Summary
Even after recovering from coronavirus disease 2019 (COVID-19), patients can experience prolonged complaints, referred to as "long COVID". Similar to reports in Caucasians, a follow-up study in Japan revealed that fatigue, dyspnea, cough, anosmia/dysgeusia, and dyssomnia are common symptoms. Although the precise mode of long COVID remains elusive, multiple etiologies such as direct organ damage by infection with severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), autoimmunity, prolonged inflammatory reactions, and psychiatric impairment seem to be involved. Notably, SARS-CoV-2 is neurotropic, and viral RNA and proteins are continuously detectable in multiple organs, including the brain. Viral proteins exert a number of different toxic effects on cells, suggesting that persistent infection is a key element for understanding long COVID. Here, we first reviewed the current status of long COVID in Japan, and then summarized literature that help us understand the molecular background of the symptoms. Finally, we discuss the feasibility of vaccination as a treatment for patients with long COVID.
Keywords: SARS-CoV-2, long COVID, persistency, viral proteins, autoantibody, vaccination
Introduction
Approximately 2 years have passed since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first reported in China in December 2019. Since then, more than 400 million people have been infected and approximately 6 million people have died from coronavirus disease 2019 (COVID-19) worldwide. After the acute phase of infection, patients can continue to suffer from various clinical symptoms that impair their quality of life, which is called "long COVID" or "long-haul COVID" (1,2). The World Health Organization recently defined this post-COVID status as "a clinical condition that occurs after 3 months of viral infection with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis" (3). A recent meta-analysis revealed that more than 50 symptoms have been identified in long COVID patients, approximately 80% of whom suffer from one or more symptoms (4). As possible causes of long COVID symptoms, multiple etiologies such as direct organ damage, constitutive inflammatory responses including autoimmunity, and psychiatric impairment seem to be involved.
A number of excellent reviews on the clinical symptoms of long COVID are available (1,2). Here, we summarize the current status of long COVID in Japan and discuss its possible modes and management by highlighting the persistence of viral infection.
Long COVID in Japan and knowledge about its main symptoms
Some individuals who were discharged from the National Center for Global Health and Medicine Hospital in Japan after recovery from COVID-19 have experienced long COVID symptoms for more than 60 days from initial symptom onset (5) (Table 1). Chronic manifestations of COVID-19 have been reported in several countries, including Spain (6), Norway (7), and Israel (8). Graded common symptoms are fatigue, dyspnea, dyssomnia, cough, chest pain, headache, and anosmia/dysgeusia. Most symptoms are prolonged from the acute phase of COVID-19 infection, although dyssomnia is a late-onset symptom that appears at least 30 days after initial symptom onset (5). Additionally, alopecia, fulminant myocarditis, and multi-system inflammatory syndrome can be observed at 6 weeks after symptom onset (9,10). Even after 1 year, a sizeable proportion of recovered COVID-19 patients still have most symptoms such as fatigue, dyspnea, arthralgia, memory loss, and concentration difficulties (11).
Table 1. Prevalence of long COVID symptoms.
| Variables | Japan | Spain | Norway | Israel |
|---|---|---|---|---|
| Number of patients | 63 | 1969 | 312 | 544 |
| Female (%) | 33.3 | 46.4 | 51.3 | 56.4 |
| Age, years, mean (SD) | 48.1 (18.5) | 61.1 (16.3) | 46 (33-58)† | 46.4 (15.5) |
| BMI, mean (SD) | 23.7 (4.0) | - | 24.6 (22.8-27.3)† | 27.6 (5.6) |
| Comorbidity (%) | ||||
| Asthma/COPD | 1.6 | 10.3 | 12.2 | 6.4 |
| Hypertension | 25.4 | 26.1 | 11.2 | 16.4 |
| Chronic heart disease | 1.6 | 11.9 | 7.1 | - |
| Diabetes | 14.3 | 12.0 | 4.2 | 8.3 |
| Symptoms (%) | ||||
| Days from symptom onset at the time of observation | 60 days | 8.4 months (1.5) | 6 months | 123 days (80-204)† |
| Fatigue | 15.9 | 61.3 | 29.9 | 75.6 |
| Dyspnea | 17.5 | 23.3 | 15.4 | 50.9 |
| Cough | 7.9 | - | 6.1 | 19.9 |
| Chest pain | - | 45.1* | - | 30.5 |
| Palpitations | - | 7.1 | 6.1 | 12.3 |
| Myalgia | - | 45.1* | - | 37.5 |
| Arthralgia | - | 45.1* | - | 9.4 |
| Paresthesia | - | 12.0 | 3.6 | 24 |
| Dyssomnia | 16.1 | 34.2 | 5.3 | 37.5 |
| Headache | - | 45.1* | 11.3 | 12.7 |
| Memory loss | - | 17.3 | 18.2 | 36.9 |
| Concentration problems | - | 7.1 | 19.0 | 39.0 |
| Anosmia/dysgeusia | 4.8 | 6.8 | 2.7 | 29.4 |
| Worse physical activity status | - | - | - | 26.1 |
| Reference (DOI) | 10.1093/ofid/ ofaa507 | 10.3390/ jcm11020413 | 10.1038/s41591- 021-01433-3 | 10.3390/ jcm11040898 |
†median (interquartile range); *pain symptoms (%) including chest pain, myalgia, arthralgia, and headache. SD, standard deviation; BMI, body mass index; COPD, chronic obstructive pulmonary disease.
Fatigue
Fatigue is the most common complaint of long COVID. However, it is observed as a postinfectious syndrome not only in long COVID but also in various viral infections such as SARS-CoV-1, Middle East respiratory syndrome (12), Epstein-Barr (EB) virus, Q fever, and Ebola virus, showing a common symptom that resembles myalgia encephalomyelitis/chronic fatigue syndrome and fibromyalgia (13). A number of follow-up studies have identified fatigue and/or dyspnea in patients with long COVID, and approximately 70% of hospitalized COVID-19 survivors feel fatigue, but the symptoms improve continuously (14,15). Female sex has been identified as a risk factor for fatigue (14). Pre-existing comorbidities, symptoms at hospitalization, and severity during hospitalization have also been suggested as risk factors (14).
To assess physical condition, diffusion capacity for carbon monoxide and the 6-min walk test with lung computed tomography (CT) scans are commonly utilized. Abnormalities of diffusion capacity for carbon monoxide, chest X-rays, and reduced distance in the 6-min walk test are observed frequently in patients who received invasive mechanical ventilation (16). CT scans have detected persistent lung pathology in 63% of patients at 3 months, with findings of bilateral ground-glass opacities and/or reticulation in the lower lung lobes, but without radiological signs of pulmonary fibrosis. Although sequential follow-up evaluations revealed an improvement of symptoms and CT abnormalities (16), it was noted that respiratory changes persist in a non-negligible number of patients (approximately one-third) who require prolonged follow-up (15). Curiously, there is no difference in lowest oxygen saturation among patients with normal and reduced 6-min walk test distance, suggesting that the difference in walk distance is not directly related to physical abnormalities, but is associated with subjective factors. These observations imply the importance of comprehensive mental and physical care for patients with fatigue (17).
Observation that there is also a non-negligible number of patients with persistent respiratory changes (15) suggests that it is important to understand how lung damage occurs after viral infection. To identify the early events of lung injury during acute viral infection, Melms et al. performed single-nucleus RNA-sequencing analysis on snap-frozen lung tissues from individuals with COVID-19. They found nine major cell types (i.e., epithelial cells, myeloid cells, fibroblasts, endothelial cells, T and natural killer lymphocytes, B lymphocytes and plasma cells, neuronal cells, mast cells, and antigen-presenting cells) in total 41 different cell types (18). Among these, alveolar type 1 (AT1) and type 2 (AT2) epithelial cells are involved in lung epithelial regeneration. AT2 cells express angiotensin converting enzyme 2 (ACE2), a receptor for SARS-CoV-2, and function as progenitors to AT1 cells. In COVID-19 lung samples, AT2 cells have decreased expression of a gene related to regeneration, whereas AT1 cells have no increased expression of a marker of late AT1 maturation. Data suggests that the regeneration program of the lung epithelium is impaired in the infected lung. Moreover, fibroblasts with high expression of the gene encoding collagen triple helix repeat containing-1, which was recently proposed as a marker of pathological fibroblasts, were identified (18).
Alopecia
Hair loss after viral infection was also reported as post-influenza alopecia in 1919, indicating that it is not specific to long COVID (19). Excessive hair loss occurs within 2-3 months after SARS-CoV-2 infection (20), and is mainly diagnosed as telogen effluvium. Telogen effluvium can be divided into acute or chronic when it continues for more than 6 months, which resolves at 6-12 months after removal of the activating factor. Proinflammatory cytokines including interleukin (IL)- 1β, IL-6, tumor necrosis factor (TNF)-α, and interferon type I and II (IFN-I/II) are proposed as activating factors of telogen effluvium. As a therapeutic approach, hydroxychloroquine and glucocorticoids as anti-inflammatory agents have been tried (21).
Anosmia and dysgeusia
Viral load during infection is significantly associated with anosmia/dysgeusia (22). Consistent with the original report that ACE2 and transmembrane protease, serine 2 (TMPRSS2), a protease of the viral spike protein facilitating viral infection, are not expressed by olfactory neurons (23), single-cell RNA-sequencing analysis of cells derived from mouse nasal tissues revealed that ACE2 is expressed in dorsally located olfactory epithelial sustentacular cells and mouse olfactory bulb pericytes (24). Recently, a novel candidate receptor for SARS-CoV-2, neuropilin-1 (NRP1), has been reported. NRP1 binds to the Arg- Arg-Ala-Arg (RRAR) motif, which is located on the carboxy-terminal end of the S1 subunit of the viral spike protein (hereafter S1 protein) (25), and facilitates the cell entry of SARS-CoV-2. Different from ACE2, NRP1 is expressed in nasal epithelial cells and is responsible for infection of the nasal epithelium. Autopsy of two patients with anosmia revealed that SARS-CoV-2 infection induced axonal damage (26).
Analysis of an experimental mouse model of human ACE2 driven by the keratin 18 (K18-hACE2) promoter (27) demonstrated that SARS-CoV-2 infection impaired the ability to smell. Immunohistochemical analysis after viral infection revealed the nucleocapsid (N) protein of SARS-CoV-2 was expressed by sustentacular cells in the olfactory epithelium. Data strongly suggested that the mice were susceptible to anosmia after viral infection (28). If infection causes a functional abnormality of sustentacular cells, it is plausible that anosmia is reversible.
Analysis of three postmortem samples of the IXth (glossopharyngeal) and Xth (vagal) cranial nerves demonstrated the expression of ACE2, NRP1, and TMPRSS2, suggesting that SARS-CoV-2 can also infect these cranial nerves and induce dysgeusia by direct tissue damage (29).
Miscellaneous symptoms
In addition to these common complaints, patients with long COVID exhibit a variety of symptoms possibly related to psychiatric disorders that include attention disorder, post-activity polypnea, nausea or vomiting, memory loss, and paresthesia (4). To provide appropriate care to patients, it is necessary to exclude organic disorders in the central nervous system (CNS), further indicating the importance of identifying the tropism of SARS-CoV-2 in the CNS.
Policy for managing long COVID patients
At present, no solid clinical management approach has been established for long COVID. However, it is recommended that all patients with persistent symptoms, particularly those with multisystem complaints or symptoms lasting beyond 12 weeks, should be referred to a specialized outpatient COVID-19 recovery clinic, if available, or a subspecialty clinic relevant to the patient's specific symptoms (30).
The need for laboratory testing in patients with long COVID might be determined by the severity of symptoms and abnormal test results during the acute phase and current symptoms. For most patients who have recovered from mild acute COVID-19, routine laboratory testing is not conducted (30). In contrast, for patients recovering from more severe illness, it is reasonable to obtain complete blood counts; blood chemistry, including electrolytes, blood urea nitrogen, and serum creatinine. Liver function studies, including serum albumin should also be included. We generally do not monitor coagulation parameters and re-test serology. This approach is supported by the World Health Organization and the Centers for Disease Control and Prevention (31,32).
As for the treatment of long COVID, we often deal with each symptom individually. For instance, The American Academy of Physical Medicine and Rehabilitation has developed a multidisciplinary collaborative consensus guidance statement on the assessment and management of fatigue following COVID-19 (33). Nevertheless, there is no established drug therapy for fatigue and non-pharmaceutical approaches such as "4-P" (Planning, Pacing, Prioritizing, and Positioning) are often recommended (34).
With regard to dysosmia and dysgeusia, there is scarce evidence for the use of specific pharmacologic agents. However, patients with persistent gustatory and/ or olfactory dysfunction may benefit from olfactory training and self-guided programs. In a 2020 meta-analysis of four studies (two randomized controlled trials and two prospective cohort studies), 286 patients with pulmonary veno-occlusive disease received olfactory training and treatment protocols lasting from 4 to 9 months (35). Olfactory training was associated with an increased chance of a clinically important olfactory improvement (odds ratio 2.77, 95% confidence interval 1.67-4.58) compared with patients who did not receive such therapy. If the symptoms fail to resolve, further evaluation by an otolaryngologist may be needed, particularly in the setting of accompanying upper airway symptoms.
In other words, most symptoms caused by long COVID might not be critical; however, they may be difficult to resolve at the same time. The characteristics of patients who should be treated and how long they should be followed up for after the acute phase of COVID-19 are currently unknown. Given the circumstances, the clinical management of long COVID becomes inevitably vague and complicated.
Knowledge about molecular background of long COVID
Neurotropism of SARS-CoV-2
To understand the underlying mode of long COVID, especially related to psychological and/or autonomous function, it is important to know whether SARS-CoV-2 can infect the CNS. Generally, coronaviruses are neurotropic (36), and human CoV-229E and -OC43, which cause the common cold, are known to infect CNS tissues (37). Notably, reverse transcription-PCR analysis of cerebrospinal fluid of patients infected with SARS-CoV-1 detected the virus (38), and immunohistochemical analysis identified viral proteins in brain tissues (39). Additionally, infectious virus was isolated from autopsied brain tissue of a patient infected with SARS-CoV-1 (39). Moreover, evidence showing the ability of SARS-CoV-2 to infect the CNS was obtained by analysis of postmortem samples (40,41). Matschke et al. found SARS-CoV-2 viral proteins in cranial nerves originating from the lower brainstem and in isolated cells of the brainstem (41), whereas Meinhardt et al. detected RNA and the spike protein of SARS-CoV-2 in the medulla oblongata where the primary respiratory and cardiovascular control center is located (40). SARS-CoV-2 can enter the nervous system by crossing the neural-mucosal interface in the olfactory mucosa. Immunohistochemical analysis with anti-spike protein antibodies identified positive staining in cortical neurons (28).
The transmission of viral infection from the nasal cavity to the CNS was confirmed in transgenic K18- hACE2 mice (28,42,43). As reported for SARS-CoV-1, SARS-CoV-2 infection was detected in disconnected regions after nasal infection, implying the possibility that the virus may enter the CNS by different routes (27). It has been proposed that S1 protein internalizes the virus through ACE2 and affects blood-brain barrier integrity, resulting in SARS-CoV-2 invasion into the CNS (44). As an alternative route via a "Trojan horse" model, infected macrophages can possibly enter the CNS and form an infectious focus (45).
Risk factors for long COVID
Recently, a longitudinal multi-omics study identified several risk factors associated with long COVID that are measured at the initial point of COVID-19 diagnosis (22). These risk factors are as follows: i) SARS-CoV-2 RNA level in the blood early in infection, an indicator of viral load; ii) the existence of certain autoantibodies; iii) type 2 diabetes; and iv) reactivation of the EB virus. Among these, the presence of type 2 diabetes has been postulated as a risk factor for the severity of COVID-19 (46). It is plausible that the severity of tissue damage caused by viral infection influences the development of long COVID. In contrast, the EB virus, which infects individuals at a young age and becomes dormant in most people, is a well-known risk factor for systemic lupus erythematosus (47). An important next step is to determine how the level of viral RNA in the blood and the presence of autoantibodies are linked to the development of long COVID.
Viral proteins
In the acute phase of infection, host tissues are exposed to viral proteins, according to the severity of viral infection. The N protein of SARS-CoV-2 promotes IFN-I signaling and the production of inflammatory cytokines (48), leading to systemic inflammation and multi-organ damage (49). Notably, N protein is detected in serum during active viral replication (50), suggesting that circulating N protein causes symptoms related to long COVID through tissue damage.
In contrast, the spike protein has a variety of activities in several types of cells. In human monocytes/ macrophages, it induces proinflammatory responses with an increase in the expression of TNF-α, major histocompatibility complex class II, nuclear factor-kappa B, and c-Jun N-terminal kinase (51,52). In pulmonary vascular cells, it activates cell growth signaling with mitogen-activated protein kinase kinase phosphorylation and participates in cardiovascular/ pulmonary abnormalities by thickening the pulmonary vascular wall (53). In microglia, the S1 subunit stimulates neuroinflammation through activation of nuclear factor-kappa B and p38 mitogen-activated protein kinase, resulting in neurological, cognitive, and neuropsychiatric symptoms (54). These observations indicate that S1 protein, which circulates in the blood (Matsunaga et al. unpublished data), could directly injure blood, vascular, and neuronal components and induce multiple symptoms of long COVID.
The potent inflammatory activity of S1 protein can be explained by the properties of an amino acid stretch homologous to superantigens. Cheng et al. found that the 678TNSPRRARSVASQ690 motif was homologous to the superantigenic 150TN-KKKATVQELD161 peptide of staphylococcal enterotoxin B (SEB) (55,56). SEB was first found as a superantigen with the ability to hyper-stimulate T cells expressing the Vβ T cell receptor gene (55). Such skewing of Vb2+ T cells was reported in severe/hyperinflammatory SARS-CoV-2 patients (57). Moreover, an anti-SEB monoclonal antibody (6D3) blocks SARS-CoV-2 infection (57). It was noted that the structural motif formed by three positively charged residues (R682, R683, and R685) in the spike protein was similar to the motif formed by K152, K153, and K154 in SEB (Figure 1, left panel, circle). The Omicron variant has mutations of two amino acids in the corresponding stretch (678TKSHRRAR RSVASQ690; mutated amino acids are underlined), but simulation of its three-dimensional structure suggested similar structural properties (Figure 1, right panel). It is necessary to observe whether infection with the Omicron variant also induces long COVID symptoms.
Figure 1.
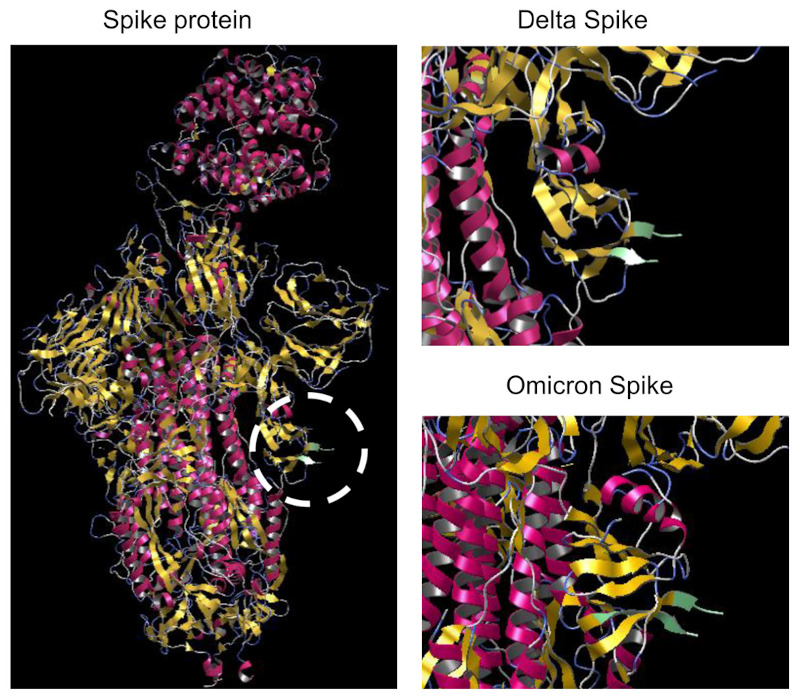
Putative three-dimensional structures of the spike proteins of the Delta and Omicron variants. Structural data were derived from the protein data bank (PDB) database (Delta variant, PDB ID: 7w92, PDB DOI: 10.2210/pdb7W92/pdb; Omicron variant (BA.1), PDB ID: 7wk4, PDB DOI: 10.2210/pdb7WK4/pdb). Simulation was originally performed for this article using the open access software myPresto version 5.0. The left panel, The structure corresponding to the proposed superantigen is highlighted using a circle. The upper and lower right panels show the superantigen structures (enlarged) of the Delta and Omicron (BA.1) variants, respectively.
Autoimmunity in COVID-19
Autoantibodies have been proposed as a risk factor for long COVID (22), and several potential mechanisms linking autoimmunity and COVID-19 have been postulated: cross-reactivity between viral proteins and autoantigens (58), molecular mimicry (59), bystander activation (60), anti-idiotypic antibodies to antiviral antibodies (61), transient immune-suppression (62), and relaxed peripheral immune-tolerance for antibody screening (63). Among these, molecular mimicry by viral proteins of host molecules has been well characterized in a number of viruses including hepatitis B virus, hepatitis C virus, EB virus, cytomegalovirus, human T lymphotropic virus type-1, and Dengue virus (64-67).
Antibodies to viral proteins
In SARS-CoV-2 proteins, six amino acid sequences similar to host proteins (DAB adaptor protein 1, apoptosis-inducing factor mitochondria associated 1, and surfeit locus protein 1) that are expressed in the brainstem respiratory pacemaker have been identified (68). SARS-CoV-2 proteins also share five amino acid peptides with pulmonary surfactant and related proteins (69). Recently, it was reported that anti-spike protein antibodies cross-react with tissue proteins, including transglutaminase 3, transglutaminase 2, anti-extractable nuclear antigen, myelin basic protein, mitochondria, nuclear antigen, alpha-myosin, thyroid peroxidase, collagen, claudin 5 and 6, and S100 calcium-binding protein B (70). Loss of immune tolerance for eliminating cells with autoantibody production is likely due to transient immunosuppression after viral infection and following immune reconstitution. In COVID-19 patients, there are marked changes in various subpopulations of immune cells, including CD4+ and CD8+ T-lymphocytes (71), memory B-lymphocytes (72), monocytes/macrophages, and dendritic cells (73).
Autoantibodies to host molecules
In long COVID patients, a variety of autoantibodies have been identified (74,75) (Table 2). Both pre-existing and infection-induced autoantibodies are related to the severity, clinical symptoms, and post-acute symptoms including autoimmune conditions of COVID-19. Patients with anti-ACE2 autoantibodies have suppressed soluble ACE2 activity in plasma and symptoms associated with vasculopathies including pulmonary hypertension (76). The reduction or loss of ACE2 activity may lead to the increased activity of angiotensin II through angiotensin II receptor type 1 in the lung, heart, and kidney, which express ACE2 at high levels, resulting in enhanced inflammation (77) and prothrombotic phenotypes (78,79). The inflammatory stimuli triggered by autoantibodies may lead to the atypical phenotypes observed in long COVID (80).
Table 2. Autoimmune diseases accompanying SARS-CoV-2 infection.
| Target of autoantibody | Disease | Common symptom | Reference (DOI) |
|---|---|---|---|
| Angiotensin II receptor I | Severe inflammation | Endothelial damage, coagulopathy | 10.1371/journal.pone.0259902 |
| Double-stranded DNA | Systemic lupus erythematosus | Immune dysregulation | 10.1007/s10067-020-05310-1 |
| Red blood cell | Autoimmune hemolytic anemia | Anemia | 10.1111/trf.16226 |
| Platelet | Autoimmune thrombocytopenia | Purpura | 10.1056/NEJMc2010472 |
| Glycolipid | Guillain-Barré syndrome | Neurological manifestation | 10.1016/S1474-4422(20)30109-5 |
| Nucleus | Kikuchi-Fujimoto disease | Cervical lymphadenopathy mild fever | 10.1111/bjh.17292 |
| Neutrophil cytoplasm | Autoimmune vasculitis | Purpura | 10.1007/s11239-020-02230-4 |
| Central nervous system | Multiple sclerosis | Chronic inflammation, demyelination | 10.1016/j.msard.2020.102377 |
| La/SSB | Multisystem inflammatory syndrome in children | Inflammation in endothelial, gastrointestinal, and immune cells | 10.3389/fped.2021.702318 |
| Phospholipid | Antiphospholipid syndrome | Thrombosis | 10.1016/j.medcli.2021.09.021 |
| ACE2 | Prothrombotic phenotype | Pulmonary hypertension, vasculopathy | 10.1371/journal.pone.0257016 |
| Jo-1 | Anti-synthetase syndrome | Myopathy, arthritis | 10.1186/s12890-020-01388-0 |
Possible involvement of viral persistence
The number of clinical reports on recurrent infections with COVID-19 has been increasing (81), explained by viral relapse/reactivation in patients with persistent infection (82,83). Consistently, analysis using autopsy samples has detected persistent SARS-CoV-2 infection in multiple sites including the trachea, heart, lymph nodes, intestine, kidney, skeletal muscle, and brain. Strikingly, infection in the brain, including the cerebral cortex, brainstem, cerebellum, thalamus, hypothalamus, corpus callosum, and basilar artery regions, was detected for up to 230 days from initial symptom onset (84). This observation is consistent with reports on recurrent shedding of SARS-CoV-2 RNA in various body fluids such as urine, feces, and nasal mucous, implying the possibility that the viral genome remains transcriptionally active for a long time (45).
The persistence of SARS-CoV-2 is not surprising because RNA-sequencing analysis of 51 tissue types collected from healthy individuals at autopsy identified human CoV-229E transcripts in various tissues such as the brain, skin, and blood (85). Moreover, a number of RNA viruses, including hepatitis C virus, Ebola virus, Zika virus, respiratory syncytial virus, and measles virus, can establish persistent infections (45,86). As the most striking example, measles virus retained in the CNS causes sclerosing subacute panencephalitis (87). A link between persistent infection and fatigue-related symptoms has also been proposed because enteroviruses and their proteins are found in tissue samples obtained from patients with myalgia/chronic fatigue syndrome (88).
In the innate immune system, mammalian cells are equipped with pattern-recognition receptors for sensing viral RNA, which depend on endosomal Toll-like receptors and cytosolic retinoic acid-inducible gene I (RIG-I)-like receptors (89). Upon pattern-recognition receptor activation, downstream signaling cascades trigger the secretion of IFN-I/III, TNF-α, IL- 1, and IL-6 (90). Recently, stimulator of interferon genes (STING), which was originally identified as a molecule that recognizes cytoplasmic DNA (89), was reported to function as an RNA-sensing molecule (91). STING cooperates with cyclic GMP-AMP synthase (cGAS), and the cGAS-STING pathway induces IFN-I production (91). Similar to SARS-CoV-1, multiple proteins of SARS-CoV-2 differentially block the pattern-recognition receptor pathway (92). Rui et al. demonstrated that protease 3CL of SARS-CoV-2 blocks the immune responses induced by the RIG-I-like receptor and cGAS-STING pathways (92). Furthermore, N protein antagonizes RIG-I-like receptor innate immune activation, whilst SARS-CoV-2 open reading frame (ORF) 3a specifically antagonizes the immune activation induced by cGAS-STING. It remains elusive how the inhibitory activity of viral proteins contributes to persistent transcription of viral RNA (91).
As an alternative mechanism for viral persistence, Zhang et al. reported that viral RNA is reverse-transcribed and integrated into the genome (93). In human cells, endogenous reverse transcriptase is derived from long interspersed element-1 (L1), an endogenous retroelement (94). L1 makes up about 17% of the human genome, and approximately 100 of the 5.0 × 105 copies present in a single cell are active for retrotransposition. L1 encodes ORF1 as well as ORF2, which has reverse transcriptase activity. It has been proposed that SARS-CoV-2 integrated into the genome is responsible for the continuous shedding of viral RNA. However, further study is required because the integration of viral DNA into the human genome was not reproducibly identified (95).
Although it remains elusive how viral proteins contribute to viral persistence, newly synthesized viral RNA and proteins will repetitively induce tissue inflammation and enhance the host immune reaction.
Therapeutic approaches for long COVID
As autoimmunity and persistent inflammatory conditions are likely to underlie the pathophysiological conditions of long COVID, Goel et al. investigated the effects of immunosuppressive agents on long COVID. Based on a 3-month follow-up analysis of 24 patients with long COVID who were administered glucocorticoid, they proposed that systemic steroids are helpful for recovery from long COVID. However, the number of enrolled patients was too small to draw a reliable conclusion (96).
There is an increasing number of reports examining the effectiveness of SARS-CoV-2 vaccination for the treatment of long COVID. Nehme et al. investigated the possible link between the improvement of long COVID after vaccination by analyzing its impact on the six main complaints (i.e., fatigue, difficulty concentrating or memory loss, impaired sense of smell or taste, shortness of breath, and headache) (97). Out of 1,596 participants with symptoms developing after SARS-CoV-2 infection, 47.1% were vaccinated once or twice, while 65.3% of 228 participants without symptoms were vaccinated. Notably, the symptoms disappeared or improved in 35.5% of the vaccinated participants, while the symptoms were stable in 28.7% and worsened in 3.3%. Vaccination with two doses was associated with a decreased prevalence of dyspnea and change in the ability to taste. Additionally, Arnold et al. studied changes of symptoms after COVID-19 vaccination (98). Out of 78 participants, 44 had received vaccination, and at least one symptom was observed in 36 of the 44 vaccinated participants. A follow-up study was conducted for 8 months after vaccination to assess its effects on 159 symptoms. Out of these symptoms, 37 (23.2%) had improved, 9 (5.6%) had worsened, and 113 (71.1%) were unchanged. The Pfizer- BioNTech and Oxford-AstraZeneca vaccines had similar effects on the symptoms. In addition to these two peer-reviewed reports on the effectiveness of vaccination on long COVID, three additional non-peer-reviewed studies reported the favorable effects of COVID-19 vaccination. In contrast, Scherlinger et al. reported that COVID-19 vaccination was tolerable, because it was not linked with severe side effects, however it was not effective for long COVID (99). An improvement of symptoms was observed in 21.8% of participants, whereas they worsened in 31%. In their study, approximately one-third of participants did not receive vaccination due to expectations that it was a contraindication for long COVID and would worsen symptoms.
Intravenous immunoglobulin therapy (IVIg) has been used for patients in the acute phase of SARS-CoV-2 infection (100). Notably, IVIg has been used to treat patients with Guillain-Barré syndrome with favorable outcomes. Although the precise mechanism underlying Guillain-Barré syndrome is unknown, it is suggested that antibodies against surface glycoproteins of the pathogen cross-react with peripheral nerves through similar native proteins and damage cellular function. Among various functions, IVIg can neutralize autoantibodies and control cell-cell interactions by blocking Fc gamma receptors on immune cells. Moreover, IVIg, when prepared from convalescent SARS-CoV-2 patients, can neutralize viral proteins, suggesting that IVIg may be effective for long COVID patients, thus warranting its evaluation in clinical trials.
Conclusion
Direct tissue damage by SARS-CoV-2 infection, constitutive inflammatory responses including autoimmunity, and psychiatric impairment have been suggested as underlying etiologies of long COVID. Although it is unclear how constitutive inflammatory responses are induced, lines of evidence support the hypothesis that persistent infection is a putative cause of continuous inflammation. Additionally, clinical trials of the effects of COVID vaccination on long COVID patients have produced favorable outcomes, further implying that a persistent viral infection is involved in long COVID. Beside vaccination, it might be worthwhile to perform clinical trials using anti-viral compounds to see whether the symptoms of long COVID can be improved.
Funding: This work was supported by Grants-in-Aid from the National Center for Global Health and Medicine (E20A2004D01, 20A2010, 20A2007D, 21A1006).
Conflict of Interest
The authors have no conflicts of interest to disclose.
References
- 1. Nalbandian A, Sehgal K, Gupta A, et al. Post-acute COVID-19 syndrome. Nat Med. 2021; 27:601-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol. 2022; 23:194-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Wo r ld Heal th Organ izat i o n. A c l i n ical cas e definition of post COVID-19 condition by a Delphi consensus, 6 October 2021. https://apps.who.int/iris/handle/10665/345824 (accessed March 9, 2022).
- 4. Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, Villapol S. More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. Sci Rep. 2021; 11:16144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Miyazato Y, Morioka S, Tsuzuki S, Akashi M, Osanai Y, Tanaka K, Terada M, Suzuki M, Kutsuna S, Saito S, Hayakawa K, Ohmagari N. Prolonged and late-onset symptoms of coronavirus disease 2019. Open Forum Infect Dis. 2020; 7:ofaa507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Fernández-de-Las-Peñas C, Martín-Guerrero JD, Pellicer- Valero ÓJ, Navarro-Pardo E, Gómez-Mayordomo V, Cuadrado ML, Arias-Navalón JA, Cigarán-Méndez M, Hernández-Barrera V, Arendt-Nielsen L. Female sex is a risk factor associated with long-term post-COVID related-symptoms but not with COVID-19 symptoms: The LONG-COVID-EXP-CM multicenter study. J Clin Med. 2022; 11:413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Blomberg B, Mohn KG, Brokstad KA, et al. Long COVID in a prospective cohort of home-isolated patients. Nat Med. 2021; 27:1607-1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yelin D, Margalit I, Nehme M, et al. Patterns of long COVID symptoms: A multi-center cross sectional study. J Clin Med. 2022; 11:898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ishikura H, Maruyama J, Hoshino K, Matsuoka Y, Yano M, Arimura T, Katano H, Kato S, Kitamura T, Nakamura Y. Coronavirus disease (COVID-19) associated delayed-onset fulminant myocarditis in patient with a history of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. J Infect Chemother. 2021; 27:1760-1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Al-Falahi Z, Al-Harthi S, Farhan H, Al Busaidi I, Al Alawi AM. Late-onset COVID-19-related multi-system inflammatory syndrome in a middle-aged man. Cureus. 2021; 13:e15855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Han Q, Zheng B, Daines L, Sheikh A. Long-term sequelae of COVID-19: A systematic review and meta-analysis of one-year follow-up studies on post-COVID symptoms. Pathogens. 2022; 11:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ahmed H, Patel K, Greenwood DC, Halpin S, Lewthwaite P, Salawu A, Eyre L, Breen A, O'Connor R, Jones A, Sivan M. Long-term clinical outcomes in survivors of severe acute respiratory syndrome and Middle East respiratory syndrome coronavirus outbreaks after hospitalisation or ICU admission: A systematic review and meta-analysis. J Rehabil Med. 2020; 52:jrm00063. [DOI] [PubMed] [Google Scholar]
- 13. Aucott JN, Rebman AW. Long-haul COVID: heed the lessons from other infection-triggered illnesses. Lancet. 2021; 397:967-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Fernández-de-Las-Peñas C, Palacios-Ceña D, Gómez- Mayordomo V, et al. Fatigue and dyspnoea as main persistent post-COVID-19 symptoms in previously hospitalized patients: Related functional limitations and disability. Respiration. 2022; 101:132-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fortini A, Rosso A, Cecchini P, Torrigiani A, Lo Forte A, Carrai P, Alessi C, Fabbrizzi F, Lovicu E, Sbaragli S, Faraone A. One-year evolution of DLCO changes and respiratory symptoms in patients with post COVID-19 respiratory syndrome. Infection. 2022; 50:513-517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sonnweber T, Sahanic S, Pizzini A, et al. Cardiopulmonary recovery after COVID-19: an observational prospective multicentre trial. Eur Respir J. 2021; 57:2003481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lam GY, Befus AD, Damant RW, Ferrara G, Fuhr DP, Stickland MK, Varughese RA, Wong EY, Smith MP. Exertional intolerance and dyspnea with preserved lung function: an emerging long COVID phenotype? Respir Res. 2021; 22:222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Melms JC, Biermann J, Huang H, et al. A molecular single-cell lung atlas of lethal COVID-19. Nature. 2021; 595:114-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hazen H. Postinfluenzal alopecia. JAMA. 1919; 72:1452. [Google Scholar]
- 20. Starace M, Iorizzo M, Sechi A, et al. Trichodynia and telogen effluvium in COVID-19 patients: Results of an international expert opinion survey on diagnosis and management. JAAD Int. 2021; 5:11-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fagan N, Meah N, York K, Bokhari L, Fletcher G, Chen G, Tobin DJ, Messenger A, Irvine AD, Sinclair R, Wall D. Shedding light on therapeutics in alopecia and their relevance to COVID-19. Clin Dermatol. 2021; 39:76-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Su Y, Yuan D, Chen DG, et al. Multiple early factors anticipate post-acute COVID-19 sequelae. Cell. 2022; 185:881-895.e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bilinska K, Jakubowska P, Von Bartheld CS, Butowt R. Expression of the SARS-CoV-2 entry proteins, ACE2 and TMPRSS2, in cells of the olfactory epithelium: Identification of cell types and trends with age. ACS Chem Neurosci. 2020; 11:1555-1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Brann DH, Tsukahara T, Weinreb C, et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19- associated anosmia. Sci Adv. 2020; 6:eabc5801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cantuti-Castelvetri L, Ojha R, Pedro LD, et al. Neuropilin-1 facilitates SARS-CoV-2 cell entry and infectivity. Science. 2020; 370:856-860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kirschenbaum D, Imbach LL, Ulrich S, Rushing EJ, Keller E, Reimann RR, Frauenknecht KBM, Lichtblau M, Witt M, Hummel T, Steiger P, Aguzzi A, Frontzek K. Inflammatory olfactory neuropathy in two patients with COVID-19. Lancet. 2020; 396:166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Winkler ES, Bailey AL, Kafai NM, et al. SARS-CoV-2 infection of human ACE2-transgenic mice causes severe lung inflammation and impaired function. Nat Immunol. 2020; 21:1327-1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Zheng J, Wong LR, Li K, Verma AK, Ortiz ME, Wohlford-Lenane C, Leidinger MR, Knudson CM, Meyerholz DK, McCray PB Jr, Perlman S. COVID-19 treatments and pathogenesis including anosmia in K18- hACE2 mice. Nature. 2021; 589:603-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Vitale-Cross L, Szalayova I, Scoggins A, Palkovits M, Mezey E. SARS-CoV-2 entry sites are present in all structural elements of the human glossopharyngeal and vagal nerves: clinical implications. bioRxiv. 2022; 2021.12.30.474580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. UpToDate. Coronavirus disease 2019 (COVID-19). https://www.uptodate.com/landing/covid19?source=promobox (accessed March 9, 2022).
- 31. World Health Organization. Support for rehabilitation: self-management after COVID-19-related illuness. 2020. https://apps.who.int/iris/bitstream/handle/10665/344472/WHO-EURO-2021-855-40590-59892-eng.pdf?sequence=1&isAllowed=y (accessed March 9, 2022).
- 32. Centers for Disease Control and Prevention. COVID-19. https://www.cdc.gov/coronavirus/2019-ncov/your-health/quarantine-isolation.html (accessed March 9, 2022).
- 33. Herrera JE, Niehaus WN, Whiteson J, Azola A, Baratta JM, Fleming TK, Kim SY, Naqvi H, Sampsel S, Silver JK, Gutierrez MV, Maley J, Herman E, Abramoff B. Multidisciplinary collaborative consensus guidance statement on the assessment and treatment of fatigue in postacute sequelae of SARS-CoV-2 infection (PASC) patients. PM R. 2021; 13:1027-1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Homerton University Hospital. Post COVID-19 patients infromation pack. Helping you to recover and manage your symptoms following COVID-19. https://gp-website-cdn-prod.s3.amazonaws.com/downloads/1589364822-a2ac4f0d54141da323f4087888276478.pdf (accessed March 9, 2022).
- 35. Kattar N, Do TM, Unis GD, Migneron MR, Thomas AJ, McCoul ED. Olfactory training for postviral olfactory dysfunction: Systematic review and meta-analysis. Otolaryngol Head Neck Surg. 2021; 164:244-254. [DOI] [PubMed] [Google Scholar]
- 36. Carod-Artal FJ. Neurological complications of coronavirus and COVID-19. Rev Neurol. 2020; 70:311-322. [DOI] [PubMed] [Google Scholar]
- 37. Arbour N, Day R, Newcombe J, Talbot PJ. Neuroinvasion by human respiratory coronaviruses. J Virol. 2000; 74:8913-8921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lau KK, Yu WC, Chu CM, Lau ST, Sheng B, Yuen KY. Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis. 2004; 10:342-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Xu J, Zhong S, Liu J, Li L, Li Y, Wu X, Li Z, Deng P, Zhang J, Zhong N, Ding Y, Jiang Y. Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis. 2005; 41:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Meinhardt J, Radke J, Dittmayer C, et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat Neurosci. 2021; 24:168-175. [DOI] [PubMed] [Google Scholar]
- 41. Matschke J, Lütgehetmann M, Hagel C, et al. Neuropathology of patients with COVID-19 in Germany: a post-mortem case series. Lancet Neurol. 2020; 19:919-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tseng CT, Huang C, Newman P, Wang N, Narayanan K, Watts DM, Makino S, Packard MM, Zaki SR, Chan TS, Peters CJ. Severe acute respiratory syndrome coronavirus infection of mice transgenic for the human Angiotensin-converting enzyme 2 virus receptor. J Virol. 2007; 81:1162-1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Netland J, Meyerholz DK, Moore S, Cassell M, Perlman S. Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol. 2008; 82:7264-7275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. DeOre BJ, Tran KA, Andrews AM, Ramirez SH, Galie PA. SARS-CoV-2 spike protein disrupts blood-brain barrier integrity via RhoA activation. J Neuroimmune Pharmacol. 2021; 16:722-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Proal AD, VanElzakker MB. Long COVID or post-acute sequelae of COVID-19 (PASC): An overview of biological factors that may contribute to persistent symptoms. Front Microbiol. 2021; 12:698169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Raveendran AV, Misra A. Post COVID-19 syndrome ("Long COVID") and diabetes: Challenges in diagnosis and management. Diabetes Metab Syndr. 2021; 15:102235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. James JA, Kaufman KM, Farris AD, Taylor-Albert E, Lehman TJ, Harley JB. An increased prevalence of Epstein-Barr virus infection in young patients suggests a possible etiology for systemic lupus erythematosus. J Clin Invest. 1997; 100:3019-3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao Y, Sui L, Wu P, Wang W, Wang Z, Yu Y, Hou Z, Tan G, Liu Q, Wang G. A dual-role of SARS-CoV-2 nucleocapsid protein in regulating innate immune response. Signal Transduct Target Ther. 2021; 6:331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Leisman DE, Ronner L, Pinotti R, Taylor MD, Sinha P, Calfee CS, Hirayama AV, Mastroiani F, Turtle CJ, Harhay MO, Legrand M, Deutschman CS. Cytokine elevation in severe and critical COVID-19: a rapid systematic review, meta-analysis, and comparison with other inflammatory syndromes. Lancet Respir Med. 2020; 8:1233-1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang Y, Ong CM, Yun C, Mo W, Whitman JD, Lynch KL, Wu AHB. Diagnostic value of nucleocapsid protein in blood for SARS-CoV-2 infection. Clin Chem. 2021; 68:240-248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Barhoumi T, Alghanem B, Shaibah H, Mansour FA, Alamri HS, Akiel MA, Alroqi F, Boudjelal M. SARS-CoV-2 coronavirus spike protein-induced apoptosis, inflammatory, and oxidative stress responses in THP-1- like-macrophages: Potential role of angiotensin-converting enzyme inhibitor (Perindopril). Front Immunol. 2021; 12:728896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shirato K, Kizaki T. SARS-CoV-2 spike protein S1 subunit induces pro-inflammatory responses via toll-like receptor 4 signaling in murine and human macrophages. Heliyon. 2021; 7:e06187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Suzuki YJ, Nikolaienko SI, Dibrova VA, Dibrova YV, Vasylyk VM, Novikov MY, Shults NV, Gychka SG. SARS-CoV-2 spike protein-mediated cell signaling in lung vascular cells. Vascul Pharmacol. 2021; 137:106823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Frank MG, Nguyen KH, Ball JB, Hopkins S, Kelley T, Baratta MV, Fleshner M, Maier SF. SARS-CoV-2 spike S1 subunit induces neuroinflammatory, microglial and behavioral sickness responses: Evidence of PAMP-like properties. Brain Behav Immun. 2022; 100:267-277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Choi Y, Lafferty JA, Clements JR, Todd JK, Gelfand EW, Kappler J, Marrack P, Kotzin BL. Selective expansion of T cells expressing V beta 2 in toxic shock syndrome. J Exp Med. 1990; 172:981-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Deacy AM, Gan SK, Derrick JP. Superantigen recognition and interactions: Functions, mechanisms and applications. Front Immunol. 2021; 12:731845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cheng MH, Porritt RA, Rivas MN, Krieger JM, Ozdemir AB, Garcia G Jr, Arumugaswami V, Fries BC, Arditi M, Bahar I. A monoclonal antibody against staphylococcal enterotoxin B superantigen inhibits SARS-CoV-2 entry in vitro. Structure. 2021; 29:951-962 e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Yamamoto K. Possible mechanisms of autoantibody production and the connection of viral infections in human autoimmune diseases. Tohoku J Exp Med. 1994; 173:75-82. [DOI] [PubMed] [Google Scholar]
- 59. Rojas M, Restrepo-Jiménez P, Monsalve DM, Pacheco Y, Acosta-Ampudia Y, Ramírez-Santana C, Leung PSC, Ansari AA, Gershwin ME, Anaya JM. Molecular mimicry and autoimmunity. J Autoimmun. 2018; 95:100-123. [DOI] [PubMed] [Google Scholar]
- 60. Pacheco Y, Acosta-Ampudia Y, Monsalve DM, Chang C, Gershwin ME, Anaya JM. Bystander activation and autoimmunity. J Autoimmun. 2019; 103:102301. [DOI] [PubMed] [Google Scholar]
- 61. Plotz PH. Autoantibodies are anti-idiotype antibodies to antiviral antibodies. Lancet. 1983; 2:824-826. [DOI] [PubMed] [Google Scholar]
- 62. Canas CA. The triggering of post-COVID-19 autoimmunity phenomena could be associated with both transient immunosuppression and an inappropriate form of immune reconstitution in susceptible individuals. Med Hypotheses. 2020; 145:110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Woodruff MC, Ramonell RP, Saini AS, et al. Relaxed peripheral tolerance drives broad de novo autoreactivity in severe COVID-19. medRxiv. 2021; 2020.10.21.20216192 [Google Scholar]
- 64. Acay A, Demir K, Asik G, Tunay H, Acarturk G. Assessment of the frequency of autoantibodies in chronic viral hepatitis. Pak J Med Sci. 2015; 31:150-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Sutton RN, Emond RT, Thomas DB, Doniach D. The occurrence of autoantibodies in infectious mononucleosis. Clin Exp Immunol. 1974; 17:427-436. [PMC free article] [PubMed] [Google Scholar]
- 66. Andersen P, Andersen HK. Smooth-muscle antibodies and other tissue antibodies in cytomegalovirus infection. Clin Exp Immunol. 1975; 22:22-29. [PMC free article] [PubMed] [Google Scholar]
- 67. Vo HTM, Duong V, Ly S, Li QZ, Dussart P, Cantaert T. Autoantibody profiling in plasma of dengue virus-infected individuals. Pathogens. 2020; 9:1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Lucchese G, Floel A. Molecular mimicry between SARS-CoV-2 and respiratory pacemaker neurons. Autoimmun Rev. 2020; 19:102556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kanduc D, Shoenfeld Y. On the molecular determinants of the SARS-CoV-2 attack. Clin Immunol. 2020; 215:108426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020; 217:108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Liu Y, Tan W, Chen H, Zhu Y, Wan L, Jiang K, Guo Y, Tang K, Xie C, Yi H, Kuang Y, Luo Y. Dynamic changes in lymphocyte subsets and parallel cytokine levels in patients with severe and critical COVID-19. BMC Infect Dis. 2021; 21:79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sosa-Hernández VA, Torres-Ruíz J, Cervantes-Díaz R, Romero-Ramírez S, Páez-Franco JC, Meza-Sánchez DE, Juárez-Vega G, Pérez-Fragoso A, Ortiz-Navarrete V, Ponce-de-León A, Llorente L, Berrón-Ruiz L, Mejía- Domínguez NR, Gómez-Martín D, Maravillas-Montero JL. B cell subsets as severity-associated signatures in COVID-19 patients. Front Immunol. 2020; 11:611004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Sanchez-Cerrillo I, Landete P, Aldave B, et al. Differential redistribution of activated monocyte and dendritic cell subsets to the lung associates with severity of COVID-19. medRxiv. 2020; 2020.05.13.20100925 [Google Scholar]
- 74. Favaloro EJ, Henry BM, Lippi G. COVID-19 and antiphospholipid antibodies: Time for a reality check? Semin Thromb Hemost. 2022; 48:72-92. [DOI] [PubMed] [Google Scholar]
- 75. Wang EY, Mao T, Klein J, et al. Diverse functional autoantibodies in patients with COVID-19. Nature. 2021; 595:283-288. [DOI] [PubMed] [Google Scholar]
- 76. Takahashi Y, Haga S, Ishizaka Y, Mimori A. Autoantibodies to angiotensin-converting enzyme 2 in patients with connective tissue diseases. Arthritis Res Ther. 2010; 12:R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jia H. Pulmonary angiotensin-converting enzyme 2 (ACE2) and inflammatory lung disease. Shock. 2016; 46:239-248. [DOI] [PubMed] [Google Scholar]
- 78. Gue YX, Gorog DA. Reduction in ACE2 may mediate the prothrombotic phenotype in COVID-19. Eur Heart J. 2020; 41:3198-3199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lupi L, Adamo M, Inciardi RM, Metra M. ACE2 down-regulation may contribute to the increased thrombotic risk in COVID-19. Eur Heart J. 2020; 41:3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Knight JS, Caricchio R, Casanova JL, et al. The intersection of COVID-19 and autoimmunity. J Clin Invest. 2021; 131:e154886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Choudhary MC, Crain CR, Qiu X, Hanage W, Li JZ. Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) sequence characteristics of coronavirus disease 2019 (COVID-19) persistence and reinfection. Clin Infect Dis. 2022; 74:237-245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Gousseff M, Penot P, Gallay L, et al. Clinical recurrences of COVID-19 symptoms after recovery: Viral relapse, reinfection or inflammatory rebound? J Infect. 2020; 81:816-846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lee JS, Kim SY, Kim TS, et al. Evidence of severe acute respiratory syndrome coronavirus 2 reinfection after recovery from mild coronavirus disease 2019. Clin Infect Dis. 2021; 73:e3002-e3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Chertow D, Stein S, Ramelli S, Grazioli A, Chung J-Y, Singh M, Yinda CK, Winkler C, Dickey J, Yiaya K. SARS-CoV-2 infection and persistence throughout the human body and brain. Research Square. 2021. DOI: 1021203/rs3rs-1139035/v1 [Google Scholar]
- 85. Kumata R, Ito J, Takahashi K, Suzuki T, Sato K. A tissue level atlas of the healthy human virome. BMC Biol. 2020; 18:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. McCarthy MK, Morrison TE. Persistent RNA virus infections: do PAMPS drive chronic disease? Curr Opin Virol. 2017; 23:8-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Sidhu MS, Crowley J, Lowenthal A, Karcher D, Menonna J, Cook S, Udem S, Dowling P. Defective measles virus in human subacute sclerosing panencephalitis brain. Virology. 1994; 202:631-641. [DOI] [PubMed] [Google Scholar]
- 88. Chia J, Chia A, Voel ler M, Lee T, Chang R. Acute enterovirus infection followed by myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and viral persistence. J Clin Pathol. 2010; 63:165-168. [DOI] [PubMed] [Google Scholar]
- 89. Ishikawa H, Barber GN. STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature. 2008; 455:674-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Vabret N, Britton GJ, Gruber C, et al. Immunology of COVID-19: Current state of the science. Immunity. 2020; 52:910-941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Webb LG, Fernandez-Sesma A. RNA viruses and the cGAS-STING pathway: reframing our understanding of innate immune sensing. Curr Opin Virol. 2022; 53:101206. [DOI] [PubMed] [Google Scholar]
- 92. Rui Y, Su J, Shen S, et al. Unique and complementary suppression of cGAS-STING and RNA sensing- triggered innate immune responses by SARS-CoV-2 proteins. Signal Transduct Target Ther. 2021; 6:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zhang L, Richards A, Barrasa MI, Hughes SH, Young RA, Jaenisch R. Reverse-transcribed SARS-CoV-2 RNA can integrate into the genome of cultured human cells and can be expressed in patient-derived tissues. Proc Natl Acad Sci U S A. 2021; 118:e2105968118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Goodier JL, Kazazian HH, Jr. Retrotransposons revisited: the restraint and rehabilitation of parasites. Cell. 2008; 135:23-35. [DOI] [PubMed] [Google Scholar]
- 95. Smits N, Rasmussen J, Bodea GO, Amarilla AA, Gerdes P, Sanchez-Luque FJ, Ajjikuttira P, Modhiran N, Liang B, Faivre J, Deveson IW, Khromykh AA, Watterson D, Ewing AD, Faulkner GJ. No evidence of human genome integration of SARS-CoV-2 found by long-read DNA sequencing. Cell Rep. 2021; 36:109530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Goel N, Goyal N, Nagaraja R, Kumar R. Systemic corticosteroids for management of 'long-COVID': an evaluation after 3 months of treatment. Monaldi Arch Chest Dis. 2021. doi:104081/monaldi20211981 [DOI] [PubMed] [Google Scholar]
- 97. Nehme M, Braillard O, Salamun J, Jacquerioz F, Courvoisier DS, Spechbach H, Guessous I. Symptoms after COVID-19 vaccination in patients with post-acute sequelae of SARS-CoV-2. J Gen Intern Med. 2022; doi: 10.1007/s11606-022-07443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Arnold DT, Milne A, Samms E, Stadon L, Maskell NA, Hamilton FW. Symptoms after COVID-19 vaccination in patients with persistent symptoms after acute infection: A case series. Ann Intern Med. 2021; 174:1334-1336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Scherlinger M, Pijnenburg L, Chatelus E, Arnaud L, Gottenberg JE, Sibilia J, Felten R. Effect of SARS-CoV-2 vaccination on symptoms from post-acute sequelae of COVID-19: Results from the nationwide VAXILONG study. Vaccines (Basel). 2021; 10:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Gupta S, Chandra A, Ray BK, Pandit A. Treatment related fluctuation and response to intravenous immunoglobulin therapy in post COVID-19 Guillain-Barre syndrome. Diabetes Metab Syndr. 2021; 15:102246. [DOI] [PMC free article] [PubMed] [Google Scholar]


