Abstract
Study Design
A retrospective case control study.
Purpose
This study aimed to assess the clinical significance of sagittal balance for predicting and managing the recollapse of cemented vertebra following percutaneous vertebroplasty (PVP) in patients with thoracolumbar osteoporotic vertebral fracture (OVF).
Overview of Literature
Recently, the recollapse of cemented vertebra following PVP for OVF has been reported. Although the risk factors for recollapse have been determined, the association between sagittal spinopelvic parameters and sagittal imbalance with recollapse has not been established.
Methods
Ambulatory patients who underwent single-level PVP for thoracolumbar OVF with a follow-up of at least 24 months were retrospectively reviewed. The patients were divided into two groups depending on the presence of symptomatic recollapse at the cemented vertebra: (1) recollapsed (RC) group and (2) noncollapsed (NC) group. The patient characteristics and radiographic measurements associated with sagittal imbalance were analyzed at each follow-up visit.
Results
Overall, 134 patients (RC group, n=28; NC group, n=106) were enrolled. The mean fracture-free interval was 3.2 months (range, 1.2–25.1 months). The multivariate binary logistic regression analysis identified low bone mineral density (p=0.047), degree of dynamic mobility within the vertebra (p=0.025), and sagittal imbalance as significant risk factors for recollapse (p=0.013; odds ratio, 5.405). The progression of sagittal imbalance and thoracolumbar kyphosis (T10–L2) was more significant in the RC and sagittal imbalance groups than in the NC group (both p=0.000).
Conclusions
Sagittal imbalance, lower bone mineral density, and dynamic mobility within the vertebra are associated with the recollapse of cemented vertebrae following PVP. Sagittal imbalance, rather than local kyphosis or thoracolumbar kyphosis, is particularly significant in that it results in more progressive collapse and sagittal deformity and is accompanied by substantial back pain and neurological deficits. Therefore, a stricter and more active management, including anti-osteoporosis medication, is required for the treatment of OVF with sagittal imbalance of the spine.
Keywords: Osteoporosis, Compression fractures, Sagittal imbalance, Thoracolumbar kyphosis, Vertebroplasty
Introduction
Osteoporotic vertebral compression fracture (OVF) is one of the most common complications of osteoporosis and has become more important as populations continue to age worldwide [1]. OVF results in substantial back pain and disability despite the use of analgesic medications. Moreover, it may lead to prolonged immobilization, which is associated with increased mortality in the elderly [2].
Percutaneous vertebroplasty (PVP) with polymethyl methacrylate is widely used to relieve back pain and reduce the need for prolonged immobilization in patients with symptomatic OVF [3–5]. Nevertheless, the recollapse of cemented vertebrae or cement loosening seldom occurs and is accompanied by the recurrence of back pain following cement augmentation [6–9]. Previous studies have identified the risk factors for the recollapse of the vertebra, namely, low bone mineral density (BMD), older age, intradiscal cement leakage, presence of multiple preexisting vertebral fractures, intravertebral vacuum cleft (IVC) sign, and fracture at the thoracolumbar junction [6,7,10–17].
The recollapse of cemented vertebrae should be carefully evaluated as it is often accompanied by an adjacent new vertebral fracture or instability, which potentially leads to further progression of kyphosis with sagittal imbalance (SI), especially at the thoracolumbar junction [17]. Several studies have indicated an association between an imbalanced spine and an increased kyphotic angle and further collapse of the affected vertebrae [10,18,19]. Moreover, when the kyphosis progresses with an accompanying neurological deficit or SI, revision surgery that involves anterior–posterior fusion should be performed [20,21] (Fig. 1). For these reasons, the prediction of further progression of thoracolumbar kyphosis associated with SI facilitates the selection of appropriate treatment. Although some studies have demonstrated the recollapse of cemented vertebrae, the associations of sagittal parameters with recollapse following PVP in patients with thoracolumbar OVF have not been clearly elucidated.
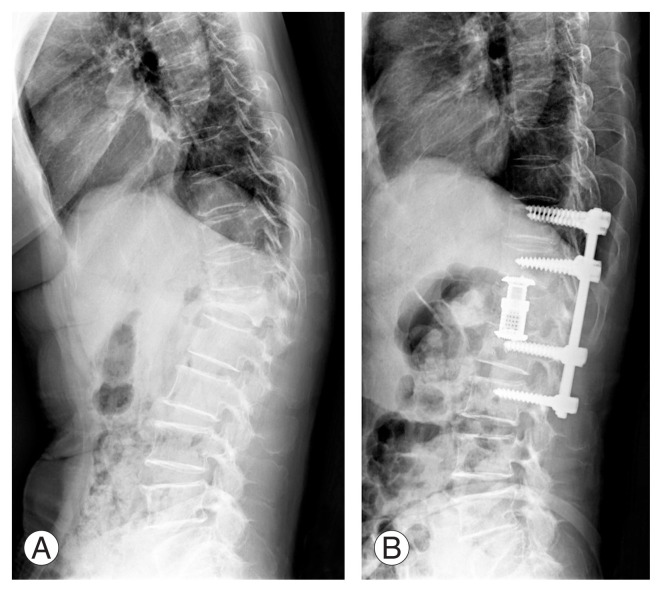
Fig. 1.
Spine lateral standing radiographs for a 68-year-old woman with kyphotic deformity at the thoracolumbar junction and severe back pain (scored 8 on a Visual Analog Scale) associated with an osteoporotic compression fracture at L1. Anterior interbody fusion using an expandable cage after corpectomy of the L1 vertebra and posterior instrumentation with fusion using an autologous bone graft were performed. (A) Preoperative radiograph. (B) A postoperative radiograph obtained at 3 months after surgery.
In this study, we evaluated the risk factors for recollapse following PVP in patients with thoracolumbar OVF and its association with sagittal spinal parameters. We hypothesized that a spine with SI and thoracolumbar kyphosis would be at a higher risk of recollapse following PVP.
Materials and Methods
This study was approved by our Institutional Review Board. Data from all patients treated during the study period was available for review and analysis. All the study participants provided informed consent prior to data collection.
In this study, we retrospectively reviewed patients who underwent single-level PVP for OVF from January 2010 to October 2017. The study inclusion criteria are as follows: (1) ambulatory with senile or postmenopausal osteoporosis; (2) recent diagnosis of OVF; (3) a BMD T-score below −2.5; (4) follow-up of more than 24 months; and (5) fracture at the thoracolumbar junction. Moreover, the exclusion criteria are as follows: (1) a neurological deficit associated with vertebral fracture, (2) another pathologic condition as a cause of osteoporosis, (3) a history of spine surgery, or (4) a history of high-energy trauma, including falls from height and motor vehicle collisions.
The lumbar spine BMD values were measured in the lateral side of the lumbar body from L2 to L4 with the use of dual X-ray absorptiometry (Lunar Prodigy Advance; GE Healthcare, Madison, WI, USA), and the corresponding T-score was calculated.
In the present study, the “recollapse” of a cemented vertebra was classified as follows: (1) new-onset back pain (a Visual Analog Scale [VAS] score of >5 on a 0–10-point scale); (2) resolution of back pain after the initial PVP for at least 4 weeks; (3) a reduction of more than 10% in cemented vertebral height based on the whole-spine lateral standing radiographs or computed tomography scans compared with that documented immediately following the initial PVP; and (4) a change in the signal intensity of the vertebral body based on magnetic resonance imaging, which was performed on those who were suggestive of recollapse (Fig. 2).
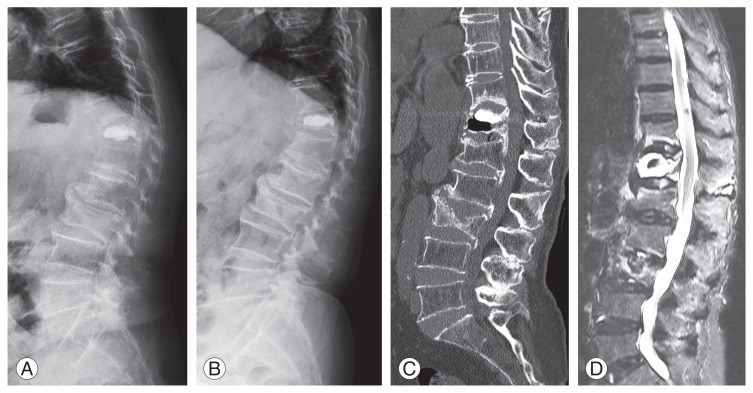
Fig. 2.
Recollapse of a vertebra after percutaneous vertebroplasty in a 73-year-old woman with the intravertebral vacuum cleft sign and instability. Local kyphosis and collapse of the vertebral body are more prominent on a standing lateral radiograph than on a supine radiograph. (A) Lateral standing radiograph. (B) Lateral supine radiograph. (C) A sagittal computed tomography scan shows the intravertebral vacuum cleft sign and recollapse of the vertebral body. (D) A fat-suppressed T2-weighted magnetic resonance image shows high signal intensity around the augmented cement in the vertebral body.
The patients were divided into two groups: (1) recollapsed group (RC group) and (2) noncollapsed group (NC group). Any new fractures of the vertebrae adjacent to the cemented vertebra were identified.
PVP was initially indicated for patients with painful OVF who were aged ≥80 years or had the IVC sign. Conservative treatment was indicated for patients younger than 80 years and did not have the IVC sign. PVP was performed if persistent pain or progression of a fracture was identified after 2 weeks of nonoperative treatment. The subcutaneous injection of teriparatide or oral bisphosphonate was prescribed at the time of identification of OVF for all patients. A thoracolumbosacral orthotic brace was utilized in all patients for at least 2 months after the procedure. PVP was performed using a transpedicular approach under the guidance of C-arm fluoroscopy. Polymethyl methacrylate (Exolent Spine; Elmdown, Milano, Italy) was injected into the cleft site or fracture site of the collapsed vertebral body while checking for cement leakage through C-arm fluoroscopy, and the amount of cement injected for each patient was retrospectively reviewed. The patients were scheduled for follow-up in our clinics at 1, 3, 6, 12, and 24 months postoperatively.
1. Clinical evaluation
Pain was evaluated based on the VAS score before and after surgery and at each follow-up visit. A higher VAS score indicates more painful symptoms. Moreover, we reviewed the patients’ characteristics, including age, sex, body mass index, BMD, and previous OVF.
2. Radiographic evaluation
Simple anteroposterior radiographs of the thoracolumbar junction and whole-spine lateral standing radiographs with the inclusion of the femoral head were obtained in all patients. By applying Cobb’s method, we measured the spinopelvic parameters, including the sagittal vertical axis (SVA) and thoracolumbar kyphosis angle (TLKA; T10–L2), in whole-spine lateral standing radiographs. The patients with an SVA of over 5 cm on a preoperative whole-spine lateral view were classified as the preoperative SI group and the others as the sagittal balance (SB) group.
We measured the local Cobb angle between the upper and lower endplates of the affected vertebra on both standing and supine lateral radiographs and calculated the degree of dynamic mobility within the vertebra by subtracting the local Cobb angle on a lateral standing radiograph from that on a simple lateral radiograph [6,22].
The cemented vertebral restoration ratio after the procedure was calculated as the ratio of the average height of the fractured vertebra subtracted from the average height of the cemented vertebra immediately after surgery on a simple lateral radiograph to the average height of the fractured vertebra.
Radiographic measurements were performed using an image analysis software (Maroview, version 5.4; Marotech, Seoul, Korea) on the hospital’s picture archiving and communication system. Measurements were obtained twice for each investigator, and the mean values were calculated. Reliability was classified as small (0–0.24), low (0.25–0.49), medium (0.50–0.69), excellent (0.70–0.89), and best (0.90–1.0), depending on the coincidence correlation coefficient in the group. The intraobserver reliability was estimated to be 0.92.
3. Statistical analysis
The independent paired t-test and chi-square test were employed to evaluate the differences in patient characteristics and radiographic measurements between the RC and NC groups. A multivariate logistic regression analysis was then conducted to identify the risk factors associated with a recollapsed vertebra after cement augmentation. Repeated measures analysis of variance was conducted to compare the progression of thoracolumbar kyphosis and SI recorded at each follow-up visit between the two study groups. All statistical analyses were conducted using the IBM SPSS ver. 20.0 (IBM Corp., Armonk, NY, USA). A p-value of <0.05 was considered statistically significant.
Results
Overall, 122 of the 256 patients who underwent PVP for thoracolumbar OVF during the study period did not meet the study’s eligibility criteria, leaving 28 patients (21%) for inclusion in the RC group and 106 in the NC group.
Table 1 presents the patient characteristics and radiographic measurements. The BMD was significantly lower in the RC group than in the NC group (p=0.000). Previous OVF was more common in the RC group (p=0.001). The dynamic mobility (indicated by the difference in the Cobb angle between supine and standing radiographs) was greater and the IVC sign was more frequent in the RC group (p=0.000 and p=0.002, respectively). SI was predominantly found in the RC group with increased TLKA and SVA (p=0.000, p=0.002, and p=0.021, respectively). Fractures of adjacent vertebra identified during follow-up were more common in the RC group (p=0.000). No significant between-group difference in the local Cobb angle, likelihood of leakage of cement into the intervertebral disc, or restoration rate following PVP was observed. The average amount of cement injected during the procedure also showed no differences between the two groups.
Table 1.
Baseline patient characteristics and radiographic measurements
| Characteristic | RC group (n=28) | NC group (n=106) | p-value |
|---|---|---|---|
| Patients’ characteristics | |||
| Age (yr) | 79.4±7.8 | 77.5±8.6 | 0.312 |
| Gender | 0.103 | ||
| Male | 2 | 23 | |
| Female | 26 | 83 | |
| Body mass index (kg/m2) | 24.1±3.9 | 22.7±3.9 | 0.079 |
| Bone mineral density (T-score) | −3.9±0.9 | −2.8±0.7 | 0.000* |
| Previous osteoporotic vertebral fracture | 14 (50) | 19 (18) | 0.001* |
| Average time to recollapse (mo) | 3.2 (1.2–25.1) | - | - |
| Radiographic measurements | |||
| Degrees of mobility (°) | 6.3±5.2 | 2.6±1.8 | 0.000* |
| Intravertebral vacuum cleft | 17 (61) | 30 (28) | 0.002* |
| Sagittal imbalance | 20 (71) | 10 (9) | 0.000* |
| Local Cobbs angle (°) | 14.3±8.1 | 15.4±6.5 | 0.183 |
| Thoracolumbar kyphosis angle (°) | 33.6±10.8 | 21.7±14.2 | 0.002* |
| Sagittal vertical axis (mm) | 10.8±5.8 | 2.1±1.8 | 0.021* |
| Adjacent vertebral fractures | 13 (46) | 11 (10) | 0.000* |
| Intervertebral disc cement leakage | 7 (25) | 20 (19) | 0.596 |
| Restoration ratio | 0.20±0.21 | 0.16±0.17 | 0.417 |
| Average amount of cement injected (mL) | 4.2 (3.0–6.5) | 4.1 (2.8–5.5) | 0.782 |
Values are presented as mean±standard deviation, number of patients (%), or mean (range), unless otherwise stated.
RC group, recollapsed group; NC group, noncollapsed group.
p<0.05 (using an independent-sample Student t-test and chi-square test).
Table 2 presents the results of the multivariate binary logistic regression analysis of possible risk factors for recollapse after cement augmentation. Lower BMD (p=0.047), degree of dynamic mobility (p=0.025), and SI (p=0.013; odds ratio, 5.405) were statistically significant risk factors associated with the recollapse of the vertebrae after the procedure.
Table 2.
Analysis of risk factors for recollapse of cemented vertebra
| Variable | Adjusted odds ratio (95% confidence interval) | p-value |
|---|---|---|
| Bone mineral density (T-score) | 0.261 (0.095–0.718) | 0.047* |
| Previous osteoporotic vertebral fracture | 2.848 (0.368–22.050) | 0.316 |
| Degrees of mobility | 1.744 (1.211–2.510) | 0.025* |
| Intravertebral vacuum cleft | 1.318 (0.082–3.230) | 0.478 |
| Sagittal imbalance | 5.405 (2.344–12.108) | 0.013* |
| Thoracolumbar kyphosis angle (T10–L2) | 1.006 (0.937–1.080) | 0.878 |
| Adjacent vertebral fractures | 2.044 (0.235–17.790) | 0.517 |
p<0.05 (using a multivariate logistic regression test).
Tables 3 and 4 present the progression of thoracolumbar kyphosis and SI in the two groups (except in patients who underwent surgical correction and fusion during follow-up) (Fig. 3). The progression of sagittal spinal deformity, including thoracolumbar kyphosis and positive SI, was identified in both groups and was more prominent and aggressive in the RC and SI groups (p=0.000 for both).
Table 3.
Comparisons of TLKA and SVA at each follow-up by RC and NC groups
| T0 | T1 | T2 | T3 | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| RC | NC | RC | NC | RC | NC | RC | NC | ||
| TLKA (°) | 33.6±10.2 | 21.7±14.2 | 34.6±10.0 | 23.1±14.6 | 38.9±9.1 | 24.3±14.7 | 44.1±7.7 | 25.7±14.4 | 0.000* |
|
| |||||||||
| SVA (mm) | 9.0±4.6 | 2.1±1.8 | 9.1±4.6 | 2.2±1.8 | 9.7±4.8 | 2.2±1.8 | 10.4±4.8 | 2.3±1.8 | 0.000* |
Values are presented as mean±standard deviation. Patients of RC group, who underwent corrective surgery and fusion with instrumentation, were excluded in this comparison.
TKLA, thoracolumbar kyphosis angle; SVA, sagittal vertical axis; T0, before vertebroplasty, T1, immediately after vertebroplasty; T2, 1 year after vertebroplasty; T3, 2 years after vertebroplasty; RC group, recollapsed group; NC group, noncollapsed group.
p<0.05 (using a repeated measure analysis of variance).
Table 4.
Comparisons of TLKA and SVA at each follow-up by SI and SB groups
| T0 | T1 | T2 | T3 | p-value | |||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|
||||||
| SI | SB | SI | SB | SI | SB | SI | SB | ||
| TLKA (°) | 32.8±10.8 | 21.7±14.2 | 33.7±10.6 | 23.2±14.7 | 37.6±10.1 | 24.4±14.8 | 43.1±8.5 | 25.6±14.5 | 0.000* |
|
| |||||||||
| SVA (mm) | 9.3±8.5 | 1.8±1.3 | 9.4±4.1 | 1.9±1.3 | 10.0±4.3 | 2.0±1.3 | 10.6±4.4 | 2.0±1.4 | 0.000* |
Values are presented as mean±standard deviation. Patients of SI group, who underwent corrective surgery and fusion with instrumentation, were excluded in this comparison.
TKLA, thoracolumbar kyphosis angle; SVA, sagittal vertical axis; T0, before vertebroplasty, T1, immediately after vertebroplasty; T2, 1 year after vertebroplasty; T3, 2 years after vertebroplasty; SI, sagittal imbalance; SB, sagittal balance.
p<0.05 (using a repeated measure analysis of variance).
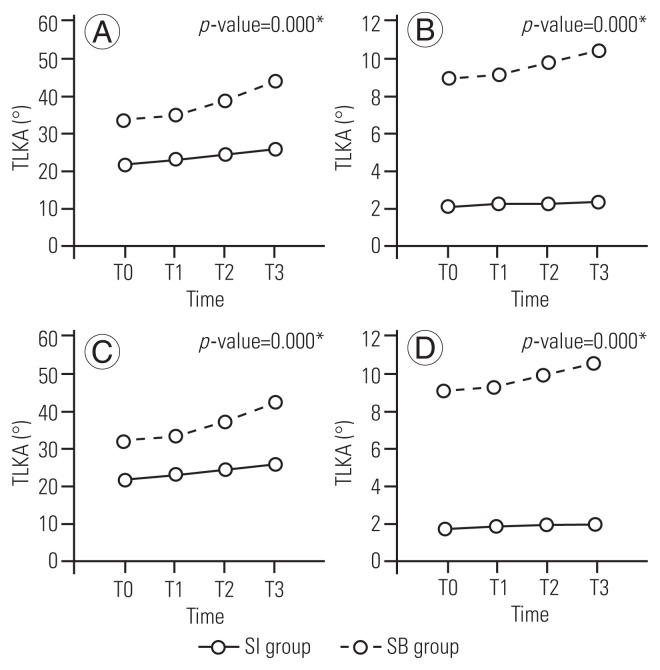
Fig. 3.
Comparison of the thoracolumbar kyphosis angle (TLKA) and sagittal vertical axis (SVA) at each follow-up visit according to the presence of sagittal imbalance. (A) Comparison of TLKA at each follow-up visit. The increase in TLKA over time is greater in the RC group than in the NC group (p=0.000). (B) Comparison of SVA at each follow-up visit. The SVA increases over time more in the RC group than in the NC group (p=0.000). (C) Comparison of TLKA at each follow-up visit. The TLKA increases over time more in the SI group than in the SB group (p=0.000). (D) Comparison of SVA at each follow-up visit. The SVA increases over time more in the SI group than in the SB group (p=0.000). Some patients in the SI group and the RC group who underwent corrective surgery and fusion with instrumentation were not included in these comparisons. RC, recollapsed; NC, non-collapsed; SI, sagittal imbalance; SB, sagittal balance; T0, before vertebroplasty, T1, immediately after vertebroplasty; T2, 1 year after vertebroplasty; T3, 2 years after vertebroplasty.
Discussion
PVP has been frequently used for OVF in elderly patients with osteoporosis and achieves substantial relief of pain and improvement in the quality of life [23]. Recently, the recollapse of the cemented vertebra has been demonstrated as a complication following cement augmentation [16,24,25]. One study identified the radiographic loss of height in the cemented vertebra in 63% of 98 patients during an average of 27 months of follow-up [26]. Another study found a symptomatic re-fracture of the cemented vertebra in 16% of 356 patients, with 52% of the re-fractures occurring within a month and 32% within 3–5 months [25]. In another study, the mean prevalence of re-fracture involving adjacent vertebrae or vertebrae remote to the cemented vertebrae (which depends on the duration of follow-up and study design) was 21.28% (ranging from 6.81% to 37.95%) [27]. In the present study, the recollapse of the cemented vertebra was identified in 28 (21%) of 134 patients during a 2-year follow-up. The patients in the RC group were prescribed with anti-osteoporosis medication, mainly subcutaneous injection of teriparatide, and were educated about the importance of strict compliance. Repeat PVP was sometimes performed for substantial back pain or accompanying adjacent fractures. Two patients of the RC group developed severe back pain, worsened SI, multiple adjacent fractures, and progressive neurological deficits and finally underwent surgical correction (Fig. 4).
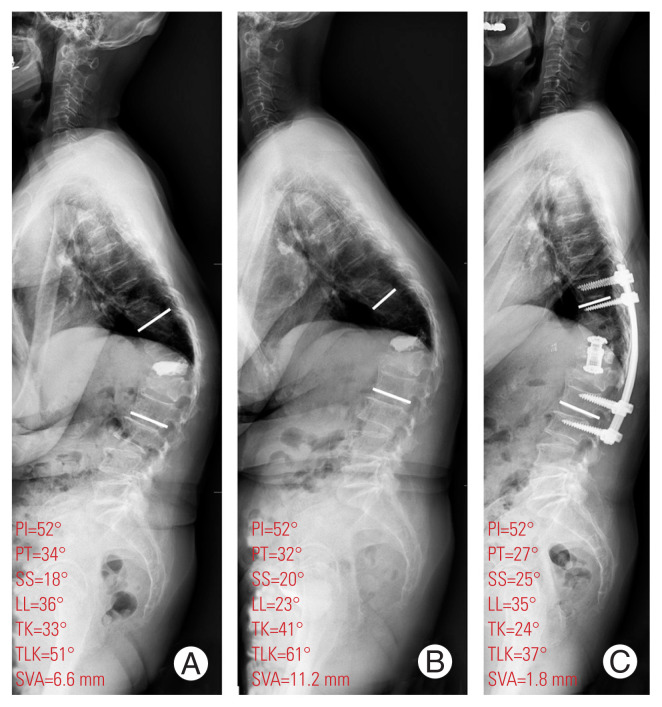
Fig. 4.
Whole-spine lateral standing radiographs for a 70-year-old woman who underwent anterior interbody fusion using cage and posterior instrumentation with posterolateral fusion for recollapse of the T12 body with the intravertebral vacuum cleft sign and substantial back pain (scored 8 on a Visual Analog Scale). (A) Recollapse of the T12 body after vertebroplasty was identified. (B) After 6 months of conservative treatment, her thoracolumbar kyphosis, sagittal imbalance, and back pain had worsened. (C) Three months after anterior and posterior fusion, sagittal balance was achieved and her back pain was improved. PI, pelvic incidence; PT, pelvic tilt; SS, sacral slope; LL, lumbar lordosis; TK, thoracic kyphosis; TLK, thoracolumbar kyphosis; SVA, sagittal vertical axis.
Previous studies have described several risk factors for recollapse following cement augmentation for OVF [10,19,24,28]. The patient-related risk factors for the re-fracture of cemented vertebrae or new fracture of an adjacent vertebra include excessive body weight, the use of steroids, advanced age, and poor compliance with anti-osteoporosis medication [7,11,15,24]. The technical factors associated with recollapse include a larger or smaller volume of injected cement and performing percutaneous balloon kyphoplasty instead of PVP [7,10]. Statistically significant radiographic risk factors have also been identified, including higher local kyphosis, intravertebral instability, and presence of the IVC sign with pseudarthrosis [6,7,10,13]. Furthermore, strong evidence has indicated that low BMD, leakage of intervertebral disc cement, fractures at the thoracolumbar junction, higher restoration rate of the vertebral height, and preexisting vertebral fractures are general risk factors [17].
In the present study, we identified three risk factors (lower BMD, dynamic mobility, and SI) for recollapse following PVP for thoracolumbar OVF. We had anticipated that the IVC sign would also be a significant risk factor as intravertebral osteonecrosis and a defect in the cancellous component of the bone would be related to gradual deformation [13]. However, this sign was not a statistically significant risk factor for recollapse. This finding suggests that significant dynamic instability accompanied by the IVC sign would be clinically important in terms of further collapse of a cemented vertebra, which is also associated with more osteoporotic bone. SI, which has not been evaluated before, would be a clinically significant factor when managing thoracolumbar OVF associated with further recollapse. This finding indicates that progressive thoracolumbar kyphosis with insufficient spinopelvic compensation may lead to stooping and further SI, which aggravates weight loading and stresses on the anterior–superior portion of the cemented vertebra or adjacent vertebrae at the thoracolumbar junction [6,29].
In the present study, the progression of the average TLKA following vertebroplasty was identified over time and was more prominent in patients with SI or a recollapsed vertebra. We demonstrated that SI was a clinically significant factor predicting further collapse and spinal deformity for patients with a recollapsed vertebra. The statistical significance of the relationship between SI and recollapse indicates the need for sagittal spinal parameters in the management of OVF, especially at the thoracolumbar junction. Therefore, when managing such patients, a whole-spine lateral standing radiograph should be checked for the presence of SI with spinopelvic decompensation and dynamic mobility within the vertebra.
In the present study, some variables were not significantly correlated with recollapse. For example, TLKA was not a risk factor for recollapse despite the difference in the average value between the two study groups, which indicates that the TLKA would not correlate quantitatively with the risk of recollapse. We believed that higher thoracolumbar kyphosis or local kyphosis would lead to vertebral recollapse following PVP. However, the degree of thoracolumbar kyphosis or local kyphosis was not clinically important. Instead, the presence of SI was a clinically significant risk factor associated with further collapse and the progression of sagittal deformity of the spine. Adjacent vertebral fractures were more common in the RC group. However, their correlation with recollapse was not statistically significant. Considering the reports indicating that adjacent fractures are associated with intradiscal cement leakage or greater restoration of the vertebral height, which was not different between our two groups, it is possible that the biomechanics of cemented vertebrae that recollapse are different from those of adjacent vertebrae that develop fractures [29,30].
A strong correlation was observed between OVF at the thoracolumbar junction and further progression of thoracolumbar kyphosis, which could be aggravated by the collapse of a vertebral body and multiple adjacent fractures as well as the severity of osteoporosis and could result in neurological and functional deficits [1]. For these reasons, prediction and prevention of the progression of kyphotic deformity or gradual SI would be necessary to preserve physiologic functions and decrease the mortality associated with OVF and other compromising conditions. A comprehensive understanding of SI and careful assessment of sagittal spinopelvic parameters on whole-spine lateral standing radiographs would be essential for the prediction of the prognosis and decision-making with regard to further treatment for such patients.
Patients who have undergone PVP a few weeks earlier should be carefully evaluated for the recollapse of the cemented vertebra; any pain usually occurs at the same site. Therefore, it may be difficult to diagnose a new fracture. The recollapse is usually associated with lower BMD and is often accompanied by an adjacent new vertebral fracture, which potentially leads to further progression of kyphosis, especially at the thoracolumbar junction [27]. In the present study, the average TLKA following vertebroplasty progressed over time, more prominently in patients with SI or a recollapsed vertebra. Therefore, we assume that the recollapse of the cemented vertebra accompanied by lower BMD or adjacent vertebral fractures would result in the progression of SI and that the SI would aggravate the collapse of the affected vertebra or lead to new adjacent vertebral fractures. Patients with SI along with a greater progression of thoracolumbar kyphosis should be managed more carefully, given that an imbalanced spine may lead to decreased quality of life and occasionally require surgical correction. During the study period, two of the 28 patients in the RC group and none of the NC group underwent additional surgeries. The two patients demonstrated a significant progression of SI and substantial back pain. After the corrective surgery, both patients showed satisfactory clinical outcomes and correction of the SI.
From a clinical point of view, corrective surgery for SI should not always be performed on patients with osteoporosis vertebral fractures with spinal imbalance. Conservative treatment with strict management for osteoporosis and careful monitoring for the progression of SB using whole-spine lateral standing radiographs and dynamic X-rays should be performed. The corrective surgery and stabilization for osteoporotic fractures with SI should be considered when the progression of substantial pain or spinal imbalance or neurologic deficits is identified.
Unlike earlier studies, we evaluated the association between SI and thoracolumbar kyphosis and recollapse following PVP. Moreover, we demonstrated the clinical significance of SB for managing the recollapsed vertebra. This study may be helpful in the understanding of recollapse despite cement augmentation and in predicting the likelihood of a need for further surgical correction.
However, this study has some limitations. First, it had a retrospective design and was conducted at a single center. Second, some variables, including the amount of cement inserted, compliance with anti-osteoporosis treatment, and individual ambulatory status, could not be evaluated by a retrospective review of medical records. Third, our stringent inclusion and exclusion criteria could have introduced a degree of selection bias. Fourth, in the present study, only vertebroplasty was employed for cement augmentation and investigated, which was mainly due to the surgeon’s preference. Finally, various factors including back pain could affect the difficulties of proper measurements of the sagittal spinal parameters based on the whole-spine lateral standing radiographs. Future research with a larger study population along with a longer-term follow-up may better identify further correlations, allowing better prediction of the requirement for further corrective surgery.
Conclusions
In conclusion, SI, dynamic mobility within the vertebra, and lower BMD are risk factors for the recollapse of a vertebra following PVP for thoracolumbar OVF. SI was a significant predictor for further collapse of a cemented vertebra and the progression of sagittal deformity in patients with a recollapsed vertebra following PVP for thoracolumbar OVF. The degrees of thoracolumbar kyphosis and local kyphosis were not significant factors for predicting further collapse or the progression of spinal deformity. Therefore, spinopelvic parameters, rather than kyphosis angle, should be carefully evaluated based on the whole-spine lateral standing radiographs for thoracolumbar OVF at each follow-up visit. More careful and active management, including patient education regarding strict adherence to anti-osteoporosis medication and counseling with regard to the potential need for re-PVP and revision surgery, is required for patients with underlying thoracolumbar kyphosis or SI.
Acknowledgments
We would like to thank for all staffs in our department of orthopedic surgery, always giving sincere advices for studies.
Footnotes
Conflict of Interest
No potential conflict of interest relevant to this article was reported.
References
- 1.Mattie R, Laimi K, Yu S, Saltychev M. Comparing percutaneous vertebroplasty and conservative therapy for treating osteoporotic compression fractures in the thoracic and lumbar spine: a systematic review and meta-analysis. J Bone Joint Surg Am. 2016;98:1041–51. doi: 10.2106/JBJS.15.00425. [DOI] [PubMed] [Google Scholar]
- 2.Bliuc D, Nguyen ND, Milch VE, Nguyen TV, Eisman JA, Center JR. Mortality risk associated with low-trauma osteoporotic fracture and subsequent fracture in men and women. JAMA. 2009;301:513–21. doi: 10.1001/jama.2009.50. [DOI] [PubMed] [Google Scholar]
- 3.Verlaan JJ, Oner FC, Slootweg PJ, Verbout AJ, Dhert WJ. Histologic changes after vertebroplasty. J Bone Joint Surg Am. 2004;86:1230–8. doi: 10.2106/00004623-200406000-00016. [DOI] [PubMed] [Google Scholar]
- 4.Wardlaw D, Cummings SR, Van Meirhaeghe J, et al. Efficacy and safety of balloon kyphoplasty compared with non-surgical care for vertebral compression fracture (FREE): a randomised controlled trial. Lancet. 2009;373:1016–24. doi: 10.1016/S0140-6736(09)60010-6. [DOI] [PubMed] [Google Scholar]
- 5.Yeom JS, Kim WJ, Choy WS, Lee CK, Chang BS, Kang JW. Leakage of cement in percutaneous transpedicular vertebroplasty for painful osteoporotic compression fractures. J Bone Joint Surg Br. 2003;85:83–9. doi: 10.1302/0301-620x.85b1.13026. [DOI] [PubMed] [Google Scholar]
- 6.Nakamae T, Yamada K, Tsuchida Y, Osti OL, Adachi N, Fujimoto Y. Risk factors for cement loosening after vertebroplasty for osteoporotic vertebral fracture with intravertebral cleft: a retrospective analysis. Asian Spine J. 2018;12:935–42. doi: 10.31616/asj.2018.12.5.935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li YX, Guo DQ, Zhang SC, et al. Risk factor analysis for re-collapse of cemented vertebrae after percutaneous vertebroplasty (PVP) or percutaneous kyphoplasty (PKP) Int Orthop. 2018;42:2131–9. doi: 10.1007/s00264-018-3838-6. [DOI] [PubMed] [Google Scholar]
- 8.Iida K, Harimaya K, Tarukado K, Tono O, Matsumoto Y, Nakashima Y. Kyphosis progression after balloon kyphoplasty compared with conservative treatment. Asian Spine J. 2019;13:928–35. doi: 10.31616/asj.2018.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sebaaly A, Nabhane L, Issa El Khoury F, Kreichati G, El Rachkidi R. Vertebral augmentation: state of the art. Asian Spine J. 2016;10:370–6. doi: 10.4184/asj.2016.10.2.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Borensztein M, Camino Willhuber GO, Posadas Martinez ML, Gruenberg M, Sola CA, Velan O. Analysis of risk factors for new vertebral fracture after percutaneous vertebroplasty. Global Spine J. 2018;8:446–52. doi: 10.1177/2192568217732988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Takahara K, Kamimura M, Moriya H, et al. Risk factors of adjacent vertebral collapse after percutaneous vertebroplasty for osteoporotic vertebral fracture in postmenopausal women. BMC Musculoskelet Disord. 2016;17:12. doi: 10.1186/s12891-016-0887-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rho YJ, Choe WJ, Chun YI. Risk factors predicting the new symptomatic vertebral compression fractures after percutaneous vertebroplasty or kyphoplasty. Eur Spine J. 2012;21:905–11. doi: 10.1007/s00586-011-2099-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heo DH, Chin DK, Yoon YS, Kuh SU. Recollapse of previous vertebral compression fracture after percutaneous vertebroplasty. Osteoporos Int. 2009;20:473–80. doi: 10.1007/s00198-008-0682-3. [DOI] [PubMed] [Google Scholar]
- 14.Voormolen MH, Lohle PN, Juttmann JR, van der Graaf Y, Fransen H, Lampmann LE. The risk of new osteoporotic vertebral compression fractures in the year after percutaneous vertebroplasty. J Vasc Interv Radiol. 2006;17:71–6. doi: 10.1097/01.RVI.0000190910.43602.3C. [DOI] [PubMed] [Google Scholar]
- 15.Cao J, Kong L, Meng F, Zhang Y, Shen Y. Risk factors for new vertebral compression fractures after vertebroplasty: a meta-analysis. ANZ J Surg. 2016;86:549–54. doi: 10.1111/ans.13428. [DOI] [PubMed] [Google Scholar]
- 16.Lin WC, Cheng TT, Lee YC, et al. New vertebral osteoporotic compression fractures after percutaneous vertebroplasty: retrospective analysis of risk factors. J Vasc Interv Radiol. 2008;19(2 Pt 1):225–31. doi: 10.1016/j.jvir.2007.09.008. [DOI] [PubMed] [Google Scholar]
- 17.Ma X, Xing D, Ma J, et al. Risk factors for new vertebral compression fractures after percutaneous vertebroplasty: qualitative evidence synthesized from a systematic review. Spine (Phila Pa 1976) 2013;38:E713–22. doi: 10.1097/BRS.0b013e31828cf15b. [DOI] [PubMed] [Google Scholar]
- 18.Black DM, Arden NK, Palermo L, Pearson J, Cummings SR. Prevalent vertebral deformities predict hip fractures and new vertebral deformities but not wrist fractures: study of Osteoporotic Fractures Research Group. J Bone Miner Res. 1999;14:821–8. doi: 10.1359/jbmr.1999.14.5.821. [DOI] [PubMed] [Google Scholar]
- 19.Kao FC, Huang YJ, Chiu PY, Hsieh MK, Tsai TT. Factors predicting the surgical risk of osteoporotic vertebral compression fractures. J Clin Med. 2019;8:501. doi: 10.3390/jcm8040501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ha KY, Kim KW, Kim YH, Oh IS, Park SW. Revision surgery after vertebroplasty or kyphoplasty. Clin Orthop Surg. 2010;2:203–8. doi: 10.4055/cios.2010.2.4.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ha KY, Kim YH, Chang DG, Son IN, Kim KW, Kim SE. Causes of late revision surgery after bone cement augmentation in osteoporotic vertebral compression fractures. Asian Spine J. 2013;7:294–300. doi: 10.4184/asj.2013.7.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McKiernan F, Jensen R, Faciszewski T. The dynamic mobility of vertebral compression fractures. J Bone Miner Res. 2003;18:24–9. doi: 10.1359/jbmr.2003.18.1.24. [DOI] [PubMed] [Google Scholar]
- 23.McKiernan F, Faciszewski T, Jensen R. Quality of life following vertebroplasty. J Bone Joint Surg Am. 2004;86:2600–6. doi: 10.2106/00004623-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 24.Ma X, Xia H, Wang J, et al. Re-fracture and correlated risk factors in patients with osteoporotic vertebral fractures. J Bone Miner Metab. 2019;37:722–8. doi: 10.1007/s00774-018-0974-4. [DOI] [PubMed] [Google Scholar]
- 25.Summa A, Crisi G, Cerasti D, Ventura E, Menozzi R, Ormitti F. Refractures in cemented vertebrae after percutaneous vertebroplasty and pain relief after a second procedure: a retrospective analysis. Neuroradiol J. 2009;22:239–43. doi: 10.1177/197140090902200216. [DOI] [PubMed] [Google Scholar]
- 26.Lin WC, Lee YC, Lee CH, et al. Refractures in cemented vertebrae after percutaneous vertebroplasty: a retrospective analysis. Eur Spine J. 2008;17:592–9. doi: 10.1007/s00586-007-0564-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Han S, Jang IT. Analysis of adjacent fractures after two-level percutaneous vertebroplasty: is the intervening vertebral body prone to re-fracture? Asian Spine J. 2018;12:524–32. doi: 10.4184/asj.2018.12.3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun G, Tang H, Li M, Liu X, Jin P, Li L. Analysis of risk factors of subsequent fractures after vertebroplasty. Eur Spine J. 2014;23:1339–45. doi: 10.1007/s00586-013-3110-0. [DOI] [PubMed] [Google Scholar]
- 29.Berlemann U, Ferguson SJ, Nolte LP, Heini PF. Adjacent vertebral failure after vertebroplasty: a biomechanical investigation. J Bone Joint Surg Br. 2002;84:748–52. doi: 10.1302/0301-620x.84b5.11841. [DOI] [PubMed] [Google Scholar]
- 30.Kim MH, Lee AS, Min SH, Yoon SH. Risk factors of new compression fractures in adjacent vertebrae after percutaneous vertebroplasty. Asian Spine J. 2011;5:180–7. doi: 10.4184/asj.2011.5.3.180. [DOI] [PMC free article] [PubMed] [Google Scholar]