Abstract
A synthetic route to embed aggregation-induced-emission-(AIE)-active luminophores in polycarbonates (PCs) in various ratios is reported. The AIE-active monomer is based on the structure of 9,10-bis(piperidyl)anthracene. The obtained PCs display good film-forming properties, similar to those observed in poly(bisphenol A carbonate) (Ba-PC). The fluorescence quantum yield (Φ) of the PC with 5 mol% AIE-active monomer was 0.04 in solution and 0.53 in solid state. Moreover, this PC is also miscible with commercially available Ba-PC at any blending ratio. A combined analysis by scanning electron microscopy and differential scanning calorimetry did not indicate any clear phase separation. These results thus suggest that even engineering plastics like polycarbonates can be functionalized with AIE luminogens without adverse effects on their physical properties.
Fluorescent polycarbonates were synthesized by embedding AIE-active diol monomers with simple structures in the polymer chain.
Introduction
The production of polymeric organic light-emitting diodes (OLEDs) requires luminophores whose luminescence efficiency does not decrease due to concentration quenching.1 In recent years, the aggregation-induced emission (AIE) phenomenon has received substantial attention on account of its potential applications in OLEDs.2,3 AIE describes a phenomenon in which organic chromophores exhibit efficient luminescence in aggregated states or under conditions where molecular motions are restricted, while their luminescence is suppressed in dilute solutions.4 This phenomenon is thus the opposite of aggregation-caused quenching (ACQ), which is encountered in most organic chromophores. The most outstanding advantage of AIE luminogens (AIEgens) is their bright solid-state luminescence. This feature arises from the weak excitonic interactions between these chromophores even in the solid state.5
Over the last two decades, AIEgens have been extensively investigated in the context of materials6 and analytical7 science. However, the structural diversity of typical AIEgens is limited. These molecules are usually sterically demanding and contain multiple rotating axes.8 When such AIEgens are employed to fabricate polymeric thin films, their physical properties, such as the glass transition temperature (Tg) and the toughness are usually affected negatively. Structurally simple AIEgens are therefore desirable in order to apply AIEgens in e.g. OLEDs and luminescent polymers. Moreover, structural simplicity would make AIEgens (i) easy to synthesize and (ii) susceptible to further modifications.6a
In a previous report, we have discovered N,N-dialkylaminoarenes as a new class of unique AIEgens,8–10 which do not depend on axis rotation to display the AIE phenomenon. These structurally simple dyes are easier to synthesize than the aforementioned bulky AIEgens and display superior fluorescence in the solid state. Moreover, they exhibit the same color in solution and in the solid state. Our mechanistic study of these unusual AIEgens opened up a new design avenue for structurally simple AIEgens, which has been corroborated by theoretical calculations.9 Our design strategy consists of introducing strong electron donors to polycyclic aromatic hydrocarbons (PAHs) so that a S1/S0 crossing (or minimum energy conical intersection (MECI)) with large amplitude modes becomes readily accessible in dilute states. This large motion is suppressed in the solid state, which leads to pronounced AIE phenomena.
By modifying these AIE dyes into bifunctional monomers, application of these AIE dyes as building blocks in polymer science becomes possible. As reported in our previous report, we have already synthesized AIE-active diamine monomers and AIE-active polyamides.11 However, the application of AIEgens in OLEDs requires the introduction of these dyes in transparent optical materials such as polymethylmethacrylates (PMMAs) and polycarbonates (PCs),12 which has not yet been accomplished. Incorporation of the AIEgen in the polymer chain has many advantages compared to making doped polymers with the AIEgen, such as the synthesized polymer not being affected much by factors such as physical ageing of the polymer, etc.
In this study, we synthesized a new type of fluorescent PCs that contain N,N-dialkylaminoarene chromophores as AIEgens in the main chain. The AIEgen was synthesized from an anthracene derivative and a piperidine derivative with a hydroxyl group. The bifunctional diol monomers were synthesized by a selective transesterification process to protect the hydroxyl group using the Ohshima–Mashima zinc cluster catalyst (ZnTAC24®),13 followed by a C–N cross-coupling reaction14,15 and a simple deprotection step. Using this bifunctional diol monomer, PCs with varying AIE-active monomer ratios were synthesized. The PCs were then cast into thin films in order to determine the transparency of these films. The miscibility of these PCs were determined by mixing them with commercially available poly(bisphenol A carbonate) Ba-PC. The optical properties of the PCs were then studied.
Results and discussion
Monomer synthesis
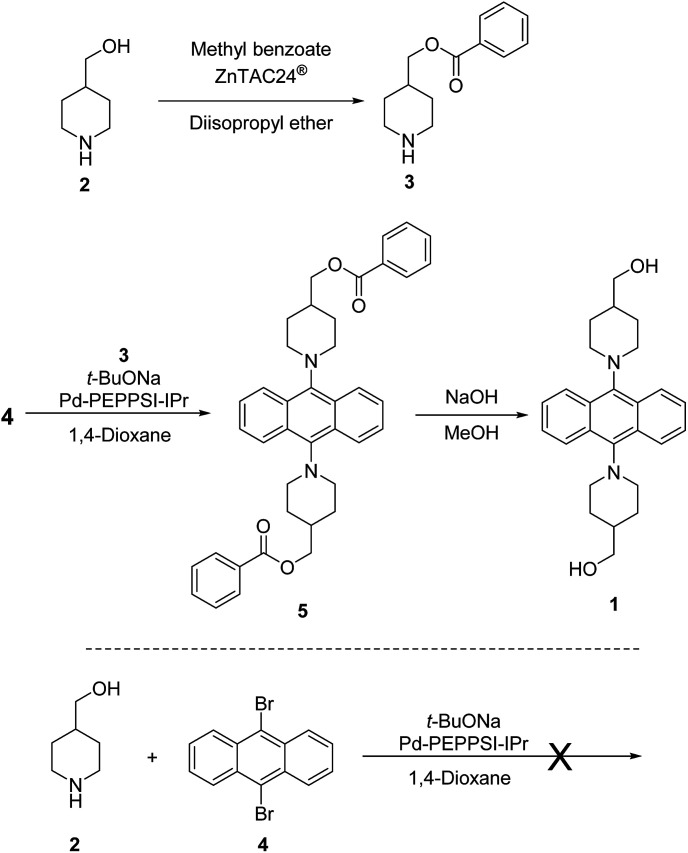
Synthesis of 9,10-bis(4-hydroxymethyl)piperidylanthracene (1) was done by introducing a reactive group into the previously reported AIEgens based on 9,10-bis(piperidyl)anthracene (BPA).8 Diol monomer 1 was synthesized according to Scheme 1. Initially, we attempted a direct synthesis using 9,10-dibromoanthracene 4 and 4-piperidinemethanol (2) with Pd-PEPPSI-IPr14 as a catalyst. This reaction was not successful, most likely due to the interference of the hydroxyl group with the C–N coupling reaction. One plausible explanation for this failure is that the hydroxyl group might coordinate to the palladium atom, which could stop the catalytic cycle. Therefore, the reaction scheme was altered to include the protection of the hydroxyl group and a final deprotection step. In this case, ZnTAC24®13 was used, given that it efficiently catalyzes the selective O-acylation. This is due to the fact that the chemoselective O-acylation using zinc clusters can be carried out under mild conditions, similar to enzymes, which allows the preservation of sensitive functional groups. Therefore, we used ZnTAC24® as a catalyst to selectively protect the hydroxyl group of 4-piperidinemethanol (2) to afford 3 in 43% yield through a transesterification process with methyl benzoate (Scheme 1). For the C–N cross-coupling reaction, we used Pd-(PEPPSI-IPr)14 or Pd(BINAP)15 as the catalyst, considering that Pd catalysts with N-heterocyclic carbene ligands are known for their high tolerance towards sterically congested systems.9 Ultimately, Pd-PEPPSI-IPr afforded better results compared to Pd-BINAP.§,16 The final step, i.e., a deprotection with sodium hydroxide, afforded 1 as a yellow solid in 41% yield.
Scheme 1. Synthesis of AIE-active monomer 9,10-bis(4-hydroxymethylpiperidyl)anthracene (1).
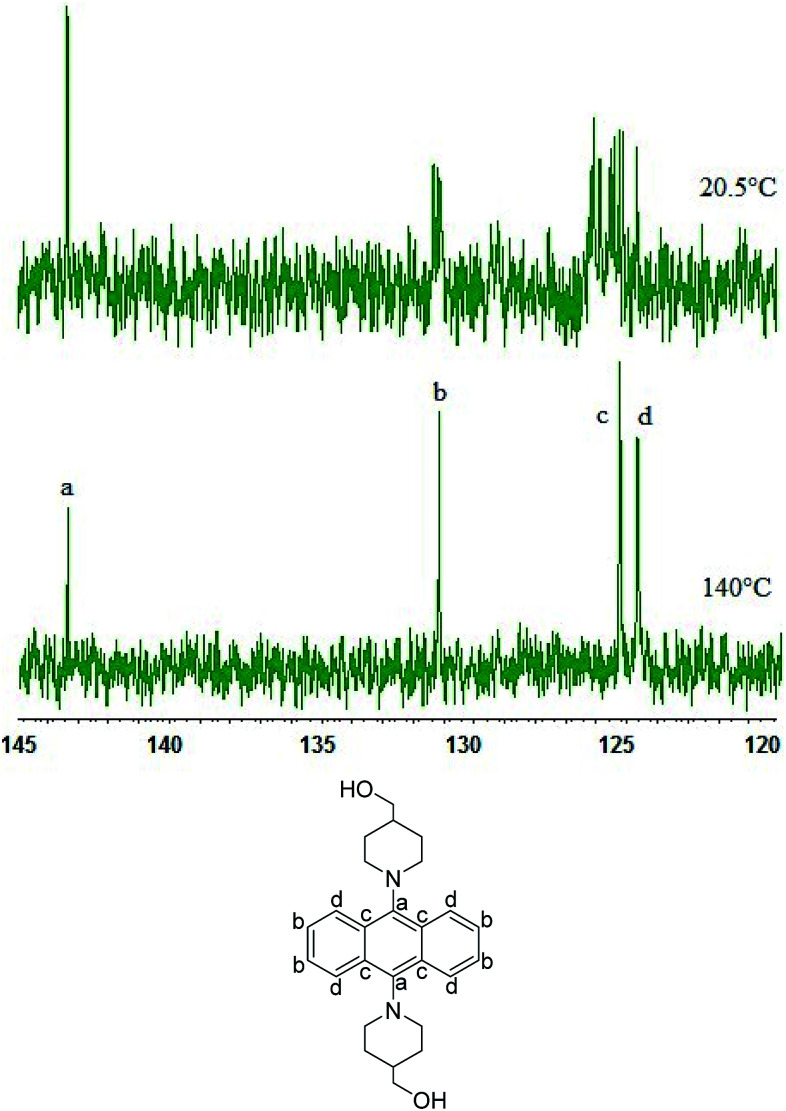
Monomer 1 was characterized by 1H and 13C NMR spectroscopy as well as FT-IR spectroscopy and high-resolution mass spectrometry (HRMS) (Fig. S2–S4†). Usually, anthracene rings with identical moieties at the para-position of the central aromatic ring, such as 9,10-bis(N,N-dimethylamino)anthracene, are expected to produce NMR spectra that exhibit doublets of doublets in the aromatic region. However, the aromatic region of the 1H NMR spectrum of 1 shows three types of peaks. Moreover, the 13C NMR spectrum shows more peaks in the aromatic region than what should be expected of 1. Variable-temperature (VT) 1H and 13C NMR experiments revealed that indicates that 1 has more than one isomer at room temperature. (Fig. S14†) In particular, measurement of 13C NMR (DMSO-d6) at 20.5 shows at least 14 peaks in aromatic region (carbon of anthracene ring). On the other hand, that of 140 °C shows 4 peaks (carbons a–d: minimum number of anthracene ring) in the aromatic region, as shown in Fig. 1. Some possible isomers of 1 include an isomer in which both hydroxyl groups are facing the same direction, and an isomer in which the hydroxyl groups are facing opposite directions. In order to unequivocally determine the precise structure of 1, a single-crystal X-ray diffraction analysis would be required; however, this is beyond the scope of this study. The FT-IR spectrum indicates the presence of a hydroxyl group, and the observed m/z value matches the calculated m/z value measured by HRMS.
Fig. 1. Magnified view of the aromatic region of the 13C NMR (125 MHz, DMSO-d6) spectrum of 1 at 20.5 °C (top) and 140 °C (bottom).
Polymer synthesis
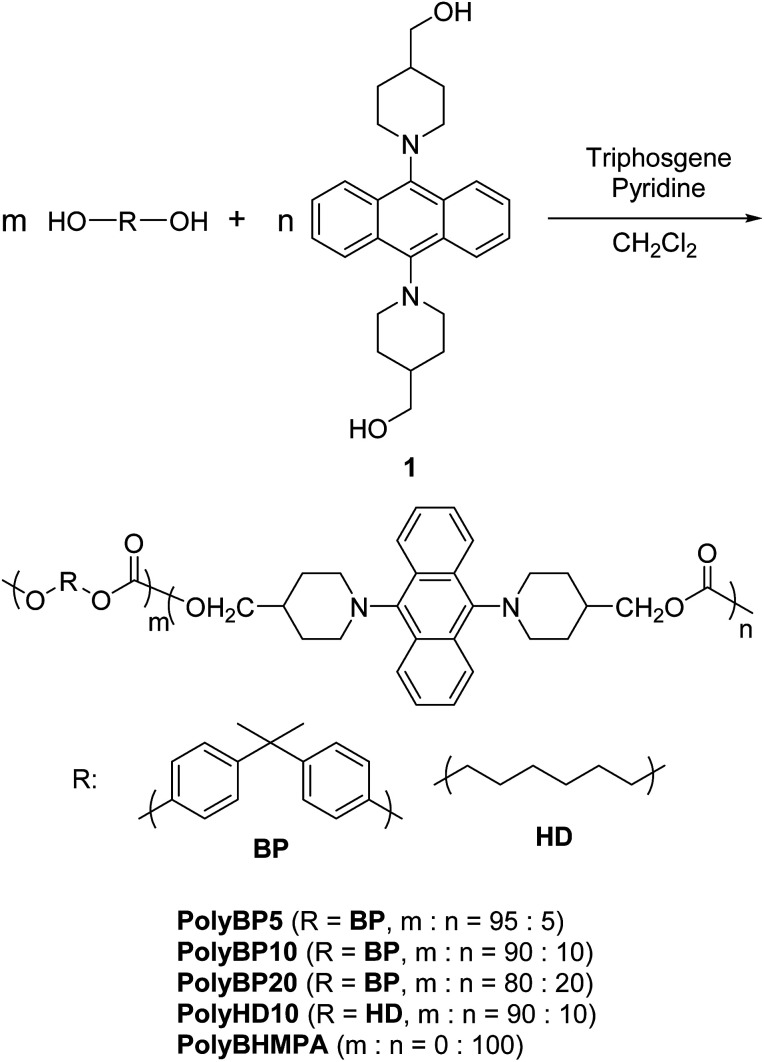
To capitalize on the excellent transparency, strength, and film-forming properties of Ba-PC, a novel design of copolymers with low AIEgen content is required. Numerous polymers have been synthesized through condensation polymerization with luminescent units in the polymer chain, which contain small doses of dyes.17 In this study, we decided to use various ratios of AIEgens in the copolymerization of triphosgene and 2,2-bis(4-hydroxyphenyl)propane (bisphenol A) as shown in Scheme 2. For comparison, PCs containing only the AIEgen or utilizing 1,6-hexanediol were also synthesized.
Scheme 2. Synthesis of PCs with varying AIEgen ratios.
The synthesis of all PCs was carried out at low temperatures in solution. The concentration of the diol monomers in dry pyridine was set to 1.0 mol L−1. Triphosgene was dissolved in dichloromethane at a concentration of 0.40 mol L−1, and added dropwise to the monomer solution at 0 °C. The reaction mixture was then allowed to stir for 30 minutes at 0 °C, and then for another 30 minutes at room temperature. Subsequently, dichloromethane was added to dissolve the precipitate formed during the reaction. The reaction mixture was then added dropwise to methanol at 0 °C in order to induce reprecipitation. The precipitate was filtered and dried in vacuo. Furthermore, in some cases, the amount of dry pyridine was increased due to the low solubility of 1. The obtained copolymers were well soluble in good solvents for Ba-PC, e.g. dichloromethane and THF.
The structures of the obtained PCs were determined by 1H NMR spectroscopy (Fig. S5 and S7–S10†). The 1H NMR spectra show that the ratios of bisphenol A to monomer 1 in the synthesized PCs are approximately identical to the starting ratios.
The yields of the synthesized PCs, the number-average molecular weight (Mn), the weight-average molecular weight (Mw), and the polydispersity index (PDI) are summarized in Table 1. The molecular weight and the degree of polymerization (DP) were determined either by gel permeation chromatography (GPC) in THF or by 1H NMR spectroscopy. The obtained results suggest that the molecular weight increases with a decreasing ratio of monomer 1 to bisphenol A. In the case of 1, decomposition did not occur during the synthesis and the purification of the PCs. Therefore, the reduction of the molecular weight of the PC due to the decomposition of 1 is highly unlikely. In the case of PolyBHMPA, the low molecular weight was therefore attributed to its low solubility during the polymerization.
Yield and physical properties of the synthesized PCs.
| PC | Yield (%) | M n | M w | PDI |
|---|---|---|---|---|
| PolyBP5 | 92 | 32 000 | 100 000 | 3.1 |
| PolyBP10 | 78 | 18 000 | 45 000 | 2.5 |
| PolyBP20 | 48 | 5900 | 15 000 | 3.0 |
| PolyHD10 | 72 | 7200 | 27 000 | 3.8 |
| PolyBHMPA | 30 | a | a | a |
PolyBHMPA: DP = 4; calculated based on the 1H NMR spectrum.
Subsequently, copolymer PolyBP5 was chosen for the analysis of its physical properties given that it is the only copolymer among the synthesized PCs with properties similar to engineering plastics, i.e., PolyBP5 exhibits a suitable molecular weight, film strength, and stability of its melting point. Therefore, it is possible to generate transparent films of PolyBP5 from spin coating or casting from dichloromethane solution.
Polymer blends
Apart from the use of the polymer films of fluorescent PCs, the use of polymer blends is also important for applications in advanced optical materials. To study the miscibility of PolyBP5 with Ba-PC, we employed differential scanning calorimetry (DSC) and scanning electron microscopy (SEM).
PolyBP5 was mixed with commercially available Ba-PC (typically: Mw = 64 000; Sigma-Aldrich) in a dichloromethane solution (1 : 1, w/w). The mixture was stirred until the polymers were completely dissolved before the polymer solution was added dropwise into methanol at 0 °C in order to induce reprecipitation of the polymer. The precipitate was filtered and dried under reduced pressure. This precipitate was then dissolved in 1,4-dioxane and used to generate a polymer blend film (thickness: ∼10 nm; measured by a Dektak surface profiler) using a spin coater.
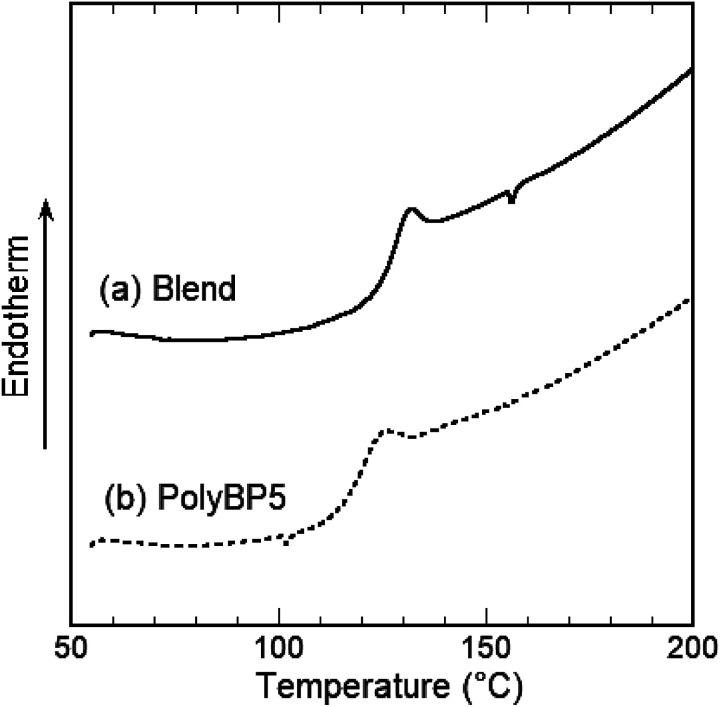
This polymer blend exhibited a jump in heat capacity ascribed to the glass transition at 127 °C. This glass transition temperature (Tg) lies between that of the commercially obtained Ba-PC (Tg = 150 °C) and that measured for PolyBP5 (Tg = 119 °C). The observed single intermediate glass transition indicates that the polymers were mixed evenly (Fig. 2).
Fig. 2. DSC thermograms of PolyBP5 and a 1 : 1 (w/w) polymer blend of PolyBP5 and Ba-PC.
The polymer blend film was then characterized by SEM in order to examine the morphology of the film. The SEM images show a clear image without any visible polymer domains (Fig. 3), which is consistent with the DSC data, that the formed film is structurally uniform. The results of the SEM and DSC measurements thus suggest the absence of any phase separation between PolyBP5 and Ba-PC. Based on these results, PolyBPs with small AIEgen ratios (5 mol%) seem ideal candidates for polymer blends, in view of their good solubility, film-forming properties and transparency, which are comparable to those of Ba-PC.
Fig. 3. SEM analysis of a 1 : 1 (w/w) polymer blend of PolyBP5 and Ba-PC.
Optical properties
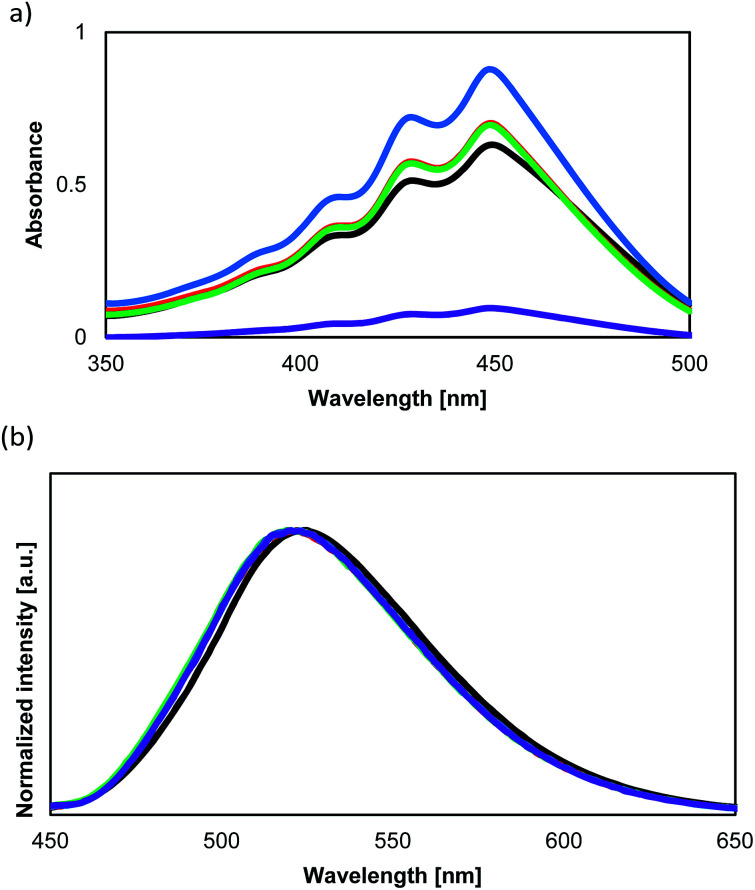
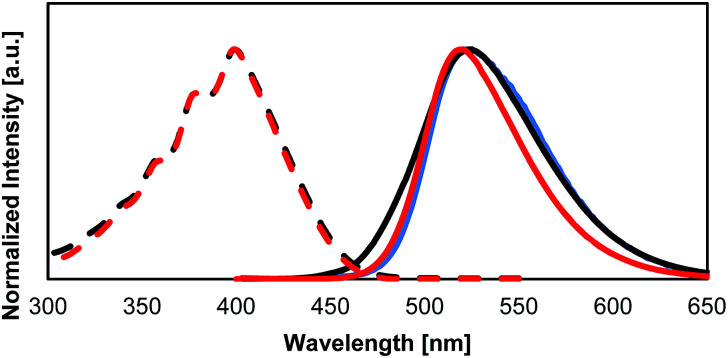
The optical absorption and fluorescent properties of the PCs synthesized in this study are summarized in Table 2 together with those of the reference compound BPA. Fig. 4a and b show the UV-vis absorption and fluorescence spectra of the PCs together with those of 1. Fig. 5 shows the UV-vis absorption and fluorescence spectra of monomer 1 and the reference compound BPA.
Optical properties of 1 and the PCs synthesized in this study.
| Compound | λ abs [nm] | λ fl [nm] | Φ THF a | Φ solid b |
|---|---|---|---|---|
| 1 | 399 | 528 | 0.03 | 0.57 |
| PolyBP5 (bulk) | 399 | 522 | 0.04 | 0.53 |
| PolyBP5 (film) | 407 | 516 | — | 0.62 |
| PolyBP10 | 399 | 519 | 0.04 | 0.54 |
| PolyBP20 | 399 | 524 | 0.04 | 0.47 |
| PolyHD10 | 399 | 524 | 0.04 | 0.23 |
| PolyBHMPA | 399 | 520 | 0.04 | 0.17 |
| PC blend film | 400 | 510 | — | 0.51 |
| 1&Ba-PC blend | 410 | 510 | — | 0.74 |
| BPAc | 399 | 528 | 0.02 | 0.86 |
Measured in THF (10−4 M) to obtain Φ at 293 K; excitation wavelength: 399 nm.
Bulk sample; excitation wavelength: 400 nm.
9,10-Bis(piperidyl)anthracene (BPA).
Fig. 4. (a) Absorption spectra of monomer 1 (black), as well as copolymers PolyBP5 (red), PolyBP10 (green), PolyBP20 (blue), and PolyBHMPA (purple). All spectra were measured in THF (10−4 M). (b) Fluorescence spectra of monomer 1 (black), as well as copolymers PolyBP5 (red), PolyBP10 (green), PolyBP20 (blue), and PolyBHMPA (purple). All spectra were measured in THF (10−4 M).
Fig. 5. Absorption and fluorescence spectra of 1 and BPA. Solid fluorescence of 1 (blue), as well as solution fluorescence of 1 (black) and BPA (red) (dashed lines: absorption spectra; solid lines: fluorescence spectra). The intensities of the spectra were normalized. All spectra were measured in THF solution (10−4 M).
The absorption and fluorescence spectra of 1 do not differ from those of previously reported BPA. However, 1 shows a similar quantum yield in solution, and a lower quantum yield than BPA in the solid state. The presence of the hydroxyl groups in 1 makes it difficult to remove all impurities from the sample. The decreased quantum yield in the solid state might be caused by these impurities. Unfortunately, it is currently not possible for us to purify 1 further with the equipment available to us.
Dispersing 1 in Ba-PC at a concentration of 0.5 wt% creates a dispersion which shows a slightly higher quantum yield compared to 1 in bulk. The dispersion solution produced a polycarbonate film which is mostly clear, indicating that the individual AIEgens are mixed evenly throughout. The higher quantum yield might be due to the fact that factors such as charge transfer cannot occur easily in the dispersion, and also the fact that there is less contamination when compared to the synthesized polycarbonates.
All the PCs display Φ values of ca. 4% in dilute solution, which is identical to that of 1 and BPA.8 Therefore, it can be concluded that in dilute solution, mobility of the luminophore units are not affected by the polymer chain of PCs. Hence, the luminophores in these PCs should undergo rapid non-radiative transitions via their S1/S0 MECI, which is stabilized by N,N-dialkylamino functional groups and consistent with our previous report.9
The quantum yield of PolyBP5 and PolyBP10 in solution and the solid state is comparable to that of 1. However, when the concentration of the AIEgen is increased to 20% (PolyBP20), the quantum yield in the solid state decreases to 47%. This phenomenon could be explained by contamination from the polymerization, given that the absorption band of PolyBP20 is broader than that of the other copolymers. Such impurities might also absorb the excitation beam, thus affecting the quantum yield.
As PolyHD10 is a viscous liquid at room temperature (Tg = −51 °C), the micro-Brownian motion of the polymer chain is less restricted than that of the PCs synthesized from bisphenol A. The unrestricted movement could make the S1/S0 MECI of the BPA luminophore easily accessible, which would result in efficient quenching.
PolyBHMPA shows the smallest Φ value in the solid-state (0.17). The absorption spectrum suggests that this might be due to a potential contamination of the sample, similar to the case of PolyBP20.
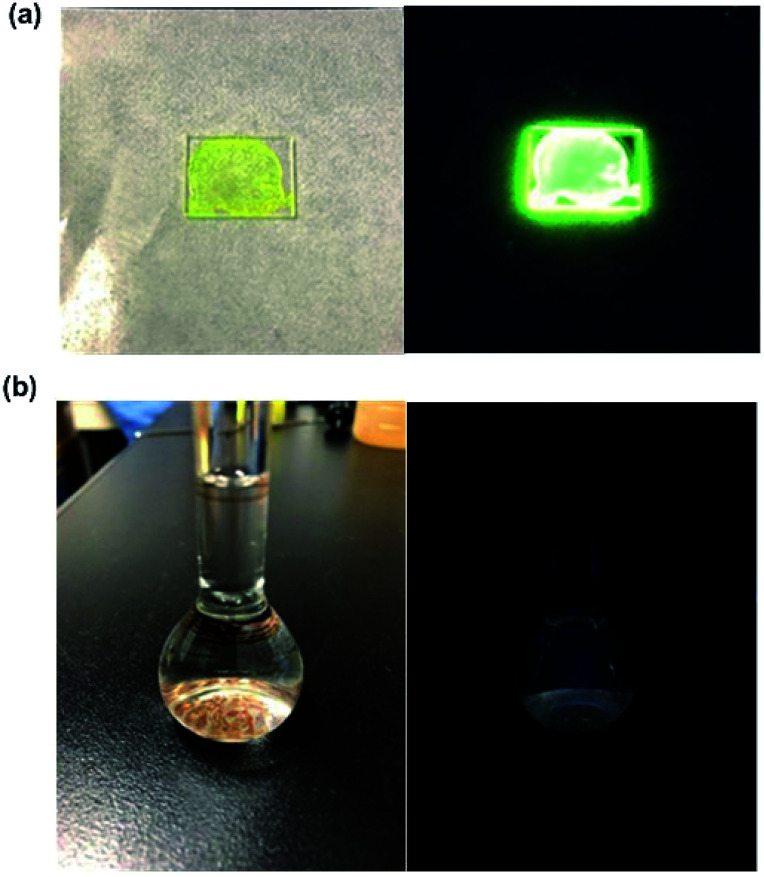
The photophysical properties of PC films of PolyBP5, which were generated using a spin coater, showed an average Φ of 0.62, which is similar to that of PolyBP5 in the bulk state. The PC blend film also showed a similar quantum yield of 0.51. The emission and fluorescence spectra are also comparable to those in the bulk. The fluorescence lifetime was found to be approximately 11.7 ns. The kf was calculated to be 4.8 × 107 s−1, and the knr was found to be 4.6 × 107 s−1. In comparison, BPA shows a fluorescence lifetime value of 9.9 ns in THF solution, and 12.1 ns (kf was calculated to be 6.7 × 107 s−1, and the knr was found to be 1.8 × 107 s−1) in colloidal suspension in a mixture of THF and water. These values indicate that non-radiative deactivation is faster, most likely due to the remaining molecular movement or the contamination in the sample (Fig. 6).
Fig. 6. (a) PolyBP5 film under ambient light (left) and under exposure to a UV lamp (λex = 365 nm) (right). (b) PolyBP5 in THF solution under ambient light (left) and under exposure to a UV lamp (λex = 365 nm) (right).
Conclusions
AIE-active PCs with varying AIE-active monomer ratios were synthesized. The photophysical properties of these PCs show that PCs with 5 mol% or 10 mol% AIE-active monomers achieve optimal luminescence. These PCs also form miscible blends with commercially available Ba-PC. The synthesized PCs exhibit good film-forming properties, which renders them suitable for applications in e.g. luminescent polymers. Embedding small ratios of AIEgens in PCs do not seem to negatively affect the physical properties of the films, and the unique properties of the AIEgen is retained in the films. Currently, we are investigating AIEgens that exhibit the three basic colors; i.e. blue, red and green in order to produce a PC with white fluorescence.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
We thank Mr Ryohei Kikuchi (SEM), Ms Masayo Ishikawa (Mass), Mr Shohei Yamazaki (DSC), and Ms Mayuko Nara (optical properties of the films) for measurements. This work is partially supported by the Grant-in-Aid for Scientific Research (B) (18H02045), Innovative Areas “π-System Figuration” (17H05145) and “Soft Crystals” (17H06371), and The Cooperative Research Program “NJRC Mater. & Dev.” from MEXT of Japan.
Electronic supplementary information (ESI) available: Experimental details, 1H and 13C NMR spectra, FT-IR chart, UV-vis and fluorescence spectra. See DOI: 10.1039/c9ra03701b
Footnotes
The choice of the catalyst depends on the substrate. The Buchwald–Hartwig C–N coupling reaction may proceed even with the presence of hydroxyl groups by choosing a different ligand and base. However, in this study, we followed the synthetic condition that was successful in our previous studies.
Notes and references
- (a) Lee J. Aizawa N. Numata M. Adachi C. Yasuda T. Adv. Mater. 2017;29:1604856. doi: 10.1002/adma.201604856. [DOI] [PubMed] [Google Scholar]; (b) Guo J. J. Zhao Z. J. Tang B. Z. Adv. Opt. Mater. 2018;6:1800264. doi: 10.1002/adom.201800264. [DOI] [Google Scholar]; (c) Albrecht K. Matsuoka K. Fujita K. Yamamoto K. Mater. Chem. Front. 2018;2:1097–1103. doi: 10.1039/C7QM00579B. [DOI] [Google Scholar]; (d) Chen S. Zeng P. J. Wang W. G. Wang X. D. Wu Y. K. Lin P. J. Peng Z. C. J. Mater. Chem. C. 2019;7:2886–2897. doi: 10.1039/C8TC06163G. [DOI] [Google Scholar]; (e) Ding D. Li K. Liu B. Tang B. Z. Acc. Chem. Res. 2013;46:2441–2453. doi: 10.1021/ar3003464. [DOI] [PubMed] [Google Scholar]; (f) Shimizu M. Hiyama T. Chem.–Asian J. 2010;5:1516–1531. doi: 10.1002/asia.200900727. [DOI] [PubMed] [Google Scholar]; (g) Uchimura M. Watanabe Y. Araoka F. Watanabe J. Takezoe H. Konishi G. Adv. Mater. 2010;22:4473–4478. doi: 10.1002/adma.201001046. [DOI] [PubMed] [Google Scholar]; (h) Niko Y. Didier P. Mely Y. Klymchenko A. S. Sci. Rep. 2016;6:18870. doi: 10.1038/srep18870. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Niko Y. Konishi G. J. Synth. Org. Chem., Jpn. 2012;70:918–927. doi: 10.5059/yukigoseikyokaishi.70.918. [DOI] [Google Scholar]; (j) Niko Y. Sugihara H. Moritomo H. Suzuki Y. Kawamata J. Konishi G. J. Mater. Chem. B. 2015;3:184–190. doi: 10.1039/C4TB01404A. [DOI] [PubMed] [Google Scholar]; (k) Chen C. H. Niko Y. Konishi G. RSC Adv. 2016;6:42962–42970. doi: 10.1039/C6RA06251B. [DOI] [Google Scholar]; (l) Wu W. C. Yeh H. C. Chan L. H. Chen C. T. Adv. Mater. 2002;14:1072–1075. doi: 10.1002/1521-4095(20020805)14:15<1072::AID-ADMA1072>3.0.CO;2-Z. [DOI] [Google Scholar]
- (a) Feng X. Qi C. X. Feng H. T. Zhao Z. Sung H. H. Y. Williams I. D. Kwok R. T. K. Lam J. W. Y. Qin A. J. Tang B. Z. Chem. Sci. 2018;9:5679–5687. doi: 10.1039/C8SC01709C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Yu L. Wu Z. B. Xie G. H. Zhong C. Zhu Z. C. Ma D. G. Yang C. L. Chem. Commun. 2018;54:1379–1382. doi: 10.1039/C7CC09925H. [DOI] [PubMed] [Google Scholar]; (c) Liu B. Q. Nie H. Lin G. W. Hu S. B. Gao D. Y. Zou J. H. Xu M. Wang L. Zhao Z. J. Ning H. L. Peng J. B. Cao Y. Tang B. Z. ACS Appl. Mater. Interfaces. 2017;9:34162–34171. doi: 10.1021/acsami.7b11422. [DOI] [PubMed] [Google Scholar]; (d) Islam A. Zhang D. D. Ouyang X. H. Yang R. J. Lei T. Hong L. Peng R. X. Duan L. Ge Z. Y. J. Mater. Chem. C. 2017;5:6527–6536. doi: 10.1039/C7TC01597F. [DOI] [Google Scholar]; (e) Furue R. Nishimoto T. Park I. S. Lee J. Yasuda T. Angew. Chem., Int. Ed. 2016;55:7171–7175. doi: 10.1002/anie.201603232. [DOI] [PubMed] [Google Scholar]; (f) Yan X. Z. Wang M. Cook T. R. Zhang M. M. Saha M. L. Zhou Z. X. Li X. P. Huang F. H. Stang P. J. J. Am. Chem. Soc. 2016;138:4580–4588. doi: 10.1021/jacs.6b00846. [DOI] [PubMed] [Google Scholar]; (g) Sasaki S. Niko Y. Igawa K. Konishi G. RSC Adv. 2014;4:33474–33477. doi: 10.1039/C4RA05871B. [DOI] [Google Scholar]
- (a) Yu X. Chen H. Shi X. Albouy P. A. Guo J. Hu J. Li M. H. Mater. Chem. Front. 2018;2:2245–2253. doi: 10.1039/C8QM00340H. [DOI] [Google Scholar]; (b) Pan L. X. Cai Y. J. Wu H. Z. Zhou F. Qin A. J. Wang Z. M. Tang B. Z. Mater. Chem. Front. 2018;2:1310–1316. doi: 10.1039/C7QM00551B. [DOI] [Google Scholar]; (c) Zhang Y. H. Xu W. Q. Kong L. W. Han B. R. Cai Z. X. Shi J. B. Tong B. Dong Y. P. Tang B. Z. Mater. Chem. Front. 2018;2:1779–1783. doi: 10.1039/C8QM00235E. [DOI] [Google Scholar]; (d) Chen X. J. Yang Z. Xie Z. L. Zhao J. Yang Z. Y. Zhang Y. Aldred M. P. Chi Z. G. Mater. Chem. Front. 2018;2:1017–1023. doi: 10.1039/C8QM00064F. [DOI] [Google Scholar]; (e) Ma L. Feng X. Wang S. Wang B. Mater. Chem. Front. 2017;1:2474–2486. doi: 10.1039/C7QM00254H. [DOI] [Google Scholar]; (f) Liu B. Pucci A. Baumgartner T. Mater. Chem. Front. 2017;1:1689–1690. doi: 10.1039/C7QM90027A. [DOI] [Google Scholar]; (g) Wan Q. Jiang R. M. Mao L. C. Xu D. Z. Zeng G. J. Shi Y. G. Deng F. J. Liu M. Y. Zhang X. Y. Wei Y. Mater. Chem. Front. 2017;1:1051–1058. doi: 10.1039/C6QM00307A. [DOI] [Google Scholar]; (h) Yang J. Li L. Yu Y. Ren Z. C. Peng Q. Ye S. H. Li Q. Q. Li Z. Mater. Chem. Front. 2017;1:91–99. doi: 10.1039/C6QM00014B. [DOI] [Google Scholar]; (i) Li Y. Lin H. C. Luo C. H. Wang Y. Q. Jiang C. L. Qi R. J. Huang R. Travas-sejdic J. Peng H. RSC Adv. 2017;7:32225–32228. doi: 10.1039/C7RA04781A. [DOI] [Google Scholar]; (j) Lin N. Zhang Q. Xia X. Liang M. Y. Zhang S. H. Zheng L. Y. Cao Q. E. Ding Z. T. RSC Adv. 2017;7:21446–21451. doi: 10.1039/C6RA28551A. [DOI] [Google Scholar]; (k) Ma C. P. He J. J. Xu B. J. Xie G. Y. Xie Z. L. Mao Z. Chi Z. G. RSC Adv. 2018;8:6252–6258. doi: 10.1039/C7RA12655G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (l) Hu J. Y. Jiang B. L. Gong Y. Y. Liu Y. L. He G. Yuan W. Z. Wei C. RSC Adv. 2018;8:710–716. doi: 10.1039/C7RA10174K. [DOI] [PMC free article] [PubMed] [Google Scholar]; (m) Yu C. Y. Hsu C. C. Weng H. C. RSC Adv. 2018;8:12619–12627. doi: 10.1039/C8RA01448E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Imoto H. Fujii R. Naka K. Eur. J. Org. Chem. 2018;6:837–843. doi: 10.1002/ejoc.201701479. [DOI] [Google Scholar]; (o) Liu J. Zhang C. H. Dong J. Zhu J. Shen C. Y. Yang G. Q. Zhang X. M. RSC Adv. 2017;7:14511–14515. doi: 10.1039/C7RA00426E. [DOI] [Google Scholar]; (p) Zhang Z. M. Miao B. X. Tang X. X. Ni Z. H. RSC Adv. 2018;8:15173–15180. doi: 10.1039/C8RA00057C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (q) Ceballos S. A. Gil S. Costero A. M. RSC Adv. 2018;8:14279–14282. [Google Scholar]; (r) Jiang Q. Zhang M. G. Wang Z. L. Song J. Yang Y. Q. Li W. C. Gu W. Xu X. Xu H. J. Wang S. F. RSC Adv. 2018;8:30055–30060. doi: 10.1039/C8RA05031G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Mei J. Leung N. L. C. Kwok R. T. K. Lam J. W. Y. Tang B. Z. Chem. Rev. 2015;115:11718–11940. doi: 10.1021/acs.chemrev.5b00263. [DOI] [PubMed] [Google Scholar]; (b) Mei J. Hong Y. N. Lam J. W. Y. Qin A. J. Tang Y. H. Tang B. Z. Adv. Mater. 2014;26:5429–5479. doi: 10.1002/adma.201401356. [DOI] [PubMed] [Google Scholar]; (c) Sasaki S. Drummen G. P. C. Konishi G. J. Mater. Chem. C. 2016;4:2731–2743. doi: 10.1039/C5TC03933A. [DOI] [Google Scholar]; (d) Chen Y. Lam J. W. Y. Kwok R. T. K. Liu B. Tang B. Z. Mater. Horiz. 2019;6:428–433. doi: 10.1039/C8MH01331D. [DOI] [Google Scholar]; (e) Luo J. D. Xie Z. L. Lam J. W. Y. Cheng L. Chen H. Y. Qiu C. F. Kwok H. S. Zhan X. W. Liu Y. Q. Zhu D. B. Tang B. Z. Chem. Commun. 2001:1740–1741. doi: 10.1039/B105159H. [DOI] [PubMed] [Google Scholar]; (f) Crespo-Otero R. Li Q. S. Blancafort L. Chem.–Asian J. 2019;14:700–714. doi: 10.1002/asia.201801649. [DOI] [PubMed] [Google Scholar]; (g) Hong Y. N. Lam J. W. Y. Tang B. Z. Chem. Commun. 2009:4332–4353. doi: 10.1039/B904665H. [DOI] [PubMed] [Google Scholar]; (h) Hong Y. N. Lam J. W. Y. Tang B. Z. Chem. Soc. Rev. 2011;40:5361–5388. doi: 10.1039/C1CS15113D. [DOI] [PubMed] [Google Scholar]; (i) Ren Y. S. Xie S. Grape E. S. Inge A. K. Ramstrom O. J. Am. Chem. Soc. 2018;140:13640–13643. doi: 10.1021/jacs.8b09843. [DOI] [PubMed] [Google Scholar]; (j) Zheng X. J. Zhu W. C. Zhang C. Zhang Y. Zhong C. Li H. Xie G. H. Wang X. J. Yang C. L. J. Am. Chem. Soc. 2019;141:4704–4710. doi: 10.1021/jacs.8b13724. [DOI] [PubMed] [Google Scholar]; (k) Fu W. Yan C. X. Guo Z. Q. Zhang J. J. Zhang H. Y. Tian H. Zhu W. H. J. Am. Chem. Soc. 2019;141:3171–3177. doi: 10.1021/jacs.8b12820. [DOI] [PubMed] [Google Scholar]; (l) Dong J. Q. Li X. Zhang K. Di Yuan Y. Wang X. Y. Zhai L. Z. Liu G. L. Yuan D. Q. Jiang J. W. Zhao D. J. Am. Chem. Soc. 2018;140:4035–4046. doi: 10.1021/jacs.7b13069. [DOI] [PubMed] [Google Scholar]; (m) Wang J. G. Gu X. G. Zhang P. F. Huang X. B. Zheng X. Y. Chen M. Feng H. T. Kwok R. T. K. Lam J. W. Y. Tang B. Z. J. Am. Chem. Soc. 2017;139:16974–16979. doi: 10.1021/jacs.7b10150. [DOI] [PubMed] [Google Scholar]; (n) Xie S. Wong A. Y. H. Kwok R. T. K. Li Y. Su H. F. Lam J. W. Y. Chen S. J. Tang B. Z. Angew. Chem., Int. Ed. 2018;57:5750–5753. doi: 10.1002/anie.201801653. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Meher N. Iyer P. K. Angew. Chem., Int. Ed. 2018;57:8488–8492. doi: 10.1002/anie.201802842. [DOI] [PubMed] [Google Scholar]; (p) Jimbo T. Tsuji M. Taniguchi R. Sada K. Kokado K. Cryst. Growth Des. 2018;18:3863–3869. doi: 10.1021/acs.cgd.8b00141. [DOI] [Google Scholar]; (q) Imoto H. Urushizaki A. Kawashima I. Naka K. Chem.–Eur. J. 2018;24:8797–8803. doi: 10.1002/chem.201801589. [DOI] [PubMed] [Google Scholar]; (r) Gon M. Morisaki Y. Chujo Y. Chem.–Eur. J. 2017;23:6323–6329. doi: 10.1002/chem.201605598. [DOI] [PubMed] [Google Scholar]; (s) Ito F. Oka N. Chem.–Asian J. 2019;14:755–759. doi: 10.1002/asia.201801563. [DOI] [PubMed] [Google Scholar]
- (a) Luo D. X. Gao D. Y. Zou J. H. Xu M. Wang L. Zhao Z. J. Qin A. J. Peng J. Ning H. L. Caoand Y. Tang B. Z. Adv. Funct. Mater. 2016;26:776783. [Google Scholar]; (b) Niko Y. Konishi G. Macromolecules. 2012;45:2327–2337. doi: 10.1021/ma3001252. [DOI] [Google Scholar]
- (a) Ruggeri G. Pucci A. Chem. Soc. Rev. 2013;42:857–870. doi: 10.1039/C2CS35414D. [DOI] [PubMed] [Google Scholar]; (b) Cheng Y. H. Liu S. J. Song F. Y. Khorloo M. Zhang H. K. Kwok R. T. K. Lam J. W. Y. He Z. K. Tang B. Z. Mater. Horiz. 2019;6:405–411. doi: 10.1039/C8MH01092G. [DOI] [Google Scholar]; (c) Chen M. Hu X. L. Liu J. K. Li B. X. Leung N. L. C. Viglianti L. Cheung T. S. Sung H. H. Y. Kwok R. T. K. Williams I. D. Qin A. J. Lam J. W. Y. Tang B. Z. Chem. Sci. 2018;9:7829–7834. doi: 10.1039/C8SC02810A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lee W. W. H. Zhao Z. Cai Y. J. Xu Z. Yu Y. Xiong Y. Kwok R. T. K. Chen Y. Leung N. L. C. Ma D. Lam J. W. Y. Qin A. J. Tang B. Z. Chem. Sci. 2018;9:6118–6125. doi: 10.1039/C8SC01377B. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Feng X. Qi C. X. Feng H. T. Zhao Z. Sung H. H. Y. Williams I. D. Kwok R. T. K. Lam J. W. Y. Qin A. J. Tang B. Z. Chem. Sci. 2018;9:5679–5687. doi: 10.1039/C8SC01709C. [DOI] [PMC free article] [PubMed] [Google Scholar]; (f) Sharber S. A. Shih K. C. Mann A. Frausto F. Haas T. E. Nieh M. P. Thomas S. W. Chem. Sci. 2018;9:5415–5426. doi: 10.1039/C8SC00980E. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Yang Z. Y. Chi Z. H. Mao Z. Zhang Y. Liu S. W. Zhao J. Aldred M. P. Chi Z. G. Mater. Chem. Front. 2018;2:861–890. doi: 10.1039/C8QM00062J. [DOI] [Google Scholar]; (h) Li Q. Li X. Wu Z. Y. Sun Y. H. Fang J. L. Chen D. Z. Polym. Chem. 2018;9:4150–4160. doi: 10.1039/C8PY00710A. [DOI] [Google Scholar]; (i) Zhao Y. J. Wu Y. Chen S. Deng H. P. Zhu X. Y. Macromolecules. 2018;51:5234–5244. doi: 10.1021/acs.macromol.8b00656. [DOI] [Google Scholar]; (j) Wang Z. Y. Feng Y. Q. Wang N. N. Cheng Y. X. Quan Y. W. Ju H. X. J. Phys. Chem. Lett. 2018;9:5296–5302. doi: 10.1021/acs.jpclett.8b02087. [DOI] [PubMed] [Google Scholar]; (k) Xu J. R. Ji W. X. Li C. Lv Y. F. Qiu Z. J. Gao L. C. Chen E. Q. Lam J. W. Y. Tang B. Z. Jiang L. Adv. Opt. Mater. 2018;6:1701149. doi: 10.1002/adom.201701149. [DOI] [Google Scholar]; (l) Huo M. Ye Q. Q. Che H. L. Wang X. S. Wei Y. Yuan J. Y. Macromolecules. 2017;50:1126–1133. doi: 10.1021/acs.macromol.6b02499. [DOI] [Google Scholar]; (m) Li Q. Q. Li Z. Adv. Sci. 2017;4:1600484. doi: 10.1002/advs.201600484. [DOI] [PMC free article] [PubMed] [Google Scholar]; (n) Ishiwari F. Hasebe H. Matsumura S. Hajjaj F. Horii-Hayashi N. Nishi M. Someya T. Fukushima T. Sci. Rep. 2016;6:24275. doi: 10.1038/srep24275. [DOI] [PMC free article] [PubMed] [Google Scholar]; (o) Gao H. Z. Xu D. F. Liu X. L. Han A. X. Zhou L. Zhang C. Yang Y. Li W. L. RSC Adv. 2017;7:1348–1356. doi: 10.1039/C6RA25871A. [DOI] [Google Scholar]; (p) Ito S. Taguchi T. Yamada T. Ubukata T. Yamaguchi Y. Asami M. RSC Adv. 2017;7:16953–16962. doi: 10.1039/C7RA01006K. [DOI] [Google Scholar]; (q) Lin L. B. Guo H. Y. Fanga X. T. Yang F. F. RSC Adv. 2017;7:20172–20177. doi: 10.1039/C7RA02338C. [DOI] [Google Scholar]; (r) Wang S. H Ye J. Han Z. Fan Z. Wang C. J. Mu C. C. Zhang W. C. He W. J. RSC Adv. 2017;7:36021–36025. doi: 10.1039/C7RA05925F. [DOI] [Google Scholar]; (s) Sagara Y. Tamaoki N. RSC Adv. 2017;7:47056–47062. doi: 10.1039/C7RA10776E. [DOI] [Google Scholar]; (t) Li W. L. Yao W. Wang J. Qiu Z. Y. Tang J. J. Yang S. Y. Zhu M. F. Xu Z. X. Hu R. Qin A. J. Tang B. Z. RSC Adv. 2017;7:41127–41135. doi: 10.1039/C7RA06527B. [DOI] [Google Scholar]
- (a) Feng G. Liu B. Acc. Chem. Res. 2018;51:1404–1414. doi: 10.1021/acs.accounts.8b00060. [DOI] [PubMed] [Google Scholar]; (b) Situ B. Gao M. He X. J. Li S. W. He B. R. Guo F. X. Kang C. M. Liu S. Yang L. Jiang M. J. Hu Y. W. Tang B. Z. Zheng L. Mater. Horiz. 2019;6:546–553. doi: 10.1039/C8MH01293H. [DOI] [Google Scholar]; (c) Qi J. Sun C. W. Zebibula A. Zhang H. Q. Kwok R. T. K. Zhao X. Y. Xi W. Lam J. W. Y. Qian J. Tang B. Z. Adv. Mater. 2018;30:1706856. doi: 10.1002/adma.201706856. [DOI] [PubMed] [Google Scholar]; (d) Hong Y. X. Wang H. Xue M. J. Zhang P. S. Liu W. Q. Chen S. Zeng R. J. Cui J. X. Gao Y. Chen J. Mater. Chem. Front. 2019;3:203–208. doi: 10.1039/C8QM00511G. [DOI] [Google Scholar]; (e) Yu H. X. Zhi J. Chang Z. F. Shen T. J. Ding W. L. Zhang X. L. Wang J. L. Mater. Chem. Front. 2019;3:151–160. doi: 10.1039/C8QM00424B. [DOI] [Google Scholar]; (f) Ni F. Zhu Z. C. Tong X. Xie M. J. Zhao Q. Zhong C. Zou Y. Yang C. L. Chem. Sci. 2018;9:6150–6155. doi: 10.1039/C8SC01485J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (g) Hu F. Cai X. L. Manghnani P. N. Wu W. B. Liu B. Chem. Sci. 2018;9:2756–2761. doi: 10.1039/C7SC04585A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (h) Jiang M. J. Gu X. G. Lam J. W. Y. Zhang Y. L. Kwok R. T. K. Wong K. S. Tang B. Z. Chem. Sci. 2017;8:5440–5446. doi: 10.1039/C7SC01400G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (i) Yuan Y. Y. Liu B. Chem. Sci. 2017;8:2537–2546. doi: 10.1039/C6SC05421H. [DOI] [PMC free article] [PubMed] [Google Scholar]; (j) Reisch A. Klymchenko A. S. Small. 2016;12:1968–1992. doi: 10.1002/smll.201503396. [DOI] [PMC free article] [PubMed] [Google Scholar]; (k) Niko Y. Arntz Y. Mely Y. Konishi G. Klymchenko A. S. Chem.–Eur. J. 2014;20:16473–16477. doi: 10.1002/chem.201405040. [DOI] [PubMed] [Google Scholar]; (l) Palma-Cando A. Woitassek D. Brunklaus G. Scherf U. Mater. Chem. Front. 2017;1:1118–1124. doi: 10.1039/C6QM00281A. [DOI] [Google Scholar]; (m) Zhang P. S. Nie X. Z. Gao M. Zeng F. Qin A. J. Wu S. Z. Tang B. Z. Mater. Chem. Front. 2017;1:838–845. doi: 10.1039/C6QM00223D. [DOI] [Google Scholar]; (n) Alam P. Dash S. Climent C. Kaur G. Choudhury A. R. Casanova D. Alemany P. Chowdhury R. Laskar I. R. RSC Adv. 2017;7:5642–5648. doi: 10.1039/C6RA24792J. [DOI] [Google Scholar]; (o) Dong W. Y. Fei T. Scherf U. RSC Adv. 2018;8:5760–5767. doi: 10.1039/C7RA13536J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (p) Li K. Zhang Y. Qiao B. Tao F. R. Li T. D. Ding Y. Q. Raymo F. M. Cui Y. Z. RSC Adv. 2017;7:30229–30241. doi: 10.1039/C7RA03956E. [DOI] [Google Scholar]; (q) Lu Z. X. Liu Y. M. Lu S. H. Li Y. Liu X. L. Qin Y. Zheng L. Y. RSC Adv. 2018;8:19701–19706. doi: 10.1039/C8RA03591A. [DOI] [PMC free article] [PubMed] [Google Scholar]; (r) Xu J. H. Yan R. Wang H. B. Du Z. L. Gu J. Cheng X. Xiong J. J. RSC Adv. 2018;8:6798–6804. doi: 10.1039/C7RA13238G. [DOI] [PMC free article] [PubMed] [Google Scholar]; (s) Du M. Huo B. L. Li M. W. Shen A. Bai X. Lai Y. R. Liu J. M. Yang Y. X. RSC Adv. 2018;8:32497–32505. doi: 10.1039/C8RA06774K. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki S. Igawa K. Konishi G. J. Mater. Chem. C. 2016;3:5940–5950. doi: 10.1039/C5TC00946D. [DOI] [Google Scholar]
- Sasaki S. Suzuki S. Sameera W. M. C. Igawa K. Morokuma K. Konishi G. J. Am. Chem. Soc. 2016;138:8194–8206. doi: 10.1021/jacs.6b03749. [DOI] [PubMed] [Google Scholar]
- (a) Sasaki S. Suzuki S. Igawa K. Morokuma K. Konishi G. J. Org. Chem. 2017;82:6865–6873. doi: 10.1021/acs.joc.7b00996. [DOI] [PubMed] [Google Scholar]; (b) Sasaki S. Konishi G. RSC Adv. 2017;7:17403–17416. doi: 10.1039/C7RA01212H. [DOI] [Google Scholar]; (c) Sasaki S. Sugita Y. Tokita M. Suenobu T. Ishitani O. Konishi G. Macromolecules. 2017;50:3544–3556. doi: 10.1021/acs.macromol.7b00213. [DOI] [Google Scholar]
- Sairi A. S. Konishi G. Asian J. Org. Chem. 2019;8:404–410. doi: 10.1002/ajoc.201900056. [DOI] [Google Scholar]
- (a) Sun J. J. Fransen S. Yu X. Q. Kuckling D. Polym. Chem. 2018;9:3287–3296. doi: 10.1039/C8PY00606G. [DOI] [Google Scholar]; (b) Venkataraman S. Mineart K. P. Prabhu V. M. Hedrick J. L. Yang Y. Y. Polym. Chem. 2018;9:2434–2437. doi: 10.1039/C8PY00406D. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Chesterman J. P. Chen F. Brissenden A. J. Amsden B. G. Polym. Chem. 2017;8:7515–7528. doi: 10.1039/C7PY01195D. [DOI] [Google Scholar]; (d) Stosser T. Li C. L. Unruangsri J. Saini P. K. Sablong R. J. Meier M. A. R. Williams C. K. Koning C. Polym. Chem. 2017;8:6099–6105. doi: 10.1039/C7PY01223C. [DOI] [Google Scholar]; (e) Beniah G. Chen X. Uno B. E. Liu K. Leitsch E. K. Jeon J. Heath W. H. Scheidt K. A. Torkelson J. M. Macromolecules. 2017;50:3193–3203. doi: 10.1021/acs.macromol.6b02513. [DOI] [Google Scholar]; (f) Nakano K. Kamada T. Nozaki K. Angew. Chem., Int. Ed. 2006;45:7274–7277. doi: 10.1002/anie.200603132. [DOI] [PubMed] [Google Scholar]; (g) Seto R. Sato T. Hosokawa K. Kojima T. Koayama Y. Konishi G. Takata T. J. Polym. Sci., Part A: Polym. Chem. 2010;48:3658–3667. doi: 10.1002/pola.24150. [DOI] [Google Scholar]; (h) Luinstra G. A. Polym. Rev. 2008;48:192–219. doi: 10.1080/15583720701834240. [DOI] [Google Scholar]
- (a) Ohshima T. Iwasaki T. Maegawa Y. Yoshiyama A. Mashima K. J. Am. Chem. Soc. 2008;130:2944–2945. doi: 10.1021/ja711349r. [DOI] [PubMed] [Google Scholar]; (b) Iwasaki T. Maegawa Y. Hayashi Y. Ohshima T. Mashima K. J. Org. Chem. 2008;73:5147–5150. doi: 10.1021/jo800625v. [DOI] [PubMed] [Google Scholar]; (c) Iwasaki T. Agura K. Maegawa Y. Hayashi Y. Ohshima T. Mashima K. Chem.–Eur. J. 2010;16:11567–11571. doi: 10.1002/chem.201000960. [DOI] [PubMed] [Google Scholar]; (d) Hayashi Y. Santoro S. Azuma Y. Himo F. Ohshima T. Mashima K. J. Am. Chem. Soc. 2013;135:6192–6199. doi: 10.1021/ja400367h. [DOI] [PubMed] [Google Scholar]
- (a) Suzuki Y. Fukui N. Murakami K. Yorimitsu H. Osuka A. Asian J. Org. Chem. 2013;2:1066–1071. doi: 10.1002/ajoc.201300162. [DOI] [Google Scholar]; (b) Sasaki S. Hattori K. Igawa K. Konishi G. J. Phys. Chem. A. 2015;119:4898–4906. doi: 10.1021/acs.jpca.5b03238. [DOI] [PubMed] [Google Scholar]
- (a) Surry D. S. Buchwald S. L. Chem. Sci. 2011;2:27–50. doi: 10.1039/C0SC00331J. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Wolfe J. P. Wagaw S. Buchwald S. L. J. Am. Chem. Soc. 1996;118:7215–7216. doi: 10.1021/ja9608306. [DOI] [Google Scholar]
- Huang W. Buchwald S. L. Chem.–Eur. J. 2016;22:14186–14189. doi: 10.1002/chem.201603449. [DOI] [PubMed] [Google Scholar]
- (a) Ego C. Marsitzky D. Becker S. Zhang J. Y. Grimsdale A. C. Mullen K. MacKenzie J. D. Silva C. Friend R. H. J. Am. Chem. Soc. 2003;125:437–443. doi: 10.1021/ja0205784. [DOI] [PubMed] [Google Scholar]; (b) Chen X. W. Liao J. L. Liang Y. M. Ahmed M. O. Tseng H. E. Chen S. A. J. Am. Chem. Soc. 2003;125:636–637. doi: 10.1021/ja0211151. [DOI] [PubMed] [Google Scholar]; (c) Uchimura M. Ishige R. Shigeta M. Arakawa Y. Niko Y. Watanabe J. Konishi G. Res. Chem. Intermed. 2013;39:403–414. doi: 10.1007/s11164-012-0658-y. [DOI] [Google Scholar]; (d) Watanabe Y. Uchimura M. Araoka F. Watanabe J. Konishi G. Takezoe H. Appl. Phys. Express. 2009;2:102501. doi: 10.1143/APEX.2.102501. [DOI] [Google Scholar]; (e) Niko Y. Kawauchi S. Otsu S. Tokumaru K. Konishi G. J. Org. Chem. 2013;78:3196–3207. doi: 10.1021/jo400128c. [DOI] [PubMed] [Google Scholar]; (f) Sumi K. Niko Y. Tokumaru K. Konishi G. Chem. Commun. 2013;49:3893–3895. doi: 10.1039/C3CC41312H. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.