Abstract
Background:
The use of heterotopic vascularized lymph node transfer (HVLNT) for the treatment of lower extremity lymphedema is still evolving. Current techniques, either place the lymph nodes in the thigh without a skin paddle or at the ankle requiring an unsightly and often bulky skin paddle for closure. We explored the feasibility of doing a below-knee transfer without a skin paddle using the medial sural vessels as recipient vessels and report our experience in 21 patients.
Methods:
A retrospective review of all patients who underwent HVLNT to the medial calf was performed. Postoperative magnetic resonance angiography (MRA) and lymphoscintigraphy (LS) were analyzed to assess lymph node viability and function after transfer.
Results:
Twenty-one patients underwent HVLNT to the medial calf. Postoperative imaging was performed at an average of 11 months after surgery. Thirteen patients had postoperative MRA, of whom 12 demonstrated viable lymph nodes. Seven patients underwent postoperative LS, of whom three demonstrated uptake in the transferred nodes. In the other four patients, the injectate failed to reach the level of the proximal calf.
Conclusion:
We provide proof of concept that HVLNT to the lower leg using the medial sural vessels without a skin paddle can result in viable and functional lymph nodes in the setting of lower extremity lymphedema.
Keywords: heterotopic vascularized lymph node transfer, lymphedema, medial sural
INTRODUCTION
In the United States, secondary lymphedema is the most common type of lower extremity lymphedema and is most often found in patients who have undergone surgery or radiation therapy as treatment for malignancy [1]. A recent meta-analysis cited the overall incidence of lymphedema in non-breast malignancy at 16%, with a much higher incidence in the lower extremity (20%) compared to the upper (5%) [2]. Lower extremity lymphedema is more difficult to treat due to its dependent location and higher venous pressures.
Vascularized lymph node transfer (VLNT) has gained popularity in the treatment of lymphedema and involves the microvascular transfer of healthy lymph nodes and perinodal tissue as a free flap based on an accompanying artery and vein. Although secondary lymphedema in the lower extremity is often due to proximal lymphatic disruption in the pelvic or inguinal region, fluid accumulation is often maximal in the distal, dependent region of the limb. In orthotopic VLNT (OVLNT), lymph nodes are transferred to the site of the lymphadenectomy to promote lymphangiogenesis in the recipient bed and to create a bridge across the site of initial injury [3–5]. However, damaged lymphatic vessels may not be able to transport lymph from the distal extremity to the newly transferred nodes. In addition, for patients who have undergone pelvic lymphadenectomy, intra-abdominal adhesions make OVLNT technically more difficult and increase the potential morbidity associated with the procedure. In heterotopic VLNT (HVLNT), lymph nodes are transferred distally to the site of maximal swelling, with the thought that transferred nodes will create a catchment effect, drawing fluid in through perinodal lymphatics. That fluid can then be returned to the venous system via permeable high endothelial venules in the nodes [6–9].
For patients with lower extremity lymphedema, swelling is often most severe below the knee; therefore, HVLNT is commonly performed at the ankle region because of its distal location and ease of access to recipient vessels. However, limited skin laxity around the ankle necessitates a bulky skin paddle and often a skin graft to allow insetting of the flap. This results in an unsightly protruding mass that interferes with wearing footwear and compression garments postoperatively. In order to avoid these major shortcomings while still situating the lymph nodes distally below the knee, we explored the feasibility of performing HVLNT to the distal medial sural vessels in the calf, without a skin paddle or skin graft for closure.
“Proof of concept” is a term used to describe the realization of a certain method or idea to demonstrate its feasibility [10]. In this study, we used objective physiologic evidence to provide proof of concept that HVLNT to the lower leg without a skin paddle can result in viable and functional lymph nodes in the lymphedematous extremity.
METHODS
Institutional review board approval was obtained and a retrospective review all patients who underwent VLNT between December 2012 and December 2015 was performed. Patients were deemed candidates for VLNT if complete decongestive therapy (CDT) failed to control progression or if they had a history of recurrent infections. All available preoperative and postoperative imaging studies were reviewed and findings documented. Means, standard deviations, medians, and ranges were used to summarize continuous variables. Frequencies and proportions were used to summarize categorical variables.
Surgical Technique
All patients were operated on in the supine position with the affected extremity abducted and flexed 90 degrees at the knee in a hemi-frog leg position. Donor lymph node flaps were harvested from either the cervical or axillary regions with axillary lymph nodes identified for harvest using Reverse Lymphatic Mapping [11]. In this technique, indocyanine green (ICG) is injected into the chest wall below the axillary donor site and the ipsilateral hand is injected with Technetium 99 (Tc99). ICG is taken up by axillary lymph nodes draining the chest wall and is visualized using an imaging system (SPY Elite System, LifeCell Corp., Branchburg, NJ) that employs a near-infrared energy source and fluoresces ICG making it visible to a camera. These nodes are then examined with a gamma probe to ensure they do not contain any Tc99. Nodes that contain Tc99 are avoided in order to preserve lymph drainage from the arm and to minimize the risk of donor site lymphedema [11]. In those patients with a cervical donor site, lymph nodes were transferred on the transverse cervical vessels. All lymph node flaps were harvested without skin paddles.
The recipient site was prepared through a longitudinal incision made over the upper medial calf. Dissection was continued down to the muscle fascia. Exploration for a perforating vessel to the skin was performed. Once identified, the perforator was dissected through the muscle to the source artery and vein. HVLNT was then performed to these vessels (Fig. 1). A hand-sewn arterial anastomosis was first performed, followed by the venous anastomosis using a coupler. At the completion of the anastomoses, ICG was administered intravenously to assess flap perfusion. Subcutaneous fat and fascia were excised from the upper medial calf to make space for the flap. The skin overlying the flap was closed primarily without tension. Closed suction drains were placed at both the donor and recipient sites prior to wound closure. Patients were kept supine during the immediate postoperative 24 hr with slight leg flexion to avoid pressure on the flap. No flap monitoring was performed.
Fig. 1.
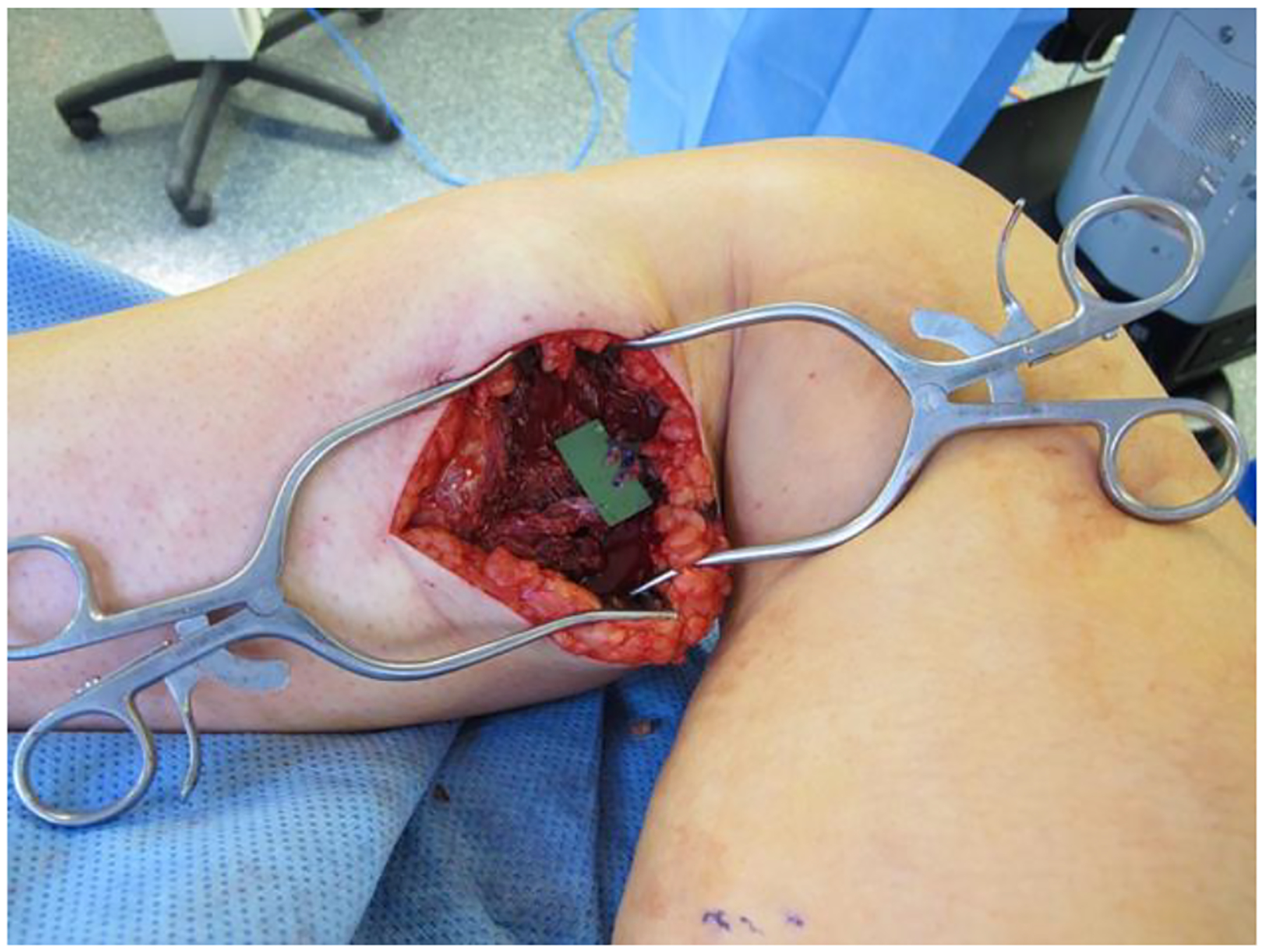
Medial sural recipient vessels prepared for microvascular anastomosis.
Imaging
Postoperatively, magnetic resonance angiography (MRA) and lymphoscintigraphy (LS) were performed to assess the long-term viability and functionality of the transferred lymph nodes. MRA was performed using the long-lasting contrast agent gadofosveset trisodium (Ablavar1) to obtain high-resolution images of the transferred lymph nodes. Lymphoscintigraphy was performed using 0.2 cc of filtered Tc99 injected into the first and second webspaces of the affected lower extremity. These were compared to preoperative imaging.
RESULTS
Twenty-one patients were identified who underwent HVLNT to the medial calf. In 17 patients, the axilla was selected as the donor site and in four patients, the cervical donor site was selected. Four cases were performed overseas, so demographic and postoperative information was not available for review. The remaining 17 patients underwent the procedure at a single institution (Mount Sinai Beth Israel, New York, NY).
Patient Characteristics
Patient characteristics are demonstrated in Table I. Means, standard deviations, medians, and ranges were used to summarize continuous variables. Frequencies and proportions were used to summarize categorical variables. Demographic information for overseas patients was not available for review. Of the 17 patients treated at our institution, 15 were female and two were male, average age was 55 years (SD = 10, range 39–70), and average BMI was 26.2 kg/m2 (SD = 4.0, range 21.4–32.9). The average duration of symptomatic lower extremity lymphedema prior to undergoing HVLNT was 13 years (SD = 11, range 2–34). There was only one case of primary lymphedema, which occurred in one of the male patients. In the secondary lymphedema group, 47% (8/17) of patients had undergone hysterectomy and/or oophorectomy with pelvic and/or inguinal lymph node dissection for uterine or cervical cancer and subsequently developed lower extremity lymphedema. Four patients had lower extremity melanoma and had undergone an excisional procedure along with inguinal lymphadenectomy. One patient had a squamous cell carcinoma of the vagina and underwent vulvectomy and inguinal lymphadenectomy. One patient developed lymphedema from severe herpes zoster infection to the ipsilateral groin region and one developed lymphedema from intravenous drug injection in the inguinal region. One patient noted onset of lower extremity swelling shortly after knee surgery, but had also undergone hysterectomy for cervical cancer 3 years prior. Therefore, the etiology of her lymphedema was unclear and perhaps multi-factorial in nature. Thirty-five percent (6/17) received radiation therapy to the lower extremity or pelvis.
TABLE I.
Patient Characteristics
| Characteristic | Mean ± SD | Median (Range) |
|---|---|---|
| Age | 55 ± 10 | 53 (39–70) |
| BMI (kg/m2) | 26.2 ± 4.0 | 24.2 (21.4–32.9) |
| Duration of symptoms (years) | 13 ± 11 | 10 (2–34) |
| N | Percent (%) | |
| Gender | ||
| Female | 15/17 | 88.2 |
| Male | 2/17 | 11.8 |
| Diagnoses and/or precipitating causes | ||
| Gynecologic malignancy with pelvic and/or IND | 9/17 | 52.9 |
| Melanoma with IND | 4/17 | 23.5 |
| Primary lymphedema | 1/17 | 5.9 |
| Herpes Zoster infection | 1/17 | 5.9 |
| IV drug abuse | 1/17 | 5.9 |
| Indeterminate etiology | 1/17 | 5.9 |
| History of radiation therapy | ||
| Yes | 6/17 | 35.3 |
| No | 11/17 | 64.7 |
IND, inguinal node dissection.
Preoperative Results
All 21 patients had preoperative MRA available for review. In all patients, the medial sural vessels were well visualized.
Intraoperative Results
In 20/21 patients, the medial sural vessels were used as recipient vessels for lymph node transfer (Fig. 2). In one patient, a single dominant perforator to the skin was seen laterally between the two heads of the gastrocnemius muscle. This perforator originated from the lesser saphenous vein and an accompanying artery (presumably the median superficial sural artery) and were used as recipient vessels for the flap. The medial sural vessels were successfully used as recipient vessels in the remaining 20 patients. We used supraclavicular lymph node flaps in four patients, all based on the transverse cervical vessels (4/21, 19.0%). In the remaining 17 patients, lymph nodes were harvested from the axillary region, with 64.7% (11/17) of these flaps based on the thoracodorsal vessels, 23.5% (4/17) on the lateral thoracic vessels, and 11.8% (2/17) utilizing a combination of both (Table II). All flaps were able to be inset at the medial calf without the use of an overlying skin paddle and with the overlying skin closed primarily (Fig. 3). Sixteen of the 17 patients operated on at our institution underwent intraoperative ICG angiography to confirm flap perfusion. The remaining patient was not imaged secondary to a machine malfunction.
Fig. 2.
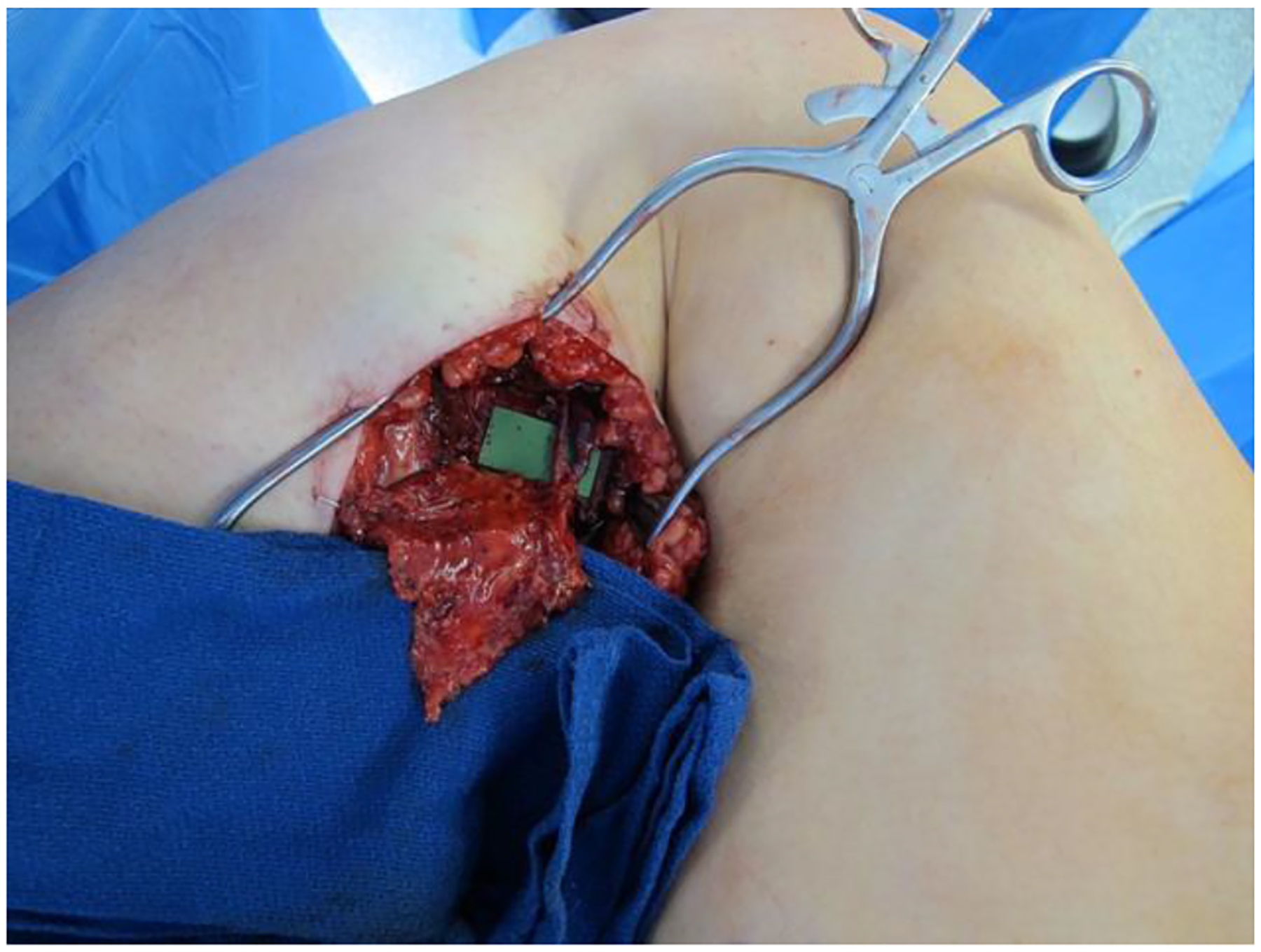
Completed anastomosis of lymph node flap to medial sural recipient vessels.
TABLE II.
Donor Lymph Node Flap Characteristics
| Lymph node donor site and donor vessel (N= 21) | N | Percent (%) |
|---|---|---|
| Cervical | 4/21 | 19.0 |
| Transverse cervical | 4/4 | 100.0 |
| Axillary | 17/21 | 81.0 |
| Thoracodorsal | 11/17 | 64.7 |
| Lateral thoracic | 4/17 | 23.5 |
| Combination thoracodorsal, lateral thoracic | 2/17 | 11.8 |
Fig. 3.
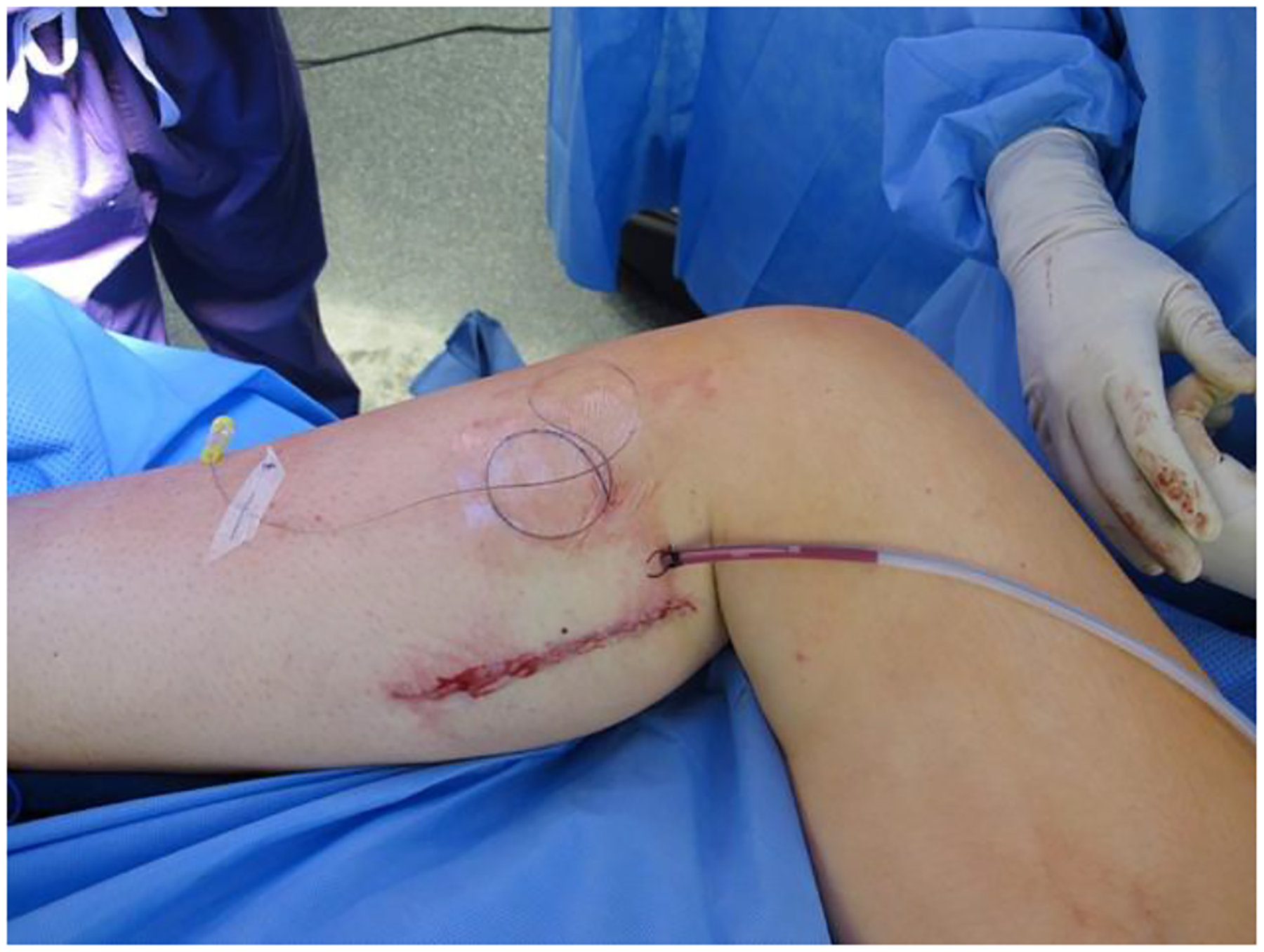
Medial calf recipient site with primary closure and no skin paddle.
Postoperative Results
At the time of this study, 13/17 patients had undergone postoperative evaluation with MRA. Postoperative results were not available for the four patients in whom we performed surgery on overseas. The average time to follow-up imaging was 11 months (SD = 4, range 1–15). A patent vascular pedicle was visualized in all. Enhancing lymph nodes within the transferred flap were seen in 92.3% (12/13) (Fig. 4). In one patient, the vascular pedicle was patent but there was no enhancement of the lymph nodes within the flap. Seven patients underwent postoperative LS with three showing spontaneous uptake in the transferred nodes (Fig. 5). In the other four patients, there was lack of Tc99 migration to level of the transferred lymph nodes within the 3-hr study period (Table III).
Fig. 4.
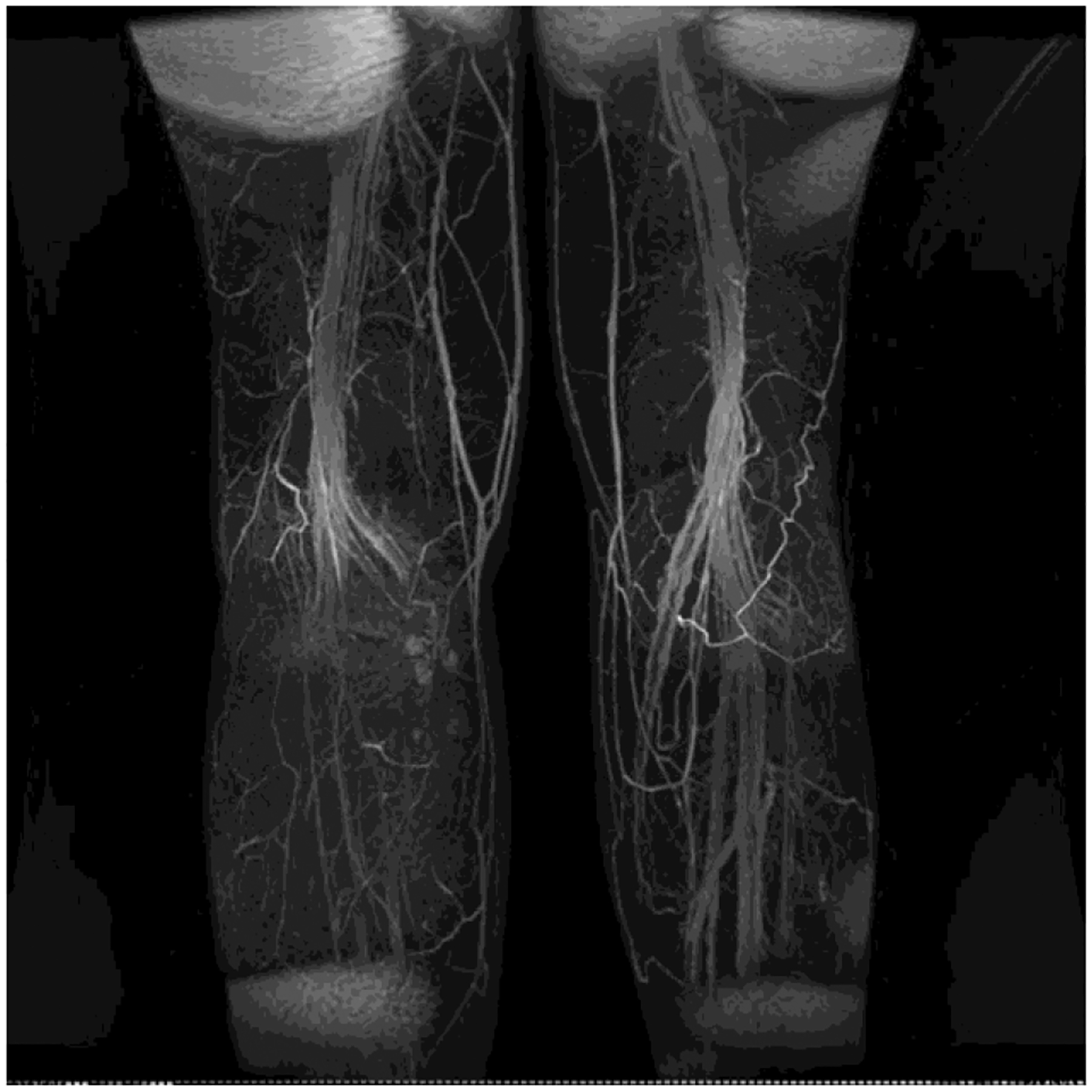
Postoperative MRA showing viable lymph nodes in transferred flap at medial calf.
Fig. 5.
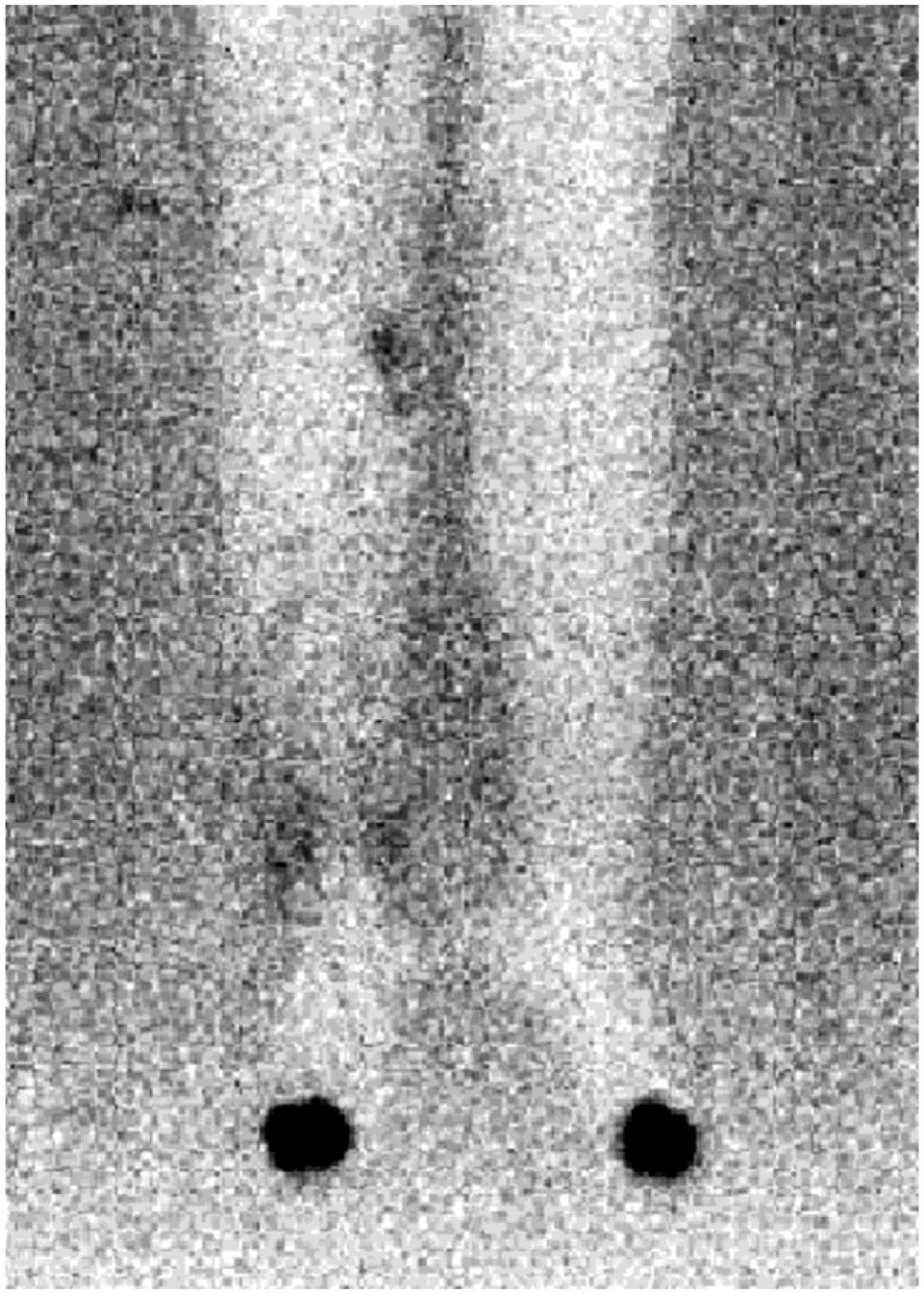
Postoperative lymphoscintigraphy showing uptake in transferred lymph nodes at medial calf.
TABLE III.
Postoperative Results
| Imaging study | Measure | N | Percent (%) |
|---|---|---|---|
| MRA (N= 13) | Patent vascular pedicle | 13/13 | 100 |
| Enhancing transferred lymph nodes | 12/13 | 92.3 | |
| Lymphoscintigraphy (N = 7) | Uptake in transferred lymph nodes | 3/7 | 42.9 |
DISCUSSION
Recipient Site Selection
Current treatments for lymphedema include both nonsurgical and surgical options. Nonsurgical CDT continues to be the first step in treatment for both primary and secondary lymphedema and includes manual lymph drainage, compression bandaging, massage, and skin care. However, operative treatment may be indicated in carefully selected patients [12]. Surgical options such as excision, suction-assisted lipectomy, lymphatic-lymphatic bypass, lymphaticovenous anastomosis, and VLNT have all been used with varying success rates [13]. Despite increasing reports of successful outcomes with VLNT, many aspects of this technique remain poorly understood.
The ideal location for VLNT has yet to be determined and both orthotopic and heterotopic placement have been reported as successful. In certain cases, such as secondary lymphedema of the lower extremity due to pelvic lymphadenectomy, heterotopic placement is currently the favored approach, as intra-abdominal transfer has yet to be evaluated.
HVLNT has been described both to the groin and the ankle regions. Becker et al. described proximal VLNT to the axilla and groin in patients with both upper and lower extremity lymphedema. They also cited the possibility of VLNT to the proximal knee using the genicular branches, but did not go into detail regarding this approach [3].
Cheng et al. described successful vascularized submandibular lymph node transfer based onthe submental vessels to the ankle in seven extremities using the dorsalis pedis as a recipient artery, but noted that use of a skin paddle is important to achieve tension-free wound closure of the fibrotic skin pocket at this site. Additionally, split-thickness skin grafts may be needed to further release wound tension and avoid compression of the pedicle [7].
The use of the medial calf as a recipient site for HVLNT has not been previously reported in the literature [14]. In fact, no other technique has been described to allow placement of lymph nodes below the knee without the use of a skin paddle or skin grafting.
We found several advantages to using the medial calf as a recipient site. The first being the ability to forego using a skin paddle or skin graft for closure while still insetting the flap in the dependent region below the knee. This location is also in close proximity to the lymphatics that course along the saphenous vein. Swelling after surgery tends to be minimal and the patient is usually able to begin wearing compression over the flap several weeks postoperatively.
Flaps transferred to the ankle require a skin paddle and often a skin graft to avoid undue tension that could compromise circulation [7]. LaPlace’s Law predicts that placement of a skin paddle will result in more swelling in the area due to the patch-like nature of the flap [15]. The bulkiness of a skin paddle is unsightly and makes it difficult to wear footwear that extends above the ankle (Fig. 6). The role of compression after distal lymph node transfer has yet to be fully elucidated; however, for most patients, even with functional improvement, limb compression remains a part of their routine and a bulky flap makes it difficult to correctly fit compression garments.
Fig. 6.
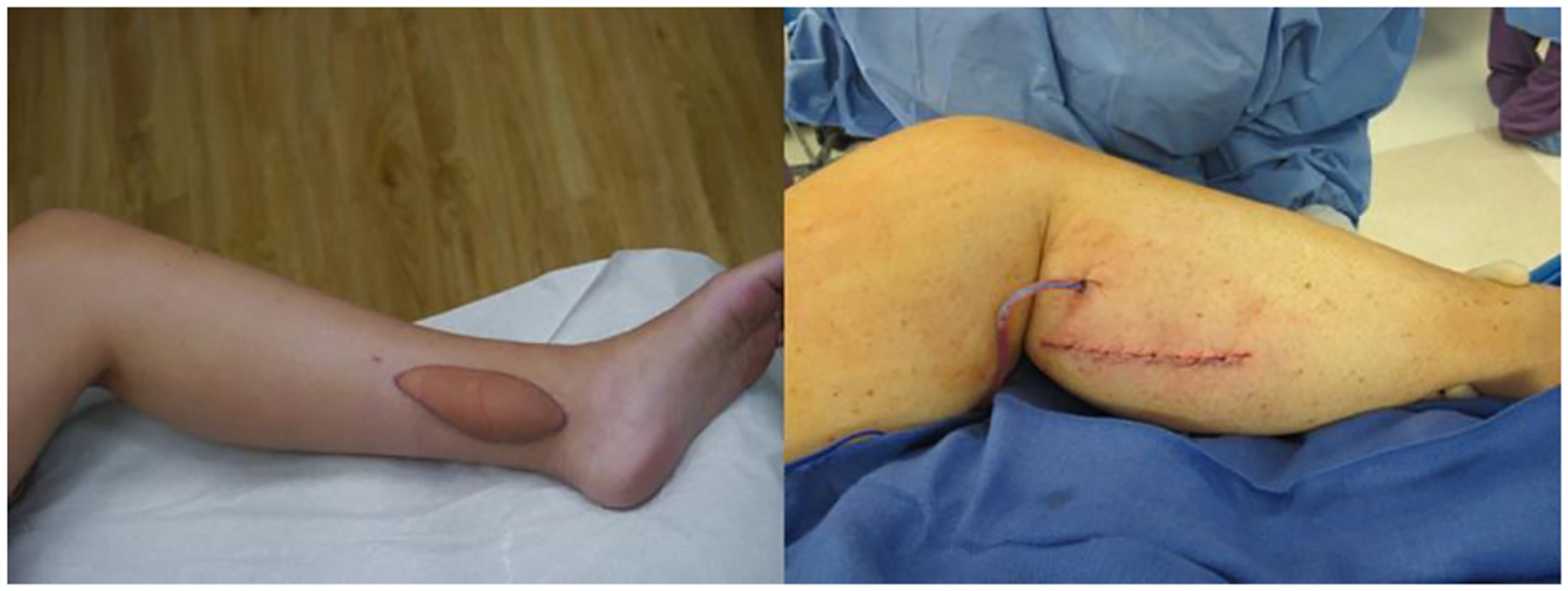
Distal lymph node transfer requiring skin paddle (left) versus primary closure (right).
The medial sural vessels have been extensively used as donor vessels for the medial sural perforator flap but there is limited experience with these vessels as recipient vessels in microvascular transfer [16,17]. These vessels can be imaged preoperatively with MRA to facilitate surgical planning. Unlike the posterior tibial or dorsalis pedis vessels, using the medial sural vessels does not risk compromising blood flow to the distal extremity [18]. The medial gastrocnemius muscle receives collateral flow from the lateral gastrocnemius muscle and functional studies show no significant decrease in gastrocnemius function from use of the medial sural vessels as recipient vessels [19,20].
From a practical perspective, the medial calf recipient site allows surgery to be performed with the patient in the supine position and to remain in this position postoperatively without direct pressure on the flap.
Physiology of Heterotopic Lymph Node Transfer
There is limited literature evaluating the postoperative physiologic function of VLNT. Becker et al. [21] reported that only 5/16 patients with upper extremity lymphedema had postoperative uptake of Tc99 on LS after orthotopic VLNT to the axilla. Regardless, the majority of patients in the study reported an improvement in symptoms. Most studies use limb circumference as the outcome benchmark, which is an unreliable measure unless controlling for variables such as changes in body weight, compression therapy regimen, time of day, temperature, and activity level. In order to avoid these confounding factors, we sought to use purely objective physiologic measures to document lymph node viability and function. We were able to show that in 12 of 13 patients who underwent postoperative MRA there were viable lymph nodes, and that in three of the seven patients who had postoperative LS there was antegrade uptake in the transferred lymph nodes, thus providing physiologic proof of concept that HVLNT to the medial calf without a skin paddle can result in viable and functioning nodes in a lymphedematous leg.
In the other patients, lack of proximal migration of the Tc99 prohibited evaluation of nodal function. This does not mean that lymph nodes were necessarily non-functional. In long-standing lymphedema, irreversible damage to the lymphatic vessels may prohibit return of transport function and limit the ability to mobilize fluid proximally without the help of compression. However, in a series of 13 distally situated upper extremity HVLNTs and seven lower extremity HVLNTs, Patel et al. [22] used ICG lymphangiography to demonstrate that retrograde lymphatic flow from the proximal limb to the distal transferred lymph node does occur, even with the patient in the supine position without the assistance of gravity. Lymphatic imaging using proximal injections to evaluate retrograde flow was not performed in this study, but retrograde flow of lymph from the proximal extremity to the distally located lymph nodes may explain how clinical improvement can occur despite a lymphoscintigraphic examination that shows a lack of proximal migration after distal injections.
Beyond facilitating the return of fluid into the circulation, lymph nodes also play an important regional immune function and it is believed that VLNT may restore regional protection against cellulitis and malignancy in patients with lymphedema [23].
Currently, the indications for VLNT and the ideal location to position the lymph nodes in the affected extremity have yet to be determined. Preoperative and postoperative imaging helps provide objective physiologic and anatomic information on the functional outcome of this treatment that may provide further insight into when and how best to perform HVLNT.
CONCLUSION
We report a new technique for VLNT in the treatment of lower extremity lymphedema using the medial sural vessels as recipient vessels. From a technical perspective, it provides a significant advantage over currently available techniques in that it allows placement of the lymph node flap below the knee in the area of maximal edema without requiring a skin paddle or skin graft for wound closure. Having the recipient site in the medial calf situates the transferred lymph nodes in proximity to the major saphenous vein lymphatics and may facilitate lymphatic connections to this system. The medial location also protects the flap from direct pressure during bedrest in the immediate postoperative period. The lack of a bulky skin paddle at the ankle is cosmetically superior and facilitates wearing compression garments and footwear postoperatively. Lymphatic imaging is an important tool in evaluating outcomes of surgical treatment of lymphedema and enabled us to provide objective physiologic evidence that lymph nodes can survive transfer to this region, function in their new location and restore regional lymphatic transport; thus demonstrating proof of concept for this approach.
Footnotes
Conflicts of interest: None.
REFERENCES
- 1.Warren AG, Brorson H, Borud LJ, et al. : Lymphedema: A comprehensive review. Ann Plast Surg 2007;59:464–472. [DOI] [PubMed] [Google Scholar]
- 2.Cormier JN, Askew RL, Mungovan KS, et al. : Lymphedema beyond breast cancer: A systematic review and meta-analysis of cancer-related secondary lymphedema. Cancer 2010;116: 5138–5149. [DOI] [PubMed] [Google Scholar]
- 3.Becker C, Vasile JV, Levine JL, et al. : Microlymphatic surgery for the treatment of iatrogenic lymphedema. Clin Plast Surg 2012; 39:385–398. [DOI] [PubMed] [Google Scholar]
- 4.Tobbia D, Semple J, Baker A, et al. : Experimental assessment of autologous lymph node transplantation as treatment of postsurgical lymphedema. Plast Reconstr Surg 2009;124: 777–786. [DOI] [PubMed] [Google Scholar]
- 5.Slavin SA, Upton J, Kaplan WD, et al. : An investigation of lymphatic function following free-tissue transfer. Plast Reconstr Surg 1997;99:730–741. [DOI] [PubMed] [Google Scholar]
- 6.Lin CH, Ali R, Chen SC, et al. : Vascularized groin lymph node transfer using the wrist as a recipient site for management of postmastectomy upper extremity lymphedema. Plast Reconstr Surg 2009;123:1265–1275. [DOI] [PubMed] [Google Scholar]
- 7.Cheng MH, Huang JJ, Nguyen DH, et al. : A novel approach to the treatment of lower extremity lymphedema by transferring a vascularized submental lymph node flap to the ankle. Gynecol Oncol 2012;126:93–98. [DOI] [PubMed] [Google Scholar]
- 8.Cheng MH, Chen SC, Henry SL, et al. : Vascularized groin lymph node flap transfer for postmastectomy upper limb lymphedema: Flap anatomy, recipient sites, and outcomes. Plast Reconstr Surg 2013;131:1286–1298. [DOI] [PubMed] [Google Scholar]
- 9.Gretz JE, Norbury CC, Anderson AO, et al. : Lymph-borne chemokines and other low molecular weight molecules reach high endothelial venules via specialized conduits while a functional barrier limits access to the lymphocyte microenvironments in lymph node cortex. J Exp Med 2000;192:1425–1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Proof of Concept. Available at: https://en.wikipedia.org/wiki/Proof_of_concept#cite_note-1. Accessed July 18, 2015.
- 11.Dayan JH, Dayan E, Smith ML: Reverse lymphatic mapping: A new technique for maximizing safety in vascularized lymph node transfer. Plast Reconstr Surg 2015;135:277–285. [DOI] [PubMed] [Google Scholar]
- 12.International Society of Lymphology. The diagnosis and treatment of peripheral lymphedema: 2013 consensus document of the International Society of Lymphology. Lymphology 2013;46:1–11. [PubMed] [Google Scholar]
- 13.Granzow JW, Soderberg JM, Kaji AH, et al. : Review of current surgical treatments for lymphedema. Ann Surg Oncol 2014;21: 1195–1201. [DOI] [PubMed] [Google Scholar]
- 14.Raju A, Chang DW: Vascularized lymph node transfer for treatment of lymphedema: A comprehensive literature review. Ann Surg 2015;261:1013–1023. [DOI] [PubMed] [Google Scholar]
- 15.Basford JR: The Law of Laplace and its relevance to contemporary medicine and rehabilitation. Arch Phys Med Rehabil 2002;83: 1165–1170. [DOI] [PubMed] [Google Scholar]
- 16.Kao HK, Chang KP, Wei FC, et al. : Comparison of the medial sural artery perforator flap with the radial forearm flap for head and neck reconstructions. Plast Reconstr Surg 2009;124:1125–1132. [DOI] [PubMed] [Google Scholar]
- 17.Hallock GG: The medial approach to the sural vessels to facilitate microanastomosis about the knee. Ann Plast Surg 1994;32: 388–393. [DOI] [PubMed] [Google Scholar]
- 18.Pyon JK, Ha BJ, Hyun WS, et al. : Sural vessels as recipient vessels for free flap transfer to the single vessel leg. J Korean Soc Plast Reconstr Surg 1999;26:366–371. [Google Scholar]
- 19.Altaf FM: The anatomical basis of the medial sural artery perforator flaps. West Indian Med J 2011;60:622–627. [PubMed] [Google Scholar]
- 20.Beumer JD, Karoo R, Caplash Y, et al. : The medial sural artery as recipient vessel and the impact on the medial gastrocnemius. Ann Plast Surg 2011;67:382–386. [DOI] [PubMed] [Google Scholar]
- 21.Becker C, Assouad J, Riquet M, et al. : Postmastectomy lymphedema: Long-term results following microsurgical lymph node transplantation. Ann Surg 2006;243:313–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel KM, Lin CY, Cheng MH: From theory to evidence: Long-term evaluation of the mechanism of action and flap integration of distal vascularized lymph node transfers. J Reconstr Microsurg 2015;31:26–30. [DOI] [PubMed] [Google Scholar]
- 23.Rabson JA, Geyer SJ, Levine G, et al. : Tumor immunity in rat lymph nodes following transplantation. Ann Surg 1982;196: 92–99. [DOI] [PMC free article] [PubMed] [Google Scholar]


