Abstract
Objective
The objective of this study is to investigate the role of serum irisin level in diagnosis of central precocious puberty (CPP) in girls and its major determinants.
Methods
This study was conducted in 67 girls with CPP, 19 girls with premature thelarche (PT) and 59 normal controls. The major determinants of irisin were assessed by multivariate linear regression (MLR) analysis. Propensity score matching (PSM) analysis was performed to minimize the bias that can result from BMI. A receiver operating characteristic curve was used to obtain the optimal threshold value of irisin.
Results
The girls with CPP and PT had higher irisin levels than controls (P < 0.05). The optimal cutoff value of irisin levels for predicting CPP was 91.88 ng/mL, with a sensitivity of 70.1% and a specificity of 72.9%. MLR analysis showed that BMI was a predictor of irisin (P < 0.05). Serum irisin levels remained higher in the CPP girls than the controls with adjustment for BMI (P < 0.05).
Conclusions
Increased serum irisin levels with CPP suggest that irisin is involved in puberty. However, due to low sensitivity and specificity, irisin level can only be used as an auxiliary indicator rather than a single diagnostic indicator of CPP.
Keywords: irisin, central precious puberty, premature thelarche, diagnosis, BMI
Introduction
Central precocious puberty (CPP) is thought to be the early onset of puberty caused by premature reactivation of the hypothalamic–pituitary–gonadal axis (HPGA). It is a common pediatric endocrine disorder characterized by rapid development of internal and external reproductive organs and secondary sexual characteristics before the age of 8 in girls and 9 in boys (1). In recent years, the age of puberty initiation has been declining all over the world, paralleled by the increasing incidence of precocious puberty (2, 3, 4). There has also been an increase in the rate of visits to patients with precocious puberty during the lockdown for the COVID-19 pandemic (5, 6). Children with early puberty may suffer accelerated skeletal maturation and impaired adult height. Early puberty development may also cause early menarche in girls. Moreover, early development and maturation of secondary sexual characteristics may lead to psychological problems or abnormal social behavior (1).
But the exact reasons that lead to the causation of early puberty are still unclear. Puberty is a complex and sophisticated developmental event in which many central and peripheral endocrine mediators are involved. These include nutritional and metabolic factors, including leptin, adiponectin, ghrelin, and insulin, which are known to have an impact on puberty initiation (7). It is known that obese boys and girls tend to undergo an earlier timing of puberty (8). A certain threshold of body fat stores (i.e. energy reserves) needs to be achieved in order to progress through puberty and reach reproductive capacity (9). At present, the diagnostic markers of CPP are still being explored, and some nutritional metabolic factors (such as leptin, adipokines, insulin-like growth factor-1 (IGF-1), insulin) have shown potential roles in the diagnosis of CPP (10, 11, 12). Recently, Kutlu et al. (13) reported the role of another novel adipomyokine, irisin, in the diagnosis of CPP, and their study implied that increased irisin levels might be useful as a diagnostic marker of CPP. However, how irisin is involved in the initiation of puberty and whether it can be used as a reliable marker for the diagnosis of CPP remains to be further explored.
In this study, we focused on the changes of serum irisin levels in girls with CPP, PT and controls in order to explore the role of irisin in the diagnosis of CPP.
Materials and Methods
Ethics
This study conformed to the Declaration of Helsinki and was approved by the Scientific Ethics Committee of The First Affiliated Hospital of Guangxi Medical University in Nanning, China (2021 (KY-E-307)). Informed consent was obtained from all subjects.
Patients
This study was conducted from September 2019 to August 2021 in the First Affiliated Hospital of Guangxi Medical University (Nanning, China). A total of 67 girls with CPP and 19 girls with PT who were followed up on time with comprehensive data were enrolled. During the same period, 59 age-matched prepubertal healthy girls who were in attendance for routine checkups were recruited as normal controls. The clinical criteria for a full diagnosis of CPP refer to the Consensus statement for the diagnosis and treatment of central precocious puberty (2015) (1): (1) breast development before 8 years of age; (2) accelerated linear growth rate (≥6 cm/year); (3) progressive bone age (BA) 1 year more than chronological age (CA); (4) the peak level of luteinizing hormone (LH) (PLH) after gonadotrophin-releasing hormone (GnRH) simulation > 5 IU/L. PT was diagnosed when the girls satisfied the following criteria: (1) early breast budding before 8 years old; (2) without the presence of other pubertal signs such as accelerated growth velocity, menarche, advanced bone age and pubic hair; (3) the PLH after GnRH simulation < 5 IU/L. In addition, girls with thyroid disease, central organic brain disease, congenital adrenal hyperplasia or a history of treatment may affect gonadotropins were excluded, as well as patients with other chronic medical conditions.
Evaluation of anthropometric and laboratory measurements
In all individuals, height, weight and Tanner staging for breast development were measured by an experienced pediatric endocrinologist. BA and uterine and ovarian ultrasounds were performed in girls with CPP and PT. BMI was calculated as weight (kg) /height (m)2.
The GnRH stimulation test was carried out by subcutaneously injecting 2.5 μg/kg (up to 100 μg) of triptorelin (Ferring GmbH), and blood samples were collected repeatedly at baseline and 30, 60, 90 and 120 min after the injection for gonadotropin measured. Girls with PLH values ≥ 5 IU/L were considered as activation of the hypothalamic–pituitary–ovarian axis. Serum LH, estradiol (E2), follicle-stimulating hormone (FSH) and insulin were tested using a chemiluminescence immunometric assay (Mindray, CL-2000i, Shenzhen, China). Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as insulin (μIU/ml) × fasting blood glucose (mmol/L)/22.5. Irisin levels were measured by ELISA (Human Irisin Elisa Kit, CUSABIO, Wuhan, China), and the minimum detectable irisin level was 0.78 ng/mL. The corresponding intra- and inter-assay coefficients of variation were <8 and <10%.
Statistical analysis
Student’s t-test and AVOVA were performed to compare normally distributed variables. Mann–Whitney U and Kruskal–Wallis H tests were used to compare non-normally distributed variables. Spearman’s correlation analysis was carried out to determine the correlation between irisin and other parameters for the cohort as a whole. Multivariate linear regression analysis was established to investigate major determinants of serum irisin levels. Patients with CPP were matched to controls by using propensity score matching analysis to minimize the potential bias that could be caused by BMI. 1:1 nearest neighbor matching without replacement was performed with a caliper set at 0.01. Receiver operating characteristic (ROC) analysis was established to obtain the best cutoff value for irisin to predict CPP, as well as the calculations for sensitivity and specificity. All data analyses were conducted by IBM SPSS statistical software version 25.0, and differences were considered to be statistically significant when P < 0.05.
Results
Anthropometric and laboratory measurements
This study included 67 patients with CPP, 19 patients with PT and 59 healthy prepubertal controls (mean age: 7.55 ± 1.39 vs 7.34 ± 1.04 vs 7.61 ± 0.92 years respectively; P = 0.677). As shown in Table 1, the serum irisin levels were shown to be highest in girls with CPP and lowest in prepubertal controls. When the groups were compared, serum irisin levels were significantly higher in girls with CPP and PT than in the controls (P < 0.05), while there was no statistical significance in irisin level between the CPP and PT groups (P > 0.05). Moreover, the height, BMI, basal LH, basal FSH and E2 levels were significantly higher in CPP girls when compared to the controls (P < 0.05). Additionally, the height, weight, BA advancement, basal and peak LH and FSH, IGF-1 and insulin were higher in CPP compared to the PT group (P < 0.05). BMI and E2 in CPP group were higher than those in PT group, with no significant differences (P > 0.05) (Table 1).
Table 1.
Anthropometric and laboratory characteristics of the study groups.
| Parameters | NC (n = 59) | PT (n = 19) | CPP (n = 67) | P |
|---|---|---|---|---|
| CA (years) | 7.61 ± 0.92 | 7.34 ± 1.04 | 7.55 ± 1.39 | 0.677 |
| BA (years) | - | 7 (7, 7.5) | 9 (7.5, 10)a | 0.000c |
| BA-CA (years) | - | −0.2 (−0.84, 0.61) | 1.1 (0.52, 1.84)a | 0.000c |
| Height (cm) | 123.20 ± 6.09 | 122.65 ± 6.80 | 129.78 ± 11.18ab | 0.000c |
| Weight (kg) | 22.4 (20.7, 25.0) | 23.9 (20.2, 26.4) | 27.1 (23.3, 32.1)ab | 0.000c |
| BMI (kg/m2) | 14.9 (14.26, 15.42) a | 15.75 (14.48, 16.48) | 15.87 (14.75, 17.19)b | 0.000c |
| Uterus volume (mL) | - | 1.50 (0.69, 2.87) | 2.72 (1.63, 4.73)a | 0.002c |
| Ovarian volume (m) | - | 1.83 (1.44, 2.48) | 2.24 (1.43, 4.11) | 0.155 |
| E2 (pmol/L) | 59.26 (23.75, 77.53)a | 92.16 (47.64, 104.92) | 108.30 (73.82, 158.63)b | 0.000c |
| B-LH (IU/L) | 0.11 (0.06, 0.16) | 0.11 (0.05, 0.17) | 1.00 (0.30, 2.14)ab | 0.000c |
| P-LH (IU/L) | - | 3.64 (2.75, 4.32) | 15.18 (9.13, 33.99)a | 0.000c |
| B-FSH (IU/L) | 1.72 (1.31, 2.16) | 2.10 (1.51, 2.60) | 3.73 (2.41, 5.71)ab | 0.000c |
| P-FSH (IU/L) | - | 13.34 (11.31, 15.68) | 17.69 (12.57, 23.47)a | 0.039c |
| P-LH/P-FSH | - | 0.27 (0.18, 0.34) | 1.11 (0.63, 1.87)a | 0.000c |
| IGF-1 (nmol/L) | - | 31.46 (24.32, 38.98) | 43.49 (32.53, 52.87)a | 0.002c |
| Insulin (pmol/L) | - | 8.14 ± 4.85 | 12.34 ± 6.21a | 0.034c |
| GLU (mmol/L) | - | 4.61 ± 0.33 | 4.46 ± 0.44 | 0.300 |
| HOMA-IR | - | 1.74 ± 0.98 | 2.53 ± 1.31 | 0.068 |
| Irisin (ng/ml) | 61.8 (38.9, 97.17)a | 100.28 (68.06, 170.63) | 159.23 (77.12, 289.46)b | 0.000c |
Values are expressed as mean ±standard deviation (s.d.) for normally distributed variables or expressed as median (interquartile ranges) for non-normally distributed variables. Statistical significance is represented as camong three groups P < 0.05, avs. PT group P < 0.05 and bvs NC group P < 0.05.
B-, base; BA, bone age; BA-CA: bone age advancement; CA, chronological age; CPP, central precocious puberty; E2, estradiol; FSH, follicle stimulating hormone; GLU, fasting glucose; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-1, insulin-like growth factor-1; LH, luteinizing hormone; NC, normal control; P-, peak; PT, premature thelarche.
ROC analysis
Based on ROC curve analysis, the area under curve (AUC) of irisin to differentiate between CPP and prepubertal controls was 0.757 (95% CI: 0.674–0.841; P < 0.0001). The irisin level of 91.88 ng/mL provided the most appropriate level with a sensitivity of 70.1% and specificity of 72.9% (Fig. 1).
Figure 1.
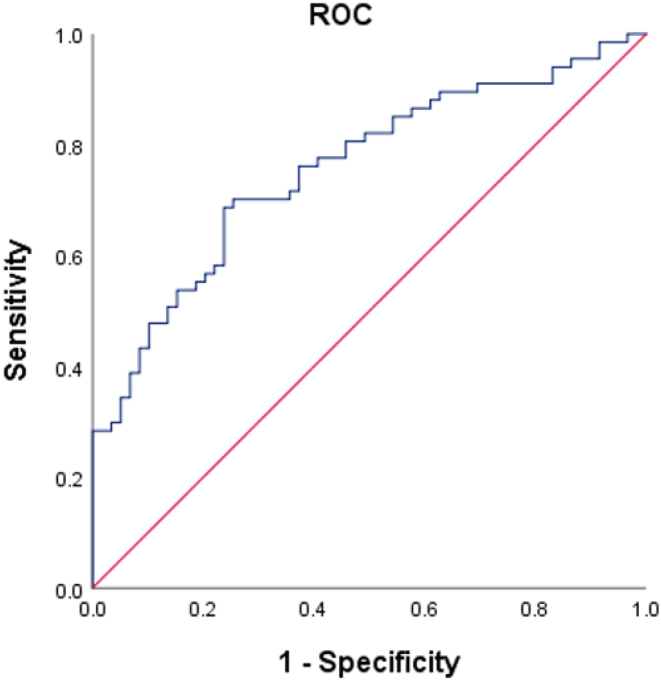
The AUC for identifying the girls with or without CPP was 0.757 (95% CI: 0.674–0.841; P < 0.0001). An irisin level of 91.88 ng/mL was found to be the most appropriate with a sensitivity of 70.1% and specificity of 72.9%.
Correlation analysis
The irisin levels showed positive correlations with age, BA, BA-CA, height, weight, BMI, ovarian volume, E2, basal LH, basal FSH, peak LH, P-LH/P-FSH, IGF-1, insulin, GLU and HOMA-IR for the cohort as a whole (P < 0.05). However, no correlation was found between irisin and uterine volume and peak FSH values (P > 0.05) (Table 2).
Table 2.
The correlation analysis between the serum irisin level and anthropometric and biochemical characteristics.
| Parameters | n | R value | P |
|---|---|---|---|
| CA (years) | 145 | 0.169 | 0.043a |
| BA (years) | 86 | 0.330 | 0.002a |
| BA-CA (years) | 86 | 0.317 | 0.003a |
| Height (cm) | 145 | 0.447 | 0.000a |
| Weight (kg) | 145 | 0.644 | 0.000a |
| BMI (kg/m2) | 145 | 0.681 | 0.000a |
| Uterus volume (mL) | 84 | 0.441 | 0.236 |
| Ovarian volume (mL) | 86 | 0.236 | 0.028a |
| E2 (pmol/L) | 145 | 0.383 | 0.000a |
| B-LH (IU/L) | 145 | 0.434 | 0.000a |
| B-FSH (IU/L) | 145 | 0.307 | 0.000a |
| P-LH (IU/L) | 86 | 0.213 | 0.049a |
| P-FSH (IU/L) | 86 | −0.112 | 0.303 |
| P-LH/P-FSH | 86 | 0.319 | 0.003a |
| TG (mmol/L) | 74 | 0.476 | 0.000a |
| IGF-1 (nmol/L) | 72 | 0.395 | 0.001a |
| Insulin (ng/mL) | 59 | 0.453 | 0.000a |
| GLU (mmol/L) | 56 | 0.311 | 0.020a |
| HOMA-IR | 56 | 0.420 | 0.001a |
The correlation coefficient was calculated with Spearman correlation analysis. aStatistical significance is represented as P< 0.05.
B-, baseline; BA, bone age; BA-CA: bone age advancement; CA, chronological age; E2, estradiol; FSH, follicle stimulating hormone; GLU, fasting glucose; HOMA-IR, homeostasis model assessment of insulin resistance; IGF-1: insulin-like growth factor-1; LH, luteinizing hormone; P-, peak; TG: triglyceride.
Multivariate linear regression analysis
Multivariate linear regression analysis indicated that only BMI was a predicting factor of irisin levels (P < 0.05), whereas other parameters had no impact on irisin levels (Table 3).
Table 3.
Determinants of serum irisin level by multivariate linear regression analysis.
| Variables | Unstandardized coefficients | Standardize coefficients | t | P | |
|---|---|---|---|---|---|
| B | Std. Error | Beta | |||
| (constant) | −663.131 | 249.131 | −2.662 | 0.011a | |
| Bone age (years) | −12.264 | 12.941 | −0.199 | −0.948 | 0.349 |
| Height (cm) | 1.056 | 2.419 | 0.092 | 0.437 | 0.665 |
| BMI (kg/m2) | 33.995 | 7.218 | 0.527 | 4.710 | 0.000a |
| B-LH (IU/L) | −7.164 | 19.946 | −0.092 | −0.359 | 0.721 |
| P-LH (IU/L) | −.068 | 1.816 | −0.015 | −0.037 | 0.970 |
| B-FSH (IU/L) | 16.769 | 9.911 | 0.294 | 1.692 | 0.098 |
| P-FSH (IU/L) | −3.385 | 2.771 | −0.224 | −1.222 | 0.228 |
| PLH/PFSH | 34.087 | 34.126 | 0.303 | 0.999 | 0.323 |
| E2 (pmol/L) | 0.012 | 0.427 | 0.003 | 0.027 | 0.978 |
| GLU (mmol/L) | 42.041 | 30.522 | 0.165 | 1.377 | 0.176 |
| HOMA-IR | 1.593 | 1.486 | 0.131 | 1.072 | 0.290 |
Dependent variable: irisin. aStatistical significance is represented as P < 0.05.
B-, baseline; E2, estradiol; FSH, follicle stimulating hormone; GLU: fasting glucose; HOMA-IR, homeostasis model assessment of insulin resistance; LH, luteinizing hormone; P-, peak.
Propensity score matching analysis
Forty-eight propensity-score matched pairs of CPP and prepubertal controls were identified. We found that serum irisin levels remained higher in CPP girls when compared to the prepubertal group after matching BMI by using propensity score matching analysis (P < 0.05) (Table 4).
Table 4.
Anthropometric and laboratory parameters of the CPP and NC groups after propensity score matching analysis.
| Parameters | NC (n = 48) | CPP (n = 48) | P |
|---|---|---|---|
| CA (years) | 7.54 ± 0.88 | 7.41 ± 1.54 | 0.619 |
| BMI (kg/m2) | 14.99 ± 1.15 | 15.23 ± 1.23 | 0.325 |
| E2 (pmol/L) | 47.24 (22.24, 71.98) | 100.11 (71.83, 157.38) | 0.000a |
| B-LH (IU/L) | 0.12 (0.07, 0.15) | 0.75 (0.23, 1.77) | 0.000a |
| B-FSH (IU/L) | 1.79 (1.42, 2.18) | 3.33 (2.00, 5.68) | 0.000a |
| Irisin (ng/L) | 58.83 (38.96, 95.12) | 110.36 (59.22, 220.33) | 0.002a |
Values are expressed as mean ±standard deviation (s.d.) for normally distributed variables or expressed as median (interquartile ranges) for non-normally distributed variables. aStatistical significance is represented as P < 0.05.
B, baseline; CA, chronological age; CPP, central precocious puberty; E2, estradiol; FSH, follicle stimulating hormone; LH, luteinizing hormone; NC, normal control.
Discussion
In the present study, we demonstrated that serum irisin levels in CPP and PT girls were higher than the healthy controls, indicating that irisin might be involved in the onset of HPGA in puberty. Previous in vitro studies showed that irisin may not only interfere with GnRH stimulation of LH and significantly stimulate E2 secretion in human ovarian granulosa cells but may also interfere with insulin’s stimulatory effect on E2 production (14). Animal experiments revealed that chronic irisin exposure might lead to disorders in the female reproductive system and had androgenic potential on the HPGA in males and stimulated the expression and release of GnRH in mouse hypothalamic cells (15, 16). Both in vitro studies and animal experiments suggested that irisin could stimulate GnRH secretion, which supported our conclusion that the level of irisin in CPP girls was higher than that in the control group. In addition, Reinehr et al. (17) also found that the irisin levels were significantly lower in prepubertal compared to pubertal obese children. Therefore, our conclusion that the level of irisin in CPP girls was higher than that in prepubertal control group had also been supported in human studies. Moreover, our study showed serum irisin level was significantly higher in PT than in the control group. PT is defined as isolated breast development before age 8 in girls without other secondary sexual characteristics (18). So far, the exact cause of PT remains unclear and may be related to transient partial activation of the HPGA and the increased sensitivity of the breast to estrogen (18, 19). In vitro studies suggested that irisin significantly stimulated E2 secretion of human ovarian granulosa cells, which supported our conclusion that the level of irisin in girls in PT group was higher than that in control group. Nevertheless, we found no differences in irisin levels between CPP and PT girls, which was inconsistent with Kutlu’s result (13). At present, the mechanism of early puberty induced by irisin might be related to the stimulation of GnRH and E2 secretion, which were not targeted in CPP and PT girls. The changes of irisin level in CPP and PT girls need to be further verified by expanding the sample size or further studies.
Previous studies (13, 17) and our study have suggested that irisin levels will increase at puberty, but another question waiting to be addressed is whether irisin can be used for the diagnosis of CPP? In our study, the area under the receiver operating characteristic curve to identify the girls with or without CPP was 0.757. The optimal cut-off value of irisin concentration was 91.88 ng/mL and the sensitivity and specificity was 70.1% and 72.9%, respectively. Due to its low sensitivity and specificity, serum irisin level could only be used as an auxiliary indicator rather than a single indicator in diagnosis of CPP. Additionally, there was no difference in irisin level between CPP and PT patients, thus serum level is unable to provide reliable diagnostic value on differentiating CPP from PT, which might be related to the small sample size of PT group.
Besides, we found that irisin level was positively correlated with BA, BA-CA, height, BMI, ovarian volume, E2, basal LH, basal FSH, peak LH, P-LH/P-FSH, IGF-1, insulin, GLU and HOMA-IR for the cohort as a whole. In previous studies of both adults and children (20, 21, 22, 23, 24, 25), circulating irisin levels were positively correlated with BMI and fat mass, while the relationship between irisin and other metabolic indexes (such as insulin, cholesterol, adiponectin and HOMA-IR) were inconsistent. Earlier studies suggested that lower circulating irisin was associated with type 2 diabetes mellitus (26). In contrast, increased levels of circulating irisin appears to be linked with improved glucose homeostasis by reducing insulin resistance (27), indicating irisin performs a possible compensatory role in glucose homeostasis regulation.
Furthermore, multivariate linear regression analysis showed that BMI was an independent influencing factor of irisin level in our study. Data originating from systematic reviews and epidemiological surveys demonstrated that girls with an excessive increased BMI were more likely to suffer from early puberty (28) and BMI standard deviation scores were significantly associated with PT in girls between the ages 4 and 8 (29). Of note, serum irisin levels remained significantly higher in CPP girls when compared to the prepubertal controls with adjustment for BMI in the present study. Thus, in addition to BMI, another factor that affects irisin levels was puberty, but whether irisin causes the initiation of puberty through modulation of adipose tissue or through other ways remains to be further clarified.
In conclusion, our preliminary study suggests that serum irisin levels increased in girls with CPP and PT, but it is not a reliable biomarker to distinguish patients with CPP from those with PT and age-matched prepubertal controls. In addition, the relationship between irisin and BMI and how the hormone participates in the adipose tissue regulatory network deserve to be further explored.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
Funding
The study was funded by the Scientific Research Project of Guangxi Health and Family Planning Commission (Grant No. Z20211173) and the First Affiliated Hospital of Guangxi Medical University starting fund for study abroad returnees (Grant No. 2010001).
Author contribution statement
Y Chen and M Li completed the experimental part of this study, analyzed data and drafted the manuscript. D Lan and J Zhong participated in study design and modified the manuscript. B Liao contributed to the specimen collection. The final version of the manuscript was approved by all the authors.
Acknowledgements
The authors are extremely grateful to all participants and thank Dr Dev Sooranna of Imperial College London for his guidance in English style of the manuscript.
References
- 1.Subspecialty Group of Endocrinologic, Hereditary and Metabolic Diseases, the Society of Pediatrics, Chinese Medical Association & Editorial Board, Chinese Journal of Pediatrics. Consensus statement for the diagnosis and treatment of central precocious puberty (2015). Zhong hua Er Ke Za Zhi 201553412–418. ( 10.3760/cma.j.issn.0578-1310.2015.06.004) [DOI] [PubMed] [Google Scholar]
- 2.Kim YJ, Kwon A, Jung MK, Kim KE, Suh J, Chae HW, Kim DH, Ha S, Seo GH, Kim HS. Incidence and prevalence of central precocious puberty in Korea: an epidemiologic study based on a national database. Journal of Pediatrics 2019208221–228. ( 10.1016/j.jpeds.2018.12.022) [DOI] [PubMed] [Google Scholar]
- 3.Bräuner EV, Busch AS, Eckert-Lind C, Koch T, Hickey M, Juul A. Trends in the incidence of central precocious puberty and normal variant puberty among children in Denmark, 1998 to 2017. JAMA Network Open 20203 e2015665. ( 10.1001/jamanetworkopen.2020.15665) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Eckert-Lind C, Busch AS, Petersen JH, Biro FM, Butler G, Bräuner EV, Juul A. Worldwide secular trends in age at pubertal onset assessed by breast development among girls: a systematic review and meta-analysis. JAMA Pediatrics 2020174 e195881. ( 10.1001/jamapediatrics.2019.5881) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li H, Yu G, Duan H, Fu J, Shu Q. Changes in children’s healthcare visits during coronavirus disease-2019 pandemic in Hangzhou, China. Journal of Pediatrics 2020224146–149. ( 10.1016/j.jpeds.2020.05.013) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stagi S, De Masi S, Bencini E, Losi S, Paci S, Parpagnoli M, Ricci F, Ciofi D, Azzari C. Increased incidence of precocious and accelerated puberty in females during and after the Italian lockdown for the coronavirus 2019 (COVID-19) pandemic. Italian Journal of Pediatrics 202046 165. ( 10.1186/s13052-020-00931-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Avendaño MS, Vazquez MJ, Tena-Sempere M. Disentangling puberty: novel neuroendocrine pathways and mechanisms for the control of mammalian puberty. Human Reproduction Update 201723737–763. ( 10.1093/humupd/dmx025) [DOI] [PubMed] [Google Scholar]
- 8.De Leonibus C, Marcovecchio ML, Chiavaroli V, de Giorgis T, Chiarelli F, Mohn A. Timing of puberty and physical growth in obese children: a longitudinal study in boys and girls. Pediatric Obesity 20149292–299. ( 10.1111/j.2047-6310.2013.00176.x) [DOI] [PubMed] [Google Scholar]
- 9.Wahab F, Shahab M, Behr R. Hypothesis: irisin is a metabolic trigger for the activation of the neurohormonal axis governing puberty onset. Medical Hypotheses 2016951–4. ( 10.1016/j.mehy.2016.08.003) [DOI] [PubMed] [Google Scholar]
- 10.Palmert MR, Radovick S, Boepple PA. Leptin levels in children with central precocious puberty. Journal of Clinical Endocrinology and Metabolism 1998832260–2265. ( 10.1210/jcem.83.7.4973) [DOI] [PubMed] [Google Scholar]
- 11.Zurita-Cruz JN, Villasís-Keever MA, Manuel-Apolinar L, Damasio-Santana L, Gutierrez-Gonzalez A, Wakida-Kusunoki G, Padilla-Rojas M, Maldonado-Rivera C, Garrido-Magaña E, Rivera-Hernández AJet al. Altered cardiometabolic profile in girls with central precocious puberty and adipokines: a propensity score matching analysis. Cytokine 2021148155660. ( 10.1016/j.cyto.2021.155660) [DOI] [PubMed] [Google Scholar]
- 12.Sørensen K, Aksglaede L, Petersen JH, Andersson AM, Juul A. Serum IGF1 and insulin levels in girls with normal and precocious puberty. European Journal of Endocrinology 2012166903–910. ( 10.1530/EJE-12-0106) [DOI] [PubMed] [Google Scholar]
- 13.Kutlu E, Özgen İT, Bulut H, Koçyiğit A, Otçu H, Cesur Y. Serum irisin levels in central precocious puberty and its variants. Journal of Clinical Endocrinology and Metabolism 2021106e247–e254. ( 10.1210/clinem/dgaa720) [DOI] [PubMed] [Google Scholar]
- 14.Poretsky L, Islam J, Avtanski D, Lin YK, Shen YL, Hirth Y, Lesser M, Rosenwaks Z, Seto-Young D. Reproductive effects of irisin: initial in vitro studies. Reproductive Biology 201717285–288. ( 10.1016/j.repbio.2017.05.011) [DOI] [PubMed] [Google Scholar]
- 15.Ulker N, Yardimci A, Kaya Tektemur N, Bulmus O, Ozer Kaya S, Gulcu Bulmus F, Turk G, Ozcan M, Canpolat S. Irisin may have a role in pubertal development and regulation of reproductive function in rats. Reproduction 2020160281–292. ( 10.1530/REP-20-0072) [DOI] [PubMed] [Google Scholar]
- 16.Wahab F, Khan IU, Polo IR, Zubair H, Drummer C, Shahab M, Behr R. Irisin in the primate hypothalamus and its effect on GnRH in vitro. Journal of Endocrinology 2019241175–187. ( 10.1530/JOE-18-0574) [DOI] [PubMed] [Google Scholar]
- 17.Reinehr T, Elfers C, Lass N, Roth CL. Irisin and its relation to insulin resistance and puberty in obese children: a longitudinal analysis. Journal of Clinical Endocrinology and Metabolism 20151002123–2130. ( 10.1210/jc.2015-1208) [DOI] [PubMed] [Google Scholar]
- 18.de Vries L, Guz-Mark A, Lazar L, Reches A, Phillip M. Premature thelarche: age at presentation affects clinical course but not clinical characteristics or risk to progress to precocious puberty. Journal of Pediatrics 2010156466–471. ( 10.1016/j.jpeds.2009.09.071) [DOI] [PubMed] [Google Scholar]
- 19.Berberoğlu M.Precocious puberty and normal variant puberty: definition, etiology, diagnosis and current management. Journal of Clinical Research in Pediatric Endocrinology 20091164–174. ( 10.4274/jcrpe.v1i4.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huh JY, Panagiotou G, Mougios V, Brinkoetter M, Vamvini MT, Schneider BE, Mantzoros CS. FNDC5 and irisin in humans: I. Predictors of circulating concentrations in serum and plasma and II. mRNA expression and circulating concentrations in response to weight loss and exercise. Metabolism: Clinical and Experimental 2012611725–1738. ( 10.1016/j.metabol.2012.09.002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jang HB, Kim HJ, Kang JH, Park SI, Park KH, Lee HJ. Association of circulating irisin levels with metabolic and metabolite profiles of Korean adolescents. Metabolism: Clinical and Experimental 201773100–108. ( 10.1016/j.metabol.2017.05.007) [DOI] [PubMed] [Google Scholar]
- 22.Elizondo-Montemayor L, Silva-Platas C, Torres-Quintanilla A, Rodríguez-López C, Ruiz-Esparza GU, Reyes-Mendoza E, Garcia-Rivas G. Association of irisin plasma levels with anthropometric parameters in children with underweight, normal weight, overweight, and obesity. BioMed Research International 201720172628968. ( 10.1155/2017/2628968) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Çatlı G, Küme T, Tuhan HÜ, Anık A, Çalan ÖG, Böber E, Abacı A. Relation of serum irisin level with metabolic and antropometric parameters in obese children. Journal of Diabetes and its Complications 2016301560–1565. ( 10.1016/j.jdiacomp.2016.07.019) [DOI] [PubMed] [Google Scholar]
- 24.Nigro E, Scudiero O, Ludovica Monaco M, Polito R, Schettino P, Grandone A, Perrone L, Miraglia Del Giudice E, Daniele A. Adiponectin profile and Irisin expression in Italian obese children: association with insulin-resistance. Cytokine 2017948–13. ( 10.1016/j.cyto.2016.12.018) [DOI] [PubMed] [Google Scholar]
- 25.Binay Ç, Paketçi C, Güzel S, Samancı N. Serum irisin and oxytocin levels as predictors of metabolic parameters in obese children. Journal of Clinical Research in Pediatric Endocrinology 20179124–131. ( 10.4274/jcrpe.3963) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu JJ, Wong MD, Toy WC, Tan CS, Liu S, Ng XW, Tavintharan S, Sum CF, Lim SC. Lower circulating irisin is associated with type 2 diabetes mellitus. Journal of Diabetes and its Complications 201327365–369. ( 10.1016/j.jdiacomp.2013.03.002) [DOI] [PubMed] [Google Scholar]
- 27.Boström P, Wu J, Jedrychowski MP, Korde A, Ye L, Lo JC, Rasbach KA, Boström EA, Choi JH, Long JZet al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nature 2012481463–468. ( 10.1038/nature10777) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li W, Liu Q, Deng X, Chen Y, Liu S, Story M. Association between obesity and puberty timing: a systematic review and meta-analysis. International Journal of Environmental Research and Public Health 201714. ( 10.3390/ijerph14101266) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Atay Z, Turan S, Guran T, Furman A, Bereket A. The prevalence and risk factors of premature thelarche and pubarche in 4- to 8-year-old girls. Acta Paediatrica 2012101e71–e75. ( 10.1111/j.1651-2227.2011.02444.x) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a