Abstract
Inherited kidney diseases (IKDs) are a large group of disorders affecting different nephron segments, many of which progress towards kidney failure due to the absence of curative therapies. With the current advances in genetic testing, the understanding of the molecular basis and pathophysiology of these disorders is increasing and reveals new potential therapeutic targets. RNA has revolutionized the world of molecular therapy and RNA-based therapeutics have started to emerge in the kidney field. To apply these therapies for inherited kidney disorders, several aspects require attention. First, the mRNA must be combined with a delivery vehicle that protects the oligonucleotides from degradation in the blood stream. Several types of delivery vehicles have been investigated, including lipid-based, peptide-based, and polymer-based ones. Currently, lipid nanoparticles are the most frequently used formulation for systemic siRNA and mRNA delivery. Second, while the glomerulus and tubules can be reached by charge- and/or size-selectivity, delivery vehicles can also be equipped with antibodies, antibody fragments, targeting peptides, carbohydrates or small molecules to actively target receptors on the proximal tubule epithelial cells, podocytes, mesangial cells or the glomerular endothelium. Furthermore, local injection strategies can circumvent the sequestration of RNA formulations in the liver and physical triggers can also enhance kidney-specific uptake. In this review, we provide an overview of current and potential future RNA-based therapies and targeting strategies that are in development for kidney diseases, with particular interest in inherited kidney disorders.
Keywords: RNA, Kidney disease, RNA-therapy, Nanoparticles
Introduction
Inherited kidney disorders represent a large spectrum of pathologies that can affect both the glomerular and/or tubular nephron segments, frequently leading to chronic kidney disease or kidney failure. So far, around 100 monogenetic kidney disorders have been described, with the number of identified genetic causes rapidly increasing due to advances in next generation sequencing and improved techniques for functional validation [1–3]. For most of these diseases, curative therapies are not available. Instead, therapeutic strategies aim to preserve kidney function and postpone disease progression [4]. Genetic causes account for around 70% of the paediatric and 10% of the adult patients receiving kidney replacement therapy. In this regard, novel molecular therapies aiming at replacement of defective genes or inhibiting genes that cause metabolic disturbances, offer new perspectives in the field [5].
RNA has revolutionized the world of molecular therapy, with the first siRNA-based drugs Onpattro (Patisiran) for hereditary transthyretin amyloidosis, Givlaari (Givosiran) for acute hepatic porphyria and Oxlumo (Lumasiran) for primary hyperoxaluria type 1 in both adult and paediatric populations, obtaining approval from the Food and Drug Administration (FDA) in 2018, 2019 and 2020, respectively [6, 7]. Furthermore, mRNA has recently proven its potential with the rapid development of mRNA-based vaccines against SARS-CoV2 [8–10]. Several ongoing clinical trials have demonstrated the potential of mRNA-based replacement therapies for cystic fibrosis (NCT03375047—phase 1/2 recruiting), heart failure (NCT03370887—phase 2 recruiting) and propionic acidemia (NCT04159103—phase 1/2 recruiting) [11–13]. However, upon systemic application, most current formulations target the liver [14]. As an alternative, local application of RNA-based therapeutics has shown much promise in the treatment of, for example, ocular pathologies, cancer, cystic fibrosis and as vaccines [8, 11, 12, 15–19]. RNA-based therapeutics for kidney diseases are lagging behind due to the challenge of targeted delivery of RNA to kidney cells. Nevertheless, several preclinical studies have utilized RNA to treat kidney fibrosis, kidney carcinoma, hyperoxaluria and glomerulonephritis, illustrating the potential of this approach to treat kidney diseases [20–25]. In this review, we describe the principles of RNA-based therapeutic approaches and discuss current and potential future therapeutic use of messenger RNA (mRNA) and small interfering RNA (siRNA) to treat kidney diseases.
Types of RNA-based therapies
The wide spectrum of inherited disorders can be subdivided into two main categories, those characterized by the activation or ectopic activity of a gene or protein (gain-of-function) and those caused by an impaired gene function (loss-of-function) and thereby lack of a functional protein. RNA-based therapies can be applied for both categories, with mRNA-based protein replacement to be used in the loss-of-function disorders and siRNA and antisense oligonucleotides (ASOs) for diseases caused by a gain-of-function [26, 27].
Protein complementation using mRNA
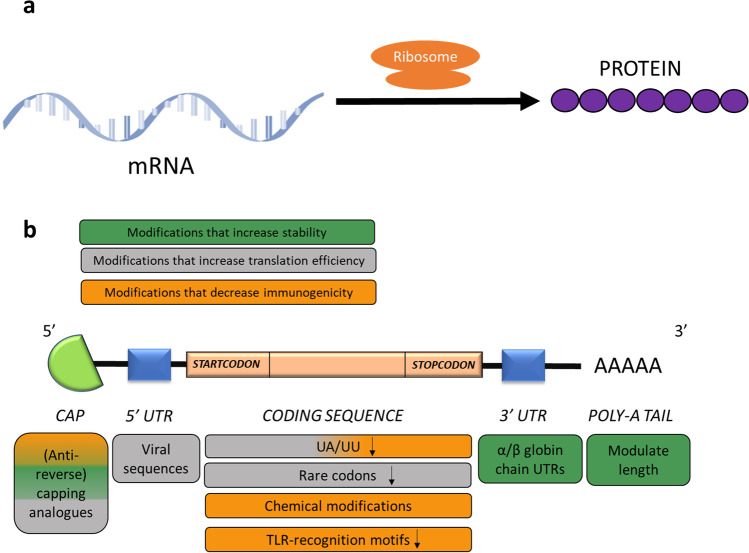
Protein complementation by using in vitro transcribed (IVT) mRNA has become an alternative for DNA-based gene replacement to treat diseases caused by the absence of functional proteins (Fig. 1a). The production of mRNA by in vitro transcription is conducted in a cell-free system, which has low manufacturing costs and is easy to standardize. For this purpose, a DNA-template with the desired protein-coding sequence is preceded by a promotor for one of three specific RNA polymerase systems (T7, T3 and SP6-polymerase). Subsequently, the primary transcript must be capped at the 5’ end and polyadenylated at the 3’ end, for which various strategies exist [11].
Fig. 1.
a In vitro transcribed mRNA is translated by ribosomes to yield proteins that can replace an absent or mutated protein [11]. b mRNA-based therapies introduce the in vitro transcribed (IVT) mature mRNA of a specific gene. A functional mRNA molecule comprises a 5’ cap structure, 5’ and 3’ UTRs, the coding sequence and a poly-A tail. Several modifications can be used to increase stability (green), modulate translation efficiency (grey) and/or decrease immunogenicity (orange). The use of (anti-reverse) capping analogues enhances protection against nuclease-mediated degradation and maintains the immune-modulatory capabilities of the 5’ cap structure. Careful consideration of the non-coding regions at the 5’ and 3’ end of the coding sequence and a 3’ poly-A tail of sufficient length (100–250 adenines) can further enhance stability and translational activity. Sequence and chemical modifications in the coding region can be applied to prevent TLR- and RIG1-activation and thus reduce immunogenicity [11, 28, 29]
The main challenges in using IVT mRNA are its immunogenic potential and rapid nuclease-mediated degradation. Both factors can be addressed through structural changes of the mRNA molecule (Fig. 1b) [30, 31].
First, instead of endogenous cap-structures, guanosine triphosphate (GTP) capping analogues that confer de-capping resistance can be employed to increase stability of the mRNA. However, problems can arise through competition of the cap analogues with normal GTP and the risk of reverse integration during synthesis. Therefore, so-called anti-reverse capping analogues (ARCAs) have been developed, which are cap-like molecules with an extra methylation on the ribose group that prevent reverse integration and increase translation efficiency [28, 32]. The 5’-cap also plays an important role in preventing the immune recognition of the mRNA by the cytosolic helicase retinoic acid-inducible gene-I (RIG-I) [29]. As a further structural element, careful consideration of the 5’ and 3’ untranslated regions (UTRs) helps to further optimize translation efficiency and intracellular half-live. At the 3’ end, modifying the sequence length or using the UTRs from the alpha- and beta- globin chains improves mRNA stability, while the use of viral UTRs at the 5’ end can increase translation efficiency [11, 33]. Finally, the length of the poly-A tail has an impact on half-life and a longer poly-A tail increases the number of available poly-A-binding protein (PABP) binding sites, an interaction that mediates gene expression and enhances mRNA stability [34, 35]. For IVT mRNA, poly-A tails can either be added post-transcriptionally, using the poly-A polymerase enzyme, or by encoding a series of thymidine residues in the DNA template [36, 37]. The ideal length of a poly-A tail has been found to be between 100 to 250 residues. Additionally, the addition of a poly-A tail with a sulfurized phosphorothioate backbone instead of the natural phosphodiester backbone, has been shown to increase mRNA stability in vitro by reducing the susceptibility to deadenylases [38]. However, for mRNA for therapeutic purposes, backbone modifications are rather the exception than the rule [11].
Next to modifications in the non-coding regions, the coding sequence in the open reading frame can also be modified to reduce the immunogenicity and enhance stability and translation efficiency (Fig. 1b). Replacing rare codons by more common variants, coding for the same amino acid, can increase translation efficiency and has been proven to be very efficient in inducing overexpression of the human erythropoietin (EPO) gene [39]. Notably, some proteins require a slow translation for a proper folding and therefore, codon optimization must be carefully evaluated in each case [32, 40]. Other approaches to reduce the immunogenicity of RNA are the elimination of structural motifs that activate receptors of the innate immune response, and the introduction of chemical modifications that render the mRNA more similar to endogenous mRNA. Uridine-rich regions can trigger toll-like receptor 7-(TLR7-) mediated immune responses, while UU and UA dinucleotides can increase mRNA decay as recognition motifs for endoribonuclease L (RNAse L). Depletion of mRNA of these motifs has been shown to increase mRNA stability and expression of eGFP, luciferase, interferon-alpha and the hepatitis B surface antigen [41–43]. As chemical modifications, naturally occurring chemically modified nucleotides, namely the incorporation of N6-methyladenosine, N5-methylcytidine, 2-thiouridine and pseudo-uridine, prevent the activation of the TLR-mediated innate immune response. The use of codon optimization, base depletion and/or chemical modifications has become standard practice in mRNA-based therapeutics and has proven to be efficient in the mRNA-based treatments that are currently tested for propionic acidemia and cystic fibrosis [43].
Gene knockdown using siRNA and ASOs
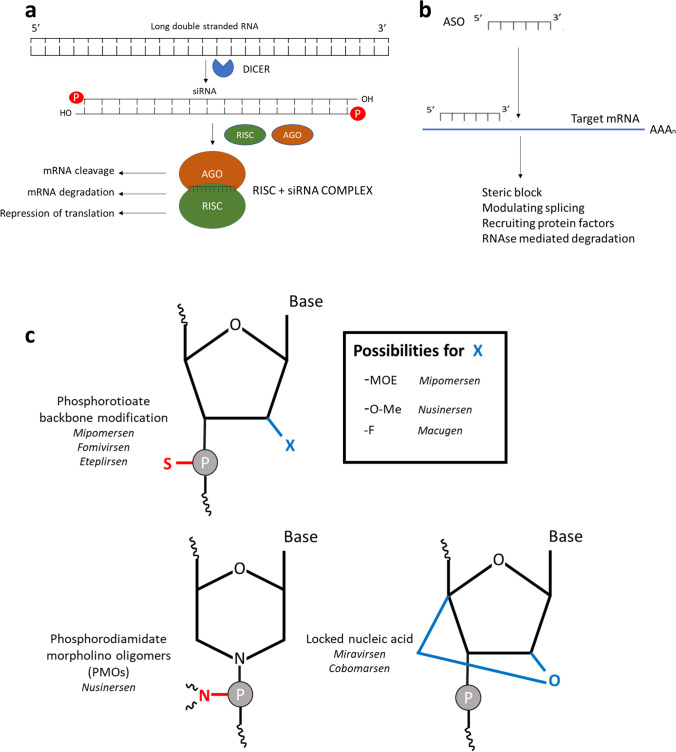
Genetic diseases that are caused by overexpression of a gene, or an accumulation of a toxic metabolite, can be treated by using antisense RNA (ASO) or siRNA. RNA therapies, outside of vaccination strategies, that have been approved by the United States Food and Drug Administration (FDA) or the European Medical Agency (EMA) so far, fall into this category. Both ASOs and siRNA-molecules are used for knocking down gene expression, with the main difference being the single-stranded nature of ASOs in comparison to the double-stranded siRNA. Once inside the cytosol, an siRNA molecule is processed by the dicer protein, loaded into the RISC complex and upon binding to its corresponding mRNA sequence, results in gene knockdown by either mRNA cleavage or translation repression (Fig. 2a). Antisense oligonucleotides can also in turn induce steric block, modulate splicing or induce RNAse-mediated degradation (GAPmers) and thus have a similar mode of action and effect (Fig. 2b) [44, 45].
Fig. 2.
a siRNA results in a knockdown of gene expression. The double-stranded precursor of the siRNA molecule is brought into the cell and subsequently cleaved by the DICER protein. The resulting single-stranded siRNA molecule binds to the Argonaut (AGO) protein and interacts with the RNA-induced silencing complex (RISC), directing the siRNA to the corresponding mRNA sequence and resulting in a knock-down of protein expression, either by cleavage, degradation or translation repression [44, 45]. b ASOs are short single-stranded RNA sequences, structurally stabilized through chemical modifications, that can lead to the knock-down of a corresponding mRNA molecule by steric block, splice modulation, or RNAse H-mediated cleavage of the mRNA [44]. c Small interfering RNA (siRNA) and antisense oligonucleotides (ASOs) can knock down gene expression. Modifications in the backbone (phosphorothioate linkage and incorporation of fluor-, amine-, halogen-, deoxy- or methyl-groups) can be used in both the siRNA precursor as well as in the single-stranded antisense oligonucleotides to increase stability. Locked nucleic acids also prevent the degradation of the single-stranded RNA molecules [46]. 2′-O-methoxyethyl (-MOE), 2′-O-methyl (-O-Me), 2’-Fluoro (-F), phosphorodiamidate morpholino oligomers (PMOs), locked nucleic acids (LNAs)
Both siRNA and ASOs can be produced in vitro by either the RNase/Dicer-mediated digestion of double stranded RNA molecules, by in vitro transcription or by chemical synthesis [45]
For therapeutic siRNA to be effective, the double-stranded RNA molecule needs to be stable, and have the appropriate GC-content and length. Any unwanted secondary structures that could interfere with the hybridisation to the target mRNA should be avoided [47, 48]. For antisense oligonucleotides, the single-stranded nature of the ASOs can result in rapid degradation. Therefore, chemical modifications of the backbone have been explored and have shown to increase stability [49].
Various stabilizing groups have been introduced into the ribose unit, with the most used modifications being 2′-O-methyl (2′-O-Me) and 2′-O-methoxyethyl (2′-MOE) RNA molecules (Fig. 2c). Furthermore, the phosphate in the backbone can be replaced by a phosphorothioate group. This modification has proven its efficacy in several FDA-approved ASO therapeutics like Fomivirsen (cytomegalovirus), Mipomersen (familial hypercholesterolemia), and Eteplirsen (Duchenne muscular dystrophy). Furthermore, backbone analogues such as phosphorodiamidate morpholino oligomers (PMOs) can be employed to protect against nuclease-mediated degradation and also enhance cellular uptake, as is the case in Nusinersen [50–52]. Modified RNA molecules are primarily excreted via the kidneys and have shown significant accumulation in the proximal tubular cells, which may lead to concentration-dependant nephrotoxicity [53, 54]. Mechanistically, it has also been shown that RNA can compete with protein ligands for the megalin–cubilin receptor complex, leading to a reversible proteinuria [55]. 2’-Fluoro-modified nucleotides have been used in the approved aptamer drug Macugen for the treatment of age-related macular degeneration even though, in this case, the working mechanism is through antibody-like target binding [56]. As a further backbone modification, the 4’C and 2’O of the ribose unit can be linked, yielding so-called locked nucleic acids (LNAs) [46]. Special types of LNAs have also been utilized in experimental ASO-based drugs like Miravirsen (hepatitis C) and Cobomarsen (T-cell lymphoma) [57].
Delivery vehicles for RNA
The main challenge for in vivo application of RNA-based therapies is the need for appropriate delivery vehicles (Table 1). While naked RNA can be used ex vivo via cell permeation methods, such as electroporation, or in vivo with local injection, the large size and anionic nature of siRNA- and mRNA-molecules generally prevents efficient cellular delivery. Nevertheless, the kidney accumulation of naked oligonucleotides has been demonstrated and this observation has been linked to the megalin binding capacity of oligonucleotides [55].
Table 1.
Delivery vehicles for nucleic acids with their (dis)advantages and applications of mRNA in the clinical setting (approved and clinical trials)
| TYPES OF DELIVERY VEHICLES FOR NUCLEIC ACIDS | ||||
|---|---|---|---|---|
| Advantages | Disadvantages | Diseases targeted by RNA therapy in the clinical setting | ||
| Cationic liposomes/cationizable LNPs | ||||
|
- Low toxicity - Low immunogenicity - Possibility of targeted delivery - Easy modification with charged groups, targeting agents, etc |
- Rapid degradation by the reticulo-endothelial system - Inability of sustained drug delivery - Difficult to reach organs other than liver |
- Hereditary transthyretin-mediated amyloidosis [58] (Patisiran) - Idiopathic pulmonary fibrosis (NCT03538301) [45] - Cystic fibrosis (NCT03375047) [12] - Heart failure (NCT03370887) [11] - Glaucoma (NCT02250612) [15] - Infectious diseases (Rabies, Zika, Cytomegalovirus, Tuberculosis, Influenza)[11] - Cancer therapy (Melanoma, Ovarian cancer, Breast cancer, …)[11] |
||
| Polypeptides | ||||
|
- Biodegradable - Easy to modify - High encapsulation efficiency - Flexible control of cationic or amphipathic nature |
- Lower efficiency (Artificial viral coat proteins) - Aggregation in salty environment (Protamines) - Low dissociation rate (Protamines) |
- Hypertrophic Scars (NCT04012099) [45] - Rabies (NCT02241135) [19] - Cancer therapy (Non-small-cell lung carcinoma, Prostate cancer, Melanoma)[11] |
||
| Cationic polymers | ||||
|
- Possibility of targeted delivery - Branched PEI shows enhanced buffering capacity for endosomal release - Cationic and amphipathic groups can be incorporated |
- Size dependant toxicity - Non-biodegradable nature |
|||
However, RNA delivery is mainly performed after complexation into nanoparticles through charge-driven interactions of a cationic delivery agent with the negatively charged oligonucleotide [11]. In this way, protection from degradation and induction of cellular uptake by endocytosis and endosomal release are achieved.
Lipid-based delivery systems
One of the most frequently used delivery methods for RNA are lipid nanoparticles (LNP) and liposomes, formed from cationic or ionizable lipids that encapsulate the RNA. Traditional liposomes comprise one or more lipid bilayers surrounding an aqueous core, while lipid nanoparticles consist of disordered micellar aggregates with an outer lipid layer [59]. The stability can be engineered through incorporation of cholesterol and neutral lipids, while the use of a poly-ethyleneglycol (PEG) layer can increase the circulation time by reducing opsonization and thus elimination by the reticuloendothelial system (RES) [60]. LNP-formulated mRNA has been explored in clinical studies for methylmalonic acidemia, ornithine transcarbamylase deficiency, cystic fibrosis, cancer immunotherapy, Fabry disease and infectious diseases. Most of these formulations contain an ionizable lipid like (6Z,9Z,28Z,31Z)-heptatriacont-6,9,28,31-tetraene-19-yl 4-(dimethylamino)butanoate (DLin-MC3-DMA) [11, 12, 19, 59, 61–63]. While a certain degree of accumulation of this type of LNP in the kidneys has been shown, they mostly direct the cargo towards the liver [64, 65]. This organ tropism is a consequence of the dissociation of the PEG-shield from the LNP, leading to opsonization of the nanoparticle with the plasma protein apolipoprotein E (ApoE), which has a high affinity for the LDL receptor on hepatocytes [66]. The siRNA-based therapeutic for a hereditary form of amyloidosis, Patisiran, uses this type of lipid vehicle to deliver an siRNA molecule to the liver [58]. Fabry disease is an X-linked lysosomal storage disorder caused by mutations in the alpha-galactosidase gene, leading to glycosphingolipid accumulation and damage to the kidney, among other organs. An intravenous injection of LNP-formulated IVT GLA mRNA in a Fabry mouse model showed increased alpha-galactosidase production in the liver, which was subsequently secreted into the circulation and successfully reduced the glycosphingolipid levels in the heart, kidney and spleen [67].
Polypeptide-based delivery systems
Because of their good biodegradability and development from endogenous substances, polypeptide-based nanoparticles are also being explored. Protamine nanoparticles are based on an arginine-rich protein that ensures a high encapsulation efficiency. These vehicles have been studied in a clinical trial of an RNA-based vaccine against rabies (NCT02241135) [19]. Cationic and/or cationic amphiphilic cell-penetrating peptides (CPPs) yield complexation and mediate membrane translocation. Some of these CPPs, like the nuclear localisation sequence peptide, showed kidney accumulation in mice [68]. However, to what degree the kidney preference also holds for nanoparticles formed from these peptides remains to be shown. While these polypeptide-based delivery vehicles have been studied extensively, their application has been restricted to cancer immunotherapy and infectious disease in animal models [11]. For example, in a mouse model for oral cancer, the combination of an epidermal growth factor receptor (EGFR)-targeting peptide and a membrane disrupting peptide successfully delivered an siRNA to downregulate the expression of protein phosphatase 2A, a known oncogene [69]. In a similar manner, the downregulation of bromodomain 4 expression was proven to be efficient in treating prostate cancer after siRNA-delivery via a PEG-based nanoparticle with a tumor-targeting peptide [70]. Another peptide-based delivery strategy, is the use of artificial viral coat proteins, which have also been formulated as virus-like particles that can encapsulate mRNA and be applied in mRNA-based vaccines for prostate cancer, but have not shown any application in kidney delivery [11].
Polymer-based delivery vehicles
Next to lipids and peptides, positively charged polymeric materials can also shield RNAs from enzymatic degradation and yield cellular uptake. Polyethyleneimine (PEI) was the first polymer that showed the potential to deliver nucleic acids, with the imine groups conferring the positive nature, nucleic acid encapsulation and facilitation of cell entry and endosomal escape. However, the high density of the positive charges also results in a high degree of toxicity, leading to the development of new and safer PEI derivatives [11]. Furthermore, some modified PEGylated polyacrylate polyplexes, like PDAEMA (poly[2 (dimethylamino)ethyl methacrylate]) have been utilized to complex RNA [71]. A notable example for polymer-based vehicles in kidney pathology is the intraperitoneal injection of a mitogen-activated protein kinase 1 (MAPK1) siRNA, encapsulated in a polyethylene glycol poly(l-lysine) (PEG-PLL) copolymer-based delivery vehicle, that could improve kidney function and ameliorate glomerular sclerosis in a mouse model of glomerulonephritis [24, 72].
The positively charged polymer chitosan also forms nanoparticles through complexation with oligonucleotides. Chitosan/hyaluronic acid-based nanoparticles are both biodegradable and biocompatible and have provided successful delivery of mRNA and siRNA in vitro and in vivo [73]. Aquaporin 1 (AQP1) siRNA-loaded chitosan nanoparticles efficiently targeted the kidneys, more specifically the proximal tubules, after intravenous injection into a mouse model. Targeting of these nanoparticles was megalin-mediated, but also determined by molecular weight, with the siRNA nanoparticles that were prepared with 40 kDa chitosan A showing the highest degree of accumulation [74].
The application of RNA-based therapies in kidney diseases
Although a few applications have been described in the section above, it remains a challenge to direct RNA-based therapies to the kidney. Kidney-targeted delivery is complicated by the complexity of organ architecture and a large number of different cell types in the kidney [5, 75]. In this section, we will discuss several relevant examples of RNA-based therapeutics targeting specific kidney cells (Table 2).
Table 2.
Examples of RNA-based therapies for kidney diseases in the clinical and preclinical setting. N-Acetylgalactosamine (GalNac), poly(ethylene glycol)-poly(l-lysine)-based nanoparticle (PEG-PLL); Lipid nanoparticle (LNP)
| Recent advancements in RNA based therapeutics for kidney diseases | ||||||
|---|---|---|---|---|---|---|
| Kidney disease | Active agent | Delivery vehicle | Administration route | Targeted structure | Status | Ref |
| Oxaluria | Glycolate oxidase siRNA | GalNAc | Subcutaneous | Liver | Approved—Lumasiran | [20] |
| Oxaluria | Lactate dehydrogenase A siRNA | GalNAc | Subcutaneous | Liver | Clinical trial—Nedosiran—NCT04555486 (not recruiting) | [25] |
|
Delayed graft function Acute kidney injury after cardiac surgery |
P53 siRNA | Naked | Intravenous | Proximal tubule | Clinical trial—Teprasiran—NCT02610296 (completed)—NCT03510897 (not recruiting) | [76] |
| Fabry disease | Α-Galactosidase mRNA | LNP | Intravenous | Liver | Preclinical | [67] |
| Ischemic and cisplatin-induced kidney injury | P53 siRNA | Naked | Intravenous | Proximal tubule | Preclinical | [77] |
| Renal ischemia reperfusion injury | Fas siRNA | Naked | Renal vein | Proximal tubule | Preclinical | [78] |
| Renal ischemia reperfusion injury | Fas siRNA | Naked | Renal artery | Proximal tubule | Preclinical | [79] |
| Renal ischemia reperfusion injury | Caspase-3 siRNA | Naked | Renal artery | Proximal tubule | Preclinical | [80] |
| Renal ischemia reperfusion injury | Caspase-3 and complement-3 siRNA | Naked | Intravenous | Proximal tubule | Preclinical | [81] |
| Kidney fibrosis | Smad4 siRNA | Naked | Intravenous | Proximal tubule | Preclinical | [21] |
| Tubulo-interstitial fibrosis | HSP47 siRNA | Gelatine microspheres | Intra-urethral | Proximal tubule | Preclinical | [82] |
| Cisplatin-induced kidney injury | Meprin-1β, Ctr1 and P53 siRNA | Carbon nanotubes | Intravenous | Proximal tubule | Preclinical | [83] |
| Acute kidney injury after unilateral urethral obstruction | Cox2 siRNA | Chitostan nanoparticle | Intravenous | Peritoneal macrophages | Preclinical | [84] |
| Acute kidney injury (folic acid-induced) | P53 siRNA | DNA tetrahedron nanovehicle | Intravenous | Proximal tubule | Preclinical | [85] |
| Acute kidney injury (polymicrobial-induced) | TLR9 siRNA | Naked | Intravenous | Proximal tubule | Preclinical | [86] |
| Polycystic kidney disease | Angiotensin ASO | Naked | Subcutaneous | Proximal tubule | Preclinical | [87] |
| Glomerulonephritis | MAPK1 siRNA | PEG-PLL | Intraperitoneal | Glomerulus | Preclinical | [24] |
| Glomerulonephritis | TGF-1β siRNA | Naked | Renal artery | Glomerulus | Preclinical | [72] |
| Immunoglobulin A nephropathy | p38α MAPK and p65 siRNA | LNP | Intravenous | Glomerulus | Preclinical | [88] |
Glomerular endothelium
The endothelial cells surrounding the glomerular capillaries form pores and are an integral part of the glomerular filtration barrier. Strategies to specifically target the glomerular endothelium remain scarce, most likely due to the low number of cell-specific receptors that can be targeted. However, the endothelium plays a central role in inflammatory diseases and during inflammation several receptors are upregulated [89]. Anti-VCAM1 antibody-modified liposomes, based on the SAINT-C18 (1-methyl-4-(cis-9-dioleyl) methyl-pyridinium-chloride) lipid were loaded with anti-cadherin siRNA to knock down endothelial expression of vascular endothelial (VE)-cadherin, which plays an important role in mediating vascular permeability. The same approach was then also applied with anti-NFκB p65 siRNA, targeting an important transcription factor in the inflammatory pathway, to locally attenuate a polysaccharide-induced inflammatory response [90]. Delivery of these siRNAs inhibited endothelial activation and expression of E-selectin, vascular cell adhesion molecule 1 (VCAM-1), intracellular adhesion molecule 1 (ICAM-1), interleukin 8 (IL-8) and IL-6 and thereby reduced inflammation [91]. Liposomes have also been directed towards the activated glomerular epithelium by means of anti-E-selectin and/or anti-P-selectin conjugation [92, 93].
Mesangial cells
The mesangial cells maintain the capillary organization and aid filtration. Particle size seems to be the most important targeting principle to direct vehicles to mesangial cells. Notably, particles that cannot be filtered, but are small enough to pass the endothelial fenestrations, can accumulate in these cells [89]. In a mouse model of glomerulonephritis, repeated intraperitoneal administration of MAPK1-siRNA-loaded PEG-PLL copolymer nanoparticles led to the suppression of glomerular MAPK1 expression, reducing glomerular sclerosis [24]. Furthermore, LNP-mediated co-delivery of p38α MAPK and p65 siRNA has also been used in a mouse model of immunoglobulin A nephropathy to reduce glomerular inflammation, and siRNA was shown to accumulate in the glomerular endothelium and mesangial cells [88]. In addition, receptor-mediated targeting has been employed. Mesangial gene transfer was achieved in mouse models of glomerulonephritis following systemic administration of liposomes that were conjugated with an anti-Thy-1 antibody, a mesangial surface antigen [77, 94].
Podocytes
Podocytes possess foot-like structures and form the epithelial counterpart of the glomerular filtration barrier. For RNA delivery, only a few studies succeeded to target these cells. One example is the use of liposomes with an anti-VCAM1 antibody to target both the endothelium and the podocytes to treat kidney inflammation in a mouse model (see above) [95, 96].
Proximal tubular cells
Extensive research has been performed to develop targeted drug delivery to the kidney tubules and RNA-based therapies to treat tubular pathologies are the most abundant. The tubular epithelium is highly susceptible to injury and targeting these cells can be used to treat an array of kidney diseases. However, nanoparticles that accommodate oligonucleotides can only cross the glomerular filtration barrier in the presence of glomerular injury, which compromises the barrier [97, 98]. Therefore, naked siRNA has also been employed as a therapeutic strategy.
Intravenously injected naked siRNA against p53 prevented kidney injury in rat models of ischemic- and cisplatin-induced acute kidney injury (AKI) through the inhibition of apoptosis [99]. Notably, Teprasiran, a naked p53 siRNA, has already found its way to the clinical trial phase and has been studied for the treatment of delayed graft function (NCT02610296—completed) after transplantation and acute kidney injury following cardiac surgery (NCT03510897—ongoing) [76]. p53 siRNA has also been coupled to carbon nanotubes, along with Meprin-1β and Ctr1 siRNA to treat cisplatin-induced kidney injury in a mouse model, and incorporated in a DNA tetrahedron nanoparticle for application in a folic acid-induced AKI mouse model [83, 85]. Tubulointerstitial fibrosis is the final pathway leading to stage 5 CKD and is characterized by interstitial myofibroblast proliferation and interstitial accumulation of extracellular matrix molecules. The inhibition of the TGFβ-Smad4 pathway, the main signalling pathway in fibrosis, by intravenous injection of naked Smad4-siRNA reduced myofibroblast proliferation and thereby the development of kidney fibrosis [21]. In polycystic kidney disease (PKD), kidney cyst enlargement is related to the activation of the renin-angiotensin system. A weekly subcutaneous injection of a naked ASO against angiotensinogen, the precursor of angiotensin, decreased angiotensinogen mRNA levels in both the kidney (40%) and the liver (60%) and slowed down cyst formation in a mouse model of PKD [87].
In order to bypass the glomerular filtration barrier, local injection strategies have also been explored. Heat Shock Protein 47 (HSP47) is a collagen-binding stress protein and an important chaperone in the procollagen secretion during fibrosis. Intra-urethral injection of a gelatine-encapsulated HSP47 siRNA prevented the progression of tubulointerstitial fibrosis in a mouse model [82]. Renal ischemia–reperfusion injury is also associated with a high degree of cell death, linked to Fas-mediated apoptosis. Protection against apoptosis has been obtained after the injection of Fas-siRNA into the renal vein in mice and through intra-arterial perfusion of siRNA in donor kidneys, before transplantation into mice [78, 79]. A similar effect could be obtained in ex vivo porcine kidneys by administration of a caspase-3 siRNA via the renal artery and after treatment with siRNA directed against complement 3 and caspase 3 in a mouse model of ischemia–reperfusion injury [80, 81]. Also, in a mouse model of polymicrobial septic AKI, intravenous injection of TLR9-siRNA was shown to reduce cell death [86].
Extra-renal targeting
Several diseases affect the kidney but have their origin in other organs that are more easily accessible. Most notably, liver targeting has been used to treat both primary oxaluria and Fabry disease. Primary hyperoxaluria type 1 is caused by mutations in the alanine-glyoxylate aminotransferase, leading to abnormally high oxalate production and subsequent crystal formation, primarily in the kidney. The siRNA mediated knockdown of glycolate oxidase, an upstream enzyme in the synthesis of oxalate, reduced the urinary oxalate concentration by up to 50% (single dose) and 98% (multiple doses) in an oxaluria mouse and rat model respectively [7, 20]. This siRNA therapeutic, Lumasiran, was further studied in a randomized, placebo-controlled clinical trial (patients older than six years—NCT03681184) and in an open-label study (patients younger than 6 years—NCT03905694). In the placebo-controlled study, the treatment group showed a 65% reduction of urinary oxalate after 2 months of treatment and normal 24-h oxalate levels by 6 months and in paediatric patients, the open-label study showed an average of 71% decrease in urinary oxalate by 6 months. The FDA approved Lumasiran for the treatment of primary hyperoxaluria type 1 in November 2020 [7, 100]. Another siRNA-based therapy for primary hyperoxaluria, Nedosiran, has also been tested in mouse models and employs the siRNA-mediated knockdown of the hepatic lactate dehydrogenase to inhibit oxalate production directly, thereby inhibiting crystal formation. Nedosiran has recently found its way into the clinical trial phase (NCT04555486) [25].
Furthermore, as described above, the treatment of Fabry disease has shown the potential of mRNA as an alternative for enzyme replacement therapy in the treatment of kidney diseases. Targeted towards the liver, this lipid nanoparticle loaded with α-galactosidase mRNA successfully restored galactosidase activity and reduced the intra-lysosomal levels of sphingolipids in a mouse model for Fabry disease [67]. In the treatment of inflammatory kidney diseases, the targeting of tissue infiltrating macrophages could be used to induce both local and systemic effects. As an example, a cyclooxygenase type 2 (COX-2) siRNA in a chitosan nanoparticle was specifically delivered to macrophages and prevented kidney injury after intra-peritoneal injection into a 3-day-old unilateral ureteral obstruction mouse model [84].
Future potential in active targeting to the kidney
Potential ligands for active targeting of RNA-loaded vehicles to the kidneys
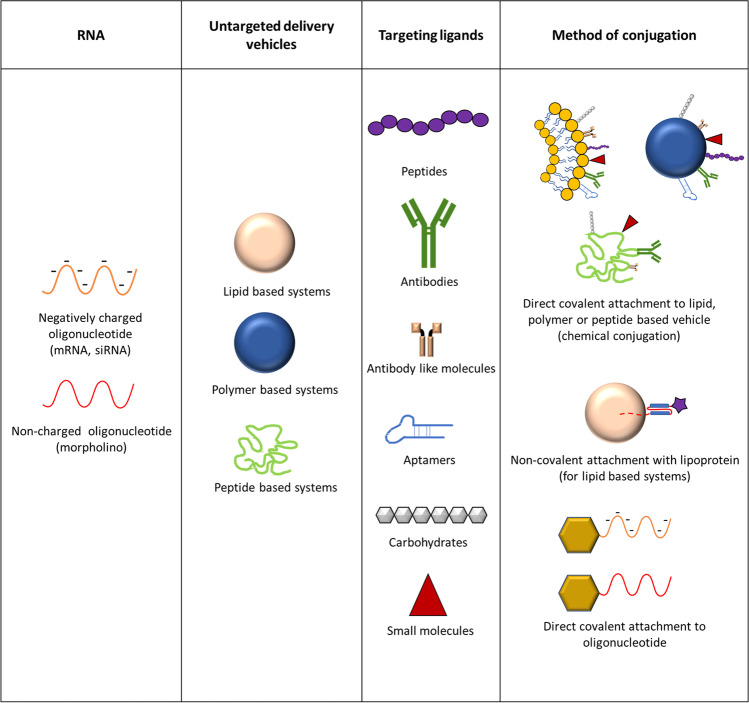
Conjugation of nanoparticles with targeting ligands can direct vehicles to the desired cell type and minimize off-target effects. Targeting ligands can be divided into 5 main categories: peptides, antibodies or antibody-like fragments, nucleic acid aptamers, carbohydrates and small molecules (Fig. 3) [101].
Fig. 3.
Strategies that can be employed for targeted delivery of RNA to specific cell types. RNA can be directly conjugated to a targeting ligand or be encapsulated in lipid, polymer and peptide-based delivery vehicles, which can be conjugated with targeting ligands
Although the clinical experience with kidney-targeted RNA-based therapies is still lacking, preclinical research has established a variety of kidney-targeting ligands, some of which could be applied to RNA-based therapies for kidney diseases (Table 3). In the next section, we will discuss which targeting ligands can potentially be applied for RNA-delivery to the kidney.
Table 3.
Overview of targeting ligands that have been employed in research for kidney-directed therapies
| Ligands for kidney-targeted drug delivery | |||||
|---|---|---|---|---|---|
| Targeted cells | Validated in/by | Targeting specificity (kidney to liver ratio)[102] | Ref | ||
| 1. Peptide sequences | |||||
| Cyclo(RGD) | Podocyte | Mouse | Medium | [103] | |
| DSHKDLK | Glomerular endothelium | In silico | [104] | ||
| PKNGSDP | Glomerular endothelium | In silico | [104] | ||
| CYFQNCPRG | Glomerular endothelium | Phage display | [105] | ||
| CLPVASC | Glomerular endothelium | Phage display | [106] | ||
| ANTPCG-PYTHDCPVKR | Proximal tubule | Mouse | Medium | [107] | |
| GVKGVQGTL | Proximal tubule | Phage display | [108] | ||
| LTCQVGRVH | Proximal tubule | Cell model | [109] | ||
| MCLPVASCGGPGVG(VPGxG)160VPGWPGSGGC | Proximal tubule | Rat + Pig | High | [110] | |
| (KKEEE)3 K | Proximal tubule | Mouse | Very High | [111] | |
| Nuclear localisation sequence (KKKRKVKε(DOTA)) | Kidney (unspecified) | Mouse | High | [68] | |
| SynB1 | Kidney (unspecified) | Mouse | Low | [110] | |
| Tat | Kidney (unspecified) | Mouse | Medium | [110] | |
| Human serum albumin (fragments) | Proximal tubule | Mouse | Low | [112] | |
| Streptavidin | Proximal tubule | Mouse, rat and rabbit | Medium | [113] | |
| Lysozyme | Proximal tubule | Rat | Medium | [114] | |
| 2. Antibodies | |||||
| Anti-Thy1 | Mesangial cells | Rat | High* | [77] | |
| Anti-E-selectin | Mesangial cells | Mouse | Very Low | [91] | |
| Anti-MHC II | Mesangial cells | Rat | Medium* | [115] | |
| Anti-CR2-Fc | Glomerular endothelium | Mouse | High* | [112] | |
| Anti-α8 integrin | Mesangial cells | Mouse | Medium* | [116] | |
| IgG | Podocytes | Mouse | Very Low | [112] | |
| Anti-CD11b | Proximal tubule | Mouse | Low* | [117] | |
| Anti-CD163 | Proximal tubule | Mouse | Very Low* | [118] | |
| Anti-VCAM1 | Podocytes and Endothelial cells | Mouse | Very Low | [90] | |
| 3. Small molecules | |||||
| Folate | Proximal tubule | Mouse | Medium | [89] | |
| Biotin | Proximal tubule | Rat | Medium | [89] | |
| 4. Carbohydrate derivates | |||||
| Glucosyl/mannosyl/2-deoxyglycosyl-conjugates | Proximal tubule | Rat | High | [119] | |
| Kidney to Liver ratio | |||||
| > 100 | Very High | ||||
| > 10 | High | ||||
| > 2 | Medium | ||||
| > 1 | Low | ||||
| < 1 | Very Low | ||||
(*) In some cases, no specific number was given that compared targeted and untargeted vehicle and the estimation of targeting efficiency was done by utilizing the biodistribution data that showed kidney accumulation (and liver accumulation) in the cited references
a) Peptide ligands
Peptide ligands are most often attached to the delivery vehicle via direct chemical conjugation, but can also be incorporated into lipid nanoparticles as lipopeptides [120]. Animal experiments have demonstrated kidney targeting for a variety of peptides that were either identified by phage display techniques or derived from natural ligands (Table 3) [104–106, 108, 109, 111–114]. For example, the cyclo(RGD) peptide targets the αvβ3 integrin receptor on the podocyte surface. Additionally, the proximal tubule cells express receptors such as megalin, cubilin and the transferrin receptor, for which targeting peptides have been described [102, 103]. Conjugation with lysozyme is a further example for receptor-mediated delivery, which leads to megalin-mediated uptake in the proximal tubule [121]. However, to our knowledge, this approach has not been applied for RNA delivery. While direct conjugation of peptides to drug molecules has been well established, not all peptides can be used for direct conjugation with RNA. Positively charged molecules, like the nuclear localization sequence peptide (KKKRKVKε(DOTA)), SynB1 and Tat, form complexes with the negatively charged RNA, which leads to formation of RNA-peptide nanoparticles instead of individual RNA conjugates. This aggregation can be avoided by initial encapsulation of the RNA in a particle followed by conjugation of peptide [107, 110, 122]. However, such a strategy could in turn compromise passage across the glomerular filtration barrier. Alternatively, the peptides can be conjugated to charge-neutral morpholino-oligonucleotides. Another complicating factor in using nanoparticles with these highly charged peptides is that they may lead to adsorption of plasma proteins which in turn would promote liver targeting [11].
b) Antibody and antibody-like molecules
A second way for targeted drug delivery is the use of specific antibodies. Antibodies can be attached to the delivery vehicle by means of Fc-binding peptides in combination with a surface linker [123]. This technique can also be used in combination with a membrane-anchoring lipoprotein to non-covalently bind the antibodies to lipid-based nanoparticles [124]. Antibodies have also been coupled directly to a β-lactam-linker functionalized siRNA, utilizing a unique reactive lysine residue in dual variable domain antibodies [125]. Although antibody-based drug delivery has been successfully applied to target specific kidney cells, for example endothelial cells with anti-VCAM1, mesangial cells with anti-MHC II and anti-α8 integrin, or proximal tubular cells with anti-CD11b and anti-CD163 (Table 3), their use can be hampered by their large size, leading to immune complex deposition on the glomerular basement membrane and subsequent risk of inflammation [90, 115–118, 126]. A potential answer to this problem might be the use of Fab fragments for conjugation or alternative small protein binders such as nanobodies or designed ankyrin repeat proteins (DARPins). These antibody-like molecules can then be attached to the nanoparticles in the same way as the full antibodies [127–129].
c) Aptamers
Aptamers are short single-stranded oligonucleotides folded upon themselves that can bind specific target molecules. Cell-internalizing DNA aptamers have been employed to specifically target inflamed kidney cells in vitro and were employed to inhibit the binding of advanced glycation end products (AGE) to their receptor (RAGE), reducing oxidative stress and inflammation [130, 131]. Aptamers can also be conjugated to the nanovehicle or directly to RNA molecules, an approach that is currently being utilized in the targeted delivery of chemotherapeutic compounds or siRNA to cancer cells. Furthermore, aptamer functionalized mSiO2/PSS/PDDA/BSA-Gd2O3 mMRI nanoprobes have been used to enhance magnetic resonance imaging (MRI) contrast by specifically targeting nucleolin-expressing renal cancer cell lines [101].
d) Carbohydrates
As well as endocytosis-mediating receptors (megalin, cubilin, transferrin receptor, etc.), the proximal tubule cells also express a variety of transporters that have been explored for targeted drug delivery. Sugar conjugates have been employed for targeted delivery of low molecular weight peptides such as, for example, oxytocin and vasopressin [119]. It should be possible to use carbohydrates like glucose and mannose as targeting ligands for siRNA, similar to the GalNAc approach for liver targeting. For mRNA delivery, the large size of carbohydrate-conjugated nanoparticles remains a challenge. Liposomes incorporating cholesterol conjugated to glucose via PEG-linkers of different length have shown some degree of kidney accumulation, but these vehicles have not been tested for RNA delivery [132].
e) Small molecules
During studies targeting the folate receptor for cancer therapy, it was noted that this approach also yielded targeting of proximal tubules. However, as for other tubular targeting strategies, the same size restrictions apply [133]. Biotin is another example of a small molecule that has been employed to target the proximal tubule [89].
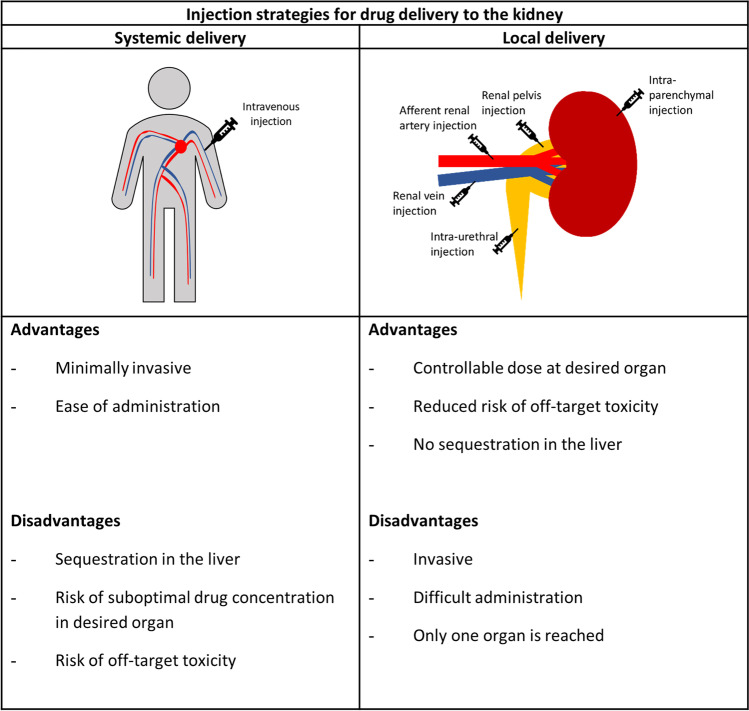
The importance of injection strategy
Although systemic injection is the least burdening mode of delivery, the accumulation and subsequent degradation of the vehicles in the liver, as well as the size restrictions imposed by the glomerular filtration barrier can pose a problem for RNA-loaded nanoparticles unless a compromised filtration barrier permits penetration. To circumvent these issues, alternative injection strategies have been studied, including renal artery, renal vein, retrograde intra-urethral and direct injection into the kidney parenchyma (Fig. 4). Some of the above-mentioned examples of kidney-targeted RNA-based therapies made use of alternative injection strategies to deliver naked RNA to the desired cells. Examples include renal artery injection of TGF-β/Smad-siRNA as treatment for glomerulonephritis and renal vein injection of a Fas-siRNA to improve the survival after ischemic reperfusion injury in a mouse model [21, 78]. Additionally, the intra-urethral injection of an HSP47-siRNA was used to treat kidney fibrosis [82].
Fig. 4.
Injection strategies for delivery of drug-carrying vehicles to the kidney with their (dis)advantages
Since these therapies utilized naked RNA molecules, it might be valuable to investigate the above-mentioned targeting ligands and nanoparticle formulations in combination with the different injection routes, as it would enable the exploration of strategies that otherwise would not reach the podocytes and tubular cells.
Physical methods to increase RNA delivery
Next to active targeting, drug vehicles can also be directed to specific cells or tissues by using physical methods. As described above, careful consideration of size and charge can already influence the targeting capacity. However, delivery vehicles can also be functionalized to respond to specific triggers such as ultrasound, electric and magnetic fields, and light [134]. Ultrasound-based drug delivery has been explored to improve targeting after systemic injection, by application of a focussed ultrasound beam to the target organ. This approach creates pressure regions and gas bubbles that result in acoustic cavitation of the vehicle and subsequent cargo release [135, 136]. In the gene delivery field, some progress has been made in utilizing this approach to increase kidney-specific plasmid DNA delivery with lipid microbubbles after intravenous injection, with no notable kidney damage . However, no such approach has been used for RNA-based therapeutics [137]. Another method of targeted delivery is electroporation, in which high voltage electric pulses transiently increase cell permeability. Electroporation has been applied to develop an siRNA-transfer system to specifically target the kidney and has been shown to aid the targeted delivery of TGF-β1-siRNA in the treatment of glomerulonephritis [72]. The application of magnetically guided oligonucleotide-loaded nanoparticles has mostly been restricted to cancer gene therapy. Infusion of ferromagnetic particles has, however, been associated with inflammatory reactions around the hilum of the kidneys [138]. Finally, light-triggered lipid-based nanoparticles can also be applied for targeted drug delivery and photo-triggerable siRNA-aptamers, which have been applied to cancer therapy, have shown a significant degree of kidney accumulation [139, 140].
Conclusion
In this review, we summarized the current state of the art regarding the potential use of RNA-based therapeutics for kidney diseases and discussed the major challenges related to the size and specific targeting. In our view, the largest short-term gains may be realized through combination of antisense oligonucleotides, using stabilized backbone chemistries, with small molecule (including small protein) targeting ligands. However, this strategy will be limited to down-regulation of gene expression whereas only mRNA can rescue expression of a missing gene product. We expect that rapid progress which has been booked in other disease fields will nourish kidney research and allow the development of successful RNA-based therapies in the future.
Author contribution
Writing—original draft preparation, T.B.; writing—review and editing, T.B., E.L., L.P.v.d.H. and R.B.; supervision, E.L., L.P.v.d.H. and R. B.; All authors have read and agreed to the published version of the manuscript.
Funding
Tjessa Bondue and Elena Levtchenko are funded by the Research Foundation—Flanders (FWO)—11A7821N and 11Y5216N.
Elena Levtchenko and Roland Brock are funded by Health Holland—GEVAL 2020–2022.
Data availability
Not applicable.
Code availability
Not applicable.
Declarations
Conflict of interest
Roland Brock is cofounder of Mercurna and RiboPro, companies that develop mRNA therapeutics (Mercurna) and offer mRNA services (RiboPro).
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hays T, Groopman EE, Gharavi AG. Genetic testing for kidney disease of unknown etiology. Kidney Int. 2020;98:590–600. doi: 10.1016/j.kint.2020.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soliman NA. Orphan Kidney Diseases. Nephron Clin Pract. 2012;120:c194–c199. doi: 10.1159/000339785. [DOI] [PubMed] [Google Scholar]
- 3.Chen TK, Knicely DH, Grams ME. Chronic Kidney Disease Diagnosis and Management: A Review. JAMA. 2019;322:1294–1304. doi: 10.1001/jama.2019.14745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Breyer MD, Susztak K. The next generation of therapeutics for chronic kidney disease. Nat Rev Drug Discov. 2016;15:568–588. doi: 10.1038/nrd.2016.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rubin JD, Barry MA. Improving Molecular Therapy in the Kidney. Mol Diagnosis Ther. 2020;24:375–396. doi: 10.1007/s40291-020-00467-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Damon Wang F, Zuroske T, Watts JK. RNA therapeutics on the rise. Nat Rev Drug Discov. 2020;19:441–442. doi: 10.1038/d41573-020-00078-0. [DOI] [PubMed] [Google Scholar]
- 7.Zhang MM, Bahal R, Rasmussen TP, Manautou JE, et al. The growth of siRNA-based therapeutics: Updated clinical studies. Biochem Pharmacol. 2021;189:114432. doi: 10.1016/j.bcp.2021.114432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Polack FP, Thomas SJ, Kitchin N, Absalon J, et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Corbett KS, Edwards DK, Leist SR, Abiona OM, et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jackson LA, Anderson EJ, Rouphael NG, Roberts PC, et al. An mRNA Vaccine against SARS-CoV-2 — Preliminary Report. N Engl J Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gómez-Aguado I, Rodríguez-Castejón J, Vicente-Pascual M, Rodríguez-Gascón A, et al. Nanomedicines to deliver mRNA: State of the art and future perspectives. Nanomaterials. 2020;10:136. doi: 10.3390/nano10020364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robinson E, MacDonald KD, Slaughter K, McKinney M, et al. Lipid Nanoparticle-Delivered Chemically Modified mRNA Restores Chloride Secretion in Cystic Fibrosis. Mol Ther. 2018;26:2034–2046. doi: 10.1016/j.ymthe.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlsson L, Clarke JC, Yen C, Gregoire F, et al. Biocompatible, Purified VEGF-A mRNA Improves Cardiac Function after Intracardiac Injection 1 Week Post-myocardial Infarction in Swine. Mol Ther Methods Clin Dev. 2018;9:330–346. doi: 10.1016/j.omtm.2018.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lorenzer C, Dirin M, Winkler AM, Baumann V, et al. Going beyond the liver: Progress and challenges of targeted delivery of siRNA therapeutics. J Control Release. 2015;203:1–15. doi: 10.1016/j.jconrel.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 15.Guzman-Aranguez A, Loma P, Pintor J. Small-interfering RNAs (siRNAs) as a promising tool for ocular therapy. Br J Pharmacol. 2013;170:730. doi: 10.1111/bph.12330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen QD, Schachar RA, Nduaka CI, Sperling M, et al. Dose-ranging evaluation of intravitreal siRNA PF-04523655 for diabetic macular edema (the DEGAS study) Invest Ophthalmol Vis Sci. 2012;53:7666–7674. doi: 10.1167/iovs.12-9961. [DOI] [PubMed] [Google Scholar]
- 17.Martínez T, González MV, Roehl I, Wright N, et al. In vitro and in vivo efficacy of SYL040012, a novel siRNA compound for treatment of glaucoma. Mol Ther. 2014;22:81–91. doi: 10.1038/mt.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu C, fei, Wang J, Delivery systems for siRNA drug development in cancer therapy. Asian J Pharm Sci. 2015;10:1–12. doi: 10.1016/j.ajps.2014.08.011. [DOI] [Google Scholar]
- 19.Armbruster N, Jasny E, Petsch B. Advances in RNA Vaccines for Preventive Indications: A Case Study of a Vaccine against Rabies. Vaccines. 2019;7:132. doi: 10.3390/vaccines7040132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liebow A, Li X, Racie T, Hettinger J, et al. An investigational RNAi therapeutic targeting glycolate oxidase reduces oxalate production in models of primary hyperoxaluria. J Am Soc Nephrol. 2017;28:494–503. doi: 10.1681/ASN.2016030338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morishita Y, Yoshizawa H, Watanabe M, Ishibashi K, et al. SiRNAs targeted to Smad4 prevent renal fibrosis in vivo. Sci Rep. 2014;4:6424. doi: 10.1038/srep06424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zisman A, Pantuck AJ, Belldegrun A. Immune and genetic therapies for advanced renal cell carcinoma. Rev Urol. 2000;2:54–60. [PMC free article] [PubMed] [Google Scholar]
- 23.Kreidberg JA. SiRNA therapy for glomerulonephritis. J Am Soc Nephrol. 2010;21:549–551. doi: 10.1681/ASN.2010020177. [DOI] [PubMed] [Google Scholar]
- 24.Shimizu H, Hori Y, Kaname S, Yamada K, et al. SiRNA-based therapy ameliorates glomerulonephritis. J Am Soc Nephrol. 2010;21:622–633. doi: 10.1681/ASN.2009030295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lai C, Pursell N, Gierut J, Saxena U, et al. Specific Inhibition of Hepatic Lactate Dehydrogenase Reduces Oxalate Production in Mouse Models of Primary Hyperoxaluria. Mol Ther. 2018;26:1983–1995. doi: 10.1016/j.ymthe.2018.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ségalat L. Loss-of-function genetic diseases and the concept of pharmaceutical targets. Orphanet J Rare Dis. 2007;2:30. doi: 10.1186/1750-1172-2-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Damase TR, Sukhovershin R, Boada C, Taraballi F, et al. The Limitless Future of RNA Therapeutics. Front Bioeng Biotechnol. 2021;9:161. doi: 10.3389/fbioe.2021.628137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jani B, Fuchs R. In vitro transcription and capping of Gaussia luciferase mRNA followed by HeLa cell transfection. J Vis Exp. 2012;61:3702. doi: 10.3791/3702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schuberth-Wagner C, Ludwig J, Bruder AK, Herzner AM, et al. A Conserved Histidine in the RNA Sensor RIG-I Controls Immune Tolerance to N1–2’O-Methylated Self RNA. Immunity. 2015;43:41–51. doi: 10.1016/j.immuni.2015.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Houseley J, Tollervey D. The Many Pathways of RNA Degradation. Cell. 2009;136:763–776. doi: 10.1016/j.cell.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 31.Mu X, Greenwald E, Ahmad S, Hur S. An origin of the immunogenicity of in vitro transcribed RNA. Nucleic Acids Res. 2018;46:5239–5249. doi: 10.1093/nar/gky177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sahin U, Karikó K, Türeci Ö. MRNA-based therapeutics-developing a new class of drugs. Nat Rev Drug Discov. 2014;13:759–780. doi: 10.1038/nrd4278. [DOI] [PubMed] [Google Scholar]
- 33.Jiang Y, Xu X-S, Russell JE. A Nucleolin-Binding 3′ Untranslated Region Element Stabilizes β-Globin mRNA In Vivo. Mol Cell Biol. 2006;26:2419–2429. doi: 10.1128/MCB.26.6.2419-2429.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mangus DA, Evans MC, Jacobson A. Poly(A)-binding proteins: multifunctional scaffolds for the post-transcriptional control of gene expression. Genome Biol. 2003;4:223. doi: 10.1186/gb-2003-4-7-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zlotorynski E. The short tail that wags the mRNA. Nat Rev Mol Cell Biol. 2017;19:2–3. doi: 10.1038/nrm.2017.120. [DOI] [PubMed] [Google Scholar]
- 36.Steinle H, Behring A, Schlensak C, Wendel HP, et al. Application of In Vitro Transcribed Messenger RNA for Cellular Engineering and Reprogramming: Progress and Challenges. Stem Cells. 2017;35:68–79. doi: 10.1002/stem.2402. [DOI] [PubMed] [Google Scholar]
- 37.Colgan DF, Manley JL. Mechanism and regulation of mRNA polyadenylation. Genes Dev. 1997;11:2755–2766. doi: 10.1101/gad.11.21.2755. [DOI] [PubMed] [Google Scholar]
- 38.Strzelecka D, Smietanski M, Sikorski P, Warminski M et al (2020) Functional and LC-MS/MS analysis of in vitro transcribed mRNAs carrying phosphorothioate or boranophosphate moieties reveal polyA tail modifications that prevent deadenylation without compromising protein expression. bioRxiv Biochem 184598
- 39.Kim CH, Oh Y, Lee TH. Codon optimization for high-level expression of human erythropoietin (EPO) in mammalian cells. Gene. 1997;199:293–301. doi: 10.1016/S0378-1119(97)00384-3. [DOI] [PubMed] [Google Scholar]
- 40.Kimchi-Sarfaty C, Oh JM, Kim IW, Sauna ZE, et al. A “silent” polymorphism in the MDR1 gene changes substrate specificity. Science. 2007;315:525–528. doi: 10.1126/science.1135308. [DOI] [PubMed] [Google Scholar]
- 41.Al-Saif M, Khabar KSA. UU/UA dinucleotide frequency reduction in coding regions results in increased mRNA stability and protein expression. Mol Ther. 2012;20:954–959. doi: 10.1038/mt.2012.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhang Z, Ohto U, Shibata T, Isobe T, et al. Structural Analyses of Toll-like Receptor 7 Reveal Detailed RNA Sequence Specificity and Recognition Mechanism of Agonistic Ligands. Cell Rep. 2018;25:3371–3381.e5. doi: 10.1016/j.celrep.2018.11.081. [DOI] [PubMed] [Google Scholar]
- 43.Freund I, Eigenbrod T, Helm M, Dalpke AH. RNA modifications modulate activation of innate toll-like receptors. Genes (Basel) 2019;10:92. doi: 10.3390/genes10020092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Watts JK, Corey DR. Silencing disease genes in the laboratory and the clinic. J Pathol. 2012;226:365–379. doi: 10.1002/path.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu B, Zhong L, Weng Y, Peng L, et al. Therapeutic siRNA: state of the art. Signal Transduct Target Ther. 2020;5:101. doi: 10.1038/s41392-020-0207-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elmén J, Thonberg H, Ljungberg K, Frieden M, et al. Locked nucleic acid (LNA) mediated improvements in siRNA stability and functionality. Nucleic Acids Res. 2005;33:439–447. doi: 10.1093/nar/gki193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Safari F, Barouji SR, Tamaddon AM. Strategies for improving siRNA-induced gene silencing efficiency. Adv Pharm Bull. 2017;7:603–609. doi: 10.15171/apb.2017.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kurreck J. siRNA Efficiency: Structure or Sequence—That Is the Question. J Biomed Biotechnol. 2006;2006:83757. doi: 10.1155/JBB/2006/83757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shen X, Corey DR. Chemistry, mechanism and clinical status of antisense oligonucleotides and duplex RNAs. Nucleic Acids Res. 2018;46:1584–1600. doi: 10.1093/nar/gkx1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Smet MD, Meenken C, van den Horn GJ. Fomivirsen – a phosphorothioate oligonucleotide for the treatment of CMV retinitis. Ocul Immunol Inflamm. 2009;7:189–198. doi: 10.1076/ocii.7.3.189.4007. [DOI] [PubMed] [Google Scholar]
- 51.Hoy SM. Nusinersen: First Global Approval. Drugs. 2017;77:473–479. doi: 10.1007/s40265-017-0711-7. [DOI] [PubMed] [Google Scholar]
- 52.Crooke ST, Geary RS. Clinical pharmacological properties of mipomersen (Kynamro), a second generation antisense inhibitor of apolipoprotein B. Br J Clin Pharmacol. 2013;76:276. doi: 10.1111/j.1365-2125.2012.04469.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Henry SP, Johnson M, Zanardi TA, Fey R, et al. Renal uptake and tolerability of a 2’-O-methoxyethyl modified antisense oligonucleotide (ISIS 113715) in monkey. Toxicology. 2012;301:13–20. doi: 10.1016/j.tox.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 54.Lidberg KA, Annalora AJ, Jozic M, Elson DJ, et al. Antisense oligonucleotide development for the selective modulation of CYP3A5 in renal disease. Sci Rep. 2021;11:4722. doi: 10.1038/s41598-021-84194-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Janssen MJ, Nieskens TTG, Steevels TAM, Caetano-Pinto P, et al. Therapy with 2′-O-Me Phosphorothioate Antisense Oligonucleotides Causes Reversible Proteinuria by Inhibiting Renal Protein Reabsorption. Mol Ther Nucleic Acids. 2019;18:298. doi: 10.1016/j.omtn.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chen S, Le BT, Chakravarthy M, Kosbar TR, et al. Systematic evaluation of 2′-Fluoro modified chimeric antisense oligonucleotide-mediated exon skipping in vitro. Sci Rep. 2019;9:6078. doi: 10.1038/s41598-019-42523-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberts TC, Langer R, Wood MJA. Advances in oligonucleotide drug delivery. Nat Rev Drug Discov. 2020;19:673–694. doi: 10.1038/s41573-020-0075-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang X, Goel V, Robbie GJ. Pharmacokinetics of Patisiran, the First Approved RNA Interference Therapy in Patients With Hereditary Transthyretin-Mediated Amyloidosis. J Clin Pharmacol. 2019;60:572–585. doi: 10.1002/jcph.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Campani V, Salzano G, Lusa S, de Rosa G. Lipid nanovectors to deliver RNA oligonucleotides in cancer. Nanomaterials. 2016;6:131. doi: 10.3390/nano6070131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Suk JS, Xu Q, Kim N, Hanes J, et al. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv Drug Deliv Rev. 2016;99:28–51. doi: 10.1016/j.addr.2015.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.McKay PF, Hu K, Blakney AK, Samnuan K, et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Prieve MG, Harvie P, Monahan SD, Roy D, et al. Targeted mRNA Therapy for Ornithine Transcarbamylase Deficiency. Mol Ther. 2018;26:801–813. doi: 10.1016/j.ymthe.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.An D, Schneller JL, Frassetto A, Liang S, et al. Systemic Messenger RNA Therapy as a Treatment for Methylmalonic Acidemia. Cell Rep. 2017;21:3548–3558. doi: 10.1016/j.celrep.2017.11.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sabnis S, Kumarasinghe ES, Salerno T, Mihai C, et al. A Novel Amino Lipid Series for mRNA Delivery: Improved Endosomal Escape and Sustained Pharmacology and Safety in Non-human Primates. Mol Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021 doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Akinc A, Querbes W, De S, Qin J, et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Zhu X, Yin L, Theisen M, Zhuo J, et al. Systemic mRNA Therapy for the Treatment of Fabry Disease: Preclinical Studies in Wild-Type Mice, Fabry Mouse Model, and Wild-Type Non-human Primates. Am J Hum Genet. 2019;104:625–637. doi: 10.1016/j.ajhg.2019.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sarko D, Beijer B, Boy RG, Nothelfer E-M, et al. The Pharmacokinetics of Cell-Penetrating Peptides. Mol Pharm. 2010;7:2224–2231. doi: 10.1021/mp100223d. [DOI] [PubMed] [Google Scholar]
- 69.Alexander-Bryant AA, Zhang H, Attaway CC, Pugh W, et al. Dual peptide-mediated targeted delivery of bioactive siRNAs to oral cancer cells in vivo. Oral Oncol. 2017;72:123–131. doi: 10.1016/j.oraloncology.2017.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suh JS, Lee HJ, Nam H, Jo BS, et al. Control of cancer stem cell like population by intracellular target identification followed by the treatment with peptide-siRNA complex. Biochem Biophys Res Commun. 2017;491:827–833. doi: 10.1016/j.bbrc.2017.05.148. [DOI] [PubMed] [Google Scholar]
- 71.Cheng C, Convertine AJ, Stayton PS, Bryers JD. Multifunctional triblock copolymers for intracellular messenger RNA delivery. Biomaterials. 2012;33:6868–6876. doi: 10.1016/j.biomaterials.2012.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Takabatake Y, Isaka Y, Imai E. In vivo transfer of small interfering RNA or small hairpin RNA targeting glomeruli. Methods Mol Biol. 2009;466:251–263. doi: 10.1007/978-1-59745-352-3_18. [DOI] [PubMed] [Google Scholar]
- 73.Cao Y, Tan YF, Wong YS, Liew MWJ, et al. Recent advances in chitosan-based carriers for gene delivery. Mar Drugs. 2019;17:381. doi: 10.3390/md17060381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gao S, Hein S, Dagnæs-Hansen F, Weyer K, et al. Megalin-mediated specific uptake of chitosan/siRNA nanoparticles in mouse kidney proximal tubule epithelial cells enables AQP1 gene silencing. Theranostics. 2014;4:1039–1051. doi: 10.7150/thno.7866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams RM, Jaimes EA, Heller DA. Nanomedicines for kidney diseases. Kidney. 2016;90:740–745. doi: 10.1016/j.kint.2016.03.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Thompson JD, Kornbrust DJ, Foy JWD, Solano ECR, et al. Toxicological and pharmacokinetic properties of chemically modified siRNAs targeting p53 RNA following intravenous administration. Nucleic Acid Ther. 2012;22:255–264. doi: 10.1089/nat.2012.0371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tomita N, Morishita R, Yamamoto K, Higaki J, et al. Targeted gene therapy for rat glomerulonephritis using HVJ-immunoliposomes. J Gene Med. 2002;4:527–535. doi: 10.1002/jgm.300. [DOI] [PubMed] [Google Scholar]
- 78.Hamar P, Song E, Kökeny G, Chen A, et al. Small interfering RNA targeting Fas protects mice against renal ischemia-reperfusion injury. Proc Natl Acad Sci U S A. 2004;101:14883–14888. doi: 10.1073/pnas.0406421101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng X, Zang GY, Jiang J, He W, et al. Attenuating ischemia-reperfusion injury in kidney transplantation by perfusing donor organs with siRNA cocktail solution. Transplantation. 2016;100:743–752. doi: 10.1097/TP.0000000000000960. [DOI] [PubMed] [Google Scholar]
- 80.Yang B, Hosgood SA, Nicholson ML. Naked small interfering RNA of caspase-3 in preservation solution and autologous blood perfusate protects isolated ischemic porcine kidneys. Transplantation. 2011;91:501–507. doi: 10.1097/TP.0b013e318207949f. [DOI] [PubMed] [Google Scholar]
- 81.Zheng X, Zhang X, Sun H, Feng B, et al. Protection of renal ischemia injury using combination gene silencing of complement 3 and caspase 3 genes. Transplantation. 2006;82:1781–1786. doi: 10.1097/01.tp.0000250769.86623.a3. [DOI] [PubMed] [Google Scholar]
- 82.Xia Z, Abe K, Furusu A, Miyazaki M, et al. Suppression of renal tubulointerstitial fibrosis by small interfering RNA targeting heat shock protein 47. Am J Nephrol. 2007;28:34–46. doi: 10.1159/000108759. [DOI] [PubMed] [Google Scholar]
- 83.Alidori S, Akhavein N, Thorek DLJ, Behling K, et al. Targeted fibrillar nanocarbon RNAi treatment of acute kidney injury. Sci Transl Med. 2016;8:331ra39–331ra39. doi: 10.1126/scitranslmed.aac9647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yang C, Nilsson L, Cheema MU, Wang Y, et al. Chitosan/siRNA Nanoparticles Targeting Cyclooxygenase Type 2 Attenuate Unilateral Ureteral Obstruction-induced Kidney Injury in Mice. Theranostics. 2015;5:110. doi: 10.7150/thno.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thai HBD, Kim K-R, Hong KT, Voitsitskyi T, et al. Kidney-Targeted Cytosolic Delivery of siRNA Using a Small-Sized Mirror DNA Tetrahedron for Enhanced Potency. ACS Cent Sci. 2020;6:2250–2258. doi: 10.1021/acscentsci.0c00763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liu L, Li Y, Hu Z, Su J, et al. Small Interfering RNA Targeting Toll-Like Receptor 9 Protects Mice against Polymicrobial Septic Acute Kidney Injury. Nephron Exp Nephrol. 2012;122:51–61. doi: 10.1159/000346953. [DOI] [PubMed] [Google Scholar]
- 87.Ravichandran K, Ozkok A, Wang Q, Mullick AE, et al. Antisense-mediated angiotensinogen inhibition slows polycystic kidney disease in mice with a targeted mutation in Pkd2. Am J Physiol Renal Physiol. 2015;308:F349–F357. doi: 10.1152/ajprenal.00478.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Y, Wu Q, Wang J, Li L, et al. Co-delivery of p38α MAPK and p65 siRNA by novel liposomal glomerulus-targeting nano carriers for effective immunoglobulin a nephropathy treatment. J Control Release. 2020;320:457–468. doi: 10.1016/j.jconrel.2020.01.024. [DOI] [PubMed] [Google Scholar]
- 89.Wang J, Masehi-Lano JJ, Chung EJ. Peptide and antibody ligands for renal targeting: Nanomedicine strategies for kidney disease. Biomater Sci. 2017;5:1450–1459. doi: 10.1039/C7BM00271H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kowalski PS, Zwiers PJ, Morselt HWM, Kuldo JM, et al. Anti-VCAM-1 SAINT-O-Somes enable endothelial-specific delivery of siRNA and downregulation of inflammatory genes in activated endothelium in vivo. J Control Release. 2014;176:64–75. doi: 10.1016/j.jconrel.2013.12.029. [DOI] [PubMed] [Google Scholar]
- 91.Adrian JE, Morselt HWM, Süss R, Barnert S, et al. Targeted SAINT-O-Somes for improved intracellular delivery of siRNA and cytotoxic drugs into endothelial cells. J Control Release. 2010;144:341–349. doi: 10.1016/j.jconrel.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 92.Constantinescu CA, Fuior EV, Rebleanu D, Deleanu M, et al. Targeted Transfection Using PEGylated Cationic Liposomes Directed Towards P-Selectin Increases siRNA Delivery into Activated Endothelial Cells. Pharmaceutics. 2019;11:47. doi: 10.3390/pharmaceutics11010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ásgeirsdóttir SA, Zwiers PJ, Morselt HW, Moorlag HE, et al. Inhibition of proinflammatory genes in anti-GBM glomerulonephritis by targeted dexamethasone-loaded AbEsel liposomes. Am J Physiol Renal Physiol. 2008;294:F554–F561. doi: 10.1152/ajprenal.00391.2007. [DOI] [PubMed] [Google Scholar]
- 94.Zuckerman JE, Gale A, Wu P, Ma R, et al. SiRNA delivery to the glomerular mesangium using polycationic cyclodextrin nanoparticles containing siRNA. Nucleic Acid Ther. 2015;25:53–64. doi: 10.1089/nat.2014.0505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu CP, Hu Y, Lin JC, Fu HL, et al. Targeting strategies for drug delivery to the kidney: From renal glomeruli to tubules. Med Res Rev. 2019;39:561–578. doi: 10.1002/med.21532. [DOI] [PubMed] [Google Scholar]
- 96.Visweswaran GRR, Gholizadeh S, Ruiters MHJ, Molema G et al (2015) Targeting rapamycin to podocytes using a vascular cell adhesion molecule-1 (VCAM-1)-harnessed SAINT-based lipid carrier system. PLoS One 10:e0138870 [DOI] [PMC free article] [PubMed]
- 97.Nair AV, Keliher EJ, Core AB, Brown D, et al. Characterizing the interactions of organic nanoparticles with renal epithelial cells in vivo. ACS Nano. 2015;9:3641–3653. doi: 10.1021/acsnano.5b00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang X, Wang H, Zhu Y, Zhang R, et al. Short-And long-Term tracking of anionic ultrasmall nanoparticles in kidney. ACS Nano. 2016;10:387–395. doi: 10.1021/acsnano.5b05066. [DOI] [PubMed] [Google Scholar]
- 99.Molitoris BA, Dagher PC, Sandoval RM, Campos SB, et al. siRNA targeted to p53 attenuates ischemic and cisplatin-induced acute kidney injury. J Am Soc Nephrol. 2009;20:1754–1764. doi: 10.1681/ASN.2008111204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.(2020) FDA Approves First Drug to Treat Rare Metabolic Disorder | FDA
- 101.Friedman A, Claypool S, Liu R. The Smart Targeting of Nanoparticles. Curr Pharm Des. 2013;19:6315–6329. doi: 10.2174/13816128113199990375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.van Asbeck AH, Dieker J, Boswinkel M, van der Vlag J, et al. Kidney-targeted therapies: A quantitative perspective. J Control Release. 2020;328:762–775. doi: 10.1016/j.jconrel.2020.09.022. [DOI] [PubMed] [Google Scholar]
- 103.Pollinger K, Hennig R, Breunig M, Tessmar J, et al. Kidney podocytes as specific targets for cyclo(RGDfC)-modified nanoparticles. Small. 2012;8:3368–3375. doi: 10.1002/smll.201200733. [DOI] [PubMed] [Google Scholar]
- 104.Jung E, Lee NK, Kang SK, Choi SH, et al. Identification of tissue-specific targeting peptide. J Comput Aided Mol Des. 2012;26:1267–1275. doi: 10.1007/s10822-012-9614-6. [DOI] [PubMed] [Google Scholar]
- 105.Susaki H, Suzuki K, Ikeda M, Yamada H, et al. Synthesis of Artificial Glycoconjugates of Arginine-Vasopressin and Their Antidiuretic Activities. Chem Pharm Bull. 1994;42:2090–2096. doi: 10.1248/cpb.42.2090. [DOI] [PubMed] [Google Scholar]
- 106.Pasqualini R, Ruoslahti E. Organ targeting in vivo using phage display peptide libraries. Nature. 1996;380:364–366. doi: 10.1038/380364a0. [DOI] [PubMed] [Google Scholar]
- 107.Geng Q, Sun X, Gong T, Zhang ZR. Peptide-drug conjugate linked via a disulfide bond for kidney targeted drug delivery. Bioconjug Chem. 2012;23:1200–1210. doi: 10.1021/bc300020f. [DOI] [PubMed] [Google Scholar]
- 108.Audigé A, Frick C, Frey FJ, Mazzucchelli L, et al. Selection of peptide ligands binding to the basolateral cell surface of proximal convoluted tubules. Kidney Int. 2002;61:342–348. doi: 10.1046/j.1523-1755.2002.00120.x. [DOI] [PubMed] [Google Scholar]
- 109.Kim YK, Kwon JT, Jiang HL, Choi YJ, et al. Kidney-specific peptide-conjugated poly(ester amine) for the treatment of kidney fibrosis. J Nanosci Nanotechnol. 2012;12:5149–5154. doi: 10.1166/jnn.2012.6372. [DOI] [PubMed] [Google Scholar]
- 110.Bidwell GL, Mahdi F, Shao Q, Logue OC, et al. A kidney-selective biopolymer for targeted drug delivery. Am J Physiol Renal Physiol. 2017;312:F54–F64. doi: 10.1152/ajprenal.00143.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wischnjow A, Sarko D, Janzer M, Kaufman C, et al. Renal Targeting: Peptide-Based Drug Delivery to Proximal Tubule Cells. Bioconjug Chem. 2016;27:1050–1057. doi: 10.1021/acs.bioconjchem.6b00057. [DOI] [PubMed] [Google Scholar]
- 112.Wu L, Chen M, Mao H, Ningning W, et al. Albumin-based nanoparticles as methylprednisolone carriers for targeted delivery towards the neonatal Fc receptor in glomerular podocytes. Int J Mol Med. 2017;39:851–860. doi: 10.3892/ijmm.2017.2902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Schechter B, Arnon R, Colas C, Burakova T, et al. Renal accumulation of streptavidin: Potential use for targeted therapy to the kidney. Kidney Int. 1995;47:1327–1335. doi: 10.1038/ki.1995.188. [DOI] [PubMed] [Google Scholar]
- 114.Zhang Z, Zheng Q, Han J, Gao G, et al. The targeting of 14-succinate triptolide-lysozyme conjugate to proximal renal tubular epithelial cells. Biomaterials. 2009;30:1372–1381. doi: 10.1016/j.biomaterials.2008.11.035. [DOI] [PubMed] [Google Scholar]
- 115.Hultman KL, Raffo AJ, Grzenda AL, Harris PE, et al. Magnetic resonance imaging of major histocompatibility class II expression in the renal medulla using immunotargeted superparamagnetic iron oxide nanoparticles. ACS Nano. 2008;2:477–484. doi: 10.1021/nn700400h. [DOI] [PubMed] [Google Scholar]
- 116.Scindia Y, Deshmukh U, Thimmalapura P-R, Bagavant H. Anti-α8 integrin immunoliposomes in glomeruli of lupus-susceptible mice: A novel system for delivery of therapeutic agents to the renal glomerulus in systemic lupus erythematosus. Arthritis Rheum. 2008;58:3884–3891. doi: 10.1002/art.24026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Shirai T, Kohara H, Tabata Y. Inflammation imaging by silica nanoparticles with antibodies orientedly immobilized. J Drug Target. 2012;20:535–543. doi: 10.3109/1061186X.2012.693500. [DOI] [PubMed] [Google Scholar]
- 118.Rubio-Navarro A, Carril M, Padro D, Guerrero-Hue M, et al. CD163-macrophages are involved in rhabdomyolysis-induced kidney injury and may be detected by MRI with targeted gold-coated iron oxide nanoparticles. Theranostics. 2016;6:896–914. doi: 10.7150/thno.14915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Suzuki K, Susaki H, Okuno S, Yamada H, et al. Specific Renal Delivery of Sugar-Modified Low-Molecular-Weight Peptides. J Pharmacol Exp Ther. 1999;288:888–897. [PubMed] [Google Scholar]
- 120.Eroğlu İ, İbrahim M. Liposome–ligand conjugates: a review on the current state of art. J Drug Target. 2020;28:225–244. doi: 10.1080/1061186X.2019.1648479. [DOI] [PubMed] [Google Scholar]
- 121.Seliverstova EV. Receptor-Mediated Endocytosis of Lysozyme in Renal Proximal Tubules of the Frog Rana Temporaria. Eur J Histochem. 2015;59:79–86. doi: 10.4081/ejh.2015.2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Asai T, Tsuzuku T, Takahashi S, Okamoto A, et al. Cell-penetrating peptide-conjugated lipid nanoparticles for siRNA delivery. Biochem Biophys Res Commun. 2014;444:599–604. doi: 10.1016/j.bbrc.2014.01.107. [DOI] [PubMed] [Google Scholar]
- 123.Almeida B, Nag OK, Rogers KE, Delehanty JB. Recent progress in bioconjugation strategies for liposome-mediated drug delivery. Molecules. 2020;25:5672. doi: 10.3390/molecules25235672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kedmi R, Veiga N, Ramishetti S, Goldsmith M, et al. A modular platform for targeted RNAi therapeutics. Nat Nanotechnol. 2018;13:214–219. doi: 10.1038/s41565-017-0043-5. [DOI] [PubMed] [Google Scholar]
- 125.Nanna AR, Kel’in AV, Theile C, Pierson JM, et al. Generation and validation of structurally defined antibody-siRNA conjugates. Nucleic Acids Res. 2020;48(5281):5293. doi: 10.1093/nar/gkaa286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Salant DJ. Immune Complex Glomerulonephritis. Front Nephrol Clin Exp Nephrol. 1998;2:271–275. doi: 10.1007/BF02480453. [DOI] [Google Scholar]
- 127.Richards DA, Maruani A, Chudasama V. Antibody fragments as nanoparticle targeting ligands: a step in the right direction. Chem Sci. 2016;8:63–77. doi: 10.1039/C6SC02403C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Verdurmen WPR, Luginbühl M, Honegger A, Plückthun A. Efficient cell-specific uptake of binding proteins into the cytoplasm through engineered modular transport systems. J Control Release. 2015;200:13–22. doi: 10.1016/j.jconrel.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 129.Tamaskovic R, Simon M, Stefan N, Schwill M et al (2012) Designed ankyrin repeat proteins (DARPins): From research to therapy. In: Methods in Enzymology. Academic Press Inc. 101–134 [DOI] [PubMed]
- 130.Ranches G, Lukasser M, Schramek H, Ploner A, et al. In Vitro Selection of Cell-Internalizing DNA Aptamers in a Model System of Inflammatory Kidney Disease. Mol Ther Nucleic Acids. 2017;8:198–210. doi: 10.1016/j.omtn.2017.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Cartón-García F, Saande CJ, Meraviglia-Crivelli D, Aldabe R, et al. Oligonucleotide-Based Therapies for Renal Diseases. Biomedicines. 2021;9:303. doi: 10.3390/biomedicines9030303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Xie F, Yao N, Qin Y, Zhang Q, et al. Investigation of glucose-modifed liposomes using polyethylene glycols with different chain lengths as the linkers for brain targeting. Int J Nanomedicine. 2012;7:163–175. doi: 10.2147/IJN.S23771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reddy JA, Abburi C, Hofland H, Howard SJ, et al. Folate-targeted, cationic liposome-mediated gene transfer into disseminated peritoneal tumors. Gene Ther. 2002;9:1542–1560. doi: 10.1038/sj.gt.3301833. [DOI] [PubMed] [Google Scholar]
- 134.Rodriguez-Devora JI, Ambure S, Shi Z-D, Yuan Y, et al. Physically facilitating drug-delivery systems. Ther Deliv. 2012;3:125. doi: 10.4155/tde.11.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.AlSawaftah NM, Awad NS, Paul V, Kawak PS, et al. Transferrin-modified liposomes triggered with ultrasound to treat HeLa cells. Sci Reports. 2021;11:1–15. doi: 10.1038/s41598-021-90349-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Awad NS, Paul V, Al-Sayah MH, Husseini GA. Ultrasonically controlled albumin-conjugated liposomes for breast cancer therapy. Artif Cells Nanomed Biotechnol. 2019;47:705–714. doi: 10.1080/21691401.2019.1573175. [DOI] [PubMed] [Google Scholar]
- 137.Omata D, Munakata L, Kageyama S, Suzuki Y, et al. Ultrasound image-guided gene delivery using three-dimensional diagnostic ultrasound and lipid-based microbubbles. J Drug Target. 2021 doi: 10.1080/1061186X.2021.1953510. [DOI] [PubMed] [Google Scholar]
- 138.Prijic S, Sersa G. Magnetic nanoparticles as targeted delivery systems in oncology. Radiol Oncol. 2011;45:1–16. doi: 10.2478/v10019-011-0001-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Yavlovich A, Smith B, Gupta K, Blumenthal R, et al. Light-sensitive Lipid-based Nanoparticles for Drug Delivery: Design Principles and Future Considerations for Biological Applications. Mol Membr Biol. 2010;27:364. doi: 10.3109/09687688.2010.507788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhao D, Yang G, Liu Q, Liu W, et al. A photo-triggerable aptamer nanoswitch for spatiotemporal controllable siRNA delivery. Nanoscale. 2020;12:10939–10943. doi: 10.1039/D0NR00301H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.
Not applicable.