Key Points
Question
Does self-monitoring of blood pressure (BP) by pregnant individuals at higher risk for preeclampsia lead to earlier detection of pregnancy hypertension compared with usual antenatal care?
Findings
In this randomized clinical trial that included 2441 pregnant individuals at increased risk for preeclampsia, self-monitoring of BP with telemonitoring compared with usual care resulted in a mean time to clinic-based detection of hypertension of 104 vs 106 days, a difference that was not statistically significant.
Meaning
Among pregnant individuals at higher risk of preeclampsia, self-monitoring of BP with telemonitoring did not lead to earlier clinic-based detection of hypertension.
Abstract
Importance
Inadequate management of elevated blood pressure (BP) is a significant contributing factor to maternal deaths. Self-monitoring of BP in the general population has been shown to improve the diagnosis and management of hypertension; however, little is known about its use in pregnancy.
Objective
To determine whether self-monitoring of BP in higher-risk pregnancies leads to earlier detection of pregnancy hypertension.
Design, Setting, and Participants
Unblinded, randomized clinical trial that included 2441 pregnant individuals at higher risk of preeclampsia and recruited at a mean of 20 weeks’ gestation from 15 hospital maternity units in England between November 2018 and October 2019. Final follow-up was completed in April 2020.
Interventions
Participating individuals were randomized to either BP self-monitoring with telemonitoring (n = 1223) plus usual care or usual antenatal care alone (n = 1218) without access to telemonitored BP.
Main Outcomes and Measures
The primary outcome was time to first recorded hypertension measured by a health care professional.
Results
Among 2441 participants who were randomized (mean [SD] age, 33 [5.6] years; mean gestation, 20 [1.6] weeks), 2346 (96%) completed the trial. The time from randomization to clinic recording of hypertension was not significantly different between individuals in the self-monitoring group (mean [SD], 104.3 [32.6] days) vs in the usual care group (mean [SD], 106.2 [32.0] days) (mean difference, −1.6 days [95% CI, −8.1 to 4.9]; P = .64). Eighteen serious adverse events were reported during the trial with none judged as related to the intervention (12 [1%] in the self-monitoring group vs 6 [0.5%] in the usual care group).
Conclusions and Relevance
Among pregnant individuals at higher risk of preeclampsia, blood pressure self-monitoring with telemonitoring, compared with usual care, did not lead to significantly earlier clinic-based detection of hypertension.
Trial Registration
ClinicalTrials.gov Identifier: NCT03334149
This randomized clinical trial compares the efficacy of self-monitoring of blood pressure with telemonitoring vs usual antenatal care in detecting hypertension among pregnant individuals at higher risk of preeclampsia.
Introduction
Elevated blood pressure (BP) has been estimated to affect approximately 10% of pregnancies worldwide and 18 million pregnancies worldwide in 2019.1,2 In the UK, inadequate management of elevated BP has previously been reported as a significant contributing factor to maternal deaths. Although maternal deaths related to high BP have reduced in the UK in recent years, preeclampsia remains important due to its influence on maternal and perinatal outcomes.3,4 Individuals who are at higher risk of preeclampsia due to risk factors such as age, high body mass index, or existing medical conditions may require more frequent monitoring.5 Blood pressure can increase rapidly in pregnancy, and hypertension may go undetected between antenatal visits.6
Self-monitoring of blood pressure (SMBP), which involves self-measurement of BP outside of the clinical setting, is now commonplace and effective at detecting and lowering BP in nonpregnant adults with hypertension.7,8,9 Self-monitoring during pregnancy has been limited to small and mostly nonrandomized feasibility studies, often without validated BP monitors.10,11 Low study quality and heterogeneity limit the conclusions that can be drawn from such studies, but initial results suggest reduced morbidity and resource use, acceptability for individuals and their clinicians, and feasibility.10,11,12,13
The Blood Pressure Monitoring in High Risk Pregnancy to Improve the Detection and Monitoring of Hypertension (BUMP 1) trial aimed to establish whether SMBP with telemonitoring, in addition to usual care, could lead to earlier detection of elevated clinic BP compared with usual care during higher-risk pregnancies.
Methods
Study Design
The trial was an unblinded randomized clinical trial of SMBP in pregnancy for the detection of elevated BP. The methods of the trial and its development have been published previously and are summarized below.14,15 The protocol is available in Supplement 1 and statistical analysis plan in Supplement 2. The West Midlands–South Birmingham NHS Research Ethics Committee (ref 17/WM/0241) provided ethical approval. All participants gave written informed consent.
Study Population
Pregnant individuals at 16 to 24 weeks' gestation with higher risk of preeclampsia were recruited by research midwives through antenatal clinics in 15 secondary care maternity units between November 2018 and September 2019. Higher risk was defined by the relevant UK guidance at the time and included 1 or more of the following risk factors for pregnancy hypertension5: age 40 years or older with a nulliparity pregnancy interval of greater than 10 years, family history of preeclampsia, history of preeclampsia or gestational hypertension, body mass index of 30 or greater, any stage of chronic kidney disease, twin pregnancy, prepregnancy diabetes, or autoimmune disease (eg, systemic lupus erythematosus or antiphospholipid syndrome). Individuals with a preexisting diagnosis of hypertension were excluded.
Randomization and Blinding
Eligible individuals were randomized (1:1 ratio) to receive either usual care or usual care plus SMBP with telemonitoring (Figure). The online randomization sequence was generated by an independent statistician using permutated varying block sizes of 4 or 6 and stratified by recruitment site and parity (0 vs ≥1). Study participants and health care professionals were unblinded due to the nature of the intervention.
Figure. Eligibility, Randomization, and Data Availability for Self-monitoring for Hypertension Among Pregnant Individuals at Risk for Preeclampsia.
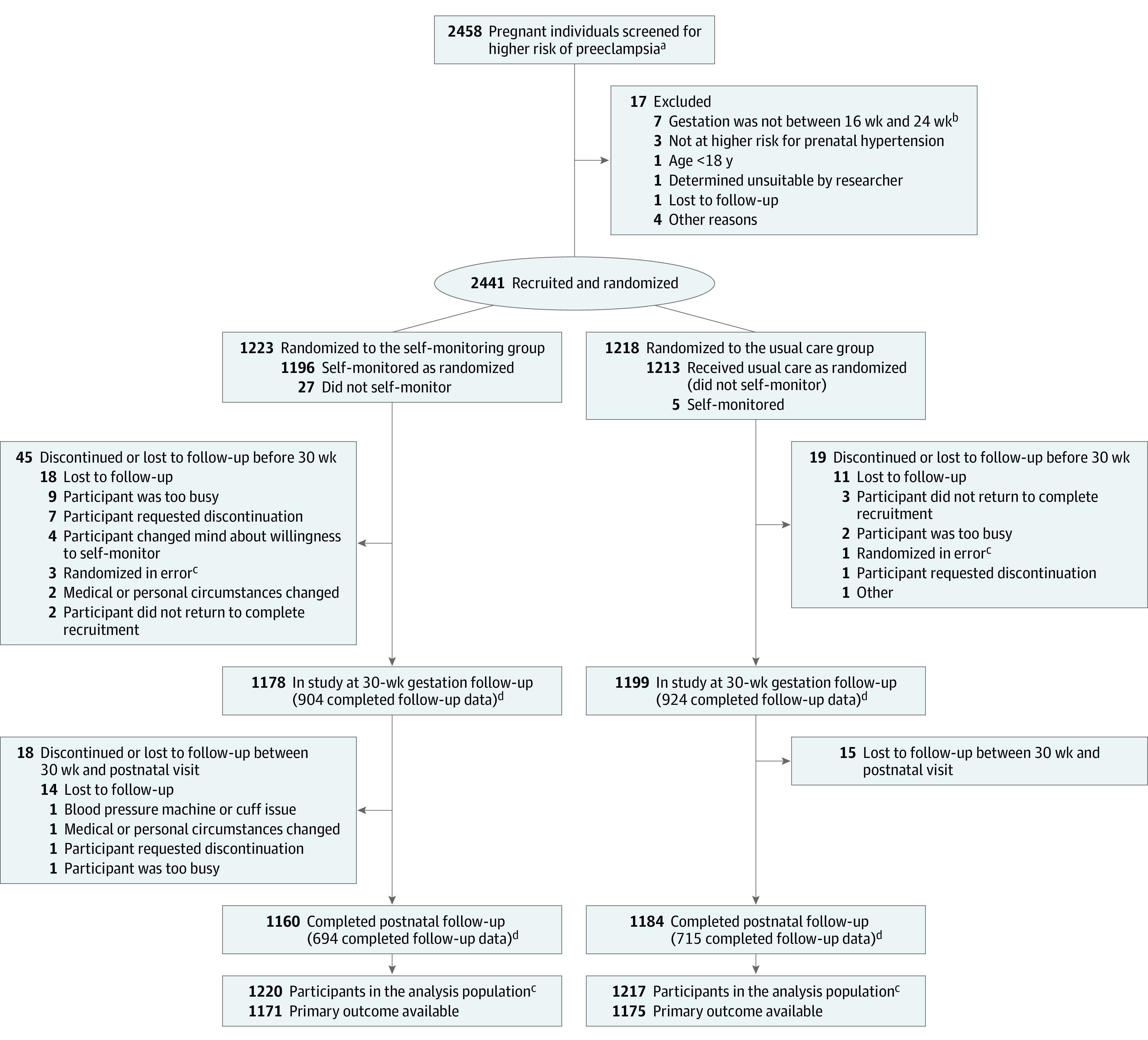
aScreening was conducted by research midwives reviewing clinical notes; therefore, the number of potentially suitable participants may underrepresent the true number. Higher risk of preeclampsia was defined by the relevant UK guidance at the time and included 1 or more of the following risk factors for pregnancy hypertension5: age ≥40 years with a nulliparity pregnancy interval >10 years, family history of preeclampsia, history of preeclampsia or gestational hypertension, body mass index of ≥30 (calculated as weight in kilograms divided by height in meters squared), any stage of chronic kidney disease, twin pregnancy, prepregnancy diabetes, and autoimmune disease (eg, systemic lupus erythematosus or antiphospholipid syndrome).
bIndicates 16 weeks 0 days to 24 weeks 0 days.
cIndividuals who were randomized in error (3 in the self-monitoring group and 1 in the usual care group) were excluded from the analysis population.
dFollow-up numbers include individuals eligible to be followed up (ie, not withdrawn or lost to follow-up) who completed questionnaires at that time. All available data were included in the analyses but some were available at only 1 follow-up point. For the self-monitoring group (n = 1223), 615 participants completed both 30-week and postnatal follow-up, 289 completed only 30-week follow-up, 79 completed only postnatal follow-up, and 240 completed neither follow-up. For the usual care group (n = 1218), 636 participants completed both 30-week and postnatal follow-up, 288 completed 30-week follow-up, 79 completed only postnatal follow-up, and 215 completed neither follow-up.
Procedures
Self-monitoring
Participants randomized to SMBP continued with usual antenatal care and in addition, were provided with a validated automated monitor (Microlife WatchBP Home).16 They were given training and written instructions for SMBP by the recruiting research midwife then enrolled on a mobile phone–based telemonitoring system with an optional paper diary.
Participants were asked to self-monitor their BP 3 times per week, to take 2 readings each time, and to manually submit the second reading by using the study app. Elevated readings triggered a request from the app for a third reading. If a participant’s third reading was also elevated, the app would trigger a message to the participant to contact their local maternity unit (eFigure 3 in Supplement 3). Initial contact was typically by telephone, and subsequent arrangements for review were at the discretion of the unit. BP thresholds were equivalent to clinic and based on pilot work and a systematic review.13,17
Each site received training on the trial and intervention from the study team. Clinicians had access to a web-based dashboard and each site was sent a summary of their participants’ results each week. The app was designed to include historic BP data, enabling participants to share their results with clinicians via their mobile phone display.
Usual prenatal care consisted of pregnant individuals attending antenatal clinic, as required (≥7 times during an uncomplicated pregnancy),18 including having their BP measured by their antenatal care team. Participants in the usual care group did not have access to the telemonitoring system and such systems were not commonplace in the UK at the time of the study.
Protocol Amendments
The overall protocol was amended significantly on 6 occasions during the trial. Two amendments affected the trial: (1) adding the external pilot; and (2) continuing recruitment until the accompanying trial14 (the same intervention was implemented in individuals with pregnancy hypertension) had finished recruitment, which had the effect of modestly increasing the sample size from 2262 to 2441.
Outcomes
The primary outcome was difference in the time from randomization to first recording of “clinic hypertension” between the randomized groups. Clinic hypertension was defined as sustained BP of 140/90 mm Hg or greater (ie, either elevated systolic BP, diastolic BP, or both) recorded by a health care professional in the clinical record in any setting up to the day before delivery. “Sustained hypertension” was defined as having at least 2 elevated BP readings within 1 week (168 hours) with no minimum time between readings, with the second reading date reported as the date of diagnosis.5 Clinic hypertension was additionally defined as having a recorded diagnosis of preeclampsia or gestational hypertension or prescription of antihypertensive medication, if either occurred before clinic hypertension was recorded. BP and other clinical data were extracted from the clinical record at the end of each participant’s participation in the trial. Additionally, the following prespecified subgroups were analyzed: eligibility for aspirin prophylaxis; gestational age at recruitment; parity; measurement of BP prior to randomization; deprivation score; race and ethnicity; educational qualifications.
Secondary outcomes were both maternal (severe hypertension [systolic BP ≥160 mm Hg and/or diastolic BP ≥110 mm Hg], serious maternal complications, and onset of labor) and perinatal (stillbirth and early neonatal death, gestation at delivery, mode of delivery, birth weight [including percentiles], small for gestational age [<10th and <3rd percentiles], and neonatal admissions). Patient-reported maternal outcomes were captured by questionnaires at baseline, 30 weeks’ gestation, and 12 weeks postnatally for illness perception (adapted Brief Illness Perception Questionnaire score range, 0-10; higher scores reflect greater confidence in ability to manage hypertension; minimal clinically important difference [MCID] not available),19 anxiety (6-item State-Trait Anxiety Inventory [STAI-6] scaled to 100; score range, 0 [no anxiety] to 100 [highest level of anxiety]; MCID = 10),20 and maternal health-related quality of life (EuroQol EQ-5D-5L; score range, −0.594 [worst quality] to +1 [best quality]; MCID = 0.037)21,22 (see eTable 1 in Supplement 3 for the full list).
In accordance with UK recommendations, self-reported race and ethnicity were recorded using standard descriptions derived from those used by UK Office for National Statistics.23
Post hoc analyses assessed fidelity of participants in the intervention group to the self-monitoring regimen as captured by the app and compared elevated BP on self-monitoring to the reference standard of elevated BP on clinic measurements.
Sample Size
An external pilot phase, which included 40 participants, tested all trial procedures prior to the main trial commencing. A sample size of 2262 (1131 per group) assuming an SD of 40 days was estimated to allow detection of an effect size of 12 days’ difference in time to detection of clinic hypertension in pregnancy between self-monitoring and control groups, with 90% power, 5% level of significance (2-sided), and assuming a 15% attrition rate. The sample size was determined via simulation, using a bootstrapping method with replacement with parameters derived from pilot work.13 Of the planned 2262 participants, 362 (16%) were expected to develop hypertension. A statistical analysis plan was agreed prior to data lock (Supplement 2).
Statistical Analysis
The primary analysis included all participants for whom data were available, according to the group to which participants were randomly allocated and regardless of any subsequent deviation from protocol. The primary outcome was analyzed using a 2-part hurdle model,24 which consisted of the following processes: (1) first the model determined whether the participants had a diagnosis of clinic hypertension using a probit model, assuming an underlying latent distribution for the probability of having clinic hypertension; and (2) the second part determined the time between randomization and clinic hypertension, conditional on having cleared the hurdle. The prespecified model adjusted for group, parity (0 or ≥1) as fixed effects, and site as random effect. However, because the model would not converge, site was subsequently fitted as a fixed effect. Sensitivity analyses included adjustment for baseline covariates that predicted missingness, multiple imputation (100 imputations) of missing values, and inclusion of only elevated BP based on BP values (ie, not clinical diagnoses or antihypertensive prescription). Prespecified subgroups were investigated through fitting a subgroup by randomized group interaction term in the model. Treatment effects of each subgroup and a test of interaction were obtained from the model.
Continuous secondary outcomes, such as birthweight and length of stay were analyzed by means of regression method, adjusting for stratification factors. Binary secondary outcomes were analyzed by means of a log binomial model. Because of the potential for type I error due to multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory.
Post hoc analyses were undertaken assessing intervention fidelity comparing app use with the protocol of thrice-weekly self-monitoring until BP increased to 135/85 mm Hg or higher, when participants were asked to monitor daily until delivery or hypertension diagnosis. Recorded hypertension in clinic or by SMBP was cross-tabulated, and the time between first elevated SMBP reading and subsequent diagnosis of hypertension was assessed using similar methods to the primary outcome analysis (second part).
All analyses were performed using STATA SE version 16.1 (StataCorp) using a 5% threshold for significance (2-sided).
Results
Of 2458 potentially eligible pregnant individuals, 2441 were randomized to either SMBP (n = 1223 [50.1%]) or usual care (n = 1218 [49.9%]), in line with the randomization algorithm (Figure). Four participants subsequently found to be ineligible after randomization were immediately withdrawn from the trial and excluded from the analysis.
From the analysis population of 2437 participants, primary outcome data were available for 96% (1171 in the self-monitoring group and 1175 in the usual care group; Figure). Baseline characteristics were well matched between groups with similar demographics and risk factors for hypertension and preeclampsia (Table 1). Mean age was 33 years, mean gestation was 20 weeks, 950 of 2346 (39%) were of parity of 1 or more, 1399 of 2346 (59%) had attained at least an undergraduate degree, and 1801 (77%) were White British, 253 (11%) Asian or Asian British, and 187 (8%) Black or Black British. Mean BP recorded before randomization was 114/69 mm Hg, and 1146 (49%) had 1 major or 2 moderate risk factors for preeclampsia.5 Prior to randomization, 639/2414 (27%) participants reported previously measuring their own BP (305 [25%] in the self-monitoring group and 334 [28%] in the usual care group).
Table 1. Baseline Characteristics of Patients in the BUMP 1 Clinical Trial, by Randomized Group.
| Characteristic | No./total (%) | |
|---|---|---|
| Self-monitoring group (n = 1220)a | Usual care group (n = 1217)a | |
| Age, mean (SD), y | 32.8 (5.7) | 33.0 (5.6) |
| Gestation at entry, mean (SD), wk | 20.3 (1.6) | 20.3 (1.6) |
| Parity: no previous births | 745 (61.1) | 742 (61.0) |
| BMI, median (IQR)b | 26.5 (22.7-32.1) | 26.1 (22.6-32.4) |
| Index of multiple deprivation quintilec | ||
| No. | 1210 | 1211 |
| 1 (most deprived) | 167 (13.8) | 170 (14.0) |
| 2 | 247 (20.4) | 239 (19.7) |
| 3 | 228 (18.8) | 258 (21.3) |
| 4 | 254 (21.0) | 244 (20.2) |
| 5 (least deprived) | 314 (26.0) | 300 (24.8) |
| Race and ethnicityd | ||
| No. | 1211 | 1007 |
| Asian or Asian British | 135 (11.1) | 118 (9.8) |
| Black or Black British | 88 (7.3) | 99 (8.2) |
| Chinese | 16 (1.3) | 11 (0.9) |
| Mixed | 55 (4.5) | 41 (3.4) |
| White (British, Irish, other) | 887 (73.3) | 914 (75.7) |
| Other | 30 (2.5) | 24 (2.0) |
| Current smoker | 57 (4.7) | 59 (4.9) |
| Highest education | ||
| No. | 1209 | 1201 |
| Tertiary education | 715 (59.1) | 684 (57.0) |
| Professional qualifications | 122 (10.1) | 120 (10.0) |
| A-level or General Certificate of Secondary Education | 294 (24.3) | 335 (27.9) |
| Vocational qualifications | 34 (2.8) | 32 (2.7) |
| No formal qualifications | 44 (3.6) | 30 (2.5) |
| Risk factors for hypertension | ||
| BMI ≥30 | 444 (46.4) | 417 (34.3) |
| Previous hypertensive disorder of pregnancy | 199 (16.3) | 220 (18.1) |
| Family history of preeclampsia | 144 (11.8) | 133 (10.9) |
| Autoimmune diseasee | 83 (6.8) | 81 (6.7) |
| Prepregnancy diabetes (type 1 or 2) | 75 (6.2) | 67 (5.5) |
| Twin pregnancy | 73 (6.0) | 67 (5.5) |
| Interval between pregnancies >10 y | 34 (2.8) | 36 (3.0) |
| Chronic kidney disease (any grade) | 9 (0.7) | 14 (1.2) |
| Blood pressure, mean (SD), mm Hgf | ||
| No. | 1161 | 1162 |
| Systolic BP at entry | 113.4 (12.8) | 113.9 (12.3) |
| Diastolic BP at entry | 68.5 (9.0) | 69.0 (9.0) |
| Health questionnaires | ||
| EQ-5D-5L index valueg | ||
| No. | 1202 | 1194 |
| Median (IQR) | 0.88 (0.77-1.00) | 0.85 (0.77-1.00) |
| STAI-6h | ||
| No. | 1201 | 1191 |
| Median (IQR) | 22.2 (5.6-33.3) | 22.2 (5.6-33.3) |
| Self-monitoring prior to trial | ||
| No. | 1209 | 1205 |
| No. (%) | 305 (25) | 334 (28) |
Abbreviations: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared); BP, blood pressure.
As shown in the Figure, 1223 (self-monitoring) and 1218 (control) were randomized; however, 3 intervention participants and 1 usual care participant were randomized in error and withdrawn immediately. Therefore, data are presented for the remaining 1220 and 1217.
Calculated as weight in kilograms divided by height in meters squared.
The index of multiple deprivation is an assessment of deprivation based on multiple weighted components including income, employment, education, health, crime, barriers to housing and services, and living environment. It is assessed at the postcode level.
Ethnicity and race self-attributed from closed list based on standard UK classification. Mixed included those self-identifying as mixed ethnicity (any combination). “Other” included any other category not listed, in which case participants were asked to specify (in the self-monitoring group: Arab: 4, Japanese: 3, Latin American: 2, Mauritian: 2, Brazilian: 1, Filipino: 1, Iraqi Kurdish: 1, Pacific Islander: 1, Tibetan Burmese Origin: 1, Turkish Kurdish: 1, Vietnamese: 1, Middle Eastern: 1, none stated: 11. Usual care group: Latin American: 6, Arab: 3, Japanese: 2, Afghan: 1, Brazilian: 1, Filipino: 1, Iranian: 1, Malaysian: 1, South East Asia: 1, South Korean: 1, Vietnamese: 1, none stated: 5).
Any autoimmune disease, eg, systemic lupus erythematosus or antiphospholipid syndrome.
At last clinic visit prior to randomization.
EQ-5D-5L: EuroQol instrument 5 Dimensions 5 Levels (index value calculated from 5 domains: mobility, self-care, usual activities, pain/discomfort, and anxiety/depression. Value calculated using cross-walk tool22 range, −0.3-1; higher is better quality of life).
STAI: short form of State-Trait Anxiety Inventory (6 items; range, 6-24; scaled to be out of 100, higher is more anxious).20
Primary Outcome
Clinic hypertension was subsequently recorded for 363 (15.5%) of those randomized, of whom 102 (4%) had preeclampsia: 179 (15.3%) in the intervention group and 184 (15.7%) in the usual care group (Table 2). The primary outcome (time to clinic hypertension defined from the clinical records) was not significantly different between individuals who self-monitored or received usual prenatal care alone (mean [SD], 104.3 [32.6] days vs 106.2 [32.0] days, respectively; mean difference, −1.6 days [95% CI, −8.1 to 4.9]; P = .64]) (Table 2). This was not materially affected by sensitivity analyses including adjustment for data missingness, multiple imputation, or when clinic hypertension was restricted to evidence of elevated professionally recorded BP only (ie, recorded diagnosis of gestational hypertension or prescription of antihypertensive medications alone was excluded) (eTable 2 in Supplement 3).
Table 2. Primary Outcome: Time From Randomization to Diagnosis of Elevated Sustained Blood Pressure.
| Self-monitoring group | Usual care group | Difference (95% CI) | P valuea | |
|---|---|---|---|---|
| Participants with primary outcome data | 1171 | 1175 | ||
| Clinic hypertension, No. (%)b | 179 (15.3) | 184 (15.7) | 0.0 (−3 to 2)c | .75 |
| Time to clinic hypertension, d | ||||
| Mean (SD) | 104.3 (32.6) | 106.2 (32.0) | −1.6 (−8.1 to 4.9) | .64 |
| Median (IQR) | 109 (90 to 127) | 115 (90 to 129) |
Self-monitoring vs usual care: threshold level of significance P = .05.
Sustained elevated blood pressure defined as 2 readings ≥140/90 mm Hg within 168 hours or a recorded diagnosis of pregnancy hypertension or prescription of an antihypertensive medication, whichever came first.
Difference in percentage of having elevated blood pressure modeled against randomized group, parity, and site.
The primary outcome was not significantly different between randomized groups in most prespecified subgroups including aspirin eligibility, gestational age at recruitment, parity, previous experience of self-monitoring, deprivation score, or educational qualifications (eTable 3, eFigure 1, and eFigure 2 in Supplement 3). There was a significant interaction for race and ethnicity, but the CIs for both individual group results crossed unity (1.0).
Secondary Outcomes
Maternal Outcomes
There was no statistically significant difference in the incidence of severe hypertension or in the incidence of preeclampsia between the groups (Table 3).
Table 3. Selected Secondary Maternal and Perinatal Outcomes by Randomized Groupa.
| No./total (%) | Adjusted absolute difference, % (95% CI)b | Adjusted risk ratio (95% CI)b | P value for treatment effect | ||
|---|---|---|---|---|---|
| Self-monitoring group | Usual care group | ||||
| Maternal | |||||
| Severe hypertension | 69/1171 (6.0) | 57/1175 (4.9) | 1.09 (−0.94 to 3.12) | 1.22 (0.87 to 1.70) | .25 |
| Preeclampsia | 51/1209 (4.2) | 51/1209 (4.2) | 0.01 (−1.84 to 1.85) | 1.00 (0.66 to 1.51) | >.99 |
| ≥1 Serious maternal complicationsc | 15/1209 (1.2) | 19/1209 (1.6) | |||
| Perinatal | |||||
| Gestation at delivery, median (IQR), wk | 39.3 (38.1 to 40.4) [1190] | 39.3 (38.0 to 40.4) [1185] | 0.14 (−0.01 to 0.30)d | ||
| Stillbirth | 5/1260 (0.4) | 3/1248 (0.2) | |||
| Neonatal death within 7 d | 2/1248 (0.2) | 0/1240 | |||
| Small for gestational age (<10th percentile) | 104/1249 (8.3) | 87/1235 (7.0) | 1.10 (−1.09 to 3.29) | 1.15 (0.87 to 1.53) | .32 |
| Infant admitted to neonatal intensive care unit | 161/1248 (12.9) | 163/1240 (13.1) | −0.64 (−3.34 to 2.05) | 0.95 (0.77 to 1.17) | .63 |
See eTables 3 and 4 in Supplement 3 for additional maternal and perinatal outcomes.
Statistical comparisons completed when >2% event rate for self-monitoring vs usual care. Log-Poisson generalized linear mixed-effects model with robust standard errors adjusted for randomized group and parity as fixed effects; and site as a random effect. Level of significance P < .05.
One or more of the following: eclampsia, transient ischemic attack or stroke, HELLP syndrome (hemolysis, elevated liver enzymes, low platelets), liver involvement (ALT or AST >70 U/L), pulmonary edema, kidney involvement (creatinine ≥90 μmol/L), and hematological involvement (platelets < x100^9/L).
Estimated median difference (95% CI) derived from quantile regression adjusted for randomized arm, parity, and site.
The incidence of serious maternal complications was 15 of 1209 (1.2%) in the self-monitoring group and 19 of 1209 (1.6%) of those receiving usual care. The prespecified threshold of sufficient events to undertake a formal statistical comparison was not met (≥2%) (Table 3). No participants in either group died.
There was no significant difference in the proportion with spontaneous onset of labor (482/1187 [41%] self-monitoring vs 493/1181 [42%] usual care; adjusted relative risk, 0.97 [95% CI, 0.88-1.07]). Indications for labor induction or prelabor cesarean delivery are presented in eTable 4 in Supplement 3.
Anxiety (measured on STAI-6)20 was not significantly different between groups at either 30 weeks’ gestation or postnatally (eTable 6 in Supplement 3). Individuals in the self-monitoring group had significantly improved scores on the adapted Brief Illness Perception Questionnaire at both 30 weeks and postnatally (eTable 7 in Supplement 3).19 Participants’ health-related quality of life based on index scores derived from the EQ-5D-5L descriptive system21 was not significantly different between groups at 30 weeks or at postnatal follow-up (eTable 8 in Supplement 3).
Perinatal Outcomes
The proportions of stillbirths and early neonatal deaths in both groups were not formally compared due to low rates of occurrence (Table 3). Mean birth weight was 3247 g in the self-monitoring group and 3264 g in the usual care group. Small for gestational age infant birth weight (<10th percentile) was present in 104 of 1249 (8.3%) self-monitoring group births vs 87 of 1235 (7.0%) usual care births (adjusted risk ratio, 1.15 [95% CI, 0.87-1.53]). Median gestation at delivery was not different between groups (39 weeks) (Table 3).
There were no significant differences in the proportions of either randomized group admitted to a neonatal unit or for subsequent length of stay (Table 3 and eTable 5 in Supplement 3). Spontaneous vaginal delivery occurred for 550 (43.7%) self-monitoring participants vs 527 (42.4%) usual care individuals (eTable 5 in Supplement 3).
Adverse Events
Eighteen serious adverse events were reported during the trial, with none judged related to the intervention by the supervising site principal investigator: 12 (1%) in the self-monitoring group (2 miscarriages [at 20 weeks and 23 weeks 6 days], 5 stillbirths, 2 neonatal deaths, 3 terminations for fetal abnormalities) and 6 (0.5%) in those receiving usual care (3 stillbirths and 3 terminations for fetal abnormalities [n = 2] and sepsis [n = 1]).
Post Hoc Outcomes
In a post hoc analysis, fidelity to the intervention by individuals randomized to self-monitoring was explored. Of the 1220 allocated to self-monitoring appropriately, 1198 self-monitored and 22 did not. The majority of participants used the app (1196 [99.8%]) with 23 (2.2%) also recording some readings in a paper diary and 2 (0.2%) exclusively using a paper diary. Because data in the paper diaries were not directly comparable to that in the app (eg, in terms of recording of timing of readings, repeat readings), those readings were excluded from further analysis. Participants followed the protocol of monitoring 3 times per week until delivery or diagnosis of clinic hypertension 76.7% of the time; if their SMBP increased to 135/85 mm Hg or higher and they were asked to monitor daily until delivery or hypertension diagnosis, this happened 71.7% of the time.
Of the 179 individuals with clinic hypertension in the intervention group, 131 (73%) had self-monitored within a week of that diagnosis and 16 (9%) had no self-monitored readings at all (Table 4). Of these, 109 of 179 individuals (61%) had an elevated SMBP of 140/90 mm Hg or higher on the same day or before the detection of clinic hypertension. The median time between first elevated SMBP and a subsequent diagnosis of hypertension was 29 days (IQR, 7-72). Of those with a clinic diagnosis of hypertension, 43 of 179 (24%) did not have an elevated SMBP (indicating likely “white coat” hypertension) at or before that time (Table 4).
Table 4. Self-monitoring vs Clinic Blood Pressure (BP) for Diagnosis of Elevated Blood Pressurea.
| Elevated BP on any home readingsb | |||
|---|---|---|---|
| Yes | No | Total | |
| Elevated BP on any health professional readingsc | |||
| Yes | 120 | 43 | 163 |
| No | 240 | 651 | 891 |
| Total | 360 | 694 | 1054 |
Including all 1054 participants randomized to self-monitoring who had both clinician and self-monitored BP recorded; 163 (15.5%) had clinic hypertension; 43 (4.1%) had white coat hypertension (elevated in clinic but not at home); 240 (22.8%) had masked hypertension (elevated at home but not in clinic).
First reading of ≥140/90 mm Hg on home BP device.
Two readings of ≥140/90 mm Hg from any clinical setting within 168 hours or new prescription of antihypertensive medication, whichever came first.
Discussion
In this randomized trial, SMBP from 20 weeks’ gestation until delivery or development of hypertension, in addition to usual care, did not lead to an earlier diagnosis of clinic hypertension, defined on the basis of routinely recorded clinical data. There were no significant differences in either maternal or perinatal outcomes or of serious adverse events. Of those individuals who self-monitored BP in the trial who were diagnosed with hypertension, the majority had self-monitored within a week of diagnosis, suggesting that these individuals would have had an opportunity to detect hypertension at home.
To our knowledge, this was the largest randomized clinical trial of blood pressure self-monitoring in individuals with higher-risk pregnancy published to date and was powered to detect clinically important differences between the groups.25 The pragmatic trial design and broad inclusion criteria make findings applicable to routine antenatal care. Participants randomized had appropriate representation of underrepresented racial and ethnic groups, but there was some evidence of overrepresentation of those with higher educational attainment in the trial overall. Follow-up was high, with more than 95% of the primary outcome data available.
A recent systematic review found 2 randomized clinical trials involving self-monitoring in antenatal care, one of which used self-monitoring as a screening test10; one UK-based group randomized 80 low-risk pregnant individuals to weekly self-monitoring with reduced routine antenatal visits and found that for individuals who self-monitored, overall clinic attendance was reduced despite an increase in unscheduled care.26 A French group randomized 57 individuals with pregnancy hypertension without proteinuria at between 18 and 36 weeks to self-monitor BP with or without transmission of the measurements to their supervising clinicians and found no significant difference between groups.27 More recently, a US group randomized 300 low-risk pregnant individuals to remote monitoring with reduced visits vs usual care. The individuals randomized to remote care had reduced obstetric input, but more nurse/midwife time was needed for providing remote care.11 Two other subsequent trials were not comparable.28,29 Two ongoing self-monitoring trials, one in high-risk pregnancies and one with a similar screening strategy to the current study, have not reported yet.30,31
Self-monitoring of BP outside of pregnancy is already widespread and has a strong evidence base.7,8,32 Prior to the current study there were few data regarding the prevalence of SMBP in pregnancy, although the CHIPS study of different BP targets in pregnancy hypertension reported 38% of hypertensive pregnant individuals as self-monitoring.33 A survey undertaken in parallel to this trial, but excluding those randomized, found that in a sample of around 5500 pregnant individuals, 17% of those without hypertension and 49% of those with hypertension were self-monitoring, often without clinician involvement.34
Limitations
This study has several limitations. First, the trial was powered to detect a 12-day earlier presentation with SMBP compared with a clinic-based diagnosis, a difference that was considered to be clinically relevant. Although very small differences cannot be ruled out, self-monitoring did not result in a clinically important or statistically significant earlier presentation of hypertension. Second, the study was not powered to detect differences in clinical outcomes. Third, the home readings for 26% of individuals with a clinic-based primary outcome of hypertension were within the normal range, so that these participants could not have presented earlier on the basis of self-monitoring. Data on prognosis of white coat hypertension remain sparse, particularly as to the relative effect of antihypertensive therapy compared with true hypertension and, therefore, appropriate management strategies are uncertain.35
Fourth, 61% of those with hypertension in the intervention group had elevated home BP prior to or concurrently with clinic BP, and for these participants, SMBP increased approximately 1 month prior to their clinic recorded hypertension. Participants received advice through the app to check such readings with a midwife but there were no data regarding the response of participants and/or clinicians to such readings. Linked qualitative work suggested that clinicians tend to favor clinic readings in the case of discordance, perhaps explaining the observed lack of effect on the primary outcome.36
Fifth, 27% of randomized individuals had self-monitored prior to randomization, which might have diluted any effect from the intervention. The study did not collect data regarding whether self-monitoring continued later in pregnancy. However, other research suggests that at least half of these participants may have continued to do self-monitoring without the knowledge of their clinical team.34 Outside of pregnancy, there is evidence that such monitoring (without clinical support) has little effect on blood pressure.8,34
Sixth, the threshold for hypertension diagnosis with SMBP in pregnancy is not established. In the current study, the same BP threshold was used for home and clinic BP (140/90 mm Hg), although participants were asked to increase the frequency of measurement once their readings reached 135/85 mm Hg. This choice was made on the basis of a systematic review of BP measurement in different settings, which suggested that self- and clinic-monitored BP were equivalent in normotensive pregnant individuals; it was also influenced by concerns from clinicians during the development phase regarding “overalerting.”13,17 A lower threshold for home readings might have led to a different result.
Conclusions
Among pregnant individuals at higher risk of preeclampsia, blood pressure self-monitoring with telemonitoring compared with usual care did not lead to significantly earlier clinic-based detection of hypertension.
Trial Protocol
Statistical Analysis Plan
Supplemental Tables and Figures
eTable 1. Secondary Outcomes
eTable 2. Primary outcome sensitivity analyses
eTable 3. Subgroup analysis
eTable 4. Additional maternal outcomes
eTable 5. Additional perinatal outcomes
eTable 6. Summary statistics of the STAI-6 short form anxiety questionnaire and the adjusted mean difference between the randomised groups
eTable 7. Summary statistics for the adapted brief illness perception questionnaire
eTable 8. Summary of maternal health-related quality of life measured using EQ-5D-5L instrument
eFigure 1. Forest plot for the subgroup analysis for the raised blood pressure (hurdle effect) outcome
eFigure 2. Forest plot for the subgroup analysis for the time to a raised blood pressure (difference in days, conditional on having a raised blood pressure) outcome
eFigure 3. Blood Pressure Interpretation Chart
The BUMP Investigators
Data Sharing Statement
References
- 1.Abalos E, Cuesta C, Grosso AL, Chou D, Say L. Global and regional estimates of preeclampsia and eclampsia: a systematic review. Eur J Obstet Gynecol Reprod Biol. 2013;170(1):1-7. doi: 10.1016/j.ejogrb.2013.05.005 [DOI] [PubMed] [Google Scholar]
- 2.Wang W, Xie X, Yuan T, et al. Epidemiological trends of maternal hypertensive disorders of pregnancy at the global, regional, and national levels: a population-based study. BMC Pregnancy Childbirth. 2021;21(1):364. doi: 10.1186/s12884-021-03809-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cantwell R, Clutton-Brock T, Cooper G, et al. Saving Mothers’ Lives: reviewing maternal deaths to make motherhood safer: 2006-2008. The Eighth Report of the Confidential Enquiries into Maternal Deaths in the United Kingdom. BJOG. 2011;118(suppl 1):1-203. doi: 10.1111/j.1471-0528.2010.02847.x [DOI] [PubMed] [Google Scholar]
- 4.Saving Lives, Improving Mothers’ Care: Lessons Learned to Inform Maternity Care From the UK and Ireland: Confidential Enquiries Into Maternal Deaths and Morbidity 2016-18. University of Oxford; 2020. [Google Scholar]
- 5.NICE . Hypertension in pregnancy: diagnosis and management (clinical guideline CG107). National Institute for Health and Care Excellence. August 25, 2010. Accessed February 8, 2022. https://www.nice.org.uk/guidance/cg107
- 6.Douglas KA, Redman CW. Eclampsia in the United Kingdom. BMJ. 1994;309(6966):1395-1400. doi: 10.1136/bmj.309.6966.1395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodgkinson JA, Lee MM, Milner S, et al. Accuracy of blood-pressure monitors owned by patients with hypertension (ACCU-RATE study): a cross-sectional, observational study in central England. Br J Gen Pract. 2020;70(697):e548-e554. doi: 10.3399/bjgp20X710381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tucker KL, Sheppard JP, Stevens R, et al. Self-monitoring of blood pressure in hypertension: a systematic review and individual patient data meta-analysis. PLoS Med. 2017;14(9):e1002389. doi: 10.1371/journal.pmed.1002389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Constanti M, Boffa R, Floyd CN, et al. Options for the diagnosis of high blood pressure in primary care: a systematic review and economic model. J Hum Hypertens. 2021;35(5):455-461. doi: 10.1038/s41371-020-0357-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kalafat E, Benlioglu C, Thilaganathan B, Khalil A. Home blood pressure monitoring in the antenatal and postpartum period: a systematic review meta-analysis. Pregnancy Hypertens. 2020;19:44-51. doi: 10.1016/j.preghy.2019.12.001 [DOI] [PubMed] [Google Scholar]
- 11.Butler Tobah YS, LeBlanc A, Branda ME, et al. Randomized comparison of a reduced-visit prenatal care model enhanced with remote monitoring. Am J Obstet Gynecol. 2019;221(6):638. [DOI] [PubMed] [Google Scholar]
- 12.Hinton L, Tucker KL, Greenfield SM, et al. Blood pressure self-monitoring in pregnancy (BuMP) feasibility study; a qualitative analysis of women’s experiences of self-monitoring. BMC Pregnancy Childbirth. 2017;17(1):427. doi: 10.1186/s12884-017-1592-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tucker KL, Taylor KS, Crawford C, et al. Blood pressure self-monitoring in pregnancy: examining feasibility in a prospective cohort study. BMC Pregnancy Childbirth. 2017;17(1):442. doi: 10.1186/s12884-017-1605-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dougall G, Franssen M, Tucker KL, et al. Blood pressure monitoring in high-risk pregnancy to improve the detection and monitoring of hypertension (the BUMP 1 and 2 trials): protocol for two linked randomised controlled trials. BMJ Open. 2020;10(1):e034593. doi: 10.1136/bmjopen-2019-034593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Band R, Hinton L, Tucker KL, et al. Intervention planning and modification of the BUMP intervention: a digital intervention for the early detection of raised blood pressure in pregnancy. Pilot Feasibility Stud. 2019;5:153. doi: 10.1186/s40814-019-0537-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chung Y, de Greeff A, Shennan A. Validation and compliance of a home monitoring device in pregnancy: microlife WatchBP home. Hypertens Pregnancy. 2009;28(3):348-359. doi: 10.1080/10641950802601286 [DOI] [PubMed] [Google Scholar]
- 17.Tucker KL, Bankhead C, Hodgkinson J, et al. How do home and clinic blood pressure readings compare in pregnancy? Hypertension. 2018;72(3):686-694. doi: 10.1161/HYPERTENSIONAHA.118.10917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.NICE . Antenatal care for uncomplicated pregnancies (clinical guideline CG62). National Institute for Health and Care Excellence. March 26, 2008. Accessed February 8, 2022. https://www.nice.org.uk/guidance/cg62
- 19.Broadbent E, Petrie KJ, Main J, Weinman J. The Brief Illness Perception questionnaire. J Psychosom Res. 2006;60(6):631-637. doi: 10.1016/j.jpsychores.2005.10.020 [DOI] [PubMed] [Google Scholar]
- 20.Marteau TM, Bekker H. The development of a six-item short-form of the state scale of the Spielberger State-Trait Anxiety Inventory (STAI). Br J Clin Psychol. 1992;31(3):301-306. doi: 10.1111/j.2044-8260.1992.tb00997.x [DOI] [PubMed] [Google Scholar]
- 21.Herdman M, Gudex C, Lloyd A, et al. Development and preliminary testing of the new five-level version of EQ-5D (EQ-5D-5L). Qual Life Res. 2011;20(10):1727-1736. doi: 10.1007/s11136-011-9903-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Hout B, Janssen MF, Feng YS, et al. Interim scoring for the EQ-5D-5L: mapping the EQ-5D-5L to EQ-5D-3L value sets. Value Health. 2012;15(5):708-715. doi: 10.1016/j.jval.2012.02.008 [DOI] [PubMed] [Google Scholar]
- 23.Ethnic group, national identity and religion. Office for National Statistics. Accessed January 28, 2022. https://www.ons.gov.uk/methodology/classificationsandstandards/measuringequality/ethnicgroupnationalidentityandreligion
- 24.Cragg JG. Some statistical models for limited dependent variables with application to the demand for durable goods. Econometrica. 1971;39(5):829-844. doi: 10.2307/1909582 [DOI] [Google Scholar]
- 25.Tran K, Padwal R, Khan N, Wright MD, Chan WS. Home blood pressure monitoring in the diagnosis and treatment of hypertension in pregnancy: a systematic review and meta-analysis. CMAJ Open. 2021;9(2):E642-E650. doi: 10.9778/cmajo.20200099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ross-McGill H, Hewison J, Hirst J, et al. Antenatal home blood pressure monitoring: a pilot randomised controlled trial. BJOG. 2000;107(2):217-221. doi: 10.1111/j.1471-0528.2000.tb11692.x [DOI] [PubMed] [Google Scholar]
- 27.Denolle T, Weber JL, Calvez C, et al. Diagnosis of white coat hypertension in pregnant women with teletransmitted home blood pressure. Hypertens Pregnancy. 2008;27(3):305-313. doi: 10.1080/10641950802000950 [DOI] [PubMed] [Google Scholar]
- 28.Pealing LM, Tucker KL, Mackillop LH, et al. ; OPTIMUM-BP Investigators . A randomised controlled trial of blood pressure self-monitoring in the management of hypertensive pregnancy. OPTIMUM-BP: a feasibility trial. Pregnancy Hypertens. 2019;18:141-149. doi: 10.1016/j.preghy.2019.09.018 [DOI] [PubMed] [Google Scholar]
- 29.Holm L, Stucke-Brander T, Wagner S, et al. Automated blood pressure self-measurement station compared to office blood pressure measurement for first trimester screening of pre-eclampsia. Health Informatics J. 2019;25(4):1815-1824. doi: 10.1177/1460458218799505 [DOI] [PubMed] [Google Scholar]
- 30.van den Heuvel JFM, Ganzevoort W, De Haan-Jebbink JM, et al. HOspital care versus TELemonitoring in high-risk pregnancy (HOTEL): study protocol for a multicentre non-inferiority randomised controlled trial. BMJ Open. 2019;9(10):e031700. doi: 10.1136/bmjopen-2019-031700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanssens D, Thijs IM, Gyselaers W; PREMOM II – consortium . Design of the Pregnancy REmote MOnitoring II study (PREMOM II): a multicenter, randomized controlled trial of remote monitoring for gestational hypertensive disorders. BMC Pregnancy Childbirth. 2020;20(1):626. doi: 10.1186/s12884-020-03291-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McManus RJ, Mant J, Franssen M, et al. ; TASMINH4 investigators . Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet. 2018;391(10124):949-959. doi: 10.1016/S0140-6736(18)30309-X [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magee LA, von Dadelszen P, Rey E, et al. Less-tight versus tight control of hypertension in pregnancy. N Engl J Med. 2015;372(5):407-417. doi: 10.1056/NEJMoa1404595 [DOI] [PubMed] [Google Scholar]
- 34.Tucker KL, Hodgkinson J, Wilson HM, et al. Current prevalence of self-monitoring of blood pressure during pregnancy: the BUMP Survey. J Hypertens. 2021;39(5):994-1001. doi: 10.1097/HJH.0000000000002734 [DOI] [PubMed] [Google Scholar]
- 35.Johnson S, Liu B, Kalafat E, Thilaganathan B, Khalil A. Maternal and perinatal outcomes of white coat hypertension during pregnancy: a systematic review and meta-analysis. Hypertension. 2020;76(1):157-166. doi: 10.1161/HYPERTENSIONAHA.119.14627 [DOI] [PubMed] [Google Scholar]
- 36.Hinton L, Hodgkinson J, Tucker KL, et al. Exploring the potential for introducing home monitoring of blood pressure during pregnancy into maternity care: current views and experiences of staff-a qualitative study. BMJ Open. 2020;10(12):e037874. doi: 10.1136/bmjopen-2020-037874 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
Supplemental Tables and Figures
eTable 1. Secondary Outcomes
eTable 2. Primary outcome sensitivity analyses
eTable 3. Subgroup analysis
eTable 4. Additional maternal outcomes
eTable 5. Additional perinatal outcomes
eTable 6. Summary statistics of the STAI-6 short form anxiety questionnaire and the adjusted mean difference between the randomised groups
eTable 7. Summary statistics for the adapted brief illness perception questionnaire
eTable 8. Summary of maternal health-related quality of life measured using EQ-5D-5L instrument
eFigure 1. Forest plot for the subgroup analysis for the raised blood pressure (hurdle effect) outcome
eFigure 2. Forest plot for the subgroup analysis for the time to a raised blood pressure (difference in days, conditional on having a raised blood pressure) outcome
eFigure 3. Blood Pressure Interpretation Chart
The BUMP Investigators
Data Sharing Statement


