Abstract
Cytotoxic lesions of the corpus callosum (CLOCCs) are a clinical-radiological spectrum of disorders secondary to several etiopathogeneses. Cytotoxic lesions of the corpus callosum are typically associated with mild clinical symptoms including fever, headache, confusion, and altered mental status. We present a case of a 51-year-old Caucasian woman who developed a reversible lesion of the splenium of the corpus callosum associated with small round-shaped white matter hyperintensities after the first dose of SARS-CoV-2 mRNA vaccine. Magnetic resonance imaging is fundamental for diagnosis and no treatment is generally required.
Keywords: Cytotoxic lesions of the corpus callosum, SARS-CoV-2, COVID-19 vaccines, magnetic resonance imaging, white matter diseases, demyelinating disorders
Introduction
Cytotoxic lesions of the corpus callosum (CLOCCs) are a clinical-radiological spectrum of disorders that can be caused by several conditions. They were formerly known as “mild encephalopathy with reversible splenial lesions,” “transient splenial lesions,” “reversible splenial lesion syndrome,” “transient focal lesions in the splenium of the corpus callosum,” “reversible splenial lesions,” and “clinically silent lesions in the splenium of the corpus callosum.” The pathophysiological mechanism of CLOCCs is still debated, but there is agreement about the role of cytotoxic edema in determining callosal lesions.1-2 Clinical symptoms are very variable and depend on the underlying condition. Magnetic resonance imaging (MRI) is essential for diagnosis and CLOCCs generally appear as a single T2-hyperintense lesion located in the splenium of the corpus callosum. Restricted diffusion and no contrast enhancement are typically associated with the finding. Cytotoxic lesions of the corpus callosum have been recently associated with coronavirus disease 2019 (COVID-19) and SARS-CoV-2 mRNA vaccine (BNT162b)3-4 and may share clinical and radiological features with acute disseminated encephalomyelitis (ADEM). 5
Case
A 51-year-old Caucasian woman with an unremarkable past recent clinical history was admitted to our emergency department with headache, malaise, and an altered mental status 5 days after she received a first dose of SARS-CoV-2 mRNA vaccine (Pfizer-BioNTech - BNT162b2 - Pfizer, Inc; Philadelphia, Pennsylvania). She also complained a 3-day history of fever, weakness, headache, and palpitations. Upon clinical examination, drowsiness, confusion, and a mild cognitive impairment were noticed. Detailed neurological examination showed gaze fixation, distal upper limb tremor, and normal language comprehension with a slight deficit in speech production, anomia, and circumlocutions. Her motor functions and deep tendon reflexes were normal. Cranial nerves were intact, and no signs of nuchal rigidity and intracranial hypertension were revealed. Her body temperature was 37.6°C, with a blood pressure of 130/80 mmHg, a pulse rate of 95 beats/min, and oxygen saturation (SpO2) of 99%. Blood tests showed neutrophilic leukocytosis (white blood cells: 10.8 103uL, with 70% of neutrophils), high levels of C-reactive protein (CRP) (5.2 mg/L), D-dimer (0.57 mg/L Fibrinogen Equivalent Units), glycemia (138 mg/dL), and LDH (241 U/L). SARS-CoV-2 real-time reverse transcriptase polymerase chain reaction (RT-PCR) analysis of nasopharyngeal swab gave a negative result. Brain computed tomography (CT) on admission (day 1) was normal. On day 2, the patient was alert, her body temperature was normal, with a blood pressure of 160/110 mmHg, a pulse rate of 115 beats/min, and oxygen saturation (SpO2) of 98%. A lumbar puncture performed on day 2 yielded clear and colorless cerebrospinal fluid (CSF), characterized by high pH (8.0), pleocytosis (lymphocytes: 95%), high levels of proteins (77.7 mg/dL) and IgG antibodies (51.3 mg/L), normal levels of glucose, low lactates (22.8 mg/dL), and negative oligoclonal bands. Electroencephalogram (EEG) showed epileptiform abnormalities in the frontal regions. Empiric therapy with levetiracetam (2000 mg/day i.v.) and acyclovir (750 mg/day i.v.) was initiated, and a brain magnetic resonance (MRI) scan was requested. The first MRI was performed on day 4 from admission on a 3T scanner (Ingenia; Philips Medical System, Best, the Netherlands). Magnetic resonance imaging revealed a single oval-shaped lesion located in the splenium of the corpus callosum, characterized by hyperintense signal on long repetition time (TR) sequences (T2-weighted and fluid-attenuated inversion recovery (FLAIR) images), restricted diffusivity on diffusion weighted images (DWI), and absent contrast enhancement (Figure 1). No signs of hemorrhage were detected. The other MRI findings were unremarkable. After a week, the patient clinically improved and was discharged. The patient underwent two MRI scans as follow-up. The first follow-up MRI, performed seventeen (17) days after the first MRI, showed a complete resolution of the lesion in the splenium of the corpus callosum (Figure 2). As an additional finding, several small round-shaped white matter hyperintensities on T2-weighted and FLAIR sequences were noted in the white matter of bilateral centrum semiovale and corona radiata. The lesions did not show restricted diffusion or contrast enhancement (Figure 3). At the second follow-up MRI scan, performed sixty-seven (67) days after the first MRI, brain findings remained unchanged. Magnetic resonance imaging scan of the spinal cord was also performed at the second follow-up, which revealed unremarkable findings (Figure 4). The patient is ongoingly kept under surveillance through clinical follow-up examinations. Her health status remains satisfactory, with no new signs or symptoms having been noticed after her recovery.
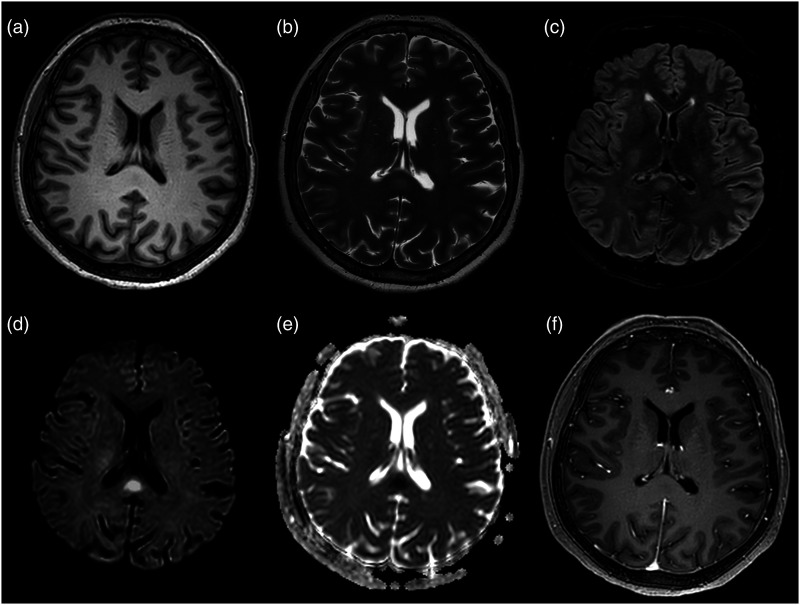
Figure 1.
First axial brain MRI on the 4th day of admission, 8 days after SARS-CoV-2 mRNA vaccine (BNT162b2). (a) T1-weighted images show a mild hypointense oval-shaped lesion in the splenium of the corpus callosum. (b, c) The lesion presents slightly hyperintense signal on T2-weighted and FLAIR images. (d, e) Hyperintense signal on b-2000 diffusion-weighted images and low apparent diffusion coefficient values are noted, consistent with restricted diffusion within the lesion. (f) Contrast-enhanced T1-weighted sequences reveal no contrast enhancement.
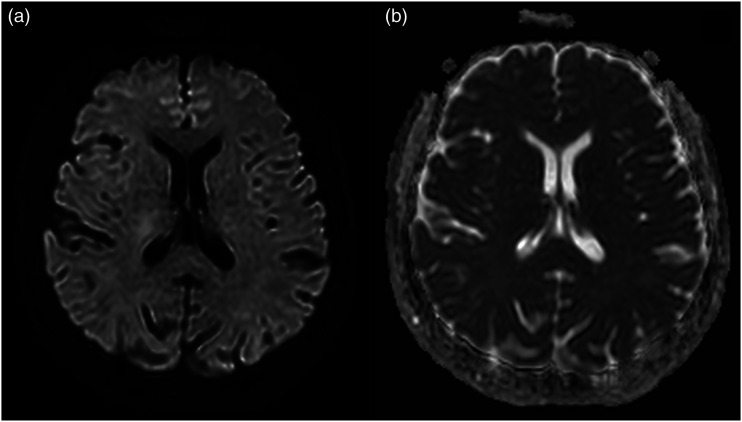
Figure 2.
Same patient as in Figure 1. Second follow-up brain MRI performed 17 days after the first MRI. (a, b) Axial b-2000 diffusion-weighted images and axial apparent diffusion coefficient demonstrate the complete disappearance of the lesion in the splenium of the corpus callosum.
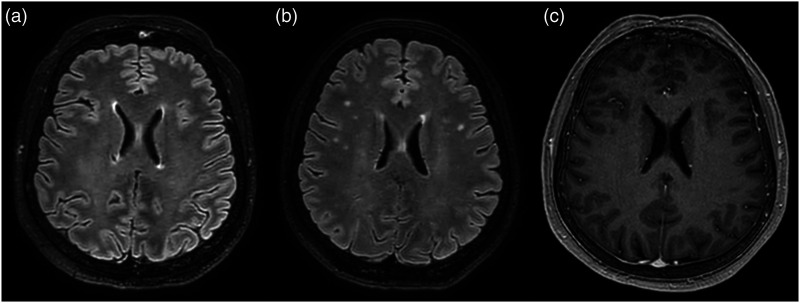
Figure 3.
(a, b) Axial fluid-attenuated inversion recovery images of the first and second MRI respectively, evidence the appearance of several small round-shaped white matter hyperintensities in the centra semiovalia, corona radiata of both cerebral hemispheres. (c) Axial contrast-enhanced T1-weighted sequence of the second MRI shows no contrast enhancement of these small white matter lesions.
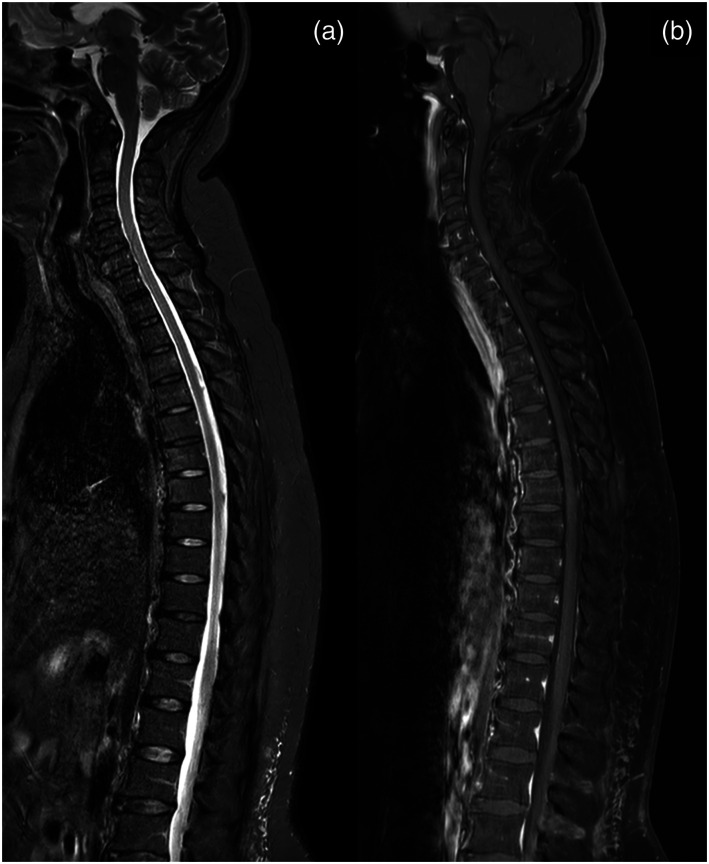
Figure 4.
Third follow-up MRI performed 67 days after the first MRI. (a, b) Sagittal T2-short tau inversion recovery and contrast-enhanced T1-weighted sequences confirm the absence of spinal cord lesions or pathological contrast enhancement of the spinal cord.
Discussion
In consideration of the onset of the symptoms after the vaccination and the neuroradiological evolution during follow-up MRIs, all differential diagnoses (ischemic lesion, diffuse axonal injury, malignancy, posterior reversible encephalopathy syndrome, and toxic and hypoglycemic encephalopathy) 6 except CLOCCs were excluded. Pathophysiologically, different entities (inflammations, drugs, trauma, and infections) may release inflammatory cytokines like interleukin 1 (IL-1), IL-6, and tumor necrosis factor-alpha (TNF‐α). IL-1 stimulates astrocytes to release glutamate and increase the concentration of extracellular glutamate. Excitotoxicity triggered by glutamate receptor activation leads to an influx of water into both neurons and astrocytes, along with the cytokine storm and the oxidative stress, resulting in intracellular swelling, namely cytotoxic edema. The corpus callosum and, particularly, the splenium, is highly involved during cytokinopathies, due to the abundant quantity of cytokine and glutamate receptors, more than in any other site of the brain. 1 Cytotoxic lesions of the corpus callosum are caused by a long list of different factors that include antiepileptic drugs, withdrawal of antiseizure drugs, epilepsy, migraine with aura, trauma, viruses (Epstein-Barr virus, rotavirus, influenza, parainfluenza, measles, mumps, cytomegalovirus, varicella-zoster, human herpesvirus‐6, human herpesvirus‐7, adenovirus, human parvovirus B19, rubella, human immunodeficiency virus, and enterovirus), bacteria (Salmonella enteritidis, Staphylococcus aureus, Legionella pneumophila, Escherichia coli, Klebsiella pneumoniae, Mycoplasma pneumoniae, Streptococcus pneumoniae, Campylobacter jejuni, and Enterococcus faecalis), malaria parasite, dengue fever, Kawasaki disease, renal failure, hemolytic uremic syndrome, autoimmune diseases, radiation therapy, metabolic diseases, mumps vaccine, malignancy, subarachnoid hemorrhage, high-altitude cerebral edema, and several pharmacological and toxic substances.1-2,7 It is important to notice that CLOCCs have been recently associated with COVID-19 and SARS-CoV-2 mRNA vaccine (BNT162b).3-4 To our knowledge, this is the second case of CLOCCs after SARS-CoV-2 vaccination described in the literature. Fever is usually a prodromic manifestation and is accompanied by headache. Seizures, diarrhea, confusion, and drowsiness may be also present. Rarely, in severe forms of CLOCCs, delirium, altered mental status, ataxia, slurred speech, nuchal rigidity, tremor, visual loss, and coma may occur. In our case, clinical manifestation was quite similar, with the clinical picture dominated by headache, fever, and confusion. In this case, initial symptoms have started 2 days after SARS-CoV-2 mRNA vaccine, a finding consistent with the data reported by Youn et al., who reported a case of cytotoxic lesions of the corpus callosum 3 days after BNT162b vaccine. 3 As regards CLOCCs correlated to other vaccinations, only mumps vaccination has been previously associated with CLOCCs and some authors have reported that the onset of symptoms ranged from 13 to 21 days after mumps vaccination, likely to the incubation period of mumps infection.8-9 Typical MRI characteristics demonstrate a transient and reversible hyperintense lesion on long TR sequences in the corpus callosum, associated with restricted diffusion on diffusion-weighted images (DWIs). The finding appears hypointense on T1-weighted sequences and does not show contrast enhancement. Full recovery and disappearance of CLOCCs typically occur within 1 month, mainly within 1 week. 10 Three different MRI patterns of CLOCCs have been described: (a) small oval-shaped lesion confined to the splenium of the corpus callosum, which is the most common site, (b) a more irregular lesion that from the splenium reaches the adjacent white matter through the callosal fibers (boomerang sign), and (c) posterior lesion extending into the anterior part of the corpus callosum. 1 In our case, the first MRI pattern is encountered. Prognosis is reported as variable because it depends on various possible underlying causes but is mostly good with full recovery. In this report, prognosis was deemed excellent with clinical restitutio ad integrum on follow-up. White matter hyperintensities in the centra semiovalia, corona radiata of both hemispheres, along with the pericallosal distribution were evidenced in the second MRI. This finding may be consistent with the disruption of the blood–brain barrier and the starting point of a demyelinating injury triggered by the vaccine. Both CLOCCs and ADEM share clinical and radiological features. 5 Sáenz-Farret et al. suggested that ADEM and CLOCCs characteristics overlap, and they may belong to a wide spectrum of the same disease. In fact, in his study, he observed that one patient filled diagnostic criteria both for ADEM and CLOCCs. 5 Takanashi et al. 11 identified three patients that presented a transient lesion of the splenium of the corpus callosum along with symmetrical lesions in the parietal or frontoparietal peripheral white matter, like our case. What differs from our paper is that white matter lesions disappeared along with the splenial lesion within a week. In ADEM, white matter lesions may be permanent. This may suggest that ADEM and CLOCCs may share a common pathophysiological trigger mechanism. The correlation between SARS-CoV-2 vaccine and demyelinating disease patterns (ADEM) has been previously described.12-15 Vogrig et al. reported a case of possible ADEM-like disorder that occurred after the administration of the first dose of SARS-CoV-2 mRNA vaccine (Pfizer-BioNTech - BNT162b2). Cao L et al. and Ozgen Kenangil et al. evidenced the onset of acute disseminated encephalomyelitis from the first dose of inactivated SARS-CoV-2 vaccine (Vero Cells, Beijing Institute of Biological Products Co., Ltd., Beijing, China) ranging from 2 weeks to 1 month. In all these cases, patient clinical outcome was favorable. Permezel et al., instead, described a fatal case of ADEM occurred after the first dose of Oxford–AstraZeneca vaccine ChAdOx1 nCoV-19 (AZD1222). No treatment is generally required for CLOCCs. Some authors have described the use of methylprednisolone and intravenous immunoglobulin to stop the cytokine storm even if no clinical benefit has been demonstrated, with full recovery of patients regardless of the therapy. 9
Conclusions
CLOCCs are rare conditions that may be caused by different disorders and may occur after SARS-CoV-2 vaccine, and in particular, mRNA vaccine, and may present with fever, confusion, and severe headache. Further studies are required to establish the real incidence of the finding, the possible correlation with ADEM-like lesions and the long-term outcome of the affected patients.
Acknowledgments
We gratefully thank Gabriella Lucidi Pressanti and Nevia Caputo for valuable discussions and for their expert advice.
Footnotes
The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Ioan P. Voicu, MD at the time of the writing of this article held a beneficial long position in BioNTech SE (BNTX) stock, either through stock ownership, options, or other derivatives.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Patient informed consent: Patient give her consent for the publication of identifiable details, which include images, case history and details within the text.
ORCID iD
Luca Procaccini https://orcid.org/0000-0001-8812-5470
References
- 1.Starkey J, Kobayashi N, Numaguchi Y, et al. Cytotoxic lesions of the corpus callosum that show restricted diffusion: mechanisms, causes, and manifestations. Radiographics 2017; 37(2): 562–576. [DOI] [PubMed] [Google Scholar]
- 2.Tetsuka S. Reversible lesion in the splenium of the corpus callosum. Brain Behavior 2019; 9(11): e01440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Youn T, Yang H. Cytotoxic lesion of the corpus callosum (CLOCCs) after SARS-CoV-2 mRNA vaccination. J Korean Med Sci 2021; 36(31): e228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gursoy G, Uzunalioglu BM, Tunc CE, et al. COVID-19 associated transient cytotoxic lesion of the corpus callosum: report of two cases and current literature review. Med Bull Haseki 2021; 59: 50–53. [Google Scholar]
- 5.Sáenz-Farret M, Cansino-Torres MA, Sandoval-Rodríguez V, et al. The spectrum of acute disseminated encephalomyelitis and mild encephalopathy with reversible splenial lesion. Case Rep Neurol Med 2019; 2019: 9272074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Park SE, Choi DS, Shin HS, et al. Splenial lesions of the corpus callosum: disease spectrum and MRI findings. Korean J Radiol 2017; 18(4): 710–721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahu A, Sankhe S, Mittal K, et al. A pictorial review on reversible splenial lesions. Indian JRadiol Imaging 2021; 31(1): 3–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hara M, Mizuochi T, Kawano G, et al. A case of clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. Brain Develop 2011; 33(10): 842–844. [DOI] [PubMed] [Google Scholar]
- 9.Takanashi J, Shiihara T, Hasegawa T, et al. Clinically mild encephalitis with a reversible splenial lesion (MERS) after mumps vaccination. J Neurol Sci 2015; 349(1–2): 226–228. [DOI] [PubMed] [Google Scholar]
- 10.Tada H, Takanashi J, Barkovich AJ, et al. Clinically mild encephalitis/encephalopathy with a reversible splenial lesion. Neurology 2004; 63(10): 1854–1858. [DOI] [PubMed] [Google Scholar]
- 11.Takanashi J, Barkovich AJ, Shiihara T, et al. Widening spectrum of a reversible splenial lesion with transiently reduced diffusion. AJNR Am J Neuroradiol 2006; 27(4): 836–838. [PMC free article] [PubMed] [Google Scholar]
- 12.Cao L, Ren L. Acute disseminated encephalomyelitis after severe acute respiratory syndrome coronavirus 2 vaccination: a case report. Acta Neurol Belg 2021: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ozgen Kenangil G, Ari BC, Guler C, et al. Acute disseminated encephalomyelitis-like presentation after an inactivated coronavirus vaccine. Acta Neurol Belgica 2021; 121(4): 1089–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogrig A, Janes F, Gigli GL, et al. Acute disseminated encephalomyelitis after SARS-CoV-2 vaccination. Clin Neurol Neurosurg 2021; 208: 106839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Permezel F, Borojevic B, Lau S, et al. Acute disseminated encephalomyelitis (ADEM) following recent Oxford/AstraZeneca COVID-19 vaccination. Forensic Sci Med Pathol 2021: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]