Abstract
Background
Maintaining plasma HIV RNA suppression below the limit of quantification is the goal of antiretroviral therapy (ART). When viral loads (VL) remain in low-level viremia (LLV), or between 201 and 999 copies/mL, the clinical consequences are still not clear. We investigated the occurrence of LLV with drug resistance and its effect on CD4 cell counts in a large Chinese cohort.
Methods
We analysed data of 6,530 ART-experienced patients (42.1 ± 10.9 years; 37.3% female) from the China’s national HIV drug resistance (HIVDR) surveillance database. Participants were followed up for 32.9 (IQR 16.7–50.5) months. LLV was defined as the occurrence of at least one viral load (VL) measurement of 50–200 copies/mL during ART. Outcomes were drug resistance associated mutations (DRAM) and CD4 cell counts levels.
Results
Among 6530 patients, 58.0% patients achieved VL less than 50 copies/mL, 27.8% with VL between 50 and 999 copies/mL (8.6% experienced LLV), and 14.2% had a VL ≥ 1000 copies/mL. Of 1818 patients with VL 50–999 copies/mL, 182 (10.0%) experienced HIVDR, the most common DRAM were M184I/V 28.6%, K103N 19.2%, and V181C/I/V 10.4% (multidrug resistance: 27.5%), and patients with HIVDR had a higher risk of CD4 cell counts < 200 cells/μL (AOR 3.8, 95% CI 2.6–5.5, p < 0.01) comparing with those without HIVDR. Of 925 patients with VL ≥ 1000 copies/mL, 495 (53.5%) acquired HIVDR, the most common DRAM were K103N 43.8%, M184I/V 43.2%, M41L 19.0%, D67N/G 16.4%, V181C/I/V 14.5%, G190A/S 13.9% and K101E 13.7% (multidrug resistance: 75.8%), and patients with HIVDR had a higher risk of CD4 cell counts < 200 cells/μL (AOR 5.8, 95% CI 4.6–7.4, p < 0.01) comparing with those without HIVDR.
Conclusion
Persistent with VL 50–999 copies/mL on ART is associated with emerging DRAM for all drug classes, and patients in this setting were at increased risk of CD4 cell counts < 200 cells/μL, which suggest resistance monitoring and ART optimization be earlier considered.
Keywords: HIV, Low-level viremia, HIV drug resistance, Drug resistance associated mutations, China
Background
The use of antiretroviral therapy (ART) has resulted in substantial reductions in HIV/AIDS-related morbidity and mortality worldwide [1–4]. Maintaining plasma HIV RNA suppression below the limit of quantification is the goal of ART, which induces persistent suppression of HIV replication and gradual recovery of CD4 cell counts [3–5]. Durable viral suppression is accomplished with sustained ART adherence in the majority of people living with HIV (PLWH). However, some PLWH develop persistent low-level viremia (LLV), which is usually defined as plasma viral load (VL) between 50 and 200 copies/mL [1, 6–8]. It’s not an uncommon finding in clinical practice, with estimates of 30% of PLWH on ART experiencing LLV [9]. It should be noted that widely followed ART guidelines diverge in their interpretation and recommended management of persistent viremia of low magnitude, reflecting the limited evidence base for these common clinical findings [1, 10, 11].
During ART, PLWH may experience small increases in VL (50–200 copies/mL) that do not reach the threshold for virological failure (VF), known as LLV [1, 10, 11]. In developed countries with easy access to VL monitoring, LLV can be a concern for both patients and physicians [12–14]. In these countries, upon detection of raised VL higher than 50 copies/mL, interventions such as resistance testing, pharmacokinetic measurement, switch of ART regimen, and adherence counselling might already be initiated [10, 15]. Studies from developed countries have demonstrated that HIV-1 drug resistance (HIVDR) testing at a plasma VL < 1000 copies/mL provides potentially clinically useful information [10, 16, 17]. They have found that patients with LLV harbor drug resistance associated mutations (DRAM) that confer resistance to the current ART regimens and decreases future therapeutic options [10, 18]. However, the available evidence might not apply to ART programmes in China, where current National Free Antiretroviral Treatment Program (NFATP) guidelines recommend that annual VL testing and interventions are only advised if VL ≥ 1000 copies/mL [19–21]. Furthermore, available studies from China performed integrase genotyping only on samples with VL ≥ 1000 copies/mL, few data are available on the emergence of such mutations in patients with LLV [22–25]. In recent years, in-house resistance assays can be performed on samples with VL below 1000 copies/mL, and the improvement of assays to quantify VL has led to progressively decrease the threshold of VF [7, 21, 26, 27]. The high threshold for VF currently used in NFATP should be reconsidered.
To date, the implications and clinical significance of LLV with drug resistance are still not clear in China. Additionally, few or no studies have analyzed the possible role of LLV with drug resistance as a tool to predict CD4 cell count < 200 cells/μL, while patients who initiate ART with a CD4 > 200 cells/μL are at reduced risk of death and serious opportunistic infections [28–32]. Whether VF thresholds should be lowered in patients from NFATP is yet to be determined. Therefore, we conducted the present study to examine the effect of LLV with drug resistance on patients’ clinical outcomes in NFATP.
Methods
Participants and definitions
We assessed all the data from China’s national HIVDR surveillance database from Jan 1, 2008, to Dec 31, 2015 (data downloaded on Dec 31, 2017). In this study, eligibility criteria were: 18 years or older; having received ART for more than 12 months; attending a participating clinic for routine HIVDR survey; having detailed viral load measurement data and CD4 cell count. In samples with VL ≥ 50 copies/mL, HIVDR genotyping was performed by in-house PCR as previously described [11, 23, 33]. HIVDR mutation analysis and viral subtype determination were performed on a 1.3 kb section of the HIV pol gene using the Stanford University HIVDR Database online sequence analysis tool: Genotypic Resistance Interpretation Algorithm (https://hivdb.stanford.edu/hivdb/by-mutations/, accessed Oct 16, 2018). NFATP guideline defines virological failure (VF) as a confirmed HIV RNA ≥ 1000 copies/mL. LLV was defined as the occurrence of at least one viral load (VL) measurement of 50–200 copies/mL during ART. The evaluation of CD4 cell count was performed after the event of LLV. The adherence questionnaire form included: (a) have you ever missed ARV drugs? (b) Have you ever missed ARV drugs because of side effects? (c) Have you ever missed ART because of excessive drugs? (d) Have you ever had difficulties taking ARV drugs at the exact time? For each question, a Likert scale: (a) never, (b) rarely, (c) sometimes, and (d) often is adopted in response. Never missing ARV drugs (or > 95% in multiple follow-up) is considered to be good adherence. Ethical approval for the study was obtained from the National Center for AIDS/STD Control and Prevention, China CDC Institutional Review Board. All individuals in this study provided written consent at the time of participation, and written informed consent was obtained from all study participants. All methods were performed in accordance with the relevant guidelines and regulations.
Statistical analysis
The significant differences in categorical variables were analyzed using χ2 test or Fisher’s exact tests. Logistic regression was used to examine the associations between LLV, DR and CD4 cell counts. Weighted analyses were used to derive representative estimates of the detection rates of patients harboring HIV with mutations conferring resistance to ARV drugs belonging to the non-nucleoside reverse transcriptase inhibitor (NNRTI), transcriptase inhibitor (NRTI) and protease inhibitor (PI) classes. Weight was calculated based on the number of patients followed in each local center. All tests of significance were two-sided, with p < 0.05 indicating that an association was statistically significant. All the statistical analyses were performed with SAS 9.4 software (SAS Institute, Cary, NC, USA).
Results
Demographic characteristics of the study population
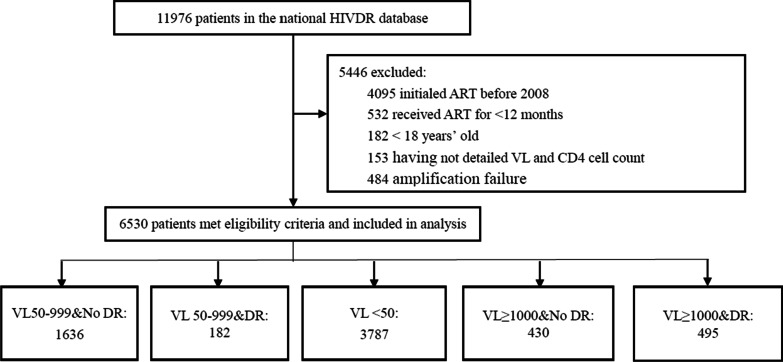
11,976 PLWH were eligible in this study; 5446 did not meet eligibility criteria, and finally 6530 PLWH were included for analysis (Fig. 1). Of all the participants, 62.7% were male, 52.3% over 40 years old, 78.6% with an education level of secondary school or less, 60.4% farmers, 74.6% married, 40.5% infected through sexual intercourse, 12.8% with CD4 cell counts < 200 cells/μL, 14.2% with a VL ≥ 1000 copies/mL. All patients received free ART treatment through the NFATP, and most patients used lamivudine (3TC) based regimens (initial: 79.8%, at survey: 78.0%). The percentage of patients who missed dose in the past month was 26.1%. For more detailed information, see Table 1.
Fig. 1.
Study profile. 11976 patients were eligible in this study; 5446 did not meet eligibility criteria, and finally 6530 patients were included for analysis
Table 1.
General characteristics of patients who initiated ART from 2008 to 2015
| Factor | N (%) | VL 50–999 and No DR (%) | VL 50–999 and DR (%) | VL < 50(%) | VL ≥ 1000 and No DR (%) | VL ≥ 1000 and DR (%) | p |
|---|---|---|---|---|---|---|---|
| Total | 6530 (100%) | 1636 (100%) | 182 (100%) | 3787 (100%) | 430 (100%) | 495 (100%) | |
| Sex | |||||||
| Male | 4097 (62.7%) | 941 (57.5%) | 110 (60.4%) | 2453 (64.8%) | 284 (66.0%) | 309 (62.4%) | < 0.01 |
| Female | 2433 (37.3%) | 695 (42.5%) | 72 (39.6%) | 1334 (35.2%) | 146 (34.0%) | 186 (37.6%) | |
| Age | |||||||
| Median years (IQR) | 41 (34–49) | 44 (37–51) | 39 (34–46) | 40 (33–48) | 41 (34–39) | 42 (35–50) | < 0.01 |
| ≤ 30 | 940 (14.4%) | 131 (8.0%) | 29 (15.9%) | 665 (17.6%) | 56 (13.0%) | 59 (11.9%) | |
| 31–40 | 2174 (33.3%) | 474 (29.0%) | 70 (38.5%) | 1305 (34.5%) | 154 (35.8%) | 171 (34.5%) | |
| > 40 | 3416 (52.3%) | 1031 (63.0%) | 83 (45.6%) | 1817 (48.0%) | 220 (51.2%) | 265 (53.5%) | |
| Education | |||||||
| Post-secondary school or more | 1400 (21.4%) | 229 (14.0%) | 25 (13.7%) | 1026 (27.1%) | 70 (16.3%) | 50 (10.1%) | < 0.01 |
| Secondary school or less | 5130 (78.6%) | 1407 (86.0%) | 157 (86.3%) | 2761 (72.9%) | 360 (83.7%) | 445 (89.9%) | |
| Occupation | |||||||
| Farmer | 3943 (60.4%) | 1197 (73.2%) | 73 (40.1%) | 2049 (54.1%) | 259 (60.2%) | 365 (73.7%) | < 0.01 |
| Other | 2552 (39.1%) | 436 (26.7%) | 108 (59.3%) | 1709 (45.1%) | 171 (39.8%) | 128 (25.9%) | |
| Marital status | |||||||
| Unmarried | 1659 (25.4%) | 326 (19.9%) | 29 (15.9%) | 1105 (29.2%) | 103 (24.0%) | 96 (19.4%) | < 0.01 |
| Married | 4871 (74.6%) | 1310 (80.1%) | 153 (84.1%) | 2682 (70.8%) | 327 (76.0%) | 399 (80.6%) | |
| Route of HIV infection | |||||||
| Sexual transmission | 2647 (40.5%) | 427 (26.1%) | 78 (42.9%) | 1917 (50.6%) | 131 (30.5%) | 94 (19.0%) | < 0.01 |
| Other | 3883 (59.5%) | 1209 (73.9%) | 104 (57.1%) | 1870 (49.4%) | 299 (69.5%) | 401 (81.0%) | |
| Initial ART regimens | |||||||
| D4T/3TC/EFV or NVP | 2953 (45.2%) | 600 (20.3%) | 87 (2.9%) | 1888 (63.9%) | 199 (6.7%) | 179 (6.1%) | < 0.01 |
| AZT/3TC/EFV or NVP | 1846 (28.3%) | 667 (36.1%) | 59 (3.2%) | 889 (48.2%) | 106 (5.7%) | 125 (6.8%) | |
| TDF/3TC/EFV or NVP | 555 (8.5%) | 45 (8.1%) | 1 (0.2%) | 454 (81.8%) | 39 (7.0%) | 16 (2.9%) | |
| Other regimens | 1176 (18.0%) | 324 (27.6%) | 35 (3.0%) | 556 (47.3%) | 86 (7.3%) | 175 (14.9%) | |
| ART regimens at survey | |||||||
| D4T/3TC/EFV or NVP | 2760 (42.3%) | 596 (21.6%) | 70 (2.5%) | 1795 (65.0%) | 161 (5.8%) | 138 (5.0%) | < 0.01 |
| AZT/3TC/EFV or NVP | 1321 (20.2%) | 269 (20.4%) | 59 (4.5%) | 825 (62.5%) | 75 (5.7%) | 93 (7.0%) | |
| TDF/3TC/EFV or NVP | 1014 (15.5%) | 149 (14.7%) | 17 (1.7%) | 762 (75.1%) | 51 (5.0%) | 35 (3.5%) | |
| Other first-line regimens | 125 (1.9%) | 19 (15.2%) | 2 (1.6%) | 61 (48.8%) | 19 (15.2%) | 24 (19.2%) | |
| PI/r-based regimens | 1310 (20.1%) | 603 (46.0%) | 34 (2.6%) | 344 (26.3%) | 124 (9.5%) | 205 (15.6%) | |
| Duration of ART (months) | |||||||
| Median months (IQR) | 33 (17–50) | 46 (23–61) | 35 (24–50) | 27 (15–48) | 28 (17–49) | 45 (22–57) | < 0.01 |
| 12–24 | 2576 (39.4%) | 444 (27.1%) | 45 (24.7%) | 1748 (46.2%) | 193 (44.9%) | 146 (29.5%) | |
| > 24 | 3954 (60.6%) | 1192 (72.9%) | 137 (75.3%) | 2039 (53.8%) | 237 (55.1%) | 349 (70.5%) | |
| CD4 cell counts (cells/μL) at survey | |||||||
| Median cells/μL (IQR) | 340 (234–443) | 353 (256–452) | 272 (189–351) | 362 (260–460) | 242 (157–328) | 165 (69–264) | < 0.01 |
| < 200 | 837 (12.8%) | 195 (11.9%) | 58 (31.9%) | 244 (6.4%) | 130 (30.2%) | 210 (42.4%) | |
| ≥ 200 | 5693 (87.2%) | 1441 (88.1%) | 124 (68.1%) | 3543 (93.6%) | 300 (69.8%) | 285 (57.6%) | |
| HIV viral load (copies/mL) at survey | |||||||
| Median log10 copies/mL (IQR) | 1.3 (1.3–2.5) | 2.2 (2.2–2.5) | 1.7 (1.6–2.9) | 1.3 (0.9–1.3) | 4.3 (3.7–4.8) | 4.4 (3.9–5.0) | < 0.01 |
| < 1000 | 5605 (85.8%) | 1636 (100.0%) | 182 (100.0%) | 3787 (100.0%) | 0 (0.0%) | 0 (0.0%) | |
| ≥ 1000 | 925 (14.2%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 430 (100.0%) | 495 (100.0%) | |
| Missed doses in the past month | |||||||
| No | 4828 (73.9%) | 993 (60.7%) | 100 (54.9%) | 3178 (83.9%) | 318 (74.0%) | 239 (48.3%) | < 0.01 |
| Yes | 1702 (26.1%) | 643 (39.3%) | 82 (45.1%) | 609 (16.1%) | 112 (26.0%) | 256 (51.7%) | |
HIV-1 drug resistance and mutations
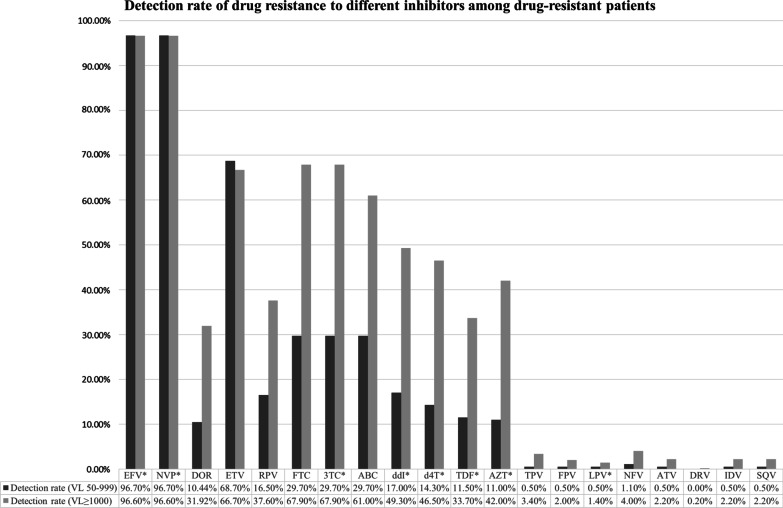
Of 1818 patients with VL 50–999 copies/mL, 182 (10.0%) had resistance to any type of HIV drugs. The detection rate of resistance to NNRTI drugs was higher than that to NRTI drugs and PI drugs. Of 925 patients with VF (VL ≥ 1000 copies/mL), 495 (53.5%) had resistance to any types of HIV drugs. The detection rate of resistance to NNRTI drugs was higher than that to NRTI drugs and PI drugs. Compared with patients with VL 50–999 copies/mL, the detection rate of NRTIs and PIs increased significantly in patients with VF (p < 0.01); however, the detection rate of NNRTIs did not have the same result in patients with VF (p = 0.97) (Fig. 2).
Fig. 2.
Detection rate of drug resistance to different inhibitors among drug-resistant patients who initiated ART from 2008 to 2015. EFV = Efavirenz; NVP = Nevirapine; DOR = Doravirine; FTC = Emtricitabine; 3TC = Lamivudine; ABC = Abacavir; DDI = Didanosine; D4T = Stavudine; TDF = Tenofovir; AZT = Azidothymidine; TPV = Tipranavir; LPV = Lopinavir; NFV = Nelfinavir; FPV = Fosamprenavir; ATV = Atazanavir; DRV = Darunavir; IDV = Indinavir; SQV = Saquinavir. *Provided through the National Free Antiretroviral Treatment Program (NFATP)
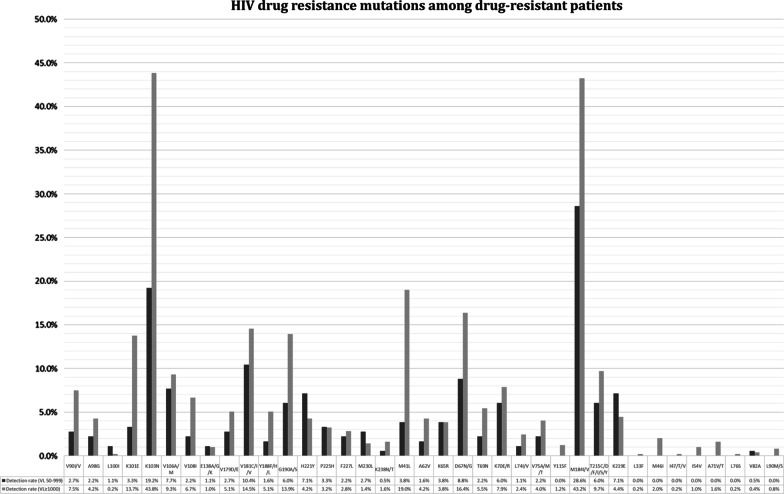
The prevalence of resistance mutations to NNRTIs, NRTIs and PIs is shown in Fig. 3. The most frequent NNRTI resistance mutations were K103N and Y181C/I/V. The most frequent NRTI resistance mutations were M184I/V. NRTI resistance mutations selected by thymidine analogues (M41L, D67N/G, K70E/R, T215C/D/F/I/S/Y and K219E) were the most frequent. The only PI mutation detected in patients with LLV was V82A (Fig. 3).
Fig. 3.
HIV drug resistance mutations among drug-resistant patients who initiated ART from 2008 to 2015
The correlation between VL 50–200 and 201–999 copies/mL, DR and CD4 cell counts < 200 cells/μL
Table 2 shows the results of logistic regression models describing the relationship between LLV, DR and CD4 cell counts < 200 cells/μL. Model 1: After adjusting sex, age, occupation, marital status, route of HIV infection, duration of ART, initial ART regimens, ART regimens at survey, and missed doses, patients with VL 50–999 copies/mL and DR (AOR 3.8, 95% CI 2.6–5.5, p < 0.01), VF and no DR (3.1, 2.4–4.1, p < 0.01), VF and DR (5.8, 4.5–7.4, p < 0.01) had higher detection rates of CD4 cell counts < 200 cells/μL, compared with VL 50–999 copies/mL and no DR. On the other hand, patients with VL < 50 copies/mL had the lowest detection rate of CD4 cell counts < 200 cells/μL (0.5, 0.4–0.6, p < 0.01). Model 2: After adjusting sex, age, occupation, marital status, route of HIV infection, duration of ART, initial ART regimens, ART regimens at survey, and missed doses, patients with VL 201–999 copies/mL and DR, VF and no DR, VF and DR had higher detection rates of CD4 cell counts < 200 cells/μL, compared with VL 50–200 copies/mL and no DR (Table 2).
Table 2.
The relationship between LLV, DR and CD4 cell counts < 200 cells/μL among patients who initiated ART from 2008 to 2015
| Factors | N | CD4 < 200 (%) | OR (95% CI) | p | AOR (95%CI) | p | |
|---|---|---|---|---|---|---|---|
| Total | 6530 | 837 | 12.8% | ||||
| Model 1 | |||||||
| VL 50–999 and No DR | 1636 | 195 | 11.9% | 1.0 | 1.0 | ||
| VL 50–999 and DR | 182 | 58 | 31.9% | 3.5 (2.4, 4.9) | < 0.01 | 3.8 (2.6, 5.5) | < 0.01 |
| VL < 50 | 3787 | 244 | 6.4% | 0.5 (0.4, 0.6) | < 0.01 | 0.5 (0.4, 0.6) | < 0.01 |
| VL ≥ 1000 and No DR | 430 | 130 | 30.2% | 3.2 (2.5, 4.1) | < 0.01 | 3.1 (2.4, 4.1) | < 0.01 |
| VL ≥ 1000 and DR | 495 | 210 | 42.4% | 5.4 (4.3, 6.9) | < 0.01 | 5.8 (4.5, 7.4) | < 0.01 |
| Model 2 | |||||||
| VL 50–200 and No DR | 537 | 38 | 7.1% | 1.0 | 1.0 | ||
| VL 201–999 and No DR | 1099 | 157 | 14.3% | 2.2 (1.5, 3.2) | 0.14 | 2.4 (1.6, 3.6) | 0.34 |
| VL 50–200 and DR | 23 | 2 | 8.7% | 1.3 (0.3, 5.5) | 0.23 | 1.2 (0.3, 5.6) | 0.21 |
| VL 201–999 and DR | 159 | 56 | 35.2% | 7.1 (4.5, 11.3) | < 0.01 | 8.0 (4.9, 13.1) | < 0.01 |
| VL < 50 | 3787 | 244 | 6.4% | 0.9 (0.6, 1.3) | < 0.01 | 0.9 (0.6, 1.2) | < 0.01 |
| VL ≥ 1000 and No DR | 430 | 130 | 30.2% | 5.7 (3.9, 8.4) | < 0.01 | 5.7 (3.8, 8.5) | < 0.01 |
| VL ≥ 1000 and DR | 495 | 210 | 42.4% | 9.7 (6.7, 14.1) | < 0.01 | 10.7 (7.2, 15.9) | < 0.01 |
CD4 cell counts < 200 cells/μL, CD4 cell counts < 200 cells/μL; OR, odds ratio; CI, confidence interval; AOR, adjusted odds ratio; Adjusted for: age, ethnicity, education, sex, marital status, occupation, route of HIV transmission, duration of ART, and ART regimens
Discussion
In this large-scale Chinese multicenter analysis, patients with VL 50–999 copies/mL occurred frequently (27.8%) and increased the risk of CD4 cell counts < 200 cells/μL. Additionally, patients were more likely to experience subsequent CD4 cell counts < 200 cells/μL if they had emergent drug resistance at the time of VL 50–999 copies/mL. To be noted, accumulation of DRAM increases when maintaining a failing ARV drug regimen with VL ≥ 50 copies/mL, leading to a loss of future therapeutic options together with a large cross-resistance between ARV drug within each ARV class.
The definitions of VF and LLV vary in different periods of each country. Substantial differences exist between guidelines in developed countries, which use VL thresholds of 50–200 copies/mL to define VF, and WHO guidelines for developing countries, which apply a more lenient threshold of 1000 copies/mL [1, 6–8]. Clinical interventions are initiated upon detection of VL > 50 copies/mL in developed countries where frequent VL monitoring is performed, and the LLV is considered in the range of 50–200 copie/mL [33]. It should be noted that the definition of LLV remains 50–999 copies/mL in China, because most commercial resistance assays can only be performed on samples with VL above a minimum of 500–1000 copies/mL [19, 27, 34, 35]. We conducted a systematic nationwide survey of HIVDR in patients with VL 50–999 copies/mL in China, and compared the results with those with VL ≥ 1000 copies/mL. Among 6530 patients, 58.0% patients achieved VL less than 50 copies/mL, 27.8% had VL 50–999 copies/mL, 14.2% had a VL ≥ 1000 copies/mL and 10.4% had HIVDR. The occurrence of LLV in this study was substantially higher than that in reports from developed countries [36–38]. The occurrence of HIVDR in this study, which was higher than previously published data from China (HIVDR genotyping was performed in samples with VL ≥ 1000 copies/mL), was also higher than that reported in developed countries [11, 22, 24, 38–41]. The observed differences in prevalence of LLV and HIVDR might therefore reflect lower overall rates of virological suppression in China. Although not applicable to all patients, a tendency towards higher rates of LLV were detected in patients with poor adherence to ART. To be noted, among the 182 patients with LLV and HIVDR, 82 (45.1%) reported having missed doses in the past month; compared with 609 (16.1%) in 3,787 patients with VL < 50 copies/mL, this suggested that poor adherence would continue to be a significant problem. Therefore, we recommend that NFATP guidelines demonstrate LLV as a remarkable warning signal to have necessary clinical action, including intensification of adherence counselling and HIVDR testing.
Our results indicate that resistance testing of samples with VL < 1000 copies/mL provides clinically relevant information. It is worth noting that the prevalence of resistance to two and three ARV drugs were 26.4% and 1.1% respectively among patients with LLV. However, this prevalence increased to 72.9% and 3.6% respectively among patients with VL ≥ 1000 copies/mL. The results support guidelines recommending resistance monitoring for all patients with VL ≥ 50 copies/mL, even if genotypic resistance tests are less efficient at low viral loads [42]. Among patients with LLV and HIVDR, the proportion of drug resistance to NNRTIs, NRTIs and PIs were 96.7%, 30.2%, and 1.7%, respectively; the proportion of drug resistance to both NNRTIs and NRTIs was 27.5%. Among patients with VL ≥ 1000 copies/mL and HIVDR, the proportion of drug resistance to NNRTIs, NRTIs and PIs were 96.8%, 77.4%, and 6.1%, respectively; the proportion of drug resistance to both NNRTIs and NRTIs was 75.8%. The results showed that most of the patients with VL ≥ 1000 copies/mL and HIVDR were resistant to two or more ARV drugs, and most of those with LLV and DR were resistant to only one ARV drug. Our finding that VL between 50 and 999 copies/mL is associated with HIVDR adds to the evidence in prior studies [21, 30].
In this study, patients with HIVDR had lower CD4 counts than those without even if they had a relatively low VL. The results also showed that poor adherence (e.g. missed doses in the past month) are influenced by CD4 cell count. Poor adherence may be the reason for the low level of CD4 counts in patients with LLV (Table 1). Earlier research has shown that more than 10% of PLWH fail to achieve normalization of CD4 cell counts during ART. These patients are referred to as “inadequate immunological responders”, who show severe immunological dysfunction [43]. Previous researches had suggested that patients with CD4 cell count > 200 cells/μL had better recovery of immune function after ART [11, 35, 44]. Claris and Delson concluded that both VL monitoring and CD4 count monitoring play important roles in improving life expectance of patients living with HIV [45]. The level of CD4 cell count is associated with the immune system [46], and the patients on ART with poor immune function, that is, CD4 cell counts < 200 cells/μL, are more likely to develop HIVDR, indicating that PLWH with LLV and HIVDR should be detected early and change their poor adherence as soon as possible.
The results of this study showed that although patients take more time to achieve a normal CD4 cell count and less time to achieve an undetectable VL, once the CD4 cell count is normal, DRAM are reduced. The patterns of DRAM observed in this survey (Fig. 2) are consistent with previous studies [11, 22, 35, 47]. M184I/V, K103N and V181C/I/V are the common DRAM reported in China [11, 35, 39]. In this study, M184I/V was the most common DRAM detected in patients with LLV, with a prevalence of 28.6%. It is worth noting that M184I/V is mainly selected by FTC or 3TC, which is one of the important backbones in the ART regimens of NFATP [48–51]. K103N was the second most common DRAM (19.2%) detected in this study and usually selected by EFV or NVP, which are the most widely used NNRTI in NFATP [11, 35, 39]. Previous research from China has shown that the reductions in virological failure and drug resistance were strongly associated with the standardized use of TDF- or AZT-based regimens in place of the D4T-based regimen [20]. The results of this study showed that the most common DRAM detected in patients with LLV were against NNRTIs, which were used widely in NFATP [52–54]. These findings indicated that LLV episodes below 1000 copies/mL while receiving ART was associated with emerging DRAM for all ARV drug classes, suggesting earlier DRAM monitoring and ART optimization, which is identical with other results of study [55].
Our study has several limitations. First, amplification of both RT and protease genes was successful in 79.8% and 97.5% of samples with VL of 50–999 and ≥ 1000 copies/mL. This difference might lead to bias. However, multivariable analysis showed that only VL independently predicted amplification failure (p < 0.01). Exposure to the different ARV drugs was well balanced between two groups in which reverse transcriptase and protease gene amplification was successful and unsuccessful (p = 0.41). Second, only patients alive at the time of the surveys were included in this study, so the statistical relationships among LLV, CD4 cell counts < 200 cells/μL and HIVDR might be reduced. Third, the lower frequency of VL monitoring used in NFATP might in part underlie the detection rates of LLV and HIVDR.
Conclusions
This study was intended to better understand the consequences of LLV on patients from NFATP. Our analyses showed that the risk of CD4 cell counts < 200 cells/μL was higher after LLV, especially when drug resistance occurred, and DRAM appeared as soon as LLV persistence. It included the largest patient data on this topic so far, covering 6,530 patients from various regions of China. Compared with previous studies from developed countries, the detected rates of LLV in China were higher and the risk of CD4 cell counts < 200 cells/μL and HIVDR after LLV was more pronounced, indicating that LLV is a serious threat to NFATP programmes in China.
Acknowledgements
We appreciate the contribution of all subjects included in this study. We are greatly grateful to the staff from provincial and county CDCs for participating in sample collection and investigation.
Abbreviations
- NFATP
National Free Antiretroviral Treatment Program
- ART
Antiretroviral therapy
- AIDS
Acquired Immune Deficiency Syndrome
- HIV
Human immunodeficiency virus
- HIVDR
HIV drug resistance
- LLV
Low-level viremia
- VF
Virological failure
- VL
Viral load
- DRAM
Drug resistance associated mutations
- NNRTI
Non-nucleoside reverse transcriptase inhibitor
- NRTI
Transcriptase inhibitor
- PI
Protease inhibitor
Author contributions
PL, YY, HX, YR and DL conceived and designed the study. PL, YY, YR and DL conducted the data analysis, literature review, and drafted the manuscript. PL, DL, YY, LL, YF, HX, GL, JL and YS were involved in the study supervision, data collection, and interpretation of the data. LL, YF, HX and YR assisted with data management and data analysis. YY and DL provided input in the revision edited the paper for English grammar. All authors contributed to the revision of the manuscript and approved the final version. All authors read and approved the final manuscript.
Funding
This study was supported by Grants from the National Natural Science Foundation of China (11971479, 82160636), Shandong medical and health science technology development program (2017WS410), Guangxi Natural Science Foundation Project (Grants 2020GXNSFAA159020), Guangxi Key Laboratory of AIDS Prevention Control and Translation [ZZH2020010], Guangxi Bagui Honor Scholarship, and Chinese State Key Laboratory of Infectious Disease Prevention and Control. The funding body did not play a role in the design of the study, data collection, analysis, interpretation of data, and in writing the manuscript. The content is solely the responsibility of the authors.
Availability of data and materials
The datasets generated and/or analysed during the current study are not publicly available due to data constraint requirements from China CDC but are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Ethical approval for the study was obtained from the National Center for AIDS/STD Control and Prevention, China CDC Institutional Review Board. All individuals in this study provided written consent at the time of participation, and written informed consent was obtained from all study participants. All methods were performed in accordance with the relevant guidelines and regulations.
Consent for publication
Not applicable.
Competing interests
The authors declares that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pengtao Liu and Yinghui You contributed equally to this paper.
Contributor Information
Pengtao Liu, Email: lpt1978@126.com.
Yinghui You, Email: yyhihy@126.com.
Lingjie Liao, Email: amicheryl@163.com.
Yi Feng, Email: fengyi@chinaaids.cn.
Yiming Shao, Email: yshao@bjmu.edu.cn.
Hui Xing, Email: xingh09@163.com.
Guanghua Lan, Email: lgh605@163.com.
Jianjun Li, Email: lijianjun810@sina.com.
Yuhua Ruan, Email: ruanyuhua92@163.com.
Dan Li, Email: ld8186898@163.com.
References
- 1.Saag MS, Gandhi RT, Hoy JF, Landovitz RJ, Thompson MA, Sax PE, Smith DM, Benson CA, Buchbinder SP, Del Rio C, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2020 recommendations of the international antiviral society-USA panel. JAMA. 2020;324(16):1651–1669. doi: 10.1001/jama.2020.17025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS. UNAIDS; [(accessed on 18 January 2022)]. Global HIV & AIDS Statistics. Available online: https://www.unaids.org/en/resources/fact-sheet.
- 3.Floyd S, Shanaube K, Yang B, Schaap A, Griffith S, Phiri M, Macleod D, Sloot R, Sabapathy K, Bond V, et al. HIV testing and treatment coverage achieved after 4 years across 14 urban and peri-urban communities in Zambia and South Africa: an analysis of findings from the HPTN 071 (PopART) trial. PLoS Med. 2020;17(4):e1003067. doi: 10.1371/journal.pmed.1003067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shanaube K, Macleod D, Chaila MJ, Mackworth-Young C, Hoddinott G, Schaap A, Floyd S, Bock P, Hayes R, Fidler S, et al. HIV care cascade among adolescents in a “Test and Treat” community-based intervention: HPTN 071 (PopART) for youth study. J Adolesc Health. 2021;68(4):719–727. doi: 10.1016/j.jadohealth.2020.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockman S, Holme MP, Makhema J, Bachanas P, Moore J, Wirth KE, Lebelonyane R, Essex M. Implementation of universal HIV testing and treatment to reduce HIV incidence in Botswana: the Ya Tsie study. Curr HIV/AIDS Rep. 2020;17(5):478–486. doi: 10.1007/s11904-020-00523-0. [DOI] [PubMed] [Google Scholar]
- 6.Gonzalez-Serna A, Swenson LC, Watson B, Zhang W, Nohpal A, Auyeung K, Montaner JS, Harrigan PR. A single untimed plasma drug concentration measurement during low-level HIV viremia predicts virologic failure. Clin Microbiol Infect. 2016;22(12):1004 e1009–1004 e1016. doi: 10.1016/j.cmi.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 7.Hermans LE, Moorhouse M, Carmona S, Grobbee DE, Hofstra LM, Richman DD, Tempelman HA, Venter WDF, Wensing AMJ. Effect of HIV-1 low-level viraemia during antiretroviral therapy on treatment outcomes in WHO-guided South African treatment programmes: a multicentre cohort study. Lancet Infect Dis. 2018;18(2):188–197. doi: 10.1016/S1473-3099(17)30681-3. [DOI] [PubMed] [Google Scholar]
- 8.EACS.18th International European AIDS Conference (EACS 2021). Available at https://www.eacsociety.org/media/final2021eacsguidelinesv11.0_oct2021.pdf.
- 9.Ryscavage P, Kelly S, Li JZ, Harrigan PR, Taiwo B. Significance and clinical management of persistent low-level viremia and very-low-level viremia in HIV-1-infected patients. Antimicrob Agents Chemother. 2014;58(7):3585–3598. doi: 10.1128/AAC.00076-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Swenson LC, Min JE, Woods CK, Cai E, Li JZ, Montaner JS, Harrigan PR, Gonzalez-Serna A. HIV drug resistance detected during low-level viraemia is associated with subsequent virologic failure. AIDS. 2014;28(8):1125–1134. doi: 10.1097/QAD.0000000000000203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu P, Tang Z, Lan G, Zhu Q, Chen H, You Y, Yang X, Liang S, Chen Y, Xing H, et al. Early antiretroviral therapy on reducing HIV transmission in China: strengths, weaknesses and next focus of the program. Sci Rep. 2018;8(1):3431. doi: 10.1038/s41598-018-21791-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grinsztejn B, Hosseinipour MC, Ribaudo HJ, Swindells S, Eron J, Chen YQ, Wang L, Ou SS, Anderson M, McCauley M, et al. Effects of early versus delayed initiation of antiretroviral treatment on clinical outcomes of HIV-1 infection: results from the phase 3 HPTN 052 randomised controlled trial. Lancet Infect Dis. 2014;14(4):281–290. doi: 10.1016/S1473-3099(13)70692-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen MS, Chen YQ, McCauley M, Gamble T, Hosseinipour MC, Kumarasamy N, Hakim JG, Kumwenda J, Grinsztejn B, Pilotto JH, et al. Prevention of HIV-1 infection with early antiretroviral therapy. N Engl J Med. 2011;365(6):493–505. doi: 10.1056/NEJMoa1105243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gunthard HF, Saag MS, Benson CA, del Rio C, Eron JJ, Gallant JE, Hoy JF, Mugavero MJ, Sax PE, Thompson MA, et al. Antiretroviral drugs for treatment and prevention of HIV infection in adults: 2016 recommendations of the international antiviral society-USA panel. JAMA. 2016;316(2):191–210. doi: 10.1001/jama.2016.8900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Widera M, Dirks M, Bleekmann B, Jablonka R, Daumer M, Walter H, Ehret R, Verheyen J, Esser S. HIV-1 persistent viremia is frequently followed by episodes of low-level viremia. Med Microbiol Immunol. 2017;206(3):203–215. doi: 10.1007/s00430-017-0494-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thompson MA, Aberg JA, Hoy JF, Telenti A, Benson C, Cahn P, Eron JJ, Gunthard HF, Hammer SM, Reiss P, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International Antiviral Society-USA panel. JAMA. 2012;308(4):387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 17.Writing G, Williams I, Churchill D, Anderson J, Boffito M, Bower M, Cairns G, Cwynarski K, Edwards S, Fidler S, et al. British HIV Association guidelines for the treatment of HIV-1-positive adults with antiretroviral therapy 2012 (Updated November 2013. All changed text is cast in yellow highlight) HIV Med. 2014;15(Suppl 1):1–85. doi: 10.1111/hiv.12119. [DOI] [PubMed] [Google Scholar]
- 18.Ruggiero A, Cozzi-Lepri A, Beloukas A, Richman D, Khoo S, Phillips A, Geretti AM, Group ES Factors associated with persistence of plasma HIV-1 RNA during long-term continuously suppressive firstline antiretroviral therapy. Open Forum Infect Dis. 2018;5(2):ofy032. doi: 10.1093/ofid/ofy032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dong K, Ye L, Leng Y, Liang S, Feng L, Yang H, Su L, Li Y, Baloch S, He F, et al. Prevalence of HIV-1 drug resistance among patients with antiretroviral therapy failure in Sichuan, China, 2010–2016. Tohoku J Exp Med. 2019;247(1):1–12. doi: 10.1620/tjem.247.1. [DOI] [PubMed] [Google Scholar]
- 20.Liu P, Liao L, Xu W, Yan J, Zuo Z, Leng X, Wang J, Kan W, You Y, Xing H, et al. Adherence, virological outcome, and drug resistance in Chinese HIV patients receiving first-line antiretroviral therapy from 2011 to 2015. Medicine (Baltimore) 2018;97(50):e13555. doi: 10.1097/MD.0000000000013555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding Y, Chen M, Wang J, Yang Y, Feng Y, Wang L, Duan S, Lin Q, Xing H, Ma Y, et al. Increase in HIV-1-transmitted drug resistance among ART-naive youths at the China-Myanmar border during 2009–2017. BMC Infect Dis. 2021;21(1):93. doi: 10.1186/s12879-021-05794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xing H, Ruan Y, Li J, Shang H, Zhong P, Wang X, Liao L, Li H, Zhang M, Xue Y, et al. HIV drug resistance and its impact on antiretroviral therapy in Chinese HIV-infected patients. PLoS ONE. 2013;8(2):e54917. doi: 10.1371/journal.pone.0054917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xing H, Wang X, Liao L, Ma Y, Su B, Fu J, He J, Chen L, Pan X, Dong Y, et al. Incidence and associated factors of HIV drug resistance in Chinese HIV-infected patients receiving antiretroviral treatment. PLoS ONE. 2013;8(4):e62408. doi: 10.1371/journal.pone.0062408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, Liu L, Sun M, Sun J, Lu H. An analysis of drug resistance among people living with HIV/AIDS in Shanghai, China. PLoS ONE. 2017;12(2):e0165110. doi: 10.1371/journal.pone.0165110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pang X, Tang K, He Q, Huang J, Fang N, Zhou X, Zhu Q, Wu X, Shen Z, Liang S. HIV drug resistance and HIV transmission risk factors among newly diagnosed individuals in Southwest China. BMC Infect Dis. 2021;21(1):160. doi: 10.1186/s12879-021-05854-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang T, Ding H, An M, Wang X, Tian W, Zhao B, Han X. Factors associated with high-risk low-level viremia leading to virologic failure: 16-year retrospective study of a Chinese antiretroviral therapy cohort. BMC Infect Dis. 2020;20(1):147. doi: 10.1186/s12879-020-4837-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu P, Xing H, Liao L, Feng Y, Leng X, Wang J, Kan W, Yan J, Li Y, Zuo Z, et al. HIV drug resistance in patients in China’s national HIV treatment programme who have been on first-line ART for at least 9 months. AIDS Res Ther. 2020;17(1):9. doi: 10.1186/s12981-020-00264-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jiang J, Qin F, Meng S, Nehl EJ, Huang J, Liu Y, Zou J, Dong W, Huang J, Chen H, et al. Effects of cotrimoxazole prophylaxis on Talaromyces marneffei infection in HIV/AIDS patients receiving antiretroviral therapy: a retrospective cohort study. Emerg Microbes Infect. 2019;8(1):367–376. doi: 10.1080/22221751.2019.1588078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu J, Wang L, Hou Y, Zhao Y, Dou Z, Ma Y, Zhang D, Wu Y, Zhao D, Liu Z, et al. Immune restoration in HIV-1-infected patients after 12 years of antiretroviral therapy: a real-world observational study. Emerg Microbes Infect. 2020;9(1):2550–2561. doi: 10.1080/22221751.2020.1840928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engsig FN, Gerstoft J, Kronborg G, Larsen CS, Pedersen G, Roge B, Jensen J, Nielsen LN, Obel N. Long-term mortality in HIV patients virally suppressed for more than three years with incomplete CD4 recovery: a cohort study. BMC Infect Dis. 2010;10:318. doi: 10.1186/1471-2334-10-318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stirrup OT, Copas AJ, Phillips AN, Gill MJ, Geskus RB, Touloumi G, Young J, Bucher HC, Babiker AG, EuroCoord CCi Predictors of CD4 cell recovery following initiation of antiretroviral therapy among HIV-1 positive patients with well-estimated dates of seroconversion. HIV Med. 2018;19(3):184–194. doi: 10.1111/hiv.12567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cunha RF, Simoes S, Carvalheiro M, Pereira JMA, Costa Q, Ascenso A. Novel antiretroviral therapeutic strategies for HIV. Molecules. 2021;26(17):5305. doi: 10.3390/molecules26175305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xing H, Ruan Y, Hsi JH, Kan W, Liao L, Leng X, Wang J, He C, Shao Y, National HWG. Reductions in virological failure and drug resistance in Chinese antiretroviral-treated patients due to lamivudine-based regimens, 2003–12. J Antimicrob Chemother. 2015;70(7):2097–2103. doi: 10.1093/jac/dkv078. [DOI] [PubMed] [Google Scholar]
- 34.Guo H, Xu X, Hu H, Zhou Y, Yang H, Qiu T, Fu G, Huan X. Low prevalence of the transmitted HIV-1 drug resistance among newly diagnosed HIV-1 individuals in Jiangsu Province, China during 2009–2011. BMC Public Health. 2015;15:120. doi: 10.1186/s12889-015-1489-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liao L, Xing H, Su B, Wang Z, Ruan Y, Wang X, Liu Z, Lu Y, Yang S, Zhao Q, et al. Impact of HIV drug resistance on virologic and immunologic failure and mortality in a cohort of patients on antiretroviral therapy in China. AIDS. 2013;27(11):1815–1824. doi: 10.1097/QAD.0b013e3283611931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Laprise C, de Pokomandy A, Baril JG, Dufresne S, Trottier H. Virologic failure following persistent low-level viremia in a cohort of HIV-positive patients: results from 12 years of observation. Clin Infect Dis. 2013;57(10):1489–1496. doi: 10.1093/cid/cit529. [DOI] [PubMed] [Google Scholar]
- 37.Fleming J, Mathews WC, Rutstein RM, Aberg J, Somboonwit C, Cheever LW, Berry SA, Gebo KA, Moore RD, Network HIVR. Low-level viremia and virologic failure in persons with HIV infection treated with antiretroviral therapy. AIDS. 2019;33(13):2005–2012. doi: 10.1097/QAD.0000000000002306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vandenhende MA, Perrier A, Bonnet F, Lazaro E, Cazanave C, Reigadas S, Chene G, Morlat P, Aquitaine ACEGo Risk of virological failure in HIV-1-infected patients experiencing low-level viraemia under active antiretroviral therapy (ANRS C03 cohort study) Antivir Ther. 2015;20(6):655–660. doi: 10.3851/IMP2949. [DOI] [PubMed] [Google Scholar]
- 39.Zuo Z, Liang S, Sun X, Bussell S, Yan J, Kan W, Leng X, Liao L, Ruan Y, Shao Y, et al. Drug resistance and virological failure among HIV-infected patients after a decade of antiretroviral treatment expansion in eight provinces of China. PLoS ONE. 2016;11(12):e0166661. doi: 10.1371/journal.pone.0166661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delaugerre C, Gallien S, Flandre P, Mathez D, Amarsy R, Ferret S, Timsit J, Molina JM, de Truchis P. Impact of low-level-viremia on HIV-1 drug-resistance evolution among antiretroviral treated-patients. PLoS ONE. 2012;7(5):e36673. doi: 10.1371/journal.pone.0036673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yuan D, Liu M, Jia P, Li Y, Huang Y, Ye L, Api L, Chen M, Yao L, Wang Z, et al. Prevalence and determinants of virological failure, genetic diversity and drug resistance among people living with HIV in a minority area in China: a population-based study. BMC Infect Dis. 2020;20(1):443. doi: 10.1186/s12879-020-05124-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amstutz A, Nsakala BL, Vanobberghen F, Muhairwe J, Glass TR, Namane T, Mpholo T, Battegay M, Klimkait T, Labhardt ND. Switch to second-line versus continued first-line antiretroviral therapy for patients with low-level HIV-1 viremia: an open-label randomized controlled trial in Lesotho. PLoS Med. 2020;17(9):e1003325. doi: 10.1371/journal.pmed.1003325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang X, Su B, Zhang X, Liu Y, Wu H, Zhang T. Incomplete immune reconstitution in HIV/AIDS patients on antiretroviral therapy: challenges of immunological non-responders. J Leukoc Biol. 2020;107(4):597–612. doi: 10.1002/JLB.4MR1019-189R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gagliardo C, Brozovich A, Birnbaum J, Radix A, Foca M, Nelson J, Saiman L, Yin M, Carras-Terzian E, West E, et al. A multicenter study of initiation of antiretroviral therapy and transmitted drug resistance in antiretroviral-naive adolescents and young adults with HIV in New York City. Clin Infect Dis. 2014;58(6):865–872. doi: 10.1093/cid/ciu003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Shoko C, Chikobvu D. A superiority of viral load over CD4 cell count when predicting mortality in HIV patients on therapy. BMC Infect Dis. 2019;19(1):169. doi: 10.1186/s12879-019-3781-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ji Y, Zhang F, Zhang R, Shen Y, Liu L, Wang J, Yang J, Tang Q, Xun J, Qi T, et al. Changes in intestinal microbiota in HIV-1-infected subjects following cART initiation: influence of CD4+ T cell count. Emerg Microbes Infect. 2018;7(1):113. doi: 10.1038/s41426-018-0117-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu P, Feng Y, Wu J, Tian S, Su B, Wang Z, Liao L, Xing H, You Y, Shao Y, et al. Polymorphisms and mutational covariation associated with death in a prospective cohort of HIV/AIDS patients receiving long-term ART in China. PLoS ONE. 2017;12(1):e0170139. doi: 10.1371/journal.pone.0170139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.When To Start C. Sterne JA, May M, Costagliola D, de Wolf F, Phillips AN, Harris R, Funk MJ, Geskus RB, Gill J, et al. Timing of initiation of antiretroviral therapy in AIDS-free HIV-1-infected patients: a collaborative analysis of 18 HIV cohort studies. Lancet. 2009;373(9672):1352–1363. doi: 10.1016/S0140-6736(09)60612-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang F, Dou Z, Ma Y, Zhang Y, Zhao Y, Zhao D, Zhou S, Bulterys M, Zhu H, Chen RY. Effect of earlier initiation of antiretroviral treatment and increased treatment coverage on HIV-related mortality in China: a national observational cohort study. Lancet Infect Dis. 2011;11(7):516–524. doi: 10.1016/S1473-3099(11)70097-4. [DOI] [PubMed] [Google Scholar]
- 50.Zhang F, Dou Z, Ma Y, Zhao Y, Liu Z, Bulterys M, Chen RY. Five-year outcomes of the China national free antiretroviral treatment program. Ann Intern Med. 2009;151(4):241–251. doi: 10.7326/0003-4819-151-4-200908180-00006. [DOI] [PubMed] [Google Scholar]
- 51.Hamers RL, Sigaloff KC, Wensing AM, Wallis CL, Kityo C, Siwale M, Mandaliya K, Ive P, Botes ME, Wellington M, et al. Patterns of HIV-1 drug resistance after first-line antiretroviral therapy (ART) failure in 6 sub-Saharan African countries: implications for second-line ART strategies. Clin Infect Dis. 2012;54(11):1660–1669. doi: 10.1093/cid/cis254. [DOI] [PubMed] [Google Scholar]
- 52.Zhang FJ, Pan J, Yu L, Wen Y, Zhao Y. Current progress of China’s free ART program. Cell Res. 2005;15(11–12):877–882. doi: 10.1038/sj.cr.7290362. [DOI] [PubMed] [Google Scholar]
- 53.Luo L, Li TS. Overview of antiretroviral treatment in China: advancement and challenges. Chin Med J (Engl) 2011;124(3):440–444. [PubMed] [Google Scholar]
- 54.Li Z, Huang Y, Ouyang Y, Xing H, Liao L, Jiang S, Shao Y, Ma L. Mutation covariation of HIV-1 CRF07_BC reverse transcriptase during antiretroviral therapy. J Antimicrob Chemother. 2013;68(11):2521–2524. doi: 10.1093/jac/dkt228. [DOI] [PubMed] [Google Scholar]
- 55.Villa G, Abdullahi A, Owusu D, Smith C, Azumah M, Sayeed L, Austin H, Awuah D, Beloukas A, Chadwick D, et al. Determining virological suppression and resuppression by point-of-care viral load testing in a HIV care setting in sub-Saharan Africa. EClinicalMedicine. 2020;18:100231. doi: 10.1016/j.eclinm.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated and/or analysed during the current study are not publicly available due to data constraint requirements from China CDC but are available from the corresponding author on reasonable request.