Abstract
Objectives.
To compare the demographic and clinical characteristics of children with Down syndrome who did and did not receive polysomnography to evaluate for obstructive sleep apnea after publication of the American Academy of Pediatrics’ guidelines recommending universal screening by age 4 years.
Study Design.
Retrospective cohort study.
Setting.
Single tertiary pediatric hospital.
Methods.
Review was conducted of children with Down syndrome born between 2007 and 2012. Children who obtained polysomnography were compared with children who did not, regarding demographic data, socioeconomic status, and comorbidities.
Results.
We included 460 children with Down syndrome; 273 (59.3%) received at least 1 polysomnogram, with a median age of 3.6 years (range, 0.1–8.9 years). There was no difference in the distribution of sex, insurance status, or socioeconomic status between children who received polysomnography and those who did not. There was a significant difference in race distribution (P = .0004) and distance from home to the medical center (P < .0001) between groups. Among multiple medical comorbidities, only children with a history of hypothyroidism (P = .003) or pulmonary aspiration (P = .01) were significantly more likely to have obtained polysomnography.
Conclusions.
Overall, 60% of children with Down syndrome obtained a polysomnogram. There was no difference between groups by payer status or socioeconomic status. A significant difference in race distribution was noted. Proximity to the medical center and increased medical need appear to be associated with increased likelihood of obtaining a polysomnogram. This study illustrates the need for improvement initiatives to increase the proportion of patients receiving guideline-based screening.
Keywords: Down syndrome, polysomnography, sleep study, obstructive sleep apnea, birth cohort, disparities
Obstructive sleep apnea (OSA) is common among children with Down syndrome (DS), with an estimated prevalence of 30% to 80%.1–4 Multiple factors are attributed to this predisposition for OSA, including adenotonsillar hypertrophy, midface and mandibular hypoplasia, relative macroglossia, glossoptosis, hypotonia, and obesity.2–5 Long-term adverse effects of OSA range from impairments in growth and neurocognitive development to the development of pulmonary hypertension and cor pulmonale.2–5
Given the high risk of OSA and the significant clinical sequelae of untreated disease, the American Academy of Pediatrics (AAP) provided recommendations regarding screening for OSA in the 2011 guidelines for health supervision for children with DS.6,7 Through early childhood (ages 1–5 years), providers are recommended to (1) discuss symptoms of OSA at each well-child visit; (2) refer all children with DS to a pediatric sleep laboratory for a polysomnogram (PSG) by 4 years of age; (3) refer any child with signs or symptoms of OSA or abnormal PSG results to a physician with expertise in pediatric sleep; and (4) discuss obesity as a risk factor for OSA.7
In a previous retrospective study, rates of PSG completion among children with DS before and after the publication of the AAP guidelines were compared.8 Nearly two-thirds of children with DS (61%) at our institution underwent a PSG overall. Although the overall rate of PSG completion did not increase significantly after the introduction of the AAP guidelines, there was a significant shift toward completion of a PSG at an earlier age, and the majority of children with DS with OSA were diagnosed before 4 years of age. Our results illustrated the importance of the current guidelines while emphasizing the need to evaluate barriers and improve adherence to completion of a screening PSG.
The objective of the current study was to investigate potential patient-level barriers to adherence to the guidelines for the evaluation of OSA in children with DS. To do so, we aimed to determine differences in demographic and clinical characteristics among children with DS who obtained a PSG as compared with those who did not following the publication of the AAP guidelines.
Methods
Study Patients
This study was designed as a retrospective cohort study. It included all pediatric patients with DS born between January 1, 2007, and December 31, 2012, who were identified per relevant ICD-9 codes (International Classification of Diseases, Ninth Revision) as patients seen within the Division of Developmental and Behavioral Pediatrics at Cincinnati Children’s Hospital Medical Center (CCHMC). The division encompasses the Thomas Center for Down Syndrome: a multidisciplinary clinic for children with DS, including developmental pediatricians, psychologists, and therapy services. The cohort of children with DS was identified and described in our previous study8 and defined by the date of birth relative to the year of publication of the AAP guidelines in 2011. A total of 460 children with DS born between 2007 through 2012 were included, as these children turned 4 years old during or after 2011, when the guidelines were introduced. Children born prior to 2007 were excluded from this study, as these children turned 4 years old prior to 2011 and would not have been exposed to the guidelines. Likewise, children with DS born after 2013 were excluded, as they had not yet turned 4 years old at the time of data collection and analysis (January 31, 2018); therefore, we were unable to evaluate the impact of these guidelines on these patients (Figure 1).
Figure 1.
The post–guidelines era cohorts, defined by year of birth relative to publication of the American Academy of Pediatrics guidelines for polysomnography for children with Down syndrome in 2011.
Data Collection
Data were extracted from the electronic medical record (Epic Hyperspace 2019; Epic Systems Corporation) with assistance provided by the Division of Biomedical Informatics. Demographic data (date of birth, sex, race, ethnicity, home address, and insurance status) and clinical data (referral for PSG, body mass index [BMI], and medical comorbidities documented in the problem list) were collected through the study period of birth through January 31, 2018.
Medical comorbidities included congenital heart disease (CHD); pulmonary disease, including pulmonary aspiration and asthma; hypothyroidism; and neuropsychiatric disorders, including attention-deficit/hyperactivity disorder (ADHD) and autism spectrum disorder (ASD).
PSG data, including age and BMI at the time of first PSG, were obtained from a comprehensive sleep study database maintained by the Division of Pulmonary and Sleep Medicine, which includes all patients who have received a PSG within the institution. For children with DS receiving a PSG, the first documented PSG was included. This study was approved by the CCHMC Institutional Review Board.
Socioeconomic Index
To quantify socioeconomic status (SES) for this study, we created a zip code–level SES index based on 6 zip code–level variables—namely, the percentage of households having an income <$10,000, requiring public assistance income, receiving food stamp and/or SNAP benefits (Supplemental Nutrition Assistance Program) in past 12 months, receiving public health insurance coverage, having no health insurance, or having an income below the poverty level in past 12 months. These data were collected from the 2013 five-year American Community Survey (US Census; https://www.census.gov/programs-surveys/acs).9 A principal components analysis was applied; the first component was called the SES index for this study. Similar methods have been described elsewhere.10 Once the SES index was derived at the zip code level, it was linked back to the individual-level study data by zip code. The index was categorized into quartiles (lowest 25th, 25th–50th, 50th–75th,>75th). Median household income was also obtained from the census data, though it was not included in the principal components analysis.
Statistical Analysis
Descriptive statistics, including frequencies with percentages and medians with ranges, were generated for the study cohort as well as for groups of children who did and did not obtain a PSG. Demographic distributions and rates of medical comorbidities were compared between groups with Fisher’s exact test and the chi-square test for categorical variables, as appropriate, and the Wilcoxon rank sum test for continuous variables. A P value <.05 indicated a statistically significant difference between groups. All data analysis was performed with SAS version 9.4 (SAS Institute).
Recognizing the possibility that children who did not obtain a PSG may have been referred for it but did not complete testing, we conducted a sensitivity analysis to evaluate differences between patients who were referred but did not complete a PSG and patients who were never referred. We applied the same statistical methods as described for the primary objective. Comparisons of continuous measures across 3 groups (PSG obtained, referred, not referred) were made with analysis of variance.
Results
Patient Characteristics
Among 460 children included in this analysis, 273 (59.3%) received at least 1 PSG, with a median age of 3.6 years (range, 0.1–8.9 years) and a mean BMI of 15.2 (SD, 2.9) at the time of first PSG. Children who obtained a PSG were slightly older (by approximately 5 months) than those who did not (Table 1).
Table 1.
Demographic Characteristics Among Children With Down Syndrome and Comparison of Those Who Did and Did Not Obtain a PSG.a
| Patient characteristic | PSG obtained (n = 273) | PSG not obtained (n = 187) | P value |
|---|---|---|---|
| Male sex | 136 (49.8) | 106 (56.7) | .15 |
| Age, y | |||
| On Jan 1, 2018, mean (SD) | 7.3 (1.9) | 6.9 (1.9) | .02 |
| At first PSG, median (range) | 3.6 (0.1–8.9) | ||
| BMI at first PSG, mean (SD) | 15.2 (2.9) | ||
| Race | |||
| Caucasian | 220 (80.6) | 144 (77) | .0004 |
| Black/African American | 21 (7.7) | 18 (9.6) | |
| Asian | 6 (2.2) | 5 (2.7) | |
| Other (includes multirace) | 22 (8.1) | 4 (2.1) | |
| Unknownb | 4 (1.5) | 16 (8.6) | |
| Ethnicity: Hispanic | 19 (7) | 8 (4.3) | .01c |
| Unknownb | 0 | 5 (2.7) | |
| Insurance status | |||
| Private insurance | 177 (64.8) | 122 (65.2) | .99 |
| Public insurance | 82 (30) | 55 (29.4) | |
| Unknown | 14 (5.1) | 10 (5.4) | |
| Median household income by zipcode, mean (SD) | 63,373 (19,449) | 63,316 (22,410) | .98 |
| SES index | |||
| Highest category (>75th) | 113 (42) | 80 (42.8) | .47 |
| Upper 50th | 67 (24.9) | 37 (19.8) | |
| Lower 50th | 44 (16.4) | 39 (20.9) | |
| Lowest category (>25th) | 45 (16.7) | 31 (16.6) |
Abbreviations: BMI, body mass index; PSG, polysomnogram; SES, socioeconomic status.
Values are presented as No. (%) unless noted otherwise.
Includes patients who refused to report race/ethnicity.
Fisher’s exact test.
Among children who did not obtain a PSG (n = 187), 176 (94.1%) had >10 clinical encounters within the institution. Only 38 patients (20.3%) were ≤4 years of age at the time of their last clinical encounter. Additionally, 42 patients (22.5%) were referred for a PSG but did not complete it during the study period; 145 patients (77.5%) neither received referral for nor completed a PSG (Supplemental Table S1, available online).
Table 1 illustrates the comparisons of demographic characteristics of children with DS who obtained a PSG and those who did not. There were no statistically significant differences between groups by sex, insurance status, or SES index. Likewise, median household income was not significantly different. A significant difference in race distribution between groups was noted (P = .0004), although the largest differences were among patients categorized as “other, including multirace” (8.8% of children who obtained a PSG vs 2.1% of children who did not) and “unknown” (1.5% vs 8.6%, respectively). As the “other” and “unknown” race categories appeared to drive the significant differences between groups, we analyzed race distribution excluding these categories. There were no differences in the distribution of the 3 remaining categories between children who obtained a PSG and those who did not (P = .70). Similarly, there was no statistical difference in ethnicity distribution between groups when the “unknown” category was excluded (P = .26).
Figure 2 shows the geographic distribution of children with DS within the cohort by home address, as well as the proportion of children with DS who obtained a PSG at CCHMC by distance from the medical center. Children living within 25 miles of the medical center were more likely to obtain a PSG than those who lived >25 miles away (72.3% vs 44.8%, P <.0001).
Figure 2.
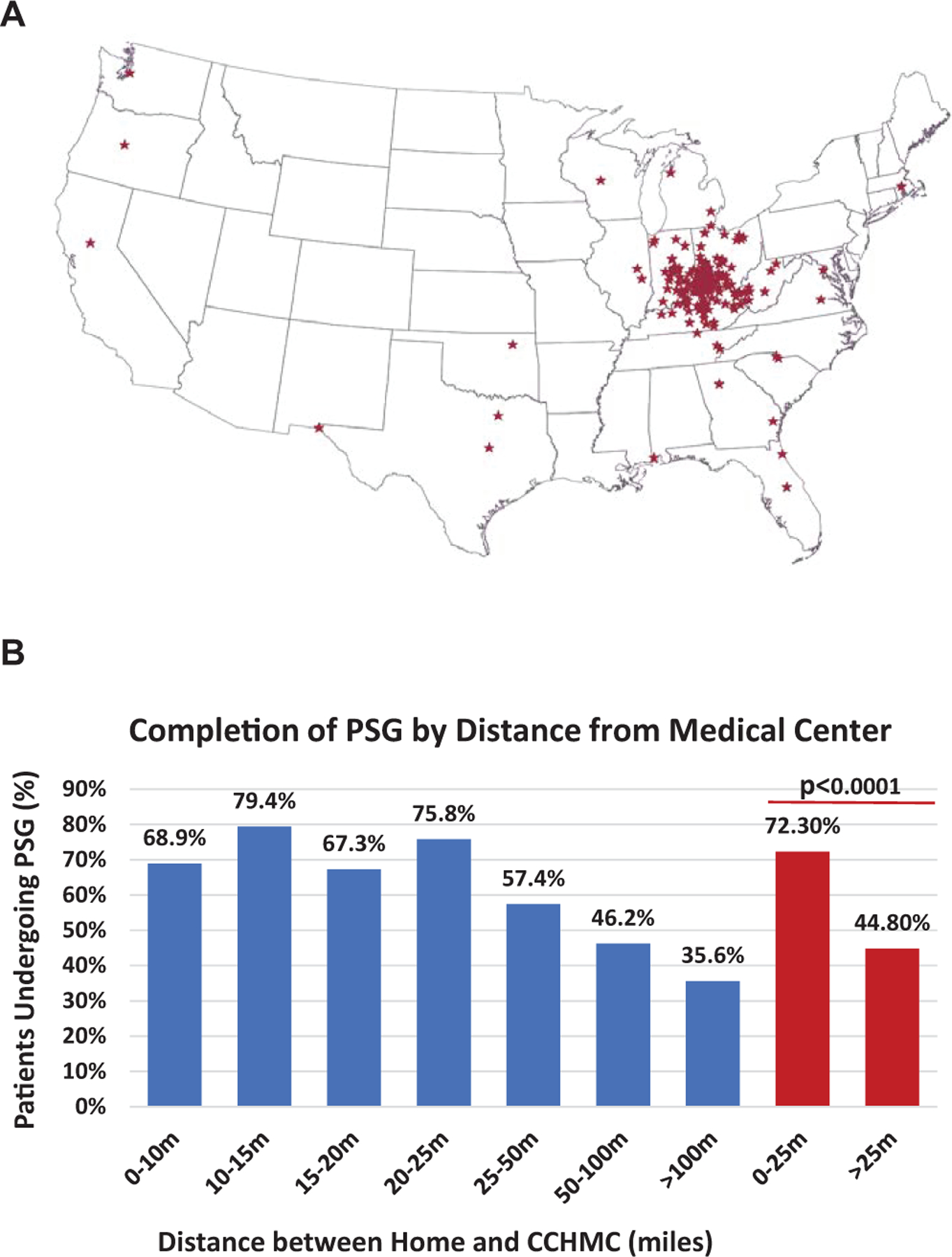
(A) Distribution of home addresses among children with Down syndrome in the study cohort. (B) Rates of completion of first polysomnogram (PSG) in children with Down syndrome by distance between home address and medical center. CCHMC, Cincinnati Children’s Hospital Medical Center.
Clinical Characteristics
Various comorbid medical conditions were commonly diagnosed, including CHD, hypothyroidism, pulmonary aspiration, reactive airway disease or asthma, ADHD, and ASD. Children with hypothyroidism (18.7% vs 8.6%, P = .003) or pulmonary aspiration (10.3% vs 3.7%, P = .01) were significantly more likely to have obtained a PSG (Table 2). There were no significant differences among children with DS and other medical comorbidities.
Table 2.
Comparison of Documented Comorbid Medical Conditions Among Children With Down Syndrome Who Did and Did Not Obtain a PSG.a
| Comorbid medical condition | PSG obtained (n = 273) | PSG not obtained (n = 187) | P value |
|---|---|---|---|
| Congenital heart disease | 59 (21.6) | 44 (23.5) | .63 |
| Hypothyroidism | 51 (18.7) | 16 (8.6) | .003 |
| Pulmonary aspiration | 28 (10.3) | 7 (3.7) | .01 |
| Asthma/reactive airway disease | 7 (2.6) | 2 (1.1) | .32b |
| Attention-deficit/hyperactivity disorder | 7 (2.6) | 1 (0.5) | .15b |
| Autism spectrum disorder | 8 (2.9) | 4 (2.1) | .77b |
Abbreviation: PSG, polysomnogram.
Values are presented as No. (%).
Fisher’s exact test.
Sensitivity Analysis
To understand potential bias among the 187 children who did not obtain a PSG, we compared those who were not referred for a PSG (n = 145) with those who were referred but did not obtain a PSG (n = 42). In general, characteristic distributions were similar between groups. Children who were referred were slightly older (approximately 10 months) and had a slightly lower median income (by nearly $9000) as compared with children who were not referred (Supplemental Table S1, available online). Children who were not referred for a PSG were less likely to live within 25 miles of the medical center as compared with children who were referred but did not obtain a PSG (30.3% vs 52.4%, P = .009). No statistical differences were noted regarding comorbidities (Supplemental Table S2).
Discussion
In this study, we evaluated a large cohort of children with DS presenting to a single tertiary care pediatric hospital through a multidisciplinary developmental-behavioral pediatrics practice. While AAP guidelines recommend universal screening for OSA with PSG in children with DS by the age of 4 years,7 only about 60% of children in our cohort received at least 1 PSG, with a median age just under 4 years at the time of the first PSG. Our study aimed to identify demographic and clinical factors associated with the rate of PSG completion to better understand potential barriers to obtaining a PSG.
While a significant difference was noted in race distribution between groups of children who did and did not obtain a PSG, these differences were noted among patients who were categorized as “other” and “unknown.” Notably, a small sample size within individual groups may limit the ability to further analyze and interpret potential differences. Although racial disparities were not clearly evident in the current cohort, prior studies have demonstrated them in the health outcomes of children with DS, with authors positing that differential access to health services across racial and ethnic groups may be a more significant contributor to variations in outcomes than underlying medical factors alone.11,12 Rates of hospital use and associated costs have been shown to be greater among children with DS of minority race or ethnicity.11 Rates of subspecialty referral were also shown to be greater among Black children with DS with CHD, though not strictly correlated with the presence and severity of underlying disease.12 While racial differences in hospital use and subspecialty referral are reported, other factors may contribute to disparities in access to care, such as medical coverage, SES, distance traveled, or unmeasured factors.
Importantly, no significant differences were noted on the basis of payer status or SES index in our study. Median household income was slightly lower among children who were not referred for a PSG. When compared with other children with special health care needs, children with DS have been shown to have a higher likelihood of unmet medical needs and a lower likelihood of established care within a medical home. The impacts of these differences are often exacerbated by the absence of insurance coverage and lower SES.13–15 Ensuring equitable access to a medical home improves care coordination and facilitates evaluation and management among primary and specialty providers to meet the medical needs of children with DS.14,16
Unsurprisingly, distance from the medical center was significant. Children who lived farther from our medical center were less likely to obtain a PSG. Furthermore, a large proportion of children not referred for PSG lived farther from the medical center. Notably, we cannot account for studies obtained at other facilities. Consistent with previous studies, access to care may be limited by travel distance. Joslyn et al reported that >20% of patients with DS encounter significant geographic restrictions to care, whereby travel to the nearest specialty care clinic may be >2 hours for a significant proportion.16 However, children living within our primary service area completed testing at a rate of about 70%. Of note, the only pediatric sleep laboratories within the service area are affiliated with our institution; therefore, we assume a low likelihood that the remaining 30% would have obtained a PSG elsewhere. These findings suggest that access to care may still be limited by presently unidentified factors.
Among children who did not obtain a PSG, those referred were slightly older than those who were not. However, the clinical significance of this small difference (<1 year) is uncertain in the context of the recommendation for clinical screening for OSA at well-child visits annually.
Children with DS and certain comorbidities, namely hypothyroidism and aspiration, were more likely to obtain a PSG. It is possible that children with DS and increased medical needs associated with these comorbidities may be more likely to have providers familiar with current guidelines and therefore adhere more frequently to recommendations.17 However, children with comorbid CHD, asthma, ADHD, or ASD were not shown to have an increased likelihood of obtaining a PSG. In the case of asthma, ADHD, and ASD, these diagnoses may have been made later in childhood than the anticipated age of recommended PSG by 4 years, and as such, even a potential association with PSG completion would be difficult to assess due to this temporal limitation.
Despite the intention of guidelines to enact changes in evaluation and care, consistent implementation of guideline-based recommendations has remained a challenge. Previous studies have illustrated low adherence rates to health care recommendations for individuals with DS.18–24 Regarding screening for OSA specifically, rates of adherence to recommendations for PSG ranged from 12% to 66%.8,19–21,24
Few studies have specifically evaluated factors associated with low adherence rates to guideline-based recommendations, yet we understand that there are several issues that likely contribute. Often, there is a lag time from publication to physician awareness and application of recommendations.18,19,25 Guideline complexity and the belief that recommendations may not change patient outcomes have also been cited.17 Degree of physician expertise in the diagnosis and management of OSA in children with DS may vary widely.18,19,25 Our study suggests that we should consider provider-level factors when trying to understand adherence, as we saw a relatively low rate of PSG referral (22%) among children who did not complete a PSG. Furthermore, extent of caregiver knowledge of OSA and interest in screening for children with DS is not well known.18,19,25 Finally, there are resource limitations for PSG, and potential patient disparities may manifest in barriers to referrals or resources for testing 17,26,27
Williams et al examined disparities related to overall low adherence to the prior version of AAP guidelines for health supervision in children with DS.17 Rates of adherence to screening recommendations for cervical spine radiographs, complete blood count, thyroid function testing, audiology evaluation, and ophthalmology examination ranged from 33% to 94%. Across all categories, adherence rates were not affected by ethnicity or race. Only adherence to recommendations for complete blood count were affected by sex and insurance status. Adherence to thyroid function testing was higher among children who received regular care from a pediatrician and who had known thyroid disease. Adherence to audiology evaluation was higher among children referred to an otolaryngologist. Differences in SES were not specifically evaluated.
Various improvement interventions, including physician education, integration of recommendations into the electronic medical record, and incorporation of care in multidisciplinary specialty clinics, have been shown to increase adherence to guideline recommendations.20,21,28 While such studies illustrate the development of reliable and sustainable quality improvement initiatives, they tend to emphasize system- and provider-level factors rather than patient-specific disparities in access to care and guideline adherence.
There are limitations to this study. This represents a retrospective analysis from a single tertiary care institution within a multidisciplinary specialty clinic setting, which limits generalization. The analysis of demographic and clinical data is limited by potential misclassification. Comorbid conditions may not be accurately recorded or maintained within the problem list throughout the study period. There is a limitation in analysis of comorbidities by the time of diagnosis, as they may be entered into or removed from the problem list at any time during the study period and may not coincide with the time of PSG. Mean BMI, as a marker for obesity and a known risk factor for OSA, could be determined for children who obtained a PSG relative to the time of completion but, without an age-matched cohort, could not be directly compared with the BMI of children who did not obtain a PSG.
This study has allowed us to begin developing institutional improvement initiatives that aim to increase adherence to guideline-based screening for OSA in children with DS. Interventions should be designed to address provider, caregiver, and patient factors alike. Future survey-based studies that focus on provider and caregiver knowledge, family preferences, and patient barriers to accessing primary and subspecialty care may inform the design of interventions that ultimately mitigate disparate care. Additional emphasis will be placed on improving the primary and subspecialty health care systems that encompass patients and families within the local community.
Conclusion
This study compares the clinical and demographic characteristics of children with DS who did and did not obtain a PSG after publication of the current AAP guidelines. Though the proportion of children who obtained a PSG was greater the nearer that they lived to the medical center, only 72% of children living within the primary service area obtained testing. There was a significant difference in race distribution between groups; however, there were no differences in payer status or SES. By understanding potential barriers to adherence, institutional and public health improvement initiatives can be designed to increase the proportion of patients receiving guideline-based care.
Supplementary Material
Funding source:
None.
Footnotes
This article was presented at the 2019 AAO-HNSF Annual Meeting & OTO Experience; September 15, 2019; New Orleans, Louisiana.
Disclosures
Competing interests: None.
Sponsorships: None.
Supplemental Material
Additional supporting information is available in the online version of the article.
References
- 1.Marcus CL, Keens TG, Bautista DB, von Pechmann WS, Davidson Ward SL. Obstructive sleep apnea in children with Down syndrome. Pediatr 1991;88(1):132–139. [PubMed] [Google Scholar]
- 2.De Miguel-Diez J, Villa-Asensi JR, Alvarez-Sala JL. Prevalence of sleep-disordered breathing in children with Down syndrome: polygraphic findings in 108 children. Sleep 2003;26(8):1006–1009. [DOI] [PubMed] [Google Scholar]
- 3.Shott SR, Amin R, Chini B, Heubi C, Hotze S, Akers R. Obstructive sleep apnea: should all children with Down syndrome be tested? Arch Otolaryngol Head Neck Surg 2006; 132:432–436. [DOI] [PubMed] [Google Scholar]
- 4.Maris M, Verhulst S, Wojciechowski M, Van de Heyning P, Boudewyns A. Prevalence of obstructive sleep apnea in children with Down syndrome. Pediatr 2016;39(3):699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fitzgerald DA, Paul A, Richmond C. Severity of obstructive sleep apnoea in children with Down syndrome who snore. Arch Dis Child 2007;92:423–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.American Academy of Pediatrics Committee on Genetics. American Academy of Pediatrics: health supervision for children with Down syndrome. Pediatr 2001;107:442–449. [DOI] [PubMed] [Google Scholar]
- 7.Bull MJ, Committee on Genetics. Clinical report—heath supervision for children with Down syndrome. Pediatr 2011;128(2): 393–406. [DOI] [PubMed] [Google Scholar]
- 8.Knollman PD, Heubi CH, Meinzen-Derr J, et al. Adherence to guidelines for screening polysomnography in children with Down syndrome. Otolaryngol Head Neck Surg 2019;161(1): 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Pol Plann 2006;21:459–468. [DOI] [PubMed] [Google Scholar]
- 10.Brokamp C, Beck AF, Goyal NK, et al. Material community deprivation and hospital utilization during the first year of life: an urban population-based cohort study. Ann Epi 2019;30:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Derrington TM, Kotelchuck M, Plummer K, Cabral H, et al. Racial/ethnic differences in hospital use and cost among a statewide population of children with Down syndrome. Res Dev Del Dis 2013;34:3276–3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Santoro SL, Esbensen AJ, Hopkin RJ, Hendershot L, Hickey F, Patterson B. Contributions to racial disparity in mortality among children with Down syndrome. J Pediatr 2016l. 174: 240–246. [DOI] [PubMed] [Google Scholar]
- 13.McGrath RJ, Stransky ML, Cooley WC, Moeschler JB. National profile of children with Down syndrome: disease burden, access to care, and family impact. J Pediatr 2011;159: 535–540. [DOI] [PubMed] [Google Scholar]
- 14.Nugent J, Gorman G, Erdie-Lalena CR. Disparities in access to healthcare transition services for adolescents with Down syndrome. J Pediatr 2018;197:214–220. [DOI] [PubMed] [Google Scholar]
- 15.Schieve LA, Boulet SL, Kogan MD, Van Naarden-Braun K, et al. A population-based assessment of the health, functional status, and consequent family impact among children with Down syndrome. Dis Health J 2011;4:68–77. [DOI] [PubMed] [Google Scholar]
- 16.Joslyn N, Berger H, Skotko BG. Geospatial analyses of accessibility to Down syndrome specialty care. J Pediatr 2020;218: 146–150.e1. [DOI] [PubMed] [Google Scholar]
- 17.Williams K, Wargowski D, Eickhoff J, Wald E. Disparities in health supervision for children with Down syndrome. Clin Pediatr 2017;56(14):1319–1327. [DOI] [PubMed] [Google Scholar]
- 18.Lin SC, Davey MJ, Horne RSC, Nixon GM. Screening for obstructive sleep apnea in children with Down syndrome. J Pediatr 2014;165:117–122. [DOI] [PubMed] [Google Scholar]
- 19.Santoro SL, Yin H, Hopkin RJ. Adherence to symptom-based care guidelines for Down syndrome. Clin Pediatr (Phila) 2017;56(2):150–152. [DOI] [PubMed] [Google Scholar]
- 20.Santoro SL, Martin LJ, Pleatman SI, Hopkin RJ. Stakeholder buy-in and physician education improve adherence to guidelines for Down syndrome. J Pediatr 2016;171:262–268. [DOI] [PubMed] [Google Scholar]
- 21.Santoro SL, Bartman T, Cua CL, Lemle S, Skotko BG. Use of electronic health record integration for Down syndrome guidelines. Pediatr 2018;142(3):e20174119. [DOI] [PubMed] [Google Scholar]
- 22.Fergeson MA, Mulvihill JJ, Schaefer GB, et al. Low adherence to national guidelines for thyroid screening in Down syndrome. Genet Med 2009;11(7):548–551. [DOI] [PubMed] [Google Scholar]
- 23.Jensen KM, Taylor LC, Davis MM. Primary care for adults with Down syndrome: adherence to preventive healthcare recommendations. J Intellect Disabil Res 2013;57(5):409–421. [DOI] [PubMed] [Google Scholar]
- 24.Hsieh A, Gilad A, Wong K, Cohen M, Levi J. Obstructive sleep apnea in children with Down syndrome: screening and effect of guidelines. Clin Pediatr 2019;58(9):993–999. [DOI] [PubMed] [Google Scholar]
- 25.Rosen D, Lombardo A, Skotko B, Davidson EJ. Parental perceptions of sleep disturbances and sleep disordered breathing in children with Down syndrome. Clin Pediatr (Phila) 2011; 50(2):121–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Skotko BG, Macklin EA, Muselli M, et al. A predictive model for obstructive sleep apnea and Down syndrome. Am J Med Genet 2017;173:889–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nehme J, LaBerge R, Pthos M, et al. Predicting the presence of sleep-disordered breathing in children with Down syndrome. Sleep Med 2017;36:104–108. [DOI] [PubMed] [Google Scholar]
- 28.Skotko BG, Davidson EJ, Weintraub GS. Contributions of a specialty clinic for children and adolescents with Down syndrome. Am J Med Genet 2013;161:430–437. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


