This case-control study assesses the sex-specific associations of demographic, clinical, and psychosocial risk factors with first acute myocardial infarction among adults younger than 55 years, overall, and by infarction subtype.
Key Points
Question
What are the sex-specific associations of demographic, clinical, and psychosocial risk factors with first acute myocardial infarction (AMI) among adults younger than 55 years, overall, and by AMI subtype?
Findings
In this matched case-control study that included 2264 patients with AMI (aged 18-55 years) and 2264 population-based controls, 7 risk factors, many potentially modifiable, accounted for 85% of the risk of first AMI in young women and men. Significant differences in risk factor profiles and risk factor associations existed by sex and by AMI subtype.
Meaning
Significant sex differences in risk factor profiles suggest the need for sex-specific strategies in risk factor modification and prevention of AMI in young adults.
Abstract
Importance
An increasing proportion of people in the US hospitalized for acute myocardial infarction (AMI) are younger than 55 years, with the largest increase in young women. Effective prevention requires an understanding of risk factors associated with risk of AMI in young women compared with men.
Objectives
To assess the sex-specific associations of demographic, clinical, and psychosocial risk factors with first AMI among adults younger than 55 years, overall, and by AMI subtype.
Design, Setting, and Participants
This study used a case-control design with 2264 patients with AMI, aged 18 to 55 years, from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study and 2264 population-based controls matched for age, sex, and race and ethnicity from the National Health and Nutrition Examination Survey from 2008 to 2012. Data were analyzed from April 2020 to November 2021.
Exposures
A wide range of demographic, clinical, and psychosocial risk factors.
Main Outcomes and Measures
Odds ratios (ORs) and population attributable fractions (PAF) for first AMI associated with demographic, clinical, and psychosocial risk factors.
Results
Of the 4528 case patients and matched controls, 3122 (68.9%) were women, and the median (IQR) age was 48 (44-52) years. Seven risk factors (diabetes [OR, 3.59 (95% CI, 2.72-4.74) in women vs 1.76 (1.19-2.60) in men], depression [OR, 3.09 (95% CI, 2.37-4.04) in women vs 1.77 (1.15-2.73) in men], hypertension [OR, 2.87 (95% CI, 2.31-3.57) in women vs 2.19 (1.65-2.90) in men], current smoking [OR, 3.28 (95% CI, 2.65-4.07) in women vs 3.28 (2.65-4.07) in men], family history of premature myocardial infarction [OR, 1.48 (95% CI, 1.17-1.88) in women vs 2.42 (1.71-3.41) in men], low household income [OR, 1.79 (95% CI, 1.28-2.50) in women vs 1.35 (0.82-2.23) in men], hypercholesterolemia [OR, 1.02 (95% CI, 0.81-1.29) in women vs 2.16 (1.49-3.15) in men]) collectively accounted for the majority of the total risk of AMI in women (83.9%) and men (85.1%). There were significant sex differences in risk factor associations: hypertension, depression, diabetes, current smoking, and family history of diabetes had stronger associations with AMI in young women, whereas hypercholesterolemia had a stronger association in young men. Risk factor profiles varied by AMI subtype, and traditional cardiovascular risk factors had higher prevalence and stronger ORs for type 1 AMI compared with other AMI subtypes.
Conclusions and Relevance
In this case-control study, 7 risk factors, many potentially modifiable, accounted for 85% of the risk of first AMI in young women and men. Significant differences in risk factor profiles and risk factor associations existed by sex and by AMI subtype. These findings suggest the need for sex-specific strategies in risk factor modification and prevention of AMI in young adults. Further research is needed to improve risk assessment of AMI subtypes.
Introduction
Approximately 800 000 people in the US are hospitalized for acute myocardial infarction (AMI) each year, of whom a third are younger than 55 years.1 The proportion of AMI hospitalizations attributable to younger individuals has been increasing steadily, making heart disease a leading cause of mortality and morbidity in this age group.2,3 However, the risk factors for development of AMI and their relative importance by sex and subtypes of AMI in younger ages are not well characterized, limiting the effectiveness of efforts to identify and treat at-risk individuals.
Although previous studies and reviews have assessed risk factors for AMI in younger individuals, they have several limitations.4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19 Most studies have exclusively included people with AMI without a control population. Therefore, they could only evaluate the prevalence of risk factors in people with AMI and were limited in making inferences about risk factors and their relative importance in young women and men.4,5,6,7,8,9,10,11 The few studies that assessed the strength of association between risk factors and AMI12,13,14,15,16,17,18,19 included only men, or only women, or older adults; ascertained AMI cases by self-report or diagnosis coding without rigorous adjudication; evaluated only a couple of risk factors at a time; or were unable to classify AMI subtypes.20,21 Developing a deeper understanding of a wide range of risk factors associated with AMI in young women and men and the strength of these associations, as well as identifying how these associations vary by sex and AMI subtype, has important implications for designing primary prevention strategies targeted to young adults.
Accordingly, we used a case-control design with 2264 patients with AMI from the VIRGO (Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients) study22 and 2264 population-based controls matched for age, sex, and race and ethnicity from the National Health and Nutrition Examination Survey (NHANES) population to examine the associations of a wide range of risk factors on first AMI in young adults and to ascertain if the associations vary by sex and AMI subtype. To the best of our knowledge, this is the largest study in the US that focused on young women and a comparable sample of similarly aged men and comprehensively evaluated the associations between a wide range of predisposing risk factors and incident AMI by sex. The novelty of this study is the design of using NHANES as a control population and then, with matching, the determination of what factors are most important for this population. We hypothesized that there were significant differences in risk factor profiles and risk factor associations by sex.
Methods
Study Population
The study population included patients with AMI from the VIRGO study22 and population-based controls matched for age, sex, and race and ethnicity from the NHANES population. Because AMI had a long latent period for disease manifestation and the incidence rate of AMI was low in young individuals, we chose a case-control study design to test multiple potential risk factors for AMI.23 This study was approved by the Institutional Review Board at Yale University and patients provided written informed consent for their study participation. The study followed the guidelines for cohort studies, described in Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guidelines for case-control studies.
The VIRGO study is the largest prospective observational study of young women and men with AMI in the US.22 We recruited all women aged 18 to 55 years presenting with an AMI to 103 geographically diverse hospitals across the US between August 2008 and January 2012. We recruited a random sample of men with AMI from these hospitals to achieve a 2:1 female-to-male enrollment ratio according to the prespecified protocol. A total of 2985 patients were finally enrolled. The characteristics of VIRGO participants were similar to those of patients with AMI in the US National Inpatient Sample.4 Consistent with the third Universal Definition of MI,24,25 we defined AMI as an increased cardiac biomarker level indicative of myocardial necrosis (at least 1 cardiac biomarker >99th percentile of the upper reference limit) within 24 hours of admission and symptoms of ischemia, electrocardiogram changes indicative of new ischemia, or imaging evidence of infarction. Independent adjudication of AMI subtypes was performed by a team of 5 physicians as previously described.26 The classification of AMI subtypes was based on physicians’ explicit review of several clinical domains: (1) risk factors and clinical presentation, (2) electrocardiography, (3) angiographic findings, (4) left ventricular systolic function, and (5) magnitude of troponin elevation, as well as the reviewers’ impression of the primary and secondary pathophysiological mechanisms underlying the AMI. We defined type 1 AMI as caused by plaque rupture, ulceration, fissuring, erosion, or dissection with resulting thrombus.25 Type 2, type 4b, and unclassified AMI were combined into the “other” category. Type 3, type 4a, and type 5 AMI were excluded from the VIRGO study (eTable 1 in the Supplement). Because we focused on patients with the first AMI, we excluded patients with previous AMI (n = 476).
Population-based controls were derived from NHANES, a series of cross-sectional surveys that provide a nationally representative sample of the noninstitutionalized population in the US.27 We excluded people with a self-reported history of cardiovascular diseases, defined as individuals with a history of coronary heart disease, angina or angina pectoris, MI, or stroke. Controls and cases were matched 1:1 on the basis of age, sex, and race and ethnicity in the main analysis. We included participants from 3 rounds of NHANES conducted between 2007 and 2012 to obtain temporally concurrent controls.
Data Collection
In VIRGO,22 information on patients’ sociodemographic characteristics, lifestyle, family history of cardiovascular diseases, and psychosocial factors was collected by standardized in-person interviews administered by trained personnel during the index AMI admission. Information on medical history, medication use on admission, physical measurements, lipid profile, and hemoglobin A1c level were collected from medical record abstraction. In NHANES, in-person interviews were conducted by trained interviewers to collect participants’ information on sociodemographic characteristics, medical history, family history of cardiovascular diseases, and psychosocial factors. Physical examination, including physical measurements and laboratory tests, was also administered by trained medical personnel in a random subsample of participants. In both VIRGO and NHANES, self-reported physical activity was measured using the Behavioral Risk Factor Surveillance Survey physical activity instrument.28 Symptoms of depression that patients experienced in the past 2 weeks were assessed during the index AMI admission using the 9-item version of the Patient Health Questionnaire (PHQ-9).29
Variable Definitions
We used consistent definitions of risk factors in VIRGO and NHANES (eTable 2 in the Supplement). In both studies, history of hypertension was defined by previous physician diagnosis or currently on antihypertensive medication. We did not use blood pressure levels because among those with AMI, blood pressure could be influenced by the characteristics of the AMI or by evidence-based therapies. Consistent with the American Diabetes Association guideline,30 history of diabetes was defined by previous physician diagnosis, or hemoglobin A1c level 6.5% or greater, or currently receiving antidiabetic medication. History of hypercholesterolemia was defined by previous physician diagnosis, or total cholesterol level 200 mg/dL or greater, or low-density lipoprotein cholesterol level 130 mg/dL or greater, or high-density lipoprotein cholesterol less than 40 mg/dL, or currently receiving lipid-lowering medication (to convert cholesterol to millimoles per liter, multiply by 0.0259). Obesity was defined as body mass index, calculated as weight in kilograms divided by height in meters squared, 30 or greater. High waist circumference was defined as waist circumference greater than 88 cm for women and greater than 102 cm for men. Smoking status was classified into current smokers, former smokers, and never smokers. Physical activity was classified into 2 levels: recommended physical activity (150 minutes or more of moderate physical activity or 75 minutes or more of vigorous physical activity per week) or insufficient physical activity or inactivity (less than 149 minutes per week of moderate physical activity or less than 74 minutes per week of vigorous physical activity).31 Consistent with previous studies, regular alcohol intake was defined as consumption 3 or more times a week.12 Family history of premature MI was defined as having any blood relatives who had an MI before the age of 50 years. Presence of depressive symptoms was defined as PHQ-9 score 10 or greater. Early menopause was defined as a woman reaching menopause before age 45 years.
Statistical Analysis
We used multivariable conditional logistic regression to quantify the association of various risk factors with AMI and adjusted for all covariates that were significant in the univariable analysis. We did not include age, sex, and race and ethnicity as covariates in the models because these variables were adjusted for by matching. Given the focus of sex-specific risk factors for AMI, we developed separate models for men and women and compared the prevalence and odds ratio (OR) of risk factors for men vs women. To test the interaction between sex and risk factors, we developed unconditional logistic regressions with interaction terms between sex and risk factors as well as interaction terms between matching factors because conditional logistic regressions did not allow matching factors to interact with explicitly modeled variables.32 We performed the sex-specific analyses for overall AMI outcome and then further stratified by type 1 AMI vs other AMI subtypes.
We estimated the combined effect of multiple risk factors by deriving the adjusted ORs for combination of risk factors through summation of their respective model coefficients and exponentiation in the multivariable logistic model. We calculated population attributable fraction (PAF), an estimation of the fraction of AMI cases in the population that were attributable to an exposure to 1 or several risk factors, using appropriate methods for case-control studies (see eMethods in the Supplement).33
Missing data were assumed to be missing at random. For covariates considered in each model, missing values were rare (<5% in the study cohort). Missing values were imputed as the most common values for categorical variables and as median values for continuous variables.34 Statistical significance was defined as a P < .05 for 2-sided tests. Analyses were conducted in R, version 4.0 (R Foundation for Statistical Computing).
Results
A total of 2264 patients with a first AMI in VIRGO and 2264 age-, sex-, and race and ethnicity–matched controls in NHANES were included, of whom the median (IQR) age was 48 (44-52) years, and 3122 (68.9%) were women; 210 (4.6%) were Hispanic individuals, 734 (16.2%) were non-Hispanic Black individuals, 3410 (75.3%) were non-Hispanic White individuals, and 174 (3.8%) were of other race or ethnicity (including Asian individuals, Native American individuals, and individuals of other races). Among patients with AMI, 1861 (82.2%) had type 1 AMI, 86 (3.8%) had type 2 AMI, and 317 (14.0%) had other subtypes; 76 (3.4%) had a history of cancer (Table 1).
Table 1. Characteristics of Study Population by Sex.
| Characteristics | No. (%)a | P value for difference between men and women among cases | |||||
|---|---|---|---|---|---|---|---|
| Men | Women | ||||||
| Cases (n = 703) | Controls (n = 703) | P value for difference between cases and controls | Cases (n = 1561) | Controls (n = 1561) | P value for difference between cases and controls | ||
| Sociodemographic characteristics | |||||||
| Age, median (IQR), y | 48 (43-52) | 48 (43-52) | >.99 | 48 (44-52) | 48 (44-53) | .90 | .53 |
| Race and ethnicity | |||||||
| Hispanic | 34 (4.8) | 34 (4.8) | >.99 | 71 (4.5) | 71 (4.5) | >.99 | <.001 |
| Non-Hispanic | |||||||
| Black | 73 (10.4) | 73 (10.4) | 294 (18.8) | 294 (18.8) | |||
| White | 572 (81.4) | 572 (81.4) | 1133 (72.6) | 1133 (72.6) | |||
| Otherb | 24 (3.4) | 24 (3.4) | 63 (4.0) | 63 (4.0) | |||
| Married/living with a partner as if married | 442 (62.9) | 469 (66.7) | .15 | 836 (53.6) | 973 (62.4) | <.001 | <.001 |
| Education | |||||||
| Less than high school | 3 (0.4) | 122 (17.4) | <.001 | 20 (1.3) | 255 (16.4) | <.001 | .17 |
| High school | 282 (40.5) | 175 (24.9) | 627 (40.4) | 347 (22.3) | |||
| More than high school | 411 (59.1) | 406 (57.8) | 904 (58.3) | 957 (61.4) | |||
| Annual household income, $ | |||||||
| <10 000 | 86 (13.1) | 40 (65.8) | <.001 | 304 (20.5) | 103 (6.9) | <.001 | <.001 |
| 10 000-99 000 | 435 (66.0) | 468 (68.2) | 1031 (69.4) | 1063 (70.9) | |||
| ≥100 000 | 138 (20.9) | 178 (25.9) | 151 (10.2) | 333 (22.2) | |||
| Health insurance | 536 (76.6) | 528 (75.1) | .56 | 1236 (79.3) | 12 474 (79.7) | .84 | .16 |
| Insurance covers prescription | 502 (72.3) | 504 (71.9) | .90 | 1171 (75.5) | 1192 (76.6) | .52 | .12 |
| Comorbidities and CVD risk factors | |||||||
| Hypertension | 412 (58.6) | 239 (34.0) | <.001 | 978 (62.7) | 444 (28.5) | <.001 | .075 |
| Diabetes | 161 (22.9) | 84 (12.6) | <.001 | 580 (37.2) | 151 (10.3) | <.001 | <.001 |
| Hypercholesterolemia | 642 (91.3) | 517 (76.9) | <.001 | 1275 (81.7) | 1033 (70.2) | <.001 | <.001 |
| Obesity (BMI ≥30) | 342 (48.6) | 237 (35.1) | <.001 | 839 (53.7) | 610 (40.6) | <.001 | .03 |
| High waist circumference (women, >88 cm; men, >102 cm) | 297 (52.1) | 310 (47.0) | .08 | 988 (81.1) | 1008 (69.6) | <.001 | <.001 |
| Smoking status | |||||||
| Never | 212 (30.2) | 352 (50.2) | <.001 | 453 (29.0) | 871 (55.8) | <.001 | .21 |
| Former | 123 (17.5) | 158 (22.5) | 236 (15.1) | 311 (19.9) | |||
| Current | 367 (52.3) | 191 (27.2) | 872 (55.9) | 378 (24.2) | |||
| Live with anyone who smokes | 248 (35.6) | 142 (20.3) | <.001 | 626 (40.3) | 297 (19.2) | <.001 | .04 |
| Regular alcohol intake | 151 (21.9) | 161 (22.9) | .72 | 161 (10.6) | 181 (11.6) | .39 | <.001 |
| Physical activity | |||||||
| Recommended | 324 (46.6) | 507 (72.1) | <.001 | 537 (34.7) | 913 (58.5) | <.001 | <.001 |
| Insufficient | 181 (26.0) | 87 (12.4) | 470 (30.4) | 268 (17.2) | |||
| Inactive | 191 (27.4) | 109 (15.5) | 542 (34.9) | 380 (24.3) | |||
| History of congestive heart failure | 9 (1.3) | 5 (0.7) | .42 | 46 (2.9) | 12 (0.8) | <.001 | .025 |
| Family history of premature MI | 202 (30.3) | 77 (11.3) | <.001 | 459 (30.9) | 222 (14.5) | <.001 | .842 |
| Family history of diabetes | 295 (42.8) | 281 (40.7) | .45 | 819 (53.2) | 650 (42.2) | <.001 | <.001 |
| Depression | 140 (20.6) | 51 (8.3) | <.001 | 588 (39.1) | 165 (12.4) | <.001 | <.001 |
| Menopause before age 45 y | 0 | 0 | NA | 91 (6.1) | 48 (3.6) | .003 | NA |
| Medication use | |||||||
| Use of statin | 129 (18.3) | 92 (13.1) | .008 | 318 (20.4) | 177 (11.3) | <.001 | .29 |
| Use of β-blocker | 96 (13.7) | 54 (7.7) | <.001 | 265 (17.0) | 107 (6.9) | <.001 | .05 |
| Use of aspirin | 119 (16.9) | 5 (0.7) | <.001 | 288 (18.4) | 8 (0.5) | <.001 | .42 |
Abbreviations: BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CVD, cardiovascular disease; MI, myocardial infarction.
All percentages were calculated by excluding missing, do not know, and patient refused.
Other races include Asian individuals, Native American individuals, and individuals of other races.
Sex-Specific Associations of Risk Factors With AMI in Young Adults
In the univariable analyses, 13 risk factors were significantly associated with higher odds of AMI among women, while 9 risk factors were significantly associated with higher odds of AMI among men (Figure 1A). Significant sex interactions were observed for diabetes, depression, hypertension, current smoking, family history of diabetes, and hypercholesterolemia, and these risk factors had stronger associations with AMI in women compared with men, except for hypercholesterolemia, which had stronger associations in men (Figure 1A).
Figure 1. Association of Risk Factors With AMI in Women vs Men.
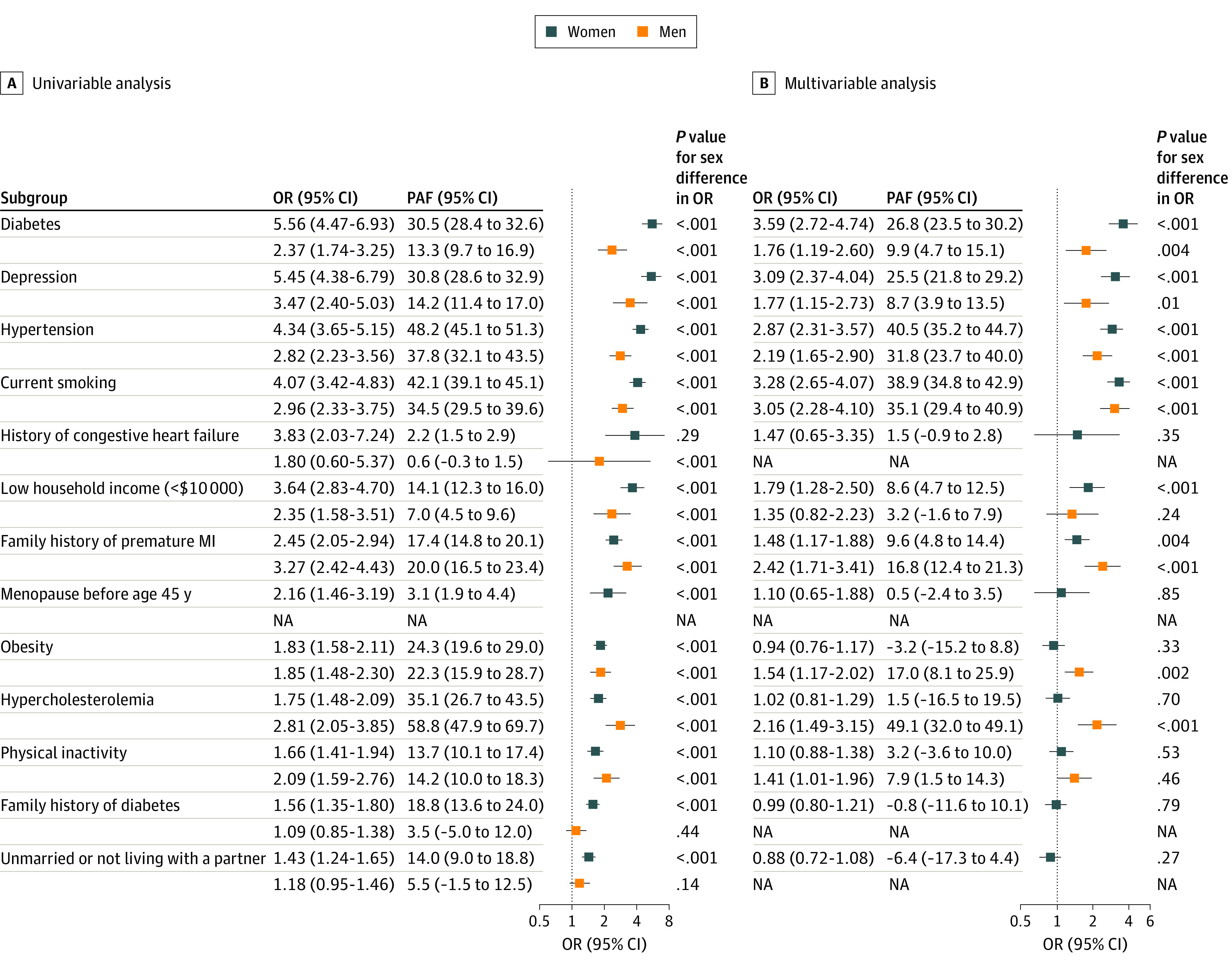
Only risk factors that were significant in the univariable analysis were included in the multivariable analysis. Age and race and ethnicity were adjusted by matching. AMI indicates acute myocardial infarction; OR, odds ratio; PAF, population attributable fraction.
After multivariable adjustment, 7 risk factors (diabetes, depression, hypertension, current smoking, family history of premature MI, low household income, hypercholesterolemia) remained statistically significant in men or women. Specifically, diabetes was associated with the highest odds of AMI among women, followed by current smoking, depression, hypertension, low household income, and family history of premature MI (Figure 1B). Current smoking was associated with the highest odds of AMI among men, followed by family history of premature MI, hypertension, hypercholesterolemia, depression, diabetes, obesity, and physical inactivity. Low household income was significantly associated with AMI in women but not in men, whereas hypercholesterolemia and physical inactivity were significantly associated with AMI in men but not in women.
The contribution of individual risk factors to the total risk of AMI varied between men and women. The PAFs of diabetes (26.8% vs 9.9%), depression (25.5 vs 8.7%), hypertension (40.5% vs 31.8%), and current smoking (38.9% vs 35.1%) were greater among women compared with men, whereas the PAFs of hypercholesterolemia (49.1% vs 1.5%) and family history of premature MI (16.8% vs 9.6%) were greater among men compared with women. Four preventable risk factors (current smoking, hypertension, diabetes, and depression) had a combined PAF of 80.2% in women compared with 63.2% in men. All 7 risk factors (diabetes, depression, hypertension, current smoking, family history of premature MI, low household income, hypercholesterolemia) had a PAF of 83.9% in women and a PAF of 85.1% in men.
Difference in Risk by AMI Subtype
The distribution of risk factors varied significantly by AMI subtype (Table 2). Compared with type 1 AMI, other types of AMI were overrepresented in young women (78.2% vs 67.0%; P < .001) and Black individuals (19.1% vs 15.6%; P = .007). Traditional cardiovascular risk factors, such as diabetes (33.7% vs 28.0%), hypercholesteremia (86.6% vs 75.7%), obesity (53.8% vs 44.7%), and current smoking (57.4% vs 42.4%), had significantly higher prevalence in patients with type 1 AMI than in those with other types of AMI (P < .05 for all; Figure 2). These risk factors were also more strongly associated with type 1 AMI for both men and women (Figure 2).
Table 2. Characteristics of Study Population Stratified by Type of AMI.
| Characteristics | No. (%)a | P value for difference between AMI subtypes among cases | |||
|---|---|---|---|---|---|
| Type 1 AMI | Other types of AMIb | ||||
| Cases (n = 1861) | Controls (n = 1861) | Cases (n = 403) | Controls (n = 403) | ||
| Sociodemographic characteristics | |||||
| Age, median (IQR), y | 48 (44-52) | 48 (44-52) | 48 (42-52) | 48 (42-52) | .13 |
| Women | 1246 (67.0) | 1246 (67.0) | 315 (78.2) | 315 (78.2) | <.001 |
| Race and ethnicity | |||||
| Hispanic | 80 (4.3) | 80 (4.3) | 25 (6.2) | 25 (6.2) | .007 |
| Non-Hispanic | |||||
| Black | 290 (15.6) | 290 (15.6) | 77 (19.1) | 77 (19.1) | |
| White | 1427 (76.7) | 1427 (76.7) | 278 (69.0) | 278 (69.0) | |
| Otherc | 64 (3.4) | 64 (3.4) | 23 (5.7) | 23 (5.7) | |
| Married/living with a partner as if married | 1061 (57.1) | 1187 (63.9) | 215 (53.3) | 264 (65.5) | .18 |
| Education | |||||
| Less than high school | 15 (0.8) | 330 (17.8) | 8 (2.0) | 80 (19.9) | .09 |
| High school | 745 (40.3) | 429 (23.1) | 164 (41.1) | 84 (20.8) | |
| More than high school | 1088 (58.9) | 1100 (59.2) | 227 (56.9) | 239 (59.3) | |
| Annual household income, $ | |||||
| <10 000 | 324 (18.3) | 117 (6.5) | 66 (17.5) | 21 (5.4) | .34 |
| 10 000-99 000 | 1197 (67.7) | 1248 (69.4) | 269 (71.2) | 277 (70.7) | |
| ≥100 000 | 246 (13.9) | 432 (24.0) | 43 (11.4) | 94 (24.0) | |
| Health insurance | 1439 (77.6) | 1464 (78.7) | 333 (82.6) | 309 (76.7) | .03 |
| Insurance covers prescription | 1366 (74.0) | 1411 (76.1) | 307 (77.3) | 296 (73.6) | .18 |
| Comorbidities and CVD risk factors | |||||
| Hypertension | 1150 (61.8) | 541 (29.1) | 240 (59.6) | 115 (28.5) | .43 |
| Diabetes | 628 (33.7) | 199 (11.5) | 113 (28.0) | 34 (9.1) | .03 |
| Hypercholesterolemia | 1612 (86.6) | 1289 (74.1) | 305 (75.7) | 256 (68.6) | <.001 |
| Obesity (BMI ≥30) | 1001 (53.8) | 666 (37.2) | 180 (44.7) | 140 (36.4) | .001 |
| High waist circumference (women, >88 cm; men, >102 cm) | 1064 (72.1) | 1054 (60.9) | 221 (70.8) | 238 (64.3) | .71 |
| Smoking status | |||||
| Never smoker | 504 (27.1) | 966 (52.0) | 161 (40.0) | 223 (55.3) | <.001 |
| Former smoker | 288 (15.5) | 398 (21.4) | 71 (17.6) | 90 (22.3) | |
| Current smoker | 1068 (57.4) | 495 (26.6) | 171 (42.4) | 90 (22.3) | |
| Live with anyone who smokes | 746 (40.3) | 380 (20.5) | 128 (32.0) | 71 (17.7) | .002 |
| Regular alcohol intake | 259 (14.2) | 308 (16.6) | 53 (13.5) | 55 (13.6) | .77 |
| Physical activity | |||||
| Recommended | 686 (37.2) | 1158 (44.3) | 175 (43.8) | 247 (61.3) | <.001 |
| Insufficient | 559 (30.3) | 296 (15.9) | 92 (23.0) | 62 (15.4) | |
| Inactive | 598 (32.4) | 407 (21.9) | 133 (33.2) | 94 (23.3) | |
| History of congestive heart failure | 41 (2.2) | 17 (0.9) | 14 (3.5) | 1 (0.2) | .19 |
| Family history of premature MI | 548 (31.0) | 245 (13.4) | 113 (29.4) | 71 (17.9) | .59 |
| Family history of diabetes | 931 (50.9) | 758 (41.3) | 183 (45.8) | 165 (41.7) | .07 |
| Depression | 603 (33.7) | 191 (11.7) | 125 (32.0) | 36 (10.4) | .56 |
| Menopause before age 45 y | 71 (6.0) | 41 (3.8) | 20 (6.6) | 9 (3.4) | .85 |
| Medication use | |||||
| Use of statin | 361 (19.4) | 203 (10.9) | 86 (21.3) | 38 (9.4) | .41 |
| Use of β-blocker | 292 (15.7) | 136 (7.3) | 69 (17.1) | 31 (7.7) | .52 |
| Use of aspirin | 339 (18.2) | 12 (0.6) | 68 (16.9) | 3 (0.7) | .57 |
Abbreviations: AMI, acute myocardial infarction; BMI, body mass index, calculated as weight in kilograms divided by height in meters squared; CAD, coronary artery disease; CVD, cardiovascular disease; MI, myocardial infarction.
All percentages are calculated by excluding missing, do not know, and patient refused.
Other types of AMI include type 2 (condition other than CAD contributes to imbalance between myocardial oxygen supply or demand), type 4b (stent thrombosis), and unclassified.
Other races include Asian individuals, Native American individuals, and individuals of other races.
Figure 2. Association of Risk Factors With Acute Myocardial Infarction (AMI) in Type 1 AMI vs Other Types of AMI, Adjusted for Age, Sex, and Race and Ethnicity.
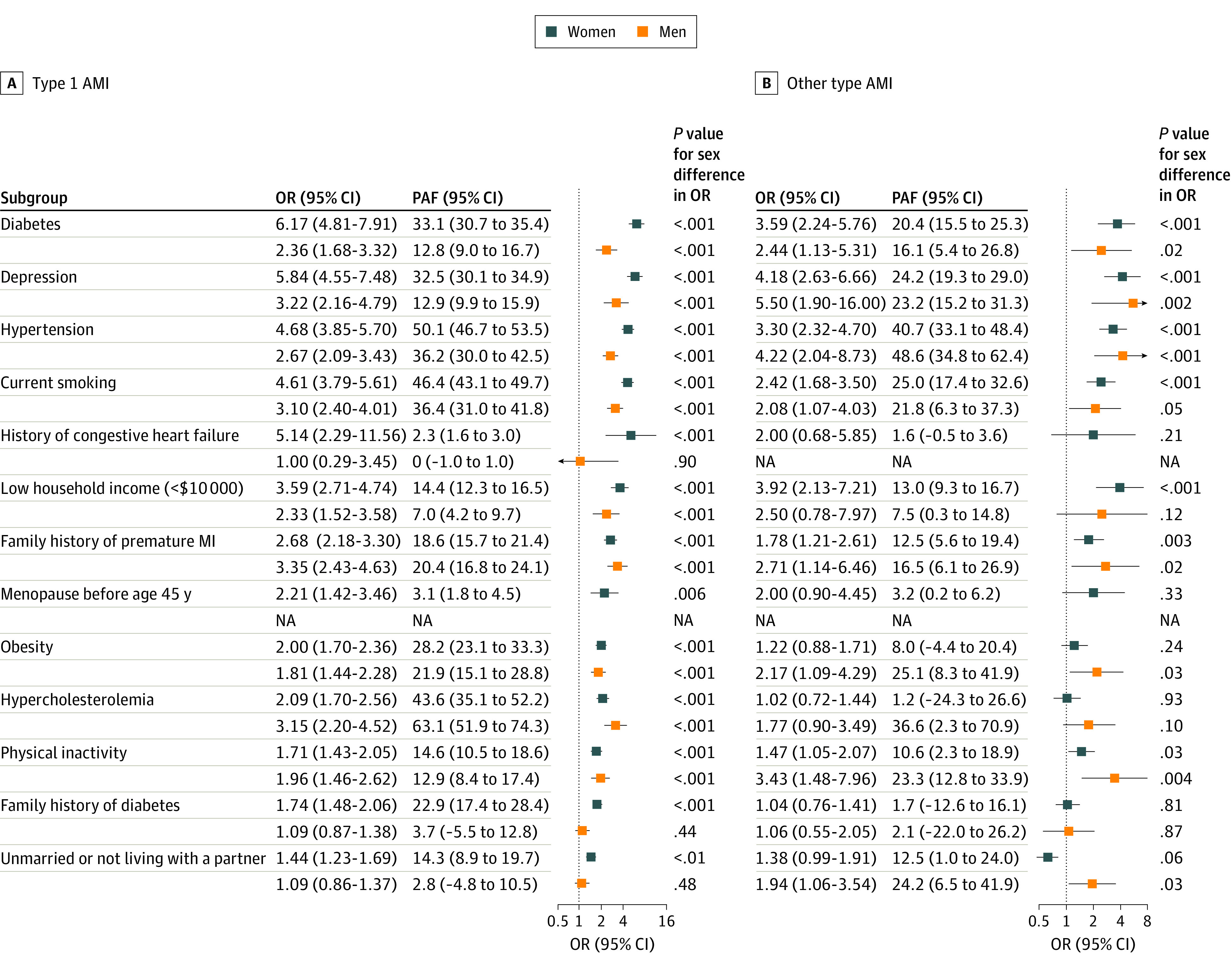
Age, sex, and race were adjusted by matching. OR indicates odds ratio; PAF, population attributable fraction.
There were sex differences in risk factor associations and PAFs by AMI subtype (Figure 2). Diabetes (OR: 6.17 vs 2.36; PAF: 33.1% vs 12.8%), depression (OR: 5.84 vs 3.22; PAF: 32.5% vs 12.9%), hypertension (OR: 4.68 vs 2.67; PAF: 50.1% vs 36.2%), current smoking (OR: 4.61 vs 3.10; PAF: 46.4% vs 36.4%), family history of diabetes (OR: 1.74 vs 1.09; PAF: 22.9% vs 3.7%), and history of congestive heart failure (OR: 5.14 vs 1.0; PAF: 2.3% vs 0%) were more strongly associated with type 1 AMI in women compared with men, whereas hypercholesterolemia (OR: 2.09 vs 3.15; PAF: 43.6% vs 63.1%) was more strongly associated with type 1 AMI in men. The risk factor associations with other types of AMI were generally similar in men and women except for physical inactivity, which was more strongly associated with men than women (OR: 1.47 vs 3.43; PAF: 10.6% vs 23.3%).
Discussion
In this case-control study, 7 risk factors (diabetes, depression, hypertension, current smoking, family history of premature MI, low household income, hypercholesterolemia) accounted for 85% of the risk of first AMI in young men and women. We further found that risk factors accounted for different risk of AMI in women compared with men, and that some of the significant factors varied by sex. Risk factors also varied by subtype of AMI, with traditional cardiovascular risk factors having higher prevalence and stronger associations for type 1 AMI compared with other types of AMI not resulting from acute plaque rupture.
This study extends the field in 3 important ways. First, compared with studies conducted in younger individuals, such as the Coronary Artery Risk Development in Young Adults (CARDIA) study and the Framingham Offspring Study,35,36,37,38 we included a much larger number of patients with AMI and examined the associations of a wide range of metabolic, familial, and social risk factors that had previously not been fully assessed, providing a more comprehensive picture of risk factors for AMI in young people. We showed that family history of premature MI has a strong association with risk of AMI in young adults, even after adjusting for other covariates. This finding is in contrast to prior observations in older populations in which the association of family history is negligible after accounting for prevalent risk factors,12 highlighting the importance of inheritable risk for AMI among younger adults.39 The associations of depression and low household income are also strong after adjusting for other risk factors, suggesting that both socioeconomic and psychological factors play an important role in the development of AMI in young individuals, particularly young women.21
Second, this study identifies significant sex differences in risk factors associated with AMI and in the strength of associations among young adults. While sex differences in the presence of risk factors among patients with AMI have been extensively studied,2,9,10 there is less evidence for the relative importance of pre-event risk factors in this young AMI population. The INTERHEART study, which focused on older populations, observed that hypertension and diabetes had stronger associations in women compared with men, and significant sex interactions were noted.12,13 We provide an independent validation of these observations in young adults and further show that current smoking and gender-related characteristics, such as depression and low income,40 were associated with a greater OR and PAF in women compared with men. Given that the prevalence of these modifiable risk factors is increasing in the US,4 the high prevalence of risk factors and the strong association with AMI in young women together will put them at a substantial risk for future cardiovascular events.
Third, based on rigorous adjudication of AMI cases, our analysis by AMI subtype provides new insights into the epidemiology of etiologically distinct classes of myocardial injury in young adults. Despite the increasing recognition of varying types of AMI in recent years, evidence on the epidemiology, including patient characterization and associated risk factors, is lacking.41 We demonstrate significant differences in risk factor profiles and their associations with outcome between subtypes of AMI. We also show that type 2 AMI—a subtype associated with higher mortality42—is overrepresented in young women and Black individuals. Moreover, given the breadth of clinical phenotypes in young women, we previously developed a new taxonomy for classifying diverse phenotypic presentations of young women with AMI. We found that AMI without obstructive coronary artery stenoses (ie, maximal stenosis of any 1 major epicardial vessel <50%) disproportionally affected young women (11.3% of women vs 2.7% of men).26
This study has clinical and public health implications. As family history of MI is a strong risk factor in young individuals, more research is needed to understand the role of familial factors in developing AMI in young adults. Some familial risk factors may portend higher risk, as recent studies have shown high polygenic score to be associated with an increased risk of AMI in this young population.39 In addition, this study identifies the need for sex-specific strategies in risk factor modification and prevention of AMI in young women. Beyond traditional risk factors, gender-related characteristics, such as psychological stressors, depression, and poverty, also play a sizeable role in women’s AMI risk, and hence a multifaceted approach targeted to these risk factors is important to improve sex- and gender-based differences in patient outcomes. The implementation of evidence-based guidelines on preventing AMI in women should be expanded to assist clinicians in decision-making, and effective strategies need to be identified to improve optimal delivery of guidelines.43 National initiatives, such as the American Heart Association Go Red for Women campaign, should also be expanded to increase awareness about cardiovascular disease risk in young women.44 Finally, our results of different risk factors associated with distinct AMI subtypes call for a more precise assessment of AMI risk in young adults. While current risk prediction tools target an aggregate diagnosis of AMI,45,46 development of more specific individual patient risk prediction for each AMI subtype will allow for effective application of preventive therapies in an individual patient. Documentation of detailed MI characterization as specified by international consensus24 is also critical to facilitate the understanding of the epidemiology, pathophysiology, and subsequent prognostic implications of different AMI subtypes in young adults.
Limitations
This study has several limitations. First, while VIRGO and NHANES samples were well matched by age, sex, and race and ethnicity, they may have differed in underlying factors that are known to be associated with AMI. The patients with AMI in VIRGO came from 103 geographically diverse hospitals across the US, and their characteristics were similar to those of patients with AMI nationally.4 Therefore, the source population that gave rise to the patients with AMI included in the study was likely to be similar to the population from which controls were selected. Second, similar to any other observational study, there is a potential for residual confounding. We minimized the confounding due to measurement error by using standardized methods for data collection and consistent definitions for variables in both patients with AMI and controls. We restricted patients with AMI to those with a first event to reduce the possibility that individuals with previous cardiovascular disease might have substantially changed risk factor levels before this event. Third, certain factors, such as diet, stress, illicit drug use, familial hyperlipidemia, history of congenital heart disease, pregnancy, and childbearing, were either not measured or were measured using inconsistent methods in VIRGO and NHANES; hence, we were unable to assess their associations with AMI in this study. As suggested by previous studies, these factors may be important contributors to AMI risk in young adults.18,19 Fourth, as with any other studies that use interviews to obtain medical history of participants, there may be recall bias in patients with AMI who tend to report family history more often than people without AMI. However, family history of AMI was measured by standardized questionnaire in both VIRGO and NHANES, and we used consistent definitions of risk factors in the 2 studies to minimize measurement error. Finally, the results of PAF need to be interpreted with caution, as patients with AMI in this study may have had different risk factor distributions compared with other patients with AMI.
Conclusions
In this case-control study, 7 risk factors, many potentially modifiable, accounted for 85% of the risk of first AMI in young individuals. Significant differences in risk factor profiles and risk factor associations existed by sex and by subtypes of AMI. These findings suggest the need for sex-specific strategies in risk factor modification and prevention of AMI in young adults. Further research is needed to improve risk assessment of AMI subtypes.
eMethods. Methods to calculate population attributable fraction for individual risk factors
eTable 1. AMI classification by the third global definition of MI
eTable 2. Comparison of variables in VIRGO and NHANES
References
- 1.Benjamin EJ, Virani SS, Callaway CW, et al. ; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee . Heart disease and stroke statistics—2018 update: a report from the American Heart Association. Circulation. 2018;137(12):e67-e492. doi: 10.1161/CIR.0000000000000558 [DOI] [PubMed] [Google Scholar]
- 2.Arora S, Stouffer GA, Kucharska-Newton AM, et al. Twenty year trends and sex differences in young adults hospitalized with acute myocardial infarction. Circulation. 2019;139(8):1047-1056. doi: 10.1161/CIRCULATIONAHA.118.037137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gupta A, Wang Y, Spertus JA, et al. Trends in acute myocardial infarction in young patients and differences by sex and race, 2001 to 2010. J Am Coll Cardiol. 2014;64(4):337-345. doi: 10.1016/j.jacc.2014.04.054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yandrapalli S, Nabors C, Goyal A, Aronow WS, Frishman WH. Modifiable risk factors in young adults with first myocardial infarction. J Am Coll Cardiol. 2019;73(5):573-584. doi: 10.1016/j.jacc.2018.10.084 [DOI] [PubMed] [Google Scholar]
- 5.Fournier JA, Sánchez-González A, Quero J, et al. Normal angiogram after myocardial infarction in young patients: a prospective clinical-angiographic and long-term follow-up study. Int J Cardiol. 1997;60(3):281-287. doi: 10.1016/S0167-5273(97)00115-0 [DOI] [PubMed] [Google Scholar]
- 6.Zimmerman FH, Cameron A, Fisher LD, Ng G. Myocardial infarction in young adults: angiographic characterization, risk factors and prognosis (Coronary Artery Surgery Study Registry). J Am Coll Cardiol. 1995;26(3):654-661. doi: 10.1016/0735-1097(95)00254-2 [DOI] [PubMed] [Google Scholar]
- 7.Barbash GI, White HD, Modan M, et al. ; The Investigators of the International Tissue Plasminogen Activator/Streptokinase Mortality Trial . Acute myocardial infarction in the young—the role of smoking. Eur Heart J. 1995;16(3):313-316. [PubMed] [Google Scholar]
- 8.Uhl GS, Farrell PW. Myocardial infarction in young adults: risk factors and natural history. Am Heart J. 1983;105(4):548-553. doi: 10.1016/0002-8703(83)90476-3 [DOI] [PubMed] [Google Scholar]
- 9.Pelletier R, Choi J, Winters N, et al. ; GENESIS-PRAXY Investigators . Sex differences in clinical outcomes after premature acute coronary syndrome. Can J Cardiol. 2016;32(12):1447-1453. doi: 10.1016/j.cjca.2016.05.018 [DOI] [PubMed] [Google Scholar]
- 10.Bucholz EM, Strait KM, Dreyer RP, et al. Editor’s Choice—Sex differences in young patients with acute myocardial infarction: a VIRGO study analysis. Eur Heart J Acute Cardiovasc Care. 2017;6(7):610-622. doi: 10.1177/2048872616661847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leifheit-Limson EC, D’Onofrio G, Daneshvar M, et al. Sex differences in cardiac risk factors, perceived risk, and health care provider discussion of risk and risk modification among young patients with acute myocardial infarction: the VIRGO study. J Am Coll Cardiol. 2015;66(18):1949-1957. doi: 10.1016/j.jacc.2015.08.859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yusuf S, Hawken S, Ounpuu S, et al. ; INTERHEART Study Investigators . Effect of potentially modifiable risk factors associated with myocardial infarction in 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):937-952. doi: 10.1016/S0140-6736(04)17018-9 [DOI] [PubMed] [Google Scholar]
- 13.Anand SS, Islam S, Rosengren A, et al. ; INTERHEART Investigators . Risk factors for myocardial infarction in women and men: insights from the INTERHEART study. Eur Heart J. 2008;29(7):932-940. doi: 10.1093/eurheartj/ehn018 [DOI] [PubMed] [Google Scholar]
- 14.Iribarren C, Go AS, Husson G, et al. Metabolic syndrome and early-onset coronary artery disease: is the whole greater than its parts? J Am Coll Cardiol. 2006;48(9):1800-1807. doi: 10.1016/j.jacc.2006.03.070 [DOI] [PubMed] [Google Scholar]
- 15.Dunder K, Lind L, Lagerqvist B, Zethelius B, Vessby B, Lithell H. Cardiovascular risk factors for stable angina pectoris versus unheralded myocardial infarction. Am Heart J. 2004;147(3):502-508. doi: 10.1016/j.ahj.2003.09.010 [DOI] [PubMed] [Google Scholar]
- 16.Stamler J, Stamler R, Neaton JD, et al. Low risk-factor profile and long-term cardiovascular and noncardiovascular mortality and life expectancy: findings for 5 large cohorts of young adult and middle-aged men and women. JAMA. 1999;282(21):2012-2018. doi: 10.1001/jama.282.21.2012 [DOI] [PubMed] [Google Scholar]
- 17.Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363:k4247. doi: 10.1136/bmj.k4247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rizk T, Blankstein R. Not all heart attacks are created equal: thinking differently about acute myocardial infarction in the young. Methodist Debakey Cardiovasc J. 2021;17(4):60-67. doi: 10.14797/mdcvj.345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gulati R, Behfar A, Narula J, et al. Acute myocardial infarction in young individuals. Mayo Clin Proc. 2020;95(1):136-156. doi: 10.1016/j.mayocp.2019.05.001 [DOI] [PubMed] [Google Scholar]
- 20.Rosengren A, Hawken S, Ounpuu S, et al. ; INTERHEART investigators . Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet. 2004;364(9438):953-962. doi: 10.1016/S0140-6736(04)17019-0 [DOI] [PubMed] [Google Scholar]
- 21.Hemingway H, Marmot M. Evidence based cardiology: psychosocial factors in the aetiology and prognosis of coronary heart disease. systematic review of prospective cohort studies. BMJ. 1999;318(7196):1460-1467. doi: 10.1136/bmj.318.7196.1460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lichtman JH, Lorenze NP, D’Onofrio G, et al. Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) study design. Circ Cardiovasc Qual Outcomes. 2010;3(6):684-693. doi: 10.1161/CIRCOUTCOMES.109.928713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Lippincott Williams & Wilkins; 2008. [Google Scholar]
- 24.Thygesen K, Alpert JS, Jaffe AS, et al. ; Executive Group on behalf of the Joint European Society of Cardiology (ESC)/American College of Cardiology (ACC)/American Heart Association (AHA)/World Heart Federation (WHF) Task Force for the Universal Definition of Myocardial Infarction . Fourth universal definition of myocardial infarction (2018). Circulation. 2018;138(20):e618-e651. doi: 10.1161/CIR.0000000000000617 [DOI] [PubMed] [Google Scholar]
- 25.Thygesen K, Alpert JS, Jaffe AS, et al. ; Joint ESC/ACCF/AHA/WHF Task Force for the Universal Definition of Myocardial Infarction . Third universal definition of myocardial infarction. Circulation. 2012;126(16):2020-2035. doi: 10.1161/CIR.0b013e31826e1058 [DOI] [PubMed] [Google Scholar]
- 26.Spatz ES, Curry LA, Masoudi FA, et al. The Variation in Recovery: Role of Gender on Outcomes of Young AMI Patients (VIRGO) classification system: a taxonomy for young women with acute myocardial infarction. Circulation. 2015;132(18):1710-1718. doi: 10.1161/CIRCULATIONAHA.115.016502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention . US National Health and Nutrition Examination Survey. Accessed May 17, 2019. https://www.cdc.gov/nchs/nhanes.htm
- 28.Yore MM, Ham SA, Ainsworth BE, et al. Reliability and validity of the instrument used in BRFSS to assess physical activity. Med Sci Sports Exerc. 2007;39(8):1267-1274. doi: 10.1249/mss.0b013e3180618bbe [DOI] [PubMed] [Google Scholar]
- 29.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med. 2001;16(9):606-613. doi: 10.1046/j.1525-1497.2001.016009606.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American Diabetes Association . 2. Classification and diagnosis of diabetes: Standards of Medical Care in Diabetes—2020. Diabetes Care. 2020;43(suppl 1):S14-S31. doi: 10.2337/dc20-S002 [DOI] [PubMed] [Google Scholar]
- 31.Haskell WL, Lee IM, Pate RR, et al. ; American College of Sports Medicine; American Heart Association . Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081-1093. doi: 10.1161/CIRCULATIONAHA.107.185649 [DOI] [PubMed] [Google Scholar]
- 32.International Agency for Research on Cancer . Statistical Methods in Cancer Research: The Analysis of Case-Control Studies. International Agency for Research on Cancer; 1980. [Google Scholar]
- 33.Mansournia MA, Altman DG. Population attributable fraction. BMJ. 2018;360:k757. doi: 10.1136/bmj.k757 [DOI] [PubMed] [Google Scholar]
- 34.Dong Y, Peng CY. Principled missing data methods for researchers. Springerplus. 2013;2(1):222. doi: 10.1186/2193-1801-2-222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fornage M, Lopez DS, Roseman JM, Siscovick DS, Wong ND, Boerwinkle E. Parental history of stroke and myocardial infarction predicts coronary artery calcification: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Eur J Cardiovasc Prev Rehabil. 2004;11(5):421-426. doi: 10.1097/01.hjr.0000129744.61087.b4 [DOI] [PubMed] [Google Scholar]
- 36.Djoussé L, Rothman KJ, Cupples LA, Levy D, Ellison RC. Effect of serum albumin and bilirubin on the risk of myocardial infarction (the Framingham Offspring Study). Am J Cardiol. 2003;91(4):485-488. doi: 10.1016/S0002-9149(02)03256-3 [DOI] [PubMed] [Google Scholar]
- 37.Lloyd-Jones DM, Nam BH, D’Agostino RB Sr, et al. Parental cardiovascular disease as a risk factor for cardiovascular disease in middle-aged adults: a prospective study of parents and offspring. JAMA. 2004;291(18):2204-2211. doi: 10.1001/jama.291.18.2204 [DOI] [PubMed] [Google Scholar]
- 38.Chami T, Kim CH. Cannabis abuse and elevated risk of myocardial infarction in the young: a population-based study. Mayo Clin Proc. 2019;94(8):1647-1649. doi: 10.1016/j.mayocp.2019.05.008 [DOI] [PubMed] [Google Scholar]
- 39.Khera AV, Chaffin M, Zekavat SM, et al. Whole-genome sequencing to characterize monogenic and polygenic contributions in patients hospitalized with early-onset myocardial infarction. Circulation. 2019;139(13):1593-1602. doi: 10.1161/CIRCULATIONAHA.118.035658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pelletier R, Ditto B, Pilote L. A composite measure of gender and its association with risk factors in patients with premature acute coronary syndrome. Psychosom Med. 2015;77(5):517-526. doi: 10.1097/PSY.0000000000000186 [DOI] [PubMed] [Google Scholar]
- 41.DeFilippis AP, Nasir K, Blaha MJ. Myocardial infarction as a clinical end point in research. Circ Res. 2019;124(12):1701-1703. doi: 10.1161/CIRCRESAHA.119.315101 [DOI] [PubMed] [Google Scholar]
- 42.DeFilippis AP, Chapman AR, Mills NL, et al. Assessment and treatment of patients with type 2 myocardial infarction and acute nonischemic myocardial injury. Circulation. 2019;140(20):1661-1678. doi: 10.1161/CIRCULATIONAHA.119.040631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mehta LS, Beckie TM, DeVon HA, et al. ; American Heart Association Cardiovascular Disease in Women and Special Populations Committee of the Council on Clinical Cardiology, Council on Epidemiology and Prevention, Council on Cardiovascular and Stroke Nursing, and Council on Quality of Care and Outcomes Research . Acute myocardial infarction in women: a scientific statement from the American Heart Association. Circulation. 2016;133(9):916-947. doi: 10.1161/CIR.0000000000000351 [DOI] [PubMed] [Google Scholar]
- 44.American Heart Association . The Go Red for Women Campaign. Accessed May 17, 2019. https://www.goredforwomen.org/
- 45.Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837-1847. doi: 10.1161/01.CIR.97.18.1837 [DOI] [PubMed] [Google Scholar]
- 46.Andrus B, Lacaille D. 2013 ACC/AHA guideline on the assessment of cardiovascular risk. J Am Coll Cardiol. 2014;63(25 Pt A):2886. doi: 10.1016/j.jacc.2014.02.606 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods. Methods to calculate population attributable fraction for individual risk factors
eTable 1. AMI classification by the third global definition of MI
eTable 2. Comparison of variables in VIRGO and NHANES


