Abstract
Aminoglycosides bind to rRNA in the small subunit of the bacterial ribosome. Mutations in the decoding region of 16S rRNA confer resistance to specific subsets of aminoglycoside antibiotics. The two major classes of 2-deoxystreptamine aminoglycosides are the 4,5- and the 4,6-disubstituted antibiotics. Antibiotics of the 4,5-disubstituted class include neomycin, paromomycin, and ribostamycin. Gentamicins and kanamycins belong to the 4,6-disubstituted class of aminoglycosides. Structural studies indicated the potential importance of position 1406 (Escherichia coli numbering) in the binding of ring III of the 4,6-disubstituted class of aminoglycosides to 16S rRNA. We have introduced a U1406-to-A mutation in a plasmid-encoded copy of E. coli 16S rRNA which has been expressed either in a mixture with wild-type ribosomes or in a strain in which all rRNA is transcribed from the plasmid-encoded rrn operon. High-level resistance to many of the 4,6-disubstituted aminoglycosides is observed only when all the rRNA contains the U1406-to-A mutation. In contrast to the partial dominance of resistance observed with other mutations in the decoding region, there is a dominance of sensitivity with the 1406A mutation. Chemical footprinting experiments indicate that resistance arises from a reduced affinity of the antibiotic for the rRNA target. These results demonstrate that although position 1406 is an important determinant in the binding and action of the 4,6-disubstituted aminoglycosides, other rRNA mutations that perturb the binding of ring I of both classes of 2-deoxystreptamine aminoglycosides confer higher levels of resistance as well as a partial dominance of resistance.
Aminoglycoside antibiotics are the archetype for RNA-directed therapeutics. The aminoglycosides can be divided into two major classes: those containing streptamine, such as streptomycin, and those containing 2-deoxystreptamine, such as the neomycins and kanamycins. Chemical probing experiments (10) and rRNA mutations (3, 4, 11, 15) that each confer resistance to only one of the two classes suggest that the two classes of aminoglycosides have different binding sites on the small ribosomal subunit, as recently confirmed by the crystal structure of the bacterial 30S subunit with streptomycin and paromomycin simultaneously bound (2). 2-Deoxystreptamine-containing aminoglycosides bind to the end of helix 44 in 16S rRNA, near the location of A-site tRNA (10).
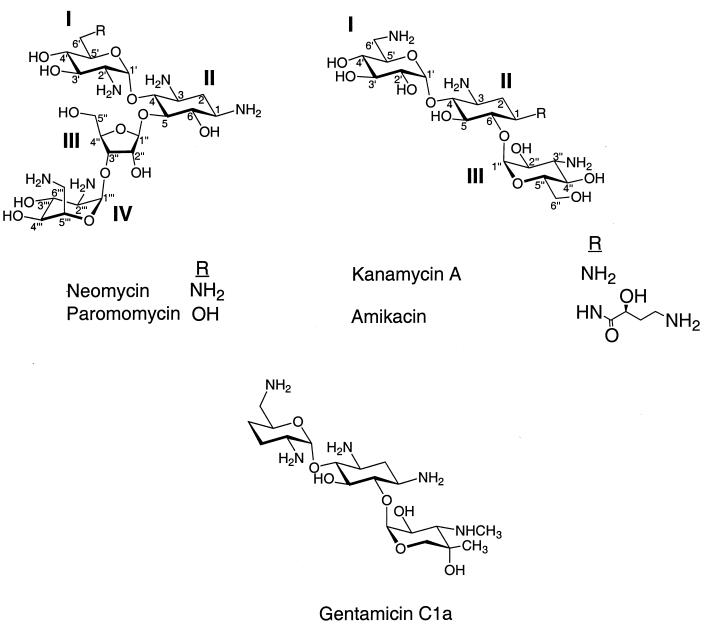
There are two classes of aminoglycosides that contain a 2-deoxystreptamine core: the 4,5- and the 4,6-disubstituted 2-deoxystreptamine antibiotics. The 4,5-disubstituted class consists of neomycin, paromomycin, and ribostamycin. The 4,6-disubstituted class consists of the kanamycins and gentamicins, including the antibiotics amikacin and G418 (Fig. 1).
FIG. 1.
Structures of 2-deoxystreptamine aminoglycosides paromomycin (4,5 disubstituted) and gentamicin C1a, kanamycin A, and amikacin (all 4,6 distubutituted).
The binding of 2-deoxystreptamine aminoglycosides to their rRNA targets has been studied with an oligonucleotide model system (9, 16). The structures of the A-site oligonucleotide bound to either paromomycin (4,5-disubstituted class) or gentamicin C1a (4,6-disubstituted class) were determined by nuclear magnetic resonance spectroscopy (5, 23). The structures show that both classes of 2-deoxystreptamine antibiotics bind in the major groove of the RNA in a binding pocket formed in part by the presence of a noncanonical A1408 · A1493 base pair and the bulged nucleotide A1492.
Paromomycin adopts an L-shaped conformation in the drug-RNA complex. Ring I is positioned near the A1408·A1493 pair at an approximately 90° angle to the rest of the antibiotic and stacks above the guanosine base at position 1491. Rings II to IV line the major groove from the U1406·U1495 base pair in the upper stem to the A1410-U1490 base pair in the lower stem. There are sequence-specific hydrogen bonds between the 1-amino group of 2-deoxystreptamine (ring II) and the O-4 of U1495 and between the 3-amino group and the N-7 of G1494. Rings III and IV are positioned near the C1409-G1491 and A1410-U1490 base pairs in the lower stem of the RNA. The hydrogen bond donors on rings III and IV do not appear to be involved in any sequence-specific contacts; rather, they appear to be making nonspecific electrostatic contacts with the phosphate backbone that contribute to the binding of the antibiotic to the RNA.
The paromomycin-RNA and gentamicin-RNA complexes reveal similarities and differences in RNA recognition by aminoglycosides. Rings I and II adopt similar orientations in both the paromomycin-RNA and gentamicin-RNA complexes, but ring III of gentamicin interacts with the upper stem of the A-site RNA, spanning the U1406 · U1495 and C1407-G1494 base pairs. The chemical groups on ring III that are common among 4,6-disubstituted-2-deoxystreptamine aminoglycosides make sequence-specific contacts with universally conserved nucleotides in the upper stem of the A-site RNA. The 2" hydroxyl group forms a hydrogen bond with either the O-2 of G1405 or the O-4 of U1406, and the 3" aminomethyl group forms a hydrogen bond with the N-7 of G1405. The 4" hydroxyl group forms a hydrogen bond with the phosphate between G1405 and U1406.
The aminoglycoside binding site in 16S rRNA is highly conserved among bacteria, archaea, and eukaryotes. However, natural variations in sequences do occur, and these affect the affinities of the drug-ribosome interaction and, thus, the efficacies of aminoglycosides as translational inhibitors. For example, position 1408 is an adenosine in all bacterial and some archaeal rRNA sequences and a guanosine in all eukaryotic cytoplasmic rRNA sequences. Mutation of A1408 to G in Escherichia coli 16S rRNA does not affect ribosome function and confers resistance to 2-deoxystreptamine aminoglycosides containing an amino group at the 6′ position of ring I (15). Cells expressing ribosomes with this mutation were resistant to aminoglycosides, with a mixed population of 60% mutant and 40% wild-type ribosomes. This partial dominance of resistance to aminoglycosides was unexpected, as sensitivity to this class of antibiotics is usually dominant in a mixed ribosome population (13, 18, 19).
The structures of aminoglycoside-rRNA complexes suggested different roles of U1406 in the binding of the 4,6- versus 4,5-disubstituted aminoglycosides to the A site. Here we report on the effects of mutation of the universally conserved nucleotide U1406 to an adenosine on cell growth in the absence of aminoglycosides and the resistance to these antibiotics conferred by this mutation. In addition, we examine the differences between hetero- and homogeneous populations of mutant ribosomes on growth rate and aminoglycoside resistance.
MATERIALS AND METHODS
Bacterial strains and plasmids.
For studies of heterogeneous ribosome populations, mutant rRNAs were expressed in E. coli strain DH1 (ATCC 33849) (7). Homogeneous ribosome populations were obtained by expression of plasmid-encoded rRNA in E. coli strain TA531 (1). Plasmid replacement was performed essentially as described previously (1), except that the absence of the original plasmid containing the wild-type rrnC operon (pHK-rrnC+) was verified by plasmid purification and sequencing of the isolated rRNA. This step was necessary, as selection for kanamycin or neomycin sensitivity was not possible with expression of ribosomes containing the U1406-to-A or A1408-to-G mutations in 16S rRNA.
Mutagenesis and expression of plasmid-encoded 16S rRNA.
The 1406A mutation was introduced into plasmid pKK3535 and was expressed from the natural rrnB promoters as described previously (14). When it was expressed in E. coli strain DH1, this mutation was introduced both with and without the additional mutation 1192U, which confers resistance to spectinomycin (17), as well as the allele-specific priming site V mutation (12). The 16S rRNA expressed in E. coli strain TA531 did not contain either the 1192U or the priming site V mutation. All mutant 16S rRNAs were expressed from the rrnB promoters of pKK3535. Quantitation of plasmid-encoded rRNA was performed as described previously (14).
Determination of MICs.
Cultures were started from single colonies and grown in Luria-Bertani (LB) medium containing 50 μg of spectinomycin per ml for 1192U plasmids or 100 μg of ampicillin per ml for 1192C plasmids. MIC tests were performed by inoculation of 5-ml cultures with a 1:1,000 dilution of overnight culture in LB medium containing spectinomycin (or ampicillin) plus a twofold series of dilutions of one of the following aminoglycosides: paromomycin, neomycin (a mixture of neomycins B and C), gentamicin C (a mixture of gentamicins C1, C1a, and C2), kanamycin A, apramycin, tobramycin, neamine, or G418.
The reported MIC is the concentration of aminoglycoside at which the growth of the cultures was completely inhibited after 24 h of incubation at 37°C.
To monitor bacterial growth, cultures were inoculated with a 1:200 dilution of an overnight culture in LB medium containing spectinomycin or ampicillin. For growth rate determinations in the presence of aminoglycosides, LB medium containing 100 μg of ampicillin per ml plus 160 μg of kanamycin A or neomycin per ml was inoculated with a 1:200 dilution of an overnight culture grown in LB medium containing 200 μg of ampicillin per ml.
Chemical modification of 30S subunits.
Preparation of 30S subunits and chemical modification experiments were performed as described previously (14).
Primer extension was performed as described previously (20) with a 21-nucleotide DNA primer complementary to nucleotides 1530 to 1509 of the wild-type E. coli 16S rRNA sequence.
RESULTS
Heterogeneous ribosome population.
Ribosomes containing the U1406-to-A mutation were expressed in E. coli DH1 from a plasmid-encoded copy of the rrnB operon (Fig. 2). When expressed from the P1 and P2 promoters of the rrnB operon in pKK3535, ∼60% of the total RNA is plasmid encoded (14) (Table 1). The doubling time of cells containing a mixture of mutant and wild-type ribosomes was identical to that of control cells expressing rRNA from a wild-type plasmid-encoded rrnB operon (42 min).
FIG. 2.
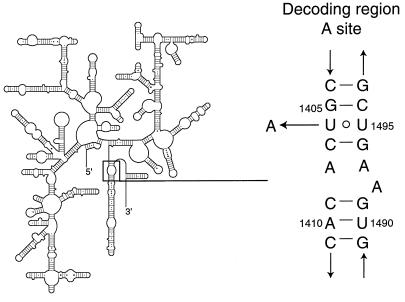
Secondary structure of 16S rRNA (6). The decoding region A site is boxed, and its sequence and secondary structure are shown on the right. The U1406-to-A mutation is indicated.
TABLE 1.
Growth rate and distribution of plasmid-encoded 1406A 16S rRNA containing the sequence at position 1192 encoding either the wild type (1192C) or spectinomycin resistance (1192U)a
| 16S rRNA sequence | Doubling time (min) | % Plasmid-encoded 16S rRNA
|
|||
|---|---|---|---|---|---|
| 30S | 70S | Disomes | Trisomes | ||
| 1406A, 1192C | 42 | 69 | 56 | 51 | 50 |
| 1406A, 1192U | 60 | 47 | 29 | 27 | 31 |
Mutant rRNAs were expressed in E. coli strain DH1. The percent plasmid-encoded 16S rRNA in each fraction is the average of at least two experiments. Data for 1406A, 1192C are from Recht et al. (14).
Functional interaction between position 1406 and the 1190 region.
Since there was no apparent effect on the growth rate of cells expressing mutant ribosomes in rich medium, the ability of the mutant ribosomes to support growth in the absence of wild-type ribosomes was examined. To test the function of the mutant ribosomes, 16S rRNA containing either the wild-type or the 1406A sequence was expressed with an additional mutation of C1192 to U that confers resistance to spectinomycin (17). The cells were either plated on medium containing 50 μg of spectinomycin per ml or placed in liquid cultures containing 50 μg of spectinomycin per ml. The plates were placed at 37 and 42°C for 48 h, and liquid cultures were placed at 37°C for 24 h. At both temperatures, expression of the wild-type (1192U) 16S rRNA supported growth on solid medium. Growth in liquid cultures was as strong as that on the solid medium. In contrast, expression of the 1406A (1192U) 16S rRNA did not support growth on spectinomycin-containing solid medium at either temperature, nor was there any growth in the liquid cultures in the presence of spectinomycin.
To determine if the combination of the 1192U mutation with 1406A impaired growth in the absence of spectinomycin, the growth of cells expressing ribosomes containing the 1406A mutation with or without the 1192U mutation was monitored. With the wild-type 1192C sequence, the doubling time was 42 min, whereas with the 1192U mutation the doubling time increased to 60 min (Table 1). The longer doubling time in the presence of the 1192U mutation but in the absence of spectinomycin indicated that the additional mutation at position 1192 was deleterious when it was combined with the 1406A mutation.
To identify what functional step of translation might be affected by the combination of 1406A and 1192U, ribosomal particles from actively growing cells expressing either 1406A (1192C) or 1406A (1192U) 16S rRNA were separated by sucrose density gradient fractionation. As shown in Table 1, the combination of 1406A with the 1192U mutation caused the mutant 16S rRNA to be underrepresented in the 70S ribosome and polysome fractions.
Effects of the 1406A mutation on aminoglycoside action.
Low-level resistance to most of the 4,6-disubstituted 2-deoxystreptamine aminoglycoside antibiotics was observed when 1406A 16S rRNA was expressed from pKK3535 in E. coli DH1 (60% plasmid-encoded 16S rRNA) (Table 2). Expression of this mutant 16S rRNA in a mixture with wild-type rRNA conferred twofold resistance to kanamycin A, gentamicin C, tobramycin, and G418. While no change in the level of resistance was observed for neomycin or paromomycin, expression of ribosomes containing the 1406A mutation caused hypersensitivity to neamine (fourfold) and to apramycin and amikacin (twofold for both) compared to the sensitivities of cells expressing plasmid-encoded wild-type 16S rRNA. No resistance to streptomycin was observed. As previously observed with sensitive strains, the aminoglycosides are bactericidal for the strains containing heterogeneous populations of ribosomes with either the 1406A or the 1408G mutation.
TABLE 2.
Comparison of the MICs of 2-deoxystreptamine aminoglycosides for E. coli strains expressing either a mixture of mutant and wild-type 16S rRNA or homogeneous mutant rRNAa
| Antibiotic | Wild-type MIC (μg/ml) | Relative resistance (fold)
|
|
|---|---|---|---|
| 1406A (60%) | 1406A (100%) | ||
| Kanamycin A | 5 | 2 | 128 |
| G418 | 5 | 2 | 128 |
| Gentamicin C | 5 | 2 | 64 |
| Tobramycin | 2.5 | 2 | 64 |
| Neomycin | 10 | 1 | 0.5 |
| Paromomycin | 10 | 1 | 0.5 |
| Amikacin | 5 | 0.5 | 0.25 |
| Apramycin | 10 | 0.5 | 0.25 |
Cells were grown in liquid LB medium containing 200 μg of ampicillin per ml plus a twofold series of dilutions of the indicated aminoglycoside. The MIC is the minimum concentration of antibiotic that fully inhibited growth after 24 h of incubation at 37°C. The values for 60% 1406A 16S rRNA are for E. coli strain DH1. The MICs for strain DH1 expressing wild-type 16S rRNA from pKK3535 are nearly identical to the MICs for strain TA531 expressing wild-type type 16S rRNA from the same plasmid.
Homogeneous ribosome population.
In a mixed population of antibiotic-resistant and -sensitive ribosomes, drug resistance is usually observed only if the mutant ribosome population makes up more than 50% of the whole population (17). The resistance arising from an A1408-to-G mutation in 16S rRNA can be enhanced by an additional C1192-to-U mutation in the G1408 rRNA and growing of the cells in the presence of 50 μg of spectinomycin per ml (15). This approach could not be applied to studies of A1406 mutant ribosomes due to the deleterious effect of the combination of the C1192-to-U and U1406-to-A mutations described above.
An E. coli strain, TA531, which derives all of its rRNA from a plasmid-encoded rRNA operon has been constructed (1). By using plasmid replacement, the plasmid-encoded wild-type rrnC operon in strain TA531 (pHK-rrnC+) can be substituted with the rrn operon of interest. We have replaced the wild-type rrnC operon with each of the following rrnB operons encoded by pKK3535: the wild-type operon and operons with U1406-to-A or A1408-to-G mutations. rRNA from each of these rrnB operons could support the growth of E. coli strain TA531. The growth rate of cells containing each of these rRNAs was determined (Table 3). There was no observable difference in the doubling time of cells expressing 16S rRNA with the 1408G mutation compared to that for cells expressing wild-type 16S rRNA. The doubling time of cells expressing 16S rRNA with the 1406A mutation was slightly longer (60 min, whereas it was 57 min for wild-type cells).
TABLE 3.
Growth rate of E. coli strain TA531 expressing wild-type or mutant rRNA from pKK3535a
| 16S rRNA sequence | Doubling time (min) with:
|
|
|---|---|---|
| No aminoglycoside | Kanamycin A at 160 μg/ml | |
| Wild type | 57 ± 2 | No growth |
| 1406A | 60 ± 1 | 90 ± 6 |
| 1408G | 57 ± 2 | 56 ± 1 |
The doubling time of cells expressing 1408G 16S rRNA in the presence of 160 μg of neomycin B per ml is 57 min. Cells were grown in liquid LB medium containing 100 μg of ampicillin per ml and the indicated aminoglycoside.
Aminoglycoside resistance with a homogeneous ribosome population.
The MICs of 4,5- and 4,6-disubstituted 2-deoxystrepatmine aminoglycosides for E. coli strain TA531 cells expressing wild-type or mutant 16S rRNA were measured in liquid medium (Table 4).
TABLE 4.
MICs of various aminoglycosides for E. coli strain TA531 expressing the indicated 16S rRNAa
| Antibiotic | MIC (μg/ml)
|
||
|---|---|---|---|
| Wild type | 1406A | 1408G | |
| Kanamycin A | 5 | 640 | <1,280 |
| Neomycin | 10 | 5 | <1,280 |
| Gentamicin C | 5 | 320 | <1,280 |
| Paromomycin | 10 | 5 | 160 |
| G418 | 5 | 640 | 5 |
| Amikacin | 5 | 1.25 | NDb |
| Apramycin | 10 | 2.5 | ND |
| Tobramycin | 2.5 | 160 | ND |
Cells were grown in liquid LB medium containing 200 μg of ampicillin per ml and a twofold dilution (from 1280 to 0.625 μg/ml) of the indicated aminoglycoside. Growth was monitored after 24 h of incubation at 37°C.
ND, not determined.
Expression of 16S rRNA containing a U1406-to-A mutation conferred high-level (128-fold) resistance to kanamycin A and G418. Moderate resistance (64-fold) to gentamicin C and tobramycin was conferred, whereas no resistance to neomycin or paromomycin was observed. Expression of the 1406A 16S rRNA caused increased sensitivity (fourfold) to amikacin and apramycin compared to that for the wild type.
Cells expressing a homogeneous population of ribosomes containing an A1408-to-G mutation in the 16S rRNA were highly resistant to kanamycin A, neomycin, and gentamicin. Moderate resistance to paromomycin was observed, but there was no resistance to G418. These results are in agreement with those previously reported for expression of a mixed population of ribosomes containing the A1408-to-G mutation when the cells were grown in the presence of spectinomycin (15).
In studies of ribosomes containing an A1408-to-G mutation, we had observed a greater than 10-fold increase in the doubling time of cells expressing a mixture of aminoglycoside-sensitive and -resistant ribosomes in the presence of sublethal concentrations of antibiotic (15). The growth rate returned to normal upon removal of aminoglycoside from the growth medium. To test if the slower growth rate was due to the presence of aminoglycoside-sensitive wild-type ribosomes in the mixture, the doubling time of E. coli strain TA531 expressing 16S rRNA with an A1408-to-G mutation was measured in the presence of 160 μg of kanamycin A or neomycin per ml. As shown in Table 3, the doubling times of cells expressing a homogeneous population of ribosomes with the 1408G mutation are identical in the presence or absence of aminoglycoside (57 min).
In contrast, although expression of a homogeneous population of ribosomes with the 1406A mutation confers resistance to kanamycin A present at up to 640 μg/ml, the doubling time of these cells increased from 60 to 90 min in the presence of 160 μg of kanamycin A per ml (Table 3).
Binding of aminoglycosides to mutant ribosomes.
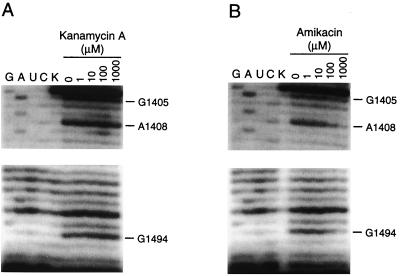
Aminoglycoside resistance could result from either a decreased affinity of the antibiotic for the ribosome or nonproductive binding of the drug to the ribosome. To determine if there was a correlation between resistance and low-affinity binding of the aminoglycoside to the ribosome, chemical footprinting experiments were performed on 70S ribosomes containing the 1406A mutation in the presence of increasing concentrations of kanamycin A. No protection from dimethyl sulfate modification is observed at G1494(N-7), and only slight protection is observed at A1408(N-1) in the presence of 100 μM kanamycin A (Fig. 3A).
FIG. 3.
Autoradiograph showing dimethyl sulfate probing experiments with 1406A ribosomes in the presence of increasing concentrations of kanamycin A (A) and amikacin (B). In all experiments ribosomes were present at 100 nM. Antibiotics were present at the indicated concentration. The lanes labeled G, A, U, and C are dideoxy sequencing reactions. K is primer extension from 16S rRNA to which no modifying agent was added.
The cells expressing 16S rRNA with the 1406A mutation are sensitive to amikacin. In contrast to the results obtained with kanamycin, both G1494(N-7) and A1408(N-1) are strongly protected from dimethyl sulfate modification in the presence of 100 μM amikacin. In addition, a weak footprint is visible at G1405(N-7) (Fig. 3B). The resistance phenotype is consistent with the footprinting data, which reflect the affinities of the antibiotics for the mutated rRNA binding site. Resistance therefore arises primarily from decreased aminoglycoside affinity for its ribosomal target.
DISCUSSION
All aminoglycosides to which expression of 16S rRNA with the 1406A mutation conferred resistance contain a 4,6-disubstituted 2-deoxystreptamine. In contrast to the resistance conferred by the 1408G mutation, the identity of the 6′ substituent of ring I was unimportant, as resistance was conferred to both gentamicin C and G418, which contain a 6′ amino and a 6′ hydroxyl, respectively. This result is consistent with the structural data, as the 6′ position of ring I is in the plane of A1408, distant from U1406 (2, 5, 23).
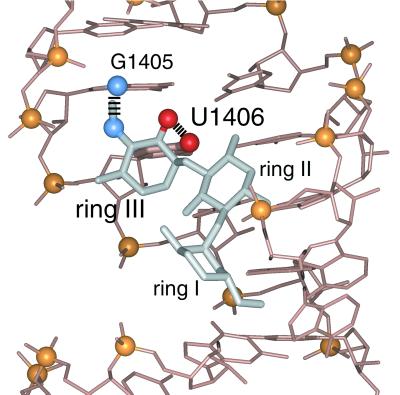
The specific resistance to 4,6-disubstituted 2-deoxystreptamine aminoglycosides induced by the A1406 mutation is explained by our prior structural studies. Gentamicin C1a can form hydrogen bonds between the 2" hydroxyl of ring III and the O-4 of U1406 (Fig. 4) (23). This likely RNA-drug contact, common to all 4,6-disubstituted aminoglycosides, is removed by the 1406A mutation, which replaces the O-4 of uridine with the N-6 amino of adenosine; in addition, there may be a steric clash between ring III of the aminoglycoside and the N-6 amino that protrudes into the major groove. Additional contacts are observed between ring III and the A-site RNA, which may account for the observation of a lower level of resistance to these aminoglycosides in the presence of the 1406A mutation compared to the level of resistance afforded by the 1408G mutation.
FIG. 4.
Contacts of ring III of gentamicin C1a with the A-site oligonucleotide (23). The 2" hydroxyl group on ring III of gentamicin C1a forms a hydrogen bond with the O-4 of U1406 (shown in bold) and the 3" aminomethyl forms a hydrogen bond with the N-7 of G1405; these interactions are shown explicitly. In addition, the ring III 4" hydroxyl forms a hydrogen bond with the phosphate between G1405 and U1406 (not highlighted). Gentamicin is gray, and the RNA is beige. Oxygen, nitrogen, and phosphorus are red, blue, and yellow, respectively.
Amikacin, which is a modified form of kanamycin A, is active against cells expressing ribosomes with a 1406A mutation. This suggests that RNA interactions by the 1-amino of 2-deoxystreptamine are sensitive to the structure of the upper stem of the A site and that the 4-amino-2-hydroxybutyryl group on amikacin may be involved in additional contacts with the rRNA that compensate for the contacts lost between ring III and the RNA upon mutation of U1406 to A. We are unable to explain the molecular basis for the hypersensitivity to apramycin of bacteria containing ribosomes with the 1406A mutation. There is currently no structure of apramycin bound to the decoding region A site, so the specific interactions between this unusual aminoglycoside and 16S rRNA are not yet known.
The chemical probing experiments in the presence of kanamycin A and amikacin demonstrate a direct correlation between high-affinity binding of antibiotic to its target RNA and antibiotic activity. By surface plasmon resonance (SPR) assay, it was demonstrated that the 4,6-disubstituted 2-deoxystreptamine aminoglycosides have a lower affinity (two- to fourfold) for a 1406A oligonucleotide than for the wild-type oligonucleotide (22). It was also observed that butirosin, a ribostamycin derivative containing the same 4-amino-2-hydroxybutyryl group as amikacin, bound with a 15-fold higher affinity to the 1406A oligonucleotide compared to the affinity for the wild-type oligonucleotide. No increased affinity for the 1406A sequence was observed for ribostamycin, demonstrating that the 4-amino-2-hydroxybutyryl group on the 1-amino group of 2-deoxystreptamine increases the affinities of aminoglycosides for the 1406A sequence. The differences in affinity observed by the SPR assay correlate nearly exactly with the resistance or sensitivity conferred by expression of ribosomes with the 1406A mutation.
The results presented here agree with the observed aminoglycoside resistance mutations in clinical bacterial isolates. As with the 1408G mutation, the aminoglycoside resistance of clinical isolates of Mycobacterium has been attributed to mutation of position 1406. Moderate resistance to kanamycin has been reported for strains of Mycobacterium smegmatis, which is the result of a U-to-A substitution at the nucleotide corresponding to position 1406 in the E. coli 16S rRNA (21).
Partial dominance of resistance is observed both with the 1408G mutation and with mutations of the C1409-G1491 base pair (3, 4, 15). The partial dominance and higher level of aminoglycoside resistance observed with these mutations compared to that observed with the 1406A mutation may be related to the site of action of these antibiotics. Binding of either paromomycin or gentamicin to the A-site oligonucleotide causes A1492 and A1493 to shift toward the minor groove. A similar shift of A1492 and A1493 is observed in the structure of the 30S subunit with paromomycin, streptomycin, and spectinomycin simultaneously bound (2). This conformational change may be the origin of the misreading phenotype caused by the aminoglycosides. Nucleotide A1408 and the 1409-1491 base pair are both in the vicinity of A1492 and A1493. The binding of ring I and the conformational change induced in A1492 and A1493 may be the essential step in aminoglycoside action. In contrast, rings II and III of aminoglycosides provide mainly sequence-specific contacts for the drug-RNA interaction. Both rRNA mutations that confer partial dominance of resistance perturb the binding site of ring I and may therefore prevent the action of the aminoglycosides more effectively than the perturbation of ring III contacts caused by the 1406A mutation does.
The exclusion of 16S rRNA containing both the 1406A and 1192U mutations from the 70S and polysome fractions suggests that ribosome function is impaired when both mutations are present. Functional interactions between the decoding region (helix 44) and the position 1200 region (helix 34) of 16S rRNA have previously been observed (8), so the combined effects of a mutation in the decoding region and at position 1192 may result in a phenotype different from that conferred by either mutation alone. In the structure of the 30S subunit, helices 34 and 44 form portions of the A site (2). There is no direct contact between these two regions of rRNA, so functional interactions between these two regions of rRNA may be communicated through the A-site tRNA, which lies between helix 34 and helix 44.
These results demonstrate the differences in binding to the ribosome by the 4,5- and 4,6-disubstituted 2-deoxystreptamine aminoglycosides. The sequence-specific contacts made by rings I and II of both classes of aminoglycosides are identical, whereas significant differences are observed for ring III. The identity of the nucleotide at position 1406 is important for the high-affinity binding and antibacterial action of the 4,6-disubstituted 2-deoxystreptamine aminoglycosides, but it appears to play only a minor role in the action of the 4,5-disubstituted 2-deoxystreptamine aminoglycosides.
ACKNOWLEDGMENTS
We thank Catherine L. Squires for the TA531 strain; Harry F. Noller and members of his laboratory for plasmids; and Stephen Douthwaite, Satoko Yoshizawa, Greg Eason, Stephen Lynch, and Kam Dahlquist for insightful discussions.
This work was supported by NIH grant GM51266.
REFERENCES
- 1.Asai T, Zaporojets D, Squires C, Squires C L. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc Natl Acad Sci USA. 1999;96:1971–1976. doi: 10.1073/pnas.96.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carter A P, Clemons W M, Brodersen D E, Morgan-Warren R J, Wimberly B T, Ramakrishnan V. Functional insights from the structure of the 30S ribosomal subunit and its interactions with antibiotics. Nature. 2000;407:340–348. doi: 10.1038/35030019. [DOI] [PubMed] [Google Scholar]
- 3.DeStasio E A, Dahlberg A E. Effects of mutagenesis of a conserved base-paired site near the decoding region of Escherichia coli 16S ribosomal RNA. J Mol Biol. 1990;212:127–133. doi: 10.1016/0022-2836(90)90309-A. [DOI] [PubMed] [Google Scholar]
- 4.DeStasio E A, Moazed D, Noller H F, Dahlberg A E. Mutations in 16S ribosomal RNA disrupt antibiotic-RNA interactions. EMBO J. 1989;8:1213–1216. doi: 10.1002/j.1460-2075.1989.tb03494.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fourmy D, Recht M I, Blanchard S C, Puglisi J D. Structure of the A site of E. coli 16S ribosomal RNA complexed with an aminoglycoside antibiotic. Science. 1996;274:1367–1371. doi: 10.1126/science.274.5291.1367. [DOI] [PubMed] [Google Scholar]
- 6.Gutell R R. Collection of small subunit (16S- and 16S-like) ribosomal RNA structures: 1994. Nucleic Acids Res. 1994;22:3502–3507. doi: 10.1093/nar/22.17.3502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 8.Hui A S, Eaton D H, deBoer H A. Mutagenesis at the mRNA decoding site in the 16S ribosomal RNA using the specialized ribosome system in Escherichia coli. EMBO J. 1988;7:4383–4388. doi: 10.1002/j.1460-2075.1988.tb03337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miyaguchi H, Narita H, Sakamoto K, Yokoyama S. An antibiotic-binding motif of an RNA fragment derived from the A-site-related region of Escherichia coli 16S rRNA. Nucleic Acids Res. 1996;24:3700–3706. doi: 10.1093/nar/24.19.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moazed D, Noller H F. Interaction of antibiotics with functional sites in 16S ribosomal RNA. Nature. 1987;327:389–394. doi: 10.1038/327389a0. [DOI] [PubMed] [Google Scholar]
- 11.Montandon P E, Wagner R, Stutz E. E. coli ribosomes with a C912 to U base change in the 16S rRNA are streptomycin resistant. EMBO J. 1986;5:3705–3708. doi: 10.1002/j.1460-2075.1986.tb04703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Powers T, Noller H F. Allele-specific structure probing of plasmid-derived 16S ribosomal RNA from Escherichia coli. Gene. 1993;123:75–80. doi: 10.1016/0378-1119(93)90542-b. [DOI] [PubMed] [Google Scholar]
- 13.Powers T, Noller H F. A functional pseudoknot in 16S ribosomal RNA. EMBO J. 1991;10:2203–2214. doi: 10.1002/j.1460-2075.1991.tb07756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Recht M I, Douthwaite S, Dahlquist K D, Puglisi J D. Effect of mutations in the A site of 16S rRNA on aminoglycoside antibiotic-ribosome interaction. J Mol Biol. 1999;286:33–43. doi: 10.1006/jmbi.1998.2446. [DOI] [PubMed] [Google Scholar]
- 15.Recht M I, Douthwaite S, Puglisi J D. Basis for prokaryotic specificity of action of aminoglycoside antibiotics. EMBO J. 1999;18:3133–3138. doi: 10.1093/emboj/18.11.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Recht M I, Fourmy D, Blanchard S C, Dahlquist K D, Puglisi J D. RNA sequence determinants for aminoglycoside binding to an A-site rRNA model oligonucleotide. J Mol Biol. 1996;262:421–436. doi: 10.1006/jmbi.1996.0526. [DOI] [PubMed] [Google Scholar]
- 17.Sigmund C D, Ettayebi M, Borden A, Morgan E A. Antibiotic resistance mutations in ribosomal RNA genes of Escherichia coli. Methods Enzymol. 1988;164:673–690. doi: 10.1016/s0076-6879(88)64077-8. [DOI] [PubMed] [Google Scholar]
- 18.Sparling P F, Davis B D. Bactericidal action of streptomycin and comparison with spectinomycin in heterozygotes of Escherichia coli. Antimicrob Agents Chemother. 1972;1:252–258. doi: 10.1128/aac.1.3.252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sparling P F, Modolell J, Takeda Y, Davis B D. Ribosomes from Escherichia coli merodiploids heterozygous for resistance to streptomycin and to spectinomycin. J Mol Biol. 1968;37:407–421. doi: 10.1016/0022-2836(68)90111-3. [DOI] [PubMed] [Google Scholar]
- 20.Stern S, Moazed D, Noller H F. Structural analysis of RNA using chemical and enzymatic probing monitored by primer extension. Methods Enzymol. 1988;164:481–489. doi: 10.1016/s0076-6879(88)64064-x. [DOI] [PubMed] [Google Scholar]
- 21.Taniguchi H, Chang B, Abe C, Nikaido Y, Mizuguchi Y, Yoshida S. Molecular analysis of kanamycin and viomycin resistance in Mycobacterium smegmatis by use of the conjugation system. J Bacteriol. 1997;179:4795–4801. doi: 10.1128/jb.179.15.4795-4801.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wong C-H, Hendrix M, Priestley E S, Greenberg W A. Specificity of aminoglycoside antibiotics for the A-site of the decoding region of ribosomal RNA. Chem Biol. 1998;5:397–406. doi: 10.1016/s1074-5521(98)90073-4. [DOI] [PubMed] [Google Scholar]
- 23.Yoshizawa S, Fourmy D, Puglisi J D. Structural origins of gentamicin antibiotic action. EMBO J. 1998;17:6437–6448. doi: 10.1093/emboj/17.22.6437. [DOI] [PMC free article] [PubMed] [Google Scholar]