Abstract
Background/Aim: A possible role of antibody-drug conjugates against tumors with low HER2-expression, leads to the emergence of a new “low-HER2” classification in breast cancer, encompassing tumors from the hormonal-receptor-positive and the triple-negative subgroups. There is a need for data (clinical trial data and real-world evidence) that will accurately describe this population, the risk of recurrence and the possible benefit of HER2 targeted therapies.
Patients and Methods: We retrospectively analyzed 949 patients from our Department databases, with hormonal receptor-positive and HER2-negative early breast cancer, for whom detailed data for immunohistochemical HER2-staining could be retrieved.
Results: HER2-low expression was detected in 66.6% of patients (472 IHC +1 and 160 IHC +2 and ISH-negative). Lobular, or mixed lobular and ductal cancers had a statistically significantly lower chance of being HER2-low when compared to pure infiltrative ductal carcinomas (53.1% vs. 69.3% respectively). HER2-low status was not prognostic for recurrence-free survival or response to neoadjuvant chemotherapy. There was a non-significant trend for increased risk of recurrence for HER2-low, compared to HER2-0, in patients with lobular or mixed lobular and ductal carcinomas (HR=2.192, 95% CI=0.819-5.912).
Conclusion: Low expression of HER2 in hormonal receptor-positive early breast cancer does not affect prognosis but may lead to a shorter progression-free-survival in lobular and mixed ductal and lobular cancers. Despite optimal management, a large proportion of hormonal receptor-positive patients will relapse. Targeting HER2 in HER2-low cancers may offer a potential additional treatment strategy to improve survival of this group.
Keywords: Breast cancer, HER2-low, prognosis, Hormone Receptor positive, lobular
Current classification of breast cancer uses immunohistochemistry (IHC) to assess the expression of Hormone Receptors (HR) and HER2 to distinguish four distinct groups: 1) HR-positive, HER2-negative, 2) HR-negative, HER2-positive, 3) HR-positive, HER2-positive, 4) HR-negative, HER2-negative. This distinction is used in combination with anatomic and patient characteristics (age, menopausal status, etc.) to guide clinical decision making and forms the core of international guidelines (1,2).
HER2 (erbB-2) is a member of the Epidermal Growth Factor Receptor (EGFR) family of transmembrane receptor tyrosine kinases which is encoded by the ERBB2 gene. Amplification of the ERBB2 gene results in HER2 overexpression, which has a central role in promoting carcinogenesis in breast cancer (3). HER2 overexpression has an established prognostic value, being associated with poor prognosis (3). Since the discovery of trastuzumab, HER2 overexpression has also gained predictive value, predicting response to HER2-targeted treatment (4). Over the years, HER2-targeted treatments have expanded to include a wide range of monoclonal antibodies (trastuzumab, pertuzumab, margetuximab), Antibody-Drug Conjugates (trastuzumab-emtansine, trastuzumab-deruxtecan), and small molecules (tucatinib, lapatinib, neratinib), with a significant positive impact on patient survival and quality of life (5). The primary predictor of responsiveness to HER2-targeted therapies in breast cancer is HER2 protein overexpression assessed by immunohistochemistry or gene amplification assessed by in situ hybridization (ISH) (ASCO/CAP guidelines) (6). IHC results for HER2 are reported as 0, +1, +2 or +3; IHC +3 is considered “positive”, IHC 0 and +1 are considered “negative”, and IHC +2 is considered “equivocal” and requires further evaluation by ISH testing. Since benefit from and access to trastuzumab requires a positive result, clinicians and pathologists have traditionally focused on the distinction of the duality between a positive and a negative result.
However, increasing evidence points to the fact that the group simply classified as HER2-negative is not homogenous, but rather includes subgroups that differ in clinical characteristics and prognosis. Several retrospective analyses have shown a prognostic significance of moderate HER2 expression (IHC +1 or IHC +2/ISH-negative, collectively referred to as HER2-low), with HER2 +2/ISH-negative patients showing shorter disease-free survival compared to HER2 0 or +1 patients (7,8), and HER2 +1 patients showing worse outcomes compared to HER2 0 patients (9). A proposed hypothesis is that there is a linear correlation between HER2 expression and tumor behavior, with HER2 +1 tumors having characteristics between HER2 0 and HER2 +2 tumors (8). In fact, compared to patients with HER2 0 or +1 tumors, patients with HER2 +2/ISH-negative tumors have been shown to present more frequently with large tumors, higher Ki-67 score and axillary lymph node involvement (7). Patients with HER2-low tumors have also been shown to exhibit significantly lower rates of pathological complete response (pCR) after neoadjuvant chemotherapy and overall survival compared to patients with HER2 0 tumors, leading to the suggestion that these two subgroups should be considered distinct and managed as such (10).
Other factors, apart from HER2 expression, should also be considered in the HER2-low group. The most prominent is the status of hormonal receptors. Outcomes in responses and survival are different among the HER2-low subgroups when stratified by HR status (8,10). Furthermore, there are differences in the molecular characterization and intrinsic subtypes of HR-/HER2-low and HR+/HER2-low tumors which should be taken into consideration (11).
The new field of HER2-low breast cancer is particularly exciting, given its potential prognostic as well as therapeutic implications, with novel HER2-targeted treatments showing efficacy also in HER2-low tumors (12). More data need to be collected before robust changes are proposed to clinical practice. For this purpose, we performed this study to examine the hypothesis that HER2 expression as assessed by IHC as 0, +1 or +2/ISH-negative has prognostic significance in HR-positive patients.
Patients and Methods
For this retrospective study, we collected data from patients treated in our center from 2007 to 2021. Patients were included provided they a) had a histologically confirmed diagnosis of breast cancer, b) had HR-positive, HER2-negative tumors for whom HER2 status as determined by IHC was recorded (0, +1, +2/ISH negative), c) had completed their initial treatment (surgery and/or chemotherapy and/or radiotherapy as indicated) and d) had started follow-up on hormonal treatment. For patients who received neoadjuvant treatment, HER2 status was recorded according to the pre-treatment core-needle biopsy. For patients who did not receive neoadjuvant treatment, HER2 status was recorded according to the surgical specimen. In patients with multiple tumors, the highest HER2 status was used for analysis. Patients that had multiple tumors were excluded if any of the tumors was HR-negative and/or HER2-positive. Patients with a previous breast cancer diagnosis were excluded as well. Histologic diagnosis and testing were performed in a laboratory accredited according to EN ISO 15189:2021 and regularly participating in an EQA IHC HER2 schemes run by UKNEQAS and NordiQC. HER2 protein expression by IHC was assessed according to the ASCO/CAP Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer guidelines following their 2008-2013 and 2018 revisions (6).
The primary endpoint of the study was the assessment of recurrence-free survival (RFS), defined as the time that elapsed between the completion of primary treatment (surgery, chemotherapy and radiotherapy) and first documentation of any breast cancer event (local or distant relapse of breast cancer) or death, stratified by immunohistochemical expression of HER2 (0, +1, +2/ISH-negative). Additional stratification was performed by nodal status (node-negative, node-positive).
The Kaplan–Meier method was used to estimate the median RFS. Log-rank tests were used to test the equality of survivor functions across groups. All data were collected from October to November, 2021 (database lock). According to the methodological features of an observational non-interventional study, all analyses were descriptive, and the results presented should be interpreted as such. All statistical analyses were performed using GraphPad Prism 9.0 software.
Continuous variables were summarized with the use of descriptive statistical measures, namely, mean value, and range (minimum, maximum), and categorical variables were displayed as frequency tables. A Chi-squared test was used to correlate the HER2 status with clinicopathological features of the disease.
Results
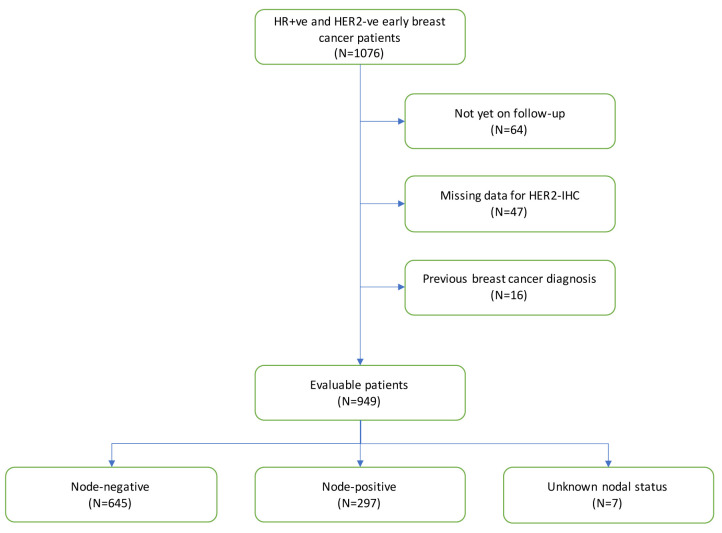
We retrospectively collected data from the medical records of patients treated at our Unit between 2007 and 2021. Out of 1,076 hormonal receptor-positive and HER2-negative patients initially screened, a total of 949 with HR-positive, HER2-negative tumors were included in the study (Figure 1). The median age at diagnosis was 51 years (range=22-85 years). Median follow-up was 2.8 years. With regards to nodal involvement, 645 of the patients were node-negative, 297 node-positive and 7 had no axillary staging. Neoadjuvant treatment was offered to 125 patients, with 113 receiving neoadjuvant chemotherapy and 12 neoadjuvant hormonal treatment. An additional 221 patients received adjuvant chemotherapy. From 949 patients, 944 received hormonal treatment; 408 with an aromatase inhibitor, 397 with tamoxifen, 79 with tamoxifen plus a gonadotropin-releasing hormone agonist (GnRHa), and 60 with an aromatase inhibitor plus an GnRHa.
Figure 1. Study population stratified by nodal status.
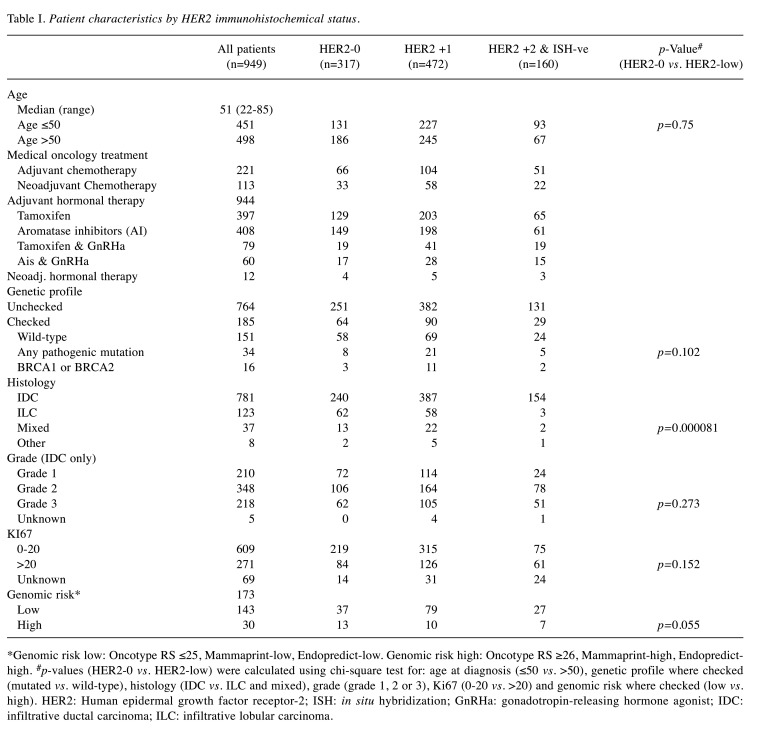
HER2 status (immunohistochemical expression zero vs. low) did not present statistically significant differences by age (up to 50 or over 50 years of age at diagnosis), genetic profile in patients that had a germline panel tested (mutated or wild-type), grade, Ki67 expression (0 to 20 or higher) or genomic risk (in patients that Oncotype, Mammaprint or Endopredict were assessed). Lobular, or mixed lobular and ductal cancers had a 53.1% chance of being HER2-low, statistically significantly lower when compared to pure infiltrative ductal carcinomas (69.3%) (Table I).
Table I. Patient characteristics by HER2 immunohistochemical status.
*Genomic risk low: Oncotype RS ≤25, Mammaprint-low, Endopredict-low. Genomic risk high: Oncotype RS ≥26, Mammaprint-high, Endopredicthigh. #p-values (HER2-0 vs. HER2-low) were calculated using chi-square test for: age at diagnosis (≤50 vs. >50), genetic profile where checked (mutated vs. wild-type), histology (IDC vs. ILC and mixed), grade (grade 1, 2 or 3), Ki67 (0-20 vs. >20) and genomic risk where checked (low vs. high). HER2: Human epidermal growth factor receptor-2; ISH: in situ hybridization; GnRHa: gonadotropin-releasing hormone agonist; IDC: infiltrative ductal carcinoma; ILC: infiltrative lobular carcinoma.
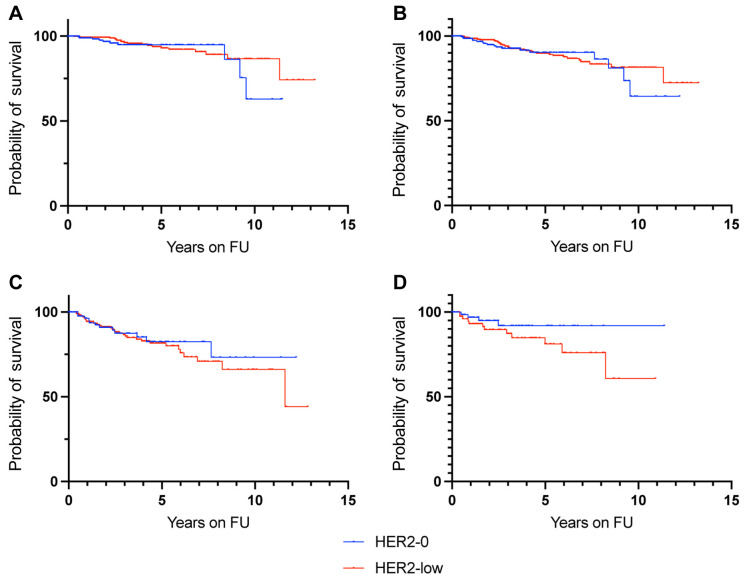
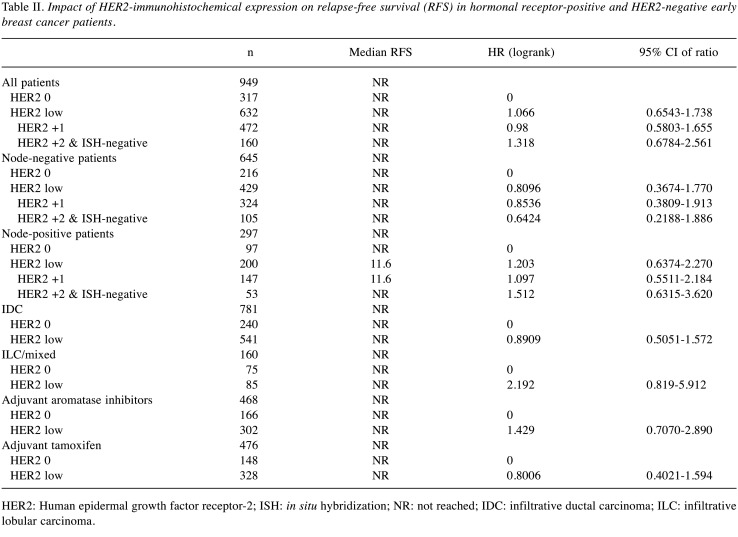
HER2 status was not prognostic for recurrence-free survival (Figure 2). When analyzed by nodal positivity, HER2 status was not prognostic in node-negative patients or in node-positive patients (Figure 3). However, HER2 +2 patients had a non-significant trend for increased risk of recurrence compared to HER2-0 patients in the node-positive population (HR=1.512, 95% CI=0.6315-3.620) (Table II). HER2-low immunohistochemical expression in lobular and mixed lobular and ductal cancers resulted in a non-significant trend for increased risk of recurrence, compared to HER2-0 patients (HR=2.192, 95% CI=0.819-5.912) (Table II and Figure 3). Additionally, HER2 status did not affect recurrence-free-survival in patients that received either tamoxifen or aromatase inhibitors (Table II and Figure 4).
Figure 2. Recurrence-free survival according to HER2 expression in all hormonal receptor-positive and HER2-negative patients (n=949). HER2: Human epidermal growth factor receptor-2; FU: follow-up.
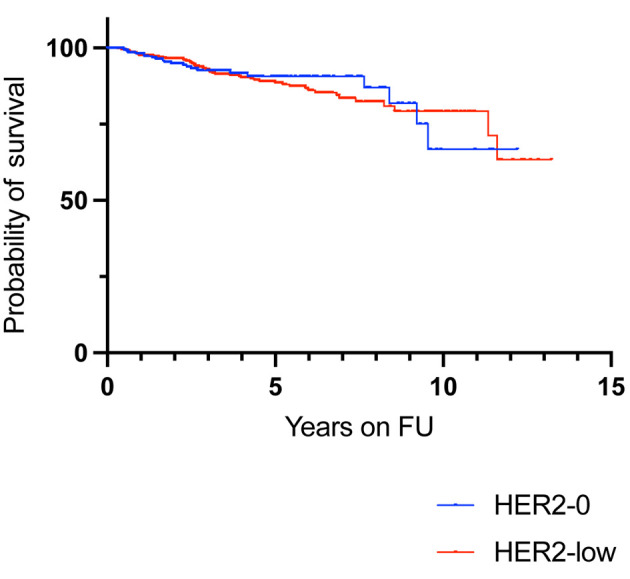
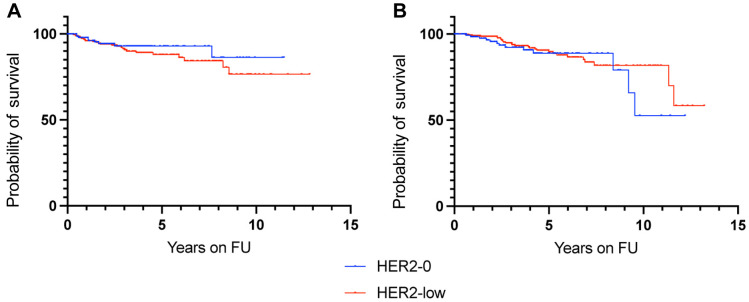
Figure 3. Kaplan-Meyer analysis for recurrence-free survival according to HER2 expression in node-negative patients (A), node-positive patients (B), patients with IDC (C) and patients with ILC and/or mixed IDC/ILC (D). HER2: Human epidermal growth factor receptor-2; FU: follow-up; IDC: infiltrative ductal carcinoma; ILC: infiltrative lobular carcinoma.
Table II. Impact of HER2-immunohistochemical expression on relapse-free survival (RFS) in hormonal receptor-positive and HER2-negative early breast cancer patients.
HER2: Human epidermal growth factor receptor-2; ISH: in situ hybridization; NR: not reached; IDC: infiltrative ductal carcinoma; ILC: infiltrative lobular carcinoma.
Figure 4. Kaplan-Meyer analysis for recurrence-free survival according to HER2 expression in patients that receive adjuvant hormonal treatment with aromatase inhibitors (A) or tamoxifen (B). HER2: Human epidermal growth factor receptor-2; FU: follow-up.
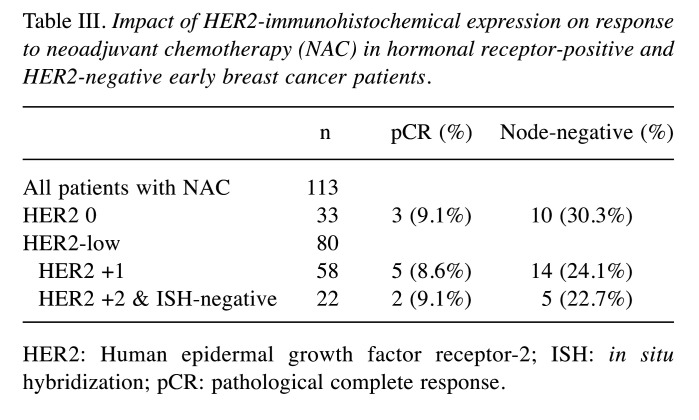
In the group of patients who received neoadjuvant chemotherapy, 10 patients had a pathologic complete response (pCR) (8.93%). HER2 IHC did not influence the outcome (Table III). Complete pathological response was not a prognostic factor for RFS (HR=1.307, p=0.715).
Table III. Impact of HER2-immunohistochemical expression on response to neoadjuvant chemotherapy (NAC) in hormonal receptor-positive and HER2-negative early breast cancer patients.
HER2: Human epidermal growth factor receptor-2; ISH: in situ hybridization; pCR: pathological complete response.
Discussion
HER2-low breast cancer, as defined by an IHC HER2 expression of +1, or +2/ISH-negative, is an emerging entity. The proposed concept is that simply classifying a tumor as HER2-negative is not sufficient, and the individual HER2-low subgroups could be distinguished on the basis of different molecular characteristics, prognosis and response to treatment.
This study was designed to assess RFS of HR-positive early breast cancer patients when stratified by HER2 expression. The retrospective analysis of data from 949 patients showed no statistically significant prognostic differences in RFS according to HER2 status. No statistically significant differences were noted in either node-negative or node-positive patients.
The prognostic significance of HER2 expression in HER2-low tumors has been evaluated retrospectively in different patient groups, with conflicting results. A large pooled analysis of patient data from four neoadjuvant clinical trials, which included 2,310 patients, showed lower rates of pathologic complete response and higher survival rates in HER2-low tumors versus HER2 0 tumors (10). In that study, the lower rates of pCR for HER2-low tumors were seen in the HR-positive subgroup but not in the HR-negative subgroup. Additional molecular analysis performed revealed that in HR-positive tumors, low positivity for HER2 was associated with a less aggressive phenotype compared to HER2 0 tumors, as evidenced by fewer grade 3 tumors, a lower Ki-67 and fewer TP53 mutations; a possible explanation suggested for the lower pCR rates in those patients is that their tumors are less aggressive and therefore less likely to respond to neoadjuvant treatment. The same study also reported survival differences, with a higher survival rate in HER2-low tumors versus HER2 0 tumors; this result was driven by the HR-negative subgroup where the differences were more pronounced, with true triple negative tumors having the worst overall survival (10). The more aggressive phenotype and worse prognosis of true triple negative tumors has been established, to the point where even more regular screening has been shown to improve the prognosis of triple negative breast cancer (13).
The clear message from these data is that HR+/HER2-low tumors and HR-/HER2-low tumors should be assessed separately. This is concordance with findings by Eggemann et al. who compared different patient groups (HER2 +2/ISH-negative Versus HER2 0 or +1) and showed statistically significant lower DFS and breast cancer-specific survival in patients with HER2 +2/ISH-negative tumors, but only in the HR-positive subgroup (8).
In our analysis, we found no statistically significant difference in DFS among HER2-low subtypes in HR-positive patients. These data are in contrast with the aforementioned studies, however similar results have also been reported. Agostinetto et al. in a retrospective analysis of 804 patients showed no significant differences in DFS and OS among HER2-low subtypes, even when paired by HR status (11). Horisawa et al. similarly reported no statistically significant differences in prognosis between HER2-low and HER2 0 patients, regardless of HR status, in a cohort of 4,918 patients (14). Based on these conflicting results, no conclusion can be reached regarding the prognostic significance of low levels of HER2 expression. The reason for the discordance among different studies is unclear. A potential explanation could be different patient populations in each study–for instance, our study included younger patients, with a median age of 51 years at diagnosis. The extent to which distinct patient characteristics are responsible for such a discordance, and a definitive answer regarding whether HER2 status is a contributing factor to DFS or not, would both be difficult to assess without prospective data stratified accordingly.
In spite of these conflicting results, the different HER2-low subgroups appear to be different at least on the molecular level. HER2-low breast cancer is comprised of a majority of HR-positive tumors (ranging from 64% to as high as 87% in the literature) (10,15). When looking at the intrinsic PAM50 subtypes in a cohort of 3,689 patients, HR-positive/HER2-low tumors showed a higher proportion of luminal subtypes compared to HER2 0 tumors, whereas HR-negative/HER2-low tumors showed a high proportion of basal subtypes across all IHC scores (15). Agostinetto et al. noted a distribution of PAM50 subtypes in HR-positive/HER2-low tumors closer to HR-positive/HER2 0 tumors than HR-negative/HER2-low tumors, but a higher proportion of HER2-enriched intrinsic subtype in HR-negative/HER2-low tumors compared to HR-negative/HER2 0 tumors (11). How these differences translate to patient prognosis has not yet been elucidated.
Further differences can be seen according to the histological subtype. In our study, lobular and mixed lobular and ductal carcinomas had a statistically significant lower chance of being HER2-low (53.1%) compared to pure ductal carcinomas (69.3%). Other studies have also reported such differences. In a retrospective analysis of 608 patients with HR-positive HER2-negative breast cancer, lobular carcinomas were significantly more common in the HER2 0 compared to the HER2-low subgroup (16). The exact association between the histological subtype and HER2-low status would require more prospective data to reach definitive conclusions. Furthermore, in our study, HER2-low expression in the subgroup of lobular and mixed lobular and ductal carcinomas was associated with an increased risk of recurrence, which was not statistically significant. As the number of lobular and mixed lobular and ductal carcinomas was comparatively small (n=160), a larger study could potentially show a prognostic significance for HER2-low expression in this relatively rare group of patients with breast cancer. HER2-positive expression has already been shown to identify clinically important subtypes of invasive lobular carcinoma with prognostic implications (17,18).
A potential limitation of our study as well as similar studies performed is their retrospective nature. More prospective studies need to be performed with patients stratified by HER2 IHC to identify the prognostic impact of intermediate HER2 expression. Such studies will allow for further translational research on the molecular and genetic differences of the HER2-low subgroups, which is becoming particularly more relevant in the era of new HER2-targeted treatments such as Antibody-Drug Conjugates that appear to be effective also in HER2-low tumors (19).
Conclusion
In this retrospective series of real-world data, no association was found between low HER2 expression and RFS in hormone receptor positive early breast cancer patients. There was a trend for increased risk of recurrence in patients with lobular and mixed lobular and ductal carcinomas with low HER2 expression and in node-positive patients with HER2 +2 expression, although neither of these trends reached statistical significance.
Conflicts of Interest
GD, EM, AI and TZ declare no relevant conflict of interest. LK has received honoraria and consultancy fees from Ipsen, BMS, Janssen, MSD and Amgen. IN has received honoraria from Roche. KP has received honoraria and consultancy fees from MSD, Gilead, AstraZeneca, Novartis, Eli Lilly, Roche, and GSK.
Authors’ Contributions
Manuscript preparation: GD, KP. Concept and design: KP. Collection of data: LK, EM, AA, IN, KP. Statistical Analysis: GD, KP. Pathology review: TZ. Manuscript reviewing and corrections: All Authors.
References
- 1.Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, Zackrisson S, Senkus E, ESMO Guidelines Committee Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol. 2019;30(8):1194–1220. doi: 10.1093/annonc/mdz173. [DOI] [PubMed] [Google Scholar]
- 2.Cardoso F, Paluch-Shimon S, Senkus E, Curigliano G, Aapro MS, André F, Barrios CH, Bergh J, Bhattacharyya GS, Biganzoli L, Boyle F, Cardoso MJ, Carey LA, Cortés J, El Saghir NS, Elzayat M, Eniu A, Fallowfield L, Francis PA, Gelmon K, Gligorov J, Haidinger R, Harbeck N, Hu X, Kaufman B, Kaur R, Kiely BE, Kim SB, Lin NU, Mertz SA, Neciosup S, Offersen BV, Ohno S, Pagani O, Prat A, Penault-Llorca F, Rugo HS, Sledge GW, Thomssen C, Vorobiof DA, Wiseman T, Xu B, Norton L, Costa A, Winer EP. 5th ESO-ESMO international consensus guidelines for advanced breastcancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 4.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, Eiermann W, Wolter J, Pegram M, Baselga J, Norton L. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 5.Tarantino P, Morganti S, Curigliano G. Targeting HER2 in breast cancer: new drugs and paradigms on the horizon. Exploration of Targeted Anti-tumor Therapy. 2021;2:139–155. doi: 10.37349/etat.2021.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, Jenkins RB, Press MF, Spears PA, Vance GH, Viale G, McShane LM, Dowsett M. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline focused update. Arch Pathol Lab Med. 2018;142(11):1364–1382. doi: 10.5858/arpa.2018-0902-SA. [DOI] [PubMed] [Google Scholar]
- 7.Rossi V, Sarotto I, Maggiorotto F, Berchialla P, Kubatzki F, Tomasi N, Redana S, Martinello R, Valabrega G, Aglietta M, Ponzone R, Montemurro F. Moderate immunohistochemical expression of HER-2 (2+) without HER-2 gene amplification is a negative prognostic factor in early breast cancer. Oncologist. 2012;17(11):1418–1425. doi: 10.1634/theoncologist.2012-0194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eggemann H, Ignatov T, Burger E, Kantelhardt EJ, Fettke F, Thomssen C, Costa SD, Ignatov A. Moderate HER2 expression as a prognostic factor in hormone receptor positive breast cancer. Endocr Relat Cancer. 2015;22(5):725–733. doi: 10.1530/ERC-15-0335. [DOI] [PubMed] [Google Scholar]
- 9.Gilcrease MZ, Woodward WA, Nicolas MM, Corley LJ, Fuller GN, Esteva FJ, Tucker SL, Buchholz TA. Even low-level HER2 expression may be associated with worse outcome in node-positive breast cancer. Am J Surg Pathol. 2009;33(5):759–767. doi: 10.1097/PAS.0b013e31819437f9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Denkert C, Seither F, Schneeweiss A, Link T, Blohmer JU, Just M, Wimberger P, Forberger A, Tesch H, Jackisch C, Schmatloch S, Reinisch M, Solomayer EF, Schmitt WD, Hanusch C, Fasching PA, Lübbe K, Solbach C, Huober J, Rhiem K, Marmé F, Reimer T, Schmidt M, Sinn BV, Janni W, Stickeler E, Michel L, Stötzer O, Hahnen E, Furlanetto J, Seiler S, Nekljudova V, Untch M, Loibl S. Clinical and molecular characteristics of HER2-low-positive breast cancer: pooled analysis of individual patient data from four prospective, neoadjuvant clinical trials. Lancet Oncol. 2021;22(8):1151–1161. doi: 10.1016/S1470-2045(21)00301-6. [DOI] [PubMed] [Google Scholar]
- 11.Agostinetto E, Rediti M, Fimereli D, Debien V, Piccart M, Aftimos P, Sotiriou C, de Azambuja E. HER2-low breast cancer: molecular characteristics and prognosis. Cancers (Basel) 2021;13(11):2824. doi: 10.3390/cancers13112824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Modi S, Park H, Murthy RK, Iwata H, Tamura K, Tsurutani J, Moreno-Aspitia A, Doi T, Sagara Y, Redfern C, Krop IE, Lee C, Fujisaki Y, Sugihara M, Zhang L, Shahidi J, Takahashi S. Antitumor activity and safety of trastuzumab deruxtecan in patients with HER2-low-expressing advanced breast cancer: results from a phase Ib study. J Clin Oncol. 2020;38(17):1887–1896. doi: 10.1200/JCO.19.02318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Inoue S, Kawaida H, Saito R, Nakayama Y, Ohmori M, Kimura A, Shoda K, Furuya S, Hosomura N, Akaike H, Kawaguchi Y, Amemiya H, Sudo M, Kono H, Ichikawa D. Clinical significance of past history of breast cancer screening for the prognosis of triple negative breast cancer. Anticancer Res. 2021;41(2):1077–1082. doi: 10.21873/anticanres.14865. [DOI] [PubMed] [Google Scholar]
- 14.Horisawa N, Adachi Y, Takatsuka D, Nozawa K, Endo Y, Ozaki Y, Sugino K, Kataoka A, Kotani H, Yoshimura A, Hattori M, Sawaki M, Iwata H. The frequency of low HER2 expression in breast cancer and a comparison of prognosis between patients with HER2-low and HER2-negative breast cancer by HR status. Breast Cancer. 2022;29(2):234–241. doi: 10.1007/s12282-021-01303-3. [DOI] [PubMed] [Google Scholar]
- 15.Schettini F, Chic N, Brasó-Maristany F, Paré L, Pascual T, Conte B, Martínez-Sáez O, Adamo B, Vidal M, Barnadas E, Fernández-Martinez A, González-Farre B, Sanfeliu E, Cejalvo JM, Perrone G, Sabarese G, Zalfa F, Peg V, Fasani R, Villagrasa P, Gavilá J, Barrios CH, Lluch A, Martín M, Locci M, De Placido S, Prat A. Clinical, pathological, and PAM50 gene expression features of HER2-low breast cancer. NPJ Breast Cancer. 2021;7(1):1. doi: 10.1038/s41523-020-00208-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mutai R, Barkan T, Moore A, Sarfaty M, Shochat T, Yerushalmi R, Stemmer SM, Goldvaser H. Prognostic impact of HER2-low expression in hormone receptor positive early breast cancer. Breast. 2021;60:62–69. doi: 10.1016/j.breast.2021.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McCart Reed AE, Kalinowski L, Simpson PT, Lakhani SR. Invasive lobular carcinoma of the breast: the increasing importance of this special subtype. Breast Cancer Res. 2021;23(1):6. doi: 10.1186/s13058-020-01384-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Flores-Díaz D, Arce C, Flores-Luna L, Reynoso-Noveron N, Lara-Medina F, Matus JA, Bargallo-Rocha E, Pérez V, Villarreal-Garza C, Cabrera-Galeana P, Mohar A. Impact of invasive lobular carcinoma on long-term outcomes in Mexican breast cancer patients. Breast Cancer Res Treat. 2019;176(1):243–249. doi: 10.1007/s10549-019-05234-8. [DOI] [PubMed] [Google Scholar]
- 19.Eiger D, Agostinetto E, Saúde-Conde R, de Azambuja E. The exciting new field of HER2-low breast cancer treatment. Cancers (Basel) 2021;13(5):1015. doi: 10.3390/cancers13051015. [DOI] [PMC free article] [PubMed] [Google Scholar]