Abstract
In humans, activated platelets contribute to sepsis complications and to multiple organ failure. In our prospective analytical study of cases of the equine systemic inflammatory response syndrome (SIRS), we adapted a standard human protocol for the measurement of activated platelets and platelet-leukocyte aggregates (PLAs) in equine platelet-leukocyte-rich plasma (PLRP) by flow cytometry, and we investigated the hypothesis that activated platelets and PLAs are increased in clinical cases of SIRS. We included 17 adult horses and ponies fulfilling at least 2 SIRS criteria, and 10 healthy equids as controls. Activation of platelets was determined by increased expression of CD62P on platelets. Activated platelets and PLAs were measured before and after in vitro activation of platelets with collagen. Median expression of CD62P on platelets was significantly increased after activation in the control group: 1.45% (interquartile range [IQR]: 1.08–1.99%) initially versus 8.78% (IQR: 6.79–14.78%, p = 0.002) after activation. The equids with SIRS had significantly more activated platelets and PLAs in native PLRP than controls: CD62P 4.92% (median, IQR: 2.21–12.41%) versus 1.45% in controls (median, IQR: 1.08–1.99%, p = 0.0007), and PLAs 4.16% (median, IQR: 2.50–8.58%) versus 2.95% in controls (median, IQR: 1.57–3.22%, p = 0.048). To our knowledge, increased platelet activation and PLAs have not been demonstrated previously with flow cytometry in clinical cases of equine SIRS.
Keywords: CD62P, equine systemic inflammatory response syndrome, flow cytometry, horses, P-selectin, platelets
Not until the early 21st century was it widely recognized that, in addition to their primary role in hemostasis, platelets are multifunctional and act as key players in inflammatory processes. Numbers of activated platelets and platelet-leukocyte aggregates (PLAs) are increased in human patients with septic shock and multiple organ dysfunction syndrome (MODS); a positive correlation has been demonstrated between platelet activation and severity of disease.25,50,53 Increased numbers of activated platelets and/or PLAs measured by flow cytometry have been described in horses after near-maximal treadmill exercise, 72 after induced infection with equine infectious anemia virus, 49 in the course of carbohydrate-induced laminitis, 71 and in vitro after incubation with lipopolysaccharides 9 and equid herpesvirus 1 (Equid alphaherpesvirus 1).60–62 Activation markers in horses with equine asthma after antigen challenge were expressed inconsistently, with increased activation in one study 29 and no difference to the control groups in 2 other studies.20,30 In experimentally induced equine endotoxemia, platelet activation was not increased at the time of leukocyte nadir. 68 Activation of secondary hemostasis with microthrombosis and organ failure in the clinical context of disseminated intravascular coagulation has been demonstrated in horses with colic15,24 and in septic foals, 2 but data are lacking regarding platelet activation from clinical cases.
The term systemic inflammatory response syndrome (SIRS) was introduced in 1991 during a human consensus conference to describe the complex pathophysiologic response to an insult such as infection, and to develop criteria for early identification of candidates for clinical trials of new treatments for sepsis. 5 The definition of SIRS has been used in several equine studies involving horses >1-y-old, with markedly different inclusion limits for the clinical parameters.3,6,16,23,36,47,54
Traditionally, washed platelets or platelet-rich plasma (PRP) have been used for the measurement of platelet function by flow cytometry.44,57 This approach is very time consuming, and sample manipulation and centrifugation increase the risk for artefactual in vitro activation 28 and thus the potential loss of platelet subpopulations. 34 Furthermore, the use of washed platelets or PRP is unsuitable for measurement of PLAs. Therefore, protocols have been established using human whole blood for immunophenotypic analysis of platelets 34 and PLAs. 4 In these protocols, activated platelets and PLAs are identified by performing dual-labeling with conjugated antibodies. In human studies, antibodies against CD41/61 are mainly used as specific platelet markers, 4 and platelet activation is demonstrated with antibodies against CD62P (synonym P-selectin), CD154 (synonym CD40L), and PAC-1.28,37,53,56 Various specific markers for leukocytes (e.g., anti-CD14, -CD64, -CD33, -CD45) have been used for measurement of human PLAs. 4 Anti-CD41/61 has been used as a specific platelet marker in equine studies, and platelet activation has been identified by increased expression of CD62P.7,20,29,30,35,55,60,72 Equine leukocytes have been identified with antibodies against CD11a/18, CD13, and CD18.7,20,35 Platelet activation has been measured in equine studies in PRP7,30,35,55,60,70 and in centrifuged platelet-leukocyte-rich plasma (PLRP).7,20,35
Our aims were first to describe a method for measurement of activated platelets and PLAs in equine PLRP by flow cytometry according to standard human protocols,4,34 and then to measure activated platelets and PLAs in clinical cases of equine SIRS. Our hypothesis was that the numbers of activated platelets and PLAs are increased in equine SIRS, with a potential impact on survival.
Materials and methods
Samples and study design
In our prospective analytical study, we collected blood samples from healthy horses and ponies (controls) and from horses and ponies with SIRS presented to the Equine Clinic, Internal Medicine, Department of Veterinary Clinical Science, Justus-Liebig-University (Giessen, Germany). Sampling was performed with owner consent for health monitoring in the control group or as part of the routine diagnostic workup of the SIRS cases prior to treatment in the equine clinic.
Adult Warmblood horses and ponies (withers height <147 cm) >3-y-old matching the hospital population were included in the control group. Controls were considered healthy based on the absence of abnormalities revealed by clinical examination, a body condition score ≥2 of 5, 12 and the absence of alterations of ≥2 of 3 inflammatory blood variables: leukocyte count (RI: 4.4–9.0 × 109/L; Advia 2120, Siemens), globulin concentration (RI: 23–42 g/L; Pentra 400, Axon Lab), and fibrinogen concentration (RI: 1.25–3.29 g/L; STA Compact, Stago). Pregnant mares were excluded based on data indicating increased platelet activation in pregnant women. 31 Similarly, horses with any medication in the past 14 d were excluded in view of the unpredictable influences of medications on platelet function. Blood samples were collected aseptically from a jugular vein with a sterile cannula (18 G; B. Braun) using a vacuum system (S-Monovette; Sarstedt) in sterile tubes with K3-EDTA (1.6 mmol/L) and lithium heparin (16 IE/mL) for the measurement of leukocytes, fibrinogen, and globulins. Tubes with trisodium citrate (0.106 mol/L, citrate:blood ratio 1:9) were used for the platelet analysis by flow cytometry because citrate is the anticoagulant used most commonly for platelet studies. 34
Adult horses and ponies >3-y-old were included in the SIRS group. SIRS cases fulfilled at least 2 of the following criteria: heart rate >52 beats per min; respiratory rate >24 breaths per min; body temperature ≥39.0°C or ≤36.0°C, and leukocyte count <3.0 × 109/L or >15.0 × 109/L (Advia 2120). The limits in our study were calculated based on the human consensus 5 reflecting the percentage increase above the upper, and decrease below the lower, limits of the physiologic parameters in horses.
Horses that were discharged from the clinic were defined as survivors, whereas non-survivors were those euthanized as a result of ineffective treatment or denied therapy based on economic reasons. Exclusion criteria were pregnancy, as in the control group, and pretreatment with acetylsalicylic acid or clopidogrel, which can induce platelet inhibition. Blood samples were collected aseptically from a jugular vein with a sterile cannula (18 G; B. Braun) or a sterile Teflon catheter (80 mm, 14 G; Walter) using a vacuum system (S-Monovette; Sarstedt) in sterile tubes with trisodium citrate (0.106 mol/L, citrate:blood ratio 1:9). The first 2 mL were discarded when sampling originated from a catheter.
Given that erythrolysis is recommended in the human protocol for the analysis of PLAs, 4 we conducted a preliminary study with citrated blood samples from 10 healthy horses to validate the effect of erythrolysis on numbers of platelets and leukocytes. PLRP was obtained as a supernatant after 30 min of undisturbed sedimentation of 10-mL citrated blood samples at room temperature (RT), as described previously. 20 Platelets and leukocytes were significantly decreased after erythrolysis, according to the manufacturer’s protocol (VersaLyse; Beckman Coulter), whereas the numbers were significantly increased in PLRP (Table 1). Therefore, PLRP was chosen as the material for flow cytometry.
Table 1.
Cell counts (Advia 2120; Siemens) in citrated whole blood within 60 min after blood sampling, after erythrolysis (according to the manufacturer’s protocol; VersaLyse, Beckman Coulter) and in platelet-leukocyte-rich plasma (after sedimentation for 30 min) in 10 healthy adult horses (median, IQR; Prism v.6, GraphPad).
| Sample | Erythrocytes (×1012/L) | Platelets (×109/L) | Leukocytes (×109/L) |
|---|---|---|---|
| Citrated whole blood | 6.87 a,b (6.40–7.06)* | 108.0b,c (93.3–113.1)† | 5.39 d,e (4.69–5.69† |
| After erythrolysis | 0.01 a (0.01–0.03)* | 26.0 c (17.5–33.5)* | 0.61 d (0.59–0.71)† |
| Platelet-leukocyte-rich plasma | 0.11 b (0.09–0.13)† | 173.5 b (130.0–212.0)† | 8.63 e (8.25–9.17)† |
p < 0.05 (Shapiro–Wilk normality test).
p ≥ 0.05 (Shapiro–Wilk normality test).
p = 0.01 (Wilcoxon test).
p < 0.001 (Wilcoxon test).
p < 0.001 (paired t-test).
Measurement of activated platelets and PLAs in PLRP
Activated platelets and PLAs were measured by dual-labeling with conjugated antibodies (all Bio-Rad, formerly AbD Serotec) as described.4,34 An allophycocyanin (APC)-conjugated (LYNX rapid antibody conjugation kit; Bio-Rad) monoclonal mouse anti-sheep antibody against CD41/61 with cross-reactivity against equine platelet CD41/617,29,30 was used as platelet marker (MCA1095GA, dilution 1:50 with modified HEPES/Tyrod buffer 4 ). Activation of platelets was determined with fluorescein isothiocyanate (FITC)-conjugated (LYNX rapid antibody conjugation kit; Bio-Rad) monoclonal mouse anti-human antibodies against CD62P (MCA2419, dilution 1:50 with modified HEPES/Tyrod buffer). The anti-CD62P has been used successfully as a marker of equine platelet activation.29,30 A FITC-conjugated monoclonal mouse anti-horse antibody against CD11a/18 (MCA1081F, dilution 1:200 with modified HEPES/Tyrod buffer) served as leukocyte marker. Matched isotype antibodies were used for negative controls: APC-conjugated polyclonal goat anti-mouse immunoglobulin (Ig) for measurements of CD41/61 expression (550826; Becton Dickinson), FITC-conjugated polyclonal goat anti-mouse Ig for CD62P (sc-2010; Santa Cruz), and FITC-conjugated polyclonal goat anti-mouse Ig for CD11a/18 (554001; Becton Dickinson).
PLRP was obtained as a supernatant after 30 min of undisturbed sedimentation of 10-mL citrated blood samples at RT, as described previously. 20 The platelet count was measured (Advia 2120) in PLRP within 30 min after undisturbed sedimentation. Samples for measurement of activated platelets were diluted 1:10 with modified HEPES/Tyrod buffer. Two plastic vials were each filled with 20 µL of diluted PLRP, 20 µL of a solution of anti-CD41/61, and 20 µL of a solution of anti-CD62P. One sample was diluted with 20 µL of modified HEPES/Tyrod buffer (native); the platelets in the second sample were activated with 20 µL of collagen (50 μg/mL, COLtest; Roche) as a positive control for activation. Samples were vortexed and incubated for 20 min in the dark at RT, and measurement started within 75 min after blood collection.
PLAs were detected, with minor modifications. Two plastic vials were each filled with 20 µL of PLRP, 20 µL of a solution of anti-CD41/61, and 20 µL of a solution of anti-CD11a/18. One sample was diluted with 20 µL of modified HEPES/Tyrod buffer (native); the platelets in the second sample were activated with 20 µL of collagen. Samples were vortexed, incubated in the dark at RT for 15 min, and were diluted after incubation with 100 µL of modified HEPES/Tyrod buffer before measurement that started within 75 min after blood collection.
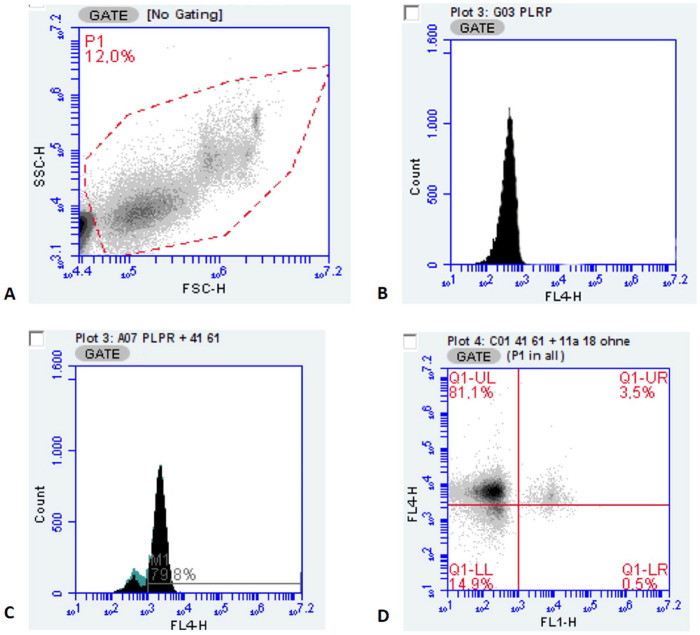
Flow cytometry was performed (Accuri C6, Accuri C6 analysis software v.1.0.264.2; Becton Dickinson). Platelets were gated on the basis of a combination of characteristic light-scatter profile in which the threshold for the forward scatter (FSC) was set at 20,000 (Fig. 1A), and fluorescence was measured with the APC-conjugated antibody against CD41/61 in FL-4 (640 nm, filter 675/25 nm; Fig. 1B, 1C). The FITC-conjugated antibodies for detection of activated platelets and PLAs were gated in the same manner in FL-1 (488 nm, filter 530/30 nm). Numbers of activated platelets and PLAs were determined as the percentage of dual-labeled events counting 20,000 CD41/61-positive events (Fig. 1D).
Figure 1.
Cytometric gating protocol. A. Platelet gate (P1) on the basis of the forward (FSC) and side scatter (SSC), threshold FSC 20,000. B. Single-parameter fluorescence histogram of the APC-conjugated polyclonal goat anti-mouse antibody as isotype-negative control for CD41/61 (FL-4: 640 nm, filter 675/25 nm). C. Single-parameter fluorescence histogram gated for APC-conjugated monoclonal mouse anti-sheep antibodies against CD41/61. D. Dotplot representing platelet expression of CD41/61 (y-axis; FL-4; Q1-UL indicating positive events for CD41/61) and expression of CD11a/18 (leukocytes; x-axis; FL-1, 488 nm, filter 530/30 nm; Q1-LR indicating positive events for CD 11a/18); dual-labeled events positive for CD41/61 and CD11a/18 representing 3.5% platelet-leukocyte aggregates in Q1-UR.
Statistics
Data were analyzed with Prism v.6 (GraphPad). The level of significance was set at p ≤ 0.05.
SIRS criteria
Differences in SIRS inclusion criteria (heart rate, respiratory rate, rectal temperature, blood leukocyte count) to compare values of the survivor group versus values of the non-survivor group were determined with the nonparametric Mann–Whitney test because values lacked Gaussian distribution after assessment for normality using the Shapiro–Wilk test (Table 2).
Table 2.
Systemic inflammatory response syndrome (SIRS) criteria in all horses and differences depending on outcome (4 non-survivors excluded from calculations as euthanized based on economic reasons; median, IQR; Mann–Whitney test).
| Parameter/variable | SIRS inclusion criteria | All (n = 17) | Survivor (n = 5) | Non-survivor (n = 8) | Outcome (p) |
|---|---|---|---|---|---|
| Heart rate (beats/min) | >52 | 62 (54–72) | 48 (46–54)* | 63 (61–86)† | 0.0008 |
| Respiratory rate (breath/min) | >20 | 28 (19–44) | 28 (15–34)* | 41 (27–72)‡ | 0.235 |
| Rectal temperature (°C) | ≤36.0 or ≥39.0 | 38.1 (37.6–39.1) | 39.1 (38.6–39.3)* | 37.8 (37.5–38.2)† | 0.044 |
| Leukocytes (×109/L) | <3.0 or >15.0 | 8.95 (3.45–15.7) | 10.10 (5.96–16.6)* | 6.34 (2.02–14.7)‡ | 0.354 |
n too small for calculation of normality test.
p < 0.05 (Shapiro–Wilk normality test).
p ≥ 0.05 (Shapiro–Wilk normality test).
Measurement of activated platelets and PLAs in the control group
Measurements of activated platelets and PLAs from the samples of the control group were assessed for normality with the Shapiro–Wilk test. Effects of in vitro activation with collagen were determined with the Wilcoxon test because values lacked Gaussian distribution after assessment for normality using the Shapiro–Wilk test (Table 3).
Table 3.
Percentages of activated platelets (CD62P-positive) and platelet-leukocyte aggregates (PLAs; CD11a/18-positive) in platelet-leukocyte-rich plasma (PLRP) and after activation with collagen (50 μg/mL) measured with Accuri C6 (Becton Dickinson) and the BD Accuri C6 analysis software in 10 healthy adult horses (median, IQR; Prism v.6, GraphPad; Wilcoxon test).
| PLRP native | PLRP with collagen | p | |
|---|---|---|---|
| CD62P (%) | 1.45 (1.08–1.99)* | 8.78 (6.79–14.78)* | 0.002 |
| CD11a/18 (%) | 2.95 (1.57–3.21)† | 1.82 (1.57–2.94)* | 0.625 |
p < 0.05 (Shapiro–Wilk normality test).
p ≥ 0.05 (Shapiro–Wilk normality test).
Measurement of activated platelets and PLAs in equine SIRS
Differences in numbers of activated platelets and PLAs between controls and the SIRS group were assessed by a 2-way ANOVA with repeated measures concerning the method (native or collagen-activated) followed by post-hoc Sidak multiple comparison tests with adjusted p-value. Before running the ANOVA, all measurements of activated platelets and PLAs in the SIRS and the control group were assessed for normality with the Shapiro–Wilk test. Given that most data, especially from the SIRS group, were not normally distributed (control group, Table 3; SIRS group PLAs with collagen p ≥ 0.05, all other p < 0.05), all data were logarithmically transformed. Residuals of the transformed data were normally distributed, according to the Shapiro–Wilk test (all p ≥ 0.05).
Measurement of activated platelets and PLAs in equine SIRS dependent on outcome
Differences in numbers of activated platelets and PLAs within the SIRS group between survivors and non-survivors were assessed using the same aforementioned methods comparing the control group and the SIRS group with normal distribution of residuals using the Shapiro–Wilk test (all p ≥ 0.05).
Results
Animals
The control group included 5 horses and 5 ponies (5 geldings, 5 mares, 5–28-y-old [median: 12.5-y-old]), and median bodyweight of 500 kg (range: 420–620 kg). Seventeen patients that were presented between September 2014 and March 2016 met the SIRS criteria and were included in the study: 10 horses and 7 ponies (10 geldings, 7 mares, median: 17-y-old [range: 5–25-y-old]). Five patients survived and were discharged, 4 of the 12 non-survivors were euthanized based on economic reasons and were excluded from the calculations regarding outcome. The most common diagnoses were diseases of the gastrointestinal tract (n = 8; 1 survivor), followed by diseases of the respiratory system (n = 5; 2 survivors), fever of unknown origin (n = 2; 1 survivor), and other diagnoses (1 non-survivor with T-cell lymphoma, 1 survivor with suspected intraabdominal abscess). A final diagnosis was made in all non-surviving horses by postmortem examination. Non-surviving horses had a significantly higher median heart rate; survivors had a significantly higher median body temperature (Table 2).
Measurement of activated platelets and PLAs in the control group
The median platelet count in PLRP was 174 × 109/L (interquartile range [IQR]: 130–212 × 109/L). The percentage of activated platelets was very low in native samples and was significantly increased after in vitro activation of platelets with collagen in all 10 individuals. The median percentage of PLAs was unaffected by activation with collagen (Table 3).
Measurement of activated platelets and PLAs in equine SIRS
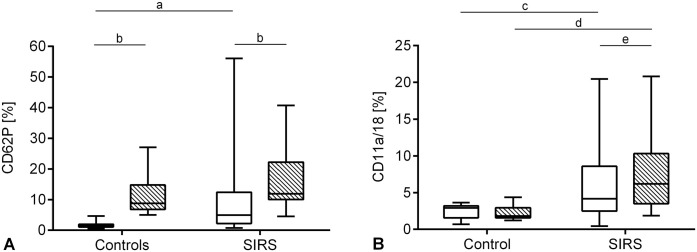
The median platelet count in PLRP was 190 × 109/L (IQR: 98–254 × 109/L). The median percentage of CD62P-positive platelets in native samples in the SIRS group was increased significantly (4.92%, IQR: 2.21–12.41%, p = 0.0007) compared to the controls; the collagen-activated samples did not differ significantly between groups (Fig. 2A). Within the SIRS group, the median percentage of CD62P was increased significantly after in vitro activation with collagen (11.9%, IQR: 10.0–22.2%, p < 0.0001; Fig. 2A).
Figure 2.
Platelet activation and platelet-leukocyte aggregates (PLAs) in controls (n = 10) and systemic inflammatory response syndrome (SIRS) group (n = 17) in native samples (white boxes) and after in vitro activation with collagen (cross-hatched boxes); boxes are median and IQR, whiskers minimum and maximum. A. Percentage of CD62P-positive platelets; a, p = 0.0007; b, p < 0.0001. B. Percentage of PLAs: c, p = 0.048; d, p = 0.0009; e, p = 0.036.
The SIRS group had a significantly higher median percentage of PLAs in native (4.16%, IQR: 2.5–8.6%, p = 0.048) and collagen-activated (6.2%, IQR: 3.5–10.3%, p = 0.0009) samples compared to the controls. In contrast to the controls, a significant increase in PLAs was measured within the SIRS group after in vitro activation with collagen (Fig. 2B).
Measurement of activated platelets and PLAs in equine SIRS dependent on outcome
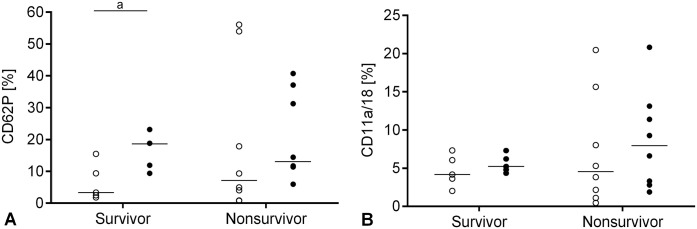
The median percentage of CD62P was increased after in vitro activation with collagen within the survivors (p = 0.03), but not within the non-survivors. There were no significant differences between non-survivors and survivors for CD62P-positive platelets either in native or in collagen-activated samples (Fig. 3A). There were no significant differences in PLAs in non-survivors compared to survivors either in native or in collagen-activated samples and within both subgroups after in vitro activation with collagen (Fig. 3B).
Figure 3.
Platelet activation and platelet-leukocyte aggregates (PLAs) in the systemic inflammatory response syndrome (SIRS) group (n = 13) depending on outcome in native samples (open circles) and after in vitro activation with collagen (black dots); bar = median. A. Individual percentage of CD62P-positive platelets; a, p = 0.03. B. Individual percentage of PLAs.
Discussion
We measured activated platelets and PLAs in PLRP by flow cytometry; activated platelets and PLAs were increased in clinical cases of equine SIRS, as hypothesized. To our knowledge, increased platelet activation in clinical cases of inflammatory diseases in horses has not been demonstrated previously by flow cytometry. Previous equine studies using flow cytometry demonstrated increased platelet activation in experimental settings after induced infection with equine infectious anemia virus 49 and in the course of carbohydrate-induced laminitis 71 ; results were inconsistent in studies of platelet activation in horses with antigen-induced equine asthma.20,29,30
We slightly modified protocols for human whole blood.4,34 Erythrolysis is recommended, especially for the measurement of PLAs 4 to avoid interference with light scattering. 64 As our preliminary observations demonstrated, platelet and leukocyte counts were decreased significantly after erythrolysis and were increased significantly in PLRP; therefore, we chose PLRP for analysis with flow cytometry. In contrast to other equine studies in which PLRP for flow cytometry was used after sedimentation and centrifugation for 2–5 min at 1,200 or 2,700 × g,7,20,35 we obtained PLRP as a supernatant after sedimentation and used the PLRP without centrifugation steps to avoid in vitro activation of platelets, as demonstrated in human samples in a previous study. 28
Contrary to the recommendations in the protocols for human platelet measurement, we did not fix the samples in our study with 1% formalin. In human samples, fixing after labeling with antibodies and activation of platelets influenced the results of flow cytometry negatively and nonlinearly. 34 Formalin-fixation before labeling and activation had unpredictable influences on the binding of antibodies in equine samples. 33 Given that all of our measurements were completed within 75 min after sampling, formalin-fixation appeared unnecessary.
Although platelets can be identified more or less reliably by their light-scattering profile, dual-labeling is recommended when measuring activated platelets and PLAs by flow cytometry. Using antibodies that are already conjugated with a fluorochrome eliminates the use of secondary antibodies, thereby avoiding washing, additional incubation, and centrifugation, reflecting procedures for artifactual in vitro activation of platelets. 34 The selection of the 2 fluorochromes should be focused on their specific emission spectrum avoiding any overlap of the curves. Hence, we used APC with a peak wavelength of 675 nm for platelet labeling and FITC with a peak wavelength of 530 nm for labeling of platelet activation and leukocytes. The antibodies used against CD41/61 and CD62P had been introduced successfully in previous equine studies,7,29,30 and cross-reactivity for both equine glycoproteins is also documented in the datasheets of the manufacturer. The anti-CD11a/18 used is a leukocyte-specific monoclonal mouse anti-equine antibody.
The amount of CD62P in the membrane of resting platelets is low, and accordingly, low percentages of dual-labeled platelets were detected in native samples. The low percentages of CD62P-positive platelets are in accord with the results of other equine studies.10,55,60–62,68 The ability of collagen to activate equine platelets was demonstrated 55 and led to a significant 6-fold increase in the percentage of dual-labeled events reflecting the increased presence of the glycoprotein in the cell membrane following activation of platelets in our study, which is in accord with the results of other equine studies.20,55 This increase was not demonstrated in the analysis of PLAs, which is consistent with the results of a human study in which in vitro activation with thrombin failed to increase the formation of PLAs in samples from healthy human donors, 13 and with the results of an equine study in which thrombin and CXCL8 were used for in vitro activation. 20
The elevated numbers of activated platelets and PLAs in our clinical cases of equine SIRS are consistent with data from human patients with sepsis, septic shock, and MODS.25,50,53 Such an increase in CD62P-positive platelets was also demonstrated in dogs with inflammatory diseases. 45 In addition to their primary role in hemostasis, platelets are multifunctional and act as key players in inflammatory processes. As supporters of the immune system, they contribute in humans and laboratory animals to increased production of IgG,22,67 they are directly bactericidal through the release of antimicrobial peptides (defensins) from α-granules, 63 and they participate in the intraerythrocytic death of malarial parasites. 43 A key function of PLAs (i.e., inducing the formation of neutrophil extracellular traps [NETs], named NETosis), mainly in capillary sinusoids of the liver and the lung in a septic mouse model, was reported in 2007. 13 These NETs are web-like structures of DNA with proteolytic activity that can trap and kill bacteria and viruses within the bloodstream.8,13,32,42 The interaction of PLAs and NETs is called immunothrombosis and is a major first line of immune defense given that it prevents the systemic dissemination of pathogens. There is some information available about NETosis in companion animals, 27 including NETosis in bronchoalveolar lavage, but not in blood in equine asthma.14,66
The percentage of activated platelets increased further after in vitro activation with collagen in the SIRS group. However, the samples from the control group averaged a 10-fold increase whereas only a 2-fold increase for CD62P was observed in the SIRS group. This reduced capability of in vitro activation is probably because of an increase in platelet activation in vivo as a result of the activation of primary and secondary hemostasis in inflammatory processes, as shown in dogs. 45 Similar observations were made in human patients with sepsis and septic shock, in whom platelet activation measured by aggregometry was significantly reduced compared to healthy controls. The authors of the latter studies suggested that samples from diseased people contained mainly platelets in a hyporesponsive state that cannot be further activated in vitro.1,17,38
In contrast to the control group, PLAs were increased significantly after in vitro activation with collagen in the SIRS group. This result is consistent with the results of a human study, in which the formation of PLAs after in vitro activation with thrombin in samples from healthy donors was increased solely after the addition of plasma from septic patients. 13 PLA formation is mainly achieved by crosslinking of activated CD41/61 in the membrane of platelets and macrophage-1 antigen (MAC-1, synonym CD11b/18) in the membrane of leukocytes activated by fibrinogen. 13 Two mechanisms might explain the failure of PLA formation in healthy subjects after in vitro activation with collagen and thrombin and conversely the formation of PLA in septic human patients or after the addition of plasma from septic human patients to plasma from healthy human controls: collagen and thrombin are unable to activate leukocytes and/or PLA formation increases as a consequence of suspected increased fibrinogen concentration in septic patients.
Platelet activation correlated positively with the severity of the disease in human patients with MODS. 26 Given that immunothrombosis is essential as a major first line of immune defense, platelet activation and PLAs contribute to the development of complications in human patients. For instance, adhesion of PLAs to endothelial cells causes cell lysis and, as a consequence, increased vessel permeability and formation of interstitial edema. 63 As a major complication, immunothrombosis results in complete vessel thrombosis, especially in the microcirculation, leading to ischemic injury as demonstrated in acute lung injury, acute kidney injury, and MODS in human patients.42,63 Suspected raised in vivo platelet activation in the non-surviving horses in our study is supported by the observation that the ability of in vitro activation by collagen was less pronounced in the non-survivors. In non-survivors, only a nonsignificant 1.5-fold increase for CD62P was achieved, whereas platelets from survivors had a significant 6-fold increase for CD62P after in vitro activation with collagen. As mentioned previously, human studies suggested that samples from diseased people contained mainly platelets in a hyporesponsive state that cannot be further activated in vitro.1,17,38
Our results suggest that the percentage of activated platelets and PLAs and the capability of in vitro activation could have an impact on outcome, and the lack of statistical significance was most likely a result of the low number of horses with SIRS, which is the main limitation of our study. Studies with higher numbers of horses with SIRS are indicated for reassessing a possible relation between platelet activation, the severity of disease, and the outcome in equine SIRS. The 29.4% survival rate of equids in our study was low. Other equine studies, in which inclusion limits for the SIRS criteria were inconsistent, had mortality rates in equine SIRS of 30–47%.47,48,54,59,65
To our knowledge, increased platelet activation and PLAs measured by flow cytometry in PLRP in clinical cases of equine SIRS has not been demonstrated previously. Platelet activation and PLAs may therefore represent valuable variables in diagnostic algorithms in equine SIRS; further studies are needed to assess the impact of platelet activation in prognostic algorithms. Although the protocol for measurement of platelet activation and PLAs in our study is simplified and more user friendly, the principle of flow cytometry is not available in most clinical settings. To our knowledge, there is no other method described for measurement of PLAs. Alternative methods for measurement of platelet activation in human patients with systemic inflammatory diseases (i.e., sepsis, SIRS, MODS) include whole blood impedance aggregometry (multiplate analyzer),1,17 measurement of soluble forms of platelet membrane glycoproteins (i.e., sP-selectin and sCD40L) with ELISAs,11,18,19,40,51 and also using ELISA measurements of platelet-specific molecules released from platelet granules during activation (i.e., platelet factor 4, microparticles, microparticle-associated tissue factor, β-thromboglobulin).41,52,58,69 Whole blood impedance aggregometry (multiplate analyzer) is a point-of-care test and was evaluated in the same cohort of equids as our study, but there were no significant differences in aggregation values between SIRS cases and controls. 21 Similarly, the soluble form of glycoproteins CD40L and CD62P could not be quantified in equine plasma samples using commercial human ELISAs. 46 Information about the use of ELISAs for measurement of platelet-specific molecules released from platelet granules in equine samples is limited. 39 Nevertheless, measurements of soluble forms of platelet membrane glycoproteins and molecules released from platelet granules during activation may represent valuable variables in diagnostic and prognostic algorithms in equine systemic inflammatory diseases and thus warrant further research.
Acknowledgments
We thank Dr. Kathrin Büttner, Unit for Biomathematics and Data Processing, Faculty of Veterinary Medicine, Justus-Liebig-University, for her support with the statistical analyses.
Footnotes
Declaration of conflicting interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Katja A. Roscher  https://orcid.org/0000-0002-3810-8004
https://orcid.org/0000-0002-3810-8004
Contributor Information
Carmen Obach-Schröck, Equine Clinic, Internal Medicine, Department of Veterinary Clinical Science (Theuerkauf, Roscher), Institute of Veterinary-Anatomy, -Histology and -Embryology (Obach-Schröck, Staszyk), Clinical Pathophysiology and Veterinary Clinical Pathology, Department of Veterinary Clinical Science (Moritz), Justus-Liebig-University, Giessen, Germany.
Carsten Staszyk, Equine Clinic, Internal Medicine, Department of Veterinary Clinical Science (Theuerkauf, Roscher), Institute of Veterinary-Anatomy, -Histology and -Embryology (Obach-Schröck, Staszyk), Clinical Pathophysiology and Veterinary Clinical Pathology, Department of Veterinary Clinical Science (Moritz), Justus-Liebig-University, Giessen, Germany.
Andreas Moritz, Equine Clinic, Internal Medicine, Department of Veterinary Clinical Science (Theuerkauf, Roscher), Institute of Veterinary-Anatomy, -Histology and -Embryology (Obach-Schröck, Staszyk), Clinical Pathophysiology and Veterinary Clinical Pathology, Department of Veterinary Clinical Science (Moritz), Justus-Liebig-University, Giessen, Germany.
Katja A. Roscher, Equine Clinic, Internal Medicine, Department of Veterinary Clinical Science (Theuerkauf, Roscher), Institute of Veterinary-Anatomy, -Histology and -Embryology (Obach-Schröck, Staszyk), Clinical Pathophysiology and Veterinary Clinical Pathology, Department of Veterinary Clinical Science (Moritz), Justus-Liebig-University, Giessen, Germany
References
- 1. Adamzik M, et al. Whole blood impedance aggregometry as a biomarker for the diagnosis and prognosis of severe sepsis. Crit Care 2012;16:R204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Armengou L, et al. Plasma D-dimer concentration in sick newborn foals. J Vet Intern Med 2008;22:411–417. [DOI] [PubMed] [Google Scholar]
- 3. Arroyo MG, et al. Factors associated with survival in 97 horses with septic pleuropneumonia. J Vet Intern Med 2017;31:894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Barnard MR, et al. Whole blood analysis of leukocyte-platelet aggregates. Curr Protoc Cytom 2003:Chapter 6:Unit 6.15. [DOI] [PubMed] [Google Scholar]
- 5. Bone RC, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992;101:1644–1655. [DOI] [PubMed] [Google Scholar]
- 6. Bonelli F, et al. Plasma procalcitonin concentration in healthy horses and horses affected by systemic inflammatory response syndrome. J Vet Intern Med 2015;29:1689–1691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Brainard BM, et al. Treatment with aspirin or clopidogrel does not affect equine platelet expression of P selectin or platelet–neutrophil aggregates. Vet Immunol Immunopathol 2012;149:119–125. [DOI] [PubMed] [Google Scholar]
- 8. Brinkmann V, et al. Neutrophil extracellular traps kill bacteria. Science 2004;303:1532–1535. [DOI] [PubMed] [Google Scholar]
- 9. Brooks AC, et al. Endotoxin-induced activation of equine platelets: evidence for direct activation of p38 MAPK pathways and vasoactive mediator production. Inflamm Res 2007;56:154–161. [DOI] [PubMed] [Google Scholar]
- 10. Brooks MB, et al. Effects of clopidogrel on the platelet activation response in horses. Am J Vet Res 2013;74:1212–1222. [DOI] [PubMed] [Google Scholar]
- 11. Cangemi R, et al. Low-grade endotoxemia, gut permeability and platelet activation in community-acquired pneumonia. J Infect 2016;73:107–114. [DOI] [PubMed] [Google Scholar]
- 12. Carroll CL, Huntington PJ. Body condition scoring and weight estimation of horses. Equine Vet J 1988;20:41–45. [DOI] [PubMed] [Google Scholar]
- 13. Clark SR, et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat Med 2007;13:463–469. [DOI] [PubMed] [Google Scholar]
- 14. Côté O, et al. Secretoglobin 1A1 and 1A1A differentially regulate neutrophil reactive oxygen species production, phagocytosis and extracellular trap formation. PLoS One 2014;9:e96217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cotovio M, et al. Detection of fibrin deposits in tissues from horses with severe gastrointestinal disorders. J Vet Intern Med 2007;21:308–313. [DOI] [PubMed] [Google Scholar]
- 16. Daniel AJ, et al. Concentrations of serum amyloid A and plasma fibrinogen in horses undergoing emergency abdominal surgery. J Vet Emerg Crit Care (San Antonio) 2016;26:344–351. [DOI] [PubMed] [Google Scholar]
- 17. Davies GR, et al. The role of whole blood impedance aggregometry and its utilisation in the diagnosis and prognosis of patients with systemic inflammatory response syndrome and sepsis in acute critical illness. PLoS One 2014;9:e108589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Di Nisio M, et al. Plasma cytokine and P-selectin levels in advanced malignancy: prognostic value and impact of low-molecular weight heparin administration. Cancer 2005;104:2275–2281. [DOI] [PubMed] [Google Scholar]
- 19. Ding H, et al. Endothelial cell injury with inflammatory cytokine and coagulation in patients with sepsis. World J Emerg Med 2013;4:285–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dunkel B, et al. Neutrophil and platelet activation in equine recurrent airway obstruction is associated with increased neutrophil CD13 expression, but not platelet CD41/61 and CD62P or neutrophil-platelet aggregate formation. Vet Immunol Immunopathol 2009;131:25–32. [DOI] [PubMed] [Google Scholar]
- 21. Ehrmann C, et al. Assessment of platelet biology in equine patients with systemic inflammatory response syndrome. J Vet Diagn Invest 2021;33:300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Elzey BD, et al. Cooperation between platelet-derived CD154 and CD4+ T cells for enhanced germinal center formation. J Leukoc Biol 2005;78:80–84. [DOI] [PubMed] [Google Scholar]
- 23. Epstein KL, et al. Thrombelastography in horses with acute gastrointestinal disease. J Vet Intern Med 2011;25:307–314. [DOI] [PubMed] [Google Scholar]
- 24. Feige K, et al. Changes in coagulation and markers of fibrinolysis in horses undergoing colic surgery. J Vet Med A Physiol Pathol Clin Med 2003;50:30–36. [DOI] [PubMed] [Google Scholar]
- 25. Gawaz M, et al. Platelet activation and interaction with leucocytes in patients with sepsis or multiple organ failure. Eur J Clin Invest 1995;25:843–851. [DOI] [PubMed] [Google Scholar]
- 26. Gawaz M, et al. Severity of multiple organ failure (MOF) but not of sepsis correlates with irreversible platelet degranulation. Infection 1995;23:16–23. [DOI] [PubMed] [Google Scholar]
- 27. Goggs R, et al. Neutrophil-extracellular traps, cell-free DNA, and immunothrombosis in companion animals: a review. Vet Pathol 2020;57:6–23. [DOI] [PubMed] [Google Scholar]
- 28. Henn V, et al. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998;391:591–594. [DOI] [PubMed] [Google Scholar]
- 29. Iwaszko A, et al. Effect of antigen challenge on dynamics of CD62P and CD41/61 expression on platelets in horses with recurrent airway obstruction (RAO). Vet Immunol Immunopathol 2018;202:172–180. [DOI] [PubMed] [Google Scholar]
- 30. Iwaszko-Simonik A, et al. Expression of surface platelet receptors (CD62P and CD41/61) in horses with recurrent airway obstruction (RAO). Vet Immunol Immunopathol 2015;164:87–92. [DOI] [PubMed] [Google Scholar]
- 31. Jakobsen C, et al. Platelet function in preeclampsia—a systematic review and meta-analysis. Platelets 2019;30:549–562. [DOI] [PubMed] [Google Scholar]
- 32. Jenne CN, et al. Neutrophils recruited to sites of infection protect from virus challenge by releasing neutrophil extracellular traps. Cell Host Microbe 2013;13:169–180. [DOI] [PubMed] [Google Scholar]
- 33. Kingston JK, et al. Effects of formaldehyde fixation on equine platelets using flow cytometric methods to evaluate markers of platelet activation. Am J Vet Res 2002;63:840–844. [DOI] [PubMed] [Google Scholar]
- 34. Krueger LA, et al. Immunophenotypic analysis of platelets. Curr Protoc Cytom 2002;Chapter 6:Unit 6.10. [DOI] [PubMed] [Google Scholar]
- 35. Lalko CC, et al. Equine platelet CD62P (P-selectin) expression: a phenotypic and morphologic study. Vet Immunol Immunopathol 2003;91:119–134. [DOI] [PubMed] [Google Scholar]
- 36. Lambert JL, et al. Association of presence of band cells and toxic neutrophils with systemic inflammatory response syndrome and outcome in horses with acute disease. J Vet Intern Med 2016;30:1284–1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lapchak PH, et al. Platelet-associated CD40/CD154 mediates remote tissue damage after mesenteric ischemia/reperfusion injury. PLoS One 2012;7:e32260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Levi M. Platelets at a crossroad of pathogenic pathways in sepsis. J Thromb Haemost 2004;2:2094–2095. [DOI] [PubMed] [Google Scholar]
- 39. López C, et al. Bacteriostatic effect of equine pure platelet-rich plasma and other blood products against methicillin-sensitive Staphylococcus aureus. An in vitro study. Vet Comp Orthop Traumatol 2014;27:372–378. [DOI] [PubMed] [Google Scholar]
- 40. Lorente L, et al. Association between serum soluble CD40 ligand levels and mortality in patients with severe sepsis. Crit Care 2011;15:R97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mavrommatis AC, et al. Coagulation system and platelets are fully activated in uncomplicated sepsis. Crit Care Med 2000;28:451–457. [DOI] [PubMed] [Google Scholar]
- 42. McDonald B, et al. Intravascular neutrophil extracellular traps capture bacteria from the bloodstream during sepsis. Cell Host Microbe 2012;12:324–333. [DOI] [PubMed] [Google Scholar]
- 43. McMorran BJ, et al. Platelets kill intraerythrocytic malarial parasites and mediate survival to infection. Science 2009;323:797–800. [DOI] [PubMed] [Google Scholar]
- 44. Michelson AD, et al. Evaluation of platelet function by flow cytometry. Methods 2000;21:259–270. [DOI] [PubMed] [Google Scholar]
- 45. Moritz A, et al. Evaluation of flow cytometric and automated methods for detection of activated platelets in dogs with inflammatory disease. Am J Vet Res 2005;66:325–329. [DOI] [PubMed] [Google Scholar]
- 46. Roscher K. Thrombozyten der Equiden - ein unterschätzter Biomarker? [Platelets in equids - an underrated biomarker?]. 1st ed. DVG Service, 2019. German. http://geb.uni-giessen.de/geb/volltexte/2019/14904/ [Google Scholar]
- 47. Roy M-F, et al. Prognostic value and development of a scoring system in horses with systemic inflammatory response syndrome. J Vet Intern Med 2017;31:582–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Ruggerone B, et al. Paraoxonase-1 activity evaluation as a diagnostic and prognostic marker in horses and foals. J Vet Intern Med 2020;34:949–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Russell KE, et al. Platelets from thrombocytopenic ponies acutely infected with equine infectious anemia virus are activated in vivo and hypofunctional. Virology 1999;259:7–19. [DOI] [PubMed] [Google Scholar]
- 50. Russwurm S, et al. Platelet and leukocyte activation correlate with the severity of septic organ dysfunction. Shock 2002;17:263–268. [DOI] [PubMed] [Google Scholar]
- 51. Sakamaki F, et al. Soluble form of P-selectin in plasma is elevated in acute lung injury. Am J Respir Crit Care Med 1995;151:1821–1826. [DOI] [PubMed] [Google Scholar]
- 52. Sartori MT, et al. Platelet-derived microparticles bearing PF4 and anti-GAGS immunoglobulins in patients with sepsis. Diagnostics (Basel) 2020;10:627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Schrottmaier WC, et al. Platelet activation at the onset of human endotoxemia is undetectable in vivo. Platelets 2016;27:479–483. [DOI] [PubMed] [Google Scholar]
- 54. Schwarz BC, et al. Diagnostic value of the neutrophil myeloperoxidase index in horses with systemic inflammation. Vet J 2012;191:72–78. [DOI] [PubMed] [Google Scholar]
- 55. Segura D, et al. Assessment of platelet function in horses: ultrastructure, flow cytometry, and perfusion techniques. J Vet Intern Med 2006;20:581–588. [DOI] [PubMed] [Google Scholar]
- 56. Shattil SJ, et al. Changes in the platelet membrane glycoprotein IIb.IIIa complex during platelet activation. J Biol Chem 1985;260:11107–11114. [PubMed] [Google Scholar]
- 57. Shattil SJ, et al. Detection of activated platelets in whole blood using activation-dependent monoclonal antibodies and flow cytometry. Blood 1987;70:307–315. [PubMed] [Google Scholar]
- 58. Soriano AO, et al. Levels of endothelial and platelet microparticles and their interactions with leukocytes negatively correlate with organ dysfunction and predict mortality in severe sepsis. Crit Care Med 2005;33:2540–2546. [DOI] [PubMed] [Google Scholar]
- 59. Stewart AJ, et al. Cortisol and adrenocorticotropic hormone concentrations in horses with systemic inflammatory response syndrome. J Vet Intern Med 2019;33:2257–2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stokol T, et al. Equid herpesvirus type 1 activates platelets. PLoS One 2015;10:e0122640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Stokol T, et al. Unfractionated and low-molecular-weight heparin and the phosphodiesterase inhibitors, IBMX and cilostazol, block ex vivo equid herpesvirus type-1-induced platelet activation. Front Vet Sci 2016;3:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Stokol T, et al. Subcutaneous administration of low-molecular-weight heparin to horses inhibits ex vivo equine herpesvirus type 1-induced platelet activation. Front Vet Sci 2018;5:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. de Stoppelaar SF, et al. The role of platelets in sepsis. Thromb Haemost 2014;112:666–677. [DOI] [PubMed] [Google Scholar]
- 64. Tarnow I, et al. Effects of physiologic agonists on canine whole blood flow cytometry assays of leukocyte–platelet aggregation and platelet activation. Vet Immunol Immunopathol 2008;123:345–352. [DOI] [PubMed] [Google Scholar]
- 65. Urayama S, et al. Blood glucose is unlikely to be a prognostic biomarker in acute colitis with systemic inflammatory response syndrome in Thoroughbred racehorses. J Equine Sci 2018;29:15–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Vargas A, et al. Neutrophil extracellular traps are downregulated by glucocorticosteroids in lungs in an equine model of asthma. Respir Res 2017;18:207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Verschoor A, et al. A platelet-mediated system for shuttling blood-borne bacteria to CD8α+ dendritic cells depends on glycoprotein GPIb and complement C3. Nat Immunol 2011;12:1194–1201. [DOI] [PubMed] [Google Scholar]
- 68. Watts AE, et al. Effects of clopidogrel on horses with experimentally induced endotoxemia. Am J Vet Res 2014;75:760–769. [DOI] [PubMed] [Google Scholar]
- 69. Wegrzyn G, et al. Biomarkers of platelet activation and their prognostic value in patients with sepsis-associated disseminated intravascular coagulopathy. Clin Appl Thromb Hemost 2021;27:1076029620943300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Weiss DJ, Evanson OA. Detection of activated platelets and platelet-leukocyte aggregates in horses. Am J Vet Res 1997;58:823–827. [PubMed] [Google Scholar]
- 71. Weiss DJ, et al. Evaluation of platelet activation and platelet-neutrophil aggregates in ponies with alimentary laminitis. Am J Vet Res 1997;58:1376–1380. [PubMed] [Google Scholar]
- 72. Weiss DJ, et al. Evaluation of platelet activation and platelet-neutrophil aggregates in Thoroughbreds undergoing near-maximal treadmill exercise. Am J Vet Res 1998;59:393–396. [PubMed] [Google Scholar]