Abstract
Traditional Chinese Medicine (TCM) has played crucial roles in treating COVID-19 in China. But its effectiveness has not yet been widely realized/recognized over the world. We performed a systematic review and meta-analysis to investigate the clinical efficacy of TCM medicine in the treatment for COVID-19. We obtained the data of COVID-19 and traditional Chinese medicine from PubMed, MEDLINE, Web of Science and other databases, and searched from January 1, 2020 to January 26, 2022 to determine the randomized controlled trials (RCTs) without language restrictions. The review includes 26 randomized clinical trials including 2981 patients. The treatment of COVID-19 by TCM combined with conventional treatment is more effective than by pure conventional treatment in many aspects, including increasing of the effective rate [OR = 2.47, 95%CI (1.85, 3.30), P < 0.00001], fever disappearance rate [OR = 3.68, 95%CI (1.95, 6.96), P < 0.0001], fatigue disappearance rate [OR = 3.15, 95%CI (1.60, 6.21), P = 0.0009], cough disappearance rate [OR = 2.89, 95%CI (1.84, 4.54), P < 0.00001], expectoration disappearance rate [OR = 5.94, 95%CI (1.98, 17.84), P = 0.001], disappearance rate of shortness of breath [OR = 2.57, 95%CI (1.13, 5.80), P = 0.02], improvement rate of CT image [OR = 2.43, 95%CI (1.86, 3.16), P < 0.00001], and reduction of the hospitalization time [MD = −3.16, 95%CI (−3.75, −2.56), P < 0.00001], and deterioration rate [OR = 0.49, 95%CI (0.29, 0.83), P = 0.007]. The findings of this meta-analysis suggest that TCM can effectively relieve symptoms, boosted patients' recovery, cut the rate of patients developing into severe conditions, and reduce the deterioration rate.
Abbreviations: TCM, Traditional Chinese Medicine; RCT, Randomized Controlled Trials; CHM, Chinese Herbal Medicine
1. Introduction
The pandemic COVID-19 caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been spreading for more than two years in the world since its outbreak in China in 2019 [[1], [2], [3]]. As of January 26, 2022, a total of 355, 802, 268 COVID-19 cases and 5,623,471 related deaths were reported in 185 counties. The novel coronavirus pneumonia has a rapid transmission speed, high mutation rate, and great difficulty in prevention and control. It has caused serious threats to and impacts on the people’ health and the world's economy. It is imperative to find an effective approach to stop the infection of the disease.
It is striking to note that Traditional Chinese Medicine (TCM) has played crucial roles in stopping the pandemic. TCM is one of the earliest medical/healing systems developed and matured more than 2000 years ago. TCM includes herbal medicine, acupuncture, moxibustion, etc., which is a fully institutionalized part of Chinese health care system. Instead of focusing on killing pathogenic factors through allopathy, or removing the lesions of tissue out of the body via surgery, TCM is engaged to tune the balance of the body, such as changing the microenvironment of the tissue and cells and strengthening the immunity of the body overall, for the cure of the diseases [4]. This feature is a superior advantage in the current situation of COVID-19 pandemic when there is still no sufficient knowledge on the pathology of the disease that hinders the development of effective drug/vaccine for the diseases.
Actually, TCM has provided effective medicinal formulae of both herbal medicine and acupuncture for treating COVID-19. For example, several effective formulae were designed and applied in the hospitals shortly after the breakout of the pandemic in China, of which Jinhua Qinggan granules, Lianhua Qingwen capsule, Qingfei Paidu decoction, Huashi Baidu formula, Xuanfei Baidu formula, were the representatives [5]. Of course, these formulae can be further improved according to the outcomes of the clinical practices. In the real practice, especially in the hospitals of modern medicine (MM) in China, a combined treatment, i.e., combining TCM medicinal formulae and conventional treatment (with modern medicine), was proposed for the treatment of COVID-19. These TCM medicines have been applied to the patients with mild, moderate, severe and critical cases of the pneumonia, which is characterized by quick, effective and safe [6]. The clinical results showed that TCM can effectively relieve symptoms, boost patients' recovery, cut the rate of patients developing into severe conditions, and reduce the mortality rate. The clinical results once again proved that the precious wisdom left by the TCM ancestors is practical and effective [7].
However, although the TCM medicinal formulae have been widely used in the treatment for COVID-19 disease in China, the efficacy of these formulae has not been systematically analyzed based on rigorous clinical data of randomized clinical trials (RCTs). In order to reveal their effectiveness, the meta-analysis of the RCTs clinical results of TCM medicine was performed in this study. We searched the PubMed, Medline, Web of Science, medRxiv, and bioRxiv databases as well as the Chinese database. We analyzed the effect of TCM medicinal formulae on the clinical outcomes from various aspects. These results provide strong clinical evidences for the efficacy of these TCM medicinal formulae.
2. Methods
This meta-analysis was performed based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [8] and some other methodological study on meta-analysis [[17], [18], [19]]. The protocol for this study has been registered in the International Prospective Register of Systematic Reviews (PROSPERO, CRD42020205856).
2.1. Data sources and searches
The following databases were searched from their inception to January 26, 2022: PubMed, Medline, Web of Science, medRxiv, bioRxiv, Wanfang Database and China National Knowledge Infrastructure (CNKI). The complete manuscripts of all relevant studies published in English and Chinese were retrieved. The key search keywords included those related to COVID-19, e.g., 2019-nCoV, SARS-CoV-2, COVID-19, new coronary pneumonia, corona virus, novel coronavirus or nCoV, and those related to TCM, e.g., Chinese Medicine, Traditional Chinese Medicine or TCM, Chinese herbal medicine or CHM.
2.2. Study selection
Search results were first screened based on the title and abstract using the keywords given in section 2.1, and the remaining articles were further screened by reading the full-text publication according to the following inclusion and exclusion criteria.
Inclusion criteria: (1) Participants: Included participants had been diagnosed with novel coronavirus pneumonia according to the Diagnosis and Treatment Protocol for COVID-19 (Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (DTPNCP) (Trial version 8)) [5]. And the patients were aged 18 years or older. (2) Interventions: TCM medicinal formulae combined with conventional treatment based on the DTPNCP protocol (Trial Version 8) [5], while the conventional treatment includes supportive care, respiratory assisted ventilation, anti-infection (mainly antiviral agents), and glucocorticoid therapy. (3) Outcome indicators: The primary outcome measures were clinical effective rate, disappearance rate of common symptoms (fever, cough, and fatigue), and improvement rate of chest CT image. And the disappearance time of symptoms, hospitalization time, biochemical markers, TCM Syndrome scale score, and the rate of deterioration were also measured.
Exclusion criteria: 1) The intervention measures were only with the treatment of Traditional Chinese Medicine or no control group with conventional treatment; 2) case report, case study; 3) incomplete or unable to extract or use the outcome index data.
2.3. Data extraction and quality assessment
Data were extracted to a predesigned spread-sheet for: First author or corresponding author, location, treatment, number of cases, outcome indicators, etc. The risk of bias were assessed by using the Cochrane risk of bias tool for the following seven domains: (1) random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessment; (5) incomplete outcome data; (6) selective reporting; (7) the other sources of bias. According to the above items, two researchers independently determined the assessment conclusions of each risk (i.e. high bias risk, low bias risk or unclear risk) and cross checked them. If there were any disagreements between them, a third reviewer was then consulted.
2.4. Data synthesis and analysis
RevMan V.5.3 software (Nordic Cochrane Centre, Copenhagen, Denmark) was adopted for the meta-analysis of the data. Odds ratio (OR) was used to present the effect sizes for dichotomous data, and mean difference (MD) or standardized mean difference (SMD) was used to present the effect sizes for continuous data, and 95% confidence interval (95% CI) was given. Q test and value were used to analyze the heterogeneity among the included studies. If the heterogeneity was not significant (p > 0.05, <50%), the fixed effect model is adopted; while if the heterogeneity was significant (p ≤ 0.05, ≥50%), the random effect model is adopted.
2.5. Treatment
TCM treatment: According to the Diagnosis and Treatment Protocol (NTPNCP, Trial Version 8) [5] made by the National Health Commission of China, the novel coronavirus infected pneumonia (NCIP) was divided into four types: mild, moderate, severe, and critical. The TCM medicinal formulae recommended were Feiyan Yihao granules, Lianhua Qingwen capsule, Jinhua Qinggan granules, Shufeng Jiedu capsule, Toujie Quwen granules, Qingfei Paidu decoction, Xuanfei Baidu formula, Maxing Xuanfei decoction, Huashi Baidu granules, Keguan-1, etc. These formulae are provided in the Supplemental Material.
Conventional treatment: Oxygen therapy, antiviral therapy, antibiotic drug treatment, immunotherapy and other therapeutic measures.
3. Results
3.1. Eligible studies
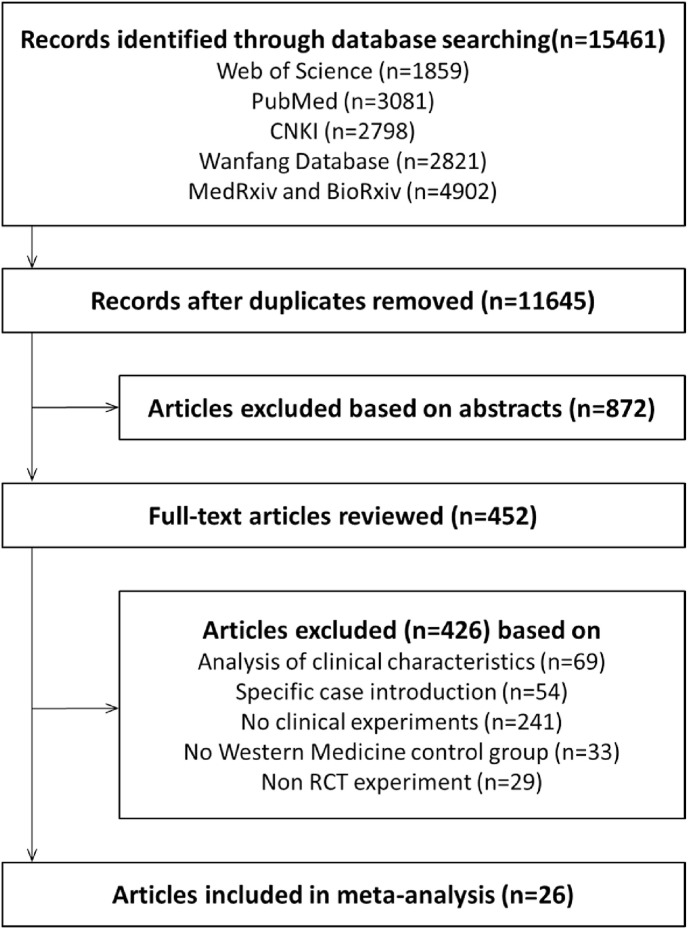
15,461 papers were preliminarily retrieved, including 2798 CNKI papers, 2821 Wanfang Database papers, 1859 Web of Science papers and 3081 PubMed papers, 4902 medRxiv and bioRxiv papers. After removing duplicate publications, 11,645 studies were remained; then the title, abstract and full text were read, according to the inclusion and exclusion criteria 26 RCTs articles were finally included for the meta-analysis [[9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34]]. Fig. 1 illustrates the specific screening process. 2981 patients with COVID-19 were included. Table 1 shows the basic information included in the meta-analysis.
Fig. 1.
Flow-chart depicting the literature search and selection strategy. A total of 26 RCTs articles were included in the meta-analysis according to the inclusion and exclusion criteria.
Table 1.
Basic information included in the analysis.
| Studies | Location | T/C | Control | Treatment | Treatment duration | Outcome indicators |
|---|---|---|---|---|---|---|
| Ai et al., 2020 [9] | Guangzhou | 33/34 | Conventional treatment | Feiyan Yihao granules + C | 7 days | 2/5/6 |
| Hu et al., 2020 [14] | Nine provinces | 139/139 | Conventional treatment | Lianhua Qingwen capsules + C | 14 days | 1/3/9 |
| Duan et al., 2020 [12] | Wuhan | 82/41 | Antiviral drugs and antibacterial drugs | Jinhua Qinggan granules + C | 5 days | 2/5/10 |
| Sun et al., 2020 [19] | Hebei | 32/25 | Interferon-α, lopinavir and tonavir tablets | Lianhua Qingke granules + C | 14 days | 2/3/5/9 |
| Jin et al., 2020 [15] | Sichuan | 20/18 | Conventional treatment | Chinese patent medicine + C | 21 days | 1/2/3/7/9 |
| Lin et al., 2020 [17] | Wenzhou | 41/41 | Conventional treatment | Xuanfei Qingre decoction + C | 14 days | 1/3/6/7/8/9/10 |
| Liu et al., 2021 [32] | Wuhan | 99/96 | Conventional treatment | Huashi Baidu granules + C | 14 days | 2/3 |
| Fu et al., 2020 [13] | Guangzhou | 37/36 | Arbidol tablets and ambroxol tablets | Toujie Quwen granules + C | 15 days | 1/5/7/8 |
| Qiu et al., 2020 [18] | Chongqing | 25/25 | Interferon-α, lopinavir and tonavir tablets | Maxing Xuanfei Jiedu decoction + C | 10 days | 3/4/9/10 |
| Yu et al., 2020 [27] | Wuhan | 147/148 | Conventional treatment | Lianhua Qingwen granules + C | 7 days | 1/3/7/8/9/ |
| Ding et al., 2020 [11] | Wuhan | 51/49 | Conventional treatment | Qingfei Touxie Fuzheng recipe + C | 10 days | 2/3/5 |
| Li et al., 2020 [16] | Shanxi | 6/6 | Conventional treatment | Qingfei Paidu decoction + C | 6 days | 1/6/7 |
| Zhang et al., 2020 [28] | Wuhan | 23/22 | Conventional treatment | Jiaweidayuanyin decoction + C | 7 days | 3/4/7 |
| Wang et al., 2020 [20] | Beijing | 24/23 | Conventional treatment | Keguan-1 +C | 14 days | 3/4 |
| Xiao et al., 2020 [25] | Wuhan | 100/100 | Antiviral drugs (abidol tablets) | Shufeng Jiedu capsules + C | 14 days | 1/3/7 |
| Wang et al., 2020 [21] | Honghu | 40/40 | Conventional treatment | Shengmai Powder combined with Shenling Baizhu Powder | unclear | 1/3/4/7/8 |
| Wang et al., 2020 [23] | Xi'an | 10/10 | Conventional treatment | Chinese medicine decoction + C | 7 days | 3/4 |
| Xiong et al., 2020 [26] | Wuhan | 22/20 | Conventional treatment | Xuanfei Baidu decoction + C | 7 days | 2/5 |
| Chen et al., 2021 [10] | Shenzhen | 28/29 | Conventional treatment | Lianhua Qingwen granules + C | Until discharged | 4 |
| Wang et al., 2021 [30] | Ji'ning | 30/30 | Conventional treatment | Chinese medicine decoction + C | 14 days | 1/4 |
| Wang et al., 2021 [22] | Hubei | 70/70 | Conventional treatment | Qingfei Paidu decoction + C | 10 days | 1/6/7/10 |
| Xiao et al., 2020 [24] | Wuhan | 94/94 | Conventional treatment | Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules + C | 14 days | 2 |
| Ye et al., 2021 [31] | Wuhan | 50/50 | Conventional treatment | Modified Shengjiang Powder + C | 6 days | 1/7 |
| Zhao et al., 2021 [34] | Wuhan | 204/204 | Conventional treatment | Huashi Baidu granules + C | 7 days | 9 |
| Zhao et al., 2021 [33] | Jingzhou | 51/45 | Conventional treatment | Antiviral Formula-1 +C | 7 days | 1/3/8/10 |
| Zheng et al., 2020 [29] | Wuhan | 65/63 | Conventional treatment | Chinese medicine decoction + C | 14 days | 1 |
Abbreviation: T/C: C: The control; T: The treatment. Outcome indicators: Primary outcome measures: 1: clinical effective rate, 2: disappearance rate of common symptoms (fever, cough, fatigue), 3: improvement rate of CT image; Secondary outcome measures: 4: disappearance time of symptoms, 5: disappearance rate of less common symptoms, 6: hospitalization time, 7: white blood cell count, 8: lymphocyte count, 9: rate of deterioration, 10: The scale score of TCM symptom.
3.2. Assessment of methodological quality
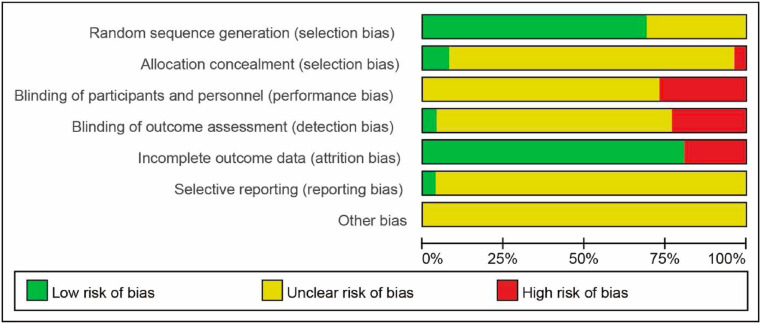
The bias risk assessment tool of Cochrane Collaboration Network is adopted for the evaluation of literature quality (Fig. 2 .). Fourteen studies illustrated specific methods of randomization, such as random number table. One study claimed use of assignment concealment. Five studies claimed no use of blinding of participants and personnel. Four studies claimed no use of blinding of outcome assessment. And there are four studies that mentioned dropouts.
Fig. 2.
Risk of bias among included trials (presented using Cochrane risk of bias assessment).
3.3. Summary of findings
Our results showed that the TCM medicine can significantly increase the effective rate and reduce the hospitalization time. Specifically, TCM can increase the rate and reduce the time of disappearance of the symptoms, such as fever, fatigue, cough, expectoration and shortness of breath; In addition, TCM can significantly improve the chest CT image, increase the TCM symptom scale score, and reduce the deterioration rate.
3.3.1. Effective rate, chest CT image, rate of deterioration
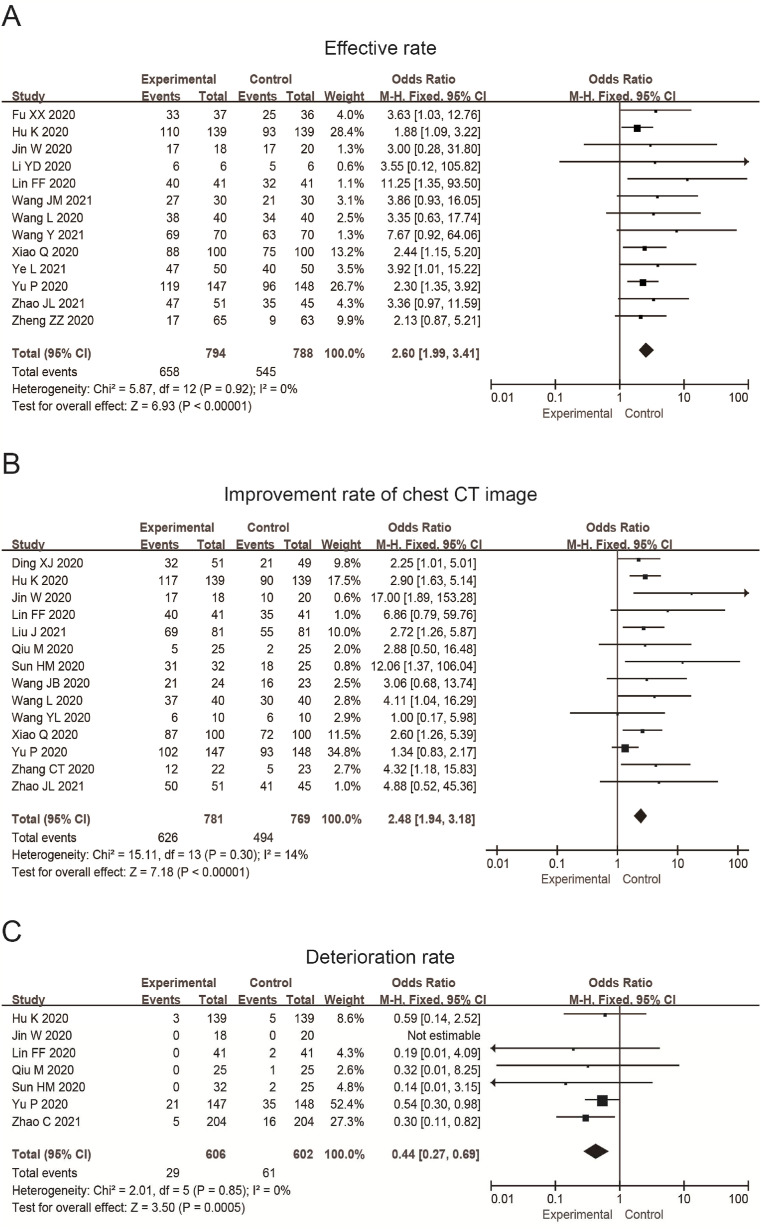
Thirteen studies reported the effective rate, in which 1582 patients were included. The results showed there was no heterogeneity among the studies (p = 0.92, = 0%), then we used the fixed effect model. The results were OR = 2.60, 95%CI (1.99, 3.41). The difference between the experimental group and control group was statistically significant (P < 0.0001) (Fig. 3 A). It is suggested that the total effective rate of the experimental group treated by the combination of TCM medicinal formulae and the conventional treatment is higher than that of the control group with the pure conventional treatment. Specifically, the effective rate of the experimental group was 82.87%, while that of the control group was 69.16%.
Fig. 3.
Forest plot shows the effective rate (A), the improvement rate of the chest CT image (B), and the rate of deterioration (C) of patients with COVID-19 treated by the TCM combined with the conventional treatment.
Fourteen studies reported the improvement rate of chest CT image, in which 1550 patients were included. The results showed that the heterogeneity among the studies was small (p = 0.30, = 14%). We then used the fixed effect model, and the results were OR = 2.48, 95%CI (1.94, 3.18). They suggested that the improvement rate of the CT image in the experimental group is much higher than that in the control group. The difference between the two groups was statistically significant (P < 0.0001) (Fig. 3B). The improvement rate was 80.15% in the experimental group and 64.24% in the control group.
Seven studies reported the rate of deterioration, and 1208 patients were included. The results showed that there was no heterogeneity among the studies (p = 0.85, = 0%). We then used the fixed effect model, and the results were OR = 0.44, 95%CI (0.27, 0.69). The deterioration rate in the experimental group is lower than that in the control group. The difference between the two groups was statistically significant (P = 0.0005) (Fig. 3C). The rate was 4.79% in the experimental group and 10.13% in the control group.
3.3.2. Fever, fatigue, cough
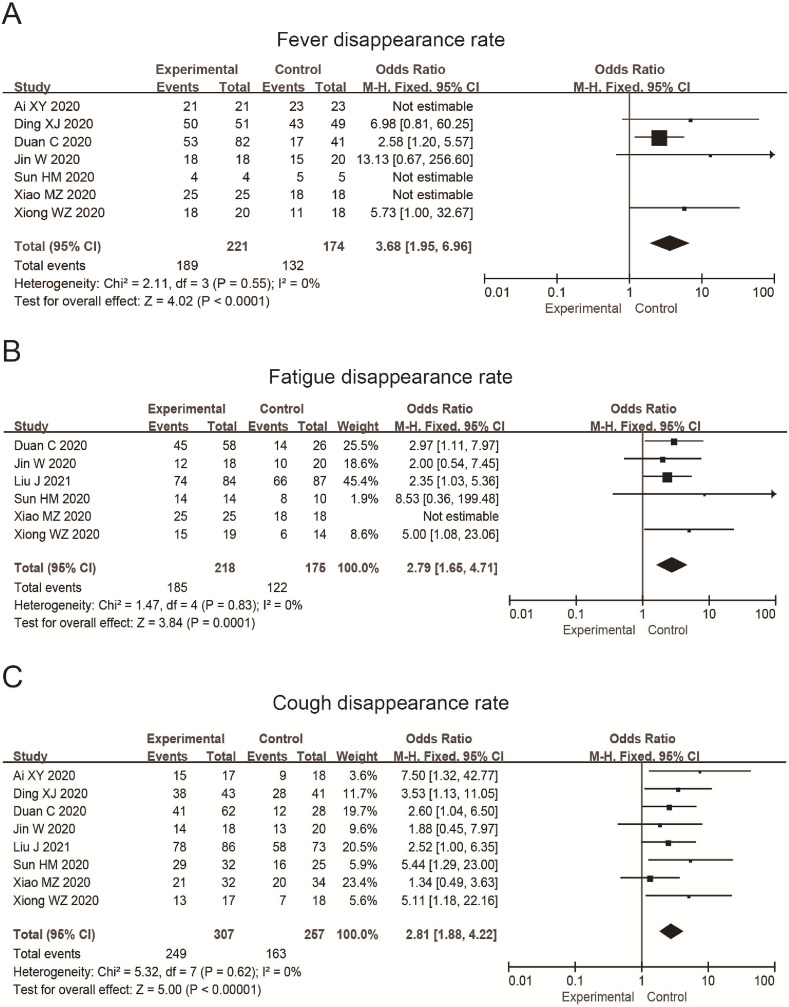
Seven studies reported the fever disappearance rate, in which 395 patients were included. The results showed that there was no heterogeneity among the studies (p = 0.55, = 0%). We used the fixed effect model, and the results were OR = 3.68, 95%CI (1.95, 6.96). The disappearance rate of fever in the experimental group is higher than that in the control group. The difference between the two groups was statistically significant (P < 0.0001) (Fig. 4 A). The rate in the experimental group was 85.52%, while that in the control group was 75.86%.
Fig. 4.
Forest plot of the disappearance rate of fever (A), fatigue (B), cough (C) of patients with COVID-19 treated by TCM combined with the conventional treatment.
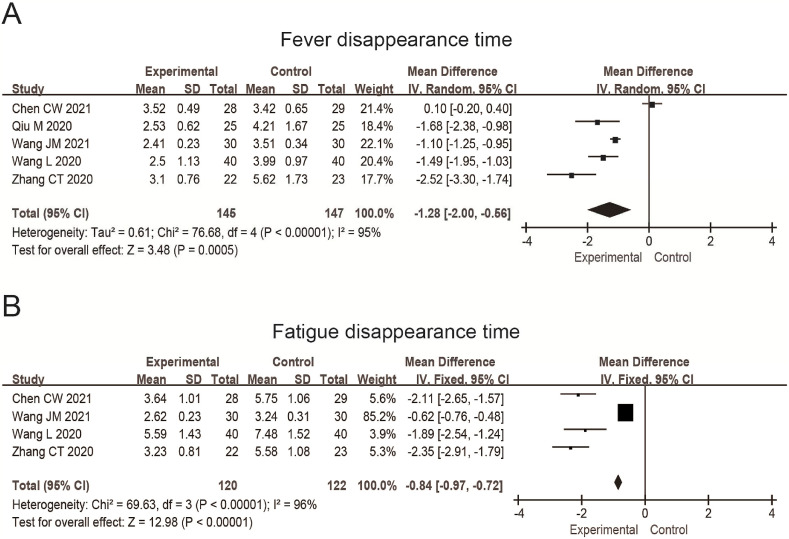
In addition, five studies reported the disappearance time of fever, in which 292 patients were included. The results were MD = −1.28, 95%CI (−2.00, −0.56), P = 0.0005 (Fig. 5 A), which showed that the fever disappearance time of the experimental group was significantly shortened by about 1.3 days compared with the control group.
Fig. 5.
Forest plot shows the disappearance time of fever (A) and fatigue (B) treated by TCM combined with the conventional treatment. The unit of the mean value is days.
Six studies reported the disappearance rate of fatigue, in which 393 patients were included. The results showed that there was no significant heterogeneity among the studies (p = 0.83, = 0%). We therefore used the fixed effect model, and the results were OR = 2.79, 95%CI (1.65, 4.71). The disappearance rate of fatigue in the experimental group is higher than that in the control group. The difference between the two groups was statistically significant (P = 0.0001) (Fig. 4B). The rate was 84.86% in the experimental group and 69.71% in the control group.
Furthermore, four studies reported the fatigue disappearance time, in which 242 patients were included. The results were MD = −0.84, 95%CI (−0.97, −0.72), P < 0.0001 (Fig. 5B), which showed that the fatigue disappearance time of the experimental group was significantly shortened by about 1.74 days compared with the control group.
Eight studies reported the cough disappearance rate, in which 564 patients were included. The results showed that there was no significant heterogeneity among the studies (p = 0.62, = 0%). We then used the fixed effect model, and the results were OR = 2.81, 95%CI (1.88, 4.22). The disappearance rate of cough in the experimental group is higher than that in the control group. The difference between the two groups was statistically significant (P < 0.0001) (Fig. 4C). It was 81.11% in the experimental group and 64.42% in the control group.
3.3.3. Expectoration, shortness of breath
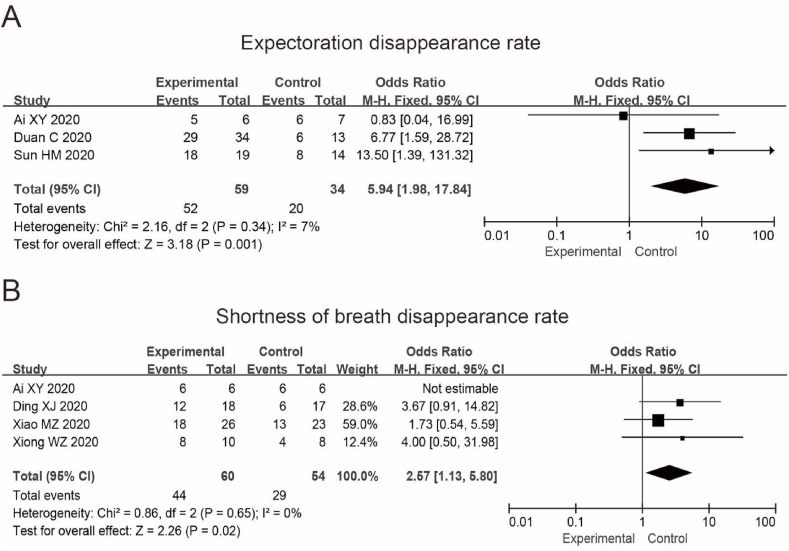
Three studies reported expectoration disappearance rate, and 93 patients were included. The results showed that the heterogeneity among the studies was small (p = 0.34, = 7%). We then used the fixed effect model, and the results were OR = 5.94, 95%CI (1.98, 17.84). The disappearance rate of expectoration in the experimental group is higher than in the control group. The difference between the two groups was statistically significant (P = 0.001) (Fig. 6 A). The disappearance rate was 88.14% in the experiment and 58.82% in the control.
Fig. 6.
Forest plot of the disappearance rate of expectoration (A) and shortness of breath (B) of patients with COVID-19 treated by TCM combined with the conventional treatment.
Four studies reported the disappearance rate of shortness of breath, in which 114 patients were included. The results showed that there was no significant heterogeneity among the studies (p = 0.65, = 0%). We then used the fixed effect model, and the results were OR = 2.57, 95%CI (1.13, 5.80). The difference between the experimental and control groups was statistically significant (P = 0.02) (Fig. 6B). The disappearance rate was 73.33% in the experimental group while 53.70% in the control group.
3.3.4. Biochemical markers
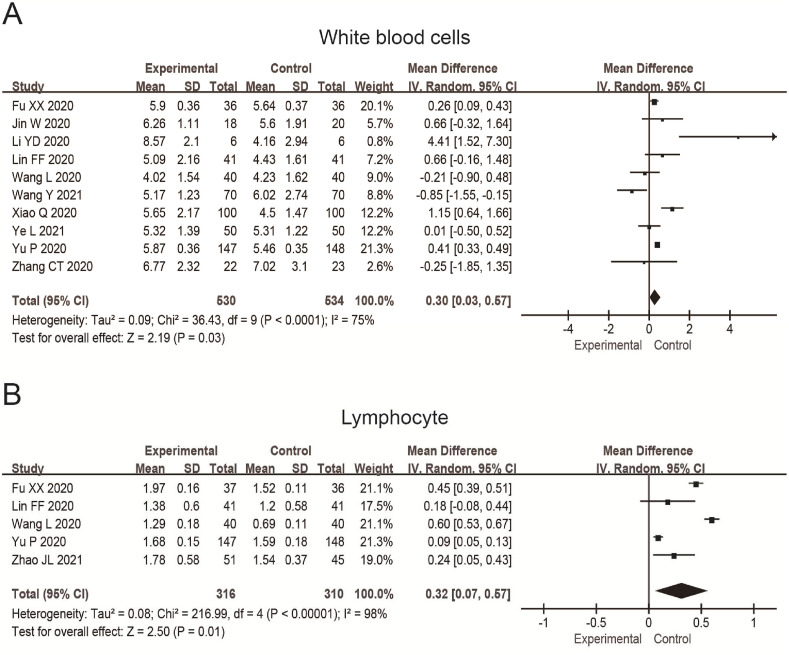
Ten studies reported the white blood cells count (WBC), and 1064 patients were included. The results were MD = 0.30, 95%CI (0.03, 0.57), P = 0.03 (Fig. 7 A). In addition, five studies reported the lymphocyte count, in which 626 patients were included. The results were MD = 0.32, 95%CI (0.07, 0.57), P = 0.01 (Fig. 7B). It showed that the value of WBC and lymphocyte count in the experimental group are higher than in the control group.
Fig. 7.
Forest plot shows the white blood cells count (A) and lymphocyte count (B) of patients with COVID-19 treated by TCM combined with the conventional treatment. The unit is 109/L.
3.3.5. Hospitalization time and TCM symptom scale score
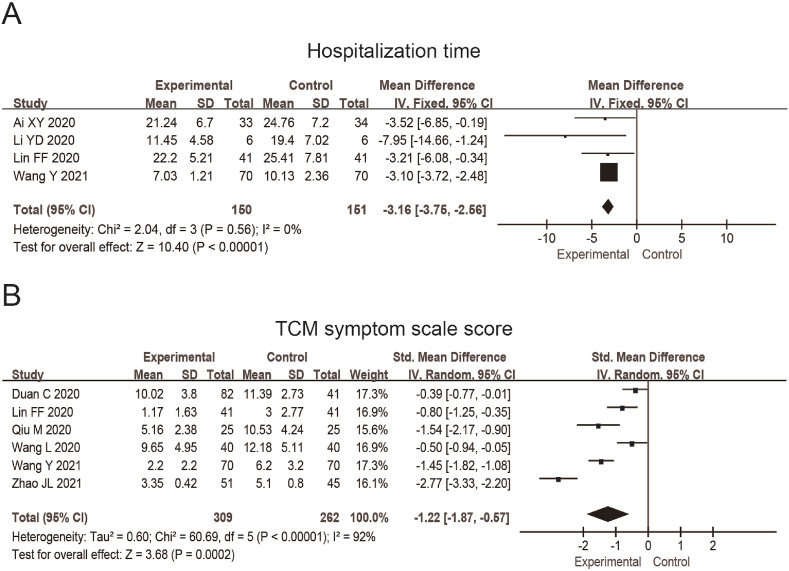
Four studies reported the hospitalization time, and 301 patients were included. The results were MD = −3.16, 95%CI (−3.75, −2.56), P < 0.0001 (Fig. 8 A), which showed that the hospitalization time of the experimental group was significantly shortened by 4.45 days compared with the control group.
Fig. 8.
Forest plot shows the hospitalization time (A) and the scale score of TCM symptom of patients (B) of patients with COVID-19 treated by TCM combined with the conventional treatment. The unit in (A) is days.
The TCM symptom scale is designed according to the Guiding Principles for Clinical Study of Chinese Medicines (Trial) [35]. The higher the scale score, the more serious the disease [36]. Six studies reported this indicator, and 571 patients were included. The results were SMD = −1.22, 95%CI (−1.87, −0.57), P = 0.0002 (Fig. 8B). The scale score in the experimental group is lower than in the control group by 2.81 points, i.e., the symptoms after treatment in the experimental group became lighter than those in the control group.
The above results suggest that the combined treatment of the TCM medicinal formulae with the conventional treatment is more effective than the pure conventional treatment for COVID-19 in this pandemic, as shown in Table 2 . The combined treatment resulted in a significantly higher rate of, and a shorter time for, symptom recovery than the control group, and for all the common symptoms, including fever, fatigue, cough, expectoration and shortness of breath, etc. Correspondingly, the laboratory examination, such as the chest CT imaging and biochemical markers, had also been significantly improved.
Table 2.
Summary of meta-analyses of the efficacy of TCM medicinal formulae.
| Outcomes | Cases | Effect Size (95%CI) | P value |
|---|---|---|---|
| Effective rate | 1582 | 2.60 (1.99, 3.41) | <0.0001 |
| Fever disappearance rate | 395 | 3.68 (1.95,6.96) | <0.0001 |
| Fatigue disappearance rate | 393 | 2.79 (1.65, 4.71) | 0.0001 |
| Cough disappearance rate | 564 | 2.81 (1.88, 4.22) | <0.0001 |
| Expectoration disappearance rate | 93 | 5.94 (1.98, 17.84) | 0.0010 |
| Disappearance rate of the shortness of breath | 114 | 2.57 (1.13, 5.80) | 0.0200 |
| CT image improvement rate | 1550 | 2.48 (1.94, 3.18) | <0.0001 |
| Rate of deterioration | 1208 | 0.44 (0.27, 0.69) | 0.0005 |
| Hospitalization time | 301 | −3.16 (−3.75, −2.56) | <0.0001 |
| Fever disappearance time | 292 | −1.28 (−2.00, −0.56) | 0.0005 |
| Fatigue disappearance time | 242 | −0.84 (−0.97, −0.72) | <0.0001 |
| White blood cells count | 1064 | 0.30 (0.03, 0.57) | 0.0300 |
| Lymphocyte count | 626 | 0.32 (0.07, 0.57) | 0.0100 |
| TCM syndrome scale score | 571 | −1.22 (−1.87, −0.57) | 0.0002 |
4. Discussion
In fact, the clinical efficacy of TCM [7] has been verified through thousands of years of practices for prevention and treatment of diseases. Instead of killing pathogenic factors or removing the lesions of tissue out of the body through allopathic approaches, TCM is engaged to tune the balance of the body and strengthen the immunity of the body overall for the cure of the diseases [37]. It has been shown that TCM and its healing modalities are excellent tools for maintaining the health and keeping the well-being of life. The advantages of TCM, particularly in this pandemic, can be understood from several aspects as follows.
Firstly, the efficacy of many TCM medicinal formulae had been proved by scientific research. For instance, Lianhua Qingwen capsule, a patented product that has been marketed for the severe acute respiratory syndrome (SARS) epidemic since 2003 in China, can significantly shorten the duration of fever, fatigue and coughing [14]. It was shown that the high rate of clinical cure and recovery of chest CT manifestations was associated with the activities of Lianhua Qingwen capsule against SARS-CoV-2 and its anti-inflammatory effects. The latest research showed that Lianhua Qingwen capsule conferred the suppression of the cytopathic effect of SARS-CoV-2 in vitro and reduced the viral loads in the cytoplasm and cellular membrane [38]. Moreover, Lianhua Qingwen could also suppress the replication of SARS-CoV-2 [39].
In addition, it is noted that the herbal drugs components, ephedra, Scutellaria baicalensis, and Poria (see Supplementary Material), the important ingredients in most of the TCM medicinal formulae for treating COVID-19, contain the anti-inflammatory polysaccharides that plays crucial roles in suppressing the cytokine storm and blocking the conversion from the mild to severe cases. Specifically, an acid component of Ephedra polysaccharide, ESP-B4, has strong protective effects on pulmonary inflammation by reducing the production of TNF-α, IL-6, IL-8 and MMP-9 [40] and inhibiting the TLR4 signaling pathway [41]. A polysaccharide from Scutellaria baicalensis can suppress NF-κB signaling and NLRP3 inflammasome activation [42]. And a polysaccharide from Poria cocos can suppress the production of IP-10, the marker of interferon (IFN)-c-induced inflammation, in a dose-dependent manner [43,44].
Secondly, as a tradition, TCM medicinal formulae have also been prescribed for prevention of infectious diseases, which are effective and convenient. The clinical practice showed that the TCM medicinal formulae are particularly suitable for the prevention and treatment of large-scale infectious diseases. A typical example is that the TCM medicinal formulae were used for preventing SARS in 2003 in Pinghu People's Hospital in Zhejiang province of China. For both the staff and inpatients in the hospital, the decoction of TCM medicine for prevention was provided for free, and about 500 people took it every day. The medicines for prevention were also recommended to many enterprises in Pinghu city, with a total of more than 150,000 people taking the medicine. Follow up survey showed that there were no fever cases, suspected cases and infected cases of SARS among those who had taken the preventive medicine [45].
Again, in this COVID-19 pandemic, the DTPNCP protocol (Trial Version 8) [5] in China recommends several medicinal formulae for the prevention of the disease, such as Huoxiang Zhengqi capsule, Jinhua Qinggan granules, Lianhua Qingwen capsule and Shufeng Jiedu capsule, for the healthy people as well as the susceptible ones who had not been diagnosed with COVID-19 but under the medical observation. Compared with those who did not take the preventive medicine, the number of people being infected with COVID-19 was significantly reduced [46]. It was reported that Changchun University of Chinese Medicine prepared a large amount of TCM preventive herbal tea in the canteen and dormitory for students. Later then, the preventive herbal tea was extended in a large scale to the universities, enterprises and institutions in Jilin Province. There are more than 100,000 people taking the herbal tea in Jilin province, and the preventive effect is significant [47].
Clinical studies [48] showed that the commonly used aromatic antifouling drugs such as musk, Atractylodes rhizome, borneol, etc., can directly act on the mucous membrane, and increase the content of secretory immunoglobulin A in respiratory tract mucosa, then produce efficient humoral and cellular immunity against harmful pathogens, and effectively repel or eliminate epidemic diseases, so that play an active preventive role. For instance, Atractylodes rhizome fumigation can effectively prevent hospital infection [49].
Thirdly, TCM is intrinsically personalized medicine. The medicinal formulae can be designed/prescribed according to the specific symptom patterns of the patients, which is one of the most important advantages of TCM. For instance, the patients may have different syndromes even with the same disease caused by the same virus (e.g. SARS-CoV-2 in this pandemic). Therefore, the medicinal formula can be adjusted according to the specific symptoms of the patients, by which the herbs can be chosen more specifically. A personalized TCM prescription will further increase the efficacy of the medicine. This character is particularly important for the patients with severe and critical conditions, e.g., complicated with other diseases. In addition, the adverse effects of TCM are small compared with the modern medicine, which has been confirmed in previous studies [50], and the relapses are few.
More importantly, as the TCM medicine is generally a type of multi-target drug, it is insensitive to the mutation of the virus, which is a distinctive feature compared to the anti-virus drug of modern medicine. This character is crucial because frequent mutations are part of the natural evolution of a virus and SARS-CoV-2 is not an exception to that rule. And the more severely a virus mutates, the less effective the vaccination campaigns might be. Therefore, TCM medicine is crucial for protecting the health of the people in this pandemic.
We realized that there are some shortcomings of this meta-analysis, for instance, some studies did not explain the use of random assignment, assignment concealment and blind method, or some studies claimed that they did not use blind method. In addition, currently only Chinese researchers carried out the clinical trial studies of the effect of TCM medicine on COVID-19. Therefore, all the articles we searched were from Chinese authors. We hope that our analyses will let the world know the efficacy of TCM medicine on COVID-19. And then more people outside China will use TCM medicine to treat COVID-19 and study its effect.
5. Conclusions
In conclusion, we showed that the COVID-19 can be treated effectively by TCM medicinal formulae combining with conventional treatment, in comparison with the pure conventional treatment. The advantages of the TCM medicine are effective, cheap, preventive, and personalized, particularly it can be promptly improved according to the change of pandemic pattern. These characteristics of the TCM medicine are particularly important for current situation that the effective anti-virus drugs are still not available yet.
Author contributions
Jiayi Xu: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Validation, Writing - original draft. Hongmei Liu: Data curation, Investigation, Formal analysis, Validation. Yubo Fan: Resources, Writing - original draft. Baohua Ji: Conceptualization, Methodology, Project administration, Validation, Resources, Writing - original draft, Writing - review & editing, Supervision.
Funding source
This research has been supported by the National Natural Science Foundation of China (Grant No. 11932017, 11772055, 11532009, and 11521062).
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.medntd.2022.100139.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Li Q., Guan X., Wu P., et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382:1199–1207. doi: 10.1056/NEJMoa2001316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D., Hu C., Zhu F., et al. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323:1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu R., Zhao X., Li J., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/s0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang J., Liu B., Ma K. Traditional Chinese medicine. Lancet. 2008;372:1938–1940. doi: 10.1016/s0140-6736(08)61354-9. [DOI] [PubMed] [Google Scholar]
- 5.National Health Commission(国家卫生健康委) Diagnosis and treatment protocol for novel coronavirus pneumonia (trial version 8) (新型冠状病毒肺炎诊疗方案 (试行第八版)) 2020. http://www.gov.cn/zhengce/zhengceku/2020-08/19/content_5535757.htm [in Chinese)]
- 6.Gong B., Zhang S., Yuan L., Chen K.Z. A balance act: minimizing economic loss while controlling novel coronavirus pneumonia. J Chin Gov. 2020;5:249–268. doi: 10.1080/23812346.2020.1741940. [DOI] [Google Scholar]
- 7.Kaptchuk T.J. Congdon and Weed; Chicago: 1983. The web that has No weaver. [Google Scholar]
- 8.Moher D., Liberati A., Tetzlaff J., Altman D.G., Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009;6 doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ai X., Lin L., Xie M., Tan X. Effect of integrated traditional Chinese and Western medicine on T lymphocyte subsets of patients with normal type of COVID-19. Guangdong Med J. 2020;41:1203–1206. doi: 10.13820/j.cnki.gdyx.20201391. [in Chinese] [DOI] [Google Scholar]
- 10.Chen C., Li X., Liu Y., Chen S. Clinical study of Lianhua Qingwen capsule in the treatment of corona virus disease 2019. Res Integ Tradit Chin West Med. 2021;13:1–4. [in Chinese] [Google Scholar]
- 11.Ding X., Zhang Y., He D., et al. Clinical effect and mechanism of Qingfei Touxie Fuzheng recipe in the treatment of COVID-19. Her Med. 2020;39:640–644. doi: 10.3870/j.issn.1004-0781.2020.05.012. [in Chinese] [DOI] [Google Scholar]
- 12.Duan C., Xia W., Zheng C., et al. Clinical observation on Jinhua Qinggan granule (金花清感颗粒) combined with conventional western medicine therapy in treating mild cases of coronavirus disease 2019. J Tradit Chin Med. 2020;61:1473–1477. doi: 10.13288/j.11-2166/r.2020.17.001. [in Chinese] [DOI] [Google Scholar]
- 13.Fu X., Lin L., Tan X. Clinical study on 37 case of COVID- 19 treated with integrated traditional Chinese and western medicine. Tradit Chin Drug Res Clin Pharmacol. 2020;31:600–604. doi: 10.19378/j.issn.1003-9783.2020.00. [in Chinese] [DOI] [Google Scholar]
- 14.Hu K., Guan W., Bi Y., et al. Efficacy and safety of Lianhuaqingwen capsules, a repurposed Chinese herb, in patients with coronavirus disease 2019: a multicenter, prospective, randomized controlled trial. Phytomedicine. 2020:153242. doi: 10.1016/j.phymed.2020.153242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jin W., Lu Y., Zhao W., et al. The efficacy of recommended treatments with integrated Chinese and western medicine for coronavirus disease 2019 (COVID-19) in Sichuan: a clinical trial observation. Pharmacol Clin Chin Mater Med. 2020;36:6–10. doi: 10.13412/j.cnki.zyyl.20201110.006. [in Chinese] [DOI] [Google Scholar]
- 16.Li Y., Zhang W. Evaluation on the clinical effect of traditional Chinese medicine and western medicine regimens on COVID-19. Guangming J Chin Med. 2020;35:1273–1275. doi: 10.3969/j.issn.1003-8914.2020.09.001. [in Chinese] [DOI] [Google Scholar]
- 17.Lin F., Huang J., Yang J., Zhang S., Xie S., Zhou F. Clinical study on the COVID-19 treated by Xuanfei Qingre prescription(宣肺清热方辅助治疗普通型新型冠状病毒肺炎临床研究) Zhejiang J Integr Tradit Chin West Med. 2020;30:977–981. [in Chinese] [Google Scholar]
- 18.Qiu M., Li Q., Zhu D., et al. Efficacy observation of maxing Xuanfei Jiedu decoction on moderate COVID-19 patients. J Emerg Tradit Chin Med. 2020;29:1129–1132. doi: 10.3969/j.issn.1004-745X.2020.07.001. [in Chinese] [DOI] [Google Scholar]
- 19.Sun H., Zhang L., Wei C., Chen J., Wang Q., Jia Z. Study on clinical efficacy of Lianhua Qingke granule in treatment of mild and ordinary COVID-19. Chin J Exp Tradit Med Formulae. 2020;26:29–34. doi: 10.13422/j.cnki.syfjx.20201438. [in Chinese] [DOI] [Google Scholar]
- 20.Wang J., Wang Z., Jing J., et al. Exploring an integrative therapy for treating COVID-19: a randomized controlled trial. Chin J Integr Med. 2020;26:648–655. doi: 10.1007/s11655-020-3426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang L., Xu M., Wang Y., Li H., Liu N., Zuo J. Clinical study on shengmai powder combined with Shenling baizhu powder in the treatment of common corona virus disease 2019. China J Tradit Chin Med Pharm. 2020;35:4268–4271. [in Chinese] [Google Scholar]
- 22.Wang Y., Chen L., Zheng L., Ku B., Yu R., Zhang X. Clinical effects of Qingfei Paidu Decoction combined with conventional treatment on patients with coronavirus disease 2019. Chin Tradit Pat Med. 2021;43:656–659. [in Chinese] [Google Scholar]
- 23.Wang Y., Yang X., Liu Y., et al. Clinical effect of the treatment of novel coronavirus pneumonia by internal administration of traditional Chinese medicine plus fumigation and absorption combined with super dose of vitamin C in treating COVID-19. J Xi’an Jiaot Univ. 2020;41:931–935. doi: 10.7652/jdyxb202006023. [in Chinese] [DOI] [Google Scholar]
- 24.Xiao M., Tian J., Zhou Y., et al. Efficacy of Huoxiang Zhengqi dropping pills and Lianhua Qingwen granules in treatment of COVID-19: a randomized controlled trial. Pharmacol Res. 2020:105126. doi: 10.1016/j.phrs.2020.105126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xiao Q., Jiang Y., Wu S., et al. Analysis of the value of traditional Chinese medicine Shufeng Jiedu capsule combined with Abidol in the treatment of mild cases of Novel coronavirus pneumonia(中药疏风解毒胶囊联合阿比多尔治疗轻症新型冠状病毒肺炎的价值分析) J Emerg Tradit Chin Med. 2020;29:756–758. doi: 10.3969/j.issn.1004-745X.2020.05.002. [in Chinese] [DOI] [Google Scholar]
- 26.Xiong W.Z., Wang G., Du J., Ai W. Efficacy of herbal medicine (Xuanfei Baidu decoction) combined with conventional drug in treating COVID-19:A pilot randomized clinical trial. Integr Med Res. 2020;9:100489. doi: 10.1016/j.imr.2020.100489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yu P., Li Y., Wan S., Wang Y. Effects of Lianhua Qingwen granules plus arbidol on treatment of mild corona virus disease-19. Chin Pharmaceut J. 2020;55:1042–1045. doi: 10.11669/cpj.2020.12.014. [in Chinese] [DOI] [Google Scholar]
- 28.Zhang C., Yang Y., You F., et al. Clinical study on COVID-19 from the perspective of “yidujiashi” theory. Pharmacol Clin Chin Mater Med. 2020;36:43–45. doi: 10.13412/j.cnki.zyyl.20200327.002. [in Chinese] [DOI] [Google Scholar]
- 29.Zheng Z., Bai Z., Li C., Ge S., Luo Y., He G. Effect of TCM syndrome differentiation on New Coronavirus pneumonia. Med J Commun. 2020;34:117–118. doi: 10.19767/j.cnki.32-1412.2020.02.005. [in Chinese] [DOI] [Google Scholar]
- 30.Wang J., Bu X., Yang S., Wang P., Wang X. Analysis of traditional Chinese medicine treatment of 60 patients with novel coronavirus pneumonia in Southwestern Shandong. Smart Healthc. 2021;31:177–179. doi: 10.19335/j.cnki.2096-1219.2021.31.057. [in Chinese] [DOI] [Google Scholar]
- 31.Ye L., Zhao H., Xu S., Chen W. Clinical study of modified Shengjiang powder in the treatment of ordinary COVID-19. Chin Foreign Med Res. 2021;18:9–13. doi: 10.14033/j.cnki.cfmr.2021.28.003. [in Chinese] [DOI] [Google Scholar]
- 32.Liu J., Yang W., Liu Y., et al. Combination of Hua Shi Bai Du granule (Q-14) and standard care in the treatment of patients with coronavirus disease 2019 (COVID-19): a single-center, open-label, randomized controlled trial. Phytomedicine. 2021;91:153671. doi: 10.1016/j.phymed.2021.153671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhao J., Yang S., Ke D., Qiu l, Jiang L. Clinical observation of the antiviral formula-1 in the treatment of novel coronavirus pneumonia in early and middle stage with cold damp and depressed lung type. Forum Tradit Chin Med. 2021;36 doi: 10.13913/j.cnki.41-1110/r.2021.06.008. [in Chinese] [DOI] [Google Scholar]
- 34.Zhao C., Li L., Yang W., et al. Chinese medicine formula Huashibaidu granule early treatment for mild COVID-19 patients: an unblinded, cluster-randomized clinical trial. Front Med. 2021;8:696976. doi: 10.3389/fmed.2021.696976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zheng X. China Medical Science and Technology Press; Beijing: 2002. Guiding Principles for clinical study of new Chinese medicines (trial) [in Chinese] [Google Scholar]
- 36.Tao J., Li X., Gao X., et al. Influencing factors of quality of life in patients with chronic hepatitis B treated with antiviral therapy. Chin Gen Pract. 2018;22:2798–2804. doi: 10.12114/j.issn.1007-9572.2018.00.459. [in Chinese] [DOI] [Google Scholar]
- 37.Cheung T. The difference and similarity between traditional Chinese and western medicine. Chin J Integr Tradit West Med. 2000;6:68–70. doi:CNKI:SUN:ZXYY.0.2000-01-026. [Google Scholar]
- 38.Li R., Hou Y., Huang J., et al. Lianhuaqingwen exerts anti-viral and anti-inflammatory activity against novel coronavirus (SARS-CoV-2) Pharmacol Res. 2020;156:104761. doi: 10.1016/j.phrs.2020.104761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhu S., Li X., Wei Y., Yang P., Qin E. Preliminary study on the inhibition of SARS-associated coronavirus in three Chinese medicine prescriptions. Lett Biotechnol. 2003;14:390–392. doi: 10.13859/j.cjz.1999.06.004. [DOI] [Google Scholar]
- 40.Liang S., Meng X., Wang Z., Liu J., Kuang H., Wang Q. Polysaccharide from Ephedra sinica Stapf inhibits inflammation expression by regulating Factor-beta 1/Smad 2 signaling. Int J Biol Macromol. 2018;106:947–954. doi: 10.1016/j.ijbiomac.2017.08.096. International Journal of Biological Macromolecules. [DOI] [PubMed] [Google Scholar]
- 41.Wang Q., Shu Z., Xing N., et al. A pure polysaccharide from Ephedra sinica treating on arthritis and inhibiting cytokines expression. Int J Biol Macromol. 2016;86:177–188. doi: 10.1016/j.ijbiomac.2016.01.010. [DOI] [PubMed] [Google Scholar]
- 42.Cui L., Wang W., Luo Y., et al. Polysaccharide from Scutellaria baicalensis Georgi ameliorates colitis via suppressing NF-kappaB signaling and NLRP3 inflammasome activation. Int J Biol Macromol. 2019;132:393–405. doi: 10.1016/j.ijbiomac.2019.03.230. [DOI] [PubMed] [Google Scholar]
- 43.Lu M., Cheng J., Lin C., Chang C. Purification, structural elucidation, and anti-inflammatory effect of a water-soluble 1,6-branched 1,3-α-d-galactan from cultured mycelia of Poria cocos. Food Chem. 2010;118:349–356. doi: 10.1016/j.foodchem.2009.04.126. [DOI] [Google Scholar]
- 44.Cao P., Wu S., Wu T., et al. The important role of polysaccharides from a traditional Chinese medicine-Lung Cleansing and Detoxifying Decoction against the COVID-19 pandemic. Carbohydr Polym. 2020;240:116346. doi: 10.1016/j.carbpol.2020.116346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hu W., Yang H., Zheng G. Prevention based on syndrome differentiation and three factors:Experience and Thinking on the application of traditional Chinese medicine to prevent SARS in Shenzhen Pinghu people’s Hospital(三因制宜辨证施防——深圳市平湖人民医院运用中医药预防SARS的体会及相关问题思考) Shenzhen J Integr Tradit Chin West Med. 2013;10:210–214. doi: 10.16458/j.cnki.1007-0893.2003.04.008. [in Chinese] [DOI] [Google Scholar]
- 46.Wang Z., An Y., Wang G., et al. Clinical application of Yuping wind powder to prevent New Coronavirus pneumonia. Tradit Chin Med. 2021;9:88–89. [in Chinese] [Google Scholar]
- 47.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/s0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yue D., Bi Y., Li X., Qin X. Chinese medicine for prevention of infl uenza and correlation study of mucosal immunity. Clin J Tradit Chin Med. 2015;27:1655–1658. doi: 10.16448/j.cjtcm.2015.0614. [in Chinese] [DOI] [Google Scholar]
- 49.He H., Sheng L., Huang X., Lv F., Huang Y. Preventive effect on nosocomial infection with herbal medicine Atractylodes chinensis disinfectant for air sterilization in cardiothoracic surgery. Chin J Nosocomiol. 2006;16:407–408. [in Chinese] [Google Scholar]
- 50.Luo X., Ni X., Lin J., et al. The add-on effect of Chinese herbal medicine on COVID-19: a systematic review and meta-analysis. Phytomedicine. 2020:153282. doi: 10.1016/j.phymed.2020.153282. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.