Abstract
Introduction
Globally, approximately half of the estimated 6.3 million under-5 deaths occur in the neonatal period (within the first 28 days of life). Kenya ranks among countries with the highest number of neonatal deaths, at 20 per 1000 live births. Improved identification and management of neonates with potentially life-threatening illness is critical to meet the WHO’s target of ≤12 neonatal deaths per 1000 live births by 2035. We developed an interactive (two-way) short messaging service (SMS) communication intervention, Mobile Solutions for Neonatal Health (Mobile women’s and children’s health (WACh) NEO), focused on the perinatal period. Mobile WACh NEO sends automated tailored SMS messages to mothers during pregnancy and up to 6 weeks post partum. Messages employ the Information-Motivation-Behaviour Skills framework to promote (1) maternal implementation of essential newborn care (ENC, including early, exclusive breast feeding, cord care and thermal care), (2) maternal identification of neonatal danger signs and care-seeking, and (3) maternal social support and self-efficacy. Participants can also send SMS to the study nurse, enabling on-demand remote support.
Methods and analysis
We describe a two-arm unblinded randomised controlled trial of the Mobile WACh NEO intervention. We will enrol 5000 pregnant women in the third trimester of pregnancy at 4 facilities in Kenya and randomise them 1:1 to receive interactive SMS or no SMS (control), and conduct follow-up visits at 2 and 6 weeks post partum. Neonatal mortality will be compared between arms as the primary outcome. Secondary outcomes include care-seeking, practice of ENC and psychosocial health. Exploratory analysis will investigate associations between maternal mental health, practice of ENC, care-seeking and SMS engagement.
Ethics and dissemination
This study received ethical approval from the University of Washington (STUDY00006395), Women and Infants Hospital (1755292-1) and Kenyatta National Hospital/University of Nairobi (P310/04/2019). All participants will provide written informed consent. Findings will be published in peer-reviewed journals and international conferences.
Trial registration number
Keywords: neonatology, depression & mood disorders, telemedicine
Strengths and limitations of this study.
To our knowledge, this is the first study to rigorously evaluate the impact of two-way short messaging service (SMS) on neonatal mortality as well as intermediate factors on the causal pathway, including maternal knowledge, preventative behaviour, care-seeking and psychosocial health.
This intervention is well suited to resource-limited settings, due to its use of ubiquitous SMS technology, automated messaging to participants, interactive messaging between participants and nurses, and no cost to the participant.
This study is large and conducted in a mix of rural and urban clinics in Kenya, increasing generalisability across geographical contexts.
Due to feasibility considerations in implementing the intervention, participants must have a mobile phone subscription through the most widely used telecom provider in Kenya; restricting to this provider may introduce bias.
Participants who are unable to read and write SMS messages are eligible but their participation in two-way SMS is reliant on an individual who can read the SMS messages to them, so engagement by low-literacy individuals may be more limited, potentially reducing generalisability.
Introduction
Half of all deaths under the age of 5 occur in the neonatal period, defined as the first 28 days of life, and 75% of these neonatal deaths occur during the first 7 days of life.1 The proportion of under-5 mortality that occurs in the neonatal period increased from 40% in 1990 to 47% in 2018.2 In Kenya, the neonatal mortality of 20 per 1000 births remains higher than the global average of 18 per 1000 births, and among women living in poverty and rural areas, the rates are substantially higher.2 3 Novel strategies are needed to prevent these deaths and attain Sustainable Development Goal 3.2.2 of reducing global neonatal mortality to less than 12 per 1000 live births by 2030.4
WHO Every Newborn Action Plan identifies priority strategies to reduce neonatal mortality, including practice of essential newborn care (ENC), prompt identification (ID) and care-seeking for newborn danger signs, and preventative treatments including vaccinations.1 5 6 ENC practices of early and exclusive breast feeding, clean cord care and thermal care have been independently shown to reduce neonatal death.7–10 For low birth weight and premature newborns, kangaroo mother care (KMC) support is additionally recommended.5 While provision of ENC can prevent neonatal illness, decisions about care-seeking when neonatal illness occurs are also key to neonatal survival.
Although ENC and appropriate care-seeking interventions show high efficacy, in general, adoption of these practices remains low, particularly in places with high neonatal mortality. Studies in Ethiopia, Uganda, Ghana, Malawi, Mali and Tanzania show suboptimal practice of ENC.11 Similarly, delays in recognising illness and deciding to seek care contribute up to 80% of neonatal and child deaths.12–15 The three delays model posits that neonatal deaths are due to delays in (1) identifying illness and deciding to seek care, (2) reaching the health facility and (3) receiving quality care once a facility is reached.16
Reasons for lack of ENC adoption and delayed care-seeking are complex, but it is thought that some can be overcome by supporting caregivers, typically mothers. From an individual perspective, a mother’s mental health, self-efficacy, socioeconomic status and social support network influence her ability and decision to practice ENC and seek care. Maternal depression has been associated with lower care-seeking and preventive practices for infants,17 18 as well as adverse infant outcomes.19–27 Social support is thought to be a mediator in the relationship between depression and low birth weight, suggesting that higher social capital helps to improve birth outcomes.28 29 Parental self-efficacy, the belief caregivers have the ability to effectively care for their newborns, is also associated with positive outcomes.30 31 Among other factors, regular close contact with a caregiver’s social support network during the early postnatal period is a major predictor of parental self-efficacy.32–34 Thus, improving the domains of maternal mental health, self-efficacy and social support may result in better neonatal outcomes.
mHealth interventions offer innovative approaches to promote ENC, reduce the delay in ID of newborn illness at home, improve timely care-seeking and provide maternal psychosocial support during the neonatal period. In Kenya, where 89% of the population own mobile phones,35 36 the Ministry of Health (MOH) has embraced mHealth interventions.37 Interactive (two-way) short messaging service (SMS) between mothers and healthcare workers (HCWs) holds promise to connect underserved communities with timely and appropriate health information targeted to reduce neonatal and maternal adverse outcomes.38 These technologies have been successfully used to deliver health information, increase communication between HCWs and patients, reinforce self-efficacy, address depressive symptoms and promote perinatal visit attendance, exclusive breast feeding and family planning uptake.39–48 However, no previous studies have rigorously evaluated the impact of two-way SMS on neonatal outcomes.39 49–53
This protocol outlines a multisite individually randomised controlled trial (RCT) to test the impact of a novel two-way SMS intervention on neonatal mortality, practice of ENC actions, and mothers’ psychosocial health in Kenya. The intervention will be compared with no SMS standard or care. Grounded in the Information-Motivation-Behavioural Skills (IMB) model of behavioural change,54 we hypothesise that the SMS intervention will reduce neonatal mortality through the mechanism of improved ID of neonatal illness, knowledge and skills for ENC provision and care-seeking, as well as improved motivation through increased social support, increased self-efficacy and reduced perinatal depression.
Methods and analysis
Objectives
Our objectives are to: (1) determine the effect of an interactive SMS messaging intervention, Mobile women’s and children’s health (WACh) NEO, on neonatal mortality, (2) determine the effect of Mobile WACh NEO on maternal implementation of ENC and care-seeking behaviour and (3) determine the effects of Mobile WACh NEO on maternal social support, self-efficacy and depression. We will additionally characterise determinants of SMS engagement and evaluate participants’ experiences with the intervention.
Trial design
The Mobile WACh NEO study is a multisite, two-arm, 1:1 individually randomised, parallel group, superiority RCT comparing peripartum interactive two-way SMS to standard of care (no SMS) control.
Study setting
The study is conducted at six healthcare facilities in Kenya. The study initially recruited at Mathare North Health Centre, Riruta Health Centre, Rachuonyo County Hospital and Bondo Sub-County Referral Hospital; to expedite enrolment, 15 months after study start, recruitment was added at two more sites, Ahero County Hospital and Kisumu County Hospital. Four facilities (Rachuonyo, Bondo, Ahero and Kisumu) are in rural locations in Western Kenya, and two facilities (Mathare and Riruta) are in urban locations in Nairobi. These sites were chosen to include Nairobi, Homa Bay, Siaya and Kisumu counties, which all have high neonatal mortality.3
Patient and public involvement
The Mobile WACh NEO study builds on a series of prior studies using a similar SMS messaging approach for a range of maternal–child health outcomes. These studies led to development of the Mobile solutions for WACh (Mobile WACh) SMS messaging platform. Participants in these studies highlighted the need for information and strategies to support the health of their newborns, in addition to their own health in pregnancy and post partum.46 In previous evaluations, there was particularly high demand for advice regarding infant illness and care-seeking. Patients were first involved in the Mobile WACh NEO project at a community advisory board meeting during piloting of the intervention.55 Patients contributed to design of the intervention by sharing their perspectives on topics of interest as well as messaging delivery approaches. They were not involved in study design, choice of outcome measures or study recruitment. Study findings will be shared with our community advisory board to inform broader dissemination of results.
Recruitment and screening
Each day, study staff obtain a list of women presenting for ANC visits at the clinic. Clinic staff share an overview of the study with ANC clients in the clinic waiting area and refer interested women at from the ANC to study staff who provide a short description of the study. If a potential participant is interested in learning more, study staff perform eligibility screening following the ANC visit. Eligible participants are ≥14 years old, pregnant at 28–36 weeks estimated gestational age, enrolled in antenatal care at a study site, have daily access to a shared or personal mobile phone with a subscriber identity module on the Safaricom network, are willing to receive SMS, plan to stay in the area for 5 months or greater, and are not enrolled in another study. These eligibility criteria were selected to include women of all ages who were able to independently consent; allow participants to receive several weeks of messages in the third trimester of pregnancy; enable efficient clinic-based recruitment and follow-up; ensure participants had access to the mobile intervention; and minimise contamination by other research interventions. Messages are delivered only through the Safaricom network, the most widely used network in Kenya, to reduce the research costs of purchasing reverse-billed SMS shortcodes on multiple networks. Literacy is not required if a woman has access to a partner or trusted person who she is comfortable having read her the messages. The approach of involving partners in receiving and sending SMS was developed in consultation with Kenyan mothers and successfully implemented in prior Mobile WACh studies.46 56
Informed consent
Informed consent is obtained by study staff. Participants provide verbal consent prior to eligibility screening and, if eligible, written consent to enrol in the RCT. When potential participants are illiterate, an impartial witness is present during the entire consent process and signs the consent form, while the participant makes a mark or thumbprint on the form. The age of consent in Kenya is 18, but pregnant women age ≥14 are considered emancipated and can consent independently, thus all eligible study participants are able to consent independently. Consent includes permission to conduct home visits or phone interviews for tracing purposes and to conduct a verbal autopsy in case of an infant death. The consent also includes permission to use deidentified participant data in future studies. A sample consent form is provided in online supplemental material S1.
bmjopen-2021-056062supp001.pdf (108.7KB, pdf)
Randomisation
Participants are randomised to two-way SMS or control, using 1:1 allocation (figure 1). A web-based randomisation service generates allocations, stratified by site, using random block sizes of 2, 4 and 6 (Randomize.net, Ottawa, Canada). The randomisation service only displays and records the allocation after staff have entered the enrolled participant ID. To ensure participants are distributed across sites, no site will enrol more than 2499 of the 5000 total women. In rare situations where randomisation using the web-based service is not possible due to technical issues, envelopes containing randomisation allocations generated by the web-based service are available. Participants and study staff are unblinded to study arm. The principal investigator (PI) and coinvestigators are blinded to study findings until study close.
Figure 1.
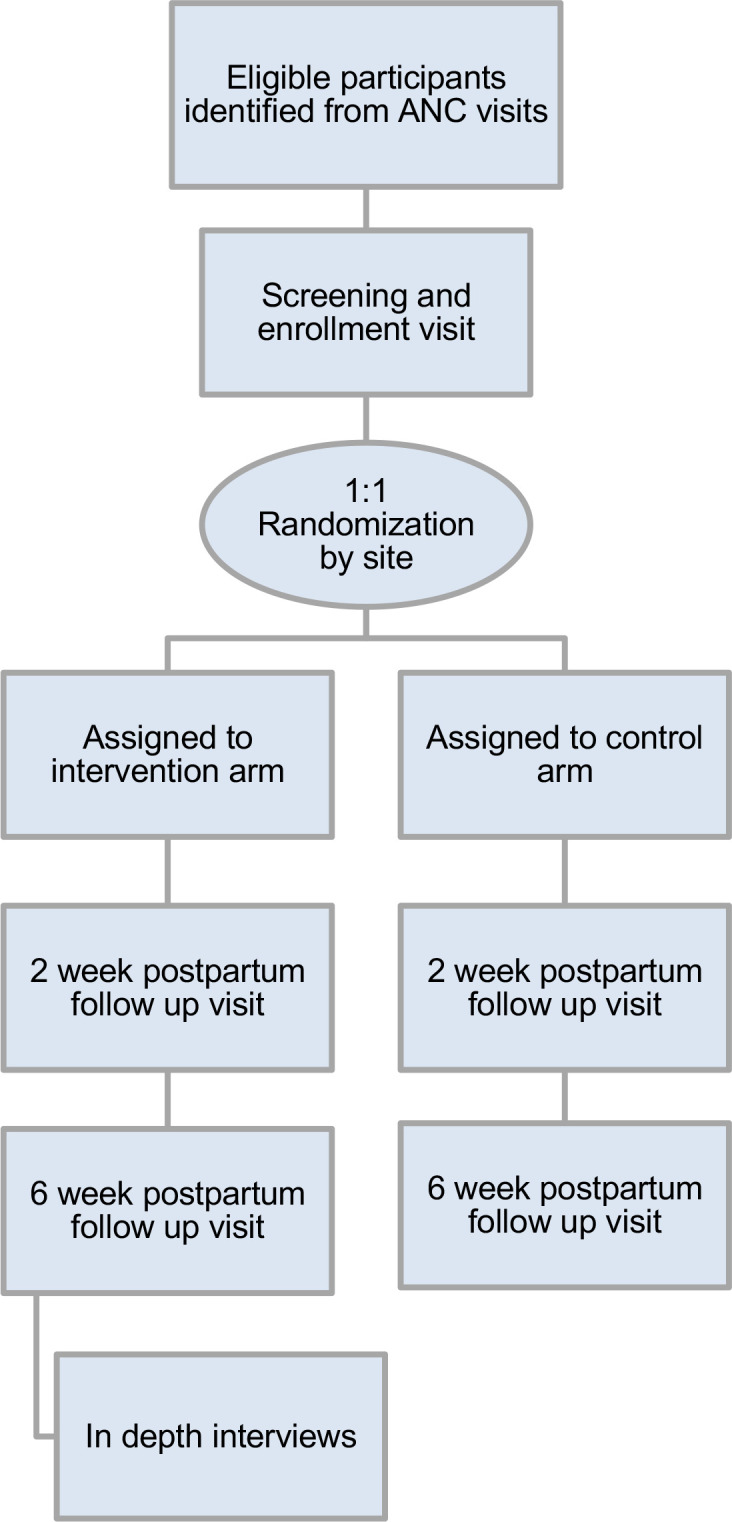
CONSORT diagram of Mobile WACh NEO Trial. The Mobile WACh NEO intervention is semiautomated interactive SMS during pregnancy and up to 6 weeks post partum. Recruitment and randomisation began in September 2020, in-depth interviews will begin in January 2022, and follow-up is anticipated to end in may 2023. ANC, antenatal care; CONSORT, Consolidated Standards of Reporting Trials; SMS, short messaging service; WACh, women’s and children’s health.
Intervention
Participants randomised to the two-way SMS arm receive a series of automated SMS messages during pregnancy and until 6 weeks post partum. Women randomised to the control arm do not receive SMS messages from Mobile WACh NEO. Participants in both arms receive standard ANC and MCH clinical services through MOH clinics with minimal in-person interactions with study personnel.
Message content is designed based on estimated gestational age or time since delivery. Messages are sent in English, Dholuo or Kiswahili depending on the participant’s preference. Participants can send SMS messages to the study at any time and study nurses respond by SMS during business hours within one business day. Sending and receiving SMS is at no cost to the participant.
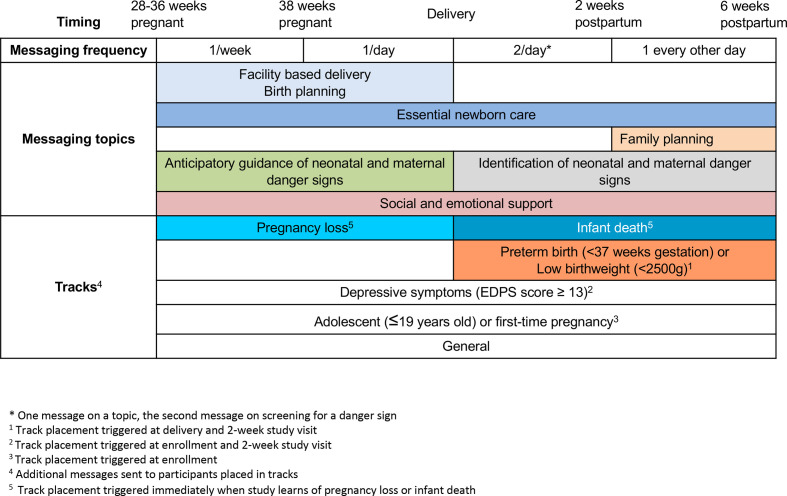
SMS messaging content during the pregnancy and postpartum periods is summarised in figure 2. From enrolment until 38 weeks gestation, automated SMS are delivered weekly, encouraging facility delivery and birth planning, and providing anticipatory guidance about neonatal danger signs and emotional support. Automated SMS are delivered at a prespecified day, time and language based on participant preferences. From 38 weeks gestation until delivery, women receive daily messages highlighting ID of neonatal danger signs and ENC practices including immediate and exclusive breast feeding, thermal and cord care. From delivery to 2 weeks post partum, mothers receive two messages per day: one message with screening questions for ID of infant danger signs and encouragement to engage with nurses by SMS if they have a concern, and one message with educational content on ENC practices, mental health and support. From 2 to 6 weeks post partum, SMSs are delivered every other day. All messages open with the participant’s preferred name, provide advice regarding an outcome of the trial, and contain a question to engage women in message content.
Figure 2.
Messaging timing, content and tracks for Mobile WACh NEO. WACh, women’s and children’s health.
Messages were adapted from prior Mobile WACh studies,46 47 56 consistent with Kenyan MOH guidelines for newborn care,57 and tested during a pilot study involving 800 peripartum women in Mathare North Health Centre and Rachuonyo County Hospital.55 The messaging curriculum was modified through consultations with obstetrics, neonatology and mental health specialists and literature review on supportive messaging. Additional messaging ‘tracks’ were created with adaptations to the messages for mother–infant dyads at elevated risk for obstetrical and neonatal complications. Those placed in high-risk tracks receive additional messages tailored to their specific risk factor. High-risk groups include (1) first-time mothers or mothers age ≤19, (2) women who screen positive for elevated depression symptoms during study data collection using the Edinburgh Postnatal Depression Scale (score ≥13) or (3) women whose infants are born premature (<37 weeks estimated gestational age) or are of low birth weight (<2.5 kg). Additionally, if a participant loses her pregnancy or her infant dies and she consents to continued messaging, she is switched to a message track that contains no content related to pregnancy or infant care but includes emotionally supportive condolence messages designed in a previous Mobile WACh study.58
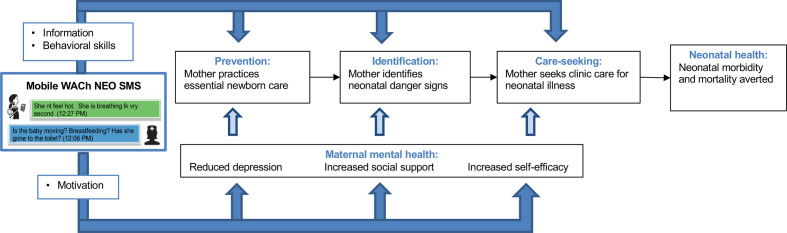
The Mobile WACh NEO SMS curriculum was developed based on the IMB theory, which holds that health behaviour is influenced by an individual’s knowledge of information about the benefits of the behaviour, their motivation to change behaviour and their skills to change and engage in new behaviour (figure 3).54 Based on this model, topics and example messages sent in the study are summarised in table 1.
Figure 3.
Conceptual framework for mobile WACh NEO. SMS, short messaging service; WACh, women’s and children’s health.
Table 1.
Example Mobile WACh NEO SMS Based on IMB theory
| Construct | Day | Example messages |
| Information | ||
|
1 week before estimated due date | {name}, this is {nurse} from {clinic}. Did you know that newborns should be taken to the health facility if they stop breastfeeding well, develop a fever, are breathing fast or become less active? How will you get to your nearest health facility in case of an emergency? |
|
Day of delivery | {name}, this is {nurse} from {clinic}. Newborns need to be kept warm. The baby is wiped right after birth. Keep the baby bare skin-to-skin with you and cover you both. Dress the baby in socks, a nappy and hat. Place the baby on your naked chest and cover both of you. This contact helps with bonding, breastfeeding and keeps the baby warm. It is very important to avoid bathing your baby in the first 2 days. Do you have questions about keeping your baby warm or the bath? |
|
2 days post partum | {name}, this is {nurse} from {clinic}. Your milk should start to ‘come in’ between birth and day 5. Breastfeed your baby often, don’t skip breastfeedings (even at night), ensure good attachment/positioning, and let baby finish the first breast before offering the other side. To decrease discomfort from swollen breasts, use cold and/or cabbage leaf compresses between feedings. If baby is having trouble attaching to the breast properly during breastfeeding due to swollen breast, express milk from the swollen breast until the nipple is soft, then try putting the baby on the breast again. |
|
3 days post partum | {name}, this is {nurse} from {clinic}. Once the baby is born you can prevent infections by washing your hands and keeping the cord clean. Do not apply any substances or bandages to the cord. Do you have any questions about taking care of the baby’s umbilical cord? |
|
4 days post partum | {name}, this is {nurse} from {clinic}. Hold your baby skin-to-skin as much as possible throughout the day. This will help them grow. How is skin-to-skin holding/kangaroo care going for you and your baby? Also be sure to monitor the baby’s umbilical cord. Do not apply any substance or bandages to it but let us know if it is red or there is discharge. |
|
4 days post partum | {name}, this is {nurse} from {clinic}. Is the baby having any trouble breathing? Do they seem very hot or cold? These are danger signs and could mean the baby is sick. Please let us know right away. |
| Motivation | ||
|
10 weeks and 5 days before expected delivery date | {name}, this is {nurse} from {clinic}. Sometimes pregnancy and motherhood can bring on sadness, anxiety or worry. This happens to many women and can cause you to feel alone, cry and have difficulty sleeping. Are you having any of these problems? |
|
24 days post partum | {name}, this is {nurse} from {clinic}. How are you and the little one doing today? We know it is a new experience and some days are much difficult than others. You and the baby are learning and growing everyday. You are doing a good job and we are here to help with any advice you might need. Please SMS us with any concerns. |
|
28 days post partum | {name}, this is {nurse} from {clinic}. How are you doing today? We know you are doing your best and we want to thank you for all the good work you put into supporting your little one. Make sure you take a few minutes to appreciate your hard work and let us know if there are areas where you are experiencing difficulties such as feeding the baby, experiencing problems in sleeping or feeding, difficulties looking after or connecting with the baby, anxious or sad feelings coming up. We are here to help. Which of these difficulties are you having? |
| Behavioural skills | ||
|
13 and 6 days before estimated delivery date | (name), this is (nurse) from (clinic). Regular, strong stomach pains are a sign of labour. If you feel this strong tightening regularly pains, leaking of fluid or bleeding, go to the facility. Do you feel any contractions? Do you have any concerns? |
|
4 days before estimated delivery date | {name}, this is {nurse} from {clinic}. Even during this time of the COVID-19 epidemic, you still need to go to the clinic for antenatal and postnatal care. Coming to the clinic, including at delivery, is still very important for you and the baby. If you have mild symptoms of COVID-19 (cough, congestion, mild fever) and have a regularly scheduled visit, please stay home. If your symptoms become severe (high fever, difficulty breathing, chest pain or trouble doing your daily activities because of respiratory illness) please seek care for your illness. Do you have any questions or concerns about the COVID-19 virus? |
|
14 days post partum | {name}, this is {nurse} from {clinic}. By 2 weeks old a newborn baby may cry up to 2 hours a day. Make sure your baby is not hungry, tired or has a dirty diaper/nappy. Pay attention to what calms your baby like singing, rocking, swaddling or sucking. Does your baby cry for more than 30 min at a time? Please let us know when you are having any problems with your baby. |
|
16 days post partum | {name}, this is {nurse} from {clinic}. Getting to know your newborn and their schedule can be hard especially when you are not getting enough sleep. It is normal to feel tired and to need help. Do you have someone to help you or have concerns that maybe the nurse can help with? |
*COVID-19 messages developed based on Kenya Ministry of Health guidelines.69
IMB, Information-Motivation-Behaviour Skills; SMS, short messaging service; WACh, women’s and children’s health.
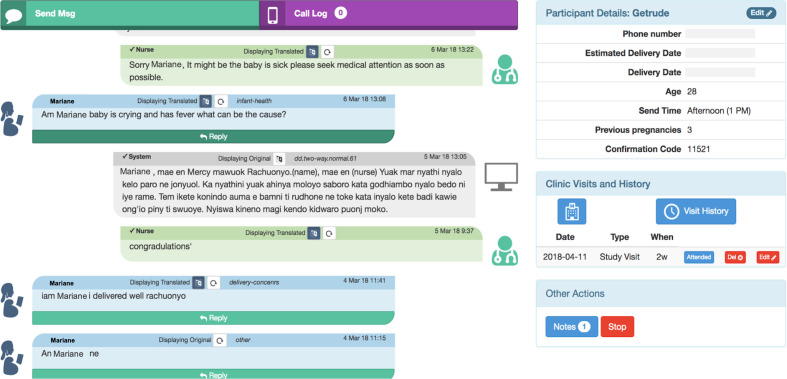
A custom human-computer hybrid software system is used to manage incoming and outgoing SMS for Mobile WACh NEO (figure 4).56 59 This online system enables two-way SMS communication and participant tracking. To facilitate management of messages, the messaging platform was developed through a human-centred design processes with Kenyan nurses to improve usability and streamline workflow.59 The interface enables nurses to review and respond to incoming participant SMS. The system also has a bank of responses to frequently asked questions, such as common pregnancy concerns, birth planning, infant feeding and infant illness, which nurses can use as a modifiable template for their responses. This allows for improved standardisation and response times. Nurses can also freely respond to participant messages or questions that arise. Nurses triage messages as they are received and respond with greater urgency to messages involving a reported maternal or neonatal danger sign, serious adverse event (SAE), or home delivery. If participants report neonatal or maternal danger signs, they are referred to facility care by study nurses. If a participant reports experiencing intimate partner violence, suicidal ideation, elevated depressive symptoms or severe food insecurity, study staff refer the participant to available local resources. Prior to study initiation, study personnel received training on use of the SMS system and topics in neonatal, maternal health and psychosocial support. Study messages are reviewed weekly by senior team members and discussed with study nurses for quality assurance.
Figure 4.
Screenshot of mobile WACh system study nurse interface. WACh, women’s and children’s health.
To ensure timely transition from antepartum to postpartum messaging at delivery, participants are encouraged to report their delivery as soon as possible by SMS or phone call. Participants without a recorded delivery by 1 day after their estimated delivery date (EDD) are contacted by phone call to confirm their delivery status. If they are not reached by phone tracing, home tracing is performed. To comply with movement restrictions during the COVID-19 pandemic, home-tracing has been suspended. If delivery is not confirmed by 43 weeks gestational age and tracing is unsuccessful, the participant automatically starts receiving postpartum messaging. If a participant reports a pregnancy or infant loss, they automatically stop receiving automated messages and are offered the option to continue receiving modified pregnancy or infant loss messages, as described above. Participants enrolled in the intervention arm can opt out of the intervention at any time or send an SMS to withdraw from the intervention. No provisions for post-trial care are offered to participants.
Outcomes
The primary outcome of the study is neonatal death, defined as death within the first 28 days after live birth. All study outcomes are outlined in table 2.
Table 2.
Mobile WACh NEO study outcomes
| Description | Indicator | Timing of ascertainment | Analysis metric | Statistical method |
| Primary outcome | ||||
| Neonatal mortality | Death during first 28 days of life | 2-week and 6-week visits, record abstraction | Comparison of proportions | Log-binomial regression |
| Secondary outcomes | ||||
| Early neonatal mortality | Death during first 7 days of life | 2-week and 6-week visits, record abstraction | Comparison of proportions | Log-binomial regression |
| Initiation of early breastfeeding | Breastfeeding in first hour of life | 2-week visit | Comparison of proportions | Log-binomial regression |
| Exclusive breastfeeding | Cessation of exclusive breast feeding in first 6 weeks of life | 2-week and 6-week visits | Comparison of time to event | Cox proportional hazards |
| Thermal care | Bath in first 24 hours of life | 2-week visit | Comparison of proportions | Log-binomial regression |
| Cord care | No application of substances to cord | 2-week visit | Comparison of proportions | Log-binomial regression |
| Home provision of Kangaroo Mother Care | Any duration skin-to-skin care on ≥10 of the first 14 days at home, among low birth weight or preterm infants | 2-week and 6-week visits | Comparison of proportions | Log-binomial regression |
| Maternal knowledge of neonatal danger signs | No of the seven danger signs or symptoms successfully named | 2-week and 6-week visits | Comparison of means | Poisson GEE |
| Appropriate care seeking | No of clinic visits with danger sign and/or hospital admissions reported in first 6 weeks | 2-week and 6-week visits | Comparison of means | Poisson regression |
| Elevated depressive symptoms | Score above ≥13 on Edinburgh Postnatal Depression Score60–62 | Enrolment, 2-week and 6-week visits | Comparison of proportions | Log-binomial GEE |
| Social support | Score using MOS Social Support Survey63 | Enrolment, 2-week and 6-week visits | Comparison of means | Linear GEE |
| Self-efficacy | Score using Karitane Parenting Confidence Scale64 | Enrolment, 2-week and 6-week visits | Comparison of means | Linear GEE |
WACh, women’s and children’s health.
Data collection and management
The participant timeline is depicted in figure 1. Participants enter the study at 28–36 weeks gestation and are randomised to either the Mobile WACh NEO or control arm at enrolment. At 2 and 6 weeks following delivery, participants attend a study visit in conjunction with their usual medical care at the clinic or complete a study visit by phone.
Data are collected at study visits at enrolment in pregnancy, 2 weeks post partum, and 6 weeks post partum. At enrolment, a tablet-based questionnaire is administered by study staff using an open-source data collection system (KoboToolbox). The questionnaire ascertains demographic characteristics, clinical and pregnancy history, family planning, experience with SMS and technology, social support, intimate partner violence, maternal and child health status, breastfeeding plans, parental self-efficacy, and depression. Standardised instruments are used to assess depressive symptoms,60–62 social support63 and parenting self-efficacy64 (table 2). Instruments are administered in the participant’s preferred language. To determine EDD at enrolment, women are asked the date of the first day of their last menstrual period. EDD is also abstracted from the MCH booklet, the primary medical record used in the peripartum period, when available. In cases of disagreement between LMP and EDD, the MCH booklet is the primary data source. Dates for follow-up visits are calculated based on actual delivery date once delivery has occurred.
At 2-week and 6-week follow-up visits, a standardised questionnaire is administered using KoboToolbox to capture infant mortality, knowledge of ENC practices, neonatal danger signs, appropriate care seeking for any instances of infant or maternal illness, depression, social support and parenting self-efficacy. The 2-week follow-up visit questionnaire additionally captures details on immediate breast feeding, delivery, thermal and cord care. Study visits are designed to align with recommended patient clinical care schedules. No biological specimens are collected.
Clinic record data about hospitalisations and deliveries are abstracted. The primary source of abstracted data for maternal or infant hospitalisation is the hospital discharge form brought by the mother to the 2-week or 6-week study visits. Abstraction of the MCH booklet brought by the mother and the maternity registry at the clinic is also performed to collect delivery details if a participant misses the 2-week or 6-week study visit(s). Abstracted data are entered into an electronic data collection system using REDCap. Data are collected digitally and uploaded daily to a secure cloud storage location accessible only by key data management personnel.
When the study is informed of an infant death, a verbal autopsy is conducted to determine the primary causes of death, using the Population Health Metrics Research Consortium shortened verbal autopsy neonatal questionnaire, translated into the participant’s preferred language, and SmartVA-Analyze V.2.0.0 application.65
If a study participant misses a 2-week or 6-week study visit by 1 week or no delivery date is recorded by 1 week after EDD, active tracing by phone is conducted. If the participant is not reached by phone after four attempts, home tracing is conducted. Participants who do not wish to come to clinic for the 2-week or 6-week study visit are offered to complete the questionnaire by phone or at home if suitable locations for ensuring participant confidentiality are available. In response to MOH guidelines and recommendations to reduce possible exposures to COVID-19, the research team modified study procedures to suspend any in-person home visits or clinic visits whose sole purpose is study data collection procedures.
Following the 6-week study visit, in-depth interviews are conducted with 60 intervention participants to gather data on their experience with the SMS curriculum. Participants are purposively sampled to participate in IDIs based on their messaging behaviour in the intervention: participants who sent a high, medium and low number of messages during the intervention will be included to understand the range of experiences with the intervention. Further, a group of participants who experienced infant hospitalisation or death will also be included. Interviews will focus on barriers and facilitators of SMS engagement to characterise high and low users, and perceived utility of remote engagement with nurses during infant illness.
Participants are enrolled in the study until 18 weeks after delivery to allow time for all follow-up activities, including in-depth interviews and verbal autopsies as needed.
AEs, including physical, psychological, social, or economic events, as well as SAE, defined as pregnancy loss, maternal or infant hospitalisation or death, will be monitored throughout the study. At each study visit, participants are asked if an AE or SAE occurred since the last visit. If an SAE is reported, study staff notify the Kenyatta National Hospital/University of Nairobi ethics review board and PI of the event within 72 hours. Any breaches of privacy or risk of breach of privacy are reported to the University of Washington Institutional Review Boards and Kenyatta National Hospital/University of Nairobi ethics review board within 24 hours.
A data safety monitoring board (DSMB) has been convened for this study, composed of five researchers in the fields of biostatistics, maternal–child health, paediatrics and mHealth. The DSMB is independent from the sponsor and has no competing interests. No other external trial monitoring or auditing is planned.
Sample size
The study is powered to detect a difference in the primary outcome of neonatal mortality. With 5000 participants randomised 1:1 to SMS and control, assuming 10% attrition and alpha=0.05, we have 80% power to detect a risk ratio of ≤0.53 assuming a neonatal mortality rate of 23 per 1000 in the control arm.3 With this sample size, assuming alpha=0.0045 (Bonferroni adjusted for 11 tests, see the Statistical analysis section), we will also have 80% power to detect the following changes in secondary outcomes: a risk ratio of 1.05 in early initiation of breast feeding assuming 80% uptake in controls,46 a risk ratio of 1.11 in application of substances to the cord or delaying first bath assuming 50% in controls,66 a risk ratio of 0.76 in elevated depression symptoms assuming 19% in controls.67 Sample size for post-RCT qualitative data collection was determined based on the number of interviews needed to achieve saturation of concepts. It is estimated that 60 interviews will be sufficient to achieve saturation and provide relevant intervention and implementation feedback.
Statistical analysis
Study outcomes and analytic approaches are summarised in table 2. The primary study outcome is neonatal mortality, defined as death within the first 28 days of life as reported in clinic records or maternal report. Risk of neonatal mortality will be compared between arms by log-binomial regression. Secondary outcomes are: neonatal mortality in the first 7 days (analysed by log-binomial regression), proportion of mothers who initiate exclusive breast feeding in the first hour of life (analysed by log-binomial regression); proportion of mothers who delay bathing for at least 24 hours after birth (analysed by log-binomial regression); proportion of mothers who apply no substances to the umbilical cord (analysed by log-binomial regression); time to cessation of breast feeding (analysed by Cox proportional hazards regression); proportion of mothers of preterm or low birth weight neonates who provide KMC (analysed by log-binomial regression); number of neonatal danger signs successfully named by the mother (analysed by generalised estimating equation Poisson regression); proportion of women with elevated depression symptoms by Edinburgh postnatal depression scale (analysed by general estimating equation log-binomial regression); social support score by the MOS Social Support Scale (analysed by generalised estimating equation linear regression); self-efficacy score in the Karitane Parenting Confidence Scale (analysed by generalised estimating equation linear regression).
All analyses will be intent-to-treat. Analysis of secondary outcomes will be corrected for multiple comparisons using the Benjamini-Hochberg method.68 Power calculations were conducted using a Bonferroni adjustment to provide a conservative estimate of the limits of detectable differences. An exploratory per-protocol analysis will be conducted excluding women who discontinue the SMS intervention. Based on previous studies in this population, we expect 10% loss to follow-up between enrolment and the 6-week study visit. Sensitivity analyses will be conducted for the primary mortality outcome assuming all participants who are lost to follow-up and missing mortality data did or did not experience neonatal mortality. An interim analysis for neonatal mortality will be performed using O’Brien-Fleming boundaries for benefit and harm when 50% of expected person time has been accrued.
The full protocol (V.2.2, October 5, 2020) is provided in online supplemental material S2. Participant-level data on primary outcomes and statistical code will be made available after publication of trial findings, on request from the authors.
bmjopen-2021-056062supp002.pdf (5.2MB, pdf)
Current study status
Participant enrolment began on 7 September 2020. Recruitment, intervention delivery and data collection are currently ongoing. Recruitment and follow-up are projected to be complete by September 2022 and May 2023, respectively.
Ethics and dissemination
The study has received ethical approval at the University of Washington (STUDY00006395), Women and Infants Hospital (1755292-1) and Kenyatta National Hospital/University of Nairobi (P310/04/2019) institutional review boards.
All participants provide written informed consent. All participants are assigned a study ID number and identified only by that ID after enrolment. Any personally identifiable information related to participants is maintained separately from study data to preserve participant confidentiality. Paper forms such as signed consent forms are stored in locked cabinets separate from forms connecting participant names and their study participant identifier. Discussions between study staff and potential participants during study visits or for purposes of data collection are conducted in a private space to maintain privacy. All study staff are trained in human subjects research and study standard operating procedures.
Dissemination of study results to health researchers, policy-makers and clinicians has potential to improve prevention strategies for neonatal mortality in Kenya and in other countries. All data collected in this study will be publicly disseminated to key policymakers at county and national level in Kenya, the public health research community in Kenya and through peer-reviewed international journals and conferences. Results from the study will be reported no later than 1 year after the completion date. Publication of the record was delayed by 4 days relative to the NIH reporting policy due to unexpected medical absence by the PI. Any protocol modifications will be recorded in the registry.
Acknowledgments
We acknowledge support from University of Washington’s Global Center for Integrated Health of Women, Adolescents, and Children (Global WACh).
Footnotes
Twitter: @AnnaHedstromMD
Contributors: KR, JAU, JK, DCW, MK, AH and BW were involved in conception and trial design. LO, PK, MM, BW, EMC and JIU were involved in data acquisition. EMC was involved in drafting of the article. KR, JAU and JIU were involved in critical revision of the article for important intellectual content. BAR provided statistical expertise. All the authors were involved in final approval of the article.
Funding: The study is funded by the National Institutes of Health, Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) through grant R01HD098105, as well as the National Institute of Mental Health and the Office of the Director through grant K18MH122978 (to KR).
Disclaimer: The funder and sponsor (University of Washington) had no role in the design of this study. They will not have any role during its execution, analyses, interpretation of the data, or decision to submit results.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. WHO . Newborns: reducing mortality. Available: https://www.who.int/news-room/fact-sheets/detail/newborns-reducing-mortality [Accessed 17 Aug 2020].
- 2. UN Inter-agency Group for Child Mortality Estimation . Levels & Trends in Child Mortality, 2019. Available: https://childmortality.org/wp-content/uploads/2019/10/UN-IGME-Child-Mortality-Report-2019.pdf
- 3. Kenya National Bureau of Statistics . Kenya demographic and health survey 2014, 2015. Available: https://dhsprogram.com/publications/publication-FR308-DHS-Final-Reports.cfm
- 4. United Nations . Global indicator framework for the sustainable development goals and targets of the 2030 agenda for sustainable development. 2020. Available: https://unstats.un.org/sdgs/indicators/GlobalIndicatorFrameworkafter2019refinement_Eng.pdf%0Ahttps://unstats.un.org/sdgs/indicators/GlobalIndicatorFramework_A.RES.71.313Annex.pdf [Accessed 14 Oct 2020].
- 5. World Health Organizaton . Every newborn: an action plan to end preventable deaths, 2014. Available: https://www.who.int/publications/i/item/9789241507448 [Accessed 14 Oct 2020].
- 6. World Health Organization . Who recommendations on newborn health guidelines, 2017. Available: https://www.who.int/publications/i/item/WHO-MCA-17.07 [Accessed 17 Aug 2020].
- 7. Bhutta ZA, Darmstadt GL, Hasan BS, et al. Community-based interventions for improving perinatal and neonatal health outcomes in developing countries: a review of the evidence. Pediatrics 2005;115:519–617. 10.1542/peds.2004-1441 [DOI] [PubMed] [Google Scholar]
- 8. Edmond K, Newton S, Hurt L, et al. Timing of initiation, patterns of breastfeeding, and infant survival: prospective analysis of pooled data from three randomised trials. Lancet Glob Health 2016;4:e266–75. 10.1016/S2214-109X(16)00040-1 [DOI] [PubMed] [Google Scholar]
- 9. Agrawal PK, Agrawal S, Mullany LC, et al. Clean cord care practices and neonatal mortality: evidence from rural Uttar Pradesh, India. J Epidemiol Community Health 2012;66:755–8. 10.1136/jech-2011-200362 [DOI] [PubMed] [Google Scholar]
- 10. Lunze K, Hamer DH. Thermal protection of the newborn in resource-limited environments. J Perinatol 2012;32:317–24. 10.1038/jp.2012.11 [DOI] [PubMed] [Google Scholar]
- 11. Bee M, Shiroor A, Hill Z. Neonatal care practices in sub-Saharan Africa: a systematic review of quantitative and qualitative data. J Health Popul Nutr 2018;37:9. 10.1186/s41043-018-0141-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bogale TN, Worku AG, Bikis GA, et al. Why gone too soon? examining social determinants of neonatal deaths in Northwest Ethiopia using the three delay model approach. BMC Pediatr 2017;17:216. 10.1186/s12887-017-0967-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Källander K, Hildenwall H, Waiswa P, et al. Delayed care seeking for fatal pneumonia in children aged under five years in Uganda: a case-series study. Bull World Health Organ 2008;86:332–8. 10.2471/BLT.07.049353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Waiswa P, Kallander K, Peterson S, et al. Using the three delays model to understand why newborn babies die in eastern Uganda. Trop Med Int Health 2010;15:964–72. 10.1111/j.1365-3156.2010.02557.x [DOI] [PubMed] [Google Scholar]
- 15. Wilmot E, Yotebieng M, Norris A, et al. Missed opportunities in neonatal deaths in Rwanda: applying the three delays model in a cross-sectional analysis of neonatal death. Matern Child Health J 2017;21:1121–9. 10.1007/s10995-016-2210-y [DOI] [PubMed] [Google Scholar]
- 16. Thaddeus S, Maine D. Too far to walk: maternal mortality in context. Soc Sci Med 1994;38:1091–110. 10.1016/0277-9536(94)90226-7 [DOI] [PubMed] [Google Scholar]
- 17. Alhusen JL, Ayres L, DePriest K. Effects of maternal mental health on engagement in favorable health practices during pregnancy. J Midwifery Womens Health 2016;61:210–6. 10.1111/jmwh.12407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grigoriadis S, VonderPorten EH, Mamisashvili L, et al. The impact of maternal depression during pregnancy on perinatal outcomes: a systematic review and meta-analysis. J Clin Psychiatry 2013;74:e321–41. 10.4088/JCP.12r07968 [DOI] [PubMed] [Google Scholar]
- 19. Jarde A, Morais M, Kingston D, et al. Neonatal outcomes in women with untreated antenatal depression compared with women without depression: a systematic review and meta-analysis. JAMA Psychiatry 2016;73:826–37. 10.1001/jamapsychiatry.2016.0934 [DOI] [PubMed] [Google Scholar]
- 20. Grote NK, Bridge JA, Gavin AR, et al. A meta-analysis of depression during pregnancy and the risk of preterm birth, low birth weight, and intrauterine growth restriction. Arch Gen Psychiatry 2010;67:1012–24. 10.1001/archgenpsychiatry.2010.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gausia K, Moran AC, Ali M, et al. Psychological and social consequences among mothers suffering from perinatal loss: perspective from a low income country. BMC Public Health 2011;11:451. 10.1186/1471-2458-11-451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Murray L, Cooper PJ, Stein A. Postnatal depression and infant development. BMJ 1991;302:978–9. 10.1136/bmj.302.6783.978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Deave T, Heron J, Evans J, et al. The impact of maternal depression in pregnancy on early child development. BJOG 2008;115:1043–51. 10.1111/j.1471-0528.2008.01752.x [DOI] [PubMed] [Google Scholar]
- 24. Huizink AC, Robles de Medina PG, Mulder EJH, et al. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry 2003;44:810–8. 10.1111/1469-7610.00166 [DOI] [PubMed] [Google Scholar]
- 25. Beijers R, Jansen J, Riksen-Walraven M, et al. Maternal prenatal anxiety and stress predict infant illnesses and health complaints. Pediatrics 2010;126:e401–9. 10.1542/peds.2009-3226 [DOI] [PubMed] [Google Scholar]
- 26. Feldman R, Granat A, Pariente C, et al. Maternal depression and anxiety across the postpartum year and infant social engagement, fear regulation, and stress reactivity. J Am Acad Child Adolesc Psychiatry 2009;48:919–27. 10.1097/CHI.0b013e3181b21651 [DOI] [PubMed] [Google Scholar]
- 27. McLearn KT, Minkovitz CS, Strobino DM. The timing of maternal depressive symptoms and mothers' parenting practices with young children: implications for pediatric practice. Pediatrics 2006;118:e174–82. 10.1542/peds.2005-1551 [DOI] [PubMed] [Google Scholar]
- 28. Wado YD, Afework MF, Hindin MJ. Effects of maternal pregnancy intention, depressive symptoms and social support on risk of low birth weight: a prospective study from southwestern Ethiopia. PLoS One 2014;9:e96304. 10.1371/journal.pone.0096304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kawachi I, Kennedy BP, Lochner K, et al. Social capital, income inequality, and mortality. Am J Public Health 1997;87:1491–8. 10.2105/AJPH.87.9.1491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Albanese AM, Russo GR, Geller PA. The role of parental self-efficacy in parent and child well-being: a systematic review of associated outcomes. Child Care Health Dev 2019;45:333–63. 10.1111/cch.12661 [DOI] [PubMed] [Google Scholar]
- 31. Bahorski JS, Childs GD, Loan LA, et al. Self-efficacy, infant feeding practices, and infant weight gain: an integrative review. J Child Health Care 2019;23:286–310. 10.1177/1367493518788466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Leahy-Warren P, McCarthy G. Maternal parental self-efficacy in the postpartum period. Midwifery 2011;27:802–10. 10.1016/j.midw.2010.07.008 [DOI] [PubMed] [Google Scholar]
- 33. Gao L-L, Chan SW-chi, Mao Q. Depression, perceived stress, and social support among first-time Chinese mothers and fathers in the postpartum period. Res Nurs Health 2009;32:50–8. 10.1002/nur.20306 [DOI] [PubMed] [Google Scholar]
- 34. Leahy Warren P, Warren PL. First-time mothers: social support and confidence in infant care. J Adv Nurs 2005;50:479–88. 10.1111/j.1365-2648.2005.03425.x [DOI] [PubMed] [Google Scholar]
- 35. Government of Kenya . Kenya integrated household budget survey 2015-2016, 2018. Available: http://statistics.knbs.or.ke/nada/index.php/catalog/88 [Accessed 17 Aug 2020].
- 36. Communications Authority of Kenya . ICT sector quarterly statistics report (January - March 2020), 2020. Available: https://ca.go.ke/wp-content/uploads/2020/07/Sector-Statistics-Report-Q3-2019-2020-.pdf [Accessed 17 Aug 2020].
- 37. Kenya Ministry of Health . Standards and guidelines for mHealth systems. Report. Kenya, 2017. https://www.health.go.ke/wp-content/uploads/2020/02/Revised-Guidelines-For-Mhealth-Systems-May-Version.pdf [Google Scholar]
- 38. Kazi AM, Carmichael J-L, Hapanna GW, et al. Assessing mobile phone access and perceptions for Texting-Based mHealth interventions among expectant mothers and child caregivers in remote regions of northern Kenya: a survey-based descriptive study. JMIR Public Health Surveill 2017;3:e5. 10.2196/publichealth.5386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lee SH, Nurmatov UB, Nwaru BI, et al. Effectiveness of mHealth interventions for maternal, newborn and child health in low– and middle–income countries: systematic review and meta–analysis. J Glob Health 2016;6:10401. 10.7189/jogh.06.010401 [DOI] [Google Scholar]
- 40. Mbuthia F, Reid M, Fichardt A. mHealth communication to strengthen postnatal care in rural areas: a systematic review. BMC Pregnancy Childbirth 2019;19:406. 10.1186/s12884-019-2531-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sondaal SFV, Browne JL, Amoakoh-Coleman M, et al. Assessing the effect of mHealth interventions in improving maternal and neonatal care in low- and middle-income countries: a systematic review. PLoS One 2016;11:e0154664. 10.1371/journal.pone.0154664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lund S, Hemed M, Nielsen BB, et al. Mobile phones as a health communication tool to improve skilled attendance at delivery in Zanzibar: a cluster-randomised controlled trial. BJOG 2012;119:1256–64. 10.1111/j.1471-0528.2012.03413.x [DOI] [PubMed] [Google Scholar]
- 43. Omole O, Ijadunola MY, Olotu E, et al. The effect of mobile phone short message service on maternal health in south-west Nigeria. Int J Health Plann Manage 2018;33:155–70. 10.1002/hpm.2404 [DOI] [PubMed] [Google Scholar]
- 44. Bangal VB, K. Borawake S, P. Gavhane S, et al. Use of mobile phone for improvement in maternal health: a randomized control trial. Int J Reprod Contracept Obstet Gynecol 2017;6:5458. 10.18203/2320-1770.ijrcog20175260 [DOI] [Google Scholar]
- 45. Jiang H, Li M, Wen LM, et al. Effect of short message service on infant feeding practice: findings from a community-based study in Shanghai, China. JAMA Pediatr 2014;168:471–8. 10.1001/jamapediatrics.2014.58 [DOI] [PubMed] [Google Scholar]
- 46. Unger JA, Ronen K, Perrier T, et al. Short message service communication improves exclusive breastfeeding and early postpartum contraception in a low‐ to middle‐income country setting: a randomised trial. BJOG: Int J Obstet Gy 2018;125:1620–9. 10.1111/1471-0528.15337 [DOI] [Google Scholar]
- 47. Harrington EK, Drake AL, Matemo D, et al. An mHealth SMS intervention on postpartum contraceptive use among women and couples in Kenya: a randomized controlled trial. Am J Public Health 2019;109:934–41. 10.2105/AJPH.2019.305051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Jones RM, Kimenju G, Subbiah S, et al. A short message service (SMS) increases postpartum care-seeking behavior and uptake of family planning of mothers in peri-urban public facilities in Kenya. PLoS One 2020;15:e0239213. 10.1371/journal.pone.0239213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Sondaal SFV, Browne JL, Amoakoh-Coleman M, et al. Assessing the effect of mHealth interventions in improving maternal and neonatal care in low- and middle-income countries: a systematic review. PLoS One 2016;11:e0154664. 10.1371/journal.pone.0154664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. WHO . WHO guideline: recommendations on digital interventions for health system strengthening. World Health Organization, 2019. http://www.who.int/reproductivehealth/publications/digital-interventions-health-system-strengthening/en/ [Google Scholar]
- 51. Zhou C, Hu H, Wang C. The effectiveness of mHealth interventions on postpartum depression: a systematic review and meta-analysis. J Telemed Telecare 2020;1357633X:2091781. [Google Scholar]
- 52. Fedha T. Impact of mobile telephone on maternal health service care: a case of Njoro division. Open J Prev Med 2014;04:365–76. 10.4236/ojpm.2014.45044 [DOI] [Google Scholar]
- 53. Lund S, Rasch V, Hemed M, et al. Mobile phone intervention reduces perinatal mortality in Zanzibar: secondary outcomes of a cluster randomized controlled trial. JMIR Mhealth Uhealth 2014;2:e15. 10.2196/mhealth.2941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Fisher WA, Fisher JD, Harman J. The information-motivation-behavioraI skills model: A general social psychological approach to understanding and promoting health behavior. In: Suls J, Wallston KA, eds. Social psychological foundations of health and illness. John Wiley & Sons, 2003: 82–106. [Google Scholar]
- 55. Unger JA, Wandika B, Ronen K. Mobile WACh NEO: engagementengagement of pregnant and postpartum women with a two-way SMS service to improve neonatal outcomes. In: Pediatric academic societies meeting. Baltimore, MD, 2019. https://www.xcdsystem.com/pas/program/2019/index.cfm?pgid=156&print=1&printmode=1 [Google Scholar]
- 56. Drake AL, Unger JA, Ronen K, et al. Evaluation of mHealth strategies to optimize adherence and efficacy of option B+ prevention of mother-to-child HIV transmission: rationale, design and methods of a 3-armed randomized controlled trial. Contemp Clin Trials 2017;57:44–50. 10.1016/j.cct.2017.03.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kenya Ministry of Public Health and Sanitation . National guidelines on essential newborn care. Nairobi, Kenya, 2015. [Google Scholar]
- 58. Unger JA, Kinuthia J, John-Stewart G. Texting Condolences: adapting mHealth programs after unexpected pregnancy and infant outcomes. JMIR Mhealth Uhealth 2017;5:e176. 10.2196/mhealth.8303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Perrier T, Dell N, DeRenzi B. Engaging pregnant women in Kenya with a hybrid computer-human SMS communication system. In: 33rd Annual ACM Conference. New York, USA: ACM Press, 2015: 1429–38. [Google Scholar]
- 60. Cox JL, Holden JM, Sagovsky R. Detection of postnatal depression. development of the 10-item Edinburgh postnatal depression scale. Br J Psychiatry 1987;150:782–6 http://www.ncbi.nlm.nih.gov/pubmed/3651732 10.1192/bjp.150.6.782 [DOI] [PubMed] [Google Scholar]
- 61. Green EP, Tuli H, Kwobah E, et al. Developing and validating a perinatal depression screening tool in Kenya blending Western criteria with local idioms: a mixed methods study. J Affect Disord 2018;228:49–59. 10.1016/j.jad.2017.11.027 [DOI] [PubMed] [Google Scholar]
- 62. Kumar M, Ongeri L, Mathai M, et al. Translation of EPDS questionnaire into Kiswahili: understanding the cross-cultural and translation issues in mental health research. J Pregnancy Child Health 2015;2. 10.4172/2376-127X.1000134 [DOI] [Google Scholar]
- 63. Sherbourne CD, Stewart AL. The MOS social support survey. Soc Sci Med 1991;32:705–14. 10.1016/0277-9536(91)90150-B [DOI] [PubMed] [Google Scholar]
- 64. Črnčec R, Barnett B, Matthey S. Development of an instrument to assess perceived self-efficacy in the parents of infants. Res Nurs Health 2008;31:442–53. 10.1002/nur.20271 [DOI] [PubMed] [Google Scholar]
- 65. Murray CJ, Lopez AD, Black R, et al. Population health metrics research Consortium gold standard verbal autopsy validation study: design, implementation, and development of analysis datasets. Popul Health Metr 2011;9:27. 10.1186/1478-7954-9-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Waiswa P, Peterson S, Tomson G, et al. Poor newborn care practices - a population based survey in eastern Uganda. BMC Pregnancy Childbirth 2010;10:9. 10.1186/1471-2393-10-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Osborn L, Ronen K, Larsen AM. Antenatal depressive symptoms in Kenyan women living with HIV: contributions of recent HIV diagnosis, stigma, and partner violence. AIDS Care 2021:9. 10.1080/09540121.2021.1981216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Benjamini Y, Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. Journal of the Royal Statistical Society: Series B 1995;57:289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- 69. Kenya Ministry of Health . COVID19 RMNH guidelines: a Kenya practical guide for continuity of reproductive, maternal, newborn and family planning care and services in the background of COVID19 pandemic. Kenya, 2020: 1–23. https://www.health.go.ke/wp-content/uploads/2020/04/KENYA-COVID19-RMNH.pdf.pdf.pdf [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2021-056062supp001.pdf (108.7KB, pdf)
bmjopen-2021-056062supp002.pdf (5.2MB, pdf)