Abstract
Over the years there has been a continuous increase in clinically relevant driver mutations in non- small cell lung cancer. Among them dysregulated activation of the MET tyrosine kinase receptor has gained importance due to the recent development of quite effective treatments.
MET dysregulation encompasses a heterogeneous array of alterations leading to the prolonged activation of the cellular MET (c-MET, or MET) receptor and downstream proliferation pathways. It can arise through several mechanisms, including gene amplification, overexpression of the receptor and/or its ligand hepatocyte growth factor and the acquisition of activating mutations
MET mutations are found in 3–5% of NSCLC, mainly adenocarcinoma, and are over-represented in the sarcomatoid subtype. De novo MET amplifications are found in 1–5% of NSCLC, also predominantly in adenocarcinoma.
Here, we will bare the biology of MET, how to diagnose clinically relevant alterations, and their rising clinical importance in light of the emergence of multiple targeted therapies, both in the context of MET as a driver of resistance and in its own right.
Precis:
MET exon 14 alterations and amplifications can be primary oncogenic drivers as well as resistance pathways, such as among patients receiving EGFR targeted therapy.MET exon 14 alterations and amplifications have differene degree of responses to targeted therapy
Keywords: MET, NSCLC, Target therapy, molecular oncology, precision medicine
Introduction
Over the course of the last decade, immunotherapy and targeted therapies have changed the prognosis of non-small cell lung cancer (NSCLC)1, 2. Today, there is a continuous increase in clinically relevant driver mutations. Mandatory molecular testing in lung adenocarcinoma according to The National Comprehensive Cancer Network (NCCN)3 and European Society of Medical Oncology (ESMO) guidelines4 includes EGFR, ALK, ROS1, BRAF, while they advise broader molecular screening for KRAS G12C, RET, ERBB2, NTRK and MET proto-oncogene receptor tyrosine kinase (MET, also known as hepatocyte growth factor receptor).
MET dysregulation encompasses a heterogeneous array of alterations leading to the prolonged activation of the cellular MET (c-MET, or MET) receptor and downstream proliferation pathways. MET mutations are found in 3–5% of NSCLC, mainly adenocarcinoma, and are over-represented in the sarcomatoid subtype5, 6. De novo MET amplifications are found in 1–5% of NSCLC7, also predominantly in adenocarcinoma.
Here, we will discuss the biology of MET, how to diagnose clinically relevant alterations, and their rising clinical importance in light of the emergence of multiple targeted therapies, both in the context of MET as a driver of resistance and in its own right.
MET biology
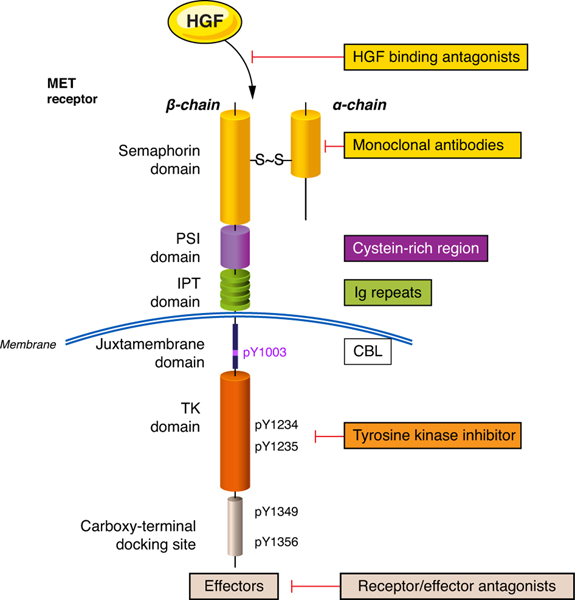
The proto-oncogene MET is located on chromosome 7q21-q31. It encodes for a precursor that is post-transcriptionally digested and glycosylated, forming extracellular alpha and transmembrane beta chains. The alpha-chain is joined to the beta-chain by disulphide bonds. The alpha-chain contains semaphorin, cysteine-rich and immunoglobulin domains. The beta-chain contains homologous domains which include a transmembrane, juxtamembrane, tyrosine kinase and carboxy-terminal docking domains (Figure 1).
Figure 1:
representation of the MET receptor and possible therapeutic targets
HGF: hepatocyte growth factor, CBL: Casitas B-lineage lymphoma, TK: tyrosine kinase, IPT: immunoglobulin-plexin transcription, PSI: plexin semaphoring integrin domain
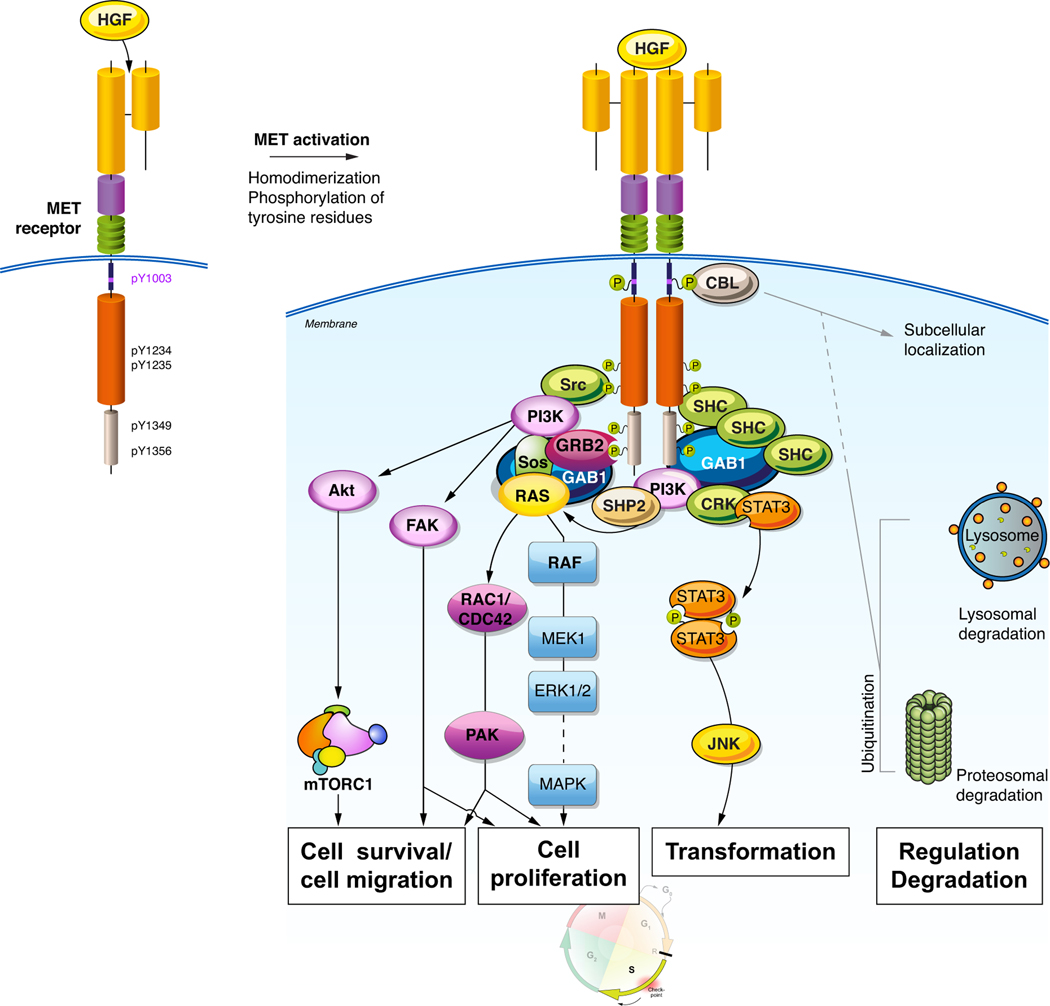
c-MET is a tyrosine kinase receptor. Upon binding with its ligand, hepatocyte growth factor (HGF), MET dimerizes, autophosphorylates and activates intracellular tyrosine kinase catalytic activity. The activation of c-MET regulates distinct downstream signalling including the PI3K/AKT/mTOR, RAS/RAF/ERK/MAPK, RAC/PAK, STAT/JNK, and FAK pathways8, 9. This signalling regulates cell survival and proliferation, migration and invasion, angiogenesis, and epithelial to mesenchymal cell transformation (Figure 2).
Figure 2:
MET signalling pathways and their effector functions
HGF: hepatocyte growth factor, CBL: Casitas B-lineage lymphoma
MET activation can result from various biological mechanisms, including protein overexpression, over-stimulation by HGF, mutations, amplifications and exon 14 skipping alterations. These distinctions may be important as treatment approaches were investigated based on different criteria used as biomarkers. For the purpose of discussing the role of MET in the current NSCLC treatment landscape, the two main subgroups are MET amplification and MET exon 14 mutations.
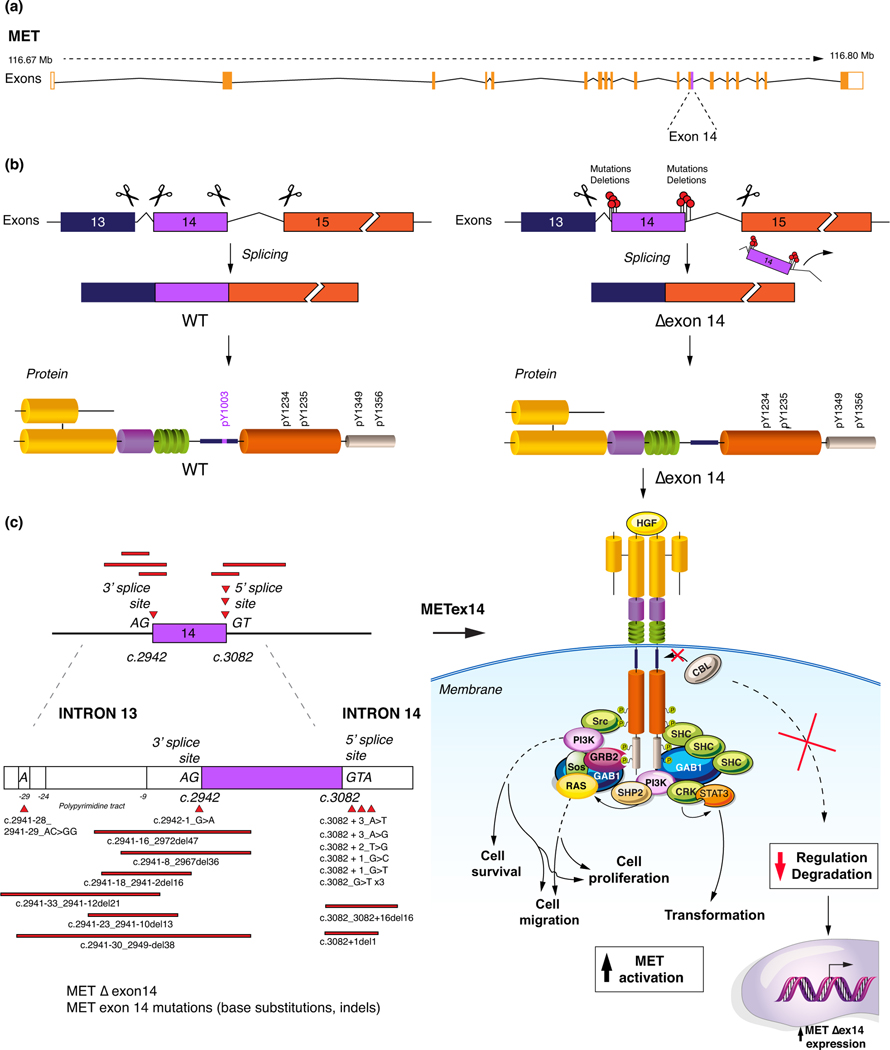
MET exon 14 partially encodes the c-MET juxtamembrane domain. This domain contains tyrosine 1003 (Y1003), the binding site of Cbl, which functions as an E3-ubiquitin protein ligase that regulates ubiquitin-mediated c-MET receptor degradation. Under normal circumstances, the introns flanking MET exon 14 are spliced out, yielding an mRNA transcript with exon 14 properly coding for the Cbl-E3 ligase binding site, thus allowing for c-MET degradation10 (Figure 3).
Figure 3:
The pathophysiology of exon 14 alterations. a) the MET structure. b) the impact of MET exon 14 alterations and development of exon 14 skipping. c) selected MET exon 14 alterations and the consequent lack of CBL binding, decreasing the MET polyubiniquitation and degradation
HGF: hepatocyte growth factor, CBL: Casitas B-lineage lymphoma, WT: wild-type, Δexon 14: exon 14 skipping.
Somatic missense mutations, insertions, deletions and insertions/deletions (indels) have been identified occurring involving or flanking MET exon 14 in lung adenocarcinoma, many of which result in aberrant splicing and skipping of exon 14 in the mRNA transcript. However, not all involve exon 14 skipping. For instance, a hotspot Y1003 mutation has an intact exon 14-encoded juxtamembrane domain save for the Cbl-binding site.
As such, these different alterations lead to a lack of Y1003-Cbl binding. This results in prolonged ligand-dependant activation of the c-MET receptor, downstream proliferation pathways, and oncogenesis. These occur in 3% of adenocarcinoma but also 13–22% of sarcomatoid lung cancers6, 11, 12 (Figure 3). Exon 14 alterations tend to be mutually exclusive with other primary oncogenic driver except for MET amplifications, copy number variants and MDM2 amplifications11, 12.
MET amplifications may drive oncogenesis in roughly 4% of TKI-naive NSCLC patients13. However, they represent a common acquired resistance mechanism to EGFR and ALK TKIs. MET amplification entails increased MET signalling through kinase activity or protein expression. In the treatment-naïve setting, in contrast to MET exon 14 alterations, amplifications are not mutually exclusive with other oncogenic drivers. While overlap is common at low amplification levels, at high levels, there is no oncogenic overlap, suggesting that only high amplification indicates a true MET-driven oncogenic state7, 14. Both can develop as secondary drivers upon progression on treatment.
Diagnosis of MET alterations (amplification, copy numbers, exon 14 skipping)
MET has become increasingly relevant as a therapeutic target over the last years, prompting the need to identify the oncogenic role each type of MET alteration and standardize the diagnostic approach. As with EGFR, non-smokers are over-represented in MET-driven NSCLC. However, a greater proportion of patients with either MET amplification (up to 77%) or exon 14 alterations (61–74%) consists of smokers than in most other driver alterations11, 15, 16.
MET exon 14 alterations have been shown to act as oncogenes and to be potentially actionable, regardless of gene amplification. Both alterations can occur concurrently in NSCLC. Overall, amplification alone appears less strongly correlated with response to MET blockade, while only high amplification, excluding polysomy, seems to drive the malignant phenotype and be predictive of meaningful clinical benefit of targeted treatment.
Amplification
Fluorescence in situ hybridization (FISH) was the first validated technique to assess MET gene copy numbers. By comparing the ratio of MET to the centromeric portion of chromosome 7 (CEP7), FISH identifies amplification, when copy numbers increase without a similar increase in CEP7. This allows for the distinction between MET copy number gains via polysomy, and true focal gene amplification. The distinction is important, as amplification but not polysomy likely represents oncogenic MET activation7, 13, 17.
Amplification has been most frequently defined as a MET:CEP7 ratio greater than 2, with ≥5 signals per cell for copy number gain, according to the scoring system established by Cappuzzo et al.14 When assessing de novo MET amplification, a MET:CEP7 ratio ≥5 appears to be the strongest predictor of a true MET-driven tumour, yet this only represents 0.34% of lung adenocarcinoma, far less than MET exon 14 skipping in such a population.
Finally, in some cases, the amplicons, which can include the entire centromere or parts thereof, does not include a balanced copy number gain of MET, resulting in a falsely low amplification ratio18.
RT-PCR evaluates copy number without providing information about the underlying cause, therefore it is unable to distinguish between polysomy and gene amplification. It carries an inherent risk of over-estimating amplification and should not be used.
Immunohistochemistry (IHC) on formalin-fixed paraffin-embedded tissue sections can be performed with a semi-quantitative assessment, mainly using the SP44 rabbit monoclonal primary antibody. The SP44 is directed against a membranous and cytoplasmic MET epitope. After staining, intensity scores are multiplied by the percentage of tumour cells to calculate a semi-quantitative histology score, (H-score), considered positive if higher than 200. However, MET protein expression and gene amplification have been shown to be poorly correlated. MET overexpression occurs in 35–72% of NSCLCs. This can be explained by the fact that the most common mechanism leading to protein overexpression is the transcriptional upregulation of the c-MET receptor without MET gene amplification19. It is also important to note that the association between MET protein overexpression and c-MET activation is not clear, despite its prognostic impact20. As such, c-MET IHC is likely unable to select tumours for a MET-directed strategy. In spite of promising phase 2 results, no phase 3 trial using IHC MET assessment has shown positive clinical outcomes by targeting MET21.
Further reinforcing the limitations of IHC screening, a recent reanalysis of tissue from 181 MET IHC positive NSCLC patients by the Lung Cancer Consortium 2 assessed the sensitivity of IHC in predicting gene amplification and MET exon 14 alterations. Nearly all IHC positive cases were negative for amplification and exon 14 mutations, suggesting that IHC is an inadequate screening tool for relevant MET alterations22.
MET exon 14
Exon 14 mutations encompass a highly heterogeneous sequence composition, with alterations occurring between exon 13 and 15. RT-PCR has been shown to be a potential tool for analysis, with 100% sensitivity and 97.4% specificity compared to NGS. Messenger RNA is extracted from the tumour sample and analysis generally includes the region between exon 13 and 15, and in case of exon 14 skipping, it is excluded from the transcript.
The molecular diagnosis of MET exon 14 skipping is most often achieved through DNA or RNA NGS. DNA sequencing can only detect a genomic variant that alters or removes a splicing site. On the other hand, RNA sequencing detects the fusion of exon 13 to 15, as this is the convergent result of any altered splicing mechanism or deletion. It therefore has the advantage of encompassing all causal genomic events that lead to exon 14 skipping23.
In addition to pre-analytical considerations, such as the vulnerability of RNA to degradation, the sensitivity of amplicon-mediated DNA-based approaches and hybrid-capture RNA-based assays appears to differ. The prevalence of MET exon 14 skipping in NSCLC was 4.2% with RNA-based NGS and 1.3% with the DNA-based approach. In samples tested with both methods, 60% of RNA positive results were false-negatives in DNA assays, the limitation appearing to be primer design unable to capture all known exon 14 skipping events23. The probe in amplicon-mediated DNA-based approaches may not cover a sufficient region of interest16. Meanwhile, RNA-based analysis is highly reliant on extracted RNA quality23. Therefore, a DNA-based hybrid NGS could be the solution to decrease false-negatives of the former and circumvent pre-analytical limitations of the latter.
MET and immuno-oncology
Tumoral c-MET signalling appears to induce the expression of programmed death ligand 1 (PD-L1) immunoregulatory proteins, as well as being associated with the expression of indoleamine-2,3-dioxygenase (IDO). It may therefore participate in immune suppression and evasion24, 25. While this could suggest an increased sensitivity to PD-1 blockade, there is little data supporting the efficacy of single-agent ICIs in MET-driven tumours. 36 MET positive patients with advanced NSCLC are included in the IMMUNOTARGET registry, evaluating the efficacy of ICIs in mutation driven NSCLC. Of the 36, 13(36%) had a MET amplification and 23 (63.8%) an exon 14 skipping mutation. 16% of these patients presented a partial response to ICIs, with a median PFS of 3.4 months. However, it is worth noting that durable responses, defined as 12 month PFS, were more frequent among MET positive patients (23.4%) than EGFR, ALK, or RET patients (5.9–7.0%). PFS was neither correlated with type of MET alteration nor smoking status26.
In a retrospective analysis of 63 patients with exon 14 alterations identified by hybrid-based NGS and treated with ICIs, 44% presented PD-L1 greater than 50% and 39% under 1%. The objective response rate (ORR) in the entire cohort was 13%, and it was 33% in the PD-L1 high (≥50%) group, while patients with negative PD-L1 had a 20% ORR. As such, PD-L1 might not be an accurate predictive biomarker for response to ICIs in patients with MET exon 14 alterations, with the limitation of the retrospective nature in a small series27.
Why certain patients appear to strictly benefit from ICIs while others do not needs further investigation. Current data from the IMMUNOTARGET registry or small case series do not support that smoking-related MET alterations, are more sensitive to ICIs, as is the case with KRAS26, 28, 29. As larger datasets emerge, perhaps more light will be shed on this topic. Furthermore, there are currently no data on chemo-immunotherapy or CTLA-4/PD(L)-1 combinations in MET alterations.
Targeting MET
When discussing targeting MET in NSCLC, it is important to specify whether MET positivity is defined as protein expression, gene amplification or exon 14 mutations. Given the ubiquity of MET protein expression, ranging between 35–72%, and the synergism between EGFR and MET pathways, attempts were made to add MET TKIs to EGFR TKIs in late lines of treatment30. The phase III ATTENTION and MARQUEE trials failed to meet their OS primary endpoint when comparing erlotinib with or without the c-MET targeting tivantinib in EGFR wild-type advanced nonsquamous NSCLC patients31. As protein expression was later found not to be a valid predictive biomarker, the lack of benefit in this fairly unselected population comes as no surprise.
Another trial using protein expression to identify MET positivity, the phase 3 METLung trial attempted to explore the benefit of onartuzumab, a MET monoclonal antibody, in combination with erlotinib. It failed to show an OS benefit compared to erlotinib with placebo, with a mOS of 6.8 and 9.1 months (HR 1.27, 95% 0.98–1.65), respectively in favour of the placebo group21.
While IHC is not a useful predictive biomarker, MET amplification first showed promise in the preliminary analysis of 12 patients from the PROFILE 1001 trial. Crizotinib showed a 33% (95% CI 10–65%) partial response rate in advanced NSCLC patients with MET amplification, and this was especially prominent among patient with MET/CEP7 ratios of 5 or higher15, who had a 50% response rate. Gene amplification thus appears predictive, contrarily to IHC, though it should be noted that the treatment assessed was not the same.
The third biomarker for MET consists of alterations in and around exon 14, the most common being exon 14 skipping. Retrospective data support that MET exon 14 skipping NSCLC confers sensitivity to direct MET-inhibitors, and among patients receiving crizotinib compared to those who did not, the mOS was 24.6 months versus 8.1 months (HR 0.11, 95% CI 0.01–0.92,p=0.04)15.
Drilon et al32 recently published promising results from a cohort of 65 evaluable NSCLC patients with MET exon 14 alterations and treated with crizotinib. This group comprised 62% of pretreated and 38% first-line patients. The ORR was 32% (95% CI 21–45%), with a median duration of response of 9.1 months and PFS of 7.3 months. Interestingly, the ORR among first-line patients was 25% (95% CI: 9.8–46.7) while it was 36.6% (22.1–53.1) among pretreated patients. There was no correlation between mutation type, splice-site and outcomes. The safety profile was as expected in previous crizotinib trials, with 29% grade 3–4 events, mainly gastro-intestinal and hepatic toxicity, oedema and blurred vision.
The Geometry mono-1 trial has evaluated capmatinib, a new generation oral selective reversible MET-inhibitor, in advanced NSCLC without EGFR or ALK alterations and harbouring MET exon 14 skipping mutations. This study assessed the efficacy as front-line and subsequent line therapy. The updated results for exon 14 mutations found an ORR of 67.9% (CI: 47.6–84.1) in the 28 patient treatment-naïve cohort and 40.6% (CI: 28.9–53.1) among 69 previously treated patients, with a median duration of response of 12.6 months (5.6-not reached) and 9.7 months (5.6–13) in each group, respectively. Furthermore, 7 out of 13 patients with brain metastases reported a partial response. These preliminary results and a favourable toxicity profile comprising mainly peripheral oedema, gastro-intestinal and creatine increase, with 31.5% grade 3 and 4.5% grade 4 adverse events, led to FDA approval for MET exon 14 skipping NSCLC33.
Data on the efficacy of capmatinib in patients with NSCLC harbouring MET amplification were released at the American Society for Clinical Oncology 2020 meeting34, 35. Amplification was defined as gene copy number ≥ 10. Among 15 treatment-naïve patients, the ORR was 40% (CI: 16.3–67.7), while it was 29% (CI: 18.7–41.2) among 69 pre-treated patients. The median DOR was 8.3 (range: 4.2–15.4) and 7.5 (range: 2.6–14.3) months in treatment-naïve and pre-treated patients, respectively. The primary endpoint was not met in these subgroups. Furthermore, among 126 pre-treated patients with gene copy numbers <10, the ORR ranged from 6.7 to 11.9%, with a linear correlation between response and gene copy numbers.
Similarly, the phase 2 VISION trial assessed tepotinib, an oral highly selective, ATP-competitive, reversible MET inhibitor. In the updated analysis, among 41 patients with a tissue-based diagnosis of MET exon 14 alterations, the ORR by independent central review was 41.5% (CI: 26.3–57.9), with a median DOR of 12.4 months (range: 1.1–18.0). Mirroring this, among 35 patients diagnosed on a liquid biopsy, the ORR was 51.4% with a DOR of 9.8 months (range: 1.1–18.0). As with capmatinib, patients responded regardless of previous therapies. The adverse event profile was also similar to that of capmatinib, without grade 4–5 events 36.
The preliminary analysis of 61 patients from a phase 2 trial (NCT02897479) evaluating savolitinib in MET exon 14 altered adenocarcinoma and pulmonary sarcomatoid carcinoma are equally promising. The ORR was 47.5% (CI: 34.6–60.7), with a median DOR that has not yet been reached and a median PFS of 6.8 months (CI: 4.2–13.8). Toxicity was in-line with other MET inhibitors37. Today, numerous MET targeting agents are in early phase trials, including TKIs, antibodies, bispecific antibodies and drug-antibody conjugates (Table 1).
Table 1:
Selected trials with different classes of MET targeting agents
| TKI | Targets | Type of TKI | MET alteration | Trial(s) | Phase | Results |
|---|---|---|---|---|---|---|
| MET-specific TKI | ||||||
| Capmatinib 1, 2 | MET | Ib | ex14, amp |
GEOMETRY-mono-1
NCT02544633 NCT03693339 |
2 | Ex14: 1L ORR : 67.9% 2L+ ORR : 40.6% 1L DOR: 11.1m, 2L+ DOR: 9.7m Amp: 1L ORR: 40%, 2L+ ORR : 29%. 1L DOR: 8.3m, 2L+ DOR: 7.5m |
| Tepotinib3 | MET | Ib | ex14 | NCT02864992 (VISION) | 2 | ORR 45.1%, DOR 12.4m |
| Savolitinib | MET | Ib | ex14 | NCT02897479 | 2 | NA |
| SAR1258444 | MET | I | amp | NCT02435121 | 2 | *ORR 18.2% |
| Bozitinib | MET | I | ex14. amp, mut |
NCT03175224
NCT02896231 NCT04258033 |
1–2 | NA |
| Glumetinib | MET | II | ex14, amp, IHC | NCT04270591 | 1–2 | NA |
| BPI-9016M | MET | II | IHC | NCT02929290 | 1a | NA |
| Multikinase inhibitors | ||||||
| Crizotinib5 | MET, ALK, ROS1 | Ia | ex14 amp |
PROFILE1001
NCT02499614 (METROS) |
2 | 1L ORR 25.0%, 2L+ ORR 36.6%, PFS 7.3m, DOR 9.1m |
| Cabozantinib (CABinMET) | MET, ROS1, VEGFR, RET, KIT, and FLT3 | II | ex14, amp | NCT03911193 | 2 | NA |
| MGCD265 Glesatinib | MET, AXL | II | amp | NCT02544633 | 2 | NA |
| Cabozantinib (CABinMET) | MET, ROS1, VEGFR, RET, KIT, and FLT3 | II | ex14, amp | NCT03911193 | 2 | NA |
| Merestinib | ROS1, AXL, RON, MERTK, FLT3, DDR1/2, MST1R, and MKNK-1/2, MET | II | ex14 | NCT02920996 | 2 | NA |
| TPX-0022 | MET, CSF1R, SRC | I | ex14, amp, fusion | NCT03993873 | 1 | NA |
| Antibodies | ||||||
| Onartuzumab + erlotinib6 | IgG1 MAb | - | NCT02031744 (NCT00854308) | 3 | ORR 8.4% vs 9.6% No PFS or OS benefit |
|
| Emibetuzumab +/− erlotinib7 | IgG4 MAb | - | IHC | NCT01900652 (CHIME) | 2 | DCR 50% vs 26%, PFS 1.6m vs 3.3m |
| Sym0158 | IgG1 MAb mixture | - | amp, ex14 | NCT02648724 | 1–2 | ORR 25%, DCR 80%, PFS 5.5m |
| Other | ||||||
| Telisotuzumab vedotin9 | Drug Ab conjugate | - | IHC, amp | NCT03539536 | 2 | *ORR 18.8%, PFS 5.7m |
| REGN5093 | MET bispecific Ab | - | ex14, amp, IHC | NCT04077099 | 1–2 | NA |
earlier phase trial results.
TKI : tyrosine-kinase inhibitor, ex 14 : exon 14 alterations, amp : amplification, ORR : objective response rate, 1L : first-line, 2L+ : second-line and beyond, DOR : mean duration of response, OS: mean overall survival, PFS : mean progression-free survival, DCR: mean disease control rate, m: months, NA: not available, IHC: immunohistochemistry, Ab: antibody, MAb: monoclonal antibody.
Wolf J. Capmatinib in patients with high-level MET-amplified advanced non–small cell lung cancer (NSCLC): results from the phase 2 GEOMETRY mono-1 study.2020 ASCO meeting.
Harry J.M. Groen WLA, Pierre Jean Souquet, Eckart Laack, Ji-Youn Han, Egbert F. Smit, Aaron Scott Mansfield, Edward B. Garon, Juergen Wolf, Daniel Shao-Weng Tan, Rebecca Suk Heist, Maeve Waldron-Lynch, Sylvie Le Mouhaer, Ngozi Nwana, Monica Giovannin. Capmatinib in patients with METex14-mutated or high-level MET-amplified advanced non–small-cell lung cancer (NSCLC): results from cohort 6 of the phase 2 GEOMETRY mono-1 study.2020 ASCO meeting
Paik PK, Veillon R, Cortot AB, et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations. Journal of Clinical Oncology. 2019;37: 9005–9005.
Angevin E, Spitaleri G, Rodon J, et al. A first-in-human phase I study of SAR125844, a selective MET tyrosine kinase inhibitor, in patients with advanced solid tumours with MET amplification. Eur J Cancer. 2017;87: 131–139.
Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nature Medicine. 2020;26: 47–51.
Spigel DR, Edelman MJ, O’Byrne K, et al. Results From the Phase III Randomized Trial of Onartuzumab Plus Erlotinib Versus Erlotinib in Previously Treated Stage IIIB or IV Non-Small-Cell Lung Cancer: METLung. J Clin Oncol. 2017;35: 412–420.
Camidge DR, Moran T, Demedts I, et al. A randomized, open-label, phase 2 study of emibetuzumab plus erlotinib (LY+E) and emibetuzumab monotherapy (LY) in patients with acquired resistance to erlotinib and MET diagnostic positive (MET Dx+) metastatic NSCLC. Journal of Clinical Oncology. 2016;34: 9070–9070.
D. Ross Camidge FJ, Alejandro Martinez-Bueno, Daniel V.T. Catenacci, Jeeyun Lee, Se-Hoon Lee, Afshin Dowlati, Kristoffer Staal Rohrberg, Alejandro Navarro, Yong Wha Moon, Mark M. Awad, Rebecca Suk Heist, Thomas Tuxen Poulsen, Arielle Yablonovitch, Lindsay Fosler, Helle Rudbaek, Frank Nygaard, Debra L. Wood, Rita P. Dalal, Enriqueta Felip;. 2020 ASCO meeting.
Strickler JH, Weekes CD, Nemunaitis J, et al. First-in-Human Phase I, Dose-Escalation and -Expansion Study of Telisotuzumab Vedotin, an Antibody–Drug Conjugate Targeting c-Met, in Patients With Advanced Solid Tumors. Journal of Clinical Oncology. 2018;36: 3298–3306.
Attempts have been made to combine TKIs for NSCLC with ICIs. Toxicity was shown to be the most important limitation – and at the present time, no signal for synergy has clearly emerged. In patients with ALK rearrangements, crizotinib was combined with nivolumab, and the trial was closed prematurely in light of a significant increase in severe hepatic dysfunction, some fatal38. It is, however, interesting to note that capmatinib has shown a preclinical immunomodulatory effect when combined with pembrolizumab, a PD-1 inhibitor, irrespective of MET dysregulations. This is under investigation in a phase 2 trial and could provide room for further trials (NCT04139317).
MET as a resistance mechanism
Early generation TKIs in EGFR and ALK often lead to on-target escape mechanisms, such as T790M in EGFR mutations and different kinase domain mutations in ALK fusions. Nonetheless, MET amplification via the ERBB3-dependent activation of PI3K are detected in up to 22% of acquired EGFR resistances to first generation targeted therapy39. MET exon 14 alterations have also emerged as resistance mechanisms to EGFR TKI therapy40. In parallel to the extension of the potency and scope of targeted therapies, resistance mechanisms have evolved, with the appearance of more off-target mutations. After third-generation TKIs for EGFR-mutant NSCLC, MET accounts for approximately 10% thereof, with amplification representing 7–15% of resistance to first-line therapy and 5–50% of resistance to second-line and above third-generation TKIs41, 42.
In patients with NSCLC with ALK rearrangements, MET amplifications were detected in 12% of patients who developed resistance to second-generation inhibitors and 22% of those progressing on lorlatinib. Early results of targeting MET in this context are promising43.
Several trials have explored the addition of a MET TKI to an EGFR TKI to overcome acquired resistance mechanisms. The phase 2 INSIGHT trial evaluated the addition of tepotinib (MET TKI) to gefitinib in EGFR mutant MET altered (IHC2–3+ or amplification) adenocarcinoma without T790M mutations at progression, compared to chemotherapy at progression. The experimental arm showed a 45.2% ORR, 4.9 month median PFS and 17.3 month OS overall, with particularly promising results in the MET amplified group, with a 66.7% ORR, 21.2 month PFS and 37.3 month OS44.
In an expansion arm of the phase Ib TATTON trial, combined osimertinib and savolitinib, a MET TKI, after T790M negative progression on first or second-generation EGFR inhibitors, have shown promising results, with a 64% ORR)45. For patients progressing on third generation EGFR therapy, the addition of osimertinib and savolitinib in MET-positive patients yields a 25% ORR and mDOR of 9.7 months. The phase 2 SAVANNAH trial aims to confirm these early results (NCT03778229). Similarly, the INSIGHT 2 trial is evaluating the addition of tepotinib to osimertinib in MET amplified adenocarcinoma, compared to chemotherapy at progression on osimertinib (NCT03940703). A similar ongoing phase I/II trial with capmatinib plus gefitinib after failure of a prior EGFR inhibitor has shown promising data, particularly with a 47% ORR among patients with high MET amplification, defined as a gene copy number ≥646.
In addition to acquired resistance while on treatment, co-occurring MET alterations can result in primary resistance to targeted therapy. Among EGFR mutated lung adenocarcinoma patients, MET copy number gains at diagnosis are not associated with primary TKI resistance, while the rarer MET amplifications appear to be predictive of poor response, and outcomes47
Type of MET inhibitors and resistance mechanisms
MET TKIs, can be divided into three broad classes. Type I TKIs, including crizotinib, tepotinib, savolitinib, bozitinib and capmatinib compete with ATP to bind to the MET receptor in its catalytically active conformation, where aspartic acid phenylalanine-glycine (DFG) motif projects into the ATP-binding site. These are further divided into type Ia inhibitors, like crizotinib, which interact with the solvent front residue G1163, and type Ib, such as capmatinib, savolitinib and tepotinib which bind to the targeted kinase domain independently from this interaction. Type II TKIs, such as multikinase inhibitors cabozantinib, glesatinib and merestinib are ATP competitive and will bind the MET receptor in its inactive DFG conformation48. Finally, type III TKIs include tivantinib, an allosteric inhibitor49.
With response rates ranging from 25 to 68%, a median duration of response between 9 and 16 months, and a favourable toxicity profile, MET TKIs offer a promising treatment option in patients with exon 14 skipping (Table 1). However, it is marred by the invariable emergence acquired resistance pathways. Resistance to type Ia MET TKI appears to be induced by mutations in residues D1228 and Y1230, by weakening the bond between the MET kinase domain and the therapeutic agent 50, 51. The solvent front G1163R mutation confers resistance to crizotinib but not type Ib nor type II inhibitors. In vitro, acquired resistance mutations to type I TKIs, which were not exposed on the surface of the ATP-binding pocket in the inactive DFG conformation remain sensitive to type II inhibitors51 49, 52. Conversely, resistance mutations at L1195 and F1200, which emerge with type II TKIs and are not exposed in the active DFG conformation, are sensitive to type I inhibitors. While changing classes of MET inhibitors appears promising for on-target mutations and focal MET amplifications, resistance pathways also include off-target alterations, mainly KRAS mutations48, 49.
Limited data are available, however, a recent retrospective analysis of 20 patients who progressed on MET TKIs for advanced NSCLC with exon 14 skipping, sheds some light on the clinical picture. 6 patients changed TKIs at progression, with 5 progressing on this subsequent line. On target resistance pathways were identified in 35% of patients, including focal MET amplification and MET kinase domain mutations. One patient presented on and off-target alterations. Off-target mechanisms of resistance included KRAS mutations or amplification of EGFR, KRAS, HER3 and BRAF and were detected in 45% of cases. In 25%, the mechanism of resistance was not identified. In patients who developed on-target resistance, 33% responded to subsequent TKIs of a different class, irrespective of sequence48.
Alterations of other pathways can also cause primary resistance to MET TKIs. For instance, PI3K-pathway alterations including PTEN loss appear to cause clinical resistance in vivo. In further in vitro analyses, sensitivity to MET inhibitors appears restored when a PI3K targeting agent is added53. Furthermore, in a study of 74 patients with MET exon 14 altered NSCLC, certain pre-TKI treatment parameters were predictive of non-response. RAS pathway activation, low (< 700 amol/μg) KRAS expression, and absence of MET protein detection in tumour tissue each conferred primary resistance to TKIs, with 0% ORR (0/6, 0/2 and 0/5 patients each), compared to 29%, 50%, and 63% ORR, respectively in patients without these factors. Similarly, acquired resistance was potentially explained by on-target alterations in MET and HGF in 22% of patients (2/9) and off-target mechanisms involving RAS, EGFR, MDM2 in 44% of patients (5/9)54.
MET therapies remain in their infancy and while resistance mechanisms are intriguing, they are not yet mature for clinical use and will require validation.
Conclusions
There has been significant progress in the field of MET inhibition. For patients with MET-driven NSCLC, the best front-line treatment is yet to be ascertained. As the identification of proper ways to diagnose the presence of MET driver alterations improves, the feasibility of large prospective, maybe randomized, trials in this rare entity emerges. Today, we would encourage participation in clinical trials, and if unavailable, to use a MET TKI depending on local approval and access.
Given the limited efficacy observed with ICIs to-date in MET altered NSCLC, we would avoid single agent ICIs in early lines. We would favour targeted therapy upfront, whenever possible, as the use of TKIs after immunotherapy could increase the risk of serious immune-related adverse events38, 55.
As we increasingly understand the mechanism of action of MET TKIs, as well as the type of resistance pathways that emerge with each type, MET treatment sequencing is becoming a fascinating field.
The MET altered NSCLC therapeutic landscape is rapidly evolving and we hope that in the near future, the METeoric rise of MET will provide therapeutic options to all affected patients.
Acknowledgments
Conflict of interests:
Dr. Friedlaender reports personal fees from BMS, from Roche, personal fees from Pfitzer, personal fees from MSD, personal fees from Astellas, outside the submitted work;
Dr. Drilon reports personal fees from Ignyta/Genentech/Roche, personal fees from Loxo/Bayer/Lilly, personal fees from Takeda/Ariad/Millenium, personal fees from TP Therapeutics, personal fees from AstraZeneca, personal fees from Pfizer, personal fees from Blueprint Medicines, personal fees from Helsinn, personal fees from Beigene, personal fees from BergenBio, personal fees from Hengrui Therapeutics, personal fees from Exelixis, personal fees from Tyra Biosciences, personal fees from Verastem, personal fees from MORE Health, personal fees from Abbvie, personal fees from 14ner/Elevation Oncology, personal fees from Axis, personal fees from Peerview Institute, personal fees from OncLive, personal fees from Paradigm Medical Communications, LLC, personal fees from Remedica Ltd., personal fees from ArcherDX, personal fees from Foundation Medicine, personal fees from PeerVoice, personal fees from Research to Practice, personal fees from Medscape, personal fees from WebMD, personal fees from Monopteros, outside the submitted work; and ASSOCIATED RESEARCH PAID TO INSTITUTION: Pfizer, Exelixis, GlaxoSmithKlein, Teva, Taiho,PharmaMar. RESEARCHßoundation Medicine. ROYALTIES:Wolters Kluwer. OTHER: Merck - Food/Beverage Puma - Food/BeverageÐerus Boehringer Ingelheim. CME HONORARIA: Medscape, OncLive, PeerVoice, Physicians Education Resources, Targeted Oncology, Research to Practice.
Dr. Banna reports personal fees from Boehringher, personal fees from Janssen-Cilag, personal fees from Roche, outside the submitted work;
Prof. Peters reports personal fees from Abbvie, personal fees from Amgen, personal fees from AstraZeneca, personal fees from Bayer, personal fees from Biocartis, personal fees from Boehringer-Ingelheim, personal fees from Bistrol-Myers Squibb, personal fees from Clovis, personal fees from Daiichi Sankyo, personal fees from Debiopharm, personal fees from Eli Lilly, personal fees from F. Hoffmann-La Roche, personal fees from Foundation Medicine, personal fees from Illumina, personal fees from Janssen, personal fees from Merck Sharp and Dohme, personal fees from Merck Serono, personal fees from Merrimack, personal fees from Novartis, personal fees from Pharma Mar, personal fees from Pfizer, personal fees from Regeneron, personal fees from Sanofi, personal fees from Seattle Genetics and Takeda, personal fees from AstraZeneca, personal fees from Boehringer-Ingelheim, personal fees from Bristol-Myers Squibb, personal fees from Eli Lilly, personal fees from F. Hoffmann-La Roche, personal fees from Merck Sharp and Dohme, personal fees from Novartis, personal fees from Pfizer, personal fees from Takeda, non-financial support from Sponsored by Amgen, non-financial support from AstraZeneca, non-financial support from Boehringer-Ingelheim, non-financial support from Bristol-Meyers Squibb, non-financial support from Clovis, non-financial support from F. Hoffmann-La Roche, non-financial support from Illumina, non-financial support from Merck Sharp and Dohme, non-financial support from Merck Serono, non-financial support from Novartis, non-financial support from Pfizer, non-financial support from Sanofi, personal fees from Bioinvent, outside the submitted work. All fees to Institution
Dr. Addeo reports personal fees from BMS, personal fees from Astrazeneca, personal fees from Roche, personal fees from Pfizer, personal fees from MSD, personal fees from Boehringer, outside the submitted work;.
References
- 1.Hirsch FR, Suda K, Wiens J, Bunn PA. New and emerging targeted treatments in advanced non-small-cell lung cancer. The Lancet. 2016;388: 1012–1024. [DOI] [PubMed] [Google Scholar]
- 2.Doroshow DB, Sanmamed MF, Hastings K, et al. Immunotherapy in Non–Small Cell Lung Cancer: Facts and Hopes. Clinical Cancer Research. 2019;25: 4592–4602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.guidelines N. NCCN NSCLC guidelines 2020
- 4.Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Annals of Oncology. 2019;30: 863–870. [DOI] [PubMed] [Google Scholar]
- 5.Aguilar EJ, Ricciuti B, Gainor JF, et al. Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Annals of Oncology. 2019;30: 1653–1659. [DOI] [PubMed] [Google Scholar]
- 6.Liu X, Jia Y, Shen Y, et al. Detection of frequent MET Exon 14 skipping events in pulmonary sarcomatoid carcinoma and response to targeted inhibition: American Society of Clinical Oncology, 2015. [Google Scholar]
- 7.Drilon A, Cappuzzo F, Ou S-HI, Camidge DR. Targeting MET in Lung Cancer: Will Expectations Finally Be MET? Journal of Thoracic Oncology. 2017;12: 15–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim ES, Salgia R. MET pathway as a therapeutic target. Journal of Thoracic Oncology. 2009;4: 444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eder JP, Vande Woude GF, Boerner SA, LoRusso PM. Novel Therapeutic Inhibitors of the c-Met Signaling Pathway in Cancer. Clinical Cancer Research. 2009;15: 2207–2214. [DOI] [PubMed] [Google Scholar]
- 10.Peschard P, Fournier TM, Lamorte L, et al. Mutation of the c-Cbl TKB Domain Binding Site on the Met Receptor Tyrosine Kinase Converts It into a Transforming Protein. Molecular Cell. 2001;8: 995–1004. [DOI] [PubMed] [Google Scholar]
- 11.Tong JH, Yeung SF, Chan AWH, et al. MET Amplification and Exon 14 Splice Site Mutation Define Unique Molecular Subgroups of Non–Small Cell Lung Carcinoma with Poor Prognosis. Clinical Cancer Research. 2016;22: 3048–3056. [DOI] [PubMed] [Google Scholar]
- 12.Frampton GM, Ali SM, Rosenzweig M, et al. Activation of MET via diverse exon 14 splicing alterations occurs in multiple tumor types and confers clinical sensitivity to MET inhibitors. Cancer Discovery. 2015;5: 850–859. [DOI] [PubMed] [Google Scholar]
- 13.Go H, Jeon YK, Park HJ, Sung S-W, Seo J-W, Chung DH. High MET gene copy number leads to shorter survival in patients with non-small cell lung cancer. Journal of Thoracic Oncology. 2010;5: 305–313. [DOI] [PubMed] [Google Scholar]
- 14.Noonan SA, Berry L, Lu X, et al. Identifying the Appropriate FISH Criteria for Defining MET Copy Number–Driven Lung Adenocarcinoma through Oncogene Overlap Analysis. Journal of Thoracic Oncology. 2016;11: 1293–1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camidge DR, Ou S-HI, Shapiro G, et al. Efficacy and safety of crizotinib in patients with advanced c-MET-amplified non-small cell lung cancer (NSCLC): American Society of Clinical Oncology, 2014. [Google Scholar]
- 16.Awad MM, Oxnard GR, Jackman DM, et al. MET exon 14 mutations in non–small-cell lung cancer are associated with advanced age and stage-dependent MET genomic amplification and c-Met overexpression. Journal of Clinical Oncology. 2016. [DOI] [PubMed] [Google Scholar]
- 17.Kawakami H, Okamoto I, Okamoto W, Tanizaki J, Nakagawa K, Nishio K. Targeting MET amplification as a new oncogenic driver. Cancers. 2014;6: 1540–1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schildhaus H-U, Schultheis AM, Rüschoff J, et al. MET Amplification Status in Therapy-Naïve Adeno- and Squamous Cell Carcinomas of the Lung. Clinical Cancer Research. 2015;21: 907–915. [DOI] [PubMed] [Google Scholar]
- 19.Finocchiaro G, Toschi L, Gianoncelli L, Baretti M, Santoro A. Prognostic and predictive value of MET deregulation in non-small cell lung cancer. Annals of translational medicine. 2015;3: 83–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peters S, Adjei AA. MET: a promising anticancer therapeutic target. Nature Reviews Clinical Oncology. 2012;9: 314–326. [DOI] [PubMed] [Google Scholar]
- 21.Spigel DR, Ervin TJ, Ramlau RA, et al. Randomized phase II trial of Onartuzumab in combination with erlotinib in patients with advanced non-small-cell lung cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2013;31: 4105–4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Guo R, Berry LD, Aisner DL, et al. MET IHC is a poor screen for MET amplification or MET Exon 14 mutations in lung adenocarcinomas: data from a tri-institutional cohort of the Lung Cancer Mutation Consortium. Journal of Thoracic Oncology. 2019;14: 1666–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Davies KD, Lomboy A, Lawrence CA, et al. DNA-Based versus RNA-Based Detection of MET Exon 14 Skipping Events in Lung Cancer. Journal of Thoracic Oncology. 2019;14: 737–741. [DOI] [PubMed] [Google Scholar]
- 24.Saigi M, Alburquerque-Bejar JJ, Mc Leer-Florin A, et al. MET-Oncogenic and JAK2-Inactivating Alterations Are Independent Factors That Affect Regulation of PD-L1 Expression in Lung Cancer. Clin Cancer Res. 2018;24: 4579–4587. [DOI] [PubMed] [Google Scholar]
- 25.Albitar M, Sudarsanam S, Ma W, et al. Correlation of MET gene amplification and TP53 mutation with PD-L1 expression in non-small cell lung cancer. Oncotarget. 2018;9: 13682–13693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mazieres J, Drilon A, Lusque A, et al. Immune checkpoint inhibitors for patients with advanced lung cancer and oncogenic driver alterations: results from the IMMUNOTARGET registry. Annals of Oncology. 2019;30: 1321–1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sabari JK, Montecalvo J, Chen R, et al. PD-L1 expression and response to immunotherapy in patients with MET exon 14-altered non-small cell lung cancers (NSCLC): American Society of Clinical Oncology, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mayenga M, Monnet I, Massiani M, et al. MA03.09 Dramatic Responses to Immune Checkpoint Inhibitors in MET Exon 14 Skipping Mutation (METex14mut) Non Small Cell Lung Cancers. Journal of Thoracic Oncology. 2019;14: S259. [Google Scholar]
- 29.Friedlaender A, Drilon A, Weiss GJ, Banna GL, Addeo A. KRAS as a druggable target in NSCLC: Rising like a phoenix after decades of development failures. Cancer treatment reviews. 2020;85: 101978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Salgia R. MET in Lung Cancer: Biomarker Selection Based on Scientific Rationale. Molecular Cancer Therapeutics. 2017;16: 555–565. [DOI] [PubMed] [Google Scholar]
- 31.Yoshioka H, Azuma K, Yamamoto N, et al. A randomized, double-blind, placebo-controlled, phase III trial of erlotinib with or without a c-Met inhibitor tivantinib (ARQ 197) in Asian patients with previously treated stage IIIB/IV nonsquamous nonsmall-cell lung cancer harboring wild-type epidermal growth factor receptor (ATTENTION study). Annals of Oncology. 2015;26: 2066–2072. [DOI] [PubMed] [Google Scholar]
- 32.Drilon A, Clark JW, Weiss J, et al. Antitumor activity of crizotinib in lung cancers harboring a MET exon 14 alteration. Nature Medicine. 2020;26: 47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. https://www.novartis.com/news/media-releases/novartis-announces-fda-approval-met-inhibitor-tabrecta-metastatic-non-small-cell-lung-cancer-metex14.
- 34.Wolf J. Capmatinib in patients with high-level MET-amplified advanced non–small cell lung cancer (NSCLC): results from the phase 2 GEOMETRY mono-1 study. 2020. ASCO meeting. [Google Scholar]
- 35.al HJMGe. Capmatinib in patients with METex14-mutated or high-level MET-amplified advanced non–small-cell lung cancer (NSCLC): results from cohort 6 of the phase 2 GEOMETRY mono-1 study.2020. ASCO meeting. [Google Scholar]
- 36.Paik PK, Veillon R, Cortot AB, et al. Phase II study of tepotinib in NSCLC patients with METex14 mutations. Journal of Clinical Oncology. 2019;37: 9005–9005. [Google Scholar]
- 37.Lu S, Fang J, Li X, et al. Phase II study of savolitinib in patients (pts) with pulmonary sarcomatoid carcinoma (PSC) and other types of non-small cell lung cancer (NSCLC) harboring MET exon 14 skipping mutations (METex14+). Journal of Clinical Oncology. 2020;38: 9519–9519. [Google Scholar]
- 38.Spigel DR, Reynolds C, Waterhouse D, et al. Phase 1/2 Study of the Safety and Tolerability of Nivolumab Plus Crizotinib for the First-Line Treatment of Anaplastic Lymphoma Kinase Translocation - Positive Advanced Non-Small Cell Lung Cancer (CheckMate 370). Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 2018;13: 682–688. [DOI] [PubMed] [Google Scholar]
- 39.Bean J, Brennan C, Shih J-Y, et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proceedings of the National Academy of Sciences. 2007;104: 20932–20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suzawa K, Offin M, Schoenfeld AJ, et al. Acquired MET Exon 14 Alteration Drives Secondary Resistance to Epidermal Growth Factor Receptor Tyrosine Kinase Inhibitor in EGFR-Mutated Lung Cancer. JCO precision oncology. 2019: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oxnard GR, Hu Y, Mileham KF, et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients With EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncology. 2018;4: 1527–1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonetti A, Sharma S, Minari R, Perego P, Giovannetti E, Tiseo M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. British Journal of Cancer. 2019;121: 725–737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dagogo-Jack I, Yoda S, Lennerz JK, et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin Cancer Res. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu Y, Cheng Y, Zhou J, et al. MA09. 09 Long-Term Outcomes to Tepotinib Plus Gefitinib in Patients with EGFR-Mutant NSCLC and MET Dysregulation: 18-Month Follow-Up. Journal of Thoracic Oncology. 2019;14: S284. [Google Scholar]
- 45.Sequist LV, Han J-Y, Ahn M-J, et al. Osimertinib plus savolitinib in patients with EGFR mutation-positive, MET-amplified, non-small-cell lung cancer after progression on EGFR tyrosine kinase inhibitors: interim results from a multicentre, open-label, phase 1b study. The Lancet Oncology. 2020;21: 373–386. [DOI] [PubMed] [Google Scholar]
- 46.Wu Y-L, Zhang L, Kim D-W, et al. Phase Ib/II study of capmatinib (INC280) plus gefitinib after failure of epidermal growth factor receptor (EGFR) inhibitor therapy in patients with EGFR-mutated, MET factor-dysregulated non-small-cell lung cancer. Journal of Clinical Oncology. 2018;36: 3101-+. [DOI] [PubMed] [Google Scholar]
- 47.Lai GGY, Lim TH, Lim J, et al. Clonal MET Amplification as a Determinant of Tyrosine Kinase Inhibitor Resistance in Epidermal Growth Factor Receptor-Mutant Non-Small-Cell Lung Cancer. J Clin Oncol. 2019;37: 876–884. [DOI] [PubMed] [Google Scholar]
- 48.Recondo G, Bahcall M, Spurr LF, et al. Molecular mechanisms of acquired resistance to MET tyrosine kinase inhibitors in patients with MET exon 14 mutant NSCLC. Clinical Cancer Research. 2020: clincanres.3608.2019. [DOI] [PubMed] [Google Scholar]
- 49.Fujino T, Kobayashi Y, Suda K, et al. Sensitivity and Resistance of MET Exon 14 Mutations in Lung Cancer to Eight MET Tyrosine Kinase Inhibitors In Vitro. Journal of Thoracic Oncology. 2019;14: 1753–1765. [DOI] [PubMed] [Google Scholar]
- 50.Heist RS, Sequist LV, Borger D, et al. Acquired resistance to crizotinib in NSCLC with MET exon 14 skipping. Journal of Thoracic Oncology. 2016;11: 1242–1245. [DOI] [PubMed] [Google Scholar]
- 51.Bahcall M, Sim T, Paweletz CP, et al. Acquired METD1228V mutation and resistance to MET inhibition in lung cancer. Cancer Discovery. 2016;6: 1334–1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tiedt R, Degenkolbe E, Furet P, et al. A drug resistance screen using a selective MET inhibitor reveals a spectrum of mutations that partially overlap with activating mutations found in cancer patients. Cancer research. 2011;71: 5255–5264. [DOI] [PubMed] [Google Scholar]
- 53.Jamme P, Fernandes M, Copin M-C, et al. Alterations in the PI3K pathway drive resistance to MET inhibitors in NSCLC harboring MET exon 14 skipping mutations. Journal of Thoracic Oncology. 2020. [DOI] [PubMed] [Google Scholar]
- 54.Guo R, Offin M, Brannon AR, et al. MET inhibitor resistance in patients with MET exon 14-altered lung cancers: American Society of Clinical Oncology, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oxnard G, Yang J-H, Yu H, et al. TATTON: A multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib or durvalumab in EGFR-mutant lung cancer. Annals of Oncology. 2020. [DOI] [PubMed] [Google Scholar]