Summary
Umbilical cord blood (UCB) transplantation is a potentially curative treatment for patients with refractory severe aplastic anaemia (SAA), but has historically been associated with delayed engraftment and high graft failure and mortality rates. We conducted a prospective phase 2 trial to assess outcome of an allogeneic transplant regimen that co-infused a single UCB unit with CD34+-selected cells from a haploidentical relative. Among 29 SAA patients [including 10 evolved to myelodysplastic syndrome (MDS)] who underwent the haplo cord transplantation (median age 20 years), 97% had neutrophil recovery (median 10 days), and 93% had platelet recovery (median 32 days). Early myeloid engraftment was from the haplo donor and was gradually replaced by durable engraftment from UCB in most patients. The cumulative incidences of grade II–IV acute and chronic graft-versus-host disease (GVHD) were 21% and 41%, respectively. With a median follow-up of 7·5 years, overall survival was 83% and GVHD/relapse-free survival was 69%. Patient- and transplant-related factors had no impact on engraftment and survival although transplants with haplo-versus-cord killer-cell immunoglobulin-like receptor (KIR) ligand incompatibility had delayed cord engraftment. Our study shows haplo cord transplantation is associated with excellent engraftment and long-term outcome, providing an alternative option for patients with refractory SAA and hypoplastic MDS who lack human leucocyte antigen (HLA)-matched donors.
Keywords: allogeneic stem-cell transplantation, haploidentical transplant, natural killer cell killer-cell immunoglobulin-like receptor ligand incompatibility, severe aplastic anaemia, umbilical cord blood
Introduction
Severe aplastic anaemia (SAA) is a life-threatening haematological disorder characterized by bone marrow (BM) failure that can clonally evolve to myelodysplastic syndrome (MDS). Long-term survival in SAA can be achieved with immunosuppressive therapy (IST) using anti-thymocyte globulin (ATG) and cyclosporin (CSA).1,2 Recent data suggest adding eltrombopag to conventional ATG/CSA improves response rate.3,4 However, approximately 30% will not respond to IST and a number of responders will relapse and/or have evolution to MDS and acute myeloid leukemia.4,5
SAA patients who are refractory to or relapse after IST and lack a human leucocyte antigen (HLA)-matched donor often proceed to the haematopoietic stem-cell transplantation (SCT) using cord blood or haploidentical donors.6–8 Haplo-transplants utilizing post-transplant cyclophosphamide have been associated with low mortality rates for patients with haematologic malignancies.9,10 Although recently published studies on haploidentical SCT for SAA show improved engraftment and survival compared to earlier studies,6,7 this approach has been associated with higher rejection rates and more than 30% one-year mortality.11–13 Transplantation using umbilical cord blood (UCBT) is a viable transplant alternative for haematological disorders, with cord blood units being rapidly available for patients needing an urgent transplant and with UCBT being associated with low rates of graft-versus-host-disease (GVHD).14 A disadvantage of UCBT is the limited number of total nucleated cells (TNC) contained within the cord blood unit, which has historically been associated with delayed engraftment and higher rates of graft failure.14–17 Although cord blood units with higher TNCs are associated with improved transplant outcome,18 such cord units are available to only a minority of adult patients undergoing transplantation.
To overcome disadvantages of UCBT, a transplant strategy was developed that co-transplants UCB and CD34+-enriched cells from a haploidentical donor for patients with haematological malignancies.19–25 In order to harness the advantages of adequately HLA-matched cord blood units being readily available for most patients needing a transplant and to expedite neutrophil recovery, we conducted a prospective study in which a single cord blood unit was co-transplanted with a relatively low number of highly purified peripheral blood CD34+ cells from a haploidentical relative in patients with SAA refractory to IST or early-stage MDS with severe neutropenia.26,27 Here, we report the outcomes and long-term follow-up results of this study.
Methods
Study design and participants
We conducted a phase 2, single-centre clinical trial (ClinicalTrials.gov: NCT00604201), between 2008 and 2020 that was approved by the National Heart, Lung, and Blood Institute’s Institutional Review Board. All patients provided written informed consent. Eligible patients included those age 4–55 years with diagnosis of SAA, SAA evolved to MDS, or hypoplastic MDS presenting with refractory anaemia (RA) or ringed sideroblasts (RARS).28 SAA patients were required to have a BM cellularity of < 30%, be transfusion-dependent for platelets and/or red blood cells, and have severe neutropenia [absolute neutrophil count (ANC) <500/μl]. MDS patients were required to have a history of SAA that clonally evolved to MDS or to have severe neutropenia and a history of one or more opportunistic infections related to neutropenia. All patients had also failed to respond to standard IST.
All patients lacked an HLA-compatible donor and were required to have: (i) a haploidentical related donor (HLA-A, -B, -C, -DR and -DQ loci); (ii) at least a 4/6 HLA-matched UCB unit available; and (iii) no evidence of historic or current donor-specific HLA antibodies (DSA) to one or more mismatched alleles expressed on the cord blood unit or in the haplo donor. More details are described in the Supporting Information.
Treatment
The conditioning regimen consisted of intravenous (IV) cyclophosphamide (60 mg/kg/day, days −7 and −6), followed by IV fludarabine (25 mg/m2/day, days −5, −4, −3, −2 and −1), equine-ATG (40 mg/kg/day, days −5, −4, −3 and −2) and 200 cGy of total body irradiation (TBI) (day −1). GVHD prophylaxis comprised tacrolimus on day −4 to 180 and mycophenolate mofetil (MMF) on day 0 to 100.
On day 0, patients received a CD34+-selected (Miltenyi CliniMACS Prodigy®, Miltenyi Biotec, Bergisch Gladbach, Germany) granulocyte stimulating growth factor (G-CSF)-mobilized haplo donor allograft combined with a single UCB unit. The haploidentical cells were infused first, immediately followed by infusion of the cord blood unit. All patients received subcutaneous G-CSF at a daily dose of 5 μg/kg from day + 1 until neutrophils ≥ 500/μl.
End-points and statistical analysis
The primary end-point was neutrophil recovery by day 42. Secondary outcomes are described in the Supporting Information. We used a Simon’s minimax two-stage design29 to test the null hypothesis that the proportion of patients achieving the primary end-point was 60% or lower. A sample size of 26 patients engrafted by day 42 out of up to 35 patients were needed for a type I error of 0·05 and 80% power if the engraftment probability was 80% or higher. The enrolment was closed after 28 out of 29 patients engrafted by day 42 because the study met the primary objective and rejected the null hypothesis for the primary end-point (P < 0·0001). All surviving patients have completed at least four years follow-up as of 1 October 2020.
Overall survival and GVHD/relapse-free survival (GFRS), were calculated by the Kaplan–Meier method.30 Cumulative incidences of engraftment, full donor chimaerism, and GVHD were estimated by considering deaths as competing risk.31 The log-rank test and the Gray’s test were used to compare survival probabilities and cumulative incidences between subgroups, respectively.32 Haematopoietic recovery and survival were correlated with transplant-related characteristics. Statistical analysis was performed using R version 4·0.2 (R Foundation for Statistical Computing, Vienna, Austria; https://www.r-project.org/).
Results
Patient characteristics
Between July 2008 and September 2016, 29 patients with SAA or SAA evolved to MDS underwent conditioning and transplantation with haploidentical CD34+ cells selected from peripheral blood combined with a single UCB unit (haplo cord transplantation). Patient characteristics prior to transplant are summarized in Table I. The median age was 20 years (range 4–48) and 15 (52%) were women. All patients had failed prior IST, and 14 (48·3%) had also failed eltrombopag therapy, with a median of three (range 1–5) prior treatment courses. With the exception of two patients treated with alemtuzumab and eltrombopag, all had received prior treatment with ATG/CSA. Nine (31%) patients had a paroxysmal nocturnal haemoglobinuria (PNH) clone with a median of 14·8% glycosylphosphatidylinositol (GPI)-negative neutrophils. The median pre-transplant ANC was 270/μl (IQR: 90–350). Patients were heavily transfused with markedly elevated ferritins (median 3495 μg/l, range 980–21465). Eleven (38%) patients were allo-immunized with HLA class I and/or class II antibodies. None of these HLA antibodies were donor-directed. The median time from diagnosis of SAA or MDS to transplantation was 39 months (range 6–139).
Table I.
Baseline patient characteristics (n = 29).
| Characteristic | SAA (n = 19) | MDS* (n = 10) | All patients (n = 29) |
|---|---|---|---|
| Age, years, median (range) | 19 (4–48) | 23 (9–40) | 20 (4–48) |
| Children (age < 18 years), n (%) | 8 (42·1) | 3 (30·0) | 11 (37·9) |
| Weight at transplant, kg, median (IQR) | 57 (44–68) | 66 (53–77) | 58 (47–74) |
| Sex, n (%) | |||
| Male | 7 (36·8) | 7 (70·0) | 14 (48·3) |
| Female | 12 (63·2) | 3 (30·0) | 15 (51·7) |
| Race, ethnicity | |||
| White | 1 (5·3) | 3 (30·0) | 4 (13·8) |
| Black/African American | 6 (31·6) | 5 (50·0) | 11 (37·9) |
| Hispanic | 7 (36·8) | 1 (10·0) | 8 (27·6) |
| Asian | 5 (26·3) | 1 (10·0) | 6 (20·7) |
| No. of prior treatment courses, median (range) | 3 (1–5) | 3 (1–4) | 3 (1–5) |
| ANC/μl, median (IQR) | 270 (65–330) | 245 (128–388) | 270 (90–350) |
| Transfusion dependence, n (%) | 19 (100) | 9 (90·0) | 28 (96·6) |
| Marrow cellularity (%), median (IQR) | 5 (5–15) | 7. 5 (5–10) | 7. 5 (5–15) |
| PNH clone detected pre-transplant | 8 (42·1) | 1 (10·0) | 9 (31·0) |
| % GPI-negative neutrophils, median (IQR) | 15·1 (11·2–47·4) | 3·9 | 14·8 (9·8–31·8) |
| Ferritin, μg/l, median (IQR) | 4501 (3107–5675) | 1946 (1055–3260) | 3495 (2261–5457) |
| Time from diagnosis to transplantation, months, median (IQR) | 39 (29–47) | 30 (14–68) | 39 (20–61) |
| HLA allo-immunization pre-transplant†, n (%) | 9 (47·4) | 2 (20·0) | 11 (37·9) |
ANC, absolute neutrophil count; GPI, glycosylphosphatidylinositol: HLA, human leucocyte antigen: IQR, interquartile range; IST, immunosuppressive therapy; MDS, myelodysplastic syndrome; PNH, paroxysmal nocturnal haemoglobinuria; SAA, severe aplastic anaemia.
Cytogenetic changes for 10 patients with MDS clonally evolved from SAA: monosomy 7 (7 patients); monosomy 7, +1, der(1;7) (1 patient); partial deletion 7 and 20 (1 patient); trisomy 8 (1 patient).
Positive for HLA class I and II antibodies in current or historical screenings. None of these HLA antibodies were donor (cord or haplo)-directed.
Among 10 patients with SAA evolved to MDS, the median BM cellularity pre-transplant was 7·5% (IQR 5% to 10%), the median age-adjusted IPSS-R score was 5·1 (IQR 4·9–5·3), the median blast% was 2% (range 1–4%), with 9/10 (90%) patients being transfusion-dependent for red blood cells and/or platelets. Prior to transplant, seven out of the eight MDS patients had failed to respond to standard IST with ATG/CSA; seven of these patients also received eltrombopag and two received alemtuzumab as part of the prior therapy. None of these patients had received other MDS therapies [immunomodulatory drugs (IMIDs), hypomethylating agents, or chemotherapies].
Transplant Characteristics
Donor and graft characteristics are shown in Table II. Haploidentical related donors were the recipient’s parents (62%), siblings (17%), children (7%), aunt (7%) and cousin (7%). Twenty-four (83%) patients were at risk of cytomegalovirus (CMV) reactivation (either patient or haplo donor IgG seropositive for CMV). Transplanted UCB units contained (prior to thawing) a median 3·5 × 107 TNCs/kg [interquartile range (IQR) 2·8–4·1], a median 1·4 × 105 CD34+ cells/kg (IQR 1·1–2·0), and a haplo CD34+-selected graft containing a median 3·2 × 106 CD34+ cells/kg (IQR, 3·0–3·3) and a median 1·0 × 103 CD3+ cells/kg (IQR, 0·6–1·7). Eighteen (62%) patients received a 4/6, 10 (35%) patients received a 5/6, and one (3%) patient received a 6/6 HLA-matched UCB-unit. High-resolution HLA typing showed that eight (28%) patients had a UCB graft matched to the recipient at ≥ 6/8 HLA alleles, 16 (55%) matched at 5/8 and five (17%) matched at less than 5/8 HLA alleles. Nine (31%) patients received grafts with killer immunoglobulin-like receptor (KIR) ligand incompatibility in the haplo-versus-cord direction (defined as the presence of a KIR ligand in the haplo donor graft that was absent in the UCB unit at HLA epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11).
Table II.
Transplant characteristics (n = 29).
| Characteristic | n (%) or median (IQR) |
|---|---|
| Relationship of haplo donor to recipient | |
| Father | 9 (31%) |
| Mother | 9 (31%) |
| Aunt | 2 (6·9%) |
| Brother | 3 (10·3%) |
| Sister | 2 (6·9%) |
| Cousin | 2 (6·9%) |
| Daughter | 1 (3·4%) |
| Son | 1 (3·4%) |
| Haplo donor age (years), median (range) | 38 (15–62) |
| Haplo CD34+ cell dose × 106/kg | 3·2 (3·0–3·3) |
| Haplo CD3+ cell dose × 103/kg | 1·0 (0·6–1·7) |
| CMV at risk | 24 (82·8%) |
| (haplo donor+ or recipient+) | |
| Cord TNC dose × 107/kg (prior to thawing) | 3·5 (2·8–4·1) |
| Cord CD34+ dose × 105/kg (prior to thawing) | 1·4 (1·1–2·0) |
| Cord HLA match (out of 6*) | |
| 6/6 | 1 (3·4%) |
| 5/6 | 10 (34–5%) |
| 4/6 | 18 (62·1%) |
| Cord HLA match (out of 8*) | |
| ≥ 6/8 | 8 (27·6%) |
| 5/8 | 16 (55·2%) |
| <5/8 | 5 (17·2%) |
| Haplo-vs-cord KIR ligand incompatibility† | 9 (31%) |
CMV, cytomegalovirus; HLA, human leucocyte antigen; IQR, interquartile range; KIR; TNC, total nucleated cells.
Out of 6 HLA (serologic HLA-A, -B, and allele level -DR loci) and out of 8 HLA alleles (high resolution HLA-A, -B, -C, and -DR loci).
Haplo-vs-cord KIR ligand incompatibility defined as the presence of a KIR ligand in the haplo donor graft that was absent in the umbilical cord blood (UCB) unit at HLA epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11.
Haematopoietic recovery
Twenty-eight patients had neutrophil recovery by day 42, with sustained engraftment associated with transfusion independence in 27 patients. The only patient who failed to engraft had an ANC > 500/μl for two consecutive days but unable to meet criteria for neutrophil recovery due to early death at day 21 from bacterial sepsis. The cumulative incidence of neutrophil recovery was 97%, and occurred at a median 10 days (range 9–28; Fig 1A). Twenty-seven (93%) patients had platelet recovery at a median 32 days (range 15–134; Fig 1B). Despite a high proportion of patients being highly HLA-allo-immunized and heavily transfused, only one patient experienced secondary graft failure. This patient underwent a salvage dual cord transplant and survives 6·5 years post-transplant.
Fig 1.
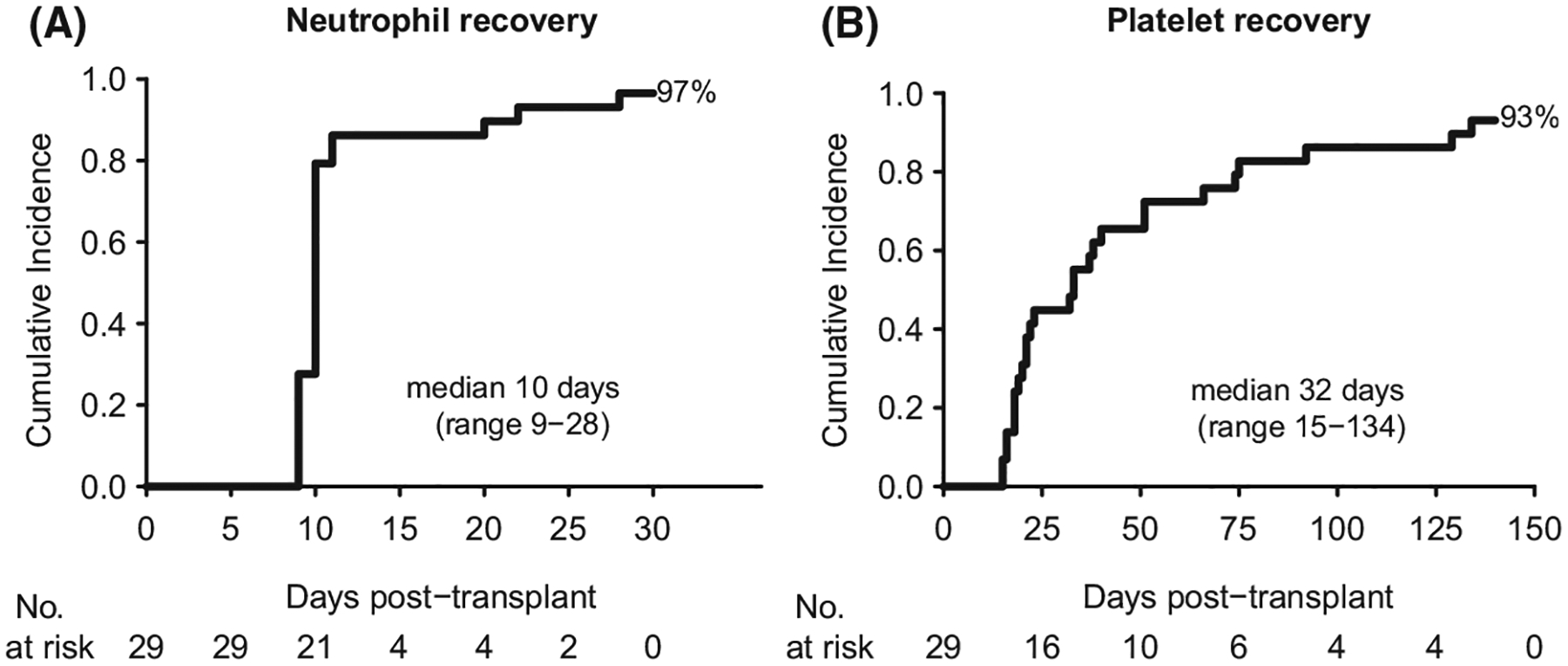
Cumulative incidence of neutrophil and platelet recovery. (A) neutrophil recovery; (B) platelet recovery.
Chimaerism analysis and cord engraftment
Chimaerism results are shown in Fig 2. Most patients (22/29, 76%) initially had high percentages of myeloid chimaerism coming from the haplo donor, followed by a gradual decrease in haplo donor myeloid chimaerism in association with increasing cord myeloid chimaerism over the next 3–6 months (Fig 2A, Figure S1A). For these patients, early haplo donor myeloid chimaerism was completely replaced by full cord myeloid chimaerism at a median 81 days (range 14–464). A total of 24 (83%) patients ultimately achieved a calculated cord ANC ≥ 500/μl (median 42 days, range 28–514) (Fig 2B). The majority of patients (23/29, 79%) also achieved full cord T-cell chimaerism (median 19 days, range 14–100: Fig 2C–D). However, in some cases, persistent mixed T-cell chimaerism from both the cord blood unit and the recipient was observed for several years following the transplant (Figure S1B). Three (10%) patients who never engrafted with the cord had persistent and sustained full haplo donor myeloid chimaerism with haplo-only engraftment occurring by day 14. Notably, these three patients had ≥ 95% recipient T-cell chimaerism for the first three months and their T-cell chimaerism from the recipient remained high or became mixed with T-cell chimaerism from the haplo donor at one year (Figure S1C). One patient with delayed cord engraftment had a unique chimaerism pattern, with myeloid chimaerism switching from haplo to cord origin in the context of T-cell chimaerism switching from cord to haplo origin (Figure S1D).
Fig 2.
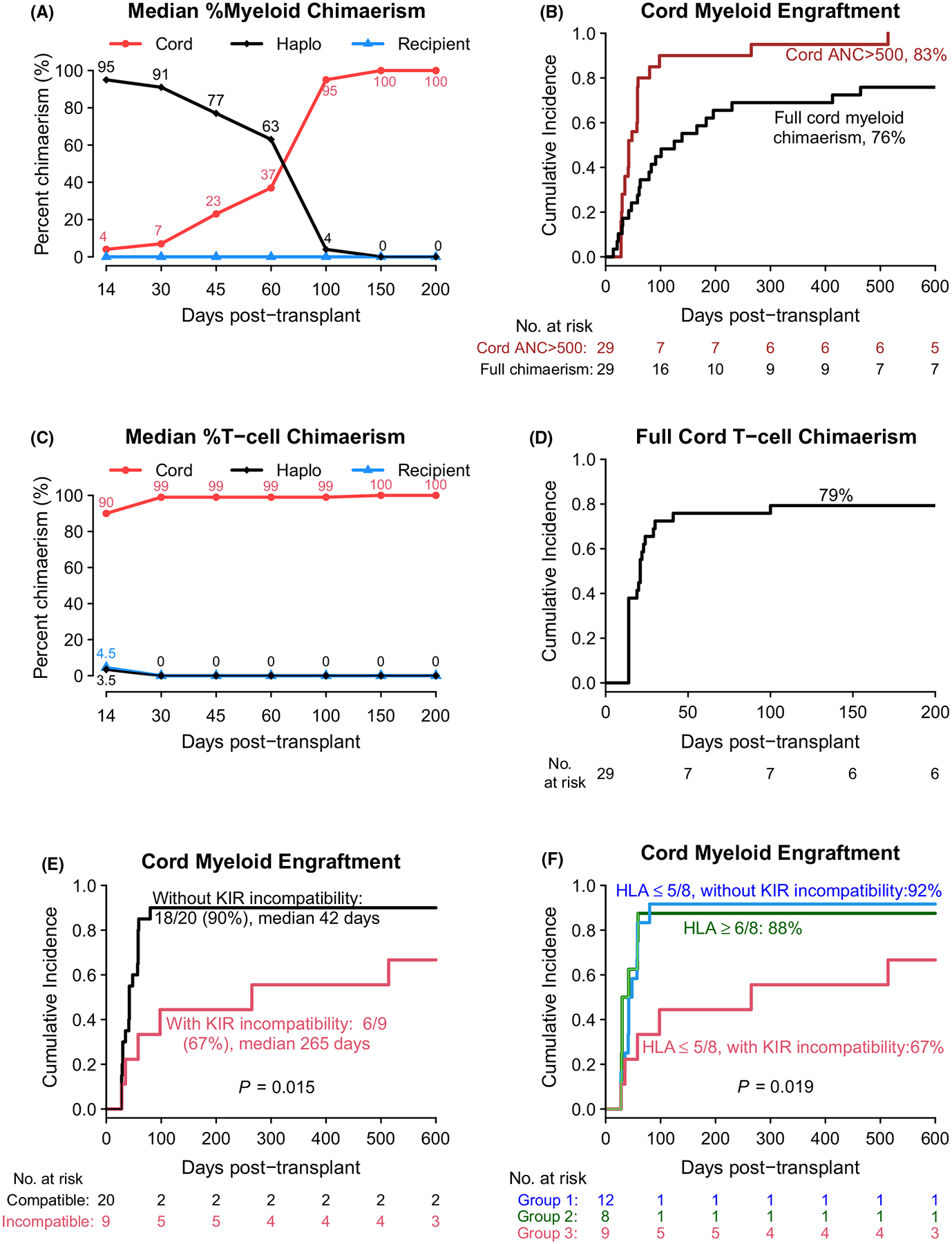
Chimaerism and cord engraftment. (A) Median per cent myeloid chimaerism; (B) cumulative incidence of cord engraftment [cord absolute neutrophil count (ANC) ≥ 500/μl] and full cord myeloid chimaerism; (C) median per cent T-cell chimaerism; (D) cumulative incidence of full cord T-cell chimaerism; (E) cumulative incidence of cord engraftment (cord ANC ≥ 500/μl) by haplo-vs-cord KIR ligand incompatibility (E) and by haplo-vs-cord KIR ligand incompatibility and cord human leucocyte antigen match to the recipient (F).
An analysis of patient- and transplant-related factors showed that age at transplant, UCB graft cell doses and HLA match did not impact the cord engraftment. However, KIR ligand incompatibility in the haplo-versus-cord direction had a negative impact on cord myeloid engraftment and appeared to delay the time until full cord myeloid chimaerism occurred. The cumulative incidence of cord engraftment was significantly higher and the time to cord engraftment was shorter in patients receiving transplants without haplo-versus-cord KIR ligand incompatibility, compared to those receiving transplants with incompatibility (90% vs 67%, median 42 vs 265 days, P = 0·015; Fig 2E). A further analysis controlling for cord HLA match to the recipient showed that haplo-versus-cord KIR incompatibility was associated with delayed cord engraftment and cord graft failure among patients receiving cord blood units that had a lower degree of HLA match (≤5 out of eight alleles, P = 0·019; Fig 2F). The analysis of time to full cord myeloid chimaerism showed similar results (Figure S2). Importantly, when chimaerism was analyzed in natural killer (NK) and myeloid subsets, haplo donor NK cells and haplo donor myeloid cells were detectable at lower levels and for a shorter duration in those without haplo-versus-cord KIR ligand incompatibility compared to those with incompatibility where both the percentage and persistence of haplo donor NK cells and myeloid cells was greater (Figure S3).
Viral reactivation, GVHD and survival
Analysis of T cells, NK cells, B cells and immunoglobulin levels showed the majority of patients had immune reconstitution by one year post-transplant (Fig 3). Most patients had at least one viral-associated infectious episode that was not life-threatening. Among those at risk, 19/24 (79%) developed CMV reactivation post-transplant (median time to first CMV reactivation 27 days, range 9–77). Epstein–Barr virus (EBV) reactivation occurred in 26 patients with 21 treated with rituximab. Three patients developed post-transplant lymphoproliferative disease (PTLD): two cases resolved following rituximab, and the third case progressed after rituximab but resolved completely following treatment with the third-party EBV-reactive cytotoxic T lymphocytes (CTLs). The rapid neutrophil recovery that occurred with this regimen may have protected patients from early transplant-related bacterial and fungal mortality: death from opportunistic bacterial or fungal infection did not occur in 28/29 patients while only one patient who was still neutropenic on day 21 died from bacteraemia (sphingomonas).
Fig 3.
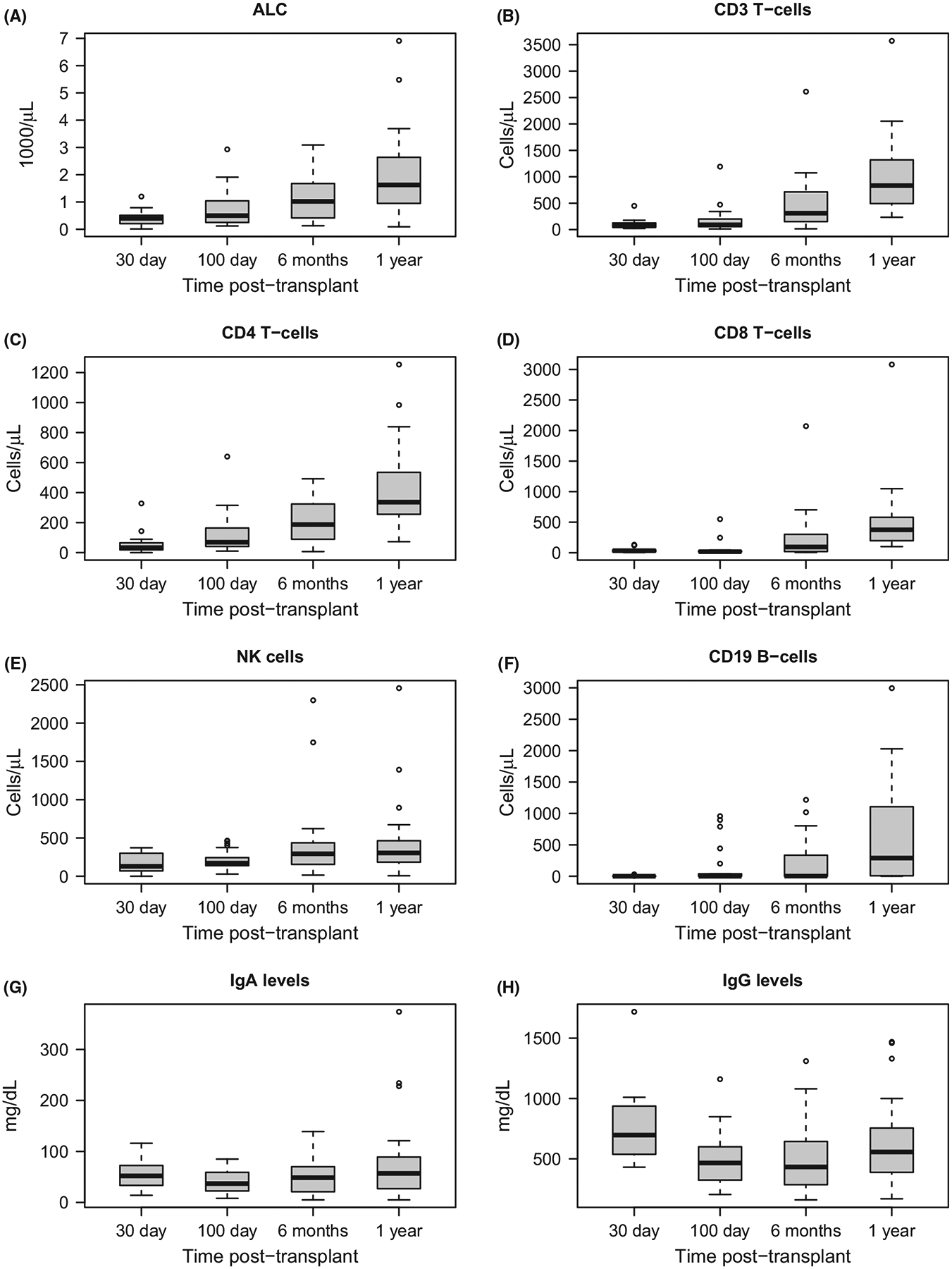
Immune reconstitution of lymphocytes, T-cells, NK- cells, B-cells and immunoglobulin levels. (A) absolute lymphocyte count, (B) CD3 T-cells, (C) CD4 T-cells, (D) CD8 T-cells, (E) NK cells, (F) CD19 B-cells, (G) IgA levels, (H) IgG levels.
The cumulative incidence of grade II–IV GVHD and grade III–IV acute GVHD were 21% and 7%, respectively (Fig 4A). There were no GVHD-related deaths and none of the acute GVHD cases were grade IV or steroid-refractory. The cumulative incidence of chronic GVHD was 41%, with 34% mild, 7% moderate and no severe cases (Fig 4B).
Fig 4.
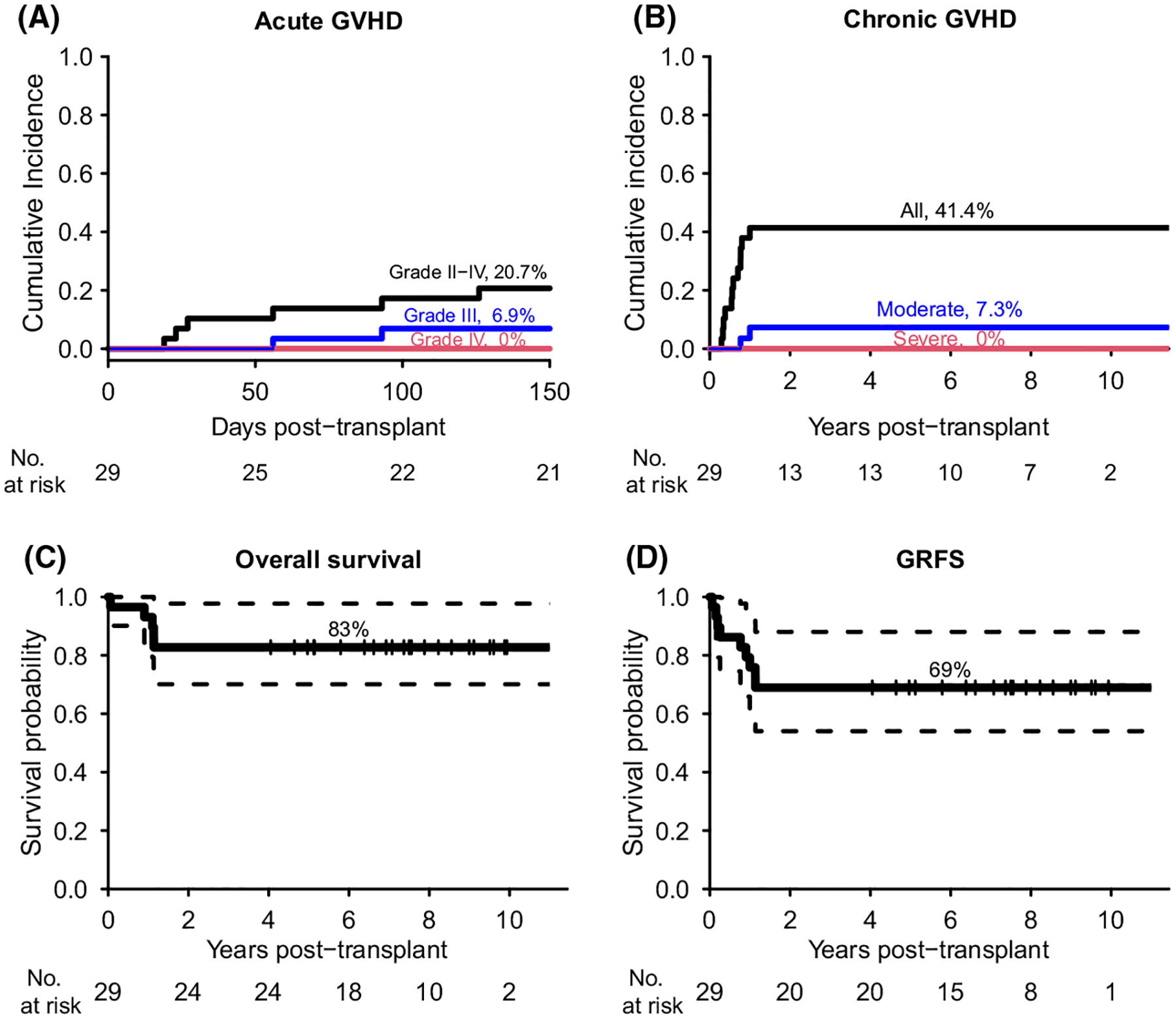
Cumulative incidence of GVHD, overall survival, and GVHD/relapse-free survival (GRFS). (A) acute GVHD, (B) chronic GVHD, (C) overall survival, (D) GRFS. Dash lines in (C-D) indicated the 95% confidence interval for overall survival and GRFS.
At day 200, 28 patients (97%) survived and the one-year overall survival was 93% (95% CI, 84–100). With a median follow-up of 7·5 years (range 4·0–12·2), 24 patients survived transfusion-free with an overall survival of 83% (95% CI, 70–98; Fig 4C). Opportunistic infection accounted for 4/5 deaths, three were viral in aetiology and occurred after day 200 [two CMV pneumonitis, one John Cunningham (JC) virus encephalitis]. One patient died of limbic encephalitis on day 402 with a brain biopsy not revealing evidence for an active infectious process. There was no relapse or secondary malignancy observed during follow-up. The one-year and seven-year GVHD-free/relapse-free survival (GRFS) were 76% (95% CI, 62–93) and 69% (95% CI, 54–88), respectively (Fig 4D). No patient- or transplant-related characteristics were statistically significantly associated with neutrophil and platelet engraftment, GVHD, and survival. With a median follow-up of 6·9 years, all the MDS patients were in remission after transplant, monitored by regular BM biopsies, with none receiving any post-transplant therapy directed at malignant clones. None of the MDS patients relapsed or had recurrence of malignant clones, and their haematopoietic recovery, chimaerism and survival were not statistically different compared to the outcomes in SAA patients (Figure S4).
Discussion
To our best knowledge, this is the largest prospective study of a single transplant regimen that combines haploidentical and cord blood transplantation for patients with SAA and hypoplastic MDS.
For patients with SAA and other BM failure syndromes who fail first-line IST and lack an HLA-matched donor, haematopoietic SCT using mismatched alternative stem-cell sources, such as cord blood or haploidentical donors, represents a viable salvage treatment option.8 Although recent studies on haplo transplants using high-dose post-transplant cyclophosphamide have been associated with very low mortality and GVHD rates,6,7 the results for engraftment and overall survival with haploidentical transplantation have varied considerably across different centres using different conditioning regimens.11–13 UCBTs have the advantage of being rapidly available and are associated with a low incidence of GVHD. However, largely due to the low stem-cell numbers, UCBTs for SAA have been associated with delayed haematopoietic recovery and high incidences of graft failure and transplant-related mortality.15,17 We designed this phase 2 study with the primary objective to investigate whether combined haplo cord transplantation would improve engraftment and expedite neutrophil recovery, which would be expected to reduce complications and mortality associated with delayed haematopoietic recovery. Because patients with SAA and hypocellular MDS share a similar pathophysiology and have previously been shown to have similar outcomes following IST and allogeneic SCT using HLA-matched siblings,28,33 we included both groups of patients in this trial. The outcome of this study fully supported our hypothesis. We observed rapid haematopoietic recovery in the cohort of 29 patients who underwent haplo cord transplantation, with 97% achieving neutrophil recovery at a median 10 days and 93% achieving platelet recovery at a median 32 days. The excellent engraftment rate observed in our study may be due in large part to the highly immunosuppressive nature of the chemotherapeutic agents (cyclophosphamide, fludarabine), ATG, and low-dose TBI utilized in the conditioning regimen.34 More importantly, compared to historical studies of cord blood transplantation in SAA patients,15–17 survival of recipients undergoing haplo cord transplants was markedly improved with 93% surviving at one year and with a median follow-up of 7·5 years, 83% survived and remained transfusion-independent. In addition, most patients responded to pre-emptive antiviral therapy and ultimately achieved immunologic recovery within one year post-transplant. Further, the incidence of grade III acute GVHD and moderate chronic GVHD were both very low at 7%, and no patients developed grade IV or steroid-refractory acute GVHD, severe chronic GVHD, or GVHD-related death. Using a composite end-point that included graft failure/relapse, severe GVHD and mortality, the estimated one-year and seven-year GRFS were 76% and 69%, respectively.
While this haplo cord transplant approach was associated with fast haematopoietic recovery and durable engraftment, engraftment patterns and profiles differed dramatically compared to conventional transplant approaches using stem cells from cord sources or from a haplo donor alone. In the majority of transplants, early T-cell chimaerism was predominantly cord in origin and was sustained. By contrast, early myeloid chimaerism after transplant was largely from the haplo donor, which gradually transitioned to full myeloid chimaerism from the UCB. These data are consistent with engrafting cord T cells mounting a gradual graft-versus-haematopoietic cell effect against engrafted haplo CD34+ progenitor cells, leading to their immunologic eradication. In three patients, early and durable engraftment was achieved only from transplanted CD34+ cells from the haploidentical donor. Taken altogether, these results suggest transplanted haploidentical CD34+ cells serve two purposes: (i) to overcome delayed haematopoietic recovery associated with transplants using UCB alone; and (ii) to work as a back-up stem cell source, which can salvage the transplant and result in sustained engraftment from the haplo donor in the event of cord graft failure.
In an analyses of factors related to haematopoietic recovery and survival, there was no evidence that patient- or transplant-related characteristics, including the patient’s age at transplant, degree of HLA matching and cell doses of the UCB and haplo donor grafts, or HLA allo-immunization, impacted transplant outcomes. However, we identified that KIR ligand incompatibility in the haplo-versus-cord direction had a significant negative impact on cord engraftment.27 In the setting of allogenic haematopoietic stem-cell transplants, prior studies have reported KIR ligand incompatibility can impact transplant outcomes, which appeared most evident with CD34+-selected haplo transplantation.35–38 In the haplo cord transplant setting, competition for engraftment exists between two separate allografts, best evidenced in cases where early myeloid haplo engraftment transitions to and is ultimately replaced by cord engraftment. We observed that when haplo-versus-cord KIR ligand incompatibility existed, cord myeloid engraftment was hindered and haplo engraftment was favoured, with high percentages of haplo donor myeloid chimaerism persisting of delayed cord myeloid chimaerism. These data suggest haplo-versus-cord KIR ligand incompatibility produced an environment where alloreactive NK cells derived from transplanted haplo donor CD34+ cells suppressed or in some cases completely eliminated cord blood stem cells (Figure S3). Our NK cell chimaerism data showing a greater number and prolonged persistence of haplo-NK cells in the context of haplo-versus-cord KIR ligand incompatibility further supports this theory. Further, in two of the three patients who never had cord engraftment, in the only patient with secondary graft failure, and in several of the cases with delayed cord engraftment (Figure S1C and D), haplo-versus-cord KIR ligand incompatibility was present. These results suggest allografts should be selected that avoid haplo-versus-cord KIR ligand incompatibility to expedite cord myeloid engraftment and reduce the risk of cord graft failure.
Our study is not without limitations. Our haplo cord transplantation approach incurs additional technical challenges and expense compared to conventional transplants, including the need to transplant two allografts including one that must be CD34+ selected. In this regard, there have been other recently reported transplant approaches based on using two sources of grafts from haploidentical haematopoietic stem-cells combined with umbilical cord-derived mesenchymal stem cells.39 Haplo-transplants using high-dose post-transplant cyclophosphamide,6,7 or when available, cord transplants using one or two UCB units with a high cell dose18 potentially represent more convenient, alternative strategies. Further, transplantation of a single UCB unit that has undergone ex vivo expansion in nicotinamide is currently being explored, potentially obviating the need for transplanting CD34+-selected cells from a haplo donor.40 Finally, our results are based on 29 patients transplanted at a single centre, and will require validation in a larger cohort and other transplant centres.
In conclusion, for IST-refractory SAA and SAA clonally evolved to MDS, transplantation of haploidentical CD34+ cells combined with cord blood achieved rapid and sustained engraftment, low rates of severe GVHD, and excellent long-term survival. This haplo cord transplantation regimen appears to represent a viable treatment option for SAA patients failing IST who lack an HLA-matched donor.
Supplementary Material
Fig S1. Engraftment kinetics in myeloid chimaerism and T-cell chimaerism. Four different patients were used to demonstrate various engraftment patterns and profiles.
Fig S2. Cumulative incidence of full cord myeloid chimaerism (A) by haplo-versus-cord KIR ligand incompatibility, (B) by haplo-versus-cord killer-cell immunoglobulin-like receptor (KIR) ligand incompatibility and cord human leucocyte antigen (HLA) match to the recipient. Haplo-versus-cord KIR ligand incompatibility is defined as the presence of a KIR ligand in the haplo donor graft that was absent in the umbilical cord blood (UCB) unit at HLA epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11.
Fig S3. Chimaerism analysis in natural killer (NK) and myeloid subsets. Haplo-versus-cord KIR ligand incompatibility is defined as the presence of a KIR ligand in the haplo donor graft that was absent in the umbilical cord blood (UCB) unit at human leucocyte antigen (HLA) epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11.
Fig S4. Transplant outcomes by disease diagnosis [severe aplastic anaemia (SAA) vs. to myelodysplastic syndrome (MDS)]. (A) Neutrophil recovery; (B) overall survival; (C) full cord myeloid chimaerism; (D) full cord T-cell chimaerism.
Acknowledgements
The authors would like to thank our patients for participating in this study.
Funding
This research was supported by the Intramural Research Program of the National Heart, Lung and Blood Institute, USA and the Commissioned Corps of United States Public Health Service, USA.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
Supporting Information
Additional supporting information may be found online in the Supporting Information section at the end of the article.
References
- 1.Scheinberg P, Nunez O, Weinstein B, Biancotto A, Wu CO, Young NS. Horse versus rabbit antithymocyte globulin in acquired aplastic anemia. N Engl J Med. 2011;365(5):430–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scheinberg P, Young NS. How I treat acquired aplastic anemia. Blood. 2012;120(6):1185–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Townsley DM, Scheinberg P, Winkler T, Desmond R, Dumitriu B, Rios O, et al. Eltrombopag added to standard immunosuppression for aplastic anemia. N Engl J Med. 2017;376(16):1540–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Young NS. Aplastic anemia. N Engl J Med. 2018;379(17):1643–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bacigalupo A How I treat acquired aplastic anemia. Blood. 2017;129 (11):1428–36. [DOI] [PubMed] [Google Scholar]
- 6.DeZern AE, Zahurak M, Symons H, Cooke K, Jones RJ, Brodsky RA. Alternative donor transplantation with high-dose post-transplantation cyclophosphamide for refractory severe aplastic anemia. Biol Blood Marrow Transplant. 2017;23(3):498–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.DeZern AE, Zahurak ML, Symons HJ, Cooke KR, Rosner GL, Gladstone DE, et al. Haploidentical BMT for severe aplastic anemia with intensive GVHD prophylaxis including posttransplant cyclophosphamide. Blood Adv. 2020;4(8):1770–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peffault de Latour R Transplantation for bone marrow failure: current issues. Hematology Am Soc Hematol Educ Program. 2016;2(1):90–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kanakry CG, Fuchs EJ, Luznik L. Modern approaches to HLA-haploidentical blood or marrow transplantation. Nat Reviews Clin Oncol. 2016;13 (1):10–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fuchs EJ. HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide. Bone Marrow Transplant. 2015;50(Suppl 2):S31–S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clay J, Kulasekararaj AG, Potter V, Grimaldi F, McLornan D, Raj K, et al. Nonmyeloablative peripheral blood haploidentical stem cell transplantation for refractory severe aplastic anemia. Biol Blood Marrow Transplant. 2014;20(11):1711–6. [DOI] [PubMed] [Google Scholar]
- 12.Esteves I, Bonfim C, Pasquini R, Funke V, Pereira NF, Rocha V, et al. Haploidentical BMT and post-transplant Cy for severe aplastic anemia: a multicenter retrospective study. Bone Marrow Transplant. 2015;50(5):685–9. [DOI] [PubMed] [Google Scholar]
- 13.Prata PH, Eikema DJ, Afansyev B, Bosman P, Smiers F, Diez-Martin JL, et al. Haploidentical transplantation and posttransplant cyclophosphamide for treating aplastic anemia patients: a report from the EBMT Severe Aplastic Anemia Working Party. Bone Marrow Transplant. 2020;55(6):1050–8. [DOI] [PubMed] [Google Scholar]
- 14.Wagner JE Jr, Eapen M, Carter S, Wang Y, Schultz KR, Wall DA, et al. One-unit versus two-unit cord-blood transplantation for hematologic cancers. N Engl J Med. 2014;371(18):1685–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peffault de Latour R, Purtill D, Ruggeri A, Sanz G, Michel G, Gandemer V, et al. Influence of nucleated cell dose on overall survival of unrelated cord blood transplantation for patients with severe acquired aplastic anemia: a study by eurocord and the aplastic anemia working party of the European group for blood and marrow transplantation. Biol Blood Marrow Transplant. 2011;17(1):78–85. [DOI] [PubMed] [Google Scholar]
- 16.Peffault de Latour R, Rocha V, Socie G. Cord blood transplantation in aplastic anemia. Bone Marrow Transplant. 2013;48(2):201–2. [DOI] [PubMed] [Google Scholar]
- 17.Yoshimi A, Kojima S, Taniguchi S, Hara J, Matsui T, Takahashi Y, et al. Unrelated cord blood transplantation for severe aplastic anemia. Biol Blood Marrow Transplant. 2008;14(9):1057–63. [DOI] [PubMed] [Google Scholar]
- 18.Peffault de Latour R, Chevret S, Jubert C, Sirvent A, Galambrun C, Ruggeri A, et al. Unrelated cord blood transplantation in patients with idiopathic refractory severe aplastic anemia: a nationwide phase 2 study. Blood. 2018;132(7):750–4. [DOI] [PubMed] [Google Scholar]
- 19.Fernandez MN, Regidor C, Cabrera R, Garcia-Marco J, Briz M, Fores R, et al. Cord blood transplants: early recovery of neutrophils from co-transplanted sibling haploidentical progenitor cells and lack of engraftment of cultured cord blood cells, as ascertained by analysis of DNA polymorphisms. Bone Marrow Transplant. 2001;28(4):355–63. [DOI] [PubMed] [Google Scholar]
- 20.Fernandez MN, Regidor C, Cabrera R, Garcia-Marco JA, Fores R, Sanjuan I, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31(6):535–44. [DOI] [PubMed] [Google Scholar]
- 21.Kwon M, Bautista G, Balsalobre P, Sanchez-Ortega I, Serrano D, Anguita J, et al. Haplo cord transplantation using CD34(+) cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant. 2014;20(12):2015–22. [DOI] [PubMed] [Google Scholar]
- 22.Liu H, Rich ES, Godley L, Odenike O, Joseph L, Marino S, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood. 2011;118(24):6438–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Magro E, Regidor C, Cabrera R, Sanjuan I, Fores R, Garcia-Marco JA, et al. Early hematopoietic recovery after single unit unrelated cord blood transplantation in adults supported by co-infusion of mobilized stem cells from a third party donor. Haematologica. 2006;91(5):640–8. [PubMed] [Google Scholar]
- 24.van Besien K, Childs R. Haploidentical cord transplantation-The best of both worlds. Semin Hematol. 2016;53(4):257–66. [DOI] [PubMed] [Google Scholar]
- 25.Van Besien K, Liu H, Jain N, Stock W, Artz A. Umbilical cord blood transplantation supported by third-party donor cells: rationale, results, and applications. Biol Blood Marrow Transplant. 2013;19(5):682–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gormley NJ, Wilder J, Khuu H, Pantin J, Donahue T, Kurlander R, et al. Co-Infusion of allogeneic cord blood with haploidentical CD34+ cellsim-proved transplant outcome for patients with severe aplastic anemiaunder-going cord blood transplantation. Blood (ASH Annual Meeting Abstracts). 2011;118(21):654. [Google Scholar]
- 27.Kotecha R, Tian X, Wilder J, Gormley N, Khuu H, Stroncek D, et al. NK Cell KIR ligand mismatches influence engraftment following combined haploidentical and umbilical cord blood (UCB) transplantation in patients with severe aplastic anemia (SAA). Blood (ASH Annual Meeting Abstracts). 2013;122(21):2038. [Google Scholar]
- 28.Calado RT. Immunologic aspects of hypoplastic myelodysplastic syndrome. Semin Oncol. 2011;38(5):667–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Simon R Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10(1):1–10. [DOI] [PubMed] [Google Scholar]
- 30.Kaplan EL, Meier P. Nonparametric-estimation from incomplete observations. J Am Stat Assoc. 1958;53(282):457–81. [Google Scholar]
- 31.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18(6):695–706. [DOI] [PubMed] [Google Scholar]
- 32.Gray RJ. A class of K-sample tests for comparing the cumulative incidence of a competive risk. Annals Statistics. 1988;16(3):1141–54. [Google Scholar]
- 33.Pantin J, Tian X, Shah AA, Kurlander R, Ramos C, Cook L, et al. Rapid donor T-cell engraftment increases the risk of chronic graft-versus-host disease following salvage allogeneic peripheral blood hematopoietic cell transplantation for bone marrow failure syndromes. Am J Hematol. 2013;88(10):874–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Deeg HJ, O’Donnell M, Tolar J, Agarwal R, Harris RE, Feig SA, et al. Optimization of conditioning for marrow transplantation from unrelated donors for patients with aplastic anemia after failure of immunosuppressive therapy. Blood. 2006;108(5):1485–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295(5562):2097–100. [DOI] [PubMed] [Google Scholar]
- 36.Ruggeri L, Mancusi A, Burchielli E, Aversa F, Martelli MF, Velardi A. Natural killer cell alloreactivity and haplo-identical hematopoietic transplantation. Cytotherapy. 2006;8(6):554–8. [DOI] [PubMed] [Google Scholar]
- 37.Davies SM, Ruggieri L, DeFor T, Wagner JE, Weisdorf DJ, Miller JS, et al. Evaluation of KIR ligand incompatibility in mismatched unrelated donor hematopoietic transplants. Killer immunoglobulin-like receptor. Blood. 2002;100(10):3825–7. [DOI] [PubMed] [Google Scholar]
- 38.Malmberg KJ, Schaffer M, Ringden O, Remberger M, Ljunggren HG. KIR-ligand mismatch in allogeneic hematopoietic stem cell transplantation. Mol Immunol. 2005;42(4):531–4. [DOI] [PubMed] [Google Scholar]
- 39.Xu L, Liu Z, Wu Y, Yang X, Cao Y, Li X, et al. Clinical evaluation of haploidentical hematopoietic combined with human umbilical cord-derived mesenchymal stem cells in severe aplastic anemia. Eur J Med Res. 2018;23 (1):12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Clara JA, Vo P, Aue G, Wilder JS, Wells B, Shalabi R, et al. Ex Vivo Nicotinamide-Expanded (NAM-Expanded) Unrelated Cord Bloodtrans-plantation (UCB) for Refractory Severe Aplastic Anemia Results in Rapid Engraftment and Expedites Immune Recovery. Biol Blood Marrow Transplant. 2019;25(3):S210–S211. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Engraftment kinetics in myeloid chimaerism and T-cell chimaerism. Four different patients were used to demonstrate various engraftment patterns and profiles.
Fig S2. Cumulative incidence of full cord myeloid chimaerism (A) by haplo-versus-cord KIR ligand incompatibility, (B) by haplo-versus-cord killer-cell immunoglobulin-like receptor (KIR) ligand incompatibility and cord human leucocyte antigen (HLA) match to the recipient. Haplo-versus-cord KIR ligand incompatibility is defined as the presence of a KIR ligand in the haplo donor graft that was absent in the umbilical cord blood (UCB) unit at HLA epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11.
Fig S3. Chimaerism analysis in natural killer (NK) and myeloid subsets. Haplo-versus-cord KIR ligand incompatibility is defined as the presence of a KIR ligand in the haplo donor graft that was absent in the umbilical cord blood (UCB) unit at human leucocyte antigen (HLA) epitopes Bw4, HLA-C group 1, HLA-C group 2, and/or HLA-A3/A11.
Fig S4. Transplant outcomes by disease diagnosis [severe aplastic anaemia (SAA) vs. to myelodysplastic syndrome (MDS)]. (A) Neutrophil recovery; (B) overall survival; (C) full cord myeloid chimaerism; (D) full cord T-cell chimaerism.


