Abstract
Artemisinin and its derivatives are endoperoxide-containing antimalarial drugs that appear to form adducts in situ with the Plasmodium falciparum translationally controlled tumor protein (TCTP) homolog. Immunoprecipitation with antibody to recombinant TCTP suggests that adducts may form with both monomeric and dimeric TCTP.
The artemisinin derivatives are an important new class of antimalarials derived from an ancient Chinese herbal remedy. Artemisinin derivatives, such as artesunate, artemether and arteether, are now widely used in Southeast Asia and other areas where multidrug-resistant parasites are found (22).
Artemisinin derivatives appear to act by a two-step mechanism. First, intraparasitic heme and/or free iron catalyze the decomposition of the endoperoxide bridge to eventually form carbon-centered free radicals. Second, these free radicals then act as alkylating agents, reacting with both intraparasitic heme and proteins. Since this mechanism was first proposed in 1991 (21), supporting evidence for it has been contributed by at least 17 research groups in 12 countries (1, 4–9, 12, 14, 15, 17, 18, 23–28, 30, 32, 34, 35).
When Plasmodium falciparum-infected erythrocytes are incubated with radiolabeled dihydroartemisinin, the radioactivity associates covalently with both parasite protein and heme (3, 16, 21). When lysates of the labeled parasites are analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), several radiolabeled protein bands can be seen on autoradiograms. These bands are seen even when low therapeutic concentrations of the drug are used, but they are not seen when labeled drug is incubated with uninfected erythrocytes or when a radiolabeled but inactive congener, [3H]deoxydihydroartemisinin, is incubated with infected erythrocytes (3).
One of the alkylated proteins has been identified as the malarial translationally controlled tumor protein (TCTP) homolog (11). TCTPs have been identified in dozens of other organisms and have been shown to bind calcium (19). In vitro, recombinant P. falciparum TCTP has been shown to bind calcium and heme and to react covalently with [3H]dihydroartemisinin in the presence of heme (10, 11). In P. falciparum, some TCTP appears to be associated with food vacuolar membranes (10). Since the food vacuole is rich in heme (29), it is possible that artemisinin might react with the TCTP molecules associated with this structure. Artemisinin-resistant strains of Plasmodium yoelii express higher levels of TCTP than do artemisinin-sensitive strains (33). However, there is no direct evidence that the alkylation of TCTP by artemisinin is responsible for the antimalarial effects of the drug.
TCTP was identified as a target for artemisinin by a process involving cutting out a labeled band from a gel and obtaining its N-terminal sequence (11). Thus, it is possible that the wrong band was sequenced and that the true target might be another protein with similar electrophoretic mobility. In order to rule out this possibility, we attempted to immunoprecipitate a [3H]dihydroartemisinin-TCTP complex with antibodies made to recombinant TCTP.
P. falciparum strain FCR3 was cultured by the method of Trager and Jensen (31) and synchronized by sorbitol lysis (20). Red cells infected with late rings and trophozoites (25 to 30% parasitemia) were incubated in culture medium in the presence of 1.5 μCi of [3H]dihydroartemisinin (1.4 Ci/mmol/ml; Moravek Biochemical, Brea, Calif.) per ml or 0.1 to 0.2 mCi of [35S]methionine (Amersham Life Science Inc., Arlington, Heights, Ill.) per ml for 3 h. Cells were pelleted by centrifugation at 1,500 × g for 5 min and washed three times with RPMI 1640 without serum. The parasites were isolated from red blood cells by saponin lysis (13) and stored at −70°C. Pelleted parasites were lysed either by incubating with 1 ml of lysis buffer (12.5 mM Tris-HCl, 150 mM NaCl, 1% Triton X-100, 1% sodium deoxycholate, 1% bovine serum albumin, 1 mM phenylmethylsulfonyl fluoride, 0.4 U of aprotinin per ml, pH 7.5 or 8) for 1 h on ice or by sonicating three times in lysis buffer without detergent (each time for 10 s followed by 2 min at 4°C). The parasite lysate was centrifuged at 3,000 × g for 10 min at 4°C, and the supernatant was saved. Then, the supernatant was recentrifuged at 10,000 × g for 30 min at 4°C. The clarified supernatant was stored at −70°C or used immediately.
One milliliter of the supernatant was precleared by incubation with 50 μl of 50% protein A-Sepharose slurry (Pharmacia Biotech) in dilution buffer (10 mM Tris-HCl, pH 8.0, containing 150 mM NaCl, 0.1% Triton X-100, 0.025% sodium azide, and 0.1% bovine serum albumin) on a rocking-platform shaker overnight at 4°C and centrifuged at 200 × g for 1 min. The pellet was discarded.
Anti-recombinant TCTP antibody and prebleed control antibody were prepared as previously described (10). The precleared lysates, approximately 105 to 107 cpm, were incubated with anti-TCTP or various amounts of control antibody (between 7.5 and 180 μg) for 3 to 4 h on a rocking-platform shaker at 4°C. Amounts of 20 to 40 μl of a 50% protein A-Sepharose slurry were added to 200 μl of precleared clarified lysate and incubated for an additional 2 h on the platform shaker at 4°C. The mixtures were centrifuged at 200 × g for 1 min, and the pellets were saved. The pellets were washed twice with 1 ml of dilution buffer: once with 1 ml of buffer A (10 mM Tris-HCl, pH 8.0, 150 mM NaCl, 0.025% sodium azide) and once with 1 ml of buffer B (50 mM Tris-HCl, pH 6.8). The supernatants were discarded. Amounts of 20 to 50 μl of SDS and sample buffer (Novex, San Diego, Calif.) were added to each pellet, incubated at 80°C for 10 min, and then microcentrifuged for 5 s. The resulting supernatants were then electrophoresed using 10% NuPAGE gels (Novex) and either stained with Coomassie blue or exposed to XAR2 autoradiography film (Eastman Kodak, Rochester, N.Y.).
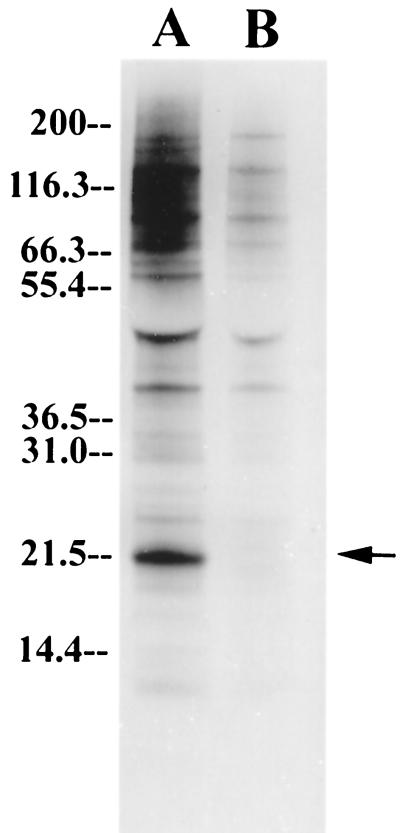
In order to evaluate our immunoprecipitation protocol, [35S]methionine-labeled parasites were first studied. Previously, when anti-TCTP antibodies were used for immunoblotting, only a single band at 22 to 23 kDa reacted, suggesting that the antibody is specific for TCTP (10). After immunoprecipitation by the same antibody, a band at 22 kDa, corresponding to monomeric TCTP, was seen, as expected, on SDS-PAGE gels (Fig. 1). This band was not seen after immunoprecipitation by control antibody. Anti-TCTP also precipitated proteins at higher molecular masses, including 45, 52, 67, and 71 kDa (Fig. 1). These bands were either absent or faint on the lane immunoprecipitated with control antibody. In light of the recent observation that recombinant rat TCTP self-aggregates (36), the bands at 45 and 67 kDa could represent dimeric and trimeric TCTPs. Alternately, they may represent aggregates of TCTP with other proteins.
FIG. 1.
Autoradiogram of [35S]methionine-labeled parasite. The parasite pellets were lysed by sonication in lysis buffer without detergent and immunoprecipitated with 90 μg of anti-TCTP and prebleed control antibody (lanes A and B, respectively). The film was exposed for 9 h.
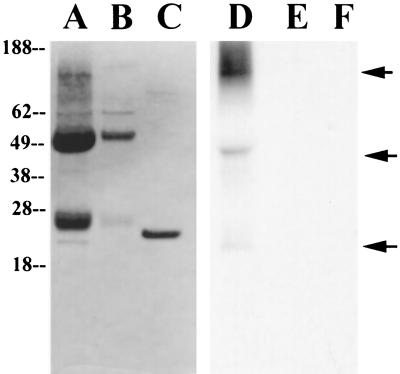
Immunoprecipitation was then carried out on lysates of [3H]dihydroartemisinin-labeled parasites (Fig. 2). After SDS-PAGE and autoradiography, the anti-TCTP immunoprecipitate contained labeled bands at 22 and 45 kDa and at a high molecular mass (lane D) which were not seen after immunoprecipitation by prebleed antisera (lane E). Recombinant TCTP, which contains a 12-amino-acid expression tag, migrated at approximately 25 kDa (lane C), as described previously (11).
FIG. 2.
Coomassie stain (lanes A, B, and C) and autoradiogram (lanes D, E, and F) of [3H]dihydroartemisinin-labeled parasites. Radiolabeled parasite pellets were lysed with lysis buffer and immunoprecipitated with 90 μg of anti-TCTP (lanes A and D) and with prebleed antibody (lanes B and E). Lanes C and F, recombinant TCTP. The film was exposed for 80 days.
Thus, both 22- and 45-kDa bands in the anti-TCTP immunoprecipitate, which probably represent monomeric and dimeric TCTPs, were labeled after whole parasites were incubated with [3H]dihydroartemisinin. Drug-derived radioactivity was also found in the high-molecular-mass region of the gel and may have several causes. First, it could be due to high-molecular-weight oligomers of TCTP or to the association of TCTP with other proteins. Second, it could be an artifact caused by artemisinin-induced cross-linking of membrane proteins previously reported (2).
These data confirm that [3H]dihydroartemisinin reacts with both monomers and dimers of TCTP in situ. This is the first evidence that TCTP self-aggregates in a normal cell. Interestingly, P. falciparum TCTP oligomers appear to be stable in SDS, while recombinant rat TCTP oligomers were found to dissociate in SDS (36). This might be due to structural differences between rat and malarial TCTPs or between native and recombinant TCTPs. Nevertheless, the formation of stable TCTP dimers might provide a clue to its biological function and to the mechanism of action of artemisinin.
Acknowledgments
This work was supported by NIH grant R21 AI 26848 (S.R.M.) and the Royal Thai Government (J.B.).
REFERENCES
- 1.Adams P A, Berman P A. Reaction between ferriprotoporphyrin IX and the antimalarial endoperoxide artesunate gives an intermediate species with enhanced redox catalytic activity. J Pharm Pharmacol. 1996;48:183–187. doi: 10.1111/j.2042-7158.1996.tb07119.x. [DOI] [PubMed] [Google Scholar]
- 2.Asawamahasakda W, Benakis A, Meshnick S R. The interaction of artemisinin with red cell membranes. J Lab Clin Med. 1994;123:757–762. [PubMed] [Google Scholar]
- 3.Asawamahasakda W, Ittarat I, Pu Y M, Ziffer H, Meshnick S R. Reaction of antimalarial endoperoxides with specific parasite proteins. Antimicrob Agents Chemother. 1994;38:1854–1858. doi: 10.1128/aac.38.8.1854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Avery M A, Mehrotra S, Bonk J D, Vroman J A, Goins D K, Miller R. Structure-activity relationships of the antimalarial agent artemisinin. 4. Effect of substitution at C-3. J Med Chem. 1996;39:2900–2906. doi: 10.1021/jm960200e. [DOI] [PubMed] [Google Scholar]
- 5.Bachi M D, Korshin E E, Hoos R, Szpilman A M. Synthesis and reactions of antimalarial bicyclic peroxides. J Heterocycl Chem. 2000;37:639–646. [Google Scholar]
- 6.Bakhshi H B, Gordi T, Ashton M. In-vitro interaction of artemisinin with intact human erythrocytes, erythrocyte ghosts, haemoglobin and carbonic anhydrase. J Pharm Pharmacol. 1997;49:223–226. doi: 10.1111/j.2042-7158.1997.tb06784.x. [DOI] [PubMed] [Google Scholar]
- 7.Benoit-Vical F, Robert A, Meunier B. In vitro and in vivo potentiation of artemisinin and synthetic endoperoxide antimalarial drugs by metalloporphyrins. Antimicrob Agents Chemother. 2000;44:2836–2841. doi: 10.1128/aac.44.10.2836-2841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Berman P A, Adams P A. Artemisinin enhances heme-catalysed oxidation of lipid membranes. Free Radic Biol Med. 1997;22:1283–1288. doi: 10.1016/s0891-5849(96)00508-4. [DOI] [PubMed] [Google Scholar]
- 9.Bharel S, Vishwakarma R A, Jain S K. Artemisinin mediated alteration of haemin to a delta-meso oxidation product: relevance to mechanism of action. J Chem Soc Perkin Trans I. 1998;1:2163–2166. [Google Scholar]
- 10.Bhisutthibhan J, Philbert M A, Fujioka M, Aikawa M, Meshnick S R. The Plasmodium falciparum translationally controlled tumor protein: subcellular localization and calcium binding. Eur J Cell Biol. 1999;78:665–670. doi: 10.1016/S0171-9335(99)80052-1. [DOI] [PubMed] [Google Scholar]
- 11.Bhisutthibhan J, Pan X Q, Hossler P A, Walker D J, Yowell C A, Carlton J, Dame J B, Meshnick S R. The Plasmodium falciparum translationally controlled tumor protein homolog and its reaction with the antimalarial drug artemisinin. J Biol Chem. 1998;273:16192–16198. doi: 10.1074/jbc.273.26.16192. [DOI] [PubMed] [Google Scholar]
- 12.Butler A R, Gilbert B C, Hulme P, Irvine L R, Renton L, Whitwood A C. EPR evidence for the involvement of free radicals in the iron-catalysed decomposition of qinghaosu (artemisinin) and some derivatives; antimalarial action of some polycyclic endoperoxides. Free Radic Res. 1998;28:471–476. doi: 10.3109/10715769809066884. [DOI] [PubMed] [Google Scholar]
- 13.Fairfield A S, Meshnick S R, Eaton J W. Malaria parasites adopt host cell superoxide dismutase. Science. 1983;221:764–766. doi: 10.1126/science.6348944. [DOI] [PubMed] [Google Scholar]
- 14.Green M D, Mount D L, Todd G D, Capomacchia A C. Chemiluminescent detection of artemisinin. Novel endoperoxide analysis using luminol without hydrogen peroxide. J Chromatogr A. 1995;695:237–242. doi: 10.1016/0021-9673(94)01236-8. [DOI] [PubMed] [Google Scholar]
- 15.Haynes R K, Vonwiller S C. The behaviour of quinghaosu (artemisinin) in the presence of heme iron(II) and (III) Tetrahedron Lett. 1996;37:257–260. [Google Scholar]
- 16.Hong Y L, Yang Y Z, Meshnick S R. The interaction of artemisinin with malarial hemozoin. Mol Biochem Parasitol. 1994;63:121–128. doi: 10.1016/0166-6851(94)90014-0. [DOI] [PubMed] [Google Scholar]
- 17.Jefford C W, Favarger F, Vicente M, Jacquier Y. The decomposition of cis-fused cyclopenteno-1,2,4-trioxanes induced by ferrous salts and some oxophilic reagents. Helv Chim Acta. 1995;78:452–458. [Google Scholar]
- 18.Kapetanaki S, Varotsis C. Ferryl-oxo heme intermediate in the antimalarial mode of action of artemisinin. FEBS Lett. 2000;474:238–241. doi: 10.1016/s0014-5793(00)01592-1. [DOI] [PubMed] [Google Scholar]
- 19.Kim M, Jung Y, Lee K, Kim C. Identification of the calcium binding sites in translationally controlled tumor protein. Arch Pharm Res. 2000;23:633–636. doi: 10.1007/BF02975253. [DOI] [PubMed] [Google Scholar]
- 20.Lambros C, Vanderberg J P. Synchronization of Plasmodium falciparum erythrocytic stages in culture. J Parasitol. 1979;65:418–420. [PubMed] [Google Scholar]
- 21.Meshnick S R, Thomas A, Ranz A, Xu C M, Pan H Z. Artemisinin (qinghaosu): the role of intracellular hemin in its mechanism of antimalarial action. Mol Biochem Parasitol. 1991;49:181–189. doi: 10.1016/0166-6851(91)90062-b. [DOI] [PubMed] [Google Scholar]
- 22.Meshnick S R. Artemisinin and related endoperoxides. In: Rosenthal P J, editor. Antimalarial chemotherapy: mechanisms of action, modes of resistance, and new directions in drug development. Totowa, N.J: Humana Press; 2001. pp. 191–201. [Google Scholar]
- 23.O'Neill P M, Bishop L P, Searle N L, Maggs J L, Ward S A, Bray P G, Storr R C, Park B K. The biomimetic iron-mediated degradation of Arteflene (Ro-42–1611), an endoperoxide antimalarial—implications for the mechanism of antimalarial activity. Tetrahedron Lett. 1997;38:4263–4266. [Google Scholar]
- 24.Paitayatat S, Tarnchompoo B, Thebtaranonth Y, Yuthavong Y. Correlation of antimalarial activity of artemisinin derivatives with binding affinity with ferroprotoporphyrin IX. J Med Chem. 1997;40:633–638. doi: 10.1021/jm960767v. [DOI] [PubMed] [Google Scholar]
- 25.Pandey A V, Tekwani B L, Singh R L, Chauhan V S. Artemisinin, an endoperoxide antimalarial, disrupts the hemoglobin catabolism and heme detoxification systems in malarial parasite. J Biol Chem. 1999;274:19383–19388. doi: 10.1074/jbc.274.27.19383. [DOI] [PubMed] [Google Scholar]
- 26.Posner G H, Cumming J N, Krasavin M. Carbon-centered radicals and rational design of new antimalarials. In: Torrence P F, editor. Biomedical chemistry. Applying chemical principles to the understanding and treatment of disease. New York, N.Y: Wiley Interscience; 2000. pp. 289–309. [Google Scholar]
- 27.Provot O, Camuzat-Dedenis B, Hamzaoui M, Moskowitz H, Mayrargue J, Robert A, Cazelles J, Meunier B, Zonhiri F, Desmaele D, d'Angelo J, Mahuteau J, Gay F, Ciceron L. Structure-activity relationships of synthetic tricyclic trioxanes related to artemisinin: the unexpected alkylative property of a 3-(methoxymethy) analog. Eur J Org Chem. 1999;8:1935–1938. [Google Scholar]
- 28.Robert A, Meunier B. Is alkylation the main mechanism of action of the antimalarial drug artemisinin? Chem Soc Rev. 1998;27:273–279. [Google Scholar]
- 29.Rosenthal P J, Meshnick S R. Hemoglobin catabolism and iron utilization by malaria parasites. Mol Biochem Parasitol. 1996;83:131–139. doi: 10.1016/s0166-6851(96)02763-6. [DOI] [PubMed] [Google Scholar]
- 30.Smith S L, Fishwick J, McLean W G, Edwards G, Ward S A. Enhanced in vitro neurotoxicity of artemisinin derivatives in the presence of haemin. Biochem Pharmacol. 1997;53:5–10. doi: 10.1016/s0006-2952(96)00591-6. [DOI] [PubMed] [Google Scholar]
- 31.Trager W, Jensen J B. Human malaria parasites in continuous culture. Science. 1976;193:673–675. doi: 10.1126/science.781840. [DOI] [PubMed] [Google Scholar]
- 32.Vattanaviboon P, Wilairat P, Yuthavong Y. Binding of dihydroartemisinin to hemoglobin H: role in drug accumulation and host-induced antimalarial ineffectiveness of alpha-thalassemic erythrocytes. Mol Pharmacol. 1998;53:492–496. doi: 10.1124/mol.53.3.492. [DOI] [PubMed] [Google Scholar]
- 33.Walker D J, Pitsch J L, Peng M M, Robinson B L, Peters W, Bhisutthibhan J, Meshnick S R. Mechanisms of artemisinin resistance in the rodent malaria pathogen Plasmodium yoelii. Antimicrob Agents Chemother. 2000;44:344–347. doi: 10.1128/aac.44.2.344-347.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei N, Sadrzadeh S M. Enhancement of hemin-induced membrane damage by artemisinin. Biochem Pharmacol. 1994;48:737–741. doi: 10.1016/0006-2952(94)90051-5. [DOI] [PubMed] [Google Scholar]
- 35.Wu W-M, Yao J-J, Jiang K, Wang Y-F. Ferrous ion induced cleavage of the peroxy bond in quinghaosu and its derivatives and the DNA damage associated with this process. Chem Commun (J Chem Soc Sect D) 1996;18:2213–2214. [Google Scholar]
- 36.Yoon T, Jung J, Kim M, Lee K M, Choi E C, Lee K. Identification of the self-interaction of rat TCTP/IgE-dependent histamine-releasing factor using yeast two-hybrid system. Arch Biochem Biophys. 2000;384:379–382. doi: 10.1006/abbi.2000.2108. [DOI] [PubMed] [Google Scholar]