, typically associated with settings in which predators are absent, selection will likely favour competitively strong animals, which in Daphnia is positively correlated with body size [41]. The response is also dependent on the type of predator: gape-limited invertebrate predators select for larger zooplankton with larger offspring [42–44], which are typically characterized by lower population growth rates. In this case, evolution induced by non-consumptive effects may not result in an adaptation to predators, but might even result in an opposite response.
In conclusion, using an experimental evolution approach with clones obtained from a resurrected natural prey population, we documented under semi-natural conditions that non-consumptive effects can induce repeatable rapid evolution in a prey population. Moreover, by integrating the clonal changes in the experimental mesocosm populations with genotypic trait values of each clone quantified in an earlier study [6], we demonstrate that the rapid evolution was adaptive and mimicked the evolutionary trajectories under changes in fish predation in the resurrected natural population. Given that rapid trait evolution may affect ecological interactions, evolution induced by non-consumptive effects adds a new dimension to the rapidly growing field of eco-evolutionary dynamics [45] between predators and their prey, by showing that non-consumptive effects, in addition to consumptive effects, have the potential to drive such dynamics.
Acknowledgements
We thank Edwin van den Berg for his help with sampling during the mesocosm experiment.
Data accessibility
All data have been deposited at Figshare [46].
Authors' contributions
C.Z.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, writing—original draft, writing—review and editing; E.G.: investigation, methodology; K.B.: resources, software; L.D.M.: conceptualization, funding acquisition, supervision, validation, writing—review and editing; R.S.: conceptualization, funding acquisition, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.
Conflict of interest declaration
We declare we have no competing interests.
Funding
Financial support came from National Natural Science Foundation of China (grant no. 42007229), China Postdoctoral Science Foundation (grant no. 2019M662337), Post-doctoral Innovation Research Program of Shandong Province and Qingdao (grant no. 236346), the Fundamental Research Funds of Shandong University (grant no. 2019HW031), Research Grants from FWO Flanders (grant no. G.0943.15) and the KU Leuven Research Fund (grant no. C16/17/002).
References
- 1.Preisser EL, Bolnick DI, Benard MF. 2005. Scared to death? The effects of intimidation and consumption in predator–prey interactions. Ecology 86, 501-509. ( 10.1890/04-0719) [DOI] [Google Scholar]
- 2.Zanette LY, White AF, Allen MC, Clinchy M. 2011. Perceived predation risk reduces the number of offspring songbirds produce per year. Science 334, 1398-1402. ( 10.1126/science.1210908) [DOI] [PubMed] [Google Scholar]
- 3.Suraci JP, Clinchy M, Dill LM, Roberts D, Zanette LY. 2016. Fear of large carnivores causes a trophic cascade. Nat. Commun. 7, 1-7. ( 10.1038/ncomms10698) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Creel S. 2018. The control of risk hypothesis: reactive vs. proactive antipredator responses and stress-mediated vs. food-mediated costs of response. Ecol. Lett. 21, 947-956. ( 10.1111/ele.12975) [DOI] [PubMed] [Google Scholar]
- 5.Losos JB, Schoener TW, Spiller DA. 2004. Predator-induced behaviour shifts and natural selection in field-experimental lizard populations. Nature 432, 505-508. ( 10.1038/nature03039) [DOI] [PubMed] [Google Scholar]
- 6.Stoks R, Govaert L, Pauwels K, Jansen B, De Meester L. 2016. Resurrecting complexity: the interplay of plasticity and rapid evolution in the multiple trait response to strong changes in predation pressure in the water flea Daphnia magna. Ecol. Lett. 19, 180-190. ( 10.1111/ele.12551) [DOI] [PubMed] [Google Scholar]
- 7.Reznick DN, Shaw FH, Rodd FH, Shaw RG. 1997. Evaluation of the rate of evolution in natural populations of guppies (Poecilia reticulata). Science 275, 1934-1937. ( 10.1126/science.275.5308.1934) [DOI] [PubMed] [Google Scholar]
- 8.Nickel BA, Suraci JP, Nisi AC, Wilmers CC. 2021. Energetics and fear of humans constrain the spatial ecology of pumas. Proc. Natl Acad. Sci. USA 118, 1-8. ( 10.1073/pnas.2004592118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zanette LY, Clinchy M. 2020. Ecology and neurobiology of fear in free-living wildlife. Annu. Rev. Ecol. Evol. Syst. 51, 297-318. ( 10.1146/annurev-ecolsys-011720-124613) [DOI] [Google Scholar]
- 10.Matthews CJD, Breed GA, LeBlanc B, Ferguson SH. 2020. Killer whale presence drives bowhead whale selection for sea ice in Arctic seascapes of fear. Proc. Natl Acad. Sci. USA 117, 6590-6598. ( 10.1073/pnas.1911761117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stoks R. 1998. Effect of lamellae autotomy on survival and foraging success of the damselfly Lestes sponsa (Odonata: Lestidae). Oecologia 117, 443-448. ( 10.1007/s004420050679) [DOI] [PubMed] [Google Scholar]
- 12.Siepielski AM, Wang J, Prince G. 2014. Nonconsumptive predator-driven mortality causes natural selection on prey. Evolution 68, 696-704. ( 10.1111/evo.12294) [DOI] [PubMed] [Google Scholar]
- 13.MacLeod KJ, Krebs CJ, Boonstra R, Sheriff MJ. 2018. Fear and lethality in snowshoe hares: the deadly effects of non-consumptive predation risk. Oikos 127, 375-380. ( 10.1111/oik.04890) [DOI] [Google Scholar]
- 14.Tollrian R, Harvell CD. 1990. The ecology and evolution of inducible defenses. Princeton, NJ: Princeton University Press. [DOI] [PubMed] [Google Scholar]
- 15.Kawecki TJ, Lenski RE, Ebert D, Hollis B, Olivieri I, Whitlock MC. 2012. Experimental evolution. Trends Ecol. Evol. 27, 547-560. ( 10.1016/j.tree.2012.06.001) [DOI] [PubMed] [Google Scholar]
- 16.Orsini L, Schwenk K, De Meester L, Colbourne JK, Pfrender ME, Weider LJ. 2013. The evolutionary time machine: using dormant propagules to forecast how populations can adapt to changing environments. Trends Ecol. Evol. 28, 274-282. ( 10.1016/j.tree.2013.01.009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hairston NG, Lampert W, Cáceres CE, Holtmeier CL, Weider LJ, Gaedke U, Fischer JM, Fox Ja, Post DM. 1999. Rapid evolution revealed by dormant eggs. Nature 401, 446. ( 10.1038/46731) [DOI] [PubMed] [Google Scholar]
- 18.Decaestecker E, Gaba S, Raeymaekers JAM, Stoks R, Van Kerckhoven L, Ebert D, De Meester L. 2007. Host–parasite ‘Red Queen’ dynamics archived in pond sediment. Nature 450, 870-873. ( 10.1038/nature06291) [DOI] [PubMed] [Google Scholar]
- 19.Cousyn C, De Meester L, Colbourne JK, Brendonck L, Verschuren D, Volckaert F. 2001. Rapid, local adaptation of zooplankton behavior to changes in predation pressure in the absence of neutral genetic changes. Proc. Natl Acad. Sci. USA 98, 6256-6260. ( 10.1073/pnas.111606798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goitom E, Kilsdonk LJ, Brans K, Jansen M, Lemmens P, De Meester L. 2018. Rapid evolution leads to differential population dynamics and top-down control in resurrected Daphnia populations. Evol. Appl. 11, 96-111. ( 10.1111/eva.12567) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Verreydt D, De Meester L, Decaestecker E, Villena MJ, Van Der Gucht K, Vannormelingen P, Vyverman W, Declerck SAJ. 2012. Dispersal-mediated trophic interactions can generate apparent patterns of dispersal limitation in aquatic metacommunities. Ecol. Lett. 15, 218-226. ( 10.1111/j.1461-0248.2011.01728.x) [DOI] [PubMed] [Google Scholar]
- 22.Peacor SD, Pangle KL, Schiesari L, Werner EE. 2012. Scaling-up anti-predator phenotypic responses of prey: impacts over multiple generations in a complex aquatic community. Proc. R. Soc. B 279, 122-128. ( 10.1098/rspb.2011.0606) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benzie JAH. 2005. Cladocera: The genus Daphnia (including Daphniopsis). Leiden, The Netherlands: Backhuys Publishers. [Google Scholar]
- 24.Jansen B, Geldof L, De Meester L, Orsini L. 2011. Isolation and characterization of microsatellite markers in the waterflea Daphnia magna. Mol. Ecol. Resour. 11, 418-421.21429157 [Google Scholar]
- 25.Orsini L, Spanier KI, De Meester L. 2012. Genomic signature of natural and anthropogenic stress in wild populations of the waterflea Daphnia magna: validation in space, time and experimental evolution. Mol. Ecol. 21, 2160-2175. ( 10.1111/j.1365-294X.2011.05429.x) [DOI] [PubMed] [Google Scholar]
- 26.Rousseeuw PJ, Croux C. 1993. Alternatives to the median absolute deviation. J. Am. Stat. Assoc. 88, 1273-1283. ( 10.1080/01621459.1993.10476408) [DOI] [Google Scholar]
- 27.Leys C, Ley C, Klein O, Bernard P, Licata L. 2013. Detecting outliers: do not use standard deviation around the mean, use absolute deviation around the median. J. Exp. Soc. Psychol. 49, 764-766. ( 10.1016/j.jesp.2013.03.013) [DOI] [Google Scholar]
- 28.Lind PR, Jeyasingh PD. 2018. Interactive effects of dietary phosphorus and iron on Daphnia life history. Limnol. Oceanogr. 63, 1181-1190. ( 10.1002/lno.10763) [DOI] [Google Scholar]
- 29.Chaturvedi A, et al. 2021. Extensive standing genetic variation from a small number of founders enables rapid adaptation in Daphnia. Nat. Commun. 12, 1-9. ( 10.1038/s41467-021-24581-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reznick D. 2016. Hard and soft selection revisited: How evolution by natural selection works in the real world. J. Hered. 107, 3-14. ( 10.1093/jhered/esv076) [DOI] [PubMed] [Google Scholar]
- 31.Sheriff MJ, Peacor SD, Hawlena D, Thaker M. 2020. Non-consumptive predator effects on prey population size: a dearth of evidence. J. Anim. Ecol. 89, 1302-1316. ( 10.1111/1365-2656.13213) [DOI] [PubMed] [Google Scholar]
- 32.Zanette LY, Clinchy M, Suraci JP. 2014. Diagnosing predation risk effects on demography: can measuring physiology provide the means? Oecologia 176, 637-651. ( 10.1007/s00442-014-3057-9) [DOI] [PubMed] [Google Scholar]
- 33.Dudeck BP, Clinchy M, Allen MC, Zanette LY. 2018. Fear affects parental care, which predicts juvenile survival and exacerbates the total cost of fear on demography. Ecology 99, 127-135. ( 10.1002/ecy.2050) [DOI] [PubMed] [Google Scholar]
- 34.Attayde JL, Hansson LA. 2001. The relative importance of fish predation and excretion effects on planktonic communities. Limnol. Oceanogr. 46, 1001-1012. ( 10.4319/lo.2001.46.5.1001) [DOI] [Google Scholar]
- 35.Abrams PA, Rowe L. 1996. The effects of predation on the age and size of maturity of prey. Evolution 50, 1052-1061. ( 10.1111/j.1558-5646.1996.tb02346.x) [DOI] [PubMed] [Google Scholar]
- 36.Riessen HP. 1999. Predator-induced life history shifts in Daphnia: a synthesis of studies using meta-analysis. Can. J. Fish. Aquat. Sci. 56, 2487-2494. ( 10.1139/f99-155) [DOI] [Google Scholar]
- 37.Walsh MR, Cooley F, Biles K, Munch SB. 2014. Predator-induced phenotypic plasticity within- and across-generations: a challenge for theory? Proc. R. Soc. B 282, 20-22. ( 10.1098/rspb.2014.2205) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lima SL, Bednekoff PA. 1999. Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am. Nat. 153, 649-659. ( 10.1086/303202) [DOI] [PubMed] [Google Scholar]
- 39.Van Doorslaer W, et al. 2009. Local adaptation to higher temperatures reduces immigration success of genotypes from a warmer region in the water flea Daphnia. Glob. Chang. Biol. 15, 3046-3055. ( 10.1111/j.1365-2486.2009.01980.x) [DOI] [Google Scholar]
- 40.Reznick DN, Bryga HA. 1996. Life-history evolution in guppies (Poecilia reticulata: Poeciliidae). V. Genetic basis of parallelism in life histories. Am. Nat. 147, 339-359. ( 10.1086/285855) [DOI] [Google Scholar]
- 41.Hall DJ, Threlkeld ST, Burns CW, Crowley PH. 1976. The size-efficiency hypothesis and the size structure of zooplankton communities. Annu. Rev. Ecol. Syst. 7, 177-208. ( 10.1146/annurev.es.07.110176.001141) [DOI] [Google Scholar]
- 42.Taylor BE, Gabriel W. 1993. Optimal adult growth of Daphnia in a seasonal environment. Funct. Ecol. 7, 513-521. ( 10.2307/2390126) [DOI] [Google Scholar]
- 43.Stibor H, Lüning J. 1994. Predator-induced phenotypic variation in the pattern of growth and reproduction in Daphnia hyalina (Crustacea: Cladocera). Funct. Ecol. 8, 97-101. ( 10.2307/2390117) [DOI] [Google Scholar]
- 44.Beckerman AP, Wieski K, Baird DJ. 2007. Behavioural versus physiological mediation of life history under predation risk. Oecologia 152, 335-343. ( 10.1007/s00442-006-0642-6) [DOI] [PubMed] [Google Scholar]
- 45.Hendry AP. 2017. Eco-evolutionary dynamics. Princeton, NJ: Princeton University Press. [Google Scholar]
- 46.Zhang C, Goitom E, Brans K, De Meester L, Stoks R. 2022. Scared to evolve? Non-consumptive effects drive rapid adaptive evolution in a natural prey population. FigShare. ( 10.6084/m9.figshare.c.5958640) [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Zhang C, Goitom E, Brans K, De Meester L, Stoks R. 2022. Scared to evolve? Non-consumptive effects drive rapid adaptive evolution in a natural prey population. FigShare. ( 10.6084/m9.figshare.c.5958640) [DOI] [PMC free article] [PubMed]
Data Availability Statement
All data have been deposited at Figshare [46].
C.Z.: conceptualization, data curation, formal analysis, funding acquisition, investigation, methodology, project administration, validation, writing—original draft, writing—review and editing; E.G.: investigation, methodology; K.B.: resources, software; L.D.M.: conceptualization, funding acquisition, supervision, validation, writing—review and editing; R.S.: conceptualization, funding acquisition, supervision, validation, writing—review and editing.
All authors gave final approval for publication and agreed to be held accountable for the work performed therein.