Abstract
Objective
Larotrectinib is a highly selective tropomyosin receptor kinase (TRK) inhibitor with demonstrated efficacy across various TRK fusion-positive solid tumours. We assessed the efficacy and safety of larotrectinib in patients with TRK fusion-positive thyroid carcinoma (TC).
Methods
We pooled data from three phase I/II larotrectinib clinical trials (NCT02576431, NCT02122913, and NCT02637687). The primary endpoint was the investigator-assessed objective response rate (ORR) per Response Evaluation Criteria in Solid Tumors v1.1. Secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety. Data cut-off: July 2020.
Results
Twenty-nine patients (median age: 60; range: 6–80) with TRK fusion-positive TC were treated. Tumour histology was papillary (PTC) in 20 (69%) patients, follicular (FTC) in 2 (7%), and anaplastic (ATC) in 7 (24%) patients. Among 28 evaluable patients, ORR was 71% (95% CI: 51–87); best responses were complete response in 2 (7%) patients, partial response in 18 (64%), stable disease in 4 (14%), progressive disease in 3 (11%), and undetermined in 1 (4%) due to clinical progression prior to the first post-baseline assessment. ORR was 86% (95% CI: 64–97) for PTC/FTC and 29% (95% CI 4–71) for ATC. Median time to response was 1.87 months (range 1.64–3.68). The 24-month DoR, PFS, and OS rates were 81, 69, and 76%, respectively. Treatment-related adverse events were mainly grades 1–2.
Conclusion
In TRK fusion-positive TC, larotrectinib demonstrates rapid and durable disease control and a favourable safety profile in patients with advanced disease requiring systemic therapy.
Significance statement
NTRK gene fusions are known oncogenic drivers and have been identified in various histologies of thyroid carcinoma, most commonly in papillary thyroid carcinoma. This is the first publication specifically studying a TRK inhibitor in a cohort of TRK fusion-positive thyroid carcinoma patients. In the current study, the highly selective TRK inhibitor larotrectinib showed durable antitumour efficacy and a favourable safety profile in patients with TRK fusion-positive thyroid carcinoma. Our findings show that patients with advanced non-medullary thyroid carcinoma who may require systemic therapy could be considered for testing for gene fusions by next-generation sequencing.
Introduction
Tropomyosin receptor kinase (TRK) proteins are a family of receptors that are vital for normal nervous system functioning (1). The three structurally related TRK receptors, TRKA, TRKB, and TRKC, are encoded by three distinct genes: NTRK1, NTRK2, and NTRK3, respectively. Recurrent NTRK gene fusion events have been reported in a diverse range of adult and paediatric cancers (2, 3, 4, 5, 6). These events have been detected at frequencies ranging from less than 1 to 25%, depending on the cancer type, and at more than 90% in some rare tumours (7). Indeed, papillary thyroid carcinoma (PTC) was one of the first tumour types in which NTRK1 fusions were identified (7, 8). NTRK gene fusions tend to be the primary oncogenic drivers in tumours that harbour them and co-occurrence with other known oncogenic alterations, including BRAF mutations, is uncommon (9).
TRK fusion-positive thyroid carcinoma (TC) is more commonly associated with a younger age of diagnosis but can be identified across the age spectrum (10, 11, 12). Depending on the population, approximately 5–25% of PTC cases in paediatric patients are reported to harbour NTRK gene fusions (10, 12, 13, 14, 15) while about 6% of adult PTCs have NTRK gene fusions (12). TRK fusion-positive TC appears to have unique morphologic characteristics, such as a follicular growth pattern, and it may be more associated with locoregional and distantly metastatic disease (10, 11, 12, 16, 17). In a recent genomic analysis of 126 patients with anaplastic thyroid carcinoma (ATC), three patients (2.4%) with ATC were found to harbour an NTRK gene fusion (18). In other recent studies, no NTRK gene fusions were identified in ATC or follicular thyroid carcinoma (FTC), although the sample size was small (11, 12).
Larotrectinib is a first-in-class, CNS-active, highly selective TRK inhibitor that is approved in more than 40 countries for adult and paediatric patients with TRK fusion-positive cancer (19, 20). Larotrectinib has demonstrated durable antitumour efficacy in a pooled analysis of 55 adults and/or children with various cancers from three phase I/II trials (21). This efficacy was sustained after further follow-up, and in an expanded patient population (n = 159), the objective response rate (ORR) was 79% (95% CI: 72–85) and the median duration of response was 35.2 months (95% CI: 22.8–not estimable) (22). Larotrectinib was well tolerated, with most adverse events (AEs) being grade 1 or 2 (22). The proportion of patients who experienced dose reductions or discontinued treatment due to treatment-related AEs was 8 and 2%, respectively (22). In the current study, we evaluate the efficacy and safety of larotrectinib in the subset of patients with TRK fusion-positive TC.
Subjects and methods
Study design
For this analysis, we included patients with measurable, locally advanced, or metastatic TC harbouring an NTRK gene fusion who were treated with larotrectinib in one of three clinical trials: a phase II ‘basket’ trial (NAVIGATE) in adults and adolescents (aged ≥12 years) with advanced solid TRK fusion-positive tumours (NCT02576431), a phase I trial in adults aged ≥18 years with advanced solid tumours (NCT02122913), and a phase I/II trial (SCOUT) in paediatric patients (aged <21 years) with advanced solid or primary CNS tumours (NCT02637687). The full methodology for these studies has been previously described (21, 22, 23); study eligibility criteria are summarised in Appendix 1 of the Data Supplement (see section on supplementary materials given at the end of this article).
In brief, larotrectinib was administered at 100 mg twice daily in adults (or 100 mg/m2 twice daily in paediatric patients) and continued until disease progression, withdrawal of the patient from the study, or unacceptable toxicity. Patients could continue treatment beyond progression if they were still benefitting in the judgement of the investigator. Tumour histology was locally assessed and NTRK gene fusions were detected by local molecular testing in Clinical Laboratory Improvement Amendments-certified or similarly accredited laboratories. All study protocols were approved by the site-specific institutional review board or independent ethics committees and were compliant with the International Ethical Guidelines for Biomedical Research Involving Human Subjects, Good Clinical Practice guidelines, the Declaration of Helsinki, and local laws. Written informed consent was obtained prior to study entry.
Study endpoints
The primary endpoint was ORR assessed by the investigator according to Response Evaluation Criteria in Solid Tumors (RECIST) version 1.1 (24). Secondary endpoints included duration of response (DoR), progression-free survival (PFS), overall survival (OS), and safety outcomes.
Study assessments
Tumours were assessed using a combination of CT, MRI, and clinical measurement at baseline, every 8 weeks for 12 months, then every 12 weeks thereafter until disease progression. For paediatric patients in the SCOUT trial, in the absence of disease progression, tumour assessment was decreased to every 6 months after 2 years of treatment.
Statistical analysis
DoR, PFS, and OS were estimated using Kaplan–Meier analysis. Confidence intervals (95% CIs) were calculated using the Clopper–Pearson method.
Results
Patient population
A total of 29 patients with TRK fusion-positive TC were identified at the data cut-off of 20 July 2020. Tumour histology, based on local assessment, was PTC in 20 (69%) patients and FTC in 2 (7%), collectively referred to as differentiated TC (DTC), and seven patients (24%) were classified as ATC (undifferentiated carcinoma). Among the ATC patients, two patients were transformed cases from PTC. Two patients classified as ATC by the local investigators had poorly differentiated TC (PDTC), as did one patient classified as PTC (Supplementary Table 1). Patient demographics and clinical characteristics are summarised in Table 1.
Table 1.
Demographics and clinical characteristics of 29 patients with advanced TRK fusion-positive thyroid carcinoma treated with larotrectinib.
| Characteristics | All | PTC/FTC | ATC |
|---|---|---|---|
| n | 29 | 22 | 7 |
| Age at study enrolment, median (range), years | 60.0 (6.0–80.0) | 58.0 (6.0–80.0) | 64.0 (49.0–77.0) |
| Paediatric (<18 years) | 2 (7) | 2 (9) | 0 |
| Adult (≥18 years) | 27 (93) | 20 (91) | 7 (100) |
| Sex, n (%) | |||
| Female | 20 (69) | 15 (68) | 5 (71) |
| Male | 9 (31) | 7 (32) | 2 (29) |
| ECOG performance status, n (%) | |||
| 0 | 12 (41) | 11 (50) | 1 (14) |
| 1 | 12 (41) | 8 (36) | 4 (57) |
| 2 | 4 (14) | 3 (14) | 1 (14) |
| 3 | 1 (3) | 0 | 1 (14) |
| Cancer subtype, n (%) | |||
| Papillary* | 20 (69) | 20 (91) | 0 |
| Follicular | 2 (7) | 2 (9) | 0 |
| Anaplastic* | 7 (24) | 0 | 7 (100) |
| Brain metastases at baseline, n (%) | |||
| Yes | 4 (14) | 4 (18) | 0 |
| No | 25 (86) | - | - |
| Prior therapies†, n (%) | |||
| Surgery | 29 (100) | 22 (100) | 7 (100) |
| Radiotherapy | 17 (59) | 12 (55) | 5 (71) |
| RAI | 23 (79) | 21 (95) | 2 (29) |
| Systemic therapy†‡ | 16 (55) | 13 (59) | 3 (43) |
| Tyrosine kinase inhibitors | 11 (38) | 11 (50) | 0 |
| Immunotherapy | 2 (7) | 2 (9) | 0 |
| Chemotherapy | 3 (10) | 0 | 3 (43) |
| Number of prior systemic therapies, n (%) | |||
| 0 | 13 (45) | 9 (41) | 4 (57) |
| 1 | 7 (24) | 4 (18) | 3 (43) |
| 2 | 7 (24) | 7 (32) | 0 |
| ≥3 | 2 (7) | 2 (9) | 0 |
| NTRK gene, n (%) | |||
| NTRK1 | 13 (45) | 10 (45) | 3 (43) |
| NTRK3 | 16 (55) | 12 (55) | 4 (57) |
*One patient classified as PTC and two patients classified as ATC had PDTC. †Patients may be counted in more than 1 category. ‡Including cabozantinib, cisplatin, doxorubicin, ipilimumab, lenvatinib, paclitaxel, pazopanib, pembrolizumab, sorafenib, sunitinib and trametinib.
ATC, anaplastic thyroid carcinoma; ECOG, Eastern Cooperative Oncology Group; FTC, follicular thyroid carcinoma; NTRK, neurotrophic tyrosine receptor kinase; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; RAI, radioactive iodine; TRK, tropomyosin receptor kinase.
The median age at study enrolment was 60 years (range: 6–80 years) and 2 (7%) patients were children (6 and 13 years old). A total of four (14%; three PTC and one FTC) patients had CNS metastases at baseline, three (75%) of whom had received prior radiation to the brain. Overall, NTRK1and NTRK3 gene fusions were identified in 45 and 55% of patients, respectively; there were no NTRK2 gene fusions. NTRKgene fusions were identified by RNA-based next-generation sequencing (NGS) in 17 (59%) patients (NTRK1: n = 8; NTRK3: n = 9), DNA NGS in 5 (17%) patients (NTRK1: n = 2; NTRK3: n = 3), and DNA and RNA NGS in 7 (24%) patients (NTRK1: n = 3; NTRK3: n = 4). The fusion partners for the 13 patients with NTRK1 gene fusions were TPM3 (n = 4), TPR (n = 4), IRF2BP2 (n = 2), NFASC (n = 1), PPL (n = 1), and DIAPH1 (n = 1), and for the 16 patients with NTRK3 gene fusions, they were ETV6 (n = 14) and EML4 (n = 2) (Supplementary Table 1).
As per study eligibility criteria, all enrolled patients had either received prior standard therapy, had a tumour for which there is no standard therapy, or in the opinion of the investigator were considered unlikely to derive clinically meaningful benefit from standard therapy. All patients who had received prior standard therapy had either progressed or were unresponsive to treatment. Prior therapies included surgery in all 29 (100%) patients, radiotherapy in 17 (59%) patients, radioactive iodine (RAI) in 23 (79%) patients, and systemic therapy in 16 (55%) patients; 9 (31%) patients had received ≥2 prior systemic therapies. Two patients (7%) had received prior immunotherapy, both with progressive disease as the best response.
Efficacy
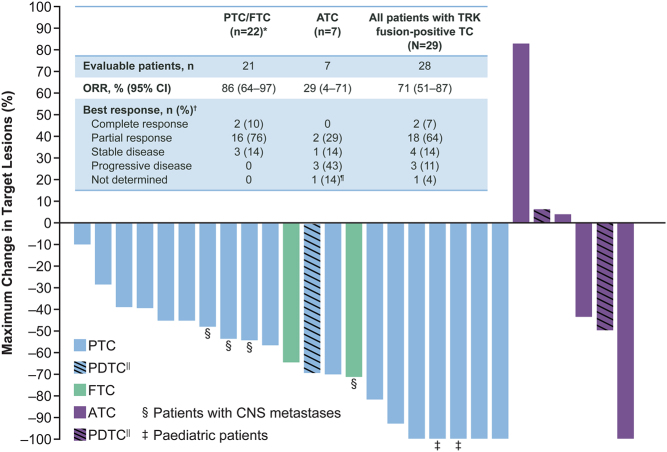
Among 28 evaluable patients, the ORR in both target and non-target lesions was 71% (95% CI: 51–87) (Fig. 1); 2 (7%) patients had a complete response (CR; including 1 paediatric patient), 18 (64%) had a partial response (PR), 4 (14%) had stable disease (SD), and 3 (11%) had progressive disease (PD). All three patients with progressive disease had ATC, including one PDTC patient. In 1 (4%) patient with ATC, response could not be determined due to clinical disease progression prior to the first post-baseline assessment. One (3%) of the patients with PTC was considered not evaluable for the assessment of tumour response as they had not been on treatment long enough for the first evaluation by the data cut-off. Among patients with an objective response (n = 20), the median time to response (RECIST v1.1) was 1.87 months (range: 1.64–3.68) (Fig. 2), which was the time of the first post-baseline assessment in most cases.
Figure 1.
Response to larotrectinib. A waterfall plot of the maximum change in target lesions following treatment with larotrectinib in patients with advanced TRK fusion-positive thyroid carcinoma. The table depicts the overall response in both target and non-target lesions, and the waterfall plot depicts the maximum change in target lesions. *One patient with papillary TC was not evaluable for assessment of tumour response. †Investigator assessment based on RECIST version 1.1. ||Three PDTCs, two in the anaplastic group and one in the papillary group. ¶One patient with anaplastic TC was evaluable, but the response could not be determined because they had clinical disease progression prior to the first tumour response assessment. ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; ORR, objective response rate; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; TC, thyroid carcinoma; TRK, tropomyosin receptor kinase. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1259.
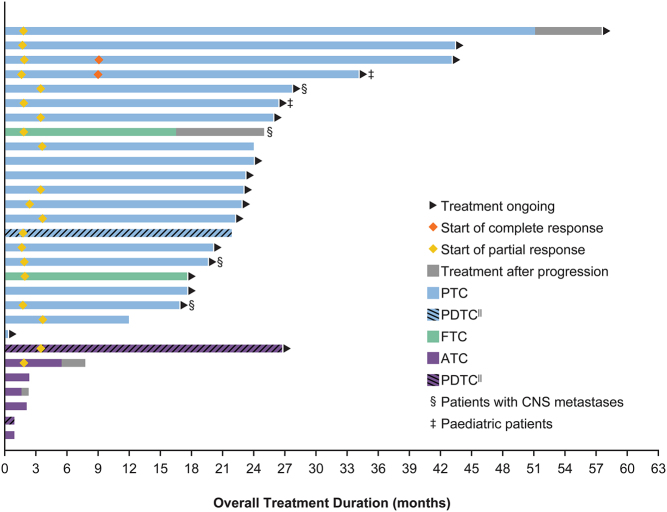
Figure 2.
Treatment duration. A swimmer plot of the treatment duration in patients with advanced TRK fusion-positive thyroid carcinoma treated with larotrectinib. ||Three PDTCs, two in the anaplastic group and one in the papillary group. ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; TRK, tropomyosin receptor kinase. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1259.
Among the 22 patients with DTC (PTC and FTC), the ORR was 86% (95% CI: 64–97). Among 19 evaluable patients with PTC, 2 had a CR (including 1 paediatric patient), 14 had a PR (including 1 PDTC patient), and 3 had SD. Both patients with FTC had a PR. Among the seven patients with ATC, the ORR in both target and non-target lesions was 29% (95% CI: 4–71); two had a PR (including one PDTC patient), one had SD, three had PD (including one PDTC patient), and in one patient the response could not be determined (Fig. 1).
Among the 13 patients with DTC who had one or more prior lines of systemic therapy, the ORR was 92%. The disease control rate (DCR) at 24 weeks for DTC (PTC and FTC) was 91% (95% CI: 71–99). The DCR at 24 weeks for ATC was 29% (95% CI: 4–71). All four patients with CNS metastases at baseline had a PR as the best overall response. Two patients had measurable intracranial disease, with intracranial tumour reductions of 14 and 46%; both had received radiotherapy ~14–15 months prior to starting larotrectinib. Of the four patients with baseline CNS metastases, one had progressed after 17 months while three had not progressed at the data cut-off, with PFS censored at 17, 20, and 27 months, respectively.
Duration of treatment ranged from 0.26+ to 57.5+ months (Fig. 2). By the data cut-off, treatment was ongoing in 19 (66%; 17 PTC, 1 FTC, and 1 ATC) patients. Six patients had progressed on treatment, of which four patients continued treatment post-progression for ≥20 days due to perceived clinical benefit. Two patients died on therapy (one with PTC/PDTC and one with ATC). Two patients discontinued treatment due to their own decision and were alive without progression at the data cut-off. One patient discontinued therapy at the recommendation of their physician and was alive at data cut-off without post-study disease assessments.
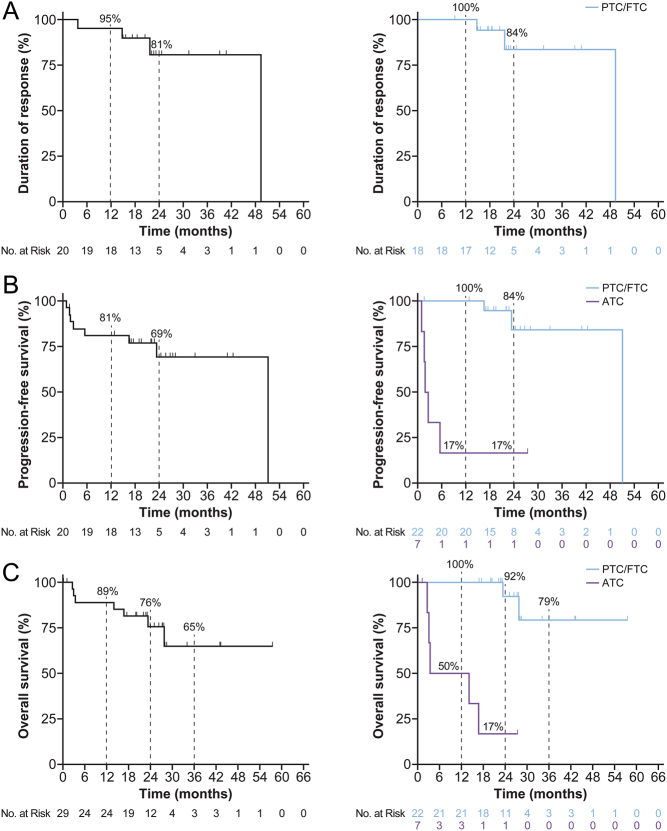
The Kaplan–Meier estimated DoR rate for all responders (n = 20) at 12 and 24 months was 95 and 81%, respectively (Fig. 3A). The PFS rate at 12 and 24 months was 81 and 69%, respectively (Fig. 3B). The proportion of patients alive after 12, 24, and 36 months was 89, 76, and 65%, respectively (Fig. 3C). Among the patients with PTC/FTC, the DoR rate at 12 and 24 months was 100 and 84%, respectively. The PFS rate at 12 and 24 months was 100 and 84%, respectively. The OS rate at 12, 24, and 36 months was 100, 92, and 79%, respectively.
Figure 3.
Duration of response and survival. Kaplan–Meier curves of (A) duration of response†, (B) progression-free survival‡, and (C) overall survival‡ in patients with advanced TRK fusion-positive thyroid carcinoma treated with larotrectinib. The left panels show data for the entire study group and the right panels show the data based on histology. †Duration of response Kaplan-Meier curve only includes the patients who experienced a response. ATC duration of response Kaplan-Meier curve is not shown due to too few patients. ‡The one patient in the ATC group with a durable response had PDTC. ATC, anaplastic thyroid carcinoma; FTC, follicular thyroid carcinoma; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; TRK, tropomyosin receptor kinase. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1259.
For patients classified as ATC, the DoR rate at 12 months was 50%. Median PFS was 2.2 months (95% CI: 0.9–NE) after a median follow-up of 27.4 months. The 12- and 24-month PFS rates were both 17%. Median OS was 8.8 months (95% CI: 2.6–NE) over a median follow-up of 27.4 months. The OS rate at 12 and 24 months was 50 and 17%, respectively. However, these data are skewed by one patient with PDTC who had a prolonged response to therapy.
Safety
Treatment-related AEs were reported by 26 (90%) patients, the most common being myalgia, fatigue, dizziness, and elevated liver transaminases. AEs that occurred in ≥15% of patients are shown in Table 2. Treatment-related AEs were mostly grade 1 or 2 and were consistent with the pooled analysis (22). Two (7%) patients experienced grade 3 AEs considered related to larotrectinib (anaemia and decreased lymphocyte count). There were two grade 5 treatment-emergent AEs (7%) that were not considered to be related to larotrectinib; one patient died from a tracheal haemorrhage and one patient died due to progressive thyroid carcinoma. A total of two patients (7%) experienced an AE leading to dose reduction, with dose reductions lasting approximately 2 and 3 weeks, respectively. No patients experienced an AE that resulted in permanent discontinuation of larotrectinib.
Table 2.
Adverse events occurring in ≥15% of patients with advanced TRK fusion-positive thyroid carcinoma treated with larotrectinib.
| Preferred term | Treatment-emergent AEs, n (%) | Treatment-related AEs, n (%) | |||||
|---|---|---|---|---|---|---|---|
| Grade 1 or 2 | Grade 3 | Grade 4 | Any grade | Grade 3 | Grade 4 | Any grade | |
| Myalgia | 12 (41) | 0 | 0 | 12 (41) | 0 | 0 | 8 (28) |
| Fatigue | 10 (34) | 0 | 0 | 10 (34) | 0 | 0 | 8 (28) |
| Nausea | 10 (34) | 0 | 0 | 10 (34) | 0 | 0 | 3 (10) |
| Constipation | 9 (31) | 0 | 0 | 9 (31) | 0 | 0 | 5 (17) |
| Cough | 8 (28) | 1 (3) | 0 | 9 (31) | – | – | – |
| Dizziness | 8 (28) | 1 (3) | 0 | 9 (31) | 0 | 0 | 8 (28) |
| Peripheral oedema | 9 (31) | 0 | 0 | 9 (31) | 0 | 0 | 4 (14) |
| ALT increased | 8 (28) | 0 | 0 | 8 (28) | 0 | 0 | 8 (28) |
| Anaemia | 4 (14) | 4 (14) | 0 | 8 (28) | 1 (3) | 0 | 2 (7) |
| AST increased | 8 (28) | 0 | 0 | 8 (28) | 0 | 0 | 8 (28) |
| Arthralgia | 7 (24) | 0 | 0 | 7 (24) | 0 | 0 | 3 (10) |
| Diarrhoea | 4 (14) | 3 (10) | 0 | 7 (24) | 0 | 0 | 3 (10) |
| Dyspnoea | 6 (21) | 1 (3) | 0 | 7 (24) | 0 | 0 | 1 (3) |
| Leukocyte count decreased | 6 (21) | 1 (3) | 0 | 7 (24) | 0 | 0 | 6 (21) |
| Lymphocyte count decreased | 3 (10) | 3 (10) | 1 (3) | 7 (24) | 1 (3) | 0 | 2 (7) |
| Vomiting | 7 (24) | 0 | 0 | 7 (24) | 0 | 0 | 2 (7) |
| Headache | 6 (21) | 0 | 0 | 6 (21) | 0 | 0 | 1 (3) |
| Pyrexia | 6 (21) | 0 | 0 | 6 (21) | 0 | 0 | 1 (3) |
| Hypoaesthesia | 5 (17) | 0 | 0 | 5 (17) | 0 | 0 | 3 (10) |
| Hypocalcaemia | 2 (7) | 2 (7) | 1 (3) | 5 (17) | 0 | 0 | 1 (3) |
| Nasal congestion | 5 (17) | 0 | 0 | 5 (17) | 0 | 0 | 1 (3) |
| Pain in extremity | 5 (17) | 0 | 0 | 5 (17) | 0 | 0 | 2 (7) |
| Rash | 5 (17) | 0 | 0 | 5 (17) | 0 | 0 | 3 (10) |
Dashes indicate AEs that were not reported to be treatment-related in any patients.
AE, adverse event; ALT, alanine aminotransferase; AST, aspartate aminotransferase; TRK, tropomyosin receptor kinase.
Case study 1
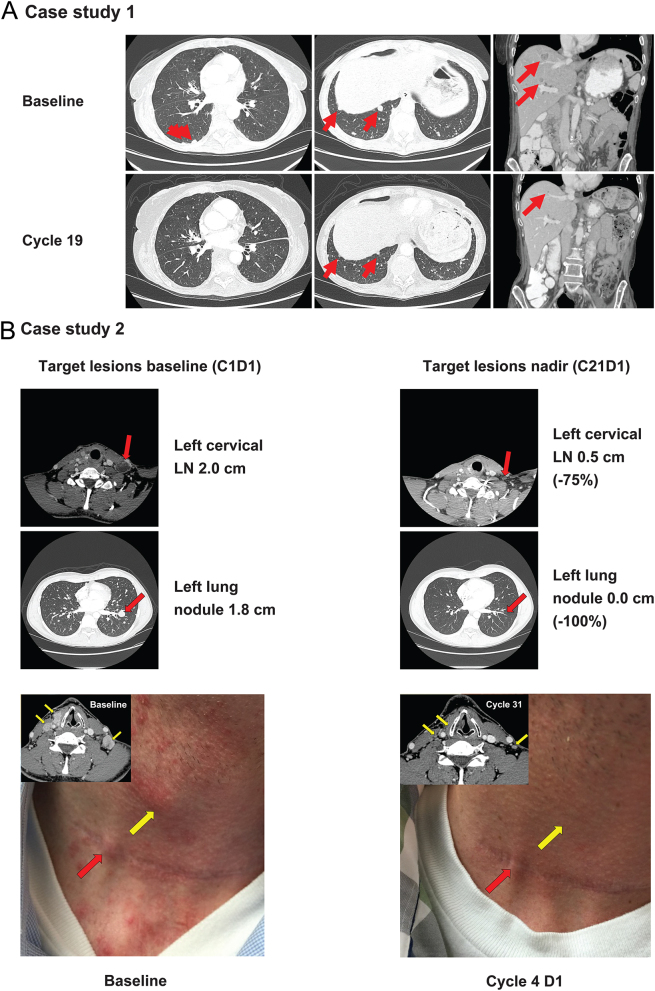
A 64-year-old woman with a history of chronic obstructive pulmonary disease (COPD) was diagnosed with PDTC derived from PTC and treated with a total thyroidectomy and RAI. One year later, she developed locoregional recurrence and bone metastases. She underwent a left-neck dissection and partial oesophagectomy, followed by external beam radiation to the neck and stereotactic radiation to multiple levels of the vertebral spine. The patient continued to progress and was enrolled in a clinical trial of radiation and immunotherapy but without response. An ETV6-NTRK3 gene fusion was detected through NGS with Oncomine Focus assay, and she began larotrectinib 100 mg twice daily. The patient achieved a PR by cycle 3, with 70% tumour reduction by cycle 19 (Fig. 4A). After 23 completed cycles, larotrectinib was suspended due to COPD complications and pneumonia, which were unrelated to larotrectinib. The patient’s disease progressed, and she died approximately 3 months later.
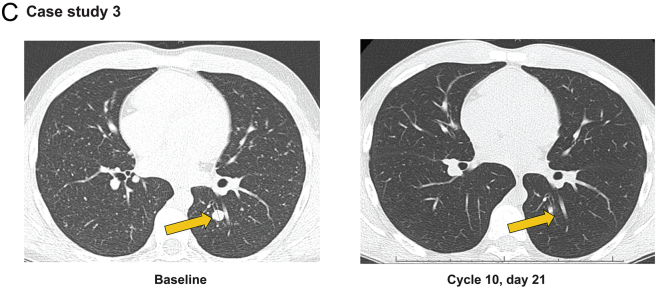
Figure 4.
Case studies of patients with advanced TRK fusion-positive thyroid carcinoma treated with larotrectinib. Contrast-enhanced CT images demonstrating response in target and non-target lesions. (A) An adult female patient was diagnosed with PDTC with an ETV6-NTRK3 gene fusion. The red arrows indicate lung and liver metastases that responded to therapy. (B) An adult male patient was diagnosed with PTC with an ETV6-NTRK3 gene fusion. The red arrows indicate target lesions, and the yellow arrows identify non-target lesions. (C) A paediatric male patient was diagnosed with PTC with an IRF2BP2-NTRK1 gene fusion and a complete response in a lung target lesion (yellow arrows). LN, lymph node; NTRK, neurotrophic tyrosine receptor kinase; PDTC, poorly differentiated thyroid carcinoma; PTC, papillary thyroid carcinoma; TRK, tropomyosin receptor kinase. A full colour version of this figure is available at https://doi.org/10.1530/EJE-21-1259.
Case study 2
A 33-year-old male, diagnosed at 27 years of age, with PTC and metastases to lungs, lymph nodes, and skin, was treated with five prior surgeries and two courses of RAI to a cumulative dose of 330 mCi/12.2 GBq. Due to RAI-refractory disease and rapid tumour progression, he was enrolled in a phase I clinical trial with pazopanib and trametinib; best response was stable disease. The patient’s tumour was found to have an ETV6-NTRK3 gene fusion through FoundationOne DNA NGS and then he started larotrectinib on the phase I trial at 100 mg twice daily in September 2015. The patient had a confirmed PR after two cycles with rapid improvement in cervical lymphadenopathy and his best response was a 92.6% reduction in target lesions after 20 cycles (Fig. 4B). The patient stopped therapy in October 2020 after 65 cycles due to the progression of disease resulting from an acquired NTRK3 solvent front mutation (p.G623R) and then switched to selitrectinib, a next-generation TRK inhibitor. The patient achieved and has maintained a durable PR on selitrectinib (currently on cycle 18 of treatment).
Case study 3
A 13-year-old male, diagnosed at 9 years of age in 2014, was diagnosed with PTC with metastases to the lungs and lymph nodes. He had two prior surgeries, complicated by post-operative hypoparathyroidism, and two RAI treatments to a cumulative dose of 382 mCi/14.1 GBq (last treatment in 2014) with persistent but diminished tumour uptake of iodine. Despite RAI, he experienced progression of pulmonary disease (RECIST progression on the largest metastasis (shown in Fig. 4C) and new micronodular lung metastases). There was no prior systemic therapy. The patient’s tumour was found to have an IRF2BP2-NTRK1 gene fusion through RNA NGS with Oncomine Focus assay, and he started treatment with larotrectinib in 2018 due to radiographic progression. The patient had a confirmed PR with 50% reduction in the target lesions after two cycles and then a CR in the target lesions after six cycles (Fig. 4C). The patient stopped therapy after 31 cycles due to an ongoing CR in the target lesions. His residual disease remains stable 10 months after cessation of larotrectinib.
Discussion
Currently, the standard-of-care treatment for unselected patients with RAI-refractory advanced DTC includes the antiangiogenic multi-kinase inhibitors lenvatinib and sorafenib, which are associated with ORRs ranging from 12 to 65% (25, 26). In phase 3 clinical trials in patients with advanced DTC, lenvatinib and sorafenib demonstrated a median PFS of 18.3 and 10.8 months, respectively. Adverse events led to discontinuation of treatment in 14% of patients receiving lenvatinib and in 19% of patients receiving sorafenib. Recommended systemic treatments for patients with ATC are primarily taxanes, doxorubicin and platinum-based therapies for BRAF WT tumours, the dabrafenib/trametinib couplet for tumours with the BRAF V600E variant, and selective ALK, RET, or TRK inhibitors if an actionable fusion protein is identified (27, 28, 29).
In the current study, the highly selective TRK inhibitor larotrectinib showed durable antitumour efficacy in both adult and paediatric patients with TRK fusion-positive TC. Sustained disease control was demonstrated with a 69% 24-month PFS rate. These data exceed the outcomes reported for the non-selective oral kinase inhibitors that primarily target the vascular endothelial growth factor receptor and are widely used in RAI-refractory DTC. The antitumour efficacy of larotrectinib was demonstrated across tumour subtypes. Among patients with DTC (PTC and FTC), the ORR was 86% (95% CI: 64–97), far exceeding that in other published studies using antiangiogenic kinase inhibitors in unselected patients with DTC. While the ORR among the seven patients classified as ATC in this analysis was lower at 29% (95% CI: 4–71), response exceeds those previously reported for cytotoxic chemotherapy, immunotherapy, and lenvatinib in this population (27, 29, 30, 31). Consistent with this, recent American Thyroid Association guidelines recommend the use of a TRK inhibitor (either larotrectinib or entrectinib) in TRK fusion-positive ATC (29).
Genomic alterations are highly prevalent in ATC compared with DTC (32). Up to 95.8% of ATC cases harbour at least one genomic alteration in receptor tyrosine kinases and the PI3K/AKT and MAPK pathways. Multiple genomic alterations, especially those in PIK3CA, play a role in the tumourigenesis and aggressiveness of ATC (33, 34) and thus may contribute to the worse responses seen in ATC patients. However, response rates with the doublet dabrafenib/trametinib in BRAF-mutated ATC are very high (35), suggesting that single-agent-targeted therapy may not be sufficient in this population (36). Further studies are required to determine the mechanisms of TRK inhibitor insensitivity in TRK fusion-positive ATC patients.
In the present analysis of larotrectinib, the treatment-related AEs were mostly grades 1–2 and 2 (7%) patients experienced an AE leading to a dose reduction, 1 patient due to paraesthesia in the shoulder, and the other due to increased alanine aminotransferase. No patients experienced an AE leading to permanent discontinuation of treatment.
The multi-kinase inhibitor entrectinib, which targets TRK, ROS1, ALK, and JAK2 is another approved therapy for TRK fusion-positive solid tumours though it is limited for patients older than 12 years (37). Entrectinib demonstrated an ORR of 53.8% in 13 patients with TC of unknown subtype in a pooled analysis of three phase I/II clinical trials (ALKA-372-001, STARTRK-1, and STARTRK-2). Median DoR was 13.2 months (95% CI: 7.0–NE) (38). While this finding is lower than the 71% ORR for larotrectinib in the present analysis, it is important to note that the entrectinib data are based on a smaller patient population (n = 13) than for larotrectinib (n = 29) and thus do not allow for a head-to-head comparison. Additionally, no paediatric patients were included in the analysis of entrectinib for TC. Across the whole efficacy-evaluable study population (n = 121), entrectinib was associated with a median DoR of 20 months (95% CI: 13.0–38.2) and median PFS of 13.8 months (95% CI: 10.1–19.9). Among the total safety population included in the pooled analysis, entrectinib was well-tolerated with most treatment-related AEs being low grade and reversible (38). In an earlier analysis of the overall safety population (n = 355), the occurrence of treatment-related AEs resulting in dose reduction, dose interruption, and treatment discontinuation was 27, 25, and 4%, respectively (39).
The findings of the current analysis support routine testing for NTRKgene fusions in patients with advanced TC in whom systemic therapies are being considered (40, 41). All gene fusion events identified in this study were in NTRK1or NTRK3, consistent with previous data indicating that fusions in TC very rarely occur in the NTRK2 gene, which is primarily associated with primary CNS tumours (1, 10, 11, 12, 14, 23, 42). Besides NTRK1and NTRK3, there are several other genomic alterations commonly associated with the pathogenesis of DTC, including the BRAFV600E mutation (occurring in 45% or more of adult PTCs), RASgene mutations, fusions involving RET, ALK, BRAF, MET, ROS1, THADA, and the PAX8-PPARGfusion (occurring primarily in FTC) (43, 44, 45, 46, 47).
There are various techniques that can be used to detect NTRK gene fusions, such as immunohistochemistry (IHC; indirectly), fluorescence in situ hybridisation (FISH), and NGS. Pan-TRK IHC is rapid and inexpensive, but it is unable to distinguish between WT and chimeric TRK protein and its sensitivity with respect to TRKC fusion proteins may be low (48); thus, it may be used as a screen to identify potential cancers with a low incidence of NTRK gene fusions that will require subsequent confirmatory tests (48). FISH is widely available but requires multiple assays (one test per NTRK gene) and additional NGS to confirm the presence of an NTRKgene fusion; it may also be most appropriate for cancers with a high incidence of NTRK gene fusions (48).
Additionally, both IHC and FISH test for single biomarkers as opposed to NGS where hundreds of gene mutations and fusions can be analysed in parallel with a relatively small amount of tissue. It has been demonstrated that DNA-based sequencing techniques may not detect all gene fusions and therefore the use of RNA-based sequencing techniques may be required to support further testing to increase the chance of identifying the NTRK gene fusions, particularly considering the large intronic regions of NTRK3 (48, 49). Of the 16 patients in this analysis with NTRK3gene fusions, 2 were EML4-NTRK3(both detected by RNA sequencing) and 14 were ETV6-NTRK3(3 detected by DNA sequencing, 7 by RNA sequencing, and 4 by both DNA and RNA sequencing). These findings support the use of RNA sequencing for detecting fusion partners with NTRK3(49).
In conclusion, larotrectinib is a highly active TRK inhibitor with a favourable safety profile in patients with TRK fusion-positive solid tumours. In TRK fusion-positive TC, larotrectinib results in rapid and durable disease control. Therefore, patients with advanced non-medullary TC who may require systemic therapy could be considered for testing for NTRK gene fusions by NGS.
Supplementary Material
Declaration of interest
S G W reports travel support from Bayer in the past and an active consulting agreement with Bayer (Vitrakvi expert programme). A D reports honoraria/advisory boards: Ignyta/Genentech/Roche, Loxo/Bayer/Lilly, Takeda/Ariad/Millenium, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Helsinn, Beigene, BergenBio, Hengrui Therapeutics, Exelixis, Tyra Biosciences, Verastem, MORE Health, Abbvie, 14ner/Elevation Oncology, Remedica Ltd., ArcherDX, Monopteros, Novartis, EMD Serono, Melendi, Liberum, Repare RX, Chugai, Merus, Chugai Pharmaceutical, Nuvalent, mBrace, AXIS, EPG Health, Harborside Nexus, Liberum, RV More, Ology, Amgen, TouchIME, Janssen; Associated research paid to institution: Pfizer, Exelixis, GlaxoSmithKline, Teva, Taiho, PharmaMar; Royalties: Wolters Kluwer; OTHER: Merck, Puma, Merus, Boehringer Ingelheim; CME honoraria: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice, Axis,Peerview Institute, Paradigm Medical Communications, WebMD, MJH Life Sciences, AXIS, EPG Health, JNCC/Harborside. J J L reports compensated consultant for Genentech, C4 Therapeutics, Blueprint Medicines, Nuvalent, Bayer, Novartis, and Turning Point Therapeutics; honorarium and travel support from Pfizer; institutional research funds from Roche/Genentech, Hengrui Therapeutics, Turning Point Therapeutics, Neon Therapeutics, Relay Therapeutics, Bayer, Elevation Oncology, Linnaeus Therapeutics, and Novartis; CME funding from OncLive, MedStar Health, and Northwell Health. M S B reports research support to the University of Pennsylvania School of Medicine from Bayer, Loxo Oncology, Genentech, Eisai, Blueprint Medicines, Lilly and Novartis; consultancy for Bayer, Loxo Oncology, Genentech, AstraZeneca, and Lilly, honoraria from Clinical Care Options, Medscape, OncLive and PeerView. R M reports advisory boards: Amgen, Bayer, BMS, Clovis, Janssen, Pfizer; Invited Speaker: Astellas, Ipsen, MSD; Local PI: Astellas, Bayer, BMS, Clovis, Regeneron; Coordinating PI: MSD. M A and A K have nothing to declare. J B reports advisory boards: Pfizer, Bayer, Kura, Janssen, Blueprint Medicine, Merck, Beigene, and Turning Point; Consulting: Pfizer, AstraZeneca, Lilly; Grant funding: BMS. M C reports advisory roles for Bayer, Astra-Zeneca, Pfizer, Servier, BMS, Roche. S K reports consultant/advisory board: Springworks Therapeutics, Gilead, EcoR1, Seagen, Mundibiopharma Ltd, Bayer, Oxford Biotherapeutics, Mirati, and HarbourBiomed; co-founder and equity holder for PathomlQ. spouse is a scientific advisor for Cadilla Pharmaceuticals and founder of Arxeon Inc. S L reports advisory role and travel grant support from Bayer and Immunocore. D O reports advisory/consultancy: AstraZeneca, Novartis, Genentech/Roche, Merck Serono, Bayer, Taiho, ASLAN, Halozyme, Zymeworks, Celgene, BeiGene, Basilea, Turning Point, Yuhan; research grant/funding (self): AstraZeneca, Novartis, Array, Eli Lilly, Servier, BeiGene, MSD, Handok. K P reports advisor for Loxo Oncology. D S reports consulting: Perthera, Ability Pharmaceuticals; honoraria: Foundation Medicine; and research funding: Agios, Bayer, Bristol-Myers Squibb, Celgene, Genentech, InCyte Loxo, OncoMed, Rafael. E S reports consulting: Bayer, Eisai, Merck, Eli Lilly, Roche and research: Roche, Fore Pharmaceuticals, Eli Lilly, Novartis. R N is an external employee of Bayer. J D S and N B are employees of Bayer. D S H reports Discloses research/grant funding from AbbVie, Adaptimmune, Amgen, AstraZeneca, Bayer, BMS, Daiichi-Sankyo, Eisai, Fate Therapeutics, Genentech, Genmab, Ignyta, Infinity, Kite, Kyowa, Lilly, Loxo Oncology, Merck, MedImmune, Mirati, MiRNA, Molecular Templates, Mologen, NCI-CTEP, Novartis, Pfizer, Seattle Genetics, Takeda; travel/accommodation/expenses from Loxo Oncology, MiRNA, ASCO, AACR, SITC, Genmab; consulting/advisory roles for Alpha Insights, Axiom, Adaptimmune, Baxter, Bayer, Genentech, GLG, Group H, Guidepoint Global, Infinity, Janssen, Merrimack, Medscape, Numab, Pfizer, Seattle Genetics, Takeda, and Trieza Therapeutics; ownership interests in Molecular Match (advisor), OncoResponse (founder), and Presagia Inc (advisor). M E C reports Bayer, Exelixis, Ignyta, Loxo Oncology, Blueprint, Eisai, Merck, Genentech.
Funding
These studies were funded by Bayer Healthcare and Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company.
Acknowledgements
The authors would like to thank the patients, their families, and all investigators involved in these studies. Medical writing support was provided by Anastasija Pesevska, PharmD and editorial support was provided by George Chappell, MSc, both of Scion, London, UK, supported by Bayer according to Good Publication Practice guidelines. The Sponsor was involved in the study design, collection, analysis, and interpretation of data, as well as data checking of information provided in the manuscript. However, ultimate responsibility for opinions, conclusions, and data interpretation lies with the authors.
References
- 1.Vaishnavi A, Le AT, Doebele RC. TRKing down an old oncogene in a new era of targeted therapy. Cancer Discovery 2015525–34. ( 10.1158/2159-8290.CD-14-0765) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ardini E, Bosotti R, Borgia AL, De Ponti C, Somaschini A, Cammarota R, Amboldi N, Raddrizzani L, Milani A, Magnaghi Pet al. The TPM3-NTRK1 rearrangement is a recurring event in colorectal carcinoma and is associated with tumor sensitivity to TRKA kinase inhibition. Molecular Oncology 201481495–1507. ( 10.1016/j.molonc.2014.06.001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen Y, Tseng SH. Targeting tropomyosin-receptor kinase fused gene in cancer. Anticancer Research 2014341595–1600. [PubMed] [Google Scholar]
- 4.Dupain C, Harttrampf AC, Urbinati G, Geoerger B, Massaad-Massade L. Relevance of fusion genes in pediatric cancers: toward precision medicine. Molecular Therapy: Nucleic Acids 20176315–326. ( 10.1016/j.omtn.2017.01.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roccato E, Miranda C, Ranzi V, Gishizki M, Pierotti MA, Greco A. Biological activity of the thyroid TRK-T3 oncogene requires signalling through Shc. British Journal of Cancer 200287645–653. ( 10.1038/sj.bjc.6600544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tognon C, Garnett M, Kenward E, Kay R, Morrison K, Sorensen PH. The chimeric protein tyrosine kinase ETV6-NTRK3 requires both Ras-ERK1/2 and PI3-kinase-Akt signaling for fibroblast transformation. Cancer Research 2001618909–8916. [PubMed] [Google Scholar]
- 7.Cocco E, Scaltriti M, Drilon A. NTRK fusion-positive cancers and TRK inhibitor therapy. Nature Reviews: Clinical Oncology 201815731–747. ( 10.1038/s41571-018-0113-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pierotti MA, Bongarzone I, Borello MG, Greco A, Pilotti S, Sozzi G. Cytogenetics and molecular genetics of carcinomas arising from thyroid epithelial follicular cells. Genes, Chromosomes and Cancer 1996161–14. () [DOI] [PubMed] [Google Scholar]
- 9.Bazhenova L, Lokker A, Snider J, Castellanos E, Fisher V, Fellous M, Nanda S, Zong J, Keating K, Jiao X. TRK fusion cancer: patient characteristics and survival analysis in the real-world setting. Targeted Oncology 202116389–399. ( 10.1007/s11523-021-00815-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kong Y, Bu R, Parvathareddy SK, Siraj AK, Siraj N, Al-Sobhi SS, Al-Dayel F, Al-Kuraya KS. NTRK fusion analysis reveals enrichment in Middle Eastern BRAF wild-type PTC. European Journal of Endocrinology 2021184503–511. ( 10.1530/EJE-20-1345) [DOI] [PubMed] [Google Scholar]
- 11.Chu YH, Wirth LJ, Farahani AA, Nose V, Faquin WC, Dias-Santagata D, Sadow PM. Clinicopathologic features of kinase fusion-related thyroid carcinomas: an integrative analysis with molecular characterization. Modern Pathology 2020332458–2472. ( 10.1038/s41379-020-0638-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pekova B, Sykorova V, Mastnikova K, Vaclavikova E, Moravcova J, Vlcek P, Lastuvka P, Taudy M, Katra R, Bavor Pet al. NTRK fusion genes in thyroid carcinomas: clinicopathological characteristics and their impacts on prognosis. Cancers 202113 1932. ( 10.3390/cancers13081932) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Potter SL, Reuther J, Chandramohan R, Gandhi I, Hollingsworth F, Sayeed H, Voicu H, Kakkar N, Baksi KS, Sarabia SFet al. Integrated DNA and RNA sequencing reveals targetable alterations in metastatic pediatric papillary thyroid carcinoma. Pediatric Blood and Cancer 202168 e28741. ( 10.1002/pbc.28741) [DOI] [PubMed] [Google Scholar]
- 14.Nies M, Vassilopoulou-Sellin R, Bassett RL, Yedururi S, Zafereo ME, Cabanillas ME, Sherman SI, Links TP, Waguespack SG. Distant metastases from childhood differentiated thyroid carcinoma: clinical course and mutational landscape. Journal of Clinical Endocrinology and Metabolism 2021106e1683–e1697. ( 10.1210/clinem/dgaa935) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Iwadate M, Mitsutake N, Matsuse M, Fukushima T, Suzuki S, Matsumoto Y, Ookouchi C, Mizunuma H, Nakamura I, Nakano Ket al. The clinicopathological results of thyroid cancer with BRAFV600E mutation in the young population of fukushima. Journal of Clinical Endocrinology and Metabolism 2020105e4328–e4336. ( 10.1210/clinem/dgaa573) [DOI] [PubMed] [Google Scholar]
- 16.Chu YH, Dias-Santagata D, Farahani AA, Boyraz B, Faquin WC, Nose V, Sadow PM. Clinicopathologic and molecular characterization of NTRK-rearranged thyroid carcinoma (NRTC). Modern Pathology 2020332186–2197. ( 10.1038/s41379-020-0574-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pekova B, Sykorova V, Dvorakova S, Vaclavikova E, Moravcova J, Katra R, Astl J, Vlcek P, Kodetova D, Vcelak Jet al. RET, NTRK, ALK, BRAF, and MET fusions in a large cohort of pediatric papillary thyroid carcinomas. Thyroid 2020301771–1780. ( 10.1089/thy.2019.0802) [DOI] [PubMed] [Google Scholar]
- 18.Xu B, Fuchs T, Dogan S, Landa I, Katabi N, Fagin JA, Tuttle RM, Sherman E, Gill AJ, Ghossein R. Dissecting anaplastic thyroid carcinoma: a comprehensive clinical, histologic, immunophenotypic, and molecular study of 360 cases. Thyroid 2020301505–1517. ( 10.1089/thy.2020.0086) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bayer AG.VITRAKVI summary of product characteristics, 2021. https://www.ema.europa.eu/en/documents/product-information/vitrakvi-epar-product-information_en.pdf [Google Scholar]
- 20.Bayer HealthCare Pharmaceuticals Inc. VITRAKVI prescribing information, 2021. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210861s006lbl.pdf [Google Scholar]
- 21.Drilon A, Laetsch TW, Kummar S, DuBois SG, Lassen UN, Demetri GD, Nathenson M, Doebele RC, Farago AF, Pappo ASet al. Efficacy of larotrectinib in TRK fusion-positive cancers in adults and children. New England Journal of Medicine 2018378731–739. ( 10.1056/NEJMoa1714448) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong DS, DuBois SG, Kummar S, Farago AF, Albert CM, Rohrberg KS, van Tilburg CM, Nagasubramanian R, Berlin JD, Federman Net al. Larotrectinib in patients with TRK fusion-positive solid tumours: a pooled analysis of three phase 1/2 clinical trials. Lancet: Oncology 202021531–540. ( 10.1016/S1470-2045(1930856-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laetsch TW, DuBois SG, Mascarenhas L, Turpin B, Federman N, Albert CM, Nagasubramanian R, Davis JL, Rudzinski E, Feraco AMet al. Larotrectinib for paediatric solid tumours harbouring NTRK gene fusions: phase 1 results from a multicentre, open-label, phase 1/2 study. Lancet: Oncology 201819705–714. ( 10.1016/S1470-2045(1830119-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney Met al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). European Journal of Cancer 200945228–247. ( 10.1016/j.ejca.2008.10.026) [DOI] [PubMed] [Google Scholar]
- 25.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YKet al. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 2014384319–328. ( 10.1016/S0140-6736(1460421-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AOet al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. New England Journal of Medicine 2015372621–630. ( 10.1056/NEJMoa1406470) [DOI] [PubMed] [Google Scholar]
- 27.Cabanillas ME, Zafereo M, Gunn GB, Ferrarotto R. Anaplastic thyroid carcinoma: treatment in the age of molecular targeted therapy. Journal of Oncology Practice 201612511–518. ( 10.1200/JOP.2016.012013) [DOI] [PubMed] [Google Scholar]
- 28.Filetti S, Durante C, Hartl D, Leboulleux S, Locati LD, Newbold K, Papotti MG, Berruti A. & ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Thyroid cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology 2019301856–1883. ( 10.1093/annonc/mdz400) [DOI] [PubMed] [Google Scholar]
- 29.Bible KC, Kebebew E, Brierley J, Brito JP, Cabanillas ME, Clark Jr TJ, Di Cristofano A, Foote R, Giordano T, Kasperbauer Jet al. 2021 American Thyroid Association guidelines for management of patients with anaplastic thyroid cancer. Thyroid 202131337–386. ( 10.1089/thy.2020.0944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Capdevila J, Wirth LJ, Ernst T, Ponce Aix S, Lin CC, Ramlau R, Butler MO, Delord JP, Gelderblom H, Ascierto PAet al. PD-1 blockade in anaplastic thyroid carcinoma. Journal of Clinical Oncology 2020382620–2627. ( 10.1200/JCO.19.02727) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wirth LJ, Brose MS, Sherman EJ, Licitra L, Schlumberger M, Sherman SI, Bible KC, Robinson B, Rodien P, Godbert Yet al. Open-label, single-arm, multicenter, phase II trial of lenvatinib for the treatment of patients with anaplastic thyroid cancer. Journal of Clinical Oncology 2021392359–2366. ( 10.1200/JCO.20.03093) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rashid M, Agarwal A, Pradhan R, George N, Kumari N, Sabaretnam M, Chand G, Mishra A, Agarwal G, Mishra SK. Genetic alterations in anaplastic thyroid carcinoma. Indian Journal of Endocrinology and Metabolism 201923480–485. ( 10.4103/ijem.IJEM_321_19) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M. Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/Akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. Journal of Clinical Endocrinology and Metabolism 2008933106–3116. ( 10.1210/jc.2008-0273) [DOI] [PubMed] [Google Scholar]
- 34.Qin Y, Wang JR, Wang Y, Iyer P, Cote GJ, Busaidy NL, Dadu R, Zafereo M, Williams MD, Ferrarotto Ret al. Clinical utility of circulating cell-free DNA mutations in anaplastic thyroid carcinoma. Thyroid 2021311235–1243. ( 10.1089/thy.2020.0296) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Subbiah V, Kreitman RJ, Wainberg ZA, Cho JY, Schellens JHM, Soria JC, Wen PY, Zielinski C, Cabanillas ME, Urbanowitz Get al. Dabrafenib and trametinib treatment in patients with locally advanced or metastatic BRAF V600-mutant anaplastic thyroid cancer. Journal of Clinical Oncology 2018367–13. ( 10.1200/JCO.2017.73.6785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hyman DM, Puzanov I, Subbiah V, Faris JE, Chau I, Blay JY, Wolf J, Raje NS, Diamond EL, Hollebecque Aet al. Vemurafenib in multiple nonmelanoma cancers with BRAF V600 mutations. New England Journal of Medicine 2015373726–736. ( 10.1056/NEJMoa1502309) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roche AG.Rozlytrek prescribing information, 2019. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/212725s000lbl.pdf [Google Scholar]
- 38.Bazhenova L, Liu SV, Lin JJ, Lu S, Drilon A, Chawla SP, Fakih M, Krzakowski M, Paz-Ares L, Blakely Cet al. 533P efficacy and safety of entrectinib in patients with locally advanced/metastatic NTRK fusion-positive (NTRK-fp) solid tumours. Annals of Oncology 202132S598–S599. ( 10.1016/j.annonc.2021.08.1055) [DOI] [Google Scholar]
- 39.Doebele RC, Drilon A, Paz-Ares L, Siena S, Shaw AT, Farago AF, Blakely CM, Seto T, Cho BC, Tosi Det al. Entrectinib in patients with advanced or metastatic NTRK fusion-positive solid tumours: integrated analysis of three phase 1–2 trials. Lancet: Oncology 202021271–282. ( 10.1016/S1470-2045(1930691-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bebb DG, Banerji S, Blais N, Desmeules P, Gill S, Grin A, Feilotter H, Hansen AR, Hyrcza M, Krzyzanowska Met al. Canadian consensus for biomarker testing and treatment of TRK fusion cancer in adults. Current Oncology 202128523–548. ( 10.3390/curroncol28010053) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perreault S, Chami R, Deyell RJ, El Demellawy D, Ellezam B, Jabado N, Morgenstern DA, Narendran A, Sorensen PHB, Wasserman JDet al. Canadian consensus for biomarker testing and treatment of TRK fusion cancer in pediatric patients. Current Oncology 202128346–366. ( 10.3390/curroncol28010038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stransky N, Cerami E, Schalm S, Kim JL, Lengauer C. The landscape of kinase fusions in cancer. Nature Communications 20145 4846. ( 10.1038/ncomms5846) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Khatami F, Larijani B, Nikfar S, Hasanzad M, Fendereski K, Tavangar SM. Personalized treatment options for thyroid cancer: current perspectives. Pharmacogenomics and Personalized Medicine 201912235–245. ( 10.2147/PGPM.S181520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yakushina VD, Lerner LV, Lavrov AV. Gene fusions in thyroid cancer. Thyroid 201828158–167. ( 10.1089/thy.2017.0318) [DOI] [PubMed] [Google Scholar]
- 45.Nozaki Y, Yamamoto H, Iwasaki T, Sato M, Jiromaru R, Hongo T, Yasumatsu R, Oda Y. Clinicopathological features and immunohistochemical utility of NTRK-, ALK-, and ROS1-rearranged papillary thyroid carcinomas and anaplastic thyroid carcinomas. Human Pathology 202010682–92. ( 10.1016/j.humpath.2020.09.004) [DOI] [PubMed] [Google Scholar]
- 46.Cancer Genome Atlas Research Network. Integrated genomic characterization of papillary thyroid carcinoma. Cell 2014159676–690. ( 10.1016/j.cell.2014.09.050) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pozdeyev N, Gay LM, Sokol ES, Hartmaier R, Deaver KE, Davis S, French JD, Borre PV, LaBarbera DV, Tan ACet al. Genetic analysis of 779 advanced differentiated and anaplastic thyroid cancers. Clinical Cancer Research 2018243059–3068. ( 10.1158/1078-0432.CCR-18-0373) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hsiao SJ, Zehir A, Sireci AN, Aisner DL. Detection of tumor NTRK gene fusions to identify patients who may benefit from tyrosine kinase (TRK) inhibitor therapy. Journal of Molecular Diagnostics 201921553–571. ( 10.1016/j.jmoldx.2019.03.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Benayed R, Offin M, Mullaney K, Sukhadia P, Rios K, Desmeules P, Ptashkin RN, Won H, Chang J, Halpenny DFet al. High yield of RNA sequencing for targetable kinase fusions in lung adenocarcinomas with no driver alteration detected by DNA sequencing and low tumor mutation burden. Clinical Cancer Research 2019254712–4722. ( 10.1158/1078-0432.CCR-19-0225) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a