Abstract
This report provides a descriptive analysis of the African swine fever (ASF) Genotype II epidemic in the affected Member States in the EU and two neighbouring countries for the period from 1 September 2020 to 31 August 2021. ASF continued to spread in wild boar in the EU, it entered Germany in September 2020, while Belgium became free from ASF in October 2020. No ASF outbreaks in domestic pigs nor cases in wild boar have been reported in Greece since February 2020. In the Baltic States, overall, there has been a declining trend in proportions of polymerase chain reaction (PCR)‐positive samples from wild boar carcasses in the last few years. In the other countries, the proportions of PCR‐positive wild boar carcasses remained high, indicating continuing spread of the disease. A systematic literature review revealed that the risk factors most frequently significantly associated with ASF in domestic pigs were pig density, low levels of biosecurity and socio‐economic factors. For wild boar, most significant risk factors were related to habitat, socio‐economic factors and wild boar management. The effectiveness of different control options in the so‐named white zones, areas where wild boar densities have been drastically reduced to avoid further spread of ASF after a new introduction, was assessed with a stochastic model. Important findings were that establishing a white zone is much more challenging when the area of ASF incursion is adjacent to an area where limited control measures are in place. Very stringent wild boar population reduction measures in the white zone are key to success. The white zone needs to be far enough away from the affected core area so that the population can be reduced in time before the disease arrives and the timing of this will depend on the wild boar density and the required population reduction target in the white zone. Finally, establishing a proactive white zone along the demarcation line of an affected area requires higher culling efforts, but has a higher chance of success to stop the spread of the disease than establishing reactive white zones after the disease has already entered in the area.
Keywords: ASF, EU, epidemiology, wild boar, domestic pigs, prevention, control, white zones
Short abstract
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2022.EN-7320/full
Summary
The European Commission requested EFSA to provide an updated analysis of the epidemiological situation of ASF in the Member States (MS) of the EU affected by African swine fever virus (ASFV) Genotype II.
Term of reference 1 (TOR 1) of the mandate requested analysis of the epidemiological data on ASF from MS and non‐EU countries affected by ASFV Genotype II, including an analysis of the temporal and spatial distribution of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention was paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
Narrative updates were provided on the ASF situation in the affected MS (Belgium, Bulgaria, Estonia, Germany, Greece, Hungary, Latvia, Lithuania, Poland, Romania and Slovakia) during the reporting period (from 1 September 2020 until 31 August 2021) and in two neighbouring countries (Serbia and Russia).
Globally, the geographical information indicates that ASF continued to circulate in wild boar in several MS in the EU and neighbouring countries and spread slowly through the wild boar populations during the reporting period. Important differences in the phases of ASF epidemic were observed among Member States. ASF was firstly confirmed in Germany on 10 September 2020 1 , spreading fast among the wild boar population.1 From the first notification until the end of reporting period, 1,872 cases were observed in wild boar in Germany, mostly near the eastern border of the country. By contrast, Belgium sent to the World Organisation for Animal Health (OIE) a self‐declaration on its ASF‐free status on 1 October 2020, 2 and no ASF outbreaks in domestic pigs nor cases in wild boar were reported in Greece in this reporting period.
The number of cases reported in wild boar in Bulgaria, Hungary, Lithuania and Poland decreased with 32, 22, 27 and 15%, respectively, compared with the previous reporting period. In Slovakia, however, almost 10 times as many cases were reported in wild boar in this reporting period, compared with the previous one. In Romania, an increase of 33% of cases reported in wild boar was observed. In Estonia, where the disease appeared to be fading out in the previous reporting period, a resurgence of the infection was observed in two counties during this reporting period, and a total increase of 19% of cases was reported in wild boar compared with the previous reporting period. After 4 years without outbreaks in domestic pigs in Estonia, an outbreak was observed in a commercial pig farm. Whereas in Slovakia, Poland and Romania, the number of outbreaks in domestic pigs increased compared with the previous reporting period; in Bulgaria, there were fewer outbreaks observed in domestic pigs, and in Lithuania, there were no outbreaks observed in domestic pigs in this reporting period.
Overall, enhanced passive surveillance (weekly testing of two dead pigs) has contributed to early detection of outbreaks in domestic pig farms. In Romania, clinical passive surveillance has led to most detections of outbreaks in smaller farms, while in the larger commercial farms, enhanced passive surveillance contributed most to the outbreak detection. The number of ASF domestic outbreaks in Romania increased by 57% in comparison with the previous reporting period. This was confirmed by the network analysis results that identified an increase in the average number of potential secondary cases coming from a single source case in this reporting period compared with the previous reporting period. In Romania, the number of outbreaks in big farms (with more than 10,000 animals) increased from 6 to 13 farms in this reporting period. Although ASF outbreaks were dispersed over the entire country, most of the outbreaks, including the large outbreaks on farms of more than 10,000 pigs, occurred in the north‐west and the south‐east of the country. As observed in previous reports, there was a clear seasonality in Romania in the outbreaks in domestic pigs, with a peak of outbreaks in summer. This peak during July and August was much more pronounced and more noticeable in farms smaller than 30 animals compared with the previous reporting period.
To provide an insight into temporal trends, time profiles were produced, showing the trend of the proportions of positive samples since the first detection based on data submitted to EFSA’s Data Collection Framework from the beginning of 2016 and up to the end of this reporting period. It can be assumed that there is a relationship between the proportion of polymerase chain reaction (PCR)‐positive samples and the incidence of ASF. This analysis revealed that, in the Baltic States, overall, the proportion of PCR‐positive samples from wild boar carcasses is declining, although a resurgence was observed in Estonia over the last reporting period. In the other countries, the proportion of PCR‐positive wild boar carcasses remained high, indicating continuing spread of the disease, without general increase in the proportion of seropositive samples in wild boar in the affected populations since the introduction of the disease. This suggests that, overall, there has been no increase in the wild boar survival rate.
There is a clear seasonality in the proportions of PCR‐positive samples from wild boar found dead with some differences between the different MS. This was based on data submitted since 1 January 2016 for Estonia, Latvia, Lithuania and Poland and on data submitted since the first day of introduction for Romania and Slovakia. While this proportion (PCR‐positive samples in wild boar) followed a decline in summer and an increase in winter in Romania and Slovakia; in Latvia and Estonia, there was a peak in the proportion of positive samples over the summer months.
In domestic pigs, there was also a seasonality observed during the spring/summer in Lithuania, Poland, Slovakia and Romania (countries that submitted sample data from domestic pigs to EFSA), with a peak of PCR‐positive samples from domestic pigs observed between May and September. Additional investigations are required to understand the reason of this observed seasonality and the differences between domestic and wild boar and between MS. Seasonality of ASF in domestic pigs in other countries that did not submit data to EFSA was not investigated.
Wild boar populations have an important impact on ASF epidemics and vice versa, as confirmed by the trends observed in the data from hunted wild boar from affected MS and neighbouring countries, which is considered as a proxy of the abundance of the wild boar. In the Baltic States, there were declining trends in the number of hunted wild boar for five seasons after ASF introduction, and a small but consistent increase during the last hunting season (2020–2021). In Bulgaria and Romania, the effects of ASF on the number of hunted wild boar started in 2018–2019 and 2020–2021, respectively, with a sharp reduction in numbers of hunted wild boar. An increasing trend in the numbers of hunted wild boar in the Central European countries has been observed since 2000, with some fluctuations.
The extent of ASF spread in the wild boar populations in each affected MS was evaluated by calculating the number of potential secondary cases that could be attributed to a single source. This number for the current reporting period was compared with the same calculations based on data from the beginning of the epidemic and on data from the previous reporting period, trying to visualise if the disease was in an expanding or declining phase in each MS. Different trends were observed: whereas in some countries, the average number of secondary cases increased (e.g. Bulgaria and Latvia), in other counties it decreased (e.g. Hungary). For other countries, there were no clear trends. In Germany, due to the recent introduction, no comparison could be made with previous reporting periods. However, the relatively high number of 6.4 cases on average per source case in wild boar indicated that the epidemic is in an expanding phase.
Term of reference 2 (TOR 2) requested a review of the previously identified risks factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic–wildlife interface with a view to strengthening biosecurity and other risk mitigation measures.
First, a systematic literature review was carried out to identify scientific studies that quantitatively evaluated possible risk factors for the occurrence of ASFV in wild boar or domestic pigs. The review identified 31 scientific articles, including the results of the risk factor analysis in this report (Sections 4.2.2–4.2.4) that quantitatively analysed 621 risk factors for ASF in domestic pig and wild boar populations. The factors most frequently found to be significantly associated with ASF in domestic populations were those related to pig farming, especially pig population density and biosecurity practices, socio‐economic factors of the farmer and population demographics, and the presence or closeness to ASF‐infected areas. Habitat‐related factors influencing wild boar suitability, socio‐economic factors and wild boar management factors, mostly wild boar density and abundance, were the factors most frequently found to be significantly associated with the risk of ASF in wild boar.
Subsequently, three different models were built to analyse the potential risk factors for ASF occurrence in wild boar populations in three different areas of the EU. First, a logistic regression model was developed to study the risk factors of ASF in wild boar in Slovakia during 2020 at district level. Second, a Besag York Mollié model was used to evaluate the risk factors of ASF in wild boar in Romania (NUTS 3 spatial resolution 2018–2021) and Baltic States [local administrative unit (LAU) level from 2018 to 2020]. The presence of ASF cases in domestic pigs was identified as a significant factor for the probability of detecting ASF‐positive PCR cases in wild boar in Romania and Slovakia. In Slovakia, the PCR‐positive results in domestic pigs were highly correlated with the density of small‐sized farms (fewer than 10 animals) per district. Environmental factors related to wild boar habitat such as the presence of croplands, urban areas and the density of waterbodies and wild boar abundance, were found to be significantly related to the PCR‐positive detection of ASF in wild boar in Romania and Slovakia. In addition, the number of hunting days was identified as a protective factor for the occurrence of ASF in wild boar in Romania, but the nature and direction of this relationship should be investigated more carefully, considering higher resolution data and additional information on the hunting methods implemented in the area. The analysis performed in the Baltic States did not identify any relevant risk factor but confirmed the significant decline in the PCR‐positive results in wild boar in the region. Additional and higher spatial resolution field data on the type of farms, biosecurity conditions and wild boar interactions would be useful to further investigate the potential relationship between domestic and wild boar.
Term of reference 3 (TOR 3) requested analysis of the data and information on the geographical areas called ‘white zones’ (WZ; zones blanches) applied by free MS to prevent the spread of the disease in wild boar. The WZ concept was originally implemented around a focal introduction of ASF in a wild boar population. The concept is a wild boar management zone that is set up as a belt at a distance from the newly ASF‐affected area, in which, among other measures, the wild boar population is reduced drastically to an a priori‐decided population density, with the aim to preventively stop the spread of the infection. The objective was to assess the effectiveness of different control options in these WZs under different scenarios. A spatially explicit, stochastic, individual‐based model was used to simulate the spread and control of ASF in the WZs. Three main questions were addressed.
First, it was questioned if there were differences when applying WZs after introduction of the disease between a neighbouring area where ASFV infection spreads in wild boar in a wide area and limited control efforts are applied compared with a WZ applied after a focal introduction of ASF. The outcomes of the model clearly demonstrated that establishing a WZ is much more challenging when the area of incursion is adjacent to a wide area with ASF spread and limited control efforts. For the same set of control measures applied in the WZ, the overall success rate was greatly reduced compared with a focal introduction.
Second, it was questioned how the WZ approach could be strengthened in the low‐control neighbourhood context. The effectiveness of the WZ approach is determined by three main parameters that should be chosen a priori: (1) the target (reduced) density of the wild boar population in the WZ; (2) the width of the WZ; and (3) the time needed to reach the target density.
For the target density of the wild boar population in the WZ, very stringent wild boar population reduction measures are the key to its success and this is more important than reducing the time to reach the target population density. Although, setting a priori a lower target population density in the WZ (e.g. less than 0.5/km2 rather than 1/km2 within 6 months in the WZ) requires that a larger number of wild boar that should be culled initially, in the end this results in a smaller overall culling effort and greater control success.
In addition, the wider the WZ is for a given target population density, the better is the expected control outcome.
Finally, the time to reach the target is related to the choice of the distance between the core area (which is the area delineating all the wild boar cases in the newly infected area, which is usually fenced) and the WZ. This distance depends on the velocity of spread of the infection through the wild boar population (which is landscape dependent) and the time planned to finalise the population reduction measures in the WZ (which is a management decision). An inappropriate distance between the core area and WZ can reduce the overall success of the WZ measures, because the infection will enter the WZ too early if the distance between core area and WZ is chosen to be too small and therefore the target density of the population has not yet been finalised before entry. In the area between the core area and the WZ population, reduction measures can also be carried out, but this is not strictly needed if resources are limited.
The third question addressed the benefits of applying proactive WZs compared with reactive WZs. When a WZ is set up, the areas all along the demarcation line adjacent to neighbouring infected areas with limited control, these are called proactive WZs, as they are set up before ASFV has entered the free area. To maintain the proactive WZ along a demarcation line, higher culling efforts are needed compared with the reactive approach but, with this approach, there are situations with a higher chance of success to stop the spread of the disease. The additional culling effort could be potentially reduced by a segmentwise release of the measures (roll‐back) in the WZ after there is no more risk of infection from the neighbouring area with limited control (e.g. after the circulation of virus has ceased in the area and surveillance efforts have been carried out to substantiate the evidence of freedom of ASFV circulation in the area; EFSA, 2021b). However, such a roll‐back of WZ segments should start only after certain exit criteria are fulfilled through surveillance. The uncertainty with the roll‐back approach is the potential translocations of ASFV infection, e.g. by humans, back into the previously cleared WZ segments.
1. Introduction
1.1. Background provided by the requestor 3
African swine fever (ASF) is an infectious lethal disease affecting domestic pigs and wild boar. It can be transmitted via direct animal contact or via dissemination of contaminated food or equipment. This disease has serious economic implications for the pigmeat and related sectors, including indirect costs related to trade restrictions. There is no vaccine or cure, despite active ongoing research. The persistence of the disease in wild boar and the limited number of control measures available represent a challenge for the whole EU agricultural sector, in particular the pig farming industry.
From the beginning of 2014 up to the time of publication of this Scientific Report, Genotype II of ASFV has been notified in Belgium, 4 Bulgaria, Czechia, Estonia, Germany, 5 Greece, Hungary, Italy, Latvia, Lithuania, Poland, Romania and Slovakia, causing very serious concerns.
The disease has also been reported in Belarus, Moldova, Serbia, Russia, Ukraine and North Macedonia, which has created a constant risk for all the Member States that share a border with these non‐EU countries. Czechia was recognised as officially ASF free in March 2019.
There is knowledge, legislation, technical and financial tools in the EU to properly face ASF. EU legislation primarily targets domestic pig and, when needed, lays down 6 specific aspects related to wild boar. The main pieces of the EU legislation relevant for ASF were at the time of the mandate: 7
Council Directive 2002/60/EC 8 of 27 June 2002 laying down specific provisions for the control of African swine fever and amending Directive 92/119/EEC as regards Teschen disease and ASF: it mainly covers prevention and control measures to be applied where ASF is suspected or confirmed either in holdings or in wild boar to control and eradicate the disease.
Commission Implementing Decision 2014/709/EU 9 of 9 October 2014 on animal health control measures on African swine fever in certain Member States and repealing Implementing Decision 2014/178/EU: it provides the animal health control measures on ASF in certain Member States by setting up a regionalisation mechanism in the EU. These measures involve mainly pigs, pig products and wild boar products. A map summarising the current regionalisation applied is available online. 10
Council Directive No 82/894/EEC 11 of 21 December 1982 on the notification of animal diseases within the Community which has the obligation for Member States to notify the Commission of the confirmation of any outbreak or infection of ASFV in pigs or wild boar.
In addition, a strategic approach to the management of ASF for the EU has been developed based on earlier scientific recommendations by EFSA. This strategy is constantly evolving based on new science available and on new experiences gained. The ASF Strategic approach is aimed to the EU countries affected by the disease and to EU countries free from the disease with a risk of introduction. 12
Some areas free from ASF, but neighbouring infected or restricted areas, are at higher risk of becoming ASF infected via natural spread of the disease through the wild boar population. Based on previous EFSA reports and on experts’ recommendations, geographical areas called white zones (WZs; zones blanches) were put in place to enable early detection (through active search of carcasses) and effectively reduce the wild boar population.
The Commission is in need of an updated epidemiological analysis based on the data collected from the Member States affected by ASFV Genotype II. This analysis should take into account the previous EFSA opinions and technical reports on ASF.
The use of the EFSA Data Collection Framework is encouraged, given it promotes the harmonisation of data collection. Any data that are available from neighbouring non‐EU countries should be used as well.
1.2. Terms of Reference as provided by the requestor
TOR 1: Analyse the epidemiological data on ASF from Member States and non‐EU countries affected by ASFV Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention should be paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
TOR 2: Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthening biosecurity and other risk mitigation measures. Risk factors involved in the occurrence of ASF in domestic pig farms in Romania should be identified.
TOR 3: Analyse the data and information on the geographical areas called WZs (zones blanches) applied by free Member States (in particular France and Luxembourg at the border with Belgium) for preventing the spread of the disease in wild boar. Assess the effectiveness of the measures and review scientific literature addressing these measures. Review and assess the robustness and effectiveness of the boundaries used for the determination/demarcation of these areas.
1.3. Interpretation of the Terms of Reference (if appropriate)
TOR 1: Analyse the epidemiological data on ASF from Member States and non‐EU countries affected by ASFV Genotype II. Include an analysis of the temporal and spatial patterns of ASF in wild boar to identify patterns (ranges and speed) of transmission and introduction of the virus in different types of domestic pig holdings. Special attention should be paid to the temporal and spatial patterns observed in domestic pig farms of different sizes in Romania.
Overview tables were provided on the ASF situation in each of the MS that were affected during the reporting period (Belgium, Bulgaria, Estonia, Germany, Greece, Hungary, Latvia, Lithuania, Poland, Romania and Slovakia) (from 1 September 2020 until 31 August 2021) and in two neighbouring countries (Serbia and Russia).
Several specific analyses were performed focused on the ASF outbreaks in large commercial farms in Romania in the last reporting period, to provide additional insights of the characteristics and patterns of the disease in those areas.
To provide an insight into potential temporal trends of the disease, time profiles were produced showing the trend of the proportions of positive samples since 1 January 2016 in Estonia, Latvia, Lithuania and Poland from the first day of introduction into these countries and after that date. Each country and possible patterns of seasonality were investigated. The possible impact of the ASF epidemic on the wild boar population in each affected MS and in four neighbouring countries (Belarus, Russia, Serbia and Ukraine) was investigated by looking at the trend of the standardised annual numbers of wild boar hunted in the last two decades. In addition, to evaluate the extent of spread of ASF in the wild boar populations in each affected MS, the numbers of potential secondary cases that could be attributed to a single source were calculated (means of bootstraps calculated with a network analysis). Furthermore, to better understand the trend of the epidemic, i.e. whether it was in an expanding phase or in decline, the number of potential secondary cases during the beginning of the epidemic was compared with that of the reporting period in each country.
TOR 2: Review the previously identified risk factors involved in the occurrence, spread and persistence of the ASF virus in the wild boar population and in the domestic/wildlife interface with a view to strengthening biosecurity and other risk mitigation measures. Risk factors involved in the occurrence of ASF in domestic pig farms in Romania should be identified.
First, a systematic literature review was developed to identify scientific studies that quantitatively assessed the factors related to the risk of ASF occurrence, spread or maintenance. The article metadata, epidemiological study characteristics and all information related to the factors analysed in the study (i.e. type of factor, significance and direction of the relationship) were extracted and analysed. These results provided useful information on the factors more frequently identified as significantly correlated with ASF in the epidemiological studies, as well as pointing out areas where additional research is required.
Next, possible risk factors for the occurrence of ASF in wild boar in Slovakia, Romania and the Baltic States were assessed, using two different methodologies depending on the data availability. On a district spatial resolution, a generalised linear model was used to evaluate potential risk factors for the occurrence of ASF in wild boar in Slovakia during 2020. In parallel, a Besag York Mollié model was used to evaluate potential risk factors for the occurrence of ASF in the wild boar populations in Romania on a NUTS 3 spatial resolution from 2018–2021, and in the Baltic States from 2017–2020 on a LAU spatial resolution.
TOR 3: Data and information were analysed on the geographical areas called WZs (zones blanches) applied by free Member States (in particular France and Luxembourg at the border with Belgium) to prevent the spread of the disease in wild boar. Effectiveness of the measures was assessed and a review of scientific literature addressing these measures was carried out. The robustness and effectiveness of the boundaries used for the determination/demarcation of these areas were reviewed and assessed.
During the analysis on the efficacy of the measures implemented in the WZ carried out for the previous reporting period (EFSA, 2021a), the concept of WZs was agreed upon as ASF‐free (negative) management areas, set up to form a belt at a distance from the newly ASF‐affected area, in which, among other measures, the wild boar population is reduced drastically to an a priori‐decided population density, with the aim to preventively stop the spread of the infection. Usually, the WZ is set up in response to a focal ASF introduction and may be expanded in response to later case detections. This TOR does address the additional uncertainty on effectiveness of the WZ if limited control measures are applied in the adjacent areas of ASF circulation.
To evaluate the effectiveness of the measures the epidemiological situation was reconstructed in a spatially explicit, stochastic individual‐based model, using varying wild boar habitat geography in Europe. Then, different parameterisation scenarios of the WZ (e.g. different width, time for the culling operation and target density) were compared against the standard WZ application surrounding a focal introduction of ASF in the same simulation landscape. Comparison was made in terms of probability to fail and culling volume. Alternatively, proactive approaches were investigated to improve the effectiveness of the WZ approach when applied in neighbourhood to an ASF‐affected area with limited control.
2. Data
2.1. ASF notification data extracted from the Animal Disease Information System Database
Data on ASF cases and outbreaks 13 in wild boar and domestic pigs, respectively, notified between 1 January 2014 and 31 August 2021, were extracted from the Animal Disease Information System (ADIS) database.
2.2. Sample‐based ASF surveillance data submitted to EFSA’s Data Collection Framework, according to SIGMA standards
The data on samples from wild boar and domestic pigs from the Laboratory Information Management System (LIMS) of the national laboratories of affected MS were collected in the EFSA’s Data Collection Framework (DCF) (EFSA, 2017) according to SIGMA standards. The data reported to the DCF by the different MS contained the information on samples tested for ASFV from 1 September 2020 to 31 August 2021. These samples were combined with the samples submitted to the DCF in previous reporting seasons starting from January 2014. Samples were tested for ASFV using PCR (testing for virus genome) and for antibodies by enzyme‐linked immunosorbent assay (ELISA) confirmed by immunoblotting (IB) or immune‐peroxidase (IPT) (tests for antibodies). It should be noted that positive antibody (Ab) ELISA test results were not systematically confirmed with confirmatory tests [IPT or wild boar (WB)] and, therefore, only the ELISA tests results were used in this report to develop comparable time profiles (Section 4.1.4) of the proportions of positive samples for the different MS. In addition, the ELISA test has not been validated for testing samples taken from carcass fluids from wild boar, and therefore the results related to wild boar found dead should be interpreted with caution. Sample‐based data of wild boar have been provided for this reporting period by Estonia, Hungary, Latvia, Poland, and Slovakia at NUTS 3 and municipality level, as well as by Romania and Lithuania at NUTS 3. For domestic pigs, data have been submitted from Estonia, Poland, Romania and Slovakia at NUTS 3 and municipality level, and from Lithuania in NUTS 3 level. Finally, it should be noted that, in most countries, the samples were pooled samples from several animals. However, there was no harmonised approach for the different countries, and therefore, the true numbers of animals from which the samples were retrieved could not be calculated.
2.3. Pig population data submitted to EFSA’s Data Collection Framework, according to SIGMA standards
Data on pig population in the MS were collected in the EFSA’s DCF (EFSA, 2017) according to SIGMA standards. The data submitted to the DCF by the different MS contained information about the number of pigs by farm, location of the premises and type of production. The level of detail of the spatial information reported varied among countries (from NUTS 3 areas to exact coordinates of the farm). All the MS affected by ASF were invited to submit one snapshot of their pig population data at the time of the year that they considered most representative for this reporting period (September 2020–August 2021). Countries that submitted pig population data to EFSA through DCF are detailed in Appendix A, including the granularity of the data submitted. These data were used in the risk assessment studies of the present report for the countries selected.
2.4. Wild boar data
Wild boar data at national level for the last two decades were provided by ENETWILD‐consortium et al. (2020). Specifically, hunting bag data were collected for the ASF‐affected countries from the national hunting associations and used to develop the linear trends of Section 4.1.6.
In addition, several MS submitted hunting data directly to EFSA, including some information on hunting methods for the risk factor analysis, as detailed in next section of the report.
2.5. Data collection and aggregation for assessing the risk factors of ASF occurrence in wild boar in Slovakia, Romania and the Baltic States
Data were collected for the risk factor analysis of ASF in wild boar in Slovakia, Romania and the Baltic States. For Slovakia, the analysis was focused on the year 2020, as historical data were not available for all the variables of interest. For Romania, data were collected from 2018 to 2021, and for the Baltic States (analysed together as a unit), data were available from 2017 to 2020. A summary of the data available for the risk factor analysis can be found in Appendix A.
From the DCF (see Sections 2.2 and 2.3), PCR test results for ASFV in wild boar and domestic pigs were collected. PCR test results in wild boar were used as outcome variable of the models, while PCR test results in domestic pig farms were used as covariate in the models for Slovakia and Romania. Data related to the potential risk factors in relation to wild boar habitat, hunting activities, pig farming system and anthropogenic factors were collected from the different sources referred to in Table 1 and aggregated per spatial unit as explained in the following sections.
Table 1.
Potential risk factors based on the available data used in the analysis of risk factors in Slovakia, Romania and the Baltic States
| Name | Acronym | Description | Explanation | VIF Slovakia | VIF Romania | VIF Baltics | Source |
|---|---|---|---|---|---|---|---|
| Potential risk factors related to wild boar habitat | |||||||
| Wild boar suitability | WB_SUIT | Percentage of area with suitable habitat for wild boar | Habitat quality could drive wild boar density | 32.7 | 26.8 | 12.6 | ENETWILD‐consortium et al. (2020) |
| Waterbodies | Water | Percentage of waterbodies in the area | Wild boar could aggregate near waterbodies | 1.7 | 4.3 | 2 | https://www.esa‐landcover‐cci.org/?q=node/158 |
| Trees | Trees | Percentage of the area covered by trees | The land cover could have an impact on wild boar behaviour, e.g. some crops attract wild boar and would facilitate aggregation and impact on transmission rates | 1.4 | 2.5 | 1.1 | https://www.esa‐landcover‐cci.org/?q=node/158 |
| Crops | Crops | Percentage of the area covered by rain‐fed crops | 2.6 | 23.4 | 2.7 | https://www.esa‐landcover‐cci.org/?q=node/158 | |
| Herbaceous | Herb | Percentage of the area that is covered by herbaceous land cover | 43.2 | 7.9 | 3.6 | https://www.esa‐landcover‐cci.org/?q=node/158 | |
| Altitude | Altitude | Average altitude | Climatic conditions could have an effect both on the survival of the virus in the environment and on the wild boar habitat | 14.1 | 39.5 | 1.8 | https://lta.cr.usgs.gov/SRTM1Arc |
| Sun | Sun | Average yearly sun radiation | 16.5 | NA | 20.7 | https://worldclim.org/version2 | |
| Snow | Snow | Average yearly snow depth | 26 | 12.4 | 3.4 | Hall and Riggs (2015) | |
| Mean temperature | Temp | Average yearly mean temperature | 4.2 | 40 | 7.5 | https://worldclim.org/version2 | |
| Potential risk factors related to hunting activities | |||||||
| Wild boar abundance | WB_DNS | Wild boar hunting bag per surface (km2) | The number of wild boar hunted is correlated with the wild boar density in the area, both having an influence on the transmission rate | 2.3 | 37.6 | 1.1 |
Ministry of Agriculture and Rural Development of the Slovak Republic Ministry of Environment, Romania Ministry of Agriculture Latvia, Ministry of Environment, Estonia Ministry of Environment, Lithuania |
| Number of hunting dogs | Dogs_DNS | Number of hunting dogs per surface (km2) | The number of dogs used for hunting is a proxy of the hunting pressure and could influence wild boar behaviour | 3.9 | 6 | NA |
Ministry of Agriculture and Rural Development of the Slovak Republic Ministry of Environment, Romania |
| WB female | WB_Fe | Number of female wild boar hunted per surface (km2) | Hunting females is a sign of hunting for population control | NA | 41.7 | NA |
Ministry of Environment, Romania |
| Number of hunters | Hunters | Number of active hunters in the season per surface (km2) | The number of hunters is a proxy of the hunting pressure and could influence wild boar behaviour | NA | 2.2 | NA |
Ministry of Environment, Romania |
| Hunting days | Hdays | Number of hunting days | The number of hunting days is a proxy of the hunting pressure and could influence wild boar behaviour | NA | 4.1 | NA |
Ministry of Environment, Romania |
| Feeding places | Feed_P | Number of feeding places for the wild boar per surface (km2) | Feeding drives wild boar population dynamics and spatial aggregation | NA | 8.5 | NA |
Ministry of Environment, Romania |
| Feeding tonnes | Feed_T | Approximate tonnes of feed for the wild boar per surface (km2) | NA | 1.8 | NA |
Ministry of Environment, Romania |
|
| Average piglet | Piglets | Average number of piglets per sow | Piglets per sow is a proxy of wild boar reproductive success | NA | 4 | NA |
Ministry of Environment, Romania |
| Potential risk factors related to the pig farming system | |||||||
| Pigs density | Pig_DNS | Density of pigs per surface (km2) | Higher density of domestic pigs implies higher susceptible population for ASF | 1.8 | NA | 1.2 |
Ministry of Agriculture and Rural Development of the Slovak Republic Ministry of Agriculture Latvia, Ministry of Rural Affairs, Estonia Ministry of Agriculture Lithuania |
| Small farms density | SmallFarm_DNS | Density of small farms (< 10 pigs) per km2 | Small farms were assumed to often implement suboptimal biosecurity measures (Ribbens et al., 2008; Correia‐Gomes et al., 2017; Nurmoja et al., 2020) | 7.2 | NA | 346.8 |
Ministry of Agriculture and Rural Development of the Slovak Republic |
| Small farm pigs density | SmallPig_DNS | Density of pigs in small farms (< 10 pigs) per km2 | 6.1 | NA | NA |
Ministry of Agriculture and Rural Development of the Slovak Republic |
|
| Potential risk factors related to ASF in domestic population | |||||||
| ASF in domestic pigs | ASF_dom | Presence of a PCR‐positive result in domestic pigs in the district | The occurrence of ASF in domestic pigs in the district can be a proxy of the level of ASF contaminations in the area, and could be related to ASF in wild boar | 2.3 | 1.7 | NA |
Ministry of Agriculture and Rural Development of the Slovak Republic Ministry of Environment, Romania |
| Potential anthropogenic risk factors | |||||||
| Human footprint index | HFP | Average human footprint index per district | A higher human activity in an area could influence the occurrence of the disease | 4.1 | 24.8 | 2.2 | Venter et al. (2018) |
| Proportion of Urban habitat | Urban | Percentage of the surface occupied by urbanised areas | 3.8 | 33.1 | 1.5 | https://efsa.onlinelibrary.wiley.com/doi/epdf/10.2903/sp.efsa.2020.EN‐1871 | |
| Bare areas | Bare | Areas where the land is not covered by semi‐natural or artificial cover | 1.6 | 1.6 | 3.1 | https://www.esa‐landcover‐cci.org/?q=node/158 | |
NA, data not available or not calculated on this spatial level; variance inflation factor (VIF). Data in red: VIF > 5: excluded from analysis due to collinearity.
For Slovakia, the spatial unit of analysis was district level (n = 79) [equivalent to Database of Global Administrative Areas (GADM) level 2], whereas in Romania the analysis was performed at county level (NUTS 3) (n = 42), and in the Baltic States at LAU region (n = 258 total). First, data were aggregated at the spatial unit of interest and some variables were transformed into density values (as indicated in Table 1), dividing them by the surface area of the spatial unit of interest. For instance, the total number of wild boar hunted in a specific district in 2020 this was divided by the km2 of that district to find the average number of wild boar hunted in that year per km2. Afterwards, data of each variable, and each year for Romania and the Baltic States, were standardised by dividing them by the maximum value of the variable in question for all the spatial units in that year.
Then, to avoid multicollinearity, the potential risk factors were assessed using the variance inflation factor (VIF) and only those potential risk factors for which the VIF value was below 5 were retained to be further used in the model building process (Imdad et al., 2016, 2019). The results are listed in Table 1 for all potential risk factors used in the three models. In addition, the heat maps in Figure 1 visualise the pairwise correlation between the variables used in both models, red indicating positive correlation and blue indicating a negative relationship.
Figure 1.
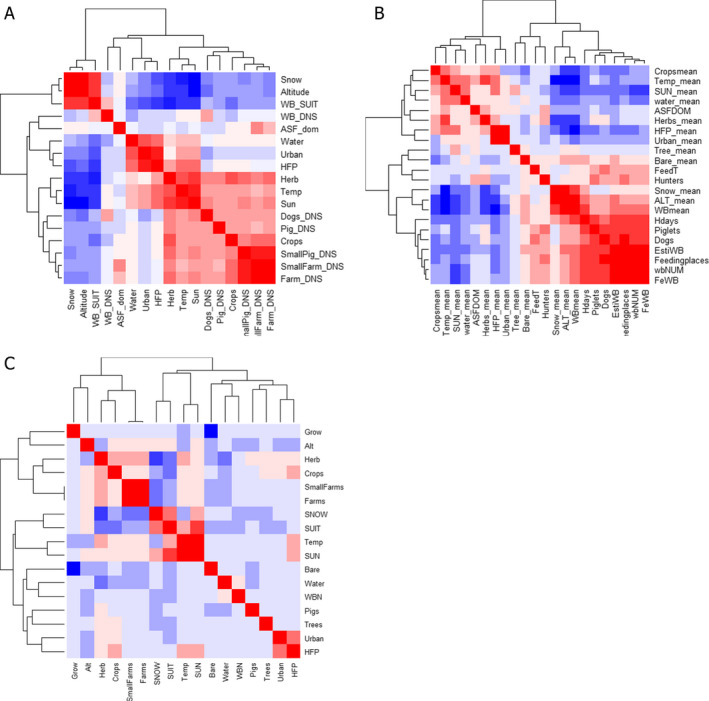
Heat map displaying the pairwise correlation between potential risk factors in Slovakia (A), Romania (B) and (C) Baltic States
2.6. Modelling the effectiveness of measures applied in dedicated zones to stop the spread of African swine fever in wild boar when bordering with a region of limited control
The wild boar habitat model by ENETWILD‐consortium et al. (2020) was used to map the spatial structure of the relative wild boar abundance distribution. The map was converted into the breeding capacity raster of 2 × 2 km (Western and Central Europe), respectively, 3 × 3 km (Eastern Europe) according to the proportionality parameter BCconversion [BreedingCapacity (per cell) = BCconversion × RelAbundance (per km2)].
Hunting bag data from Germany and the Baltic States were used to validate the conversion of the ENETWILD input data.
3. Methodologies
3.1. Descriptive epidemiology – TOR 1
3.1.1. Update the ASF situation in EU Member States and neighbouring countries
A short update on the ASF epidemic in each of the 11 affected EU MS, and in two neighbouring countries, was provided for the current reporting period.
Based on the data extracted from ADIS (see Section 2.1), the total numbers of cases and outbreaks since the first confirmation and for the reporting period were provided; maps were created in R using package ggplot2; and a video was made for displaying the spatio‐temporal spread of the disease using package gganimate.
Based on the ASF surveillance data submitted to the DCF (see Section 2.2) by the EU MS and neighbouring countries, summary information was provided on the active and passive surveillance activities in wild boar and domestic pigs, for the whole country and for the NUTS 3 areas with at least one positive test ELISA or PCR result for ASF. The following definitions were used in the update on the surveillance activities:
Active surveillance of domestic pigs: testing of samples taken from apparently healthy pigs before transport from and within restricted zones, and samples taken from pigs in the protection and surveillance zone of confirmed outbreaks.
-
Passive surveillance in domestic pigs included both the enhanced passive surveillance and the clinical passive surveillance activities in domestic pigs.
-
–
Enhanced passive surveillance in domestic pigs: the routine testing of two pigs found dead per epidemiological unit per week for ASFV (Regulation 2021/605).
-
–
Clinical passive surveillance in domestic pigs: testing of pigs because of clinical suspicion of ASFV infection.
-
–
Active surveillance in wild boar: testing of samples taken from wild boar that were hunted or killed in road accidents. 14
Passive surveillance in wild boar: testing of samples taken from wild boar that were found dead or wild boar that were killed because of ASF clinical signs.
In addition, a short summary was provided by the affected EU MS and two neighbouring countries on the new developments of the epidemic during the reporting period; if specific prevention and control measures were implemented during the reporting period in addition to those already laid down in the EU legislation; as well as a summary of the findings during the epidemiological investigations on outbreak farms (if available).
A list of potential risk factors, based on previously investigated risk factors (Boklund et al., 2020; EFSA, 2020), were provided to the affected countries and who calculated the proportions of affected farms that observed the presence of the potential risk factor during the epidemiological investigations on outbreak farms. If exact proportions could not be calculated, it was indicated that the numbers were estimations. It should be noted that information on the proportions of the presence of those potential risk factors on farms where the disease was not observed was not available, which would be essential to make any inference on association of the risk factor and the presence of the disease. Nonetheless, the information on the proportions of the presence of those potential risk factors was included, as it could provide an insight into which potential risk factors were rarely or never observed.
3.1.2. Time profile of proportions of positive samples tested with Ab ELISA or PCR in wild boar hunted and found dead
The proportion of positive samples reported through the DCF (either tested by PCR or Ab ELISA) was calculated as the number of positive samples divided by the total number of samples tested (either through active or passive surveillance) per month, in the affected MS. As there was no consistent reporting of results of the IB or IPT confirmatory tests, the results of the ELISA tests were used as results for the serology results. Generalised linear mixed models including restricted cubic splines (Perperoglou et al., 2019) were fitted to estimate the average profiles describing the global trends of the PCR‐ or Ab ELISA‐positive samples. Confidence bands are also presented to show uncertainties in the estimation of the smoothing curves.
The time profiles were provided per country displaying the proportion of positive samples from only the affected NUTS 3 areas, where at least one positive case has been found, from the first positive detection in that NUTS 3 area onwards. The time profiles were created on NUTS 3 level from year 2016 onwards. The affected regions only contributed to the estimation of proportion of positive samples in the months after the first infection was found in that country.
3.1.3. Seasonality of proportions of positive samples in wild boar hunted and found dead
The seasonal patterns of the numbers of cases reported through EFSA’s DCF were analysed. Therefore, the data were aligned according to geographical location (NUTS 3 region where the sample was taken), the sampling date and the final test result (for this analysis, a sample was considered an ASF case in a wild boar if it tested PCR positive). ELISA‐positive results were not considered given that seropositivity reflects historical rather than recent infection, and because surviving animals can be assumed to remain seropositive for a long time. Each NUTS 3 region was included from the month on which the first positive sample was reported for that NUTS 3 region, i.e. the starting date. Previous negative reports for that region were excluded from the analysis. Generalised linear mixed models including restricted cubic splines (Perperoglou et al., 2019) were used to estimate the average profiles describing the global trends of the PCR‐positive samples. Confidence bands (CI 95%) are also presented to show uncertainties in the estimation of the smoothing curves.
3.1.4. Trend of yearly wild boar density in previously or currently affected EU Member States and selected neighbouring countries
Wild boar data from countries where ASF were reported in wild boar in Europe were aggregated at country level and standardised to facilitate the identification of potential trends in population density. Temporal graphs were created for four groups of geographic locations: (1) Baltic States (Estonia, Latvia, Lithuania), (2) Central European ASF‐affected countries (Belgium, Czechia, Germany, Hungary, Slovakia and Poland), (3) South‐East European ASF‐affected countries (Romania, Bulgaria and Serbia) and (4) East European counties (Belarus, Russia and Ukraine). A graphical indication was added in each graph to represent the moment ASF was firstly reported in wild boar in the region.
3.1.5. Secondary cases network
The location and confirmation date of each case reported through ADIS was used to build the Directed Acyclic Graph (DAG) representing the network connecting nodes and directed edges (Thulasiraman and Swamy, 1992). This network represents the potential parent–child relationship between the nodes. The wild boar cases reported were sorted by confirmation date. Starting from the first reported case (considered to be the source), the distance to each subsequent case that occurred in a window of 60 days (observations a month apart from each other were calculated using great circle distance), in accordance with Barongo et al. (2015). Only those that were in a band of 1 km to the closest outbreak (represented by the blue band around the red point) from the potential source (represented by the red point) were considered to be linked to the source outbreak. This figure was in line with a median velocity of spread calculated for Belgium, Czechia, Estonia, Hungary, Latvia, Lithuania and Poland between 2.9 and 11.7 km/year (EFSA, 2020), or ~ 0.25–1 km per month. Once a source was identified, no other source could be linked to the recipient node. A schematic representation of the procedure followed is presented in Figure 2.
Figure 2.
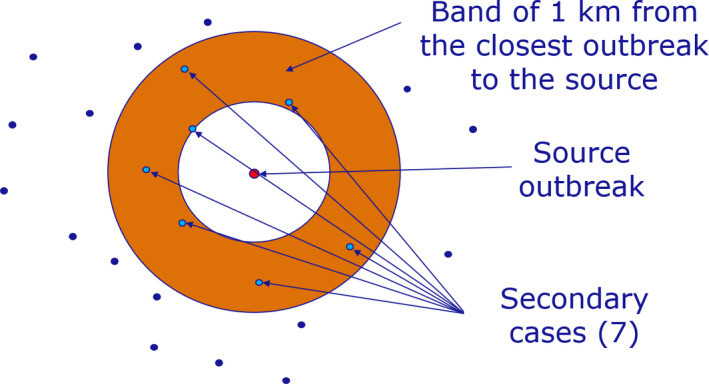
Schematic representation to build the DAG network and calculate the number of nodes connected to each source of infections
Once the DAG was built, the number of edges coming out from all sources of infections were calculated and this information was used to build a frequency table of notifications that could be classified as potential secondary infections to the source cases. Finally, bootstrapping (a total of 100,000 bootstraps) was used to quantify uncertainty around the mean of secondary cases obtained from the DAG built.
3.2. Risk factor analysis – TOR 2
3.2.1. Literature review
A literature review was performed to identify the factors (either risk or protective factors) associated with the occurrence, reoccurrence, spread and persistence of ASF in domestic pigs and wild boar populations according to the EFSA Guidance document (EFSA, 2010). The review and extraction data protocol has been developed by EFSA in accordance with the Working Group (WG).
3.2.2. Generalised linear model to analyse risk factors for the occurrence of ASF in wild boar in Slovakia
The data reported by Slovakia through the DCF were used to build a binary indicator per district, which takes the value 0 if no sample was PCR positive for ASF in wild boar was reported for that district, and 1 if at least one PCR‐positive sample was reported in the year 2020 for that district. For each district, data available on hunting activity (total wild boar hunted and number of dogs used), pig farming (total pig density and total farms density, as well as density of small farms (< 10 animals) and density of pigs in small farms per district), ASF occurrence in domestic pigs in the district (as binary variable based on the presence/absence of a PCR‐positive test result in domestic pigs per district), environmental information (temperature, croplands, herbaceous cover, urban areas, waterbodies, altitude and suitability scores) as well as information on human footprint index in the area, were used to explore the potential effect on the probability of observing ASF in wild boar in the district. Details of data sources can be found in Table 1.
Only 1‐year data were available for Slovakia for all the covariates of interest at the desired spatial resolution of the analysis (district). Therefore, a generalised linear model was chosen to analyse the risk factors for ASF occurrence in wild boar in Slovakia and explore the effect of the covariates. A backward selection procedure was applied to eliminate covariates in the model that were not significantly (p > 0.05) associated with the presence of at least one ASF PCR‐positive result in wild boar in a district. The significant factors and their odds ratios are presented in Table 7 and the proportion of ASF PCR‐positive results in wild boar in Slovakia per district is presented in a choropleth map (Figure 1).
Table 7.
Results of generalised linear model to estimate the probability to observe a PCR‐positive results in wild boar in Slovakia districts based on analysis a set of potential covariates
| Odds ratios | p‐value Wald | p‐value Likelihood ratio | |
|---|---|---|---|
| Crops | 800.15 (11.62, 55,084.74) | 0.002 | < 0.001 |
| ASF in domestic pigs | 18.56 (1.75, 196.58) | 0.015 | 0.003 |
| Wild boar abundance | 116.36 (1.08, 12,570.05) | 0.046 | 0.032 |
| Urban habitat surface | 0 (0, 0.01) | 0.032 | 0.018 |
| Number of hunting dogs a ) | 0 (0, 0.96) | 0.048 | 0.034 |
Hunting dogs in Slovakia are only used to hunt wild boar in areas without ASF.
3.2.3. Besag York Mollié model to analyse risk factors for the occurrence of African swine fever in wild boar in Romania and the Baltic States
For Romania, 4 years of data at the desired spatial resolution were available and, for the Baltic countries, 3 years. Therefore, the Besag York Mollié (BYM) model was chosen for both analyses to be able to include the time effect in the analyses. BYM is a lognormal Poisson model, which includes both an intrinsic conditional autoregression for spatial smoothing and an ordinary random‐effects component for non‐spatial heterogeneity. Details on the models used can be found in EFSA (2017) and in the Zenodo repository (Varewyck et al., 2017).
Section 2.5 describes how the data were aggregated and assessed for collinearity to ensure independence between the covariates used in the model. Then, the model was fitted with the remaining potential risk factors (Table 1) aggregated per county, to evaluate their effect on the probability of occurrence of ASF in wild boar in the different counties (NUTS 3) in Romania and in the Baltic States. Using a backward elimination procedure, the potential risk factors were removed one by one, if their significance level was p > 0.05, given their lack of significant contribution to model the probability of presence of ASF cases in the county.
3.3. Modelling the effectiveness of measures applied in dedicated zones (‘white zones’) to stop the spread of African swine fever in wild boar when bordering with a region of limited control
In a white zone (WZ), measures are undertaken to reduce the wild boar population to prevent the situation that ASF would spread further from an adjacent ASF‐affected area. These measures are preparing the WZ to act as buffer towards other ASF‐free areas where at that time no control measures are implemented. The intended functionality of the WZ inherently foresees that ASF might enter but, given that the preparation of the WZ is appropriate, the infection is not expected to spread beyond the wild boar population in the WZ. In other words, a WZ (or sometimes called ASF‐free management area or negative area) still remains functional, even if no longer ‘white’, ‘ASF‐free’ or ‘negative’. Inside an effective WZ eventually the infection is expected to fade out. Nonetheless, in practice, WZs usually will be extended (precautionary), once ASF enters the originally delineated white zone.
These principles are basic to the methodology described in the following sections and the assessment of the capability of WZ measures to control the spread of ASF.
3.3.1. Principle scenario
The problem addresses a specific epidemiological situation, which is new compared with previous literature and EFSA outputs. Figure 3 illustrates the general situation and sets definitions of concepts used in this report.
Figure 3.
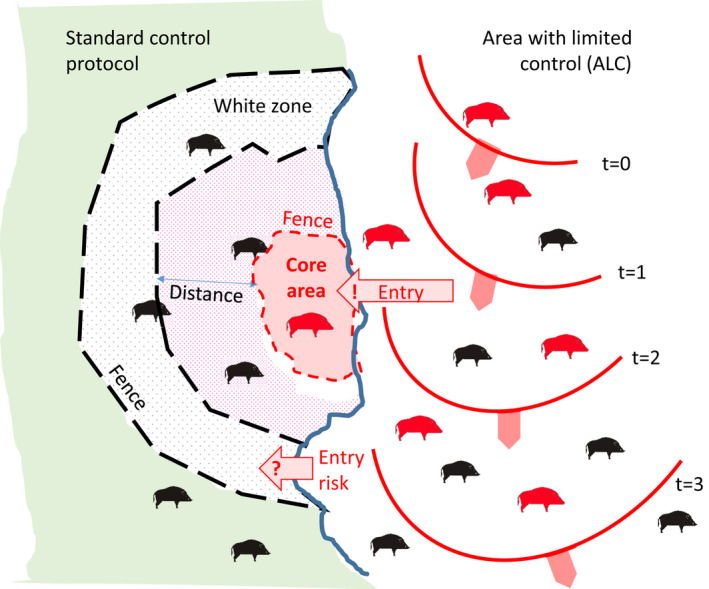
- The wild boar habitat is separated into an area with less intensive control measures, and an area with intensive control measures. The separation line between the two areas (bold blue line) symbolises the presence of a certain demarcation (e.g. a fence). Red animals symbolise infection and the red waves the advance of the epidemic over time. In the scenario, mobile or fixed fences (black dashed lines) are set up as a response to an ASF intrusion in the wild boar habitat.
Although there will be different configurations, the basic principle is reflected in Figure 3. The total wild boar management area is divided by the blue demarcation line (which might include a physical barrier, e.g. a fence) into an area where no or less intensive, and therefore, insufficient control measures are implemented [right; below called an adjacent area with limited control measures (ALC)], and an area where more intensive control measures have been put in place (left). In the left part, different control schemes are applied, combining available measures for the control of ASF in a wild boar population, i.e. fencing, intensive population reduction and carcass removal. Within the right part, the limited control of ASF in wild boar results over time in continued circulation and spread of ASF within the population from upper to lower down in the figure along the blue demarcation line between the right and left parts.
Within the left part, the standard control measures are implemented (based on the measures applied in EU Member States after a focal introduction), which include an – usually fenced – infected core area (CA) trying to contain animals inside and avoid outwards movements and the WZ – also usually fenced – prepared for intensive population reduction in short time.
The outer green area symbolises the part that should be protected from ASF incursion by the set of measures applied to the WZ. Additionally, the distance between the CA and WZ needs to be determined (arrow in Figure 3). However, as reported by EFSA (2021b), there is the risk that the WZ does not halt the infection, if it was placed too close to the CA, because the target WB population density to be reached with the measures in the WZ is not accomplished in the time before the infection arrives at the WZ (Lange et al., 2021).
The problem of this epidemiological situation is, however, that, unlike for a focal introduction, there is a risk that the disease may enter from all over the length of the bordering demarcation line with the ALC. The main question, therefore, is whether the approaches that have proven to be effective after a focal introduction (in Belgium and Czechia), i.e. a CA surrounded by WZ at a certain distance, can be similarly parametrised on size and intensity of the measures in this specific epidemiological situation.
The schematic representation indicates the variable parameters that will be analysed as follows. Three parameters specify the effort put into the WZ implementation and determine the probability of stopping ASF inside the WZ: (1) the width of the WZ, (2) the targeted wild boar density inside the WZ and (3) the time interval within which the targeted wild boar density must be reached.
Additionally, the WZ can be surrounded by a fence, which may have unknown fence permeability. The distance between the WZ and the CA results from the velocity of spread of the ASFV infection and the WZ parameter of the time interval until the measures are implemented inside the WZ. Carcass search and removal is optionally considered.
3.3.2. Spatially explicit stochastic model
The detailed situation per MS was implemented in a spatially explicit stochastic individual‐based model. The model is developed to simulate spread and control of ASF in wild boar in structured landscapes of wild boar habitat. The tool was used in support of previous EFSA output on ASF in wild boar and, in particular, for a principal assessment of the capacity to manage ASF spread in alternative scenarios [i.e. large‐scale front, EFSA AHAW Panel (2015) and EFSA (2017), or focal introduction EFSA (EFSA, 2018)]. The disease component of the model was updated with knowledge on ASFV infection and epidemiology as reviewed in EFSA (2021b). The updated standardised model documentation (ODD protocol; Grimm et al., 2006; Grimm et al., 2010) is available from https://ecoepi.eu/ASFWB/WZ.
A model framework has been developed and applied in the context of multiple infectious diseases of wild boar, i.e. CSF, FMD and ASF. The model consists of (1) an ecological component detailing processes and mechanisms related to the ecology, sociology and behaviour of wild boar in natural free‐roaming populations of the species Sus scrofa; (2) an epidemiological component reflecting individual disease course characteristics and transmission pathways including direct contact transmission on different spatial scales and environmental transmission caused by ground contamination or contacts with carcasses of succumbed infected host animals; and (3) a management component implementing surveillance and control scenarios in a spatio‐temporal explicit manner. The model is stochastic in relation to all three components and parametrised using reported distributions from published literature including variability and uncertainty. The model population emerges from birth and death probabilities depending on habitat quality on the level of individual social groups.
The component representing wild boar ecology was validated independently of ASF in terms of habitat use predicted by the model rules on reproduction, breeding capacity and subadult dispersal. Validity of predictions was field verified with spatial distribution of opportunistic sightings of wild boar in Denmark (Moltke‐Jordt et al., 2016). Moreover, the model was shown to accurately predict geographical disease spread and time of infection circulation if the modes of infection and transmission are conceptually understood (EFSA AHAW Panel, 2012; Dhollander et al., 2016).
The model uses habitat maps to represent population distribution and dynamics. These maps determine local reproduction and density variations (ENETWILD‐consortium et al., 2020). Maximum abundance or density is calibrated to estimations provided by the MS (EFSA, 2021a). Finally, the data provided by the MS on hunting records and carcasses found in and around the WZ were used to validate or adjust the population numbers emerging from the model habitats.
On the geographic landscape, the spread of ASF is initialised according to the ADIS notifications and simulated considering relevant human‐mediated translocation events until the date when the WZ was set up. From this time forwards, ASF spread is simulated, and control efforts applied to the WZ including fencing, ASF‐related excess hunting, depopulation activities and carcass search/removal. The purpose is to investigate each WZ under the epidemiological situation for which it was set up, and in which one possible outcome was already known from the field.
Model output is compiled to derive:
the likelihood of the observed outcome with a particular WZ (post hoc);
the probability of successful control over time of the applied measures.
Dynamic visualisations of example simulation output are available from https://ecoepi.eu/ASFWB/WZ.
3.3.2.1. Transmission model of ASF infections in wild boar
The basic principle of transmission relates to the number of adjacent/in contact animals and carcasses using event probabilities, i.e. each infectious object provides a chance of transmission to every susceptible animal sufficiently close.
Wild boar are acknowledged to organise in a matriarchal structure with female groups of strong kinship and satellite solitary movement and temporary aggregation of males with sow groups. Consequently, the wild boar–ASF system comprises three potential modes of transmission, i.e. between live animals of the same social group (within‐group transmission), between live animals of different groups (between‐group transmission) and between carcasses of animals that have succumbed to the infection and live animals (carcass‐mediated transmission). The conceptual framework of multimodus transmission was set up during past usage of the model (Kramer‐Schadt et al., 2009; Lange and Thulke, 2017; Lange et al., 2018) and recently validated by an ecological study of contact frequency within and between social groups (Podgórski et al., 2017). Details of the modes of transmission related to ASFV were studied also by Pepin et al. (2020).
Parametrisation of the modes of transmission is based on multiple sources. Quantitative experimental data are accessible for within‐group transmission, i.e. animals in permanent contact with groupmates (transmission trials; see review in EFSA, 2021b). Between‐group transmission was parameterised relative to the within‐group transmission and reversely calibrated against the speed of propagation (Lange et al., 2018). Evidence on the role of carcasses of animals dying as consequence of an ASF infection is very experimental, including the potentially contaminated soil underneath (Probst et al., 2017, 2020). Given the assumption that carcass‐mediated transmission is relevant, insights exist on the likely volume of carcass‐based transmission in the spread of ASF (Pepin et al., 2020). Based on the reverse parametrisation procedure by Lange and Thulke (2017), ubiquitous access to dead animals (i.e. not hiding or retreating due to morbidity) but very seldom contacts that may warrant transmission (blood, secretions or body fluids), has to be modelled to reconstruct observed spatial spread patterns.
3.3.2.2. Simulation protocol
The simulations were performed using data from real habitat geography. Habitat maps for selected terrestrial Europe (Figure 4) are derived from the habitat model according to the ENETWILD‐consortium et al. (2020). The landscape is calibrated to generate the ENETWILD information in terms of spring population density in the WZ before ASF.
Figure 4.
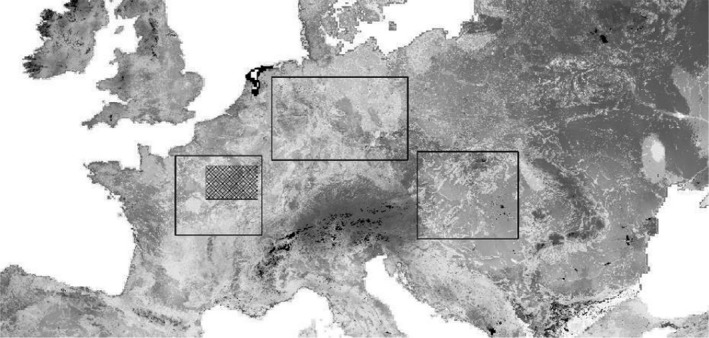
- Without randomised simulation landscape, the model refers to one arbitrarily chosen but fixed rectangle of the middle box.
For every scenario and parameter combinations, either 300 model runs were performed on one a selected landscape (fixed landscape) or one model run was performed per 1,000 randomly selected landscapes (randomised landscape; Figure 4). In all simulations, however, the size of the simulation area was fixed and equal. This facilitates the direct comparison of model outputs regarding space–time interaction in a finite simulation area.
The infection was released in the north‐eastern part of the simulation landscape (Figure 5). The simulated infection expands in westward and southward directions approaching continuously the demarcation line (see Figure 3). When the infection was close enough to the demarcation line, the WZ was set up, and measures were implemented so that after the preset time horizon, a preset target population density would be reached.
Figure 5.
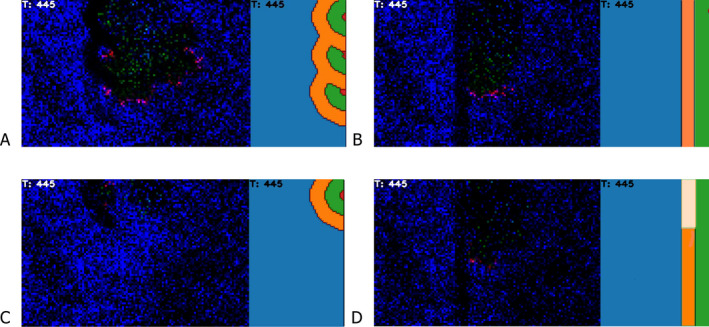
Snapshot of the spatial model output (left per scenario) and the associated layout of the control measures (right) for the different scenarios A, B, C and D
The population reduction measures are implemented with two parameters: (1) the duration of campaigns, which is the time per measure to achieve a specified population density target, and (2) the interval between campaigns determining after what time a repeated campaign with the same target will be reapplied to maintain the population inside the WZ at the target density. Fences are optionally constructed around both the CA and the WZ. Fences are simulated with different permeabilities of 0, 10 and 100% (see Lange and Thulke, 2015).
Scenario A: ‘Reactive WZ next to ALC’: Every new introduction is responded by the establishment of a new individual concentrical WZ.
Scenario B: ‘Proactive WZ’: As soon as the infection enters the green rectangle, a WZ is implemented as block from top to bottom and maintained until the end of the simulation.
Scenario C: ‘Reactive WZ focal’: As in Scenario A, but at the moment of entry of ASF and the establishment of the WZ, further transmission in the adjacent limited control part is supressed to compare the effect of the measures in the WZ with ALC to those implemented after a focal introduction.
Scenario D: ‘Proactive WZ roll‐back’: As in Scenario B, but with segmentwise release of the measures in those segments in the WZ, where there is no more infection observed for a long time.
The left side in each panel shows the model landscape of wild boar (bright blue spots = uninfected wild boar; the red spots = infectious wild boar, green spots = seropositive wild boar and black spots = succumbed or hunted wild boar), brightness of colours relates to the number of individuals in the location.
The right sides of the panels show the layout of the management zones at the moment when the spatial snapshot was taken, i.e. the core area (red), the WZ (orange) with the distance between CA and WZ reflected in green.
The simulation considers four scenarios (Figure 5). In Scenario A (= reactive WZ), measures are implemented for every introduction of ASF into the left part of the simulation area (Figure 3). Several introductions can happen during one simulation run, which can be expected to be due to the limited control measures and continued spread in the right part.
Comparing Scenario A with Scenario C facilitates the comparison of the two epidemiological situations [neighbouring affected ALC (Scenario A) vs. focal introduction, initially without ASFV infections outside of the CA (Scenario C)]. The outcomes to compare are the overall failure rate of the measures and the number of wild boar culled. In Scenario C, transmission is disabled in the right part of the simulation landscape (i.e. the limited control part) right after the fifth wild boar in the other, the left part of the landscape, was infected. The purpose is to mimic a focal introduction.
Scenario B (= Proactive Scenario) immediately places a block‐like WZ along the complete demarcation line as soon as the infection enters the green rectangle. The block‐like WZ was maintained until the end of the simulation. In Scenario D (= proactive WZ roll‐back), the WZ was rolled back segmentwise, depending on the case distribution in the neighbouring affected ALC measures. The simulation landscape was compartmentalised into horizontal segments (spanning ‘perpendicular’ to the direction of spread). If a segment became ASF‐free after being ASF positive, the part of the proactive WZ intersecting with the segment was released from measures (rolled back).
4. Assessment
4.1. Descriptive epidemiology – TOR 1
4.1.1. Update on the ASF situation in the EU
Table 2 displays the notifications in the previous reporting period (from 1 September 2019 to 31 August 2020) and the current reporting period (from 1 September 2020 to 31 August 2021). In the current reporting period, ASF was confirmed in Germany on 10 September 2020. Belgium submitted a self‐declaration to the World Organisation for Animal Health (OIE) to obtain the Freedom of Disease status on 1 October 2020. There were no outbreaks in domestic pigs nor cases in wild boar reported in Greece, and only cases were found in wild boar in Hungary and Lithuania during the reporting period. In Romania, an increase in outbreaks of 57% was observed compared with the previous reporting period, with a noticeable increase in outbreaks in large commercial pig farms, which is dealt with in more detail in Section 4.1.3.
Table 2.
Number of African swine fever virus genotype II outbreaks in domestic pigs and cases in wild boar notified to the Animal Disease Information System up to 31 August 2021
| Country* | Date of first confirmation in the country | Date of obtaining Freedom of Disease status | Number of outbreaks a in domestic pigs in period | Number of cases b in wild boar in period | ||||
|---|---|---|---|---|---|---|---|---|
| Previous reporting period | Current reporting period | Previous reporting period | Current reporting period | |||||
| EU | ||||||||
| 1 | Belgium | 13/9/2018 (WB) | 1/10/2020 | 0 | 0 | 6 | 0 | |
| 2 | Bulgaria | 31/8/2018 (DP) | NA | 27 | 5 | 476 | 326 | |
| 3 | Czechia | 26/6/2017 (WB) | 19/4/2019 | 0 | 0 | 0 | 0 | |
| 4 | Estonia | 8/9/2014 (WB) | NA | 0 | 1 | 59 | 70 | |
| 5 | Germany | 10/9/2020 (WB) | NA | 0 | 3 | 0 | 1,872 | |
| 6 | Greece | 5/2/2020 (DP) | NA | 1 | 0 | 0 | 0 | |
| 7 | Hungary | 21/4/2018 (WB) | NA | 0 | 0 | 3,934 | 3,082 | |
| 8 | Latvia | 26/6/2014 (DP and WB)** | NA | 3 | 2 | 310 | 319 | |
| 9 | Lithuania | 24/1/2014 (WB) | NA | 7 | 0 | 244 | 177 | |
| 10 | Poland | 17/2/2014 (WB) | NA | 80 | 101 | 3,621 | 3,070 | |
| 11 | Romania | 31/7/2017 (DP) | NA | 1,043 | 1,637 | 810 | 1,081 | |
| 12 | Slovakia | 24/7/2019 (DP) | NA | 11 | 13 | 164 | 1,605 | |
| Neighbouring EU | ||||||||
| 1 | Ukraine | 07/1/2017 (DP) | NA | 22 | 17 | 5 | 3 | |
| 2 | Serbia | 31/7/2019 (DP) | NA | 13 | 36 | 41 | 71 | |
| 2 | Russia | 1/1/2001(DP) | NA | 234 | 171 | 150 | 306 | |
| Total | 1,443 | 1,987 | 9,849 | 11,982 | ||||
DP: domestic pigs; WB: wild boar.
An outbreak of ASF in domestic pigs refers to one or more confirmed cases detected in a pig holding.
Both seropositive and virus‐positive wild boar are included among ‘cases’.
Only countries where ASFV Genotype II outbreaks or cases have been reported to the ADIS up to 31 August 2021 are listed in the table, and only the outbreaks and cases reported to ADIS are listed.
The first cases in wild boar and outbreak in domestic pig were detected on the same day.
In Germany, although the disease was introduced at the beginning of reporting period, 1,872 cases were already reported in wild boar within the first year.
Figures 6 and 7 display all the ASF cases in wild boar and outbreaks in pigs in the EU and neighbouring countries, reported to the ADIS up to 31 August 2021. The maps show spread of the disease over the years since it entered the EU in 2014.
Figure 6.
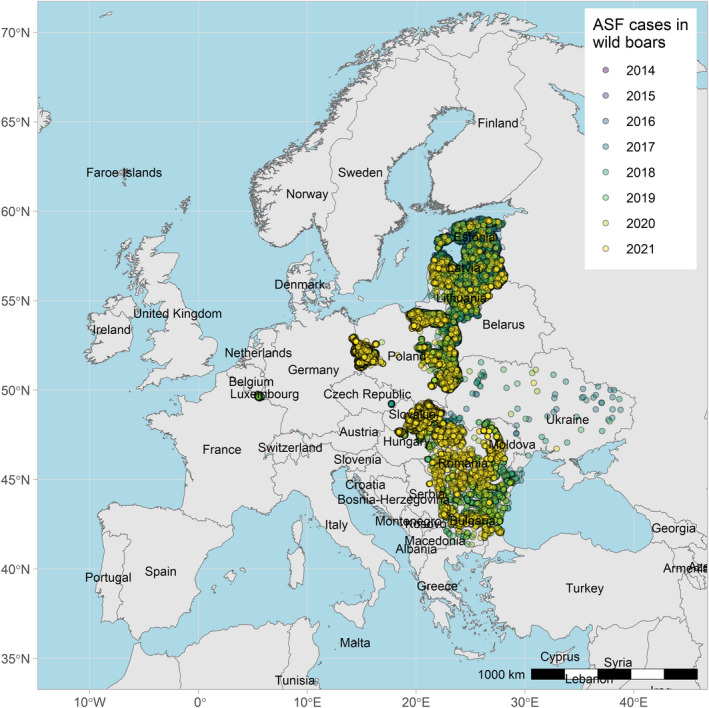
ASF cases in wild boar in the EU and neighbouring countries, reported to the Animal Disease Information System up to 31 August 2021
Figure 7.
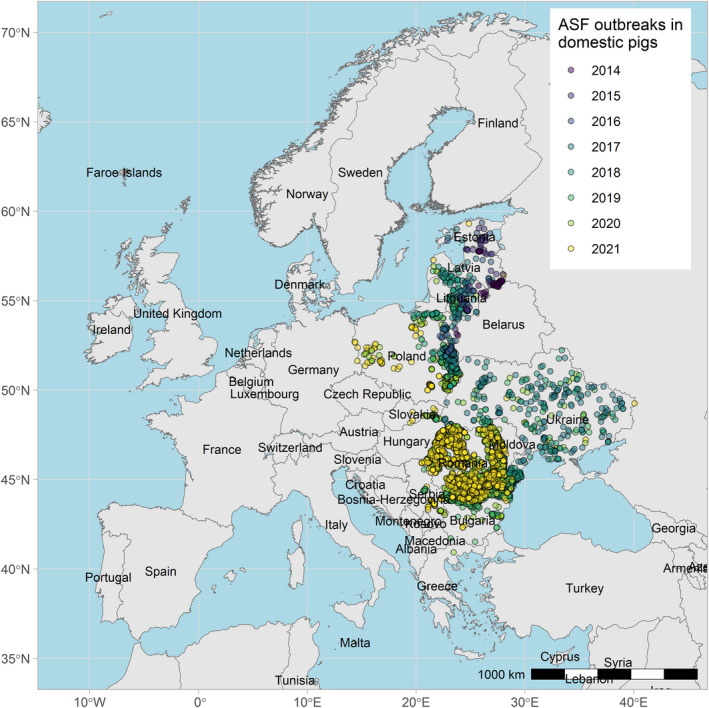
ASF outbreaks in the EU and neighbouring countries in domestic pigs reported to Animal Disease Information System up to 31 August 2021
4.1.2. Update on the ASF situation in the individual affected Member States and neighbouring countries
4.1.2.1. Belgium
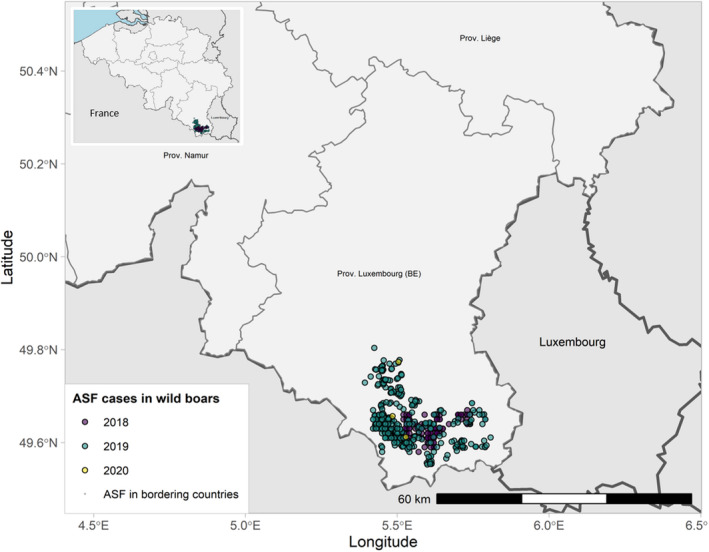
Figure 8 ASF cases in wild boar in Belgium reported to the Animal Disease Information System up to 31 August 2021
| Surveillance in NUTS 3 areas with at least one ASF notification during the reporting period | No cases of ASF in wild boar nor outbreaks in domestic pigs were reported during the reporting period. |
| New developments of the epidemic in the reporting period | Belgium is officially free from African swine fever for all Suidae since 1 October 2020 in accordance with Chapter 15 of the OIE Terrestrial Code. |
| Specific prevention and control measures implemented during the reporting period |
Measures implemented after regaining Freedom of Disease (FOD) status 1. Preventive measures and surveillance in domestic pigs Measures are carried out by the Federal Agency for the Safety of the Food Chain (FASFC) in the whole country and the Belgian legislation has been adapted. Several measures that were put in place during the crisis (enhanced passive surveillance, strict biosecurity procedures and active surveillance) have been maintained. The annual evaluation of the biosecurity measures in each pig holding (BioCheck) is mandatory since January 2021. Restocking of pig farms in the former infected area is allowed since January 2021. 2. Preventive measures and surveillance in wild boar Measures are carried out by the regional authorities (Public Service of Wallonia). 2.1. From the early days of the outbreak, infected forests had been completely closed for walking and professional activities. From 2020 onwards, the bans were progressively lifted by maintaining biosecurity rules. Free access to the forest was allowed in April 2021 with respect to the forestry code. 2.2. Active and scheduled search for dead wild boar was still organised by the regional authorities over the two former ASF zones until end of March. From April 2021, the search effort was maintained until end of August but with a lower search rate associated with fences inspection activities (A. Licoppe, personal communication). Places where alive wild boar were detected by the network of cameras were targeted for search. 2.3. All fences (approximately 300 km in and around the two former ASF zones) were maintained, checked and repaired during the reporting period. The three carcass collection centres set up at the beginning of the crisis remained functional until end of March 2021. From April 2021, the veterinary teams kept open only the main centre in Virton. 2.4. The regional authorities maintained the depopulation measures in the two former ASF zones during the reporting period and the regional legislation was adapted (Walloon government decree, July 2020). Night shooting and trapping were suspended on 31 August 2020 in anticipation of the hunting season. The results of driven hunts were poor for different reasons, including low densities of wild boar, lack of motivation of the hunters and the COVID‐19 crisis. In mid‐November 2020, night patrols were re‐launched in the agriculture area. Both night shots and trapping were intensified in February/March and stopped from 1 April 2021. Night shooting could be re‐activated by the regional authorities in case of resurgence or to impose the active surveillance. In conclusion, during the reporting period (September 2020 to August 2021), 280 wild boar were analysed, and all virological results were negative (Sciensano, B. Cay, personal communication). |
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
| Spread of the disease |
|
|
| NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance in whole country (samples tested for ASF) during reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 52 | 0 | PCR | 4,832 | 0 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 228 | 0 | PCR | 32,682 | 0 | |
| ELISA | ND | ND | ELISA | 1,416 | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Surveillance in NUTS 3 areas with at least one ASF notification samples tested for ASF) during the reporting period** | No notification during the reporting period | |||||
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
**: Sample data, as described in Section 2.2 were not submitted to EFSA’s Data Collection Framework, but aggregated data have been provided directly by the Belgian WG member to complete this table.
Passive surveillance in WB in the whole country (total 52): n = 8 (former Part I/II), n = 23 (the rest of Wallonia) and n = 21 (Flanders).
Other isolated bones (n = 27) were found in the former Part I/II due to active search but they could not longer be analysed because of advanced decay.
Active surveillance in WB (total 228): n = 220 (shot at night, trapped, culled or road victims in the former Part I/II) and n = 8 (sanitary shots in the rest of Wallonia).
4.1.2.2. Bulgaria
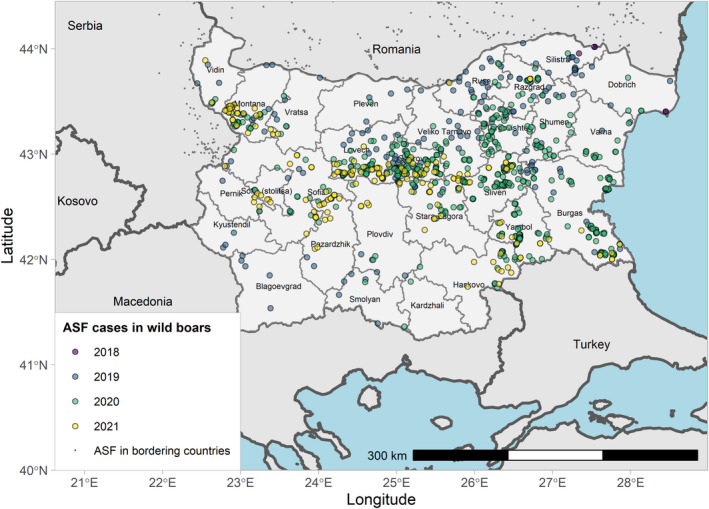
Figure 9 ASF cases in wild boar in Bulgaria reported to the Animal Disease Information System up to 31 August 2021
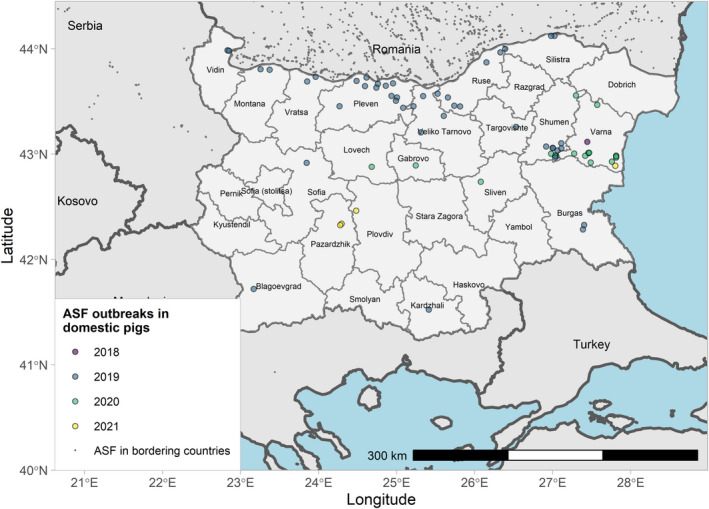
Figure 10 ASF outbreaks in Bulgaria in domestic pigs reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period |
Overall, there were fewer outbreaks observed in domestic pig farms in the current reporting period. There were five outbreaks in the reporting period: two outbreaks in industrial farms, one outbreak on a family farm, one case in an East Balkan pig farm and one outbreak in a backyard farm. In total, 13,732 animals were affected. The ASF outbreaks in domestic pigs were all confirmed in the autumn, between August and early October. A declining trend of the detection of ASF‐positive wild boar cases was observed as well, compared with the data of the previous reporting period. A decrease in the cases in north Bulgaria was recorded; Geographically, ASF‐positive wild boar were confirmed in the middle part of the country, in contrast with the situation in the previous reporting period, when ASF was observed mainly in the northern part of the country. This could be due to the movement of wild boar from places affected by African swine fever to previously virus‐free areas. |
| Specific prevention and control measures implemented during the reporting period |
|
| Epidemiological investigations in infected farms during the reporting period |
The main factors playing role as a driver for virus introduction in domestic pig farms were due to human‐mediated spread and low levels of biosecurity. In the industrial farms, good biosecurity infrastructure was in place in both infected farms, but weaknesses were detected in the management and personnel behaviour. Despite this fact, the disease spreads very slowly in the holdings (only one and two epidemiological units infected on the first and second affected farm, respectively). The outbreaks in commercial farms were detected as part of the enhanced passive surveillance, which prove the key role of this surveillance system for early detection of the disease. In both commercial farms infected by ASF, there were dead wild boar found nearby (1.5 km). Neither home slaughter nor swill feeding was carried out on these farms. Introduction of ASF in the East Balkan pig holding and backyard farms was most likely to be due to the breaches in implementation of biosecurity measures. |
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 367 | 328 | PCR | 20,475 | 37 | |
| ELISA | 8 | 0 | ELISA | 148 | 0 | |
| ELISA + IPT | ND | ND | ELISA + IPT | ND | ND | |
| ELISA + IB | ND | ND | ELISA + IB | ND | ND | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 12,671 | 1,167 | PCR | 19,889 | 0 | |
| ELISA | 4,729 | 1 | ELISA | 380 | 0 | |
| ELISA + IPT | ND | ND | ELISA + IPT | ND | ND | |
| ELISA + IB | ND | ND | ELISA + IB | ND | ND | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 138 | 130 | PCR | 269 | 6 | |
| ELISA | ND | ND | ELISA | 0 | NA | |
| ELISA + IPT | ND | ND | ELISA + IPT | NA | NA | |
| ELISA + IB | ND | ND | ELISA + IB | NA | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 2325 | 19 | PCR | 114 | 0 | |
| ELISA | ND | ND | ELISA | 0 | NA | |
| ELISA + IPT | ND | ND | ELISA + IPT | NA | NA | |
| ELISA + IB | ND | ND | ELISA + IB | NA | NA | |
4.1.2.3. Estonia
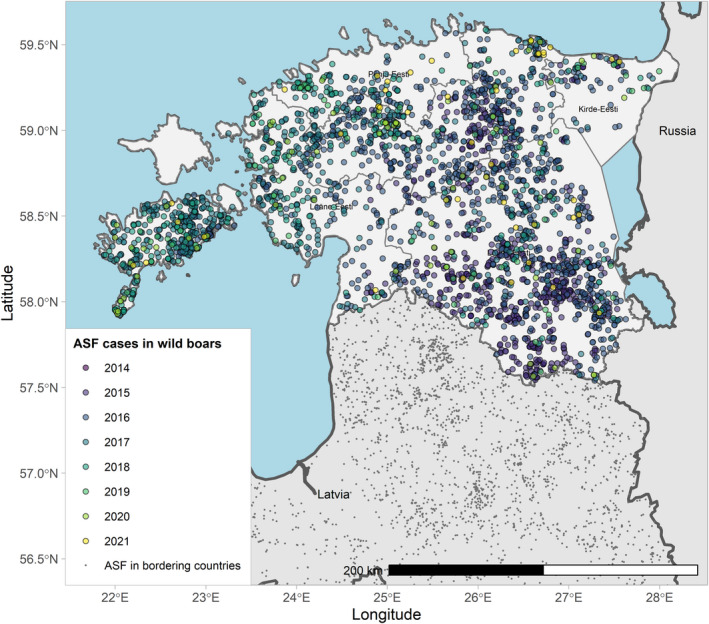
Figure 11 ASF cases in wild boar in Estonia reported to the Animal Disease Information System up to 31 August 2021
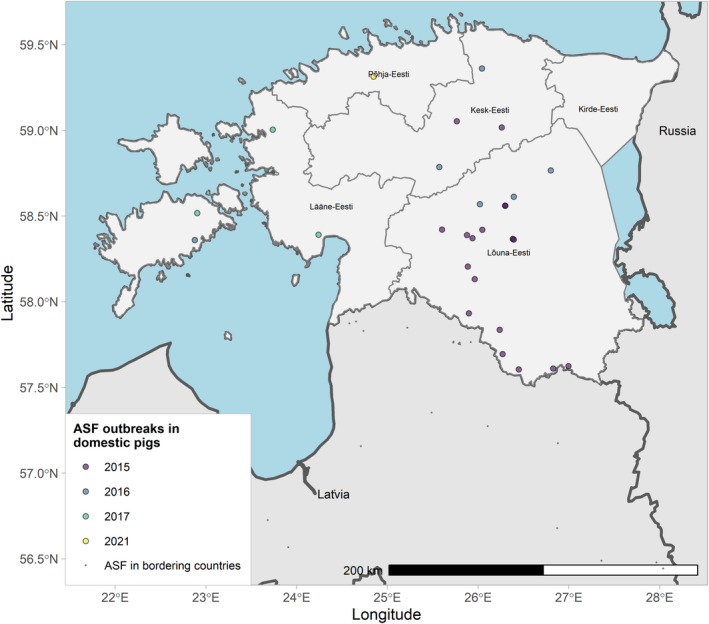
Figure 12 ASF outbreaks in Estonia in domestic pigs reported to Animal Information Notification System up to 31 August 2021
| ELISA + IB | |
| New developments of the epidemic in the reporting period |
After re‐emergence of PCR‐positive wild boar cases in central Estonia (Rapla county) in August 2020, another focus of PCR‐positive wild boar was detected in December 2020 in Lääne‐Viru county 120 km to the north‐east from the previous one (~40 months since last PCR‐positive finding in this county). By the summer of 2021, the Rapla county focus had expanded and reached the neighbouring Harju county in the north. In August 2021, after 4 years without outbreaks in domestic pigs, one commercial domestic pig farm in Harju county became infected in the area with active spread of the virus among wild boar. The disease situation in wild boar may be an indication of a start of the second wave of ASF spread, as the population of wild boar has substantially increased since 2019. |
| Specific prevention and control measures implemented during the reporting period | None |
| Epidemiological investigations in infected farms during the reporting period |
One outbreak occurred during the reporting period. On this outbreak farm, the following observations were made:
There were minor breaches in implementation of biosecurity measures |
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 65 | 20 | PCR | 3,959 | 3 | |
| ELISA | 6 | 1 | ELISA | 158 | 0 | |
| ELISA + IPT | 1 | 1 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 10,081 | 12 | PCR | 1,011 | 0 | |
| ELISA | 10,069 | 75 | ELISA | 61 | 0 | |
| ELISA + IPT | 75 | 64 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 28 | 20 | PCR | 1,213 | 3 | |
| ELISA | 2 | 1 | ELISA | 14 | 0 | |
| ELISA + IPT | 1 | 1 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 1,975 | 12 | PCR | ND | ND | |
| ELISA | 1,976 | 27 | ELISA | ND | ND | |
| ELISA + IPT | 27 | 23 | ELISA + IPT | ND | ND | |
| ELISA + IB | 0 | NA | ELISA + IB | ND | ND | |
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
4.1.2.4. Germany
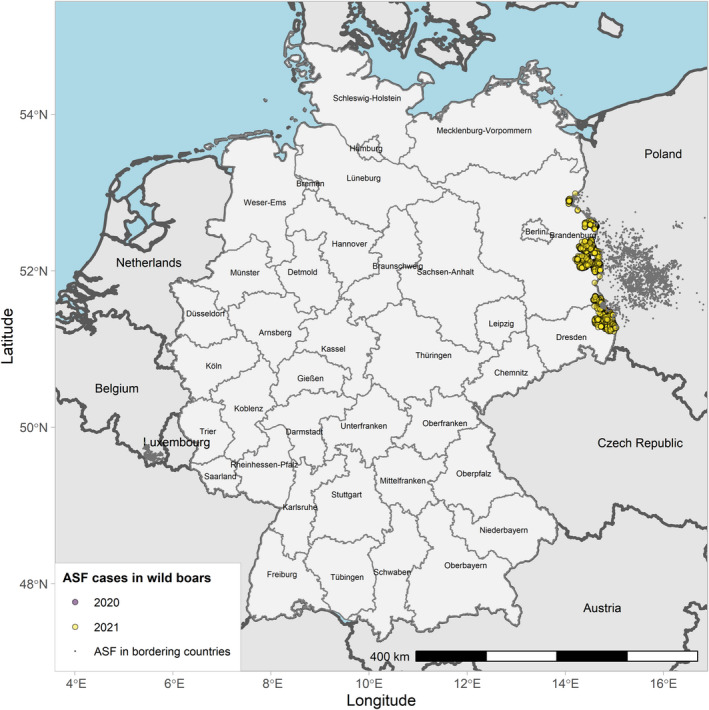
Figure 13 ASF cases in wild boar in Germany reported to the Animal Disease Information System up to 31 August 2021
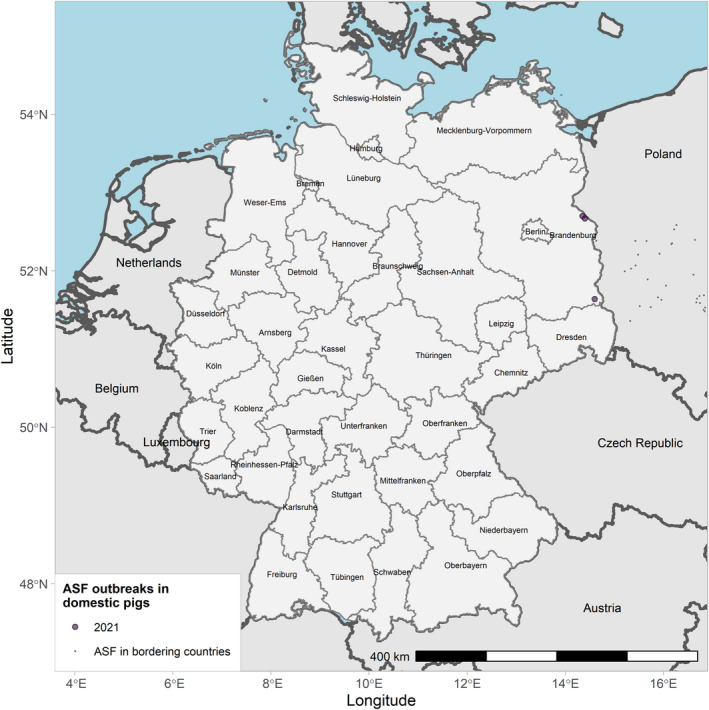
Figure 14 ASF outbreaks in Germany in domestic pigs reported to Animal Disease Information System up to 31 August 2021
| New developments of the epidemic in the reporting period |
Since ASF emerged in western Poland in November 2019, surveillance efforts, in particular examination of wild boar found dead, were intensified in the regions of Germany bordering with Poland. The first case of ASF in wild boar in Germany was detected at approximately 6 km from the Polish border by passive surveillance and confirmed on 10 September 2020. Phylogenetic analysis of the ASFV whole‐genome sequence generated from material of the first carcass detected in Germany revealed that it groups with ASFV Genotype II including all sequences from Eastern Europe, Asia and Belgium. In the weeks and months after the first finding, there were several independent virus entries caused by continuous infection pressure along the border with Poland. Several different clusters could be identified along the border. The course within the individual clusters in Germany was comparable to that in Czechia and Belgium, but the overall situation showed clear differences, due to the multitude of clusters. According to the estimated minimum post‐mortem intervals (PMI), which were estimated from the decomposition of the wild boar carcasses, ASFV was most likely to be introduced into Germany in the beginning of July 2020, at the latest (Sauter‐Louis et al., 2021). |
| Specific prevention and control measures implemented during the reporting period |
After the first case of ASF was detected in wild boar in Germany in September 2020, it was obvious that the successful control measures from Czechia and Belgium were used as orientation for the German control strategy with the aim of preventing further spread of the disease. Therefore, the first control measures focused on defining affected areas, intensifying the search for carcasses and fencing the areas at risks. However, in contrast with Czechia and Belgium, where ASF was locally introduced in a single event, Germany faces a constant infection pressure along the border with the affected region in Poland and the disease has not only been introduced at one point, but in several locations along the border with Germany and on several occasions. Nevertheless, the control measures applied are like those in Belgium and Czechia, including setting up restriction zones, erecting fences (mobile electric fences first, later replaced with solid fences) around the core areas, carcass search (using humans, specially trained dogs, drones, helicopters) and removal of these carcasses as well as reduction of wild boar population around the affected areas. |
| Epidemiological investigations in infected farms during the reporting period |
|
|
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
Date first confirmation: 10/9/2020 Cases since first confirmation: 1,872 Date first case reported in the reporting period: 10/9/2020 Cases during reporting period: 1,872 |
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period** | Passive surveillance in WB* | Surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 10,148 | 1,827 | PCR | 66,197 | 8 | |
| ELISA | 652 | 66 | ELISA | 65 | 1 | |
| ELISA + IPT | 10 | 10 | ELISA + IPT | 1 | 1 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | ||||||
| Diagnostic test(s) used | Total samples | Positive samples | ||||
| PCR | 84,036 | 200 | ||||
| ELISA | 2,772 | 0 | ||||
| ELISA + IPT | 0 | NA | ||||
| ELISA + IB | 0 | NA | ||||
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 3,863 | 1,827 | PCR | 2238 | 7 | |
| ELISA | 624 | 66 | ELISA | 0 | NA | |
| ELISA + IPT | 10 | 10 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 33,329 | 200 | PCR | 10,670 | 1 | |
| ELISA | 2412 | 0 | ELISA | 65 | 1 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 1 | 1 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
4.1.2.5. Greece
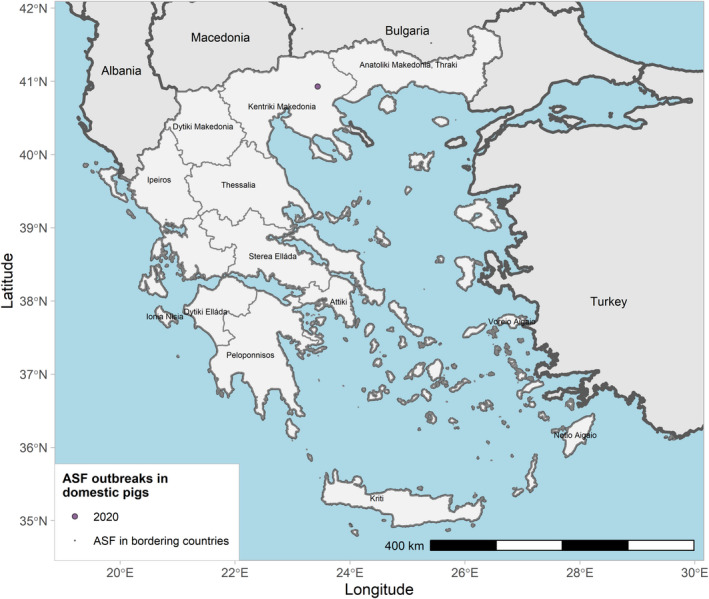
Figure 15 ASF outbreaks in Greece in domestic pigs reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period |
Following the only outbreak of ASF in Serres (5 May 2020), no other outbreaks of the disease have occurred in Greece. |
| Specific prevention and control measures implemented during the reporting period |
On wild boar population management and according to the European Union strategic approach, the Joint Ministerial Decision No 147/21886/2021 has been issued on epizootic surveillance, prevention and control programme for ASF in wild boars. The establishment of a framework for cooperation between competent authorities and bodies for the management of the wild boar population is included here. To this purpose, Regional Coordinating Bodies are to be set up, and these will monitor the activities of the official hunting groups and the trend of the wild boar population decrease strategy on regional level. Also, the Ministerial Decision No 1102/182415/2021 has been issued, entitled ‘Programme of epizootic surveillance, prevention and control for ASF in swine farms, controls on biosecurity requirements, as well as measures and sanctions in swine farms with the aim to prevent ASF virus introduction and spread’. In this Decision, biosecurity measures and requirements are thoroughly described. Moreover, regular checks on biosecurity measures in swine farms in the entire Greek territory is to be carried out by official veterinarians. Farms are selected, based on risk factors such as their distance to affected area and forest areas, their numbers of pigs in the holding and previous compliance records with the legislation. These on‐farm visits include: 1. Provision of information to swine farmers on ASF. 2. Inspection on identification of animals and their registration in the online National Database. 3. Record keeping controls in swine farms, especially on updates of the farm registration records including movements. 4. Clinical examination of animals and collection of samples if necessary. 5. Biosecurity checks – a questionnaire designed for this purpose is to be filled and submitted by the official veterinarian to the CCA. 6. In cases where biosecurity measures described here are not implemented, a timetable for applying corrective actions is set by the Veterinary Authorities, re‐evaluation is performed in scheduled on‐farm visits and sanctions are posed if corrective measures are then assessed as insufficient. |
| Epidemiological investigations in infected farms during the reporting period | No epidemiological investigations have been conducted, as no new outbreaks have occurred in Greece since February 2020. |
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
| NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 12 | 0 | PCR | 13 | 0 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| Active surveillance in WB* | Active surveillance in WB* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 31 | 0 | PCR | 15 | 0 | |
| ELISA | 0 | NA | ELISA | 626 | 0 | |
| ELISA + IPT | NA | NA | ELISA + IPT | NA | NA | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
| Surveillance in NUTS 3 areas with at least one ASF notification during the reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | NA | NA | PCR | NA | NA | |
| ELISA | NA | NA | ELISA | NA | NA | |
| Active surveillance in WB* | Active surveillance in WB* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | NA | NA | PCR | NA | NA | |
| ELISA | NA | NA | ELISA | NA | NA | |
4.1.2.6. Hungary
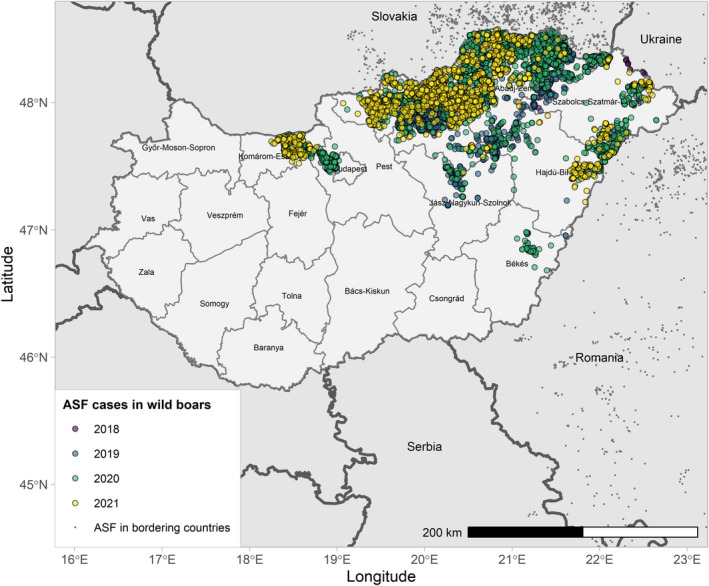
Figure 16 ASF cases in wild boar in Hungary reported to the Animal Disease Information System up to 31 August 2021
| New developments of the epidemic in the reporting period |
In Hungary, ASF has been diagnosed only in the wild boar population. On 10 August 2021, ASF was confirmed in a dead wild boar in the north of a new county, Fejér, only a few kilometres away from the Komárom‐Esztergom county. The possible source of infection was the natural spread of wild boars from Komárom‐Esztergom county. Since then, no new cases in Fejér county have been detected. |
| Specific prevention and control measures implemented during the reporting period | On 21 January 2021, the National Action Plan on wild boar management was published. The main strategic goal is to reduce the wild boar density to 0.5 wild boar/km2 by 28/2/2025. A network of advisers has been set up by the National Disease Control Centre. The advisers make on‐site visits across the country to help reaching this goal. |
| Epidemiological investigations in infected farms during the reporting period | No ASF outbreaks have occurred in Hungary up to the end of the current reporting period. |
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 4,808 | 3,397 | PCR | 12,118 | 0 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IPT | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 59,747 | 781 | PCR | 234,266 | 0 | |
| ELISA | 3,270 | 35 | ELISA | 0 | ND | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | ND | |
| ELISA +IB | 0 | NA | ELISA + IPT | 0 | ND | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 4,379 | 3,397 | PCR | 8,879 | 0 | |
| ELISA | 0 | NA | ELISA | 0 | ND | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | ND | |
| ELISA +IB | 0 | NA | ELISA +IB | 0 | ND | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 24,489 | 781 | PCR |
234,266 |
0 | |
| ELISA | 2578 | 35 | ELISA | 0 | ND | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | ND | |
| ELISA +IB | 0 | NA | ELISA + IB | 0 | ND | |
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
4.1.2.7. Latvia
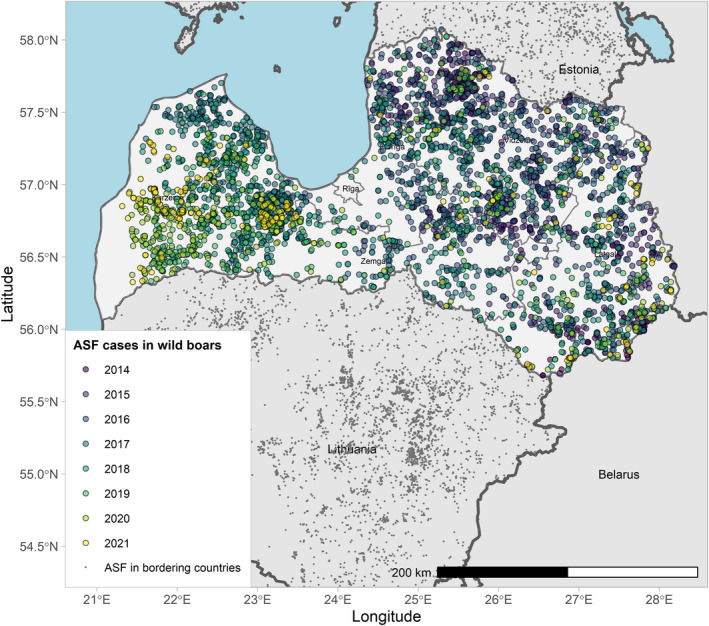
Figure 17 ASF cases in wild boar in Latvia reported to the Animal Disease Information System up to 31 August 2021
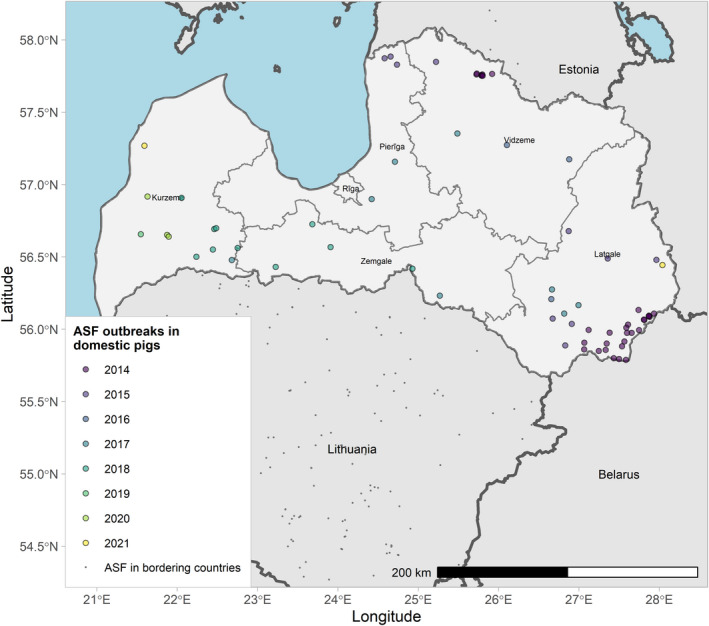
Figure 18 ASF outbreaks in Latvia in domestic pigs reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period |
ASF still persists in a wild boar population in the western part of Latvia, where it could be considered as a frontline of the infection, as most of PCR‐positive cases in wild boar are detected there. In July 2021, one ASF outbreak was also confirmed in a commercial farm with 2000 pigs in the same area. After few years of having mostly seropositive results in wild boar (very few active infections detected) in the eastern part of Latvia (Oļševskis et al., 2020), several PCR‐positive wild boar (in both hunted and found dead) were detected, starting from June 2021 in bordering areas with Russian Federation and Belarus. Considering the high number of new PCR‐positive cases in wild boar in a short period (few weeks), these are most likely to be re‐introductions of ASFV in an area that started to recover gradually since 2019. These re‐introductions have caused further ASF spread in the local wild boar population and just few weeks after first detections of PCR‐positive wild boars, ASF was confirmed in a small backyard farm in the same area. |
| Specific prevention and control measures implemented during the reporting period | None. |
| Epidemiological investigations in infected farms during the reporting period |
Two outbreaks have occurred during the reporting period:
|
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 237 | 110 | PCR | 3,407 | 3 | |
| ELISA | 6 | 2 | ELISA | 3 | 2 | |
| ELISA + IPT | 2 | 1 | ELISA + IPT | 3 | 2 | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 20,889 | 51 | PCR | 1,889 | 0 | |
| ELISA | 20,854 | 743 | ELISA | 0 | NA | |
| ELISA + IPT | 743 | 215 | ELISA + IPT | 0 | NA | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 190 | 110 | PCR | 3,217 | 3 | |
| ELISA | 5 | 2 | ELISA | 3 | 2 | |
| ELISA + IPT | 2 | 1 | ELISA + IPT | 3 | 2 | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 8,923 | 51 | PCR | 1,889 | 0 | |
| ELISA | 8,897 | 341 | ELISA | 0 | NA | |
| ELISA + IPT | 341 | 138 | ELISA + IPT | 0 | NA | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
4.1.2.8. Lithuania
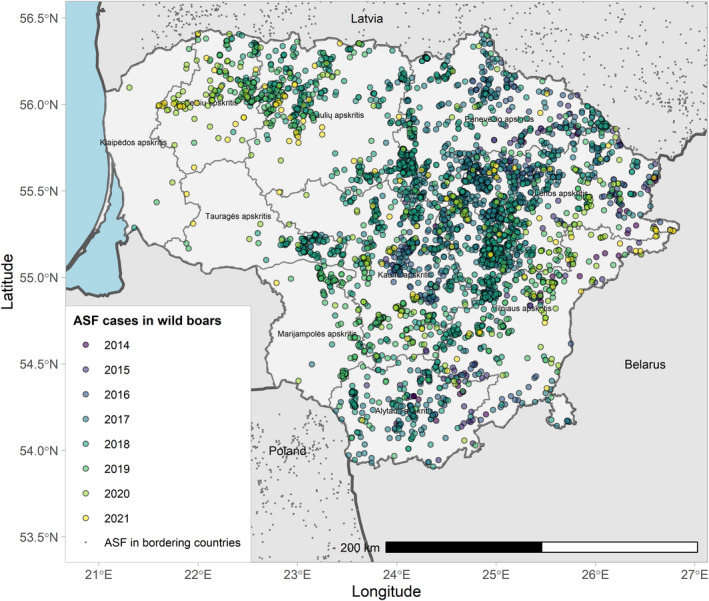
Figure 19 ASF cases in wild boar in Lithuania reported to the Animal Disease Information System up to 31 August 2021
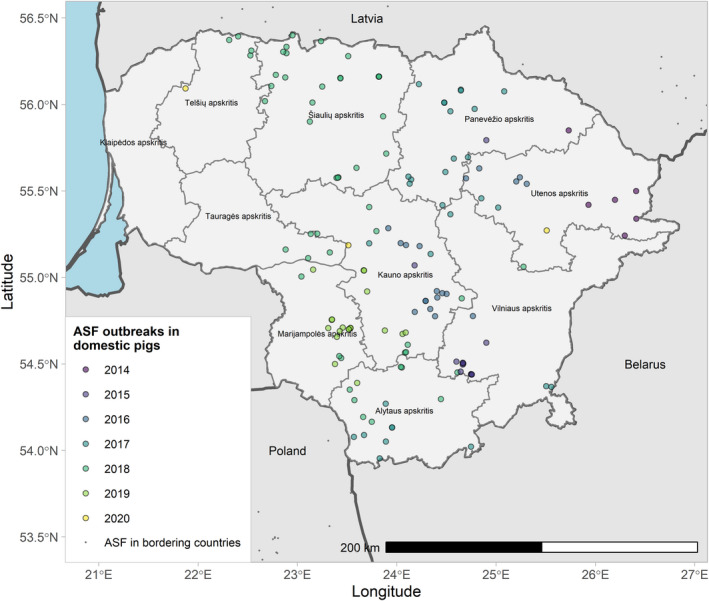
Figure 20 ASF outbreaks in domestic pigs in Lithuania reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period | In the reporting period from 1 September 2020 until 31 August 2021, in Lithuania 185 ASFV‐positive wild boar were found in 177 locations (164 wild boar hunted and 21 found dead). The low number of found dead wild boar suggests a decrease in the active circulation of the ASF virus and/or a decrease in the numbers of wild boar, making it difficult to find carcasses. Most ASF cases in wild boar were detected from the hunted animals being seropositive. Due to COVID‐19 restrictions in 2020, driven hunts were minimised and, in some regions, not preformed and only individual hunts were allowed. This led to reduced disturbance of wild boar. At the beginning of 2021, ASF virus was detected only in two regions of Lithuania and most hunters decided to actively allow the wild boar population to increase again on their hunting grounds, as according to their personal understanding ASF has disappeared. This may have led to increased spread of ASF. By the end of this reporting period, on 31 August 2021, wild boar tested PCR positive in 12 regions of Lithuania. This suggests that in addition to the increased wild boar density, human‐driven activities may have influenced the fast ASFV dispersal in the 12 regions of Lithuania. |
| Specific prevention and control measures implemented during the reporting period |
In the reporting period from 1 September 2020 until 31 August 2021, African swine fever outbreaks in domestic pigs were not reported in Lithuania. The last ASF outbreak in domestic pigs was detected on 10 August 2020 under the framework of passive surveillance in a non‐commercial holding with three pigs. Despite the pandemic situation caused by COVID‐19 and quarantine conditions with movement restrictions inside the country, the domestic pig sector was a priority, ensuring free trade inside and outside the country with the commodities of pigs under strict veterinary control. All movements were allowed from holdings that were fully complying with the national biosecurity requirements, namely those that were inspected twice per year by the official veterinarian; where passive surveillance is carried out (each week 2 to 10 dead pigs are sent for virological testing to the National Reference Laboratory). In exceptional cases, if small commercial pig farms were not inspected twice per year, by the official veterinarian, active surveillance was carried out before the pigs were moved out from the farm. In such cases, the active surveillance was not financed by the government, but the owner paid for the test and in case of a negative result the certificate or commercial document was issued via the Traces system. Due to the COVID‐19 restrictions, movement between the different types of farm was decreased, personal protection equipment was used more often. Furthermore, veterinary control and recurrent awareness campaign were in place, pig keepers had more knowledge about ASF and all these factors prevented the ASF introduction into domestic pig holdings. |
| Epidemiological investigations in infected farms during the reporting period | Not applicable: no outbreaks during the reporting period |
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period* | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 96 | 18 | PCR | 5,143 | 0 | |
| ELISA | 10 | 0 | ELISA | 5,033 | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 12,924 | 24 | PCR | 0 | NA | |
| ELISA | 12,823 | 148 | ELISA | 0 | NA | |
| ELISA + IPT | 0 | 0 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 49 | 18 | PCR | 2,287 | 0 | |
| ELISA | 3 | 0 | ELISA | 2,250 | NA | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 6,497 | 24 | PCR | 0 | NA | |
| ELISA | 6,423 | 96 | ELISA | 0 | NA | |
| ELISA + IPT | 0 | 0 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
Surveillance in whole country during a reporting period was the same as surveillance in NUTS 3 areas with at least one ASF notification.
4.1.2.9. Poland
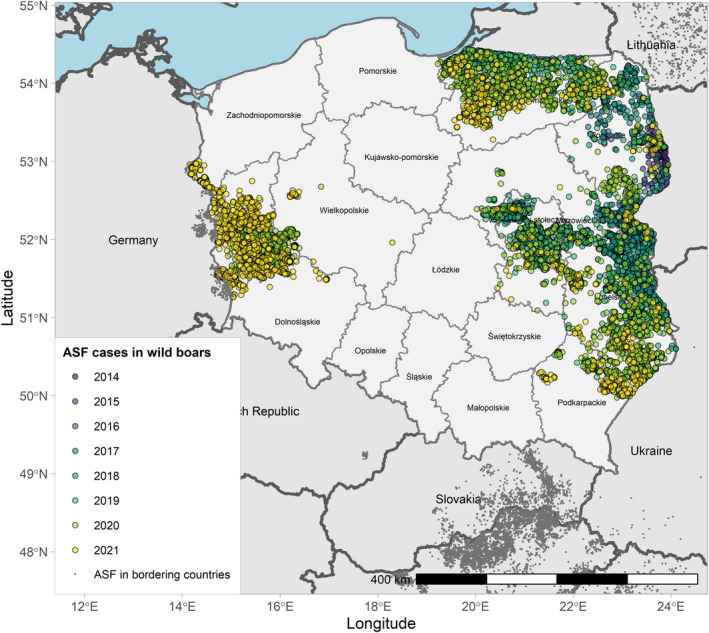
Figure 21 ASF cases in wild boar in Poland reported to the Animal Disease Information System up to 31 August 2021
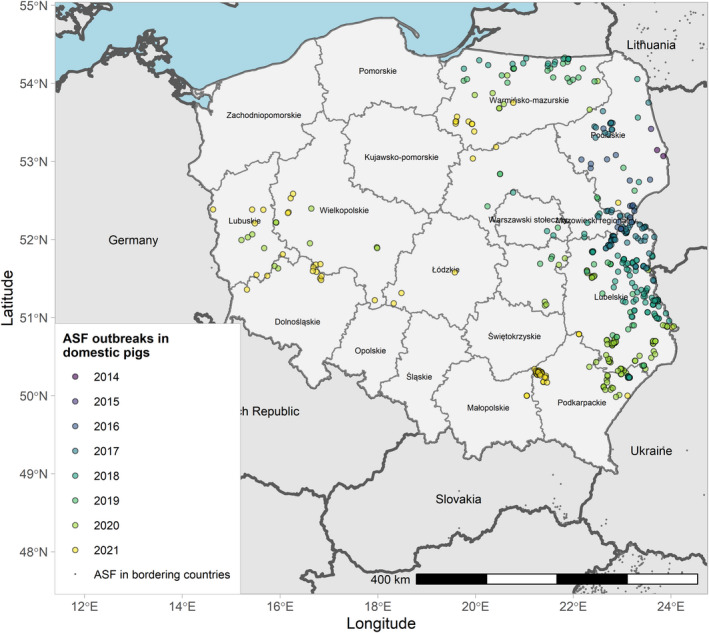
Figure 22 ASF outbreaks in domestic pigs in Poland reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period | During the reporting period, in Poland, three new clusters of ASF emerged in western Poland, namely in Lubuskie, Wielkopolskie and Dolnoslaskie voivodeship. The potential source of ASF introduction into the population of wild boar in these areas remains unclear, since the nearest ASF outbreaks occurred in central Poland (Mazowieckie voivodeship) distanced over 300 km away. One of the potential factors determining the fast emergence of the disease in the voivodeships next to Lubuskie was the high wild boar and forests density. As the consequence of ASF occurrence in the wild boar population in 2021, new clusters of ASF in domestic pigs have been identified in western Poland, resulting in 24 affected holdings. Additionally, ASF was diagnosed in three pig farms in the centre of the country (Lodzkie voivodeship), despite the lack of previous reports of ASF in wild boar at the same territory. From the epidemiological investigations, the most probable source of ASFV introduction in this area was illegal movements of pigs purchased from ASF‐affected area. |
| Specific prevention and control measures implemented during the reporting period | Fencing of the newly affected areas along with repellents (butyric acid) has been applied. Moreover, drones have been used for the estimation of wild boar density at the newly identified areas in western Poland. |
| Epidemiological investigations in infected farms during the reporting period |
There were 101 outbreaks during the reporting period, where the following observations were made:
|
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
Click on the LINK to see the spread of the disease since the first confirmation 49% of the NUTS 3 areas in the country had at least one case or outbreak of ASF reported in the reporting period |
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Surveillance in DP | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 10,358 | 5,552 | PCR | 1,712,616 | 1,713 | |
| ELISA | 94 | ND | ELISA | 76,928 | 77 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 84 | 33 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | ||||||
| Diagnostic test(s) used | Total samples | Positive samples | ||||
| PCR | 119,336 | 477 | ||||
| ELISA | 63,986 | 192 | ||||
| ELISA + IPT | 184 | 69 | ||||
| ELISA + IB | 0 | NA | ||||
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Surveillance in DP | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 1,463 | 982 | PCR | 1,545,381 | 1,545 | |
| ELISA | ND | ND | ELISA | 75,724 | 76 | |
| ELISA + IPT | ND | ND | ELISA + IPT | 82 | 33 | |
| ELISA + IB | NA | NA | ELISA + IB | NA | NA | |
| 0 Active surveillance in WB* | ||||||
| Diagnostic test(s) used | Total samples | Positive samples | ||||
| PCR | 84,724 | 424 | ||||
| ELISA | 56,309 | 169 | ||||
| ELISA + IPT | 180 | 126 | ||||
| ELISA + IB | NA | NA | ||||
ND: no data provided to EFSA; NA: Not applicable.
4.1.2.10. Romania
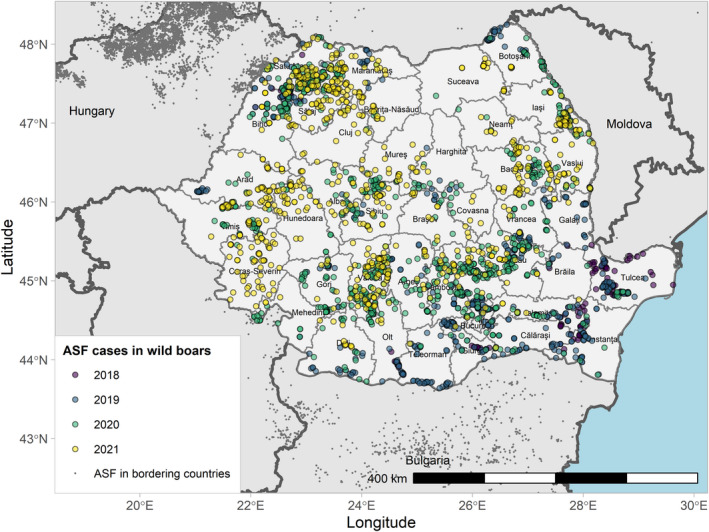
Figure 23 ASF cases in wild boar in Romania reported to the Animal Disease Information System up to 31 August 2021
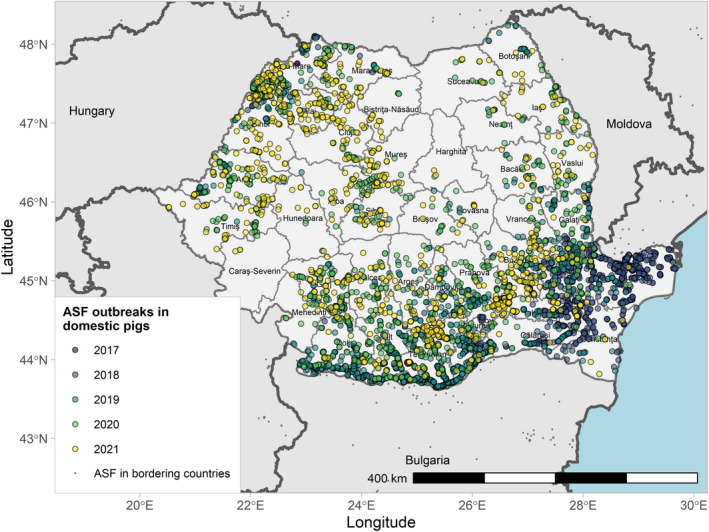
Figure 24 ASF outbreaks in Romania in domestic pigs reported to Animal Information Notification System up to 31 August 2021
| New developments of the epidemic in the reporting period |
Backyard farms: Because suspected outbreaks are not always immediately notified; due to non‐compliance with restrictions, illegal pig movements and low biosecurity measures, these establishments remain as vulnerable as before to the possible introduction of disease. Commercial farms: The ASF outbreaks in backyard farms leading to prohibitions of movements of pigs also applied on commercial farms caused important animal health and welfare issues and enormous economic losses to farmers. In affected commercial establishments, the introduction of the virus remained unnoticed by the farmer or the veterinarian for extended time. Under unfavourable circumstances, the high‐risk period was several weeks or even months, and during this period, the virus could spread within the farm or to other farms. |
| Specific prevention and control measures implemented during the reporting period |
In addition to the control measures set up according to the European Union legislation, NSVFSA informed on the epidemiological situation all Prefectures (Head of County Disease Control Centre) in the counties where ASF was confirmed in professional commercial pig holdings. It was requested to improve the Action Plan by setting concrete deadlines to reduce the risk of ASF dissemination. Consultancy visits on biosecurity measures on small farms are being performed by veterinarians where the farmers are reminded of their obligation to notify any health issue (10 consultations/month/contracted veterinarian). Regular traffic controls are organised by the veterinary authorities, and they reveal persistent non‐compliance with the rules on the movement of live pigs and their products. |
|
Epidemiological investigations in infected farms during the reporting period |
There were 1,637 outbreaks during the reporting period where the following observations have been made:
|
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 1,482 | 643 | PCR | 44,691 | 3,550 | |
| ELISA | 265 | 17 | ELISA | 222,329 | 2,369 | |
| ELISA + IPT | 2 | 2 | ELISA + IPT | 51 | 46 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 32,690 | 1,115 | PCR | 0 | NA | |
| ELISA | 28,443 | 653 | ELISA | 0 | NA | |
| ELISA + IPT | 21 | 19 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 1,471 | 643 | PCR | 43,730 | 3,550 | |
| ELISA | 254 | 16 | ELISA | 215,962 | 1,869 | |
| ELISA + IPT | 2 | 2 | ELISA + IPT | 51 | 46 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 31,639 | 1,115 | PCR | 0 | NA | |
| ELISA | 27,620 | 611 | ELISA | 0 | NA | |
| ELISA + IPT | 20 | 20 | ELISA + IPT | 0 | NA | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
4.1.2.11. Russia
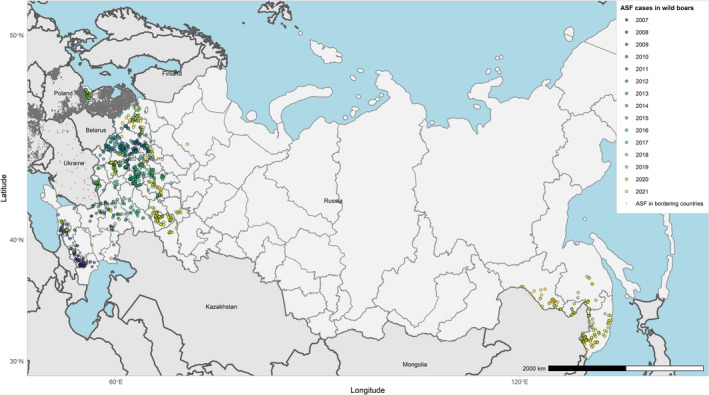
Figure 25 ASF cases in wild boar in Russia up to 31 August 2021
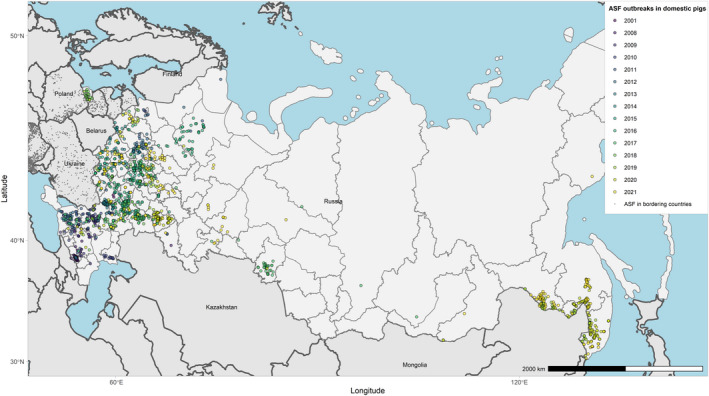
Figure 26 ASF outbreaks in domestic pigs in Russia up to 31 August 2021
| New developments of the epidemic in the reporting period |
During the reporting period, the spread of the disease had the same characteristics as in previous periods: distant spread of the virus followed by the formation of local epizootics due to the involvement of the unprotected sector and wild boar. One of these stable clusters is on the territory of Russian Far East bordering with China. Two new types of virus have yet been found in this region. The main efforts to control the disease are aimed at increasing the biosecurity of pig farms, reducing the number of unprotected households and also reducing the wild boar population. Temporal dynamics are still in an upward trend, which is likely to be reflected by new outbreaks in the unprotected pig sector. |
| Specific prevention and control measures implemented during the reporting period | The number of domestic pigs kept in backyards has reduced significantly. |
| Epidemiological investigations in infected farms during the reporting period |
There were 152 outbreaks during the reporting period. On 92 of them, the following observations were made:
|
| Reporting period | 1 September 2020 to 31 August 2021 | Reporting period |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease Regions with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period** | Passive surveillance in WB* | Passive surveillance in DP* |
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 2,290 | 112 | PCR | 128,573 | 248 | |
| ELISA | 0 | NA | ELISA | 1,210 | 0 | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 32,313 | 79 | PCR | 337,090 | 286 | |
| ELISA | 10,069 | 75 | ELISA | 74,271 | 0 | |
| Surveillance in regions with at least one ASF notification during the reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 348 | 112 | PCR | 41,863 | 248 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 15,749 | 79 | PCR | 126,321 | 286 | |
| ELISA | 16 | 0 | ELISA | 44,475 | 0 | |
4.1.2.12. Slovakia
Figure 27 ASF cases in wild boar in Slovakia reported to the Animal Disease Information System up to 31 August 2021
Figure 28 ASF outbreaks in domestic pigs in Slovakia reported to the Animal Disease Information System up to 31 August 2021
| Reporting period | 1 September 2020 to 31 August 2021 |
| New developments of the epidemic in the reporting period |
During the reporting period, ASF has spread gradually in the wild boar population from the south‐east (border with Hungary and Ukraine) northwards towards the Polish border and towards the central part of the country. The fastest progress of ASF in the wild population has been recorded in forested mountains with lower altitude (up to 1,000 m) and without natural or artificial barriers. The large Danube River between Slovakia and Hungary appears to be a temporary effective barrier to the ASF progression. Two weeks after the introduction of ASF into the Slovak Republic, we recorded the first cases of the disease in wild boars, i.e. caught wild boar without clinical signs of ASF. After an initial significant decrease in the number of wild boar, we expect a gradual increase in the wild boar population in the area with the first occurrence of ASF, the south‐eastern part of Slovakia. In domestic pigs, the disease was diagnosed on one large commercial farm in the district Lucenec (close to Hungarian border) and in several small holdings. All these small holdings were located in the ASF‐infected area in different parts of country (east, central and south), The occurrence of ASF in domestic pig farms seems to have a ‘connection’ with the presence of the virus in the environment, considering that ASF occurred in farms that were all located in areas where previously only wild boar cases were observed (i.e. Part II areas subsequently transferred into Part III according to CIR 2021/605/EC). |
| Specific prevention and control measures implemented during the reporting period | There was no specific point to be raised for this point and control measures as laid down in the EU legal framework were implemented for the control and prevention of further spread of ASF. |
|
Epidemiological investigations in infected farms during the reporting period |
There were 13 outbreaks during the reporting period, where the following observations have been made:
|
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
|
Spread of the disease NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 4,289 | 3,337 | PCR | 3,952 | 67 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| ELISA + IPT | 1,076 | 203 | ELISA + IPT | 1,172 | 17 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 27,624 | 140 | PCR | 796 | 0 | |
| ELISA | 0 | NA | ELISA | 0 | NA | |
| ELISA + IPT | 27,514 | 88 | ELISA + IPT | 596 | N0 | |
| ELISA + IB | 0 | NA | ELISA + IB | 0 | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 3,936 | 3,337 | PCR | 2,445 | 67 | |
| ELISA | ND | ND | ELISA | 0 | NA | |
| ELISA + IPT | 864 | 203 | ELISA + IPT | 1,166 | 17 | |
| ELISA + IB | ELISA + IB | 0 | NA | |||
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 22,135 | 140 | PCR | 746 | 0 | |
| ELISA | ND | ND | ELISA | |||
| ELISA + IPT | 22,085 | 88 | ELISA + IPT | 546 | 0 | |
| ELISA + IB | 0 | NA | ELISA + IB | |||
ND: no data provided to EFSA; NA: Not applicable.
*: See Section 3.1.1 for definitions.
4.1.2.13. Serbia
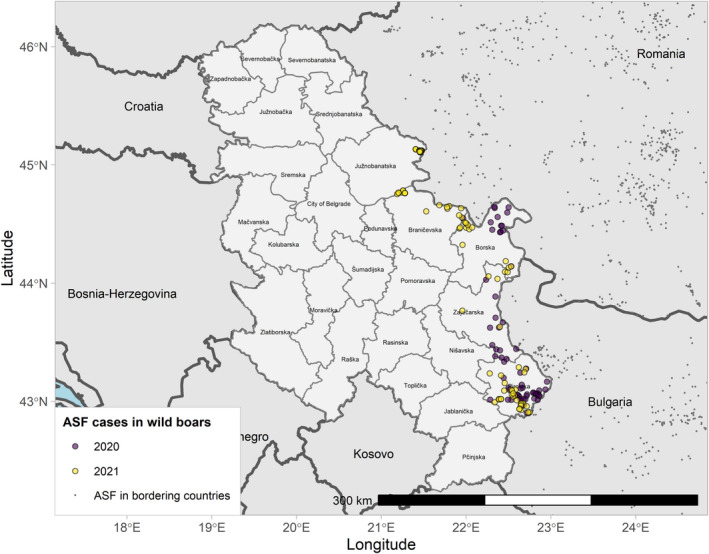
Figure 29 ASF cases in wild boar in Serbia reported to the Animal Disease Information System up to 31 August 2021
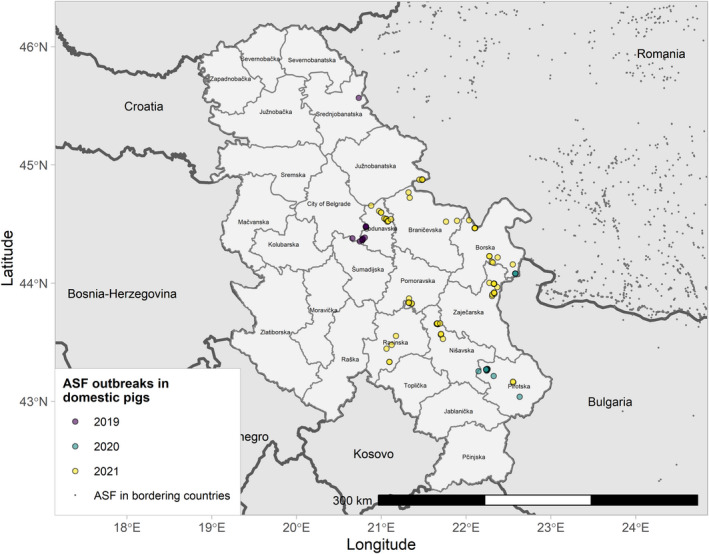
Figure 30 ASF outbreaks in domestic pigs in Serbia reported to the Animal Disease Information System up to 31 August 2021
| New developments of the epidemic in the reporting period |
In 2020, ASF cases in wild boar occurred in two districts in Serbia: Borski (bordering Romania and Bulgaria) and Pirotski (bordering Bulgaria). In 2021, the disease has further spread to the neighbouring districts, Braničevski, Južnobanatski and Zaječarski, resulting in five affected districts in Serbia during the reporting period. In 2020, ASF occurred in domestic pigs, only in the Pirotski district. In 2021, the disease has spread further to domestic pigs in nine districts in Serbia (Pirotski, Borski, Nišavski, Pomoravski, Rasinski, Zaječarski, Braničevski, Podunavski and Južnobanatski). In 2021, a commercial pig farm in Halovo, with more than 18,000 pigs, was infected in the Zaječarske district. There were no other outbreaks in commercial farms. In 2021, in total, 20,322 pigs were culled due to ASF. Based on the implemented measures and the results of the final diagnostic tests in addition, the ordinances on declaration of ASF epidemics in the Pomoravski and Rasinski administrative districts were officially revoked, and the outbreaks were considered to be resolved. |
| Specific prevention and control measures implemented during the reporting period |
Specific prevention and control measures for ASF in Serbia are laid down in four main legislative documents: 1. Surveillance plan on African and classical swine fever in domestic pigs in the Republic of Serbia IN 2020/2021 2. Final surveillance and diagnostic testing plan in infected areas with African swine fever (protection and surveillance zone) in domestic pigs 3. Biosecurity instructions for the users of hunting grounds (hunting clubs) 4. Methods for monitoring of Classical swine fever and African swine fever in wild boar populations in 2020 and 2021. These documents can be found on the websites of the Veterinary directorate http://www.vet.minpolj.gov.rs and the Forestry directorate http://upravazasume.gov.rs |
|
Epidemiological investigations in infected farms during the reporting period |
There were 36 outbreaks during the reporting period. Although quantitative data from the outbreak investigation are not available, the following observations were made for these outbreaks:
|
| Reporting period | 1 September 2020 to 31 August 2021 | |
| Host | WILD BOAR (WB) | DOMESTIC PIGS (DP) |
| Cases/Outbreaks |
|
|
| Spread of the disease |
|
|
| NUTS 3 areas with at least one ASF notification during the reporting period |
|
|
| Surveillance (samples tested for ASF) in whole country during reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 240 | 191 | PCR | 288 | 130 | |
| ELISA | ND | 7 | ELISA | ND | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 7 | 7 | ELISA + IB | 0 | NA | |
| Active surveillance in WB* | Active surveillance in DP* | |||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 4,950 | 68 | PCR | 9,736 | 14 | |
| ELISA | ND | 2 | ELISA | ND | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 2 | 2 | ELISA + IB | 0 | NA | |
| Surveillance (samples tested for ASF) in NUTS 3 areas with at least one ASF notification during the reporting period** | Passive surveillance in WB* | Passive surveillance in DP* | ||||
| Diagnostic test(s) used | Total samples | Positive samples | Diagnostic test(s) used | Total samples | Positive samples | |
| PCR | 215 | 191 | PCR | 183 | 130 | |
| ELISA | ND | 7 | ELISA | ND | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 7 | 7 | ELISA + IB | 0 | NA | |
| Passive surveillance in WB* | Passive surveillance in DP* | |||||
| PCR | 3,298 | 68 | PCR | 1,407 | 14 | |
| ELISA | ND | 2 | ELISA | ND | 0 | |
| ELISA + IPT | 0 | NA | ELISA + IPT | 0 | NA | |
| ELISA + IB | 2 | 2 | ELISA + IB | 0 | NA | |
4.1.3. Special focus on the outbreaks in large commercial pig farms in Romania in the last two reporting periods
4.1.3.1. Occurrence of outbreaks in large commercial pig farms
In the previous reporting period (1 September 2019 until 31 August 2020), in total 1,043 outbreaks of ASF in domestic pigs were reported to ADIS in Romania, compared with 1,637 outbreaks in the current reporting period (1 September 2020 until 31 August 2021), which represent an increase in outbreaks of 57%. The total number of pigs affected by the outbreaks in the previous reporting period were 242,758 pigs compared with 368,297 pigs in the last reporting period, which represents an increase of animals affected by the outbreaks of 52%.
When comparing the frequency distribution of the number of outbreaks according to farm size in both periods (Figure 31), 95% of all the outbreaks were farms with fewer than 78 pigs and 96 pigs in the previous and current reporting periods, respectively.
Figure 31.
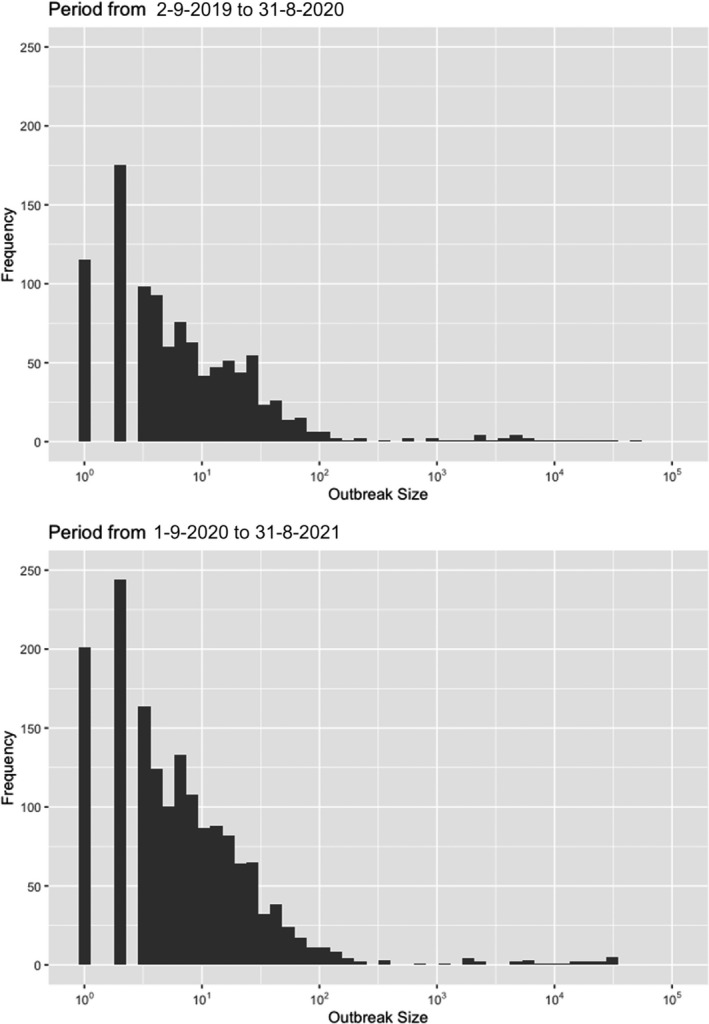
- Source: ADIS.
The number of outbreaks in farms with more than 10,000 animals increased from 6 to 13 farms from the previous to the current reporting period, representing 0.6 to 0.8% of all the outbreaks in both periods.
However, when comparing the yearly incidence of outbreaks in the current reporting period per farm size class (Table 3), it can be seen that the incidence in the smallest farm size class is almost 80 times smaller (0.003) compared with the incidence in the largest farm size class (0.236), and in the farm size class between 100 and 1,000 animals (0.207). Pig population data were only available for the current reporting period; therefore, the comparison of incidence in these farm size classes with the previous reporting period could not be performed.
Table 3.
Annual incidence of ASF outbreaks per farm size class in Romania in the reporting period 1 September 2020–31 August 2021
| Farm sizes | Number of farms | Number of outbreaks | Incidence (95% confidence interval) |
|---|---|---|---|
| x ≤ 30 | 444,722 | 1,460 | 0.0033 (0.0031–0.0035) |
| 30 < x ≤ 100 | 1,650 | 123 | 0.0745 (0.0623–0.0883) |
| 100 < x ≤ 1,000 | 135 | 28 | 0.2074 (0.1425–0.2856) |
| 1,000 < x ≤ 10,000 | 168 | 13 | 0.0774 (0.0418–0.1287) |
| x ≥ 10,000 | 55 | 13 | 0.2364 (0.1323–0.3702) |
Source of number of outbreaks: ADIS; Source of number of or farms: Pig population data submitted by Romania at farm level through EFSA DCF according to SIGMA standards for the reporting period (1 September 2020 to 31 August 2021).
4.1.3.2. Spatial distribution of large outbreaks in pig holdings
Figure 32 shows the spatial distribution of outbreaks in Romania in the current and previous reporting period. Outbreaks in large farms with more than 10,000 pigs are dispersed over the entire country. However, it appears that most outbreaks (including the large outbreaks) are concentrated in the south‐east and north‐west of the country. It appears also that there are more outbreaks in the north‐west in the current reporting period compared with the previous reporting period.
Figure 32.
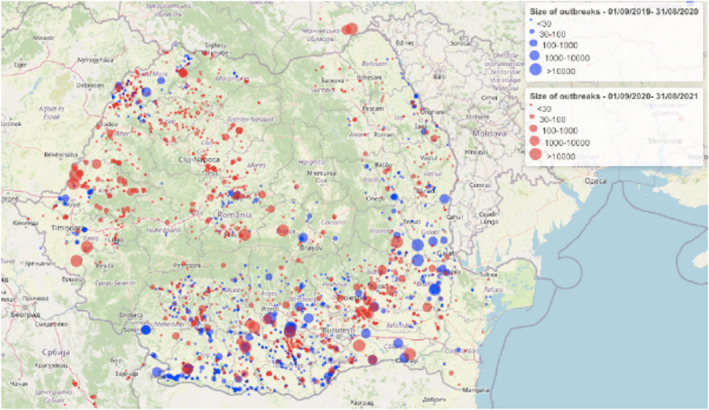
- Source: ADIS. Size of outbreaks expressed in number of pigs on the farm.
4.1.3.3. Number of secondary outbreaks from a single source farm (extent of spread)
The purpose of this investigation was to evaluate, if there was a change in the numbers of notifications that could be classified as secondary outbreaks linked to a single source outbreak, and to compare this for the previous and the current reporting period. Although this average number of notifications that could be classified as secondary outbreaks (means of bootstraps calculated with a network analysis) is not to be mixed up with the current reproduction number (R0), it can be considered as a proxy for the ‘extent of spread’ in the evaluated time period, and it therefore allows comparison between periods in the epidemic. Comparing the average number of notifications classified as potential secondary outbreaks per source outbreak in the two reporting periods in Romania demonstrated an increase from 2.2 to 2.5 (Figure 33). This demonstrates that the extent of spread increased in this reporting period compared with the previous reporting period, indicating that the epidemic in Romania is still expanding.
Figure 33.
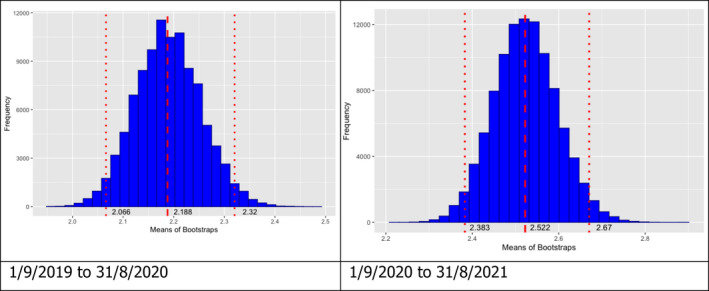
- Source: ADIS.
4.1.3.4. Temporal distribution of outbreaks in large commercial farms
Figure 34 displays the weekly number of ASF outbreaks reported to ADIS during the last two reporting periods. Overall, the number of outbreaks reported in the current reporting period is slightly higher than in the previous reporting period throughout the year. As observed in other reporting periods, there is a clear seasonality in the outbreaks, with a peak of outbreaks in summer. However, there is an important increase observed in the number of reported outbreaks in this reporting period in the summer months (July 2021 to September 2021) when comparing with the previous reporting period.
Figure 34.
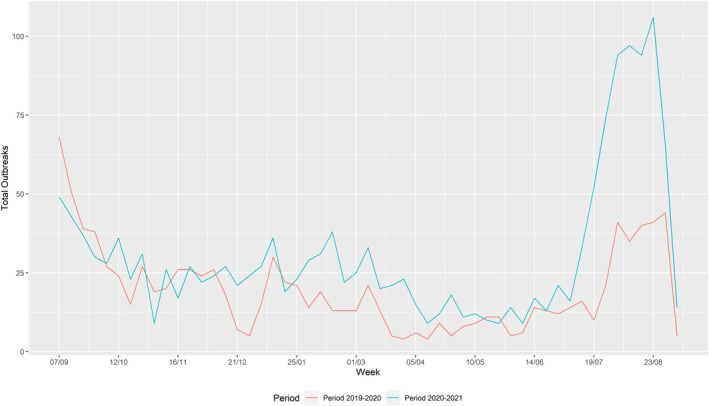
- Source: ADIS. Period 2019–2020 = 1 September 2019 to 31 August 2020; Period 2020–2021 = 1 September 2020 to 31 August 2021.
As the outbreak information reported to ADIS does not contain information on the type of farm, it was decided to categorise the farms according to the farm size, as an approximation for non‐commercial farms and commercial farms. Consequently, the outbreaks were classified in small farms with fewer than 30 pigs vs. farms with more than 30 pigs; according to the same cut‐off implemented in an ongoing case–control study in Romania.
Figure 35 reveals that the difference in the total numbers of outbreaks between the reporting periods during the summer peak was more pronounced in the farms with fewer than 30 pigs. And the seasonal peak seemed less prominent in the larger farm class.
Figure 35.
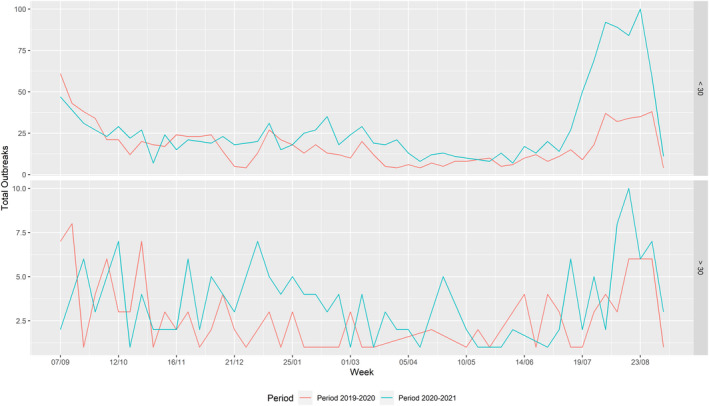
- Source: ADIS, Period 2019–2020 = 1 September 2019 to 31 August 2020; Period 2020–2021 = 1 September 2020 to 31 August 2021. Week 1 in graph corresponds to first week of September 2019 and 2020, respectively.
4.1.3.5. Different likelihoods of outbreaks in commercial farms in Romanian counties in the reporting period (1 September 2020 to 31 August 2021)
As the type of farm (commercial or non‐commercial farm) is not reported to ADIS, and the outbreak reference numbers reported to ADIS do not match the farm identification numbers reported to the DCF, a detailed analysis of the incidence in the different farm types could not be carried out. Also, the geo‐coordinates of the individual pig farms were not reported to the DCF, so a spatial risk factor analysis could not be performed.
Figure 36 displays the number of outbreaks per 1,000 pig farms reported to ADIS in the 42 counties of Romania in the current reporting period; the number of farms reported to the DCF in 2021 was used for this calculation. The incidence of outbreaks in the reporting period is higher in the counties in the north‐west and south‐east of the country. The high number of outbreaks reported in this reporting period in these areas was also observed in Figure 32. The pie chart in Figure 36 represents the total number of farms in the county, and the fraction of non‐commercial farms and commercial farms. The fraction of commercial farms in the north‐east is relatively higher than in the southwest of the country. Figure 37 displays the number of susceptible pigs in affected pig farms in this reporting period (as reported to ADIS) per 1,000 pigs present in each of the 42 counties in Romania; the number of pig farms reported to the DCF in 2021 was used for this calculation. The pie chart represents the total number of pigs per county, and the fraction of pigs reared in non‐commercial farms and commercial farms.
Figure 36.
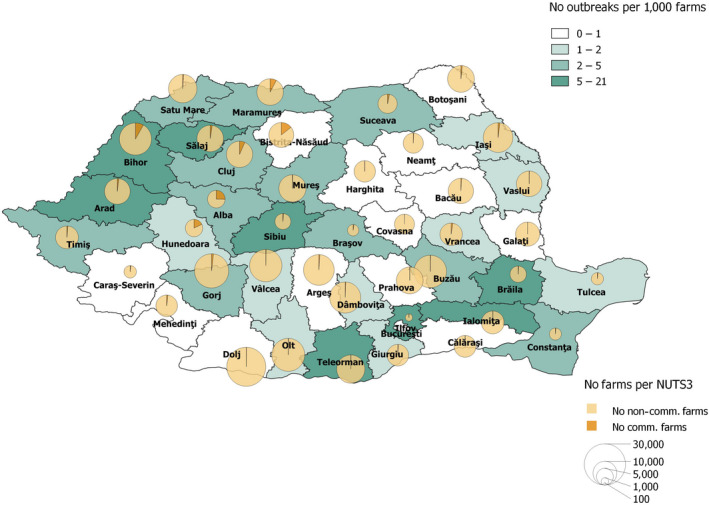
Numbers of outbreaks in pig farms (reported to ADIS) per 1,000 farms (reported to the DCF) in the reporting period per county in Romania
Figure 37.
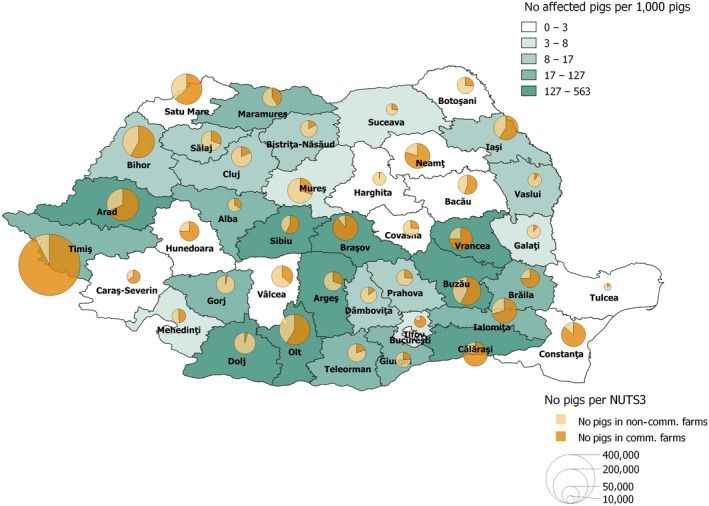
The numbers of susceptible animals in pig farms (reported to ADIS) more than 1,000 pigs (reported to the DCF) in the reporting period per county in Romania
4.1.4. Time profile of proportions of positive samples tested with Ab ELISA or PCR from wild boar detected through active or passive surveillance
Figures 38A–44A show the observed proportions of positive samples of wild boar detected through passive surveillance (wild boar that were found dead or hunted wild boar that showed ASF clinical signs), tested either by PCR or Ab ELISA. Only samples tested since 2016 are shown. Figures 38B–44B show the same proportions, but only from active surveillance (apparently healthy wild boar that were hunted or killed in road accident).
Figure 38.
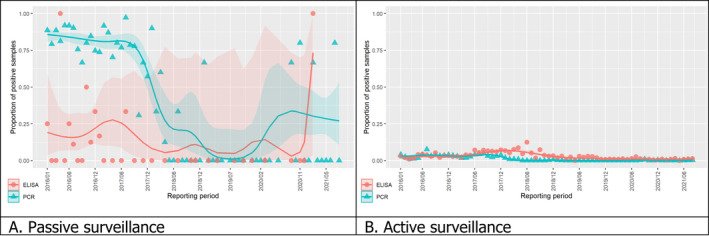
Proportion of ASF‐positive samples over the tested samples (by Ab ELISA and PCR) from all wild boar in the ASF‐affected areas of Estonia (1 January 2016 to 31 August 2021)
Figure 44.
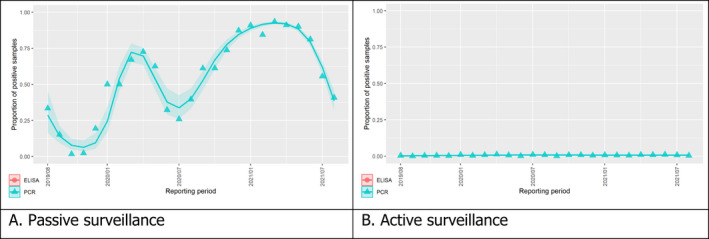
Proportion of ASF‐positive samples over the tested samples (by Ab ELISA and PCR) from all wild boar in the ASF‐affected areas of Slovakia (24 July 2019 to 31 December 2021)
As for the previous reporting period, in the affected areas the proportion of wild boar testing positive through passive surveillance tested by PCR has generally been much higher than the proportions testing positive to Ab ELISA, albeit the seasonality.
In the Baltic States, overall, the proportions of PCR‐positive samples from wild boar carcasses are declining, although a resurgence has occurred in Estonia over the last reporting period. In the other countries, the proportions of PCR‐positive wild boar carcasses remain high, indicating continuation of the spread of the disease.
There are clear seasonal fluctuations observed in the proportions of PCR‐positive samples from wild boar carcasses, which are further investigated in Section 4.1.5. There was no general increase in the proportion of Ab ELISA‐positive samples over time. The wide confidence intervals around the proportion of ELISA‐positive samples of the plots on passive surveillance are due to scarce data (small numbers of samples tested with ELISA).
4.1.4.1. Wild boar
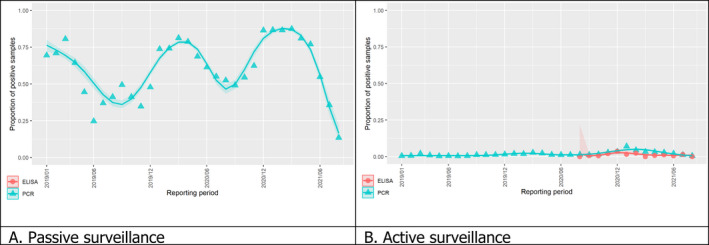
Figure 39 Proportion of ASF‐positive samples over the tested samples (only by PCR) from all wild boar in the ASF‐affected areas of Hungary (21 April 2018 to 31 August 2021)
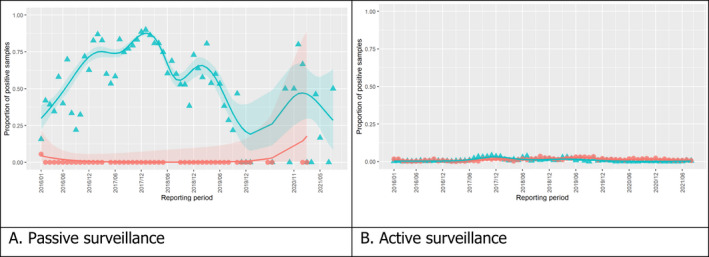
Figure 40 Proportion of ASF‐positive samples over the tested samples by Ab ELISA and PCR from all wild boar in the ASF‐affected areas of Lithuania (1 January 2016 to 31 August 2021)
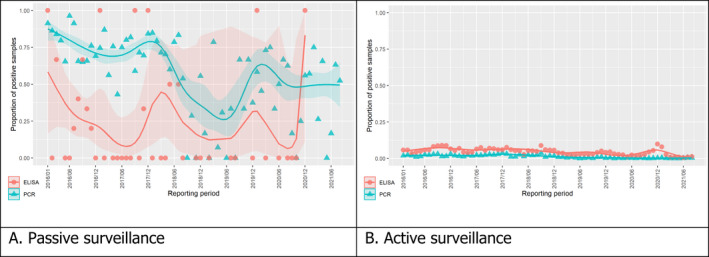
Figure 41 Proportion of ASF‐positive samples over the tested samples (by Ab ELISA and PCR) from all wild boar in the ASF‐affected areas of Latvia (1 January 2016 to 31 August 2021)
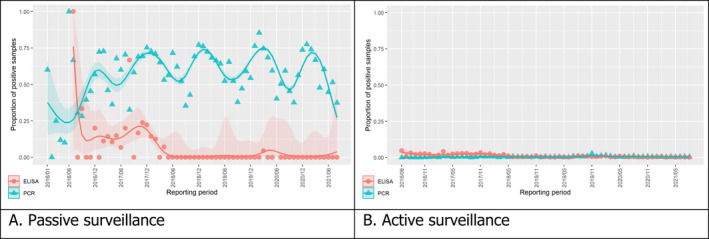
Figure 42 Proportion of ASF‐positive samples over the tested samples (by Ab ELISA and PCR) from all wild boar in the ASF‐affected areas of Poland (1 January 2016 to 31 August 2021)
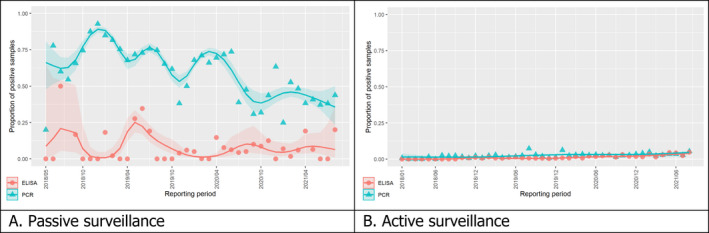
Figure 43 Proportion of ASF‐positive samples over the tested samples (by Ab ELISA and PCR) from all wild in the ASF‐affected areas of Romania (31 July 2017 to 31 December 2021)
Figure 45 shows the proportion of positive samples (by Ab ELISA and PCR) over the tested samples from all domestic pigs in the ASF‐affected areas of Lithuania (A), Poland (B) and Romania (C) and Slovakia (D). The proportions of PCR‐positive samples remain very low throughout the years in domestic pigs, although seasonal peaks of proportions of the tested samples being positive of up to 50% are observed in Romania.
4.1.4.2. Domestic pigs
Figure 45.
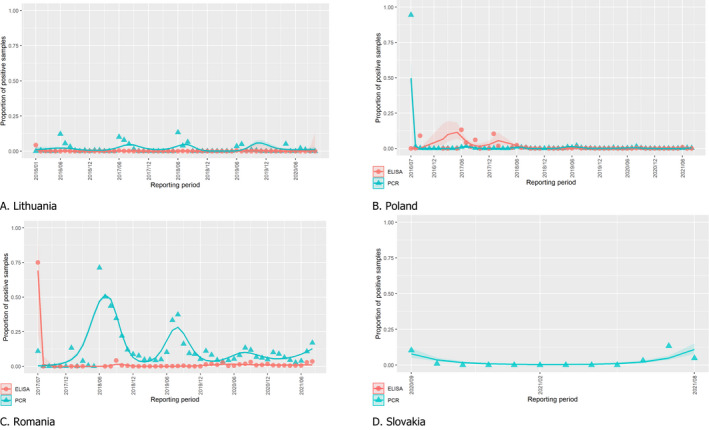
Proportion of ASFV‐positive samples (by Ab ELISA and PCR) over the tested samples from all domestic pigs in the ASF‐affected areas of: (A) Lithuania (1 January 2016 to 31 August 2021), (B) Poland (1 January 2016 to 31 August 2021), (C) Romania (31 July 2017 to 31 August 2021), (D) Slovakia (24 July 2019 to 31 August 2021)
4.1.5. Seasonality of African swine fever outbreaks and cases
4.1.5.1. Domestic pigs
Figure 46 shows the average proportions of PCR‐positive domestic pigs in Lithuania (A), Poland (B), Romania (C) and Slovakia (D) aggregated by calendar month and NUTS 3 region through passive surveillance. Higher proportions of positive samples are observed in the summer months, in line with the seasonality previously observed for outbreaks in domestic pig farms. Seasonality in domestic pigs in countries that did not submit data to EFSA’s DCF was not investigated.
Figure 46.
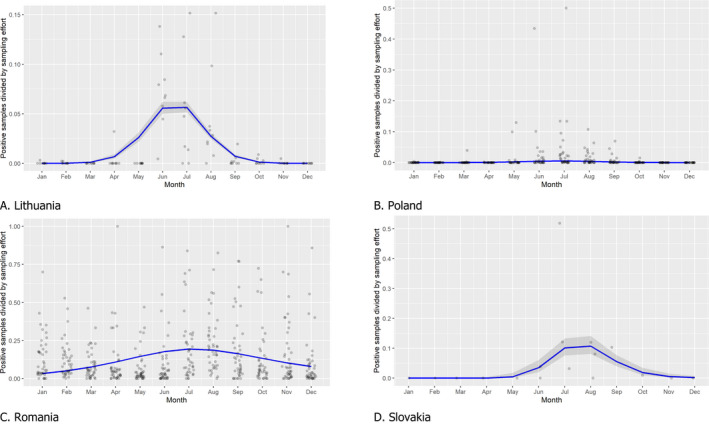
Average proportions of domestic pigs testing positive to ASF (PCR) by calendar month in NUTS 3 regions of: (A) Lithuania (1 January 2016 to 31 August 2021), (B) Poland (1 January 2016 to 31 August 2021), (C) Romania (31 July 2017 to 31 August 2021), (D) Slovakia (24 July 2019 to 31 August 2021)
Figures 47–53 show the proportions of PCR‐positive samples from wild boar tested through active and passive surveillance activities. The proportions of PCR‐positive samples from active surveillance remain low throughout the year without visible seasonal patterns.
Figure 47.
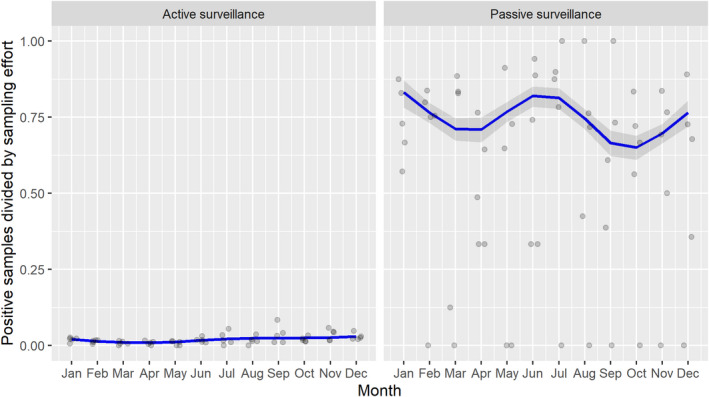
Average proportion of wild boar testing positive for ASF (PCR) in Estonia aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
Figure 53.
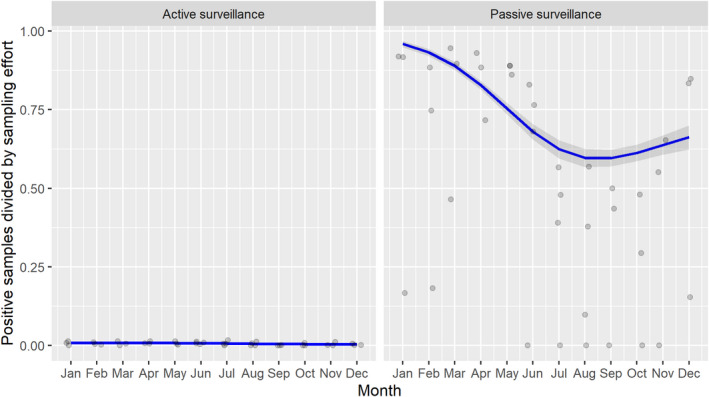
Average proportion of wild boar testing positive to ASF (PCR) in Slovakia aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
Conversely, there is a clear seasonality in the proportions of PCR‐positive samples taken from wild boar through passive surveillance, although the patterns are slightly different for the different MS. This pattern is not synchronised with the seasonal pattern observed in domestic pigs, displayed in Figure 46, where a clear peak of proportions of PCR‐positive samples between May and September is observed in Lithuania, Romania, Poland and Slovakia. In wild boar found dead (passive surveillance), the proportions of PCR‐positive samples clearly declines during summer months in Romania and Slovakia and increases during the colder months of the year. The peak of proportions PCR‐positive samples observed in wild boar in Latvia and Estonia, however, seem to be in the summer months.
Further investigations are needed studying if the surveillance regimes could have affected the observed seasonality in wild boar found dead, or to study what could be the possible underlying drivers for the observed seasonality.
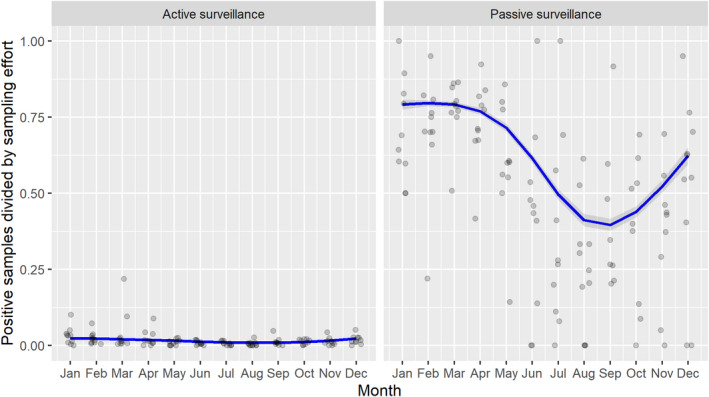
Figure 48 Average proportion of wild boar testing positive to ASF (PCR) in Hungary aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
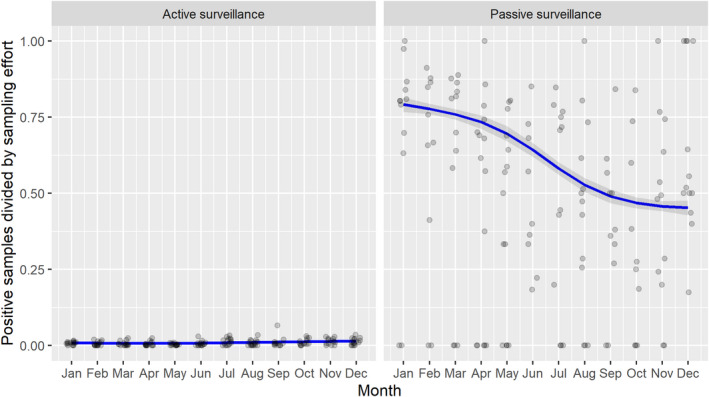
Figure 49 Average proportion of wild boar testing positive to ASF (PCR) in Lithuania aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
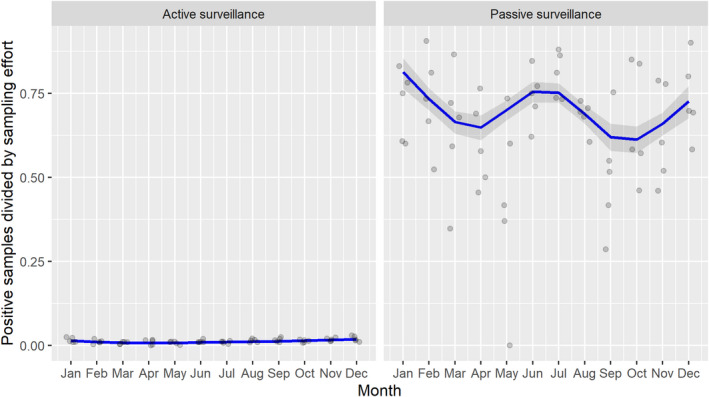
Figure 50 Average proportion of wild boar testing positive to ASF (PCR) in Latvia aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
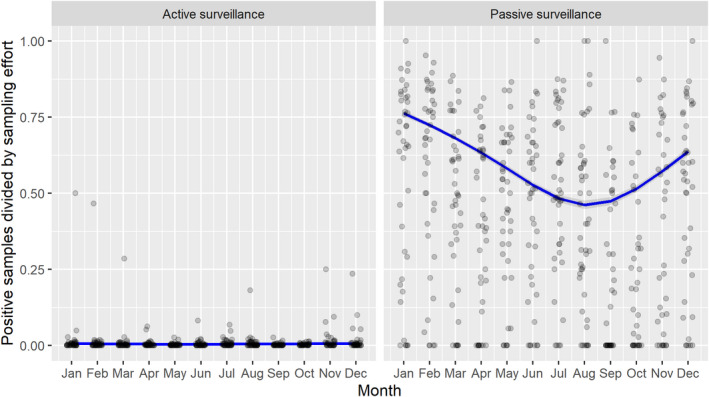
Figure 51 Average proportion of wild boar testing positive to ASF (PCR) in Poland aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
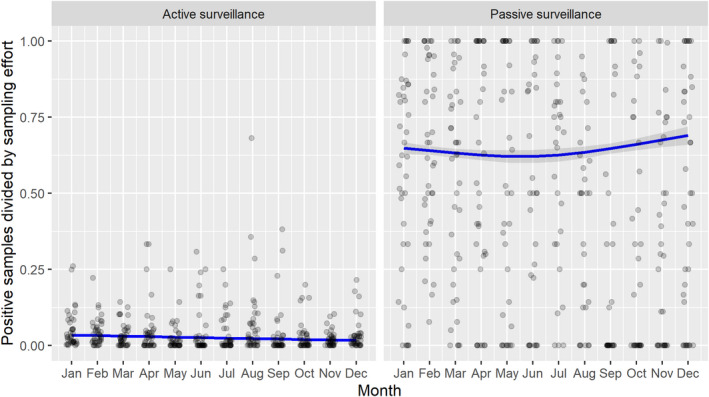
Figure 52 Average proportion of wild boar testing positive to ASF (PCR) in Romania aggregated by calendar month and NUTS 3 region for hunted wild boar (active surveillance, left figure) or wild boar found dead (passive surveillance, right figure)
4.1.6. Annual wild boar hunting harvest in ASF‐affected countries
Figure 54 shows the trend of the annual number of wild boar hunted in the European countries affected by ASF in wild boar divided by geographical region and standardised for visualisation purposes. The first graph on the top left focuses on the three Baltic States, where an initial steady increase in the numbers of hunted wild boar can be seen from 2000 onwards, followed by a rapid decline of the number of wild boar hunted since 2014–2015, when ASF was firstly detected in the area. However, in the last hunting season, a small but clear increase have been detected the three Baltic States.
Figure 54.
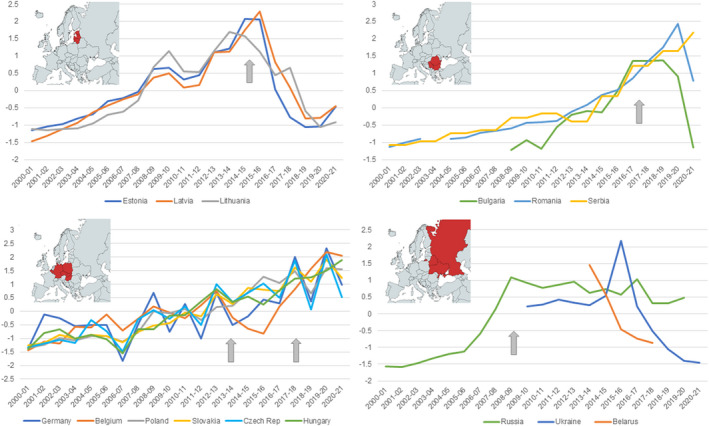
- Grey arrows show the year when ASF was firstly reported in the region.
Similarly, in the south‐eastern affected countries (graph on the top right of Figure 54), an important increase in the numbers of hunted wild boar was observed from 2000 (or 2008 for Bulgaria as no data were available beforehand). In Serbia, this steady growth continued until the last hunting season, while in Bulgaria and Romania, important declines were observed from 2018–2019 and 2020–2021, respectively, a couple of years after ASF introduction.
In Central European countries (graph on the bottom left), the hunting data followed a general increase trend with some fluctuations observed since 2000. Two arrows were added in this graph, representing when the disease was firstly reported in Poland in 2014, and when the other countries of the geographical region became infected, starting with Czechia in 2017. No clear homogenous trend on the wild boar hunted figures were observed in this region after the introduction of the disease.
Finally, in Eastern Europe, ASF‐affected countries (bottom right) the data were scarcer but point a clear reduction of hunting data in Ukraine and Belarus from 2016 and 2014, respectively. In Russia, a small but constant decrease of the hunting populations is observed since 2008.
4.1.7. Secondary cases network
As for the previous reporting period, the purpose of this investigation was to evaluate if there was a development in the numbers of potential secondary cases that could be attributed to a single source and to compare this for the first year of the epidemic, the previous reporting period and the current reporting period. Although this potential number of secondary cases (means of bootstraps calculated with a network analysis) is not to be interpreted as the true reproduction number, it can be considered as a proxy for the extent of spread in the evaluated period, and it therefore allows comparison between periods in the epidemic in the same country. This can be useful to help understanding the trend of epidemic, i.e. if it is still in the expanding phase, or if it is rather fading out (EFSA, 2021b).
Different trends can be observed in the average number of secondary cases observed (Table 4). For instance, in Bulgaria and Latvia, an increased average number of secondary cases was observed, and this decreased for some countries (e.g. Hungary) and for other countries no clear trend was observed.
Table 4.
Average number of potential secondary cases in wild boar in the EU Member States affected within different reporting periods
| Country | Date first notification | Average number of potential secondary cases in the year after the first notification (95%CI) | Date last notification in PREVIOUS reporting period | Average number of potential secondary cases in PREVIOUS reporting period | Date last notification in CURRENT reporting period | Average number of potential secondary cases in CURRENT reporting period |
|---|---|---|---|---|---|---|
| Latvia | 26/6/2014 | 2.01 (1.84–2.17) | 31/8/2020 | 1.78 (1.63–1.93) | 31/8/2021 | 1.97 (1.82–2.14) |
| Lithuania | 24/1/2014 | 2.00 (1.65–2.42) | 31/8/2020 | 1.83 (1.69–1.98) | 3/8/2021 | 1.78 (1.60–1.96) |
| Hungary | 21/4/2018 | 3.15 (2.94–3.40) | 31/8/2020 | 3.08 (2.97–3.17) | 29/8/2021 | 2.81 (2.73–2.88) |
| Estonia | 8/9/2014 | 3.5 (3.09–3.96) | 28/8/2020 | 1.63 (1.33–1.97) | 6/8/2021 | 1.57 (1.33–1.84) |
| Bulgaria | 23/10/2018 | 1.73 (1.48–2.01) | 31/8/2020 | 1.87 (1.75–1.99) | 27/8/2021 | 2.12 (1.95–2.30) |
| Romania | 29/5/2018 | 2.14 (1.98–2.29) | 31/8/2020 | 2.00 (1.89–2.10) | 30/8/2021 | 1.92 (1.84–2.01) |
| Poland | 29/5/2018 | 1.65 (1.30–2.04) | 31/8/2020 | 2.45 (2.39–2.52) | 31/8/2021 | 2.31 (2.25–2.38) |
| Slovakia | 8/8/2019 | 2.00 (1.5–2.5) | 31/8/2020 | 2.36 (2.08–2.65) | 31/8/2021 | 2.40 (2.32–2.49) |
| Germany a ) | 10/9/2020 | 6.40 (6.02–6.78) | NA | NA | NA | NA |
| Serbia | 31/7/2019 | ND | ND | ND | 18/3/2021 | 1.68 (1.44–1.94) |
| Overall range |
1.65–3.96* (1.30–6.6.78) |
1.63–3.08 (1.33–3.17) |
1.57–2.81 (1.33–2.88) |
ND: no sufficient data available for network analysis.
Germany was excluded from the overall range, as there was only 1 year of infection in the country.
In Germany, the first notification was during this reporting period, and therefore, no comparison can be made with previous reporting periods. However, the relatively high number of 6.4 potential secondary cases on average per source case, indicates that the epidemic is in its full expanding phase. Overall, the average number of potential secondary cases calculated for a single source (ASF case in wild boar) was fewer than four cases per source case for all the other countries for any of the periods investigated.
Although a 10‐fold number of cases in wild boar was observed in Slovakia in this reporting period compared with the previous period, the average number of secondary cases from a single source case did not increase. This is illustrated in Figure 55. On average, each node leads to 2.4 nodes in both periods. In the previous reporting period, the network started with only five cases in the first month of the reporting period, while in this reporting period the starting point of the epidemic was much higher, reporting 25 cases instead.
Figure 55.
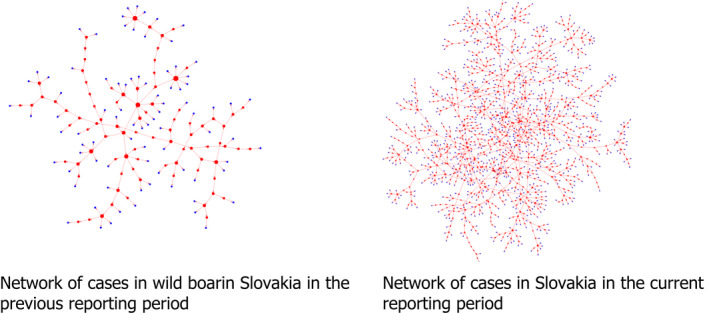
Network of cases in wild boar in the previous and current reporting periods
4.2. Risk factor analysis – TOR 2
4.2.1. Update from systematic literature review
The literature search retrieved 349 articles related to the interest on quantitative risk factors associated with ASF in domestic and/or wild boar population. The study selection process was carried out according to the PRISMA statement (Moher et al., 2009) and is reported in the flow chart shown in Figure 56.
Figure 56.
- *: The results from the risk factor analyses of the present epidemiological report were added to the 30 articles included in the review.
Thirty‐one articles (30 articles extracted from the SLR plus the results from the present EFSA epidemiological report: Sections 3.2.2 and 3.2.3) were included in the analyses, from which data were extracted in a standardised form. Of these, 22 articles (33 studies) described risk factors on ASF occurrence in domestic pigs, eight articles (24 studies) described risk factors on ASF occurrence in wild boar, and one article (two studies) described risk factors on ASF occurrence in both populations.
The main findings on the area where the study was carried out, the population of interest and the risk factors analysed in the studies are described below. Most of the articles included in the review were focused on ASF in Europe (60%), standing out were Estonia and Italy (Sardinia) with five articles each on quantitative risk factor analysis.
The factors potentially involved in the occurrence, spread or persistence of ASF analysed in the various articles were numerous (n = 621) and very heterogeneous, including factors related to pig farming (e.g. pig population density, farm management, biosecurity), wild boar management (e.g. hunting‐related variables and wild boar abundance), habitat‐related factors (e.g. vegetation, waterbodies and climatic conditions), socio‐economic factors (e.g. human population density, demographic characteristics and access to laboratory services) and the presence of the virus and vectors in the area.
The risk factors found to be significant for ASF presence/spread/occurrence in the selected articles were grouped in subcategories and broader categories to be able to analyse the results of the literature review. For each category and subcategory of risk factors, frequency indicators were calculated including the number of articles, where those factors were analysed, and the number of studies (as an article may include more than one study, e.g. focusing on different study areas or using different methodologies). All this information is summarised in Table 5 for domestic pigs and in Table 6 for wild boars, including the references where those factors were analysed.
Table 5.
Results of the systematic review of the quantitative risk factor analysis of ASF in domestic pigs
| Risk factor category 1 | Risk factor category 2 | Risk factor category 3 | N significant risk factors | N studies | N articles | Refs |
|---|---|---|---|---|---|---|
| Pig farming | Biosecurity | Access to farm/pens | 5 | 3 | 3 | Fasina et al. (2012), Kabuuka et al. (2014), Awosanya et al. (2015), Nantima et al. (2015), Dione et al. (2017), Boklund et al. (2020), Bisimwa et al. (2021), Gao et al. (2021) |
| Carcasses/waste management | 3 | 2 | 2 | |||
| Cleaning and disinfection | 2 | 2 | 2 | |||
| Contact with other farms | 1 | 1 | 1 | |||
| Disease‐related measures | 3 | 2 | 2 | |||
| Food and water control | 4 | 4 | 4 | |||
| Hygiene practices | 1 | 1 | 1 | |||
| Introduction of new pigs | 1 | 1 | 1 | |||
| Pests management | 1 | 1 | 1 | |||
| Vectors management | 1 | 1 | 1 | |||
| Farm management | Herd size | 3 | 3 | 3 | Nantima et al. (2015), Dione et al. (2017), Cappai et al. (2018), Boklund et al. (2020), Chambaro et al. (2020), Nurmoja et al. (2020), Bisimwa et al. (2021) | |
| Housing type | 2 | 2 | 1 | |||
| Management of pigs | 2 | 2 | 2 | |||
| Management of pigs breeding | 1 | 1 | 1 | |||
| Treatments | 2 | 2 | 2 | |||
| Feed/water/bedding | Bedding | 1 | 1 | 1 | Boklund et al. (2020) | |
| Feeding | 1 | 1 | 1 | |||
| Non‐compliance | ASF rules | 1 | 1 | 1 | Martínez‐López et al. (2015), Cappai et al. (2018), Jurado et al. (2018) | |
| Census | 2 | 2 | 2 | |||
| Free‐range grazing and illegal breeding | 1 | 1 | 1 | |||
| Pig population density | Farm density | 12 | 7 | 5 | Gulenkin et al. (2011), Martínez‐López et al. (2015), Vergne et al. (2016), Huang et al. (2017), Cappai et al. (2018), Jurado et al. (2018), Mur et al. (2018), Loi et al. (2019), Andraud et al. (2021) | |
| Pig population density | 18 | 12 | 8 | |||
| Pig trade | 6 | 5 | 5 | Nantima et al. (2015), Cappai et al. (2018), Jurado et al. (2018), Bisimwa et al. (2021), Glazunova et al. (2021) | ||
| Pig farming Total | 74 | 26 | 20 | |||
| Socio‐economic factors | Demographic characteristics | Farmer demographics | 11 | 3 | 4 | Dione et al. (2017), Cappai et al. (2018), Loi et al. (2019) |
| Population demographics | 10 | 2 | 3 | |||
|
Human population density |
Population density | 7 | 4 | 7 | Gulenkin et al. (2011), Martínez‐López et al. (2015), Vergne et al. (2016), Huang et al. (2017), Cappai et al. (2018), Andraud et al. (2021) | |
| Road/rail density | 8 | 4 | 7 | |||
| Urban areas | 1 | 1 | 1 | |||
| Access to laboratory services | 3 | 2 | 2 | Vergne et al. (2016), Cappai et al. (2018) | ||
| Socio‐economic factors Total | 40 | 11 | 8 | |||
| Habitat | Altitude | Altitude | 1 | 1 | 1 | Martínez‐López et al. (2015) |
| Climatic conditions | Seasonality | 1 | 1 | 1 | Awosanya et al. (2015) | |
| Fauna | Other wild animals | 1 | 1 | 1 | Dione et al. (2017), Huang et al. (2017) | |
| Wild suids | 1 | 1 | 1 | |||
|
Vegetation |
Crops | 1 | 1 | 1 | Okoth et al. (2013), Boklund et al. (2020), Andraud et al. (2021) | |
| Protected area | 1 | 1 | 1 | |||
| Trees | 3 | 3 | 1 | |||
|
Waterbodies |
Wetlands | 3 | 3 | 1 | Gulenkin et al. (2011), Cappai et al. (2018), Andraud et al. (2021) | |
| Waterbodies | 6 | 6 | 3 | |||
| Habitat total | 18 | 12 | 9 | |||
| Area | Presence of pig‐related activities | Abattoir | 1 | 1 | 1 | Fasina et al. (2012) |
| Country | Country | 2 | 1 | 1 | Nantima et al. (2015) | |
|
ASFV‐infected area |
Distance to affected areas | 3 | 2 | 1 | Boklund et al. (2020), Cappai et al. (2018), Fasina et al. (2012), Huang et al. (2017), Nurmoja et al. (2020), Vergne et al. (2017) | |
| Presence of infection | 11 | 8 | 6 | |||
| Area total | 17 | 10 | 7 | |||
|
WB management |
WB abundance |
WB abundance | 1 | 1 | 1 | Jurado et al. (2018), Boklund et al. (2020) |
| WB density | 3 | 3 | 2 | |||
| WB management Total | 4 | 4 | 3 | |||
|
Vectors |
Ticks |
In the area | 1 | 1 | 1 | Kabuuka et al. (2014), Huang et al. (2017) |
| On pigs/in the farm | 1 | 1 | 1 | |||
| Vectors total | 2 | 2 | 2 | |||
| TOTAL | 155 | 33 | 22 |
Table 6.
Results of the systematic review of the quantitative risk factor analysis of ASF in wild boar
| Risk factor category 1 | Risk factor category 2 | Risk factor category 3 | N significant risk factors | N studies | N articles | Refs |
|---|---|---|---|---|---|---|
| Habitat | Vegetation | Trees | 3 | 3 | 3 | EFSA (2021a), Loi et al. (2019), Podgórski et al. (2020), current report) |
| Crops | 1 | 1 | 1 | |||
| Growth | 1 | 1 | 1 | |||
| Waterbodies | Waterbodies | 2 | 2 | 1 | EFSA (2022) | |
| WB suitability | Suitable wild boar area | 2 | 2 | 1 | Lim et al. (2021) | |
| Altitude | Altitude | 1 | 1 | 1 | EFSA (2022) | |
| Climatic conditions | Seasonality | 1 | 1 | 1 | Podgórski et al. (2020) | |
| Habitat total | 11 | 9 | 5 | |||
| Socio‐economic factors | Demographic characteristics | Population demographics | 7 | 1 | 1 | Loi et al. (2019) |
| Human population density | Human footprint index | 2 | 2 | 2 | EFSA (2018, 2021a,b, current report) | |
| Road/rail density | 1 | 1 | 1 | |||
| Urban areas | 2 | 2 | 2 | |||
| Socio‐economic factors total | 12 | 6 | 4 | |||
| WB management | Hunting‐related variables | Number day hunted | 1 | 1 | 1 | Current report |
| Number hunting dogs | 1 | 1 | 1 | |||
| WB abundance | WB abundance | 2 | 2 | 2 | Dione et al. (2017), EFSA (2018, 2021a,b), Podgórski et al. (2020, current report) | |
| WB density | 4 | 3 | 3 | |||
| WB management total | 8 | 6 | 5 | |||
| Area | ASFV‐infected area | Distance to affected areas | 3 | 3 | 2 | Podgórski et al. (2020), Lim et al. (2021), EFSA (2022) |
| Presence of infection | 2 | 2 | 1 | |||
| Area total | 5 | 5 | 3 | |||
| Pig farming | Pig population density | Farm density | 1 | 1 | 1 | Dione et al. (2017), EFSA (2018, 2021a,b), current report) |
| Pig population density | 3 | 3 | 3 | |||
| Pig farming total | 4 | 4 | 4 | |||
| Total | 40 | 13 | 8 |
4.2.1.1. Domestic pigs
The two main risk factor categories found to be significant for the occurrence of ASF in domestic pigs were those related to pig farming (this category of factors was found to be significant n = 74 times; from now onwards ‘n’ will refer to the number of times a category or subcategory of factors were found to be significantly correlated with ASF) and socio‐economic factors (n = 40).
In relation to pig farming, the two subcategories most frequently identified as significantly correlated with ASF were the pig population density (n = 30) and factors related to biosecurity (n = 22). Pig population density has been analysed in many ways, including the density of farms (n = 12) and the density of pig population (n = 17), either in general or by specific farming/production types (e.g. confined farms, commercial farms, breeding farms, free‐ranging farms). No specific trend was observed in relation to the type of farm most found to be significant for the risk of ASF in domestic pigs.
Biosecurity is a very wide category encompassing factors related to the access to the farm or pens, practices related to the management of carcasses and waste, pest and vectors managements, feed and water biosecurity practices, as well as protocols for introduction of new pigs or personnel. Importantly, this subcategory includes factors that resulted protective and could be considered as good biosecurity practices (e.g. controlled access to farm and pens, the routine cleaning of the pig pen and vector control), as well as risk factors related to poor biosecurity practices (e.g. inadequate carcass management, swill feeding and no separation of sick pigs).
Other factors found to be significant in the context of pig farming were the management of the farm (herd size and housing system); the non‐compliance with rules (i.e. ASF control programmes, illegal breeding and periodical census activities); the origin of feed/ source of water and bedding material; and pig‐trade‐related factors.
On socio‐economic factors, demographic characteristics were very frequently found to be significant (n = 21), followed by human population density indicators (n = 15) and access to the laboratory services (n = 3). The demographics subcategory includes risk factors related to the farmer (n = 11), such as lower age and education level, as well as population demographic factors (n = 10), like the deprived conditions of a region or unemployment rates, which were found to be at increased ASF risk. However, it is important to emphasise that this category of factors was analysed almost exclusively in studies focused on Sardinia, where specific swine production types and a long history of ASF is present. Therefore, extrapolation of these results to other areas should be done cautiously. Human population density has been analysed as the true population density or using proxies (presence of roads, urban areas, human footprint index). However, contradictory results were obtained in different studies as whereas an increasing human population density was found to be generally a risk, in one case human population density was identified as a protective factor for ASF in domestic pigs (Cappai et al., 2018).
In addition to pig farming and anthropogenic factors, other types of factors less frequently found to be significant for ASF in domestic pigs were those related to the habitat (n = 18), the area itself including previous ASFV infection or presence of abattoirs (n = 17), wild boar management (n = 4) and the presence of vectors (n = 2). Together with pig and farm density and demographics, the presence of ASFV infection in the area was one of the risk factor category 3 most often found to be significant for the occurrence of ASF (n = 11). This emphasises the importance of short distance spread in the epidemiology of the disease in domestic pigs and the need to identify the specific routes and materials most involved in those short distance infections.
Factors related to habitat (i.e. vegetation, waterbodies, fauna and climatic conditions) were significant in a considerable number of studies for the occurrence of ASF in domestic pigs. However, whether that relates directly to the presence of ASF in domestic pigs or indirectly through the wild boar potentially affected population is unclear.
4.2.1.2. Wild boar
For wild boar, the total number of risk factors found to be significantly correlated with the risk of ASF was much smaller than for the domestic pigs (40 in wild boar vs. 155 in domestic pigs). The risk factor categories most frequently found to be significantly correlated with the risk of ASF in wild boar were those related to the socio‐economic factors (n = 12), habitat‐related factors (n = 11) and wild boar management‐related factors (n = 8) as detailed in Table 6.
The factors included in the habitat category were related to the vegetation, waterbodies, wild boar suitability areas and climate conditions. All of those are clearly related to the presence or absence of wild boar populations. As socio‐economic factors, numerous population demographic factors (n = 7) were found to be significantly correlated with ASF in wild boar. However, all those seven factors (employment rate, criminality rate, thefts, etc.) were analysed in the same study and therefore other studies should be done to verify the importance of these factors in other areas.
Interestingly, only eight wild boar management factors, including hunting‐related activities or wild boar abundance, were found to be significant for the risk of ASF in wild boar. This could be partly explained by the extreme difficulties existing to obtain good quality, standardised data on wild boar populations and management of those populations. In this sense, the work of ENETWILD is essential for building the networks and creating harmonised ways of collecting data. Only by the collection of and analysis of good quality data, will it be possible to identify the practices that pose a risk or protect from ASF spread.
Finally, the factors related to pig farms and ASF presence in the area were found to be significant five and four times, respectively. However, as in the domestic pigs, the significance of pig farming is most probably related to the risk in domestic pigs affecting indirectly the risk in wild boar.
4.2.2. Risk factors for the occurrence of ASF in wild boar in the different districts of Slovakia, analysis with Generalised Linear model
Figure 57 shows the percentage of ASF PCR‐positive wild boar samples per reported district in Slovakia in 2020. The eastern and central south of the country concentrated the positive PCR samples from wild boar, while most of the western districts and some scattered districts in the east and surrounding did not report any positive PCR samples in wild boar during 2020.
Figure 57.
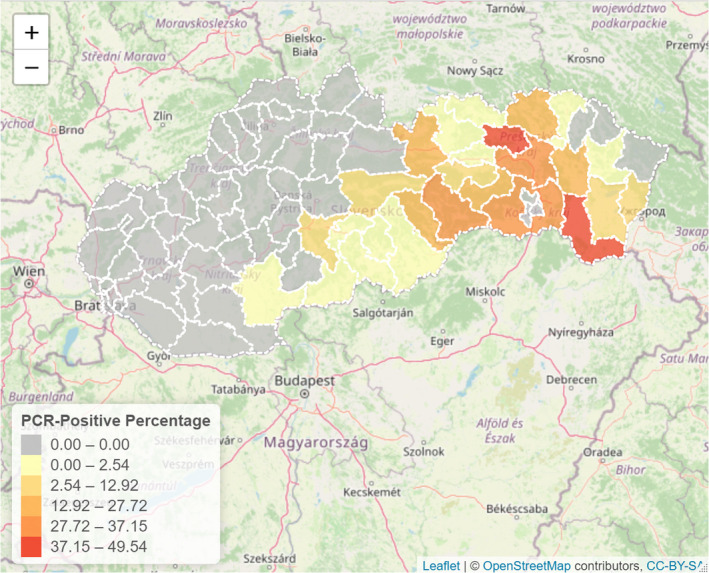
Proportion of ASF PCR‐positive reported per district in 2020 in Slovakia
A generalised linear model was used to estimate the probability to observe ASF PCR‐positive results in wild boar per district (Table 7) based on a set of potential covariates previously identified. Covariates with a variance inflation factor (VIF) > 5 were excluded from the later analysis due to collinearity (Section 2.5), although a manual check was performed to ensure that at least one variable per category was included in the model. The final model obtained, using backward selection procedure, is presented in Table 7.
After discarding many variables due to multicollinearity issues, the generalised linear model found that the probability to find at least one PCR‐confirmed ASF case in wild boar in a district was mainly influenced by the habitat (croplands), wild boar abundance and the presence of ASF PCR‐positive samples in domestic pigs in the district.
The model results indicate relationship between the presence of ASF in pig farms in the district and the detection of ASF in wild boar. The PCR‐positive results in domestic pigs for ASF were correlated with the density of small size swine farms in the district, considering those as farms with fewer than 10 animals per establishment. The density of small farms was originally excluded from the initial model due to collinearity with other farming variables (VIF 7.1) and ASF in domestic pigs (see Figure 1). However, considering the importance of this factor, previously pointed out by other studies as a relevant risk factor (see Section 4.2.1), a second model was run including the small farm density as a covariable. No significant results were obtained for this factor, suggesting that additional factors than the size of the farm contribute to the risk of ASF in domestic pigs as well as in wild boar in the district.
In addition, wild boar abundance (estimated based on hunting bag data) and the density of crops in the district, are correlated with the wild boar suitability and climatic factors, among others. The model identified urban habitat and the number of dogs used for hunting activities in the district as protective factors for the probability of ASF detection in wild boar in Slovakia. The percentage of surface occupied by urbanised areas could act as a protective factor as in those areas highly urbanised the density of wild boar is expected to be lower. Previously, the number of hunting dogs (per km2) has been considered as a proxy of the hunting pressure in an area (Vajas et al., 2020). However, in this case, the Veterinary Authorities of Slovakia informed EFSA that in areas where ASF is present, dogs are not used for hunting, but only to track injured game and must be kept on a belt. Therefore, the result of the analysis confirms the effectiveness of this national policy on hunting, but do not make it possible to infer any additional conclusion from it.
4.2.3. Risk factors for the occurrence of ASF in wild boar in different counties of Romania, analysis with a Besag York Mollié model (BYM)
For Romania, detailed data were available for four consecutive years at NUTS 3 level (2018–2021). Therefore, instead of a generalised linear model (GLM), the risk factors were analysed using a BYM that considers the year effect. The probability to obtain a PCR‐positive test result in samples of wild boar shot or found dead in each of the counties (NUTS 3) of Romania from 2018 to 2021 as generated by the BYM model is presented in Figure 58. At the beginning of the epidemic (2018), the ASF‐positive cases in wild boar were highly concentrated in the south‐eastern region of the country. However, as the epidemic evolved from 2019, the probability of having a positive result in wild boar samples was homogenously distributed in Romania, with quite high probabilities (more than 0.8) in most of the counties. As can be observed in the maps, it was much more likely to obtain a positive result in 2019 than in 2018, and in 2021 than in 2020.
Figure 58.
Average probability to obtain a PCR‐positive test result in samples from wild boar in the different counties of Romania, from 2018 to 2021
The results of the BYM identified the ASF PCR‐positive results in domestic pigs in the county as a significant risk factor for the presence of ASF in wild boar, while the number of hunting days and the density of waterbodies per district were protective factors. In addition, there was a significant year effect in 2019 compared with 2018, as it is observed in Figure 58, when the probability of finding ASF PCR‐positive results in wild boar was much higher than in the previous year. The lack of historical data on pig population in Romania did not allow the study of the correlation between ASF in pigs and the density of farms or any type of farm size. However, the results of the model indicate that, in Romania in the counties where active circulation of ASF virus exists in the domestic population, there is a higher risk for wild boar to be infected, and/or vice versa. The direction of this interaction cannot be elucidated from current results, and still more field studies are needed in this area (Table 8).
Table 8.
Outcomes of Bayesian hierarchical model (BYM) after stepwise elimination of non‐significant variables to estimate the probability to obtain a PCR‐positive test result in samples of wild boar shot or found dead in each of the counties (NUTS 3) of Romania from 2018 to 2021
| Mean | 0.025 quantile | 0.975 quantile | |
|---|---|---|---|
| Effect year 2019 | 2.9 | 1.3 | 4.9 |
| Effect year 2020 | 7.9 | −7.5 | 33.3 |
| Effect year 2021 | 1.3 | ‐0.2 | 2.9 |
| Number of hunting days | −2.7 | −5.4 | −0.2 |
| ASF in domestic pigs | 3.3 | 1.8 | 5.1 |
| Waterbodies | −3.7 | −6.8 | −0.6 |
From all the hunting variables available for Romania (see Table 1), only four were included in the model analysis (i.e. feeding tonnes, number of hunters, hunting days and wild boar piglets observed per year), as all the other factors presented high VIF, been intrinsically correlated. Finally, only the hunting days resulted significant in the model. However, considering the high correlation existing between this variable and all the other hunting variables (Figure 1B), hunting days could be considered a good proxy of the hunting pressure in the area. This suggests that intense hunting helps to reduce the probability of recording positive PCR results in wild boar in Romania. The exact effect of the different hunting methods requires additional information and analysis.
In this model, the density of waterbodies was identified as a protective factor. As previously discussed in the literature review, this variable has been identified in previous studies as a significant factor for ASF occurrence in wild boar and domestic pigs. However, contradictory results have been obtained by different authors. Whereas in some analysis water resulted as a risk factor (in the Baltic States in this report), in others (including this model) it acted as a protective factor. This fact can be highly influenced by the latitude and climatic conditions of the area analysed. Therefore, the influence of water and ASF is complex, as many other parameters can act as confounders, which makes it difficult to draw clear conclusions from this result.
In contrast with current results, in a previous ASF epidemiological report (EFSA, 2021a), the analysis of risk factors for ASF in wild boar at NUTS 3 level in Romania (2017–2019) only identified the human footprint index as significant factor. However, the previous year analysis did not include the additional hunting information included this year, nor the presence of ASF in domestic pig population as potential risk factors. This can explain the differences in outcomes, and the results reinforce the importance of the message previously convened that higher spatial and temporal resolution data are needed to improve the risk factor analysis.
Despite the use of different methodologies (GLM vs. BYM) and datasets (historical vs. 1 year, different spatial units and different hunting variables), the results of Slovakia and Romania provided similar results, suggesting a relationship between ASF in domestic pigs and in wild boar, as pointed out also in the descriptive section of this report. Additional data on higher resolution on the type of farms, biosecurity conditions and detailed field data would be useful to further investigate the potential relationship between domestic and wild boar.
These results are in line with the results obtained in the risk factors analysis in previous ASF epidemiological report (EFSA, 2021a), that identified wild boar abundance, environmental factors and backyard pigs as the main drivers for ASF in wild boar in Romania.
4.2.4. Risk factors for the occurrence of ASF in wild boar in the Baltic States, analysis with a Besag York Mollié model (BYM)
The Bayesian hierarchical model in the Baltic States at LAU level for the years 2017–2020 identified the density of waterbodies and the mean altitude per LAU as significant risk factors, and the human foot index as a protective factor. A significant year effect was observed for all the years of the analysis (2018, 2019 and 2020), as the probability of PCR‐positive results in wild boar clearly decreased during the period of the study, as already discussed in the TOR1 of the present report.
None of the other parameters related to the wild boar abundance, hunting bags and domestic pig population were significant in the model. Several factors could have influenced this outcome. First, the data were obtained from different sources between countries and within countries for different years (see Appendix A), with potential different biases for data collection [i.e. some years wild boar hunted data were directly obtained from the veterinary competent authorities, but in some others, these data were obtained through ENETWILD‐consortium and colleagues (2020)]. Second, some of the relevant variables used in the other models, such as the ASF‐positive PCR in domestic pigs or additional information on hunting activities, were not included in the analysis, as these data were not available for all years for all three countries. Therefore, more detailed studies that focus in smaller areas with higher resolution harmonised data are recommended in the future to explore the risk factors of ASF in the Baltic States.
4.3. Modelling the effectiveness of ASF measures applied in white zones bordering a region with ASF in the wild boar population where limited control measures are implemented – TOR3
The assessment addresses a particular epidemiological situation: WZ measures are implemented in wild boar populations neighbouring an area with ASF in the wild boar population where no or inadequate control measures are in place. These neighbouring areas are called, for simplicity, from here onwards neighbouring areas with limited control (ALC). The effectiveness of the WZ is potentially hampered by the continued spread of the infection in the ALC, i.e. along the demarcation line in Figure 3. The model‐based simulation of the WZ approach in an area adjacent to an ALC revealed three main insights, which will be explained in more detail in the next sections.
First, the effectiveness of the WZ approach was greatly reduced when applied in an affected area next to an ALC. This was assessed by comparing the outcome of control measures simulated in the same geographical environment either next to an ALC (Scenario A in Figure 5) or in the absence of neighbouring infections (similar to a focal introduction, Scenario C in Figure 5).
Second, applying WZ measures in an affected area next to an ALC does require substantially greater efforts to stop the infection compared with the measures applied in the WZ after a focal introduction, such as previously addressed in Czechia or Belgium. This was assessed by comparing the failure rate of the Scenarios A and C for alternative WZ parameters, i.e. by changing the width of the WZ, the target density of population reduction and the distance between WZ and CA as recommended in previous EFSA’s Epidemiological Report (EFSA, 2021a). Moreover, the gain of adding fences and carcass removal to the WZ measures was considered by varying their effectiveness (permeability of fences; intensity of carcass removal).
Third, the proactive establishment of a WZ along the entire demarcation line with the ALC (proactive WZ) was beneficial compared with the reactive WZs, with concentrical constructions of WZ in response to successive entries of the infection from the ALC. However, the proactive WZ did require a larger culling effort. This could be compensated by a risk‐based release of the measures in segments of the WZ (proactive WZ roll‐back), if the WZ segment is free of ASF and the epidemic front did already pass the adjacent segment of the ALC.
4.3.1. Reactive WZ establishment
4.3.1.1. Comparing limited control neighbourhood and focal introduction
The application of the WZ protocol next to an ALC is complicated due to the continued risk of entry along the demarcation line of the ALC (Figure 3). Therefore, the efficiency of the overall WZ strategy next to an ALC as measured by the probability to fail was substantially reduced (Figure 59). When comparing the probability of the WZ to fail in proximity to an ALC (red bars) with the focal situation (green bars), an increase is seen for each of the three widths of simulated WZ (A, B, C) and for two target densities (here achieved within 6 months after establishment of the WZ). The increase is most pronounced for the single simulation landscape (solid bars), but the tendency of the outcome is the same, when considering 1,000 randomly located simulation landscapes from European wild boar habitat (striped bars; Figure 4).
Figure 59.

- The diagrams illustrate the effect of the width of the WZ (A = 4 km, B = 8 km, C = 13 km) for two alternative depopulation scenarios (x‐axis; 0.5 vs. 1 animal per km² after 6 months, respectively, buffer width 8 km). Red bars show the probability to fail for the WZ applied in an area adjacent to a region with limited control, and green bars the focal situation. Solid bars represent one selected geographical area and striped bars summarise results for randomly selected simulation areas. Parameterisation of the WZ is listed on top of the diagram.
Figure 59 also highlights, by comparing the same bar across the three diagrams, the effect of the width of the WZ on its effectiveness (i.e. the reduction in failure rate) is more pronounced in the scenario with the lower target density compared with that with the higher. For example, with target population of one animal per km2, the failure rate is largest in Figure 59a. Then the doubled (B) or threefold width (C) of the WZ reduce the average failure rate only from 84% to 80% and 73% for the selected region (solid bar). In comparison with the strategy that achieves 0.5 animals per km² at the same time, the reduction of failures is 67% to 41% to 23%. Therefore, the relative efficiency in the former is 0.13 vs. 0.66 in the latter (i.e. realised/possible reduction).
4.3.1.2. Parameters strengthening the efficiency of the white zone approach in the limited control neighbourhood situation
Figure 59 revealed the increased risk to fail with the WZ approach when applied in an area next to an ALC. In difference to the focal approach, the largest WZ width did not reduce the probability to fail to below 20%. Therefore, it was useful to understand more interactions of typical strategy parameters in the particular epidemiological context near an ALC. Three parameters were investigated: (i) no carcass removal in difference to removing carcasses in the WZ, the area between CA and WZ and the CA, (ii) no fencing versus fencing around WZ, and (iii) alternative time horizons until population reduction to a set target is achieved.
Figure 60.
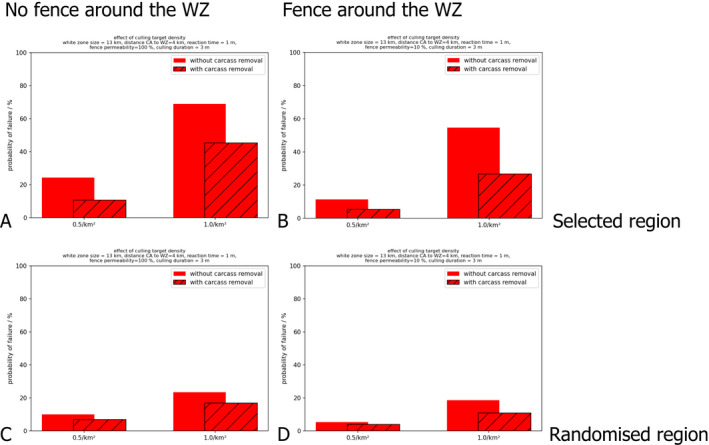
Model output of the probability of overall failure of the white zone strategy for scenarios combining fencing the white zone (A, C without fence; B, D with fence around the white zone) and carcass removal (solid bars without, stripped bars with 40% carcass removal)
Parameterisation is listed on top of the diagram.
Figure 60 shows the model output of the probability of overall failure of the WZ strategy for the selected simulation landscape (a, b) and the randomised landscapes (c, d). Per column the diagrams illustrate the effect of fencing the WZ (a, c without fence; b, d with fence around the WZ). The data are shown for two alternative depopulation scenarios (x‐axis; 0.5 vs. 1 animal per km2 after 3 months with a buffer zone of 4 km). The solid bars represent the data without carcass removal, and the diagonally striped bars assume 40% carcass removal in the simulation area (CA + between CA and WZ + WZ).
For the same target density value, the combination of both measures (striped bar right in b) results in largest improvement against the outcome without the two options (solid bar right in a). Interestingly the striped bar in a (adding carcass removal of 40%) is always lower than the solid bar in b (adding fences without carcass removal). Figure 60(c, d) illustrate the equivalent message for the average of randomly selected landscapes although the relative effect is always lower given the generally lower probability to fail in the overall average.
4.3.1.3. Distance between core area and white zone at time of declaration
When setting a WZ, it is critical to decide on the right distance to the CA; usually fenced area containing all notified ASFV infections and meant also to contain all yet‐not‐detected infections. The suitable choice of the distance between CA and WZ is to ensure that the time to achieve the planned population reduction inside the WZ fits the period the infection would need to spread towards the WZ after an accidental escape from the CA.
Figure 61 illustrates the effect of different combinations of the distance from CA to the WZ and the time to achieve the target of depopulation (A 17 km and 12 months, B 8 km and 6 months, C 4 km and 3 months). The analysis is shown for two alternative depopulation scenarios (x‐axis; target population density 0.5 vs. 1 animal per km2). Solid bars represent one selected geographical area and hashed bars summarise results for randomly selected simulation areas.
Figure 61.
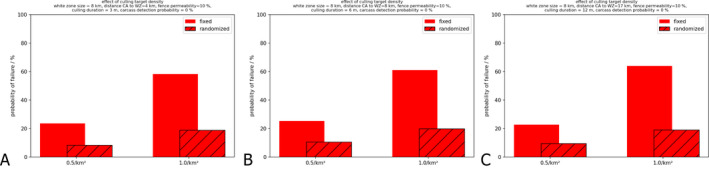
- Parameterisation of the WZ is listed on top of the diagram.
In the scenarios shown in Figure 61 the distance between the WZ and the CA was increased from 4 km, 8 km to 17 km. However, the time until the target density after depopulation was achieved was adjusted to the set distance, i.e. dependent on the local speed of propagation in the geography of the simulation landscape.
The outcome in terms of probability to fail with the strategy did not reveal noteworthy differences between the combinations of distance from CA to WZ (4, 8 and 17 km) and depopulation time (3, 6, 12 months); neither for the two target densities nor for the landscape selection. Indeed, the result implies that the suitable alignment between distance of WZ from CA and time to reach depopulation target is sufficient to enable the minimal failure level (given the width of the WZ and other measures like fencing and carcass removal efforts are the same). The aligned distance between WZ and CA results from multiplying the approximate speed of propagation in an area (or reasonable upper bounds) with the time planned for culling efforts to reach the target density in the WZ, i.e. how far the infection will advance during implementation of culling measures. However, logically, the effort to manage carcasses increases the wider the distance between WZ and CA, while the time left to reach the target population level inside the WZ shortens the shorter the distance chosen. If distance between WZ and CA and time to reach the target population density are aligned, then there is room for practically driven decisions on which pair of distance and culling time is preferred.
4.3.1.4. Target population density
The intuitive expectation was that an a priori stricter target population density in the WZ, would result in more animals being culled. In the initial implementation phase of the WZ (until the target density was reached), this was confirmed from the model analysis. However, independently of the width of the WZ, the final cumulative culling effort was always lower for the a priori stricter target (Figure 62). Given that the WZ must be sustained for longer than the initial time anticipated to reach the target population density, an a priori stricter target pays back. This is reasonable, because the fewer animals that survive the initial implementation phase, the slower is a possible recovery between repeated culling campaigns. Therefore, the cumulated culling effort is smaller if a stricter target population density is maintained (e.g. 0.5/km2 instead of 1/km2).
Figure 62.
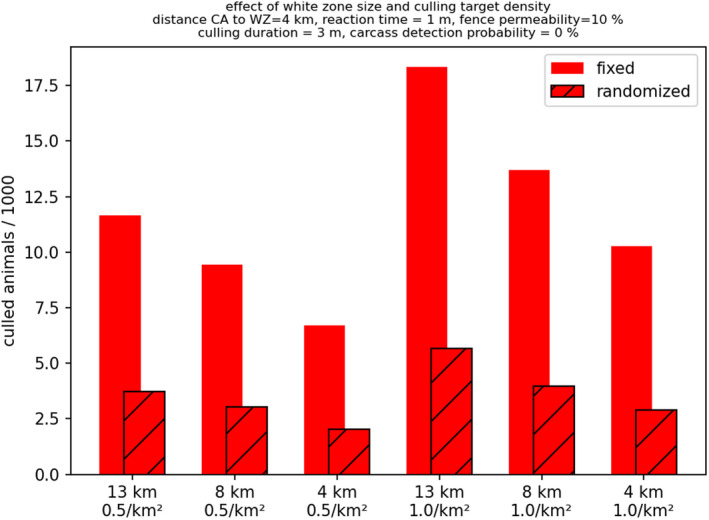
- Output is shown for the single (solid bars) and the randomly selection of simulation landscape per model run (striped bars). Parameterisation is listed on top of the diagram.
4.3.1.5. Summary of analysis of the reactive white zone approach aside a limited control neighbourhood
Figure 63 summarises the main outcomes of the simulation experiments for the WZ application adjacent to an ALC. The x‐axis provides the effort in terms of culled animals, the y‐axis summarises the outcome of the strategy application in terms of failure rate, the colour of the data points refers to target density aspect (red: 1.0 and green: 0.5 animals/km2), the direction of the triangles refers to the width of the WZ, i.e. 4, 8 and 13 km.
Figure 63.
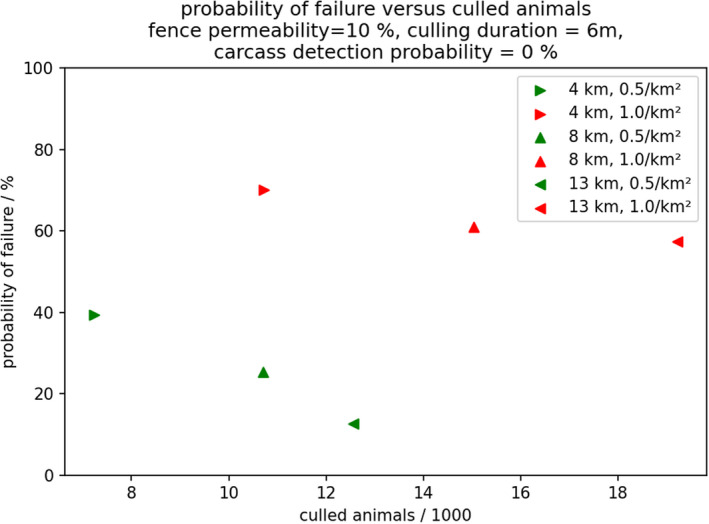
Model output for the fixed landscape relating the culling effort and the probability of failure per white zone parameters WZ width and target density of population reduction
4.3.2. Proactive white zone establishment
Section 4.3.1 considered the reactive establishment of multiple independent WZs in response to the sequel of entries of ASFV infections along the demarcation line with the ALC. In contrast, the following simulations add the perspective of a proactive WZ set up along the complete demarcation line directly when the first entry happens. It is expected to find a reduced probability to fail, because there is an early prepared WZ packed to the borderline and independent of the spatial vicinity to the risk of circulating ASFV infections. It is also expected to find increased culling effort associated with the proactive WZ, because the surface of the WZ is possibly larger than the one built from several reactive WZs. The following section addresses the respective quantitative response from the model simulations.
4.3.2.1. Comparison of failure rate between reactive and proactive white zones next to a neighbourhood with limited control measures in place
Figure 64 shows the comparison of the reactive (red) and proactive WZ in terms of probability to fail with the strategy. The bars represent the average outcome of simulations with a particular width of the WZ (x‐axis 4, 8 and 13 km).
Figure 64.
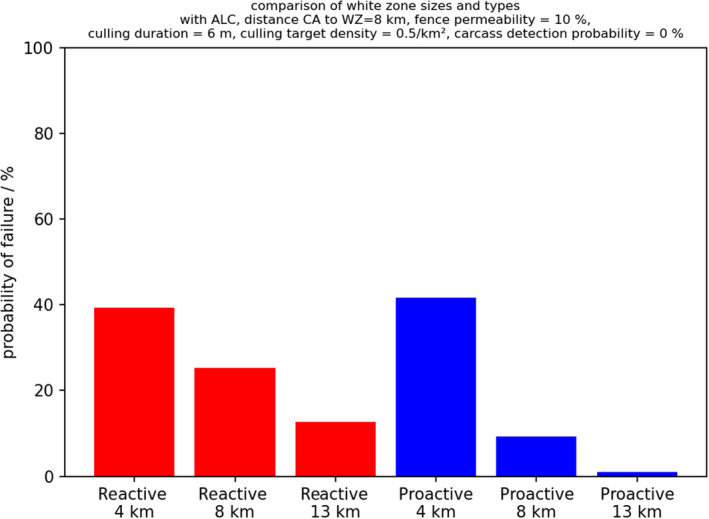
Model output for the fixed landscape of the probability of overall failure of reactive (red) and proactive (blue) white zone scenarios as a function of the width of the white zone. Parameterisation is listed on top of the diagram
The outcome improved when replacing the reactive WZ by the proactive approach. Figure 64 shows the model outcome for different widths of the WZ. A failure rate below 10% or even close to zero is seen for the wider WZ (8 and 13 km). The smaller WZ (4 km) does not show noteworthy reduction in the probability of failure. The simulations in Figure 64 are based on optimistic configurations, i.e. achieving a strict target population density of 0.5 wild boar per km² and fenced WZ. Therefore, the unchanged outcome at 4‐km width suggests that this is an insufficient dimension of the WZ, even with a proactively prepared WZ. The risk for the infection to cross a 4‐km wide area is obviously high enough to disqualify it as an appropriate choice. In other landscapes or with less stringent WZ parameters there was not always an improvement when applying the proactive approach.
4.3.2.2. Proactive WZ and segmental roll‐back
The proactive WZ, implies extra costs, given that the population in the enlarged WZ area must be continuously maintained at the target density. Comparing the final surface for the reactive vs. proactive WZ approach, an area of 4,000 km² vs. 3,400 km² was enclosed, while in total 28.000 vs. 68.000 wild boar had to be culled. The threefold increase in culling effort may render the proactive WZ very costly; particularly when the width of the WZ has to be increased.
The drawback of the proactive WZ results from the continuous culling efforts all over the total WZ, even when there is no more infection in parts of the ALC and the adjacent compartment of the WZ (Figure 3). Therefore, it was tested whether the segmentwise release of the WZ in response to current distribution of infection could compensate the increase in culling effort required with the proactive WZ approach (‘roll‐back’).
Figure 65 puts together the comparison of the proactive WZ and proactive WZ with roll‐back approach. The outcome of the comparison of blue vs. green data series underscores that the proactive WZ with roll‐back did result in similar success rate as the reactive WZ (comparison of equal directed triangles against the y‐axis), while the culling effort is reduced to 25% of the former with the latter (equally directed triangles against the x‐axis).
Figure 65.
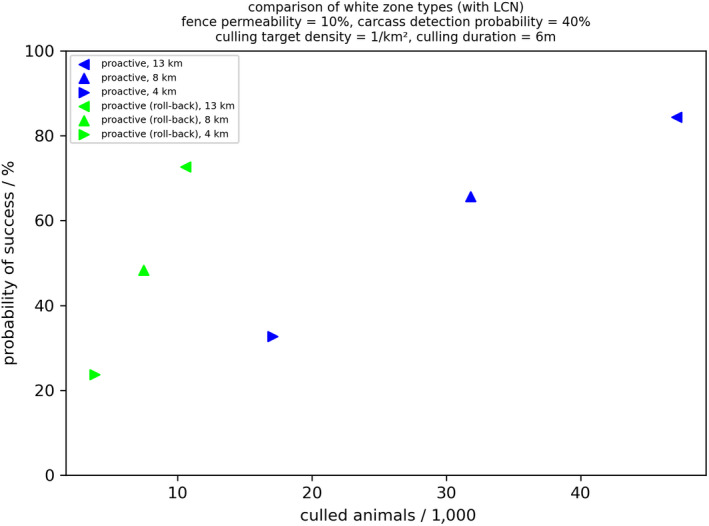
Overview of model output comparing the overall success achieved when simulating the proactive white zone (blue triangles) and the proactive white zone + roll‐back (green triangles). Triangle format represents the width of the white zone (i.e. 13, 8 and 4 km)
5. Conclusions
5.1. Descriptive epidemiology – TOR 1
5.1.1. Update the ASF situation in affected Member States and neighbouring countries during this reporting period
Overall, ASF continued to circulate in wild boar in several MS in the EU and neighbouring countries and spread slowly through the wild boar populations.
ASF was confirmed in Germany at the border with Poland. The first notification was on 10 September 2020 in wild boar. Since then, 1,872 cases were observed in wild boar and three outbreaks were observed in domestic pigs until the end of the reporting period.
Belgium made a self‐declaration of Freedom of ASF on 1 October 2020 to the OIE.
In Greece, no outbreaks in domestic pigs nor cases in wild boar were reported.
In Hungary and Lithuania, only cases in wild boar were reported.
The number of cases reported in wild boar in Bulgaria, Hungary, Lithuania and Poland decreased by 32, 22, 27 and 15% compared with the previous reporting period.
In Slovakia, almost 10 times as many cases were reported in wild boar compared with the previous period.
In Romania, an increase of 33% of cases reported in wild boar was observed.
In Estonia, where the disease appeared to be fading out in the previous reporting period, a resurgence of the infection was observed in two counties, and a total increase of 19% of cases reported in wild boar. After 4 years without outbreaks in domestic pigs, one outbreak was observed in a commercial pig farm.
In Latvia, an increase of ASF cases in wild boar was observed in the eastern part of the country suggesting ASFV re‐introduction from neighbouring non‐EU countries in summer 2021.
Whereas in Slovakia, Poland and Romania, the number of outbreaks in domestic pigs increased compared with the previous reporting period; in Bulgaria and Lithuania, there were fewer outbreaks observed in domestic pigs.
Enhanced passive surveillance (the routine testing of two pigs found dead per epidemiological unit per week for ASFV) has contributed to early detection of outbreaks in domestic pigs.
In Romania, clinical passive surveillance has led to most detections of outbreaks in smaller farms, while in the larger commercial farms enhanced passive surveillance contributed most to outbreak detection.
5.1.2. Special focus on the outbreaks in large commercial pig farms in Romania in the last two reporting periods
The number of outbreaks in farms with more than 10,000 animals increased from 6 to 13 farms from the previous to the current reporting period, representing 0.6–0.8% of the total number of outbreaks in both periods, respectively.
In the current reporting period, the year incidence of affected farms in the smallest farm size class (farms with fewer than 30 pigs) was 0.003 compared with 0.236 in the largest farm size class (farms with more than 10,000 pigs).
Although dispersed over the entire country, most ASF outbreaks in domestic pig farms, including the outbreaks on farms of more than 10,000 pigs, occurred in the north‐west and the south‐east of the country in this reporting period.
Based on a secondary case network output, the overall extent of spread of the disease between domestic pig farms increased in this reporting period compared with the previous reporting period.
As observed in previous reports, there is a clear seasonality in the outbreaks in domestic pigs, with a peak of outbreaks in summer. In the small farms, the seasonal peak in July and August was much more pronounced compared with the previous reporting period.
5.1.3. Time profile of proportions of positive samples tested with Ab ELISA or PCR in wild boar hunted and found dead
Based on data submitted to EFSA’s Data Collection Framework from the beginning of 2016 and since the first detection in the country, up to the end of this reporting period, in the affected areas:
In the Baltic States, overall, the proportions of PCR‐positive samples from wild boar carcasses are declining, although a resurgence has occurred in Estonia over the last reporting period. Indicating…
In the other countries, the proportions of PCR‐positive wild boar carcasses remain high, indicating continuing spread of the disease.
There has been no general increase in the proportion of seropositive samples in wild boar in the affected populations. This suggests that, overall, there is no increase in the survival rate.
It can be assumed that there is a relationship between the proportion of PCR‐positive samples and the incidence of ASF.
5.1.4. Seasonality of African swine fever outbreaks and cases
There is a clear seasonality in the proportions of PCR‐positive samples from wild boar found dead, although the patterns are slightly different in the different MS.
There is a clear decline in summer and an increase in winter in Romania and Slovakia in the proportion of PCR‐positive samples from wild boar found dead.
In Latvia and Estonia, by contrast, the peak of proportions PCR‐positive samples observed in wild boar seem to be in the summer and winter, but there is a decline in spring and autumn.
There is a clear peak observed in the proportions of PCR‐positive samples from domestic pigs between May and September in Lithuania, Poland, Slovakia and Romania, which as expected, coincides with the observed seasonality of outbreaks in domestic pig establishments.
5.1.5. Trend yearly number of wild boar hunted in affected countries
In the Baltic States, a rapid decline of wild boar hunted from 2014 to 2019 was observed after the introduction of ASF in the region, and a small but consistent increase was detected in the three countries in the last hunting season (2020–2021).
In Bulgaria and Romania, an important change in the increasing trend of the annual hunted wild boar was observed starting in 2018–2019 and 2020–2021, respectively. Since then, a sharp reduction of number of wild boar hunted was observed in both countries.
An increasing trend in the numbers of hunted wild boar in the Central European countries was observed since 2000, with some fluctuations.
5.1.6. Trend of the extent of spread of the disease in wild boar, based on a secondary case network
Different trends were observed in the average number of secondary cases observed from a single source case. In some countries, an increased average number of secondary cases was observed (e.g. Bulgaria and Latvia), and in other countries, it decreased (e.g. Hungary). For other countries, there was no clear trends.
In Germany, due to the recent introduction no comparison can be made with previous reporting periods. However, the relatively high number of 6.4 cases on average per source case in wild boar indicates that the epidemic is in an expanding phase.
5.2. Risk factor analysis – TOR 2
5.2.1. Systematic literature review
The systematic literature review identified 31 scientific articles that quantitatively analysed 621 risk factors for ASF in domestic pig (34 studies) and wild boar population (25 studies). The analytical results of the present report were also included.
The categories of factors most frequently identified as significant for the occurrence of ASF in domestic populations were those related to pig farming, especially pig population density and biosecurity practices (which includes protective and risk factors); socio‐economic factors related to the farmer and population demographics; and the presence or closeness to ASF‐infected areas.
The factors most frequently observed to be significantly associated with the risk of ASF in wild boar were the habitat‐related factors (vegetation, water, climate and other factors related to wild boar habitat suitability); socio‐economic factors such as human population demographics; and wild boar management factors, especially wild boar density and abundance.
5.2.2. Risk factors for the occurrence of ASF in wild boar in Slovakia, Romania and Baltic States
There was a significant relationship between the presence of ASF cases in domestic pigs and the probability of detecting ASFV‐positive PCR cases in wild boar in the analysis done in Romania and Slovakia.
The PCR‐positive results in domestic pigs in Slovakia were highly correlated with the density of small size farms (fewer than 10 animals) per district.
Environmental factors, such as the presence of croplands, urban areas, the density of waterbodies and wild boar abundance, were found to be significantly related to the PCR‐positive detection of ASF in wild boar.
The number of hunting days was identified as a protective factor for the occurrence of ASF in wild boar in Romania. However, the nature and direction of this relationship should be investigated more carefully, considering higher resolution data and additional information on the hunting methods implemented in the area.
The analysis performed in the Baltic States did not identify any relevant risk factor but confirmed the significant decline in the PCR‐positive results in wild boar in the region.
Additional and higher spatial resolution field data on the type of farms and biosecurity conditions would be useful to further investigate the potential relationship between domestic and wild boar.
5.3. Evaluation of measures applied in ASF‐free areas adjacent to areas with affected wild boar using a stochastic model
5.3.1. Is there a difference when applying white zones after introduction from an adjacent area with limited control measures in place compared with a white zone applied after a focal introduction of ASF?
The WZ concept was originally implemented around a focal introduction of ASF in a wild boar population.
It is a wild boar management zone that is set up as a belt at a distance from the newly ASF‐affected area, in which, among other measures, the wild boar population is reduced drastically to an a priori‐decided population density, with the aim to preventively stop the spread of the infection.
The application of the WZ approach is challenged when the area of incursion is adjacent to a region where ASFV infections is widespread in wild boar and limited control efforts are applied. For the same set of control measures applied in the WZ, the overall success rate is greatly reduced, when the WZs are applied along an adjacent ALC measures in place compared with after a focal introduction of ASF.
5.3.2. How the white zone approach may be strengthened in the context of an adjacent area with limited control measures in place?
The effectiveness of the WZ approach is determined by three main parameters that should be chosen a priori when planning the WZ: the width of the WZ, the target density of the wild boar population reduction in the WZ and the time needed to reach the target.
Stringent wild boar population reduction measures in the WZ are the key to successful application of the WZ approach and more important than reducing the time to reach the target population density.
When a lower target population density is set to be reached in the WZ a larger number of wild boar must be culled initially (the culling effort is at the initial phase of implementation of the WZ). However, maintaining a lower population density (e.g. less than 0.5/km2 rather than 1/km2) will, in the end, result in a lower total culling effort and greater control success.
The wider a WZ is for a given target population density, the better is the expected control outcome.
The choice of the distance between the CA and the WZ must respect the velocity of spread of the infection through the WB population (landscape dependent) and the time anticipated to finalise the population reduction measures in the WZ (management decision).
The area between the CA and the WZ can also be treated by population reduction, but this is not strictly needed if resources are limited.
An inappropriate distance between the CA and WZ can reduce the overall success of the WZ measures, because the infection will enter the WZ too early if the distance between CA and WZ is chosen too small, and therefore, the population reduction has not yet been finalised before entry.
5.3.3. What are the benefits of applying proactive white zones compared with reactive white zones
For the WZ approach applied adjacent to neighbouring ALC, it might be beneficial if set up all along the demarcation line (called proactive WZs).
To maintain the proactive WZ along the demarcation line, higher culling efforts are needed compared with the reactive approach.
The additional culling effort could be potentially compensated with a segmentwise release of the measures in the WZ (roll‐back) after the ASFV infection risk moved on in the neighbouring ALC measures in place.
Roll‐back should start only after certain exit criteria are fulfilled through surveillance and the status of the infection in the respective part of the ALC is clarified. The uncertainty with rolling‐back is potential reoccurrence of ASFV infection, e.g. by humans, back into the previously cleared segments.
Abbreviations
- ADIS
Animal Disease Information System
- ALC
Area with limited control
- ASF
African swine fever
- ASFV
African swine fever virus
- BYM
Besag York Mollié
- CA
Core area
- DAG
Directed Acyclic Graph
- DCF
Data Collection Framework
- EFSA
European Food Safety Authority
- ELISA
Enzyme‐linked immunosorbent assay
- FOD
Freedom of Disease
- GLM
Generalised linear model
- HG
Hunting ground
- LAU
Local administrative unit
- LIMS
Laboratory Information Management System
- MS
Member States
- PCR
Polymerase chain reaction
- PMI
Post‐mortem intervals
- RF
Risk factor
- VIF
Variance inflation factor
- WB
Wild boar
- WG
Working Group
- WZ
White zone
Appendix A – Data availability for risk factor analysis
A.1. Data availability
| Bulgaria | Estonia | Germany | Hungary | Latvia | Lithuania | Poland | Romania | Slovakia | |
|---|---|---|---|---|---|---|---|---|---|
| Wb (ASF PCR data) | – |
LAU (2014–2021) |
– |
HG a (2020/2021) |
Coordinates (2014–2021) |
Coordinates (2016–2021) |
LAU (2020/2021) |
NUTS 3 (2017–2021) |
HG (2020/2021) |
| Domestic pigs (ASF PCR data) | – | LAU (2020/2021) | – | – | – |
Coordinates (2016–2020) NUTS 3 (2021) |
LAU (2020/2021) |
NUTS 3 (2017–2021) |
Coordinates (2020/2021) |
| Pig farming data |
Coordinates (2020/2021) |
Coordinates (2014–2020) | – | – |
Coordinates (2014–2019) Parish (2020/2021) |
LAU (2014–2016) Coordinates (2017–2021) |
NUTS 3 (2020/2021) |
NUTS 3 (2020/2021) |
LAU (2020/2021) |
| Wild boar hunting bag | – |
HG (2015–2021) |
– | – |
HG (2015–2017)EW HG (2019–2021) |
HG (2014–2017) EW NUTS 3 (2018) HG (2019–2020) |
HG (2015–2020)EW |
HG (2016–2021) |
District (2020/2021) |
| Dogs + other hunting data | – | – | – | – | – |
HG (2019–2020) |
– |
HG (2016–2021) |
District (2020/2021) |
EW: data provided by the ENETWILD‐consortium et al. (2020).
Note: hunting ground (HG) has a higher spatial resolution than local administrative unit (LAU), and LAU has a higher resolution than the Nomenclature of Territorial Units for Statistics level 3 (NUTS 3).
Suggested citation: EFSA (European Food Safety Authority) , Baños JV, Boklund A, Gogin A, Gortázar C, Guberti V, Helyes G, Kantere M, Korytarova D, Linden A, Masiulis M, Miteva A, Neghirla I, Oļševskis E, Ostojic S, Petr S, Staubach C, Thulke H‐H, Viltrop A, Wozniakowski G, Broglia A, Abrahantes Cortiñas J, Dhollander S, Mur L, Papanikolaou A, Van der Stede Y, Zancanaro G and Ståhl K, 2022. Scientific report on the epidemiological analyses of African swine fever in the European Union. EFSA Journal 2022;20(5):7290, 106 pp. 10.2903/j.efsa.2022.7290
Requestor: European Commission
Question number: EFSA‐Q‐2020‐00427
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgements: EFSA wishes to thank all European competent institutions, Member State bodies and the other organisations that provided data for this scientific output. In addition, EFSA would like to thank Chinchio Eleonora for all the support she provided to draft this report and most in particular the work she did on the systematic review for the risk factors and the maps. EFSA also would like to thank Aminalragia Roxani for all the support she provided to the data providers to submit data to the data collection framework and for the support with the data extraction and analysis. And finally, EFSA would like to thank Lombardo Ludovico for the creation of the videos and his support with the maps.
Waiver: In accordance with Article 21 of the Decision of the Executive Director on Competing Interest Management a waiver was granted to seven experts of the Working Group. Pursuant to Article 21(6) of the aforementioned Decision, the concerned experts were allowed to take part in the discussions and in the drafting of the scientific output but were not allowed to take up the role of chair within that time frame. Any competing interests are recorded in the respective minutes of the meetings of the working group of the AHAW Panel.
Approved: 30 March 2022
This publication is linked to the following EFSA Journal article: http://onlinelibrary.wiley.com/doi/10.2903/sp.efsa.2022.EN-7320/full
Notes
At the moment of enforcement, ASF had been introduced into mainland Italy (January 2022), but these data were not included in the reporting period.
Background provided in the original mandate, received on 23/04/2020.
According to the self‐declaration of Belgium, submitted on 1 October 2020, Belgium declared the African swine fever‐free status in all swine species https://www.fasfc.be/sites/default/files/content/explorer/Animals/ASF/OIE/2020_12_Belgium_ASF_self‐declaration_ENG.pdf)’.
ASFV entered Germany on 10 September 2020, and was added in the above text after reception of this mandate.
ASFV entered Italy on 7 January 2022, and was added in the above text after reception of this mandate.
To ease communication, the terms ‘domestic pigs’ and ‘wild boar’ will be used in this report. They refer to kept and wild porcine animal of the species of ungulates of the family Suidae listed in Annex III to Regulation (EU) 2016/429.
From 21 April 2021, Regulation (EU) 2016/429 (the Animal Health Law) is the new applicable legal basis for the prevention and control of relevant for ASF.
https://ec.europa.eu/food/system/files/2020-04/ad_control-measures_asf_wrk-doc-sante-2015-7113.pdf
As the mandate was received before Regulation (EU) 2016/429 had been implemented, in this report an outbreak of ASF in domestic pigs refers to one or more confirmed cases detected in a pig holding. A case of ASF in wild boar refers to one or more confirmed cases detected in wild boar.
Although wild boar killed in road accidents are usually found dead, they were included in this report under active surveillance as they were considered more likely not to show clinical signs before being killed by traffic.
References
- Andraud M, Bougeard S, Chesnoiu T and Rose N, 2021. Spatiotemporal clustering and Random Forest models to identify risk factors of African swine fever outbreak in Romania in 2018–2019. Science Reports, 11, 2098. 10.1038/s41598-021-81329-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awosanya EJ, Olugasa B, Ogundipe G and Grohn YT, 2015. Sero‐prevalence and risk factors associated with African swine fever on pig farms in southwest Nigeria. BMC Veterinary Research, 11, 133. 10.1186/s12917-015-0444-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barongo MB, Ståhl K, Bett B, Bishop RP, Fèvre EM, Aliro T, Okoth E, Masembe C, Knobel D and Ssematimba A, 2015. Estimating the basic reproductive number (R0) for African swine fever virus (ASFV) transmission between pig herds in Uganda. PLoS One, 10, e0125842. 10.1371/journal.pone.0125842 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisimwa PN, Dione M, Basengere B, Mushagalusa CA, Steinaa L and Ongus J, 2021. Risk factors of African swine fever virus in suspected infected pigs in smallholder farming systems in South‐Kivu province, Democratic Republic of Congo. Journal of Veterinary Science, 22, e35. 10.4142/jvs.2021.22.e35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boklund A, Dhollander S, Chesnoiu Vasile T, Abrahantes JC, Botner A, Gogin A, Gonzalez Villeta LC, Gortázar C, More SJ, Papanikolaou A, Roberts H, Stegeman A, Ståhl K, Thulke HH, Viltrop A, Van der Stede Y and Mortensen S, 2020. Risk factors for African swine fever incursion in Romanian domestic farms during 2019. Science Reports, 10, 10215. 10.1038/s41598-020-66381-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappai S, Rolesu S, Coccollone A, Laddomada A and Loi F, 2018. Evaluation of biological and socio‐economic factors related to persistence of African swine fever in Sardinia. Preventive Veterinary Medicine, 152, 1–11. 10.1016/j.prevetmed.2018.01.004 [DOI] [PubMed] [Google Scholar]
- Chambaro HM, Sasaki M, Sinkala Y, Gonzalez G, Squarre D, Fandamu P, Lubaba C, Mataa L, Shawa M, Mwape KE, Gabriël S, Chembensofu M, Carr MJ, Hall WW, Qiu Y, Kajihara M, Takada A, Orba Y, Simulundu E and Sawa H, 2020. Evidence for exposure of asymptomatic domestic pigs to African swine fever virus during an inter‐epidemic period in Zambia. Transboundary and Emerging Diseases, 67, 2741–2752. 10.1111/tbed.13630 [DOI] [PubMed] [Google Scholar]
- Correia‐Gomes C, Henry MK, Auty HK and Gunn GJ, 2017. Exploring the role of small‐scale livestock keepers for national biosecurity—The pig case. Preventive Veterinary Medicine, 145, 7–15. 10.1016/j.prevetmed.2017.06.005 [DOI] [PubMed] [Google Scholar]
- Dhollander S, Belsham GJ, Lange M, Willgert K, Alexandrov T, Chondrokouki E, Depner K, Khomenko S, Özyörük F, Salman M, Thulke HH and Bøtner A, 2016. Assessing the potential spread and maintenance of foot‐and‐mouth disease virus infection in wild ungulates: general principles and application to a specific scenario in Thrace. Transboundary and Emerging Diseases, 63, 165–174. 10.1111/tbed.12240 [DOI] [PubMed] [Google Scholar]
- Dione MM, Akol J, Roesel K, Kungu J, Ouma EA, Wieland B and Pezo D, 2017. Risk factors for African swine fever in smallholder pig production systems in Uganda. Transboundary and Emerging Diseases, 64, 872–882. 10.1111/tbed.12452 [DOI] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2010. Application of systematic review methodology to food and feed safety assessments to support decision making. EFSA Journal 2010;8(6):1637, 90 pp. 10.2903/j.efsa.2010.1637 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Depner K, Gortazar C, Guberti V, Masiulis M, More S, Oļševskis E, Thulke HH, Viltrop A, Woźniakowski G, Cortiñas Abrahantes J, Gogin A, Verdonck F and Dhollander S, 2017. Epidemiological analyses of African swine fever in the Baltic States and Poland: (Update September 2016–September 2017). EFSA Journal 2017;15(11):5068, 59 pp. 10.2903/j.efsa.2017.5068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Boklund A, Cay B, Depner K, Földi Z, Guberti V, Masiulis M, Miteva A, More S, Olsevskis E, Šatrán P, Spiridon M, Stahl K, Thulke HH, Viltrop A, Wozniakowski G, Broglia A, Cortinas Abrahantes J, Dhollander S, Gogin A, Verdonck F, Amato L, Papanikolaou A and Gortázar C, 2018. Epidemiological analyses of African swine fever in the European Union (November 2017 until November 2018). EFSA Journal 2018;16(11):5494, 106 pp. 10.2903/j.efsa.2018.5494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Anette B, Anette B, Theodora CV, Klaus D, Daniel D, Vittorio G, Georgina H, Daniela K, Annick L, Aleksandra M, Simon M, Edvins O, Sasa O, Helen R, Mihaela S, Karl S, Hans‐Hermann T, Grigaliuniene V, Arvo V, Richard W, Grzegorz W, José AC, Sofie D, Andrey G, Corina I, Alexandra P, González VLC and Christian GS, 2020. Epidemiological analyses of African swine fever in the European Union (November 2018 to October 2019). EFSA Journal 2020;18(1):5996, 107 pp. 10.2903/j.efsa.2020.5996 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , Desmecht D, Gerbier G, Gortázar Schmidt C, Grigaliuniene V, Helyes G, Kantere M, Korytarova D, Linden A, Miteva A, Neghirla I, Olsevskis E, Ostojic S, Petit T, Staubach C, Thulke H‐H, Viltrop A, Richard W, Wozniakowski G, Abrahantes Cortiñas J, Broglia A, Dhollander S, Lima E, Papanikolaou A, Van der Stede Y and Ståhl K, 2021a. Scientific Opinion on the epidemiological analysis of African swine fever in the European Union (September 2019 to August 2020). EFSA Journal 2021;19(5):6572, 101 pp. 10.2903/j.efsa.2021.6572 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , Nielsen SS, Alvarez J, Bicout DJ, Calistri P, Depner K, Drewe JA, Garin‐Bastuji B, Gonzales Rojas JL, Gortazar Schmidt C, Herskin M, Michel V, Miranda Chueca MÁ, Pasquali P, Roberts HC, Sihvonen LH, Spoolder H, Stahl K, Velarde A, Winckler C, Abrahantes JC, Dhollander S, Ivanciu C, Papanikolaou A, Van der Stede Y, Blome S, Guberti V, Loi F, More S, Olsevskis E, Hans Thulke HH and Viltrop A, 2021b. ASF Exit Strategy: providing cumulative evidence of the absence of African swine fever virus circulation in wild boar populations using standard surveillance measures. EFSA Journal 2021;19(3):6419, 72 pp. 10.2903/j.efsa.2021.6419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2012. Scientific opinion on foot‐and‐mouth disease in Thrace. EFSA Journal 2012;10(4):2635, 91 pp. 10.2903/j.efsa.2012.2635 [DOI] [Google Scholar]
- EFSA AHAW Panel (EFSA Panel on Animal Health and Welfare) , 2015. Scientific opinion on African swine fever. EFSA Journal 2015;13(7):4163, 92 pp. 10.2903/j.efsa.2015.4163 [DOI] [Google Scholar]
- ENETWILD‐consortium , Acevedo P, Croft S, Smith G, Blanco‐Aguiar JA, Fernández‐López J, Scandura M, Apollonio M, Ferroglio E, Keuling O, Sange M, Zanet S, Brivio F, Podgorski T, Petrovic K, Soriguer R and Vicente J, 2020. Update of occurrence and hunting yield‐based data models for wild boar at European scale: new approach to handle the bioregion effect. EFSA Supporting Publications 2020;17(5):EN‐1871. 10.2903/sp.efsa.2020.EN-1871 [DOI] [Google Scholar]
- Fasina FO, Agbaje M, Ajani FL, Talabi OA, Lazarus DD, Gallardo C, Thompson PN and Bastos AD, 2012. Risk factors for farm‐level African swine fever infection in major pig‐producing areas in Nigeria, 1997–2011. Preventive Veterinary Medicine, 107, 65–75. 10.1016/j.prevetmed.2012.05.011 [DOI] [PubMed] [Google Scholar]
- Gao L, Sun X, Yang H, Xu Q, Li J, Kang J, Liu P, Zhang Y, Wang Y and Huang B, 2021. Epidemic situation and control measures of African Swine Fever Outbreaks in China 2018–2020. Transboundary and Emerging Diseases, 68, 2676–2686. 10.1111/tbed.13968 [DOI] [PubMed] [Google Scholar]
- Glazunova AA, Korennoy FI, Sevskikh TA, Lunina DA, Zakharova OI, Blokhin AA, Karaulov AK and Gogin AE, 2021. Risk factors of African swine fever in domestic pigs of the samara region. Russian Federation. Frontiers in Veterinary Science, 8, 723375. 10.3389/fvets.2021.723375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm V, Berger U, Bastiansen F, Eliassen S, Ginot V, Giske J, Goss‐Custard J, Grand T, Heinz SK, Huse G, Huth A, Jepsen JU, Jørgensen C, Mooij WM, Müller B, Pe’er G, Piou C, Railsback SF, Robbins AM, Robbins MM, Rossmanith E, Rüger N, Strand E, Souissi S, Stillman RA, Vabø R, Visser U and DeAngelis DL, 2006. A standard protocol for describing individual‐based and agent‐based models. Ecological Modelling, 198, 115–126. 10.1016/j.ecolmodel.2006.04.023 [DOI] [Google Scholar]
- Grimm V, Berger U, DeAngelis DL, Polhill JG, Giske J and Railsback SF, 2010. The ODD protocol: a review and first update. Ecological Modelling, 221, 2760–2768. 10.1016/j.ecolmodel.2010.08.019 [DOI] [Google Scholar]
- Gulenkin VM, Korennoy FI, Karaulov AK and Dudnikov SA, 2011. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Preventive Veterinary Medicine, 102, 167–174. 10.1016/j.prevetmed.2011.07.004 [DOI] [PubMed] [Google Scholar]
- Hall DK and Riggs GA, 2015. Modis/Terra Snow Cover Monthly p. L3 Global 0.05Deg CMG, version 6. Boulder. National Astronautics and Space Administration National Snow and Ice Data Center Distributed Active Archive Center. 10.5067/modis/mod10cm.006 [DOI] [Google Scholar]
- Huang ZY, van Langevelde F, Honer KJ, Naguib M and de Boer WF, 2017. Regional level risk factors associated with the occurrence of African swine fever in West and East Africa. Parasites and Vectors, 10, 16. 10.1186/s13071-016-1953-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad MU, Aslam M and Altaf S, 2016. mctest: An R package for detection of collinearity among regressors. R Journal, 8, 499–509. [Google Scholar]
- Imdad MU, Aslam M, Altaf S and Ahmed M, 2019. Some new diagnostics of multicollinearity in linear regression model. Sains Malaysiana, 48, 2051–2060. 10.17576/jsm-2019-4809-26 [DOI] [Google Scholar]
- Jurado C, Fernández‐Carrión E, Mur L, Rolesu S, Laddomada A and Sánchez‐Vizcaíno JM, 2018. Why is African swine fever still present in Sardinia? Transboundary and Emerging Diseases, 65, 557–566. 10.1111/tbed.12740 [DOI] [PubMed] [Google Scholar]
- Kabuuka T, Kasaija PD, Mulindwa H, Shittu A, Bastos AD and Fasina FO, 2014. Drivers and risk factors for circulating African swine fever virus in Uganda, 2012–2013. Research in Veterinary Science, 97, 218–225. 10.1016/j.rvsc.2014.07.001 [DOI] [PubMed] [Google Scholar]
- Kramer‐Schadt S, Fernández N, Eisinger D, Grimm V and Thulke H‐H, 2009. Individual variations in infectiousness explain long‐term disease persistence in wildlife populations. Oikos, 118, 199–208. 10.1111/j.1600-0706.2008.16582.x [DOI] [Google Scholar]
- Lange M, Guberti V and Thulke H‐H, 2018. Understanding ASF spread and emergency control concepts in wild boar populations using individual‐based modelling and spatio‐temporal surveillance data. EFSA Supporting Publications 2018;15(11):EN‐1521. 10.2903/sp.efsa.2018.EN-1521 [DOI] [Google Scholar]
- Lange M, Reichold A and Thulke H‐H, 2021. Modelling wild boar management for controlling the spread of ASF in the areas called white zones (zones blanche). EFSA Supporting Publications, 2021;18(5):EN‐6573, 32 pp. 10.2903/sp.efsa.2021.EN-6573 [DOI] [Google Scholar]
- Lange M and Thulke HH, 2015. Mobile barriers as emergency measure to control outbreaks of African swine fever in wild boar. In: Thulke HH and Verheyen K (eds.). Proceedings of the SVEPM, Ghent, pp. 122–132.
- Lange M and Thulke H‐H, 2017. Elucidating transmission parameters of African swine fever through wild boar carcasses by combining spatio‐temporal notification data and agent‐based modelling. Stochastic Environmental Research and Risk Assessment, 31, 379–391. 10.1007/s00477-016-1358-8 [DOI] [Google Scholar]
- Lim JS, Vergne T, Pak SI and Kim E, 2021. Modelling the spatial distribution of ASF‐positive wild boar carcasses in South Korea using 2019–2020 national surveillance data. Animals (Basel), 11. 10.3390/ani11051208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi F, Laddomada A, Coccollone A, Marrocu E, Piseddu T, Masala G, Bandino E, Cappai S and Rolesu S, 2019. Socio‐economic factors as indicators for various animal diseases in Sardinia. PLoS One, 14, e0217367. 10.1371/journal.pone.0217367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martínez‐López B, Perez AM, Feliziani F, Rolesu S, Mur L and Sánchez‐Vizcaíno JM, 2015. Evaluation of the risk factors contributing to the African swine fever occurrence in Sardinia. Italy. Frontiers in Microbiology, 6, 314. 10.3389/fmicb.2015.00314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group 2009. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA statement. PLoS Medicine, 6, e1000097. 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moltke‐Jordt A, Lange M, Kramer‐Schadt S, Nielsen LH, Nielsen SS, Thulke HH, Vejre H and Alban L, 2016. Spatiotemporal modeling of the invasive potential of wild boar‐a conflict‐prone species‐using multi‐source citizen science data. Preventive Veterinary Medicine, 124, 34–44. 10.1016/j.prevetmed.2015.12.017 [DOI] [PubMed] [Google Scholar]
- Mur L, Sánchez‐Vizcaíno JM, Fernández‐Carrión E, Jurado C, Rolesu S, Feliziani F, Laddomada A and Martínez‐López B, 2018. Understanding African Swine Fever infection dynamics in Sardinia using a spatially explicit transmission model in domestic pig farms. Transboundary and Emerging Diseases, 65, 123–134. 10.1111/tbed.12636 [DOI] [PubMed] [Google Scholar]
- Nantima N, Ocaido M, Ouma E, Davies J, Dione M, Okoth E, Mugisha A and Bishop R, 2015. Risk factors associated with occurrence of African swine fever outbreaks in smallholder pig farms in four districts along the Uganda‐Kenya border. Tropical Animal Health and Production, 47, 589–595. 10.1007/s11250-015-0768-9 [DOI] [PubMed] [Google Scholar]
- Nurmoja I, Mõtus K, Kristian M, Niine T, Schulz K, Depner K and Viltrop A, 2020. Epidemiological analysis of the 2015–2017 African swine fever outbreaks in Estonia. Preventive Veterinary Medicine, 181, 104556. 10.1016/j.prevetmed.2018.10.001 [DOI] [PubMed] [Google Scholar]
- Okoth E, Gallardo C, Macharia JM, Omore A, Pelayo V, Bulimo DW, Arias M, Kitala P, Baboon K, Lekolol I, Mijele D and Bishop RP, 2013. Comparison of African swine fever virus prevalence and risk in two contrasting pig‐farming systems in South‐West and Central Kenya. Preventive Veterinary Medicine, 110, 198–205. 10.1016/j.prevetmed.2012.11.012 [DOI] [PubMed] [Google Scholar]
- Oļševskis E, Schulz K, Staubach C, Seržants M, Lamberga K, Pūle D, Ozoliņš J, Conraths FJ and Sauter‐Louis C, 2020. African swine fever in Latvian wild boar—a step closer to elimination. Transboundary and Emerging Diseases, 67, 2615–2629. 10.1111/tbed.13611 [DOI] [PubMed] [Google Scholar]
- Pepin KM, Golnar AJ, Abdo Z and Podgórski T, 2020. Ecological drivers of African swine fever virus persistence in wild boar populations: Insight for control. Ecology and Evolution, 10, 2846–2859. 10.1002/ece3.6100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perperoglou A, Sauerbrei W, Abrahamowicz M and Schmid M, 2019. A review of spline function procedures in R. BMC Medical Research Methodology, 19, 46. 10.1186/s12874-019-0666-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podgórski T, Apollonio M and Keuling O, 2017. Contact rates in wild boar populations: implications for disease transmission. The Journal of Wildlife Management, 82, 1210–1218. 10.1002/jwmg.21480 [DOI] [Google Scholar]
- Podgórski T, Apollonio M and Keuling O, 2018. Contact rates in wild boar populations: implications for disease transmission. Journal of Wildlife Management, 82, 1210–1218. 10.1002/jwmg.21480 [DOI] [Google Scholar]
- Podgórski T, Borowik T, Łyjak M and Woźniakowski G, 2020. Spatial epidemiology of African swine fever: Host, landscape and anthropogenic drivers of disease occurrence in wild boar. Preventive Veterinary Medicine, 177, 104691. 10.1016/j.prevetmed.2019.104691 [DOI] [PubMed] [Google Scholar]
- Probst C, Gethmann J, Amendt J, Lutz L, Teifke JP and Conraths FJ, 2020. Estimating the postmortem interval of wild boar carcasses. Veterinary Sciences, 7, 6. 10.3390/vetsci7010006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst C, Globig A, Knoll B, Conraths FJ and Depner K, 2017. Behaviour of free ranging wild boar towards their dead fellows: potential implications for the transmission of African swine fever. Royal Society Open Science, 4, 170054. 10.1098/rsos.170054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribbens S, Dewulf J, Koenen F, Mintiens K, De Sadeleer L, de Kruif A and Maes D, 2008. A survey on biosecurity and management practices in Belgian pig herds. Preventive Veterinary Medicine, 83, 228–241. 10.1016/j.prevetmed.2007.07.009 [DOI] [PubMed] [Google Scholar]
- Sauter‐Louis C, Forth JH, Probst C, Staubach C, Hlinak A, Rudovsky A, Holland D, Schlieben P, Göldner M, Schatz J, Bock S, Fischer M, Schulz K, Homeier‐Bachmann T, Plagemann R, Klaaß U, Marquart R, Mettenleiter TC, Beer M, Conraths FJ and Blome S, 2021a. Joining the club: first detection of African swine fever in wild boar in Germany. Transboundary and Emerging Diseases, 68, 1744–1752. 10.1111/tbed.13890 [DOI] [PubMed] [Google Scholar]
- Sauter‐Louis C, Schulz K, Richter M, Staubach C, Mettenleiter TC and Conraths FJ, 2021. African swine fever: why the situation in Germany is not comparable to that in the Czech Republic or Belgium. Transboundary and Emerging Diseases, Advance Online Publication, 10.1111/tbed.14231 [DOI] [PubMed] [Google Scholar]
- Thulasiraman K and Swamy MNS, 1992. 5.7 Acyclic Directed Graphs. graphs: Theory and algorithms, John Wiley & Son. [Google Scholar]
- Vajas P, Calenge C, Richard E, Fattebert J, Rousset C, Saïd S and Baubet E, 2020. Many, large and early: hunting pressure on wild boar relates to simple metrics of hunting effort. Science of the Total Environment, 698, 134251. 10.1016/j.scitotenv.2019.134251 [DOI] [PubMed] [Google Scholar]
- Varewyck M, Verbeke T and Cortinas Abrahantes J, 2017. Exploratory analysis for spatiotemporal epidemiological data WEB app (spatial). Zenodo, 10.5281/zenodo.889551 [DOI] [Google Scholar]
- Venter O, Sanderson EW, Magrach A, Allan JR, Beher J, Jones KR, Possingham HP, Laurance WF, Wood P, Fekete BM, Levy MA and Watson JE, 2018. Last of the Wild Project, Version 3 (LWP‐3): 2009 Human Footprint, 2018 Release. NASA Socioeconomic Data and Applications Center (SEDAC), Palisades, NY. 10.7927/h46t0jq4. Accessed 20 November 2021. [DOI]
- Vergne T, Korennoy F, Combelles L, Gogin A and Pfeiffer DU, 2016. Modelling African swine fever presence and reported abundance in the Russian Federation using national surveillance data from 2007 to 2014. Spatial and Spatio‐Temporal Epidemiology, 19, 70–77. 10.1016/j.sste.2016.06.002 [DOI] [PubMed] [Google Scholar]
- Vergne T, Gogin A and Pfeiffer DU, 2017. Statistical exploration of local transmission routes for African swine fever in pigs in the Russian Federation, 2007–2014. Transboundary and Emerging Diseases, 64, 504–512. 10.1111/tbed.12391 [DOI] [PubMed] [Google Scholar]


