Abstract
Liposomal amphotericin B was immunosuppressive on target cell lysis in vitro and on protection mediated by cytotoxic CD8 T cells in murine listeriosis. When dosages usually used for therapy in humans were compared, the immunosuppressive effect of 5 mg of liposomal amphotericin B/kg of body weight/day was similar to that of standard amphotericin B at 1 mg/kg/day, but a dosage of liposomal amphotericin B of 1 mg/kg/day was not suppressive in vivo.
Amphotericin B is the most important drug for the treatment of life-threatening fungal infections in the immunocompromised host (2, 3). Amphotericin B is a polyene macrolide which binds to ergosterol and other sterol components of the fungal cell membrane (2). Apart from other detrimental effects which limit the maximum dosage applicable in humans to 1 mg/kg of body weight/day, amphotericin B inhibits cellular functions of the immune system, including mitogen and antigen-induced proliferation of T and B cells in vitro (1, 7, 10, 13) as well as the cytolytic function of cytotoxic T cells (4). When higher dosages must be used the reduction of undesirable side effects is indispensable.
One means to achieve this goal is incorporation into liposomes. This has been shown to alter the pharmacokinetic properties of amphotericin B and the relative efficiencies in different organs because liposomes are accumulated in the organs of the reticuloendothelial system, including the liver (2, 8, 15). In spite of the alterations in the pharmakokinetic properties incorporation in liposomes has been shown to ameliorate most of the side effects of the substance so that higher dosages, usually 3 to 5 mg/kg/day may be used for the treatment of critically ill patients. Similar dosages of 1 mg/kg/day for liposomal amphotericin and standard amphotericin B may be used in special situations including prophylaxis (1, 2, 15).
In this study we compared the suppression of cytotoxic T-cell function by dosages of standard and liposomal amphotericin B commonly used in therapeutic situations. We used in vitro and in vivo models for Listeria monocytogenes infection which are established models for the study of cytotoxic-T-cell-dependent immunity (11). Because CD8 T cells specific for the protein p60 of L. monocytogenes have been shown to confer efficient protection against L. monocytogenes (6) we used a CD8 T-cell line specific for the peptide p60 fragment including residues 449 to 457 (p60 449–457) to investigate the effect of liposomal incorporation on the suppressive effect of amphotericin B.
Amphotericin B (CAS 1397-89-3) complexed with deoxycholate was obtained from Bristol-Myers Squibb (Munich, Germany). Liposomal amphotericin B (AmBisome) was obtained from Nexstar, Munich, Germany. CD8 T cells specific for p60 449-457 were derived and cultured from L. monocytogenes-infected BALB/c mice as described (4, 5) and used in the in vitro and animal experiments. In vitro cytolytic assays were performed as described (4) with 103 51Cr-labeled P815 target cells per well. Peptide-specific cytolysis was tested after 60 min of incubation of target cells with p60 449–457. All targets were tested in triplicate in a 4-h cytolytic assay with an effector-to-target cell ratio of 10:1. For in vivo experiments female BALB/cOlaHsd (H-2d) mice were purchased from Harlan-Winkelmann (Borchen, Germany) and used at 8 to 12 weeks of age. Naive mice were adoptively transferred intravenously (i.v.) with 5 × 106 CD8 T cells. Immediately after transfer mice were challenged i.v. with 5 × 103 CFU of L. monocytogenes 1/2a EGD. Treatment with standard and liposomal amphotericin B was performed i.v. 30 min before and 24 and 48 h after challenge. Amphotericin was used at a dose of 1 mg/kg, which was the highest dose tolerated by the mice. Liposomal amphotericin B was used at doses of 1, 2, 5, 7, and 10 mg/kg, which were well tolerated. Control animals received isotonic saline instead of amphotericin B and CD8 T cells, respectively. Mice were killed, the organs were homogenized in distilled water 72 h after challenge and the number of CFU was determined as described (4). Extraction of amphotericin B from the organ homogenates for high-performance liquid chromatography (HPLC) was performed as described (9). The statistical significance of the results of the in vivo experiments was checked with Tukey's test for multiple comparisons at the 0.05 significance level using WINKS statistical analysis software (Texasoft, Cedar Hill, Tex.).
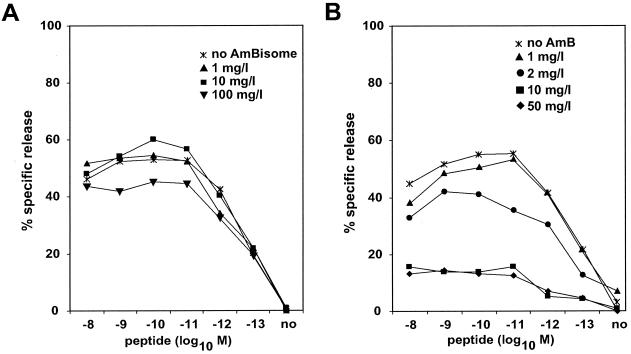
Figure 1 shows the relative amphotericin B-mediated inhibition of target cell lysis. The presence of amphotericin B in deoxycholate suppressed target cell lysis in a concentration-dependent manner (Fig. 1B). The lowest amphotericin B concentration which resulted in more than 25% inhibition of target cell lysis was 2 μg/ml. In contrast, a concentration of 100 μg/ml was necessary to produce a comparable effect when amphotericin B was incorporated into liposomes (Fig. 1A).
FIG. 1.
Effect of liposomal amphotericin B (AmBisome) (panel A) or standard amphotericin B (AmB) (panel B) on specific lysis of 51Cr-labeled P815 cells loaded with p60 449-457 by a corresponding peptide-specific CD8 T-cell line.
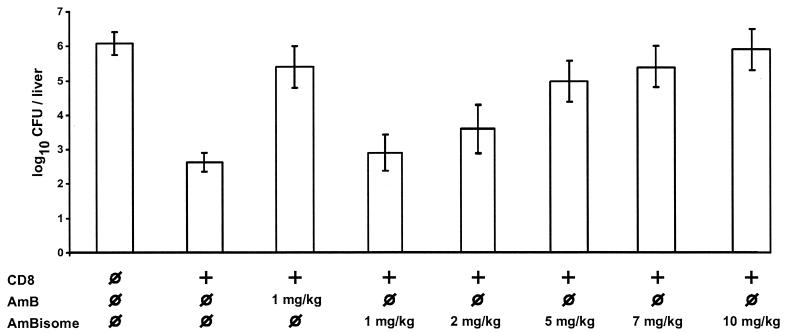
In mice, protection mediated by transferred CD8 T cells was significantly reduced after treatment with standard amphotericin B at the maximum dose (1 mg/kg) used in humans (Fig. 2). Comparable immunosuppression was observed with a liposomal amphotericin B dosage of 5 mg/kg/day (Fig. 2), which is currently used for treatment of proven infections with aspergilli and Candida spp. (2, 12, 14). After treatment with a liposomal amphotericin B dosage of 1 and 2 mg/kg/day, immunosuppression was less pronounced because there was a significant difference between CFU seen in transferred mice treated with amphotericin B and CFU seen in transferred mice treated with liposomal amphotericin B at either 1 or 2 mg/kg/day.
FIG. 2.
Effect of incorporation into liposomes on amphotericin B-mediated inhibition of CD8 T-cell-mediated antilisterial protection. Mice received 5 × 106 p60 449-457-specific CD8 T cells (+) or isotonic saline ( ) immediately before challenge with L. monocytogenes. Five mice for each group were treated i.v. with the indicated dose of amphotericin B (AmB) or liposomal amphotericin B (AmBisome) or with isotonic saline (
) immediately before challenge with L. monocytogenes. Five mice for each group were treated i.v. with the indicated dose of amphotericin B (AmB) or liposomal amphotericin B (AmBisome) or with isotonic saline ( ) 30 min before and 24 and 48 h after challenge with L. monocytogenes. Results are expressed as mean log10 CFU ± standard deviation (error bars). There was a significant difference (P < 0.05) between CFU when transferred mice treated with standard amphotericin B or liposomal amphotericin B were compared with transferred mice which received isotonic saline instead of either formulation of amphotericin B.
) 30 min before and 24 and 48 h after challenge with L. monocytogenes. Results are expressed as mean log10 CFU ± standard deviation (error bars). There was a significant difference (P < 0.05) between CFU when transferred mice treated with standard amphotericin B or liposomal amphotericin B were compared with transferred mice which received isotonic saline instead of either formulation of amphotericin B.
During therapy of mice with liposomal amphotericin B, concentrations of amphotericin B in the liver as determined by HPLC were higher than concentrations obtained with amphotericin B (4.0 ± 0.6 μg/g) and increased in a dose-dependent manner. In mice treated with the 1- and 2-mg/kg liposomal preparation, concentrations of 14.3 ± 2.2 and 22.0 ± 1.9 μg/g of liver, respectively, were measured after 3 days of treatment. Concentrations in mice treated with 5-, 7-, and 10-mg/kg liposomal amphotericin B were 51.3 ± 2.6, 64.3 ± 3.8, and 85.0 ± 2.0 μg/g of liver, respectively. Although these concentrations were lower than the suppressive concentration of liposomal amphotericin B observed in vitro, one may assume that more amphotericin B was released from the liposomes in vivo than in vitro and that suppressive concentrations were reached in the liver tissue already during treatment with the dosage of 5 mg/kg/day.
In summary, at commonly used dosages, standard and liposomal amphotericin B produced comparable effects on cytotoxic-T-cell function in vivo. Lower dosages of liposomal amphotericin B of 1 and 2 mg/kg/day that might be suitable in some situations were less suppressive.
REFERENCES
- 1.Boggs J M, Chang N H, Goundalkar A. Liposomal amphotericin B inhibits in vitro T-lymphocyte response to antigen. Antimicrob Agents Chemother. 1991;35:879–885. doi: 10.1128/aac.35.5.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coukell A J, Brogden R N. Liposomal amphotericin B. Therapeutic use in the management of fungal infections and visceral leishmaniasis. Drugs. 1998;55:585–612. doi: 10.2165/00003495-199855040-00008. [DOI] [PubMed] [Google Scholar]
- 3.Edwards J E, Jr, Bodey G P, Bowden R A, Buchner T, de Pauw B E, Filler S G, Ghannoum M A, Glauser M, Herbrecht R, Kauffman C A, Kohno S, Martino P, Meunier F, Mori T, Pfaller M A, Rex J H, Rogers T R, Rubin R H, Solomkin J, Viscoli C, Walsh T J, White M. International conference for the development of a consensus on the management and prevention of severe candidal infections. Clin Infect Dis. 1997;25:43–59. doi: 10.1086/514504. [DOI] [PubMed] [Google Scholar]
- 4.Geginat G, Kretschmar M, Walter S, Junker D, Hof H, Nichterlein T. Suppression of acquired immunity against Listeria monocytogenes by amphotericin B-mediated inhibition of CD8 T cell function. J Infect Dis. 1999;180:1186–1194. doi: 10.1086/315007. [DOI] [PubMed] [Google Scholar]
- 5.Geginat G, Nichterlein T, Kretschmar M, Schenk S, Hof H, Lalic-Multhaler M, Goebel W, Bubert A. Enhancement of the Listeria monocytogenes p60-specific CD4 and CD8 T cell memory by nonpathogenic Listeria innocua. J Immunol. 1999;162:4781–4789. [PubMed] [Google Scholar]
- 6.Harty J T, Pamer E G. CD8 T lymphocytes specific for the secreted p60 antigen protect against Listeria monocytogenes infection. J Immunol. 1995;154:4642–4650. [PubMed] [Google Scholar]
- 7.Hauser W E, Jr, Remington J S. Effect of amphotericin B on natural killer cell activity in vitro. J Antimicrob Chemother. 1983;11:257–262. doi: 10.1093/jac/11.3.257. [DOI] [PubMed] [Google Scholar]
- 8.Hiemenz J W, Walsh T J. Lipid formulations of amphotericin B: recent progress and future directions. Clin Infect Dis. 1996;22(Suppl. 2):S133–144. doi: 10.1093/clinids/22.supplement_2.s133. [DOI] [PubMed] [Google Scholar]
- 9.Kretschmar M, Nichterlein T, Hannak D, Hof H. Effects of amphotericin B incorporated into liposomes and in lipid suspensions in the treatment of murine candidiasis. Drug Res. 1996;46:711–715. [PubMed] [Google Scholar]
- 10.Mehta R T, Mehta K, Lopez-Berestein G, Juliano R L. Effect of liposomal amphotericin B on murine macrophages and lymphocytes. Infect Immun. 1985;47:429–433. doi: 10.1128/iai.47.2.429-433.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.North R J, Dunn P L, Conlan J W. Murine listeriosis as a model of antimicrobial defense. Immunol Rev. 1997;158:27–36. doi: 10.1111/j.1600-065x.1997.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 12.Rex J H, Walsh T J, Sobel J D, Filler S G, Pappas P G, Dismukes W E, Edwards J E. Practice guidelines for the treatment of candidiasis. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:662–678. doi: 10.1086/313749. [DOI] [PubMed] [Google Scholar]
- 13.Schindler J J, Warren R P, Allen S D, Jackson M K. Immunological effects of amphotericin B and liposomal amphotericin B on splenocytes from immune-normal and immune-compromised mice. Antimicrob Agents Chemother. 1993;37:2716–2721. doi: 10.1128/aac.37.12.2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens D A, Kan V L, Judson M A, Morrison V A, Dummer S, Denning D W, Bennett J E, Walsh T J, Patterson T F, Pankey G A. Practice guidelines for diseases caused by Aspergillus. Infectious Diseases Society of America. Clin Infect Dis. 2000;30:696–709. doi: 10.1086/313756. [DOI] [PubMed] [Google Scholar]
- 15.Wong-Beringer A, Jacobs R A, Guglielmo B J. Lipid formulations of amphotericin B: clinical efficacy and toxicities. Clin Infect Dis. 1998;27:603–618. doi: 10.1086/514704. [DOI] [PubMed] [Google Scholar]