Abstract
Purpose:
We had previously reported on the safety and the recommended phase 2 dose (RP2D) of olaparib in combination with the PI3Kα-specific inhibitor alpelisib in patients with high-grade serous ovarian cancer as studied in a phase 1b trial (NCT01623349). Here we report on the breast cancer cohort from that study.
Experimental Design:
Eligible patients had recurrent triple-negative breast cancer (TNBC), or recurrent breast cancer of any subtype with a germline BRCA mutation and were enrolled to a dose escalation or expansion cohort. After definition of the RP2D, secondary end points included safety and objective response rate (ORR). Exploratory analyses were performed using circulating free DNA (cfDNA).
Results:
17 patients with TNBC were enrolled with a median of 3 prior lines of chemotherapy. The most common treatment-related grade 3–4 adverse events were hyperglycemia (18%) and rash (12%). The ORR was 18% (23% for patients treated at the RP2D) and 59% had disease control. The median duration of response was 7.4 months. Analysis of cfDNA tumor fractions (TFx) revealed that patients with TFx<15% after completion of the first cycle had a longer progression-free survival compared to those with TFx>15% (6.0 months vs 0.9 months, p=0.0001).
Conclusions:
Alpelisib in combination with olaparib is tolerable in patients with pre-treated TNBC, with evidence of activity in non-BRCA carriers. CfDNA provided important prognostic information. Results highlight potential synergistic use of a PI3Ki to sensitize HR-proficient (BRCA wild-type) TNBC to PARPi and suggest the potential to expand the use of PARPi beyond BRCA-mutant tumors.
Keywords: triple-negative breast cancer, PARPi, PI3Ki, ctDNA
Background
Triple-negative breast cancer (TNBC) accounts for 20% of all breast cancers, and is associated with inferior survival outcomes.(1) In contrast to the efficacy of targeted therapies against hormone-receptor positive and HER2-positive breast cancers, and despite the recent approval of an antibody-drug conjugate and a checkpoint inhibitor, cytotoxic chemotherapy remains the backbone of treatment for TNBC.
Therapeutic Poly (ADP-ribose) polymerase (PARP) inhibition is approved for metastatic HER2-negative breast cancer with a germline BRCA1/2 mutation (gBRCAm). In this setting, which applies to less than 5% of metastatic breast cancer patients, olaparib prolongs median progression-free survival (PFS) and improves quality of life in comparison to non-platinum chemotherapy.(2,3) BRCA-associated breast cancers have a defect in homologous recombination (HR) repair resulting in sensitivity to PARPi and other DNA damaging treatments by a variety of synthetic lethal mechanisms. Recent data suggest that patients with germline PALB2 mutations or patients with somatic BRCA alterations may also benefit from PARP inhibition.(4,5) The identification of patients beyond BRCA carriers, whose breast cancers have homologous recombination deficiency (HRD) and may therefore benefit from therapies like PARPi, remains a critical goal.
BRCA1/2-associated and sporadic TNBC share many features, such as high-grade histology, extensive copy number alterations, TP53 mutations, PI3K pathway activation, and defects in DNA damage repair including homologous recombination (HR). Transcriptionally, they are typically basal-like by hierarchical clustering, with these cancers sharing a pattern of genomic instability characterized by allelic loss.(6,7) This genomic instability sensitizes cancer cells to DNA-damaging agents including PARPi. However, in prior studies of olaparib monotherapy, minimal activity has been observed.(8) Phosphatidylinositol 3-kinase (PI3K) inhibitors (PI3Ki) have also demonstrated limited activity in breast cancer patients without a PIK3CA mutation.(9,10)
Preclinical work suggests that PI3Ki impair homologous recombination repair (HR) pathways, which sensitizes HR-proficient cancers to PARPi.(11) The addition of a PI3Ki to PARPi had been shown to improve treatment outcomes in preclinical models of both BRCA1-proficient(11) and BRCA1-deficient tumors.(12) In BRCA wildtype models PI3Ki led to increased DNA-damage with poly(ADP)-ribosylation and transcriptional down-regulation of BRCA1, sensitizing tumor cells to PARPi.(11) In BRCA1-deficient tumors, a similar increase in DNA damage was observed, as well as inhibition of tumor angiogenesis,(12) providing a rationale for this combination in TNBC irrespective of BRCA mutation status. Previously, we reported on the safety and efficacy of the combination of olaparib and the PI3Ki buparlisib in ovarian and breast cancer, but dose escalation of buparlisib was limited by CNS toxicity in the form of depression and anxiety.(13) More recently, Konstantinopoulos et al reported on the ovarian cancer cohort in this trial which tested the α-specific PI3K inhibitor alpelisib (BYL719) in combination with olaparib.(14) Here we report the safety and preliminary evidence of efficacy of olaparib in combination with alpelisib in patients with TNBC and/or BRCA-associated breast cancer.
Methods
Study design and participants
In this phase 1b trial, eligible patients had a confirmed diagnosis of recurrent TNBC (estrogen and progesterone receptor < 1% by IHC and HER2 IHC 1+ or negative by ISH) or confirmed diagnosis of recurrent breast cancer of any subtype with a known germline BRCA mutation. Other inclusion criteria included age 18 years or older, Eastern Cooperative Oncology Group performance status of 1 or lower, estimated life expectancy of greater than 4 months, measurable disease per RECIST 1.1 criteria, normal organ function, and ability to provide informed consent. Patients with major comorbidities were excluded. There was no limit on number of previous systemic therapies; patients with recurrent, metastatic, TNBC must have had at least one chemotherapy regimen for metastatic breast cancer or have developed metastatic breast cancer within 1 year of completion of adjuvant chemotherapy. Previous use of PARPi and PI3Ki was allowed for patients in the dose-escalation cohort but not for patients in the dose-expansion cohort. The clinical trial was approved by the institutional review boards of all participating institutions and the FDA. The full protocol is available in the supplementary information. All procedures involving human participants were carried out in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients before enrollment in the study. This study is registered with ClinicalTrials.gov, number NCT01623349.
Procedures
Olaparib and alpelisib were administered orally as tablet formulations in 28-day cycles. Four dose levels were planned: starting dose level alpelisib 250 mg once daily plus olaparib 100 mg twice daily (dose level 0); alpelisib 250 mg once daily plus olaparib 200 mg twice daily (dose level 1); alpelisib 300 mg once daily plus olaparib 200 mg twice daily (dose level 2); and alpelisib 200 mg once daily plus olaparib 200 mg twice daily (dose level 3). The dose expansion cohort used the dose level 3 schedule. Treatment continued until progression or unacceptable toxicity. Safety monitoring and dose modifications and reductions followed prespecified rules (appendix pp 94–129). Tumor assessment by RECIST v1.1 occurred every 8 weeks (two cycles) and included assessment of chest, abdomen, and pelvis via CT or MRI scan. After completion of cycle 16, tumor assessment was performed every 12 weeks. Toxicity was assessed by Common Terminology Criteria for Adverse Events (CTCAE), version 4.03.
Statistical Analysis
Progression-free survival, duration of response, and overall survival analyses were summarized with the Kaplan-Meier product limit estimator. 95% CIs were reported for outcome events (such as progression for progression-free survival, or death for overall survival) at landmark times and for median progression-free and overall survival using Greenwood’s formula. Patient demographics and adverse event frequencies were summarized with descriptive statistics. All statistical analyses were done with R (version 3.6.1).
Tumor Sequencing
Archival tissue was retrieved and massively parallel sequencing was performed using the OncoPanel platform as previously described.(15) Briefly, OncoPanel is a targeted 447 gene next generation sequencing panel selected based on clinical actionability in cancer that is designed for the detection of single-nucleotide variants, indels, copy number alterations, and structural variants. The method is validated and has a sensitivity of 98% for single-nucleotide variants, 84% for indels, and 74% for structural variants.
Circulating tumor DNA
Patient blood was collected from all patients at baseline (C1D1), at the end of the first cycle of treatment (C2D1), and at the end of treatment (EOT). Circulating free DNA (cfDNA) was extracted from plasma and germline DNA (gDNA) was extracted from the buffy coat. Ultra-low pass whole-genome sequencing (ULPWGS) was performed to screen for tumor content in all 17 breast cancer patients and tumor fractions scores (TFx) using ichorCNA were generated as previously described.(16)
Data Availability
The authors support the dissemination of research data that has been generated, and increased cooperation between investigators. The data that support the findings of this study are available. Raw data for this study were generated at the Dana-Farber Cancer Center (Boston, MA) and Broad Institute of MIT and Harvard (Cambridge, MA). Derived data supporting the findings of this study are available as Supplementary Data (Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, and Table S7) and on Mendeley Data at the following link: (DOI: 10.17632/ch24bpf65r.1). Further de-identified individual participant data will be provided according to institutional procedures. Requests must include a description of the nature of the proposed research and extent of data requirements. Data recipients are required to enter a formal data sharing agreement that describes the conditions for release and requirements for data transfer, storage, archiving, publication, and intellectual property. Requests are reviewed by the study team in terms of scientific merit and ethical considerations, including patient consent.
Results
A total of 17 patients with TNBC were enrolled from Jan 2015 to Dec 2016 at four centers within the Dana-Farber Harvard Cancer Center Consortium. Four participants were enrolled into the dose-escalation cohort (dose levels 0–2) and 13 in the dose-expansion cohort (dose level 3). At the time of data cutoff (April 2020), all 17 patients were off treatment and off study with a median follow up time of 11.8 months (IQR: 4.8–19.6). The demographics and baseline characteristics of the study population are summarized in Table 1; the median age was 51 years (range 33 – 66) and 3 (18%) participants were carriers of a pathogenic BRCA1/2 mutation. Most of the enrolled patients were heavily pretreated, with a median of 3 (range: 1 – 6) prior lines of chemotherapy for metastatic disease.
Table 1.
Demographic characteristics
| Overall | Overall | ||
|---|---|---|---|
| (n=17) | (n=17) | ||
| Age | Cancer stage at diagnosis | ||
| Mean (SD) | 52 (10.0) | Early stage | 9 (53%) |
| Median [Min, Max] | 51 [33.0, 65.6] | Locally advanced | 2 (12%) |
| Race | De novo metastatic | 4 (24%) | |
| White | 14 (82.4%) | Missing | 2 (11%) |
| Black or African American | 2 (11.8%) | ||
| Other | 1 (5.9%) | ||
| Ethnicity | Number of prior lines of chemotherapy for advanced disease | ||
| Hispanic or Latino | 1 (5.9%) | Median [Min, Max] | 3.00 [1, 6] |
| Non-Hispanic | 16 (94.1%) | Prior platinum exposure | 8 (47%) |
| ECOG performance status | Prior taxane exposure | 14 (82%) | |
| Fully active | 12 (70.6%) | ||
| Restricted | 5 (29.4%) | ||
| Germline BRCA mutation status | |||
| Carrier | 3 (17.6%) | ||
| Non-Carrier | 11 (64.7%) | ||
| Unknown | 3 (17.6%) | ||
| Phase | |||
| Escalation | 4 (23.5%) | ||
| Expansion | 13 (76.5%) |
Treatment-related grade 3–4 adverse events occurring in at least 10% of all patients were hyperglycemia (18%) and rash (12%). The most common all-grade toxicities included fatigue (71%), anorexia (59%), hyperglycemia (59%), and nausea (53%). Table S1 lists all treatment-related adverse events that occurred in at least 10% of all patients and all grade 3–4 adverse events. No treatment-related deaths occurred. Two participants had dose-limiting toxicities of hyperglycemia and febrile neutropenia. One patient in the dose escalation cohort at dose level 3 had to stop therapy due to hyperglycemia at C1D21.
Within this study cohort, 3 patients (18%, 95% CI: 3.8–43.4) achieved a partial response and 7 (41%) had stable disease as best response according to RECIST 1.1 (Figure 1). None of the 3 patients who achieved partial response had a known germline BRCA mutation, and one of the 3 had previously progressed on carboplatin. Of the 7 patients with stable disease, 3 (18%) had stable disease for more than 6 months (Figure 2). The objective response rate (ORR, complete or partial response) was 18%, the clinical benefit rate (response or stable disease for at least 6 months) was 35% (95% CI: 14.2–61.7), and the disease control rate (response or stable disease for at least 3 months) was 53% (95% CI: 27.8–77). All patients with partial response were at the recommended phase 2 dose (RP2D) of alpelisib 200 mg daily plus olaparib 200 mg twice daily; thus, the ORR at the RP2D was 23% (3/13) (95% CI: 5–53.8). Tumor shrinkage of any magnitude was observed in 35% (6/17) of patients. The median duration of response was 7.4 months. The median progression-free survival was 3.6 months (95% CI: 1.8–9.2), and the progression-free survival at 6 months was 31.2% (95%CI: 11.4–53.6). The median overall survival was 11.8 months (95% CI: 4.2–19.6). Progression-free and overall survival were similar for patients with and without a germline BRCA mutation (Figure S1).
Figure 1.
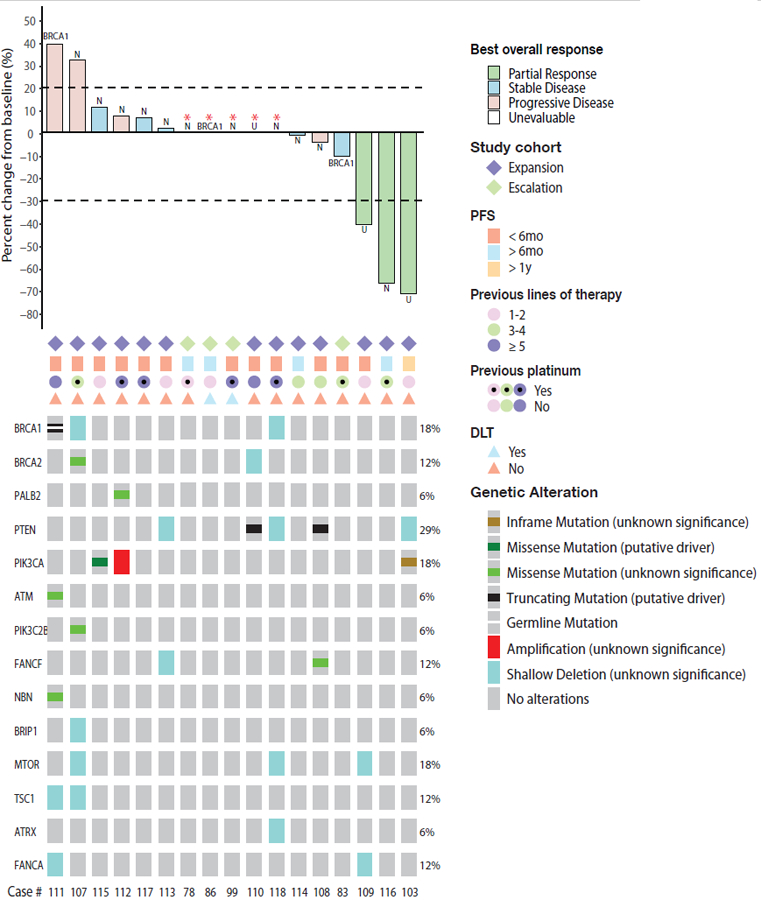
Intensity of responses and genomic alterations in the PI3K pathway or DNA damage repair in patients with metastatic breast cancer on this study. In the waterfall plot (top), there are 5 blank bars with asterisks (patients 78, 86, 99, 110, 118). Three of them (patients 99, 110, and 118) had DLT or progression in the brain before the first restaging scan so target lesion was unevaluable. Two participants (patients 78 and 86) did not have evaluable target lesions. Mutations in FANCB, FANCM, and TSC2 were not found. U = “Unknown BRCA status”. N = “Non-BRCA carrier”.
Figure 2.
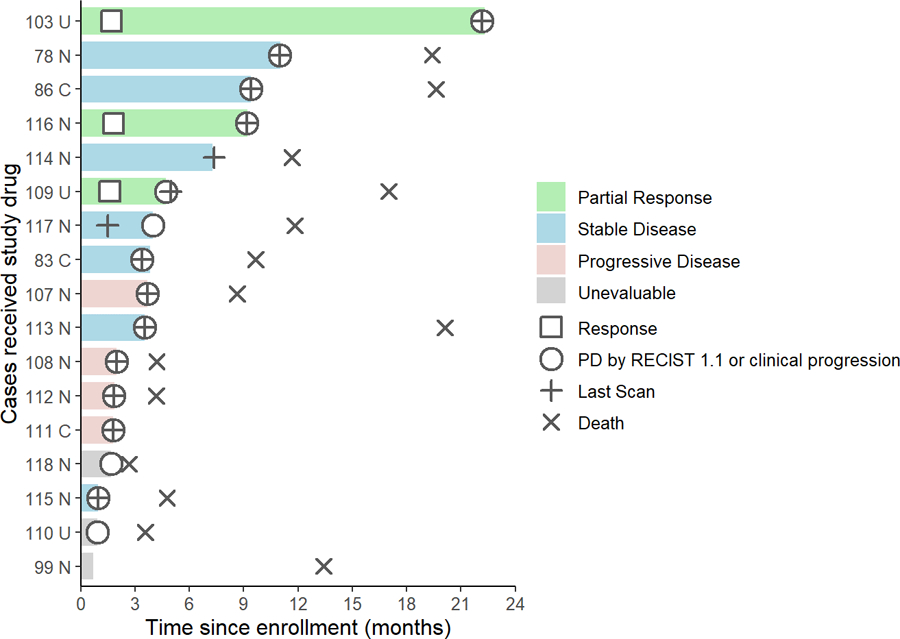
Duration of responses of 17 patients with TNBC treated with alpelisib plus olaparib. Interval from date of enrollment to date of progression or death. Patients are labelled according to their best response per RECIST 1.1 criteria. Three patients (patients 99, 110, and 118) had DLT or progression in the brain before the first restaging scan so target lesion was unevaluable. U = “Unknown BRCA status”. N = “Non-BRCA carrier”. C = “BRCA carrier”. PD = “Progressive disease”.
We examined archival tumor tissue to identify potential mutations predictive of response to the combination of olaparib and alpelisib. Alterations in HR and PI3K pathway genes are shown in Figure 1. None of the responders were known BRCA carriers or had a deleterious somatic mutation of a gene in the HR or PI3K pathways.
Exploratory analyses of circulating tumor DNA (ctDNA) were also performed. The tumor fraction (TFx) median and mean at C1D1 were 9% and 15% (range: 0 – 43%). The estimation of TFx is more reliable when above 3%,(16) and we found that 15 of the 17 patients (88%) had TFx ≥ 3%. In our cohort, 7 of the 17 patients (41%) had TFx ≥ 10%, a level above which exome sequencing is thought to be feasible.(17) At C1D1, five (29%) of the 17 patients had TFx ≥ 25%, and 12/17 (71%) had TFx < 25%.TFx ≥ 25% was associated with inferior outcomes; the mPFS for those with TFx ≥ 25% was 1.6 months versus 4.7 months for those with TFx < 25% (p < 0.0001) (Figure 3a). At C2D1, we identified 6/17 patients (35%) with TFx ≥ 15%, with a mPFS of 0.9 months, compared to those with 6.0 months in those with TFx < 15% (p = 0.0001) (Figure 3b). Overall, a TFx < 15% at C2D1 predicted the longest mPFS.
Figure 3.
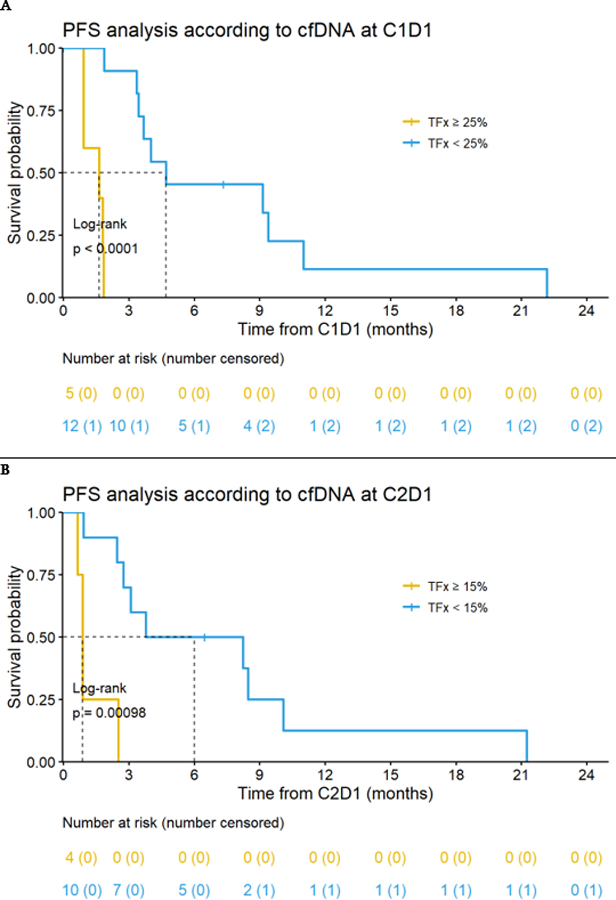
Impact of ctDNA analysis of tumor fraction (TFx) on progression-free survival (PFS). A. At C1D1, the median PFS was 1.6 months for patients with TFx ≥ 25% and 4.7 months for patients with TFx < 25% (p < 0.0001) B. At C2D1, the median PFS was 0.9 months for patients with TFx ≥ 15% and 6.0 months for patients with TFx < 15% (p = 0.0001).
Discussion
In this TNBC cohort from a parent phase 1 trial, the combination of alpelisib plus olaparib was well tolerated with few grade 3–4 adverse events. Hyperglycemia is a known on-target side effect of alpelisib and is readily managed in the outpatient setting with medication. The combination showed activity in heavily pretreated metastatic TNBC, with notable rates of clinical benefit and disease control without a preference for tumors with germline BRCA mutations or PIK3CA mutations.
Our previously experience with the combination of olaparib and the pan-PI3K-inhibitor buparlisib in advanced high-grade serous ovarian and TNBC showed that response to therapy was not restricted to patients harboring a germline BRCA mutation.(13) Further development of buparlisib, however, was abandoned due to its unfavorable safety profile with significant central nervous system toxicity. Possibly due to selective inhibition of the alpha-subunit of PI3K, alpelisib in combination with olaparib has been better tolerated than buparlisib in ovarian cancer(14) and in our breast cancer cohort.
Both PI3Ki and PARPi have established activity in the management of advanced breast cancer. After activity was observed in PIK3CA-mutant advanced HR+HER2- breast cancer with the combination of alpelisib and fulvestrant,(9) the SOLAR-1 trial Phase III study demonstrated a doubling in median PFS (11.0 months vs 5.7 months) in PIK3CA-mutant advanced HR+/HER2- breast cancer with this combination.(18) Alpelisib was subsequently approved by the FDA in 2019 to be used in combination with fulvestrant in PIK3CA-mutant HR+HER2- BC. In a Phase I/II study which included 12 evaluable patients with TNBC, the combination of alpelisib and chemotherapy (nab-paclitaxel) showed an ORR of 59% in advanced HER2- BC with a similar rate for those with TNBC. In that study, the population was not as heavily pretreated (70% of patients had ≤1 prior systemic therapy) and 40% of patients had an activating PIK3CA mutation, which was associated with longer PFS.(19)
Antitumor activity of PARPi has been demonstrated in metastatic breast cancer in those with a germline BRCA mutation.(2,3) Beyond germline BRCA carriers, TBCRC 048 also showed responses to olaparib monotherapy in patients with somatic BRCA mutations or germline PALB2 mutations.(20) The absence of activity among the three BRCA germline carriers enrolled in this study could have been related to restoration of HR after prior chemotherapy exposure;(21) which included platinum for one patient and veliparib for another. Experiences with PARPi monotherapy in metastatic BRCA wildtype TNBC have not suggested significant activity, although responses have been observed in the treatment-naïve preoperative setting.(22)
In the current study, the response rate was higher than expected from either agent as monotherapy, supporting the preclinical findings of synergistic activity of the combination of PARPi and PI3Ki and the hypothesis that PI3Ki sensitize tumors to PARP inhibition, in the absence of a PIK3CA mutation.(12) To our knowledge, the current report is the first to demonstrate responses in BRCA-proficient TNBC to a PARPi in the advanced disease setting.
This combination is appealing for use in TNBC for several reasons. First, it is attractive to consider the option of a targeted approach in a disease otherwise primarily treated with chemotherapy. Second, this combination utilizes oral agents that avoid toxicities associated with chemotherapy, including alopecia. Although conclusions cannot be drawn from cross trial observations, results in our cohort compare favorably to the control arm of the ASCENT trial, where in the arm with chemotherapy of physician’s choice a median PFS of 1.7 months and an ORR of 5% were observed in a patient population similar to this cohort.(23) The development of even more precise biomarkers could refine patient selection in the future. A multi-institutional clinical trial evaluating the combination of olaparib and alpelisib for HER2-negative breast cancer is being designed and will be performed via a cooperative group (Alliance). This combination for ovarian cancer is currently being investigated in the EPIK-O study (NCT04729387).
An exploratory, hypothesis-generating analysis of ctDNA revealed that TFx at C1D1 and C2D1 have prognostic value with higher levels at both time points associated with shorter PFS, supporting previous findings.(17) Notably, detection of meaningful TFx was possible in the majority of patients in our cohort. Tumor fraction estimations are analytically validated with a lower limit of detection of 3% and are particularly reliable above 10%, which gives us confidence that the cutoffs of 25% and 15% identified for prognostication at C1D1 and C2D1 are not only clinically meaningful but also technically feasible.(16) To date, FDA approval for liquid biopsies is restricted to metastatic HR+HER2- BC after progression on endocrine therapy. In these cases, ctDNA can be used to detect specific PIK3CA mutations that are predictive of a response to alpelisib in combination with fulvestrant.(18)
Conclusions
This early-phase trial of olaparib plus alpelisib in a cohort of patients with TNBC showed that the combination was safe and tolerable. Although conclusions are limited by the small number of patients, the combination demonstrated activity in a heavily pretreated population, with response and clinical benefit rates of 18% and 35%, respectively. TNBC remains a challenging subtype of breast cancer, and progress is needed to identify novel therapies that can improve outcomes for patients. Based on these preliminary clinical data on the synergy of this combination irrespective of BRCA status, further studies to explore the use of PARP inhibitors beyond BRCA mutant tumors are warranted. Larger prospective randomized studies and biomarker development are planned to identify patients who are most likely to benefit from this all-oral combination.
Supplementary Material
Translational Relevance Statement.
Triple-negative breast cancer (TNBC) is associated with inferior survival outcomes and cytotoxic chemotherapy remains the mainstay of treatment. The defect in homologous recombination (HR) repair encountered in tumors in carriers of a germline BRCA1/2 mutation (gBRCAm) results in sensitivity to PARPi by a variety of synthetic lethal mechanisms. Preclinical work suggests that PI3Ki impair homologous recombination repair (HR) pathways, which sensitizes HR-proficient cancers (BRCA wild-type) to PARPi. In this study, we find that alpelisib in combination with olaparib is tolerable in patients with pre-treated TNBC, with evidence of activity in non-BRCA carriers. The response rate of 23% in those treated at the RP2D highlights potential synergistic use of a PI3Ki to sensitize HR-proficient TNBC to PARPi and suggest the potential to expand the use of PARPi beyond BRCA-mutant tumors.
Acknowledgements
Stand Up to Cancer-American Association for Cancer Research Dream Team Translational Research Grant, Grant Number SU2C-AACR-DT0209 (GMW, GIS, UAM and LCC). National Institutes of Health (NIH) Research Project Grant 1R01CA226776–01 (GMW). DF/HCC Specialized Program of Research Excellence (SPORE) in Breast Cancer NIH P50 CA168504 (GIS, EPW, GMW, ELM). NIH R35 CA197588 (LCC). Grants from the Breast Cancer Research Foundation (GMW, UAM, EPW,LCC). National Cancer Institute P50 CA240243–01A1 (UAM). Grants from the Mary Kay Ash Foundation and Breast Cancer Alliance (GMW).
Footnotes
Disclosures
FB reports personal fees from Curio Science, outside of the submitted work. LCC is a co-founder and member of the senior advisory board of Volastra Therapeutics. He is a founder, former member of the senior advisory board of Agios Pharmaceuticals and served as member of the senior advisory board of Ravenna Pharmaceuticals (previously Petra Pharmaceuticals). These companies are developing novel therapies for cancer. LCC holds equity in Volastra, Agios, and Ravenna. LCC’s laboratory also received some financial support from Ravenna Pharmaceuticals. No drugs from these companies are discussed in this manuscript. GIS reports grants and personal fees from Eli Lilly, Pfizer, Merck/EMD Serono, and Sierra Oncology; and personal fees from Roche, Bicycle Therapeutics, Fusion Pharmaceuticals, G1 Therapeutics, Cybrexa Therapeutics, Bayer, Ipsen, Astex, Almac, Boehringer Ingelheim, Artios, Atrin, Concarlo Holdings, Syros, Zentalis, CytomX Therapeutics and Blueprint Medicines outside the submitted work. In addition, he holds a patent entitled, “Dosage regimen for sapacitabine and seliciclib,” also issued to Cyclacel Pharmaceuticals, and a pending patent, entitled, “Compositions and Methods for Predicting Response and Resistance to CDK4/6 Inhibition,” together with Liam Cornell. VAA has patent applications filed with the Broad Institute and is a member of the scientific advisory boards of AGCT GmbH and Bertis Inc, which were not involved in this study. EPW reports institutional research funding from Genentech/Roche, serving in advisory boards at Leap, and receiving personal fees from Athenex, Carrick Therapeutics, Genentech/Roche, Gilead, GSK, Jounce, Leap, and Syros. PAK reports institutional research funding from AstraZeneca and Novartis during the conduct of the study. PAK also reports serving in advisory boards at AstraZeneca, Pfizer, and Merck outside the submitted work. UAM reports personal fees from Astrazeneca, Merck, Novartis, Blueprint Medicine, and NextCure. GMW reports institutional research funding from Glaxo Smith-Kline, has a patent US 20090258352 A1, “Pin1 as a marker for abnormal cell growth” licensed to Cell Signaling (R&D Systems) and received grant funding from Merck & Co outside of the submitted work. ELM also reports serving as consultant at Lilly, Novartis, AstraZeneca, and Gilead. The other authors declare no competing interests.
References
- 1.Hudis CA, Gianni L. Triple‐Negative Breast Cancer: An Unmet Medical Need. The Oncologist 2011;16:1–11. [DOI] [PubMed] [Google Scholar]
- 2.Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. The New England Journal of Medicine 2017;377:523–33. [DOI] [PubMed] [Google Scholar]
- 3.Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee K-H, et al. Talazoparib in Patients with Advanced Breast Cancer and a Germline BRCA Mutation. N Engl J Med 2018;379:753–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Farmer H, McCabe N, Lord CJ, Tutt ANJ, Johnson DA, Richardson TB, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature Nature Publishing Group; 2005;434:917–21. [DOI] [PubMed] [Google Scholar]
- 5.Konstantinopoulos PA, Ceccaldi R, Shapiro GI, D’Andrea AD. Homologous Recombination Deficiency: Exploiting the Fundamental Vulnerability of Ovarian Cancer. Cancer Discov 2015;5:1137–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Verhoog LC, Brekelmans CT, Seynaeve C, van den Bosch LM, Dahmen G, van Geel AN, et al. Survival and tumour characteristics of breast-cancer patients with germline mutations of BRCA1. Lancet 1998;351:316–21. [DOI] [PubMed] [Google Scholar]
- 7.Perou CM, Sørlie T, Eisen MB, van de Rijn M, Jeffrey SS, Rees CA, et al. Molecular portraits of human breast tumours. Nature 2000;406:747–52. [DOI] [PubMed] [Google Scholar]
- 8.Gelmon KA, Tischkowitz M, Mackay H, Swenerton K, Robidoux A, Tonkin K, et al. Olaparib in patients with recurrent high-grade serous or poorly differentiated ovarian carcinoma or triple-negative breast cancer: a phase 2, multicentre, open-label, non-randomised study. Lancet Oncol 2011;12:852–61. [DOI] [PubMed] [Google Scholar]
- 9.Juric D, Janku F, Rodón J, Burris HA, Mayer IA, Schuler M, et al. Alpelisib Plus Fulvestrant in PIK3CA-Altered and PIK3CA-Wild-Type Estrogen Receptor-Positive Advanced Breast Cancer: A Phase 1b Clinical Trial. JAMA oncology 2019;5:e184475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baselga J, Im S-A, Iwata H, Cortés J, De Laurentiis M, Jiang Z, et al. Buparlisib plus fulvestrant versus placebo plus fulvestrant in postmenopausal, hormone receptor-positive, HER2-negative, advanced breast cancer (BELLE-2): a randomised, double-blind, placebo-controlled, phase 3 trial. The Lancet Oncology 2017;18:904–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ibrahim YH, García-García C, Serra V, He L, Torres-Lockhart K, Prat A, et al. PI3K Inhibition Impairs BRCA1/2 Expression and Sensitizes BRCA-Proficient Triple-Negative Breast Cancer to PARP Inhibition. Cancer Discovery 2012;2:1036–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Juvekar A, Burga LN, Hu H, Lunsford EP, Ibrahim YH, Balmañà J, et al. Combining a PI3K Inhibitor with a PARP Inhibitor Provides an Effective Therapy for BRCA1-Related Breast Cancer. Cancer Discovery 2012;2:1048–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Matulonis UA, Wulf GM, Barry WT, Birrer M, Westin SN, Farooq S, et al. Phase I dose escalation study of the PI3kinase pathway inhibitor BKM120 and the oral poly (ADP ribose) polymerase (PARP) inhibitor olaparib for the treatment of high-grade serous ovarian and breast cancer. Ann Oncol 2017;28:512–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konstantinopoulos PA, Barry WT, Birrer M, Westin SN, Cadoo KA, Shapiro GI, et al. Olaparib and α-specific PI3K inhibitor alpelisib for patients with epithelial ovarian cancer: a dose-escalation and dose-expansion phase 1b trial. Lancet Oncol 2019;20:570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sholl LM, Do K, Shivdasani P, Cerami E, Dubuc AM, Kuo FC, et al. Institutional implementation of clinical tumor profiling on an unselected cancer population. JCI Insight 2016;1:e87062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Adalsteinsson VA, Ha G, Freeman SS, Choudhury AD, Stover DG, Parsons HA, et al. Scalable whole-exome sequencing of cell-free DNA reveals high concordance with metastatic tumors. Nat Commun 2017;8:1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stover DG, Parsons HA, Ha G, Freeman SS, Barry WT, Guo H, et al. Association of Cell-Free DNA Tumor Fraction and Somatic Copy Number Alterations With Survival in Metastatic Triple-Negative Breast Cancer. J Clin Oncol 2018;36:543–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.André F, Ciruelos E, Rubovszky G, Campone M, Loibl S, Rugo HS, et al. Alpelisib for PIK3CA-Mutated, Hormone Receptor–Positive Advanced Breast Cancer. New England Journal of Medicine 2019;380:1929–40. [DOI] [PubMed] [Google Scholar]
- 19.Sharma P, Abramson VG, O’Dea AP, Nye L, Mayer IA, Pathak HB, et al. Clinical and biomarker results from phase I/II study of PI3K inhibitor alpelisib plus nab-paclitaxel in HER2-negative metastatic breast cancer. Clin Cancer Res 2021; [DOI] [PMC free article] [PubMed]
- 20.Tung NM, Robson ME, Ventz S, Santa-Maria CA, Marcom PK, Nanda R, et al. TBCRC 048: A phase II study of olaparib monotherapy in metastatic breast cancer patients with germline or somatic mutations in DNA damage response (DDR) pathway genes (Olaparib Expanded). JCO American Society of Clinical Oncology; 2020;38:1002–1002. [DOI] [PubMed] [Google Scholar]
- 21.Waks AG, Cohen O, Kochupurakkal B, Kim D, Dunn CE, Buendia Buendia J, et al. Reversion and non-reversion mechanisms of resistance to PARP inhibitor or platinum chemotherapy in BRCA1/2-mutant metastatic breast cancer. Ann Oncol 2020;31:590–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eikesdal HP, Yndestad S, Elzawahry A, Llop-Guevara A, Gilje B, Blix ES, et al. Olaparib monotherapy as primary treatment in unselected triple negative breast cancer. Ann Oncol 2021;32:240–9. [DOI] [PubMed] [Google Scholar]
- 23.Bardia A, Hurvitz SA, Tolaney SM, Loirat D, Punie K, Oliveira M, et al. Sacituzumab Govitecan in Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine Massachusetts Medical Society; 2021;384:1529–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors support the dissemination of research data that has been generated, and increased cooperation between investigators. The data that support the findings of this study are available. Raw data for this study were generated at the Dana-Farber Cancer Center (Boston, MA) and Broad Institute of MIT and Harvard (Cambridge, MA). Derived data supporting the findings of this study are available as Supplementary Data (Table S1, Table S2, Table S3, Table S4, Table S5, Table S6, and Table S7) and on Mendeley Data at the following link: (DOI: 10.17632/ch24bpf65r.1). Further de-identified individual participant data will be provided according to institutional procedures. Requests must include a description of the nature of the proposed research and extent of data requirements. Data recipients are required to enter a formal data sharing agreement that describes the conditions for release and requirements for data transfer, storage, archiving, publication, and intellectual property. Requests are reviewed by the study team in terms of scientific merit and ethical considerations, including patient consent.


