Abstract
Endometriosis is a chronic neuro-inflammatory disorder the defining feature of which is the growth of tissue (lesions) that resembles the endometrium outside the uterus. Estimates of prevalence quote rates of ~10% of women of reproductive age, equating to at least 190 million women world-wide. Genetic, hormonal and immunological factors have all been proposed as contributing to risk factors associated with the development of lesions. Twin studies report the heritable component of endometriosis as ~50%. Genome-wide association studies (GWAS) have identified single nucleotide polymorphisms (SNPs) that appear over-represented in patients with endometriosis, particularly those with more extensive disease (stage III/IV). In different sample populations, there has been replication of SNPs near genes involved in oestrogen and other steroid regulated pathways including ESR1 (oestrogen receptor alpha), GREB1, HOXA10, WNT4 and MAPK kinase signalling. Comparisons with GWAS conducted on other patient cohorts have found links with reproductive traits (age at menarche) and disorders (fibroids, endometrial and ovarian cancer) and common co-morbidities (migraine, depression, asthma). In summary, genetic analyses have provided new insights into the hormone-regulated pathways that may contribute to increased risk of developing endometriosis some of which may act in early life. New studies are needed to clarify the relationship between the many SNPs identified, the genes that they regulate and their contribution(s) to development of different forms of endometriosis. We hope that more advanced methods allowing integration between GWAS, epigenetic and tissue expression data will improve risk analysis and reduce diagnositic delay.
Lay summary
Endometriosis is a debilitating reproductive disorder affecting ~10% of reproductive-age women, and those assigned female at birth, which causes a range of symptoms including chronic pain and infertility. The reason why some, but not all these individuals, develop the lesions that characterise the disease are poorly understood, but recently attention has focused on genetic risk factors to explain why the incidence is higher in some families. Studies on large cohorts of patients with comparison of their DNA to women without endometriosis or with other disorders have documented changes in genes associated with steroid hormone production or action. The results provide further evidence that endometriosis shares genetic risk factors with other disorders of the reproductive system and a platform for new ideas related to risk, biomarkers and therapies.
Key Words: women’s health, endometriosis, genome-wide association studies (GWAS), single nucleotide polymorphism (SNP), oestrogens, androgens
Introduction
Endometriosis is a complex, heterogeneous, chronic, incurable disorder the hallmark of which is growth of tissue ‘lesions’ that have histological features resembling the intrauterine (eutopic) endometrium outside the uterine cavity (Horne & Saunders 2019, Zondervan et al. 2020). Estimates of prevalence typically quote rates of ~10% of women of reproductive age, equating to 190 million individuals world-wide (Zondervan et al. 2020). This is likely to be an underestimate as many women, or those assigned as female at birth, may remain undiagnosed, and ‘lesions’ have also been found in asymptomatic fertile women (Shafrir et al. 2018). Prevalence rates can be as high as 50% in women seeking treatment for infertility (Meuleman et al. 2009): rates in adolescents with pelvic pain range from 49% to 75% (Shafrir et al. 2018). A recent review highlighted the profound negative impact on the lives of individuals with the disorder (Missmer et al. 2021).
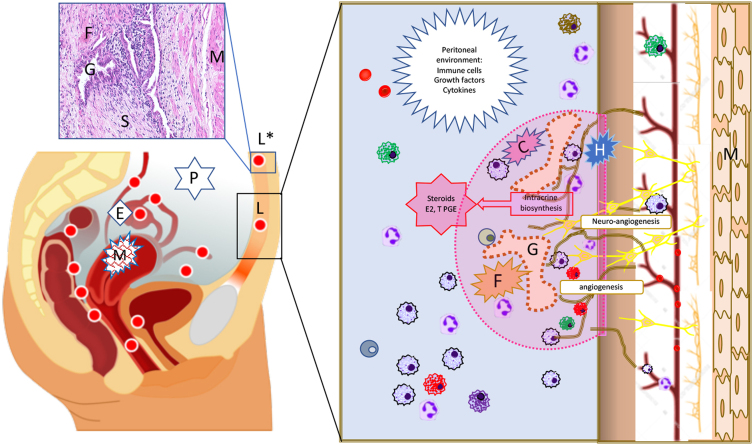
Endometriosis lesions are most commonly found within the pelvic cavity (Fig. 1) (Zondervan et al. 2020) but may also occur in other sites including the thorax and nervous system (Andres et al. 2020). The location, type, degree of invasion, extent of disease and associated adhesions have been used to ‘stage’ the disease with the most widely adopted scheme being that proposed by the American Society of Reproductive Medicine. This scheme proposes classification of endometriosis lesions into four stages I to IV: stage I – mild, stage II – minimal, stage III – moderate, stage IV – severe. The assignment of stage is based on visual analysis at time of surgery and a points-based system with the majority of peritoneal disease scored as stage I/II and more extensive disease associated with adhesions and deep nodules as stage III or IV.
Figure 1.
Location and histology of endometriosis lesions. Endometriosis lesions are predominantly found in the pelvic cavity where they may be associated with the peritoneal wall (superficial peritoneal), the ovary (cysts/endometriomas) or as nodules (deep endometriosis) associated with areas of fibrosis and adhesions between bowel, bladder and vagina (lower left diagram shown as red circles). A histological section of a superficial peritoneal lesion (*) stained with H&E is shown above the diagram of the pelvic cavity. The lesion is supported by the peritoneal wall which has a layer of smooth muscle (M), and it contains stromal fibroblasts (S), myofibroblasts (fibrosis, F) and a gland surrounded by epithelial cells (G). The right-hand panel shows a diagrammatic representation of a lesion (surrounded by dotted pink line) mirroring the histology of the H&E image complemented by representation of additional cell types including nerves (yellow), blood vessels (brown) and immune cells (variety of colours). It also shows processes that contribute to lesion survival (angiogenesis), growth of nerves in lesions (neuroangiogenesis) and creation of a unique environment that has high concentrations of steroids (intracrine biosynthesis).
Endometriosis is associated with a wide range of symptoms including pelvic pain (which may be more severe during menstruation), painful sex, heavy menstrual bleeding, bladder and bowel symptoms as well as those shared with other chronic pain conditions such as fatigue and depression. Several reports have recorded poor correlation between patient-reported pain and endometriosis stage (Vercellini et al. 2007). Some commentators have suggested endometriosis should be considered a ‘syndrome’ with greater emphasis on symptoms rather than lesion subtype/location (Saunders & Horne 2021). Results from genomic and other studies (discussed below) suggest there may also be differences in the aetiology of deep and ovarian disease compared with peritoneal, superficial lesions.
In this narrative review, I will consider the evidence that genetic changes in gene pathways associated with hormone production or action may contribute to the risk of endometriosis and other disorders that may have shared genetic risk factors. Opportunities to use the information to improve non-surgical diagnosis or personalised therapies will be briefly discussed.
Why is endometriosis usually referred to as a ‘hormone-dependent disorder’?
Endometriosis only occurs spontaneously in menstruating species including humans and some primates. Explanations for the formation of endometriosis lesions, particularly those in the pelvis, have largely focused on a theory, first proposed in the 1920s, that tissue fragments including stem/progenitor cells and immune cells transferred via fallopian tubes at the time of menstruation (retrograde flow) survive and become attached to the peritoneal wall and other sites (Horne & Saunders 2019, Zondervan et al. 2020). Other theories include transfer via the vasculature and (Yovich et al. 2020) and coelomic metaplasia (Zondervan et al. 2020).
The human endometrium is a complex multicellular tissue that is exquisitely sensitive to the actions of sex steroids with cycles of proliferation, differentiation breakdown, shedding (menstruation) and repair orchestrated by changes in circulating concentrations of endocrine hormones secreted by the ovaries (Gibson et al. 2020). Within the ovaries, follicular maturation, and subsequent expression of steroid enzymes such as aromatase (metabolises androgens to oestrogens) within the granulosa cells (Turner et al. 2002), is stimulated by the pituitary hormones LH and FSH. G-protein coupled receptors that bind FSH (FSHR) are expressed in the granulosa cells, and there are reports that FSHR polymorphisms are associated with increased risk of endometriosis in fertile women (Andre et al. 2018).
The actions of sex steroids, including oestrogens, progestins and androgens, are mediated by receptors encoded by genes (ESR1, ESR2, PR, ARrespectively) that are members of a large family of ligand-activated transcription factors. Studies using cell-based assays and mouse models have highlighted a key role for ESR1 (oestrogen receptor alpha) in regulation of endometrial cell proliferation and stromal–epithelial cell signalling (Winuthayanon et al. 2017). Progesterone acting via PR, in combination with other factors including cAMP, plays a critical role in the differentiation (decidualisation) of endometrial stromal fibroblasts resulting in remodelling of their cytoskeleton (shape), changes in gene expression and a novel secretory profile (Gellersen & Brosens 2003). Decidualisation is associated with increased synthesis and secretion of factors such as interleukin 15 that regulate recruitment of immune cells including the CD56+ uterine natural killer cells (uNK) which play a key role in remodelling of the vasculature (Gibson et al. 2015). Androgens and AR also play an important role in regulation of endometrial function and endometrial pathologies (Gibson et al. 2020) with strong expression of AR in stromal fibroblasts and variable expression in epithelial cells in normal endometrium. Steroid metabolism within lesions results in high levels of testosterone which are independent of stage of the cycle (Huhtinen et al. 2014). Expression of AR and 5 alpha reductase enzymes capable of metabolising testosterone to the highly potent steroid dihydrotestosterone in lesions has also been reported (Carneiro et al. 2008).
Studies on the role of steroids in the pathogenesis of endometriosis have included evidence of changes in the function of the endometrium in women with endometriosis, measurement of steroids in the peritoneal fluid and detailed analysis of expression of steroid receptors, metabolising enzymes and steroid concentrations in the lesions themselves (Fig. 1). The results of these studies have consistently reported differences between samples from women with or without endometriosis that involve steroid hormone production or action including altered responses to progesterone (‘progesterone resistance’) (Burney et al. 2007). There is also a large body of work that has identified changes in expression of enzymes and the creation of an oestrogen-dominated microenvironment within the lesions which has provided the rationale for the use of enzyme inhibitors, including those targeting the aromatase enzyme, as therapeutics (Dunselman et al. 2014).
Readers interested in learning more about the many studies exploring the role of steroid receptors in endometriosis are encouraged to read the comprehensive review by Yilmaz and Bulun that summarises papers published up to 2018 (Yilmaz & Bulun 2019).
Evidence that endometriosis is a heritable disease
Patients often report cases of endometriosis in close relatives. A study using questionnaires explored the incidence of endometriosis in female monozygotic and dizygotic twins in the Australian National Health and Medical Research Council Twin Register. More than 3000 twins responded with 215 twins recording a diagnosis of endometriosis; when available medical and pathology reports were included, the authors concluded ~51% of the variance of the latent liability to endometriosis could be due to additive genetic influences (Treloar et al. 1999). In a subsequent study, the same group published a key paper based on genetic linkage analysis of 1176 families in Australia and the UK with at least 2 affected individuals (Treloar et al. 2005). They used a positional-cloning approach that starts with linkage analysis to identify genomic regions likely to harbour genes that contributed to disease predisposition. They identified significant linkage (MLS = 3.16) to a novel susceptibility locus on chromosome 10q26. Notably, a subsequent GWAS study (described below) identified a number of genetic polymorphisms in this region associated with endometriosis using larger numbers of patient samples (Painter et al. 2011a). A recent study used population data in Korea to quantify the familial risk of endometriosis among full siblings (19,195 women with 1126 cases) to examine interactions between family history, smoking, age at menarche and BMI (Kim et al. 2021). This study endorsed the findings of earlier studies showing increased risk associated with having an affected sibling which was higher in twins.
Genomic studies on samples from women with and without a diagnosis of endometriosis have identified gene polymorphisms that appear associated with disease risk
A large number of studies have focused on specific changes in gene expression in lesions and/or endometrium from women with endometriosis (Table 1; reviewed by Yilmaz & Bulun 2019, Zondervan et al. 2020, Saunders & Horne 2021). To complement these data, other investigations have looked for polymorphisms in steroid receptor genes. For example, Kitawaki et al. examined the distribution of PVUII genotypes (PP, Pp and pp) in ESR1 using DNA from blood samples (203 women, 109 with a diagnosis of endometriosis) reaching the conclusion that the Pp/pp variants were higher in women with endometriosis, fibroids or adenomyosis (Kitawaki et al. 2001). Based on analysis of 72 women with deep endometrial disease, van Kaam and colleagues reported the presence of the PR gene polymorphic allele +331A was associated with reduced risk of endometriosis compared to a healthy population (n = 102) (van Kaam et al. 2007). Negative findings in other studies are in part believed to be due to low numbers of patients versus controls something that has now been addressed using large-scale unbiased genome scanning (genome-wide association studies, GWAS). In support of this approach, Zondervan and colleagues highlighted the technical developments including the generation of data in the 1000 genomes project and improved statistical analysis that have made GWAS analysis more robust and a good platform for unbiased analysis of genetic changes (Zondervan et al. 2016). For complex diseases such as endometriosis, where changes in the levels of expression of individual gene may only make a small overall contribution to the aetiology of the disorder, very large numbers of individuals need to be evaluated. To ensure that valid comparisons are made it is also important that the endometriosis phenotype of patients has been recorded using robust clinical criteria and controls are drawn from populations with identical ancestry. A summary of key GWAS conducted on endometriosis patients (including stage of disease where this is known) is given in Table 2, and the results that appear to implicate steroid regulated pathways in the development of the disease are discussed in more detail below.
Table 1.
Summary of key studies that have provided evidence for altered steroid biosynthesis and/or action in eutopic endometrium or endometriosis lesions from patients.
| Title/topic | Methods | Results | References |
|---|---|---|---|
| Expression of ESR1 vs ESR2 in endometriosis lesions compared with endometrium | Fibroblasts isolated from endometrioma and endometrium, immunohistochemistry, evaluation of methylation status of ESR2 promoter | ESR2 overexpressed in lesions compared with ESR1; mechanism involving altered methylation | Xue et al. (2007) |
| Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis | Endometrial tissue biopsies from 21 women with endometriosis and 16 women without, cycle stage determined. Affymetrix arrays+gene ontogeny | Phase-dependent changes in gene expression in both tissue sets. Patient samples – enrichment of genes involved in proliferation in early secretory phase dysregulation of P target genes in secretory phase | Burney et al. (2007) |
| Gene expression profile for ectopic vs eutopic endometrium provides new insights into endometriosis oncogenic potential | Paired samples of endometriomas and endometrium (12 women, luteal phase). Nimgen microarrays, validation of 20 genes, pathway analysis (DAVID) | Cluster-dependent modulation of HOX genes Altered cell cycle genes (suppressed?) |
Borghese et al. (2008) |
| Prostaglandin E2 via SF-1 coordinately regulates transcription of steroidogenic genes necessary for oestrogen synthesis in endometriosis | Stromal cells isolated from wall of endometriomas (17) and endometrium (16), extra-ovarian tissue from differentgroup of women (13). RT-PCR steroid enzyme mRNAs, ChIP assays | StAR, P450scc, P450c17, P450arom are higher in ectopic samples. SF1 is high and binds promoter of StAR | Yilmaz and Bulun (2019) |
| Intra-tissue steroid profiling and enzyme analysis confirms differences in steroid metabolism in the endometrium and endometriosis lesions | RT-PCR analysis of enzymes in lesions vs endometrium, LC-MS/MS for direct measurement of steroid concentrations | Altered tissue steroid concentrations in endometriosis; altered expression of various steroidogenic enzymes HSD3B2 high CYP11A1 low |
Huhtinen et al. (2012) |
Table 2.
Larger GWAS studies that have identified common SNP variants associated with endometriosis.
| Patients | Controls | SNPs | Genes/pathways | Reference |
|---|---|---|---|---|
| 3194 surgically confirmed | Population controls 7060 (UK/Australia) | 7p15.2 strongest in subgroup with stage III/IV disease; rs12700667 | Intergenic region upstream of NFE2L3, HOXA10/A11 | Painter et al. (2011a) |
| 3223 women with surgically confirmed endometriosis, 1090 women without endometriosis | Population controls 7060 | Examined 11,984 SNPs on chromosome 10. Signal 10q26 rs11592737 replicated |
CYP2C19 | Painter et al. (2011b) |
| 4604 women with surgically confirmed endometriosis | 9393 women of Japanese and European ancestry | rs12700667 replicated in Japanese; rs7521902 at 1p36.12; rs13394619 at 2p25 |
NFE2L3, HOXA10 WNT4 GREB1 |
Nyholt et al. (2012) |
| Meta-analysis of 11,506 cases: stage III/IV 2859 | 32,678 (European and Japanese) | 8 of 9 loci have stronger effect in stage II/IV; rs1537377; rs13394619 rs12700667; rs7521902; rs7739264 | CDKN2B-AS1; GREB1; WNT4; VEZT; ID4 | Rahmioglu et al. (2014) |
| 3908 diagnosis of endometriosis | 8568 women of Japanese and European ancestry | rs6542095, rs3783550 rs3783525 | IL4 locus | Sapkota et al. (2015b) |
| 2594 Australians with positive diagnosis | 4496 controls | rs3820282; rs12038474; + blood eQTL | Interactions with promoters of LINC00339, CDC42 (silencer) | Powell et al. (2016) |
| 3194 (surgical confirmed) stage I/II 1686; stage III/IV 1364 | 7060 controls European ancestry | rs144240142 (intronic MAP3K4) | MAPK signalling pathway; ECM glycoprotein | Uimari et al. (2017) |
| Metanalysis 17,045 cases | 191,596 controls | FN1, CCDC170, ESR1, SYNE1 and FSHB | Steroid receptors and steroid signalling | Sapkota et al. (2017) |
| Pooled GWAS, endometrioma, Han Chinese women 50 primary and 1448 for validation | 1540 (had secondary infertility or fibroids) | 10 novel loci | Most significant: IGF1R (signalling); Meis homeobox | Wang et al. (2017) |
In their 2011 studies, Painter and colleagues identified SNPs on 7p15.2 (Painter et al. 2011a) and 10q26 (Painter et al. 2011b) in regions of the genome that appeared associated with HOXA10 and CYP2C19C. The finding of a SNP associated with HOXA10 was exciting as there is a large body of work that has highlighted the importance of HOX genes in development of the female reproductive tract and differentiation of the endometrium in preparation for pregnancy discussed below (Du & Taylor 2015).
In a follow-up study, the authors re-analysed 80 SNPs highlighting rs4244285, a functional SNP in exon 5 of CYP2C19,that abrogates its function through the creation of an alternative splice site and another functional SNP in the CYP2C19promoter. The authors proposed that variants of CYP2C19 may contribute to endometriosis susceptibility in both familial and sporadic cases (Painter et al. 2014). CYP2C19 is a member of the cytochrome P450 enzyme superfamily often implicated in drug metabolism by the liver. In the context of endometriosis, it is notable that this enzyme acts as an expoxygenase that can convert arachidonic acid to four epoxyeicosatrienoic acid regioisomers which have diverse impacts on blood vessels and inflammation (Spector 2009). Whilst there is no specific evidence for a role for CYP2C19 in endometrium or endometriosis, it is notable that expoxygenase activity has been studied in the context of macrophage activity in wounding and fibrosis both processes relevant to development of endometriosis lesions (Guo 2018).
Several primary GWAS and subsequent meta-analyses have reported associations between endometriosis and SNPs near genes involved in signalling pathways implicated in endometrial tissue function (Table 2). For example, Nyholt et al. (2012) identified rs7521902 at 1p36.12 near WNT4; thereafter, the group conducted fine mapping of 1p36 region spanning WNT4, CDC42 and LINC00339 finding three additional SNPs located in DNA sequences with potential overlap with binding sites for FOXA1, FOXA2, ESR1 and ESR2 (Luong et al. 2013). In their meta-analyses, which incorporated data from eight of the GWAS conducted before 2014, Rahmiglou and co-investigators confirmed significance for SNPs associated with WNT4, CREB1 and VEZT. They also highlighted the stronger effect sizes among women diagnosed with more extensive or ovarian disease (stage III/IV) for eight of the SNPs (Rahmioglu et al. 2014). Other studies have also reported that the most robust findings are found if results are stratified and sorted according to disease stage (Sapkota et al. 2015a ).
A meta-analysis with more than 17,000 patients and 191,000 controls identified 5 novel SNPs associated with steroid signalling pathways as well as 5 secondary association signals, including two at the ESR1 locus, resulting in 19 independent SNPs which the authors postulated might contribute to 5.19% of variance in endometriosis (Sapkota et al. 2017). Whilst these studies have revealed some promising leads, the population studied has largely been limited to women with European ancestry although the 7p15.2 SNP was also replicated in Japanese women (Nyholt et al. 2012). In a small study Wang et al. focused on endometriomas in Han Chinese women finding the most significant signalling pathway was that associated with IGF1 receptor (Wang et al. 2017) which is interesting as macrophage-derived IGF1 has recently been highlighted as a nerve sensing factor in endometriosis-associated pain (Forster et al. 2019).
Replication and meta-analysis of previous GWAS confirmed vezatin as a locus having a strong association with endometriosis in Italian patients (Pagliardini et al. 2015). Immunostaining of endometrium suggested the protein was in multiple cell types and not altered according to cycle stage; in the same study, the authors focused on the 12q22 region and explored whether the SNPs found in this region that are associated with the VEZT gene (rs10859871) had an impact on expression in endometrial tissue samples (eQTL analysis) (Holdsworth-Carson et al. 2016). A total of 11 coding variants of VEZT(including 1 novel variant) were identified from an endometriosis cohort consisting of 2594 cases and 4496 controls, but they did not find any definitive evidence of a change in VEZT protein expression in subset of endometrial tissue samples (n = 122) concluding further validation was needed of a relationship between SNP and gene expression levels.
Using in vitro approaches and blood eQTL analysis, a SNP at rs12038474 was found to be located in transcriptional silencer for CDC42 and to increase its expression in reporter assays (Powell et al. 2016). CDC42 is a member of the Rho family of GTPase signalling molecules, and its overexpression in some cancers has been implicated in increased cell migration. Other studies have reported that stromal and stem cells from women with endometriosis have an altered phenotype associated with enhanced migration and suggested this may involve Rho/ROCK signalling pathways (Yotova et al. 2011). Therapies targeting CDC42-dependent pathways are being explored as treatments for cancers with high mortality such as non-small cell lung cancer (Tan et al. 2020). Expression of CDC42 was previously investigated using immunostaining of eutopic and ectopic endometrium in 19 patients with ovarian endometriosis (Goteri et al. 2006) with some weak evidence of increased expression in secretory phase endometrium in those with disease.
Evidence from genomic studies supporting a role for sex steroids in the aetiology of endometriosis
Evidence from GWAS (Table 2) appear consistent with a role for genetic mutations in genes implicated in steroid regulation of the endometrium in modifying the risk of developing endometriosis. Follow-up studies, reviewed briefly below, have been conducted which have further strengthened this evidence and complemented studies on individual hormone-dependent gene expression in endometrial cells and tissues (Burney et al. 2007).
In a meta-analysis, Nyholt and collaborators used data from 11 GWAS case–control data sets with more than 17,000 endometriosis cases (Table 2) (Sapkota et al. 2017). They replicated previous reported loci and identified five novel SNPs significantly associated with genes involved in sex steroid hormone signalling pathways including FSH beta (FSHB), fibronectin (FN1) and CCDC170 a gene implicated in breast cancer risk (Dunning et al. 2016) and five secondary association signals, including two at the ESR1 locus. Given the importance of FSH in regulating production of oestrogens by the ovarian granulosa cells, the finding of SNPs associated with the FSHB gene provides a further link between endometriosis risk and oestrogen action(s). Notably in their paper, Sapkota et al. (2017) reported that their data were supported by independent samples from the UK Biobank (Ruth et al. 2016) and the index SNP was in high linkage disequilibrium with other SNPs associated with FSH concentrations.
SNPs associated with the ESR1 gene and GREB1, an early response gene that is regulated by oestrogens as well as androgens in hormone-dependent cancers (Cheng et al. 2018), were reported in endometriosis GWAS including large-scale meta-analysis (Rahmioglu et al. 2014, Sapkota et al. 2017), although not all studies have replicated findings of an association with the rs11674184 SNP of the GREB1gene (Matalliotaki et al. 2019). Oestrogen receptors play a key role in regulation of endometrial function, and it is notable that many studies have recorded dysregulation of ESR1/ESR2 with overexpression of the latter in endometriosis lesions (Table 1) which has been attributed to changes in methylation status of ESR2 (Xue et al. 2007) rather than genomic SNPs.
In a recent paper, Marla et al. (2021) examined hormonal and genetic regulation of genes in the ESR1 region in endometrium and explored the effect of endometriosis risk variants. The authors noted that variants in the ESR1 region of SNPs associated with endometriosis risk were not the same as the ESR1 SNPs associated with age at first birth, age at menarche or breast cancer which is something that needs to be born in mind when linking risks to pathways.
SNPs associated with HOXA10 (7p15.2) have been reported in more than one GWAS (Painter et al. 2011a, Nyholt et al. 2012). Expression of HOXA10 is steroid regulated in adult endometrium: two EREs that can bind either ESR1 or ESR2 in vitro have been identified in the regulatory region of the gene highlighting a direct link to oestrogens and oestrogen receptor action in endometrial cells (Akbas et al. 2004). The abundance of HOXA10 protein in endometrial stromal cells increases as they decidualise in response to progesterone and it plays a key role in regulating other genes implicated in regulation of metabolism, DNA replication and repair, cell junction, and lysosome and signal transduction (Wang et al. 2021). Miss-expression of HOXA10 has been reported to contribute to infertility (Ashary et al. 2020) and mice with deletion of Hoxa10 have severe defects in decidualisation and implantation (Gao et al. 2015). More recent studies have suggested altered expression of HOXA10 might also be a risk factor for adenomyosis (abnormal invasion of endometrium into the myometrium) which is often found as co-morbidity with endometriosis consistent with these conditions sharing common risk factors (Bulun et al. 2021).
Members of the Wnt gene family are well established as regulators of endometrial cell function with important roles in epithelial–mesenchymal interactions (Tulac et al. 2003). SNPs associated with WNT4 (at 1p36.12) have been reported in several GWAS (Rahmioglu et al. 2014). This gene encodes a secreted signalling factor that regulates both development of endometrial glands and progesterone signalling during decidualisation (Franco et al. 2011, Hayashi et al. 2011). In a study comparing expression of WNT4 in eutopic and ectopic endometrium of 30 patients with endometrium from 30 controls, some evidence was presented for downregulation in ectopic endometrium and in eutopic endometrium of patients compared with controls (Liang et al. 2016) although more extensive studies to link SNPs to gene expression are required.
Whilst the evidence described above appears to add weight to the link between genetic changes in regions of the genome that appear to be associated with genes involved in steroid regulation of endometrium/endometriosis lesions, it is important to acknowledge that steroids can have pleiotrophic effects that span development as well as adult life making cause and effect difficult to unravel particularly in a disorder with a complex presentation. Much larger-scale studies are now required to expand on the link between SNPs and the regulation of gene expression that is specific to endometrium (Mortlock et al. 2020).
Genomic studies have revealed links between endometriosis reproductive traits and other disorders
The rapid increase in large-scale GWAS has opened up the opportunity to compare SNPs in women with endometriosis with those identified as associated with reproductive traits and reproductive or other disorders (Table 3).
Table 3.
GWAS studies from reproductive and other disorders that have identified SNPs in common with endometriosis.
| Condition | Cohort for non-endo condition | SNP overlap with endometriosis | Target genes/pathways | References |
|---|---|---|---|---|
| Fibroids | 35,474 cases and 267,505 female controls of European ancestry | 1p36.12, rs7412010; 2p25.1, rs35417544; 6q25.2, rs58415480; 11p14.1, rs11031006 | WNT4, CDC42, GREB1, ESR1, FSHB | Gallagher et al. (2019) |
| Age at menarche | 395 patients (endo), 981 controls | 52 SNPs previously identified for age at menarche: 16 SNPs overlap with endo; rs6589964 | 28 genes in G alpha signalling pathway; LHCGRseveral SNPs (strong); BSX– increases affinity for FOXA transcription factors | Ponomarenko et al. (2020) |
| Endometrial cancer | 4 data sets: 6459 patients, 32,624 controls | 13 loci incl rs2475335 located in PTPRD | STAT3 pathway | Painter et al. (2018) |
| Endometrial cancer | Data from O’Mara et al. (2018) with replication using UK Biobank 12,270 cases/46,126 controls | 4 regions identified with 17q21.32 demonstrating evidence of a shared genetic risk signal; 3q21.3? novel | Potential genes?: CBX1, MIR1203, SKAP1 SNX11 |
Kho et al. (2021) |
| Ovarian cancer | 10,065 cases and 21,663 controls | Clear cell carcinoma showed the strongest genetic correlation with endometriosis | ?? | Lu et al. (2015) |
| Obesity/leanness | BMI (GIANT; 123,865 individuals) and WHRadjBMI (GIANT: 77,167 individuals) | 7p15.2; KIFAP3 and CAB39L are novel associations for both traits | Wnt pathway (3 genes) | Rahmioglu et al. (2015) |
| Migraine | 22 GWAS, 59,674 migraine cases and 316,078 controls (sex considered as a covariant) | SNPs near SLC35G6, TRIM32, ARL14EP | IL1R binding, PI3K-Akt-mTOR-signalling, MAPK signalling, TNF-α signalling | Adewuyi et al. (2020) |
| Depression | 170,756 cases of depression 329,443 controls of European ancestry | 20 independent loci, 8 novel | Causal relationship?; Gastric mucosal abnormality | Adewuyi et al. (2021) |
| Asthma | UK Biobank 26,332 cases of asthma/ 375,505 controls; TAGC consortium 19,954 cases/107715 controls | UKB comparison 14 independent loci, 5 putative novel (3 replicated in TAGC) | Biological pathways including thyroid hormone signalling, androgen biosynthetic process | Adewuyi et al. (2022) |
Younger age at menarche has been implicated in increased risk of developing endometriosis as having short menstrual cycles and low BMI whereas having more children is associated with lower risk (Shafrir et al. 2018). GWAS studies have shed light on the heritable factors that may contribute to these characteristics with comparisons made to endometriosis data sets. For example, a large-scale GWAS has identified a genetic component to age at first birth and number of children with 12 loci including an SNP associated with ESR1(rs4851269) (Barban et al. 2016). There is also evidence from GWAS studies for a shared genetic risk factors between ovarian ageing and premature ovarian failure (McGrath et al. 2021). A preprint article (https://doi.org/10.1101/401448) which has not yet been peer reviewed reported a GWAS of endometriosis-related infertility, including 2969 cases and 3770 controls; they did not show genome-wide significance for any SNPs associated with endometriosis-related infertility although they recorded three SNPs at or near genes implicated in female fertility in model organisms.
To identify loci for age at menarche, a meta-analysis of 32 GWAS in 87,802 women of European descent, with replication in up to 14,731 women, was performed resulting in the identification of more than 30 new SNP loci (Elks et al. 2010). Notably three of these were in or near genes implicated in hormonal regulation (INHBA, PCSK2, RXRG). A more recent small-scale study took 52 of the candidate SNPs for age at menarche and their gene–gene and gene–environment interactions and analysed whether they were associated with endometriosis using samples from 395 patients and 981 controls (Ponomarenko et al. 2020). They found 16 SNPs that were associated with endometriosis and evidence for a link with the G protein signalling pathway. One of the most well-established associations with age at menarche is body size with early studies indicating this is regulated by genetic factors rather than diet (Stark et al. 1989). It is therefore of note that Rahmioglu and colleagues have reported a significant enrichment of common SNPs when comparing data sets based on fat distribution and endometriosis (Rahmioglu et al. 2015) including shared genes associated with the WNT signalling pathway (Table 3). A recent analysis using two-sample randomization also found evidence that reduced body weight/BMI and variants that expose women to more episodes of menstruation might be mediating genetic susceptibility to endometriosis (Garitazelaia et al. 2021) which backs up epidemiological and other genetic data including GWAS discussed above.
Comparisons have made between SNP data from endometriosis patients and those from women with fibroids (leiomyomata) (Gallagher et al. 2019). A meta-analysis reported that genes associated with endometriosis that were involved in hormone signalling (WNT4/CDC42, GREB1, ESR1, FSHB) were also associated with diagnosis of fibroids. The authors reported that there was at least a doubling of risk for a diagnosis of fibroids among those with a history of endometriosis suggesting overlapping genetic origins. Notably, candidate genes identified for age at menarche are also associated with presence of fibroids. A recent study reported that of the 23 loci associated with fibroids, 16 were associated with either age at menarche (7 SNPs) or height and/or BMI (13 SNPs) (Ponomarenko et al. 2020). One of the SNPs was associated with at least two of the three phenotypes being rs4374421 (associated with LHCGR)consistent with an important role for hormones/receptors in regulation of multiple reproductive phenotypes.
Epidemiological and array studies have identified an increased risk of developing some forms of ovarian cancer in women with endometriosis (Lu et al. 2015). Analysis of endometriosis and endometrial cancer SNP data sets (Painter et al. 2018) highlighted 13 distinct loci associated with both endometriosis and endometrial cancer. The study suggested that endometriosis and endometrial cancer have a moderate, but significant, shared genetic aetiology. Recently, Japanese researchers performed GWAS studies of two benign gynaecologic diseases (endometriosis, fibroids) and three reproductive cancers (ovarian, endometrial and cervical) using data of 46,837 subjects and 39,556 matched female controls from the Japan Biobank Project (Masuda et al. 2020). They reported genetic correlations were relatively strong between ovarian cancer and endometriosis and reported a weaker association between endometriosis and fibroids as well as SNPs in endometrial and ovarian cancer unique to Japanese and/or East Asians. In a recent study, Australian researchers also identified genetic risk regions shared between endometriosis, endometrial cancer and fibroids and a novel genome-wide significant endometrial cancer risk locus at 1p36.12, contained biologically relevant genes, including WNT4 discussed above (Kho et al. 2021) (Table 3). In this study, the authors used a GWAS data set from endometrial cancers and an expanded data set of 12,906 cases to identify 9 new SNPs with complementary analysis of epigenomic marks in cell lines showing greater overlap in oestrogen-treated cells (O’Mara et al. 2018). Comparison with the endometriosis SNP data is available in a preprint (bioRxiv. https://doi.org/10.1101/406967). Whilst stringent analysis using replication data sets failed to replicate some earlier data, the authors did report finding four shared genetic risk regions, three of which (9p21.3, 15q15.1 and 17q21.32) have previously been independently associated with risk of both diseases (Sapkota et al. 2017, O’Mara et al. 2018).
The finding of common SNPs between endometriosis and migraine (Adewuyi et al. 2020) is interesting because they align with reports that migraine is more common in women than men and many women report worse symptoms during menstruation suggestive of an impact of hormones. The co-morbidity of endometriosis with migraine has been reported in a number of epidemiological studies (Yang et al. 2012). Notably, in a twin-based study of 815 monozygotic and 457 dizygotic female twin pairs, Nyholt and colleagues reported a significant additive genetic correlation and bivariate heritability between migraine and endometriosis (Nyholt et al. 2009). Meta-analysis of endometriosis and migraine GWAS data sets did not find novel genome-wide significant SNPs nor evidence of a causal link however they did identify some SNPs associated with genetically controlled biological mechanisms which might explain the co-occurrence of the two disorders. These included several signalling pathways previously noted in GWAS studies on endometriosis such as IL1R, MAP kinase and Akt-mTOR (Adewuyi et al. 2020).
Depression and fatigue are symptoms commonly reported by women with endometriosis (Saunders & Horne 2021). A meta-analysis of endometriosis and depression GWAS (sample size 709,111) identified 20 independent genome-wide significant loci of which 8 were novel (Adewuyi et al. 2021). Genes overlapping the two traits were significantly enriched for the biological pathways ‘cell-cell adhesion’, ‘inositol phosphate metabolism’, ‘Hippo-Merlin signalling dysregulation’ and ‘gastric mucosa abnormality’.
New data highlighting shared genetic traits with asthma also align with the strong association between endometriosis and inflammatory processes (Adewuyi et al. 2022) (Table 3). Notably in their paper the authors highlighted the many lines of evidence that exposure to high levels of oestrogens increases the risk of asthma (Keselman & Heller 2015, Keselman et al. 2017) providing a plausible biological link between the two conditions.
Have genomic studies provided any new diagnostic or therapeutic opportunities?
Genetic changes identified by GWAS or other methods based on sequencing of DNA arise in the germline, and their impact may therefore be at any time during formation, differentiation or function of a differentiated tissue. The results from these approaches need to be complemented by analysis of cells recovered from lesions or the endometrium of women with endometriosis that can provide information on somatic mutations, epigenetic changes and transcriptomes. One of the main reasons genetic studies were carried out was in anticipation they might lead to the development of screening panels for genes implicated in endometriosis reducing the need for surgical diagnosis. A recent study on Korean women which explored familial cases of endometriosis found shared risk factors/SNPs suggested women with an affected sibling, early menarche, low BMI or who smoked could be considered an at-risk population (Kim et al. 2021). This study shows the power of combining information from several studies to move the field forward towards the goal of personalised risk assessment. Notably this study was conducted in Asia whereas nearly all the other GWAS have largely focused on populations with European ancestry: there is clearly an urgency to increase the ethnic diversity of populations studied in GWAS for all reproductive traits and disorders. Another notable limitation of many of the findings from existing GWAS is that the most significant findings with the most robust statistical significance have only been associated with more extensive disease (stages III/IV). This may suggest genetic changes play a more important role in the aetiology of the disorder in this subset of women, but we cannot conclude this is the case without additional data from well phenotyped individuals with a stage I/II diagnosis.
A study using whole genome sequencing of members of an affected family with ovarian endometriosis highlights the power of this approach to identify novel mutations that might explain familial cases (Albertsen et al. 2019). The rapidly reducing cost of whole genome sequencing is likely to increase the use of this approach for analysis of at-risk families and could be one way to increase early diagnosis and better integrate GWAS data into diagnostic pathways. Another approach that shows promise involves analysis of levels of long non-coding RNAs or miRNAs in blood (Moustafa et al. 2020) or saliva (Balogova et al. 2022) which may also be useful in stratification of stages (Maier & Maier 2021).
Some useful insights that may accelerate new therapies have come from comparisons between endometriosis-associated SNPs and those associated with other traits and disorders. For example, a recent study linking risks of asthma and endometriosis (Adewuyi et al. 2022) is consistent with an important role for inflammatory processes that may be exacerbated by oestrogens in both conditions (Reyes-Garcia et al. 2021, Saunders & Horne 2021). In asthma, androgens can negatively regulate inflammation and Adewuyi and colleagues suggested that androgen receptor modulators might be explored as therapies for both conditions (Adewuyi et al. 2022). This suggestion is one that has been made in the context of endometriosis as a way of overcoming the negative side effects of the pain medication Danazol (Gibson et al. 2020); however, as our recent studies in mice demonstrate more studies on the impact of SARMs on endometirum are needed before they can be widely adopted in women (Simitsidellis et al. 2019).
In the case of migraine there is already discussion surrounding repurposing of drugs used to treat migraine for treatment of endometriosis-associated pain (Saunders & Horne 2021). Likewise reports that GWAS analysis of data sets related to depression and comparison to those of endometriosis identified a link to ‘gastric mucosa abnormality’ (Adewuyi et al. 2021) are consistent with new evidence that the gut–brain axis can play a role in pain pathways (Muller et al. 2020). These findings are likely to stimulate further studies on dietary modification as a non-drug therapy for both conditions.
The strong association between inflammation and endometriosis also means we can learn from new genomics-led approaches to identify targets and accelerate drug repurposing that have been applied to asthma and autoimmune disorders such as Sjogen’s syndrome (Fang et al. 2019). With data sets of priority targets now available (Fang & Knight 2022), comparisons using endometriosis datasets are a possibility and may yield new targets including those involved in crosstalk between inflammation and steroid signalling pathways.
Conclusions and future perspectives
The endometrium is a tissue in which both steroids and inflammatory processes are implicated in normal function, and in disorders such as endometriosis, so the apparent association between genetic variants that have an impact on steroid receptor expression and/or steroid signalling and endometriosis risk appears to back up what we already know about the characteristics of the disorder (Saunders & Horne 2021). Challenges remain in linking changes in specific gene expression with causation and/or aetiology which is not surprising given the heterogeneity of the disease and the complex interrelationships between steroids (biosyntheisis/metabolism), steroid signalling pathways and changes in tissue function.
Montgomery and colleagues have argued that we may achieve additional breakthroughs in our understanding of the role(s) of gene mutations in the origins and pathogenesis of the disorder (Montgomery et al. 2020) by expanding our studies on somatic mutations in epithelial cells within the eutopic endometrium many of which may arise early in life (Lac et al. 2019): this is clearly an important area for future work.
Steroid regulation of the endometrium and endometriosis lesions which may contribute to risk and progression of the disorder will also be influenced by epigenetic changes to the genome and large-scale studies exploring DNA methylation data from women with endometriosis and comparisons to controls are now underway. These studies have expanded on those analysing the impact of methylation on expression of individual genes such as ESR2 (Xue et al. 2007). Mortlock et al. analysed DNA methylation data from endometrium and blood samples of 66 women reporting genetic regulation of methylation in endometrium across the menstrual cycle that was not observed in blood and novel disease-related methylation quantitative trait loci including one near GREB1(Mortlock et al. 2019). Another study used stromal cells isolated from eutopic endometrium during the proliferative phase as well as in vitro cultures with E2 and/or progesterone in combination with analysis of the DNA methylome to compare epigenetic landscape and see if this was altered in endometriosis patients (Houshdaran et al. 2020). The authors reported finding pre-existing aberrant DNA methylation signatures in the cells from women with endometriosis and that these were not uniform throughout the patient group with those found in women with stage IV disease associated with a blunting of response to E2 treatment.
Another regulatory pathway that has been investigated in the context of hormone regulation of endometrium/endometriosis is that of non-coding RNAs (Vashisht et al. 2020). Whilst outside the scope of this review the miRNA field is a rapidly expanding one with some promising results linking miRNAs to disease mechanisms (Stejskalova et al. 2021). Further studies on epigenetic changes in the genome and non-coding RNA pathways are anticipated but they also need to be closely integrated with the insights from genomic studies.
In summary, the rapid explosion in the use of unbiased genomic approaches such as GWAS has led to a large body of data that consistently reports mutations in areas of the genome that appear associated with genes that regulate hormone-dependent gene expression (receptors, enzymes, transcription factors). These changes may explain some of the genetic risk associated with developing this disorder and other co-morbidities reported by patients. The next challenge is to integrate these data with changes in cell/tissue function and to use them as a platform for improvements in diagnosis, development of new therapies and care pathways.
Declaration of interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of this review.
Funding
Work in the author’s laboratory directed at understanding the role of sex steroids has been funded by the Medical Research Council UK (MR/N024524/1, G1100356/1).
References
- Adewuyi EO, Sapkota YInternational Endogene Consortium Iec, andMe Research Team, International Headache Genetics Consortium IHGC, Auta A, Yoshihara K, Nyegaard M, Griffiths LR, Montgomery GWet al. 2020Shared molecular genetic mechanisms underlie endometriosis and migraine comorbidity. Genes 11 268. ( 10.3390/genes11030268) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adewuyi EO, Mehta D, Sapkota YInternational Endogene Consortium, 23andMe Research Team, Auta A, Yoshihara K, Nyegaard M, Griffiths LR, Montgomery GWet al. 2021Genetic analysis of endometriosis and depression identifies shared loci and implicates causal links with gastric mucosa abnormality. Human Genetics 140529–552. ( 10.1007/s00439-020-02223-6) [DOI] [PubMed] [Google Scholar]
- Adewuyi EO, Mehta D, Sapkota Y, Yoshihara K, Nyegaard M, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo Iet al. 2022Genetic overlap analysis of endometriosis and asthma identifies shared loci implicating sex hormones and thyroid signalling pathways. Human Reproduction 37366–383. ( 10.1093/humrep/deab254) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbas GE, Song J, Taylor HS.2004A HOXA10 estrogen response element (ERE) is differentially regulated by 17 beta-estradiol and diethylstilbestrol (DES). Journal of Molecular Biology 3401013–1023. ( 10.1016/j.jmb.2004.05.052) [DOI] [PubMed] [Google Scholar]
- Albertsen HM, Matalliotaki C, Matalliotakis M, Zervou MI, Matalliotakis I, Spandidos DA, Chettier R, Ward K, Goulielmos GN.2019Whole exome sequencing identifies hemizygous deletions in the UGT2B28 and USP17L2 genes in a threegeneration family with endometriosis. Molecular Medicine Reports 191716–1720. ( 10.3892/mmr.2019.9818) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andre GM, Martins Trevisan C, Pedruzzi IN, Fernandes RFM, Oliveira R, Christofolini DM, Bianco B, Barbosa CP.2018The impact of FSHR gene polymorphisms Ala307Thr and Asn680Ser in the endometriosis development. DNA and Cell Biology 37584–591. ( 10.1089/dna.2017.4093) [DOI] [PubMed] [Google Scholar]
- Andres MP, Arcoverde FVL, Souza CCC, Fernandes LFC, Abrao MS, Kho RM.2020Extrapelvic endometriosis: a systematic review. Journal of Minimally Invasive Gynecology 27373–389. ( 10.1016/j.jmig.2019.10.004) [DOI] [PubMed] [Google Scholar]
- Ashary N, Laheri S, Modi D.2020Homeobox genes in endometrium: from development to decidualization. International Journal of Developmental Biology 64227–237. ( 10.1387/ijdb.190120dm) [DOI] [PubMed] [Google Scholar]
- Balogova S, Darai E, Noskovicova L, Lukac L, Talbot JN, Montravers F.2022Interference of known or suspected endometriosis in reporting FDG PET/CT performed in another indication. Clinical Nuclear Medicine 47305–313. ( 10.1097/RLU.0000000000004049) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barban N, Jansen R, De Vlaming R, Vaez A, Mandemakers JJ, Tropf FC, Shen X, Wilson JF, Chasman DI, Nolte IMet al. 2016Genome-wide analysis identifies 12 loci influencing human reproductive behavior. Nature Genetics 481462–1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borghese B, Mondon F, Noel JC, Fayt I, Mignot TM, Vaiman D, Chapron C.2008Gene expression profile for ectopic versus eutopic endometrium provides new insights into endometriosis oncogenic potential. Molecular Endocrinology 222557–2562. ( 10.1210/me.2008-0322) [DOI] [PubMed] [Google Scholar]
- Bulun SE, Yildiz S, Adli M, Wei JJ.2021Adenomyosis pathogenesis: insights from next-generation sequencing. Human Reproduction Update 271086–1097. ( 10.1093/humupd/dmab017) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burney RO, Talbi S, Hamilton AE, Vo KC, Nyegaard M, Nezhat CR, Lessey BA, Giudice LC.2007Gene expression analysis of endometrium reveals progesterone resistance and candidate susceptibility genes in women with endometriosis. Endocrinology 1483814–3826. ( 10.1210/en.2006-1692) [DOI] [PubMed] [Google Scholar]
- Carneiro MM, Morsch DM, Camargos AF, Reis FM, Spritzer PM.2008Androgen receptor and 5alpha-reductase are expressed in pelvic endometriosis. BJOG 115113–117. ( 10.1111/j.1471-0528.2007.01521.x) [DOI] [PubMed] [Google Scholar]
- Cheng M, Michalski S, Kommagani R.2018Role for growth regulation by estrogen in breast cancer 1 (GREB1) in hormone-dependent cancers. International Journal of Molecular Sciences 19 2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Taylor HS.2015The role of hox genes in female reproductive tract development, adult function, and fertility. Cold Spring Harbor Perspectives in Medicine 6 a023002. ( 10.1101/cshperspect.a023002) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunning AM, Michailidou K, Kuchenbaecker KB, Thompson D, French JD, Beesley J, Healey CS, Kar S, Pooley KA, Lopez-Knowles Eet al. 2016Breast cancer risk variants at 6q25 display different phenotype associations and regulate ESR1, RMND1 and CCDC170. Nature Genetics 48374–386. ( 10.1038/ng.3521) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunselman GA, Vermeulen N, Becker C, Calhaz-Jorge C, D’Hooghe T, De Bie B, Heikinheimo O, Horne AW, Kiesel L, Nap Aet al. 2014. ESHRE guideline: management of women with endometriosis. Human Reproduction 29400–412. ( 10.1093/humrep/det457) [DOI] [PubMed] [Google Scholar]
- Elks CE, Perry JR, Sulem P, Chasman DI, Franceschini N, He C, Lunetta KL, Visser JA, Byrne EM, Cousminer DLet al. 2010Thirty new loci for age at menarche identified by a meta-analysis of genome-wide association studies. Nature Genetics 421077–1085. ( 10.1038/ng.714) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang H, Knight JC.2022Priority index: database of genetic targets in immune-mediated disease. Nucleic Acids Research 50D1358–D1367. ( 10.1093/nar/gkab994) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang HConsortium U-D, De Wolf H, Knezevic B, Burnham KL, Osgood J, Sanniti A, Lledo Lara A, Kasela S, De Cesco Set al. 2019A genetics-led approach defines the drug target landscape of 30 immune-related traits. Nature Genetics 511082–1091. ( 10.1038/s41588-019-0456-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forster R, Sarginson A, Velichkova A, Hogg C, Dorning A, Horne AW, Saunders PTK, Greaves E.2019Macrophage-derived insulin-like growth factor-1 is a key neurotrophic and nerve-sensitizing factor in pain associated with endometriosis. FASEB Journal 3311210–11222. ( 10.1096/fj.201900797R) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco HL, Dai D, Lee KY, Rubel CA, Roop D, Boerboom D, Jeong JW, Lydon JP, Bagchi IC, Bagchi MKet al. 2011WNT4 is a key regulator of normal postnatal uterine development and progesterone signaling during embryo implantation and decidualization in the mouse. FASEB Journal 251176–1187. ( 10.1096/fj.10-175349) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher CS, Mäkinen N, Harris HR, Rahmioglu N, Uimari O, Cook JP, Shigesi N, Ferreira T, Velez-Edwards DR, Edwards TLet al. 2019Genome-wide association and epidemiological analyses reveal common genetic origins between uterine leiomyomata and endometriosis. Nature Communications 10 4857. ( 10.1038/s41467-019-12536-4) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F, Bian F, Ma X, Kalinichenko VV, Das SK.2015Control of regional decidualization in implantation: role of FoxM1 downstream of Hoxa10 and cyclin D3. Scientific Reports 5 13863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garitazelaia A, Rueda-Martinez A, Arauzo R, De Miguel J, Cilleros-Portet A, Mari S, Bilbao JR, Fernandez-Jimenez N, Garcia-Santisteban I.2021A systematic two-sample Mendelian randomization analysis identifies shared genetic origin of endometriosis and associated phenotypes. Life 11 24. ( 10.3390/life11010024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellersen B, Brosens J.2003Cyclic AMP and progesterone receptor cross-talk in human endometrium: a decidualizing affair. Journal of Endocrinology 178357–372. ( 10.1677/joe.0.1780357) [DOI] [PubMed] [Google Scholar]
- Gibson DA, Greaves E, Critchley HO, Saunders PT.2015Estrogen-dependent regulation of human uterine natural killer cells promotes vascular remodelling via secretion of CCL2. Human Reproduction 301290–1301. ( 10.1093/humrep/dev067) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson DA, Simitsidellis I, Collins F, Saunders PTK.2020Androgens, oestrogens and endometrium: a fine balance between perfection and pathology. Journal of Endocrinology 246R75–R93. ( 10.1530/JOE-20-0106) [DOI] [PubMed] [Google Scholar]
- Goteri G, Ciavattini A, Lucarini G, Montik N, Filosa A, Stramazzotti D, Biagini G, Tranquilli AL.2006Expression of motility-related molecule Cdc42 in endometrial tissue in women with adenomyosis and ovarian endometriomata. Fertility and Sterility 86559–565. ( 10.1016/j.fertnstert.2006.01.031) [DOI] [PubMed] [Google Scholar]
- Guo SW.2018Cancer driver mutations in endometriosis: variations on the major theme of fibrogenesis. Reproductive Medicine and Biology 17369–397. ( 10.1002/rmb2.12221) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi K, Yoshioka S, Reardon SN, Rucker 3rd EB, Spencer TE, Demayo FJ, Lydon JP, Maclean JA.2011Wnts in the neonatal mouse uterus: potential regulation of endometrial gland development. Biology of Reproduction 84308–319. ( 10.1095/biolreprod.110.088161) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holdsworth-Carson SJ, Fung JN, Luong HT, Sapkota Y, Bowdler LM, Wallace L, Teh WT, Powell JE, Girling JE, Healey Met al. 2016Endometrial vezatin and its association with endometriosis risk. Human Reproduction 31999–1013. ( 10.1093/humrep/dew047) [DOI] [PubMed] [Google Scholar]
- Horne AW, Saunders PTK.2019SnapShot: endometriosis. Cell 1791677, .e1–1677.e1. ( 10.1016/j.cell.2019.11.033) [DOI] [PubMed] [Google Scholar]
- Houshdaran S, Oke AB, Fung JC, Vo KC, Nezhat C, Giudice LC.2020Steroid hormones regulate genome-wide epigenetic programming and gene transcription in human endometrial cells with marked aberrancies in endometriosis. PLoS Genetics 16 e1008601. ( 10.1371/journal.pgen.1008601) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huhtinen K, Stahle M, Perheentupa A, Poutanen M.2012Estrogen biosynthesis and signaling in endometriosis. Molecular and Cellular Endocrinology 358146–154. ( 10.1016/j.mce.2011.08.022) [DOI] [PubMed] [Google Scholar]
- Huhtinen K, Saloniemi-Heinonen T, Keski-Rahkonen P, Desai R, Laajala D, Stahle M, Hakkinen MR, Awosanya M, Suvitie P, Kujari Het al. 2014Intra-tissue steroid profiling indicates differential progesterone and testosterone metabolism in the endometrium and endometriosis lesions. Journal of Clinical Endocrinology and Metabolism 99E2188–E2197. ( 10.1210/jc.2014-1913) [DOI] [PubMed] [Google Scholar]
- Keselman A, Heller N.2015Estrogen signaling modulates allergic inflammation and contributes to sex differences in asthma. Frontiers in Immunology 6 568. ( 10.3389/fimmu.2015.00568) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keselman A, Fang X, White PB, Heller NM.2017Estrogen signaling contributes to sex differences in macrophage polarization during asthma. Journal of Immunology 1991573–1583. ( 10.4049/jimmunol.1601975) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kho PF, Mortlock SEndometrial Cancer Association Consortium, International Endometriosis Genetics Consortium, Rogers PAW, Nyholt DR, Montgomery GW, Spurdle AB, Glubb DM, O’Mara TAet al. 2021Genetic analyses of gynecological disease identify genetic relationships between uterine fibroids and endometrial cancer, and a novel endometrial cancer genetic risk region at the WNT4 1p36.12 locus. Human Genetics 1401353–1365. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee HS, Kazmi SZ, Hann HJ, Kang T, Cha J, Choi S, Swan H, Kim H, Lee YSet al. 2021Familial risk for endometriosis and its interaction with smoking, age at menarche and body mass index: a population-based cohort study among siblings. BJOG 1281938–1948. ( 10.1111/1471-0528.16769) [DOI] [PubMed] [Google Scholar]
- Kitawaki J, Obayashi H, Ishihara H, Koshiba H, Kusuki I, Kado N, Tsukamoto K, Hasegawa G, Nakamura N, Honjo H.2001Oestrogen receptor-alpha gene polymorphism is associated with endometriosis, adenomyosis and leiomyomata. Human Reproduction 1651–55. ( 10.1093/humrep/16.1.51) [DOI] [PubMed] [Google Scholar]
- Lac V, Nazeran TM, Tessier-Cloutier B, Aguirre-Hernandez R, Albert A, Lum A, Khattra J, Praetorius T, Mason M, Chiu Det al. 2019Oncogenic mutations in histologically normal endometrium: the new normal? Journal of Pathology 249173–181. ( 10.1002/path.5314) [DOI] [PubMed] [Google Scholar]
- Liang Y, Li Y, Liu K, Chen P, Wang D.2016Expression and significance of WNT4 in ectopic and eutopic endometrium of human endometriosis. Reproductive Sciences 23379–385. ( 10.1177/1933719115602763) [DOI] [PubMed] [Google Scholar]
- Lu Y, Cuellar-Partida G, Painter JN, Nyholt DRAustralian Ovarian Cancer Study, International Endogene Consortium (IEC), Morris AP, Fasching PA, Hein A, Burghaus Set al. 2015Shared genetics underlying epidemiological association between endometriosis and ovarian cancer. Human Molecular Genetics 245955–5964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luong HT, Painter JN, Shakhbazov K, Chapman B, Henders AK, Powell JE, Nyholt DR, Montgomery GW.2013Fine mapping of variants associated with endometriosis in the WNT4 region on chromosome 1p36. International Journal of Molecular Epidemiology and Genetics 4193–206. [PMC free article] [PubMed] [Google Scholar]
- Maier IM, Maier AC.2021miRNAs and lncRNAs: potential non-invasive biomarkers for endometriosis. Biomedicines 9 1662. ( 10.3390/biomedicines9111662) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marla S, Mortlock S, Houshdaran S, Fung J, Mckinnon B, Holdsworth-Carson SJ, Girling JE, Rogers PAW, Giudice LC, Montgomery GW.2021Genetic risk factors for endometriosis near estrogen receptor 1 and coexpression of genes in this region in endometrium. Molecular Human Reproduction 27 gaaa082. ( 10.1093/molehr/gaaa082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda T, Low SK, Akiyama M, Hirata M, Ueda Y, Matsuda K, Kimura T, Murakami Y, Kubo M, Kamatani Yet al. 2020GWAS of five gynecologic diseases and cross-trait analysis in Japanese. European Journal of Human Genetics 2895–107. ( 10.1038/s41431-019-0495-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matalliotaki C, Matalliotakis M, Rahmioglu N, Mavromatidis G, Matalliotakis I, Koumantakis G, Zondervan K, Spandidos DA, Goulielmos GN, Zervou MI.2019Role of FN1 and GREB1 gene polymorphisms in endometriosis. Molecular Medicine Reports 20111–116. ( 10.3892/mmr.2019.10247) [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath IM, Mortlock S, Montgomery GW.2021Genetic regulation of physiological reproductive lifespan and female fertility. International Journal of Molecular Sciences 22 2556. ( 10.3390/ijms22052556) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuleman C, Vandenabeele B, Fieuws S, Spiessens C, Timmerman D, D’Hooghe T.2009High prevalence of endometriosis in infertile women with normal ovulation and normospermic partners. Fertility and Sterility 9268–74. ( 10.1016/j.fertnstert.2008.04.056) [DOI] [PubMed] [Google Scholar]
- Missmer SA, Tu FF, Agarwal SK, Chapron C, Soliman AM, Chiuve S, Eichner S, Flores-Caldera I, Horne AW, Kimball ABet al. 2021Impact of endometriosis on life-course potential: a narrative review. International Journal of General Medicine 149–25. ( 10.2147/IJGM.S261139) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montgomery GW, Mortlock S, Giudice LC.2020Should genetics now be considered the pre-eminent etiologic factor in endometriosis? Journal of Minimally Invasive Gynecology 27280–286. ( 10.1016/j.jmig.2019.10.020) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock S, Restuadi R, Levien R, Girling JE, Holdsworth-Carson SJ, Healey M, Zhu Z, Qi T, Wu Y, Lukowski SWet al. 2019Genetic regulation of methylation in human endometrium and blood and gene targets for reproductive diseases. Clinical Epigenetics 11 49. ( 10.1186/s13148-019-0648-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortlock S, Kendarsari RI, Fung JN, Gibson G, Yang F, Restuadi R, Girling JE, Holdsworth-Carson SJ, Teh WT, Lukowski SWet al. 2020Tissue specific regulation of transcription in endometrium and association with disease. Human Reproduction 35377–393. ( 10.1093/humrep/dez279) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa S, Burn M, Mamillapalli R, Nematian S, Flores V, Taylor HS.2020Accurate diagnosis of endometriosis using serum microRNAs. American Journal of Obstetrics and Gynecology 223557.e1–557.e11. ( 10.1016/j.ajog.2020.02.050) [DOI] [PubMed] [Google Scholar]
- Muller PA, Schneeberger M, Matheis F, Wang P, Kerner Z, Ilanges A, Pellegrino K, Del Marmol J, Castro TBR, Furuichi Met al. 2020Microbiota modulate sympathetic neurons via a gut-brain circuit. Nature 583441–446. ( 10.1038/s41586-020-2474-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR, Gillespie NG, Merikangas KR, Treloar SA, Martin NG, Montgomery GW.2009Common genetic influences underlie comorbidity of migraine and endometriosis. Genetic Epidemiology 33105–113. ( 10.1002/gepi.20361) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt DR, Low SK, Anderson CA, Painter JN, Uno S, Morris AP, MacGregor S, Gordon SD, Henders AK, Martin NGet al. 2012Genome-wide association meta-analysis identifies new endometriosis risk loci. Nature Genetics 441355–1359. ( 10.1038/ng.2445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Mara TA, Glubb DM, Amant F, Annibali D, Ashton K, Attia J, Auer PL, Beckmann MW, Black A, Bolla MKet al. 2018Identification of nine new susceptibility loci for endometrial cancer. Nature Communications 9 3166. ( 10.1038/s41467-018-05427-7) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliardini L, Gentilini D, Sanchez AM, Candiani M, Vigano P, Di Blasio AM.2015Replication and meta-analysis of previous genome-wide association studies confirm vezatin as the locus with the strongest evidence for association with endometriosis. Human Reproduction 30987–993. ( 10.1093/humrep/dev022) [DOI] [PubMed] [Google Scholar]
- Painter JN, Anderson CA, Nyholt DR, Macgregor S, Lin J, Lee SH, Lambert A, Zhao ZZ, Roseman F, Guo Qet al. 2011aGenome-wide association study identifies a locus at 7p15.2 associated with endometriosis. Nature Genetics 4351–54. ( 10.1038/ng.731) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JN, Nyholt DR, Morris A, Zhao ZZ, Henders AK, Lambert A, Wallace L, Martin NG, Kennedy SH, Treloar SAet al. 2011bHigh-density fine-mapping of a chromosome 10q26 linkage peak suggests association between endometriosis and variants close to CYP2C19. Fertility and Sterility 952236–2240. ( 10.1016/j.fertnstert.2011.03.062) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JN, Nyholt DR, Krause L, Zhao ZZ, Chapman B, Zhang C, Medland S, Martin NG, Kennedy S, Treloar Set al. 2014Common variants in the CYP2C19 gene are associated with susceptibility to endometriosis. Fertility and Sterility 102496–502.e5. ( 10.1016/j.fertnstert.2014.04.015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Painter JN, O’Mara TA, Morris AP, Cheng THT, Gorman M, Martin L, Hodson S, Jones A, Martin NG, Gordon Set al. 2018Genetic overlap between endometriosis and endometrial cancer: evidence from cross-disease genetic correlation and GWAS meta-analyses. Cancer Medicine 71978–1987. ( 10.1002/cam4.1445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponomarenko I, Reshetnikov E, Polonikov A, Verzilina I, Sorokina I, Elgaeva EE, Tsepilov YA, Yermachenko A, Dvornyk V, Churnosov M.2020Candidate genes for age at menarche are associated with endometriosis. Reproductive Biomedicine Online 41943–956. ( 10.1016/j.rbmo.2020.04.016) [DOI] [PubMed] [Google Scholar]
- Powell JE, Fung JN, Shakhbazov K, Sapkota Y, Cloonan N, Hemani G, Hillman KM, Kaufmann S, Luong HT, Bowdler Let al. 2016Endometriosis risk alleles at 1p36.12 act through inverse regulation of CDC42 and LINC00339. Human Molecular Genetics 255046–5058. ( 10.1093/hmg/ddw320) [DOI] [PubMed] [Google Scholar]
- Rahmioglu N, Nyholt DR, Morris AP, Missmer SA, Montgomery GW, Zondervan KT.2014Genetic variants underlying risk of endometriosis: insights from meta-analysis of eight genome-wide association and replication datasets. Human Reproduction Update 20702–716. ( 10.1093/humupd/dmu015) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmioglu N, Macgregor S, Drong AW, Hedman ÅK, Harris HR, Randall JC, Prokopenko IInternational Endogene Consortium (IEC), The GIANT Consortium, Nyholt DR, Morris APet al. 2015Genome-wide enrichment analysis between endometriosis and obesity-related traits reveals novel susceptibility loci. Human Molecular Genetics 241185–1199. ( 10.1093/hmg/ddu516) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes-Garcia J, Montano LM, Carbajal-Garcia A, Wang YX.2021Sex hormones and lung inflammation. Advances in Experimental Medicine and Biology 1304259–321. ( 10.1007/978-3-030-68748-9_15) [DOI] [PubMed] [Google Scholar]
- Ruth KS, Beaumont RN, Tyrrell J, Jones SE, Tuke MA, Yaghootkar H, Wood AR, Freathy RM, Weedon MN, Frayling TMet al. 2016Genetic evidence that lower circulating FSH levels lengthen menstrual cycle, increase age at menopause and impact female reproductive health. Human Reproduction 31473–481. ( 10.1093/humrep/dev318) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota Y, Attia J, Gordon SD, Henders AK, Holliday EG, Rahmioglu N, Macgregor S, Martin NG, Mcevoy M, Morris APet al. 2015aGenetic burden associated with varying degrees of disease severity in endometriosis. Molecular Human Reproduction 21594–602. ( 10.1093/molehr/gav021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota Y, Low SK, Attia J, Gordon SD, Henders AK, Holliday EG, Macgregor S, Martin NG, Mcevoy M, Morris APet al. 2015bAssociation between endometriosis and the interleukin 1a (IL1A) locus. Human Reproduction 30239–248. ( 10.1093/humrep/deu267) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapkota Y, Steinthorsdottir V, Morris AP, Fassbender A, Rahmioglu N, De Vivo I, Buring JE, Zhang F, Edwards TL, Jones Set al. 2017Meta-analysis identifies five novel loci associated with endometriosis highlighting key genes involved in hormone metabolism. Nature Communications 8 15539. ( 10.1038/ncomms15539) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders PTK, Horne AW.2021Endometriosis: etiology, pathobiology, and therapeutic prospects. Cell 1842807–2824. ( 10.1016/j.cell.2021.04.041) [DOI] [PubMed] [Google Scholar]
- Shafrir AL, Farland LV, Shah DK, Harris HR, Kvaskoff M, Zondervan K, Missmer SA.2018Risk for and consequences of endometriosis: a critical epidemiologic review. Best Practice and Research: Clinical Obstetrics and Gynaecology 511–15. ( 10.1016/j.bpobgyn.2018.06.001) [DOI] [PubMed] [Google Scholar]
- Simitsidellis I, Esnal-Zuffiaure A, Kelepouri O, O’Flaherty E, Gibson DA, Saunders PTK.2019Selective androgen receptor modulators (SARMs) have specific impacts on the mouse uterus. Journal of Endocrinology 242227–239. ( 10.1530/JOE-19-0153) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spector AA.2009Arachidonic acid cytochrome P450 epoxygenase pathway. Journal of Lipid Research 50 (Supplement) S52–S56. ( 10.1194/jlr.R800038-JLR200) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark O, Peckham CS, Moynihan C.1989Weight and age at menarche. Archives of Disease in Childhood 64383–387. ( 10.1136/adc.64.3.383) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stejskalova A, Fincke V, Nowak M, Schmidt Y, Borrmann K, Von Wahlde MK, Schafer SD, Kiesel L, Greve B, Gotte M.2021Collagen I triggers directional migration, invasion and matrix remodeling of stroma cells in a 3D spheroid model of endometriosis. Scientific Reports 114115. ( 10.1038/s41598-021-83645-8) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan HY, Ho VW, Chan YT, Zhang C, Wang N, Xia W, Feng Y.2020Combination of Gentiana rhodantha and Gerbera anandria in the BL02 formula as therapeutics to non-small cell lung carcinoma acting via Rap1/cdc42 signaling: a transcriptomics/bio-informatics biological validation approach. Pharmacological Research 155104415. ( 10.1016/j.phrs.2019.104415) [DOI] [PubMed] [Google Scholar]
- Treloar SA, O’Connor DT, O’Connor VM, Martin NG.1999Genetic influences on endometriosis in an Australian twin sample. sueT@qimr.edu.au. Fertility and Sterility 71701–710. ( 10.1016/s0015-0282(9800540-8) [DOI] [PubMed] [Google Scholar]
- Treloar SA, Zhao ZZ, Armitage T, Duffy DL, Wicks J, O’Connor DT, Martin NG, Montgomery GW.2005Association between polymorphisms in the progesterone receptor gene and endometriosis. Molecular Human Reproduction 11641–647. ( 10.1093/molehr/gah221) [DOI] [PubMed] [Google Scholar]
- Tulac S, Nayak NR, Kao LC, Van Waes M, Huang J, Lobo S, Germeyer A, Lessey BA, Taylor RN, Suchanek Eet al. 2003Identification, characterization, and regulation of the canonical Wnt signaling pathway in human endometrium. Journal of Clinical Endocrinology and Metabolism 883860–3866. ( 10.1210/jc.2003-030494) [DOI] [PubMed] [Google Scholar]
- Turner KJ, Macpherson S, Millar MR, Mcneilly AS, Williams K, Cranfield M, Groome NP, Sharpe RM, Fraser HM, Saunders PT.2002Development and validation of a new monoclonal antibody to mammalian aromatase. Journal of Endocrinology 17221–30. ( 10.1677/joe.0.1720021) [DOI] [PubMed] [Google Scholar]
- Uimari O, Rahmioglu N, Nyholt DR, Vincent K, Missmer SA, Becker C, Morris AP, Montgomery GW, Zondervan KT.2017Genome-wide genetic analyses highlight mitogen-activated protein kinase (MAPK) signaling in the pathogenesis of endometriosis. Human Reproduction 32780–793. ( 10.1093/humrep/dex024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Kaam KJ, Romano A, Schouten JP, Dunselman GA, Groothuis PG.2007Progesterone receptor polymorphism +331G/A is associated with a decreased risk of deep infiltrating endometriosis. Human Reproduction 22129–135. ( 10.1093/humrep/del325) [DOI] [PubMed] [Google Scholar]
- Vashisht A, Alali Z, Nothnick WB.2020Deciphering the role of miRNAs in endometriosis pathophysiology using experimental endometriosis mouse models. Advances in Anatomy, Embryology, and Cell Biology 23279–97. ( 10.1007/978-3-030-51856-1_5) [DOI] [PubMed] [Google Scholar]
- Vercellini P, Fedele L, Aimi G, Pietropaolo G, Consonni D, Crosignani PG.2007Association between endometriosis stage, lesion type, patient characteristics and severity of pelvic pain symptoms: a multivariate analysis of over 1000 patients. Human Reproduction 22266–271. ( 10.1093/humrep/del339) [DOI] [PubMed] [Google Scholar]
- Wang W, Li Y, Li S, Wu Z, Yuan M, Wang T, Wang S.2017Pooling-based genome-wide association study identifies risk loci in the pathogenesis of ovarian endometrioma in Chinese Han women. Reproductive Sciences 24400–406. ( 10.1177/1933719116657191) [DOI] [PubMed] [Google Scholar]
- Wang Y, Hu S, Yao G, Sun Y.2021Identification of HOXA10 target genes in human endometrial stromal cells by RNA-seq analysis. Acta Biochimica et Biophysica Sinica 53365–371. ( 10.1093/abbs/gmaa173) [DOI] [PubMed] [Google Scholar]
- Winuthayanon W, Lierz SL, delaRosa KC, Sampels SR, Donoghue LJ, Hewitt SC, Korach KS.2017Juxtacrine activity of estrogen receptor alpha in uterine stromal cells is necessary for estrogen-induced epithelial cell proliferation. Scientific Reports 78377. ( 10.1038/s41598-017-07728-1) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Q, Lin Z, Cheng YH, Huang CC, Marsh E, Yin P, Milad MP, Confino E, Reierstad S, Innes Jet al. 2007Promoter methylation regulates estrogen receptor 2 in human endometrium and endometriosis. Biology of Reproduction 77681–687. ( 10.1095/biolreprod.107.061804) [DOI] [PubMed] [Google Scholar]
- Yang MH, Wang PH, Wang SJ, Sun WZ, Oyang YJ, Fuh JL.2012Women with endometriosis are more likely to suffer from migraines: a population-based study. PLoS ONE 7 e33941. ( 10.1371/journal.pone.0033941) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yilmaz BD, Bulun SE.2019Endometriosis and nuclear receptors. Human Reproduction Update 25473–485. ( 10.1093/humupd/dmz005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yotova IY, Quan P, Leditznig N, Beer U, Wenzl R, Tschugguel W.2011Abnormal activation of Ras/Raf/MAPK and RhoA/ROCKII signalling pathways in eutopic endometrial stromal cells of patients with endometriosis. Human Reproduction 26885–897. ( 10.1093/humrep/der010) [DOI] [PubMed] [Google Scholar]
- Yovich JL, Rowlands PK, Lingham S, Sillender M, Srinivasan S.2020Pathogenesis of endometriosis: look no further than John Sampson. Reproductive Biomedicine Online 407–11. ( 10.1016/j.rbmo.2019.10.007) [DOI] [PubMed] [Google Scholar]
- Zondervan KT, Rahmioglu N, Morris AP, Nyholt DR, Montgomery GW, Becker CM, Missmer SA.2016Beyond endometriosis genome-wide association study: from genomics to phenomics to the patient. Seminars in Reproductive Medicine 34242–254. ( 10.1055/s-0036-1585408) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondervan KT, Becker CM, Missmer SA.2020Endometriosis. New England Journal of Medicine 3821244–1256. ( 10.1056/NEJMra1810764) [DOI] [PubMed] [Google Scholar]



 This work is licensed under a
This work is licensed under a