Abstract
Background:
A proportion of people with treatment-resistant schizophrenia fail to show improvement on clozapine treatment. Knowledge of the sociodemographic and clinical factors predicting clozapine response may be useful in developing personalised approaches to treatment.
Methods:
This retrospective cohort study used data from the electronic health records of the South London and Maudsley (SLaM) hospital between 2007 and 2011. Using the Least Absolute Shrinkage and Selection Operator (LASSO) regression statistical learning approach, we examined 35 sociodemographic and clinical factors’ predictive ability of response to clozapine at 3 months of treatment. Response was assessed by the level of change in the severity of the symptoms using the Clinical Global Impression (CGI) scale.
Results:
We identified 242 service-users with a treatment-resistant psychotic disorder who had their first trial of clozapine and continued the treatment for at least 3 months. The LASSO regression identified three predictors of response to clozapine: higher severity of illness at baseline, female gender and having a comorbid mood disorder. These factors are estimated to explain 18% of the variance in clozapine response. The model’s optimism-corrected calibration slope was 1.37, suggesting that the model will underfit when applied to new data.
Conclusions:
These findings suggest that women, people with a comorbid mood disorder and those who are most ill at baseline respond better to clozapine. However, the accuracy of the internally validated and recalibrated model was low. Therefore, future research should indicate whether a prediction model developed by including routinely collected data, in combination with biological information, presents adequate predictive ability to be applied in clinical settings.
Keywords: Refractory psychosis, health records, machine learning, zaponex, clorazil
Introduction
It is estimated that up to a third of mental healthcare service-users with schizophrenia do not show an adequate response to conventional antipsychotics, or drugs for psychosis (Lally et al., 2016; Siskind et al., 2021). Clozapine is the only drug for psychosis recommended by existing guidelines for the management of treatment-resistant schizophrenia (TRS) (Lally and Gaughran, 2019; NICE, 2015; Siskind et al., 2016), with evidence showing it is associated with greater symptomatic improvement (Siskind et al., 2016) and a reduction in hospitalisation when compared to other antipsychotics (Kesserwani et al., 2019; Land et al., 2017). An episode of relapse and hospitalisation is estimated to cost over £25,000 (Munro et al., 2011), and considering the quality-adjusted life-years gained, clozapine is also the most cost-effective medication for TRS (Jin et al., 2020). However, clozapine initiation is on average delayed by 4 years (Howes et al., 2012), and there is increasing evidence that such delays are associated with a poorer response (Shah et al., 2020; Yoshimura et al., 2017). Despite its better efficacy, a recent meta-analysis estimated that, after 3 months of treatment with clozapine, between 63% and 71% of people will not show an adequate response (Siskind et al., 2017). An adequate response to clozapine is commonly defined as a reduction in symptoms of over 20% (Siskind et al., 2017), with an absolute severity below mild (Howes et al., 2017; Lieberman et al., 1994). Failure to show an adequate response to clozapine has been termed ultra-treatment resistance or clozapine-resistance (Howes et al., 2017; Lieberman et al., 1994; Shah et al., 2020).
Establishing predictors of response to clozapine could have important clinical implications for identifying clozapine-resistance earlier and initiating the augmentation of another antipsychotic or considering cessation if the expected benefits do not outweigh the side effects (Lally and Gaughran, 2019). Moreover, identifying the sociodemographic and clinical profile of people at high risk of inadequate response to clozapine may help the selection of a subgroup of patients who could be the target for pharma-led novel compound development (Vickers et al., 2006).
In a recent meta-analysis, younger age at clozapine initiation, paranoid subtype of schizophrenia and fewer negative symptoms at baseline were associated with better response (Okhuijsen-Pfeifer et al., 2020). Furthermore, another recent systematic review identified that longer duration of illness, fewer hospitalisations and fewer antipsychotic trials before clozapine initiation were associated with a better response (Griffiths et al., 2021). However, a key problem with the meta-analysis of risk factors is that individual studies adjust for different covariates making it difficult to address confounding (Griffiths et al., 2021; Okhuijsen-Pfeifer et al., 2020). Moreover, the consideration of clinical factors has been limited to examining the features of psychosis (e.g. psychosis subtype or length of illness), and as a result, comorbidities have received less attention.
Capitalising on the richness of information present in the clinical health records of the South London and Maudsley (SLaM) NHS Foundation Trust, this study aims to identify the sociodemographic and clinical predictors of response to clozapine at 3 months of treatment. To analyse an extensive range of sociodemographic and clinical factors, we used statistical learning approaches, which perform better than traditional regression analysis when the objective is to maximise predictive power, as opposed to investigating aetiological relationships (Hastie et al., 2009; Tibshirani, 1996).
Methods
Setting
This retrospective cohort study used data from SLaM electronic health records (EHRs). SLaM is one of the largest secondary mental healthcare providers in Europe (Stewart et al., 2009). The catchment area of this NHS Trust includes four London boroughs: Southwark, Lewisham, Lambeth and Croydon, with a population of over 1.3 million. Access to data was possible via the Clinical Record Interactive Search (CRIS) (Perera et al., 2016; Stewart et al., 2009). CRIS was established in 2007–2008, following the full implementation of EHRs in SLaM in 2006. At the time of data extraction, CRIS provided access to the de-identified information (in both EHRs structured and free-text fields) of over 230,000 individuals (Legge et al., 2016). CRIS has been approved for secondary data analysis by the Oxford C Research Ethics Committee (18/SC/0372) (Perera et al., 2016; Stewart et al., 2009).
The retrieval of information in the free-text fields is facilitated by a range of Natural Language Processing (NLP) algorithms (CRIS NLP Service, 2021; Jackson et al., 2017; Perera et al., 2016; Stewart et al., 2009). These NLP algorithms distinguish positive versus negative references to a concept of interest located within the medical record, thus outperforming a simple keyword search (Jackson et al., 2017; Perera et al., 2016). Researchers can also retrieve anonymised excerpts of free text from medical records. These can be used to validate and/or confirm results from NLP and to derive scores for validated instruments, such as the Clinical Global Impression (CGI) scale.
Sample inclusion criteria
SLaM service-users who met the following inclusion criteria (1) had a primary diagnosis of a schizophrenia spectrum disorder (International Classification of Diseases, 10th Revision (ICD-10): F20–F29), (2) were aged between 18 and 65 years, (3) had their first trial of clozapine between 1 January 2007 and 31 December 2011 and (4) were still taking clozapine after 3 months of treatment. This cohort has been used in previous studies focusing on reasons for clozapine discontinuation (Legge et al., 2016) and antipsychotic polypharmacy before clozapine initiation (Thompson et al., 2016). For further information on sample derivation, please see Legge and colleagues’ study (Legge et al., 2016).
Outcome measures
Response to treatment was manually rated using the CGI scale, which is a validated clinical tool to assess the patients’ overall severity of disease (CGI; Busner and Targum, 2007). Three months of treatment is the recommended length to assess the efficacy of a clozapine trial (Howes et al., 2017; Lieberman et al., 1994). Service-users’ clinical condition was retrospectively rated using information from a variety of EHRs (such as ward round notes, outpatient intervention teams notes and correspondence), which was entered around the time of clozapine initiation and 3 months later. The assessment was completed by four researchers, with an observed inter-rater reliability of .71 for the severity subscale (Thompson et al., 2016). The CGI–severity scale (CGI-S) ratings considered (1) the presence of psychotic symptoms, (2) the presence of negative symptoms, (3) the frequency of their occurrence, (4) the intensity or severity of symptoms and (5) the effect of symptoms on functioning in major areas of the service-users’ life (work, study, home and relationships). Severity ratings range from 1 (normal, not all ill) to 7 (among the most extremely ill patients). We calculated change in severity by subtracting the CGI-S ratings at baseline from those at 3 months; thus, higher values represented less improvement. It is estimated that a 1-point reduction in the CGI-S is equivalent to a 10-point reduction in Brief Psychiatric Rating Scale (BPRS) and 15-point reduction in the Positive and Negative Syndrome Scale (PANSS) scores (Leucht et al., 2006); this is clinically considered a minimal improvement
We primarily assessed response by a change in the score of severity of symptoms, as it is most commonly done (Griffiths et al., 2021; Lieberman et al., 1994); however, the response was also rated using the CGI Improvement subscale. The findings regarding the CGI Improvement scale are presented in the supplementary material (Shah et al., 2020).
Exposure variables
Sociodemographic and clinical potential predictors of response to clozapine were measured as close as possible to the time of clozapine initiation, within the 6 months before. Sociodemographic information included gender, age at clozapine initiation, ethnicity and deprivation. Ethnicity was grouped into White (British, Irish and other White Backgrounds), Black (African, Caribbean, White and Black African, White and Black Caribbean, and any Other Black background) and Other (Bangladeshi, Chinese, Indian, Pakistani, White and Asian, any Other Asian background, any Other Mixed Background, any Other ethnic group or ethnicity not stated). The neighbourhood deprivation score of the area where the person was living at the time of clozapine initiation was based on the English Indices of Deprivation 2010 (Department for Communities and Local Government, 2011).
Clinical exposures included the ICD-10 psychiatric diagnoses present in the health records within the 6 months before clozapine initiation, the clinical condition assessment using the Health of the Nation Outcome Scale (HoNOS) and the length of illness. Psychiatric diagnosis was identified from information in the EHR structured fields and information in free-text fields using NLP applications designed for that purpose (CRIS NLP Service, 2021). Where more than one diagnosis was recorded, a diagnostic hierarchy was used: in patients with diagnoses of both schizoaffective disorder (F25) and schizophrenia (F20), schizoaffective disorder was taken as the diagnosis. The other codes for psychotic disorders (F21–F24, F28–F29) were only used in patients with no instances of F20 or F25. Psychiatric comorbidities mentioned in records within 6 months before clozapine initiation were also included in the analyses. These comprised personality disorders (F60–F61); any substance use disorders (F10–F14, F16, F18–F19); developmental disorders (F70–F79, F80–F84, F88, F90); anxiety-related disorders (F40–F43); and mood disorders (F30–F39, F42.1). The HoNOS (Pirkis et al., 2005; Wing et al., 1998) was considered as evidence of service-users’ psychiatric symptoms, problematic behaviour, level of impairment and problems in social functioning. The HoNOS is completed regularly as part of routine clinical care in SLaM. The 12 items of the scale are rated between 0 (no problem), 1 (minor problem requiring no action), 2 (mild problem but definitely present), 3 (moderately severe problem) and 4 (severe to very severe problem). Due to low numbers in some of the categories, we collapsed the score into 0 (no problem), 1 (minor problem requiring no action) and 2–4 (mild to very severe problem) (Hayes et al., 2012). The most relevant HoNOS score was selected using a hierarchy in reference to the date of clozapine initiation: the closest date to initiation within the 3 months before; if none available, up to 1 week after; if also unavailable, the latest date between 3 months and 1 year before clozapine initiation; if also unavailable, HoNOS was coded as missing. The length of psychotic illness at the time of clozapine initiation was calculated based on the information in case notes. Depending on the information available, it could refer to first psychotic symptoms, prescription of a drug for psychosis, diagnosis of psychosis or the first contact with services.
Several measures of service use in the 6 months before clozapine initiation were included in the models, namely: the number of days as an inpatient, the number of days where there was at least one face-to-face contact with outpatient intervention teams (maximum one contact per day) and the number of outpatient intervention team events (not restricted to one per day and including events where there was non-contact with the patient). To adjust for differences in the period receiving care in SLaM, these measures were divided by the number of days that the person was under active treatment with SLaM (active days) during the 6 months before clozapine initiation. Furthermore, for the regression models, due to skewed values, these ratios were log-transformed. Other measures of service use, in the 6 months before clozapine initiation, included having received care from an early intervention service for psychosis, having received care from a psychiatric intensive care unit, the number of compulsory medical hospitalisations under the Mental Health Act 1983 (MHA) (HM Government, 1983), having been detained under a forensic section of the MHA and having been conveyed to a place of safety by police from a public place or private premises, all measured in the 6 months before clozapine initiation.
To assess possible non-adherence to treatment in the 6 months before the clozapine trial, we analysed evidence of treatment with a Long-Acting Injection (LAI; depot) drug for psychosis or having been submitted to supervised pharmacological treatment in the community, a Community Treatment Order (CTO) under the MHA (Barnes et al., 2020; Kadra et al., 2016; MH Government, 2007).
Statistical analysis
We examined predictors of clozapine non-response using a linear Least Absolute Shrinkage and Selection Operator (LASSO) regression (Tibshirani, 1996). We chose regularised regression over traditional statistical methods to minimise the variance of prediction and overfitting and to perform automatic variable selection (Hastie et al., 2009). We followed the standard guidelines for model building available at the time of study initiation, including the steps proposed by Steyerberg and Vergouwe (2014). We reported the results according to the TRIPOD statement (Collins et al., 2015). The missing data were imputed through K-nearest neighbours (Jonsson and Claes, 2004), using the Gower distance (Gower, 1971), and LASSO regression was fitted with 20-time repeated 10-fold cross-validation tuning on a grid of 100 tuning parameters, minimising the mean squared error (MSE). The model’s discriminative performance was evaluated with a pseudo-R2 defined as , with being the outcome (Breiman, 2001). Calibration, a measure of the agreement between observed values and predictions, was assessed with the calibration slope and the calibration-in-the-large. The calibration slope regards the slope of the line with the best fit obtained by regressing the observed outcome on the predicted outcome; the calibration-in-the-large is the difference between the mean of the observed outcome and the mean of the predicted outcome (Steyerberg, 2019). All measures of performance were internally validated using 100-time repeated 10-fold cross-validation optimism-correction after the method of Harrell (2015). When the calibration slope departed from the ideal slope of 1, the estimated coefficients were recalibrated by multiplying them by the corrected calibration slope; the intercept of the model was recalibrated by multiplying it by the corrected calibration slope and by adding the corrected calibration-in-the-large to the result (Steyerberg, 2019). Calibration plots of observed outcomes against predictions (uncalibrated line) and against recalibrated predictions (recalibrated line) are presented. Analyses were undertaken in R software using the following packages: glmnet (Friedman et al., 2010), caret (Kuhn, 2008), pROC (Robin et al., 2019), StatMatch (D’Orazio, 2015) and c060 (Sill et al., 2014).
Results
Participants
There were 316 service-users with a schizophrenia spectrum disorder who had their first trial of clozapine between 2007 and 2011 (Legge et al., 2016). Of these, 242 (76.6%) were treated for longer than 3 months and were considered eligible for this study. Men comprised 67% of the cohort, and people with minority ethnic background 59%. At the time of clozapine initiation, the median age of the sample was 35.9 years, and the median length of psychotic illness was 8 years. Eighty-seven per cent were diagnosed with schizophrenia (F20), and 24.4% had a comorbid mood disorder (F30–F39). At the time of clozapine initiation, 51% were hospitalised (and 30% of the whole sample was hospitalised involuntarily under the MHA). Sixty-eight per cent had received a depot medication in the 6 months before clozapine initiation. Missing data on at least one variable were present in 58 (28%) cases. Table 1 shows the sample characteristics, clinical factors, service use and missing data.
Table 1.
Descriptive statistics for the exposures included in the models.
| Exposures | N = 242 | Missing data |
|---|---|---|
| Continuous | Median (25th–75th p) | Count (%) |
| Categorical | Count (%) | |
| Sociodemographic information | ||
| Age (years) | 35.9 (28.2–44.0) | 0 |
| Gender | ||
| Men (R) | 162 (66.9) | |
| Women | 80 (33.1) | 0 |
| Ethnicity | ||
| Black (R) | 118 (48.8) | 0 |
| White | 100 (41.3) | |
| Other | 24 (9.9) | |
| Neighbourhood deprivation score | 31.2 (23.9–37.0) | 13 (5.4) |
| Main diagnosis | ||
| Schizophrenia spectrum diagnosis | ||
| Schizophrenia (R) (ICD-10: F20) | 211 (87.2) | 0 |
| Schizoaffective (ICD-10: F25) | 26 (10.7) | |
| Other prolonged psychosis (ICD-10: F21–F24.9, F28–F29.9) | 5 (2.1) | |
| Length of illness (years) | 8.0 (3.5–14.0) | 11 (4.6) |
| Comorbidities (diagnosis in the 6 months before) | ||
| Personality disorder (ICD-10: F60–F61) | 27 (11.2) | 0 |
| Any substance use disorder (ICD-10: F10–F14, F16, F18–F19) | 36 (14.9) | 0 |
| Developmental disorder (ICD-10: F70–F79, F80–F84, F88, F90) | 9 (3.7) | 0 |
| Anxiety-related disorder (ICD-10: F40–F43) | 8 (3.3) | 0 |
| Mood disorder (ICD-10: F30–F39, F42.1) | 59 (24.4) | 0 |
| The Health of the Nation Outcome Scales (closest to clozapine initiation but between 1 year before and 1 week after) | ||
| 1. Overactive, agitated behaviour | ||
| No problem (R) | 93 (38.4) | 29 (12.0) |
| Minor problem, no action | 55 (22.7) | |
| Significant problem | 65 (26.9) | |
| 2. Non-accidental self-injury | ||
| No problem (R) | 168 (69.4) | 29 (12.0) |
| Minor problem | 24 (9.9) | |
| Significant problem | 21 (8.7) | |
| 3. Drinking or drug-taking | ||
| No problem (R) | 146 (60.3) | 32 (13.2) |
| Minor problem | 18 (7.4) | |
| Significant problem | 46 (19.0) | |
| 4. Cognitive problems | ||
| No problem (R) | 100 (41.3) | 32 (13.2) |
| Problem | 55 (22.7) | |
| Significant problem | 55 (22.7) | |
| 5. Physical illness or disability | ||
| No problem (R) | 151 (62.4) | 29 (12.0) |
| Minor problem | 37 (15.3) | |
| Significant problem | 25 (10.3) | |
| 6. Hallucinations and delusions | ||
| No problem (R) | 19 (7.9) | 33 (13.6) |
| Minor problem | 24 (9.9) | |
| Significant problem | 166 (68.6) | |
| 7. Depressed mood | ||
| No problem (R) | 94 (38.8) | 30 (12.4) |
| Minor problem | 65 (26.9) | |
| Significant problem | 53 (21.9) | |
| 8. Other mental and behavioural problems | ||
| No problem (R) | 56 (23.1) | 31 (12.8) |
| Minor problem | 41 (16.9) | |
| Significant problem | 114 (47.1) | |
| 9. Relationship problems | ||
| No problem (R) | 69 (23.1) | 31 (12.8) |
| Minor problem | 64 (16.9) | |
| Significant problem | 78 (47.1) | |
| 10. Activities of daily living | ||
| No problem (R) | 83 (34.3) | 29 (12.0) |
| Minor problem | 55 (22.7) | |
| Significant problem | 75 (31.0) | |
| 11. Living conditions | ||
| No problem (R) | 111 (45.9) | 39 (16.1) |
| Minor problem | 44 (18.2) | |
| Significant problem | 48 (19.8) | |
| 12. Occupation and activities | ||
| No problem (R) | 69 (28.5) | 39 (16.1) |
| Minor problem | 56 (23.1) | |
| Significant problem | 78 (32.2) | |
| 8.a Other mental and behavioural problems. Type: | ||
| Phobic, anxiety, obsessive-compulsive | 50 (20.7) | 29 (12.0) |
| Mental strain/tension | 39 (16.1) | 29 (12.0) |
| Dissociative, somatoform | 13 (5.4) | 29 (12.0) |
| Eating, sleep, sexual | 43 (17.8) | 29 (12.0) |
| Service use (in 6 months before clozapine initiation) | ||
| Days with face-to-face clinical contacts with outpatient intervention teams/active days | 0.07 (0.03–0.11) | 0 |
| Days in hospitalisation/active days | 0.29 (0.04–0.73) | 0 |
| Number of outpatient intervention teams events/active days | 0.15 (0.08–0.27) | 0 |
| Received care from a psychiatric intensive care unit | 26 (10.7) | 0 |
| Received care from an early intervention service for psychosis | 33 (13.6) | 0 |
| Conveyed to a place of safety by police (MHA, police sections) | 17 (7.0) | 0 |
| Detained under the forensic section of the (MHA Part 3 sections) | 24 (9.9) | 0 |
| Count of compulsory medical hospitalisations (MHA Part 2 sections) | 0 (0–56.8) | 0 |
| Non-adherence (in the 6 months before clozapine initiation) | ||
| Supervised community treatment (Community treatment order) | 3 (1.2) | 0 |
| Long-Acting Injection (depot) drug for psychosis | 165 (68.2) | 0 |
| Clinical Global Impression Scale | ||
| CGI severity at baseline | 5.0 (5.0–6.0) | 0 |
p: percentile; R: reference category; MHA: Mental Health Act 1983; HoNOS: Health of the Nation Outcome Scales; CGI: Clinical Global Impression; ICD-10: International Classification of Diseases, 10th Revision.
Predictors of response to clozapine
After 3 months of clozapine initiation, 55% of the cases showed a minimal response (i.e., 1-point reduction in severity) and 22% showed no change in the severity of symptoms (Table 2). Moreover, only 18% were observed to be within the threshold of mild severity (Howes et al., 2017; Lieberman et al., 1994). According to the LASSO regression, three factors were identified as predictors of better response to clozapine: higher severity at baseline, female gender and having a comorbid mood disorder (Table 3). We checked the robustness of high severity predicting better response by examining potential ceiling, floor and regression to the mean effects. No such effects were observed.
Table 2.
Clinical Global Impression–Severity scale scores at baseline and at 3 months of treatment with clozapine.
| CGI–Severity | At baseline | At 3 months | Change in severity of symptoms | |
|---|---|---|---|---|
| Scores | n (%) | n (%) | Scores | n (%) |
| 1 (normal) | 0 | 0 | −4 (reduction) | 1 (0) |
| 2 (borderline ill) | 0 | 3 (1) | −3 | 7 (3) |
| 3 (mildly ill) | 0 | 42 (17) | −2 | 38 (20) |
| 4 (moderately ill) | 37 (15) | 129 (53) | −1 | 133 (55) |
| 5 (markedly ill) | 129 (53) | 60 (25) | 0 (no change) | 53 (22) |
| 6 (severely ill) | 75 (31) | 8 (3) | ||
| 7 (extremely ill) | 1 (0) | 0 | ||
| Mdn (IQR) | 5 (5, 6) | 4 (4, 5) | −1 (−1, −1) | |
CGI: Clinical Global Impression; IQR: interquartile range.
Table 3.
LASSO regression selected predictors for severity change at 3 months.
| Severity change (higher scores indicate poorer response: −4 to −1 = reduction in severity; 0 = no change) | Mean change | Recalibrated coefficients |
|---|---|---|
| (Intercept) | 0.695 | 0.9200 |
| Baseline CGI–Severity score | −0.358 | −0.4907 |
| Female gender | −0.167 | −0.2287 |
| Comorbid mood disorder | −0.030 | −0.0412 |
| Model performance | Apparent | Corrected |
| Pseudo R2 | 0.21 | 0.18 |
| Calibration slope | 1.36 | 1.37 |
| Calibration-in-the-large | 0.00 | −0.01 |
CGI: Clinical Global Impression; LASSO: Least Absolute Shrinkage and Selection Operator.
The internally validated pseudo-R2 was 0.18, which indicates these three factors should explain 18% of the variance in response to clozapine in unseen samples of the same clinical population. The optimism-corrected calibration slope was 1.37, indicating underfitting of the model if the model is applied to unseen data (Table 3). The calibration plot shows that the recalibrated model presents a calibration line closer to the ideal calibration line (45° line) than the calibration line of the non-calibrated model (Figure 1).
Figure 1.
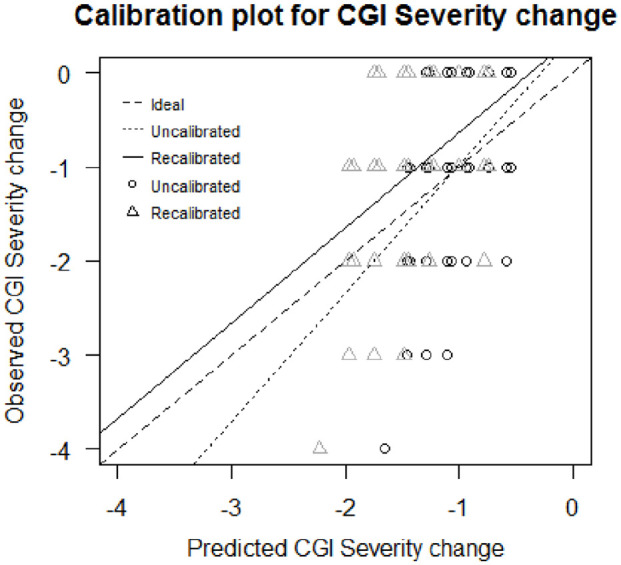
Calibration plot of the model predicting change in the severity of symptoms. For the same y-coordinate, the circles’ x-coordinate is the predicted outcome through the uncalibrated model and the triangles’ x-coordinate is the predicted outcome through the recalibrated model.
Discussion
This study aimed to identify sociodemographic and clinical predictors of response to clozapine at 3 months of treatment. Of the 242 SLaM service-users who had a 3-month trial of clozapine, 22% showed no change in severity, while 55% showed a minimal response, and only 18% were considered to be mildly ill or better (Howes et al., 2017; Leucht et al., 2006; Lieberman et al., 1994). These findings are in line with previous observations that up to 71% of people treated with clozapine will not show an adequate response in the short term (Lieberman et al., 1994; Siskind et al., 2017).
Regarding the sociodemographic predictors of clozapine response, women appear to show a better response than men. A similar result was seen in Usall et al. (2007) study, where the outcome was also measured using the CGI, and also in Shah et al. (2020), where late non-response to clozapine was more frequent in men. However, the direction of the effect of gender differences in clozapine response is not consistent across studies (Griffiths et al., 2021; Okhuijsen-Pfeifer et al., 2020; Yoshimura et al., 2017). CGI captures a broader range of domains (e.g. daily activities) than the traditional symptom-based measures (e.g. PANSS). It may be that women are more likely to show response in these other domains, hence their superior response on the CGI.
In previous studies, both younger age and shorter duration of illness at the time of the clozapine trial have been associated with better response (Griffiths et al., 2021; Okhuijsen-Pfeifer et al., 2020). Age did not emerge as a predictor of response in our study. However, it is notable that our cohort was older than in many studies (median age 35.9), and the median time to clozapine treatment was rather long, at 8 years. There is evidence to suggest that a shorter length of illness and younger age at onset are associated with best responses only when clozapine is introduced within the first 3 years of illness (Yoshimura et al., 2017). It may be that our study’s relatively older cohort contained a large proportion of people who had missed this window, diluting the effect of length of illness and age.
Concerning other clinical factors, more severe symptoms at baseline predicted a better response to clozapine. In a recent meta-analysis of observational studies, no significant associations between global baseline scores (from studies using CGI, BPRS and PANSS, analysed separately) and response were observed; only fewer negative symptoms (using PANSS) were associated with better response (Okhuijsen-Pfeifer et al., 2020). Also, in a meta-analysis of randomised controlled trials, comparing the efficacy of clozapine versus other antipsychotics, it was observed that higher baseline mean psychosis score predicted greater response for clozapine in the long term (⩾3 months), but not in the short term (<3 months) (Siskind et al., 2016). According to the nature of the scoring of the scale, and attending to the fact that complete remission of symptoms in schizophrenia is rare, it is possible that the finding of high severity at baseline predicting better response is due to the most severe cases at baseline showing a larger reduction in symptoms after 3 months.
Having a comorbid mood disorder predicted a greater reduction in severity following treatment with clozapine. Research on the association between response to clozapine and psychiatric comorbidities is scarce. However, in one previous study, no differences in psychiatric comorbidities between long-term clozapine responders and non-responders were reported (Shah et al., 2020). Depressive disorders seem to be more prevalent in TRS samples (Jönsson et al., 2019; Wimberley et al., 2016), but, to our knowledge, this is the first time a comorbid mood disorder is associated with a better response to clozapine. This finding is in line with the evidence that clozapine has some efficacy in mood disorders, including bipolar disorder (Li et al., 2015).
The novelty of some of this study’s observed associations, or the lack thereof, warrants caution in the conclusions to be drawn. The use of statistical learning statistical methods that are focused on prediction instead of the traditional statistical methods, which are fit for analyses of existing (past) associations, may be related to the divergence in findings between this study and the previous literature. Moreover, most past research has adopted a different way to measure response, namely, for the dichotomisation of response levels (Okhuijsen-Pfeifer et al., 2020). The impact of these methodological differences is unknown, so it is imperative that future research using similar methods is conducted in order to establish a more solid knowledge.
Limitations and strengths
This study had several limitations that need to be borne in mind when interpreting the findings. First, given that this study used secondary data, we were restricted to the information that is routinely collected in SLaM healthcare provision. Second, previous studies showed that clozapine dose, the number of previous antipsychotic trials and delays in clozapine initiation are key predictors of clozapine response, and these factors were not analysed in the present study (Nielsen et al., 2012; Shah et al., 2020; Yoshimura et al., 2017). Third, the statistical models were not tested in other samples for external validity, given the lack of data. Finally, and most importantly, our analysis may be underpowered. In contrast to what is recommended by a large part of the literature (Hastie et al., 2009; Tibshirani, 1996; Wang et al., 2020), regularised regression methods using the penalty optimising the error (the best penalty) do not always resolve problems associated with limited sample size relative to the number of variables (Van Calster et al., 2020), especially if the irrepresentable condition (where relevant variables should not strongly correlate with irrelevant variables) does not hold (Zhao and Yu, 2006). Therefore, developing LASSO prediction models using the best penalty with small samples and a relatively large number of predictors could potentially lead to overfitting of the models and poor model performance. Riley et al. (2019) suggest a sample size calculation for linear prediction models to avoid overfitting. According to their research, a model like the one we have developed, including 42 parameters, expecting to have an adjusted Cox–Snell R2 of 0.2 with our outcome mean and standard deviation, would need a minimum sample size of 1602 to avoid overfitting, which is much larger than our study sample size. It is also true that Riley et al. (2019) do not provide a sample size calculation adapted to regularised regression models (which do not retain all the variables in the model), and there is no explicit guidance about the sufficient sample size to avoid overfitting for LASSO regression. Nonetheless, in the present study, we have corrected the models’ overfitting/underfitting with internal validation and recalibration, which leads to the best predictive performance possible using LASSO with these data. We consider that the alternative of using different statistical learning methods could lower the interpretability of the results.
A key strength of this study is the representativeness of the population studied and the likely low selection bias. In the United Kingdom, almost all people with severe mental illness receive free medical care through the NHS, and SLaM is the only NHS provider of secondary care for mental health in its catchment area. Another strength of this study is investigating a vast range of sociodemographic, clinical factors and service-use events available in clinical records. Similarly, the depth and size of the information on the electronic records have enabled us to combine extensive information to inform the CGI ratings.
Conclusions
Our findings suggest that women, people with a comorbid mood disorder and those who are most ill, according to the CGI-S, respond better to treatment with clozapine. Sociodemographic and clinical factors may have insufficient predictive power alone for the development of clinical predictive algorithms (as these factors are associated with only 18% of variance in response); however, future research could determine whether this could potentially be useful in combination with information regarding genetic factors and other biomarkers.
Supplemental Material
Supplemental material, sj-docx-1-jop-10.1177_02698811221078746 for Using a statistical learning approach to identify sociodemographic and clinical predictors of response to clozapine by Daniela Fonseca de Freitas, Giouliana Kadra-Scalzo, Deborah Agbedjro, Emma Francis, Isobel Ridler, Megan Pritchard, Hitesh Shetty, Aviv Segev, Cecilia Casetta, Sophie E Smart, Johnny Downs, Søren Rahn Christensen, Nikolaj Bak, Bruce J Kinon, Daniel Stahl, James H MacCabe and Richard D Hayes in Journal of Psychopharmacology
Footnotes
Authors’ Note: Nikolaj Bak is now affiliated to Cyclerion Therapeutics, Massachusetts, USA.
Declaration of conflicting interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: DFdF, GKS, EF and IR have received research funding from Janssen and H. Lundbeck A/S. IR received research funding from H. Lundbeck A/S. RDH and HS have received research funding from Roche, Pfizer, Janssen and Lundbeck. SES is employed on a grant held by Cardiff University from Takeda Pharmaceutical Company Ltd. for work unrelated to the analysis reported here. SRC, NB and BK were employees of H. Lundbeck A/S at the time of the study. JHM has received research funding from H. Lundbeck A/S.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: The study was funded from a researcher-initiated grant to JHM from H. Lundbeck A/S, as part of STRATA, a large multi-centre research collaborative programme funded by the Medical Research Council (MRC; Grant number MR/L011794). This work utilised the Clinical Record Interactive Search (CRIS) platform funded and developed by the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. DFdF, GKS, DA, EF, IR, MP, HS, DS, JHM and RDH received salary support from the National Institute for Health Research (NIHR) Biomedical Research Centre at South London and Maudsley NHS Foundation Trust and King’s College London. JD is supported by an NIHR Clinician Science Fellowship award (CS-2018-18-ST2-014) and has received support from an MRC Clinical Research Training Fellowship (MR/L017105/1) and a Psychiatry Research Trust Peggy Pollak Research Fellowship in Developmental Psychiatry. The views expressed are those of the authors and not those of the funding agencies.
ORCID iDs: Daniela Fonseca de Freitas  https://orcid.org/0000-0002-8876-4595
https://orcid.org/0000-0002-8876-4595
Giouliana Kadra-Scalzo  https://orcid.org/0000-0003-3182-905X
https://orcid.org/0000-0003-3182-905X
Isobel Ridler  https://orcid.org/0000-0003-2196-4733
https://orcid.org/0000-0003-2196-4733
Sophie E Smart  https://orcid.org/0000-0002-6709-5425
https://orcid.org/0000-0002-6709-5425
Richard D Hayes  https://orcid.org/0000-0003-4453-244X
https://orcid.org/0000-0003-4453-244X
Supplemental material: Supplemental material for this article is available online.
References
- Barnes TRE, Drake R, Paton C, et al. (2020) Evidence-based guidelines for the pharmacological treatment of schizophrenia: Updated recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 34(1): 3–78. [DOI] [PubMed] [Google Scholar]
- Breiman L. (2001) Random forests. Machine Learning 45(1): 5–32. [Google Scholar]
- Busner J, Targum SD. (2007) The clinical global impressions scale: Applying a research tool in clinical practice. Psychiatry 4(7): 28–37. [PMC free article] [PubMed] [Google Scholar]
- Collins GS, Reitsma JB, Altman DG, et al. (2015) Transparent reporting of a multivariable prediction model for individual prognosis or diagnosis (TRIPOD): The TRIPOD statement. BMJ 350: g7594. [DOI] [PubMed] [Google Scholar]
- CRIS NLP Service (2021) Library of production-ready applications, v1.6. Available at: https://www.maudsleybrc.nihr.ac.uk/facilities/clinical-record-interactive-search-cris/cris-natural-language-processing/
- Department for Communities and Local Government (2011) The English Indices of Deprivation 2010. Available at: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/6871/1871208.pdf
- D’Orazio M. (2015) Integration and imputation of survey data in R: The StatMatch package. Romanian Statistical Review 63(2): 57–68. Available at: http://www.revistadestatistica.ro/wp-content/uploads/2015/04/RRS2_2015_A06.pdf [Google Scholar]
- Friedman J, Hastie T, Tibshirani R. (2010) Regularization paths for generalized linear models via coordinate descent. Journal of Statistical Software 33(1): 1–22. Available at: http://www.jstatsoft.org/v33/i01/ [PMC free article] [PubMed] [Google Scholar]
- Gower JC. (1971) A general coefficient of similarity and some of its properties. Biometrics 27(4): 857–871. [Google Scholar]
- Griffiths K, Millgate E, Egerton A, et al. (2021) Demographic and clinical variables associated with response to clozapine in schizophrenia: A systematic review and meta-analysis. Psychological Medicine 51: 376–386. [DOI] [PubMed] [Google Scholar]
- Harrell FE. (2015) Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, and Survival Analysis (2nd edn, Springer Series in Statistics). Cham: Springer. [Google Scholar]
- Hastie T, Tibshirani R, Friedman J. (2009) The Elements of Statistical Learning: Data Mining, Inference, and Prediction (2nd edn, Springer Series in Statistics). New York: Springer. [Google Scholar]
- Hayes RD, Chang C-K, Fernandes AC, et al. (2012) Functional status and all-cause mortality in serious mental illness. PLoS ONE 7(9): e44613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HM Government (1983) Mental Health Act 1983. Available at: http://www.legislation.gov.uk/ukpga/1983/20/contents
- Howes OD, McCutcheon R, Agid O, et al. (2017) Treatment-resistant schizophrenia: Treatment response and resistance in psychosis (TRRIP) working group consensus guidelines on diagnosis and terminology. American Journal of Psychiatry 174(3): 216–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howes OD, Vergunst F, Gee S, et al. (2012) Adherence to treatment guidelines in clinical practice: Study of antipsychotic treatment prior to clozapine initiation. British Journal of Psychiatry 201(6): 481–485. [DOI] [PubMed] [Google Scholar]
- Jackson RG, Patel R, Jayatilleke N, et al. (2017) Natural language processing to extract symptoms of severe mental illness from clinical text: The Clinical Record Interactive Search Comprehensive Data Extraction (CRIS-CODE) project. BMJ Open 7(1): e012012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H, Tappenden P, MacCabe JH, et al. (2020) Evaluation of the cost-effectiveness of services for schizophrenia in the UK across the entire care pathway in a single whole-disease model. JAMA Network Open 3(5): e205888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jönsson L, Simonsen J, Brain C, et al. (2019) Identifying and characterizing treatment-resistant schizophrenia in observational database studies. International Journal of Methods in Psychiatric Research 28: e1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonsson P, Claes W. (2004) An evaluation of k-nearest neighbour imputation using Likert data. In: 10th international symposium on software metrics, Chicago, IL, 11–17 September, pp. 108–188. New York: IEEE. [Google Scholar]
- Kadra G, Stewart R, Shetty H, et al. (2016) Predictors of long-term (⩾ 6 months) antipsychotic polypharmacy prescribing in secondary mental healthcare. Schizophrenia Research 174(1–3): 106–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesserwani J, Kadra G, Downs J, et al. (2019) Risk of readmission in patients with schizophrenia and schizoaffective disorder newly prescribed clozapine. Journal of Psychopharmacology 33(4): 449–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn M. (2008) Building predictive models in R using the caret package. Journal of Statistical Software 28(5): 1–26. Available at: http://www.jstatsoft.org/v28/i05/paper27774042 [Google Scholar]
- Lally J, Ajnakina O, Di Forti M, et al. (2016) Two distinct patterns of treatment resistance: Clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychological Medicine 46(15): 3231–3240. [DOI] [PubMed] [Google Scholar]
- Lally J, Gaughran F. (2019) Treatment resistant schizophrenia – Review and a call to action. Irish Journal of Psychological Medicine 36(4): 279–291. [DOI] [PubMed] [Google Scholar]
- Land R, Siskind D, McArdle P, et al. (2017) The impact of clozapine on hospital use: A systematic review and meta-analysis. Acta Psychiatrica Scandinavica 135(4): 296–309. [DOI] [PubMed] [Google Scholar]
- Legge SE, Hamshere M, Hayes RD, et al. (2016) Reasons for discontinuing clozapine: A cohort study of patients commencing treatment. Schizophrenia Research 174(1–3): 113–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leucht S, Kane JM, Etschel E, et al. (2006) Linking the PANSS, BPRS, and CGI: Clinical implications. Neuropsychopharmacology 31(10): 2318–2325. [DOI] [PubMed] [Google Scholar]
- Li X-B, Tang Y-L, Wang C-Y, et al. (2015) Clozapine for treatment-resistant bipolar disorder: A systematic review. Bipolar Disorders 17(3): 235–247. [DOI] [PubMed] [Google Scholar]
- Lieberman JA, Safferman AZ, Pollack S, et al. (1994) Clinical effects of clozapine in chronic schizophrenia: Response to treatment and predictors of outcome. American Journal of Psychiatry 151(12): 1744–1752. [DOI] [PubMed] [Google Scholar]
- MH Government (2007) Mental Health Act 2007. Available at: https://www.legislation.gov.uk/ukpga/2007/12/contents
- Munro J, Osborne S, Dearden L, et al. (2011) Hospital treatment and management in relapse of schizophrenia in the UK: Associated costs. The Psychiatrist 35(3): 95–100. [Google Scholar]
- NICE (2015) Psychosis and schizophrenia in adults (QS8). Available at: https://www.nice.org.uk/guidance/qs80
- Nielsen J, Nielsen RE, Correll CU. (2012) Predictors of clozapine response in patients with treatment-refractory schizophrenia: Results from a Danish register study. Journal of Clinical Psychopharmacology 32(5): 678–683. [DOI] [PubMed] [Google Scholar]
- Okhuijsen-Pfeifer C, Sterk AY, Horn IM, et al. (2020) Demographic and clinical features as predictors of clozapine response in patients with schizophrenia spectrum disorders: A systematic review and meta-analysis. Neuroscience and Biobehavioral Reviews 111: 246–252. [DOI] [PubMed] [Google Scholar]
- Perera G, Broadbent M, Callard F, et al. (2016) Cohort profile of the South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLaM BRC) Case Register: Current status and recent enhancement of an Electronic Mental Health Record-derived data resource. BMJ Open 6(3): 1–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirkis JE, Burgess PM, Kirk PK, et al. (2005) A review of the psychometric properties of the Health of the Nation Outcome Scales (HoNOS) family of measures. Health and Quality of Life Outcomes 3: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley RD, Snell KIE, Ensor J, et al. (2019) Minimum sample size for developing a multivariable prediction model: PART II – Binary and time-to-event outcomes. Statistics in Medicine 38(7): 1276–1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin X, Turck N, Hainard A, et al. (2019) pROC: Display and Analyze ROC Curves (R Package Version 1). Available at: https://cran.r-project.org/web/packages/pROC/pROC.pdf
- Shah P, Iwata Y, Brown EE, et al. (2020) Clozapine response trajectories and predictors of non-response in treatment-resistant schizophrenia: A chart review study. European Archives of Psychiatry and Clinical Neuroscience 270(1): 11–22. [DOI] [PubMed] [Google Scholar]
- Sill M, Hielscher T, Becker N, et al. (2014) c060: Extended Inference with Lasso and Elastic-Net Regularized Cox and Generalized Linear Models. Journal of Statistical Software 62(5): 1–22. [Google Scholar]
- Siskind D, McCartney L, Goldschlager R, et al. (2016) Clozapine v . first- and second-generation antipsychotics in treatment-refractory schizophrenia: Systematic review and meta-analysis. British Journal of Psychiatry 209(5): 385–392. [DOI] [PubMed] [Google Scholar]
- Siskind D, Orr S, Sinha S, et al. (2021) Rates of treatment-resistant schizophrenia from first-episode cohorts: Systematic review and meta-analysis. The British Journal of Psychiatry. Epub ahead of print 11 May. DOI: 10.1192/bjp.2021.61. [DOI] [PubMed] [Google Scholar]
- Siskind D, Siskind V, Kisely S. (2017) Clozapine response rates among people with treatment-resistant schizophrenia: Data from a systematic review and meta-analysis. The Canadian Journal of Psychiatry 62(11): 772–777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart R, Soremekun M, Perera G, et al. (2009) The South London and Maudsley NHS Foundation Trust Biomedical Research Centre (SLAM BRC) case register: Development and descriptive data. BMC Psychiatry 9(1): 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steyerberg EW. (2019) Clinical Prediction Models: A Practical Approach to Development, Validation, and Updating (2nd edn, Statistics for Biology and Health). Cham: Springer. [Google Scholar]
- Steyerberg EW, Vergouwe Y. (2014) Towards better clinical prediction models: Seven steps for development and an ABCD for validation. European Heart Journal 35(29): 1925–1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JV, Clark JM, Legge SE, et al. (2016) Antipsychotic polypharmacy and augmentation strategies prior to clozapine initiation: A historical cohort study of 310 adults with treatment-resistant schizophrenic disorders. Journal of Psychopharmacology 30(5): 436–443. [DOI] [PubMed] [Google Scholar]
- Tibshirani R. (1996) Regression Shrinkage and Selection via the Lasso. Journal of the Royal Statistical Society: Series B (Methodological) 58(1): 267–288. [Google Scholar]
- Usall J., Suarez D., Haro J. M. (2007). Gender differences in response to antipsychotic treatment in outpatients with schizophrenia. Psychiatry Research 153(3): 225–231. [DOI] [PubMed] [Google Scholar]
- Van Calster B, van Smeden M, De Cock B, et al. (2020) Regression shrinkage methods for clinical prediction models do not guarantee improved performance: Simulation study. Statistical Methods in Medical Research 29(11): 3166–3178. [DOI] [PubMed] [Google Scholar]
- Vickers AJ, Kramer BS, Baker SG. (2006) Selecting patients for randomized trials: A systematic approach based on risk group. Trials 7(1): 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Mukherjee S, Richardson S, et al. (2020) High-dimensional regression in practice: An empirical study of finite-sample prediction, variable selection and ranking. Statistics and Computing 30(3): 697–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimberley T, Støvring H, Sørensen HJ, et al. (2016) Predictors of treatment resistance in patients with schizophrenia: A population-based cohort study. The Lancet Psychiatry 3(4): 358–366. [DOI] [PubMed] [Google Scholar]
- Wing JK, Beevor AS, Curtis RH, et al. (1998) Health of the nation outcome scales (HoNOS): Research and development. British Journal of Psychiatry 172: 11–18. [DOI] [PubMed] [Google Scholar]
- Yoshimura B, Yada Y, So R, et al. (2017) The critical treatment window of clozapine in treatment-resistant schizophrenia: Secondary analysis of an observational study. Psychiatry Research 250: 65–70. [DOI] [PubMed] [Google Scholar]
- Zhao P, Yu B. (2006) On model selection consistency of Lasso. Journal of Machine Learning Research 7: 2541–2563. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-jop-10.1177_02698811221078746 for Using a statistical learning approach to identify sociodemographic and clinical predictors of response to clozapine by Daniela Fonseca de Freitas, Giouliana Kadra-Scalzo, Deborah Agbedjro, Emma Francis, Isobel Ridler, Megan Pritchard, Hitesh Shetty, Aviv Segev, Cecilia Casetta, Sophie E Smart, Johnny Downs, Søren Rahn Christensen, Nikolaj Bak, Bruce J Kinon, Daniel Stahl, James H MacCabe and Richard D Hayes in Journal of Psychopharmacology


