Abstract
Objective
To present an overview of different approaches and recent advances for long-term preservation of germ cells and gonadal tissues at ambient temperatures.
Methods
Review of the existing literature.
Results
Preserving viable spermatozoa, eggs, embryos, and gonadal tissues for the long term is critical in human fertility treatment and for the management of animal populations (livestock, biomedical models, and wild species). The need and number of banked germplasms are growing very fast in all disciplines, but current storage options at freezing temperatures are often constraining and not always sustainable. Recent research indicates that structures and functions of gametes or gonadal tissues can be preserved for the long term using different strategies based on dehydration and storage at supra-zero temperatures. However, more studies are needed in rehydration and reanimation of germplasms (including proper molecular and cellular evaluations).
Conclusions
While a lot of research is still warranted to optimize drying and rehydration conditions for each sample type and each species, alternative preservation methods will change the paradigm in fertility preservation and biobanking. It will transform the way we maintain and manage precious biomaterials for the long term.
Lay summary
Living sperm cells, eggs, embryos, and reproductive tissues can be preserved at freezing temperatures for human fertility treatments and used to manage breeding in livestock, laboratory animals, and wild species through assisted reproduction. These cells can be stored in cell banks and demand for them is growing fast. However, current long-term storage options at freezing temperatures are expensive. Instead of using low temperatures, recent research indicates that these cells can be dried and stored above freezing temperatures for an extended amount of time. While a lot of research is still needed to optimize how different samples are dried and rehydrated, alternative methods of preserving cells will make fertility preservation and cell banking easier. It will also transform the way we keep and manage samples for the long term.
Key Words: fertility preservation, biobanking, gametes, gonadal tissues, desiccation, non-cryogenic storage
Introduction: values and limitations of cryo-banking in fertility preservation
Preserving viable biomaterials of good quality for the long term is essential in many scientific disciplines. To protect, preserve, or even extend fertility, there is a specific interest in preserving spermatozoa, eggs, embryos, and gonadal tissues (so-called germplasms) in human reproductive medicine, livestock production, laboratory animal management, and wild species conservation (Saragusty et al. 2020). The need and demand for reliable and sustainable germplasm storage are exponentially increasing for fertility treatments in humans and animals (in association with the use of assisted reproductive technologies, ART) (Comizzoli et al. 2018, Holt & Comizzoli 2021).
Currently, biophysical and biochemical activities can be suspended at freezing temperatures in cells and tissues. This ensures long-term longevity and quality of living biomaterials (Hubel et al. 2014, Wolkers & Oldenhof 2021). However, during cryopreservation, germplasms must go through a series of stresses including exposure to toxic cryoprotectant(s), detrimental ice formation during cooling/freezing, possible variations of temperature during storage (risk of accelerated degradation of the samples), and then thawing/warming (risk of devitrification and/or ice recrystallization). Furthermore, sensitivity and response to those stresses vary among species as well as between tissues, cells, organelles, and DNA. We have learned this from years of research in diverse animal models (Comizzoli & Wildt 2013, 2017, Holt & Comizzoli 2021). While vitrification was reported in the mid-1980s to overcome issues related to ice crystal formation in mouse embryos (Rall & Fahy 1985), little attention has been directed to alternative ways for long-term storage of germplasms.
In addition, electrical ultra-cold freezers and liquid nitrogen containers require constant monitoring, complex maintenance, alarm systems, and specialized rooms fitted with backup power and controlled environment. Unfortunately, facilities with sustained supply of electrical power and liquid nitrogen are expensive and not always affordable or readily available in some regions of the world. In addition to possible issues of cross-contaminations in liquid nitrogen (Bajerski et al. 2021), cryo-storage systems are prone to failures – from equipment breakdown to human error – which, recently, has led to dramatic sample losses in human fertility clinics and research laboratories (Pomeroy et al. 2019, Letterie & Fox 2020). To address the limitations mentioned above, researchers have been exploring for many years alternative solutions to safely store germplasms for later use in fertility preservation programs. The objective of the review is to present different approaches and recent advances toward long-term preservation of germ cells and gonadal tissues at ambient temperatures.
Principles and different approaches for long-term storage of germplasms at ambient temperatures
Principles of dehydration
To explore alternative preservation strategies, scientists have been inspired by a vast array of organisms (microbes, fungi, plants, seeds, and animals) that have evolved to survive nearly complete dehydration in nature, sometimes for years or decades. No other strategy in nature is as efficient as dehydration for long-term stabilization. Certain nematodes, tardigrades, insects, and brine shrimp survive extreme cellular water loss via a natural process called ‘anhydrobiosis’ – a term first used by Alfred Giard in 1894 (Keilin 1959, Crowe 2012). Cellular and molecular structures and functions can then be preserved in the dehydrated state above freezing temperatures.
Studies of anhydrobiotic organisms have provided several candidate genes related to the production of ‘xero-protectants’ and conveying tolerance to extreme conditions (Belott et al. 2020, Czernik et al. 2020, Voronina et al. 2020, Anderson & Hand 2021). Unfortunately, desiccation genes or related analogs are not present in vertebrate genomes. It is therefore mandatory to directly supply ‘xero-protectants’ to the cells from vertebrate species before removing the water content (Loi et al. 2021).
In addition to slowing down metabolism and producing critical components, one of the keys to reach and survive dry conditions relies on the organisms’ capacity to synthesize and accumulate intracellular disaccharides (mainly trehalose or sucrose) while losing water content (Wolkers & Oldenhof 2021). After introduction to the cells, major advantages of natural disaccharides like trehalose are their low toxicity and high glass-transition temperature (possibility to vitrify at non-freezing temperatures) compared to conventional cryoprotectants such as dimethyl sulfoxide, ethylene glycol, or 1,2-propanediol (Chen et al. 2000).
Numerous studies have demonstrated the superior ability of the non-reducing trehalose to stabilize key cellular components, including membranes, proteins, and DNA upon desiccation (Crowe 2012, Zhang et al. 2016, Brogna et al. 2021, Wolkers & Oldenhof 2021). Three mechanisms have been proposed to explain the desiccation-tolerant properties of trehalose (Hibshman et al. 2020). First, the water replacement hypothesis suggests that the disaccharides substitute water molecules during desiccation to form hydrogen bonds with the natural biomacromolecules (such as lipid membrane, proteins, or nucleic acids), which maintains their native 3D/ordered confirmations and structures (Jain & Roy 2009, Golovina et al. 2010). As water plays a fundamental role in maintaining protein structure and function, the second hypothesis (water entrapment hypothesis) states that an extremely thin layer of water surrounding surfaces of macromolecules remains entrapped in a shell formed by trehalose to maintain their native 3D/ordered confirmations and structures during desiccation, protecting proteins from damage (Wolkers et al. 2010, Olsson et al. 2019). Lastly, removal of water leads to increase in trehalose viscosity and transforms it from the liquid state to a glassy state (a process known as vitrification) (Crowe et al. 1998). This amorphous glass likely facilitates immobilization of cellular structures and promotes quiescence of enzymatic activities. While the glass-transition property is not unique to trehalose, it has higher glass-transition temperature compared to other disaccharides, potentially allowing stable preservation at higher temperatures, such as ambient temperatures. The three mechanisms are not mutually exclusive and likely operate synergistically to achieve the protective effect of trehalose against desiccation stress. In addition to trehalose, the production of late embryogenesis abundant (LEA) proteins and heat shock proteins (HSPs) have also been observed in a variety of anhydrobiotic organisms. These proteins that form functional 3D conformations upon dehydration, often in conjunction with trehalose, likely act as chaperons to stabilize cellular components (Hincha & Thalhammer 2012, Li et al. 2012, Kim et al. 2018, Czernik et al. 2020).
Dehydration methods and storage options for germplasms
Note that excellent illustrations of the methods described below are available in recent reviews (Loi et al. 2021, Weng 2021).
Lyophilization (freeze-drying) is the most widely used method for germplasm desiccation (Saragusty et al. 2020, Loi et al. 2021), mainly for sperm cells. The process includes freezing samples followed by primary drying through sublimation of ice at freezing temperature under vacuum and secondary drying through desorption by slowly elevating temperature under vacuum (Weng 2021). Protectants and supplements used in this procedures include trehalose (Martins et al. 2007, Sánchez-Partida et al. 2008, Ito et al. 2019, Shahmoradi et al. 2021), buffers like EGTA (Liu et al. 2004, Martins et al. 2007, Ringleb et al. 2013, Palazzese et al. 2020) or EDTA (Mercati et al. 2020), fetal bovine serum (Choi et al. 2011), or media/buffer only (Keskintepe et al. 2002, Kwon et al. 2004). Sperm cells from many species have been preserved using that method (Patrick et al. 2017a, Saragusty et al. 2020). Embryos or live birth have been obtained from freeze-dried spermatozoa stored at non-freezing temperatures in mouse (Wakayama & Yanagimachi 1998, Kusakabe & Tateno 2011, Ito et al. 2019), pig (Kwon et al. 2004), monkey (Sánchez-Partida et al. 2008), sheep (Olaciregui et al. 2017, Anzalone et al. 2018, Arav et al. 2018), horse (Choi et al. 2011), or cattle (Keskintepe et al. 2002). All studies had to use intra-cytoplasmic sperm injection (ICSI) as sperm cells are not motile after rehydration. Most common storage containers for lyophilized sperm cells are glass ampoules/vials (Fig. 1). Recently, it has been demonstrated in mouse that freeze-drying on weighing paper and then storing in between plastic sheet had comparable outcomes, which further simplifies storage (Ito et al. 2021). Freeze-drying of sperm cells in trehalose has also been successful in humans (Gianaroli et al. 2012, Keskintepe & Eroglu 2021). Recently, partial freeze/dried human spermatozoa was successfully rehydrated and utilized for ICSI, leading to the production of normal euploid human blastocysts (Alexandrova et al. 2020).
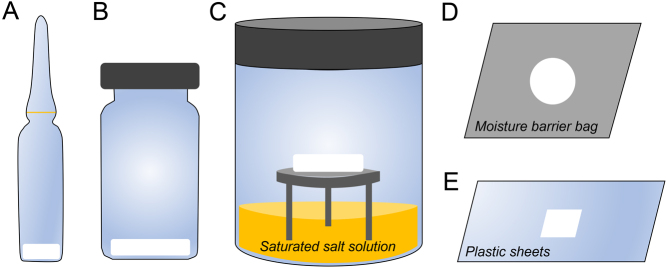
Figure 1.
Different types of storage containers for dried samples. Glass ampoule (A), glass vial (B), salt sorption jars (C), moisture-barrier bag (D), plastic sheet (E). All devices are of comparable size, except for the jars that are larger in general.
Regarding the female gamete, freeze-dried porcine nuclei (germinal vesicles) can resume meiosis after transfer into an enucleated oocyte (Dang-Nguyen et al. 2018). Further analysis demonstrated that a small portion of dried germinal vesicles stored in glass vial at non-freezing temperatures retained intact nuclear envelope and/or DNA. Freeze-drying of ovarian tissue has recently been attempted in sheep. Results showed high levels of RNA degradation and morphological alteration (Bebbere et al. 2021). Nonetheless, it provided clues for subsequent improvement and storage options. Currently, there are no reports on freeze-drying of embryos or testicular tissues yet.
Passive air drying or evaporative drying have been attempted in sperm cells and germinal vesicles. Long-term storage after evaporative drying was successful in mouse sperm cells (Li et al. 2007). Air drying and ambient temperatures also induce conformational changes of nucleic acids and stallion sperm chromatin in trehalose preservation formulations (Brogna et al. 2021). Comparable observations about the loss of DNA integrity have also been reported in air-dried llama spermatozoa (Carretero et al. 2020). On the female side, cat germinal vesicles that were air-dried in trehalose and stored at 4°C for up to 8 weeks largely retained DNA and membrane integrity. A portion of these nuclei were capable to resume meiosis after transfer to fresh cytoplasts (Graves-Herring et al. 2013).
Convective drying is a different method that utilizes dry gas (nitrogen) to facilitate active water evaporation and vapor removal. It is one of the earliest methods tested to bypass freezing to achieve sample desiccation. The approach has been used for desiccation of mouse spermatozoa, which resulted in fetus production (Bhowmick 2003, McGinnis 2005, Liu et al. 2012). The spermatozoa in these studies were dried on glass slides and stored either in vacuum-sealed bags (Bhowmick 2003, McGinnis 2005) or salt-sorption jars (Liu et al. 2012) at 4°C or ambient temperatures (Fig. 1).
Microwave-assisted drying is one of the most recent strategies to accelerate water evaporation while staying within physiological temperatures. It was first explored to desiccate live mammalian cells (mouse macrophage cells) in 2008, showing viability after rehydration (Chakraborty et al. 2008). Drying kinetics and sample uniformity were thoroughly characterized (Chakraborty et al. 2008, Cellemme et al. 2013). It was later translated to the cat spermatozoa, with morphology and DNA integrity being maintained after drying on coverslips. Even after immediate rehydration, developmental potential was reduced (Patrick et al. 2017b). In recent studies using storage in moisture-barrier bags (Fig. 1) at −20°C, DNA integrity was unchanged in the first 3 months and only moderately decreased after longer storage (5–16 months). Developmental potential was sustained after up to 16 months of storage (Lee et al. 2021). Other studies focused on the cat germinal vesicle that could be dried on glass fiber filter paper to a moisture level that was compatible with supra-zero temperature storage. DNA integrity was mostly maintained after storage for up to 8 weeks at either 4°C or ambient temperatures in moisture-barrier bags (Elliott et al. 2015). Epigenetic alteration (decreased H3K4me3) was observed after germinal vesicle drying as well as an increase in structural damage of nuclear envelope and chromatin but to a lesser extent compared to cryopreservation (Lee & Comizzoli 2019). The feasibility of applying this drying technique to gonadal tissue has also been explored. Drying of cat ovarian tissue showed limited impact on morphology but altered transcriptional activity and gene expression (Lee & Comizzoli 2019, Lee et al. 2019, Amelkina & Comizzoli 2020). Recently, drying of cat testicular tissues showed that structural integrity and cell viability could be maintained at an acceptable level (Silva et al. 2020).
Other dehydration methods have been explored but there are no reports on germplasms or even live cells yet. Spin drying combines convective evaporation with water removal by centrifugal force to form an ultra-thin layer of samples for achieving rapid drying. During passive drying in trehalose droplets, a thin glassy film may form at the trehalose/air interface to dramatically slow down the evaporative desiccation of trehalose solution (He et al. 2008, Chakraborty et al. 2011). The method was first developed to overcome this caveat and provide more homogenous desiccation and effective drying was then confirmed by Raman spectroscopy (Abazari et al. 2014). Spin drying of mammalian cells (CHO cells) retained membrane integrity in >95% of cells (Chakraborty et al. 2011); however, functional survival of live cells has not been reported. Lastly, light-assisted drying (LAD) utilizes near-infrared laser light to facilitate samples drying. Initial reports suggested maintenance of protein functionality after drying (Young et al. 2018). The authors also showed that LAD-processed samples largely remained in glassy state after storage at ambient temperatures at low relative humidity (Furr et al. 2020).
Overall, these encouraging results clearly show that structures and, more importantly, functions of gametes and gonadal tissues can be suspended in trehalose glass after desiccation and potentially be preserved for the long term at supra-zero temperatures. In the meantime, we also learned that there is still a need for environmental control during storage (temperature and relative humidity levels). As mentioned above, storage containers for ambient temperature storage vary according to the methods (Fig. 1). Glass ampoules (Kamada et al. 2018) or glass vials (Dang-Nguyen et al. 2018) have been traditionally used to store lyophilized germplasms. The main inconvenience is that ampoules are breakable. Salt-sorption jars have been experimented to control the percentage of relative humidity (Liu et al. 2012), but their size is not practical for long-term storage. More recently, moisture-barrier bag (Elliott et al. 2015, Lee et al. 2021) or plastic sheets (Ito et al. 2021) have been used. Besides the advantage of gaining space for storage, they also are easier to ship from one location to the other.
Research directions to optimize germplasm preservation at ambient temperatures
Studies on rehydration and recovery from the desiccation stress
So far, most studies focused on optimizing the drying process to reduce the damage, and little is known about rehydration conditions, which is a critical step (Loi et al. 2021). Commonly, rehydration is achieved by simply adding the volume of water lost back to the sample. One study reported that stepwise rehydration with serial dilution of trehalose after microwave-assisted drying of cat germinal vesicle did not seem to mitigate drying-induced epigenetic alteration (Lee & Comizzoli 2019). Recent studies in anhydrobiosis provided valuable insight into the molecular regulation of the recovery process. For instance, a high-throughput mass spectrometry analysis in Chironomus discovered that, while trehalose is crucial during desiccation, glucosamine appears to be essential for recovery (Thorat et al. 2017). Transcriptome and/or proteomic analysis revealed that several DNA repair systems including homologous recombination, nucleotide excision repair, non-homologous end joining are active during rehydration/recovery phase in an anhydrobiotic cell line and a desiccation-resistant bacterium (Ujaoney et al. 2017, Yamada et al. 2018). Certain HSPs also are upregulated by rehydration processes in fly pupae and springtails, suggesting distinct roles (Hayward et al. 2004, Sørensen et al. 2010).
Other research needs
Despite encouraging advances in storage at ambient temperatures, more research is needed. As mentioned above, the scientific and technical evidence of an optimal dehydration method is missing (including the species-specificities of the approaches). Although natural ‘anhydrobiosis’ is inspiring, none of the small organisms mentioned earlier undergo freezing followed by low-pressure sublimation of ice. Thus, there is an urgent need to optimize the dehydration process and storage containers with devices adapted to the type and size of each sample for each animal species. A comprehensive list of necessary evaluations in rehydrated samples is provided in Fig. 2. So far, most studies have focused on structures/components and functions of germplasms although biosynthesis and metabolism have not been thoroughly explored. Too little research has been conducted on gene expression (Fig. 2), which should be one of the highest priorities in the coming years to develop optimal protocols for different species.
Figure 2.
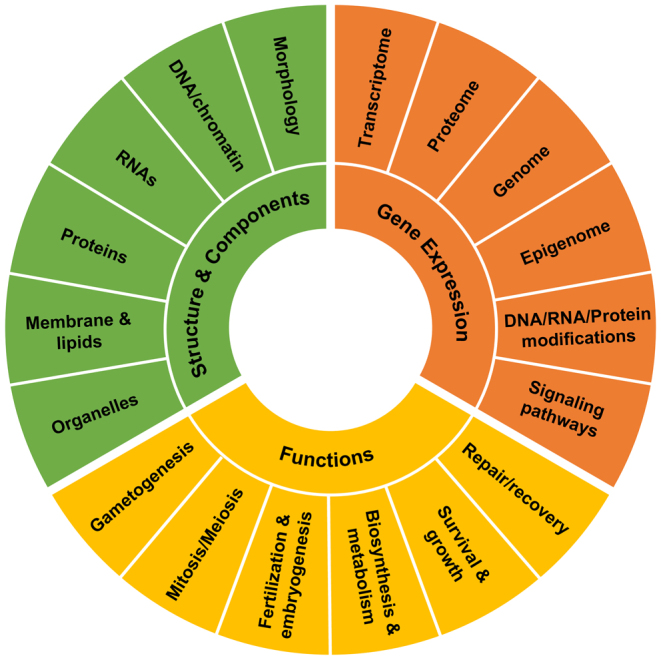
Full list of required evaluations for rehydrated samples.
Collaborative efforts with bioengineers have been fruitful to develop dehydration and storage methods. Continued work at the intersection of these disciplines will lead to the best solutions for germplasms. Although motile sperm cells would be desirable (to allow in vitro fertilization), progresses in ICSI are made rapidly as shown by the increasing success of embryonic development following injection of dried/rehydrated spermatozoa. Even minor technical improvements affect the efficiency dramatically (Palazzese et al. 2020). Further research in experimental animal models also is warranted before translating new knowledge to other species, including humans. While there might be a growing interest in human reproductive medicine in adopting dry storage for human spermatozoa (Gianaroli et al. 2012), many more data are required on the production of live offspring with freeze-dried spermatozoa, including their health later in life, even at the epigenetic/genetic level to exclude long-term side effects on DNA caused by desiccation and storage at ambient temperatures (Fig. 2). Lastly, studies on germplasms in the following areas will help to make progress faster: trehalose delivery, including through nanoparticles (Rao et al. 2015, Zhang et al. 2019); adaptation of drying technologies to large and complex biological samples (tissues, organs); and genome-wide evaluations (transcriptome, epigenome).
Conclusions and future perspectives about operations of biobanks at ambient temperatures for humans or animal species
Regardless of the drying and storage approaches that are chosen for fertility preservation, we will still have to ask the same essential questions to any new emerging banking effort: What are we storing? Why are we storing it? What storage container are we using? How many do we want to store? For how long? However, storage at ambient temperatures will lead to different biobanking logistics and operations in terms of processes, maintenance, and curation (Comizzoli et al. 2022). Desiccating and storing germplasms at ambient temperatures would be highly advantageous. It would decrease the costs related to processing and storage of samples by simplifying the preservation methods, reducing the need for specialized space/infrastructures, and avoiding liquid nitrogen purchase. Biosecurity (prevention of pathogen transmission) of storage at supra-zero temperatures in individual containers should be higher than for samples placed within the same liquid nitrogen vat. Transport of biomaterials between locations will be easier, with patients even having the option of at-home-storage for their own samples. Lastly, while moisture content will have to be maintained to a low level to prevent degradation during storage, samples will be more resilient to variations of temperatures than frozen samples.
Even though there will be less constraints in terms of the location, new storage facilities at ambient temperatures will still require environmental control, sample accessioning, and safety. New sample holders and identification/ labeling methodologies will also be needed for dried samples. In sum, a whole set of standard operating procedures will have to be developed. As mentioned, ambient temperature storage will also help to develop the concept of de-centralized biobanks (or home-storage) that involves less liability than centralized biobanks. Samples would be closer to the end-users and could be easily stored for a short duration. However, new sets of ethical aspects and issues of proper use (risk of parallel markets) may have to be anticipated.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
For P C and P C L, this publication was supported by the Office of The Director, National Institutes of Health under Award Number R01OD023139. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. X H is supported by NSF CBET-1831019 and NIH R01EB023632.
Author contribution statement
P C conceived the idea for the article, undertook the literature search, wrote the manuscript, and approved the manuscript for submission. X H conceived the idea for the manuscript, undertook editorial changes, and approved it for submission. P C L conceived the idea for the article, undertook the literature search, wrote sections of the manuscript, developed the figures, and approved the manuscript for submission.
References
- Abazari A, Chakraborty N, Hand S, Aksan A, Toner M.2014A Raman microspectroscopy study of water and trehalose in spin-dried cells. Biophysical Journal 107 2253. ( 10.1016/j.bpj.2014.09.032) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandrova S, Arav A, Hood K, Natan Y, Zhang J, Pasquale P.2020Successful production of normal euploid human blastocysts derived from sperm after partial freeze drying, rehydration and ICSI: towards developing a novel method for safe storage of biological samples. Fertility and Sterility 114e37–e38. [Google Scholar]
- Amelkina O, Comizzoli P.2020Initial response of ovarian tissue transcriptome to vitrification or microwave-assisted dehydration in the domestic cat model. BMC Genomics 21 828. ( 10.1186/s12864-020-07236-z) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson JM, Hand SC.2021Transgenic expression of late embryogenesis abundant proteins improves tolerance to water stress in Drosophila melanogaster. Journal of Experimental Biology 224 jeb238204. ( 10.1242/jeb.238204) [DOI] [PubMed] [Google Scholar]
- Anzalone DA, Palazzese L, Iuso D, Martino G, Loi P.2018Freeze-dried spermatozoa: an alternative biobanking option for endangered species. Animal Reproduction Science 19085–93. ( 10.1016/j.anireprosci.2018.01.010) [DOI] [PubMed] [Google Scholar]
- Arav A, Idda A, Nieddu SM, Natan Y, Ledda S.2018High post-thaw survival of ram sperm after partial freeze-drying. Journal of Assisted Reproduction and Genetics 351149–1155. ( 10.1007/s10815-018-1145-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bajerski F, Nagel M, Overmann J.2021Microbial occurrence in liquid nitrogen storage tanks: a challenge for cryobanking? Applied Microbiology and Biotechnology 1057635–7650. ( 10.1007/s00253-021-11531-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bebbere D, Arav A, Nieddu SM, Burrai GP, Pietro SS, Patrizio P, Ledda S.2021Molecular and histological evaluation of sheep ovarian tissue subjected to lyophilization. Animals 11 3407. ( 10.3390/ani11123407) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belott C, Janis B, Menze MA.2020Liquid-liquid phase separation promotes animal desiccation tolerance. PNAS 11727676–27684. ( 10.1073/pnas.2014463117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhowmick S, Zhu L, McGinnis L, Lawitts J, Nath BD, Toner M, Biggers J.2003Desiccation tolerance of spermatozoa dried at ambient temperature: production of fetal mice. Biology of Reproduction 681779–1786. ( 10.1095/biolreprod.102.009407) [DOI] [PubMed] [Google Scholar]
- Brogna R, Fan J, Sieme H, Wolkers WF, Oldenhof H.2021Drying and temperature induced conformational changes of nucleic acids and stallion sperm chromatin in trehalose preservation formulations. Scientific Reports 11 14076. ( 10.1038/s41598-021-93569-y) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carretero MI, Chaves MG, Arraztoa CC, Fumuso FG, Gambarotta MC, Neild DM.2020Air-drying llama sperm affects DNA integrity. Frontiers in Veterinary Science 7597952. ( 10.3389/fvets.2020.597952) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cellemme SL, Van Vorst M, Paramore E, Elliott GD.2013Advancing microwave technology for dehydration processing of biologics. Biopreservation and Biobanking 11278–284. ( 10.1089/bio.2013.0024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty N, Biswas D, Parker W, Moyer P, Elliott GD.2008A role for microwave processing in the dry preservation of mammalian cells. Biotechnology and Bioengineering 100782–796. ( 10.1002/bit.21801) [DOI] [PubMed] [Google Scholar]
- Chakraborty N, Chang A, Elmoazzen H, Menze MA, Hand SC, Toner M.2011A spin-drying technique for Lyopreservation of mammalian cells. Annals of Biomedical Engineering 391582–1591. ( 10.1007/s10439-011-0253-1) [DOI] [PubMed] [Google Scholar]
- Chen T, Fowler A, Toner M.2000Literature review: Supplemented phase diagram of the trehalose-water binary mixture. Cryobiology 40277–282. ( 10.1006/cryo.2000.2244) [DOI] [PubMed] [Google Scholar]
- Choi YH, Varner DD, Love CC, Hartman DL, Hinrichs K.2011Production of live foals via intracytoplasmic injection of lyophilized sperm and sperm extract in the horse. Reproduction 142529–538. ( 10.1530/REP-11-0145) [DOI] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE.2013Mammalian fertility preservation through cryobiology: value of classical comparative studies and the need for new preservation options. Reproduction, Fertility, and Development 2691–98. ( 10.1071/RD13259) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Wildt DE.2017Cryobanking biomaterials from wild animal species to conserve genes and biodiversity: relevance to human biobanking and biomedical research. In Biobanking of Human Biospecimens, pp. 217–235. Cham: Springer. [Google Scholar]
- Comizzoli P, Paulson EE, McGinnis LK.2018The mutual benefits of research in wild animal species and human-assisted reproduction. Journal of Assisted Reproduction and Genetics 35551–560. ( 10.1007/s10815-018-1136-2) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comizzoli P, Loi P, Patrizio P, Hubel A.2022Long-term storage of gametes and gonadal tissues at room temperatures: the end of the ice age ? Journal of Assisted Reproduction and Genetics 39321–325. ( 10.1007/s10815-021-02392-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe JH.2012Introduction: stabilization of dry biological materials. Biopreservation and Biobanking 10375–375. ( 10.1089/bio.2012.1043) [DOI] [PubMed] [Google Scholar]
- Crowe JH, Carpenter JF, Crowe LM.1998The role of vitrification in anhydrobiosis.. Annual Review of Physiology 6073–103. ( 10.1146/annurev.physiol.60.1.73) [DOI] [PubMed] [Google Scholar]
- Czernik M, Fidanza A, Luongo FP, Valbonetti L, Scapolo PA, Patrizio P, Loi P.2020Late embryogenesis abundant (LEA) proteins confer water stress tolerance to mammalian somatic cells. Cryobiology 92189–196. ( 10.1016/j.cryobiol.2020.01.009) [DOI] [PubMed] [Google Scholar]
- Dang-Nguyen TQ, Nguyen HT, Nguyen MT, Somfai T, Noguchi J, Kaneko H, Kikuchi K.2018Maturation ability after transfer of freeze-dried germinal vesicles from porcine oocytes. Animal Science Journal 891253–1260. ( 10.1111/asj.13067) [DOI] [PubMed] [Google Scholar]
- Elliott GD, Lee PC, Paramore E, Van Vorst M, Comizzoli P.2015Resilience of oocyte germinal vesicles to microwave-assisted drying in the domestic cat model. Biopreservation and Biobanking 13164–171. ( 10.1089/bio.2014.0078) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furr DP, Elliott GD, Young MA, McKeough RQ, Trammell SR.2020Light-assisted drying for anhydrous preservation of biological samples: optical characterization of the trehalose preservation matrix. Biomedical Optics Express 11801–816. ( 10.1364/BOE.376630) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaroli L, Magli MC, Stanghellini I, Crippa A, Crivello AM, Pescatori ES, Ferraretti AP.2012DNA integrity is maintained after freeze-drying of human spermatozoa. Fertility and Sterility 971067, .e1–1073.e1. ( 10.1016/j.fertnstert.2012.02.014) [DOI] [PubMed] [Google Scholar]
- Golovina EA, Golovin A, Hoekstra FA, Faller R.2010Water replacement hypothesis in atomic details: effect of trehalose on the structure of single dehydrated POPC bilayers. Langmuir 2611118–11126. ( 10.1021/la100891x) [DOI] [PubMed] [Google Scholar]
- Graves-Herring JEJE, Wildt DEDE, Comizzoli P.2013Retention of structure and function of the cat germinal vesicle after air-drying and storage at suprazero temperature. Biology of Reproduction 88 139. ( 10.1095/biolreprod.113.108472) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward SAL, Rinehart JP, Denlinger DL.2004Desiccation and rehydration elicit distinct heat shock protein transcript responses in flesh fly pupae. Journal of Experimental Biology 207963–971. ( 10.1242/jeb.00842) [DOI] [PubMed] [Google Scholar]
- He X, Fowler A, Menze M, Hand S, Toner M.2008Desiccation kinetics and biothermodynamics of glass forming trehalose solutions in thin films. Annals of Biomedical Engineering 361428–1439. ( 10.1007/s10439-008-9518-8) [DOI] [PubMed] [Google Scholar]
- Hibshman JD, Clegg JS, Goldstein B.2020Mechanisms of desiccation tolerance: themes and variations in brine shrimp, roundworms, and tardigrades. Frontiers in Physiology 11592016. ( 10.3389/fphys.2020.592016) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hincha DK, Thalhammer A.2012LEA proteins: IDPs with versatile functions in cellular dehydration tolerance. Biochemical Society Transactions 401000–1003. ( 10.1042/BST20120109) [DOI] [PubMed] [Google Scholar]
- Holt WV, Comizzoli P.2021Genome resource banking for wildlife conservation: promises and caveats. Cryoletters 42309–320. [PubMed] [Google Scholar]
- Hubel A, Spindler R, Skubitz APN.2014Storage of human biospecimens: selection of the optimal storage temperature. Biopreservation and Biobanking 12165–175. ( 10.1089/bio.2013.0084) [DOI] [PubMed] [Google Scholar]
- Ito D, Wakayama S, Kamada Y, Shibasaki I, Kamimura S, Ooga M, Wakayama T.2019Effect of trehalose on the preservation of freeze-dried mice spermatozoa at room temperature. Journal of Reproduction and Development 65 353–359. ( 10.1262/jrd.2019-058) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito D, Wakayama S, Emura R, Ooga M, Wakayama T.2021Mailing viable mouse freeze-dried spermatozoa on postcards. iScience 24102815. ( 10.1016/j.isci.2021.102815) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jain NK, Roy I.2009Effect of trehalose on protein structure. Protein Science 1824–36. ( 10.1002/pro.3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamada Y, Wakayama S, Shibasaki I, Ito D, Kamimura S, Ooga M, Wakayama T.2018Assessing the tolerance to room temperature and viability of freeze-dried mice spermatozoa over long-term storage at room temperature under vacuum. Scientific Reports 810602. ( 10.1038/s41598-018-28896-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilin D.1959The problem of anabiosis or latent life: history and current concept. Proceedings of the Royal Society of London: Series B, Biological Sciences 150149–191. ( 10.1098/rspb.1959.0013) [DOI] [PubMed] [Google Scholar]
- Keskintepe L, Eroglu A.2021Preservation of mammalian sperm by freeze-drying. In Methods in Molecular Biology, pp. 721–730. Humana Press Inc. [DOI] [PubMed] [Google Scholar]
- Keskintepe L, Pacholczyk G, Machnicka A, Norris K, Curuk MA, Khan I, Brackett BG.2002Bovine blastocyst development from oocytes injected with freeze-dried spermatozoa. Biology of Reproduction 67409–415. ( 10.1095/biolreprod67.2.409) [DOI] [PubMed] [Google Scholar]
- Kim SX, Çamdere G, Hu X, Koshland D, Tapia H.2018Synergy between the small intrinsically disordered protein Hsp12 and trehalose sustain viability after severe desiccation. eLife 7e38337. ( 10.7554/eLife.38337) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakabe H, Tateno H.2011Characterization of chromosomal damage accumulated in freeze-dried mouse spermatozoa preserved under ambient and heat stress conditions. Mutagenesis 26447–453. ( 10.1093/mutage/ger003) [DOI] [PubMed] [Google Scholar]
- Kwon IK, Park KE, Niwa K.2004Activation, pronuclear formation, and development in vitro of pig oocytes following intracytoplasmic injection of freeze-dried spermatozoa. Biology of Reproduction 711430–1436. ( 10.1095/biolreprod.104.031260) [DOI] [PubMed] [Google Scholar]
- Lee PC, Comizzoli P.2019Desiccation and supra-zero temperature storage of cat germinal vesicles lead to less structural damage and similar epigenetic alterations compared to cryopreservation. Molecular Reproduction and Development 861822–1831. ( 10.1002/mrd.23276) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Adams DM, Amelkina O, White KK, Amoretti LA, Whitaker MG, Comizzoli P.2019Influence of microwave-assisted dehydration on morphological integrity and viability of cat ovarian tissues: first steps toward long-term preservation of complex biomaterials at supra-zero temperatures. PLoS ONE 14 e0225440. ( 10.1371/journal.pone.0225440) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee PC, Zahmel J, Jewgenow K, Comizzoli P.2021Desiccated cat spermatozoa retain DNA integrity and developmental potential after prolonged storage and shipping at non-cryogenic temperatures. Journal of Assisted Reproduction and Genetics 39141–151. ( 10.1007/s10815-021-02337-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letterie G, Fox D.2020Lawsuit frequency and claims basis over lost, damaged, and destroyed frozen embryos over a 10-year period. F&S Reports 1 78–82. ( 10.1016/j.xfre.2020.06.007) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li MW, Biggers JD, Elmoazzen HY, Toner M, McGinnis L, Lloyd KCK.2007Long-term storage of mouse spermatozoa after evaporative drying. Reproduction 133919–929. ( 10.1530/REP-06-0096) [DOI] [PubMed] [Google Scholar]
- Li S, Chakraborty N, Borcar A, Menze MA, Toner M, Hand SC.2012Late embryogenesis abundant proteins protect human hepatoma cells during acute desiccation. PNAS 10920859–20864. ( 10.1073/pnas.1214893109) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JL, Kusakabe H, Chang CC, Suzuki H, Schmidt DW, Julian M, Pfeffer R, Bormann CL, Tian XC, Yanagimachi Ret al. 2004Freeze-dried sperm fertilization leads to full-term development in rabbits. Biology of Reproduction 701776–1781. ( 10.1095/biolreprod.103.025957) [DOI] [PubMed] [Google Scholar]
- Liu J, Lee GY, Lawitts JA, Toner M, Biggers JD.2012Preservation of mouse sperm by convective drying and storing in 3-O-methyl-D-glucose. PLoS ONE 7e29924. ( 10.1371/journal.pone.0029924) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loi P, Anzalone DA, Palazzese L, Dinnyés A, Saragusty J, Czernik M.2021Dry storage of mammalian spermatozoa and cells: state-of-the-art and possible future directions. Reproduction, Fertility and Development 3382–90. ( 10.1071/RD20264) [DOI] [PubMed] [Google Scholar]
- Martins CF, Báo SN, Dode MN, Correa GA, Rumpf R.2007Effects of freeze-drying on cytology, ultrastructure, DNA fragmentation, and fertilizing ability of bovine sperm. Theriogenology 671307–1315. ( 10.1016/j.theriogenology.2007.01.015) [DOI] [PubMed] [Google Scholar]
- McGinnis LK, Zhu L, Lawitts JA, Bhowmick S, Toner M, Biggers JD.2005Mouse sperm desiccated and stored in trehalose medium Without freezing. Biology of Reproduction 73627–633. ( 10.1095/biolreprod.105.042291) [DOI] [PubMed] [Google Scholar]
- Mercati F, Domingo P, Pasquariello R, Dall’Aglio C, Di Michele A, Forti K, Cocci P, Boiti C, Gil L, Zerani Met al. 2020Effect of chelating and antioxidant agents on morphology and DNA methylation in freeze-drying rabbit (Oryctolagus cuniculus) spermatozoa. Reproduction in Domestic Animals 5529–37. ( 10.1111/rda.13577) [DOI] [PubMed] [Google Scholar]
- Olaciregui M, Luño V, Domingo P, González N, Gil L.2017In vitro developmental ability of ovine oocytes following intracytoplasmic injection with freeze-dried spermatozoa. Scientific Reports 71096. ( 10.1038/s41598-017-00583-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson C, Genheden S, García Sakai V, Swenson J.2019Mechanism of trehalose-induced protein stabilization from neutron scattering and modeling. Journal of Physical Chemistry: B 1233679–3687. ( 10.1021/acs.jpcb.9b01856) [DOI] [PubMed] [Google Scholar]
- Palazzese L, Anzalone DA, Turri F, Faieta M, Donnadio A, Pizzi F, Pittia P, Matsukawa K, Loi P.2020Whole genome integrity and enhanced developmental potential in ram freeze-dried spermatozoa at mild sub-zero temperature. Scientific Reports 10 18873. ( 10.1038/s41598-020-76061-x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick J, Comizzoli P, Elliott G.2017aDry preservation of spermatozoa: considerations for different species. Biopreservation and Biobanking 15158–168. ( 10.1089/bio.2016.0087) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick JL, Elliott GD, Comizzoli P.2017bStructural integrity and developmental potential of spermatozoa following microwave-assisted drying in the domestic cat model. Theriogenology 10336–43. ( 10.1016/j.theriogenology.2017.07.037) [DOI] [PubMed] [Google Scholar]
- Pomeroy KO, Reed ML, LoManto B, Harris SG, Hazelrigg WB, Kelk DA.2019Cryostorage tank failures: temperature and volume loss over time after induced failure by removal of insulative vacuum. Journal of Assisted Reproduction and Genetics 362271–2278. ( 10.1007/s10815-019-01597-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rall WF, Fahy GM.1985Ice-free cryopreservation of mouse embryos at -196°C by vitrification. Nature 313573–575. ( 10.1038/313573a0) [DOI] [PubMed] [Google Scholar]
- Rao W, Huang H, Wang H, Zhao S, Dumbleton J, Zhao G, He X.2015Nanoparticle-mediated intracellular delivery enables cryopreservation of human adipose-derived stem cells using trehalose as the sole cryoprotectant. ACS Applied Materials and Interfaces 75017–5028. ( 10.1021/acsami.5b00655) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ringleb J, Waurich R, Wibbelt G, Streich WJ, Jewgenow K.2013Prolonged storage of epididymal spermatozoa does not affect their capacity to fertilise in vitro-matured domestic cat (Felis catus) oocytes when using ICSI. Reproduction, Fertility and Development 23 818. [DOI] [PubMed] [Google Scholar]
- Sánchez-Partida LG, Simerly CR, Ramalho-Santos J.2008Freeze-dried primate sperm retains early reproductive potential after intracytoplasmic sperm injection. Fertility and Sterility 89742–745. ( 10.1016/j.fertnstert.2007.02.066) [DOI] [PubMed] [Google Scholar]
- Saragusty J, Anzalone DA, Palazzese L, Arav A, Patrizio P, Gosálvez J, Loi P.2020Dry biobanking as a conservation tool in the Anthropocene. Theriogenology 150130–138. ( 10.1016/j.theriogenology.2020.01.022) [DOI] [PubMed] [Google Scholar]
- Shahmoradi E, Baheiraei N, Halvaei I.2021Trehalose attenuates detrimental effects of freeze-drying on human sperm parameters. Biopreservation and Biobanking 2031–37. ( 10.1089/bio.2020.0167) [DOI] [PubMed] [Google Scholar]
- Silva HVR, Da Silva AM, Lee PC, Brito BF, Silva AR, Da Silva LDM, Comizzoli P.2020Influence of microwave-assisted drying on structural integrity and viability of testicular tissues from adult and prepubertal domestic cats. Biopreservation and Biobanking 18415–424. ( 10.1089/bio.2020.0048) [DOI] [PubMed] [Google Scholar]
- Sørensen JG, Heckmann LH, Holmstrup M.2010Temporal gene expression profiles in a Palaearctic springtail as induced by desiccation, cold exposure and during recovery. Functional Ecology 24838–846. [Google Scholar]
- Thorat L, Oulkar D, Banerjee K, Gaikwad SM, Nath BB.2017High-throughput mass spectrometry analysis revealed a role for glucosamine in potentiating recovery following desiccation stress in Chironomus. Scientific Reports 7 3659. ( 10.1038/s41598-017-03572-5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ujaoney AK, Padwal MK, Basu B.2017Proteome dynamics during post-desiccation recovery reveal convergence of desiccation and gamma radiation stress response pathways in Deinococcus radiodurans. Biochimica et Biophysica Acta: Proteins and Proteomics 18651215–1226. ( 10.1016/j.bbapap.2017.06.014) [DOI] [PubMed] [Google Scholar]
- Voronina TA, Nesmelov AA, Kondratyeva SA, Deviatiiarov RM, Miyata Y, Tokumoto S, Cornette R, Gusev OA, Kikawada T, Shagimardanova EI.2020New group of transmembrane proteins associated with desiccation tolerance in the anhydrobiotic midge Polypedilum vanderplanki. Scientific Reports 10 11633. ( 10.1038/s41598-020-68330-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakayama T, Yanagimachi R.1998Development of normal mice from oocytes injected with freeze-dried spermatozoa. Nature Biotechnology 16639–641. ( 10.1038/nbt0798-639) [DOI] [PubMed] [Google Scholar]
- Weng L.2021Technologies and applications toward preservation of cells in a dry state for therapies. Biopreservation and Biobanking 19332–341. [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Oldenhof H.2021Principles underlying cryopreservation and freeze-drying of cells and tissues. Methods in Molecular Biology 21803–25. ( 10.1007/978-1-0716-0783-1_1) [DOI] [PubMed] [Google Scholar]
- Wolkers WF, Oldenhof H, Glasmacher B.2010Dehydrating phospholipid vesicles measured in real-time using ATR Fourier transform infrared spectroscopy. Cryobiology 61108–114. ( 10.1016/j.cryobiol.2010.06.001) [DOI] [PubMed] [Google Scholar]
- Yamada TG, Suetsugu Y, Deviatiiarov R, Gusev O, Cornette R, Nesmelov A, Hiroi N, Kikawada T, Funahashi A.2018Transcriptome analysis of the anhydrobiotic cell line Pv11 infers the mechanism of desiccation tolerance and recovery. Scientific Reports 817941. ( 10.1038/s41598-018-36124-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young MA, Antczak AT, Wawak A, Elliott GD, Trammell SR.2018Light-assisted drying for protein stabilization. Journal of Biomedical Optics 23 1. [DOI] [PubMed] [Google Scholar]
- Zhang M, Oldenhof H, Sieme H, Wolkers WF.2016Freezing-induced uptake of trehalose into mammalian cells facilitates cryopreservation. Biochimica et Biophysica Acta 18581400–1409. ( 10.1016/j.bbamem.2016.03.020) [DOI] [PubMed] [Google Scholar]
- Zhang Y, Wang H, Stewart S, Jiang B, Ou W, Zhao G, He X.2019Cold-responsive nanoparticle enables intracellular delivery and rapid release of trehalose for organic-solvent-free cryopreservation. Nano Letters 199051–9061. ( 10.1021/acs.nanolett.9b04109) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a