Abstract
Background:
The Children’s Oncology Group (COG) adopted cisplatin/5FU/vincristine (C5V) as standard therapy after legacy study INT-0098 showed statistically equivalent survival, but less toxicity, when compared to cisplatin/doxorubicin (CD). Subsequent experience demonstrated doxorubicin to be effective in patients with recurrent disease after C5V, suggesting that it could be incorporated to intensify therapy for patients with advanced disease.
Methods:
In this COG non-randomized, phase 3 trial, we aimed to primarily explore the feasibility and toxicity of the novel therapeutic C5VD regimen with the addition of doxorubicin to C5V for patients considered to be intermediate-risk (IR). Patients were eligible if they had unresectable, non-metastatic disease. Patients with a complete resection at diagnosis with local pathologic evidence of small cell undifferentiated histology were also eligible for assessment of feasibility.
Results:
102 evaluable patients enrolled between September 14, 2009 and March 12, 2012. Delivery of C5VD was feasible and tolerable with a mean percentage of the target dose of cisplatin delivered (96%, 95%CI: 94-97%), 5-FU (96%, 95%CI: 94-97%), doxorubicin (95%, 95%CI: 93-97%) and vincristine (90%, 95%CI: 87-93%). Toxicity was within expectations with death as a first event in one patient. The most common adverse events were febrile neutropenia (n=55, 54%), infection (n=48, 47%), mucositis (n=31, 30%), hypokalemia (n=39, 38%) and elevated aspartate aminotransferase (n= 28, 27%). Five-year event-free and overall-survival for the 93 patients who did not have complete resection at diagnosis was 88% (95%CI:79-93%) and 95% (95%CI:87-98%) respectively.
Conclusions:
The addition of doxorubicin to the previous standard regimen of C5V was feasible, tolerable, and efficacious suggesting C5VD as a good regimen for future clinical trials.
Keywords: hepatoblastoma, cisplatin, doxorubicin, toxicity, pediatric liver tumor, pediatric liver transplant, PRETEXT
PRECIS:
The addition of doxorubicin to our previous standard regimen of C5V is a feasible, tolerable, and efficacious novel treatment regimen (C5VD). This regimen is a reasonable option for future clinical trials, especially those including patients with unresectable disease at diagnosis.
Introduction
Hepatoblastoma (HB) is the most common malignant liver tumor in children and typically presents in the first few years of life.1,2 Surgical resection of the primary tumor is the foundation of curative therapy but only about one third of newly diagnosed children have conventionally resectable tumors at diagnosis.3,4 Historically, approximately two-thirds of such tumors are amenable to conventional resection following several cycles of neoadjuvant chemotherapy.5,6
Over the last two decades, advances in both surgical techniques and chemotherapeutics, including the use of cisplatin (C) and doxorubicin (D), have resulted in increased survival for children with HB.1-4 However, these most active drugs are dose limited due to renal, auditory, and cardiac toxicities.7,8 5-flououracil (5), vincristine (V), carboplatin, ifosfamide, and etoposide have also been used in various combinations with modest improvements in outcomes.2 Cisplatin/5-flourouracil/vincristine (C5V) has been the standard regimen used in the North American cooperative group/Children’s Oncology Group (COG) protocols while the International Childhood Liver Tumors Strategy Group (SIOPEL) has routinely included cisplatin in combination with doxorubicin (PLADO).4,8 Successive trials from these groups as well as the Japanese Liver Tumor Group (JPLT) and the German Society for Pediatric Oncology (GPOH) have tried to optimize use of these agents to maximize tumor response, increase resection rates, and improve survival9-11 but since disease stratification and risk classification used in each trial have varied, no regimen has been shown to be clearly superior.
The Pediatric Intergroup Study INT-0098 randomized patients to receive treatment with 4-8 cycles of either C5V or CD to compare the efficacy of these two regimens.12 Tumor progression accounted for 86% of the events among patients treated with C5V, but only 50% for those treated with CD. Even though tumor responses to CD were superior, this arm was associated with a higher percentage of drug-associated cardiac toxicities and there was no statistical difference in outcome. Thus, COG adopted treatment with C5V as the standard.13,14 The SIOPEL4 high risk (HR) study of dose compressed cisplatin plus doxorubicin had significant toxicity but did show improved surgical resection rates compared to SIOPEL 3HR; both included subgroups of “intermediate-risk” patients included in this paper.15,16 Despite all of the advances in chemotherapy, surgical techniques, and imaging, a cohort of patients can only be rendered free of primary disease with the use of orthotopic liver transplantation (OLT).
The AHEP0731 protocol included a specific primary aim to test the feasibility and tolerability of the addition of doxorubicin to the standard C5V regimen for the neoadjuvant treatment of patients with initially unresectable, non-metastatic, intermediate-risk HB. Secondary objectives included assessment of efficacy including improved resection rates and outcomes for patients unresectable at diagnosis.
Methods
Study Design and Participants:
The AHEP0731 trial was designed to test a risk-based treatment approach for children with HB, to diminish toxicity in low-risk patients,17 to assess regimen feasibility and outcomes in intermediate-risk patients, and to identify new agents that could be used in high-risk patients.18,19 Eligible patients were assigned on the basis of Evans surgical stage, tumor histology, and serum alpha-fetoprotein (AFP) levels at diagnosis to one of four different risk treatment categories.
Patients with newly diagnosed, previously untreated unresectable, non-metastatic Evans stage III HB or stage I or II resected HB with SCU histology as determined by the institutional pathologist were stratified at enrollment as intermediate-risk and were eligible for this study stratum (see protocol in the Appendix). Eligible patients were younger than 21 years of age at the time of enrollment, had not received previous chemotherapy or other tumor directed therapy, had normal bone marrow, kidney, liver, and cardiac function and had at least 50% Karnofsky (patients > 16 years) or Lansky (patients < 16 years) performance status and an AFP level (> 100 ng/ml).
The National Cancer Institute, the Pediatric Central IRB, and the institutional review boards of the participating institutions approved the protocol. Written informed consent was obtained for all patients prior to treatment according to Department of Health and Human Services guidelines.
Procedures
Staging:
Patients were staged for risk classification using COG staging guidelines prior to the initiation of chemotherapy. Modified PRETreatment EXTent of disease (PRETEXT) grouping was also performed at diagnosis by institution for treatment purposes (See Supplemental File 1).
Chemotherapy:
Patients were to receive 6 cycles of C5VD: cisplatin (100 mg/m2/dose or 3.3 mg/kg/dose for < 10 kg) intravenously over 6 hours on day 1; doxorubicin (30 mg/m2/dose or 1 mg/kg/dose for < 10 kg) intravenously over 15 minutes on days 1 and 2; 5-fluorouracil (600 mg/m2/dose or 20 mg/kg/dose for < 10 kg) intravenous push on day 2; and vincristine (1.5 mg/m2/day, maximum dose 2 mg, or 0.05 mg/kg/day) intravenous push on days 2, 9, and 16. Dexrazoxane (300 mg/m2/dose or 10 mg/kg/dose for patients less than 10 kg), intravenous push was given prior to doxorubicin during the last two cycles of C5VD when the cumulative dose was > 300 mg/m2. All cycles were intended to last 21 days. The use of granulocyte colony stimulating factor was not mandated. Dose reductions and modifications were allowed per protocol (Appendix).
Toxicity:
The individual occurrences of toxicities were graded according to National Cancer Institute Common Toxicity criteria v3/4 guidelines. All eligible patients contributed to this analysis. Toxicities of grade 3 or higher were tabulated for each reporting period, where a reporting period represented 2 cycles of chemotherapy. The number of patients with any grade 3 or higher toxicity during protocol therapy was also calculated.
Surgery:
Study patients could have resection of the primary tumor at any point after the start of chemotherapy, either by partial liver resection or by total hepatectomy followed by OLT. The protocol defined the optimal time for surgical resection between chemotherapy cycles 4 and 5 but this timeline was not protocol mandated. Patients were removed from protocol chemotherapy, but remained on study if: 1) the primary tumor was not resected following 4 cycles of chemotherapy; 2) the patient was unable to resume post resection chemotherapy within 42 days following resection.
Pathology:
If local pathologic review determined the presence of SCU components in a patient without metastatic disease than the patient was eligible for the intermediate-risk strata. Central pathologic review was to be performed for all biopsied and resected specimens to assess tumor histology.
Evaluation of Response:
Baseline physical exams, organ function, AFP levels, and imaging studies (including a CT or MRI of the abdomen, ultrasound of the liver, and CT of the chest) were performed prior to initiating therapy. Repeat imaging studies (CT or MRI of the abdomen) were performed after two and four cycles of C5VD. AFP levels were to be obtained prior to beginning each cycle.
All eligible patients were considered in the assessment of overall response to therapy. For the purpose of this study, a complete response (CR) was considered to be the resolution of all lesions according to institutional evaluation and a normal AFP level. A partial response (PR) was considered as either: 1) a ≥ 30% decrease according to RECIST criteria; or 2) a serum AFP level concentration decline of ≥ 90% (≥ 1 log10) in the absence of disease progression. Any patient who had her or his primary liver tumor completely resected prior to disease progression or death was considered a complete responder (CR) with respect to measurable disease at that time without the need for a subsequent scan per protocol guidelines criteria. All patients considered for biochemical overall response evaluation had an AFP at the end of chemotherapy. If, at any time after the start of chemotherapy, the patient’s serum AFP was considered within normal limits the patient was considered an AFP complete responder (AFP-CR). If a patient did not achieve AFP-CR, but serum AFP declined by at least 90% (≥ 1 log10) compared with the “initial’ AFP level, the patient was considered per protocol as an AFP partial responder on the first date this was confirmed.
Statistical Design and Analysis:
Feasibility of Delivery of C5VD:
The primary measure of feasibility was the rate of death as a first analytic event. All patients who received at least one dose of the C5VD regimen were considered evaluable for this endpoint. Any patient who died on protocol therapy or within 30 days of the termination of protocol therapy of a cause considered possibly, probably, or likely related to systemic chemotherapy (and not disease itself) was to be considered to have experienced an on-protocol-therapy death. Target accrual for the trial was 99 eligible patients. If five or fewer of the 99 patients experienced death as a first analytic event, the regimen will be considered feasible for further development. If the regimen is associated with a death-event rate of 3%, the regimen would be considered feasible with probability 0.92. If the regimen is associated with a death-event rate of 10%, the regimen would be considered not feasible with probability 0.94. The design accommodated enrollment of up to 109 patients to account for possible ineligible patients.
Since the addition of doxorubicin to C5V could affect the delivery of all the agents in the combination, the study characterized the amount of each agent that could be delivered during the first four cycles of therapy (see Supplemental File 1).
Survival:
Event-free survival (EFS) was measured from the time of patient enrollment until the last follow-up or an analytic event was observed, whichever occurred first. Analytic events for this cohort were: (1) progression of disease or occurrence of disease at new sites; (2) treatment failure defined as the presence of disease after planned chemotherapy; (3) death from any cause prior to disease progression; or (4) diagnosis of a second malignancy.20 Overall survival (OS) was defined from the time of enrollment until death from any cause or last follow-up, whichever came first. Median follow-up was calculated according to the KM-PF method of Schemper and Smith.21 The proportion of patients who were event-free or alive as a function of time since enrollment was estimated using the method of Kaplan and Meier (Supplemental File 1).22
This trial is registered with ClinicalTrials.gov, number NCT00980460 and is now permanently closed to accrual.
Results
A total of 105 patients were enrolled on study between September 14, 2009 and March 12, 2012. Outcome current to February 10, 2020 was used in this analysis. Three patients were deemed ineligible for the study: one did not meet organ function requirements, one was identified on central review to have a different diagnosis, and one had metastatic disease at diagnosis leaving 102 patients for this primary feasibility analysis (Figure 1). Of the remaining patients, the median age at enrollment was 16 months (range: 0-189 months) (Table 1). There were 59 male and 43 female eligible patients and the median AFP at diagnosis was 184,715 ng/ml (range: 363 - 3,380,000 ng/ml).
Figure 1.
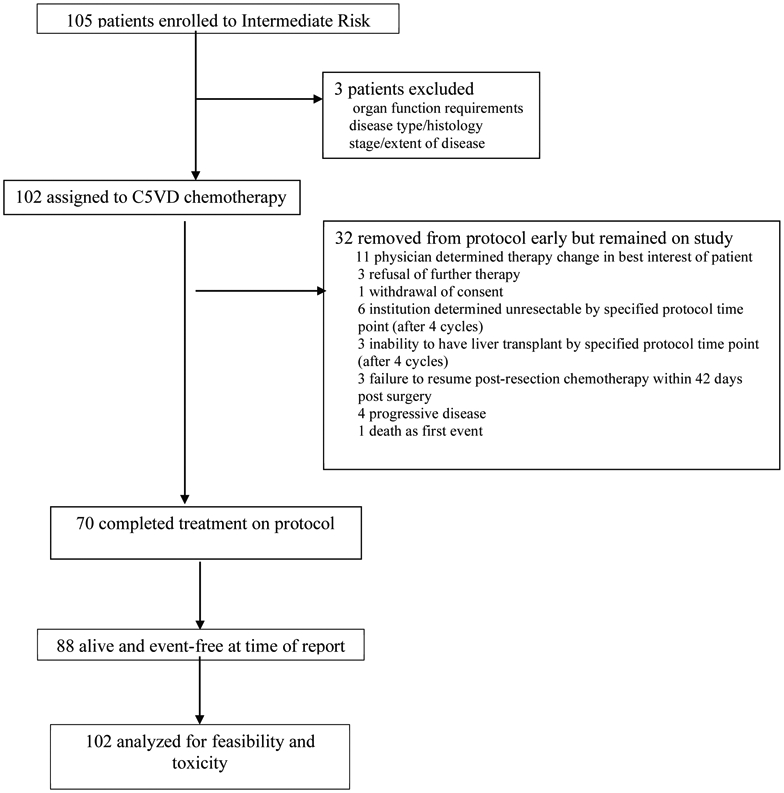
AHEP-0731 Intermediate Risk Stratum CONSORT Diagram
Table 1.
Patient Demographics for Patients with Intermediate-risk Hepatoblastoma
| Number (percent) | |
|---|---|
| Age | 0-189 months (16 mo) |
| ≤ 1 year | 39 (38) |
| >1 to 2 years | 50 (49) |
| >3 to 7 years | 9 (9) |
| > 7 years | 4 (4) |
| Sex | |
| Male | 59 (58) |
| Female | 43 (42) |
| STAGE | |
| I | 6 (6) |
| II | 3 (3) |
| III | 93 (91) |
| PRETEXT GROUP | |
| I | 3 (3) |
| II | 31 (30) |
| III | 54 (53) |
| IV | 14 (14) |
| MULTIFOCAL | |
| Yes | 16 (16) |
| No | 86 (84) |
| EXTRAHEPATIC | |
| Yes | 1 (1) |
| No | 101 (99) |
| Pathology | |
| SCU | 21 (21) |
| Non-SCU | 79 (77) |
| Not available | 2 (2) |
| AFP | 363 – 3,380,000 (184715) |
| 100 to < 1000 ng/ml | 3 (3) |
| ≥ 1000 to 999,999 ng/ml | 83 (81) |
| ≥ 1,000,000 ng/ml | 9 (9) |
| Inevaluable# | 7 (7) |
Inevaluable AFP includes 6 patients with initial AFP values that were not specific/diluted to an exact value (i.e. > 36,000 ng/ml) and one patient who did not have AFP assessed prior to the start of chemotherapy.
Toxicity:
Febrile neutropenia (54%), infections (47%), hypokalemia (38%) and other electrolyte abnormalities, and mucositis (30%) were the most common grade 3, 4 or 5 toxicities as expected (Table 2). Targeted therapeutic toxicities of ototoxicity (18%), cardiac toxicity (5%) and motor or sensory neuropathy (6%) also demonstrated tolerance of therapy. Interestingly, transaminitis was noted more commonly during cycles 3 and 4 when the majority of surgeries occurred. There was no mandated reporting of grade 1-2 events.
Table 2.
Toxicities (Grade 3, 4, and 5) of Patients with Intermediate-risk Hepatoblastoma
| Cycles | ||||
|---|---|---|---|---|
| 1-2 (N=102) |
3-4 (N=93) |
5-6 (N=74) |
||
| Percent | Percent | Percent | ||
| Organ Systems | Toxicity Type | 20.6 | 29.0 | 33.8 |
| None | None | |||
| Blood/Lymphatic | Anemia | 2.0 | 1.4 | |
| Disseminated intravascular coagulation | 1.1 | |||
| Febrile neutropenia | 38.2 | 23.7 | 29.7 | |
| Cardiac | Left ventricular systolic dysfunction | 1.0 | ||
| Cardiac arrest | 1.1 | |||
| Right ventricular dysfunction | 1.0 | 1.1 | ||
| Ventricular tachycardia | 1.0 | |||
| Ear/Labyrinth | Hearing impaired | 1.0 | 12.9 | 9.5 |
| Gastrointestinal | Abdominal distension | 1.0 | ||
| Abdominal pain | 5.9 | 2.2 | 2.7 | |
| Colitis | 1.0 | 1.1 | 1.4 | |
| Anal mucositis | 1.0 | |||
| Diarrhea | 5.9 | 4.3 | 6.8 | |
| Ascites | 1.0 | |||
| Malabsorption | 1.1 | |||
| Mucositis oral | 27.5 | 7.5 | 12.2 | |
| Nausea | 4.9 | 3.2 | 2.7 | |
| Constipation | 2.0 | |||
| Vomiting | 11.8 | 8.6 | 5.4 | |
| Dental caries | 1.0 | |||
| Typhlitis | 1.0 | 1.1 | ||
| Duodenal obstruction | 1.0 | |||
| Esophageal hemorrhage | 1.0 | |||
| Gastritis | 1.0 | |||
| Ileus | 2.9 | |||
| Oral pain | 3.9 | |||
| Small intestinal mucositis | 1.0 | |||
| Small intestinal obstruction | 1.0 | |||
| General/Administration | Fever | 3.9 | 6.8 | |
| Other general disorders and administration site conditions | 1.1 | |||
| Pain | 2.0 | 3.2 | 1.4 | |
| Multi-organ failure | 1.1 | |||
| Hepatobiliary | Biliary fistula | 1.0 | ||
| Other hepatobiliary disorders | 1.4 | |||
| Hepatic hemorrhage | 2.0 | |||
| Portal vein thrombosis | 1.0 | |||
| Infections/Infestations | Biliary tract infection | 1.0 | ||
| Abdominal infection | 1.1 | |||
| Catheter related infection | 2.9 | 2.2 | 4.1 | |
| Bladder infection | 2.2 | |||
| Enterocolitis infectious | 3.9 | 2.2 | 2.7 | |
| Duodenal infection | 1.0 | |||
| Other infections and infestations | 15.7 | 10.8 | 16.2 | |
| Upper respiratory infection | 3.2 | 1.4 | ||
| Eye infection | 1.1 | |||
| Wound infection | 1.4 | |||
| Sepsis | 2.9 | 2.2 | ||
| Urinary tract infection | 2.0 | 1.1 | ||
| Lung infection | 2.2 | |||
| Peritoneal infection | 1.1 | |||
| Skin infection | 1.1 | |||
| Small intestine infection | 1.1 | |||
| Injury/Poisoning/Procedural | Biliary anastomotic leak | 1.0 | ||
| Postoperative hemorrhage | 1.0 | |||
| Investigations | Alanine aminotransferase increased | 3.9 | 20.4 | 6.8 |
| Activated partial thromboplastin time prolonged | 2.2 | |||
| Aspartate aminotransferase increased | 8.8 | 24.7 | 6.8 | |
| Alkaline phosphatase increased | 1.4 | |||
| Blood bilirubin increased | 1.0 | 5.4 | 1.4 | |
| Creatinine increased | 1.0 | 1.1 | 1.4 | |
| GGT increased | 1.0 | 4.3 | 2.7 | |
| Neutrophil count decreased | 2.0 | 3.2 | 4.1 | |
| Fibrinogen decreased | 2.2 | |||
| Ejection fraction decreased | 1.4 | |||
| Weight loss | 2.0 | 3.2 | 1.4 | |
| Other investigations | 1.4 | |||
| Platelet count decreased | 1.1 | 2.7 | ||
| Metabolism/Nutrition | Acidosis | 1.0 | 3.2 | |
| Anorexia | 16.7 | 7.5 | 8.1 | |
| Alkalosis | 1.1 | |||
| Dehydration | 6.9 | 4.3 | 2.7 | |
| Hyperglycemia | 6.9 | 5.4 | 4.1 | |
| Hyperkalemia | 5.9 | 2.2 | 5.4 | |
| Hypermagnesemia | 3.2 | 1.4 | ||
| Hypernatremia | 1.0 | |||
| Hypocalcemia | 1.0 | 3.2 | 4.1 | |
| Hypokalemia | 21.6 | 12.9 | 18.9 | |
| Hypoalbuminemia | 3.2 | |||
| Hypomagnesemia | 2.9 | 4.3 | 5.4 | |
| Hyponatremia | 11.8 | 6.5 | 5.4 | |
| Hypophosphatemia | 9.8 | 6.5 | 6.8 | |
| Tumor lysis syndrome | 2.0 | |||
| Musculoskeletal/Connective | Arthralgia | 1.0 | ||
| Generalized muscle weakness | 1.1 | 1.4 | ||
| Back pain | 1.0 | |||
| Bone pain | 1.0 | |||
| Muscle weakness lower limb | 1.0 | |||
| Nervous | Oculomotor nerve disorder | 2.0 | 1.1 | |
| Abducens nerve disorder | 1.1 | |||
| Peripheral motor neuropathy | 1.0 | 4.3 | 2.7 | |
| Peripheral sensory neuropathy | 2.0 | 3.2 | ||
| Syncope | 1.1 | |||
| Psychiatric | Agitation | 2.0 | 1.1 | 1.4 |
| Hallucinations | 1.1 | |||
| Renal/Urinary | Acute kidney injury | 6.9 | 2.7 | |
| Other renal and urinary disorders | 1.0 | 1.1 | 1.4 | |
| Renal calculi | 1.0 | |||
| Respiratory/Thoracic/Mediastinal | Atelectasis | 2.0 | ||
| Dyspnea | 1.1 | 1.4 | ||
| Bronchospasm | 1.4 | |||
| Hypoxia | 6.9 | 2.2 | 1.4 | |
| Pleural effusion | 1.0 | 1.1 | ||
| Pulmonary edema | 1.0 | |||
| Stridor | 1.1 | |||
| Respiratory failure | 2.0 | |||
| Skin/Subcutaneous | Erythema multiforme | 1.0 | ||
| Other skin and subcutaneous tissue disorders | 1.0 | 1.4 | ||
| Rash maculo-papular | 2.9 | |||
| Vascular | Hypertension | 4.9 | 1.1 | |
| Hematoma | 1.1 | |||
| Hypotension | 1.0 | 1.1 | 1.4 | |
| Other vascular disorders | 1.0 | 1.1 | ||
| Eye | Other eye disorders | 1.1 | ||
| Surgical/Medical | Other surgical and medical procedures | 1.1 | ||
N=Number of Patients
Delivery of chemotherapy
Delivery of C5VD was possible with a mean percentage of the target dose of cisplatin delivered during cycles 1-4 of 96% (95% CI: 94-97%), 5-FU of 96% (95% CI: 94-97%), doxorubicin of 95% (95% CI: 93-97%) and vincristine of 90% (95% CI: 87-93%).
Response:
101 eligible patients were evaluable for best overall response to therapy, 92 (91%) demonstrated a complete response, another 7 (7%) as a partial response and 2 (2%) had stable disease. One patient was lost to followup and was not fully evaluable.
Outcome:
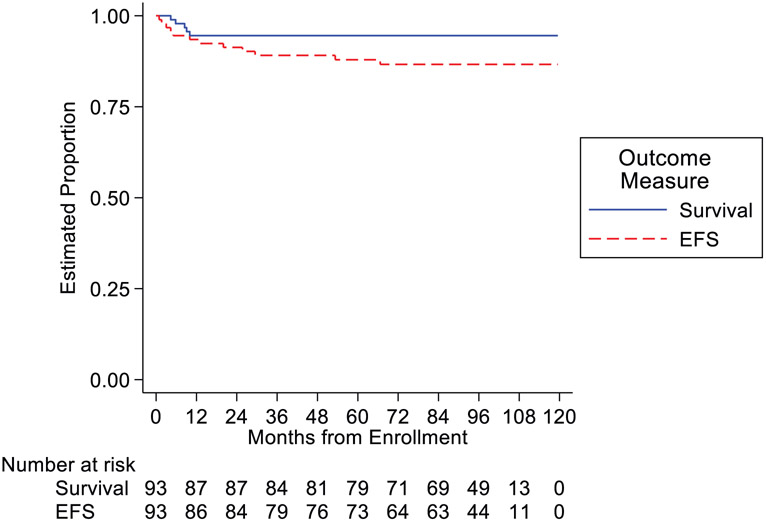
For the 93 patients who did not have complete resection at diagnosis, five-year EFS was 88% (95% confidence interval-CI: 79-93%) while five-year OS was 95% (95% CI: 87-98%) (Figure 2). The median potential follow-up time for EFS was 97 months; the 25th and 75th percentiles for potential follow-up were 86 months and 102 months. There was no significant difference in risk for an event according to patient sex or age.
Figure 2.
Event-Free and Overall Survival of Patients with Unresectable non-Metastatic Hepatoblastoma
A total of ten patients have had disease relapse at the time of study analysis. The sites of recurrence were pulmonary (n = 4), liver (n= 3), combined pulmonary and liver recurrence (n=2) and nodal (n=1). Two patients have had secondary malignancies and only one patient experienced death as a first analytic event at 4 months after the start of protocol therapy. According to protocol criteria, the regimen is considered to be feasible to administer to the target population.
PRETEXT Group and Surgery:
PRETEXT groups at diagnosis were I (n=3), II (n=31), III (n=54), and IV (n=14). Ninety-two patients had an upfront biopsy, nine had a primary resection (PRETEXT I=3, PRETEXT II=5, PRETEXT III=1), and one patient was deemed too sick for an initial biopsy. Following chemotherapy, a total of 65 patients were able to undergo a partial liver resection. Thirty-three additional patients underwent complete liver resection and OLT. Therefore, a total of 98 patients (96%) had a resection performed. Three patients who underwent OLT could not resume protocol therapy within 42 days of surgery, and nine patients did not have surgery following cycle 4 all while awaiting transplant. Operative procedures performed included: right lobectomy (n=29 [44%]), left lobectomy (n=14 [21%]), right trisegmentectomy (n=9 [14%]), non-anatomic wedge resection (n=5 [8%]), left lateral segmentectomy (n=5 [8%], and mesohepatectomy (n=3 [5%]).
Pathology:
Twenty-one patients were noted to have SCU on central review at diagnosis ranging from 1-25% of the overall tumor, 4 have recurred and three are dead of disease. SCU histology was not predictive of the risk for an event with a detailed description to be reported elsewhere.
Discussion
The AHEP0731 trial accomplished the primary objective and demonstrates that C5VD is a feasible and tolerable regimen for patients with non-metastatic or resected SCU histology HB. Toxicities were within the scope of expectations, and chemotherapy could be delivered as intended in the vast majority of patients. Specifically, enrolled patients experienced low rates of cardiac toxicity.23 The impact of the use of dexroxazane during the last two C5VD cycles is difficult to discern and this trial was not designed nor does it shed light on its use in future studies. The routine use of dexroxazane should be considered for use in future trials for all doses depending on the planned cumulative dosing. EFS of 88% at 5 years is excellent for patients with unresectable, non-metastatic disease at diagnosis.
This patient cohort experienced favorable secondary outcomes of response rates, surgical resection rates, and outcomes. Differences in risk-stratification and disease classification make comparison to other trials difficult. When evaluating treatment regimens such as C5VD for patients with unresectable disease, the question remains as to whether improvements observed here are more a result of enhanced chemotherapeutic efficacy or increased resection rates. In reality, it is difficult to determine this for any regimen and is likely due in part to both.
INT-0098 demonstrated the impact of doxorubicin on outcome as tumor progression accounted for 86% of the events among patients treated with C5V, but only 50% of the events for those treated with CD.12 However, the history of COG protocols demonstrates that there is a substantial subgroup of patients who can be resected and cured without doxorubicin using C5V alone.1 The SIOPEL 3 results using cisplatin monotherapy for standard-risk patients supports this as well.24 Ultimately, the contribution of 5-flourouracil and vincristine to the efficacy of North American HB treatment regimens has been debated but toxicities related to these agents are relatively mild with >90% of intended chemotherapy being delivered in the majority of patients. Modifications of vincristine were seen most commonly. However, this is very much consistent with modifications observed in trials of other solid tumors such as rhabdomyosarcoma and Wilms tumor which also use weekly vincristine as administered here. This does support efforts to determine the true contribution of vincristine to HB therapy.
International cooperative group trials have focused on the use of cisplatin and doxorubicin but have used various doses and durations of infusion for both drugs.4,8,11 For safety reasons, the COG has adopted standard dosing for doxorubicin to be given over 15 minutes unless there is clear evidence that a different infusion schema, and specifically continuous infusion, is of proven benefit. The administration of doxorubicin in the majority of previous HB trials has been by continuous infusion.1,8 However, there are no data that establish continuous infusion doxorubicin as more efficacious or safer than bolus administration. Prolonged administration of doxorubicin requires hospitalization and results in more mucositis and myelosuppression. Additionally, there is no documented evidence that continuous infusion doxorubicin results in diminished cardiac toxicity.25 The doxorubicin dosing given over 15 minutes in this study was well tolerated. In addition, the 95% drug DOX drug delivery, feasibility, and high CR/PR rates and excellent outcomes in this trial suggest that the shorter infusion time is a reasonable strategy although this may need to be further evaluated in a randomized trial.
C5VD was a feasible and well tolerated regimen and resulted in excellent outcomes. The data here suggest that this is a regimen that should continue to be explored in future trials because of the favorable resection rates, survival, and toxicity profile.
Supplementary Material
ACKNOWLEDGENTS:
The study team is indebted to Fran Laurie, Sandy Kessel, Richard Hanusik, Loretta Nelson, and the rest of the staff of the Quality Assurance Review Center for collection of scans, compilation of data, and facilitation of radiographic central review sessions.
RESEARCH SUPPORT:
Research is supported by the Imaging and Radiation Oncology Core (IROC-RI, formerly QARC) Grant U10 CA29511, the National Clinical Trials Network Operations Center Grant U10CA180886 and Statistics & Data Center Grant U10CA180899 from the National Cancer Institute, National Institutes of Health, Bethesda, MD, USA and the St. Baldrick’s Foundation, Monrovia CA, USA. A complete listing of grant support for research conducted by CCG and POG before initiation of the COG grant in 2003 is available online at: http://www.childrensoncologygroup.org/admin/grantinfo.htm
Footnotes
CONFLICT OF INTEREST DISCLOSURE:
HMK is employed by EMD Serono, a division of Merck KGaA. This work was done prior to this employment. AJT reported grants from the Cystic Fibrosis Foundation, Guerget, and Siemens. He reports royalties from Applied Radiology and Elsevier outside the submitted work. MDK reports personal fees from Merck Sharpe and Dohme outside the submitted work. No potential conflict of interest relevant to this article was reported by any of the remaining authors.
REFERENCES:
- 1.Trobaugh-Lotrario AD, Katzenstein HM. Chemotherapeutic approaches for newly diagnosed hepatoblastoma: past, present, and future strategies. Pediatr Blood Cancer 2012, 59: 809–812. [DOI] [PubMed] [Google Scholar]
- 2.Aronson DC, Meyers RL. Malignant tumors of the liver in children. Sem Pediatr Surg 2016, 25: 265–275. [DOI] [PubMed] [Google Scholar]
- 3.Katzenstein HM, Langham MR, Malogolowkin MH, et al. Minimal adjuvant chemotherapy for children with hepatoblastoma resected at diagnosis (AHEP0731): a Children’s Oncology Group, multicentre, phase 3 trial. Lancet Oncol 2019, 20: 719–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czauderna P, Lopez-Terrada D, Hiyama E, et al. Hepatoblastoma state of the art: pathology, genetics, risk stratification, and chemotherapy. Curr Opin Pediatr 2014, 26:19–28. [DOI] [PubMed] [Google Scholar]
- 5.Lake CM, Tiao GM, Bondoc AJ. Surgical management of locally advanced and metastatic hepatoblastoma. Semin Pediatr Surg 2019, 28:150856. [DOI] [PubMed] [Google Scholar]
- 6.Meyers RL, Tiao G, de ville de Goyet, et al. Hepatoblastoma state of the art: PRETEXT, surgical resection guidelines and the role of liver transplantation. Curr Opin Pediatr 2014, 26: 29–36. [DOI] [PubMed] [Google Scholar]
- 7.Brock PR, Maibach R, Childs M, et al. Sodium thiosulfate for protection from cisplatin induced hearing loss. N Engl J Med 2018, 25: 2376–2385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perilongo G, Malogolowkin M, Feusner J. Hepatoblastoma clinical research: Lessons learned and future challenges. Pediatr Blood Cancer 2012. 59: 818–82. [DOI] [PubMed] [Google Scholar]
- 9.Hishiki T, Matsunaga T, Sasaki F, et al. Outcome of hepatoblastoma treated using the Japanese Study Group for Pediatric Liver Tumor (JPLT) protocol- 2: report from the JPLT. Pediatr Surg Int 2011; 27:1–8 [DOI] [PubMed] [Google Scholar]
- 10.Fuchs J, Rydzynski J, Hecker H et al. The influence of preoperative chemotherapy and surgical technique in the treatment of hepatoblastoma: a report from the German Cooperative Liver Tumour Studies HB 89 and HB 94. Eur J Pediatr Surg 2002, 12: 255–61. [DOI] [PubMed] [Google Scholar]
- 11.Haeberle B, Maxwell R, vonSchweinitz D, Schmid I. High dose chemotherapy with autologous stem cell transplantation in hepatoblastoma does not improve outcome. Results of the GPOH study HB99; Klin Padiatr 2019. 231: 283–290. [DOI] [PubMed] [Google Scholar]
- 12.Ortega JA, Douglass EC, Feusner JH et al. Randomized comparison of cisplatin/vincristin/5-fluorouracil and cisplatin/doxorubicin for the treatment of pediatric hepatoblastoma (HB): A report from the Children’s Cancer Group and the Pediatric Oncology Group. J Clin Oncol 2000; 18: 2665–2675. [DOI] [PubMed] [Google Scholar]
- 13.Malogolowkin MH, Katzenstein HM, Krailo M, et al. Intensified platinum therapy is an ineffective strategy for improving outcome in pediatric patients with advanced hepatoblastoma. J Clin Oncol 2006; 24: 2879–84. [DOI] [PubMed] [Google Scholar]
- 14.Katzenstein HM, Chang KW, Krailo MD, et al. Amifostine does not prevent platinum-induced hearing loss associated with treatment of children with hepatoblastoma; A report of the Intergroup Hepatoblastoma Study P9645 as part of Childrens Oncology Group. Cancer 2009; 115: 5828–5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zsiros J, Maibach R, Shafford E, et al. Successful treatment of childhood high-risk hepatoblatoma with dose-intensive multiagent chemotherapy and surgery: Final results of the SIOPEL-3HR study. J Clin Oncol 2010; 28: 2584–2590. [DOI] [PubMed] [Google Scholar]
- 16.Zsiros J, Brugieres L, Brock P, et al. Dose-dense cisplatin-based chemotherapy and surgery for children with high risk hepatoblastoma (SIOPEL 4): A prospective, single-arm, feasibility study. Lancet Oncol 2013, 14: 834–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weldon CB, Madenci AL, Tiao GM, et al. Evaluation of the diagnostic biopsy approach for children with hepatoblastoma: A report from the children's oncology group AHEP 0731 liver tumor committee. J Pediatr Surg 2020; 55: 655–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katzenstein HM, Furman WL, Malogolowkin MH, et al. Upfront window vincristine/irinotecan treatment of high risk hepatoblastoma: a report from the children’s oncology group AHEP 0731 study committee. Cancer 2017, 123: 2360–2367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O’Neill AF, Towbin AJ, Krailo MD, et al. Characterization of pulmonary metastases in children with hepatoblastoma treated on Children’s Oncology Group protocol AHEP 0731 (The treatment of children with all stages of hepatoblastoma): a report from the Children’s Oncology Group. J Clin Oncol 2017. 35: 3465–3473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalbfleisch JD and Prentice RL. The statistical analysis of failure time data. John Wiley and Sons, New York, 2002. [Google Scholar]
- 21.Schemper M and Smith TL. A note on quantifying follow-up in studies of failure time. Controlled Clin Trials 1996. 17: 343–346. [DOI] [PubMed] [Google Scholar]
- 22.Kaplan EL and Meier P. Nonparametric estimation from incomplete observations. J Amer Statist Assoc 1958. 53: 457–481. [Google Scholar]
- 23.Malogolowkin MH, Katzenstein HM, Krailo M, et al. Redefining the role of doxorubicin for the treatment of children with hepatoblastoma. J Clin Oncol 2008; 26: 2379–2383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perilongo G, Maibach R, Shafford E, et al. Cisplatin versus cisplatin plus doxorubicin for standard risk hepatoblastoma. N Engl J Med 2009; 361: 1662–1670. [DOI] [PubMed] [Google Scholar]
- 25.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol 2013. 10: 697–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.