Abstract
Numerous vaccines have been developed to address the COVID-19 pandemic, the most frequently administered are the Moderna and Pfizer-BioNTech (Pfizer) mRNA COVID-19 vaccines. Of 177 million individuals that have full vaccination status, there have been 1,148 cases of myocarditis or pericarditis reported. At this time, the relationship between myopericarditis and the Pfizer mRNA COVID-19 vaccine has not been well established in current literature due to the novelty of the vaccine. We discuss a 16-year-old male who presented to the emergency department with chest pain 48 hours after receiving his second dose of the Pfizer COVID-19 vaccine. His laboratory and electrocardiogram findings were consistent with acute myopericarditis and work-up did not reveal an obvious etiology. After starting anti-inflammatory therapies, the patient’s symptoms and laboratory markers improved and the patient was discharged from the hospital expected to make a full recovery. This case demonstrates the rapid recovery with no sequelae following this adverse effect, highlighting that the benefits of the COVID-19 vaccination most likely outweigh the risks.
Keywords: COVID-19 vaccine, myopericarditis, pediatric, adverse effect
Introduction
Though over 605,000 people in the United States have died from the SARS-CoV-2 virus, there remains significant hesitation amongst many Americans due to concerns of safety and testing. As of late September 2021, the Center for Disease Control (CDC) reported only 177 million (53.2%) of Americans are fully vaccinated against COVID-19. 10.4 million of these individuals that are fully vaccinated are in the 12 to 17 year age group [1]. With the emergence of variants of COVID-19, it is vital that individuals are informed of the major benefits and risks of receiving these vaccines and preventing the further spread of disease.
This case report will focus on the Pfizer COVID-19 mRNA vaccine which utilizes the host’s translation of this injected mRNA into a virus-specific surface spike protein that elicits a humoral immune response. This provides a primer for a more robust immunologic response if natural infection with the virus occurs or is bolstered by the recommended second dose administered at least 21 days after the initial vaccination [2]. The COVID-19 vaccine has shown to be an effective preventative measure with few serious adverse effects. Though rare, cases of myocarditis and pericarditis have been reported following COVID-19 vaccination [3]. At this time, the relationship between myopericarditis with the Pfizer mRNA COVID-19 vaccine has not been well established in current literature due to the novelty of the vaccine. Currently, research is being done to determine if the relationship between the mRNA vaccines and the previously mentioned heart sequelae is one of association or causation.
Case report
In June 2021, a 16-year-old male with a history of generalized anxiety disorder and recent right ankle synovitis felt to be related to ankle ligament reconstruction presented to the emergency department for a one-day history of midsternal chest pain exacerbated by deep breathing. The day prior to presentation, he vomited twice and was febrile to 40.5 C, attributing these initial symptoms to his second dose of the Pfizer COVID-19 vaccine approximately 48 hours prior to admission. The patient denied any changes in his regimen of buspirone or escitalopram oxalate, new medications, sick contacts, insect bites, recent travel, or recent illness. He denied fever on day of admission, headache, changes in vision, diarrhea, palpitations, edema, cough, rash, and dysuria. The patient denied any prior history of cardiac issues. The patient’s family history is not indicative of cardiac risk factors or disease at a young age.
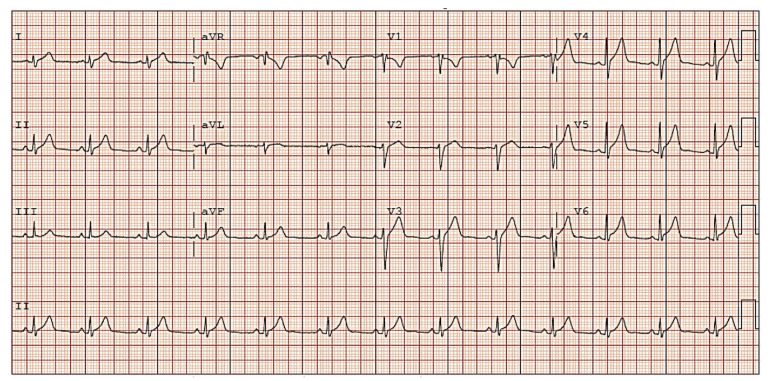
At the time of admission, the patient appeared nontoxic, mildly uncomfortable, and anxious without significant tachycardia, hypotension, or hypoxemia. His physical examination, including a cardiovascular exam, was overall unremarkable other than midsternal tenderness to palpation. Original differential diagnoses included anxiety-induced chest pain, costochondritis, myocarditis, pericarditis, pulmonary embolism, chest wall trauma, and others. Initial labs were notable for elevations of C-reactive protein, troponin, brain natriuretic peptide, D-dimer, and leukocyte count (Table I). His SARS-CoV-2 IgG was negative. Rapid viral PCR testing for several viruses, including COVID-19, was negative. The initial electrocardiogram showed diffuse ST elevations consistent with acute pericarditis (Figure 1).
Table I.
Inpatient laboratory test values
| Laboratory test | Reference range | Day 1 01:35 |
Day 1 06:24 |
Day 1 13:07 |
Day 1 18:55 |
Day 2 01:20 |
Day 2 06:22 |
Day 2 12:00 |
Day 2 17:41 |
Day 2 23:52 |
Day 3 06:05 |
Day 3 11:54 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Troponin [ng/ml] | < 0.03 | 18.69 | 24.55 | 18.02 | 15.63 | 19.15 | 18.46 | 14.27 | 14.47 | 16.04 | 13.82 | 8.02 |
| C-reactive protein [mg/l] | 4.6 – 24.2 | 79.7 | - | - | - | - | - | - | - | - | - | - |
| D-dimer [ug/ml] | < 0.50 | 0.60 | - | - | - | - | - | - | - | - | - | - |
| BNP [pg/ml] | < 20 | 21 | - | - | - | - | 119 | - | - | - | - | - |
| WBC [k/ul] | 3.8 – 9.8 | 11.4 | - | - | - | - | - | - | - | - | - | - |
BNP - Brain natriuretic peptide, WBC - leukocyte count
Fig. 1.
Initial electrocardiogram showing diffuse ST segment elevations
His chest x-ray was within normal limits, and the CT angiogram of his chest was nondiagnostic for a pulmonary embolism. An echocardiogram revealed no effusion and normal function. It was determined following initial work-up this presentation is most consistent with acute myopericarditis.
In consultation with infectious disease and cardiology, NSAIDs and colchicine were initiated along with prophylactic pantoprazole. Trending of troponin values every 6 hours was recommended with a peak on hospital day 1 of 24 ng/mL (Table I). His chest pain began to improve on Day 2 of hospitalization though with some new complaints of nausea and epigastric abdominal pain that was attributed to his new medications and resolved with timing medications with meals. His brain natriuretic peptide increased to 119 pg/mL on hospital day 2, prompting a repeat echocardiogram which was stable and normal (Table I).
With a stable, downward trend of his troponin, resolved symptoms, and lack of hemodynamic instability on echocardiogram or vital signs, the patient was discharged home on colchicine 0.6 mg orally twice daily, ibuprofen 600 mg orally every 8 hours, and pantoprazole 40 mg daily with discouragement of competitive sports until outpatient follow-up with pediatric cardiology occurred. A Vaccine Adverse Events Reporting System (VAERS) report was made prior to discharge. The patient was asymptomatic at both follow-up visits with pediatric cardiology; his post-discharge day 2 and day 10 troponins were down to 0.27 ng/mL and negative at 0.02 ng/mL respectively. Due to his negative troponin and lack of remaining symptoms or adverse effects, he was determined to have made a full recovery.
Discussion
Since the initiation of mRNA COVID-19 vaccine administration, 1,148 cases of myocarditis or pericarditis following vaccination have been reported to the VAERS. Of these, 674 were confirmed by the CDC and Food and Drug Administration (FDA) via medical chart reviews and follow up [3]. The highest reporting rates of myocarditis following the COVID-19 vaccine were in males aged 12-17 years [4]. A recent analysis conducted in the PubMed/Medline database found a total of 15 patients in case studies who had myocarditis following COVID-19 vaccination. In each reported case, clinical symptoms resolved within 6 days with preservation of the cardiac function and no complications [5].
Though the precise underlying mechanism of myocarditis following mRNA COVID-19 vaccine administration is unknown, theories attempting to explain this adverse effect have been described in the literature [6, 7]. In development of mRNA vaccines, modifications were made to the mRNA component to make it less immunogenic within the patient. It is possible that in certain individuals who likely have a genetic predisposition, these modifications drive activation of an abnormal immune response to the mRNA rather than decreasing it. This abnormal response could initiate inflammatory cascades and pathways resulting in a systemic reaction causing inflammation in many tissues [6]. Another theory involves molecular mimicry between the spike protein of SARS-CoV-2 and self-antigens within the host. Antibodies against the spike protein are made by the host in the expected response to the vaccine, but these antibodies have been experimentally shown to react against similar peptide sequences found within the host, including alpha-myosin which is found in cardiac muscle [7]. It is possible this reaction triggers preexisting dysregulated pathways in certain individuals resulting in inflammation of myocardial and other tissues [6].
This patient likely experienced inflammation of his myocardium and pericardium secondary to his Pfizer COVID-19 vaccine that he received 48 hours prior as testing for other potential etiologies were negative. According to VAERS, of the 12.8 million individuals in the US age 12-17 years who received at least one dose of the COVID-19 vaccine, 420 cases of myocarditis and 110 cases of pericarditis have been reported [2, 8]. Of these cases, 369 myocarditis cases and 96 pericarditis cases occurred in males, accounting for 87.74% of all cases. The pediatric age group of 6-17 years accounts for 28.95% and 11.49% of the total reported cases of myocarditis and pericarditis respectively [8]. This data demonstrates that the pediatric population is at notable risk for developing myocarditis or pericarditis following the COVID-19 vaccine with a preponderance of affected males.
While most complaints of pediatric chest pain are non-cardiac in origin, this case demonstrates myocarditis and pericarditis following COVID-19 vaccination should be considered with this chief complaint. The case highlights the value of a detailed history and exam to drive decisions regarding ancillary studies to discern potential etiologies and determine optimal treatments. This patient responded well to the prescribed interventions with continued stability on outpatient follow-up echocardiography and improvement in laboratory values. It should be noted in discussion that this patient was overall healthy with no significant risk factors for poor outcomes prior to development of myopericarditis, potentially influencing his rapid recovery. As further data is gathered on any potential short- or long-term complications from myopericarditis diagnosed after mRNA COVID-19 vaccination, this will help further steer discussion regarding the risk of this rare complication relative to the benefits of immunity from this prevalent disease with significant morbidity and mortality.
Conclusion
This case describes the clinical presentation, course, and findings in a case of myopericarditis within 48 hours of COVID-19 vaccination. Myopericarditis should be considered as a potential adverse effect when weighing the risks and benefits of administering the COVID-19 vaccine, especially in pediatric males. Although initially concerning, this patient’s case, and similar reported cases, proved to be non-life-threatening and resulted in a full recovery. Therefore, the COVID-19 vaccine should not be avoided in the pediatric male population simply due to the risk of myopericarditis. With the risk of long-term illness, multisystem inflammatory syndrome in children, and possible death from developing COVID-19, this case has provided further support for COVID-19 vaccination producing far more benefits than risks to the population.
Acknowledgements
The authors would like to acknowledge the patient and the patient’s family for being willing to share this case to improve understanding of this case in medicine. Informed consent was obtained from the patient and patient’s legal guardians for publication of this case report.
Conflicts of interest
The authors declare that they have no competing interests.
Disclaimer
The views expressed in this case report are the authors’ own.
Disclosure statement
The authors have no relevant financial or nonfinancial relationships to disclose.
References
- 1.Centers for Disease Control and Prevention. COVID Data Tracker Weekly Review. [ https://www.cdc.gov/coronavirus/2019-ncov/covid-data/covidview/index.html available at 01.11.2022]
- 2.Centers for Disease Control and Prevention. Understanding How COVID-19 Vaccines Work. [ https://www.cdc.gov/coronavirus/2019-ncov/vaccines/different-vaccines/how-they-work.html available at 01.11.2022]
- 3.Centers for Disease Control and Prevention. Selected Adverse Events Reported after COVID-19 Vaccination. [ https://www.cdc.gov/coronavirus/2019-ncov/vaccines/safety/adverse-events.html available at 01.11.2022]
- 4.Gargano JW, Wallace M, Hadler SC, et al. Use of mRNA COVID-19 vaccine after reports of myocarditis among vaccine recipients: Update from the Advisory Committee on Immunization Practices—United States. MMWR Morb Mortal Wkly Rep. 2021;70(27):977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salah HM, Mehta JL. COVID-19 vaccine and myocarditis. Am J Cardiol. 2021;157:146–148. doi: 10.1016/j.amjcard.2021.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozkurt B, Kamat I, Hotez PJ. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144(6):471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vojdani A, Kharrazian D. Potential antigenic cross-reactivity between SARS-CoV-2 and human tissue with a possible link to an increase in autoimmune diseases. Clin Immunol. 2020;217:108480. doi: 10.1016/j.clim.2020.108480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.U.S. Department of Health and Human Services. Vaccine Adverse Event Reporting System. [ https://vaers.hhs.gov/ available at 01.11.2022]