Abstract
Coronavirus disease 2019 (COVID-19) is a remarkably contagious and pathogenic viral infection arising from the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which first appeared in Wuhan, China. For the time being, COVID-19 is not treated with a specific therapy. The Food and Drug Administration (FDA) has approved Remdesivir as the first drug to treat COVID-19. However, many other therapeutic approaches are being investigated as possible treatments for COVID-19. As part of this review, we discussed the development of various drugs, their mechanism of action, and how they might be applied to different cases of COVID-19 patients. Furthermore, this review highlights an update in the emergence of new prophylactic or therapeutic vaccines against COVID-19. In addition to FDA or The World Health Organization (WHO) approved vaccines, we intended to incorporate the latest published data from phase III trials about different COVID-19 vaccines and provide clinical data released on the networks or peer-review journals.
Abbreviations: ACE2, Angiotensin-converting enzyme 2; Arb, Arbidol; ARDS, Acute respiratory distress syndrome; COVID-19, Coronavirus disease 2019; ER, Endoplasmic reticulum; ERGIC, Endoplasmic reticulum Golgi intermediate compartment; FDA, Food and Drug Administration; gRNA, Genomic RNA; HIV, Human immunodeficiency virus; mAbs, Monoclonal antibodies; MERS-CoV, The Middle East respiratory syndrome 20 coronavirus; ORFs, Open reading frames; RBD, Receptor binding domain; RdRp, RNA-dependent RNA polymerase; RTC, Replicase transcriptase complex; SARS-CoV-2, Severe acute respiratory syndrome of coronavirus 2; sgRNA, Subgenomic RNA; VLPs, Virus-like particles; WHO, World Health Organization; WMT, Washed microbiota transplantation
Keywords: Antiviral agents, COVID-19, Pandemics, Pneumonia, SARS-CoV-2, Vaccines
1. Introduction
Coronavirus has been one of the significant intracellular parasites which predominantly attack the human respiratory system. Severe acute respiratory syndrome (SARS)-CoV and Middle East Respiratory Syndrome (MERS)-CoV were two earlier coronaviral (CoV) outbreaks, described previously as a severe health concern (Rothan and Byrareddy, 2020). A series of unclear etiology pneumonia was proclaimed in Wuhan City, the Chinese province of Hubei, in December 2019 (Arabi et al., 2020). On December 31, an emergency response team was dispatched to Wuhan by the Chinese Disease Management and Preventing Centre. Possible causes including influenza, avian influenza, adenovirus, SARS-CoV, and MERS-CoV were eliminated one by one. (Zhang, 2020; Zu et al., 2020).
Eventually, the World Health Organization (WHO) identified this disorder as Coronavirus Disease 2019 (COVID-19), and the responsible pathogen was collocated as SARS-CoV-2 by the International Committee on Taxonomy on Viruses (Gorbalenya, 2020). Infections cause diverse clinical manifestations, shifting from moderate presentations to severe respiratory attacks, and though asymptomatic infection is possible. Then, it was circulated outside China and produced a pandemic in plenty of other countries (Biswas et al., 2020). The number of reported infections globally was over 515,755,796 with around 6,271,647 mortalities and over 470,499,907 recoveries until May 5, 2022 (Worldometers, 2022).
The search for therapeutic agents that can be administrated primarily for severe cases of infection remains a priority. Many research groups in many countries have ventured to generate an antiviral drug against the SARS-CoV-2; More than 80 firms and academic research groups are now conducting 3370 registered studies for COVID-19 pharmaceutical testing and vaccine development. Repurposing available drugs have been explored due to its urgent need for effective treatments. Unfortunately, the COVID-19 infection is not currently treated with medication; For this reason, it is necessary to understand the effect of antiviral medicines on the treatment of mild, moderate, severe, and critical forms of the disease so that patients with COVID-19 can get the best possible therapy. According to the World Health Organization, vaccines are one of the most efficient ways to prevent diseases by boosting the immune system's ability to guard against infectious organisms like bacteria and viruses. In this study, we examine prospective therapies and mechanisms, as well as permitted and approved vaccine platforms and associated side effects.
2. Therapeutic strategies
Despite the recent approval of a few vaccines against SARS-CoV-2 infections by the regulatory authorities, there are currently no possible curative approaches available to manage this pandemic. To make matters harder, policymakers and developers must deal with issues such as vaccine distribution and production in appropriate quantities, as well as vaccine pricing and deployment (Wouters et al., 2021; Singh et al., 2017; Thakur and Bailey, 2019). Governments have used a variety of pharmaceutical therapies to treat the condition, including antiviral agents that have been shown to be beneficial for COVID-19 as well as several types of oxygen therapy, and mechanical respiration. The COVID-19 pandemic necessitates the rapid creation of effective and efficient treatments and medications, which can be accomplished by following three principles: (i) The first strategy entails researching and evaluating the treatment effectiveness of currently accessible antivirals. (Sanders et al., 2020; Alam et al., 2021). (ii) Molecular repositories, archives, and libraries which can help with high processing capacity and ongoing examination of a large number of possible medicines (Gurung et al., 2021; Lu, 2020). (iii) Targeted therapy planned to disrupt the viral signaling (cellular and molecular pathways) as well as the immune response (Lu, 2020; Hussman, 2020; McGowan et al., 2020). Therefore, as the exploration for novel effective therapeutic choices continue and will necessitate additional time and efforts, experts are pursuing the feasibility of using already available pharmaceuticals to treat COVID-19 together with the discovery and provision of new vaccines (Khan et al., 2021). As a result, already approved drugs, such as antiviral (Vlachakis et al., 2020) and antimalarial medications (Rolain et al., 2007) which inhibit viral entry (Bonam et al., 2020) membrane fusion (Azad et al., 2009), endocytosis, and SARS-CoV-2 activity, followed by preferred broad-spectrum antibiotics and immunotherapeutic drugs, are the main categories of therapies that appear to be useful in COVID-19 treatment and are currently being preferred in different countries (Indari et al., 2021; Clinicaltrials, 2020c). These medicines have the advantage of having been licensed for the treatment of other viral infections, so their metabolic features, doses, potential efficacy, and adverse effects are all known. Nevertheless, since these medicines are broad-spectrum, they seem unable to destroy CoVs directly, and potential adverse effects must be taken into account (Rahimkhoei et al., 2021).
2.1. Viral entry, viral membrane fusion, and endocytosis inhibitors
Cell entry inhibitors have been successfully utilized to treat viral infections because they have the intrinsic ability to prevent viral and cellular membranes from fusing together (Kandeel et al., 2021). These inhibitors include antibodies, small molecules, as well as peptide inhibitors.
2.1.1. Antibodies
SARS-CoV-2 antibodies bind to SARS-CoV-2 RBD directly, disrupt SARS-CoV-2 RBD-hACE2 receptor interactions, and efficiently neutralize SARS-CoV-2 S protein pseudotyped virus infection (Chen et al., 2020d). The use of the following SARS-CoV-2-targeting mAbs for the treatment of COVID-19 has been allowed for particular patients by an Emergency Use Authorization (EUA).
2.1.1.1. REGEN-COV
On November 21, 2020, the FDA approved an EUA for the cure of mild to moderate COVID-19 in adults and children with REGEN-COV (casirivimab and imdevimab, administered together) (Administration, 2022a).
2.1.1.2. Sotrovimab
On May 26, 2021, the FDA granted an EUA for sotrovimab in adults and children with mild-to-moderate COVID-19 (Administration, 2021).
2.1.1.3. Bamlanivimab and etesevimab
On February 9, 2021, the FDA approved the emergency use of bamlanivimab and etesevimab in adults and children with mild to moderate arthritis. COVID-19 (Administration, 2022b).
2.1.2. Small molecules
2.1.2.1. Camostat mesylate
Furin cleaves the SARS-CoV-2 spike (S) protein into its S1, and S2 subunits at a polybasic cleavage site, and TMPRSS2 and CatL proteolytic activity are needed for S protein activation. Camostat mesylate, a medicinal drug used in Japan to treat chronic pancreatitis, can inhibit TMPRSS2 activity. Thus, it could be able to stop pseudotyped SARS-CoV-2 from infecting Calu-3 cells (Hoffmann et al., 2020a).
2.1.2.2. Nafamostat mesylate
Nafamostat mesylate activity was around 15-fold more significant than that of camostat mesylate, with an EC50 of 5 nM, and it could hinder TMPRSS2-dependent cell membrane entrance (Hoffmann et al., 2020b). Furthermore, Docking on US-FDA licensed drugs that target the targets TMPRSS2 and ACE2 shows that lopinavir and valrubicin have the ability to block SARS-CoV-2 entry into the host by inhibiting both targets (Baby et al., 2021).
2.1.2.3. SID-26681509
Aside from TMPRSS2 inhibitors, SID-26681509, a small molecule compound-based CatL-like protease inhibitor, was found to prevent 76% of SARS-CoV-2 S pseudovirions from accessing 293/hACE2 cells (Ou et al., 2020).
2.1.2.4. Dalbavancin
Dalbavancin, another glycopeptide antibiotic, showed inhibitory activity as well. Furthermore, at 30 μM, the broad-spectrum cysteine protease inhibitor E64D prevented 92.5% of SARS-CoV-2 pseudovirions from entering the body (Zhang et al., 2020; Ou et al., 2020).
2.1.2.5. Metformin
Metformin was first released as an anti-influenza medicine, with glucose-lowering as one of its adverse effects. This medication phosphorylates and changes the structure of the ACE2 receptor, potentially reducing viral entrance. Accordingly, a retrospective study by Pan Luo et al. discovered that Metformin treatment reduced mortality rates in COVID-19 diabetic patients (Sharma et al., 2020; Luo et al., 2020).
2.1.2.6. Arbidol
Arbidol (Arb) has an Antiviral function toward influenza A and B, providing viral fusion inhibition with the targeted membrane. It prevents the penetration of viruses into the cell(Boriskin et al., 2008). A study showed that SARS-CoV-2 could be effectively inhibited in vitro by a 10–30 μM concentration of Arbidol (Zhu et al., 2020). However, its efficiency and safety, on the other hand, have yet to be proven.
2.1.2.7. Oseltamivir
Oseltamivir is another medicine approved for the treatment of influenza A and B. It works by inhibiting viral neuraminidase, preventing viral particles from being released from host cells and thereby decreasing the propagation of the virus in the respiratory system (Davies, 2010). In patients with COVID-19, a trial found that combining oseltamivir with hydroxychloroquine may be beneficial (Ramatillah and Isnaini, 2021). Moreover, multiple clinical trials (e.g., NCT04303299, NCT04516915, NCT04338698, and others) are currently being conducted to determine the efficacy of oseltamivir in treating SARS-CoV-2 infection.
2.1.3. Peptides
Currently, peptide drugs are the main subject of fusion inhibitor research. The most recent updates on these kinds of therapeutics are discussed below.
2.1.3.1. Peptides preventing the fusion core formation
2019-nCoV-HR2P, for instance, is a peptide that can prevent fusion core formation by supplying the heptad repeat domains (HR1 and HR2) of the SARS-CoV-2 spike (S) (Xia et al., 2020b; Kandeel et al., 2020). More examples include the 36-mer peptide (peptide #2) derived from SARS-CoV-2 HR2 (Kandeel et al., 2021) and the EK1 peptide as an HR1-derived peptide (Xia et al., 2020b). The C-terminus of the EK1 peptide was conjugated with cholesterol. The anti-CoV activity of the resulting lipopeptide EK1C4 was 150- and 240-fold higher than that of the EK1 peptide against pseudotyped SARS-CoV-2 infection and S-mediated membrane fusion, respectively (Xia et al., 2020a; Outlaw et al., 2020).
2.1.3.2. Peptides preventing ACE2 binding of the SARS-CoV-2 spike protein (RBD)
Due to RBD-binding interaction motifs, AHB1 and AHB2 neutralized SARS-CoV-2. Moreover, LCB1 and LCB3, which bind the RBD with lower dissociation constants and neutralize SARS-CoV-2 in the picomolar range, have been discovered (Cao et al., 2020b).
2.1.3.3. Peptides targeting ACE2
Watson and colleagues created SARS-BLOCK™ peptides that resemble the SARS-CoV-2 RBD domain and used biolayer interferometry to determine their binding to ACE2 and neutralizing antibodies (Watson et al., 2020). Beddingfield and colleagues also investigated the antiviral activity of ATN-161, a fibronectin-derived anticancer peptide that could bind directly to the integrin, the S protein's RGD motif, and the KGD motif in ACE2 (Integrins have been found to bind to the RGD motif in the SARS-CoV-2 S protein, as well as a KGD sequence in the ACE2 protein) (Beddingfield et al., 2021).
2.1.3.4. Peptides targeting proteolytic S protein activation
Decanoyl-RVKRchloromethylketone (dec-RVKR-cmk), a peptidomimetic furin inhibitor, blocks the activation of various viral glycoproteins. SARS-CoV-2 S processing was shown to be hindered by dec-RVKR-cmk (Cheng et al., 2020). Moreover, MI-1851, a peptidomimetic furin inhibitor, was investigated by Bestle and colleagues. It is demonstrated that MI-1851 could stop SARS-CoV-2 S protein cleavage, according to Western blotting (Bestle et al., 2020). Besides, the bovine lung polypeptide aprotinin is a broad-spectrum serine protease inhibitor that inhibits TMPRSS2 and thus hinders SARS-CoV-2 replication. MI-432 and MI-1900, peptidomimetic inhibitors, were found to be more effective than aprotinin at reducing viral titers (Bestle et al., 2020). It has previously been demonstrated that α1-antitrypsin inhibits TMPRSS2's proteolytic activity. As a result, by inhibiting TMPRSS2-mediated S protein activation, α1-antitrypsin may be able to prevent SARS-CoV-2 infection (Azouz et al., 2020).
In the absence of TMPRSS2 and/or furin after endocytosis, CatL activation of S proteins is most anticipated. Teicoplanin, a glycopeptide antibiotic widely used to treat gram-positive bacterial infections, has been shown to suppress CatL and decrease SARS-CoV and MERS-CoV infection (Zhang et al., 2020). Peptide P9 is also one chemical that inhibits CatL activity. P9 binds to SARS-CoV-2 particles directly and prevents endosome acidification, thus interfering with CatL activity indirectly (Zhao et al., 2020).
2.1.3.5. Human angiotensin-converting soluble recombinant enzyme 2 (hrsACE2)
Coronavirus (CoV) S protein attaches to the ACE2 receptor. Administration of recombinant soluble ACE2 can block/prevent binding of the novel coronavirus (Mohamed et al., 2020).
2.1.4. Convalescent plasma
Research indicates that convalescent plasma from patients who have recovered from viral infections can be used as a therapy without emerging significant adverse effects. Hence, evaluating the safety and effectiveness of convalescent plasma transfusion in patients diagnosed with SARS-CoV-2 may be valuable. One conceivable elucidation of the efficacy of convalescent plasma treatment is that the antibodies from convalescent plasma might eliminate viraemia by amending host humoral resistance to SARS-CoV-2 (Chen et al., 2020b). On August 23, 2020, the FDA granted an EUA for convalescent plasma to treat inpatients with COVID 19 (FDA, 2021).
2.2. Viral activity inhibitors
2.2.1. Molnupiravir
Molnupiravir, also known as Lagevrio, inhibits viral reproduction by causing major changes in viral RNA replication through RNA-dependent RNA polymerase (RdRp). It has shown in nonclinical models that it can treat infections triggered by a number of RNA viruses, including highly pathogenic coronaviruses and influenza viruses, as well as encephalitic alphaviruses including Venezuelan, Eastern, and Western equine encephalitis viruses. It is changed to β-D-N4-Hydroxycytidine 5′-triphosphate, a ribonucleoside analog with a cytidine-like appearance (NHC-TP). Instead of using real cytidine, the virus's enzyme integrates NHC-TP into newly produced RNA during replication. This results in viral error catastrophe, or lethal mutagenesis, in which the virus accumulates more mutations in all downstream copies than it can tolerate. (Painter et al., 2021).
2.2.2. Lopinavir
Lopinavir, a type 1 aspartate protease inhibitor of the human immunodeficiency virus (HIV), also having in vitro inhibitory effect against SARS-CoV (Que et al., 2003; Oldfield and Plosker, 2006). As well as lopinavir, ritonavir is also formulated to increase the half-life of lopinavir. Ritonavir is an antiviral agent designed to inhibit the action of HIV protease. Thus, when applied in conjunction with other drugs, it helps treat HIV infections. Ritonavir inhibits cytochrome P450 3A, which reduces lopinavir's metabolic rate. Thus, Lopinavir–ritonavir lowers the risk of severe clinical effects (ARDS or death) and viral load in SARS patients (Cao et al., 2020a; Chu et al., 2004). Lopinavir/ritonavir was initially assumed to be effective against the SARS-CoV-2; however, later findings proved the drugs were ineffective or even harmful strictly prohibited from further use (Siemieniuk et al., 2020; Kim et al., 2020).
2.2.3. Nirmatrelvir
Nirmatrelvir is an antiviral medication manufactured by Pfizer that works as an orally active 3CL protease inhibitor. In December 2021, the US-FDA granted an EUA for the combination of nirmatrelvir and ritonavir for the treatment of coronavirus infection. COVID-19. The trademark for the co-packaged medications is Paxlovid. (Ahmad et al., 2021; Businesswire, 2022).
2.2.4. Nelfinavir
Nelfinavir is a potent HIV-1 protease inhibitor that has been shown to exhibit antiviral properties against the SARS-CoV-2 virus. In animal studies, however, encouraging outcomes have still not been observed. (Ohashi et al., 2020). Darunavir, a second-generation HIV protease inhibitor, has been proven to suppress viral replication at a dose of 300 μM by Chinese researchers (Dong et al., 2020). Glycyrrhizin also decreases virus uptake and penetration in the early stages of propagation, according to studies. This chemical has a reduced toxicity risk than other protease inhibitors (e.g., Ribavirin) (LuoLiu and Li, 2020).
2.2.5. Remdesivir
Remdesivir (GS-5734) is an inhibitor of HIV reverse transcriptase, which also has wide-spectrum effects in cell cultures and animal models on RNA viruses, including MERS and SARS (Lim et al., 2020). Animal-based studies have shown that Remdesivir decreases viral lung loads of MERS-CoV in mice, increases lung capacity, and reduces damage to lung tissue. (Wang et al., 2020). Despite the fact that Remdesivir was approved by the FDA as the initial medication choice for COVID-19 in adults and pediatrics aged 12 and above and weighing at least 40 kg, the WHO stated that Remdesivir had little to no impact on the 28-day or in-hospital mortality of patients with COVID-19. Within individuals not receiving ventilation, a 5-day regimen will likely deliver more significant benefits and less side effects with lower drug expenditures than a 10-day course (Lei et al., 2020; Corum et al., 2020; FasterCures, 2020).
2.2.6. Ivermectin
Ivermectin was initially developed as an anti-parasitic medicine; however, it further gained anti-HIV1 application as it could halt the replication of the virus. Thus, researchers further opted for testing the drug on the SARS-CoV-2. Preliminary investigations, which took place in Australia, demonstrated the high efficiency of the drug in hindering viral replication. In-vitro analysis of the cell's supernatant showed a 93% decline in the amount of viral RNA and a 99.80% decline in cell-associated viral RNAs.
In vitro testing of ivermectin's antiviral activity on SARS-CoV-2 infected cells indicated a 93% reduction in viral RNA detectable in the supernatant and a 99.80% reduction in cell-associated viral RNA in Australia (Ahmed et al., 2021). The most well-established of the several mechanisms via which ivermectin functions is as a blocker of nuclear transport mediated by the importin α/β1 heterodimer, which is responsible for the translocation of various viral species proteins (HIV-1, SV40), which are necessary for their replication.
2.2.7. Ganovo
Ganovo (Danoprevir) is an authorized hepatitis C medicine, an inhibitor of NS3/4A protease (Drugbank, 2020). It should be noted that the last clinical phase of this drug combination has recently passed (Clinicaltrials, 2020b; Chen et al., 2020a). Danoprevir in combination with ritonavir, is effective against COVID-19 according to the data. The first negative reverse real-time PCR (RT-PCR) test occurred on average 2 days after starting danoprevir/ritonavir treatment, with a range of 1 to 8 days, and the first distinct absorption in CT scans on average 3 days, with a range of 2 to 4 days. After taking danoprevir augmented by ritonavir for 4 to 12 days, all 11 patients were discharged from the hospital (Chen et al., 2020a).
2.2.8. Favipiravir
Favipiravir is an antiviral drug that works against RNA viruses by inhibiting RdRp activity. In animal studies, Favipiravir has demonstrated activity against influenza viruses, yellow fever virus, foot-and-mouth disease virus, West Nile virus, and several other flaviviruses, arenaviruses, bunyaviruses, and alphaviruses as well (Furuta et al., 2009). The initial data of a clinical trial on the effectiveness of Favipiravir for treatment of COVID-19 on February 14, 2020, showed a higher degree of antiviral activity relative to that of Lopinavir/Ritonavir. There were also no notable side effects observed in the treatment group with Favipiravir (Kramer et al., 2020).
2.2.9. α-lipoic acid (ALA)
ALA has been beneficial at inhibiting HIV-1 replication (Baur et al., 1991). This new coronavirus ALA may also be used as an alternative therapy (Zhang and Liu, 2020). ALA's putative positive effects in SARS-CoV-2 infection include cell entrance inhibition, anti-inflammatory properties, maintaining a high GSH/GSSG ratio in the endogen, and reduction of mitochondrial oxidative damage, according to multiple lines of information (Dragomanova et al., 2021).
2.3. Immunotherapeutic drugs
2.3.1. Monoclonal antibodies
2.3.1.1. Siltuximab and tocilizumab
Siltuximab and Tocilizumab can be used as the treatments in patients with severe lung lesions, developing ARDS, systemic cytokine release syndrome, and displaying elevated levels of IL-6 in laboratory research (National Health Commission and State Administration of Traditional Chinese Medicine, 2020; Biocentury, 2020).
2.3.1.2. Sintilimab
Sintilimab is a human anti-PD-1 antibody and has an immune-modulatory function in COVID-19 patients (Biocentury, 2020).
Considerable safety in mice inflicting a lethal MERS-CoV attack is attained by passive immunization with poorly and strongly neutralizing antibodies. These antibodies can thus signify a creative approach to strengthen humoral defense against emerging CoVs by targeting different epitopes and functions of S proteins. Regeneron is seeking to define mAbs as unique and efficient to COVID-19 (National Health Commission and State Administration of Traditional Chinese Medicine, 2020). Other studies have shown that routine therapy with intravenous immunoglobulin (IVIG) can decrease hospitalization, promote patient recovery and reduce ventilation usage. This form of therapy is in progress in clinical trials (NCT04261426) (Xie et al., 2020).
2.3.2. Corticosteroids and immune modulators
Systematic corticosteroids (methylprednisolone or dexamethasone) are recommended to alleviate inflammatory-induced lung damage (Chen et al., 2020c; National Health Commission and State Administration of Traditional Chinese Medicine, 2020). The dosage of glucocorticoids should be determined by the intensity of the systemic response, presence or absence of ARDS, the degree of shortness of breath, and the chest imaging findings. However, steroids and methylprednisolone tend to prolong viral shedding in patients with MERS-CoV, WHO suggests that this medication not be used for the treatment of COVID-19 unless, for patients with ARDS (Vetter et al., 2020; Dong et al., 2020).
2.3.3. Interferon-based COVID-19 therapy
Interferons are not advised for treating individuals with severe COVID-19, except in clinical studies. They can only reduce viral load during the initial phase of the infection, which may help to lessen symptoms and duration of the disease. Numerous clinical trials for various Type I interferon formulations (α-2b, β-1a, β-1b) and Type III (λ) interferon formulations have been registered so far (Outlaw et al., 2020). In one trial, nebulized interferon α-2b resulted in quicker viral clearance from the upper respiratory tract as well as decreased inflammatory processes (Calabrese et al., 2020). Interferon β-1a (together with hydroxychloroquine and Lopinavir-Ritonavir or Atazanavir-Ritonavir) resulted in a greater Day 14 discharge rate and reduced Day 28 mortality rate for severe COVID-19 in another research (Davoudi-Monfared et al., 2020). Interferon β -1b in combination with lopinavir-Ritonavir was found to be effective in easing symptoms and reducing viral shedding days and hospital stay in mild to moderate COVID-19 cases (Hung et al., 2020). In addition, prescribing peginterferonI-λ1 for COVID-19 outpatients improves viral clearance by Day 7, particularly in individuals with high baseline viral loads (Feld et al., 2020).
2.4. Broad-spectrum antibiotics
In addition to a single or combined treatment, antibiotics including azithromycin, cephalosporins, vancomycin, capreomycin, carbapenem, quinolones, and cefepime were administered to cover specific or atypical pathogens. Of necessity, antibiotics or antifungal medicines are crucial for patients suffering from secondary infection according to drug sensitivity and bacterial or fungal culture (Chen et al., 2020c; Clinicaltrials, 2020c).
2.5. Antimalarial agents
2.5.1. Chloroquine and hydroxychloroquine
Chloroquine and hydroxychloroquine are among antimalarial medicines that have antiviral activity against HIV, mainly by inhibiting the penetration of viruses into host cells. Another antiviral mechanism contributes to the post-translation modification of freshly synthesized proteins by inhibiting glycosylation (Rolain et al., 2007). A Systematic Review on both the effectiveness and protection of Chloroquine for COVID-19 treatment has shown that this drug appears to be successful in limiting SARS-CoV-2 replication (Cortegiani et al., 2020).
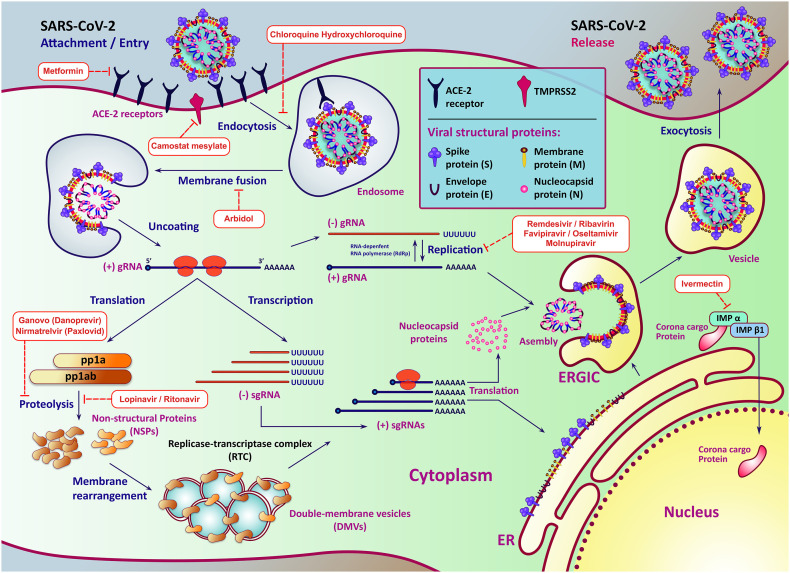
Chloroquine has also been suggested as an immune modulator as a potentially effective antiviral treatment for COVID-19 infections in China (Lim et al., 2020). Interestingly, in COVID-19 patients, a combination of Hydroxychloroquine (a less toxic analog of chloroquine) and azithromycin was shown to eradicate SARS-CoV-2 nasopharyngeal carriage (Gautret et al., 2020). Despite the foregoing, the WHO and FDA have classed this medicine as an unpromising therapy. They have ordered a clinical investigation due to its significant adverse effects on the heart and other organs (Corum et al., 2020; FasterCures, 2020). Fig. 1 depicts the SARS-CoV-2 life-cycle as well as the virus inhibitory mechanism of several major antiviral drugs.
Fig. 1.
SARS-CoV-2 life-cycle and the inhibitory mechanism of some very important antiviral drugs. After attachment to the receptor, the virus will next penetrate the cytosol of the host cell. The next process in the coronavirus lifespan involves the translation of the replicase gene from genomic RNA (gRNA) of the virion, which produces two co-terminal polyproteins, pp1a and pp1ab. Then, almost all the NSPs combine into the Replicase-Transcriptase Complex (RTC) to make an area appropriate for the synthesis of RNA and are conclusively responsible for the subgenomic RNA (sgRNA) replication and transcription. sgRNAs act as mRNAs for the structural and accessory genes existing downstream of the replicase polyprotein. Both gRNAs and sgRNAs are generated by negative-strand intermediate RNAs. The viral structural proteins, S, E, and M, are translated then introduced into the endoplasmic reticulum (ER) after replication and synthesis of the (+) sgRNA. Such proteins pass into the endoplasmic reticulum – Golgi intermediate compartment (ERGIC) along the secretory process. Then, the genome of the virus enclosed by N protein bud within ERGIS membranes, which consists of other viral structural proteins creating mature virions. The particles are transported through vesicles to the cell surface after assembly and released by exocytosis.
3. Promising vaccine modalities
A global outbreak spread across the globe in 2020 has led to substantial investment and focus on creating a vaccine (Gates, 2020). On January 11, 2020, the genetic sequence of SARS-CoV-2 was released, prompting intensified worldwide R&D effort to establish a vaccine against COVID-19 (Thanh et al., 2020). The Coalition for Epidemic Preparedness Innovations (CEPI) predicted a vaccine's production would cost $2 billion (CEPI, 2020).
Research groups around the globe have opted for various approaches that could lead to viable vaccines, many of which are now in clinical trials. The majority of the vaccines revolved around the Spike protein as it was responsible for cell penetration and demonstrated immunological value.
At least nine distinct technology platforms are being researched and developed to build a COVID-19 vaccine. The coronavirus spike protein and its variations are the major antigens of COVID-19 infection in most vaccine candidates in clinical trials. In general, The antiviral vaccines are based on one of the following categories: inactive (Whole-Virion-Killed vaccine), live attenuated viruses, non-replicating (recombinant vector vaccine/ viral vectors), virus-like particles (VLPs), Nucleic acid (DNA and RNA) vaccines, and protein-based (protein subunit: nanoparticle vaccine) vaccines (Liu et al., 2020).
Thirty-One vaccines have reflected high efficacy (overall roughly 90%) in phase III of clinical trials and were able to gain EUA by at least one official agency until January 11, 2022; however, only 10 out of the 31 vaccines were approved by WHO. These vaccines are either based on newly developed RNA vaccines (e.g., Moderna and Pfizer) or based on recombinant production of viral proteins which possess immunological importance (e.g., Sputnik V and Oxford/AstraZeneca). Alternatively, some contain inactivated forms of the virus (e.g., Sinopharm, Sinovac, and Bharat Biotech (Covaxin)), and some, such as Novavax and Covovax, contain isolated and purified viral proteins.
The authorized vaccines contain Nucleic acid (RNA) vaccines, protein Subunit vaccines, Inactivated vaccines, and Non-Replicating Viral vectors (see Table 1 ) (Tracker, 2021).
Table 1.
The vaccines granted EUA by WHO and/or approved by at least one nation.
| Vaccine name | Type | Status | Efficacy | Adverse effect(s) | Reference |
|---|---|---|---|---|---|
| Moderna | mRNA based | Approved | 94.1% | Pain, Swelling, Redness at the injection site, Chills or shivers, Headache, Fatigue | (Vejthani-Hospital, 2021) |
| Pfizer–BioNTech | mRNA based | Approved | 81.8–95% | Pain, Swelling, Redness at the injection site, Chills or shivers, Headache, Fatigue | (Vejthani-Hospital, 2021) |
| Bharat Biotech: Covaxin | Inactivated | Approved | 78% | Pain, Swelling, Redness at the injection site, Vomiting, Nausea, Headache, Fatigue | (Clinicaltrials, 2020a), (Biotech, 2020) |
| Sinopharm (Beijing) | Inactivated | Approved | 79.3% | Pain, Swelling, Redness at the injection site, Headache, Muscle pain, Rash, Fever | (Vejthani-Hospital, 2021) |
| Sinovac: CoronaVac | Inactivated | Approved | 49.6–50.7% | Pain at the injection site, Headache, Numbness | (Vejthani-Hospital, 2021) |
| Oxford/AstraZeneca | Non-Replicated Viral Vector | Approved | 70.4–82.4% | Pain at the injection site, Chills or shivers, Fever, Headache, Fatigue, Muscle pain, Thrombosis | (Vejthani-Hospital, 2021) |
| Janssen (Johnson & Johnson) | Non-Replicated Viral Vector | Approved | 66.9% | Pain at the injection site, Headache, Fever, Muscle pain, Fatigue, Nausea | (Clinicaltrials, 2021e), (Vejthani-Hospital, 2021) |
| Covishield | Non-Replicated Viral Vector | Approved | 70% | Swelling, Tenderness, Heat, Itching, Pain, Redness, Body pain, Cold, Mild fever, and headache | (MPNRC, 2022) |
| Novavax | Protein subunit | Approved | 89.3% | Pain at the injection site, Headache, Fever, Fatigue, Nausea, Swollen lymph nodes | (Vejthani-Hospital, 2021) |
| Covovax | Protein subunit | Approved | 96.4% | Local pain, Tenderness, Headache, Fatigue, Muscle pain/myalgia, and fever. | (Translation, 2021) |
| ZF2001 | Protein subunit | Phase III | Fever, Headache, Fatigue, Nausea, Vomiting, Diarrhea, Muscle pain (non-inoculated site), Cough, Acute allergic reactions. | (Clinicaltrials, 2021c) | |
| BECOV2A | Protein subunit | Phase III | – | Swelling, Irritability, Pain, Sweating, Body ache, Mild fever, and headache | (http://ctri.nic.in/, 2022), (Edudwar, 2022) |
| CanSino:Ad5-nCoV | Non-Replicated Viral Vector | Phase III | 68.83% | Fever, Redness, Swelling, and pain in the vaccination site | (Clinicaltrials, 2021b), (Wego, 2021) |
| CIGB-66 | Protein subunit | Phase III | – | Fever | (RPCEC, 2021a), (Nature, 2021a) |
| KoviVac | Inactivated | Phase I/II | – | – | (Edudwar, 2022) |
| EpiVacCorona | Protein subunit | Phase III | 79% | – | (Clinicaltrials, 2021g), (RUSSIAN-NEWS-AGENCY, 2021) |
| Sputnik Light | Non-Replicated Viral Vector | Phase III | 79.4% | Mild pain at the injection site, Fever, Headaches, Fatigue, Muscle aches | (Clinicaltrials, 2021h), (Today, 2021) |
| Sputnik V | Non-Replicated Viral Vector | Phase III | 91.1–91.5% | Pain, Swelling, Redness at the injection site, Headache, Muscle pain, Rash, Fatigue | (Clinicaltrials, 2021a), (Vejthani-Hospital, 2021) |
| ERUCOV-VAC | Inactivated | Phase III | – | – | (Clinicaltrials, 2022a) |
| Soberana 02 | Protein subunit | Phase III | 71.0% | Mild pain at the injection site, Intermittent Headache, Fever, and general malaise | (RPCEC, 2021b), (Medrxiv, 2021), (Exterior, 2021) |
| Soberana Plus | Protein subunit | Phase III | 92.4% | Local pain and redness in the injection Site, Mild general malaise | (RPCEC, 2021b), (Finlay, 2021) |
| QazVac | Inactivated | Phase III | 70%–96% | Mild pain at the injection site, Fever | (Clinicaltrials, 2021d), (Zakarya et al., 2021) |
| MVC-COV1901 | Protein subunit | Phase III | 80%–90% | Mild pain at the injection site, Malaise or fatigue, Fever | (Clinicaltrials, 2022c), (Hsieh et al., 2021) |
| KCONVAC | Inactivated | Phase III | – | – | (Clinicaltrials, 2021i) |
| FAKHRAVAC (MIVAC) | Inactivated | Phase III | – | – | (IRCT, 2021a) |
| Razi Cov Pars | Protein subunit | Phase III | 90% | Not serious | (IRCT, 2021b), (Tehran-Times, 2021) |
| COVIran Barekat | Inactivated | Phase II/III | 79% | Not serious | (IRCT, 2021c), (Iran-Press, 2021) |
| Sinopharm (Wuhan) | Inactivated | Phase III | – | – | (ChiCTR, 2020) |
| Takeda: TAK-919 | mRNA based | Phase II/III | – | – | (Clinicaltrials, 2021f) |
| COVAX-19 | Protein subunit | Phase III | 60% | Not serious | (Clinicaltrials, 2022b), (Arena, 2021) |
| ZyCoV-D | DNA based | Phase III | 67% | Not serious | (CTRI, 2021), (Nature, 2021b) |
3.1. RNA vaccines
mRNA vaccines gained immediate attention from the scientific communities as they showed outstanding outcomes and were the first of their kind. The mRNAs which contain the information to code target proteins are encapsulated in lipid nanoparticles that have been specifically designed to be absorbed by antigen-presenting cells. Ensuing absorption, the mRNAs are subject to translations, and the polypeptides are presented to immune cells via MHC molecules. One of the main features of mRNA vaccines is the use of non-canonical nucleic acids, which confer high stability. The Moderna and Pfizer–BioNTech vaccines are the only two commercially available mRNA-based vaccines that illustrated high performance in the face of all SARS-CoV-2 variants.
When RNA is injected into a tissue, it acts as messenger RNA (mRNA), causing the cells to synthesize the foreign protein and triggering an adaptive immune response. The coformulation of mRNA into lipid nanoparticles protects the RNA strands and aids their uptake into cells (Krammer, 2020; Park et al., 2021). The first COVID-19 vaccines to be approved in the United Kingdom, the United States, and the European Union were RNA vaccines (CDC, 2021). The Pfizer–BioNTech COVID-19 vaccine and the Moderna COVID-19 vaccine are both approved vaccines of this sort (Tracker, 2021).
3.2. Inactivated vaccines
Here, although the whole viral particles are used, they lose their virulence after they are killed via heat subjection or formaldehyde supplementation while maintaining their propensity to elicit a sufficient level of immune response. This is the most traditional way of producing vaccines, and many of the vaccines in the market contain inactivated viral particles such as Bharat and Sinopharm.
Authorized vaccines of this type are the Bharat Biotech: Covaxin, Sinopharm (Beijing): BBIBP-CorV, Sinopharm (Wuhan), Sinovac: CoronaVac, Covaxin, CoviVac, Shifa Pharmed Industrial Co and QazCovid-in) (Tracker, 2021).
3.3. Non-replicating viral vector vaccines
This form of vaccine entails injecting viral-based expression vectors, yet incapable of producing any form of a virus, harboring a payload DNA that codes for a target protein capable of inducing the immune response. Several commercial COVID-19 vaccines such as Oxford/AstraZeneca, Janssen (Johnson & Johnson), and Sputnik V are produced accordingly. Although they all encode the whole Spike protein of the virus, they differ in the type of viral vector, administration dose, and immunization schedule.
An adenovirus shell carrying DNA that encodes a SARS-CoV-2 protein is used in these vaccines. The COVID-19 vaccines based on viral vectors are non-replicating, which means they do not produce new virus particles but instead produce the antigen, which evokes a systemic immune response (Motamedi et al., 2021). Four companies (Astra Zeneca/University of Oxford (AZ/Ox), CanSino Biologics, Gamaleya Research Institute, and Johnson & Johnson/Janssen (J&J)) have used non-replicating adenoviral vectors to drive the production of the full-length SARS-CoV-2 spike glycoprotein in order to elicit an immunological response. The chosen vector strain and immunization schedules are the distinctions between them.
The Oxford/AstraZeneca vaccine exploits adenoviral vectors derived from chimpanzees. As the vector is derived from another species, it ensures that no illness is caused following immunization. CanSino (Ad5-nCoV) is based on the Ad5 human adenovirus vectors, and the Johnson/Janssen makes use of a relatively rare adenovirus (Ad26) among humans. In contrast, Sputnik V is constituted of a combination of Ad26 and Ad5 viral vectors. Of note, Johnson/Janssen vaccine, in contrast to others, does not follow the prime-boost immunization scheme.
Except for J&J, everyone uses a prime-boost technique. The adenoviral vector used in AZ/Ox (also known as AZ1222 or Covishield in India) infects chimps but not humans. J&J (Ad26.COV2.S) employs a mutant adenovirus Ad26 that is not generally (or only rarely) encountered by the human population, whereas CanSino (Ad5-nCoV) uses a recombinant form of Ad5 adenovirus that naturally transmits in people. Gamaleya (Sputnik V) employs a mix of prime and boost with Ad26 and Ad5 (Funk et al., 2021).
3.4. Protein subunit vaccines
Here, instead of employing the whole viral particles, protein antigens of the target virus are recombinantly produced and processed prior to injection and immunization. This allows the immune cells to recognize the immunogenic epitopes, either conformational or linear epitopes depending on the vaccine design, of the protein antigen and respond accordingly. ZF2001, Abdala, EpiVacCorona, and MVC-COV1901 vaccines are among this type.
Among them, Moderna, Pfizer/BioNTech, Janssen (Johnson & Johnson), Oxford/AstraZeneca, Covishield (Oxford/AstraZeneca formulation), Sinopharm, Sinovac, Novavax, Covovax, and Covaxin Approved for Use by WHO. In total, 326 vaccine candidates are in various stages of development, with 103 in clinical research, including 30 in Phase I trials, 30 in Phase I–II trials, 10 in Phase II, 25 in Phase III trials, and 8 in Phase IV development (Shrotri et al., 2021; Tracker, 2021).
4. Conclusion
A number of treatment options are available for COVID-19 to prevent its spread and limit its morbidity and mortality. The present review paper covers the latest information on the emergence of the COVID-19 treatment and/or vaccine. Since the safety profile of antiviral and immunomodulatory drugs is known, repurposing them is a good strategy. SARS-CoV-2 has, however, underscored yet again the urgent need for developing broad-spectrum antiviral drugs to prevent further infection both for coronaviruses and other viral infections in the future. To assess the potential benefit for the patients undergoing COVID-19 treatment, it is imperative that clinical trials be transparent and ultimately reported. Moreover, preclinical and clinical therapy studies need to be based on clear in vitro and in vivo scientific evidence. However, studies suggest that specific antiviral drugs from previous coronavirus forms may be efficient toward SARS-CoV-2.
Five vaccine firms have conducted clinical trials on SARS-CoV-2 up to this point. Even though the SARS-CoV-2 virus is spreading, vaccinated people will experience a limited number of infections. The number of infections does not determine a vaccine's effectiveness. Every vaccine is only partially effective, but the effectiveness of COVID-19 is relatively high. Furthermore, vaccination prevents new strains while protecting those at the highest risk of severe illness. There are also possibilities that these vaccines will invoke long-term immunity and defend humans from the disease of this novel coronavirus. As long as the SARS-CoV-2 virus continues to circulate, a limited number of infections will occur in people who have completed the recommended vaccination schedule.
Declaration of competing interest
The authors have no conflicts of interest to declare.
Edited by May Yin LEE
References
- Administration U.S.F.A.D. Vol. 100. 2021. Emergency Use Authorization 100 [Online]. Available: Emergency Use Authorization. [Google Scholar]
- Administration U.S.F.A.D. Emergency Use Authorization 091 [Online] 2022. https://www.fda.gov/media/145610/download Available:
- Administration U.S.F.A.D. Emergency Use Authorization 094 [Online] 2022. https://www.fda.gov/media/145801/download Available:
- Ahmad B., Batool M., Ain Q.U., Kim M.S., Choi S. Exploring the binding mechanism of PF-07321332 SARS-CoV-2 protease inhibitor through molecular dynamics and binding free energy simulations. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22179124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S., Karim M.M., Ross A.G., Hossain M.S., Clemens J.D., Sumiya M.K., Phru C.S., Rahman M., Zaman K., Somani J.J.I.J.O.I.D. A Five-day Course of Ivermectin for the Treatment of COVID-19 May Reduce the Duration of Illness. Vol. 103. 2021. pp. 214–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alam S., Kamal T., Sarker M., Zhou J., Rahman S., Mohamed I.J.F.I.P., Org W.F. Therapeutic effectiveness and safety of repurposing drugs for the treatment of COVID-19: position standing in 2021. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.659577.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arabi Y.M., Murthy S., Webb S. COVID-19: a novel coronavirus and a novel challenge for critical care. Intensive Care Med. 2020:1–4. doi: 10.1007/s00134-020-05955-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arena C.T. Vaxine to file for Australian approval of protein subunit Covid-19 vaccine [Online] 2021. https://www.clinicaltrialsarena.com/analysis/vaxine-australia-approval-covid-19-vaccine/
- Azad M., Kaviani S., Soleimani M., Noruzinia M., Hajfathali A. Common polymorphism’s analysis of thiopurine S-methyltransferase (TPMT) in Iranian population. Cell J. 2009;11(3):311–316. [Google Scholar]
- Azouz N.P., Klingler A.M., Callahan V., Akhrymuk I.V., Elez K., Raich L., Henry B.M., Benoit J.L., Benoit S.W., Noe F., Kehn-Hall K., Rothenberg M.E. bioRxiv; 2020. Alpha 1 Antitrypsin Is an Inhibitor of the SARS-CoV-2-Priming Protease TMPRSS2. 2020.05.04.077826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baby K., Maity S., Mehta C.H., Suresh A., Nayak U.Y., Nayak Y. SARS-CoV-2 entry inhibitors by dual targeting TMPRSS2 and ACE2: an in silico drug repurposing study. Eur. J. Pharmacol. 2021;896 doi: 10.1016/j.ejphar.2021.173922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur A., Harrer T., Peukert M., Jahn G., Kalden J., Fleckenstein B. Alpha-lipoic acid is an effective inhibitor of human immuno-deficiency virus (HIV-1) replication. Klin. Wochenschr. 1991;69:722–724. doi: 10.1007/BF01649442. [DOI] [PubMed] [Google Scholar]
- Beddingfield B.J., Iwanaga N., Chapagain P.P., Zheng W., Roy C.J., Hu T.Y., Kolls J.K., Bix G.J. The integrin binding peptide, ATN-161, as a novel therapy for SARS-CoV-2 infection. JACC Basic Transl. Sci. 2021;6:1–8. doi: 10.1016/j.jacbts.2020.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bestle D., Heindl M.R., Limburg H., Van Lam Van T., Pilgram O., Moulton H., Stein D.A., Hardes K., Eickmann M., Dolnik O., Rohde C., Klenk H.-D., Garten W., Steinmetzer T., Böttcher-Friebertshäuser E. Vol. 3. 2020. TMPRSS2 and Furin Are Both Essential for Proteolytic Activation of SARS-CoV-2 in Human Airway Cells. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biocentury First up for COVID-19: nearly 30 clinical readouts before end of April [Online] 2020. https://www.biocentury.com/article/304658/nearly-30-trials-for-covid-19-could-start-to-yield-data-in-the-next-couple-of-months Available:
- Biotech B. Covaxin, SARS-CoV-2 vaccine by Bharat Biotech [Online] 2020. https://www.bharatbiotech.com/images/covaxin/covaxin-factsheet2.pdf Available:
- Biswas A., Bhattacharjee U., Chakrabarti A.K., Tewari D.N., Banu H., Dutta S. Emergence of novel coronavirus and COVID-19: whether to stay or die out? Crit. Rev. Microbiol. 2020;46:182–193. doi: 10.1080/1040841X.2020.1739001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonam S.R., Kaveri S.V., Sakuntabhai A., Gilardin L., Bayry J. Adjunct immunotherapies for the management of severely ill COVID-19 patients. Cell Rep. Med. 2020;1 doi: 10.1016/j.xcrm.2020.100016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boriskin Y., Leneva I., Pecheur E.-I., Polyak S. Arbidol: a broad-spectrum antiviral compound that blocks viral fusion. Curr. Med. Chem. 2008;15:997–1005. doi: 10.2174/092986708784049658. [DOI] [PubMed] [Google Scholar]
- Businesswire Pfizer Receives U.S. FDA Emergency Use Authorization for Novel COVID-19 Oral Antiviral Treatment [Online] 2022. https://www.businesswire.com/news/home/20211221005795/en/Pfizer-Receives-U.S.-FDA-Emergency-Use-Authorization-for-Novel-COVID-19-Oral-Antiviral-Treatment Available:
- Calabrese L.H., Lenfant T., Calabrese C. Interferon therapy for COVID-19 and emerging infections: prospects and concerns. Cleve. Clin. J. Med. 2020;87 doi: 10.3949/ccjm.87a.ccc066. PMID: 33219050. [DOI] [PubMed] [Google Scholar]
- Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G., Ruan L., Song B., Cai Y., Wei M., Li X., Xia J., Chen N., Xiang J., Yu T., Bai T., Xie X., Zhang L., Li C., Yuan Y., Chen H., Li H., Huang H., Tu S., Gong F., Liu Y., Wei Y., Dong C., Zhou F., Gu X., Xu J., Liu Z., Zhang Y., Li H., Shang L., Wang K., Li K., Zhou X., Dong X., Qu Z., Lu S., Hu X., Ruan S., Luo S., Wu J., Peng L., Cheng F., Pan L., Zou J., Jia C., Wang J., Liu X., Wang S., Wu X., Ge Q., He J., Zhan H., Qiu F., Guo L., Huang C., Jaki T., Hayden F.G., Horby P.W., Zhang D., Wang C. A trial of lopinavir-ritonavir in adults hospitalized with severe Covid-19. N. Engl. J. Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L., Goreshnik I., Coventry B., Case J.B., Miller L., Kozodoy L., Chen R.E., Carter L., Walls A.C., Park Y.-J., Strauch E.-M., Stewart L., Diamond M.S., Veesler D., Baker D. De Novo Design of Picomolar SARS-CoV-2 Miniprotein Inhibitors. Vol. 370. 2020. pp. 426–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC COVID-19 ACIP Vaccine Recommendations [Online] 2021. https://www.cdc.gov/vaccines/hcp/acip-recs/vacc-specific/covid-19.html Available:
- CEPI COVID-19 vaccine doses shipped by the COVAX Facility head to Ghana, marking beginning of global rollout [Online] 2020. https://cepi.net/covid-19/ Available:
- Chen H., Zhang Z., Wang L., Huang Z., Gong F., Li X., Chen Y., Wu J.J. First clinical study using HCV protease inhibitor danoprevir to treat COVID-19 patients. Medicine. 2020;99 doi: 10.1097/MD.0000000000023357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Xiong J., Bao L., Shi Y. Convalescent plasma as a potential therapy for COVID-19. Lancet Infect. Dis. 2020;20:398–400. doi: 10.1016/S1473-3099(20)30141-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Li R., Pan Z., Qian C., Yang Y., You R., Zhao J., Liu P., Gao L., Li Z., Huang Q., Xu L., Tang J., Tian Q., Yao W., Hu L., Yan X., Zhou X., Wu Y., Deng K., Zhang Z., Qian Z., Chen Y., Ye L. Human monoclonal antibodies block the binding of SARS-CoV-2 spike protein to angiotensin converting enzyme 2 receptor. Cell Mol. Immunol. 2020;17:647–649. doi: 10.1038/s41423-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y.W., Chao T.L., Li C.L., Chiu M.F., Kao H.C., Wang S.H., Pang Y.H., Lin C.H., Tsai Y.M., Lee W.H., Tao M.H., Ho T.C., Wu P.Y., Jang L.T., Chen P.J., Chang S.Y., Yeh S.H. Furin inhibitors block SARS-CoV-2 spike protein cleavage to suppress virus production and cytopathic effects. Cell Rep. 2020;33 doi: 10.1016/j.celrep.2020.108254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CHICTR A Phase III clinical trial for inactivated novel coronavirus pneumonia (COVID-19) vaccine (Vero cells) [Online] 2020. http://www.chictr.org.cn/showprojen.aspx?proj=56651
- Chu C.M., Cheng V.C., Hung I.F., Wong M.M., Chan K.H., Chan K.S., Kao R.Y., Poon L.L., Wong C.L., Guan Y., Peiris J.S., Yuen K.Y., Group H.U.S.S. Role of lopinavir/ritonavir in the treatment of SARS: initial virological and clinical findings. Thorax. 2004;59:252–256. doi: 10.1136/thorax.2003.012658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ClinicalTrials n Efficacy and Safety Clinical Trial of an Investigational COVID-19 Vaccine (BBV152) in Adult Volunteers [Online] 2020. https://clinicaltrials.gov/ct2/show/NCT04641481 Available:
- ClinicalTrials Ganovo (Danoprevir) [Online] 2020. https://clinicaltrials.gov/ct2/show/NCT04291729
- ClinicalTrials Proactive Prophylaxis With Azithromycin and Chloroquine in Hospitalized Patients With COVID-19 (ProPAC-COVID) [Online] 2020. https://clinicaltrials.gov/ct2/show/NCT04322396 Available: [DOI] [PMC free article] [PubMed]
- ClinicalTrials Clinical Trial of Efficacy, Safety, and Immunogenicity of Gam-COVID-Vac Vaccine Against COVID-19 (RESIST) [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT04530396 Available:
- ClinicalTrials Clinical Trial of Recombinant Novel Coronavirus Vaccine (Adenovirus Type 5 Vector) Against COVID-19 [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT04540419 Available:
- ClinicalTrials Clinical Trials of the Consistency and Non-inferiority Bridging Between Batches of Recombinant New Coronavirus Vaccine (CHO Cells) [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT05091411 Available:
- ClinicalTrials Immunogenicity, Efficacy and Safety of QazCovid-in® COVID-19 Vaccine [Online] 2021. https://www.clinicaltrials.gov/ct2/show/NCT04691908?term=NCT04691908&draw=2&rank=1 Available:
- ClinicalTrials Sisonke (Together): OPEN LABEL TRIAL COVID-19 (Sisonke) [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT04838795 Available:
- ClinicalTrials A Study of TAK-919 in Healthy Japanese Adults (COVID-19) [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT04677660 Available:
- ClinicalTrials Study of the Tolerability, Safety, Immunogenicity and Preventive Efficacy of the EpiVacCorona Vaccine for the Prevention of COVID-19 [Online] 2021. https://www.clinicaltrials.gov/ct2/show/NCT04780035?term=vaccine&cond=Covid19&draw=2 Available:
- ClinicalTrials Study to Evaluate Efficacy, Immunogenicity and Safety of the Sputnik-Light (SPUTNIK-LIGHT) [Online] 2021. https://www.clinicaltrials.gov/ct2/show/NCT04741061?id=NCT04733807+OR+NCT04718467+OR+NCT04706156+OR+NCT04743947+OR+NCT04715997+OR+NCT04732468+OR+NCT04713488+OR+NCT04741061&draw=2&rank=2&load=cart Available:
- ClinicalTrials A Study to Evaluate the Efficacy, Safety and Immunogenicity of SARS-CoV-2 Vaccine (Vero Cells), Inactivated in Healthy Adults Aged 18 Years and Older (COVID-19) [Online] 2021. https://clinicaltrials.gov/ct2/show/NCT04852705?term=vaccine&recrs=abdf&cond=COVID-19&phase=0123&sort=nwst&draw=2 Available:
- ClinicalTrials Efficacy, Immunogenicity, and Safety of the Inactivated COVID-19 Vaccine (TURKOVAC) Versus the CoronaVac Vaccine [Online] 2022. https://www.clinicaltrials.gov/ct2/show/NCT04942405
- ClinicalTrials Phase III Clinical Trial of CinnaGen COVID-19 Vaccine (SpikoGen) [Online] 2022. https://clinicaltrials.gov/ct2/show/NCT05005559 Available:
- ClinicalTrials A Study to Evaluate Immunogenicity and Safety of MVC-COV1901 Compared With AZD1222 Against COVID-19 in Adults [Online] 2022. https://clinicaltrials.gov/ct2/show/NCT05011526 Available:
- Cortegiani A., Ingoglia G., Ippolito M., Giarratano A., Einav S. A systematic review on the efficacy and safety of chloroquine for the treatment of COVID-19. J. Crit. Care. 2020;57:279–283. doi: 10.1016/j.jcrc.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corum J., Wu K.J., Zimmer C. The New York Times; 2020. Coronavirus Drug and Treatment Tracker. [Google Scholar]
- CTRI Trial Registration [Online] 2021. http://ctri.nic.in/Clinicaltrials/showallp.php?mid1=51254&EncHid=&userName=ZyCoV-D Available:
- Davies B.E. Pharmacokinetics of oseltamivir: an oral antiviral for the treatment and prophylaxis of influenza in diverse populations. J. Antimicrob. Chemother. 2010;65 Suppl 2:ii5-ii10. doi: 10.1093/jac/dkq015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davoudi-Monfared E., Rahmani H., Khalili H., Hajiabdolbaghi M., Salehi M., Abbasian L., Kazemzadeh H., Yekaninejad M.S. A randomized clinical trial of the efficacy and safety of interferon beta-1a in treatment of severe COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.01061-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong L., Hu S., Gao J. Discovering drugs to treat coronavirus disease 2019 (COVID-19) Drug Discov. Ther. 2020;14:58–60. doi: 10.5582/ddt.2020.01012. [DOI] [PubMed] [Google Scholar]
- Dragomanova S., Miteva S., Nicoletti F., Mangano K., Fagone P., Pricoco S., Staykov H., Tancheva L. Therapeutic potential of alpha-lipoic acid in viral infections, including COVID-19. Antioxidants (Basel) 2021;10 doi: 10.3390/antiox10081294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DrugBank Danoprevir [Online] 2020. https://www.drugbank.ca/drugs/DB11779 Available:
- Edudwar Biological E Vaccine (Corbevax) Registration: Dates, Efficacy, Doses Cost, Side Effects [Online] 2022. https://www.edudwar.com/biological-e-vaccine-corbevax-registration/ Available:
- Exterior R.D.D.C.E.E. Cuba's Soberana Plus against Covid-19 is showing good results [Online] 2021. http://misiones.minrex.gob.cu/en/articulo/cubas-soberana-plus-against-covid-19-showing-good-results
- Fastercures M.I. COVID-19 treatment and vaccine tracker [Online] 2020. https://covid-19tracker.milkeninstitute.org Available:
- FDA Convalescent Plasma EUA Letter of Authorization [Online]. Available. 2021. https://www.fda.gov/media/141477/download
- Feld J.J., Kandel C., Biondi M.J., Kozak R.A., Zahoor M.A., Lemieux C., Borgia S.M., Boggild A.K., Powis J., McCready J. medRxiv; 2020. Peginterferon-lambda for the Treatment of COVID-19 in Outpatients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finlay SOBERANA®Plus [Online] 2021. https://www.finlay.edu.cu/blog/wp-content/uploads/2021/12/Commercial-file-SOBERANA-Plus-Eng.pdf
- Funk C.D., Laferriere C., Ardakani A. Target product profile analysis of COVID-19 vaccines in phase III clinical trials and beyond: an early 2021 perspective. Viruses. 2021;13:418. doi: 10.3390/v13030418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuta Y., Takahashi K., Shiraki K., Sakamoto K., Smee D.F., Barnard D.L., Gowen B.B., Julander J.G., Morrey J.D. T-705 (favipiravir) and related compounds: novel broad-spectrum inhibitors of RNA viral infections. Antivir. Res. 2009;82:95–102. doi: 10.1016/j.antiviral.2009.02.198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gates B. Responding to Covid-19 - a once-in-a-century pandemic? N. Engl. J. Med. 2020;382:1677–1679. doi: 10.1056/NEJMp2003762. Available: [DOI] [PubMed] [Google Scholar]
- Gautret P., Lagier J.-C., Parola P., Meddeb L., Mailhe M., Doudier B., Courjon J., Giordanengo V., Vieira V.E., Dupont H.T. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int. J. Antimicrob. Agents. 2020:105949. doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E. BioRxiv; 2020. Severe Acute Respiratory Syndrome-related Coronavirus–The Species and Its Viruses, a Statement of the Coronavirus Study Group. Available: [Google Scholar]
- Gurung A.B., Ali M.A., Lee J., Farah M.A., Al-Anazi K.M. An updated review of computer-aided drug design and its application to COVID-19. Biomed. Res. Int. 2021;2021:8853056. doi: 10.1155/2021/8853056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Kleine-Weber H., Schroeder S., Kruger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Muller M.A., Drosten C., Pohlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(271–280) doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M., Schroeder S., Kleine-Weber H., Muller M.A., Drosten C., Pohlmann S. Nafamostat mesylate blocks activation of SARS-CoV-2: new treatment option for COVID-19. Antimicrob. Agents Chemother. 2020;64 doi: 10.1128/AAC.00754-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsieh S.M., Liu M.C., Chen Y.H., Lee W.S., Hwang S.J., Cheng S.H., Ko W.C., Hwang K.P., Wang N.C., Lee Y.L., Lin Y.L., Shih S.R., Huang C.G., Liao C.C., Liang J.J., Chang C.S., Chen C., Lien C.E., Tai I.C., Lin T.Y. Safety and immunogenicity of CpG 1018 and aluminium hydroxide-adjuvanted SARS-CoV-2 S-2P protein vaccine MVC-COV1901: interim results of a large-scale, double-blind, randomised, placebo-controlled phase 2 trial in Taiwan. Lancet Respir. Med. 2021;9:1396–1406. doi: 10.1016/S2213-2600(21)00402-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- http://ctri.nic.in/ Trial Registeration [Online] 2022. http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=59772&EncHid=&userName=corbevax Available:
- Hung I.F., Lung K.C., Tso E.Y., Liu R., Chung T.W., Chu M.Y., Ng Y.Y., Lo J., Chan J., Tam A.R., Shum H.P., Chan V., Wu A.K., Sin K.M., Leung W.S., Law W.L., Lung D.C., Sin S., Yeung P., Yip C.C., Zhang R.R., Fung A.Y., Yan E.Y., Leung K.H., Ip J.D., Chu A.W., Chan W.M., Ng A.C., Lee R., Fung K., Yeung A., Wu T.C., Chan J.W., Yan W.W., Chan W.M., Chan J.F., Lie A.K., Tsang O.T., Cheng V.C., Que T.L., Lau C.S., Chan K.H., To K.K., Yuen K.Y. Triple combination of interferon beta-1b, lopinavir-ritonavir, and ribavirin in the treatment of patients admitted to hospital with COVID-19: an open-label, randomised, phase 2 trial. Lancet. 2020;395:1695–1704. doi: 10.1016/S0140-6736(20)31042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussman J.P. Cellular and molecular pathways of COVID-19 and potential points of therapeutic intervention. Front. Pharmacol. 2020;11:1169. doi: 10.3389/fphar.2020.01169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Indari O., Jakhmola S., Manivannan E., Jha H.C. An Update on Antiviral Therapy Against SARS-CoV-2: How Far Have We Come? Front. Pharmacol. 2021;12:632–677. doi: 10.3389/fphar.2021.632677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iran-Press COV-Iran Barakat has no side effect: Official [Online] 2021. https://iranpress.com/content/40144/cov-iran-barakat-has-side-effect-official Available:
- IRCT Comparison of the safety and efficacy of Fakhravac and Sinopharm SARS-CoV-2 vaccines, in adults aged 18 and over; a phase III randomized, non-inferiority clinical trial [Online] 2021. https://en.irct.ir/trial/57980 Available:
- IRCT Comparison of the safety and efficacy of Razi SARS-CoV-2 recombinant Spike protein (Razi Cov Pars) and Sinopharm vaccines in adults aged 18 and over, a phase III randomised, double blind, non-inferiority clinical trial [Online] 2021. https://www.irct.ir/trial/58143 Available:
- IRCT A double-blind, randomized, placebo-controlled Phase II/III Clinical trial to evaluate the safety and efficacy of COVID-19 inactivated vaccine (Shifa-Pharmed) in a population aged 18 to 75 years [Online] 2021. https://en.irct.ir/trial/54881 Available:
- Kandeel M., Yamamoto M., Al-Taher A., Watanabe A., Oh-Hashi K., Park B.K., Kwon H.J., Inoue J.I., Al-Nazawi M. Small molecule inhibitors of Middle East respiratory syndrome coronavirus fusion by targeting cavities on heptad repeat trimers. Biomol. Ther. (Seoul) 2020;28:311–319. doi: 10.4062/biomolther.2019.202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandeel M., Yamamoto M., Tani H., Kobayashi A., Gohda J., Kawaguchi Y., Park B.K., Kwon H.J., Inoue J.I., Alkattan A. Discovery of new fusion inhibitor peptides against SARS-CoV-2 by targeting the spike S2 subunit. Biomol. Ther. (Seoul) 2021;29:282–289. doi: 10.4062/biomolther.2020.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan Z., Karataş Y., Ceylan A., Rahman H. COVID-19 and Therapeutic Drugs Repurposing in Hand: The Need for Collaborative Efforts. Le Pharmacien Hospitalier et Clinicien. 2021;56:3–11. [Google Scholar]
- Kim P.S., Read S.W., Fauci A.S. Therapy for early COVID-19: a critical need. JAMA. 2020;324:2149–2150. doi: 10.1001/jama.2020.22813. [DOI] [PubMed] [Google Scholar]
- Kramer D.G., Da Silva M.J.L., Da Silva G.S.E., De Moura A.M.M.A., Junior G.B.C., De Sousa A.M., Da Silva A.E.A. Favipiravir as a potential drug in the treatment of COVID-19. Int. J. Res. Granthaalayah. 2020;8:7–12. [Google Scholar]
- Krammer F. SARS-CoV-2 vaccines in development. Nature. 2020;586:516–527. doi: 10.1038/s41586-020-2798-3. Available: [DOI] [PubMed] [Google Scholar]
- Lei C., Qian K., Li T., Zhang S., Fu W., Ding M., Hu S. Neutralization of SARS-CoV-2 spike pseudotyped virus by recombinant ACE2-ig. Nat. Commun. 2020;11:2070. doi: 10.1038/s41467-020-16048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim J., Jeon S., Shin H.Y., Kim M.J., Seong Y.M., Lee W.J., Choe K.W., Kang Y.M., Lee B., Park S.J. Case of the index patient who caused tertiary transmission of COVID-19 infection in Korea: the application of Lopinavir/Ritonavir for the treatment of COVID-19 infected pneumonia monitored by quantitative RT-PCR. J. Korean Med. Sci. 2020;35 doi: 10.3346/jkms.2020.35.e79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S. ACS Publications; 2020. Research and Development on Therapeutic Agents and Vaccines for COVID-19 and Related Human Coronavirus Diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci. Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Luo P., Qiu L., Liu Y., Liu X.-L., Zheng J.-L., Xue H.-Y., Liu W.-H., Liu D., Li J. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am. J. Trop. Med. Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. tpmd200375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luoliu P., Li J. Pharmacologic perspective: glycyrrhizin may be an efficacious therapeutic agent for COVID-19. Int. J. Antimicrob. Agents. 2020;105995 doi: 10.1016/j.ijantimicag.2020.105995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGowan E.M., Haddadi N., Nassif N.T., Lin Y. Targeting the SphK-S1P-SIPR Pathway as a Potential Therapeutic Approach for COVID-19. Int. J. Mol. Sci. 2020;21:7189. doi: 10.3390/ijms21197189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrxiv . 2021. Efficacy and Safety of SOBERANA 02, a COVID-19 Conjugate Vaccine in Heterologous Three Doses Combination. [Google Scholar]
- Mohamed A.E.-A.T., Al-Sabi A., Stockand J.D. Human recombinant soluble ACE2 (hrsACE2) shows promise for treating severe COVID19. 2020;5 doi: 10.1038/s41392-020-00374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motamedi H., Ari M.M., Dashtbin S., Fathollahi M., Hossainpour H., Alvandi A., Moradi J., Abiri R. An update review of globally reported SARS-CoV-2 vaccines in preclinical and clinical stages. Int. Immunopharmacol. 2021;96 doi: 10.1016/j.intimp.2021.107763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MPNRC Covishield vaccine Registration, Efficacy rate, Side Effects, Dose Gap [Online] 2022. https://www.mpnrc.org/covishield-vaccine-registration/ Available:
- National Health Commission and State Administration of Traditional Chinese Medicine Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7) [Online] 2020. http://www.kankyokansen.org/uploads/uploads/files/jsipc/protocol_V7.pdf Available:
- Nature Cuba's bet on home-grown COVID vaccines is paying off [Online] 2021. https://www.nature.com/articles/d41586-021-03470-x Available: [DOI] [PubMed]
- Nature India's DNA COVID vaccine is a world first – more are coming [Online] 2021. https://www.nature.com/articles/d41586-021-02385-x Available: [DOI] [PubMed]
- Ohashi H., Watashi K., Saso W., Shionoya K., Iwanami S., Hirokawa T., Shirai T., Kanaya S., Ito Y., Kim K.S. bioRxiv; 2020. Multidrug Treatment With Nelfinavir and Cepharanthine Against COVID-19. [Google Scholar]
- Oldfield V., Plosker G.L. Lopinavir/ritonavir: a review of its use in the management of HIV infection. Drugs. 2006;66:1275–1299. doi: 10.2165/00003495-200666090-00012. [DOI] [PubMed] [Google Scholar]
- Ou X., Liu Y., Lei X., Li P., Mi D., Ren L., Guo L., Guo R., Chen T., Hu J., Xiang Z., Mu Z., Chen X., Chen J., Hu K., Jin Q., Wang J., Qian Z. Characterization of spike glycoprotein of SARS-CoV-2 on virus entry and its immune cross-reactivity with SARS-CoV. Nat. Commun. 2020;11:1620. doi: 10.1038/s41467-020-15562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Outlaw V.K., Bovier F.T., Mears M.C., Cajimat M.N., Zhu Y., Lin M.J., Addetia A., Lieberman N.A.P., Peddu V., Xie X., Shi P.Y., Greninger A.L., Gellman S.H., Bente D.A., Moscona A., Porotto M. Inhibition of coronavirus entry in vitro and ex vivo by a lipid-conjugated peptide derived from the SARS-CoV-2 spike glycoprotein HRC domain. MBio. 2020;11 doi: 10.1128/mBio.01935-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter W.P., Holman W., Bush J.A., Almazedi F., Malik H., Eraut N., Morin M.J., Szewczyk L.J., Painter G.R. Human safety, tolerability, and pharmacokinetics of molnupiravir, a novel broad-spectrum oral antiviral agent with activity against SARS-CoV-2. Antimicrob. Agents Chemother. 2021;65 doi: 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K.S., Sun X., Aikins M.E., Moon J.J. Non-viral COVID-19 vaccine delivery systems. Adv. Drug Deliv. Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Que T., Wong V., Yuen K. Treatment of severe acute respiratory syndrome with lopinavir/ritonavir: a multicentre retrospective matched cohort study. Hong Kong Med. J. 2003;9:399–406. [PubMed] [Google Scholar]
- Rahimkhoei V., Jabbari N., Nourani A., Sharifi S., Akbari A.J.C.B., Function . Potential Small-molecule Drugs as Available Weapons to Fight Novel Coronavirus (2019-nCoV): A review. Vol. 39. 2021. pp. 4–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramatillah D.L., Isnaini S. Treatment profiles and clinical outcomes of COVID-19 patients at private hospital in Jakarta. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolain J.M., Colson P., Raoult D. Recycling of chloroquine and its hydroxyl analogue to face bacterial, fungal and viral infections in the 21st century. Int. J. Antimicrob. Agents. 2007;30:297–308. doi: 10.1016/j.ijantimicag.2007.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothan H.A., Byrareddy S.N. The epidemiology and pathogenesis of coronavirus disease (COVID-19) outbreak. J. Autoimmun. 2020;109 doi: 10.1016/j.jaut.2020.102433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RPCEC ABDALA Clinical Study - Phase III [Online] 2021. https://rpcec.sld.cu/en/trials/RPCEC00000359-En Available:
- RPCEC SOBERANA 02 - Phase III [Online] 2021. https://rpcec.sld.cu/trials/RPCEC00000354-En Available:
- Russian-News-Agency EpivacCorona vaccine's immunological efficacy proves to be 79% — newspaper [Online] 2021. https://tass.com/society/1322797 Available:
- Sanders J.M., Monogue M.L., Jodlowski T.Z., Cutrell J.B. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. 2020;323:1824–1836. doi: 10.1001/jama.2020.6019. [DOI] [PubMed] [Google Scholar]
- Sharma S., Ray A., Sadasivam B. Metformin in COVID-19: a possible role beyond diabetes. Diabetes Res. Clin. Pract. 2020;164 doi: 10.1016/j.diabres.2020.108183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrotri M., Swinnen T., Kampmann B., Parker E.P.K. An interactive website tracking COVID-19 vaccine development. Lancet Glob. Health. 2021;9:e590–e592. doi: 10.1016/S2214-109X(21)00043-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemieniuk R.A., Bartoszko J.J., Ge L., Zeraatkar D., Izcovich A., Kum E., Pardo-Hernandez H., Qasim A., Martinez J.P.D., Rochwerg B., Lamontagne F., Han M.A., Liu Q., Agarwal A., Agoritsas T., Chu D.K., Couban R., Cusano E., Darzi A., Devji T., Fang B., Fang C., Flottorp S.A., Foroutan F., Ghadimi M., Heels-Ansdell D., Honarmand K., Hou L., Hou X., Ibrahim Q., Khamis A., Lam B., Loeb M., Marcucci M., McLeod S.L., Motaghi S., Murthy S., Mustafa R.A., Neary J.D., Rada G., Riaz I.B., Sadeghirad B., Sekercioglu N., Sheng L., Sreekanta A., Switzer C., Tendal B., Thabane L., Tomlinson G., Turner T., Vandvik P.O., Vernooij R.W., Viteri-Garcia A., Wang Y., Yao L., Ye Z., Guyatt G.H., Brignardello-Petersen R. Drug treatments for covid-19: living systematic review and network meta-analysis. BMJ. 2020;370 doi: 10.1136/bmj.m2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R.K., Dhama K., Karthik K., Tiwari R., Khandia R., Munjal A., Iqbal H.M.N., Malik Y.S., Bueno-Mari R. Advances in diagnosis, surveillance, and monitoring of Zika virus: an update. Front. Microbiol. 2017;8:2677. doi: 10.3389/fmicb.2017.02677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tehran-Times Nasal dose of COV Pars reduces virus transmission by 90% [Online] 2021. https://www.tehrantimes.com/news/465937/Nasal-dose-of-COV-Pars-reduces-virus-transmission-by-90 Available:
- Thakur N., Bailey D. Advances in diagnostics, vaccines and therapeutics for Nipah virus. Microbes Infect. 2019;21:278–286. doi: 10.1016/j.micinf.2019.02.002. [DOI] [PubMed] [Google Scholar]
- Thanh L.T., Andreadakis Z., Kumar A., Gómez R.R., Tollefsen S., Saville M., Mayhew S. The COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020;19:305–306. doi: 10.1038/d41573-020-00073-5. [DOI] [PubMed] [Google Scholar]
- Today M.N. Sputnik Light COVID-19 vaccine: What we know [Online] 2021. https://www.medicalnewstoday.com/articles/sputnik-light-covid-19-vaccine-what-we-know Available:
- Tracker C.V. Approved vaccines [Online] 2021. https://covid19.trackvaccines.org/vaccines/approved/ Available:
- Translation O.O.A.T.D.C.S.F.S.D. 2021. https://setkab.go.id/en/bpom-issues-emergency-use-authorization-for-covovax-vaccine/ Available:
- Vejthani-Hospital COVID-19 vaccines [Online] 2021. https://www.vejthani.com/2021/05/covid-19-vaccines/ Available:
- Vetter P., Eckerle I., Kaiser L. British Medical Journal Publishing Group; 2020. Covid-19: A Puzzle With Many Missing Pieces. [DOI] [PubMed] [Google Scholar]
- Vlachakis D., Papakonstantinou E., Mitsis T., Pierouli K., Diakou I., Chrousos G., Bacopoulou F. Molecular mechanisms of the novel coronavirus SARS-CoV-2 and potential anti-COVID19 pharmacological targets since the outbreak of the pandemic. Food Chem. Toxicol. 2020;146 doi: 10.1016/j.fct.2020.111805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M., Cao R., Zhang L., Yang X., Liu J., Xu M., Shi Z., Hu Z., Zhong W., Xiao G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson A., Ferreira L., Hwang P., Xu J., Stroud R. 2020. Peptide Antidotes to SARS-CoV-2 (COVID-19). 2020.08.06.238915. [Google Scholar]
- WEGO CanSino Vaccine in Pakistan — Everything You Want to Know About the Vaccine [Online] 2021. https://blog.wego.com/cansino-vaccine-pakistan/ Available:
- Worldometers COVID live [Online] 2022. https://www.worldometers.info/coronavirus/ Available:
- Wouters O.J., Shadlen K.C., Salcher-Konrad M., Pollard A.J., Larson H.J., Teerawattananon Y., Jit M. Challenges in ensuring global access to COVID-19 vaccines: production, affordability, allocation, and deployment. Lancet. 2021;397:1023–1034. doi: 10.1016/S0140-6736(21)00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Liu M., Wang C., Xu W., Lan Q., Feng S., Qi F., Bao L., Du L., Liu S., Qin C., Sun F., Shi Z., Zhu Y., Jiang S., Lu L. Inhibition of SARS-CoV-2 (previously 2019-nCoV) infection by a highly potent pan-coronavirus fusion inhibitor targeting its spike protein that harbors a high capacity to mediate membrane fusion. Cell Res. 2020;30:343–355. doi: 10.1038/s41422-020-0305-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia S., Zhu Y., Liu M., Lan Q., Xu W., Wu Y., Ying T., Liu S., Shi Z., Jiang S., Lu L. Fusion mechanism of 2019-nCoV and fusion inhibitors targeting HR1 domain in spike protein. Cell Mol. Immunol. 2020;17:765–767. doi: 10.1038/s41423-020-0374-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Y., Cao S., Dong H., Li Q., Chen E., Zhang W., Yang L., Fu S., Wang R. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81:318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakarya K., Kutumbetov L., Orynbayev M., Abduraimov Y., Sultankulova K., Kassenov M., Sarsenbayeva G., Kulmagambetov I., Davlyatshin T., Sergeeva M., Stukova M., Khairullin B. Safety and immunogenicity of a QazCovid-in(R) inactivated whole-virion vaccine against COVID-19 in healthy adults: a single-centre, randomised, single-blind, placebo-controlled phase 1 and an open-label phase 2 clinical trials with a 6 months follow-up in Kazakhstan. EClinicalMedicine. 2021;39:101078. doi: 10.1016/j.eclinm.2021.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y. Analysis of Epidemiological characteristics of new coronavirus pneumonia. Chin. J. Epidemiol. 2020;41:1–7. [Google Scholar]
- Zhang L., Liu Y. Potential interventions for novel coronavirus in China: a systemic review. J. Med. Virol. 2020;92:479–490. doi: 10.1002/jmv.25707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Ma X., Yu F., Liu J., Zou F., Pan T., Zhang H. 2020. Teicoplanin Potently Blocks the Cell Entry of 2019-nCoV. 2020.02.05.935387. [Google Scholar]
- Zhao H., To K.K.W., Sze K.H., Yung T.T., Bian M., Lam H., Yeung M.L., Li C., Chu H., Yuen K.Y. A broad-spectrum virus- and host-targeting peptide against respiratory viruses including influenza virus and SARS-CoV-2. Nat. Commun. 2020;11:4252. doi: 10.1038/s41467-020-17986-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Lu Z., Xu T., Chen C., Yang G., Zha T., Xue Y. Arbidol monotherapy is superior to lopinavir/ritonavir in treating COVID-19. J. Infect. 2020;81:e21–e23. doi: 10.1016/j.jinf.2020.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zu Z.Y., Jiang M.D., Xu P.P., Chen W., Ni Q.Q., Lu G.M., Zhang L.J. Coronavirus disease 2019 (COVID-19): a perspective from China. Radiology. 2020;296:E15–E25. doi: 10.1148/radiol.2020200490. [DOI] [PMC free article] [PubMed] [Google Scholar]