Abstract
An Endo-European Reference Network guideline initiative was launched including 16 clinicians experienced in endocrinology, pediatric and adult and 2 patient representatives. The guideline was endorsed by the European Society for Pediatric Endocrinology, the European Society for Endocrinology and the European Academy of Andrology. The aim was to create practice guidelines for clinical assessment and puberty induction in individuals with congenital pituitary or gonadal hormone deficiency. A systematic literature search was conducted, and the evidence was graded according to the Grading of Recommendations, Assessment, Development and Evaluation system. If the evidence was insufficient or lacking, then the conclusions were based on expert opinion. The guideline includes recommendations for puberty induction with oestrogen or testosterone. Publications on the induction of puberty with follicle-stimulation hormone and human chorionic gonadotrophin in hypogonadotropic hypogonadism are reviewed. Specific issues in individuals with Klinefelter syndrome or androgen insensitivity syndrome are considered. The expert panel recommends that pubertal induction or sex hormone replacement to sustain puberty should be cared for by a multidisciplinary team. Children with a known condition should be followed from the age of 8 years for girls and 9 years for boys. Puberty induction should be individualised but considered at 11 years in girls and 12 years in boys. Psychological aspects of puberty and fertility issues are especially important to address in individuals with sex development disorders or congenital pituitary deficiencies. The transition of these young adults highlights the importance of a multidisciplinary approach, to discuss both medical issues and social and psychological issues that arise in the context of these chronic conditions.
Introduction
Puberty normal physiology
Puberty is the physiological process during which secondary sexual characteristics develop. The physical changes, driven by marked hormonal alterations, are not only limited to the development of secondary sexual characteristics but also include changes in body composition, brain, cardiovascular and skeletal development. In parallel, the adolescent matures psychosocially and emotionally. The onset of puberty depends on genetic, nutritional and environmental factors (1). Oestrogen and testosterone are produced and secreted in response to pituitary gonadotropins (luteinising hormone (LH) and follicle-stimulating hormone (FSH)), which are under the control of hypothalamic gonadotropin-releasing hormone (GnRH). There is a broad range for the age at pubertal onset.
In girls, the earliest visible sign of central puberty is usually breast budding, described as breast stage 2 (B2) on the Tanner scale (2). The average attainment age for breast stage 2 is less than 10 years and should appear before the age of 13 years (3, 4). Menarche usually occurs 2–3 years after the onset of puberty, after peak height velocity, which usually occurs between B3 and B4 (2, 5, 6). However, individual variation is large.
In boys, the first sign of pubertal development is an increase in testis volume, ≥4 mL assessed by orchidometer, which occurs at an average age of 11 years, and should appear before the age of 14 years. This is typically followed by the development of pubic and axillary hair (7). Peak height velocity usually coincides with a testis volume of about 10–12 mL (7). After this, voice breaking occurs (8).
Mini-puberty
Mini-puberty is a period which describes the activation of the hypothalamic-pituitary-gonadal hormone axis (HPG axis) in the first months of life. The gonadotropin levels show a postnatal LH surge during the first 24 h followed by an increase at around 1 week and a peak between 1 and 3 months of age (9, 10). Compared to full-term infants, those born preterm or small for gestational age show an exaggerated postnatal HPG axis activity. In boys, HPG activation coincides with a testosterone peak, with penile and testicular growth and with final testicular descent if not already in the scrotum at birth. In girls, it coincides with an increase in oestradiol, infant breast development, uterine growth and maturation of ovarian follicles.
The complete biological significance of mini-puberty is still to be elucidated, but it affects brain development, may be responsible for differences in body composition and have long-term consequences for gonadal function (11, 12). After the age of 3 months, gonadotropins decrease towards the age of 6 months, although FSH levels in girls can remain elevated until 3–4 years of age. The infant activation of the HPG hormone axis is silenced from the age of 3–6 months until reactivation at the onset of puberty. Therefore, these first months of life provide a window of opportunity for investigating the HPG hormone axis (13, 14, 15). The analysis of basal gonadotropin and gonadal hormones at the age of 1–3 months of life is helpful when investigating infants with suspected central or primary hypogonadism.
Deficiencies of sexual development; DSD and gonadotropin deficiency
Disorders or differences of sex development (DSD) is the umbrella term for congenital conditions associated with atypical chromosomal, gonadal or phenotypic sex development and can be divided into three main categories, that is, chromosomal DSD, 46,XY DSD and 46,XX DSD (16) (see Table 1). Chromosomal DSD with atypical sex chromosomes comprises conditions such as Turner syndrome (45,X and other karyotypic variants) in girls or Klinefelter syndrome (47,XXY) in boys. Children with mixed gonadal dysgenesis (MGD) conditions such as 45,X/46,XY mosaicism are brought up either as boys or girls, depending on several factors including the degree of prenatal masculinisation. In 46,XY DSD, there may be a problem in gonadal development, testosterone/DHT synthesis or action. It includes patients with complete gonadal dysgenesis, typically with a female phenotype at birth, no pubertal development and a uterus. It also includes patients with partial gonadal dysgenesis with varying degrees of masculinisation at birth as well as during puberty; some of their physical features overlap with those of patients with 46,XY partial androgen insensitivity (PAIS). 46,XY complete androgen insensitivity (CAIS) is associated with a female phenotype but without a uterus due to the production of anti-Müllerian hormone (AMH) by the testis during fetal development. 46,XY disorders of androgen synthesis are also associated with reduced masculinisation at birth, and, in some conditions such as 17β-hydroxysteroid dehydrogenase deficiency and 5α-reductase deficiency, the activation of isoenzymes in parallel to the increase of testosterone levels during puberty can result in spontaneous virilisation. The 46,XX DSD category includes the relatively frequent occurrence of patients with androgen excess due to congenital adrenal hyperplasia caused by 21-hydroxylase deficiency and also patients with 46,XX DSD with testis development due to, for example, the presence of sex-determining factor of Y (SRY) on one of the X chromosomes or on other chromosomes. Ovotesticular DSD is characterised by the presence of both testicular and ovarian tissue, with a karyotype that can be 46,XX, 46,XY or mosaic.
Table 1.
Deficiencies of sexual development: DSD nomenclature and hypogonadotropic hypogonadism.
| Sex chromosome DSD | 46,XY DSD | 46,XX DSD | Hypogonadotropic hypogonadism |
|---|---|---|---|
| (A) 45,X | (A) Disorders of gonadal (testicular) development | (A) Disorders of gonadal (ovarian) development | Congenital |
| Turner syndrome and variants |
|
|
|
| (B) 47,XXY | (B1) Disorders of androgen synthesis | (B1) Androgen excess | Acquired |
| Klinefelter syndrome and variants |
|
|
|
| (B2) Disorders of androgen action | (B2) Fetoplacental | ||
|
|
||
| (C) 45,X/46,XY, MGD | (C) Other | (C) Other | |
|
|
||
| (D) 46,XX/46,XY | |||
| chimeric, ovotesticular DSD |
AR, androgen receptor; CAIS, complete androgen insensitivity syndrome; CGD, complete gonadal dysgenesis; MGD, mixed gonadal dysgenesis; PAIS, partial androgen insensitivity syndrome; PGD, partial gonadal dysgenesis.
Individuals with DSD may be identified in the neonatal period because of atypical external genitalia, lack of neonatal minipuberty or may be diagnosed in puberty/adolescence when pubertal development is delayed, incomplete, absent or atypical (16). Girls may seek medical attention because of the absence of breast development and/or primary amenorrhoea or with increasing virilisation during puberty. Boys may present with short height, slow or non-progressing pubertal development and/or gynaecomastia. There is an increased risk of malignancy in patients with 46,XY DSD, especially if gonads are intra-abdominal, and patients may present with gonadal tumour-secreting steroid hormones, complicating the diagnosis and may even be the presenting symptom (17). In some cases, early gonadectomy may have been performed due to a high malignancy risk of the presence of a Y chromosome together with under-masculinisation (18).
Finally, deficiencies of sexual development requiring treatment also include patients with impaired gonadal function due to gonadotropin deficiency and congenital hypogonadotropic hypogonadism (CHH), which can be divided into normosmic CHH and Kallmann syndrome (CHH and deficient sense of smell). CHH can also be a part of multiple pituitary hormone deficiencies or part of a syndrome such as CHARGE or Waardenburg syndrome. Phenotypes associated with CHH apparently shortly after birth or during childhood are midline defects such as cleft lip and/or palate, dental anomaly, anomaly of digits, congenital hearing impairment, anosmia/hyposmia, microphallus/cryptorchisism and family history of CHH.
The role of hormonal replacement therapy during puberty
It is not uncommon that testosterone or oestrogen replacement therapy (ERT) is required in adolescents with DSD or pituitary deficiency (see Table 2) (21). The overall aim of the therapy is to ensure that secondary sexual characteristics and maturation of the body and the brain occur at a similar pace to peers. This may also be the case even if puberty has started spontaneously but ceases to progress appropriately.
Table 2.
Spontaneous puberty, sex hormone replacement and possibility of fertility in subjects with DSD conditions and hypogonadotropic hypogonadism.
| Spontaneous puberty | Sex hormone replacement | Fertility options | References | |
|---|---|---|---|---|
| Dysgenetic gonads | ||||
| CGD, 46,XX and XY females | No | Yes | If uterus present, oocyte donation | 22, 23, 24 |
| PGD:males, 46,XY | 57–85% | 17–25% | Azoospermia microTESE and ICSI; oigozoospermia ICSI | |
| PGD: females, 46,XX | Not reported | Yes | If uterus present, oocyte donation | |
| MGD: males | EMS < 5, 63%EMS > 5, all | EMS < 5, allEMS >5, 25% | Azoospermic in 80%; microscopic focal spermatogenesis in 25%; ICSI; sperm donation |
22, 25, 26 |
| MGD: females | Possible | Yes | Uterus present, ART | |
| Turner syndrome | In 21–50% if mosaicism; menarche 15–30% | Yes Almost all |
Uterus present, ART If mosaicism, spontaneous pregnancy possible, 7% |
27, 28 |
| Klinefelter syndrome | Yes, normal start of puberty. Regression of testis | Yes, usually late in puberty or after puberty | Azoospermia, micro_TESE sometimes possible due to areas of preserved spermatogenesis | 29, 30, 31, 32 |
| 46,XX males | Yes; 13% with cryptorchidism or hypospadias | May need hormone replacement; >90% elevated FSH and LH1/3 have low testosterone | Sperm donation | 33 |
| OvoTestis | Possible if gonadal tissue is present | Depends on the presence of gonadal tissue | Uterus in 31% spontaneous pregnancy described if ovarian tissue and 46,XX | 26, 34 |
| Males | ||||
| Females | ||||
| 46,XY DSD | ||||
| Steroid production | If gonads retained | Impaired spermatogenesis, TESE, ICSI ART | 35, 36, 37 | |
| 5αR | Yes | Common | ||
| 17β-HSD | Yes | Common | ||
| CAIS | Yes | Yes | No | 38 |
| PAIS: males | EMS <5, 67%EMS >5, all | Yes, 83% | Azoo-oligozoospermia | |
| PAIS: females | Yes, virilising | Yes | No | |
| Hypopituitarism | ||||
| Isolated | Seldom, spontaneous puberty may be late | Yes FSH/LH |
Possible with FSH/LH treatment | See Table 5 |
| MPHD | Variable | Variable | Possible | |
| CDGP | Yes | No Initially at times |
Yes | |
| MRKH | Yes | no | Uterus transplantation, research basis | 39 |
CDGP, constitutional delay of growth and puberty; CGD, complete gonadal dysgenesis; EMS, external masculinisation score 1–10 (1 lowest, 10 highest) (40); MGD, mixed gonadal dysgenesis; MPHD, multiple pituitary hormone deficiency; MRKH, Mayer–Rockytansky–Kuster–Hauser syndrome, PGD, partial gonadal dysgenesis.
Regardless of the cause of hypogonadism, appropriate oestrogen or testosterone replacement will be required for puberty induction and puberty progression. This should mimic the physiological process inducing the secondary sexual characteristics, growth plate maturation and psychological functioning. In those with no identified cause of hypogonadism, the lack of pubertal features at the age of 13 years in girls and 14 years in boys should prompt investigations and may indicate the need for pharmacological puberty induction. In those with an identified cause of hypogonadism, puberty should be induced over a period of 2–4 years until a satisfactory outcome, usually when an adult dose has been reached. Sex steroid hormones are imperative for somatic and psychological wellbeing, also in the longer perspective, due to their effects on bone mineral density (BMD), haematopoiesis and cardiovascular, sexual and metabolic health.
Aromatase inhibitors in puberty
Aromatase inhibitors inhibit the formation of oestrogens from androgens, leading to oestrogen depletion. Given that oestrogens mediate the growth spurt in both sexes and contribute to epiphyseal closure, it was hypothesised that oestrogen depletion would improve adult height in boys (41). Aromatase inhibitors have been given to prepubertal or pubertal boys for 1–2 years, to increase predicted adult height (42, 43). In girls, aromatase inhibitors have been used in combination with GnRH analogues attempting to increase adult height in early maturing girls (44) and in the experimental treatment of congenital adrenal hyperplasia (45). Aromatase inhibitors have been used in gynaecomastia, McCune–Albright syndrome, aromatase overexpression in patients with large calcifying Sertoli cell tumours and familial male-limited precocious puberty (46). The most common side effect is the loss of BMD.
In boys, aromatase inhibitors given prior to the onset of puberty do not seem to change the timing (47). In contrast, the situation is different once the central restraint of gonadotropin secretion is diminished at the onset of puberty when puberty is mainly controlled by the sex steroid-mediated feedback from the gonads. In both sexes, this feedback is mediated by oestrogen, and consequently, oestrogen depletion in boys at that time leads to increased gonadotropin and testosterone levels.
Biochemical hormonal testing
The assessment of the HPG axis includes the quantification of serum concentrations of gonadotropins, FSH and LH, as well as gonadal sex steroids oestradiol (E2) and testosterone (T). In addition, gonadal peptides like inhibin B, AMH (48) and insulin-like factor 3 (49) may add useful information about the gonadal Sertoli and Leydig cell function, respectively. AMH is also used as a marker of ovarian reserve.
When puberty starts, the secretion of FSH and LH is very low and increases with pulsatility only at night time in peripubertal children. Ultrasensitive FSH and LH assays (i.e. detection limit < 0.1 IU/L) are required to separate prepubertal from pubertal children based on their serum concentrations (48). A basal LH concentration above 0.3 IU/L is considered evidence of pituitary activation, whereas a lower or even undetectable LH concentration does not exclude pubertal onset. Therefore, a short intravenous GnRH test may be needed, with a stimulated LH above 5 IU/L which is considered a pubertal response. Importantly, peak LH may reach values of almost 10 IU/L in 1–3 years old prepubertal children (50). The GnRH test is often used to diagnose central precocious puberty but has limited additional value in delayed puberty (51). Reference ranges covering all ages including the pubertal transition period are available for FSH and LH using ultrasensitive assays (52).
The quantification of E2 and T at low concentrations found in pre- and peripubertal boys and girls requires specific and sensitive assays. E2 quantification is needed in the evaluation of girls with premature thelarche, precocious and delayed puberty, boys and men with gynaecomastia, patients with suspected hypogonadism, as well as monitoring of hypogonadal girls during puberty induction with oestrogen. High accuracy, specificity and precision, as well as standardisation of E2 assays, are mandatory according to the Endocrine Society (53, 54), as well as to European guidelines (55). Some diurnal variation in E2 has been shown using an ultrasensitive immunoassay (56). Sensitive mass spectrometry-based methods, such as the liquid chromatography-tandem mass spectrometry (LC-MS/MS) methods, are now accepted as state-of-the-art methods for the quantitative analysis of T and E2 (57). Reference ranges for E2 and T determined by LC-MS/MS are available (58, 59).
Puberty induction general issues to consider
Psychological function and social development
Puberty is associated with numerous physical, psychological and social changes. Recent functional MRI studies on brain development in adolescence elucidated the impact of steroids on neuropsychological maturation (60, 61). A better understanding of sex steroid action on the changes in cognitive, emotional and social functioning during the transition from childhood to adulthood has raised awareness that atypical pubertal development is not only harmful to physical maturation and health in general but also creates a delay in intellectual, emotional and social capacities. Therefore, the management of puberty and puberty induction should include the monitoring of psychosocial functioning.
Informing the child
The child needs to be informed about the medical condition in a continuous and age-appropriate process (62). By discussing how to inform the child already at an early stage, parents will be able to prepare themselves. Parents need to understand and be prepared for the physical, psychological and social changes during adolescence in order to become aware of the challenges their child will come across. Advisory booklets or informative websites on how to prepare and support their child will empower parents and make it easier for them to communicate with their child about medical condition and the challenges they may encounter (see websites (https://www.dsdfamilies.org/charity; https://www.dsdteens.org/; http://www.accordalliance.org/dsdguidelines/parents.pdf; https://www.fairview.org/patient-education/40119; https://www.connecticutchildrens.org/wp-content/uploads/2017/02/DSD_Resources.pdf)). In particular, for adolescents with DSD, timely information and sex education are important. Psychological counselling, dedicated educational websites and contacts with other patients may be helpful.
All DSD conditions per se may intensify parental focus on their child’s behaviour, particularly with respect to assigned gender at birth in children with DSD. Some parents seek reinforcement of the decisions made and may start worrying when they experience insufficient reassurance. In psychological counselling, information on psychological aspects of child development including play behaviour, development of gender preferences and development of knowledge on sex and gender will be helpful to take away the turmoil.
Importantly, gender role behaviour, interests and preferences are mostly neither possible nor desirable to change (63, 64, 65) and cannot be used to predict gender identity (66). Acceptance of behaviour is needed to enable the child to develop a positive self-esteem that is necessary to cope with the challenges that children with DSD will meet in puberty and adulthood. Children need support from their parents. Parents who feel shame, shyness or inability to cope or to protect will need support and reinforcement of their parental competency.
Gender development
Prenatal androgen exposure and action influence future gender role behaviour and interests. This has been shown in studies of patients with 46,XX CAH and in children with 46,XY DSD raised male or female with different levels of prenatal androgen exposure (67, 68, 69, 70). The influence of prenatal androgens on gender identity is less clear. It should be emphasised that gender role behaviour does not imply gender identity; follow-up studies indicate that, as adults, the vast majority of individuals with a DSD developed a gender identity in agreement with the gender assigned at birth (71, 72, 73, 74, 75, 76, 77, 78). Many individuals who changed gender may not only have been prenatally exposed to androgens but also in the first 6 months after birth (during the mini-puberty) and during puberty (73, 79, 80). Patients living in countries outside Europe and Northern America often have limited possibilities for medical evaluation and treatment, leading to a delay in clinical management (76, 81, 82). Reports on the psychosocial implications of this delay in clinical management indicate that patients face social stigmatisation (83, 84, 85). The assessment of gender development should be conducted before the hormonal induction of puberty.
Transition
For all patients with chronic disorders, the transfer to adult care represents a major change. In pediatric care, young people generally have a long-standing relationship and are comfortable with the team who is familiar with their illness and their personal and social history; however, consultations do not always meet the needs of the adolescents and their necessary empowerment (86, 87, 88). International recommendations have been developed to support a successful transition from paediatric to adult care (89, 90, 91). However, the majority of the recommendations focus on paediatric preparation and advocate for ongoing information about transition throughout the care pathway, the inclusion of the family, consideration of developmental aspects, patient education, coordination with primary care and adaptation of the timing of transfer to the individual’s situation. A prepared, coordinated transition has a positive impact on patients’ health, experience of care and use of care (92).
A transition program can be tailored to the individual’s needs and future clinical management and is preferably drafted in collaboration with the medical specialist who will become the patient’s doctor in adulthood (89, 90, 91, 93). The program should test the adolescent’s knowledge and skills, encourage the adolescent to discuss daily life challenges with the medical team and/or parents and challenge the adolescent to set goals for independence (94).
The transition of young adults with gonadal dysfunction, whatever the reason, illustrates the importance of a multidisciplinary approach, to discuss both medical issues (about hormonal replacement therapy, long-term consequences in terms of sexuality, fertility) and social and psychological issues that arise in the context of these chronic conditions (89, 95).
Methods
Guideline working group
These guidelines were developed on behalf of the European Reference Network on Rare Endocrine Conditions (Endo-ERN). The following societies were represented: the European Society of Endocrinology (ESE) (Dekkers), the European Society for Paediatric Endocrinology (ESPE) (Vd Akker and Gawlik), CH/SOD association (Vitali) and the Turner Syndrome Support Society (Smyth). The working group had one in-person meeting (December 2019) and one virtual meeting (June 2020). All participants completed conflict of interest forms.
A draft of the guideline was reviewed by five experts in the field (see ‘Acknowledgments’ section) and has been distributed to all Endo-ERN members for comments. In addition, the following societies and networks were asked to review the guidelines: ESPE, ESE and the European Academy of Andrology. Furthermore, patient representatives in the Endo-ERN were involved in the whole process.
Target group and aims
This guideline was developed for health care providers who may see patients with DSD or hypogonadotropic hypogonadism (HH) in need of treatment to induce or sustain puberty. In general, these patients should preferably be treated by a multidisciplinary team of experts in an Endo-ERN Reference Centre and their affiliated regional healthcare providers. General practitioners and patients might also find the guideline useful. Additionally, the guideline can serve as a source document for the preparation of patient information leaflets and educational materials.
In clinical practice, when making treatment decisions, both the recommendations and the clinical judgement of the treating physician should be taken into account in a patient-centred, shared-decision process. Recommendations are not meant to replace clinical acumen. Certain recommendations may not be feasible in individual countries and must be interpreted in the context of available resources.
Summary of methods used for guideline development
This guideline used Grading of Recommendations, Assessment, Development and Evaluation (GRADE) as a methodological base (96). The first step was to define clinical questions; the second step was to perform a systematic literature search. After including all relevant articles for each clinical question, we rated the quality of the evidence and estimated an average effect for specific outcomes if possible. The quality of the evidence behind the recommendations is classified as very low (+OOO), low (++OO), moderate (+++O) or strong (++++) per outcome (97). Formal evidence syntheses were performed and graded only for recommendations addressing our initial clinical questions. Not all recommendations were formally graded.
For the recommendations, we considered the quality of the evidence, the balance of desirable and undesirable outcomes and individual values and preferences (patient preferences, goals for health, costs, management inconvenience, feasibility of implementation, etc.) (98). The recommendations are worded as ‘recommend’ (strong recommendation) or ‘suggest’ (weak recommendation). The meaning of a strong recommendation is that all reasonably informed persons (clinicians, politicians and patients) would want the management in accordance with the recommendation. For a weak recommendation, most persons would still act in accordance with the guideline but a substantial number would not (99). Importantly, one cannot abstain from making recommendations when there is scarce evidence, as treatment decisions will have to be made anyway. Recommendations are accompanied by an explanation of why the recommendation was made.
Clinical questions, eligibility criteria and endpoint definition
At the start of this guideline process, six clinical questions were formulated, for which we performed a systematic literature search and review (See Supplementary Appendix 1 for details, see section on supplementary materials given at the end of this article).
Question I: What is the optimal treatment to induce or sustain puberty in males with partial gonadal dysgenesis?
Question II: What is the optimal treatment to induce or sustain puberty in males with HH?
Question III: What is the optimal treatment to induce or sustain puberty in females with partial gonadal dysgenesis?
Question IV: What is the optimal treatment to induce or sustain puberty in females with HH?
Question V: What is the optimal treatment to induce or sustain puberty in patients with CAIS?
Question VI: What is the optimal treatment to induce or sustain puberty in patients with PAIS?
Description of search and selection of literature
We performed a literature search using five electronic medical databases in February 2020 (PubMed, Embase, Web of Science, COCHRANE and Emcare). No language restrictions were imposed. Due to similarities in treatments of interest and clinical outcomes, one overarching search strategy was used for all six clinical questions. References of included articles were checked to identify potentially relevant articles. Only articles studying a minimum of ten patients (to avoid including small studies with a high risk of selection bias), which directly compared at least two treatments to induce or sustain puberty (or one treatment vs placebo), were eligible for inclusion (Fig. 1).
Figure 1.
Flowchart of included papers.
In total, we identified 6212 papers with our search strategy. For questions I, V and VI (puberty treatment in male patients with partial gonadal dysgenesis, CAIS and partial androgen insensitivity syndrome), after a formal search and assessment of potentially relevant papers, no papers were found. For question II (puberty treatment in males with HH, we included four papers. For question III (puberty treatment in females with partial gonadal dysgenesis), we included 22 papers, of which 1 was also included for question IV. For question IV (puberty treatment in females with HH), we included one paper, which was also included for question III. A flow diagram of study inclusion is presented in Fig. 1.
Summary and interpretation of the evidence from the systematic literature review
Clinical question I What is the optimal treatment to induce or sustain puberty in boys with partial gonadal dysgenesis?
We found no studies on treatment to induce or sustain puberty in male patients with partial gonadal dysgenesis.
Clinical question II What is the optimal treatment to induce or sustain puberty in boys with hypogonadotropic hypogonadism?
We included four studies on treatment to induce or sustain puberty in males with HH (100, 101, 102, 103), see Supplementary Appendix 2 Table 1 for the GRADE table and Supplementary Appendix 2 Table 2 for details of included studies and individual study outcomes. The four studies compared the use of i.m. testosterone vs no treatment (100), monthly i.m. testosterone vs weekly i.m. hCG (101), high- vs low-dose GnRH (102) and GnRH vs hCG (103). The following outcomes were investigated: Tanner stage, penile length, testicular volume, spermatogenesis, BMD, height, weight and BMI. Due to the small number of included studies, and the large variation in treatments used, no firm conclusion regarding optimal treatment to induce or sustain puberty in male HH can be drawn.
Clinical question III What is the optimal treatment to induce or sustain puberty in female patients with partial gonadal dysgenesis?
We included 22 studies on treatment to induce or sustain puberty in female patients with partial gonadal dysgenesis, of which 1 was also included for clinical question IV; see Supplementary Appendix 3 Table 1 for the GRADE evidence table and Supplementary Appendix 3 Table 2 for study details including individual study outcomes. There were 10 randomised trials, 1 non-randomised trial and 11 cohort studies. In total, the studies included 1472 patients (there may be partial overlap in some study populations). Various oestrogen treatment regimens were compared: oestrogen vs no oestrogen, early (age 12–14 years) vs late (age 14–17 years) start of oestrogen, individualised vs fixed dose, oral vs transdermal administration and high vs low oestrogen dose.
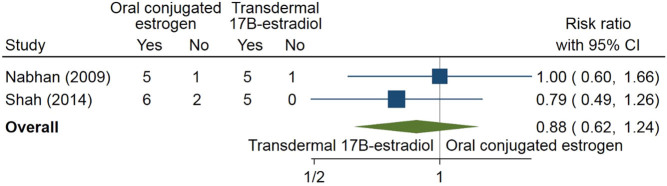
Eight different outcomes were studied: Tanner stage, menarche, uterine size, BMD, height, weight, BMI and liver function. In only four instances at least two studies describe the same outcome (e.g. height) presented in the same way (e.g. in cm) for the same comparison. In Fig. 2, the number of patients to reach Tanner stage B3 during the study period for oral conjugated oestrogen vs transdermal 17β oestradiol was meta-analysed for two studies (104, 105); no firm evidence for superiority, defined as patients reaching Tanner stage B3 or not, of one of the treatment modalities was found.
Figure 2.
Oral-conjugated oestrogen vs transdermal 17β oestradiol for reaching Tanner stage B3. Yes denotes having reached Tanner stage 3; no denotes having not reached Tanner stage 3: the risk ratio expresses the probability ratio for reaching Tanner stage B3.
In Fig. 3, final height was compared, in a placebo-controlled study, between patients with Turner treated with oestrogen (ethinyl E2) vs patients, not on oestrogen in a meta-analysis using two studies (one split into patients on additionally a high or low dose of growth hormone) (106, 107). Patients on oestrogen had a lower final height than patients on placebo (difference −2.9 cm; 95% CI: −4.9 to −0.8 cm); it should be noted that these patients were treated with growth hormone.
Figure 3.
Oestrogen as add-on therapy for final height.
In Fig. 4, final height was compared between early (age 10–12 years) and late (from age 12 years) start of oestrogen treatment (EE2 or E2) in a meta-analysis using six studies (108, 109, 110, 111, 112, 113). Early start of treatment did not result in a clearly lower final height than the late start of treatment (difference −1.0 cm; 95% CI: −4.0 to 1.9 cm).
Figure 4.
Early (age 10–12 years) and late (from age 12 years) start of oestrogen for final height.
In Fig. 5, BMI was compared between early (age 10–12 years) and late (from age 12 years) start of oestrogen treatment in a meta-analysis using two studies (108, 111). The early start of treatment resulted in a lower, though not significantly, BMI than the late start of treatment (difference −0.9 points; 95% CI: −2.7 points to 0.9 points).
Figure 5.
Early (age 10–12 years) and late (from age 12 years) start of oestrogen for BMI.
For all formal comparisons, the number of studies was low, with broad CIs and genuine uncertainty regarding the effect estimates.
Clinical question IV What is the optimal treatment to induce or sustain puberty in female patients with hypogonadotropic hypogonadism?
We included 1 paper with 20 females with HH and treatment to induce or sustain puberty (105), which was also included for clinical question III and which did not present separate data for both patient categories. The GRADE evidence table is shown in Supplementary Appendix 4 Table 1, including details of the study and its outcomes. Patients used either oral 17β oestradiol, transdermal 17β oestradiol or oral-conjugated equine oestrogen. Patients using oral or transdermal 17β oestradiol more often reached Tanner breast stage 3 and had lower height and weight at the end of the study period than patients using oral-conjugated equine oestrogen. The number of patients is too small to draw firm conclusions on preferred treatment.
Clinical question V What is the optimal treatment to induce or sustain puberty in women with complete androgen insensitivity syndrome?
We found no studies on treatment to induce or sustain puberty in patients with CAIS.
Clinical question VI What is the optimal treatment to induce or sustain puberty in patients with partial androgen insensitivity syndrome?
We found no studies on treatment to induce or sustain puberty in patients with partial androgen insensitivity syndrome.
Recommendations and rationales
General
R 1.1We recommend that children and adolescents who need pubertal induction or sex hormone replacement to sustain puberty should be treated by a multidisciplinary team including a paediatric endocrinologist, adult endocrinologist, psychologist, urologist, gynaecologist, geneticist, surgeon and nurse specialist depending on the situation and specific requirements.
Rationale
Puberty and sexual maturation are sensitive and private matters and may be especially sensitive for individuals with a DSD. When the diagnosis is known since the neonatal period, the child and the parents can be informed and prepared well in advance of the time of puberty. If the diagnosis is made due to delayed, partial or absent pubertal development, the situation is more complex. In all cases, it is preferred that a specialised team with a multidisciplinary approach is involved (62).
The medical diagnosis requires patients and parents to cope with uncertainties regarding health and future development and to understand a complicated medical condition often associated with societal beliefs about gender and ‘normalcy’. As DSD conditions are rare, the expertise of medical professionals, also regarding many psychosocial aspects related to DSD, is essential. Discussing psychosocial aspects of DSD facilitates the acceptance of the condition and empowers parents to support their child whenever needed.
Puberty induction in girls
Introduction
Accumulating data show that initiation of puberty at an age comparable with peers is essential for normal physiologic development, including secondary sex characteristics, bone, muscle, and social, sexual and psychologic development. Delayed pubertal induction, which is often the case in patients without pubertal development, may have longstanding consequences. An orderly and timely induction of puberty with the 17β-oestradiol in some form is the appropriate approach. The use of combined oral contraceptives to induce puberty is not recommended. However, there are currently still many uncertainties regarding the optimal pubertal induction regimen. Most of the recommendations below regarding oestrogen dosing are based on clinical experience and data in girls with Turner syndrome. Literature sources are scant regarding other types of female hypogonadism. Clinical experience in combination with the evidence from Turner syndrome data can, to some extent, be extrapolated to these groups.
In whom to consider puberty induction?
R. 2.1We recommend that an expert evaluation should be performed on any girl who does not show any sign of puberty by the age of 13 years, any girl who, by some definition, has delayed puberty (puberty s.d. score <−2) and all girls with a known condition/diagnosis that poses a risk of hypogonadism from the age of 8 years.
Rationale
Start of puberty in girls is typically defined by two signs of oestrogen action, the start of breast development, Tanner scale B2 and increased growth velocity. On average, this takes place at 10 years of age, the lower and upper limits of the normal range being after 8 years and before 13 years, respectively. Curve nomogram for pubertal progression exists (114):
Evaluation in girls with pubertal delay includes a detailed medical and family history. Laboratory investigations of gonadotropin levels and sex steroids will aid in differentiating between primary and HH. Primary gonadal failure such as complete or partial gonadal dysgenesis is confirmed by increased FSH and LH. Ultrasound or MRI should be performed to identify a uterus or Müllerian structures; however, a small uterus before oestrogen exposure may be missed by imagery. Bone age determination may be informative.
Karyotype or chromosomal array should be considered in all girls with delayed puberty and/or short stature to detect sex chromosomal abnormalities. The most common cause of primary ovarian failure is Turner syndrome due to 45,X karyotype or 45,X/46,XX or 45,X/46,XY mosaicism. These girls may have additional symptoms associated to the syndrome including cardiac anomalies. With the presence of Y chromosome material, there may be some degree of prenatal virilisation. Girls with complete gonadal dysgenesis can have 46,XY karyotype and a uterus (Swyer syndrome). Girls with ovo-testis may have 46,XX, 46,XY or mosaicism. Puberty may start spontaneously but come to a halt or be accompanied by virilisation due to the testicular component of the ovo-testis. The presence of Y material and under-masculinisation increases the risk of germ cell tumours.
The absence of menarche despite otherwise normal pubertal development should prompt a pelvic ultrasound to identify the uterus or Müllerian structures and to confirm or exclude Mayer–Rokitansky–Küster–Hauser syndrome or the Mullerian aplasia, renal anomalies, cervicothoracic somite dysplasia association (39).
Girls with 46,XY karyotype and deficiencies in testosterone or dihydrotestosterone synthesis virilise during puberty if their gonads have not been removed before puberty due to the activation of isoenzymes (21). They require a prompt diagnostic workup and psychological evaluation to discuss clinical management.
Girls with a known diagnosis predisposing or clearly resulting in primary gonadal failure or hypogonadal hypogonadism should be seen from the age of 8 years or earlier. This follow-up allows the DSD team to identify the individuals’ and family’s needs for clinical and psychological support throughout puberty and adolescence. This also allows time for the team to explain the diagnosis and prepare and inform the patient and parents about treatment decisions.
Girls with HH with low FSH, LH and anosmia such as in Kallmann syndrome or without anosmia require investigations to differentiate from constitutional delay or systemic diseases causing HPG axis suppression (115, 116).
R. 2.2We recommend to individualise but consider puberty induction at the age of 11 in most girls with gonadal dysgenesis or other syndromes with the absence of spontaneous puberty, who do not show signs of puberty and have confirmed hypogonadism after testing (++OO).
Rationale
Regardless of the cause, in female hypogonadism with insufficient pubertal progression, appropriate oestrogen replacement therapy (ERT) mimicking the physiology in timing and pace is the mainstay of puberty induction. The aim is to maintain the signs of puberty and obtain long-term effects by normalising uterine growth, attaining peak bone mass, influencing normal development of the brain and influencing metabolism, as well as sexual and psychological functioning. In the present context, we discuss conditions such as gonadal dysgenesis, HH (also transient forms), Turner syndrome, Prader Willi syndrome and others.
R 2.3We recommend that spontaneous puberty and treatable causes of hypogonadism should be ruled out before starting puberty induction in girls.
Rationale
The reasons for the lack of expected pubertal progression can be transient and/or need causal treatment. Differential diagnosis of female delayed puberty should take into account the constitutional delay of growth and puberty (CDGP) and maturational delay in the HPG axis secondary to an underlying non-reproductive condition. CDGP is responsible for a third of all cases of pubertal delay in teenage girls (117) and is mainly considered as part of the spectrum of normal puberty. To diagnose CDGP, congenital HH (CHH) needs to be ruled out. However, in some cases, the initial work-up fails to provide an unequivocal diagnosis. Current studies show that CDGP and CHH have distinct genetic profiles which may aid in discriminating between these conditions (118). Other causes of hypogonadism, as well as other pituitary deficiencies, should be treated but should not delay puberty induction.
R. 2.4We recommend to start individualised puberty induction in girls at the age of 11 years in cases where there are no signs of pubertal development and a diagnosis of hypogonadism is confirmed, or at the age of 13 years if the constitutional delay is suspected. (+OOO)
Rationale
ERT should be initiated around 11 years of age in cases with known hypogonadism which is slightly later than average. However, starting puberty induction at a younger age resulted in a lower final height (Fig. 4) and a lower BMI (Fig. 5) than starting puberty induction at a later age. The main goal of all therapeutic protocols for puberty induction in hypogonadism should be to achieve an endocrine milieu similar to natural processes with gradual increase of the oestrogen dose to mimic physiological pubertal development and obtain an appropriate adult phenotype with respect to uterine (when present), breast and bone development, body composition, as well as adult stature. In order to imitate the natural dynamic of puberty advancement, an incremental increase in the dose is recommended over a period of 2–3 years until satisfactory physical effect, usually an adult dose, has been reached (119, 120).
The age for the start of puberty treatment should be individualised. In individuals with tall stature and a tall final height prognosis, an earlier start of puberty treatment may be considered, for example in girls with 46,XY DSD. It may have to be delayed if CDGP is suspected and the results of a diagnostic work-up have to be awaited. In case of CDGP, treatment may be initiated as a short course of 3–6 months using low doses of oestradiol. Doses can be increased in order to mimic the normal course of puberty, but continuous monitoring for spontaneous resumption of progress and gonadotropin secretion is required.
The age of puberty induction also affects other aspects of life. Historically, much-delayed induction of puberty to hypothetically increase final height had long-lasting negative effects on sexual life in young adult women with Turner syndrome (TS) (121, 122).
R 2.5When premature ovarian insufficiency is seen, that is if appropriate progression fails, we recommend to start sex hormone replacement treatment also in girls who had a normal spontaneous start of puberty.
Rationale
Young women with spontaneous pubertal development and menstruations but markedly elevated gonadotropins need regular follow-up to start ERT before symptoms of premature ovarian insufficiency appear.
A considerable proportion of females with TS have spontaneous thelarche and/or menarche (20–40% show some degree of pubertal development, with menarche in approximately 16–20% and regular menstrual cycles in 6% of cases) (123, 124, 125, 126). A recent meta-analysis showed rates of menarche of 32 and 20% dependent on specific karyotype (127). Nevertheless, 80% or more of girls and women with TS require or will require ERT to initiate, progress or maintain pubertal development (128). As indicated by some observations, FSH level below 10 mIU/mL at 12 years and below 6.7 mIU/mL during mid-childhood (between 6 and 10 years) could be seen as an indicator of spontaneous puberty and the possibility of cyclical menstruation, but typically premature ovarian insufficiency within a few years will ensue (123, 129).
A similar situation may occur for females with mixed gonadal dysgenesis or partial gonadal dysgenesis. In females with ovo-testicular DSD and well-developed ovarian tissue, it often remains functional and menstruations have been reported to occur in 50% of cases (26). In individuals with spontaneous puberty and risk of premature ovarian failure, it is important to follow-up pubertal progression and measure gonadotropins in order to estimate ovarian reserve and assess the need for oestrogen replacement. AMH could be used as an additional laboratory marker. Values of AMH below –2 s.d. (4 pmol/L) predicted failure to enter puberty (130).
Inhibin B measurements have also been used to assess ovarian reserve (130, 131, 132). Undetectable inhibin B levels measured prior to pubertal onset were found in all patients with Turner syndrome and premature ovarian failure (133). However, due to its limited specificity (more than 1/3 of healthy girls have undetectable inhibin B levels during the menstrual cycle (follicular phase)), using inhibin B as a screening test to assess longitudinal ovarian reserve raises concerns (134). AMH appears to be a more reliable marker in this respect.
In case of premature ovarian insufficiency, decision regarding the dosing and the type and route of hormonal replacement therapy should be based on patient’s pubertal stage and the aim to mimic physiology.
Treatment approach and monitoring
R 2.6We recommend to use 17β-oestradiol for puberty induction or to sustain puberty in girls (++OO).
Rationale
Different types of sex hormones are used for ERT (see Table 3 for details). Especially for pubertal induction, bioidentical human oestrogens (oestradiol/17β-oestradiol E2) are the dominant formulations used presently and are preferred to non-bioidentical (e.g. ethinyl E2, conjugated equine oestrogens, dienestrol, and mestranol), synthetic or derived from animal sources. We acknowledge that different formulations may not be available in all countries.
Table 3.
Oestrogen and progesterone preparations that can be used for pubertal induction and replacement therapy. This s not meant to be exhaustive, multiple preparations and brand names are available throughout the world.
| Preparation | Doses available | Starting dose of puberty | Increase approximately every 6 months* to adult dosing | Considerations for use |
|---|---|---|---|---|
| E2: transdermal options TD (some brands examples) | 3–7 µg/day | 25–100 µg/day | See text on applying patches | |
| Menostar | 14 µg | Part of patch twice weekly | Only used for low dosing situations, not fully hypogonadal replacement | The easiest way to give a low dose |
| Vivelle Dot | 25, 37.5, 50, 75, 100 µg | Part of patch twice weekly or 1 patch per month (no patch for 3 weeks)** | 25–100 µg twice weekly | Designed for twice weekly but can be given once per week to increase the dose slower |
| Vivelle Mini | 25, 37.5, 50, 75, 100 µg | Part of patch twice weekly or 1 patch per month (no patch for 3 weeks)** | 25–100 µg twice weekly | Smaller size patch, but not smaller dosing |
| Generic (different brands in different countries; e.g. Oesclim Estradot, Evorel, Systen, Climara, Demestril) | 25, 37.5, 50, 75, 100 µg | Part of patch twice weekly or 1 patch per month (no patch for 3 weeks)** | 25–100 µg twice weekly | Estradot: too small to properly cut into low doses and not stable in elevated temperature |
| Estraderm | 25, 50, 100 µg | Part of patch twice weekly or 1 patch per month (no patch for 3 weeks)** | 25–100 µg twice weekly | Reservoir form cannot be used to initiate puberty |
| Estraderm MX | ||||
| Divigel 0.1% | 0.5 and 1.0 mg E2/sachet | Too potent for pubertal initiation | 1–2 sachets daily | Cannot use to initiate puberty |
| Estragel 0.06% | 0.75 mg E2/pump | Too potent for pubertal initiation | 1–3 pumps daily | Cannot use to initiate puberty |
| E2: oral options | 5 µg/kg/day | |||
| 17β-oestradiol (e.g.: Estrace, Cetura; Zumenon, Ormone, Estrofem mite, Estrofem) | 0.5, 1, 2, 4 mg | Part of a pill daily*** | 1–4 mg/day | The cheapest option, brands vary by country |
| Oestradiol valerate (e.g. Climaval, Progynova) | ||||
| Ethinyloestradiol (EE2) | 2 µg/day | 10–20 µg/day | Not available in many countries | |
| Premarin (CEE) | 0.3, 0.625, 0.9, 1.25 mg | Part of pill daily | 0.625–1.25 mg/day | Not available in many countries |
| Depot options | ||||
| Depot E2 (cypionate) | 5 mg/mL | 0.2 mg/mL | 2 mg/mL | Not available in Europe |
| Adding Gestagen options | Not needed to initiate puberty | Add once bleeding occurs or after 2 years | ||
| Medroxyprogesterone acetate (e.g. Provera) | 10 mg/tablet | Give with E2, or alone for 10 days/cycle | ||
| Dydrogesterone (Duphaston) | 10 mg/tablet | Give with E2, or alone for 10 days/cycle | ||
| Micronised progesterone (e.g. Prometrium, Utrogestan, Progesterone Besins) | 100 and 200 mg /tablet | Give with E2, or alone for 10 days/cycle | Utrogestan: before going to bed, lactose-free, indication: galactosemia | |
| Progesterone (e.g. Luttagen, Luteina) | ||||
| Jaydess, Kyleena, Mirena | Intrauterine device | Give with E2 | ||
| Combined E2/Gestagen sequential patch | Do not use to initiate puberty | |||
| Climara Pro | E20.045 mg/levonorgestrel 0.015 mg/24 h | 1 patch weekly | ||
| Combipatch | E20.045 mg/norethidrone 0.14 or 0.25 mg/24 h | 1 patch weekly | ||
| Evo-Sequi | E250 µg/norethisterone acetate 170 µg/24 h | 1 patch twice weekly | ||
| Systen Sequi | ||||
| Combined E2/Gestagen sequential pills | Do not use to initiate puberty | |||
| Trisequens | E22 mg/norethisterone acetate 1 mg | 1 pill/day | ||
| Divina plus | Oestradiol valerate 2 mg/medroxyprogesterone acetate 10 mg | 1 pill/day | ||
| Femoston 1/10 or 2/10 | Tablet 1–14: 1–2 mg E2; Tablet 15–28: 1–2 mgE2+ 10 mg dydrogesterone |
1 tablet/day | ||
| Femoston Continu | All tablets: 1 mg E2/5 mg dydrogesterone | 1 tablet/day | ||
| Oral contraceptive pills | Ethinyl estradiol and progestins | Do not use it to initiate puberty | ||
| Loestrin (norethindrone) | Less progestational, less androgenic, low estrogenic | |||
| Lo-ovral (norgestrel) | More progestational, intermediate androgenic, low estrogenic | |||
| Orthotricylcen (norgestimate) | Less androgenic but progestational and more estrogenic | |||
*Detailed comments are in the text; **To avoid cutting (in daily practice, we cut the patches and inform our patients how to cut them; however, there is no manual in the product’s label); ***The preparation with the appropriate dose should be prepared by a pharmacist.
The optimal type, route of administration and dose of E2 used for female puberty initiation are not well established. In line, no clear advantage was found for any type of hormone treatment for puberty induction or to sustain puberty in girls. Patients using 17β-oestradiol had a higher chance to reach Tanner stage B3 during the study period (Fig. 2) than those with conjugated oestrogen, although the difference did not reach statistical significance.
Oestrogens can be used orally or transdermally for puberty induction. Their use is complicated by the lack of oestrogen formulations dedicated to younger patients. Thus, paediatricians have to deal with oestrogen formulations aimed for use by adult women (off-label). The possibility to split a transdermal patch, and thereby split the dose, facilitates mimicking the spontaneous increase in concentration, as well as the diurnal pattern of serum oestradiol in early puberty (135, 136).
Compared with oral E2, transdermal forms resulted in oestradiol, estrone and bioestrogen concentrations closer to normal in the high-dose transdermal group (137). The normalisation of gonadotropins was comparable after oral and transdermal oestrogen (138) but observed only after high-dose transdermal treatment (137). Data regarding the increase in the uterine size during oestrogen therapy are inconclusive, and only a few studies show that adult uterine volume can be achieved by using oral-conjugated oestrogens or oral contraceptives (139). Higher oral 17β-oestradiol dose for 5 years (2 mg vs 4 mg) in the years immediately after pubertal induction led to more girls with TS achieving a normal uterine size (140). See also Supplementary Appendix 3 Table 1.
The metabolic effects of transdermal and oral routes of oestrogen delivery are similar concerning multiple endpoints (bone mineralisation, body composition, BMI, lipids, glucose, insulin tolerance, protein turnover and lipolysis) (141, 142, 143), although it should be noted that firm evidence is lacking (see Supplementary Appendix 3 Table 1 for details).
Available evidence points towards a liver protective effect of E2 supplementation (144, 145, 146). Results regarding the influence of different routes of oestrogen therapy on IGF1 concentration are inconsistent (137, 142). Bone age advancement, one of the major concerns during oestrogen therapy, was less significant with transdermal oestrogen (147). Moreover, transdermal E2 compared with conjugated oral oestrogens resulted in faster bone accrual (spine) (104).
A randomised trial comparing transdermal and oral 17β-oestradiol and oral-conjugated oestrogen therapy in adolescents with ovarian failure did not show differences in fibrinogen and antithrombin activity, glucose and insulin, liver enzymes activity, lipids concentration, plasma renin, as well as IGFBP3 and IGF1 levels (105).
In TS, the long-term risk of breast cancer after long-term oral or transdermal oestrogen remains much lower than among control women (148).
R 2.7We recommend a follow-up frequency with a minimum of once every 3–6 months during pubertal induction or sex hormone replacement to sustain puberty in girls.
Rationale
When treatment is started, baseline values should be noted for parameters such as weight, height, Tanner stage, blood pressure, bone age and hormone measurements. The individual response to treatment and pubertal progress should be followed. Physical examination, including weight, height, blood pressure and Tanner stage should be assessed every 3–6 months to ensure the progress of puberty induction for each patient. Detailed history and discussion to monitor the compliance and resolve patient’s doubts regarding side effects of the therapy and its practical aspects (e.g. dosing, storage) (135) should take place during every visit. In case Müllerian structure is present, pelvic ultrasonography before the start of puberty induction, before adding progesterone or at the time of the first breakthrough bleeding is required. In the context of increasing options for fertility treatment, adequate uterine development and regular monitoring of its dimensions are recommended (119). The dosing should be adjusted accordingly (see below).
R 2.8We suggest titrating/adjusting the oestrogen therapy dose based on the appropriate progression of puberty during puberty induction or sex hormone replacement in girls. (+OOO)
Rationale
The main goal of all therapeutic protocols for puberty induction in hypogonadism should be to achieve an endocrine milieu similar to natural processes. For this purpose, a gradual increase over a period of 2–3 years until an adult dose of oestrogen is reached seems mandatory. Researchers used different schemes depending on their experiences, preferable administration route, patient’s age, medicine pharmacodynamics/pharmacokinetics and local availability. If the dose is increased too fast, it may have a negative influence on, for example, breast development or growth (149). However, no studies were found comparing titration or adjustment of hormonal therapy for puberty induction based on puberty progression.
For the initiation of oestrogen treatment with nocturnally administered E2 patches, the starting doses can be as low as 0.05–0.07 µg/kg, to mimic E2 levels during gonadarche. In older girls, when breast development is of high priority, the starting dose can be 0.08–0.12 µg/kg. Serum E2 levels of 17–23 pmol/L were found for doses of 0.05–0.07 µg/kg and E2 levels of 26–39 pmol/L on doses of 0.08–0.12 µg/kg (150). In turn, a 5-year study with oestradiol gel showed that the initial percutaneous dose of 0.1 mg ending at 1.5 mg leads to mean serum oestradiol concentrations increasing from 22.2 pmol/L at baseline to 162.2 pmol/L and mean FSH levels decreased from 77.4 IU/L at baseline to 19.2 IU/L after 5 years (151), which indicates that this dose is too small. Regular monitoring of oestradiol or FSH or LH during hormonal replacement therapy may be useful to guide treatment in addition to the full clinical picture, especially if mass-spectrometry is available for E2 measurements in the low ranges.
Most of the published experiences are from studies on patients with Turner syndrome (104, 126, 136, 151, 152, 153, 154). However, due to differences in puberty induction protocols, comparison between protocols is difficult. Nevertheless, the dynamic of breast development seems similar in most of the studies: stage B2 was reached during the first months and B4 during or after approximately 2–2.5 years (104, 152, 154, 155), a pace that is comparable to the pace of spontaneous puberty (2, 5, 6).
Low-dose oral oestradiol therapy given as a fixed dose (0.2 mg/day during the first year followed by 0.5 mg/day during the second year) is well-tolerated, not interfering with growth, and produces satisfactory pubertal development in patients with TS not inferior to individualised dose (5–15 μg/kg/day during 2 years) (153). A number of different oestrogen dose titration models for female puberty induction have been proposed (119, 156).
R 2.9We recommend that progesterone is added after puberty induction or during sex hormone replacement to sustain puberty in girls after breakthrough bleeding, after at least 2 years of treatment. (+OOO)
Rationale
If a uterus is present, progesterone must be added at some point because of the risk of endometrial cancer associated with long-term unopposed oestrogen (157). Progestins, synthetic progestagens, are most frequently used. Progesterone is the only bioidentical progestagen. Progestagens, similarly to oestrogens, can be used orally, vaginally, transdermally, intranasally or intramuscularly. There is a lack of valuable data concerning the optimal progesterone induction scheme; no studies were found comparing the addition of progesterone after or during puberty induction.
If optimal breast and uterine maturation has been achieved, it is assumed that progesterone should be added at the time of the first breakthrough bleeding or at least 2 years after oestrogen therapy initiation. In most protocols, a 10-day treatment given cyclically is preferred, as no evidence exists for the optimal duration (from min. 5 to max. 14 days). Oral, natural micronised progesterone (100–200 mg daily), oral dydrogesterone (10 mg daily) or medroxyprogesterone acetate (5–10 mg daily) or norethisterone (1 mg daily) and other preparations can be used (156, 158).
R 2.10We recommend to change pubertal induction treatment of oestrogen and progesterone to permanent adult sex hormone replacement therapy at the end of pubertal induction.
Rationale
The 2- to 3-year puberty induction is followed by the regimen of sex hormone therapy required in a young woman. Similar to puberty induction, transdermal or oral E2 should be the first choice; however, other, less recommended, options include EE2, depot E2 or equine-conjugated oestrogens (the last two are available and are used in the United States). Suggested adult dosing for the preparations is presented in Table 3 (119, 120). Decision regarding the optimal adult regimen is based on clinical features (effectiveness, tolerance), E2 levels and economic considerations. The presence of a uterus obliges to include progestogen in the regimen. However, there are no data clearly indicating the optimal route and regimen in female hypogonadism. Progestagens can be given cyclically or continuously, orally as a single or a component of contraceptive pills, transdermally combined with oestradiol and by intrauterine devices.
R 2.11Puberty induction in late-diagnosed patients must be individualised. A faster than normal increase in oestrogen doses can be considered in such cases.
Rationale
There are controversies concerning the optimal model of female puberty induction in patients with a delayed diagnosis or postponed initiation of oestrogen treatment. Late-onset puberty inductions need individualised approach to optimise the overall outcome with respect to patient’s wishes, height, secondary sex characteristics development and psychosocial endpoints.
The model of a faster increase in oestrogen dosing was presented in single studies regarding patients with Turner syndrome. In one such pilot study using a 1-year protocol with transdermal E2 therapy in 14-year-old girls (first 6 months 25 μg/day, thereafter 37.5 μg/day), a progressive increase in the puberty stage was observed; Tanner stage 3 or 4 was reached after 1 year in all and breakthrough bleeding in four of six girls (104). Another study with patients older than 14 years of age and a comparable simple protocol (12.5 μg/24 h for the first 2 months, thereafter 25.0 μg/24 h until breakthrough bleeding) suggested that easy-to-use fixed-dose regimen for late-onset puberty induction allowed for a satisfactory rate of pubertal stage progression and did not influence the growth potential (152).
Intentional delaying pubertal induction did not improve final height (112, 113). However, postponing pubertal induction in girls who are diagnosed particularly late and in whom short stature is a major concern should be discussed with the family (112).
Puberty induction in boys
Introduction
The initiation of puberty at an age comparable with peers is essential for normal physiologic development, including secondary sex characteristics, bone, muscle, and social, sexual and psychological development. In general, constitutional delay of pubertal development is more common among boys than girls, which may contribute to an even further delay of diagnosis and treatment with pubertal induction in boys with gonadal deficiency due to a DSD. In some cases, puberty may start but not progress properly, in which case a hormonal substitution is needed. A timely induction of puberty with testosterone in some form is appropriate. In case of pituitary deficiency with HH, alternative approaches are also possible (see R. 4).
In whom to consider puberty induction?
R. 3.1We recommend expert evaluation of puberty in any boy who has delayed puberty as defined by a puberty s.d. score <−2 or no signs of puberty at the age of 14 years or fails to show adequate progression in puberty.
Rationale
The start of puberty in boys is defined by testicular growth ≥4 mL. The timing of puberty is physiologic if the age at which it occurs is within 2 s.d. of a reference population (puberty nomograms exist for Caucasian boys (159)). Although puberty usually starts before the age of 14 years in boys, its timing is influenced by several factors including ethnicity, genetics and factors such as obesity and nutrition (160).
Expert evaluation of puberty in boys with suspected pubertal delay starts with a detailed history (161) and the initial evaluation aims to differentiate CDGP from other forms of hypogonadism that may be permanent or secondary to other systemic diseases (116, 162, 163). Clinically, the pubertal stage is measured by scoring Tanner stages and testis volume using a Prader orchidometer. Growth velocity, biochemical testing of gonadotropins and sex steroids and radiological evaluation of bone age by X-ray of the left hand give additional information. The family history on the timing of pubertal development is essential in the evaluation process. CDGP is the most common cause of delayed puberty in teenage boys (~63%), with a majority (50–75%) of them having at least one parent with a history of delayed puberty (117, 164). The diagnosis of CDGP can only be made after exclusion of conditions such as CHH or primary gonadal failure that leads to permanent hypogonadism, as well as chronic illnesses, or a tumour that may lead to a variable extent of delayed growth and puberty (115, 116). Depending on age, gonadal failure can be confirmed biochemically by hypergonadotropic hypogonadism. A history of cryptorchidism (especially bilateral), micro-penis, midline defects, hypo or anosmia, deafness or renal anomalies increases the likelihood of CHH. Furthermore, complications such as failed orchidopexies in boys with CHH can produce a complex biochemical picture that needs careful interpretation. CDGP can often be difficult to distinguish from CHH which may also be associated with a positive family history (115, 116). In addition, to a variable degree, CHH may be associated with other clinical features and an expert evaluation should explore this (115, 116). Clinically, CDGP is transient and confirmed by a spontaneous progression of puberty.
Although a karyotype or a chromosomal microarray is not routinely performed in boys with delayed puberty, this should be considered in those boys who have hypergonadotropic hypogonadism and do not have a predisposing condition such as a past history of testicular damage. Approximately 25% of boys with XY DSD have additional congenital malformations (165) and these boys are more likely to have copy number variations (166). Sex chromosome abnormalities, such as Klinefelter syndrome or 45,X/46,XY, may be associated with several distinct clinical features including behavioural problems, cardiovascular problems and increased sitting height percentage. Klinefelter is associated with tall stature and 45,X/46,XY with short stature (167). Thus, boys with delayed puberty and a past history of atypical genitalia, congenital malformations, hypergonadotropic hypogonadism or clinical features of sex chromosome disorders should also have a karyotype or a chromosomal microarray.
R 3.2All boys with a history of a condition that places them at risk of hypogonadism should undergo pubertal assessment from the age of 9 years.
Rationale
Several conditions that are associated with primary hypogonadism may present in early infancy with atypical genitalia. This includes sex chromosome disorders, disorders of gonadal development, disorders of androgen synthesis and lastly, disorders of androgen action. In these boys, a firm aetiological diagnosis of their DSD that is supported by endocrine and molecular genetic tests allows targeted follow-up that is appropriate for that specific diagnosis. In some conditions such as anorchia, in the absence of a specific aetiological diagnosis, the prospect of not undergoing spontaneous puberty is also unequivocally clear. The likelihood of developing hypogonadism is clearer for some conditions than for others, but ensuring that all these boys are followed up as per current recommendations in a standardised manner will ensure improved knowledge in the future (168). Klinefelter syndrome is the most common cause of congenital male hypogonadism, and although these boys typically enter puberty at a normal age, they often have signs of gonadal dysfunction including gynaecomastia, small, firm testes, elevated gonadotropins and relatively low testosterone levels (169, 170). These boys may also, in rare cases, have a past history of atypical genitalia and an ongoing history of learning difficulties or difficulties in social adjustment.
In 45,X/46,XY sex chromosome mosaicism, spontaneous puberty has been reported to occur in 80% but may be less likely in those who present in the neonatal period with atypical genitalia. Over 50% of this ‘early presentation’ group (diagnosed due to genital anomalies and/or short stature) required testosterone supplementation in puberty compared to 15% in the late presentation group (diagnosed in adulthood due to infertility work up) (25). In partial gonadal dysgenesis, a disorder of gonadal development, absent pubertal development has been reported in 10–15% of adolescents (22). In the rest who have spontaneous initiation of puberty, the gonadal function may not be sufficient to sustain pubertal development, and most of the adolescents with partial gonadal dysgenesis demonstrated elevated gonadotropins with only about half having a serum testosterone in the normal range after puberty (22).
In patients with steroidogenic factor 1 ( SF1) deficiency, Leydig cell function can be effective in puberty, even in patients who were severely under-virilised at birth (171). Testicular function may deteriorate over time. Boys with 46,XX testicular DSD who present with atypical genitalia often have primary hypogonadism. On the other hand, men who present to an assisted conception service and are discovered to have 46,XX karyotype rarely have a history of atypical genitalia in infancy but are infertile, due to the absence of the azoospermia factor genes on the Y chromosome (33, 172). Testicular function in boys with ovotesticular DSD can be quite variable and depend on the androgen-producing capacity of the testicular component of the gonads (34).
In conditions that are due to a genetic disorder of androgen synthesis, boys can undergo a variable range of spontaneous virilisation in puberty but may require topical or systemic androgen supplementation. In many patients with DSD, especially partial androgen insensitivity syndrome or Klinefelter syndrome, the affected boy may display additional features such as gynaecomastia, disordered growth or even precocious puberty.
The monitoring of pubertal development includes both clinical measurements and laboratory assessments. Clinically, assessment of growth, Tanner stage and testicular volume with a Prader orchidometer are useful to detect pubertal onset and laboratory assessment includes the assessment of LH, FSH, total testosterone measurements, inhibin B and AMH. In some forms of primary testicular failure, a differential effect of the Leydig cell and Sertoli cell compartments within the testis may make the assessment of testicular volume misleading. In cases of selective Sertoli cell failure and/or lack of germ cells, testicular volume usually remains small although testosterone production may be active. Boys with partial hypogonadism may also show signs of precocious puberty (173, 174).
As a greater number of infants with XY DSD are being raised as boys nowadays (175), it is likely that there will be a greater number of boys with a wide range of partial forms of hypogonadism who approach pubertal age and will need regular support. Thus, to see these boys at around 9 years allows the DSD team to gauge the need for clinical and psychological support as the patient proceeds through adolescence. Starting pubertal monitoring at a relatively early age provides the physician time to specify and explain the diagnosis and to make shared decisions with patient and parents on therapeutic options.
R. 3.3We recommend that all boys with permanent hypogonadism should undergo pubertal induction.
Rationale
Androgens are required to induce secondary sexual characteristics, achieve optimal adult male body composition including bone mineral content and muscle mass and promote physical and social well-being. Testosterone plays an important role in socio-emotional and cognitive development (176, 177). In boys with pre-existing behavioural or intellectual disabilities, pubertal induction should be performed carefully and in close collaboration with a mental health specialist. Parents and caretakers of such boys should be informed about the effects of testosterone on impulsivity. In addition, pubertal induction should be accompanied by sexual education and by the provision of resources for sex education elaborated for individuals with intellectual disability. Studies show that this specific patient group remains too often insufficiently educated and prepared about sexual and emotional life situations (178).
In boys with HH, there is a wide range of pubertal induction regimens, including the use of gonadotropins that are available, see section on ‘hypogonadotropic hypogonadism’. There is no clear evidence that one regimen is superior to another and there is no clear evidence that supports the preferential use of any of these regimens instead of testosterone replacement for the purpose of achieving virilisation in boys with HH. The preparations used will depend on the preferences of the patient, ease of use as well as local availability and if fertility is considered.
R. 3.4In boys who are known to have a diagnosis with a high risk of hypogonadism, we recommend that pubertal induction can be started by the age of 12 years if there are no signs of pubertal development. (+OOO)
Rationale
The aim of pubertal induction in boys with hypogonadism should be to mimic normal physiology. Attention needs to be paid to the age-appropriate development of secondary sexual characteristics, growth acceleration, normal body proportions achievement, changes in body composition, avoidance of psychosocial consequences of delayed growth and puberty. Late-onset of puberty has been associated with a higher risk of anxiety and depression (179) and increased cardiometabolic risk (180). There are also ethnic and familial variations in the onset and tempo of puberty, which may need to be considered when deciding to start pubertal induction in an individual patient. For instance, pubertal onset as determined by age at testicular volume of at least 4 mL varies from 10.6 years in the Chinese population to 11.4–11.7 years in Hong Kong, the Netherlands, Greece and Denmark (181).
Surveys performed in the context of managing CDGP have shown that in paediatric endocrinology, pubertal induction in boys is usually started after the chronological age of 14 years (169). However, no studies were found comparing different age groups for the start of puberty induction in boys with (high risk of) hypogonadism (see section on Systematic literature review). Factors that may influence the timing of induction may include the bone age and the sociocultural perspective (182). In boys with conditions that predispose to permanent hypogonadism, such as bilateral anorchia or known CHH, surveys of practice suggest that pubertal induction may often be initiated at an age younger than 12 years and even as young as 10 years (164, 183). These studies also show that in several boys with DSD conditions associated with hypogonadism, testosterone therapy may be initiated at a later age possibly due to a partial hypogonadism in these boys.
R. 3.5We recommend that pubertal induction in boys with hypogonadism should be performed with testosterone (++OO)
Rationale
There is a wide range of testosterone preparations that are available for use in boys with hypogonadism (see Table 4). Recent studies show that in boys with early onset hypogonadism, i.m. forms of testosterone remain the commonest form of preparations (164). Studies are scarce that compare different forms of testosterone (see section 4.1), but there is no clear evidence that one form of testosterone is superior to another (184, 185). One study comparing testosterone vs no treatment to induce puberty found increased BMD in patients using testosterone compared to the control group (100). One study comparing testosterone vs hCG for puberty induction showed inconclusive results regarding Tanner stage progression, smaller testicular volume and similar height after use of testosterone compared to hCG (101).
Table 4.
| Starting dose for pubertal induction | Adult dose | Advantages | Disadvantages | |
|---|---|---|---|---|
| Intramuscular | ||||
| Testosterone enanthate, cypionate or mixture of T esters | 25–50 mg monthly. Increase of 50 mg every 6–12 months | 150–250 mg every 2–4 weeks | Good adherence; most data and clinical experience to support use in adolescents | Not physiological; painful |
| Testosterone undecanoate, i.m. injection | No data available | 750–1000 mg every 10–14 weeks | Stable serum T concentrations; less frequent injections | Painful injections; expensive; lack of data in adolescents |
| Transdermal | ||||
| Testosterone gel | 2%: 0.5 g 10 mgT/day | 2%: 2–4 g 40–80 mgT/day | Mimics normal physiology; pain free | Potential transfer to another individual |
| Testosterone patch (Scrotal) | No data available | 4–6 mg/day | Mimics normal physiology | Skin irritation; patch is too large for prepubertal boys; lack of data in adolescents |
| Testosterone patch (non-scrotal) | 2.5 mg × 12–24 h/day or 5 mg × 8 h/day | 2.5–5 mg/day | Mimics normal physiology | Skin irritation; lack of data in adolescents |
| Oral | ||||
| Testosterone undecanoate (Restandol) | 40 mg alternate day or daily | 40–80 mg 2–3 times daily | Oral; pain free | Multiple doses needed per day; variable absorption |
| Testosterone undecanoate (Jatenzo) | No data available | 158–396 mg twice daily | Oral; pain free | Multiple dose; GI side effects; concerns rehypertension |
| Buccal testosterone | No data available | 30 mg twice daily | Mimics normal physiology | Altered taste; gum irritation |
| Subcutaneous | ||||
| Testosterone cypionate | 25 mg subcut every alternate week | 50–70 mg subcut every week | Less painful than i.m.; can be administered at home | Lack of data in hypogonadal boys |
| Testosterone pellets | 8–10 mg/kg once | 3–4 pellets (75 mg each) every 4–6 months | Adherence | Risk of extrusion of pellet, fibrosis, infection Cost |
| Intranasal | ||||
| Intranasal testosterone | No data available | 11 mg tid | Non-invasive; easy to administer; no transference | Altered taste |
In healthy boys, testosterone measured by LC-MS/MS starts to increase a year before the appearance of pubic hair growth, at 11.5 years on average. When measured by RIA, plasma testosterone starts to increase at a testicular volume between 3 and 6 mL with clear evidence of diurnal variation at a median age of 12.5 years (186, 187). While mimicking the gradual physiological rise in testosterone remains the objective of pubertal induction, it is unlikely that the wide range of pharmacological preparations that are currently available possess the pharmacodynamic and kinetic properties to achieve completely normal pubertal development, especially in the early stage of puberty. As an example, measured plasma testosterone after 25 mg testosterone reached an adult range for 1 day and for 2 days after 50 mg testosterone enanthate (188).
For suggested testosterone formulations and doses, see Table 4. The addition of DHEAS in boys with hypogonadism is not recommended. Although the benefits of mimicking normal physiology or, indeed, the adverse effects of not mimicking normal physiology have not been studied thoroughly, it is clear from boys with precocious puberty that quick progress is associated with adverse behaviour as well as restricted growth prognosis. Thus, the dose of testosterone should be increased gradually to mimic normal growth and pubertal development. It is common to begin treatment with low-dose testosterone (i.e. 50 mg testosterone enanthate every month, 10 mg transdermal testosterone every other day or 40 mg oral testosterone undecanoate daily) and then gradually increase to the adult dose. The speed of this gradual increase will also need to be individualised based on the age as well as the mental maturity of the boy.
R. 3.6We recommend that all boys who receive pubertal induction therapy should have a structured endocrine assessment at baseline and at follow-up.
Rationale
Regular clinical follow-up is generally performed every 3–6 months to assess the effectiveness of testosterone therapy by assessing pubertal and skeletal maturation and height velocity. In the guidelines for adults on testosterone replacement therapy (TRT), monitoring is recommended and standardised (191, 192). There is clear evidence that systematic monitoring in adolescents on testosterone is performed to a variable extent (164) and it is possible that this variation is due to the wide range of conditions that necessitate TRT in adolescents. Recently, monitoring schemes designed to assess the effectiveness as well as safety of TRT have been proposed (183). With greater knowledge of a wider range of effects of testosterone and the advent of several newer forms of testosterone replacement, the need for careful monitoring is becoming greater. A structured assessment can also be used to screen for adverse effects as well as titrate replacement. It should include the assessment of adverse signs such as local reactions, gynaecomastia, priapism, increased haematocrit, deranged liver function and inappropriate behavioural changes. Table 4 highlights the advantages and disadvantages of specific preparations and further details can also be found in recent publications (183, 189, 190). While there is no evidence that dose titration against a marker such as a haematocrit is necessary for pubertal induction, the regular assessment of haematocrit would be advisable, especially as the adolescent reaches a steady adult replacement dose (183). The care pathway can also ensure age-appropriate explanations and discussions about both endocrine and reproductive testicular functions with the appropriate experts in the multidisciplinary team. With increasing maturity, these discussions may cover issues regarding sexual function and fertility including biological fathering and other modes of fathering. Physiological variations in steroid metabolism as well as androgen sensitivity when combined with the wide range of preparations that are available increase the likelihood of inter-individual differences in how boys will respond to replacement therapy and there is a need for clinically relevant markers of androgen action.
R. 3.7We suggest adjusting the puberty induction treatment or changing the route of administration in case of relevant adverse effects/complications.
Rationale
The occurrence of relevant adverse effects is rare with testosterone (193). For injectable forms of TRT, priapism has rarely been reported. Testosterone undecanoate, the long-acting preparation, should not be used for induction of puberty or progression through the early stages of puberty, as it is likely to advance bone age too rapidly with a potential for truncating final height.
When ingested orally, testosterone is absorbed well from the gut but is effectively metabolised and inactivated in the liver before it reaches the target organs (‘first pass-effect’). Testosterone induces liver enzymes that are responsible for its own metabolism.
Testosterone gel provides a possibility for more physiologic serum testosterone levels and it can closely mimic natural diurnal variation in testosterone concentrations. It has only minor systemic side effects. However, published data on transdermal testosterone in pubertal induction are scarce. In many countries, testosterone formulations are not licenced for the induction of puberty.
Transdermal gels are available in different formulations: 1% testosterone strength and the metered-dose gel formulation of 2% or 2.5% testosterone strength. Topical testosterone has been repeatedly reported to cause undesirable exogenous testosterone exposure through passive transfer to members of the patients’ household (194). Due to the alcoholic enhancer used and the occlusive nature of the systems, the application is associated with skin irritation in up to 60% of the subjects.
R 3.8We suggest monitoring potential gynaecomastia in boys to timely discuss treatment options.
Rationale
Testosterone therapy can lead to gynaecomastia through its aromatisation to oestradiol; hence, regular monitoring of this potential side effect is requested. Gynaecomastia occurs in up to a third of patients on gonadotropins (195) or testosterone (196) and usually (although not invariably) occurs during supraphysiological replacement doses. The risk and severity of gynaecomastia can be reversed by adjusting the dosage. It should be remembered that 50% of all adolescents develop gynaecomastia which typically presents in early puberty and lasts for 6–12 months.
Similarly to physiological puberty, the most likely cause of gynaecomastia during TRT is a relative imbalance between oestrogen and androgen levels in the serum or at the tissue level (197). During the early proliferative phase, manifested clinically as breast pain and tenderness, medical therapy may be attempted (198). Medical treatment of gynaecomastia aims to correct the oestrogen–androgen imbalance by three possible pathways: (i) blocking the effects of oestrogens on the breast (e.g. clomiphene, tamoxifen, raloxifene), (ii) administering androgens (e.g. danazol or DHT) and (iii) inhibiting oestrogen production (e.g. anastrozole, testolactone). Almost all data dealing with these treatments are based on uncontrolled studies, and in addition, the evaluation of their efficacy is further complicated by the fact that gynaecomastia may resolve spontaneously (199, 200). It must be noted that none of these therapies are approved for the treatment of gynaecomastia. It seems to be reasonable to try a 3 months trial in selected cases of recent-onset gynaecomastia (201) because it offers rapid relief from pain, regardless of the magnitude of the response. If the gynaecomastia has been present for >1 year, it is unlikely to regress substantially, either spontaneously or with medical therapy, because fibrotic tissue is usually present. In such circumstances, surgical s.c. mastectomy, ultrasound-assisted liposuction and suction-assisted lipectomy are the best options for cosmetic improvement of Tanner stage III and above (202, 203).
R 3.9Treatment in patients with a late diagnosis, without pubertal development or for whom the pubertal development has come to a halt, should be individualised.
Rationale
Patients who come to diagnosis at a later age, towards the late teenage years or as young adults, may have no pubertal development or a spontaneous start of puberty but a slow or no pubertal progress. This is often the case in young men with Klinefelter syndrome and can be the case in individuals with 45,X/46,XY, gonadal dysgenesis or androgen insensitivity.
Adult endocrinologists often see patients with CHH in late adolescence or early adulthood with the main complaint of lack of pubertal development (204). In such cases, the therapeutic approach is often more incisive than for younger patients, involving higher initial testosterone doses than those used by paediatric endocrinologists (200–250mg testosterone enanthate monthly, then every 2–3 weeks) to induce more rapid virilisation (205).
Pubertal induction in patients with hypogonadotropic hypogonadism
Introduction
HH is caused by congenital or acquired defects of the hypothalamic and/or pituitary compartment of the HPG axis. CHH forms can be isolated, part of an overlapping syndrome (such as CHARGE) or part of combined pituitary hormone deficits. Kallmann syndrome is associated with hypo- or anosmia. All these forms have strong genetic backgrounds (206). Acquired forms of HH, mainly associated with other pituitary hormone deficits, are usually the results of neoplastic, metabolic, immune, infectious, infiltrative, inflammatory or iatrogenic causes affecting the hypothalamic-pituitary region. HH can also be related to functional causes, which are usually characterised by a reversible negative effect on the HPG axis activation/functionality. These include chronic and/or inflammatory systemic illness, malnutrition (e.g. anorexia nervosa), extreme exercise or stressful conditions and opioid use.
Pubertal induction has to be considered in all cases with permanent HH after the diagnostic procedure has been carefully followed. The goals of the therapy for pubertal induction in male and female HH are not different from the ones reported for primary hypogonadism, although in this specific cohort of patients, the possibility of future fertility can be considered and, in males, specific treatment regimens can allow for the maturation of testes (207, 208). Briefly, the main goals will be the attainment of optimal masculinisation/feminisation and secondary sexual characteristics; the achievement of pubertal growth and an optimal target height; the prevention of osteoporosis by accruing normal bone mass and mineralisation; to allow for a normal psychosocial development; and in certain cases, to gain fertility (20, 207, 208, 209).
Timing of puberty induction
In principle, the timing for pubertal induction in patients with a diagnosis of permanent HH does not differ from what has been recommended for boys and girls with hypogonadism. However, it should be pointed out that the main differential diagnosis of CHH is CDGP. It is a more common situation and also more frequent in boys compared to girls. This differential diagnosis is one of the greatest challenges for the clinician evaluating a pubertal delay (116). Unfortunately, to date, we do not have specific indicators that allow for a clear distinction between these two clinical conditions although a history of micropenis, bilateral cryptorchism, midline defect, hypo/anosmia, deafness or renal anomalies should raise the suspicion of CHH. For this reason, in patients with pubertal delay and uncertainty regarding the diagnosis of CHH or CDGP, it may sometime be necessary to delay the initiation of induction even beyond 14 years. Nonetheless, the decision must always be made according to the clinical situation in each individual case and in concern with the patient him/herself and his/her parents (20, 205, 209).
Treatment approach and monitoring
Recommendations regarding treatment approach and monitoring for puberty induction in girls (R 2.1–2.9) apply equally to those with HH. Pubertal induction in girls with HH using gonadotropins has not been described, although gonadotropin–ovulation–induction is a standard fertility treatment in adult life.
R 4.1We suggest discussing two treatment options for pubertal induction in boys with secondary hypogonadism: testosterone or gonadotropins. See Table 5 for the so far reported different treatment options using gonadotrophins. (+OOO)
Table 5.
Treatment with gonadotropins for puberty induction or induction of spermatogenesis.
| Age (years) | n | First-phase therapy dose frequency | Treatment length (months) | Second phase or unique phase therapy dose frequency | Treatment length (months) | Reference | |
|---|---|---|---|---|---|---|---|
| rFSH or hMG | hCG or GnRH | ||||||
| 18–34.5 | 16 | hCG (n = 12): 1.5–10.000 UI/w | 1–14 | hMG: 225–750 UI; ?/week | hCG: 3–15 000 IU; ?/week | 18–28 | (221) |
| 12.8–13.2 | 3 | rFSH: 1.5 U/kg; 3/week | – | 12 | (219) | ||
| 13–15.2 | 37 | hCG: 1500 UI 2/w s.c. | 6 | hMG: 75 UI; 2/week | hCG: 1500 UI 2/week s.c. | 24 | (222) |
| 13.7–21.1 | 14 | rFSH: 75–100 UI alternative day/week | hCG: 1000–1500 UI; alternative day/week | na | (217) | ||
| 18–31 | 8 | rFSH: 150 UI/day s.c. | 1 | rFSH: 150 UI/day s.c.; n = 7 | hCG: 1500 IU i.m.; 2/week | 2 | (223) |
| 10.4–17.7 | 14 | rFSH: 1.5 U/kg (180–450); 3/week s.c. | 2–32; n = 9 >12 | rFSH: (no dose specified) | hCG: 500–4000 s.c./week; 1–3/week (?) | (215) | |
| 14.5–31 | 19 | n= 8: testostosterone | 6–36 | rFSH; 150–300 UI 3/week* | hCG: 500–1500 UI; 2/week (increments 6 months) | 9 | (218) |
| Adults | 18 | rFSH: 75–150 IU/day | 4 | GnRH pulsatile | 24 | (214) | |
| 18–45 | 67 | Groups A & B: hCG 2000 UI; 2/week | 6 | Group A: uFSH 75 UI; 3/week Group B: uFSH 75 UI; 3/week 3 months alternative |
Groups A & B: hCG 2000 UI; 2/week | 18 | (224) |
| Groups | 60 | (225) | |||||
| A: mean 15.5 | 34 | hCG: 250–500 UI 2/week s.c. | 250–500 UI increase every 6 months | rFSH: 75–150 UI 3/week (max 150 UI) | hCG: max 2500 UI 3/week | 24 ± 7 | |
| B: mean 18.8 | 26 | Testosterone (previous to study) | 18–68.4; mean 30 | rFSH: 75–150 UI 3/week (max 150 UI) | Start 1500 IU s.c. 2/week → max 2500 UI 3/week | 22 ± 6 | |
| 14.8–15.1 | 2 | rFSH: 66.7–112.5 UI; 3/week | 6–7 | rFSH: 66.7 UI; 3/week | hCG: 500-1500 UI; 3/week | 33–34 | (220) |
| 14–23 | 19 | FSH (13/19 patients): 75 IU e/week | 4 | FSH: 75 IU 3/week | hCG: 250–2000; 2/week (increments 6 months) | 24 | (216) |
hCG, human chorionic gonadotropin; hMG, human menopausal gonadotropin; rFSH, recombinant follicle stimulating hormone; GnRH, gonadotropin releasing hormone.
Rationale
Pubertal induction in a boy can be carried out through two different possible approaches: the use of testosterone analogues similar to the treatment of primary hypogonadism or the use of exogenous gonadotropins. Preparations, doses and aim of the two possible treatments are indicated in R 3.1–3.5 and Tables 4 and 5. Monitoring of patients with HH undergoing gonadotropin treatment does not differ from the recommendation for pubertal induction with testosterone (see R 3.5–R 3.8).
To date only a few randomised studies have been published comparing these different treatment strategies and the patients recruited in these studies are often quite heterogeneous. It is therefore difficult to conclude which treatment strategy is more effective or preferred (210). A systematic review provided no firm evidence for an optimal induction approach (see section on Systematic literature review) (100, 101, 102, 103). The two options, testosterone or gonadotropins (Table 5), should be presented and discussed with the patient and his parents, also considering the availability of the different treatment options in the patient’s country of residence.
The most common treatment for pubertal induction in males with HH involves, as for primary hypogonadism, the use of i.m. injection of testosterone esters (enanthate, propionate or cypionate) (20, 205, 209, 210, 211). Although effective in inducing pubertal signs and symptoms, testosterone formulations neither stimulate testicular growth nor will they induce spermatogenesis. It is still unclear whether testosterone therapy for pubertal induction hinders future testis development or likelihood of spermatogenesis if gonadotropins are used in adulthood (212, 213).
Alternatively, a potential testicular development and fertility in males with HH can be achieved with puberty induction through the use of gonadotropins (212, 214, 215, 216). This might offer adolescent patients a significant psychological reassurance and may also enhance their self-confidence (20, 205). On the other hand, although this has to be verified and demonstrated, it might also allow for the early identification of potential ‘poor responders’ (i.e. patients with severe oligozoospermia and a total sperm count <5 millions/mL) and thus give them the opportunity to consider a preventive and prophylactic sperm cryopreservation for future use in case of a worse spermatic response in adulthood. Different treatment protocols with gonadotropins can be used to induce puberty in adolescent males with HH: exogenous hCG alone or in combination with recombinant FSH (214, 215, 216, 217, 218). However, consistent evidence suggests that the combination therapy with recombinant FSH (rFSH)/hCG is significantly more effective than hCG alone both for inducing spermatogenesis and increasing testicular volume. Furthermore, some evidence suggests that a pre-treatment with rFSH followed by the combination with hCG or GnRH is even more effective in optimising the Sertoli cell maturation and is able to induce spermatogenesis even in extremely small testes, and cryopreservation of sperm subsequently allows for enhancing the future fertility of these patients (214, 215, 219, 220). Indeed, this approach mimics the physiological elevation of FSH during spontaneous pubertal development.
Treatment with gonadotropins, whatever the protocol used, requires multiple weekly injections, although s.c. injections can be given. This aspect should be discussed with the patient and parents before the final decision.
R 4.2We suggest considering switching gonadotropins to testosterone in boys with HH after pubertal induction.
Rationale
If pubertal induction is made by the administration of rFSH and hCG in order to mimic physiology, we recommend switching to testosterone treatment in boys with HH after the completion of pubertal induction. Possible spermatogenesis should be considered. Although there is not enough evidence to recommend one treatment before the other, there are theoretical arguments based on the limited knowledge available on testis and Sertoli cell maturation with regard to fertility. During early puberty, the proliferation of immature Sertoli cells occurs under the stimulus of FSH. Hence, treatment with FSH stimulates the proliferation of immature Sertoli cells and spermatogonia (214, 219, 226). On the other hand, testosterone induces the differentiation of Sertoli cells via androgen receptor which begins to be expressed after the first 5 years of life (211, 227). The combination of rFSH and hCG therapy for 6–24 months results in testicular growth in almost all and spermatogenesis in 80–95% of patients without undescended testes (216, 217, 228, 229, 230). The use of hCG without FSH priming could, therefore, in theory, cause premature differentiation of the pool of Sertoli and germ cells, thereby diminishing the chance of proliferation leading to lower fertility potential in adulthood. The shift from FSH/hCG therapy to testosterone should occur after sperm analysis, and in the case of sufficient sperm in the ejaculate, sperm cryopreservation (banking) should be considered, particularly in severe oligospermia. The reason behind this is that even if repeated gonadotropin treatment has been reported to induce quicker initiation of spermatogenesis (231, 232), it is not certain. In cases with azoospermia, which then appear as ‘poor responders’ to gonadotropin stimulation, testicular sperm extraction (TESE) procedure might be considered, however, with uncertain outcome, before switching to TRT, especially in patients who are old and mature enough for this.
Furthermore, since ‘reversal’ of CHH has been described (144) in 5–20% of patients with CHH, a brief therapy withdrawal (i.e. 4 months) after the conclusion of pubertal induction with gonadotropins and before the start of TRT might be considered in order to verify a spontaneous recovery of the HPG axis.
Puberty induction in Klinefelter
Introduction
Klinefelter syndrome is the most common cause of congenital male hypogonadism. However, boys with this syndrome typically enter puberty at a normal age. With advancing adolescent age, the gonadal dysfunction becomes more marked and may be associated with a failure to progress in puberty (169). The diagnosis of Klinefelter syndrome affecting 1:600 men is often overlooked (233, 234) with less than 10% diagnosed before puberty, probably due to the variable and sometimes subtle phenotype, especially in the mosaic forms. During or after puberty, boys with Klinefelter syndrome typically present with gynaecomastia and they have elevated FSH and low testosterone levels, although testosterone can also be at the lower end of the normal range with elevated LH (167, 169). Patients have an increased incidence of cryptorchidism. In puberty, the testes grow initially and then regress, and in adulthood, testes are small and firm. There is a wide spectrum of the phenotype, many are never even diagnosed while some can have psychosocial difficulties and learning disabilities. The diagnosis is confirmed by karyotyping.
We place a high value on the recommendation to treat adult patients with Klinefelter syndrome and low testosterone levels as well as accompanying symptoms of androgen deficiency with an adequately dosed testosterone replacement (Table 4).
Timing of puberty induction
The general recommendations as formulated under R 3.1 and 3.3 (in whom to consider pubertal induction) apply to Klinefelter syndrome, although the most common situation is a normal start of puberty followed by a slow progression of puberty with small testes and development of gynaecomastia.
Treatment approach and monitoring
With regard to the timing of puberty, an individual prediction of pubertal onset for patients with Klinefelter syndrome can be estimated from the parents’ pubertal history. Whether early testosterone replacement, when LH starts to rise above normal levels (>+2 s.d.), should be started is still not clear. Some advocate for early treatment as soon as LH starts to rise in order to prevent the adverse body composition, osteopenia/osteoporosis, impaired physical and psychological development, educational achievements and social integration which are commonly observed in the untreated newly diagnosed young adult (170, 235, 236, 237, 238). However, others think that testosterone supplementation should be postponed until testosterone levels fall below the normal range (<−2 s.d.) (239). Some in the expert group advocated for aiming at securing sperm for cryopreservation before starting testosterone supplementation, while others, in the absence of scientific evidence for such an approach, advocated for commencing testosterone supplementation as soon as LH starts to rise.
The recommendations regarding treatment and monitoring R 3.5–3.9 apply equally to Klinefelter.
R. 5.3We suggest performing sperm analysis if physically and mentally possible before the start of testosterone treatment in boys with Klinefelter Syndrome and spontaneous start of puberty.
Rationale
Randomised–controlled trials are needed to elucidate the influence of testosterone replacement therapy on pubertal development and spermatogenesis in patients with Klinefelter Syndrome. There are also no randomised–controlled trials evaluating the possible deleterious impact of testosterone treatment on successful sperm retrieval or its possible effects on reproductive outcomes in men with Klinefelter Syndrome. Testosterone preparations may theoretically suppress any remaining spermatogenesis if the endogenous LH drive is reduced; however, this has not been shown in clinical trials. Some advocate for assessing fertility status before the start of testosterone supplementation, while others do not. Therefore, individual decisions should be made until more evidence is gathered. In those patients who have no spermatozoa in their ejaculate, fertility preservation can be attempted through TESE. According to a recent literature review, spermatozoa can be found by TESE in up to half of the patients with Klinefelter syndrome aged 16–30 years old, of whom many had been treated with testosterone (240), although real-life studies report much lower values (241). Therefore, a TESE procedure for fertility preservation should not be performed in Klinefelter syndrome patients younger than 16 years and it can be postponed at least until the age of 30 since no significant differences were observed in TESE outcome in this age interval.
The use of hCG or anti-oestrogens (aromatase inhibitors or selective oestrogen receptor modulators) on compassionate care grounds may have a positive effect on gonadotropin secretion even in the hypergonadotropic state as seen in Klinefelter syndrome (242). Theoretically, this may result in higher levels of intra-testicular as well as circulating testosterone and maintained (or even augmented) spermatogenesis. However, no controlled trials are available and evidence is poor (243). Hence, such therapies cannot be recommended in general.
No data have been published on the possible positive or negative effects of testosterone treatment in boys with Klinefelter syndrome and compensated hypergonadotropic hypogonadism. The guideline group acknowledges that different strategies are employed. Some suggest that in the absence of clinical signs and symptoms of hypogonadism, these boys should not be treated with testosterone (239). In some earlier studies, no benefit of TRT was observed in preventing obesity/overweight (238), and no significant differences in bone structure or bone biomarkers were reported in patients with Klinefelter with and without testosterone therapy (244). However, a recent study advocates testosterone treatment in cases with elevated LH in order to potentially positively affect the muscle mass, the BMD and neuropsychological functioning (170). In young adults with Klinefelter syndrome, a recent placebo-controlled, randomised trial has shown that 6 months of testosterone treatment led to decreased total body and abdominal fat mass, as well as expected increases in haemoglobin and IGF-I (245). In adults, individualised transdermal and injection testosterone therapy resulted in comparable treatment efficacy when evaluating androgen-responsive parameters (246).
Androgen insensitivity syndrome (PAIS and CAIS)
Androgen insensitivity syndrome (AIS) is a rare inherited condition caused by X-linked pathogenic mutations in the androgen receptor (AR) gene (38, 247). There are now more than 1000 mutations reported (248), and the genotype–phenotype correlation is highly variable. The estimated prevalence is 1–5 per 100 000 births (249, 250). The phenotype depends on the residual AR function. According to the residual sensitivity to testosterone, the external genitalia will be variably virilised (251, 252, 253). In general, three subgroups of AIS are defined.
A complete absence of androgen receptor action leads to the clinical picture of CAIS. In these girls, the external genitalia are typically female. They have testes, which secrete testosterone with no (or markedly diminished) effect on the androgen receptor and Sertoli cells that produce AMH resulting in the regression of Mullerian structures prenatally. Thus, the uterus, fallopian tubes and upper part of the vagina are absent. Furthermore, Wolffian structures do not develop due to the insensitivity to testosterone. The testes are typically located within the abdomen, inguinal canal or in the labia majora (250). Girls and women present during puberty with absence of menarche but with normal breast development due to the presence of androgens which are aromatised to oestrogens. Some girls (up to 50%) present with a bilateral inguinal hernia during infancy/childhood (254, 255, 256). Women with CAIS do not develop pubic and axillary hair. A blind-ending vagina is observed in almost all patients (from 2.5 to 8 cm long). Women with CAIS have a female gender identity and are female in their gender role behaviour (66, 68).
Reduced AR residual activity results in PAIS with variable virilisation of the external genitalia during fetal life. Individuals generally present with ambiguous genitalia at birth. Earlier studies described a grading scheme (grade 1–5) for the description of virilisation (249). The residual activity of the AR also leads to a variable degree of virilisation of the external genitalia and the presence of gynaecomastia at the onset of puberty. Unfortunately, the phenotype–genotype correlation is poor (257). However, it has been shown that the degree of virilisation at birth estimated by the external masculinisation score (EMS) is a good predictor of the degree of virilisation at puberty (175, 258, 259). In only 22% of the cases with a clinical diagnosis of PAIS, a pathogenic variant in the AR is found, making it a more complex and a highly variable clinical and diagnostic challenge (258, 260).
The mildest form of androgen insensitivity called mild androgen insensitivity syndrome is typically diagnosed in men with infertility without atypical genitalia (261, 262, 263). A detailed description of this form is outside the scope of this guideline.
Gonadectomy in patients with complete androgen insensitivity syndrome
Patients with CAIS and intraabdominal testes might be at increased risk of developing gonadal germ cell cancers (GGCC), mainly seminomas, but this risk seems to be low before/during puberty (264, 265, 266). Historically, nearly all patients with CAIS underwent gonadectomy before puberty because of this risk. Delaying gonadectomy allows for spontaneous pubertal development which is thought to be more satisfactory to the individual and also allows the patient to be fully involved in the shared decision-making to remove their gonads or not. There is an ongoing debate about the need to perform gonadectomy after puberty since the risk of invasive GGCC development is still uncertain. In a recent publication by Tack et al., an algorithm was presented, in which they suggest performing gonadectomy after puberty in individuals with CAIS and only after careful counselling. Yearly follow-up if the gonads are retained was advised including self-examination and imaging (ultrasound/MRI) (265). However, self-examination is only possible when the gonads are located in the inguinal region.
Women with CAIS and intact gonads have an increased risk of developing low BMD due to bone insensitivity to testosterone and relative oestrogen deficiency. After gonadectomy, the risk of developing low BMD further increases. Treatment with sex steroids aims to prevent osteoporosis and other metabolic complications (267, 268, 269, 270, 271, 272, 273). Effects on cardiovascular health, quality of life, sexual health and mortality are less well studied (274, 275).
Pubertal induction in CAIS
In whom to consider puberty induction?
R 6.1We recommend allowing girls with CAIS and retain gonads to go through spontaneous puberty.
Rationale
Puberty treatment is not indicated in girls with retained testes. In subjects with CAIS, androgen-responsive tissues including the pituitary gland are insensitive to testosterone, resulting in persistently elevated LH concentrations. Testosterone levels are within or above the normal male range in patients with retained testes. Testosterone is peripherally aromatised to oestrogen resulting in endogenous oestrogen levels which are above the male range but generally lower than the normal female range (276, 277). These oestrogens induce breast development during puberty. In addition, this endogenous oestradiol has positive effects on the skeleton, brain and body habitus in patients with CAIS (269). The patients develop no or scarce pubic and axillary hair. It is generally perceived that in patients with CAIS and intact gonads, spontaneous puberty occurs at the same age as described in the general population although one study reported the start of breast development at a median age of 12–13 years (277). Furthermore, in patients with CAIS, final height is generally above the mean female final height but below the average height of the male population (273, 278, 279).
In individuals with intact gonads, oestrogen treatment can be considered in those with delayed puberty, particularly in individuals with tall stature with the aim to reduce final height. It is known that high dosages of oestrogens used during puberty can be successfully used to reduce final height in girls with constitutional tall stature (280, 281). However, there are no studies reporting the effect of oestrogen treatment (dosage and timing) on the final height in patients with CAIS.
Timing of puberty induction, treatment approach and monitoring
R. 6.2 We recommend to start puberty induction at the age of 11 years in girls with CAIS who underwent gonadectomy. We recommend the same treatment protocols (for pubertal induction in girls with CAIS who have been gonadectomised ) as for other girls (R2.2–2.3, R2.6–2.8, R2.11). (+OOO)
Rationale
In girls with CAIS who have been gonadectomised, pubertal induction should start at the age of 11 years in accordance with the current treatment recommendations to mimic normal pubertal development.
Treatment with sex hormones in patients with CAIS is generally only indicated when the gonads have been removed. The timing of puberty should be similar to other indications for pubertal induction in girls.
Patients with CAIS treated with transdermal oestrogens were reported to have better bone health than those treated with oral formulations (269). The risks and benefits of oral vs transdermal E2 are likely to be similar to other cohorts in whom these therapies have been studied more comprehensively, with regard to liver and cardiovascular adverse effects (274). However, the benefits and disadvantages with regard to bone health and sexual function may differ for patients with other diagnoses.
There are still conflicting data concerning the optimal dose of hormonal replacement therapy following puberty induction. Plasma oestradiol levels are positively associated with BMD. Therefore, routine monitoring of oestrogen concentrations by LC-MS/MS and bone density is recommended. Because of the absence of a uterus, the addition of treatment with progestins is generally not required. There is not enough evidence for a beneficial effect on brain, bone or other tissues to recommend the addition of progestin treatment. The use of testosterone rather than oestrogens in an attempt to mimic the hormonal levels of women with CAIS and intact gonads has been studied, particularly with regard to sexual function (274, 282, 283).
An unsolved aspect of the clinical care of young adults with CAIS and intact gonads is that although estrogen treatment is generally not recommended, low E2 plasma levels and BMD can be seen and thus, there may be a need for E2 or testosterone supplementation in these patients (282).
R 6.3We recommend treatment monitoring every 3–6 months during puberty induction.
Rationale
Regular follow-up with clinical parameters (breast development, height), patient’s satisfaction and bone density. We suggest yearly measurements of FSH and LH as well as E2 levels similarly to (R2).
Pubertal induction in PAIS
In whom to consider puberty induction?
R. 7.1We recommend that in all patients with PAIS, evaluation of gender identity should take place before considering puberty induction.
Rationale
The clinical picture of PAIS is variable and depends on the residual androgen receptor activity and other modifying partially unknown factors. The genital phenotype ranges from hypospadias to female appearance (284) (Table 1). Furthermore, the degree of virilisation at birth estimated by the EMS has been shown to be a good predictor of virilisation at puberty (175, 258, 259). The external genitalia score (EGS) developed for evaluation in a wider spectrum of genital differences may be used in the future (285).
Careful counselling by a multidisciplinary team is therefore required before any decision about hormonal treatment is taken. An experienced psychologist should evaluate the patients’ gender identity development, preferably from the age of 8 years. Structured psychological assessment of gender should be conducted.
In case there are uncertainties about gender identity, puberty can be delayed by the use of GnRH agonists. Delay of puberty can be helpful in order to gain time to find out which gender will suit the individual best. Psychological follow-up is needed to evaluate gender development over time and keep an eye on the adolescent’s mental health (emotional problems such as social phobia or depression need to be identified and treated). Recognising that gender identity is a non-binary phenomenon can facilitate satisfaction with one’s gender. Detailed information is provided under ‘Psychological Aspects’.
However, pubertal treatment is essential for long-term wellbeing in all patients, and a decision regarding hormonal treatment eventually has to be made.
Evaluation of pubertal development
R. 7.2We recommend that careful evaluation of patients’ endocrine profile, phenotype and genotype is undertaken and that this information is used to consider the potential for virilisation during puberty (in patients with a male or undecided/binary identity).
Rationale
To evaluate the degree of pubertal development in PAIS, we recommend monitoring clinical signs of virilisation, height, full hormonal evaluation, degree of masculinisation at birth (EMS/EGS) and the genetic variant.
At the onset of puberty, the hypothalamic GnRH pulse generator initiates pulsatile GnRH secretion resulting in pulsatile FSH and LH secretion from the pituitary gland. The resulting testosterone secretion from the Leydig cells in the testes fails to exert negative feedback to the hypothalamic-pituitary axis due to insufficient central AR action. However, the increased circulating testosterone is aromatised to oestradiol which exerts normal negative feedback on FSH levels that typically remain within the normal range. Thus, the LH/FSH ratio is typically relatively high in CAIS and PAIS (15, 52). Oestradiol circulates in high-normal concentrations (compared to the male reference range) resulting in gynaecomastia in many boys with PAIS (286, 287). The testicular Sertoli cell markers inhibin B and AMH are within the normal prepubertal male range (48). In the physiological situation, inhibin B increases with pubertal onset, whereas AMH decreases when the Sertoli cells differentiate and start to express AR. With rising intratesticular testosterone levels, AMH secretion is inhibited in a subject with normal AR function, whereas a boy with PAIS continues to have prepubertal levels of AMH due to lack of inhibition. Inhibin B is usually within normal male ranges in boys with PAIS (286).
It is difficult to predict the course of puberty in patients with PAIS. A study analysed EMS at birth in 27 PAIS patients in relation to the pubertal outcome and found that only six of nine patients with EMS < 5 at birth underwent spontaneous male onset of puberty, whereas all 18 patients with EMS ≥ 5 at birth experienced a spontaneous male onset of puberty. In contrast to the clinical findings, the functional analysis of AR variants did not appear to predict pubertal outcome (258). It remains to be seen to which degree the early (peripubertal) changes in reproductive hormones will guide the clinician in the prediction of spontaneous virilisation.
Treatment approach and monitoring
R. 7.3In children with PAIS identifying as as girls, the general recommendations for pubertal induction in CAIS as formulated under R 6.2–6.3 apply.
Rationale
In children with PAIS who have been raised as girls and identify as girls and who have not been gonadectomised, gonadectomy can be considered before or at the very beginning of puberty, to avoid virilisation and gender discomfort/dysphoria in addition to considerations regarding malignancy risk (38, 39, 44, 45, 46, 49). Pubertal induction as recommended for other conditions in which pubertal induction is necessary applies.
R 7.4In boys with PAIS, we suggest considering additional testosterone treatment in mid puberty depending on the clinical and biochemical assessment of pubertal development. If clinical signs of hypoandrogenism such as micropenis and gynaecomastia are present, we suggest treating with the addition of testosterone for 6 months and then evaluating the effect. (+OOO)
Rationale
The clinical phenotype of individuals with PAIS who identify as males is highly variable from micropenis to severe hypospadias and/or bifid scrotum. It is a theoretical consideration that the inherent androgen insensitivity can be overcome by adding supraphysiological testosterone levels on top of the endogenous testosterone secretion. Anecdotally, patients report a beneficial effect on genital growth and wellbeing, but no randomised trials exist. Transdermal testosterone and long-acting i.m. injections have been tried (287). Clearly, serum testosterone concentrations cannot be used to monitor efficacy, which relies exclusively on clinical improvement and general wellbeing. Biochemically, haematocrit is suggested as a safety parameter. LH has also been suggested as a surrogate marker for optimal dosing of testosterone in males with PAIS.
The majority of males with PAIS develop gynaecomastia (38, 277, 288), which may theoretically either worsen (because of increased E2) or improve (because of lowered LH-induced aromatase activity) with pharmacological testosterone treatment (286). Breast cancer has only been described in a few cases (289). The effect of medical treatment on gynaecomastia is variable and most males decide to undergo mastectomy. Based on the beneficial effects of oestrogen receptor blocking in pubertal gynaecomastia, this treatment modality could also be considered in male patients with PAIS (290). Successful use of tamoxifen, a selective oestrogen receptor blocker, to reduce gynaecomastia was described in two patients with PAIS (291). However, long-term studies are lacking, and whether or not there is a role for oestrogen blockers to prevent gynecomastia remains to be studied.
Other signs of under-virilisation in patients with PAIS raised as boys, such as hypospadias and cryptorchidism, have been surgically corrected during infancy or childhood in most patients.
Future perspectives
Most of the recommendations in this guideline are based on expert clinical experience and often a long-term clinical experience of treating a large number of individuals. Studies on pubertal induction specifically in individuals with a DSD are scarce. It is clear that further studies are needed. Just to mention a few, issues directly related to hormone treatment with improved formulations and optimal route of administration as well as timing and progression of puberty are needed. The function and importance of the mini-puberty are still largely unknown. The issue of fertility and how the possibility for future fertility can be improved for individuals with different diagnoses are attracting increased attention. Does the treatment with FSH and LH/hCG before the start of testosterone increase the possibility of future fertility in CHH? Studies on optimisation of sex hormone replacement in AIS and how to avoid long-term consequences for these patients are warranted. Animal studies and stem-cell research may open possibilities for the development of novel technologies involving Leydig cell transplantation in male hypogonadism in the future (292).
Diagnostic difficulties to distinguish CHH vs CDGP are still largely unsolved and a clinical recurrent issue. Improved tools for identifying a halt in pubertal development would be of benefit for many patients. Improved psychosocial support for patients is essential.
Conclusion
Disorders of sexual development comprise a large array of diagnoses and patients with CHH and DSD present different symptoms and needs, especially during puberty. The care for these individuals and their families needs to be individualised and requires the involvement of specialised multidisciplinary teams.
Supplementary Material
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this guidance.
Funding
This work did not receive any specific grant from any funding agency in the public, commercial, or not-for-profit sector.
Acknowledgements
The authors acknowledge valuable comments and contributions to the manuscript from the following reviewers: Claire Bouvattier, Department of Pediatric endocrinology and diabetes, hôpital Bicêtre, Assistance Publique – Hôpitaux de Paris, Le Kremlin Bicêtre, France; Silvano Bertelloni, Uo Pediatria 1 – Dipartimento Materno-infantile, Azienda Ospedaliero-Universitaria Pisana, Pisa, Italy; Sophie Christin-Maitre, Department diabetology and reproductive medicine, St Antoine Hospital, Sorbonne University, Paris, France; Richard Quinton Department of Endocrinology, Diabetes & Metabolism, Newcastle-upon-Tyne Hospitals, NE1 4LP, UK and Translational & Clinical Research Institute, University of Newcastle-upon-Tyne, UK; Giulia Rastrelli, Department of Experimental and Clinical Biomedical Sciences ‘Mario Serio’, University of Florence and Andrology Womens Endocrinology Unit Careggi Teaching Hospital Florence, Italy.
References
- 1.Parent AS, Teilmann G, Juul A, Skakkebaek NE, Toppari J, Bourguignon JP. The timing of normal puberty and the age limits of sexual precocity: variations around the world, secular trends, and changes after migration. Endocrine Reviews 200324668–693. ( 10.1210/er.2002-0019) [DOI] [PubMed] [Google Scholar]
- 2.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Archives of Disease in Childhood 196944291–303. ( 10.1136/adc.44.235.291) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sørensen K, Aksglaede L, Petersen JH, Juul A. Recent changes in pubertal timing in healthy Danish boys: associations with body mass index. Journal of Clinical Endocrinology and Metabolism 201095263–270. ( 10.1210/jc.2009-1478) [DOI] [PubMed] [Google Scholar]
- 4.Sørensen K, Mouritsen A, Aksglaede L, Hagen CP, Mogensen SS, Juul A. Recent secular trends in pubertal timing: implications for evaluation and diagnosis of precocious puberty. Hormone Research in Paediatrics 201277137–145. ( 10.1159/000336325) [DOI] [PubMed] [Google Scholar]
- 5.Taranger J, Engström I, Lichtenstein H, Svennberg- Redegren I. VI. Somatic pubertal development. Acta Paediatrica 197665 (S258)121–135. ( 10.1111/j.1651-2227.1976.tb14766.x) [DOI] [PubMed] [Google Scholar]
- 6.Biro FM, Huang B, Crawford PB, Lucky AW, Striegel-Moore R, Barton BA, Daniels S. Pubertal correlates in black and white girls. Journal of Pediatrics 2006148234–240. ( 10.1016/j.jpeds.2005.10.020) [DOI] [PubMed] [Google Scholar]
- 7.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Archives of Disease in Childhood 19704513–23. ( 10.1136/adc.45.239.13) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Busch AS, Hollis B, Day FR, Sørensen K, Aksglaede L, Perry JRB, Ong KK, Juul A, Hagen CP. Voice break in boys-temporal relations with other pubertal milestones and likely causal effects of BMI. Human Reproduction 2019341514–1522. ( 10.1093/humrep/dez118) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bizzarri C, Cappa M. Ontogeny of hypothalamus-pituitary gonadal axis and minipuberty: an ongoing debate? Frontiers in Endocrinology 202011 187. ( 10.3389/fendo.2020.00187) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanciotti L, Cofini M, Leonardi A, Penta L, Esposito S. Up-to-date review about minipuberty and overview on hypothalamic-pituitary-gonadal axis activation in fetal and neonatal life. Frontiers in Endocrinology 20189 410. ( 10.3389/fendo.2018.00410) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hines M, Spencer D, Kung KT, Browne WV, Constantinescu M, Noorderhaven RM. The early postnatal period, mini-puberty, provides a window on the role of testosterone in human neurobehavioural development. Current Opinion in Neurobiology 20163869–73. ( 10.1016/j.conb.2016.02.008) [DOI] [PubMed] [Google Scholar]
- 12.Papadimitriou DT, Chrysis D, Nyktari G, Zoupanos G, Liakou E, Papadimitriou A, Mastorakos G. Replacement of male mini-puberty. Journal of the Endocrine Society 201931275–1282. ( 10.1210/js.2019-00083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jespersen K, Ljubicic ML, Johannsen TH, Christiansen P, Skakkebaek NE, Juul A. Distinguishing between hidden testes and anorchia: the role of endocrine evaluation in infancy and childhood. European Journal of Endocrinology 2020183107–117. ( 10.1530/EJE-20-0041) [DOI] [PubMed] [Google Scholar]
- 14.Aksglaede L, Davis SM, Ross JL, Juul A. Minipuberty in Klinefelter syndrome: current status and future directions. American Journal of Medical Genetics: Part C, Seminars in Medical Genetics 2020184320–326. ( 10.1002/ajmg.c.31794) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Johannsen TH, Main KM, Ljubicic ML, Jensen TK, Andersen HR, Andersen MS, Petersen JH, Andersson AM, Juul A. Sex differences in reproductive hormones during mini-puberty in infants with normal and disordered sex development. Journal of Clinical Endocrinology and Metabolism 20181033028–3037. ( 10.1210/jc.2018-00482) [DOI] [PubMed] [Google Scholar]
- 16.Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, Elford S, Hughes IA, Izatt L, Krone Net al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (revised 2015). Clinical Endocrinology 201684771–788. ( 10.1111/cen.12857) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Faure-Conter C, Orbach D, Fresneau B, Verité C, Bonneau J, Thebaud E, Poirée M, Thouvenin S, Pluchart C, Mure PYet al. Disorder of sex development with germ cell tumors: which is uncovered first? Pediatric Blood and Cancer 202067 e28169. ( 10.1002/pbc.28169) [DOI] [PubMed] [Google Scholar]
- 18.Lucas-Herald AK, Bryce J, Kyriakou A, Ljubicic ML, Arlt W, Audi L, Balsamo A, Baronio F, Bertelloni S, Bettendorf Met al. Gonadectomy in conditions affecting sex development: a registry-based cohort study. European Journal of Endocrinology 2021184791–801. ( 10.1530/EJE-20-1058) [DOI] [PubMed] [Google Scholar]
- 19.Mendonca BB, Leite MV, de Castro M, Kino T, Elias LL, Bachega TA, Arnhold IJ, Chrousos GP, Latronico AC. Female pseudohermaphroditism caused by a novel homozygous missense mutation of the GR gene. Journal of Clinical Endocrinology and Metabolism 2002871805–1809. ( 10.1210/jcem.87.4.8379) [DOI] [PubMed] [Google Scholar]
- 20.Young J, Xu C, Papadakis GE, Acierno JS, Maione L, Hietamäki J, Raivio T, Pitteloud N. Clinical management of congenital hypogonadotropic hypogonadism. Endocrine Reviews 201940669–710. ( 10.1210/er.2018-00116) [DOI] [PubMed] [Google Scholar]
- 21.Nordenström A.Puberty in individuals with a disorder of sex development. Current Opinion in Endocrine and Metabolic Research 20201442–51. ( 10.1016/j.coemr.2020.05.004) [DOI] [Google Scholar]
- 22.Andrade JGR, Fabbri-Scallet H, Dos Santos AP, Cools M, Werner R, Hiort O, de Mello MP, Guerra-Júnior G, Maciel-Guerra AT. Clinical findings and follow-up of 46,XY and 45,X/46,XY testicular dysgenesis. Sexual Development 201913171–177. ( 10.1159/000504239) [DOI] [PubMed] [Google Scholar]
- 23.Gabriel Ribeiro de Andrade J, Marques-de-Faria AP, Fabbri HC, de Mello MP, Guerra-Júnior G, Maciel-Guerra AT. Long-term follow-up of patients with 46,XY partial gonadal dysgenesis reared as males. International Journal of Endocrinology 20142014480724. ( 10.1155/2014/480724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gomes NL, Lerário AM, Machado AZ, Moraes DR, Silva TED, Arnhold IJP, Batista RL, Faria Júnior JAD, Costa EF, Nishi MYet al. Long-term outcomes and molecular analysis of a large cohort of patients with 46,XY disorder of sex development due to partial gonadal dysgenesis. Clinical Endocrinology 201889164–177. ( 10.1111/cen.13717) [DOI] [PubMed] [Google Scholar]
- 25.Ljubicic ML, Jørgensen A, Acerini C, Andrade J, Balsamo A, Bertelloni S, Cools M, Cuccaro RT, Darendeliler F, Flück CEet al. Clinical but not histological outcomes in males with 45,X/46,XY mosaicism vary depending on reason for diagnosis. Journal of Clinical Endocrinology and Metabolism 20191044366–4381. ( 10.1210/jc.2018-02752) [DOI] [PubMed] [Google Scholar]
- 26.Kim YM, Oh A, Kim KS, Yoo HW, Choi JH. Pubertal outcomes and sex of rearing of patients with ovotesticular disorder of sex development and mixed gonadal dysgenesis. Annals of Pediatric Endocrinology and Metabolism 201924231–236. ( 10.6065/apem.2019.24.4.231) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gravholt CH, Viuff MH, Brun S, Stochholm K, Andersen NH. Turner syndrome: mechanisms and management. Nature Reviews: Endocrinology 201915601–614. ( 10.1038/s41574-019-0224-4) [DOI] [PubMed] [Google Scholar]
- 28.Bernard V, Donadille B, Zenaty D, Courtillot C, Salenave S, Brac de la Perrière A, Albarel F, Fèvre A, Kerlan V, Brue Tet al. Spontaneous fertility and pregnancy outcomes amongst 480 women with Turner syndrome. Human Reproduction 201631782–788. ( 10.1093/humrep/dew012) [DOI] [PubMed] [Google Scholar]
- 29.Chang S, Skakkebaek A, Davis SM, Gravholt CH. Morbidity in Klinefelter syndrome and the effect of testosterone treatment. American Journal of Medical Genetics: Part C, Seminars in Medical Genetics 2020184344–355. ( 10.1002/ajmg.c.31798) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zitzmann M, Aksglaede L, Corona G, Isidori AM, Juul A, T’Sjoen G, Kliesch S, D’Hauwers K, Toppari J, Słowikowska-Hilczer Jet al. European academy of andrology guidelines on Klinefelter Syndrome Endorsing Organization: European Society of Endocrinology. Andrology 20219145–167. ( 10.1111/andr.12909) [DOI] [PubMed] [Google Scholar]
- 31.Boeri L, Palmisano F, Preto M, Sibona M, Capogrosso P, Franceschelli A, Ruiz-Castañé E, Sarquella-Geli J, Bassas-Arnau L, Scroppo FIet al. Sperm retrieval rates in non-mosaic Klinefelter patients undergoing testicular sperm extraction: what expectations do we have in the real-life setting? Andrology 20208680–687. ( 10.1111/andr.12767) [DOI] [PubMed] [Google Scholar]
- 32.Corona G, Pizzocaro A, Lanfranco F, Garolla A, Pelliccione F, Vignozzi L, Ferlin A, Foresta C, Jannini EA, Maggi Met al. Sperm recovery and ICSI outcomes in Klinefelter syndrome: a systematic review and meta-analysis. Human Reproduction Update 201723265–275. ( 10.1093/humupd/dmx008) [DOI] [PubMed] [Google Scholar]
- 33.Chen T, Tian L, Wu F, Xuan X, Ma G, Tang R, Lu J. Clinical and genetic analysis in males with 46,XX disorders of sex development: a reproductive centre experience of 144 cases. Andrologia 201951 e13232. ( 10.1111/and.13232) [DOI] [PubMed] [Google Scholar]
- 34.Verkauskas G, Jaubert F, Lortat-Jacob S, Malan V, Thibaud E, Nihoul-Fékété C. The long-term followup of 33 cases of true hermaphroditism: a 40-year experience with conservative gonadal surgery. Journal of Urology 2007177726–731; discussion 731. ( 10.1016/j.juro.2006.10.003) [DOI] [PubMed] [Google Scholar]
- 35.Avendaño A, Paradisi I, Cammarata-Scalisi F, Callea M. 5-α-Reductase type 2 deficiency: is there a genotype-phenotype correlation? A review. Hormones 201817197–204. ( 10.1007/s42000-018-0013-9) [DOI] [PubMed] [Google Scholar]
- 36.Marzuki NS, Idris FP, Kartapradja HD, Harahap AR, Batubara JRL. Characterising SRD5A2 gene variants in 37 Indonesian patients with 5-alpha-reductase type 2 deficiency. International Journal of Endocrinology 201920197676341. ( 10.1155/2019/7676341) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hiort O, Marshall L, Birnbaum W, Wünsch L, Holterhus PM, Döhnert U, Werner R. Pubertal development in17beta-hydroxysteroid dehydrogenase type 3. Hormone Research in Paediatrics 201787354–358. ( 10.1159/000453613) [DOI] [PubMed] [Google Scholar]
- 38.Mongan NP, Tadokoro-Cuccaro R, Bunch T, Hughes IA. Androgen insensitivity syndrome. Best Practice and Research: Clinical Endocrinology and Metabolism 201529569–580. ( 10.1016/j.beem.2015.04.005) [DOI] [PubMed] [Google Scholar]
- 39.Herlin MK, Petersen MB, Brännström M. Mayer-Rokitansky-Küster-Hauser (MRKH) syndrome: a comprehensive update. Orphanet Journal of Rare Diseases 202015 214. ( 10.1186/s13023-020-01491-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmed SF, Khwaja O, Hughes IA. The role of a clinical score in the assessment of ambiguous genitalia. BJU International 200085120–124. ( 10.1046/j.1464-410x.2000.00354.x) [DOI] [PubMed] [Google Scholar]
- 41.Wickman S, Sipilä I, Ankarberg-Lindgren C, Norjavaara E, Dunkel L. A specific aromatase inhibitor and potential increase in adult height in boys with delayed puberty: a randomised controlled trial. Lancet 20013571743–1748. ( 10.1016/S0140-6736(0004895-9) [DOI] [PubMed] [Google Scholar]
- 42.McGrath N, O’Grady MJ. Aromatase inhibitors for short stature in male children and adolescents. Cochrane Database of Systematic Reviews 2015CD010888. ( 10.1002/14651858.CD010888.pub2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Varimo T, Toiviainen-Salo S, Raivio T, Kerttula L, Dunkel L, Hero M. Letrozole monotherapy in pre- and early-pubertal boys does not increase adult height. Frontiers in Endocrinology 201910 201. ( 10.3389/fendo.2019.00201) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Papadimitriou DT, Dermitzaki E, Papagianni M, Papaioannou G, Papaevangelou V, Papadimitriou A. Anastrozole plus leuprorelin in early maturing girls with compromised growth: the ‘GAIL’ study. Journal of Endocrinological Investigation 201639439–446. ( 10.1007/s40618-015-0399-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merke DP, Keil MF, Jones JV, Fields J, Hill S, Cutler GB. Flutamide, testolactone, and reduced hydrocortisone dose maintain normal growth velocity and bone maturation despite elevated androgen levels in children with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2000851114–1120. ( 10.1210/jcem.85.3.6462) [DOI] [PubMed] [Google Scholar]
- 46.Hero M, Varimo T, Raivio T. Aromatase inhibitors in puberty. Current Opinion in Endocrine and Metabolic Research 20201437–41. ( 10.1016/j.coemr.2020.04.001) [DOI] [Google Scholar]
- 47.Hero M, Norjavaara E, Dunkel L. Inhibition of estrogen biosynthesis with a potent aromatase inhibitor increases predicted adult height in boys with idiopathic short stature: a randomized controlled trial. Journal of Clinical Endocrinology and Metabolism 2005906396–6402. ( 10.1210/jc.2005-1392) [DOI] [PubMed] [Google Scholar]
- 48.Johannsen TH, Andersson AM, Ahmed SF, de Rijke YB, Greaves RF, Hartmann MF, Hiort O, Holterhus PM, Krone NP, Kulle Aet al. Peptide hormone analysis in diagnosis and treatment of differences of sex development: joint position paper of EU COST Action ‘DSDnet’ and European Reference Network on Rare Endocrine Conditions. European Journal of Endocrinology 2020182P1–P15. ( 10.1530/EJE-19-0831) [DOI] [PubMed] [Google Scholar]
- 49.Albrethsen J, Johannsen TH, Jørgensen N, Frederiksen H, Sennels HP, Jørgensen HL, Fahrenkrug J, Petersen JH, Linneberg A, Nordkap Let al. Evaluation of serum insulin-like factor 3 quantification by LC-MS/MS as a biomarker of Leydig cell function. Journal of Clinical Endocrinology and Metabolism 2020105 dgaa145. ( 10.1210/clinem/dgaa145) [DOI] [PubMed] [Google Scholar]
- 50.Vestergaard ET, Schjørring ME, Kamperis K, Petersen KK, Rittig S, Juul A, Kristensen K, Birkebæk NH. The follicle-stimulating hormone (FSH) and luteinizing hormone (LH) response to a gonadotropin-releasing hormone analogue test in healthy prepubertal girls aged 10 months to 6 years. European Journal of Endocrinology 2017176747–753. ( 10.1530/EJE-17-0042) [DOI] [PubMed] [Google Scholar]
- 51.Mosbah H, Bouvattier C, Maione L, Trabado S, De Filippo G, Cartes A, Donzeau A, Chanson P, Brailly-Tabard S, Dwyer AAet al. GnRH stimulation testing and serum inhibin B in males: insufficient specificity for discriminating between congenital hypogonadotropic hypogonadism from constitutional delay of growth and puberty. Human Reproduction 2020352312–2322. ( 10.1093/humrep/deaa185) [DOI] [PubMed] [Google Scholar]
- 52.Ljubicic ML, Jespersen K, Aksglaede L, Hagen CP, Petersen JH, Andersen HR, Linneberg A, Main KM, Andersson AM, Johannsen THet al. The LH/FSH ratio is not a sex-dimorphic marker after infancy: data from 6417 healthy individuals and 125 patients with differences of sex development. Human Reproduction 2020352323–2335. ( 10.1093/humrep/deaa182) [DOI] [PubMed] [Google Scholar]
- 53.Stanczyk FZ, Clarke NJ. Measurement of estradiol – challenges ahead. Journal of Clinical Endocrinology and Metabolism 20149956–58. ( 10.1210/jc.2013-2905) [DOI] [PubMed] [Google Scholar]
- 54.Handelsman DJ, Wartofsky L. Requirement for mass spectrometry sex steroid assays in the Journal of Clinical Endocrinology and Metabolism. Journal of Clinical Endocrinology and Metabolism 2013983971–3973. ( 10.1210/jc.2013-3375) [DOI] [PubMed] [Google Scholar]
- 55.Kulle A, Krone N, Holterhus PM, Schuler G, Greaves RF, Juul A, de Rijke YB, Hartmann MF, Saba A, Hiort Oet al. Steroid hormone analysis in diagnosis and treatment of DSD: position paper of EU COST Action BM 1303 ‘DSDnet’. European Journal of Endocrinology 2017176P1–P9. ( 10.1530/EJE-16-0953) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Norjavaara E, Ankarberg C, Albertsson-Wikland K. Diurnal rhythm of 17 beta-estradiol secretion throughout pubertal development in healthy girls: evaluation by a sensitive radioimmunoassay. Journal of Clinical Endocrinology and Metabolism 1996814095–4102. ( 10.1210/jcem.81.11.8923866) [DOI] [PubMed] [Google Scholar]
- 57.Keevil BG.LC-MS/MS analysis of steroids in the clinical laboratory. Clinical Biochemistry 201649989–997. ( 10.1016/j.clinbiochem.2016.04.009) [DOI] [PubMed] [Google Scholar]
- 58.Frederiksen H, Johannsen TH, Andersen SE, Albrethsen J, Landersoe SK, Petersen JH, Andersen AN, Vestergaard ET, Schorring ME, Linneberg Aet al. Sex-specific estrogen levels and reference intervals from infancy to late adulthood determined by LC-MS/MS. Journal of Clinical Endocrinology and Metabolism 2020105754–768. ( 10.1210/clinem/dgz196) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Søeborg T, Frederiksen H, Mouritsen A, Johannsen TH, Main KM, Jørgensen N, Petersen JH, Andersson AM, Juul A. Sex, age, pubertal development and use of oral contraceptives in relation to serum concentrations of DHEA, DHEAS, 17α-hydroxyprogesterone, Δ4-androstenedione, testosterone and their ratios in children, adolescents and young adults. Clinica Chimica Acta: International Journal of Clinical Chemistry 20144376–13. ( 10.1016/j.cca.2014.06.018) [DOI] [PubMed] [Google Scholar]
- 60.Crone EA, Dahl RE. Understanding adolescence as a period of social-affective engagement and goal flexibility. Nature Reviews: Neuroscience 201213636–650. ( 10.1038/nrn3313) [DOI] [PubMed] [Google Scholar]
- 61.Goddings AL, Beltz A, Peper JS, Crone EA, Braams BR. Understanding the role of puberty in structural and functional development of the adolescent brain. Journal of Research on Adolescence 20192932–53. ( 10.1111/jora.12408) [DOI] [PubMed] [Google Scholar]
- 62.Nordenström A, Thyen U. Improving the communication of healthcare professionals with affected children and adolescents. Endocrine Development 201427113–127. ( 10.1159/000363636) [DOI] [PubMed] [Google Scholar]
- 63.Berenbaum SA.Effects of early androgens on sex-typed activities and interests in adolescents with congenital adrenal hyperplasia. Hormones and Behavior 199935102–110. ( 10.1006/hbeh.1998.1503) [DOI] [PubMed] [Google Scholar]
- 64.Pasterski VL, Geffner ME, Brain C, Hindmarsh P, Brook C, Hines M. Prenatal hormones and postnatal socialization by parents as determinants of male-typical toy play in girls with congenital adrenal hyperplasia. Child Development 200576264–278. ( 10.1111/j.1467-8624.2005.00843.x) [DOI] [PubMed] [Google Scholar]
- 65.Wong WI, Pasterski V, Hindmarsh PC, Geffner ME, Hines M. Are there parental socialization effects on the sex-typed behavior of individuals with congenital adrenal hyperplasia? Archives of Sexual Behavior 201342381–391. ( 10.1007/s10508-012-9997-4) [DOI] [PubMed] [Google Scholar]
- 66.Hines M.Human gender development. Neuroscience and Biobehavioral Reviews 202011889–96. ( 10.1016/j.neubiorev.2020.07.018) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jürgensen M, Hiort O, Holterhus PM, Thyen U. Gender role behavior in children with XY karyotype and disorders of sex development. Hormones and Behavior 200751443–453. ( 10.1016/j.yhbeh.2007.01.001) [DOI] [PubMed] [Google Scholar]
- 68.Hines M, Ahmed SF, Hughes IA. Psychological outcomes and gender-related development in complete androgen insensitivity syndrome. Archives of Sexual Behavior 20033293–101. ( 10.1023/a:1022492106974) [DOI] [PubMed] [Google Scholar]
- 69.Nordenström A, Servin A, Bohlin G, Larsson A, Wedell A. Sex-typed toy play behavior correlates with the degree of prenatal androgen exposure assessed by CYP21 genotype in girls with congenital adrenal hyperplasia. Journal of Clinical Endocrinology and Metabolism 2002875119–5124. ( 10.1210/jc.2001-011531) [DOI] [PubMed] [Google Scholar]
- 70.Frisén L, Nordenström A, Falhammar H, Filipsson H, Holmdahl G, Janson PO, Thorén M, Hagenfeldt K, Möller A, Nordenskjöld A. Gender role behavior, sexuality, and psychosocial adaptation in women with congenital adrenal hyperplasia due to CYP21A2 deficiency. Journal of Clinical Endocrinology and Metabolism 2009943432–3439. ( 10.1210/jc.2009-0636) [DOI] [PubMed] [Google Scholar]
- 71.Wisniewski AB, Migeon CJ, Gearhart JP, Rock JA, Berkovitz GD, Plotnick LP, Meyer-Bahlburg HF, Money J. Congenital micropenis: long-term medical, surgical and psychosexual follow-up of individuals raised male or female. Hormone Research 2001563–11. ( 10.1159/000048083) [DOI] [PubMed] [Google Scholar]
- 72.Mazur T.Gender dysphoria and gender change in androgen insensitivity or micropenis. Archives of Sexual Behavior 200534411–421. ( 10.1007/s10508-005-4341-x) [DOI] [PubMed] [Google Scholar]
- 73.Cohen-Kettenis PT.Gender change in 46,XY persons with 5alpha-reductase-2 deficiency and 17beta-hydroxysteroid dehydrogenase-3 deficiency. Archives of Sexual Behavior 200534399–410. ( 10.1007/s10508-005-4339-4) [DOI] [PubMed] [Google Scholar]
- 74.Richter-Appelt H, Discher C, Gedrose B. Gender identity and recalled gender related childhood play-behaviour in adult individuals with different forms of intersexuality. Anthropologischer Anzeiger 200563241–256. ( 10.1127/anthranz/63/2005/241) [DOI] [PubMed] [Google Scholar]
- 75.Jurgensen M, Kleinemeier E, Lux A, Steensma TD, Cohen-Kettenis PT, Hiort O, Thyen U, Köhler B. & DSD Network Working Group. Psychosexual development in adolescents and adults with disorders of sex development – results from the German Clinical Evaluation Study. Journal of Sexual Medicine 2013102703–2714. ( 10.1111/j.1743-6109.2012.02751.x) [DOI] [PubMed] [Google Scholar]
- 76.Ediati A, Juniarto AZ, Birnie E, Drop SL, Faradz SM, Dessens AB. Gender development in Indonesian children, adolescents, and adults with disorders of sex development. Archives of Sexual Behavior 2015441339–1361. ( 10.1007/s10508-015-0493-5) [DOI] [PubMed] [Google Scholar]
- 77.Callens N, Van Kuyk M, van Kuppenveld JH, Drop SLS, Cohen-Kettenis PT, Dessens AB. & Dutch Study Group on DSD. Recalled and current gender role behavior, gender identity and sexual orientation in adults with disorders/differences of sex development. Hormones and Behavior 2016868–20. ( 10.1016/j.yhbeh.2016.08.008) [DOI] [PubMed] [Google Scholar]
- 78.Kreukels BPC, Kohler B, Nordenstrom A, Roehle R, Thyen U, Bouvattier C, de Vries ALC, Cohen-Kettenis PT. & dsd-LIFE group. Gender dysphoria and gender change in disorders of sex development/intersex conditions: results from the dsd-LIFE study. Journal of Sexual Medicine 201815777–785. ( 10.1016/j.jsxm.2018.02.021) [DOI] [PubMed] [Google Scholar]
- 79.Berenbaum SA, Beltz AM. Sexual differentiation of human behavior: effects of prenatal and pubertal organizational hormones. Frontiers in Neuroendocrinology 201132183–200. ( 10.1016/j.yfrne.2011.03.001) [DOI] [PubMed] [Google Scholar]
- 80.Hines M.Gender development and the human brain. Annual Review of Neuroscience 20113469–88. ( 10.1146/annurev-neuro-061010-113654) [DOI] [PubMed] [Google Scholar]
- 81.Warne GL, Raza J. Disorders of sex development (DSDs), their presentation and management in different cultures. Reviews in Endocrine and Metabolic Disorders 20089227–236. ( 10.1007/s11154-008-9084-2) [DOI] [PubMed] [Google Scholar]
- 82.Zainuddin AA, Grover SR, Shamsuddin K, Mahdy ZA. Research on quality of life in female patients with congenital adrenal hyperplasia and issues in developing nations. Journal of Pediatric and Adolescent Gynecology 201326296–304. ( 10.1016/j.jpag.2012.08.004) [DOI] [PubMed] [Google Scholar]
- 83.Chowdhury TK, Kabir M, Chowdhury MZ, Hutson JM, Banu T. The challenges in diagnosis and gender assignment in disorders of sex development presenting to a pediatric surgical unit in a developing country: the role of laparoscopy and simple tests for gender identity. Journal of Pediatric Urology 2014101255–1260. ( 10.1016/j.jpurol.2014.06.021) [DOI] [PubMed] [Google Scholar]
- 84.Ediati A, Faradz SM, Juniarto AZ, van der Ende J, Drop SL, Dessens AB. Emotional and behavioral problems in late-identified Indonesian patients with disorders of sex development. Journal of Psychosomatic Research 20157976–84. ( 10.1016/j.jpsychores.2014.12.007) [DOI] [PubMed] [Google Scholar]
- 85.Ediati A, Juniarto AZ, Birnie E, Okkerse J, Wisniewski A, Drop S, Faradz SMH, Dessens A. Social stigmatisation in late identified patients with disorders of sex development in Indonesia. BMJ Paediatrics Open 20171 e000130. ( 10.1136/bmjpo-2017-000130) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hepburn CM, Cohen E, Bhawra J, Weiser N, Hayeems RZ, Guttmann A. Health system strategies supporting transition to adult care. Archives of Disease in Childhood 2015100559–564. ( 10.1136/archdischild-2014-307320) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.van Staa AL, Jedeloo S, van Meeteren J, Latour JM. Crossing the transition chasm: experiences and recommendations for improving transitional care of young adults, parents and providers. Child: Care, Health and Development 201137821–832. ( 10.1111/j.1365-2214.2011.01261.x) [DOI] [PubMed] [Google Scholar]
- 88.Monaghan M, Hilliard M, Sweenie R, Riekert K. Transition readiness in adolescents and emerging adults with diabetes: the role of patient-provider communication. Current Diabetes Reports 201313900–908. ( 10.1007/s11892-013-0420-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Godbout A, Tejedor I, Malivoir S, Polak M, Touraine P. Transition from pediatric to adult healthcare: assessment of specific needs of patients with chronic endocrine conditions. Hormone Research in Paediatrics 201278247–255. ( 10.1159/000343818) [DOI] [PubMed] [Google Scholar]
- 90.American Academy of Pediatrics, American Academy of Family Physicians & American College of Physicians-American Society of Internal Medicine. A consensus statement on health care transitions for young adults with special health care needs. Pediatrics 20021101304–1306. ( 10.1542/peds.110.S3.1304) [DOI] [PubMed] [Google Scholar]
- 91.Kaufman M, Pinzon J. & Canadian Paediatric Society, Adolescent Health Committee. Transition to adult care for youth with special health care needs. Paediatrics and Child Health 200712785–793. ( 10.1093/pch/12.9.785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Nagra A, McGinnity PM, Davis N, Salmon AP. Implementing transition: ready steady go. Archives of Disease in Childhood: Education and Practice Edition 2015100313–320. ( 10.1136/archdischild-2014-307423) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Le Roux E, Menesguen F, Tejedor I, Popelier M, Halbron M, Faucher P, Malivoir S, Pinto G, Léger J, Hatem Set al. Transition of young adults with endocrine and metabolic diseases: the ‘TRANSEND’ cohort. Endocrine Connections 20211021–28. ( 10.1530/EC-20-0520) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Colver A, McConachie H, Le Couteur A, Dovey-Pearce G, Mann KD, McDonagh JE, Pearce MS, Vale L, Merrick H, Parr JRet al. A longitudinal, observational study of the features of transitional healthcare associated with better outcomes for young people with long-term conditions. BMC Medicine 201816 111. ( 10.1186/s12916-018-1102-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bachelot A, Vialon M, Baptiste A, Tejedor I, Elie C, Polak M, Touraine P. Impact of transition on quality of life in patients with congenital adrenal hyperplasia diagnosed during childhood. Endocrine Connections 20176422–429. ( 10.1530/EC-17-0094) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, Norris S, Falck-Ytter Y, Glasziou P, DeBeer Het al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 201164383–394. ( 10.1016/j.jclinepi.2010.04.026) [DOI] [PubMed] [Google Scholar]
- 97.Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, Vist GE, Falck-Ytter Y, Meerpohl J, Norris Set al. GRADE guidelines: 3. Rating the quality of evidence. Journal of Clinical Epidemiology 201164401–406. ( 10.1016/j.jclinepi.2010.07.015) [DOI] [PubMed] [Google Scholar]
- 98.Andrews J, Guyatt G, Oxman AD, Alderson P, Dahm P, Falck-Ytter Y, Nasser M, Meerpohl J, Post PN, Kunz Ret al. GRADE guidelines: 14. Going from evidence to recommendations: the significance and presentation of recommendations. Journal of Clinical Epidemiology 201366719–725. ( 10.1016/j.jclinepi.2012.03.013) [DOI] [PubMed] [Google Scholar]
- 99.Andrews JC, Schünemann HJ, Oxman AD, Pottie K, Meerpohl JJ, Coello PA, Rind D, Montori VM, Brito JP, Norris Set al. Going from evidence to recommendation-determinants of a recommendation’s direction and strength. Journal of Clinical Epidemiology 201366726–735. ( 10.1016/j.jclinepi.2013.02.003) [DOI] [PubMed] [Google Scholar]
- 100.Arisaka O, Arisaka M, Nakayama Y, Fujiwara S, Yabuta K. Effect of testosterone on bone density and bone metabolism in adolescent male hypogonadism. Metabolism: Clinical and Experimental 199544419–423. ( 10.1016/0026-0495(9590046-2) [DOI] [PubMed] [Google Scholar]
- 101.Bistritzer T, Lunenfeld B, Passwell JH, Theodor R. Hormonal therapy and pubertal development in boys with selective hypogonadotropic hypogonadism. Fertility and Sterility 198952302–306. ( 10.1016/s0015-0282(1660859-2) [DOI] [PubMed] [Google Scholar]
- 102.Delemarre-Van de Waal HA.Induction of testicular growth and spermatogenesis by pulsatile, intravenous administration of gonadotrophin-releasing hormone in patients with hypogonadotrophic hypogonadism. Clinical Endocrinology 199338473–480. ( 10.1111/j.1365-2265.1993.tb00342.x) [DOI] [PubMed] [Google Scholar]
- 103.Gong C, Liu Y, Qin M, Wu D, Wang X. Pulsatile GnRH is superior to hCG in therapeutic efficacy in adolescent boys with hypogonadotropic hypogonadodism. Journal of Clinical Endocrinology and Metabolism 20151002793–2799. ( 10.1210/jc.2015-1343) [DOI] [PubMed] [Google Scholar]
- 104.Nabhan ZM, Dimeglio LA, Qi R, Perkins SM, Eugster EA. Conjugated oral versus transdermal estrogen replacement in girls with Turner syndrome: a pilot comparative study. Journal of Clinical Endocrinology and Metabolism 2009942009–2014. ( 10.1210/jc.2008-2123) [DOI] [PubMed] [Google Scholar]
- 105.Shah S, Forghani N, Durham E, Neely EK. A randomized trial of transdermal and oral estrogen therapy in adolescent girls with hypogonadism. International Journal of Pediatric Endocrinology 20142014 12. ( 10.1186/1687-9856-2014-12) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Quigley CA, Crowe BJ, Anglin DG, Chipman JJ. Growth hormone and low dose estrogen in Turner syndrome: results of a United States multi-center trial to near-final height. Journal of Clinical Endocrinology and Metabolism 2002872033–2041. ( 10.1210/jcem.87.5.8477) [DOI] [PubMed] [Google Scholar]
- 107.Nilsson KO, Albertsson-Wikland K, Alm J, Aronson S, Gustafsson J, Hagenäs L, Häger A, Ivarsson SA, Karlberg J, Kriström Bet al. Improved final height in girls with Turner’s syndrome treated with growth hormone and oxandrolone. Journal of Clinical Endocrinology and Metabolism 199681635–640. ( 10.1210/jcem.81.2.8636281) [DOI] [PubMed] [Google Scholar]
- 108.Ruszala A, Wojcik M, Zygmunt-Gorska A, Janus D, Wojtys J, Starzyk JB. Prepubertal ultra-low-dose estrogen therapy is associated with healthier lipid profile than conventional estrogen replacement for pubertal induction in adolescent girls with Turner syndrome: preliminary results. Journal of Endocrinological Investigation 201740875–879. ( 10.1007/s40618-017-0665-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chernausek SD, Attie KM, Cara JF, Rosenfeld RG, Frane J. Growth hormone therapy of Turner syndrome: the impact of age of estrogen replacement on final height. Genentech, Inc., Collaborative Study Group. Journal of Clinical Endocrinology and Metabolism 2000852439–2445. ( 10.1210/jcem.85.7.6684) [DOI] [PubMed] [Google Scholar]
- 110.Demetriou E, Emans SJ, Crigler Jr JF. Final height in estrogen-treated patients with Turner syndrome. Obstetrics and Gynecology 198464459–464. ( 10.1097/00006254-198505000-00014) [DOI] [PubMed] [Google Scholar]
- 111.Hasegawa Y, Ariyasu D, Izawa M, Igaki-Miyamoto J, Fukuma M, Hatano M, Yagi H, Goto M. Gradually increasing ethinyl estradiol for Turner syndrome may produce good final height but not ideal BMD. Endocrine Journal 201764221–227. ( 10.1507/endocrj.EJ16-0170) [DOI] [PubMed] [Google Scholar]
- 112.Gault EJ, Cole TJ, Casey S, Hindmarsh PC, Betts P, Dunger DB, Donaldson MDC. Effect of oxandrolone and timing of pubertal induction on final height in Turner syndrome: final analysis of the UK randomised placebo-controlled trial. Archives of Disease in Childhood 202110674–76. ( 10.1136/archdischild-2019-317695) [DOI] [PubMed] [Google Scholar]
- 113.Rosenfield RL, Devine N, Hunold JJ, Mauras N, Moshang T, Root AW. Salutary effects of combining early very low-dose systemic estradiol with growth hormone therapy in girls with Turner syndrome. Journal of Clinical Endocrinology and Metabolism 2005906424–6430. ( 10.1210/jc.2005-1081) [DOI] [PubMed] [Google Scholar]
- 114.Lindhardt Johansen M, Hagen CP, Mieritz MG, Wolthers OD, Heuck C, Petersen JH, Juul A. Pubertal progression and reproductive hormones in healthy girls with transient thelarche. Journal of Clinical Endocrinology and Metabolism 20171021001–1008. ( 10.1210/jc.2016-2871) [DOI] [PubMed] [Google Scholar]
- 115.Bollino A, Cangiano B, Goggi G, Federici S, Duminuco P, Giovanelli L, Galazzi E, Vezzoli V, Persani L, Bonomi M. Pubertal delay: the challenge of a timely differential diagnosis between congenital hypogonadotropic hypogonadism and constitutional delay of growth and puberty. Minerva Pediatrica 202072278–287. ( 10.23736/S0026-4946.20.05860-0) [DOI] [PubMed] [Google Scholar]
- 116.Persani L, Bonomi M, Cools M, Dattani M, Dunkel L, Gravholt CH, Juul A. ENDO-ERN expert opinion on the differential diagnosis of pubertal delay. Endocrine 202171681–688. ( 10.1007/s12020-021-02626-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Sedlmeyer IL, Palmert MR. Delayed puberty: analysis of a large case series from an academic center. Journal of Clinical Endocrinology and Metabolism 2002871613–1620. ( 10.1210/jcem.87.4.8395) [DOI] [PubMed] [Google Scholar]
- 118.Raivio T, Miettinen PJ. Constitutional delay of puberty versus congenital hypogonadotropic hypogonadism: genetics, management and updates. Best Practice and Research: Clinical Endocrinology and Metabolism 201933101316. ( 10.1016/j.beem.2019.101316) [DOI] [PubMed] [Google Scholar]
- 119.Gravholt CH, Andersen NH, Conway GS, Dekkers OM, Geffner ME, Klein KO, Lin AE, Mauras N, Quigley CA, Rubin Ket al. Clinical practice guidelines for the care of girls and women with Turner syndrome: proceedings from the 2016 Cincinnati International Turner Syndrome Meeting. European Journal of Endocrinology 2017177G1–G70. ( 10.1530/EJE-17-0430) [DOI] [PubMed] [Google Scholar]
- 120.Klein KO, Rosenfield RL, Santen RJ, Gawlik AM, Backeljauw PF, Gravholt CH, Sas TCJ, Mauras N. Estrogen replacement in Turner syndrome: literature review and practical considerations. Journal of Clinical Endocrinology and Metabolism 20181031790–1803. ( 10.1210/jc.2017-02183) [DOI] [PubMed] [Google Scholar]
- 121.Cardona Attard C, Cameron-Pimblett A, Puri D, La Rosa C, Talaulikar VS, Davies MC, Learner HI, Liao LM, Conway GS. Relationship and sexual experiences in women with early-onset oestrogen deficiency: comparison between women with Turner syndrome and premature ovarian insufficiency. Clinical Endocrinology 202093473–481. ( 10.1111/cen.14271) [DOI] [PubMed] [Google Scholar]
- 122.Carel JC, Elie C, Ecosse E, Tauber M, Léger J, Cabrol S, Nicolino M, Brauner R, Chaussain JL, Coste J. Self-esteem and social adjustment in young women with Turner syndrome – influence of pubertal management and sexuality: population-based cohort study. Journal of Clinical Endocrinology and Metabolism 2006912972–2979. ( 10.1210/jc.2005-2652) [DOI] [PubMed] [Google Scholar]
- 123.Hankus M, Soltysik K, Szeliga K, Antosz A, Drosdzol-Cop A, Wilk K, Zachurzok A, Malecka-Tendera E, Gawlik AM. Prediction of spontaneous puberty in Turner syndrome based on mid-childhood gonadotropin concentrations, karyotype, and ovary visualization: a longitudinal study. Hormone Research in Paediatrics 20188990–97. ( 10.1159/000485321) [DOI] [PubMed] [Google Scholar]
- 124.Pasquino AM, Passeri F, Pucarelli I, Segni M, Municchi G. Spontaneous pubertal development in Turner’s syndrome. Italian Study Group for Turner’s syndrome. Journal of Clinical Endocrinology and Metabolism 1997821810–1813. ( 10.1210/jcem.82.6.3970) [DOI] [PubMed] [Google Scholar]
- 125.Negreiros LP, Bolina ER, Guimarães MM. Pubertal development profile in patients with Turner syndrome. Journal of Pediatric Endocrinology and Metabolism 201427845–849. ( 10.1515/jpem-2013-0256) [DOI] [PubMed] [Google Scholar]
- 126.Folsom LJ, Slaven JE, Nabhan ZM, Eugster EA. Characterization of spontaneous and induced puberty in girls with Turner syndrome. Endocrine Practice 201723768–774. ( 10.4158/EP161738.OR) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Dabrowski E, Jensen R, Johnson EK, Habiby RL, Brickman WJ, Finlayson C. Turner syndrome systematic review: spontaneous thelarche and menarche stratified by karyotype. Hormone Research in Paediatrics 201992143–149. ( 10.1159/000502902) [DOI] [PubMed] [Google Scholar]
- 128.Viuff MH, Berglund A, Juul S, Andersen NH, Stochholm K, Gravholt CH. Sex hormone replacement therapy in Turner syndrome: impact on morbidity and mortality. Journal of Clinical Endocrinology and Metabolism 2020105 dgz039. ( 10.1210/clinem/dgz039) [DOI] [PubMed] [Google Scholar]
- 129.Aso K, Koto S, Higuchi A, Ariyasu D, Izawa M, Miyamoto Igaki J, Hasegawa Y. Serum FSH level below 10 mIU/mL at twelve years old is an index of spontaneous and cyclical menstruation in Turner syndrome. Endocrine Journal 201057909–913. ( 10.1507/endocrj.k10e-092) [DOI] [PubMed] [Google Scholar]
- 130.Lunding SA, Aksglaede L, Anderson RA, Main KM, Juul A, Hagen CP, Pedersen AT. AMH as predictor of premature ovarian insufficiency: a longitudinal study of 120 Turner syndrome patients. Journal of Clinical Endocrinology and Metabolism 2015100E1030–E1038. ( 10.1210/jc.2015-1621) [DOI] [PubMed] [Google Scholar]
- 131.Hagen CP, Aksglaede L, Sørensen K, Main KM, Boas M, Cleemann L, Holm K, Gravholt CH, Andersson AM, Pedersen ATet al. Serum levels of anti-Müllerian hormone as a marker of ovarian function in 926 healthy females from birth to adulthood and in 172 Turner syndrome patients. Journal of Clinical Endocrinology and Metabolism 2010955003–5010. ( 10.1210/jc.2010-0930) [DOI] [PubMed] [Google Scholar]
- 132.Visser JA, Hokken-Koelega AC, Zandwijken GR, Limacher A, Ranke MB, Flück CE. Anti-Müllerian hormone levels in girls and adolescents with Turner syndrome are related to karyotype, pubertal development and growth hormone treatment. Human Reproduction 2013281899–1907. ( 10.1093/humrep/det089) [DOI] [PubMed] [Google Scholar]
- 133.Hagen CP, Main KM, Kjaergaard S, Juul A. FSH, LH, inhibin B and estradiol levels in Turner syndrome depend on age and karyotype: longitudinal study of 70 Turner girls with or without spontaneous puberty. Human Reproduction 2010253134–3141. ( 10.1093/humrep/deq291) [DOI] [PubMed] [Google Scholar]
- 134.Gravholt CH, Naeraa RW, Andersson AM, Christiansen JS, Skakkebaek NE. Inhibin A and B in adolescents and young adults with Turner’s syndrome and no sign of spontaneous puberty. Human Reproduction 2002172049–2053. ( 10.1093/humrep/17.8.2049) [DOI] [PubMed] [Google Scholar]
- 135.Ankarberg-Lindgren C, Gawlik A, Kriström B, Mazzanti L, Ruijgrok EJ, Sas TCJ. Estradiol matrix patches for pubertal induction: stability of cut pieces at different temperatures. Endocrine Connections 20198360–366. ( 10.1530/EC-19-0025) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Ankarberg-Lindgren C, Elfving M, Wikland KA, Norjavaara E. Nocturnal application of transdermal estradiol patches produces levels of estradiol that mimic those seen at the onset of spontaneous puberty in girls. Journal of Clinical Endocrinology and Metabolism 2001863039–3044. ( 10.1210/jcem.86.7.7667) [DOI] [PubMed] [Google Scholar]
- 137.Taboada M, Santen R, Lima J, Hossain J, Singh R, Klein KO, Mauras N. Pharmacokinetics and pharmacodynamics of oral and transdermal 17β estradiol in girls with Turner syndrome. Journal of Clinical Endocrinology and Metabolism 2011963502–3510. ( 10.1210/jc.2011-1449) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jospe N, Orlowski CC, Furlanetto RW. Comparison of transdermal and oral estrogen therapy in girls with Turner’s syndrome. Journal of Pediatric Endocrinology and Metabolism 19958111–116. ( 10.1515/jpem.1995.8.2.111) [DOI] [PubMed] [Google Scholar]
- 139.Bakalov VK, Shawker T, Ceniceros I, Bondy CA. Uterine development in Turner syndrome. Journal of Pediatrics 2007151528–53, 531.e1. ( 10.1016/j.jpeds.2007.04.031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Cleemann L, Holm K, Fallentin E, Møller N, Kristensen B, Skouby SO, Leth-Esbensen P, Jeppesen EM, Jensen AK, Gravholt CH. Effect of dosage of 17β-estradiol on uterine growth in Turner syndrome – a randomized controlled clinical pilot trial. Journal of Clinical Endocrinology and Metabolism 2020105 dgz061. ( 10.1210/clinem/dgz061) [DOI] [PubMed] [Google Scholar]
- 141.Alves ST, Gallichio CT, Guimarães MM. Insulin resistance and body composition in Turner syndrome: effect of sequential change in the route of estrogen administration. Gynecological Endocrinology 200622590–594. ( 10.1080/08916930600929586) [DOI] [PubMed] [Google Scholar]
- 142.Mauras N, Shulman D, Hsiang HY, Balagopal P, Welch S. Metabolic effects of oral versus transdermal estrogen in growth hormone-treated girls with turner syndrome. Journal of Clinical Endocrinology and Metabolism 2007924154–4160. ( 10.1210/jc.2007-0671) [DOI] [PubMed] [Google Scholar]
- 143.Reinehr T, Lindberg A, Toschke C, Cara J, Chrysis D, Camacho-Hübner C. Weight gain in Turner syndrome: association to puberty induction? Longitudinal analysis of KIGS data. Clinical Endocrinology 20168585–91. ( 10.1111/cen.13044) [DOI] [PubMed] [Google Scholar]
- 144.Viuff MH, Stochholm K, Grønbaek H, Berglund A, Juul S, Gravholt CH. Increased occurrence of liver and gastrointestinal diseases and anaemia in women with Turner syndrome – a nationwide cohort study. Alimentary Pharmacology and Therapeutics 202153821–829. ( 10.1111/apt.16277) [DOI] [PubMed] [Google Scholar]
- 145.Roulot D, Degott C, Chazouillères O, Oberti F, Calès P, Carbonell N, Benferhat S, Bresson-Hadni S, Valla D. Vascular involvement of the liver in Turner’s syndrome. Hepatology 200439239–247. ( 10.1002/hep.20026) [DOI] [PubMed] [Google Scholar]
- 146.Gravholt CH, Poulsen HE, Ott P, Christiansen JS, Vilstrup H. Quantitative liver functions in Turner syndrome with and without hormone replacement therapy. European Journal of Endocrinology 2007156679–686. ( 10.1530/EJE-07-0070) [DOI] [PubMed] [Google Scholar]
- 147.Çakır ED, Sağlam H, Eren E, Özgür T, Tarım ÖF. Retrospective evaluation of pubertal development and linear growth of girls with Turner syndrome treated with oral and transdermal estrogen. Journal of Pediatric Endocrinology and Metabolism 2015281219–1226. ( 10.1515/jpem-2014-0007) [DOI] [PubMed] [Google Scholar]
- 148.Viuff MH, Stochholm K, Lin A, Berglund A, Juul S, Gravholt CH. Cancer occurrence in Turner syndrome and the effect of sex hormone substitution therapy. European Journal of Endocrinology 202118479–88. ( 10.1530/EJE-20-0702) [DOI] [PubMed] [Google Scholar]
- 149.Naeraa RW, Nielsen J, Kastrup KW. Growth hormone and 17 beta-oestradiol treatment of Turner girls – 2-year results. European Journal of Pediatrics 199415372–77. ( 10.1007/BF01959210) [DOI] [PubMed] [Google Scholar]
- 150.Ankarberg-Lindgren C, Kriström B, Norjavaara E. Physiological estrogen replacement therapy for puberty induction in girls: a clinical observational study. Hormone Research in Paediatrics 201481239–244. ( 10.1159/000356922) [DOI] [PubMed] [Google Scholar]
- 151.Piippo S, Lenko H, Kainulainen P, Sipilä I. Use of percutaneous estrogen gel for induction of puberty in girls with Turner syndrome. Journal of Clinical Endocrinology and Metabolism 2004893241–3247. ( 10.1210/jc.2003-032069) [DOI] [PubMed] [Google Scholar]
- 152.Gawlik AM, Hankus M, Szeliga K, Antosz A, Gawlik T, Soltysik K, Drosdzol-Cop A, Wilk K, Kudela G, Koszutski Tet al. Late-onset puberty induction by transdermal estrogen in Turner syndrome girls – a longitudinal study. Frontiers in Endocrinology 20189 23. ( 10.3389/fendo.2018.00023) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Labarta JI, Moreno ML, López-Siguero JP, Luzuriaga C, Rica I, Sánchez-del Pozo J, Gracia-Bouthelier R. & Spanish Turner working group. Individualised vs fixed dose of oral 17β-oestradiol for induction of puberty in girls with Turner syndrome: an open-randomised parallel trial. European Journal of Endocrinology 2012167523–529. ( 10.1530/EJE-12-0444) [DOI] [PubMed] [Google Scholar]
- 154.Bannink EM, van Sassen C, van Buuren S, de Jong FH, Lequin M, Mulder PG, de Muinck Keizer-Schrama SM. Puberty induction in Turner syndrome: results of oestrogen treatment on development of secondary sexual characteristics, uterine dimensions and serum hormone levels. Clinical Endocrinology 200970265–273. ( 10.1111/j.1365-2265.2008.03446.x) [DOI] [PubMed] [Google Scholar]
- 155.van Pareren YK, de Muinck Keizer-Schrama SM, Stijnen T, Sas TC, Jansen M, Otten BJ, Hoorweg-Nijman JJ, Vulsma T, Stokvis-Brantsma WH, Rouwé CWet al. Final height in girls with turner syndrome after long-term growth hormone treatment in three dosages and low dose estrogens. Journal of Clinical Endocrinology and Metabolism 2003881119–1125. ( 10.1210/jc.2002-021171) [DOI] [PubMed] [Google Scholar]
- 156.Donaldson M, Kristrom B, Ankarberg-Lindgren C, Verlinde S, van Alfen-van der Velden J, Gawlik A, van Gelder MMHJ, Sas T. & on behalf of the European Society for Paediatric Endocrinology Turner Syndrome Working Group. Optimal pubertal induction in girls with Turner syndrome using either oral or transdermal estradiol: a proposed modern strategy. Hormone Research in Paediatrics 201991153–163. ( 10.1159/000500050) [DOI] [PubMed] [Google Scholar]
- 157.Shifren JL, Gass ML. & NAMS Recommendations for Clinical Care of Midlife Women Working Group. The North American Menopause Society recommendations for clinical care of midlife women. Menopause 2014211038–1062. ( 10.1097/GME.0000000000000319) [DOI] [PubMed] [Google Scholar]
- 158.Matthews D, Bath L, Högler W, Mason A, Smyth A, Skae M. Hormone supplementation for pubertal induction in girls. Archives of Disease in Childhood 2017102975–980. ( 10.1136/archdischild-2016-311372) [DOI] [PubMed] [Google Scholar]
- 159.Lawaetz JG, Hagen CP, Mieritz MG, Blomberg Jensen M, Petersen JH, Juul A. Evaluation of 451 Danish boys with delayed puberty: diagnostic use of a new puberty nomogram and effects of oral testosterone therapy. Journal of Clinical Endocrinology and Metabolism 20151001376–1385. ( 10.1210/jc.2014-3631) [DOI] [PubMed] [Google Scholar]
- 160.Dwyer AA, Phan-Hug F, Hauschild M, Elowe-Gruau E, Pitteloud N. TRANSITION IN ENDOCRINOLOGY: Hypogonadism in adolescence. European Journal of Endocrinology 2015173R15–R24. ( 10.1530/EJE-14-0947) [DOI] [PubMed] [Google Scholar]
- 161.Palmert MR, Dunkel L. Clinical practice. Delayed puberty. New England Journal of Medicine 2012366443–453. ( 10.1056/NEJMcp1109290) [DOI] [PubMed] [Google Scholar]
- 162.Bozzola M, Bozzola E, Montalbano C, Stamati FA, Ferrara P, Villani A. Delayed puberty versus hypogonadism: a challenge for the pediatrician. Annals of Pediatric Endocrinology and Metabolism 20182357–61. ( 10.6065/apem.2018.23.2.57) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Basaria S.Male hypogonadism. Lancet 20143831250–1263. ( 10.1016/S0140-6736(1361126-5) [DOI] [PubMed] [Google Scholar]
- 164.Lucas-Herald AK, Mason E, Beaumont P, Mason A, Shaikh MG, Wong SC, Ahmed SF. Single-centre experience of testosterone therapy for boys with hypogonadism. Hormone Research in Paediatrics 201890123–127. ( 10.1159/000490738) [DOI] [PubMed] [Google Scholar]
- 165.Cox K, Bryce J, Jiang J, Rodie M, Sinnott R, Alkhawari M, Arlt W, Audi L, Balsamo A, Bertelloni Set al. Novel associations in disorders of sex development: findings from the I-DSD Registry. Journal of Clinical Endocrinology and Metabolism 201499E348–E355. ( 10.1210/jc.2013-2918) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Nixon R, Cerqueira V, Kyriakou A, Lucas-Herald A, McNeilly J, McMillan M, Purvis AI, Tobias ES, McGowan R, Ahmed SF. Prevalence of endocrine and genetic abnormalities in boys evaluated systematically for a disorder of sex development. Human Reproduction 2017322130–2137. ( 10.1093/humrep/dex280) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Bonomi M, Rochira V, Pasquali D, Balercia G, Jannini EA, Ferlin A. & Klinefelter ItaliaN Group (KING). Klinefelter syndrome (KS): genetics, clinical phenotype and hypogonadism. Journal of Endocrinological Investigation 201740123–134. ( 10.1007/s40618-016-0541-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Flück C, Nordenström A, Ahmed SF, Ali SR, Berra M, Hall J, Köhler B, Pasterski V, Robeva R, Schweizer Ket al. Standardised data collection for clinical follow-up and assessment of outcomes in differences of sex development (DSD): recommendations from the COST action DSDnet. European Journal of Endocrinology 2019181545–564. ( 10.1530/EJE-19-0363) [DOI] [PubMed] [Google Scholar]
- 169.Wikström AM, Dunkel L. Klinefelter syndrome. Best Practice and Research: Clinical Endocrinology and Metabolism 201125239–250. ( 10.1016/j.beem.2010.09.006) [DOI] [PubMed] [Google Scholar]
- 170.Gravholt CH, Chang S, Wallentin M, Fedder J, Moore P, Skakkebæk A. Klinefelter syndrome: integrating genetics, neuropsychology, and endocrinology. Endocrine Reviews 201839389–423. ( 10.1210/er.2017-00212) [DOI] [PubMed] [Google Scholar]
- 171.Tantawy S, Lin L, Akkurt I, Borck G, Klingmüller D, Hauffa BP, Krude H, Biebermann H, Achermann JC, Köhler B. Testosterone production during puberty in two 46,XY patients with disorders of sex development and novel NR5A1 (SF-1) mutations. European Journal of Endocrinology 2012167125–130. ( 10.1530/EJE-11-0944) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Krausz C, Casamonti E. Spermatogenic failure and the Y chromosome. Human Genetics 2017136637–655. ( 10.1007/s00439-017-1793-8) [DOI] [PubMed] [Google Scholar]
- 173.Maqdasy S, Barres B, Salaun G, Batisse-Lignier M, Pebrel-Richard C, Kwok KHM, Labbé A, Touraine P, Brugnon F, Tauveron I. Idiopathic central precocious puberty in a Klinefelter patient: highlights on gonadotropin levels and pathophysiology. Basic and Clinical Andrology 202030 19. ( 10.1186/s12610-020-00117-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Gong C, Li L, Chen J, Li W. Central precocious puberty as a prelude to hypogonadism in a patient with Klinefelter syndrome. Pediatric Investigation 20193127–130. ( 10.1002/ped4.12136) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kolesinska Z, Ahmed SF, Niedziela M, Bryce J, Molinska-Glura M, Rodie M, Jiang J, Sinnott RO, Hughes IA, Darendeliler Fet al. Changes over time in sex assignment for disorders of sex development. Pediatrics 2014134e710–e715. ( 10.1542/peds.2014-1088) [DOI] [PubMed] [Google Scholar]
- 176.Eisenegger C, Haushofer J, Fehr E. The role of testosterone in social interaction. Trends in Cognitive Sciences 201115263–271. ( 10.1016/j.tics.2011.04.008) [DOI] [PubMed] [Google Scholar]
- 177.Bos PA, Panksepp J, Bluthé RM, van Honk J. Acute effects of steroid hormones and neuropeptides on human social-emotional behavior: a review of single administration studies. Frontiers in Neuroendocrinology 20123317–35. ( 10.1016/j.yfrne.2011.01.002) [DOI] [PubMed] [Google Scholar]
- 178.Roden RC, Schmidt EK, Holland-Hall C. Sexual health education for adolescents and young adults with intellectual and developmental disabilities: recommendations for accessible sexual and reproductive health information. Lancet: Child and Adolescent Health 20204699–708. ( 10.1016/S2352-4642(2030098-5) [DOI] [PubMed] [Google Scholar]
- 179.Graber JA.Pubertal timing and the development of psychopathology in adolescence and beyond. Hormones and Behavior 201364262–269. ( 10.1016/j.yhbeh.2013.04.003) [DOI] [PubMed] [Google Scholar]
- 180.Day FR, Elks CE, Murray Aet al. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Scientific Reports 2015511208.( 10.1038/srep11208) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tinggaard J, Mieritz MG, Sørensen K, Mouritsen A, Hagen CP, Aksglaede L, Wohlfahrt-Veje C, Juul A. The physiology and timing of male puberty. Current Opinion in Endocrinology, Diabetes, and Obesity 201219197–203. ( 10.1097/MED.0b013e3283535614) [DOI] [PubMed] [Google Scholar]
- 182.Drobac S, Rubin K, Rogol AD, Rosenfield RL. A workshop on pubertal hormone replacement options in the United States. Journal of Pediatric Endocrinology and Metabolism 20061955–64. ( 10.1515/jpem.2006.19.1.55) [DOI] [PubMed] [Google Scholar]
- 183.Stancampiano MR, Lucas-Herald AK, Russo G, Rogol AD, Ahmed SF. Testosterone therapy in adolescent boys: the need for a structured approach. Hormone Research in Paediatrics 201992215–228. ( 10.1159/000504670) [DOI] [PubMed] [Google Scholar]
- 184.Lampit M, Hochberg Z. Androgen therapy in constitutional delay of growth. Hormone Research 200359270–275. ( 10.1159/000070624) [DOI] [PubMed] [Google Scholar]
- 185.Ahmed SF, Tucker P, Mayo A, Wallace AM, Hughes IA. Randomized, crossover comparison study of the short-term effect of oral testosterone undecanoate and intramuscular testosterone depot on linear growth and serum bone alkaline phosphatase. Journal of Pediatric Endocrinology and Metabolism 200417941–950. ( 10.1515/jpem.2004.17.7.941) [DOI] [PubMed] [Google Scholar]
- 186.Ankarberg-Lindgren C, Norjavaara E. Changes of diurnal rhythm and levels of total and free testosterone secretion from pre to late puberty in boys: testis size of 3 ml is a transition stage to puberty. European Journal of Endocrinology 2004151747–757. ( 10.1530/eje.0.1510747) [DOI] [PubMed] [Google Scholar]
- 187.Mouritsen A, Søeborg T, Johannsen TH, Aksglaede L, Sørensen K, Hagen CP, Mieritz MG, Frederiksen H, Andersson AM, Juul A. Longitudinal changes in circulating testosterone levels determined by LC-MS/MS and by a commercially available radioimmunoassay in healthy girls and boys during the pubertal transition. Hormone Research in Paediatrics 20148212–17. ( 10.1159/000358560) [DOI] [PubMed] [Google Scholar]
- 188.Nieschlag E, Cüppers HJ, Wiegelmann W, Wickings EJ. Bioavailability and LH-suppressing effect of different testosterone preparations in normal and hypogonadal men. Hormone Research 19767138–145. ( 10.1159/000178721) [DOI] [PubMed] [Google Scholar]
- 189.Kresch E, Patel M, Lima TFN, Ramasamy R. An update on the available and emerging pharmacotherapy for adults with testosterone deficiency available in the USA. Expert Opinion on Pharmacotherapy 2021221761–1771. ( 10.1080/14656566.2021.1918101) [DOI] [PubMed] [Google Scholar]
- 190.Mason KA, Schoelwer MJ, Rogol AD. Androgens during infancy, childhood, and adolescence: physiology and use in clinical practice. Endocrine Reviews 202041 bnaa003. ( 10.1210/endrev/bnaa003) [DOI] [PubMed] [Google Scholar]
- 191.Corona G, Goulis DG, Huhtaniemi I, Zitzmann M, Toppari J, Forti G, Vanderschueren D, Wu FC. European Academy of Andrology (EAA) guidelines on investigation, treatment and monitoring of functional hypogonadism in males: endorsing organization: European Society of Endocrinology. Andrology 20208970–987. ( 10.1111/andr.12770) [DOI] [PubMed] [Google Scholar]
- 192.Bhasin S, Brito JP, Cunningham GR, Hayes FJ, Hodis HN, Matsumoto AM, Snyder PJ, Swerdloff RS, Wu FC, Yialamas MA. Testosterone therapy in men with hypogonadism: an Endocrine Society clinical practice guideline. Journal of Clinical Endocrinology and Metabolism 20181031715–1744. ( 10.1210/jc.2018-00229) [DOI] [PubMed] [Google Scholar]
- 193.Rogol AD, Swerdloff RS, Reiter EO, Ross JL, ZumBrunnen TL, Pratt GA, Brennan JJ, Benesh J, Kan-Dobrosky N, Miller MG. A multicenter, open-label, observational study of testosterone gel (1%) in the treatment of adolescent boys with Klinefelter syndrome or anorchia. Journal of Adolescent Health 20145420–25. ( 10.1016/j.jadohealth.2013.07.021) [DOI] [PubMed] [Google Scholar]
- 194.Kunz GJ, Klein KO, Clemons RD, Gottschalk ME, Jones KL. Virilization of young children after topical androgen use by their parents. Pediatrics 2004114282–284. ( 10.1542/peds.114.1.282) [DOI] [PubMed] [Google Scholar]
- 195.Schopohl J, Mehltretter G, von Zumbusch R, Eversmann T, von Werder K. Comparison of gonadotropin-releasing hormone and gonadotropin therapy in male patients with idiopathic hypothalamic hypogonadism. Fertility and Sterility 1991561143–1150. ( 10.1016/S0015-0282(1654730-X) [DOI] [PubMed] [Google Scholar]
- 196.Seftel A.Testosterone replacement therapy for male hypogonadism: part III. Pharmacologic and clinical profiles, monitoring, safety issues, and potential future agents. International Journal of Impotence Research 2007192–24. ( 10.1038/sj.ijir.3901366) [DOI] [PubMed] [Google Scholar]
- 197.Kanakis GA, Nordkap L, Bang AK, Calogero AE, Bártfai G, Corona G, Forti G, Toppari J, Goulis DG, Jørgensen N. EAA clinical practice guidelines-gynecomastia evaluation and management. Andrology 20197778–793. ( 10.1111/andr.12636) [DOI] [PubMed] [Google Scholar]
- 198.Rhoden EL, Morgentaler A. Treatment of testosterone-induced gynecomastia with the aromatase inhibitor, anastrozole. International Journal of Impotence Research 20041695–97. ( 10.1038/sj.ijir.3901154) [DOI] [PubMed] [Google Scholar]
- 199.Treves N.Gynecomastia; the origins of mammary swelling in the male: an analysis of 406 patients with breast hypertrophy, 525 with testicular tumors, and 13 with adrenal neoplasms. Cancer 1958111083–1102. () [DOI] [PubMed] [Google Scholar]
- 200.Gruntmanis U, Braunstein GD. Treatment of gynecomastia. Current Opinion in Investigational Drugs 20012643–649. [PubMed] [Google Scholar]
- 201.Braunstein GD.Clinical practice. Gynecomastia. New England Journal of Medicine 20073571229–1237. ( 10.1056/NEJMcp070677) [DOI] [PubMed] [Google Scholar]
- 202.Rohrich RJ, Ha RY, Kenkel JM, Adams Jr WP. Classification and management of gynecomastia: defining the role of ultrasound-assisted liposuction. Plastic and Reconstructive Surgery 2003111909–923; discussion 924–905. ( 10.1097/01.PRS.0000042146.40379.25) [DOI] [PubMed] [Google Scholar]
- 203.Tashkandi M, Al-Qattan MM, Hassanain JM, Hawary MB, Sultan M. The surgical management of high-grade gynecomastia. Annals of Plastic Surgery 20045317–20; discussion 21. ( 10.1097/01.sap.0000112347.30612.f4) [DOI] [PubMed] [Google Scholar]
- 204.Bonomi M, Vezzoli V, Krausz C, Guizzardi F, Vezzani S, Simoni M, Bassi I, Duminuco P, Di Iorgi N, Giavoli Cet al. Characteristics of a nationwide cohort of patients presenting with isolated hypogonadotropic hypogonadism (IHH). European Journal of Endocrinology 201817823–32. ( 10.1530/EJE-17-0065) [DOI] [PubMed] [Google Scholar]
- 205.Boehm U, Bouloux PM, Dattani MT, de Roux N, Dodé C, Dunkel L, Dwyer AA, Giacobini P, Hardelin JP, Juul Aet al. Expert consensus document: European Consensus Statement on congenital hypogonadotropic hypogonadism – pathogenesis, diagnosis and treatment. Nature Reviews: Endocrinology 201511547–564. ( 10.1038/nrendo.2015.112) [DOI] [PubMed] [Google Scholar]
- 206.Cangiano B, Swee DS, Quinton R, Bonomi M. Genetics of congenital hypogonadotropic hypogonadism: peculiarities and phenotype of an oligogenic disease. Human Genetics 202114077–111. ( 10.1007/s00439-020-02147-1) [DOI] [PubMed] [Google Scholar]
- 207.Young J.Approach to the male patient with congenital hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism 201297707–718. ( 10.1210/jc.2011-1664) [DOI] [PubMed] [Google Scholar]
- 208.Shiraishi K, Oka S, Matsuyama H. Assessment of quality of life during gonadotrophin treatment for male hypogonadotrophic hypogonadism. Clinical Endocrinology 201481259–265. ( 10.1111/cen.12435) [DOI] [PubMed] [Google Scholar]
- 209.Dunkel L, Quinton R. Transition in endocrinology: induction of puberty. European Journal of Endocrinology 2014170R229–R239. ( 10.1530/EJE-13-0894) [DOI] [PubMed] [Google Scholar]
- 210.Delemarre EM, Felius B, Delemarre-van de Waal HA. Inducing puberty. European Journal of Endocrinology 2008159 (Supplement 1) S9–S15. ( 10.1530/EJE-08-0314) [DOI] [PubMed] [Google Scholar]
- 211.Swee DS, Quinton R. Managing congenital hypogonadotrophic hypogonadism: a contemporary approach directed at optimizing fertility and long-term outcomes in males. Therapeutic Advances in Endocrinology and Metabolism 2019102042018819826889. ( 10.1177/2042018819826889) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Rohayem J, Hauffa BP, Zacharin M, Kliesch S, Zitzmann M. & ‘ German Adolescent Hypogonadotropic Hypogonadism Study Group ’. Testicular growth and spermatogenesis: new goals for pubertal hormone replacement in boys with hypogonadotropic hypogonadism? A multicentre prospective study of hCG/rFSH treatment outcomes during adolescence. Clinical Endocrinology 20178675–87. ( 10.1111/cen.13164) [DOI] [PubMed] [Google Scholar]
- 213.Rastrelli G, Corona G, Mannucci E, Maggi M. Factors affecting spermatogenesis upon gonadotropin-replacement therapy: a meta-analytic study. Andrology 20142794–808. ( 10.1111/andr.262) [DOI] [PubMed] [Google Scholar]
- 214.Dwyer AA, Sykiotis GP, Hayes FJ, Boepple PA, Lee H, Loughlin KR, Dym M, Sluss PM, Crowley WF, Pitteloud N. Trial of recombinant follicle-stimulating hormone pretreatment for GnRH-induced fertility in patients with congenital hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism 201398E1790–E1795. ( 10.1210/jc.2013-2518) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Raivio T, Wikström AM, Dunkel L. Treatment of gonadotropin-deficient boys with recombinant human FSH: long-term observation and outcome. European Journal of Endocrinology 2007156105–111. ( 10.1530/eje.1.02315) [DOI] [PubMed] [Google Scholar]
- 216.Cangiano B, Goggi G, Federici S, Bresesti C, Cotellessa L, Guizzardi F, Vezzoli V, Duminuco P, Persani L, Bonomi M. Predictors of reproductive and non-reproductive outcomes of gonadotropin mediated pubertal induction in male patients with congenital hypogonadotropic hypogonadism (CHH). Journal of Endocrinological Investigation 2021442445–2454. ( 10.1007/s40618-021-01556-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Barrio R, de Luis D, Alonso M, Lamas A, Moreno JC. Induction of puberty with human chorionic gonadotropin and follicle-stimulating hormone in adolescent males with hypogonadotropic hypogonadism. Fertility and Sterility 199971244–248. ( 10.1016/s0015-0282(9800450-6) [DOI] [PubMed] [Google Scholar]
- 218.Zacharin M, Sabin MA, Nair VV, Dabadghao P. Addition of recombinant follicle-stimulating hormone to human chorionic gonadotropin treatment in adolescents and young adults with hypogonadotropic hypogonadism promotes normal testicular growth and may promote early spermatogenesis. Fertility and Sterility 201298836–842. ( 10.1016/j.fertnstert.2012.06.022) [DOI] [PubMed] [Google Scholar]
- 219.Raivio T, Toppari J, Perheentupa A, McNeilly AS, Dunkel L. Treatment of prepubertal gonadotrophin-deficient boys with recombinant human follicle-stimulating hormone. Lancet 1997350263–264. ( 10.1016/S0140-6736(0562227-1) [DOI] [PubMed] [Google Scholar]
- 220.Kohva E, Huopio H, Hero M, Miettinen PJ, Vaaralahti K, Sidoroff V, Toppari J, Raivio T. Recombinant human FSH treatment outcomes in five boys with severe congenital hypogonadotropic hypogonadism. Journal of the Endocrine Society 201821345–1356. ( 10.1210/js.2018-00225) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Saal W, Happ J, Cordes U, Baum RP, Schmidt M. Subcutaneous gonadotropin therapy in male patients with hypogonadotropic hypogonadism. Fertility and Sterility 199156319–324. ( 10.1016/s0015-0282(1654493-8) [DOI] [PubMed] [Google Scholar]
- 222.Bouvattier C, Tauber M, Jouret B, Chaussain JL, Rochiccioli P. Gonadotropin treatment of hypogonadotropic hypogonadal adolescents. Journal of Pediatric Endocrinology and Metabolism 199912 (Supplement 1) 339–344. [PubMed] [Google Scholar]
- 223.Young J, Chanson P, Salenave S, Noël M, Brailly S, O’Flaherty M, Schaison G, Rey R. Testicular anti-Mullerian hormone secretion is stimulated by recombinant human FSH in patients with congenital hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism 200590724–728. ( 10.1210/jc.2004-0542) [DOI] [PubMed] [Google Scholar]
- 224.Zhang M, Tong G, Liu Y, Mu Y, Weng J, Xue Y, Luo Z, Xue Y, Shi L, Wu Xet al. Sequential versus continual purified urinary FSH/hCG in men with idiopathic hypogonadotropic hypogonadism. Journal of Clinical Endocrinology and Metabolism 20151002449–2455. ( 10.1210/jc.2014-3802) [DOI] [PubMed] [Google Scholar]
- 225.Rohayem J, Sinthofen N, Nieschlag E, Kliesch S, Zitzmann M. Causes of hypogonadotropic hypogonadism predict response to gonadotropin substitution in adults. Andrology 2016487–94. ( 10.1111/andr.12128) [DOI] [PubMed] [Google Scholar]
- 226.Cortes D, Müller J, Skakkebaek NE. Proliferation of Sertoli cells during development of the human testis assessed by stereological methods. International Journal of Andrology 198710589–596. ( 10.1111/j.1365-2605.1987.tb00358.x) [DOI] [PubMed] [Google Scholar]
- 227.Chemes HE, Rey RA, Nistal M, Regadera J, Musse M, González-Peramato P, Serrano A. Physiological androgen insensitivity of the fetal, neonatal, and early infantile testis is explained by the ontogeny of the androgen receptor expression in Sertoli cells. Journal of Clinical Endocrinology and Metabolism 2008934408–4412. ( 10.1210/jc.2008-0915) [DOI] [PubMed] [Google Scholar]
- 228.Bouloux P, Warne DW, Loumaye E. & FSH Study Group in Men ’ s Infertility. Efficacy and safety of recombinant human follicle-stimulating hormone in men with isolated hypogonadotropic hypogonadism. Fertility and Sterility 200277270–273. ( 10.1016/s0015-0282(0102973-9) [DOI] [PubMed] [Google Scholar]
- 229.Efficacy and safety of highly purified urinary follicle-stimulating hormone with human chorionic gonadotropin for treating men with isolated hypogonadotropic hypogonadism. European Metrodin HP Study Group. Fertility and Sterility 199870256–262. ( 10.1016/s0015-0282(9800156-3) [DOI] [PubMed] [Google Scholar]
- 230.Jones TH, Darne JF. Self-administered subcutaneous human menopausal gonadotrophin for the stimulation of testicular growth and the initiation of spermatogenesis in hypogonadotrophic hypogonadism. Clinical Endocrinology 199338203–208. ( 10.1111/j.1365-2265.1993.tb00994.x) [DOI] [PubMed] [Google Scholar]
- 231.Liu PY, Baker HW, Jayadev V, Zacharin M, Conway AJ, Handelsman DJ. Induction of spermatogenesis and fertility during gonadotropin treatment of gonadotropin-deficient infertile men: predictors of fertility outcome. Journal of Clinical Endocrinology and Metabolism 200994801–808. ( 10.1210/jc.2008-1648) [DOI] [PubMed] [Google Scholar]
- 232.Liu PY, Gebski VJ, Turner L, Conway AJ, Wishart SM, Handelsman DJ. Predicting pregnancy and spermatogenesis by survival analysis during gonadotrophin treatment of gonadotrophin-deficient infertile men. Human Reproduction 200217625–633. ( 10.1093/humrep/17.3.625) [DOI] [PubMed] [Google Scholar]
- 233.Berglund A, Viuff MH, Skakkebæk A, Chang S, Stochholm K, Gravholt CH. Changes in the cohort composition of turner syndrome and severe non-diagnosis of Klinefelter, 47,XXX and 47,XYY syndrome: a nationwide cohort study. Orphanet Journal of Rare Diseases 201914 16. ( 10.1186/s13023-018-0976-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bojesen A, Juul S, Gravholt CH. Prenatal and postnatal prevalence of Klinefelter syndrome: a national registry study. Journal of Clinical Endocrinology and Metabolism 200388622–626. ( 10.1210/jc.2002-021491) [DOI] [PubMed] [Google Scholar]
- 235.Simm PJ, Zacharin MR. The psychosocial impact of Klinefelter syndrome – a 10 year review. Journal of Pediatric Endocrinology and Metabolism 200619499–505. [PubMed] [Google Scholar]
- 236.Forti G, Corona G, Vignozzi L, Krausz C, Maggi M. Klinefelter’s syndrome: a clinical and therapeutical update. Sexual Development 20104249–258. ( 10.1159/000316604) [DOI] [PubMed] [Google Scholar]
- 237.Mehta A, Clearman T, Paduch DA. Safety and efficacy of testosterone replacement therapy in adolescents with Klinefelter syndrome. Journal of Urology 2014191 (Supplement) 1527–1531. ( 10.1016/j.juro.2013.09.015) [DOI] [PubMed] [Google Scholar]
- 238.Wosnitzer MS, Paduch DA. Endocrinological issues and hormonal manipulation in children and men with Klinefelter syndrome. American Journal of Medical Genetics: Part C, Seminars in Medical Genetics 2013163C16–26. ( 10.1002/ajmg.c.31350) [DOI] [PubMed] [Google Scholar]
- 239.Rohayem J, Nieschlag E, Zitzmann M, Kliesch S. Testicular function during puberty and young adulthood in patients with Klinefelter’s syndrome with and without spermatozoa in seminal fluid. Andrology 201641178–1186. ( 10.1111/andr.12249) [DOI] [PubMed] [Google Scholar]
- 240.Franik S, Hoeijmakers Y, D’Hauwers K, Braat DD, Nelen WL, Smeets D, Claahsen-van der Grinten HL, Ramos L, Fleischer K. Klinefelter syndrome and fertility: sperm preservation should not be offered to children with Klinefelter syndrome. Human Reproduction 2016311952–1959. ( 10.1093/humrep/dew179) [DOI] [PubMed] [Google Scholar]
- 241.Garolla A, Selice R, Menegazzo M, Valente U, Zattoni F, Iafrate M, Prayer-Galetti T, Gardiman MP, Ferlin A, Di Nisio Aet al. Novel insights on testicular volume and testosterone replacement therapy in Klinefelter patients undergoing testicular sperm extraction. A retrospective clinical study. Clinical Endocrinology 201888711–718. ( 10.1111/cen.13572) [DOI] [PubMed] [Google Scholar]
- 242.Colpi GM, Francavilla S, Haidl G, Link K, Behre HM, Goulis DG, Krausz C, Giwercman A. European Academy of Andrology Guideline Management of oligo-astheno-teratozoospermia. Andrology 20186513–524. ( 10.1111/andr.12502) [DOI] [PubMed] [Google Scholar]
- 243.Mehta A, Bolyakov A, Roosma J, Schlegel PN, Paduch DA. Successful testicular sperm retrieval in adolescents with Klinefelter syndrome treated with at least 1 year of topical testosterone and aromatase inhibitor. Fertility and Sterility 2013100970–974. ( 10.1016/j.fertnstert.2013.06.010) [DOI] [PubMed] [Google Scholar]
- 244.Shanbhogue VV, Hansen S, Jørgensen NR, Brixen K, Gravholt CH. Bone geometry, volumetric density, microarchitecture, and estimated bone strength assessed by HR-pQCT in Klinefelter syndrome. Journal of Bone and Mineral Research 2014292474–2482. ( 10.1002/jbmr.2272) [DOI] [PubMed] [Google Scholar]
- 245.Høst C, Bojesen A, Erlandsen M, Groth KA, Kristensen K, Jurik AG, Birkebæk NH, Gravholt CH. A placebo-controlled randomized study with testosterone in Klinefelter syndrome: beneficial effects on body composition. Endocrine Connections 201981250–1261. ( 10.1530/EC-19-0323) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kabilan A, Skakkebæk A, Chang S, Gravholt CH. Evaluation of the efficacy of transdermal and injection testosterone therapy in Klinefelter syndrome: a real-life study. Journal of the Endocrine Society 20215 bvab062. ( 10.1210/jendso/bvab062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Hornig NC, de Beaufort C, Denzer F, Cools M, Wabitsch M, Ukat M, Kulle AE, Schweikert HU, Werner R, Hiort Oet al. A recurrent germline mutation in the 5’UTR of the androgen receptor causes complete androgen insensitivity by activating aberrant uORF translation. PLoS ONE 201611 e0154158. ( 10.1371/journal.pone.0154158) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gottlieb B, Beitel LK, Nadarajah A, Paliouras M, Trifiro M. The androgen receptor gene mutations database: 2012 update. Human Mutation 201233887–894. ( 10.1002/humu.22046) [DOI] [PubMed] [Google Scholar]
- 249.Quigley CA, De Bellis A, Marschke KB, el-Awady MK, Wilson EM, French FS. Androgen receptor defects: historical, clinical, and molecular perspectives. Endocrine Reviews 199516271–321. ( 10.1210/edrv-16-3-271) [DOI] [PubMed] [Google Scholar]
- 250.Berglund A, Johannsen TH, Stochholm K, Viuff MH, Fedder J, Main KM, Gravholt CH. Incidence, prevalence, diagnostic delay, and clinical presentation of female 46,XY disorders of sex development. Journal of Clinical Endocrinology and Metabolism 20161014532–4540. ( 10.1210/jc.2016-2248) [DOI] [PubMed] [Google Scholar]
- 251.Lanciotti L, Cofini M, Leonardi A, Bertozzi M, Penta L, Esposito S. Different clinical presentations and management in complete androgen insensitivity syndrome (CAIS). International Journal of Environmental Research and Public Health 201916 1268. ( 10.3390/ijerph16071268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Werner R, Zhan J, Gesing J, Struve D, Hiort O. In-vitro characterization of androgen receptor mutations associated with complete androgen insensitivity syndrome reveals distinct functional deficits. Sexual Development 2008273–83. ( 10.1159/000129692) [DOI] [PubMed] [Google Scholar]
- 253.Pizzo A, Lagana AS, Borrielli I, Dugo N. Complete androgen insensitivity syndrome: a rare case of disorder of sex development. Case Reports in Obstetrics and Gynecology 20132013232696. ( 10.1155/2013/232696) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Audi L, Fernandez-Cancio M, Carrascosa A, Andaluz P, Torán N, Piró C, Vilaró E, Vicens-Calvet E, Gussinyé M, Albisu MAet al. Novel (60%) and recurrent (40%) androgen receptor gene mutations in a series of 59 patients with a 46,XY disorder of sex development. Journal of Clinical Endocrinology and Metabolism 2010951876–1888. ( 10.1210/jc.2009-2146) [DOI] [PubMed] [Google Scholar]
- 255.Tyutyusheva N, Mancini I, Baroncelli GI, D’Elios S, Peroni D, Meriggiola MC, Bertelloni S. Complete androgen insensitivity syndrome: from bench to bed. International Journal of Molecular Sciences 202122 1264. ( 10.3390/ijms22031264) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Deeb A, Hughes IA. Inguinal hernia in female infants: a cue to check the sex chromosomes? BJU International 200596401–403. ( 10.1111/j.1464-410X.2005.05639.x) [DOI] [PubMed] [Google Scholar]
- 257.Deeb A, Mason C, Lee YS, Hughes IA. Correlation between genotype, phenotype and sex of rearing in 111 patients with partial androgen insensitivity syndrome. Clinical Endocrinology 20056356–62. ( 10.1111/j.1365-2265.2005.02298.x) [DOI] [PubMed] [Google Scholar]
- 258.Lek N, Tadokoro-Cuccaro R, Whitchurch JB, Mazumder B, Miles H, Prentice P, Bunch T, Zielińska K, Metzler V, Mongan NPet al. Predicting puberty in partial androgen insensitivity syndrome: use of clinical and functional androgen receptor indices. EBiomedicine 201836401–409. ( 10.1016/j.ebiom.2018.09.047) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Lee YF, Shyr CR, Thin TH, Lin WJ, Chang C. Convergence of two repressors through heterodimer formation of androgen receptor and testicular orphan receptor-4: a unique signaling pathway in the steroid receptor superfamily. PNAS 19999614724–14729. ( 10.1073/pnas.96.26.14724) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.De Bellis A, Quigley CA, Marschke KB, el-Awady MK, Lane MV, Smith EP, Sar M, Wilson EM, French FS. Characterization of mutant androgen receptors causing partial androgen insensitivity syndrome. Journal of Clinical Endocrinology and Metabolism 199478513–522. ( 10.1210/jcem.78.3.8126121) [DOI] [PubMed] [Google Scholar]
- 261.Zuccarello D, Ferlin A, Vinanzi C, Prana E, Garolla A, Callewaert L, Claessens F, Brinkmann AO, Foresta C. Detailed functional studies on androgen receptor mild mutations demonstrate their association with male infertility. Clinical Endocrinology 200868580–588. ( 10.1111/j.1365-2265.2007.03069.x) [DOI] [PubMed] [Google Scholar]
- 262.Ferlin A, Vinanzi C, Garolla A, Selice R, Zuccarello D, Cazzadore C, Foresta C. Male infertility and androgen receptor gene mutations: clinical features and identification of seven novel mutations. Clinical Endocrinology 200665606–610. ( 10.1111/j.1365-2265.2006.02635.x) [DOI] [PubMed] [Google Scholar]
- 263.Vockel M, Riera-Escamilla A, Tüttelmann F, Krausz C. The X chromosome and male infertility. Human Genetics 2021140203–215. ( 10.1007/s00439-019-02101-w) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Weidler EM, Linnaus ME, Baratz AB, Goncalves LF, Bailey S, Hernandez SJ, Gomez-Lobo V, van Leeuwen K. A management protocol for gonad preservation in patients with androgen insensitivity syndrome. Journal of Pediatric and Adolescent Gynecology 201932605–611. ( 10.1016/j.jpag.2019.06.005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Tack LJW, Maris E, Looijenga LHJ, Hannema SE, Audi L, Köhler B, Holterhus PM, Riedl S, Wisniewski A, Flück CEet al. Management of gonads in adults with androgen insensitivity: an international survey. Hormone Research in Paediatrics 201890236–246. ( 10.1159/000493645) [DOI] [PubMed] [Google Scholar]
- 266.Chaudhry S, Tadokoro-Cuccaro R, Hannema SE, Acerini CL, Hughes IA. Frequency of gonadal tumours in complete androgen insensitivity syndrome (CAIS): a retrospective case-series analysis. Journal of Pediatric Urology 201713498.e1–498.e6. ( 10.1016/j.jpurol.2017.02.013) [DOI] [PubMed] [Google Scholar]
- 267.Soule SG, Conway G, Prelevic GM, Prentice M, Ginsburg J, Jacobs HS. Osteopenia as a feature of the androgen insensitivity syndrome. Clinical Endocrinology 199543671–675. ( 10.1111/j.1365-2265.1995.tb00533.x) [DOI] [PubMed] [Google Scholar]
- 268.Bertelloni S, Baroncelli GI, Federico G, Cappa M, Lala R, Saggese G. Altered bone mineral density in patients with complete androgen insensitivity syndrome. Hormone Research 199850309–314. ( 10.1159/000023296) [DOI] [PubMed] [Google Scholar]
- 269.Gava G, Mancini I, Orsili I, Bertelloni S, Alvisi S, Seracchioli R, Meriggiola MC. Bone mineral density, body composition and metabolic profiles in adult women with complete androgen insensitivity syndrome and removed gonads using oral or transdermal estrogens. European Journal of Endocrinology 2019181711–718. ( 10.1530/EJE-19-0383) [DOI] [PubMed] [Google Scholar]
- 270.Marcus R, Leary D, Schneider DL, Shane E, Favus M, Quigley CA. The contribution of testosterone to skeletal development and maintenance: lessons from the androgen insensitivity syndrome. Journal of Clinical Endocrinology and Metabolism 2000851032–1037. ( 10.1210/jcem.85.3.6428) [DOI] [PubMed] [Google Scholar]
- 271.Sobel V, Schwartz B, Zhu YS, Cordero JJ, Imperato-McGinley J. Bone mineral density in the complete androgen insensitivity and 5alpha-reductase-2 deficiency syndromes. Journal of Clinical Endocrinology and Metabolism 2006913017–3023. ( 10.1210/jc.2005-2809) [DOI] [PubMed] [Google Scholar]
- 272.Danilovic DL, Correa PH, Costa EM, Melo KF, Mendonca BB, Arnhold IJ. Height and bone mineral density in androgen insensitivity syndrome with mutations in the androgen receptor gene. Osteoporosis International 200718369–374. ( 10.1007/s00198-006-0243-6) [DOI] [PubMed] [Google Scholar]
- 273.Han TS, Goswami D, Trikudanathan S, Creighton SM, Conway GS. Comparison of bone mineral density and body proportions between women with complete androgen insensitivity syndrome and women with gonadal dysgenesis. European Journal of Endocrinology 2008159179–185. ( 10.1530/EJE-08-0166) [DOI] [PubMed] [Google Scholar]
- 274.Birnbaum W, Marshall L, Werner R, Kulle A, Holterhus PM, Rall K, Köhler B, Richter-Unruh A, Hartmann MF, Wudy SAet al. Oestrogen versus androgen in hormone-replacement therapy for complete androgen insensitivity syndrome: a multicentre, randomised, double-dummy, double-blind crossover trial. Lancet: Diabetes and Endocrinology 20186771–780. ( 10.1016/S2213-8587(1830197-9) [DOI] [PubMed] [Google Scholar]
- 275.Berglund A, Johannsen TH, Stochholm K, Viuff MH, Fedder J, Main KM, Gravholt CH. Morbidity, mortality, and socioeconomics in females with 46,XY disorders of sex development: a nationwide study. Journal of Clinical Endocrinology and Metabolism 20181031418–1428. ( 10.1210/jc.2017-01888) [DOI] [PubMed] [Google Scholar]
- 276.Hughes IA, Deeb A. Androgen resistance. Best Practice and Research: Clinical Endocrinology and Metabolism 200620577–598. ( 10.1016/j.beem.2006.11.003) [DOI] [PubMed] [Google Scholar]
- 277.Melo KF, Mendonca BB, Billerbeck AE, Costa EM, Inácio M, Silva FA, Leal AM, Latronico AC, Arnhold IJ. Clinical, hormonal, behavioral, and genetic characteristics of androgen insensitivity syndrome in a Brazilian cohort: five novel mutations in the androgen receptor gene. Journal of Clinical Endocrinology and Metabolism 2003883241–3250. ( 10.1210/jc.2002-021658) [DOI] [PubMed] [Google Scholar]
- 278.Zachmann M, Prader A, Sobel EH, Crigler JF, Ritzén EM, Atarés M, Ferrandez A. Pubertal growth in patients with androgen insensitivity: indirect evidence for the importance of estrogens in pubertal growth of girls. Journal of Pediatrics 1986108694–697. ( 10.1016/s0022-3476(8681043-5) [DOI] [PubMed] [Google Scholar]
- 279.Papadimitriou DT, Linglart A, Morel Y, Chaussain JL. Puberty in subjects with complete androgen insensitivity syndrome. Hormone Research 200665126–131. ( 10.1159/000091592) [DOI] [PubMed] [Google Scholar]
- 280.Weimann E, Bergmann S, Bohles HJ. Oestrogen treatment of constitutional tall stature: a risk-benefit ratio. Archives of Disease in Childhood 199878148–151. ( 10.1136/adc.78.2.148) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Drop SL, De Waal WJ, De Muinck Keizer-Schrama SM. Sex steroid treatment of constitutionally tall stature. Endocrine Reviews 199819540–558. ( 10.1210/edrv.19.5.0345) [DOI] [PubMed] [Google Scholar]
- 282.Doehnert U, Bertelloni S, Werner R, Dati E, Hiort O. Characteristic features of reproductive hormone profiles in late adolescent and adult females with complete androgen insensitivity syndrome. Sexual Development 2015969–74. ( 10.1159/000371464) [DOI] [PubMed] [Google Scholar]
- 283.Bertelloni S, Dati E, Baroncelli GI, Hiort O. Hormonal management of complete androgen insensitivity syndrome from adolescence onward. Hormone Research in Paediatrics 201176428–433. ( 10.1159/000334162) [DOI] [PubMed] [Google Scholar]
- 284.Batista RL, Costa EMF, Rodrigues AS, Gomes NL, Faria JA, Nishi MY, Arnhold IJP, Domenice S, Mendonca BB. Androgen insensitivity syndrome: a review. Archives of Endocrinology and Metabolism 201862227–235. ( 10.20945/2359-3997000000031) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.van der Straaten S, Springer A, Zecic A, Hebenstreit D, Tonnhofer U, Gawlik A, Baumert M, Szeliga K, Debulpaep S, Desloovere Aet al. The External Genitalia Score (EGS): a European multicenter validation study. Journal of Clinical Endocrinology and Metabolism 2020105 dgz142. ( 10.1210/clinem/dgz142) [DOI] [PubMed] [Google Scholar]
- 286.Hellmann P, Christiansen P, Johannsen TH, Main KM, Duno M, Juul A. Male patients with partial androgen insensitivity syndrome: a longitudinal follow-up of growth, reproductive hormones and the development of gynaecomastia. Archives of Disease in Childhood 201297403–409. ( 10.1136/archdischild-2011-300584) [DOI] [PubMed] [Google Scholar]
- 287.Lucas-Herald A, Bertelloni S, Juul A, Bryce J, Jiang J, Rodie M, Sinnott R, Boroujerdi M, Lindhardt Johansen M, Hiort Oet al. The long-term outcome of boys with partial androgen insensitivity syndrome and a mutation in the androgen receptor gene. Journal of Clinical Endocrinology and Metabolism 20161013959–3967. ( 10.1210/jc.2016-1372) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Bouvattier C, Mignot B, Lefevre H, Morel Y, Bougnères P. Impaired sexual activity in male adults with partial androgen insensitivity. Journal of Clinical Endocrinology and Metabolism 2006913310–3315. ( 10.1210/jc.2006-0218) [DOI] [PubMed] [Google Scholar]
- 289.Poujol N, Lobaccaro JM, Chiche L, Lumbroso S, Sultan C. Functional and structural analysis of R607Q and R608K androgen receptor substitutions associated with male breast cancer. Molecular and Cellular Endocrinology 199713043–51. ( 10.1016/s0303-7207(9700072-5) [DOI] [PubMed] [Google Scholar]
- 290.Lawrence SE, Faught KA, Vethamuthu J, Lawson ML. Beneficial effects of raloxifene and tamoxifen in the treatment of pubertal gynecomastia. Journal of Pediatrics 200414571–76. ( 10.1016/j.jpeds.2004.03.057) [DOI] [PubMed] [Google Scholar]
- 291.Saito R, Yamamoto Y, Goto M, Araki S, Kubo K, Kawagoe R, Kawada Y, Kusuhara K, Igarashi M, Fukami M. Tamoxifen treatment for pubertal gynecomastia in two siblings with partial androgen insensitivity syndrome. Hormone Research in Paediatrics 201481211–216. ( 10.1159/000356923) [DOI] [PubMed] [Google Scholar]
- 292.Li L, Papadopoulos V. Advances in stem cell research for the treatment of primary hypogonadism. Nature Reviews: Urology 202118487–507. ( 10.1038/s41585-021-00480-2) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



 This work is licensed under a
This work is licensed under a