Abstract
Effective therapeutic options are still lacking for uveal melanoma (UM) patients who develop metastasis. Metastatic traits of UM are linked to BRCA1-associated protein 1 (BAP1) mutations. Cell metabolism is re-programmed in UM with BAP1 mutant UM, but the underlying mechanisms and opportunities for therapeutic intervention remain unclear. BAP1 mutant UM tumors have an elevated glycolytic gene expression signature, with increased expression of pyruvate dehydrogenase (PDH) complex and PDH kinase (PDHK1). Furthermore, BAP1 mutant UM cells showed higher levels of phosphorylated PDHK1 and PDH that was associated with an upregulated glycolytic profile compared to BAP1 wild type UM cells. Suppressing PDHK1-PDH phosphorylation decreased glycolytic capacity and cell growth, and induced cell cycle arrest of BAP1 mutant UM cells. Our results suggest that PDHK1-PDH phosphorylation is a causative factor of glycolytic phenotypes found in BAP1 mutant UM and propose a therapeutic opportunity for BAP1 mutant UM patients.
Keywords: pyruvate dehydrogenase, glycolysis, BAP1, uveal melanoma
Introduction
Uveal melanoma (UM) originates from melanocytes in the choroid, iris, and ciliary body regions of the eye (1). The incidence of UM is ~ 2,000 cases per year in the US (1, 2). Despite effective treatments for primary UM, ~50% of patients develop metastases with typical survival of less than one year (1). Therapies that are effective in cutaneous melanoma, including BRAF or MEK small molecule inhibitors and immune checkpoint inhibitors, have invariably yielded poor responses in UM (3–6), highlighting an urgent need to develop new therapeutic options for metastatic UM (7).
As cancer cells become malignant, they undergo metabolic adaptation. Oncogene activation and/or tumor suppressor inactivation are known to regulate metabolic enzyme expression and/or activity (8). For instance, oncogenic KRAS and BRAF signaling leads to elevated glutamine and glucose utilization (9, 10), and loss of PTEN leads to aerobic glucose utilization (11). It is important to investigate the interactions between cancer-associated genes and metabolic alterations to understand the cancer pathogenesis and to develop more effective therapeutic avenues.
BRCA1-associated protein 1 (BAP1) is a tumor suppressor gene located on chromosome 3p21.1 that is frequently deleted in metastatic UM, mesothelioma and clear cell renal cell carcinoma (ccRCC) (12–14). Inactivating mutations in BAP1 are strongly associated with an elevated susceptibility to metastasis, with ~ 80% of UM metastases and metastasizing tumors reported to carry a loss-of-BAP1 mutation (12, 13, 15). Recent studies show that BAP1 loss in cancer cells causes pivotal changes in cellular metabolism (16–21). For example, mesothelioma cells that carry germline BAP1 mutations have upregulated aerobic glycolysis (17), and BAP1 mutant ccRCC tumor samples display elevated glycolysis (22). In UM, BAP1 deficient tumors demonstrate high levels of oxidative phosphorylation (OXPHOS) and altered metabolism (20, 23). However, the mechanism behind how BAP1 mutations cause an upregulation of glucose utilization remain unclear.
Pyruvate dehydrogenase complex (PDC) is composed of pyruvate dehydrogenase (PDH, E1), dihydrolipoamide acetyltransferase (E2, DLAT), and dihydrolipoamide dehydrogenase subunit (E3, DLD) (24). It is responsible for converting pyruvate to acetyl-CoA and its activity is reversibly controlled through phosphorylation of PDH by PDH kinases (PDHKs) and PDH phosphatases (PDPs) (24). Multiple allosteric regulators (i.e. ATP/ADP ratio) are involved in PDH phosphorylation, leading to its inactivation (24, 25). Additionally, PDHK1 phosphorylation and activation are also associated with increased PDH phosphorylation and glycolysis (26). PDH inactivation and/or PDHKs overexpression is one of the main leading factors of increased aerobic glycolysis in cancer cells; thus, targeting PDHK-PDC offers a potential therapeutic vulnerability (25, 27).
Here, we show that BAP1 mutant UM tumors have an elevated glycolytic gene signature and high PDC and PDHK1 gene expression compared to BAP1 wild type UM tumors. Consistent with patient samples, BAP1 mutant UM cells display higher phosphorylated PDHK1-PDH, leading to increased glycolytic profiles compared to BAP1 wild type UM cells. Suppression of PDH phosphorylation significantly decreases UM cell growth and glycolytic abilities of BAP1 mutant UM cells, but not BAP1 wild type UM cells. Our results suggest that PDH inactivation causes increased glucose utilization in BAP1 mutant UM. Moreover, these findings suggest a potential targetable vulnerability of BAP1 mutant UM tumors.
Results
Genes involved in glycolysis are elevated in BAP1 mutant UM
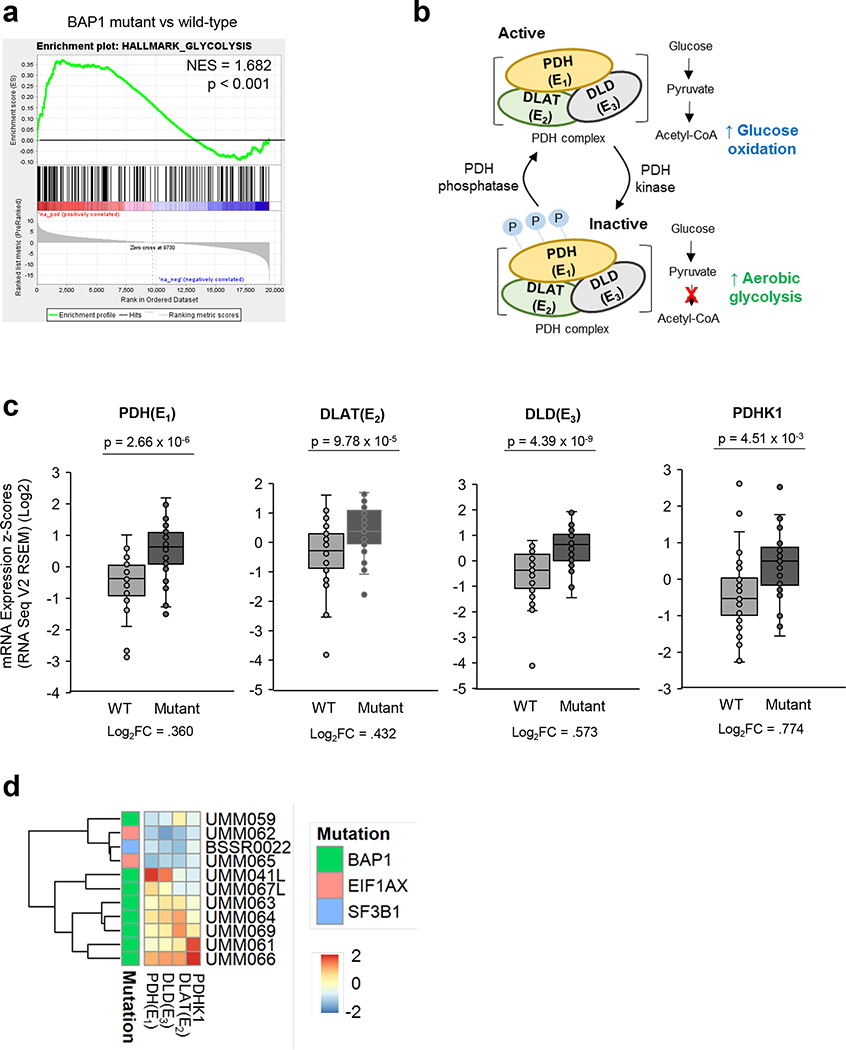
To identify how loss of BAP1 affects UM glycolysis, we analyzed UM cases in The Cancer Genome Atlas (TCGA) (15). UM samples were divided into BAP1 wild type (n=40) and BAP1 mutant/altered groups (n=40). We utilized the glycolytic gene set from the mSigDB Hallmark collection (28) to perform gene set enrichment analysis (GSEA). We observed that the BAP1 mutant group has upregulated enrichment compared to the BAP1 wild type group (Fig. 1a and Supplementary Fig 1a). Based on the oxidative phosphorylation (OXPHOS) gene signature, there are two subgroups in BAP1 mutant UM group, OXPHOSlow and OXPHOShigh (20); thus, we further performed glycolysis GSEA according to these subgroups. We found that both OXPHOSlow and OXPHOShigh groups within BAP1 mutant UM group exhibit markedly elevated enrichment compared to the BAP1 wild type group (Supplementary Fig 1a).
Figure 1. BAP1 mutant UM tumors have elevated mRNA levels related to PDC and PDHK1.
UVM RNA-seq V2 gene expression data from the TCGA were retrieved from the latest Broad GDAC Firehose data run (stddata_2016_01_28). Based on BAP1 mutation and copy loss, samples were stratified into BAP1 mutant and wild type groups. Differential expression analysis was performed between BAP1 mutant (n=40) and wild type (n=40) and used for performing GSEA. a. GSEA enrichment plots of the glycolysis hallmark gene set for comparison in BAP1 mutant vs wild type group. b. PDH complex is composed of PDH (E1), DLAT (E2) and DLD (E3). Phosphorylation of PDH inactivates its activity, elevating aerobic glycolysis. c. UVM RNA sequencing (RNA-seq) V2 gene expression of PDH (E1), DLAT(E2), DLD (E3) and PDHK1 d. A heatmap showing z-scores for the average expression of genes in malignant cells from single cell RNA-seq of UM tumors.
PDH phosphorylation/inactivation and PDHK1 overexpression are critical factors of elevated aerobic glycolysis in cancer cells (Fig. 1b) (29). Next, we compared expression of PDC components, including PDH, DLAT and DLD, and PDHK1 between BAP1 mutant and wild type UM patient samples. From TCGA data, BAP1 mutant UM samples displayed significantly increased transcript levels of PDH, DLAT, DLD and PDHK1 compared to BAP1 wild type UM samples (Fig. 1c). Furthermore, single cell RNA sequencing showed that BAP1 mutant UM tumors have higher PDH, DLAT, DLD and PDHK1 expression compared to BAP1 wild type UM tumors in these patient samples (Fig. 1d). Together, these observations suggest that the phosphorylation of PDH is enhanced and may result in an increase in glycolysis in BAP1 mutant UM tumors.
BAP1 mutant UM cells have increased PDHK1-PDH phosphorylation
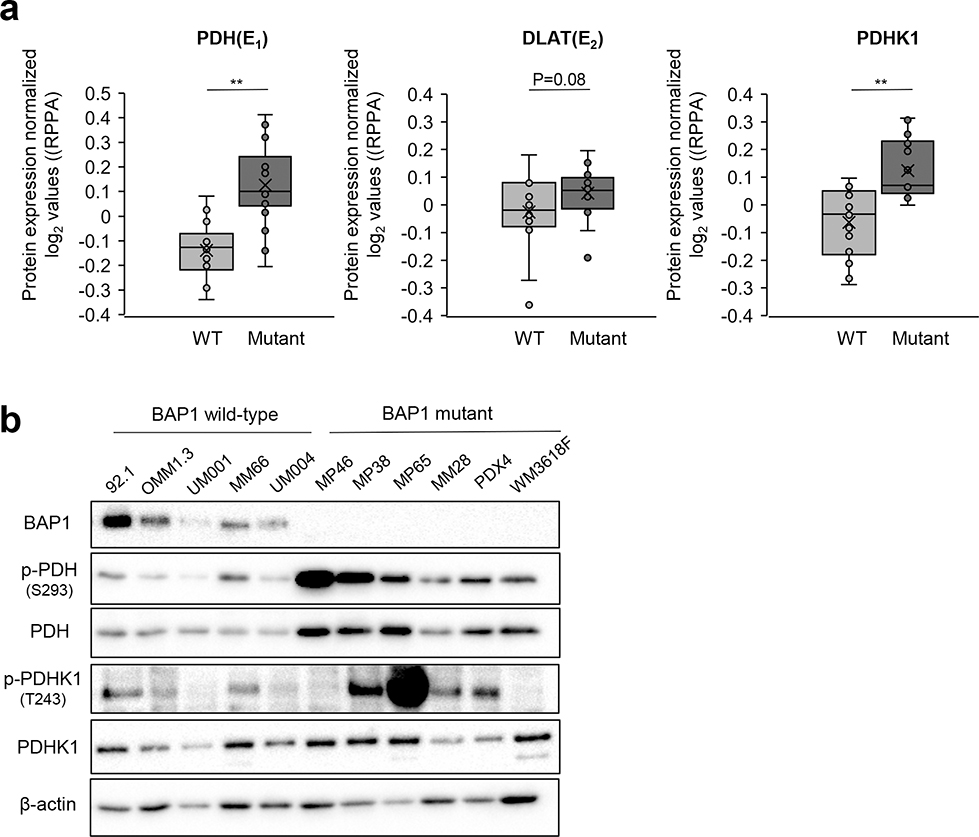
Next, we analyzed PDH activity and PDHK1 levels in UM cell lines. We performed Reverse Phase Protein Array (RPPA) of UM cell panels to compare PDH, DLAT and PDHK1 protein expression between BAP1 wild type and BAP1 mutant cells. Consistent with patient data, we observed that BAP1 mutant cells have significantly upregulated PDH, DLAT and PDHK1 levels compared to BAP1 wild type cells (Fig. 2a). In addition, BAP1 mutant UM cells showed increased total and phosphorylated PDH levels compared to BAP1 wild type UM cells, while the expression of PDHK1 and DLAT remained unaltered (Fig. 2b and Supplementary Fig. 1b). Moreover, knockdown of BAP1 by siRNA transfection increased the phosphorylation of PDH in a BAP1 wild type cell line, UM004 (Supplementary Fig. 1c). Several factors induce PDH phosphorylation, including allosteric regulators (i.e. elevated ATP/ADP ratio) and PDHK1 phosphorylation (24, 26). To identify factors that regulate increased PDH phosphorylation in BAP1 mutant UM cells, we compared the ATP/ADP ratio in BAP1 wild type (MM66) and mutant (MP46 and MP65) UM cells. We observed that BAP1 mutant cells display a significantly lower ATP/ADP ratio but higher phosphorylated PDHK1 than BAP1 wild type UM cells (Supplementary Fig. 1d and Fig. 2b). These observations suggest that PDHK1 phosphorylation may cause PDH phosphorylation in BAP1 mutant UM cells.
Figure 2. BAP1 mutant UM cells have elevated PDHK1-PDH phosphorylation.
a. Average expression of PDH (E1), DLAT (E2) and PDHK1 between BAP1 wild type (92.1, OMM1.3, UM001, UM004, UM002B and MM66) and mutant (MP46, MP65, MP38, MM28, PDX4 and WM3618F) UM cells were analyzed by RPPA analysis. RPPA data were used to determine antibodies that are significantly different between mutant and wild-type cell lines. Comparison of average normalized log2 values were performed by the two-sample t-test method and assumed unequal variance. Data points are shown as individual lysate collections **p<0.01. b. Validation of protein expression by Western blot.
PDH phosphorylation results in increased glycolytic profiles of BAP1 mutant UM cells
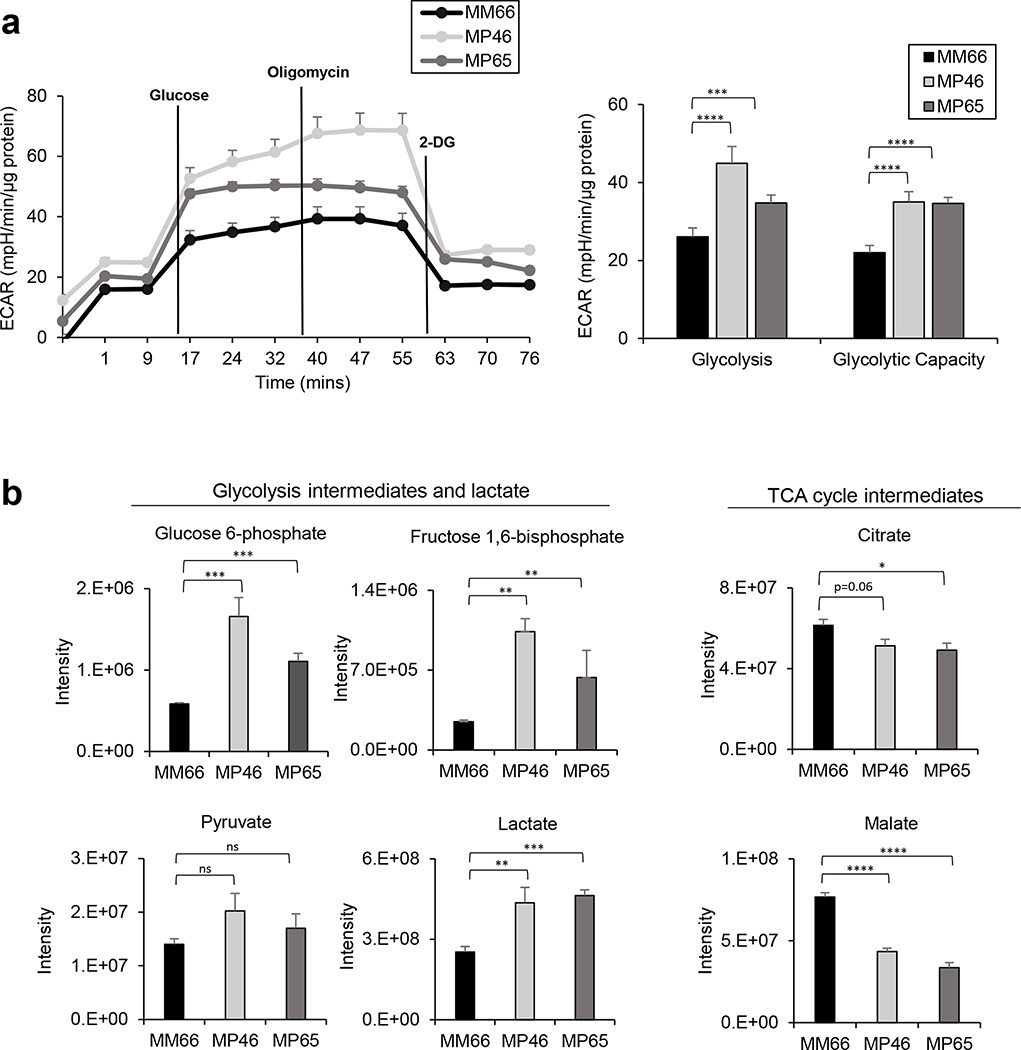
Since PDH inactivation is associated with elevated aerobic glucose utilization (29), we compared glycolytic capacities in MM66, MP46 and MP65 cells using the Seahorse Extracellular Flux Analyzer. We observed that BAP1 mutant MP46 and MP65 cells have significantly higher glycolysis and glycolytic capacity than BAP1 wild type MM66 cells (Fig. 3a). Aerobic glycolysis increases lactate production and decreases TCA cycle intermediate levels (17); therefore, we compared the intermediates of glycolysis and TCA cycle in MM66 cells, MP46 and MP65 cells. Both BAP1 mutant MP46 and MP65 cells displayed significantly higher levels of glycolysis intermediates (glucose-6-phosphate, fructose 1,6-bisphosphate and pyruvate) and lactate compared to BAP1 wild type MM66 cells (Fig. 3b). In addition, MP46 and MP65 cells exhibited significantly lower levels of TCA cycle intermediates, including citrate and malate compared to MM66 cells (Fig. 3b), whereas MP46 cells exhibit a higher succinate level than MM66 and MP65 cells (Supplementary Fig. 1d). These data suggest that BAP1 mutant UM cells have increased aerobic glycolysis compared to BAP1 wild type UM cells.
Figure 3. BAP1 mutant UM cells have increased glycolytic profiles.
a. Glycolytic capacity of MM66, MP46 and MP65 cells was compared by ECAR using the Seahorse analyzer. Data were normalized to protein level and analyzed via Agilent Seahorse XF report generators. Data are shown as mean ± SEM (n=12). b. Levels of metabolite of MM66, MP46 and MP65 cells in glycolysis and TCA cycle are shown. Data from whole cell extractions (n=6) are displayed as mean ± SEM (n=6). *p<0.05, **<0.01, ***<0.001, ****<0.0001 and not significant (ns).
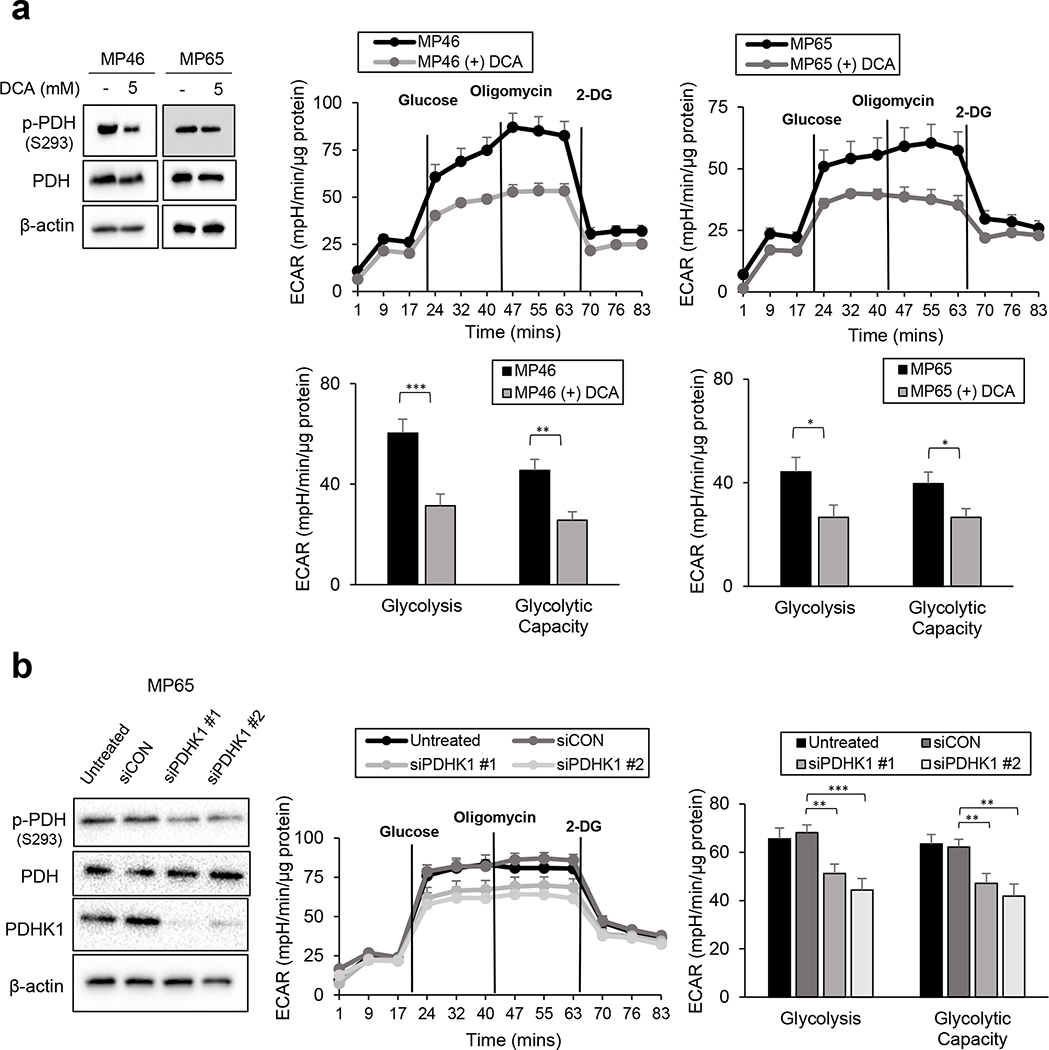
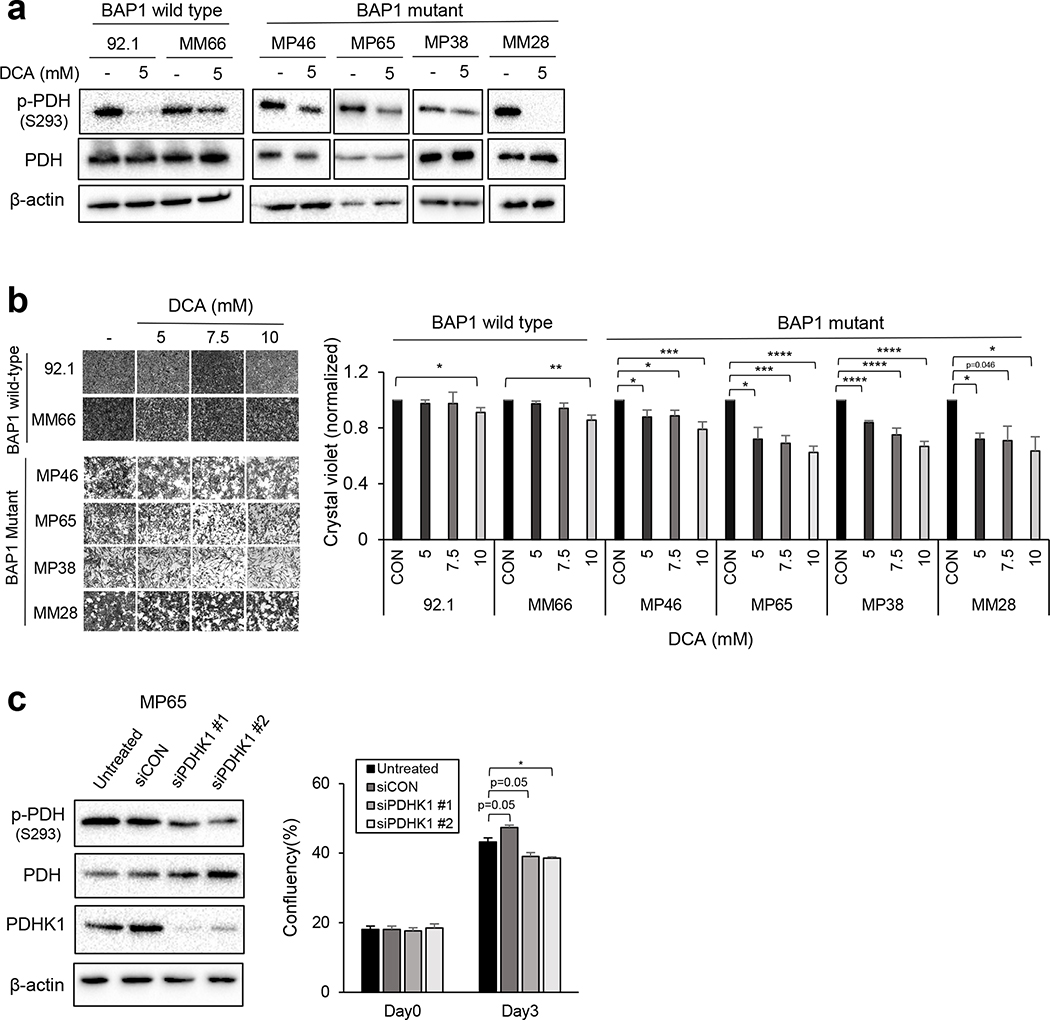
We examined whether PDH phosphorylation is responsible for the glycolytic profiles of BAP1 mutant cells. To suppress PDH phosphorylation, MP46 and MP65 cells were pre-treated with dichloroacetate (DCA, a PDHK inhibitor), and glycolytic profiles were measured. DCA treatment significantly lowered PDH phosphorylation (Fig. 4a) and decreased glycolysis and glycolytic capacity in both MP46 and MP65 cells (Fig. 4a). The treatment of DCA significantly increased oxygen consumption rate (OCR) parameters in MP46 cells, while the OCR parameters of MP65 cells displayed slight elevations (Supplementary Fig. 1e). Consistent with above results, molecular knockdown of PDHK1 (siPDHK1) markedly decreased PDH phosphorylation, as well as glycolysis and glycolytic capacity in MP65 cells. (Fig. 4b). We also observed increased expression of PDH in BAP1 mutant UM cells (Fig 2b), which might be also related to the elevation of PDH phosphorylation along with PDHK1 expression in BAP1 mutant UM cells. Thus, we performed PDHA1 knockdowns and observed that PDHA1 siRNAs decreased phosphorylation of PDH in MP65 cells (Supplementary Fig. 1f). There was a trend of reduction in glucose utilization in MP65 cells following PDHA1 knockdown, although it was not statistically significant (Supplementary Fig. 1f). These results suggest that PDH phosphorylation causes the elevation of glycolytic flux in BAP1 mutant UM cells.
Figure 4. Suppression of PDH phosphorylation decreased the glycolytic capacity of BAP1 mutant UM cells.
a. MP46 and MP65 cells were pre-treated with DCA (5mM) for 24 hours and glycolytic capacity was measured using the Seahorse instrument. Efficiency of DCA was determined by detecting downregulation of p-PDH in western blot analysis. b. Effect of siPDHK1 on MP65 cells glycolytic ability was measured by the Seahorse instrument. Knockdown of PDHK1 was confirmed by western blot. Data were normalized to protein level and analyzed via Agilent Seahorse XF report generators. Data are shown as mean ± SEM (n=12). *p<0.05, **<0.01, ***<0.001, ****<0.0001 and not significant (ns).
Suppression of PDH phosphorylation induces cell cycle arrest in BAP1 mutant UM cells.
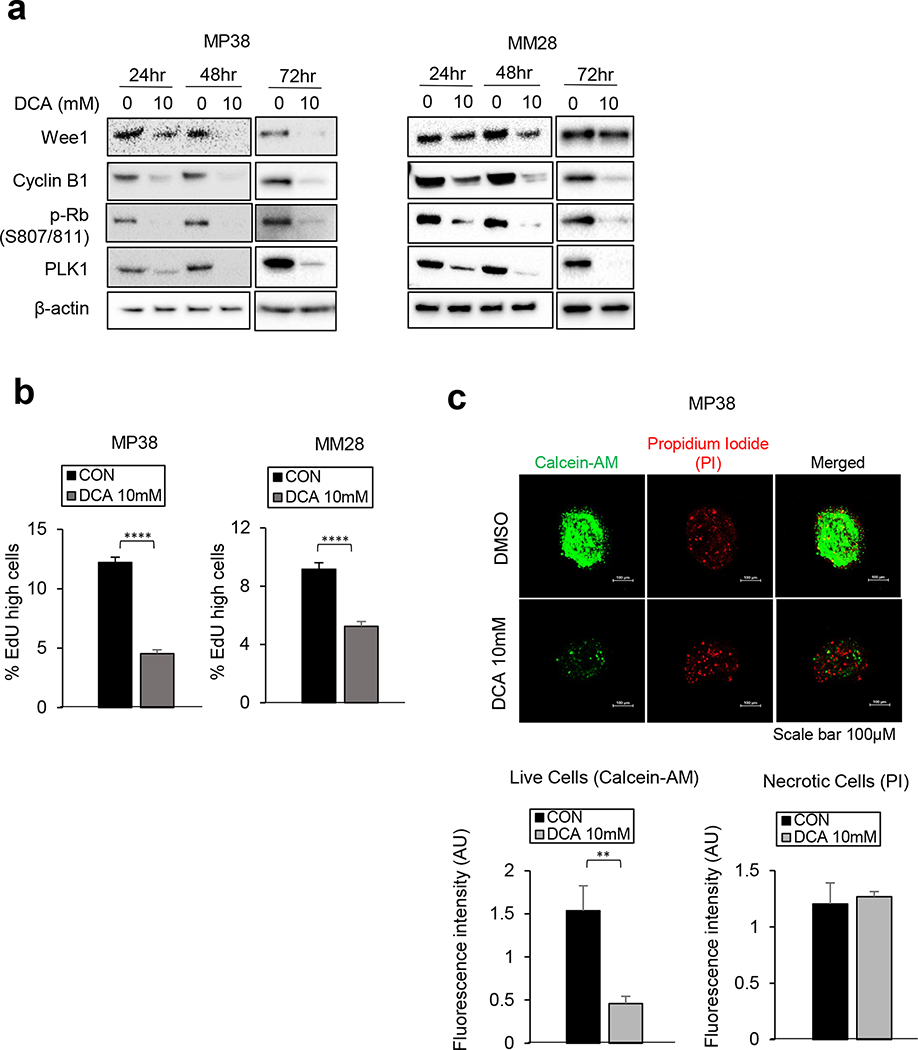
Targeting PDHK with inhibitors such as DCA, results in tumor growth inhibition by shifting energy metabolism toward OXPHOS instead of glycolysis (24, 30). To test whether the upregulated PDH phosphorylation of BAP1 mutant cells is a potential therapeutic vulnerability of BAP1 mutant cells, we treated BAP1 wild type and mutant cells with DCA. DCA treatment significantly reduced PDH phosphorylation in both BAP1 wild type cells (92.1 and MM66) and BAP1 mutant cells (MP46, MP65, MP38 and MM28) (Fig. 5a). In addition, we observed that DCA treatment inhibits BAP1 mutant cell growth in a dose-dependent manner; whereas it has little effect on BAP1 wild type cells (Fig. 5b). As expected, knockdown of PDHK1 (siPDHK1) also significantly decreased phosphorylation of PDH and BAP1 mutant MP65 cell growth (Fig. 5c). We observed that DCA treatment downregulates expression of proteins involved in cell cycle progression, including Wee1, cyclin B1, phospho-Rb (S807/811), and PLK1 in BAP1 mutant cells (Fig. 6a). To confirm the effects of DCA on cell cycle progression, EdU incorporation assays were performed in BAP1 mutant cells. We observed that treatment with DCA significantly decreases EdU incorporation percentage, indicating suppression of S-phase entry (Fig. 6b). Moreover, DCA significantly suppressed the viability of 3D spheroids of MP38 cells (Fig. 6c). These results indicate that inhibition of PDH phosphorylation by PDHK1 inhibitions decreases BAP1 mutant UM cell growth via cell cycle arrest.
Figure 5. Inhibition of PDH phosphorylation reduces cell survival of BAP1 mutant UM cells.
a. Efficiency of DCA in UM cells was determined by detecting downregulation of p-PDH after 5mM DCA treatment for 48 hours. b. BAP1 wild type (92.1 and MM66) and mutant (MP46, MP65, MP38 and MM28) cells were treated with DCA (5, 7.5 or 10 mM) for 3 days. Changes of cell viability were measured by crystal violet staining. Quantification bar graph represents relative fold change of cell viability with DCA treatments in cells. Representative images of crystal violet are shown. Scale bar: 100 μm. Data are shown as mean ± SEM from biological replicate experiments (n=4). c. Effect of siPDHK1 on MP65 cell growth was analyzed through Incucyte Live Cell Analysis Imaging System. Data are shown as mean ± SEM from biological replicate experiments. *p<0.05, **<0.01, ***<0.001, ****<0.0001 and not significant (ns).
Figure 6. Suppression of PDH phosphorylation leads to the cell cycle arrest in BAP1 mutant UM cells.
Knockdown of PDHK1 was confirmed by western blot. MP38 and MM28 cells were treated with DCA (10mM) for 24, 48, and 72 hours. Then, cells were collected for a. a nalysis of protein expressions related to cell cycle progression, and b. EdU incorporation assay (72hr, n=3). c. MP38 cells were treated with DCA (10mM) for 72 hours. Cell viability assays of 3D tumor spheroids were performed. The representative figures of MP38 cells 3D spheroids are shown. For the quantification, 3D tumor spheroids were stained with calcein-AM (7μM) and propidium iodide (PI, 10μg/mL) for live cells and necrotic/dead cells, respectively (n=3). Magnification: 200X, Scale bar: 100μm. Data are shown as mean ± SEM from biological replicate experiments. *p<0.05, **<0.01, ***<0.001, ****<0.0001 and not significant (ns).
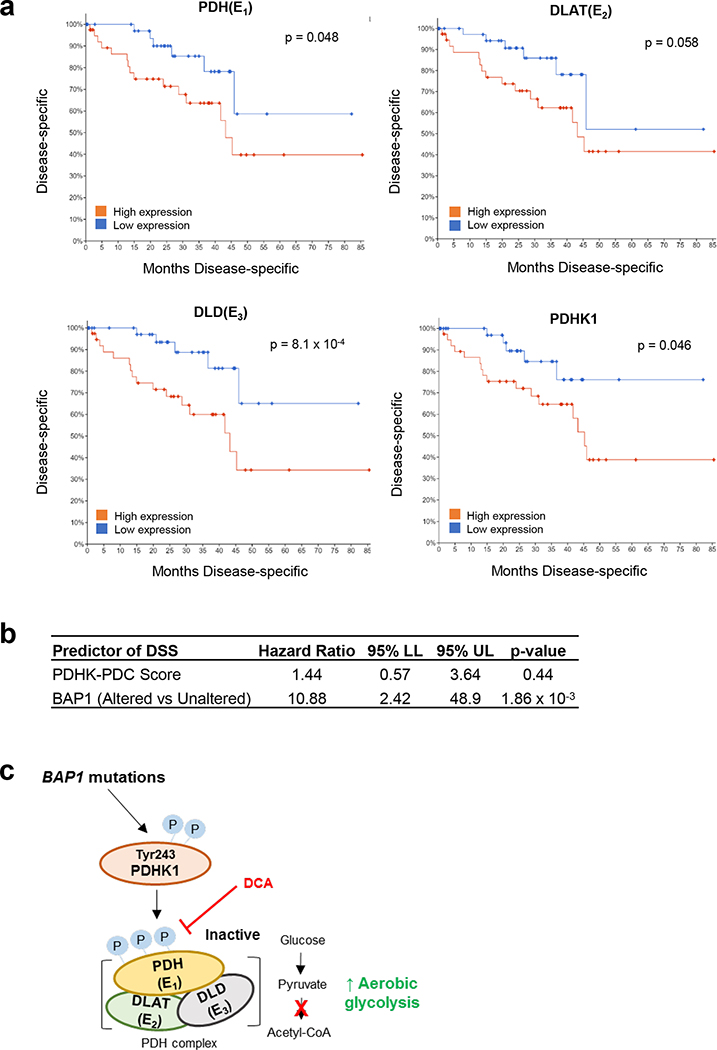
Increased PDC and PDHK1 expression is associated with poor prognosis in UM patients.
Our observations indicate that BAP1 mutant UM has high PDC and PDHK1 phosphorylation, leading to a glycolytic phenotype compared to BAP1 wild type UM. To investigate the clinical relevance, we analyzed UM patient survival based on expression of these genes. Analysis of UM-specific survival outcomes showed a significantly poorer outcome with high expression of PDC components, including PDH, DLAT and DLD, and PDHK1 (Fig. 7a). BAP1 mutations are significantly associated with poor prognosis in UM (12, 31). Thus, to see whether observations in Fig. 7a are simply due to BAP1 status, we performed multivariate analysis. When controlling for BAP1 status there is a trend for a higher PDHK-PDC activity score associating with poor disease-specific survival, although data are not statistically significant (Fig. 7b). After stratifying BAP1 mutant patients by median expression, the higher expression of PDC and PDHK1 groups displayed a trend towards poorer disease-specific survival compared to lower gene expression groups (Supplementary Fig. 2a). Altogether, elevated PDC and PDHK1 expression is linked to worse prognosis of UM patients.
Figure 7. UM patient survival curve based on PDC and PDHK1 expression.
a. Analysis of TCGA data retrieved from the TCGA Pan-Cancer Clinical Data Resource (TCGA-CDR) for UM patient disease specific survival based on PDH (E1), DLAT (E2), DLD (E3) and PDHK1 expression are shown. Expression is stratified into high (n=40) or low (n=40) by median expression of each gene. Logrank test was used to determine significance in overall survival (https://wiki.nci.nih.gov/plugins/servlet/mobile#content/view/24279961). b. Cox proportional hazards (PH) model analysis for the association between disease-specific survival, PDHK-PDC score and BAP1 alteration status are shown. Patients were stratified into BAP1 altered groups based on BAP1 mutation and copy loss data. PDHK-PDC scores were calculated by averaging the z-score value for PDH, DLAT, DLD and PDHK1. c. A summary mechanism of BAP1 mutant-driven glycolysis in UM. BAP1 mutations induces phosphorylation of PDHK1-PDH axis, elevating aerobic glucose utilization of BAP1 mutant UM cells. Inhibition of PDH phosphorylation reduces BAP1 mutant UM cell growth via cell cycle arrest.
Discussion
In this study, we found that BAP1 mutant UM tumors have an upregulated glycolytic gene set expression and elevated gene expression of PDC components, including PDH, DLAT and DLD, and PDHK1 compared to BAP1 wild type UM tumors. By incorporating metabolomic and molecular analyses, we demonstrate that PDHK1-PDH phosphorylation leads to the elevation of glycolytic capacity in BAP1 mutant UM cells compared to BAP1 wild type UM cells (Fig. 7b). Moreover, we shown that inhibition of PDH phosphorylation reduces BAP1 mutant UM cell growth by induction of cell cycle arrest.
There has been significant progress in the understanding of UM genetics (32); however, effective treatment options for advanced UM are still poorly established. Since BAP1 mutations are highly correlated with metastatic UM and poor survival outcomes (33, 34), understanding the scope of functions performed by BAP1 is important to develop new therapeutic options. While the roles of BAP1 roles in cellular metabolism are well understood (35, 36), its metabolic functions are comparatively under studied. Recent publications addressed BAP1 function in several critical cellular metabolism pathways, including lipid and glucose homeostasis, and mitochondrial functions (16, 18, 20, 23, 37). Moreover, BAP1 mutations are associated with increased aerobic glycolysis in mesothelioma and ccRCC (17, 22). However, the mechanisms that drive increased glycolysis in BAP1 UM mutant tumors remain unclear.
We found that BAP1 mutant UM tumors have an upregulated glycolysis gene signature compared to BAP1 wild type UM tumors from TCGA UM patient data. Inactivation of PDH is one major reason for elevated glycolysis in cancer cells (29). By analyzing TCGA and single cell RNA sequencing data, we observed that BAP1 mutant UM samples have higher gene expression of PDC components and PDHK1 compared to BAP1 wild type UM samples. Consistent with patient data, we observed that BAP1 mutant UM cells display markedly higher phosphorylation of PDHK1-PDH, leading to elevated glycolytic profiles compared to BAP1 wild type UM cells. We also observed BAP1 mutant UM cells have significantly increased total PDH expression compared to BAP1 wild type UM cells, and knockdown of PDHA1 markedly reduced PDH phosphorylation. These findings suggest that further examination of PDH complex activity, detection of non-phosphorylated PDH, and the roles of elevated total PDH expression may be important to understand why BAP1 mutant UM cells has both increased total and phosphorylated PDH. Although our observations indicate that PDHK1-PDH phosphorylation is the causative factor for heightened glycolytic profiles in BAP1 mutant UM cells, the regulatory mechanism of PDHK1-PDH phosphorylation by BAP1 still needs to be investigated in future studies. Recently, it was reported that O-GlcNAcylation of phosphoglycerate kinase 1 leads its translocation to the mitochondria, resulting in PDHK1-PDH phosphorylation and elevation of glucose usage to support tumor growth (38). BAP1 indirectly O-GlcNacylates several proteins (i.e. HCF1) by recruiting O-linked N-acetylglucosamine transferase (OGT) (39, 40). Future studies should interrogate the role of BAP1 deubiquitinating activity in modulating PDHK1-PDH phosphorylation.
Targeting the PDHK-PDH axis inhibits tumor growth and improves cancer therapies by inhibiting glucose utilization (25, 27, 41). For example, dephosphorylation of PDH by DCA enhanced the efficacy of reovirus-induced cancer cell death in pre-clinical models (41). We found that both pharmacological (DCA treatment) and molecular PDHK1 knockdown significantly decrease BAP1 mutant UM cell growth through cell cycle arrest. By contrast, effects of targeting PDH inactivation were modest in BAP1 wild type UM cells. These observations suggest that inhibition of the PDHK1-PDH axis could be a novel therapeutic option for BAP1 mutant UM. In the future, the efficacy of PDHK1-PDH inhibition needs to be evaluated in an in vivo model. Moreover, future studies analyzing the potential synergic effects of PDHK1-PDH axis inhibition and other therapies, including MEK inhibitors, anti-LAG3 antibodies, and bispecific immune antibodies e.g. IMCgp100 may improve the poor responses of UM to current therapeutic options.
By sustaining increased glucose utilization, cancer cells meet their bioenergetic and biosynthetic requirements to support high proliferation rates and survive under stressful environments (i.e. lower oxygen and nutrient availability) (42, 43). We observed that BAP1 mutant UM cells have higher glycolytic profiles than BAP1 wild type UM cells. As BAP1 mutations are strongly correlated with metastatic UM (34, 44), it is possible that this unique metabolic feature of BAP1 mutant UM cells allows them to survive better under stressful conditions and/or local tumor microenvironments. Indeed, an elevated total glycolytic activity was significantly linked to poor survival outcomes of UM patients (45). An interesting future study will be to determine how BAP1 regulates PDHK1-PDH phosphorylation under the stressful conditions, such as hypoxia compared to non-stressful conditions. A recent study in breast cancer cells showed that AMP-dependent kinase (AMPK) activates the PDH complex which promoted cell survival under glucose deprivation and in vivo lung metastasis (46). Our laboratory found elevated levels of active AMPK and its downstream effector, acetyl coA carboxylase (ACC), in BAP1 mutant UM (21). These findings suggest that the AMPK/PDH axis may represent a mechanism mediating UM metastasis in BAP1 mutant UM but this needs to be explored in the context of UM. It has been reported that upregulation of glycolysis supports several processes of cancer metastasis, including local invasion and colonization (47, 48). We found that higher expression of PDH complex and PDHK1 are related to a trend of worse survival in BAP1 mutant UM, even though it was not statistically significant. Lastly, UM metastases display remarkable hepatic tropism (49). PDHK1-PDH phosphorylation-driven glycolytic metabolism is required for liver metastasis of breast cancer (50). Therefore, studying the effects of BAP1 mutant-driven glycolytic profiles through PDHK1-PDH phosphorylation may broaden our understanding of metabolic roles of BAP1 in metastasis and hepatic tropism of UM.
In conclusion, this study provides evidence of the metabolic functions of BAP1 in UM and a major leading factor of increased glycolytic profiles of BAP1 mutant UM by integrating bioinformatics, single cell RNA seq, metabolomics, and molecular approaches. We showed that BAP1 mutant tumors have high phosphorylation of PDHK1-PDH, which subsequently induced glycolytic profiles in UM (Fig. 6b). Targeting phosphorylation of PDHK1-PDH resulted in cell cycle arrest, decreasing BAP1 mutant UM cell growth. Our findings suggest that BAP1 plays a critical role in UM cellular metabolism. Moreover, targeting BAP1 mutant-driven metabolic alterations might be a novel therapeutic avenue for BAP1 mutant UM.
Materials and Methods
TCGA data Analysis:
TCGA uveal melanoma RNA-seq V2 expression data were retrieved from the latest Broad GDAC Firehose data run (stddata__2016_01_28). BAP1 mutation and copy number data were collected using the RTCGA package (version 1.10.0). Samples with a copy number segment mean below −0.5 were classified as copy loss of BAP1. Samples were stratified into BAP1 wild type and mutant groups based on mutation of BAP1 and copy loss data. Analysis of differential expression was conducted between BAP1 wild type (n=40) and mutant (n=40) groups using the DESeq2 package (version 1.20.0) (51). Gene Set Enrichment Analysis (GSEA) (52, 53) was used to determine enriched pathways in the MSigDB Hallmark gene set collection (28). Patient clinical outcome data were collected from the TCGA Pan-Cancer Clinical Data Resource (TCGA-CDR) repository (54). A PDHK-PDC activity score was calculated for each sample using the average z-score value for PDH, DLAT, DLD and PDHK genes present in the pyruvate dehydrogenase complex gene ontology accession GO:0045254 (55). Multivariable survival analysis methods described in (54) were used to determine the association between disease-specific survival, PDHK-PDC score and BAP1 mutant status. In brief, the Cox proportional hazards regression model was used with assumptions validated by a test of Schoenfeld residuals. A p-value cutoff of < 0.05 was used to determine statistical significance. Data analyses were conducted in R (http://www.R-project.org/). For Kaplan-Meier plots, the log-rank test was used to determine differences between BAP1 status, samples stratified by the median expression, or by BAP1 status then median expression. Kaplan-Meier plots were generated to display the distribution of disease-specific survival outcomes for each log-rank test.
Single Cell RNA Analysis:
Uveal melanoma scRNA Seq data were obtained from the GEO database (Accession GSE139829). The Seurat package (v3.1.4) was used to analyze the data (56). Malignant cells were identified using the methods described previously (31). Raw count data for malignant cells were normalized using the SCTransform method (57) with regression based on the percent mitochondrial content. The pheatmap package (v1.0.12) was used to generate heatmaps. Data analyses were performed in R (http://www.R-project.org/).
Cell Culture and Cell Line Modification:
OMM1.3 and 92.1 cells were provided from Dr. Bruce Ksander (Schepens Eye Research Institute, Boston, MA) and Dr. Martine Jager (Leiden University, Leiden, Netherlands) laboratories, respectively. Culture conditions have been described previously (58, 59). MP46, MP65, MP38, MM28 and MM66 were provided from Dr. Sergio Roman Roman’s lab (Institute Curie, Paris, France) and cultured as reported previously (60). PDX4, UM001, UM004 and UM002B were derived from human UM metastases and established as cell lines at Thomas Jefferson University (58, 59). WM3618F was purchased from Rockland Immunochemicals (Limerick, PA) and cultured routinely in 1× MCDB media containing 10% heat-inactivated FBS, CaCl2, 50 IU penicillin and 50 μg/mL streptomycin. GNAQ,GNA11 or BAP1 mutations were confirmed by Sanger sequencing from Dr. Sergio Roman-Roman’s (60) and Dr. Takami Sato’s laboratory. The test for mycoplasma of all cell lines were regularly performed and authentication of cell lines was confirmed by STR analysis (February 2021).
Inhibitors.
Sodium dichloroacetate (DCA) was purchased from Acros Organics (Geel, Belgium). DCA was newly dissolved in PBS for every experiment.
siRNA Transfection:
Cells were transfected for 72 hours with 25 nM of siRNAs by using Lipofectamine RNAiMAX (Invitrogen, Carlsbad, CA). siRNA sequence (Dharmacon, Lafayette, CO): PDHK1 #1, GAUCAGAAACCGACACAAU PDHK1 #2, GCCAGAAUGUUCAGUACUU, BAP1 #1, GAGUUCAUCUGCACCUUUA BAP1 #3, GAUGAUACGUCCGUGAUUG and control, UGGUUUACAUGUCGACUAA
Flux Experiment:
XFe24 Analyzer (Agilent, Santa Clara, CA) was used to measure the ECAR. The detailed protocol is described in (20). Before performing analyses, the cells incubated with the Seahorse XF RPMI base media containing 2 mM glutamine for 1 hour in non-CO2 incubator at 37°C. The glycolysis stress test kit (#103020-100) was purchased from Agilent (Santa Clara, CA) and the concentration of injection compounds was glucose (10 mM), oligomycin (1 μM) and 2-DG (50 mM). Data were normalized to protein concentration and analyzed by Agilent Seahorse XF report generator.
Reverse Phase Protein Array (RPPA):
Cell lysates were prepared as described previously (61) and RPPA analysis was performed at the MD Anderson Functional proteomic core facility (Houston, TX). Selected protein expressions including PDHK1, PDH (E1), and DLAT were analyzed as explained (62). Relative protein levels were quantified using SuperCurve fitting and normalized for protein loading. Comparison of average normalized log2 values were performed by the two-sample t-test method and assumed unequal variance. Data points are shown as individual lysate collections **p<0.01.
Western Blot:
Cell lysates were prepared in Laemmli sample buffer containing β-ME, separated by SDS-PAGE and transferred to PVDF membranes. Membranes were blocked with 1% BSA and incubated with primary antibodies overnight at 4°C. Proteins were probed using HRP secondary antibodies and chemiluminescence substrate (Pierce, Rockford, IL) on a ChemiDoc Imaging System (Bio-Rad, Hercules, CA). Primary antibodies were used (1:1000): BAP1 (#13271) PDHK1 (#3820), and HSP90 (#4877) from Cell Signaling Technology (Danvers, MA); p-PDH (Ser293, ab92696), PDH (ab110330) and DLAT (ab66511) from Abcam (Cambridge, UK); p-PDHK1 (Tyr293, #11597) from Signalway Antibody (College Park, MD) and β-actin (#A2066, 1:3000) rom Sigma-Aldrich (St. Louis, MO).
Cell Viability Assay:
Cell viability was analyzed through Incucyte Live Cell Analysis Imaging System (Essen Bioscience, Ann Arbor, MI) or crystal violet staining assay. For IncuCyte imaging analysis, images of cells were taken every 2 hours. Percent of confluency was calculated using IncuCyte zoom software. For crystal violet staining, the cells were washed with PBS and stained with 0.2 % crystal violet in 10% buffered formalin. Dried-plates were scanned and 100X magnification images were taken using the Nikon Eclipse Ti-e microscope and NIS-Elements AR 3.00 software (Nikon, Japan).
3D Spheroid Assay:
For tumor 3D spheroids formation, 2.5 × 104 uveal melanoma cells/mL were plated on 1.5% agar bed (w/v) for 3 days. Then, 3D spheroids were transferred and embedded into collagen I solution. The next day, collagen-embedded 3D tumor spheroids were treated, as described above. Pictures were obtained using a Nikon A1R confocal microscope.
S Phase Entry Assay:
Cells cultured in 6-well plates and treated. Before 16 hours of treatment end, 10 μM EdU was added and then cells processed using the Click-iTTM Plus EdU Alexa FluorTM 594 flow cytometry assay kit (Life Technologies, Carlsbad, CA) based on company protocol.
Metabolites Screening:
Metabolomic analysis was conducted at the Proteomics and Metabolomics Facility at the Wistar Institute (Philadelphia, USA). LC-MS analysis was performed on a ThermoFisher Scientific Q Exactive HF-X mass spectrometer equipped with a HESI II probe and coupled to a ThermoFisher Scientific Vanquish Horizon UHPLC system as described previously (63), and more detail methods and processes to extract polar metabolites and analyses are described in (64). Comparison between cell types were based on evaluation of six biological replicates per cell line and data were normalized with cell numbers.
Statistical Analysis:
The results of cell viability assay was presented as mean and standard error of the mean (SEM) of at least three biological replicates (n=3). RPPA analysis was performed by using three biological replicates of each cell lines and analyses of metabolites were performed by using six biological replicates. Statistical significance was calculated using the unpaired t-test.
Supplementary Material
Acknowledgements
The Cancer Center Support Grant 5P30CA056036-17 to the SKCC supported the Microscopy Shared Resource facility. We thank Dr. Bruce Ksander (Schepens Eye Research Institute, Boston, MA), Dr. Martine Jager (Leiden University, Leiden, Netherlands) and Dr. Sergio Roman-Roman (Institute Curie, Paris, France) for cell lines. We are grateful to Dr. Michael Durante (University of Miami Miller School of Medicine, Miami, FL) for his help with single cell RNA Seq data and Dr. Hsin-Yao Tang (The Wistar Institute, Philadelphia, PA) for metabolomic analyses. This work was supported by National Institutes of Health (NIH)/National Cancer Institute (NCI) grants R01 CA196278, R01 CA253977 and P01 CA114046 to A.E.A. This work also was supported by a Melanoma Research Alliance team science award (#559058) to A.E.A and J.W.H. The Wistar Proteomics and Metabolomics Facility was supported by P30CA010815, P01CA140043 and S10OD023586. Further support was from American Association for Cancer Research (AACR)/Ocular Melanoma Foundation (OMF) awarded to A.H.
Footnotes
Conflict of interest: A.E. Aplin reports receiving a commercial research grant from Pfizer Inc. (2013–2017) and has ownership interest in patent number 9880150. J.W. Harbour is the inventor of intellectual property related to prognostic testing for uveal melanoma. He is a paid consultant for Castle Biosciences, licensee of this intellectual property, and he receives royalties from its commercialization. The other authors disclose no potential conflicts of interest.
References
- 1.Krantz BA, Dave N, Komatsubara KM, Marr BP, Carvajal RD. Uveal melanoma: epidemiology, etiology, and treatment of primary disease. Clin Ophthalmol. 2017;11:279–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Singh N, Bergman L, Seregard S, Singh AD. Uveal melanoma: Epidemiologic aspects. Ocul Oncol Pathol: Springer; 2014. p. 75–87. [Google Scholar]
- 3.Chua V, Aplin AE. Novel therapeutic strategies and targets in advanced uveal melanoma. Curr Opin Oncol. 2018;30(2):134–41. [DOI] [PubMed] [Google Scholar]
- 4.Heppt MV, Amaral T, Kähler KC, Heinzerling L, Hassel JC, Meissner M, et al. Combined immune checkpoint blockade for metastatic uveal melanoma: a retrospective, multi-center study. J Immunother Cancer. 2019;7(1):299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Carvajal RD, Piperno-Neumann S, Kapiteijn E, Chapman PB, Frank S, Joshua AM, et al. Selumetinib in combination with dacarbazine in patients with metastatic uveal melanoma: a phase III, multicentre, randomised trial (SUMIT). J Clin Oncol. 2018;36(12):1232–9. [DOI] [PubMed] [Google Scholar]
- 6.Chua V, Mattei J, Han A, Johnston L, LiPira K, Selig SM, et al. The Latest on Uveal Melanoma Research and Clinical Trials: Updates from the Cure Ocular Melanoma (CURE OM) Science Meeting (2019). Clin Cancer Res. 2021;27(1):28–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Han A, Schug ZT, Aplin AE. Metabolic Alterations and Therapeutic Opportunities in Rare Forms of Melanoma. Trends in cancer. 2021;7(8):671–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nanda CS, Venkateswaran SV, Patani N, Yuneva M. Defining a metabolic landscape of tumours: genome meets metabolism. Br J Cancer. 2019:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son J, Lyssiotis CA, Ying H, Wang X, Hua S, Ligorio M, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496(7443):101–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hutton JE, Wang X, Zimmerman LJ, Slebos RJ, Trenary IA, Young JD, et al. Oncogenic KRAS and BRAF drive metabolic reprogramming in colorectal cancer. Mol Cell Proteom. 2016;15(9):2924–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen C-Y, Chen J, He L, Stiles BL. PTEN: tumor suppressor and metabolic regulator. Front Endocrinol. 2018;9:338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harbour JW, Onken MD, Roberson ED, Duan S, Cao L, Worley LA, et al. Frequent mutation of BAP1 in metastasizing uveal melanomas. Science. 2010;330(6009):1410–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Field MG, Durante MA, Anbunathan H, Cai LZ, Decatur CL, Bowcock AM, et al. Punctuated evolution of canonical genomic aberrations in uveal melanoma. Nat Commun. 2018;9(1):116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carbone M, Harbour JW, Brugarolas J, Bononi A, Pagano I, Dey A, et al. Biological mechanisms and clinical significance of BAP1 mutations in human cancer. Cancer Discov. 2020;10(8):1103–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Robertson AG, Shih J, Yau C, Gibb EA, Oba J, Mungall KL, et al. Integrative analysis identifies four molecular and clinical subsets in uveal melanoma. Cancer cell. 2017;32(2):204–20. e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Han A, Purwin TJ, Aplin AE. Roles of the BAP1 Tumor Suppressor in Cell Metabolism. Cancer Res. 2021;81(11):2807–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bononi A, Yang H, Giorgi C, Patergnani S, Pellegrini L, Su M, et al. Germline BAP1 mutations induce a Warburg effect. Cell Death Differ. 2017;24(10):1694–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baughman JM, Rose CM, Kolumam G, Webster JD, Wilkerson EM, Merrill AE, et al. NeuCode proteomics reveals Bap1 regulation of metabolism. Cell Rep. 2016;16(2):583–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Y, Shi J, Liu X, Feng L, Gong Z, Koppula P, et al. BAP1 links metabolic regulation of ferroptosis to tumour suppression. Nat Cell Biol. 2018;20(10):1181–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Han A, Purwin TJ, Bechtel N, Liao C, Chua V, Seifert E, et al. BAP1 mutant uveal melanoma is stratified by metabolic phenotypes with distinct vulnerability to metabolic inhibitors. Oncogene. 2021;40(3):618–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chua V, Han A, Bechtel N, Purwin TJ, Hunter E, Liao C, et al. The AMP-dependent kinase pathway is upregulated in BAP1 mutant uveal melanoma. Pigment Cell Melanoma Res. 2021. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen GK, Mellnick VM, Yim AK-Y, Salter A, Ippolito JE. Synergy of sex differences in visceral fat measured with CT and tumor metabolism helps predict overall survival in patients with renal cell carcinoma. Radiology. 2018;287(3):884–92. [DOI] [PubMed] [Google Scholar]
- 23.Chattopadhyay C, Oba J, Roszik J, Marszalek JR, Chen K, Qi Y, et al. Elevated Endogenous SDHA Drives Pathological Metabolism in Highly Metastatic Uveal Melanoma. Investig Ophthalmol Vis Sci. 2019;60(13):4187–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Saunier E, Benelli C, Bortoli S. The pyruvate dehydrogenase complex in cancer: An old metabolic gatekeeper regulated by new pathways and pharmacological agents. Int J Cancer. 2016;138(4):809–17. [DOI] [PubMed] [Google Scholar]
- 25.Zhang W, Zhang S-L, Hu X, Tam KY. Targeting tumor metabolism for cancer treatment: is pyruvate dehydrogenase kinases (PDKs) a viable anticancer target? Int J Biol Sci. 2015;11(12):1390–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li X, Jiang Y, Meisenhelder J, Yang W, Hawke DH, Zheng Y, et al. Mitochondria-translocated PGK1 functions as a protein kinase to coordinate glycolysis and the TCA cycle in tumorigenesis. Mol Cell. 2016;61(5):705–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stacpoole PW. Therapeutic targeting of the pyruvate dehydrogenase complex/pyruvate dehydrogenase kinase (PDC/PDK) axis in cancer. J Natl Cancer Inst. 2017;109(11). [DOI] [PubMed] [Google Scholar]
- 28.Liberzon A, Birger C, Thorvaldsdóttir H, Ghandi M, Mesirov JP, Tamayo P. The molecular signatures database hallmark gene set collection. Cell Syst. 2015;1(6):417–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang S, Hulver MW, McMillan RP, Cline MA, Gilbert ER. The pivotal role of pyruvate dehydrogenase kinases in metabolic flexibility. Ann Nutr Metab. 2014;11(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zachar Z, Marecek J, Maturo C, Gupta S, Stuart SD, Howell K, et al. Non-redox-active lipoate derivates disrupt cancer cell mitochondrial metabolism and are potent anticancer agents in vivo. J Mol Med. 2011;89(11):1137–48. [DOI] [PubMed] [Google Scholar]
- 31.Durante MA, Rodriguez DA, Kurtenbach S, Kuznetsov JN, Sanchez MI, Decatur CL, et al. Single-cell analysis reveals new evolutionary complexity in uveal melanoma. Nat Commun. 2020;11(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johansson PA, Brooks K, Newell F, Palmer JM, Wilmott JS, Pritchard AL, et al. Whole genome landscapes of uveal melanoma show an ultraviolet radiation signature in iris tumours. Nat Commun. 2020;11(1):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kalirai H, Dodson A, Faqir S, Damato B, Coupland S. Lack of BAP1 protein expression in uveal melanoma is associated with increased metastatic risk and has utility in routine prognostic testing. Br J Cancer. 2014;111(7):1373–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Karlsson J, Nilsson LM, Mitra S, Alsén S, Shelke GV, Sah VR, et al. Molecular profiling of driver events in metastatic uveal melanoma. Nat Commun. 2020;11(1):1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Di Nunno V, Frega G, Santoni M, Gatto L, Fiorentino M, Montironi R, et al. BAP1 in solid tumors. Future Oncol. 2019;15(18):2151–62. [DOI] [PubMed] [Google Scholar]
- 36.Wang A, Papneja A, Hyrcza M, Al-Habeeb A, Ghazarian D. Gene of the month: BAP1. J Clin Pathol. 2016;69(9):750–3. [DOI] [PubMed] [Google Scholar]
- 37.Hebert L, Bellanger D, Guillas C, Campagne A, Dingli F, Loew D, et al. Modulating BAP1 expression affects ROS homeostasis, cell motility and mitochondrial function. Oncotarget. 2017;8(42):72513–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie H, Ju H, Fan J, Shi X, Cheng Y, Cang X, et al. O-GlcNAcylation of PGK1 coordinates glycolysis and TCA cycle to promote tumor growth. Nat Commun. 2020;11(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Forma E, Jóźwiak P, Bryś M, Krześlak A. The potential role of O-GlcNAc modification in cancer epigenetics. Cell Mol Biol Lett. 2014;19(3):438–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ruan H-B, Han X, Li M-D, Singh JP, Qian K, Azarhoush S, et al. O-GlcNAc transferase/host cell factor C1 complex regulates gluconeogenesis by modulating PGC-1α stability. Cell Metab. 2012;16(2):226–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kennedy BE, Murphy JP, Clements DR, Konda P, Holay N, Kim Y, et al. Inhibition of pyruvate dehydrogenase kinase enhances the antitumor efficacy of oncolytic reovirus. Cancer Res. 2019;79(15):3824–36. [DOI] [PubMed] [Google Scholar]
- 42.DeBerardinis RJ, Chandel NS. Fundamentals of cancer metabolism. Sci Adv. 2016;2(5):e1600200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kreuzaler P, Panina Y, Segal J, Yuneva M. Adapt and conquer: Metabolic flexibility in cancer growth, invasion and evasion. Mol Metab. 2020;33:83–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shain AH, Bagger MM, Yu R, Chang D, Liu S, Vemula S, et al. The genetic evolution of metastatic uveal melanoma. Nat Genet. 2019;51(7):1123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eldredge-Hindy H, Ohri N, Anne PR, Eschelman D, Gonsalves C, Intenzo C, et al. Yttrium-90 microsphere brachytherapy for liver metastases from uveal melanoma: clinical outcomes and the predictive value of fluorodeoxyglucose positron emission tomography. J Clin Oncol. 2016;39(2):189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cai Z, Li CF, Han F, Liu C, Zhang A, Hsu CC, et al. Phosphorylation of PDHA by AMPK Drives TCA Cycle to Promote Cancer Metastasis. Mol Cell. 2020;80(2):263–78 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei Q, Qian Y, Yu J, Wong CC. Metabolic rewiring in the promotion of cancer metastasis: mechanisms and therapeutic implications. Oncogene. 2020;39(39):6139–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pascual G, Domínguez D, Benitah SA. The contributions of cancer cell metabolism to metastasis. Dis Model Mech. 2018;11(8):dmm032920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Meir T, Dror R, Yu X, Qian J, Simon I, Pe’er J, et al. Molecular characteristics of liver metastases from uveal melanoma. Investig Ophthalmol Vis Sci. 2007;48(11):4890–6. [DOI] [PubMed] [Google Scholar]
- 50.Dupuy F, Tabariès S, Andrzejewski S, Dong Z, Blagih J, Annis MG, et al. PDK1-dependent metabolic reprogramming dictates metastatic potential in breast cancer. Cell Metab. 2015;22(4):577–89. [DOI] [PubMed] [Google Scholar]
- 51.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15(12):550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mootha VK, Lindgren CM, Eriksson K-F, Subramanian A, Sihag S, Lehar J, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34(3):267–73. [DOI] [PubMed] [Google Scholar]
- 53.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. PNAS. 2005;102(43):15545–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J, Lichtenberg T, Hoadley KA, Poisson LM, Lazar AJ, Cherniack AD, et al. An Integrated TCGA Pan-Cancer Clinical Data Resource to Drive High-Quality Survival Outcome Analytics. Cell. 2018;173(2):400–16 e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Attrill H, Gaudet P, Huntley RP, Lovering RC, Engel SR, Poux S, et al. Annotation of gene product function from high-throughput studies using the Gene Ontology. Database (Oxford). 2019;2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Butler A, Hoffman P, Smibert P, Papalexi E, Satija R. Integrating single-cell transcriptomic data across different conditions, technologies, and species. Nat Biotechnol. 2018;36(5):411–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Stuart T, Butler A, Hoffman P, Hafemeister C, Papalexi E, Mauck WM III, et al. Comprehensive integration of single-cell data. Cell. 2019;177(7):1888–902. e21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Chua V, Orloff M, Teh JL, Sugase T, Liao C, Purwin TJ, et al. Stromal fibroblast growth factor 2 reduces the efficacy of bromodomain inhibitors in uveal melanoma. EMBO Mol Med. 2019;11(2):e9081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lapadula D, Farias E, Randolph CE, Purwin TJ, McGrath D, Charpentier TH, et al. Effects of Oncogenic Galphaq and Galpha11 Inhibition by FR900359 in Uveal Melanoma. Mol Cancer Res. 2019;17(4):963–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Amirouchene-Angelozzi N, Nemati F, Gentien D, Nicolas A, Dumont A, Carita G, et al. Establishment of novel cell lines recapitulating the genetic landscape of uveal melanoma and preclinical validation of mTOR as a therapeutic target. Mol Oncol. 2014;8(8):1508–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tibes R, Qiu Y, Lu Y, Hennessy B, Andreeff M, Mills GB, et al. Reverse phase protein array: validation of a novel proteomic technology and utility for analysis of primary leukemia specimens and hematopoietic stem cells. Mol Cancer Ther. 2006;5(10):2512–21. [DOI] [PubMed] [Google Scholar]
- 62.Cheng H, Chua V, Liao C, Purwin TJ, Terai M, Kageyama K, et al. Co-targeting HGF/cMET Signaling with MEK Inhibitors in Metastatic Uveal Melanoma. Mol Cancer Ther. 2017;16(3):516–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Agarwal E, Altman BJ, Seo JH, Ghosh JC, Kossenkov AV, Tang H-Y, et al. Myc-mediated transcriptional regulation of the mitochondrial chaperone TRAP1 controls primary and metastatic tumor growth. J Biol Chem. 2019;294(27):10407–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li J, Agarwal E, Bertolini I, Seo JH, Caino MC, Ghosh JC, et al. The mitophagy effector FUNDC1 controls mitochondrial reprogramming and cellular plasticity in cancer cells. Sci Signal. 2020;13(642):eaaz8240. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.