Abstract
Atopic dermatitis (AD) is a prevalent condition impacting up to 25% of children and 8% of adults worldwide. An estimated 20% of those with AD comprise a subset of patients with treatment-resistant AD, for whom traditional therapeutics and management strategies are unsuccessful. Physical symptoms significantly impact quality of life for patients and caregivers. The condition is chronic and may persist throughout the lifespan with recurrent episodes. Novel AD therapeutics offer new opportunities to resolve symptoms of treatment-resistant more effectively AD. Recently developed pharmacological agents were developed with an appreciation of AD as a heterogeneous condition. New advances include topical, oral, and injectable therapeutics with novel mechanisms of action. In addition, advances in clinical practice, including the application of digital tools, can promote a personalized medicine approach. For example, teledermatology for chronic conditions such as AD have been embraced by clinicians and patients; communicating symptoms via photographs can augment patient symptom trackers and aid trigger identification. Digital tools can also be used to increase medication adherence and improve patient/caregiver engagement. Integrating the above is a personalized medicine approach. Advanced therapeutics with novel mechanisms of action, integrated with digital tools, and trends toward patient-centered medicine can assist this chronic, heterogeneous condition via precision medicine and better treat treatment-resistant AD.
Keywords: Atopic dermatitis, Treatment resistance, Biologics, JAK inhibitors, Phototherapy, Personalized medicine
INTRODUCTION
Atopic dermatitis (AD) is a prevalent condition, impacting approximately 10%–25% of children and 2%–8% of adults worldwide [1,2]. Signs and symptoms of AD may include cutaneous erythema, inflammation, pruritus, and xerosis [3,4,5]. Many individuals with AD experience childhood onset which may persist into adulthood, subside, or recur intermittently throughout the lifespan [6]. Other individuals experience adult-onset AD, which may occur at any age, including after the age of 60 years [6]. AD often impacts quality of life for patients and caregivers, causing sleep disturbance, fatigue, and emotional distress, in addition to the physical manifestations [3,7,8,9].
Approximately 20% of AD patients have treatment-resistant AD for which traditional therapeutics have had limited success [7]. AD is a heterogeneous condition influenced by hereditary, environmental, and immunological factors [3,7,8,9,10,11]. The diverse phenotypes and endotypes of AD demonstrate variability across traits such as age and ethnicity [7,10,11]. Recently developed AD pharmaceuticals with novel mechanisms of action offer targeted therapeutics for a personalized medicine approach [10,11,12].
Several new topical, oral, and injectable pharmaceuticals have received approval in Europe, Japan, and the USA; moreover, clinical trials are underway for additional therapeutics for which approval is anticipated. Such medications are promising options for cases of treatment-resistant AD in which traditional therapeutics are of limited efficacy or pose a high risk of adverse effects (AEs) [7].
This article will discuss recently developed pharmacological agents and the promise they offer for treatment-resistant AD. Advances in clinical practice such as the application of digital tools will be incorporated. Optimizing teledermatology, electronic health records, symptom trackers, trigger diaries, and patient registries may promote patient/caregiver engagement, treatment adherence, trigger identification, and the assessment of AD subtypes. This precision medicine approach will help identify the best treatment strategy and application of new therapeutics for each patient.
OVERVIEW OF AD THERAPEUTICS
Current treatments for AD include topical, oral, and injectable therapeutics, in addition to phototherapy, wet wrap therapy, and other treatments. Acute AD flares are typically treated with topical corticosteroids, topical phosphodiesterase 4 (PDE4) inhibitors, or topical calcineurin inhibitors [13]. For severe, recurrent, or refractory AD, dupilumab is a first line therapy, approved in 2017 for adults and 2020 for children and adolescents (aged 6–17 years) [13,14]. Second line therapies include systemic glucocorticoids (very short-term treatment up to 1 week may be an option for severe exacerbation in exceptional cases), cyclosporine, or phototherapy [13]. Third line therapies include the off-label use of methotrexate, mycophenolate mofetil, and azathioprine, however, the use of these systemics is limited due to a high risk of AEs [13].
Approximately 33% of pediatric AD patients and 50% of adult AD patients experience moderate-to-severe AD which for which topical treatment and phototherapy are ineffective [1,15]. Systemic corticosteroids should be used rarely but may be indicated for such patients if other treatments are unavailable, particularly during acute flares [16]. The decision to use systemics requires ruling out coexisting and/or mimicking conditions, confirming patient trigger avoidance, and confirming patient treatment adherence [15]. The significant proportion of AD patients for whom the condition is refractory, and the risk of AEs with systemics, demonstrate the need for improved therapeutics.
Several new pharmacological agents have recently received approval in Europe, Japan, and the USA, including topical formulations (ruxolitinib, tofacitinib, delgocitinib), oral formulations (abrocitinib, baricitinib, upadacitinib) and injectable formulations (lebrikizumab, nemolizumab, omalizumab, risankizumab, tralokinumab, ustekinumab) (Table 1). In addition, promising clinical trials are underway studying the efficacy of many more therapeutic agents to treat moderate-to-severe AD (Table 2). Significant research is ongoing to explore treating AD through the off-label use of medications approved for other atopic conditions.
Table 1. Oral, topical, and injectable therapies for treatment-resistant atopic dermatitis.
| Route of administration | Drug name | Mechanism of action | Patient population (age) |
|---|---|---|---|
| Oral | Abrocitinib | JAK 1 Inhibitor | Adults (≥18 yr) |
| Children, adolescents (12–17 yr) | |||
| Baricitinib | JAK 1 & 2 Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (2–17 yr) | |||
| Upadacitinib | JAK 1 Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (6 mo–17 yr) | |||
| Ruxolitinib | JAK Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (12–17 yr) | |||
| Topical | Tofacitinib | JAK Inhibitor | Adults (≥18 yr) |
| Delgocitinib | JAK Inhibitor | Adults (≥18 yr) | |
| Injectable | Lebrikizumab | IL-13 Inhibitor | Adults (≥18 yr) |
| Children, adolescents (12–17 yr) | |||
| Nemolizumab | IL-31 Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (12–17 yr) | |||
| Omalizumab | Immunoglobulin E (IgE) | Adults (≥18 yr) | |
| Children, adolescents (4–17 yr) | |||
| Risankizumab | IL-23 Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (12–17 yr) | |||
| Tralokinumab | IL-13 Inhibitor | Adults (≥18 yr) | |
| Children, adolescents (12–17 yr) | |||
| Ustekinumab | IL-12 Inhibitor | Adults (≥18 yr) | |
| IL-23 Inhibitor |
JAK, Janus kinase; IL, interleukin.
Table 2. Pharmaceuticals in clinical trials (phase II+) for atopic dermatitis treatment.
| Route of administration | Drug name/ID | Mechanism of action | Patient population (age) |
|---|---|---|---|
| Oral | Asimadoline | Selective kappa opioid receptor agonist | Adults (≥18 yr) |
| Difelikefalin | Kappa opioid receptor agonist | Adults (18–80 yr) | |
| DS107 | CD40 expression inhibitor | Adults (≥18 yr) | |
| Etrasimod | Sphingosine 1 phosphate receptor modulator | Adults (18–70 yr) | |
| Gusacitinib | JAK and SYK dual inhibitor | Adults (18–75 yr) | |
| SHR0302 | JAK 1 inhibitor | Adults (≥18 yr) | |
| Children, adolescents (12–17 yr) | |||
| Injectable | Astegolimab | Anti-ST2 antibody (inhibits IL-33 binding) | Adults (18–75 yr) |
| Benralizumab | Anti-interleukin-5 receptor alpha antibody | Children, adolescents (12–17 yr) | |
| Bermikimab | Anti-interleukin-1 alpha antibody | Adults (≥18 yr) | |
| CBP-201 | Anti-interleukin-4 receptor antibody | Adults (18–75 yr) | |
| FB825 | Antibody to CεmX domain of membrane form IgE | Adults (18–70 yr) | |
| GBR830 | Anti-OX40 receptor antibody | Adults (≥18 yr) | |
| KHK4083 | Anti-OX40 receptor antibody | Adults (≥18 yr) | |
| KY1005 | Anti-OX40 ligand antibody | Adults (18–75 yr) | |
| MEDI3506 | Anti-IL-33 antibody | Adults (18–75 yr) | |
| REGN3500 | Anti-IL-33 antibody | Adults (18–75 yr) | |
| Spesolimab | Anti-IL-36 receptor antibody | Adults (18–75 yr) | |
| Tezepelumab | Anti-TSLP antibody | Adults (18–75 yr) | |
| Topical | ALX-101 | Liver X receptor agonist | Adults (18–75 yr) |
| Children, adolescents (12–17 yr) | |||
| ARQ-252 | JAK 1 inhibitor | Adults (≥18 yr) | |
| ATI-1777 | JAK 1 and 3 inhibitor | Adults (18–65 yr) | |
| Brepocitinib | JAK 1 and Tyk inhibitor | Adults (18–75 yr) | |
| Cerdulatinib | JAK and SYK dual inhibitor | Adults (≥18 yr) | |
| Difamilast | PDE4 inhibitor | Adults (18–70 yr) | |
| Children, adolescents (10–17 yr) | |||
| DS107 | CD40 expression inhibitor | Adults (≥18 yr) | |
| EB01 | Phospholipase A2 inhibitor | Adults (18–70 yr) | |
| Lotamilast | PDE4 inhibitor | Adults (≥18 yr) | |
| Q301 | Leukotriene inhibitor | Adults (18–70 yr) | |
| Children, adolescents (12–17 yr) | |||
| Tapinarof | Therapeutic aryl hydrocarbon receptor modulating agent | Adults (18–65 yr) | |
| Children, adolescents (12–17 yr) |
JAK, Janus kinase; SYK, spleen tyrosine kinase; IL, interleukin; IgE, Immunoglobulin E; TSLP, thymic stromal lymphopoietin; Tyk, tyrosine kinase; PDE4 Inhibitor, phosphodiesterase-4 Inhibitor.
clinicaltrials.gov -- https://nationaleczema.org/research/eczema-treatment-research/.
Recent advances in AD therapeutics
Oral therapeutics
Baricitinib
Baricitinib is an oral selective Janus kinase (JAK)1/JAK2 inhibitor studied for moderate-to-severe AD in children and adolescents (aged 2–17 years) and in adults [17,18,19], with U.S. Food and Drug Administration (FDA) approval for the treatment of AD anticipated in near future. However, Baricitinib is already approved by many regulatory bodies and countries including European Medicines Agency (EMA), Korea, and Japan for adult cases with moderate-to-severe AD who are candidates for systemic therapy. It is already FDA approved for moderately to severely active rheumatoid arthritis in adults [17,18,19].
Abrocitinib
Abrocitinib is an orally administered JAK1 inhibitor employed for moderate-to-severe AD in adolescents (aged 12–17 years) and in adults [20,21]. Abrocitinib was safe and effective in adults and adolescents with moderate-to-severe AD when used as solitary agent in once daily dosing [21]. Even a recent study assessed the efficacy of abrocitinib in patients who received prior dupilumab [20]. This study bolsters the safety and efficacy vignette of abrocitinib for moderate-to-severe cases of AD notwithstanding previous dupilumab response status [20]. Abrocitinib is approved by FDA for the treatment of refractory, moderate-to-severe AD whose disease is not adequately controlled with other systemic agents, including biologics or when use of those therapies is not advisable.
Upadacitinib
Upadacitinib is an orally administered JAK1 inhibitor utilized for moderate-to-severe AD in adolescents (aged 12–17 years) and adults [22,23]. It is recently FDA approved for refractory, moderate-to-severe cases of AD in adults and children 12 years and older whose disease process was not responding to previous treatments or AD is not controlled by other systemic medications including biologics or when those other medications are not recommended [22,23]. A favorable benefit-risk profile and efficacy were observed in recently concluded 2 long-term, randomized clinical trials of upadacitinib’s efficacy and safety in moderate-to-severe AD [22]. Clinicians should be encouraged to use upadacitinib in adults as well as adolescent resistant cases of AD in view of these recent encouraging results.
Topical therapeutics
Ruxolitinib
Ruxolitinib in the cream form has been approved by FDA for the short-term and noncontinuous treatment of mild-to-moderate AD in nonimmunocompromised patients aged 12 years and older whose AD is not adequately controlled with other topical medications or when those medicines are not advisable. It is the first and only FDA approved topical form JAK inhibitor [24].
Tofacitinib
Tofacitinib is a topically administered JAK inhibitor studied for moderate-to-severe AD in adults [25]. It is currently FDA and EMA approved to treat juvenile idiopathic arthritis in children and adolescents (aged 2–17 years) and rheumatoid arthritis, psoriatic arthritis, and ulcerative colitis in adults [26].
Delgocitinib
Delgocitinib is a topically administered JAK inhibitor studied for moderate-to-severe AD in children and adolescents (aged 2–17 years) and adults [27,28]. It is approved for the treatment of AD in adults in Japan, with FDA and EMA approval for the treatment of AD anticipated [27,28].
Injectable therapeutics
Lebrikizumab
Lebrikizumab (subcutaneous injection) is an anti-interleukin 13 (IL-13) antibody studied for moderate-to-severe AD in adolescents (aged 12–17 years) and adults [29,30]. It is not yet FDA approved, while phase 3 clinical trials have been promising [29,30].
Nemolizumab
Nemolizumab (subcutaneous injection) is monoclonal antibody that targets interleukin 31 (IL-31), studied for moderate-to-severe AD in adolescents (aged 12–17 years) and in adults. It is not yet FDA approved, while phase 3 clinical trials have been promising [31].
Tralokinumab
Tralokinumab (subcutaneous injection) is fully human, high-affinity monoclonal antibody that selectively blocks the signaling of IL-13. Tralokinumab is approved by FDA for moderate-to-severe AD in adults whose AD is not controlled with topical remedies or when those therapies are not advisable [32].
Ustekinumab
Ustekinumab (subcutaneous injection) is human interleukin 12 (IL-12) and interleukin 23 (IL-23) antagonist studied for the treatment of severe and/or chronic AD in adults [33]. Ustekinumuab is FDA approved for the treatment of moderate-to-severe psoriasis in children and adolescents (aged 6–17 years), and adults with psoriasis, psoriatic arthritis, Crohn disease, and ulcerative colitis [34].
Risankizumab
Risankizumab (subcutaneous injection) is monoclonal antibody that blocks IL-23 that is FDA approved for the treatment of moderate-to-severe plaque psoriasis in adults [35]. Phase 2 studies are underway to evaluate risankizumab for the treatment of moderate-to-severe AD in adolescents (aged 12–17 years) and in adults [36].
Omalizumab
Omalizumab (subcutaneous injection) is a biologic that targets immunoglobulin E (IgE) production, studied for the treatment of AD in children and adolescents (aged 4–17 years) as well as in adults [37]. Omalizumab is FDA approved to treat allergic asthma in children and adolescents (aged 6–17 years) and in adults; chronic idiopathic urticaria in adolescents (aged 12–17 years) and in adults, and nasal polyps in adults [38]. Patients with AD may have coexisting atopic conditions such allergic asthma for which omalizumab can be administered. While the potential efficacy of omalizumab to treat AD in such patients is noteworthy, the use of omalizumab to treat AD is presently controversial [39].
Clinical evaluations and treatment strategies
The clinical evaluation for treatment-resistant AD involves confirming the diagnosis of AD, classifying it as treatment resistant, and selecting appropriate treatment and prevention strategies. Patient/caregiver engagement and education are essential, particularly for treatment adherence, trigger avoidance, and trigger identification [40].
Confirming the diagnosis of AD
Confirming the diagnosis of AD involves assessing for a personal/family history of atopy; the physical examination; ruling out coexisting or mimicking conditions; and reviewing trigger exposures [40]. The diagnosis of AD is based on clinical decision-making as there is no biomarker to distinguish AD from other diseases. An elevated serum IgE level is present in some, but not all individuals with AD [40,41]. Patients with AD typically experience pruritus, xerosis, inflammation, erythema, and characteristic morphological patterns [40,41]. Several disease severity and clinical outcome scales are available, including: body surface area of AD involvement; Eczema Area and Severity Index; Investigator's Global Assessment; and SCORing Atopic Dermatitis (SCORAD). The application of these instruments in clinical settings is limited, however, assessments such as the SCORAD index may be useful for assessing disease states and treatment efficacy [40,41].
Possible coexisting and mimicking conditions include psoriasis, seborrheic dermatitis, atopic contact dermatitis, nummular dermatitis, scabies, or other conditions [3,41]. It is important to rule out the presence of infections such as Staphylococcus aureus, impetigo, and tinea [41]. Finally, is important to rule out hypersensitivity reactions to medications, irritation from topical formulations, or drug allergies which may stem from active or inactive ingredients, such as drug dyes [42].
Classifying AD as treatment resistant
The condition may be classified as treatment-resistant AD if the patient's symptoms and clinical score are unchanged or aggravated after 2 weeks of treatment, provided that the patient has been treatment adherent and symptoms are not caused by ongoing trigger exposures [40].
It is recommended that clinicians seek information on the duration and relapsing/remitting nature of symptoms [40,41], as this can be correlated with treatment adherence and trigger avoidance to establish causal associations. AD that is persistent or recurrent due to ongoing or intermittent trigger exposure may respond to treatment after the patient adheres to triggers avoidance strategies [40,41]. Similarly, non-adherence or partial adherence with treatment may explain the frequent recurrence or persistence of symptoms.
Environments harboring triggers
AD triggers are found in many environments, including the home, workplace, school, automobiles, mass transit, medical settings, fitness facilities, and recreational settings. The patient may experience exposure to one or more AD trigger(s). In the home, AD triggers may be found in grooming products such as soaps, hair care products, moisturizers, sunscreens, cosmetics, or perfumes, laundry detergents and/or household cleaners. Fabrics such as clothing, bedding, and towels may themselves trigger AD, or harbor allergic triggers such as pet dander or dust mites. Aeroallergens have sources such as animal dander/saliva from pets or rodents. Animals, including house pets (cats, dogs) or those found in agriculture/farming environments (pigs, horses), may transmit allergens via contact or inhalation of aeroallergens.
Certain foods, milk, alcoholic beverages, and infant formula may also cause AD, however, the role of food allergies in AD remains controversial [40]. While dietary restrictions to prevent AD are burdensome, they are typically within the control of the patient/caregiver. AD may be triggered by medications, as well as inactive ingredients such as dyes, compounding agents, or the vehicle for topical formulations [42]. Medical supplies or over the counter health care products may also be implicated, such as gloves, face masks, or tape/adhesives.
Adherence with trigger avoidance recommendations
AD symptoms may be caused by ongoing, intermittent, or recurrent exposure(s) to one or multiple trigger(s). In a given AD patient, recurrent skin rashes may follow a pattern of recurrent trigger exposure(s). Trigger avoidance includes proactively identifying new triggers in addition to avoiding known triggers. Patient/caregiver education should provide information that AD as a chronic condition; that a patient may develop reactions to new triggers which were not previously problematic; and that latency—a delay from the time of exposure to the appearance of the skin rash—may obscure trigger attribution [43]. Many environments may harbor a wide range of triggers such as aeroallergens, cleansers, fabrics, foods, and grooming products [43,44,45].
Trigger identification tools
Patient symptom trackers and trigger exposure diaries may enhance patient engagement, prompt trigger identification, and promote adherence. As AD is a chronic, relapsing/remitting condition, recurrent or persistent rashes are common. Similarly, identifying new triggers that underly an outbreak is not uncommon, and proactive trigger identification is an important component of prevention. Trigger diaries may be organized by environment (e.g., home, school, workplace, fitness center, swimming pool) or suspected causative agents (e.g., foods, cleansers, animals). Exposures should be recorded with date and time; the trigger exposure diary should be correlated with a symptom tracker to assess for a causative association.
Patients/caregivers may have experience using these tools, particularly if they suffer coexisting conditions of atopy (e.g., asthma) for which such tools are common. Digital versions of these tools may increase engagement and the ability to capture detail. Photos diaries depicting skin rashes may demonstrate discoloration, body surface area impacted, changes over time, and be correlated in time and place to trigger exposures [3]. Photo diaries may be particularly useful in demonstrating the presence or absence of a response to treatment. As AD is a heterogeneous, chronic condition, ongoing patient/caregiver engagement is important, and can be enhanced with digital tools.
DIGITAL TOOLS AND PRECISION MEDICINE
Precision medicine involves patient engagement to develop treatment strategies, including prescribing therapeutics, targeted to the individual patient's condition [46]. Clinicians and patients have embraced telemedicine for the treatment of AD [47]. Virtual visits, combined with face-to-face consultations and lab tests when needed, have been found to provide health care for AD that is equally effective to onsite visits at a lower cost [46,47]. Referrals may be appropriate for more specialized consultations (e.g., oncology) or diagnostic testing (e.g., biopsy) [47,48].
The expansion of telemedicine visits amid the coronavirus disease 2019 (COVID-19) pandemic has included teledermatology utilization when clinics were closed [49]. A retrospective records analysis conducted by the department of dermatology at Massachusetts General Hospital of Harvard University, Boston, USA found that after clinics reopened, the proportion of telehealth visits declined by less than 50%, with telehealth engagement remaining particularly high for patients with common dermatoses such as AD [49]. The fact that teledermatology utilization levels remained high despite the availability of onsite visits demonstrates strong provider and patient acceptance that will likely endure beyond the pandemic [47,48,49].
Adherence with treatment recommendations
Patient/caregiver adherence with treatment generally includes preventive skin care (moisturizers, emollients) and prescribed therapeutics. Obstacles to adherence may include AEs, cost, steroid phobia, inconvenience, and/or cognitive/physical limitations. Notably, a significant cause of treatment failure is the practice of applying topicals too sparingly; this may be due to cost or insufficient knowledge on the part of the patient/caregiver. The onset of AD often occurs during childhood, and a significant proportion of patients are children and adolescents. While it may be practical for caregivers to monitor medication adherence, a significant element of a patient's treatment plan involves trigger identification and avoidance, which is more difficult to monitor. Caregivers must be aware of potential triggers in the child or adolescent's environments (e.g., home, school, recreational facilities), as well as trigger avoidance habits.
A patient-centered approach may be effective in removing adherence obstacles and developing a treatment plan with alternatives better suited to the patient/caregiver abilities and preferences. Additionally, increased patient/caregiver education may stress the relationship of consistent adherence to improved outcomes, and thereby gain increased adherence. Only when the patient is adherent with both treatment recommendations and trigger avoidance strategies can a determination be made as to whether the AD is truly treatment resistant. A patient-centered approach may incorporate self-administered tools that increase patient/caregiver engagement. Such tools include the Patient Oriented Eczema Measure; Patient Oriented SCORing Atopic Dermatitis; Atopic Dermatitis Control Tool [47].
Implementation
Telemedicine encounters may be synchronous, asynchronous, or hybrid virtual visits [47,48]. Synchronous virtual visits offer the advantage of real-time, spontaneous clinician/patient interactions that are similar to onsite clinic visits. They may occur via video platforms that allow clinicians and patients to see and hear one another, or via an audio only connection [47,48]. One disadvantage is that synchronous virtual visits must be scheduled at a mutually convenient time for provider and patient, as occurs with traditional office visits. Asynchronous virtual visits, also called "store-and-forward," offer scheduling freedom, yet do not allow the clinician to obtain additional information from the patient spontaneously [47,48]. Hybrid virtual visits allow clinicians and patients to supplement synchronous or asynchronous appointments with further questions, information, or the transmission of images [47,48]. This is particularly useful for devices such as smart phones which may preclude the transfer of images during the call [47,48].
Photographic images
Most patients/caregivers can use cameras, phones, or other devices to capture and transmit clear digital images efficiently. Photos can document the appearance of AD skin rashes. A photos series can document changes over time and be correlated with a symptom tracker, trigger exposure diary, and treatment adherence diary to evaluate the impact of trigger exposures or treatments.
Photographic images can be taken by the patient/caregiver, or by an allied health professional in the community, e.g., a physician's assistant or nurse practitioner in a nursing facility, school nurse, or corporate nurse. Photos can be transmitted digitally from patient/caregiver to physician, and onward to a specialist (e.g., oncologist) if needed. Photo image quality is important: photos should have clear focus, high resolution, and good lighting. Several pictures should be taken to depict detail and body surface area impacted [47,48].
Telemedicine may indeed be inadequate for some evaluations, for example, examining body areas that are hair-bearing or conditions requiring palpation. However, if clinics are closed due to COVID-19, or a patient requires specialty care unavailable locally, telemedicine may offer clinical access that would otherwise be unavailable [47,48,49].
Digital tools may enhance patient and caregiver engagement and promote adherence. Comprehensive databases and registries with patient demographics, clinical traits, triggers, symptoms, and responses to treatment could facilitate research to further improve management strategies for AD. There remain many gaps in knowledge and unmet needs, for example, there are needs for uniform definitions and standardized terminology to facilitate database analyses. There are also no comprehensive multinational databases for AD presently [3,50]. Developing digital tools for AD research and surveillance may build on the success of other conditions of atopy (e.g., asthma) for which registries and retrospective records analyses are useful.
These digital tools and patient engagement methods are particularly useful for clinicians managing AD with a shared decision-making model, by which doctor, and patient consider clinical evidence together with patient priorities to develop a treatment plan [51]. This patient-centered approach is well-suited to AD treatment plans, as AD is a heterogeneous, chronic condition heavily impacted by lifestyle and environmental factors, with multiple treatment options available [50].
Identifying the potential root-cause of treatment resistance in patients of AD
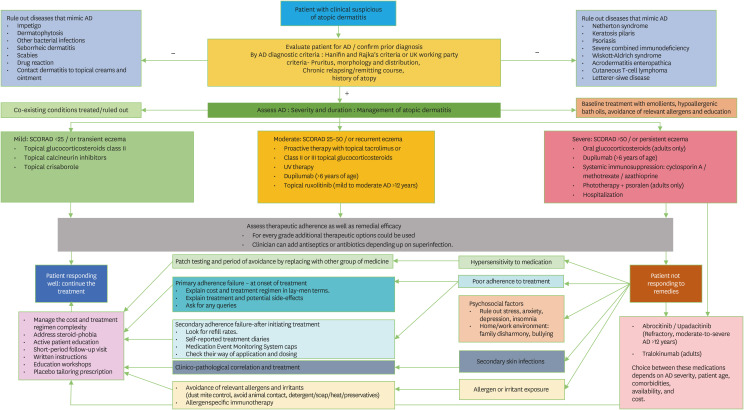
Although this article has thrown light on every aspect of treatments for AD, finding the ultimate reason of treatment failure is an integral portion of therapeutic strategy in these cases. Poor adherence to prescribed treatment has been identified as the most common reason of treatment failure and hence the resistance [40]. Other crucial things to address in treatment-resistance patients of AD are psychosomatic aspects as pruritis-scratch cycle negatively impact the quality of life. Regular clinical dermatological examination can identify any secondary cutaneous infection in atopic skin. Early identification of infection can help in targeting microbes to prevent deterioration. Comprehensive allergy work-up will help in focusing on allergens which were not considered in prior management. Widening the vision with targeted histories of occupational setting, cosmetic utilization, soap and diet will benefit the clinician in closing on the ultimate culprit allergen [40]. Once treatment resistance is addressed, SCORAD recounting and recategorization are recommended. In extremely rare circumstances, even after correcting all possible causes of resistance and employing medications; if patient is not responding then clinicians should consider second line immunosuppressive medicines, biopsy, and second opinion with dermato-immunologist (Fig. 1).
Fig. 1. Flowchart for the management of atopic dermatitis. *Oral glucocorticoids have largely unfavorable benefit/risk ratio in the treatment of atopic dermatitis. Short-term treatment up to 1 week may be an option for severe exacerbation in exceptional cases). AD, Atopic dermatitis; SCORAD, SCORing Atopic Dermatitis.
CONCLUSIONS AND FUTURE DIRECTIONS
Recent advances in the development of targeted therapeutics offer new promise for patients with treatment-resistant AD. These therapeutics were developed with recognition of the heterogeneity of AD and enable a precision medicine approach to optimize patient outcomes. Patient and caregiver engagement as essential components of developing an efficacious treatment plan tailored to the individual patient. Digital tools and telemedicine may also be employed to enhance patient and caregiver engagement. Progress toward standardized terminology and developing databases and registries will facilitate research to enhance scientific understanding of AD and patient subgroups. Emerging therapeutics currently under development are expected to offer improved outcomes for treatment-resistant AD.
ACKNOWLEDGEMENTS
The author acknowledges the medical writing, and editorial work of Dr. Daniel Bennett and Dr. Parth Naik.
References
- 1.Drucker AM, Ellis AG, Bohdanowicz M, Mashayekhi S, Yiu ZZN, Rochwerg B, Di Giorgio S, Arents BWM, Burton T, Spuls PI, Küster D, Siegels D, Schmitt J, Flohr C. Systemic immunomodulatory treatments for patients with atopic dermatitis: a systematic review and network meta-analysis. JAMA Dermatol. 2020;156:659–667. doi: 10.1001/jamadermatol.2020.0796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barbarot S, Auziere S, Gadkari A, Girolomoni G, Puig L, Simpson EL, Margolis DJ, de Bruin-Weller M, Eckert L. Epidemiology of atopic dermatitis in adults: Results from an international survey. Allergy. 2018;73:1284–1293. doi: 10.1111/all.13401. [DOI] [PubMed] [Google Scholar]
- 3.Kowalska-Olędzka E, Czarnecka M, Baran A. Epidemiology of atopic dermatitis in Europe. J Drug Assess. 2019;8:126–128. doi: 10.1080/21556660.2019.1619570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eczema Resource Center [Internet] Washington (DC): American Academy of Dermatology Association; 2021. [cited 2021 Mar 9]. Available from: https://www.aad.org/public/diseases/eczema. [Google Scholar]
- 5.Eckert L, Gupta S, Gadkari A, Mahajan P, Gelfand JM. Burden of illness in adults with atopic dermatitis: Analysis of National Health and Wellness Survey data from France, Germany, Italy, Spain, and the United Kingdom. J Am Acad Dermatol. 2019;81:187–195. doi: 10.1016/j.jaad.2019.03.037. [DOI] [PubMed] [Google Scholar]
- 6.Lam M, Zhu JW, Maqbool T, Adam G, Tadrous M, Rochon P, Drucker AM. Inclusion of older adults in randomized clinical trials for systemic medications for atopic dermatitis: a systematic review. JAMA Dermatol. 2020;156:1240–1245. doi: 10.1001/jamadermatol.2020.2940. [DOI] [PubMed] [Google Scholar]
- 7.Renert-Yuval Y, Guttman-Yassky E. New treatments for atopic dermatitis targeting beyond IL-4/IL-13 cytokines. Ann Allergy Asthma Immunol. 2020;124:28–35. doi: 10.1016/j.anai.2019.10.005. [DOI] [PubMed] [Google Scholar]
- 8.Ramirez FD, Chen S, Langan SM, Prather AA, McCulloch CE, Kidd SA, Cabana MD, Chren MM, Abuabara K. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155:556–563. doi: 10.1001/jamadermatol.2018.5641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Simpson EL, Guttman-Yassky E, Margolis DJ, Feldman SR, Qureshi A, Hata T, Mastey V, Wei W, Eckert L, Chao J, Arnold RJG, Yu T, Vekeman F, Suárez-Fariñas M, Gadkari A. Association of inadequately controlled disease and disease severity with patient-reported disease burden in adults with atopic dermatitis. JAMA Dermatol. 2018;154:903–912. doi: 10.1001/jamadermatol.2018.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Litman T. Personalized medicine-concepts, technologies, and applications in inflammatory skin diseases. APMIS. 2019;127:386–424. doi: 10.1111/apm.12934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Czarnowicki T, He H, Krueger JG, Guttman-Yassky E. Atopic dermatitis endotypes and implications for targeted therapeutics. J Allergy Clin Immunol. 2019;143:1–11. doi: 10.1016/j.jaci.2018.10.032. [DOI] [PubMed] [Google Scholar]
- 12.Kim J, Kim BE, Leung DYM. Pathophysiology of atopic dermatitis: clinical implications. Allergy Asthma Proc. 2019;40:84–92. doi: 10.2500/aap.2019.40.4202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Johnson BB, Franco AI, Beck LA, Prezzano JC. Treatment-resistant atopic dermatitis: challenges and solutions. Clin Cosmet Investig Dermatol. 2019;12:181–192. doi: 10.2147/CCID.S163814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim NS, Maliyar K, Oliveira L, O’Toole A, Gooderham MJ. Real-world experience of dupilumab in the treatment of moderate-to-severe atopic dermatitis. Int J Dermatol. 2020;59:e361–e363. doi: 10.1111/ijd.15053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Simpson EL, Bruin-Weller M, Flohr C, Ardern-Jones MR, Barbarot S, Deleuran M, Bieber T, Vestergaard C, Brown SJ, Cork MJ, Drucker AM, Eichenfield LF, Foelster-Holst R, Guttman-Yassky E, Nosbaum A, Reynolds NJ, Silverberg JI, Schmitt J, Seyger MMB, Spuls PI, Stalder JF, Su JC, Takaoka R, Traidl-Hoffmann C, Thyssen JP, van der Schaft J, Wollenberg A, Irvine AD, Paller AS. When does atopic dermatitis warrant systemic therapy? Recommendations from an expert panel of the International Eczema Council. J Am Acad Dermatol. 2017;77:623–633. doi: 10.1016/j.jaad.2017.06.042. [DOI] [PubMed] [Google Scholar]
- 16.Drucker AM, Eyerich K, de Bruin-Weller MS, Thyssen JP, Spuls PI, Irvine AD, Girolomoni G, Dhar S, Flohr C, Murrell DF, Paller AS, Guttman-Yassky E. Use of systemic corticosteroids for atopic dermatitis: International Eczema Council consensus statement. Br J Dermatol. 2018;178:768–775. doi: 10.1111/bjd.15928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.US National Library of Medicine. Baricitinib study [Internet] Bethesda (MD): ClinicalTrials.gov; [updated 2021 Mar 4]. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT03952559?term=Baricitinib&age=0&draw=4&rank=2. [Google Scholar]
- 18.Radi G, Simonetti O, Rizzetto G, Diotallevi F, Molinelli E, Offidani A. Baricitinib: the first jak inhibitor approved in europe for the treatment of moderate to severe atopic dermatitis in adult patients. Healthcare (Basel) 2021;9:1575. doi: 10.3390/healthcare9111575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reich K, Kabashima K, Peris K, Silverberg JI, Eichenfield LF, Bieber T, Kaszuba A, Kolodsick J, Yang FE, Gamalo M, Brinker DR, DeLozier AM, Janes JM, Nunes FP, Thyssen JP, Simpson EL. Efficacy and safety of baricitinib combined with topical corticosteroids for treatment of moderate to severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:1333–1343. doi: 10.1001/jamadermatol.2020.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi VY, Bhutani T, Fonacier L, Deleuran M, Shumack S, Valdez H, Zhang F, Chan GL, Cameron MC, Yin NC. Phase 3 efficacy and safety of abrocitinib in adults with moderate-to-severe atopic dermatitis after switching from dupilumab (JADE EXTEND) J Am Acad Dermatol. 2022:S0190-9622(22)00608-9. doi: 10.1016/j.jaad.2022.04.009. [DOI] [PubMed] [Google Scholar]
- 21.Silverberg JI, Simpson EL, Thyssen JP, Gooderham M, Chan G, Feeney C, Biswas P, Valdez H, DiBonaventura M, Nduaka C, Rojo R. Efficacy and safety of abrocitinib in patients with moderate-to-severe atopic dermatitis: a randomized clinical trial. JAMA Dermatol. 2020;156:863–873. doi: 10.1001/jamadermatol.2020.1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simpson EL, Papp KA, Blauvelt A, Chu CY, Hong HC, Katoh N, Calimlim BM, Thyssen JP, Chiou AS, Bissonnette R, Stein Gold LF, Wegzyn C, Hu X, Liu M, Liu J, Tenorio AR, Chu AD, Guttman-Yassky E. Efficacy and safety of upadacitinib in patients with moderate to severe atopic dermatitis: analysis of follow-up data from the measure up 1 and measure up 2 randomized clinical trials. JAMA Dermatol. 2022;158:404–413. doi: 10.1001/jamadermatol.2022.0029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guttman-Yassky E, Thaçi D, Pangan AL, Hong HC, Papp KA, Reich K, Beck LA, Mohamed MF, Othman AA, Anderson JK, Gu Y, Teixeira HD, Silverberg JI. Upadacitinib in adults with moderate to severe atopic dermatitis: 16-week results from a randomized, placebo-controlled trial. J Allergy Clin Immunol. 2020;145:877–884. doi: 10.1016/j.jaci.2019.11.025. [DOI] [PubMed] [Google Scholar]
- 24.Papp K, Szepietowski JC, Kircik L, Toth D, Eichenfield LF, Leung DYM, Forman SB, Venturanza ME, Sun K, Kuligowski ME, Simpson EL. Efficacy and safety of ruxolitinib cream for the treatment of atopic dermatitis: Results from 2 phase 3, randomized, double-blind studies. J Am Acad Dermatol. 2021;85:863–872. doi: 10.1016/j.jaad.2021.04.085. [DOI] [PubMed] [Google Scholar]
- 25.US National Library of Medicine. Tofacitinib study [Internet] Bethesda (MD): ClinicalTrials.gov; [updated 2015 Oct 15]. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT02001181?term=Tofacitinib&draw=8&rank=68. [Google Scholar]
- 26.U.S. Food and Drug Administration. Drugs@FDA: Tofacitinib [Internet] Silver Spring (MD): US Department of Health and Human Services; [cited 2021 Mar 9]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. [Google Scholar]
- 27.Nakagawa H, Nemoto O, Igarashi A, Saeki H, Kaino H, Nagata T. Delgocitinib ointment, a topical Janus kinase inhibitor, in adult patients with moderate to severe atopic dermatitis: a phase 3, randomized, double-blind, vehicle-controlled study and an open-label, long-term extension study. J Am Acad Dermatol. 2020;82:823–831. doi: 10.1016/j.jaad.2019.12.015. [DOI] [PubMed] [Google Scholar]
- 28.Dhillon S. Delgocitinib: first approval. Drugs. 2020;80:609–615. doi: 10.1007/s40265-020-01291-2. [DOI] [PubMed] [Google Scholar]
- 29.US National Library of Medicine. Lebrikizumab study [Internet] Bethesda (MD): ClinicalTrials.gov; 2020. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/show/NCT04392154?term=Lebrikizumab&draw=2&rank=1. [Google Scholar]
- 30.Guttman-Yassky E, Blauvelt A, Eichenfield LF, Paller AS, Armstrong AW, Drew J, Gopalan R, Simpson EL. Efficacy and safety of lebrikizumab, a high-affinity interleukin 13 inhibitor, in adults with moderate to severe atopic dermatitis: a phase 2b randomized clinical trial. JAMA Dermatol. 2020;156:411–420. doi: 10.1001/jamadermatol.2020.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US National Library of Medicine. Nemolizumab study [Internet] Bethesda (MD): ClinicalTrials.gov; 2020. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/results?term=Nemolizumab&Search=Apply&age_v=&gndr=&type=&rslt= [Google Scholar]
- 32.Wollenberg A, Blauvelt A, Guttman-Yassky E, Worm M, Lynde C, Lacour JP, Spelman L, Katoh N, Saeki H, Poulin Y, Lesiak A, Kircik L, Cho SH, Herranz P, Cork MJ, Peris K, Steffensen LA, Bang B, Kuznetsova A, Jensen TN, Østerdal ML, Simpson EL ECZTRA 1 and ECZTRA 2 study investigators. Tralokinumab for moderate-to-severe atopic dermatitis: results from two 52-week, randomized, double-blind, multicentre, placebo-controlled phase III trials (ECZTRA 1 and ECZTRA 2) Br J Dermatol. 2021;184:437–449. doi: 10.1111/bjd.19574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.US National Library of Medicine. Ustekinumab study [Internet] Bethesda (MD): ClinicalTrials.gov; 2016. [Accessed March 14, 2021]. Available from: https://clinicaltrials.gov/ct2/results?term=Ustekinumab&cond=Dermatitis%2C+Atopic&age_v=&age=1&age=2&gndr=&type=&rslt=&Search=Apply. [Google Scholar]
- 34.U.S. Food and Drug Administration. Drugs@FDA: Ustekinumab [Internet] Silver Spring (MD): US Department of Health and Human Services; 2021. [cited 2021 Mar 9]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. [Google Scholar]
- 35.U.S. Food and Drug Administration. Drugs@FDA: Risankizumab [Internet] Silver Spring (MD): US Department of Health and Human Services; 2021. [cited 2021 Mar 9]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. [Google Scholar]
- 36.US National Library of Medicine. Risankizumab study [Internet] Bethesda (MD): ClinicalTrials.gov; 2020. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/results?cond=Dermatitis%2C+Atopic&term=Risankizumab+&cntry=&state=&city=&dist= [Google Scholar]
- 37.US National Library of Medicine. Omalizumab study [Internet] Bethesda (MD): ClinicalTrials.gov; 2015. [cited 2021 Mar 9]. Available from: https://clinicaltrials.gov/ct2/results?term=Omalizumab&cond=Dermatitis%2C+Atopic&Search=Apply&age_v=&gndr=&type=&rslt= [Google Scholar]
- 38.U.S. Food and Drug Administration. Drugs@FDA: Omalizumab [Internet] Silver Spring (MD): US Department of Health and Human Services; 2021. [cited 2021 Mar 9]. Available from: https://www.accessdata.fda.gov/scripts/cder/daf/index.cfm?event=BasicSearch.process. [Google Scholar]
- 39.Wu AC. Omalizumab for atopic dermatitis: overtreatment or lifesaver? JAMA Pediatr. 2020;174:15–16. doi: 10.1001/jamapediatrics.2019.4509. [DOI] [PubMed] [Google Scholar]
- 40.Wollenberg A, Christen-Zäch S, Taieb A, Paul C, Thyssen JP, de Bruin-Weller M, Vestergaard C, Seneschal J, Werfel T, Cork MJ, Kunz B, Fölster-Holst R, Trzeciak M, Darsow U, Szalai Z, Deleuran M, von Kobyletzki L, Barbarot S, Heratizadeh A, Gieler U, Hijnen DJ, Weidinger S, De Raeve L, Svensson Å, Simon D, Stalder JF, Ring J European Task Force on Atopic Dermatitis/EADV Eczema Task Force. ETFAD/EADV Eczema task force 2020 position paper on diagnosis and treatment of atopic dermatitis in adults and children. J Eur Acad Dermatol Venereol. 2020;34:2717–2744. doi: 10.1111/jdv.16892. [DOI] [PubMed] [Google Scholar]
- 41.Siegfried EC, Hebert AA. Diagnosis of atopic dermatitis: mimics, overlaps, and complications. J Clin Med. 2015;4:884–917. doi: 10.3390/jcm4050884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Medications and Drug Allergic Reactions [Internet] Milwaukee (WI): American Academy of Allergy, Asthma & Immunology; 2021. [cited 2021 Mar 9]. Available from: https://www.aaaai.org/conditions-and-treatments/library/allergy-library/medications-and-drug-allergic-reactions. [Google Scholar]
- 43.Tamagawa-Mineoka R, Katoh N. Atopic dermatitis: identification and management of complicating factors. Int J Mol Sci. 2020;21:2671. doi: 10.3390/ijms21082671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cid BJ, Perez-Mateluna G, Iturriaga C, Zambrano MJ, Vives MI, Valenzuela PM, Borzutzky A. Is there an association between indoor allergens and the severity of atopic dermatitis? Int J Dermatol. 2019;58:433–439. doi: 10.1111/ijd.14281. [DOI] [PubMed] [Google Scholar]
- 45.Dhar S, Srinivas SM. Food allergy in atopic dermatitis. Indian J Dermatol. 2016;61:645–648. doi: 10.4103/0019-5154.193673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Joyner MJ, Paneth N. Seven questions for personalized medicine. JAMA. 2015;314:999–1000. doi: 10.1001/jama.2015.7725. [DOI] [PubMed] [Google Scholar]
- 47.Simpson C, Lipoff J. Making teledermatology work for you: how to choose the right platform [Internet] Washington (DC): American Academy of Dermatology; 2020. [cited 2021 Mar 9]. Available from: https://aadhighlights.com/summaries-podcasts/article/16-telemedicine-in-the-time-of-covid. [Google Scholar]
- 48.EADV Teledermatology Task Force [Internet] Lugano (CH): European Academy of Dermatology and Venereology; 2019. [cited 2021 Mar 9]. Available from: https://www.eadv.org/task-forces. [Google Scholar]
- 49.Su MY, Smith GP, Das S. Trends in teledermatology use during clinic reopening after COVID-19 closures. J Am Acad Dermatol. 2021;84:e213–e214. doi: 10.1016/j.jaad.2020.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hall R, Lebwohl MG, Bushmakin AG, Simpson EL, Gooderham MJ, Wollenberg A, Gater A, Wells JR, Cappelleri JC, Hsu MA, Papacharalambous J, Peeva E, Tallman AM, Zhang W, Chen L. Development and content validation of pruritus and symptoms assessment for atopic dermatitis (PSAAD) in adolescents and adults with moderate-to-severe AD. Dermatol Ther (Heidelb) 2021;11:221–233. doi: 10.1007/s13555-020-00474-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Coughlin CC, Politi MC. Shared decision-making in dermatologic care: a call for more training and resources. JAMA Dermatol. 2021;157:271–272. doi: 10.1001/jamadermatol.2020.5360. [DOI] [PubMed] [Google Scholar]