Abstract
Nearly half of all adults with type 2 diabetes mellitus (T2DM) live in India and China. These populations have an underlying predisposition to deficient insulin secretion, which has a key role in the pathogenesis of T2DM. Indian and Chinese people might be more susceptible to hepatic or skeletal muscle insulin resistance, respectively, than other populations, resulting in specific forms of insulin deficiency. Cluster-based phenotypic analyses demonstrate a higher frequency of severe insulin-deficient diabetes mellitus and younger ages at diagnosis, lower β-cell function, lower insulin resistance and lower BMI among Indian and Chinese people compared with European people. Individuals diagnosed earliest in life have the most aggressive course of disease and the highest risk of complications. These characteristics might contribute to distinctive responses to glucose-lowering medications. Incretin-based agents are particularly effective for lowering glucose levels in these populations; they enhance incretin-augmented insulin secretion and suppress glucagon secretion. Sodium–glucose cotransporter 2 inhibitors might also lower blood levels of glucose especially effectively among Asian people, while α-glucosidase inhibitors are better tolerated in east Asian populations versus other populations. Further research is needed to better characterize and address the pathophysiology and phenotypes of T2DM in Indian and Chinese populations, and to further develop individualized treatment strategies.
Subject terms: Type 2 diabetes, Diabetes
Prevalence of type 2 diabetes mellitus (T2DM) is increasing substantially in India and China. This Review discusses the epidemiology, phenotypes and pathogenesis of T2DM in India and China and evaluates options for optimal pharmacological management.
Key points
India and China account for nearly half of the global number of people with type 2 diabetes mellitus (T2DM), and incidence rates are rising rapidly among young people.
Indian and Chinese people seem to have increased susceptibility to reduced insulin secretion, which probably has a stronger or more frequent role than insulin resistance in the pathogenesis of T2DM than in other populations.
In many Indian people, deficient first-phase insulin secretion and increased hepatic insulin resistance result in impaired fasting glucose, while in many Chinese people, deficient first-phase and second-phase insulin secretion and increased skeletal muscle insulin resistance result in impaired glucose tolerance.
Cluster-based phenotypic analyses demonstrate a higher frequency of severe insulin-deficient diabetes mellitus and demonstrate generally younger ages at diagnosis, lower β-cell function, lower insulin resistance and lower BMI among Indian people and Chinese people compared with European people.
Dipeptidyl peptidase 4 inhibitors and glucagon-like peptide 1 receptor agonists improve pancreatic β-cell function and reduce blood levels of glucose especially effectively in Indian and Chinese people compared with other populations.
Sodium–glucose cotransporter 2 inhibitors might lower blood levels of glucose more effectively in Asian people than in European people, while α-glucosidase inhibitors are better tolerated among east Asian people than in other populations.
Introduction
Type 2 diabetes mellitus (T2DM) is a complex, heritable and heterogeneous condition that manifests in a range of phenotypes1. Characterizing these phenotypes could reveal insights into the underlying pathophysiology, which is relevant in individualizing clinical management to each patient2,3. There is keen interest in personalizing the treatment of T2DM using a ‘precision medicine’ approach that integrates epidemiological, phenotypic, genetic and molecular data4,5. However, much of the evidence in this arena has been limited to studies in populations of European descent.
Of the estimated 463 million adults with diabetes mellitus globally, >90% have T2DM and nearly half live in two large countries: India and the People’s Republic of China (henceforth referred to as China)6. Indian and Chinese people exhibit differences in the characteristics of T2DM compared with people of European descent, including a younger age at diagnosis, greater tendency towards β-cell failure and leaner body habitus7–9. In these populations, novel analytical approaches have revealed phenotypic variations in young-onset T2DM (defined in this Review as age at diagnosis <40 years10) and other subtypes of T2DM, and have revealed unique responses to certain glucose-lowering medications (for example, sodium–glucose cotransporter 2 (SGLT2) inhibitors and α-glucosidase inhibitors). Examining how the pathophysiology and phenotypes of T2DM vary among Indian and Chinese people could generate valuable insights to inform the development, refinement and broader implementation of precision medicine strategies.
In this Review, we synthesize the latest evidence regarding the epidemiology, pathophysiology, phenotypes and glycaemic management of T2DM among Indian and Chinese populations and their diasporas, and identify novel avenues of research to advance therapeutic approaches. Because the vast majority (98.0–99.7%11,12) of Chinese and Indian adults with diabetes mellitus have T2DM, we included population-based studies of diabetes mellitus in general, as these are likely to be representative of T2DM. We also include studies that report pertinent findings for Asian people in general (including people from India, China and other Asian regions) if country-specific or ethnicity-specific data were unavailable. Atypical forms of diabetes mellitus (including fibrocalculous pancreatic diabetes mellitus13,14) are beyond the scope of this Review.
Epidemiology
Prevalence
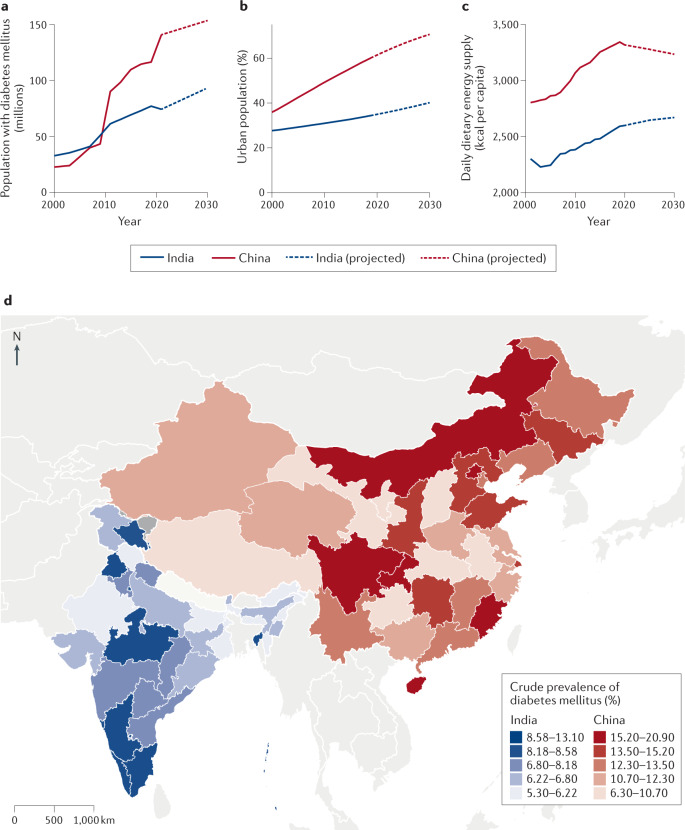
In 2021, the estimated age-adjusted prevalence of diabetes mellitus was 10.6% (95% CI 9.2–12.0%) in China and 9.6% (95% CI 8.5–10.6%) in India, accounting for 145 million and 74 million people, respectively, or 41% of the global number of adults with diabetes mellitus6 (Fig. 1). The large burden of diabetes mellitus in India and China has been shaped by various behavioural, geographical, socioeconomic and cultural factors in each country (Box 1).
Fig. 1. Burden of diabetes mellitus and its correlates in India and China.
a | Estimated number of adults with diabetes mellitus (millions) aged 20–79 years in India and China from 2000 with projections to 2030. b | Percentage of population residing in urban areas in India and China from 2000 with projections to 2030. c | Average daily dietary energy supply in India and China from 2000 with projections to 2030. Dietary energy supply indicates the amount of calories available for consumption in a given country; measures of individual caloric intake are not available at the national population level253,254. d | Crude prevalence of diabetes mellitus in the states of India (2016) and provinces of China (2015–2017). Crude prevalence rates are shown to illustrate spatial distribution. Rates are not directly comparable between countries owing to differences in the age distribution between countries (comparable age-standardized data unavailable). In China and India, the rising prevalence of diabetes mellitus is driven by rapid urbanization, increased caloric intake and reduced physical activity255–258. The high crude prevalence and number of people with diabetes mellitus in China relative to India is driven in part by China’s older age distribution259,260. Panel a is derived from data from the International Diabetes Federation Atlas6. Panel b is derived from data from the United Nations, Department of Economic and Social Affairs, & Population Division261. Panel c is derived from data from the Food and Agriculture Organization of the United Nations253,254. Panel d is derived from data from Tandon et al.262 and Li et al.53.
Box 1 Comparing contextual factors affecting T2DM in India and China.
Health-related behaviours
Rapid economic growth, unprecedented urbanization, sedentary behaviour, insufficient sleep, increased carbohydrate and saturated fat consumption and reduced fibre consumption drive an increase in type 2 diabetes mellitus (T2DM) prevalence255–258.
Packaged foods and beverages in India and China have the highest energy density and saturated fat content in a comparison with 12 other countries92.
High rates of tobacco use among men (China: 50.5%; India: 19.0%)266,267 are associated with increased risk of T2DM268 and its complications268,269.
Geographical and socioeconomic factors
Owing to the high population and large size of China and India, both countries have wide variations in prevalence: the highest rates are in southern India (Tamil Nadu, Kerala: 12.1–12.3%)262 and northern China (Beijing, Tianjin, Hebei, Shanxi, Inner Mongolia: 12.7%)270.
Diabetes mellitus is most prevalent among wealthy271,272 and urban populations (India: urban 11.2%, rural 5.2%48; China: urban 13.1%; rural 8.7%270).
Public health-care systems are limited by inadequate public investment, especially in India relative to China (1% versus 5% of gross domestic product, respectively)273.
Limited health services and high risk of unaffordable health-care costs contribute to disparities in outcome among rural and low socioeconomic status populations compared with wealthier, urban populations274–277.
Culture, tradition and religion
Linguistic, ethnic and sociocultural variation across India and China (especially ethnic minorities that are not Han Chinese) shape lived experiences278,279 and should inform T2DM interventions276,277.
Religious fasting (such as Ramadan and Navaratri) affects diet and physical activity; optimal T2DM management requires education and individualization280,281.
Ayurveda (Indian traditional medicine) and traditional Chinese medicine are often used as holistic, patient-focused approaches to address T2DM.
Traditional Chinese medicine and Ayurveda include components such as litchi (also called lychee)282 and pomegranate283 to address T2DM284. Litchi toxicity causes hypoglycaemia in children285, and some components can alter the gut microbiome286.
Incidence
In India, the incidence of total diabetes mellitus has been estimated within local and regional studies. In Tamil Nadu, the Chennai Urban Population Study reported an incidence of 20.2 per 1,000 person-years for the period 1996–1997 (n = 513)15. The Chennai Urban Rural Epidemiology Study (2001–2013) estimated an incidence of 33.1 per 1,000 person-years (n = 1,376)16. Among adults aged 20–44 years in Delhi and Chennai, the Centre for Cardiometabolic Risk Reduction in South Asia Study (CARRS) (2010–2018) reported incidence rates of 14.2 (men) and 14.8 (women) per 1,000 person-years (n = 6,676)17,18. In Puducherry, the incidence was 21.5 per 1,000 person-years in 2007 (n = 1,223)19, while in urban Kerala, the incidence was 24.5 per 1,000 person-years between 2007 and 2017 (n = 869)20.
In China, T2DM incidence trends have been estimated in local and regional studies using population-based data. In Zhejiang province, the incidence of T2DM increased from 193.8 to 443.7 (men) and 212.9 to 370.5 (women) per 100,000 person-years over the period 2007–2017 (ref.21). In Hong Kong, the incidence of young-onset T2DM increased from 75.4 to 110.8 (men) and 45.0 to 62.1 (women) per 100,000 population over the period 2002–2015 (ref.11). Among those aged 40–59 years in Hong Kong, incidence increased from 697.0 to 852.7 (men) and 569.7 to 639.8 (women) per 100,000 person-years between 2005 and 2012 and 2005 and 2011, respectively, while incidence declined among those aged ≥60 years.
International comparisons of T2DM incidence are limited by differences in age standardization, time period during which incidence rates were measured, living environments and methodologies. In a comparative study using similar paediatric registries (age <20 years), the incidence of young-onset T2DM was lower in Chennai and New Delhi (0.5 per 100,000 population) than in the US SEARCH for Diabetes study (5.9 per 100,000 population). However, this difference could have been due to greater diagnostic delay in India than in the USA22. Multi-ethnic studies in high-income countries suggest that T2DM incidence is higher among populations of Indian origin than in populations of Chinese origin in the global diaspora. A Canadian population-based study reported nearly double the incidence of T2DM among individuals of south Asian origin (defined here as ethnic origin from India, Pakistan, Bangladesh or Sri Lanka) compared with individuals of Chinese origin23. Young-onset T2DM incidence among individuals of south Asian origin was triple that of individuals of Chinese origin aged 20–29 years (147.2 versus 46.9 per 100,000 patient-years), and more than double that of individuals of European origin aged 20–29 years (147.2 versus 67.1 per 100,000 patient-years)24. In a population-based international comparative study, T2DM incidence was highly heterogeneous among Chinese Canadians. People who had emigrated from the Chinese mainland, Hong Kong and Taiwan had respectively higher, similar and lower T2DM incidence rates compared with their origin populations25.
Pathophysiology
Insulin resistance and impairments in insulin secretion are crucial factors in the pathophysiology of T2DM26,27 (Box 2). Insulin is secreted by pancreatic β-cells in response to rising arterial concentrations of glucose28. First-phase insulin release peaks at 2–4 min after the initial rise in arterial levels of glucose and drops sharply by 10–15 min, while second-phase insulin release is more gradual, achieving a steady state at 2–3 h after the initial rise in arterial levels of glucose28. Under conditions of insulin resistance, β-cells are stimulated to secrete more insulin than under conditions of normal insulin sensitivity28. Insufficient insulin secretion, especially in the presence of insulin resistance, glucolipotoxicity and obesity-related inflammation, results in hyperglycaemia and, eventually, T2DM29.
Box 2 Indices of β-cell function.
The gold standard for quantifying insulin secretion and insulin resistance are the hyperglycaemic and euglycaemic–hyperinsulinaemic clamps, respectively287. During these tests, the concentration of glucose in the blood is ‘clamped’ at a constant concentration to reduce the confounding effects of prevailing blood levels of glucose on insulin action or secretion287. However, clamps are intensive and impractical to implement in large studies. Simpler surrogate measures have been developed using fasting blood tests that assess fasting levels of insulin and glucose in a steady state. The Homeostatic Model Assessment (HOMA) method is a mathematical model that indirectly estimates β-cell function (HOMA-β) or hepatic and peripheral insulin resistance (HOMA-IR) on the basis of fasting plasma levels of glucose and insulin or C-peptide concentrations288. There are various versions of the HOMA model288. The HOMA method can also quantify insulin sensitivity (HOMA-S), which is the reciprocal of HOMA-IR288. HOMA-β is modestly correlated with the gold standard (r = 0.69), whereas HOMA-IR is more strongly correlated with the gold standard (r = 0.88)288,289. It is important to note that HOMA and clamps reflect steady and dynamic states, respectively, and could therefore be viewed as complementary measures288. Other methods of measuring in vivo insulin secretion have been derived using stimulated measurements with intravenous glucose, oral glucose and mixed-meal tolerance tests. These methods are reviewed elsewhere289,290. In particular, the oral disposition index is a measure of insulin secretion corrected for insulin sensitivity, and this test generally yields similar results to the clamp studies289.
In large epidemiological studies, the decline in β-cell function over time might be indirectly quantified as glycaemic deterioration121,122,291,292. The rate of glycaemic deterioration is equivalent to the slope of HbA1c over time, after accounting for glucose-lowering drugs using statistical adjustment121. As with HOMA methods288, glycaemic deterioration cannot be estimated among people treated with exogenous insulin121.
β-Cell dysfunction and insulin resistance
Although the natural history of T2DM has been traditionally described as a process of increasing age-related insulin resistance followed by declining β-cell function and insufficient insulin secretion, it has been postulated that people predisposed to T2DM probably have a primary genetic factor that impairs β-cell function26,28,30. This hypothesis is based partly on studies that demonstrate reduced first-phase and second-phase insulin secretion among healthy, normoglycaemic and insulin-sensitive first-degree relatives of people with T2DM28. Accordingly, T2DM might develop among genetically predisposed people when obesity or other factors such as age increase insulin resistance to overwhelm their intrinsically limited β-cell insulin secretion.
Several studies in south Asian populations suggested that people of Indian origin develop early β-cell failure in response to increased insulin resistance. This concept was demonstrated in the prospective British Whitehall study of 5,749 white civil servants and 230 civil servants of south Asian origin without diabetes mellitus at baseline (1991–2009)31. In this cohort, the Homeostatic Model Assessment of insulin sensitivity (HOMA-S) was lower among people of south Asian origin than in white people, but declined with age in both groups. However, the increase in the Homeostatic Model Assessment of β-cell function (HOMA-β), which might reflect a compensatory response to insulin resistance, was much greater and peaked around 15 years earlier (at age 50–60 years) in people of south Asian origin than in white people. During the study, fasting blood levels of glucose remained constant among white people but increased linearly with time among people of south Asian origin, indicating that compensatory insulin secretion was insufficient to overcome insulin resistance. These differences were partially explained by higher rates of obesity, less healthy dietary patterns and lower socioeconomic status in the south Asian versus the white population. Similar findings were reported in the Netherlands32 and in the Mediators of Atherosclerosis in South Asians Living in America (MASALA) and the Multiethnic Study of Atherosclerosis (MESA) cohorts of US adults with or without diabetes mellitus33.
By contrast, many people of Chinese origin seem to exhibit progressive β-cell failure despite declining insulin resistance over time. In the MASALA and MESA cohorts, HOMA-β dropped sharply during adulthood up to age 60 years, followed by a steadier decline after this age, in the presence of steadily declining Homeostatic Model Assessment of insulin resistance (HOMA-IR) across all ages33. In a systematic review, east Asian people (defined here as having an ethnic origin from China, Japan, South Korea or Taiwan) had high sensitivity to insulin but a severely limited innate capacity to secrete insulin34. This limited capacity meant that even a small reduction in insulin secretion (for example, due to ageing or β-cell exhaustion) would result in T2DM.
Multi-ethnic comparisons
In the MASALA and MESA cohorts33, HOMA-β was statistically significantly lower in south Asian people than in Chinese people33. Insulin secretion among south Asian and Chinese people was the lowest among all ethnic groups. South Asian people had a higher HOMA-IR than white or Chinese people, especially at the ages of 40–60 years33. After age 60 years, white people had the highest insulin resistance among these ethnic groups. In the CARRS cohort of adults in India and Pakistan without diabetes mellitus at baseline, HOMA-β was statistically significantly lower in these south Asian people compared with Pima Indian people35 and Black, but not white, people in the USA36 after adjusting for age and BMI. In the Singapore Adults Metabolism Study, β-cell function among people of Indian origin was not statistically significantly different from that of people of Chinese origin among lean (BMI <23 kg/m2) individuals without diabetes mellitus. People of Indian origin also had higher insulin resistance (measured using the euglycaemic–hyperinsulinaemic clamp) than people of Chinese origin37.
Relative contributions of β-cell dysfunction and insulin resistance
Studies published during the past year suggest that decreased insulin secretion among Indian people could be more important than insulin resistance in predicting progression to T2DM. In the CARRS cohort, fewer Indian and Pakistani participants had obesity, and Indian and Pakistani participants had a lower HOMA-IR compared with age-matched Pima Indian people35, white people36 and Black people in the USA36. HOMA-IR also had a weaker association with incident diabetes mellitus among the south Asian CARRS cohort (adjusted hazard ratio (HR) 2.28–3.58)35 compared with Pima Indian people (HR 4.40, 95% CI 3.49–5.56)35. This association between HOMA-IR and incident diabetes mellitus was also weaker in the CARRS cohort of south Asian people (HR 2.67, 95% CI 2.05–3.48)36 compared with Black people (HR 14.55, 95% CI 11.11–19.07)36 or white people (HR 34.64, 95% CI 27.63–43.42)36. Interestingly, higher HOMA-β was associated with lower diabetes mellitus incidence among all of these populations. This association was weaker among south Asian people (HR 0.44, 95% CI 0.34–0.58) compared with Black people (HR 0.08, 95% CI 0.06–0.11) and white people (HR 0.05, 95% CI 0.04–0.06)36, but was similar among south Asian people (HR 0.35, 95% CI 0.28–0.44) and Pima Indian people (HR 0.34, 95% CI 0.26–0.43)35. In the MASALA study, insulin secretion, measured using the oral disposition index (Box 2), was associated with progression to pre-diabetes or T2DM after a mean of 2.5 years, while the insulin sensitivity index did not significantly predict incident pre-diabetes or T2DM after accounting for the level of visceral adipose tissue38. In Chennai, the Diabetes Community Lifestyle Improvement programme reported that the contribution of impaired β-cell function to impaired fasting glucose (IFG) and impaired glucose tolerance (IGT) was greater than that of insulin resistance, suggesting that β-cell dysfunction was the main contributor to IFG and IGT39.
Studies in Chinese populations report mixed findings on the relative contributions of impaired insulin secretion and insulin resistance to T2DM pathogenesis. In Shanghai, cross-sectional studies reported worse β-cell function in people with T2DM compared with individuals with pre-diabetes, whereas insulin sensitivity was similar across groups40,41. Studies in Nanjing city42 and Zhejiang province43 found that both β-cell function and insulin resistance were worse among people with T2DM compared with people with pre-diabetes or normal glucose tolerance. Furthermore, in Zhejiang, insulin resistance was associated with increased odds ratio of pre-diabetes, whereas impaired β-cell function was associated with increased odds ratio of T2DM43. The nationwide China Cardiometabolic Disease and Cancer Cohort Study of 94,952 people found that a greater proportion of incident diabetes mellitus cases were attributable to HOMA-IR (24.4%, 95% CI 23.6–25.2%) than to HOMA-β (12.4%, 95% CI 11.2–13.7%)44 and that small increases in body weight were associated with increased risks of diabetes mellitus44. Although these studies seem to be conflicting, their interpretation is limited by potential confounders, including socioeconomic and health-related behavioural changes over time and the lack of dynamic insulin secretion indices.
Distinct pathways of T2DM development
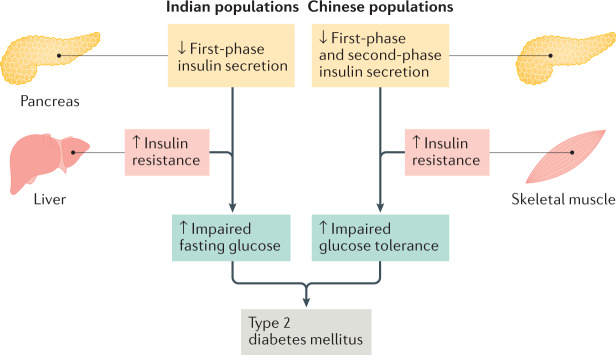
An indirect approach to characterizing the development of T2DM involves examining epidemiological patterns of IFG and IGT, which are different states of pre-diabetes associated with distinct metabolic abnormalities and a high risk of progression to T2DM45–47 (Fig. 2). In India, there is a preponderance of isolated IFG relative to isolated IGT (prevalence rate of 6.5%, 95% CI 6.3–6.7% and 2.8%, 95% CI 2.7–3.0%, respectively, in most states)48. This pattern is similar to white, Black and Hispanic populations in the USA, in which IFG is more common than IGT49. IFG is characterized by hepatic insulin resistance causing increased hepatic gluconeogenesis and reduced first-phase insulin secretion38,46,50. These changes are consistent with the observation of elevated HOMA-IR, a key indicator of hepatic insulin resistance, among people with IFG versus those with normoglycaemia46. Increased hepatic lipid accumulation might have an especially important role in Indian people51. In a multi-ethnic study of lean, healthy men in the USA, increased insulin resistance among people of Indian origin (relative to people who are not Asian and people of east Asian origin) was associated with increased hepatic triglyceride content, suggesting a predisposition to hepatic steatosis and hepatic insulin resistance52.
Fig. 2. Distinctive pathways of T2DM development commonly observed among Indian and Chinese populations.
This conceptualization is based on the epidemiological patterns of impaired fasting glucose (IFG) versus impaired glucose tolerance (IGT) prevalence observed in India and China, the known pancreatic β-cell defects associated with IFG and IGT and evidence from single-ethnic and multi-ethnic studies. In Indian populations, β-cell dysfunction often causes reduced first-phase insulin secretion. In the presence of increased insulin resistance, especially hepatic insulin resistance, Indian people have an increased risk of developing IFG, which might ultimately lead to type 2 diabetes mellitus (T2DM). In Chinese populations, β-cell dysfunction might predispose to globally impaired insulin secretion (reduced first-phase and second-phase insulin secretion). In the presence of insulin resistance, especially skeletal muscle insulin resistance, Chinese people have an increased risk of developing IGT, which might lead to T2DM. Hepatic insulin resistance and adiposity can be more severe in Indian people than in Chinese people, thus exacerbating insulin deficiency and causing relatively early (before age 40 years) and increased incidence of T2DM. Given the heterogeneous nature of T2DM pathogenesis, these features sometimes intersect within or vary between Indian and Chinese individuals.
By contrast, China has a much higher prevalence of isolated IGT (11.5%, 95% CI 10.5–12.7) than isolated IFG (2.4%, 95% CI 2.0–2.9)53. IGT is characterized by skeletal muscle insulin resistance and changes in both first-phase and second-phase insulin secretion46,50,54, a finding that has been specifically confirmed in Chinese people55. Hyperinsulinaemic–euglycaemic clamp studies have demonstrated that insulin-stimulated glucose disposal, a key indicator of muscle insulin sensitivity, is reduced in IGT across ethnicities46. Furthermore, IGT could be associated with an increased risk of cardiometabolic disease compared with IFG among Chinese people56,57 and other populations54. IFG and IGT might arise from distinct β-cell abnormalities50 and confer different risks of progression to T2DM54. These issues require further characterization in Chinese and Indian populations.
Pathophysiological mechanisms
Genetic architecture
Genome-wide association studies (GWAS) have revealed insights into the pathophysiological mechanisms of T2DM, which is a complex polygenic disease. Most of the >500 currently identified genetic loci for T2DM58–60 are related to β-cell function or mass61. Most common genetic variants are similar across populations of south Asian, east Asian and European ancestry, suggesting that genetic susceptibility to T2DM is mostly shared across ethnicities62–65. However, some alleles vary in frequency across populations, and ancestry-specific genetic variants might partly explain some features of T2DM among Indian and Chinese populations. A south Asian GWAS that involved 5,700 Indian participants identified six novel genetic loci, including HNF4A (linked to defective insulin secretion in maturity-onset diabetes of the young type 1), GRB14 (which encodes a protein that binds to the insulin receptor and inhibits signalling) and ST6GAL1 (which encodes a protein that might influence insulin action)64. Other novel loci include SGCG (which encodes a protein expressed in skeletal muscle) among Indian Punjabi Sikh people66 and TMEM163 (which encodes a synaptic vesicle membrane protein linked to insulin secretion) among Indian people65. In the UK Biobank, people of south Asian origin had a lower frequency of genetic variants predisposing to subcutaneous adiposity compared with the white population, which might predispose them to increased visceral adiposity and adipocyte hypertrophy, resulting in β-cell glucolipotoxicity67. Because the population of India is particularly genetically complex owing to endogamy and diverse sociolinguistic groupings68, large sample sizes are required to ensure adequately powered GWAS and to stratify by Indian subpopulation69.
The unique genetic variants for T2DM in Indian populations seem to be distinct from those in Chinese populations. Large GWAS have confirmed that genetic loci such as PAX4 (which encodes a pancreatic islet transcription factor), PSMD6 and ZFAND3 (which encode proteins involved in insulin secretion), NID2 (associated with lipodystrophy traits or body adipose tissue distribution after adjustment for BMI) and ALDH2 (which encodes an enzyme for alcohol metabolism associated with T2DM in men) are unusually frequent among east Asian people60,63,70, who might have limited β-cell regenerative capacity71. In particular, polymorphisms of PSMD6 and PAX4 (which appears to be monomorphic in people who are not east Asian60) might reduce insulin secretion and β-cell functional mass, respectively72. These polymorphisms might also predict responses to rosiglitazone in Chinese populations (rosiglitazone is no longer used in most regions over concerns about increased cardiovascular risk73)74,75. Other studies suggest that genetic variants might predict responses to metformin and time to insulin therapy76, but further pharmacogenetic studies are required77.
Early life environment
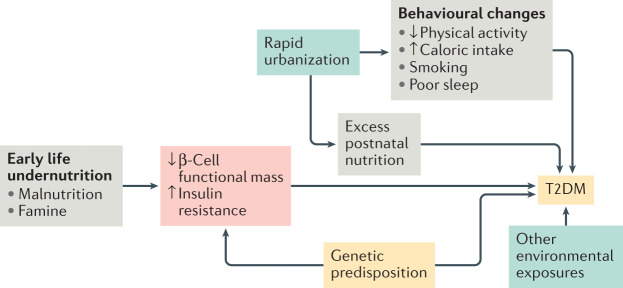
According to the developmental origins of health and disease ‘thrifty phenotype’ hypothesis, intrauterine and early life exposure to malnutrition followed by an energy-rich setting in later life results in a high risk of T2DM78 (Fig. 3). Major events such as the great Bengal famine (1943)79 and Chinese famine (1959–1961)80 affected millions of Indian and Chinese people, respectively. Fetal and childhood exposure to the Chinese famine, followed by high socioeconomic status during adulthood, were associated with increased T2DM incidence in several studies80,81. The effect of famine and malnutrition might be mediated by epigenetic changes82,83, decreased pancreatic β-cell proliferation and increased insulin resistance compared with individuals not exposed to famine or malnutrition84. In India, the prospective Pune Maternal Nutrition Study demonstrated that maternal undernutrition and vitamin B12 deficiency predicted low birthweight, childhood adiposity and childhood insulin resistance85. However, paternal T2DM is also associated with low birthweight86. This observation suggests that a genetic predisposition to insulin deficiency also partly explains the relationship between low birthweight and T2DM risk (the fetal insulin hypothesis)87. Conversely, maternal hyperglycaemia and obesity are associated with higher birthweight and increased risk of T2DM82 compared with mothers without hyperglycaemia or obesity during pregnancy. This association was demonstrated in the prospective Mysore Parthenon Birth Cohort Study, which reported increased childhood adiposity and insulin resistance among offspring of mothers with T2DM compared with those from mothers without T2DM88.
Fig. 3. Key factors influencing the development of T2DM.
According to the developmental origins of health and disease hypothesis, fetal or early life exposure to undernutrition results in low birthweight and adaptive responses characterized by reduced β-cell secretion and increased insulin resistance. Genetic predisposition to type 2 diabetes mellitus (T2DM) probably also contributes to these changes, as proposed by the fetal insulin hypothesis. In the context of the rapid pace of urbanization in India and China, the postnatal environment often provides excess nutrition, which further exacerbates the risk of T2DM. Health-related behaviours such as low levels of physical activity, high caloric intake, poor sleep and smoking independently raise the risk of T2DM255–258. Some genetic variants that predispose to T2DM seem to be unique among populations of east and south Asian ancestry. However, currently identified genetic variants explain <10% of the estimated 30–70% heritability of T2DM263–265. This figure is not exhaustive; concepts have been simplified here for the purposes of illustration.
Islet amyloid
Amyloid deposits within the pancreatic islets might have a role in T2DM pathogenesis by accelerating β-cell apoptosis26,89,90. An estimated 40–100% of individuals with T2DM have islet amyloid deposits90. A Chinese autopsy study demonstrated that individuals with T2DM were statistically significantly more likely to have islet amyloid deposits at autopsy than individuals without T2DM91. High dietary fat intake (which appears to be particularly common in India and China92) might increase islet secretion of amyloid89,90. Genetic studies investigating a link between T2DM risk and mutations of the islet amyloid polypeptide (also known as amylin) gene have shown that the S20G mutation is associated with T2DM and an earlier age at T2DM diagnosis (age <40 years) among Chinese93 and other east Asian populations90, but not in European-origin and Indian populations90,94. Animal studies suggest that this mutation might reduce the number and function of β-cells95.
Gut microbiome
Emerging evidence suggests that gut microbial composition might affect T2DM risk by modulating inflammation and affecting gut permeability, insulin resistance, carbohydrate processing and fatty acid oxidation96. Local differences in diet and environment probably contribute to unique gut microbial signatures among Indian97 and Chinese98 populations. These differences could affect T2DM risk and responses to glucose-lowering drugs97,98.
Infection
Infections increase insulin resistance by activating inflammatory mediators (for example, tumour necrosis factor-α (TNFα) and C-reactive protein) and could have a role in T2DM pathogenesis99,100. Hepatitis B infection is common in India and China, but, unlike hepatitis C infection, has not been consistently associated with T2DM in these populations101,102. Coronavirus disease 2019 (COVID-19) infection increases insulin resistance among previously normoglycaemic individuals and might predispose to future T2DM development103. Limited evidence suggests that tuberculosis and its treatments might increase the risk of T2DM104,105. Helicobacter pylori infection has been inconsistently linked to T2DM in Chinese populations106,107.
Phenotypes
Subgroups of diabetes mellitus
In the Swedish All New Diabetics in Scania (ANDIS) cohort108, all incident cases of diabetes mellitus were classified into five subgroups using a cluster analysis based on specific phenotypic characteristics: age at diagnosis, BMI, glutamic acid decarboxylase (GAD) antibodies, HOMA-β and HOMA-IR. The severe autoimmune diabetes mellitus subgroup consisted of people with type 1 diabetes mellitus (T1DM) and latent autoimmune diabetes mellitus of adulthood, and T2DM was divided into subgroups hypothesized to relate to disease aetiology: severe insulin-deficient diabetes mellitus, severe insulin-resistant diabetes mellitus, mild obesity-related diabetes mellitus and mild age-related diabetes mellitus108.
Although this method of classification has been criticized as oversimplified109, comparing the clinical characteristics across subgroups and populations suggests distinct phenotypes of diabetes mellitus in Indian and Chinese people (Table 1). In India, a cluster-based analysis was performed among people with diabetes mellitus (disease duration <5 years) identified within private clinics across nine states110. Although GAD antibodies were unavailable to identify severe autoimmune diabetes mellitus, when compared with the ANDIS cohort, severe insulin-deficient diabetes mellitus occurred more often (26.2% versus 17.5%), at a younger age (mean 42.5 years versus 56.7 years), at a lower BMI (mean 24.9 kg/m2 versus 28.9 kg/m2) and with lower levels of β-cell function and insulin resistance within this Indian cohort. Individuals within the remaining subgroups were also younger at diagnosis, with lower BMI and lower β-cell function compared with the ANDIS cohort. In particular, the Indian subgroup with severe insulin resistance also had low β-cell function, a characteristic not observed in the Swedish subgroup with severe insulin-resistant diabetes mellitus (mean HOMA-β 64.5 versus 150.5). Indian people with obesity-related diabetes mellitus also had higher insulin resistance compared with Swedish people in this subgroup (mean HOMA-IR 4.1 versus 3.4). Although selection bias was a potential limitation, these findings appeared consistent when validated among a larger community-dwelling population sampled across 15 Indian states110.
Table 1.
Phenotypic characteristics among subgroups of diabetes mellitus identified in Indian, Chinese and Swedish cohorts
| Parameter | Sweden | India | Chinaa | |||
|---|---|---|---|---|---|---|
| ANDIS | INSPIRED110 | INDIAB110 | Li et al.111 | CNDMDS114 | Xiong et al.112 b | |
| Sample size (n) | 8,980 | 19,084 | 2,204 | 15,772 | 2,316 | 5,414 |
| Newly diagnosed | Yes | No | No | Yes | Yes | No |
| Disease duration (years; mean (s.d.)) | 0 (0.0) | <5 | Unknown | 0 (0.0) | 0 (0.0) | 8.6 (6.3) |
| Setting | Clinic | Clinic | Survey | Clinic | Survey | Clinic |
| Location | Scania | Nine states | Fifteen states | National | National | Hunan |
| Severe autoimmune diabetes mellitus | ||||||
| Frequency (%) | 6.4 | NA | NA | 6.2 | NA | 3.7 |
| Age at diagnosis (years; mean (s.d.)) | 50.5 (17.9) | NA | NA | 42.7 (14.0) | NA | 49.3 (12.1) |
| HbA1c (%; mean (s.d.)) | 9.5 (2.8) | NA | NA | 10.7 (5.3) | NA | 8.9 (2.4) |
| BMI (kg/m2; mean (s.d.)) | 27.5 (6.4) | NA | NA | 22.0 (3.8) | NA | 24.3 (3.9) |
| HOMA-β (mean (s.d.)) | 56.7 (44.7) | NA | NA | 21.9 | NA | 84.0 (112.8) |
| HOMA-IR (mean (s.d.)) | 2.2 (1.6) | NA | NA | 0.7 | NA | 3.7 (7.9) |
| Severe insulin-deficient diabetes mellitus | ||||||
| Frequency (%) | 17.5 | 26.2 | 27.4 | 24.8 | 13.5 | 41.2 |
| Age at diagnosis (years; mean (s.d.)) | 56.7 (11.1) | 42.5 (10.8) | 40.1 (9.8) | 50.5 (11.6) | 52.4 (11.9) | 46.6 (10.7) |
| HbA1c (%; mean (s.d.)) | 11.5 (1.8) | 10.7 (2.1) | 10.0 (2.1) | 12.5 (4.0) | NA | 10.2 (1.9) |
| BMI (kg/m2; mean (s.d.)) | 28.9 (4.8) | 24.9 (3.5) | 22.7 (3.1) | 22.5 (2.6) | 25.4 (3.2) | 25.0 (3.7) |
| HOMA-β (mean (s.d.)) | 47.6 (28.9) | 38.8 (26.9) | NA | 20.2 | NA | 32.2 (19.5) |
| HOMA-IR (mean (s.d.)) | 3.2 (1.7) | 2.8 (1.6) | NA | 1.1 | NA | 1.3 (0.8) |
| Severe insulin-resistant diabetes mellitusc | ||||||
| Frequency (%) | 15.3 | 12.1 | 7.6 | 16.6 | 8.6 | NA |
| Age at diagnosis (years; mean (s.d.)) | 65.3 (9.3) | 42.1 (9.8) | 45.4 (10.2) | 51.8 (11.0) | 47.4 (13.4) | NA |
| HbA1c (%; mean (s.d.)) | 7.1 (3.6) | 9.1 (1.9) | 9.0 (2.0) | 7.2 (3.6) | NA | NA |
| BMI (kg/m2; mean (s.d.)) | 33.9 (5.2) | 26.5 (3.1) | 25.0 (2.9) | 27.0 (3.2) | 27.8 (4.3) | NA |
| HOMA-β (mean (s.d.)) | 150.5 (47.2) | 64.5 (37.7) | NA | 98.6 | NA | NA |
| HOMA-IR (mean (s.d.)) | 5.5 (2.7) | 3.8 (1.9) | NA | 2.2 | NA | NA |
| Moderate obesity-related diabetes mellitusc | ||||||
| Frequency (%) | 21.6 | 25.9 | 30.3 | 21.6 | 32.7 | NA |
| Age at diagnosis (years; mean (s.d.)) | 49.0 (9.5) | 46.5 (10.4) | 48.2 (9.6) | 39.1 (10.2) | 52.1 (11.7) | NA |
| HbA1c (%; mean (s.d.)) | 7.4 (3.6) | 8.3 (1.8) | 7.9 (1.8) | 10.1 (4.1) | NA | NA |
| BMI (kg/m2; mean (s.d.)) | 35.7 (5.4) | 32.6 (4.1) | 29.9 (3.6) | 27.9 (3.0) | 29.3 (2.7) | NA |
| HOMA-β (mean (s.d.)) | 95.0 (32.5) | 100.8 (51.5) | NA | 36.0 | NA | NA |
| HOMA-IR (mean (s.d.)) | 3.4 (1.2) | 4.1 (1.5) | NA | 1.8 | NA | NA |
| Mild age-related diabetes mellitus | ||||||
| Frequency (%) | 39.1 | 35.8 | 34.8 | 30.9 | 45.1 | 55.1 |
| Age at diagnosis (years; mean (s.d.)) | 67.4 (8.6) | 50.2 (10.3) | 55.5 (9.8) | 54.8 (9.8) | 53.2 (12.7) | 53.6 (10.0) |
| HbA1c (%; mean (s.d.)) | 6.7 (3.1) | 7.2 (1.2) | 6.7 (1.2) | 7.6 (3.5) | NA | 7.1 (1.1) |
| BMI (kg/m2; mean (s.d.)) | 27.9 (3.4) | 25.9 (2.9) | 23.4 (2.8) | 23.4 (2.6) | 23.3 (2.3) | 24.7 (3.5) |
| HOMA-β (mean (s.d.)) | 86.6 (26.4) | 94.1 (43.1) | NA | 48.8 | NA | 82.6 (42.6) |
| HOMA-IR (mean (s.d.)) | 2.6 (0.8) | 2.6 (0.8) | NA | 1.3 | NA | 1.5 (0.8) |
Cohorts included people with newly diagnosed or established diabetes mellitus as indicated. Among cohorts with established diabetes mellitus, age at diagnosis was ascertained retrospectively, and biomarkers were measured at variable times after diagnosis (indicated by disease duration). Homeostatic Model Assessment (HOMA) values included in this table were all calculated using C-peptide values. ANDIS, All New Diabetics in Scania; HOMA-β, Homeostatic Model Assessment of β-cell function; HOMA-IR, Homeostatic Model Assessment of insulin resistance; INDIAB, Indian Council of Medical Research-India Diabetes study; INSPIRED, India–Scotland Partnership for Precision Medicine in Diabetes project; CNDMDS, China National Diabetes and Metabolic Disorders Study; NA, not available; s.d., standard deviation. aThe study by Wang et al.113 was excluded from this table owing to data unavailability. bStandardized by sex. cThe subgroup originally characterized by Anjana et al.110 as ‘combined insulin resistant and deficient diabetes’ in both Indian cohorts is referred to in this table and the text as severe insulin-resistant diabetes mellitus for consistency and ease of comparison. Similarly, the subgroup originally characterized as ‘insulin-resistant obese diabetes’ is referred to as moderate obesity-related diabetes mellitus. The distinctive characteristics of these subgroups are reviewed in the text.
In China, cluster-based analyses similarly revealed low levels of HOMA-β, HOMA-IR and BMI across subgroups, with younger ages at diagnosis and an increased frequency of severe insulin-deficient diabetes mellitus in some111–113, but not all114, cohorts compared with the ANDIS cohort. In a 2020 study (the largest study conducted in clinics across China)111, all subgroups had lower levels of HOMA-β, HOMA-IR and BMI compared with the ANDIS cohort. Severe autoimmune diabetes mellitus occurred at a younger age (mean 42.7 years) than in Swedish people (mean 50.5 years). As with Indian people, severe insulin-deficient diabetes mellitus was more common (24.8% versus 17.5%) than in the ANDIS cohort and occurred >5 years earlier in life. Severe insulin-resistant and moderate obesity-related diabetes mellitus were diagnosed at least a decade earlier in life than in ANDIS. Finally, the mild age-related diabetes mellitus subgroup was less frequent and was diagnosed a decade earlier compared with ANDIS. In the China National Diabetes and Metabolic Disorders Study (CNDMDS), people with newly diagnosed diabetes mellitus were identified in a nationally representative survey, but autoantibodies were unavailable and surrogate measures were used for HbA1c and C-peptide114. The findings were broadly similar to those of the 2020 study111, except for lower proportions of severe insulin-deficient diabetes mellitus and severe insulin-resistant diabetes mellitus, higher proportions of moderate obesity-related diabetes mellitus and mild age-related diabetes mellitus, and older age at diagnosis of moderate obesity-related diabetes mellitus114. A similar study among hospital inpatients in Beijing reported younger age at diagnosis for severe insulin-deficient diabetes mellitus, but disease durations were unreported113. In Hunan, a clinic-based study of people with long disease duration was unable to replicate the ANDIS subgroups112.
In comparison with the Chinese cohorts, it appears that Indian people have generally higher levels of insulin resistance across subgroups, along with higher levels of insulin secretion across most subgroups110–112,114. Severe insulin-deficient diabetes mellitus presented earlier in life (around age 40 years), at a similar BMI, but with higher HOMA-β and HOMA-IR in the Indian cohort than in the Chinese cohorts110–112,114. Severe insulin-resistant diabetes mellitus also presented earlier in life, but with much lower HOMA-β in the context of higher HOMA-IR in Indian people compared with Chinese people110–112,114. Moderate obesity-related diabetes mellitus occurred with a similar frequency but with higher BMI, HOMA-β and HOMA-IR values among Indian cohorts compared with Chinese cohorts110–112,114. Mild age-related diabetes mellitus featured much higher HOMA-β and HOMA-IR among Indian people, but was otherwise generally similar to that seen in Chinese people110–112,114.
These Indian and Chinese cohorts generally featured younger ages at diagnosis compared with the ANDIS cohort108,110–112,114. Although these findings seem to conflict with previous multi-ethnic studies in high-income countries that report a higher incidence of young-onset T2DM among people of Indian origin relative to people of European or Chinese origin23,24, immigrant populations are heterogeneous and vary from the populations from which they emigrated according to socioeconomic status and other factors25. Moreover, young-onset T2DM incidence in Canada among men who have emigrated from China has risen over the past decade to surpass that seen in people who are not Chinese Canadian as of 2017 (ref.25). Although selection bias could have inflated the proportion of younger individuals in the clinic-based or hospital-based Chinese studies, new cases of diabetes mellitus in the nationally representative CNDMDS cohort were similarly diagnosed at younger ages compared with the ANDIS cohort111,112. Further research is required to confirm whether these data reflect a skewed distribution towards earlier age at T2DM diagnosis in Chinese people.
One US multi-ethnic cluster-based study included people aged ≥45 years in the MASALA and MESA cohorts examined on average 5.7 years after diagnosis with diabetes mellitus (standard deviation (s.d.) 7.8 years), without data on GAD antibodies115. People taking insulin were excluded from the cluster analysis and manually classified on the basis of age at diagnosis. Although the results were not directly comparable to those of ANDIS, five unique subgroups were identified: older age at onset (43%), severe hyperglycaemia (26%), severe obesity (20%), younger age at onset (1%) and requiring insulin medication use (9%). People of Chinese origin were more likely to be classified in the older age at onset subgroup than people of other ethnicities, and less likely to be classified in the severe obesity subgroups, while people of south Asian origin were most likely to be classified in the severe hyperglycaemia subgroup. Although the authors suggested that different metabolic processes might be occurring among south Asian and Chinese populations, further multi-ethnic studies are required to explore this possibility.
Young-onset type 2 diabetes mellitus
Phenotypes of T2DM have also been classified by age at diagnosis10,116. Young-onset T2DM is especially important in Indian and Chinese populations because of their tendency to be diagnosed at an early age (<40 years)110–112,114, as well as the aggressive course and poor outcomes of this condition10.
Rapid β-cell dysfunction
In the Treatment Options for Type 2 Diabetes in Adolescents and Youth (TODAY) study of 699 mostly Black, white and Hispanic youth with young-onset T2DM (mean age 13.8–14.1 years) randomized to receive metformin with or without rosiglitazone or basal insulin, the oral disposition index declined by 20–35% per year, which is a much faster decline in insulin secretion than that seen in older populations117. For example, the United Kingdom Prospective Diabetes Study (UKPDS; mean age 52–53 years) reported that HOMA-β declined by approximately 7% per year in people with newly diagnosed T2DM118,119. In A Diabetes Outcome Progression Trial (ADOPT), HOMA-β declined by approximately 7–11% per year in mostly white, Black and Hispanic adults with T2DM (mean age 56.3–57.9 years) randomized to receive rosiglitazone, metformin or glyburide monotherapy120. The percentage decline in HOMA-β was equivalent to a glycaemic deterioration rate of around 0.07–0.14% per year120, which is similar in magnitude to that of a Scottish observational cohort121.
In China, multiple lines of evidence have confirmed the accelerated rates of β-cell decline in young-onset T2DM. In a Hong Kong population-based study of adults aged 18–75 years, the steepest rates of glycaemic deterioration were among those with young-onset T2DM, especially those diagnosed at age 20 years122. Aggressive glycaemic deterioration in young-onset T2DM contributed to persistently increased HbA1c levels and more than triple the cumulative exposure to hyperglycaemia from diagnosis until age 75 years, compared with usual-onset T2DM (defined here as age at diagnosis ≥40 years)122. A national Chinese cross-sectional survey of people with newly diagnosed T2DM showed that HOMA-β was similar across ages at diagnosis, but the difference in HOMA-β between people with T2DM and age-matched control individuals was much greater among people with young-onset than in people with usual-onset T2DM123. Furthermore, people with young-onset T2DM had the most abnormal HOMA-IR and oral disposition index out of all the groups in the study123. Similarly, a study conducted in India of 82 people aged <25 years with either young-onset T2DM, pre-diabetes or normoglycaemia showed that T2DM was associated with a lower oral disposition index, but not insulin resistance, after adjustment for age and obesity124.
High risk of complications
The Emerging Risk Factors Collaboration estimated that people with usual-onset T2DM might die up to 6 years earlier than those without T2DM125, but an analysis of the Swedish National Diabetes Registry estimated that those diagnosed with young-onset T2DM at age 15 years might lose 12 years of life compared with people without T2DM126. This increased risk of mortality is driven by an elevated risk of T2DM complications compared with usual-onset T2DM127–130.
In China, a national cross-sectional study of 200,000 people with T2DM who were identified using the China National HbA1c Surveillance System reported that those with young-onset T2DM had an increased prevalence of cardiovascular disease (OR 1.91, 95% CI 1.81–2.02)131. In a subset of this population, the prevalence of diabetic kidney disease was also higher among people with young-onset T2DM (5.1%) than those with usual-onset T2DM (1.5%; OR 1.69, 95% CI 1.46–1.95)132. In Hong Kong, young-onset T2DM was associated with an increased risk of cardiovascular disease (HR 1.48, 95% CI 1.17–1.88) and diabetic kidney disease (HR 1.35, 95% CI 1.12–1.62) versus usual-onset T2DM133. Furthermore, young-onset T2DM was associated with an increased risk of hospitalization owing to mental illness, with 36.1% of all hospitalizations of people with young-onset T2DM before age 40 years attributable to psychiatric conditions, including psychotic disorders and mood disorders134. In these studies, the increased risk of complications associated with a younger age at diagnosis was not entirely explained by longer disease duration131–134. The prevalence of complications in China varies along a north to south gradient, with higher rates among northern Chinese populations than southern Chinese populations for both young-onset and usual-onset T2DM135.
In India, the epidemiology of complications in young-onset T2DM is described at the local and regional level. In a large diabetes mellitus centre in Chennai, 267 people with young-onset T2DM diagnosed before age 25 years had a similar odds ratio of having diabetic nephropathy (OR 1.07, 95% CI 0.66–1.73) and higher odds ratio of having diabetic retinopathy (OR 2.02, 95% CI 1.38–2.95) compared with 267 people with usual-onset T2DM diagnosed after age 50 years, and metabolic risk factors were more poorly controlled in the young-onset T2DM group136. In the same centre, a study of people diagnosed with diabetes mellitus at age 10–25 years found that people with young-onset T2DM had double the odds ratio of having any microvascular or macrovascular complication (OR 2.11, 95% CI 1.27–3.51) compared with people who had T1DM137. A subsequent study estimated that the incidence of diabetic nephropathy was 13.8 per 1,000 person-years in T2DM compared with 6.2 per 1,000 person-years in T1DM (attained age <20 years)138.
Few international studies have systematically compared complication rates specifically among people with young-onset T2DM in India and China. In a cross-sectional study of people with T2DM from outpatient clinics across Asia (n = 5,646 (China, public clinics), 15,341 (Hong Kong, public clinics), 9,107 (India, private clinics)), the Joint Asia Diabetes Evaluation programme found that Indian people with young-onset T2DM had a higher prevalence of ischaemic heart disease (India, 8.3%; China, 2.8%; Hong Kong, 5.8%) and diabetic kidney disease (India, 19.7%; China, 1.3%; Hong Kong, 5.7%) than Chinese people; however, differences in disease duration (median: 9.0 years (India), 4.0 years (China) and 14.0 years (Hong Kong)) might have had a role139. The prevalence of stroke was higher among Chinese people than in Indian people with young-onset T2DM (China, 1.5%; Hong Kong, 3.3%; India, 0.7%)139.
Emerging management strategies
Clinical recommendations for management of young-onset T2DM lack evidence because most intervention studies have been conducted in people with usual-onset T2DM140,141. In China, short-term intensive insulin therapy is a standard treatment option for newly diagnosed T2DM142. Younger age at diagnosis predicted a reduced rate of glycaemic relapse (HR 0.75, 95% CI 0.58–0.95 for every 10 years) after 2 weeks of continuous subcutaneous insulin infusion among 124 Chinese adults with newly diagnosed T2DM (mean age 47.33 ± 9.45 years)143. A retrospective analysis of a randomized controlled trial that compared subcutaneous insulin infusion therapy with or without metformin among people with newly diagnosed T2DM showed that people with young-onset T2DM treated with metformin required lower doses of insulin than people with usual-onset T2DM. This finding suggests that people with young-onset T2DM might be more sensitive to metformin than people with usual-onset T2DM144. By contrast, an observational study of adults in Hong Kong showed that metformin alone or in combination with other drugs was associated with a similar degree of glycaemic lowering among people with young-onset T2DM and usual-onset T2DM122. In the TODAY study, metformin monotherapy was associated with treatment failure in 51.7% of people with young-onset T2DM diagnosed at age 10–17 years, with lower treatment failure rates when metformin was combined with rosiglitazone (38.6%) or behavioural intervention (46.6%)145. In the Restoring Insulin Secretion study, treatment for 3 months with insulin glargine followed by 9 months with metformin improved β-cell function compared with metformin monotherapy among adults diagnosed with T2DM at ages 20–65 years, but not among those diagnosed at ages 10–19 years146.
Additionally, early combination therapy with multiple glucose-lowering agents147 is a promising strategy for young-onset T2DM. In a 5-year randomized clinical trial, early treatment with metformin and the dipeptidyl peptidase 4 (DPP4) inhibitor vildagliptin led to greater and more durable glycaemic lowering compared with a sequential strategy of metformin followed by augmentation with vildagliptin148,149. These effects were similar in Asian people and people who are not Asian148, and the benefit was even greater in young-onset T2DM than usual-onset T2DM (48% versus 24% reduction in risk of treatment failure)150.
Pharmacological glycaemic management
Reducing the incidence of microvascular and macrovascular complications requires optimal glycaemic control, a fundamental cornerstone of multifactorial T2DM management151. Multiple classes of non-insulin pharmacological agent are commonly used to treat hyperglycaemia. In accordance with their distinctive pathophysiology and phenotypes, Indian and Chinese people with T2DM respond differently to many of these medications compared with other populations (Table 2).
Table 2.
Characteristics and consensus recommendations for the use of non-insulin glucose-lowering drugs in Indian and Chinese populations
| Drug class | Drug action | Glycaemic lowering | Other characteristics | Consensus guidelines and recommendations | ||
|---|---|---|---|---|---|---|
| India217 | China142 | USA and Europe218 | ||||
| Metformin (biguanide) | Activates AMP-activated protein kinase, reduces hepatic glucose production, enhances insulin-mediated glucose uptake, increases glucose utilization and GLP1 secretion in the gut without weight gain or hypoglycaemia219–221 | Probably efficacious in Asian people153, but there have been no studies comparing the effects of metformin in Asian populations and populations that were not Asian | Improves β-cell function among Indian154 and Chinese155 people | First-line monotherapy (HbA1c <9%) or as component of dual oral therapy (asymptomatic HbA1c >9%)217 | Preferred first-line monotherapy222 | First-line monotherapy218 |
| α-Glucosidase inhibitors | Delay carbohydrate absorption in the small bowel, thus reducing postprandial glucose excursion and postprandial insulin secretion223 | Glycaemic-lowering efficacy is similar to that of metformin156 and DPP4 inhibitors157 | Fewer gastrointestinal adverse effects (specifically diarrhoea) in east Asian populations than people who are not Asian163 | Second-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217 | Less preferred as first-line monotherapy than metformin142 | Not specifically recommended218 |
| Real-world glucose-lowering effectiveness might be better in Chinese (and other east and southeast Asian) people than Indian (and other south Asian) and European people161 | Better weight reduction than metformin or DPP4 inhibitor among Chinese people156,157 | Second-line or third-line therapy in combination with metformin142 | ||||
| Sulfonylureas | Reduce blood levels of glucose in a glucose-independent manner by binding to the SUR and closing the ATP-sensitive potassium channels, thus stimulating β-cell insulin secretion165 | Gliclazide reduces blood levels of glucose efficaciously in Asian populations and populations that were not Asian172 | Glyburide accelerates β-cell decline, especially in severe insulin-deficient diabetes mellitus (no studies in Asian people)116 | First-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217 | Less preferred as first-line monotherapy than metformin142 | Second-line or third-line therapy in situations in which cost is a major issue218 |
| Probably no effect on the risk of cardiovascular disease224 | Greater glycaemic-lowering effectiveness than thiazolidinediones for men with BMI <30 kg/m2 among people of European origin (no studies in Asian people)174 | Probably do not increase the risk of cardiovascular disease across Asian populations and populations that are not Asian225 | Consider as an initial monotherapy (especially gliclazide167) if a patient has a contraindication or intolerance to metformin166 | Second-line or third-line therapy in combination with metformin142 | ||
| DPP4 inhibitors | Inhibit DPP4, an enzyme that catalyses the degradation of incretins such as GLP1 and gastric inhibitory polypeptide175,176 | Sitagliptin has greater glucose-lowering efficacy in Asian populations than in populations that are not Asian; these182 benefits to Asian populations might apply to all DPP4 inhibitors185,186 | Less improvement in β-cell function among Asian populations compared with populations that were not Asian187 | First-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217 | Second-line or third-line therapy in combination with metformin142 | Second-line or third-line therapy for individuals with a compelling need to minimize hypoglycaemia218 |
| No effect on cardiovascular events224,226 | Higher insulin resistance associated with less-effective glucose lowering among people of European origin (no studies in Asian people)183 | |||||
| Thiazolidinediones | Decrease insulin resistance by activating the peroxisome proliferator-activated receptor-γ, stimulating differentiation of pre-adipocytes to adipocytes, shifting adipose tissue distribution from visceral to subcutaneous, ameliorating glucolipotoxicity and inflammation227 | Probably efficacious in Asian people189, but no studies comparing effects with people who were not Asian | Improved insulin sensitivity and pancreatic β-cell function among Indian190 and Chinese people191 | Second-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217 | Second-line or third-line therapy in combination with metformin142 | Second-line or third-line therapy if cost is a major issue or if there is a compelling need to minimize hypoglycaemia218 |
| Might reduce the risk of cardiovascular complications in Chinese people197 | ||||||
| Increased risk of heart failure, fracture and bladder carcinoma (pioglitazone)194,228–230 | Less efficacious in preventing T2DM among south Asian people than in people who were not Asian192 | Does not increase the risk of hospitalization due to heart failure among east Asian people193 | ||||
| SGLT2 inhibitors | Lower blood levels of glucose by inhibiting SGLT2-mediated glucose reabsorption in the kidney in an insulin-independent manner231,232 | Similar200,201 or more186 efficacious glucose lowering in Asian people compared with people who were not Asian | Similar200,201 or more186 efficacious weight lowering in Asian people versus people who were not Asian | First-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217,233 | Second-line or third-line therapy in combination with metformin142 | Recommended independently of HbA1c for patients with established ASCVD (or indicators of high ASCVD risk), heart failure or chronic kidney disease218 |
| Reduce weight234–236, blood pressure231, all-cause and cardiovascular mortality234–237, non-fatal myocardial infarction234–237, heart failure hospitalization237–239, progression of diabetic kidney disease234–237,240; increased risk of diabetic ketoacidosis241,242, genital mycotic infections237,242 and lower-extremity amputations241,242 | Similar cardiovascular risk reduction in Asian people compared with white people202 | Consider as first-line monotherapy if a patient has intolerance or a contraindication to metformin233 | Recommended in addition to metformin for patients with established ASCVD, high risk of ASCVD or chronic kidney disease | Second-line or third-line therapy for individuals with a compelling need to minimize hypoglycaemia218 | ||
| SGLT2 inhibitors are preferred over GLP1 receptor agonists for patients with heart failure243 | Second-line or third-line therapy for individuals with a compelling need to minimize weight gain or promote weight loss218 | |||||
| GLP1 receptor agonists | Activate the GLP1 receptor with multiple actions, including delayed absorption of nutrients in the stomach and small bowel, decreased appetite and food consumption, weight loss, glucose-dependent insulin secretion and suppression of glucagon, resulting in reduction in hyperglycaemia239,244. | Similarly efficacious glucose lowering in Asian people and people who are not Asian186 | Greater cardiovascular risk reduction in Asian people compared with white people202 | Second-choice second-line therapy in combination with metformin for asymptomatic HbA1c >9%217 | Second-line or third-line therapy in combination with metformin142 | Recommended independently of HbA1c for patients with established ASCVD (or indicators of high ASCVD risk)218 |
| Reduced cardiovascular mortality and all-cause mortality, non-fatal myocardial infarction, non-fatal stroke and diabetic kidney disease progression237,245–251 | More effective glucose lowering among Chinese people with shorter disease duration and higher baseline HbA1c213 | Consider as monotherapy if a patient has metformin intolerance, obesity, established ASCVD or high risk of ASVCD215 | Recommended in addition to metformin for established ASCVD, high risk of ASCVD or chronic kidney disease with contraindication to SGLT2 inhibitors, regardless of baseline HbA1c243,252 | Second-line or third-line therapy for individuals with a compelling need to minimize hypoglycaemia218 | ||
| Associated with gastrointestinal adverse effects, such as nausea237. | Second-line or third-line therapy for individuals with a compelling need to minimize weight gain or promote weight loss218 | |||||
AMP, adenosine monophosphate; ASCVD, atherosclerotic cardiovascular disease; ATP, adenosine triphosphate; DPP4, dipeptidyl peptidase 4; GLP1, glucagon-like peptide 1; SGLT2, sodium–glucose cotransporter 2; SUR, sulfonylurea receptor; T2DM, type 2 diabetes mellitus.
Metformin
The efficacy of metformin as a treatment for T2DM was first demonstrated in the UKPDS study152. Subsequent studies have verified that metformin lowers glucose levels efficaciously among Chinese and south Asian people153, and improves β-cell function among Indian people154 and Chinese155 people without obesity. However, no studies to our knowledge have directly compared its effects across Chinese, Indian and other populations.
α-Glucosidase inhibitors
In China, the Metformin and Acarbose in Chinese as the Initial Hypoglycaemic Treatment study reported that acarbose was not inferior to metformin in HbA1c lowering (mean difference 0.01%, 95% CI −0.12% to 0.14%), and resulted in better weight reduction and improved levels of lipids compared with metformin156. A meta-analysis comparing acarbose with DPP4 inhibitors also reported that acarbose had superior weight loss efficacy with a similar degree of glucose lowering157. In western populations with IGT, acarbose was found to reduce the risk of developing T2DM (HR 0.75, 95% CI 0.63–0.90) compared with placebo158. A meta-analysis of randomized controlled trials reported that acarbose had a greater HbA1c-lowering effect among east Asian populations than in western populations (1.26% (s.d. 1.20%) versus 0.62% (s.d. 1.28%))159. This difference was postulated to be driven by the fairly large proportion of starch content in east Asian diets160. An observational study of 21 countries also found that east and southeast (defined here as having an ethnic origin from Indonesia, Philippines, Thailand, Vietnam, Malaysia, Cambodia or Singapore) Asian people had larger HbA1c reductions with acarbose compared with south Asian people and European people161. These findings conflict with other systematic reviews reporting similar HbA1c-lowering effects in east Asian people and western people162,163. The incidence of diarrhoea as an adverse effect might be less common in Asian people than people who are not Asian163. This finding may be most applicable to east Asian people because the Asian populations included within this meta-analysis were predominantly east Asian. In a trial among Chinese people with coronary artery disease and IGT, acarbose prevented the onset of diabetes mellitus but did not reduce the frequency of cardiovascular events164.
Sulfonylureas
Although sulfonylureas can cause hypoglycaemia and weight gain165, they are cheap and are the second most common oral glucose-lowering medication in India166–168 and China169,170 after metformin. Glycaemic improvements with sulfonylureas might be less durable compared with improvements with other classes of glucose-lowering medication owing to accelerated β-cell failure, especially with older sulfonylureas165. In ADOPT, the incidence of elevated fasting blood levels of glucose >10.0 mmol/l after a median of 4.0 years of treatment was 34% for glyburide compared with 21% and 15% for metformin and rosiglitazone, respectively120. A cluster-based analysis of the ADOPT cohort showed that glycaemic deterioration was the steepest among those with severe insulin-deficient diabetes mellitus treated with glyburide, while those with mild age-related diabetes mellitus showed the greatest benefit116. An intensive glucose-lowering strategy using modified-release gliclazide, a modern sulfonylurea, successfully lowered HbA1c to 6.5% after a median of 5 years in the Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation (ADVANCE) trial171. A sub-analysis of the ADVANCE trial showed that HbA1c lowering was similar among Asian people (defined in this study as people from China, India, Malaysia and the Philippines) (−0.78%, 95% CI −0.83% to −0.74%) and eastern European people (−0.71%, 95% CI −0.78% to −0.64%)172. Secondary analyses of clinical trials suggest that sulfonylureas lower blood levels of glucose more effectively among men of European origin without obesity, and less effectively among women of European origin with obesity, compared with thiazolidinediones173,174. These findings have not been replicated in Indian and Chinese populations.
Dipeptidyl peptidase 4 inhibitors
DPP4 inhibitors lower blood levels of glucose by restoring first-phase insulin secretion in a glucose-dependent manner and suppressing abnormally elevated glucagon levels175,176, thus targeting two crucial issues in T2DM and IGT177. These features of DPP4 inhibitors are especially important in the context of β-cell dysfunction among Indian and Chinese people. Although the initial uptake of DPP4 inhibitors was slow in China170,178 and India168, their popularity seems to be rapidly increasing178–180, especially with the availability of low-cost tenegliptin in India181. In the Trial Evaluating Cardiovascular Outcomes with Sitagliptin study, east Asian people had the greatest initial reduction in HbA1c (−0.60%) compared with other groups (≤0.55% reduction)182. This greater effect in east Asian people has been attributed to the restoration of first-phase insulin secretion176. Lower levels of insulin resistance among east Asian people111,112,114 relative to people of European origin108 could also have a role, as insulin resistance is associated with less-effective glucose lowering with DPP4 inhibitors among people of European origin183. Further research is required to confirm whether these findings extend to Indian people184. A meta-analysis that compared randomized controlled trials including mostly Asian participants with trials that included mostly participants who were not Asian found that DPP4 inhibitors lowered HbA1c better in Asian people than in people who were not Asian (between-group HbA1c difference –0.26%, 95% CI –0.36% to –0.17%)185. Another meta-analysis that compared randomized controlled trials that included mostly white participants with trials that included mostly Asian participants reported similar HbA1c lowering in white people (−0.49%, 95% CI −0.59% to −0.38%) and Asian people (−0.62%, 95% CI −0.8% to −0.45%)186, but a sensitivity analysis restricted to studies 12–52 weeks in duration showed greater HbA1c lowering among Asian people (mostly east Asian people; −0.73%, 95% CI −0.88% to −0.57%) versus white people (−0.49%, 95% CI −0.59% to −0.39%)186. An additional meta-analysis reported better glucose lowering with DPP4 inhibitors in Asian people compared with white people (between-group HbA1c difference –0.18%, 95% CI –0.32% to –0.04%), but improvements in HOMA-β were smaller in Asian people than in white people187. Similar findings have been described with DPP4 inhibitor and metformin combination therapy188.
Thiazolidinediones
A meta-analysis reported that pioglitazone and rosiglitazone are efficacious in lowering HbA1c among Chinese, Indian and other Asian people189, but, to our knowledge, there are no studies comparing Chinese, Indian or Asian people in general with people who are not Asian. In a UK study of 177 people of Indian descent with T2DM, rosiglitazone reduced HbA1c by 1.16% compared with placebo and improved insulin sensitivity and β-cell function190. Similar findings have been described with pioglitazone in China191. In the multi-ethnic Diabetes Reduction Assessment with Ramipril and Rosiglitazone Medication trial, rosiglitazone was less efficacious in reducing the incidence of diabetes mellitus in south Asian people than in people of other ethnicities192. An international observational study reported that pioglitazone and rosiglitazone are associated with increased hospitalization for heart failure in Australia and Canada, but not in east Asian countries or regions193. In European populations, pioglitazone reduces all-cause mortality, myocardial infarction and stroke among people with T2DM194,195, especially those with insulin resistance196. In China, an observational study suggested that pioglitazone was associated with a 30% reduction in myocardial infarction, heart failure and stroke197. Pioglitazone is commonly used in China (5.6–17.0% of people with T2DM who are treated with oral agents)169,170 and India (regularly prescribed by 56.2% of Indian physicians)198,199.
SGLT2 inhibitors
Several meta-analyses of randomized controlled trials have shown that SGLT2 inhibitors are probably similarly200,201 or more186 effective in reducing blood levels of glucose and body weight, and similarly effective in reducing the incidence of adverse cardiovascular events202, in Asian people and people who were not Asian or white people. It is unknown whether these effects might differ between Indian people and Chinese people186,200. Although SGLT2 inhibitors are widely recommended among Asian populations203–205, data on the real-world use of SGLT2 inhibitors in China and India are scarce. In Hong Kong, the use of SGLT2 inhibitors increased rapidly from 2015 to 2016 (0.04% to 0.4% of non-insulin glucose-lowering medications)178. In India, the availability of low-cost remogliflozin and future generic options will probably expand the uptake of this class168,206.
GLP1 receptor agonists
In a secondary analysis of a randomized controlled trial, the glucagon-like peptide 1 (GLP1) receptor agonist liraglutide lowered blood levels of glucose by a similar degree among south Asian people and western European people207. A meta-analysis comparing 19 studies including predominantly white participants with three studies including predominantly Asian participants found that the glucose-lowering efficacy of GLP1 receptor agonists was similar across both groups186. A similar meta-analysis reported that the glucose-lowering effectiveness seemed to be similar among Asian people and people who are not Asian regardless of baseline weight208.
Previous meta-analyses comparing cardiovascular risk reduction among Asian people and people who are not Asian reported mixed findings209,210, but an updated meta-analysis of six cardiovascular outcome trials for GLP1 receptor agonists reported that reductions in major adverse cardiovascular events were greater among Asian people than in white people202. Emerging evidence suggests that GLP1 receptor agonists could be more effective when initiated early in the course of disease among people with adequate β-cell function211. In the prospective observational Predicting Response to Incretin Based Agents study, long duration of diabetes mellitus, low C-peptide concentrations, treatment with insulin and positive GAD or islet antibodies were associated with reduced lowering of blood levels of glucose211. However, a post hoc analysis of A Study in Participants With Type 2 Diabetes Mellitus found that dulaglutide lowered blood levels of glucose similarly among people with high and low titres of GAD antibodies212. In China, a retrospective cohort study reported that those with a shorter duration of T2DM and a higher baseline HbA1c had greater reductions in levels of glucose with exenatide213, a finding that has been replicated in Korean people214. Similar studies have not been conducted among Indian people. GLP1 receptor agonists are not commonly used in India168 or China170,178 owing to barriers such as high costs215.
Conclusions
Although the massively growing burden of T2DM in India and China has been strongly driven by rapid urbanization, these populations also have an inherent, shared predisposition to deficient insulin secretion. This susceptibility confers an increased risk of severe insulin-deficient diabetes mellitus occurring even among lean people at young ages <40 years. Other subtypes of T2DM also seem to present earlier in life and with lower β-cell function, lower insulin resistance and lower BMI among Chinese people and most Indian people compared with European people. These distinctions are key to deciphering the unique responses to glucose-lowering medications among Indian and Chinese populations. In particular, incretin-based therapies have a key role in enhancing pancreatic β-cell function in these populations, providing effective glucose lowering with DPP4 inhibitors and providing highly effective glucose lowering with GLP1 receptor agonists if initiated early in the course of disease when β-cell function is still fairly well preserved. Informed by these differences, future precision medicine strategies should be adapted, recalibrated and rigorously validated among Indian and Chinese populations. In particular, better treatments tailored to phenotype are required for those diagnosed at the youngest ages, and further work is required to identify effective early combination therapies. Future research will need to integrate basic sciences, clinical, epidemiological, genetic and other sources of data216 to better characterize and treat the unique pathophysiology and phenotypes of T2DM in India and China, and throughout their global diasporas.
Acknowledgements
C.K. was supported by the Canadian Institutes of Health Research and the South Asian Network Supporting Awareness and Research. The authors thank S. H. (Hana) Fu, Centre for Global Health Research, for her assistance in preparing the map and thank X. Zou for providing the numerical data underlying Figure A from her study114 for inclusion in Table 1.
Glossary
- Person-years
The unit of measurement that accounts for the total amount of time that individuals are at risk of developing a condition, often used in the denominators of incidence rates.
- Glucolipotoxicity
Toxicity to the pancreatic β-cells arising from chronically elevated levels of glucose and fatty acids.
- Endogamy
The practice of marrying within one’s own social group.
- Lipodystrophy
A rare group of disorders characterized by partial or generalized loss of adipose tissue.
- Latent autoimmune diabetes mellitus of adulthood
A type of diabetes mellitus that presents in adulthood with immunogenetic markers of type 1 diabetes mellitus, but that does not require insulin at the time of diagnosis.
- Glycaemic deterioration rate
An epidemiological measure of the speed of progression of diabetes mellitus, calculated as the slope of HbA1c over time after statistical adjustment for glucose-lowering drugs (this rate cannot be determined for people treated with insulin).
- Glycaemic relapse
The recurrence of type 2 diabetes mellitus (T2DM) in an individual who previously had T2DM in remission.
Author contributions
C.K. researched data for the article, wrote the article, contributed substantially to the discussion of content and reviewed and/or edited the manuscript before submission. K.M.V.N, J.C.N.C., P.J. and B.R.S. contributed substantially to the discussion of content and reviewed and/or edited the manuscript before submission.
Peer review
Peer review information
Nature Reviews Endocrinology thanks Sanjay Kalra, who co-reviewed with Madhur Verma, and Yingli Lu for their contribution to the peer review of this work.
Competing interests
C.K. reports consulting fees and honoraria from Sanofi, Abbott, and AstraZeneca. The other authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Philipson LH. Harnessing heterogeneity in type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020;16:79–80. doi: 10.1038/s41574-019-0308-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Del Prato S. Heterogeneity of diabetes: heralding the era of precision medicine. Lancet Diabetes Endocrinol. 2019;7:659–661. doi: 10.1016/S2213-8587(19)30218-9. [DOI] [PubMed] [Google Scholar]
- 3.Gloyn AL, Drucker DJ. Precision medicine in the management of type 2 diabetes. Lancet Diabetes Endocrinol. 2018;6:891–900. doi: 10.1016/S2213-8587(18)30052-4. [DOI] [PubMed] [Google Scholar]
- 4.Prasad RB, Groop L. Precision medicine in type 2 diabetes. J. Intern. Med. 2019;285:40–48. doi: 10.1111/joim.12859. [DOI] [PubMed] [Google Scholar]
- 5.Chung WK, et al. Precision medicine in diabetes: a consensus report From the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Dia Care. 2020;43:1617–1635. doi: 10.2337/dci20-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.IDF. IDF Diabetes Atlas, 10th Edition Committee. IDF Diabetes Atlas (International Diabetes Federation, 2021). An authoritative source of country-specific estimates of the prevalence of diabetes mellitus.
- 7.Gujral UP, Weber MB, Staimez LR, Narayan KMV. Diabetes among non-overweight individuals: an emerging public health challenge. Curr. Diab. Rep. 2018;18:60. doi: 10.1007/s11892-018-1017-1. [DOI] [PubMed] [Google Scholar]
- 8.Gujral UP, et al. Cardiometabolic abnormalities among normal-weight persons from five racial/ethnic groups in the United States: a cross-sectional analysis of two cohort studies. Ann. Intern. Med. 2017;166:628–636. doi: 10.7326/M16-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gujral UP, Narayan KMV. Diabetes in normal-weight individuals: high susceptibility in nonwhite populations. Diabetes Care. 2019;42:2164–2166. doi: 10.2337/dci19-0046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magliano DJ, et al. Young-onset type 2 diabetes mellitus — implications for morbidity and mortality. Nat. Rev. Endocrinol. 2020;16:321–331. doi: 10.1038/s41574-020-0334-z. [DOI] [PubMed] [Google Scholar]
- 11.Luk AOY, et al. Secular trends in incidence of type 1 and type 2 diabetes in Hong Kong: a retrospective cohort study. PLOS Med. 2020;17:e1003052. doi: 10.1371/journal.pmed.1003052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jayawardena R, et al. Prevalence and trends of the diabetes epidemic in South Asia: a systematic review and meta-analysis. BMC Public Health. 2012;12:380. doi: 10.1186/1471-2458-12-380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Unnikrishnan R, Anjana RM, Mohan V. Diabetes mellitus and its complications in India. Nat. Rev. Endocrinol. 2016;12:357–370. doi: 10.1038/nrendo.2016.53. [DOI] [PubMed] [Google Scholar]
- 14.Bavuma C, et al. Atypical forms of diabetes mellitus in Africans and other non-European ethnic populations in low- and middle-income countries: a systematic literature review. J. Glob. Health. 2019;9:020401. doi: 10.7189/jogh.09.020401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mohan V, Deepa M, Anjana RM, Lanthorn H, Deepa R. Incidence of diabetes and pre-diabetes in a selected urban south Indian population (CUPS-19) J. Assoc. Physicians India. 2008;56:152–157. [PubMed] [Google Scholar]
- 16.Anjana RM, et al. Incidence of diabetes and prediabetes and predictors of progression among Asian Indians: 10-year follow-up of the Chennai Urban Rural Epidemiology Study (CURES) Diabetes Care. 2015;38:1441–1448. doi: 10.2337/dc14-2814. [DOI] [PubMed] [Google Scholar]
- 17.Narayan KMV, et al. 1597-P: Incidence of diabetes in young adult south Asians compared with Pima Indians. Diabetes. 2019;68(Suppl. 1):1597. doi: 10.2337/db19-1597-P. [DOI] [Google Scholar]
- 18.Luhar S, et al. Lifetime risk of diabetes in metropolitan cities in India. Diabetologia. 2021;64:521–529. doi: 10.1007/s00125-020-05330-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghorpade A, et al. Diabetes in rural Pondicherry, India: a population-based study of the incidence and risk factors. WHO South East Asia J. Public. Health. 2013;2:149. doi: 10.4103/2224-3151.206761. [DOI] [PubMed] [Google Scholar]
- 20.Vijayakumar G, et al. Incidence of type 2 diabetes mellitus and prediabetes in Kerala, India: results from a 10-year prospective cohort. BMC Public. Health. 2019;19:140. doi: 10.1186/s12889-019-6445-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang M, et al. Incidence and time trends of type 2 diabetes mellitus among adults in Zhejiang Province, China, 2007-2017. J. Diabetes Res. 2020;2020:e2597953. doi: 10.1155/2020/2597953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jensen ET, et al. Comparison of the incidence of diabetes in United States and Indian youth: an international harmonization of youth diabetes registries. Pediatr. Diabetes. 2021;22:8–14. doi: 10.1111/pedi.13009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khan NA, et al. Ethnicity and sex affect diabetes incidence and outcomes. Diabetes Care. 2011;34:96–101. doi: 10.2337/dc10-0865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ke C, Sohal P, Qian H, Quan H, Khan NA. Diabetes in the young: a population-based study of South Asian, Chinese and White people. Diabet. Med. 2015;32:487–496. doi: 10.1111/dme.12657. [DOI] [PubMed] [Google Scholar]
- 25.Ke C, Luk AO, Chan JCN, Wei X, Shah BR. Migration and diabetes incidence among Chinese adults in Canada, China, Hong Kong, and Taiwan: an international population-based comparative study from 2000 to 2017. Diabetes Res. Clin. Pract. 2021;180:109062. doi: 10.1016/j.diabres.2021.109062. [DOI] [PubMed] [Google Scholar]
- 26.Kahn SE. The relative contributions of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 27.Weyer C, Bogardus C, Mott DM, Pratley RE. The natural history of insulin secretory dysfunction and insulin resistance in the pathogenesis of type 2 diabetes mellitus. J. Clin. Invest. 1999;104:787–794. doi: 10.1172/JCI7231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gerich JE. Is reduced first-phase insulin release the earliest detectable abnormality in individuals destined to develop type 2 diabetes? Diabetes. 2002;51:S117–S121. doi: 10.2337/diabetes.51.2007.S117. [DOI] [PubMed] [Google Scholar]
- 29.Kahn SE, et al. Quantification of the relationship between insulin sensitivity and β-cell function in human subjects: evidence for a hyperbolic function. Diabetes. 1993;42:1663–1672. doi: 10.2337/diab.42.11.1663. [DOI] [PubMed] [Google Scholar]
- 30.Holman RR, Clark A, Rorsman P. β-Cell secretory dysfunction: a key cause of type 2 diabetes. Lancet Diabetes Endocrinol. 2020;8:370. doi: 10.1016/S2213-8587(20)30119-4. [DOI] [PubMed] [Google Scholar]
- 31.Ikehara S, et al. Age trajectories of glycaemic traits in non-diabetic South Asian and white individuals: the Whitehall II cohort study. Diabetologia. 2015;58:534–542. doi: 10.1007/s00125-014-3448-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jainandunsing S, et al. Failing beta-cell adaptation in South Asian families with a high risk of type 2 diabetes. Acta Diabetol. 2015;52:11–19. doi: 10.1007/s00592-014-0588-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanaya AM, et al. Understanding the high prevalence of diabetes in U.S. South Asians compared with four racial/ethnic groups: The MASALA and MESA studies. Diabetes Care. 2014;37:1621–1628. doi: 10.2337/dc13-2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kodama K, et al. Ethnic differences in the relationship between insulin sensitivity and insulin response. Diabetes Care. 2013;36:1789–1796. doi: 10.2337/dc12-1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Narayan KMV, et al. Incidence of diabetes in South Asian young adults compared to Pima Indians. BMJ Open Diabetes Res. Care. 2021;9:e001988. doi: 10.1136/bmjdrc-2020-001988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Narayan KMV, et al. Incidence and pathophysiology of diabetes in South Asian adults living in India and Pakistan compared with US blacks and whites. BMJ Open Diabetes Res. Care. 2021;9:e001927. doi: 10.1136/bmjdrc-2020-001927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tan VMH, et al. Ethnic differences in insulin sensitivity and beta-cell function among Asian men. Nutr. Diabetes. 2015;5:e173. doi: 10.1038/nutd.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gujral UP, Narayan KMV, Kahn SE, Kanaya AM. The relative associations of β-cell function and insulin sensitivity with glycemic status and incident glycemic progression in migrant Asian Indians in the United States: the MASALA study. J. Diabetes Complications. 2014;28:45–50. doi: 10.1016/j.jdiacomp.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Staimez LR, et al. Evidence of reduced β-cell function in asian indians with mild dysglycemia. Diabetes Care. 2013;36:2772–2778. doi: 10.2337/dc12-2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Qian L, et al. Early insulin secretion failure leads to diabetes in Chinese subjects with impaired glucose regulation. Diabetes Metab. Res. Rev. 2009;25:144–149. doi: 10.1002/dmrr.922. [DOI] [PubMed] [Google Scholar]
- 41.Qian L, et al. Metabolic characteristics of subjects with normal glucose tolerance and 1-h hyperglycaemia. Clin. Endocrinol. 2008;69:575–579. doi: 10.1111/j.1365-2265.2008.03209.x. [DOI] [PubMed] [Google Scholar]
- 42.Bi Y, et al. Decreased beta cell function and insulin sensitivity contributed to increasing fasting glucose in Chinese. Acta Diabetol. 2012;49:51–58. doi: 10.1007/s00592-010-0194-4. [DOI] [PubMed] [Google Scholar]
- 43.Cai X, et al. Differential role of insulin resistance and β-cell function in the development of prediabetes and diabetes in middle-aged and elderly Chinese population. Diabetol. Metab. Syndr. 2019;11:24. doi: 10.1186/s13098-019-0418-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang T, et al. Association of insulin resistance and β-cell dysfunction with incident diabetes among adults in China: a nationwide, population-based, prospective cohort study. Lancet Diabetes Endocrinol. 2020;8:115–124. doi: 10.1016/S2213-8587(19)30425-5. [DOI] [PubMed] [Google Scholar]
- 45.Cefalu WT. “Prediabetes”: are there problems with this label? No, we need heightened awareness of this condition! Diabetes Care. 2016;39:1472–1477. doi: 10.2337/dc16-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Abdul-Ghani MA, Tripathy D, DeFronzo RA. Contributions of β-cell dysfunction and insulin resistance to the pathogenesis of impaired glucose tolerance and impaired fasting glucose. Diabetes Care. 2006;29:1130–1139. doi: 10.2337/dc05-2179. [DOI] [PubMed] [Google Scholar]
- 47.Chow EYK, Chan JCN. Insulin resistance versus β-cell dysfunction in type 2 diabetes: where public and personalised health meet. Lancet Diabetes Endocrinol. 2020;8:92–93. doi: 10.1016/S2213-8587(19)30421-8. [DOI] [PubMed] [Google Scholar]
- 48.Anjana RM, et al. Prevalence of diabetes and prediabetes in 15 states of India: results from the ICMR–INDIAB population-based cross-sectional study. Lancet Diabetes Endocrinol. 2017;5:585–596. doi: 10.1016/S2213-8587(17)30174-2. [DOI] [PubMed] [Google Scholar]
- 49.Andes LJ, Cheng YJ, Rolka DB, Gregg EW, Imperatore G. Prevalence of prediabetes among adolescents and young adults in the United States, 2005–2016. JAMA Pediatr. 2020;174:e194498. doi: 10.1001/jamapediatrics.2019.4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kanat M, et al. Distinct β-cell defects in impaired fasting glucose and impaired glucose tolerance. Diabetes. 2012;61:447–453. doi: 10.2337/db11-0995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Narayan KMV, Kanaya AM. Why are South Asians prone to type 2 diabetes? A hypothesis based on underexplored pathways. Diabetologia. 2020;63:1103–1109. doi: 10.1007/s00125-020-05132-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Petersen KF, et al. Increased prevalence of insulin resistance and nonalcoholic fatty liver disease in Asian-Indian men. PNAS. 2006;103:18273–18277. doi: 10.1073/pnas.0608537103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li Y, et al. Prevalence of diabetes recorded in mainland China using 2018 diagnostic criteria from the American Diabetes Association: national cross sectional study. BMJ. 2020;369:m997. doi: 10.1136/bmj.m997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Abdul-Ghani M, DeFronzo RA, Jayyousi A. Prediabetes and risk of diabetes and associated complications: impaired fasting glucose versus impaired glucose tolerance does it matter? Curr. Opin. Clin. Nutr. Metab. Care. 2016;19:394–399. doi: 10.1097/MCO.0000000000000307. [DOI] [PubMed] [Google Scholar]
- 55.Lin Z, et al. High-normal 2 h glucose is associated with defects of insulin secretion and predispose to diabetes in Chinese adults. Endocrine. 2015;48:179–186. doi: 10.1007/s12020-014-0244-8. [DOI] [PubMed] [Google Scholar]
- 56.Li Y, et al. Insulin resistance, beta-cell function, adipokine profiles and cardiometabolic risk factors among Chinese youth with isolated impaired fasting glucose versus impaired glucose tolerance: the BCAMS study. BMJ Open Diab Res. Care. 2020;8:e000724. doi: 10.1136/bmjdrc-2019-000724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu L, et al. Impact of impaired fasting glucose and impaired glucose tolerance on arterial stiffness in an older Chinese population: the Guangzhou Biobank Cohort Study–CVD. Metab. Clin. l Exp. 2010;59:367–372. doi: 10.1016/j.metabol.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 58.Mahajan A, et al. Fine-mapping type 2 diabetes loci to single-variant resolution using high-density imputation and islet-specific epigenome maps. Nat. Genet. 2018;50:1505–1513. doi: 10.1038/s41588-018-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Vujkovic M, et al. Discovery of 318 new risk loci for type 2 diabetes and related vascular outcomes among 1.4 million participants in a multi-ancestry meta-analysis. Nat. Genet. 2020;52:680–691. doi: 10.1038/s41588-020-0637-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Spracklen CN, Sim X. Progress in defining the genetic contribution to type 2 diabetes in individuals of east asian ancestry. Curr. Diab Rep. 2021;21:17. doi: 10.1007/s11892-021-01388-2. [DOI] [PubMed] [Google Scholar]
- 61.McCarthy MI. Genomics, type 2 diabetes, and obesity. N. Engl. J. Med. 2010;363:2339–2350. doi: 10.1056/NEJMra0906948. [DOI] [PubMed] [Google Scholar]
- 62.Chen J, et al. The trans-ancestral genomic architecture of glycemic traits. Nat. Genet. 2021;53:840–860. doi: 10.1038/s41588-021-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Spracklen CN, et al. Identification of type 2 diabetes loci in 433,540 East Asian individuals. Nature. 2020;582:240–245. doi: 10.1038/s41586-020-2263-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kooner JS, et al. Genome-wide association study in individuals of South Asian ancestry identifies six new type 2 diabetes susceptibility loci. Nat. Genet. 2011;43:984–989. doi: 10.1038/ng.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tabassum R, et al. Genome-wide association study for type 2 diabetes in indians identifies a new susceptibility locus at 2q21. Diabetes. 2013;62:977–986. doi: 10.2337/db12-0406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Saxena R, et al. Genome-wide association study identifies a novel locus contributing to type 2 diabetes susceptibility in sikhs of Punjabi origin from India. Diabetes. 2013;62:1746–1755. doi: 10.2337/db12-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yaghootkar H, Whitcher B, Bell JD, Thomas EL. Ethnic differences in adiposity and diabetes risk–insights from genetic studies. J. Intern. Med. 2020;288:271–283. doi: 10.1111/joim.13082. [DOI] [PubMed] [Google Scholar]
- 68.Bose A, Platt DE, Parida L, Drineas P, Paschou P. Integrating linguistics, social structure, and geography to model genetic diversity within India. Mol. Biol. Evol. 2021;38:1809–1819. doi: 10.1093/molbev/msaa321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sharma V, et al. Replication of newly identified type 2 diabetes susceptible loci in northwest Indian population. Diabetes Res. Clin. Pract. 2017;126:160–163. doi: 10.1016/j.diabres.2017.02.013. [DOI] [PubMed] [Google Scholar]
- 70.Cho YS, et al. Meta-analysis of genome-wide association studies identifies eight new loci for type 2 diabetes in east Asians. Nat. Genet. 2012;44:67–72. doi: 10.1038/ng.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kou K, Saisho Y, Satoh S, Yamada T, Itoh H. Change in β-cell mass in Japanese nondiabetic obese individuals. J. Clin. Endocrinol. Metab. 2013;98:3724–3730. doi: 10.1210/jc.2013-1373. [DOI] [PubMed] [Google Scholar]
- 72.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. The Pax4 gene is essential for differentiation of insulin-producing β cells in the mammalian pancreas. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 73.Wallach JD, et al. Updating insights into rosiglitazone and cardiovascular risk through shared data: individual patient and summary level meta-analyses. BMJ. 2020;368:I7078. doi: 10.1136/bmj.l7078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen M, et al. Association of PAX4 genetic variants with oral antidiabetic drugs efficacy in Chinese type 2 diabetes patients. Pharmacogenomics J. 2014;14:488–492. doi: 10.1038/tpj.2014.18. [DOI] [PubMed] [Google Scholar]
- 75.Chen M, et al. A variant of PSMD6 is associated with the therapeutic efficacy of oral antidiabetic drugs in Chinese type 2 diabetes patients. Sci. Rep. 2015;5:10701. doi: 10.1038/srep10701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jiang G, et al. Obesity, clinical, and genetic predictors for glycemic progression in Chinese patients with type 2 diabetes: a cohort study using the Hong Kong Diabetes Register and Hong Kong Diabetes Biobank. PLoS Med. 2020;17:e1003209. doi: 10.1371/journal.pmed.1003209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rathmann W, Bongaerts B. Pharmacogenetics of novel glucose-lowering drugs. Diabetologia. 2021;64:1201–1212. doi: 10.1007/s00125-021-05402-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hales CN, Barker DJP. Type 2 (non-insulin-dependent) diabetes mellitus: the thrifty phenotype hypothesis. Diabetologia. 1992;35:595–601. doi: 10.1007/BF00400248. [DOI] [PubMed] [Google Scholar]
- 79.Sen, A. Poverty and Famines: An Essay on Entitlement and Deprivation (Clarendon Press, 1981).
- 80.Li Y, et al. Exposure to the chinese famine in early life and the risk of hyperglycemia and type 2 diabetes in adulthood. Diabetes. 2010;59:2400–2406. doi: 10.2337/db10-0385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang N, et al. Is exposure to famine in childhood and economic development in adulthood associated with diabetes? J. Clin. Endocrinol. Metab. 2015;100:4514–4523. doi: 10.1210/jc.2015-2750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fernandez-Twinn DS, Hjort L, Novakovic B, Ozanne SE, Saffery R. Intrauterine programming of obesity and type 2 diabetes. Diabetologia. 2019;62:1789–1801. doi: 10.1007/s00125-019-4951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Song C, et al. Ten SNPs may affect type 2 diabetes risk in interaction with prenatal exposure to chinese famine. Nutrients. 2020;12:3880. doi: 10.3390/nu12123880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol. Rev. 2005;85:571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 85.Krishnaveni GV, Yajnik CS. Developmental origins of diabetes — an Indian perspective. Eur. J. Clin. Nutr. 2017;71:865–869. doi: 10.1038/ejcn.2017.87. [DOI] [PubMed] [Google Scholar]
- 86.Franks PW, McCarthy MI. Exposing the exposures responsible for type 2 diabetes and obesity. Science. 2016;354:69–73. doi: 10.1126/science.aaf5094. [DOI] [PubMed] [Google Scholar]
- 87.Hughes AE, Hattersley AT, Flanagan SE, Freathy RM. Two decades since the fetal insulin hypothesis: what have we learned from genetics? Diabetologia. 2021;64:717–726. doi: 10.1007/s00125-021-05386-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Krishnaveni GV, et al. Intrauterine exposure to maternal diabetes is associated with higher adiposity and insulin resistance and clustering of cardiovascular risk markers in Indian children. Diabetes Care. 2010;33:402–404. doi: 10.2337/dc09-1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kahn SE, Andrikopoulos S, Verchere CB. Islet amyloid: a long-recognized but underappreciated pathological feature of type 2 diabetes. Diabetes. 1999;48:241–253. doi: 10.2337/diabetes.48.2.241. [DOI] [PubMed] [Google Scholar]
- 90.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol. Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 91.Zhao H-L, et al. Prevalence and clinicopathological characteristics of islet amyloid in chinese patients with type 2 diabetes. Diabetes. 2003;52:2759–2766. doi: 10.2337/diabetes.52.11.2759. [DOI] [PubMed] [Google Scholar]
- 92.Dunford EK, et al. A comparison of the healthiness of packaged foods and beverages from 12 countries using the Health Star Rating nutrient profiling system, 2013–2018. Obes. Rev. 2019;20:107–115. doi: 10.1111/obr.12879. [DOI] [PubMed] [Google Scholar]
- 93.Lee SC, et al. The islet amyloid polypeptide (amylin) gene S20G mutation in Chinese subjects: evidence for associations with type 2 diabetes and cholesterol levels. Clin. Endocrinol. 2001;54:541–546. doi: 10.1046/j.1365-2265.2001.01244.x. [DOI] [PubMed] [Google Scholar]
- 94.McCarthy MI, et al. The islet amyloid polypeptide gene and non-insulin-dependent diabetes mellitus in South Indians. Diabetes Res. Clin. Pract. 1992;18:31–34. doi: 10.1016/0168-8227(92)90052-S. [DOI] [PubMed] [Google Scholar]
- 95.Meier DT, et al. The S20G substitution in hIAPP is more amyloidogenic and cytotoxic than wild-type hIAPP in mouse islets. Diabetologia. 2016;59:2166–2171. doi: 10.1007/s00125-016-4045-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gurung M, et al. Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine. 2020;51:102590. doi: 10.1016/j.ebiom.2019.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alvarez-Silva C, et al. Trans-ethnic gut microbiota signatures of type 2 diabetes in Denmark and India. Genome Med. 2021;13:37. doi: 10.1186/s13073-021-00856-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Forslund K, et al. Disentangling type 2 diabetes and metformin treatment signatures in the human gut microbiota. Nature. 2015;528:262–266. doi: 10.1038/nature15766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fernández-Real J-M, et al. Burden of infection and insulin resistance in healthy middle-aged men. Diabetes Care. 2006;29:1058–1064. doi: 10.2337/dc05-2068. [DOI] [PubMed] [Google Scholar]
- 100.Chakraborty S, Bhattacharyya R, Banerjee D. Infections: a possible risk factor for type 2 diabetes. Adv. Clin. Chem. 2017;80:227–251. doi: 10.1016/bs.acc.2016.11.004. [DOI] [PubMed] [Google Scholar]
- 101.Rangarajan J, et al. A study of seroprevalence of hepatitis B and C in type 2 diabetes patients in a tertiary care teaching institute in South India. Int. J. Contemp. Med. 2016;3:4. [Google Scholar]
- 102.Liu Y, et al. Association of hepatitis B surface antigen seropositivity and hepatitis B surface antibody seropositivity with diabetes: a cross-sectional study based on two Chinese populations in Guangdong, China. BMJ Open. 2020;10:e028968. doi: 10.1136/bmjopen-2019-028968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Montefusco L, et al. Acute and long-term disruption of glycometabolic control after SARS-CoV-2 infection. Nat. Metab. 2021;3:774–785. doi: 10.1038/s42255-021-00407-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yorke E, et al. The bidirectional relationship between tuberculosis and diabetes. Tuberc. Res. Treat. 2017;2017:e1702578. doi: 10.1155/2017/1702578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dooley KE, Chaisson RE. Tuberculosis and diabetes mellitus: convergence of two epidemics. Lancet Infect. Dis. 2009;9:737–746. doi: 10.1016/S1473-3099(09)70282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Han X, et al. Helicobacter pylori infection is associated with type 2 diabetes among a middle- and old-age Chinese population. Diabetes Metab. Res. Rev. 2016;32:95–101. doi: 10.1002/dmrr.2677. [DOI] [PubMed] [Google Scholar]
- 107.Ko GTC, et al. Helicobacter pylori infection in Chinese subjects with type 2 diabetes. Endocr. Res. 2001;27:171–177. doi: 10.1081/ERC-100107178. [DOI] [PubMed] [Google Scholar]
- 108.Ahlqvist E, et al. Novel subgroups of adult-onset diabetes and their association with outcomes: a data-driven cluster analysis of six variables. Lancet Diabetes Endocrinol. 2018;6:361–369. doi: 10.1016/S2213-8587(18)30051-2. [DOI] [PubMed] [Google Scholar]
- 109.van Smeden M, Harrell FE, Dahly DL. Novel diabetes subgroups. Lancet Diabetes Endocrinol. 2018;6:439–440. doi: 10.1016/S2213-8587(18)30124-4. [DOI] [PubMed] [Google Scholar]
- 110.Anjana RM, et al. Novel subgroups of type 2 diabetes and their association with microvascular outcomes in an Asian Indian population: a data-driven cluster analysis: the INSPIRED study. BMJ Open Diabetes Res. Care. 2020;8:e001506. doi: 10.1136/bmjdrc-2020-001506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li X, et al. Validation of the Swedish diabetes re-grouping scheme in adult-onset diabetes in China. J. Clin. Endocrinol. Metab. 2020;105:dgaa524. doi: 10.1210/clinem/dgaa524. [DOI] [PubMed] [Google Scholar]
- 112.Xiong X, et al. Identification of two novel subgroups in patients with diabetes mellitus and their association with clinical outcomes: a two-step cluster analysis. J. Diabetes Invest. 2021;12:1346–1358. doi: 10.1111/jdi.13494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wang W, et al. Application of new international classification of adult-onset diabetes in Chinese inpatients with diabetes mellitus. Diabetes Metab. Res. Rev. 2020;37:e3427. doi: 10.1002/dmrr.3427. [DOI] [PubMed] [Google Scholar]
- 114.Zou X, Zhou X, Zhu Z, Ji L. Novel subgroups of patients with adult-onset diabetes in Chinese and US populations. Lancet Diabetes Endocrinol. 2019;7:9–11. doi: 10.1016/S2213-8587(18)30316-4. [DOI] [PubMed] [Google Scholar]
- 115.Bancks MP, et al. Association of diabetes subgroups with race/ethnicity, risk factor burden and complications: the MASALA and MESA studies. J. Clin. Endocrinol. Metab. 2021;106:e2106–e2115. doi: 10.1210/clinem/dgaa962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dennis JM, Shields BM, Henley WE, Jones AG, Hattersley AT. Disease progression and treatment response in data-driven subgroups of type 2 diabetes compared with models based on simple clinical features: an analysis using clinical trial data. Lancet Diabetes Endocrinol. 2019;7:442–451. doi: 10.1016/S2213-8587(19)30087-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.TODAY Study Group. Effects of metformin, metformin plus rosiglitazone, and metformin plus lifestyle on insulin sensitivity and β-cell function in TODAY. Diabetes Care. 2013;36:1749–1757. doi: 10.2337/dc12-2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.U. K. Prospective Diabetes Study Group. U.K. Prospective Diabetes Study 16: Overview of 6 years’ therapy of type II diabetes: a progressive disease. Diabetes. 1995;44:1249–1258. doi: 10.2337/diab.44.11.1249. [DOI] [PubMed] [Google Scholar]
- 119.Matthews DR, Cull CA, Stratton IM, Holman RR, Turner RC. UKPDS 26: Sulphonylurea failure in non-insulin-dependent diabetic patients over six years. Diabet. Med. 1998;15:297–303. doi: 10.1002/(SICI)1096-9136(199804)15:4<297::AID-DIA572>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- 120.Kahn SE, et al. Glycemic durability of rosiglitazone, metformin, or glyburide monotherapy. N. Engl. J. Med. 2006;355:2427–2443. doi: 10.1056/NEJMoa066224. [DOI] [PubMed] [Google Scholar]
- 121.Donnelly LA, et al. Rates of glycaemic deterioration in a real-world population with type 2 diabetes. Diabetologia. 2018;61:607–615. doi: 10.1007/s00125-017-4519-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ke C, et al. Age at diagnosis, glycemic trajectories, and responses to oral glucose-lowering drugs in type 2 diabetes in Hong Kong: a population-based observational study. PLOS Med. 2020;17:e1003316. doi: 10.1371/journal.pmed.1003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zou X, et al. The characteristics of newly diagnosed adult early-onset diabetes: a population-based cross-sectional study. Sci. Rep. 2017;7:46534. doi: 10.1038/srep46534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Mohan V, et al. Associations of β-cell function and insulin resistance with youth-onset type 2 diabetes and prediabetes among Asian Indians. Diabetes Technol. Ther. 2013;15:315–322. doi: 10.1089/dia.2012.0259. [DOI] [PubMed] [Google Scholar]
- 125.The Emerging Risk Factors Collaboration. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N. Engl. J. Med. 2011;364:829–841. doi: 10.1056/NEJMoa1008862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sattar N, et al. Age at diagnosis of type 2 diabetes mellitus and associations with cardiovascular and mortality risks. Circulation. 2019;139:2228–2237. doi: 10.1161/CIRCULATIONAHA.118.037885. [DOI] [PubMed] [Google Scholar]
- 127.Pavkov ME, et al. Effect of youth-onset type 2 diabetes mellitus on incidence of end-stage renal disease and mortality in young and middle-aged Pima Indians. JAMA. 2006;296:421–426. doi: 10.1001/jama.296.4.421. [DOI] [PubMed] [Google Scholar]
- 128.Nanayakkara N, et al. Age, age at diagnosis and diabetes duration are all associated with vascular complications in type 2 diabetes. J. Diabetes Complications. 2018;32:279–290. doi: 10.1016/j.jdiacomp.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 129.Steinarsson AO, et al. Short-term progression of cardiometabolic risk factors in relation to age at type 2 diabetes diagnosis: a longitudinal observational study of 100,606 individuals from the Swedish National Diabetes Register. Diabetologia. 2018;61:599–606. doi: 10.1007/s00125-017-4532-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.TODAY Study Group. Long-term complications in youth-onset type 2 diabetes. N. Engl. J. Med. 2021;385:416–426. doi: 10.1056/NEJMoa2100165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Huo X, et al. Risk of non-fatal cardiovascular diseases in early-onset versus late-onset type 2 diabetes in China: a cross-sectional study. Lancet Diabetes Endocrinol. 2016;4:115–124. doi: 10.1016/S2213-8587(15)00508-2. [DOI] [PubMed] [Google Scholar]
- 132.Li L, et al. Prevalence of microvascular diseases among tertiary care Chinese with early versus late onset of type 2 diabetes. J. Diabetes Complications. 2015;29:32–37. doi: 10.1016/j.jdiacomp.2014.08.010. [DOI] [PubMed] [Google Scholar]
- 133.Chan JCN, et al. Premature mortality and comorbidities in young-onset diabetes: a 7-year prospective analysis. Am. J. Med. 2014;127:616–624. doi: 10.1016/j.amjmed.2014.03.018. [DOI] [PubMed] [Google Scholar]
- 134.Ke C, Shah BR, Stukel TA, Chan JCN, Luk A. Excess burden of mental illness and hospitalization in young-onset type 2 diabetes. Ann. Intern. Med. 2019;171:78–79. doi: 10.7326/L19-0243. [DOI] [PubMed] [Google Scholar]
- 135.Wang L, et al. Greater macrovascular and microvascular morbidity from type 2 diabetes in northern compared with southern China: a cross-sectional study. J. Diabetes Invest. 2020;11:1285–1294. doi: 10.1111/jdi.13262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Unnikrishnan R, et al. Younger-onset versus older-onset type 2 diabetes: clinical profile and complications. J. Diabetes Complications. 2017;31:971–975. doi: 10.1016/j.jdiacomp.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 137.Amutha A, et al. Incidence of complications in young-onset diabetes: comparing type 2 with type 1 (the young diab study) Diabetes Res. Clin. Pract. 2017;123:1–8. doi: 10.1016/j.diabres.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 138.Amutha A, et al. Clinical profile and incidence of microvascular complications of childhood and adolescent onset type 1 and type 2 diabetes seen at a tertiary diabetes center in India. Pediatr. Diabetes. 2021;22:67–74. doi: 10.1111/pedi.13033. [DOI] [PubMed] [Google Scholar]
- 139.Yeung RO, et al. Metabolic profiles and treatment gaps in young-onset type 2 diabetes in Asia (the JADE programme): a cross-sectional study of a prospective cohort. Lancet Diabetes Endocrinol. 2014;2:935–943. doi: 10.1016/S2213-8587(14)70137-8. [DOI] [PubMed] [Google Scholar]
- 140.Ke C, Shah BR, Luk AO, Ruggiero ED, Chan JCN. Cardiovascular outcomes trials in type 2 diabetes: time to include young adults. Diabetes, Obes. Metab. 2020;22:3–5. doi: 10.1111/dom.13874. [DOI] [PubMed] [Google Scholar]
- 141.Sargeant JA, et al. Adults with early-onset type 2 diabetes (aged 18–39 years) are severely underrepresented in diabetes clinical research trials. Diabetologia. 2020;63:1516–1520. doi: 10.1007/s00125-020-05174-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Jia W, et al. Standards of medical care for type 2 diabetes in China 2019. Diabetes Metab. Res. Rev. 2019;35:e3158. doi: 10.1002/dmrr.3158. [DOI] [PubMed] [Google Scholar]
- 143.Wang H, et al. Predictors of long-term glycemic remission after 2-week intensive insulin treatment in newly diagnosed type 2 diabetes. J. Clin. Endocrinol. Metab. 2019;104:2153–2162. doi: 10.1210/jc.2018-01468. [DOI] [PubMed] [Google Scholar]
- 144.Li F, et al. Young onset type 2 diabetic patients might be more sensitive to metformin compared to late onset type 2 diabetic patients. Sci. Rep. 2017;7:16382. doi: 10.1038/s41598-017-16658-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.TODAY Study Group. et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N. Engl. J. Med. 2012;366:2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.RISE Consortium. Effects of treatment of impaired glucose tolerance or recently diagnosed type 2 diabetes with metformin alone or in combination with insulin glargine on β-cell function: comparison of responses in youth and adults. Diabetes. 2019;68:1670–1680. doi: 10.2337/db19-0299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Campbell SA, Light PE, Simpson SH. Costarting sitagliptin with metformin is associated with a lower likelihood of disease progression in newly treated people with type 2 diabetes: a cohort study. Diabet. Med. 2020;37:1715–1722. doi: 10.1111/dme.14154. [DOI] [PubMed] [Google Scholar]
- 148.Matthews DR, et al. Glycaemic durability of an early combination therapy with vildagliptin and metformin versus sequential metformin monotherapy in newly diagnosed type 2 diabetes (VERIFY): a 5-year, multicentre, randomised, double-blind trial. Lancet. 2019;394:1519–1529. doi: 10.1016/S0140-6736(19)32131-2. [DOI] [PubMed] [Google Scholar]
- 149.Ji L, et al. Early combination versus initial metformin monotherapy in the management of newly diagnosed type 2 diabetes mellitus–an East Asian perspective. Diabetes Obes. Metab. 2021;23:3–17. doi: 10.1111/dom.14205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Chan JCN, et al. Early combination therapy delayed treatment escalation in newly diagnosed young-onset type 2 diabetes: a subanalysis of the VERIFY study. Diabetes, Obes. Metab. 2021;23:245–251. doi: 10.1111/dom.14192. [DOI] [PubMed] [Google Scholar]
- 151.Gæde P, Lund-Andersen H, Parving H-H, Pedersen O. Effect of a multifactorial intervention on mortality in type 2 diabetes. N. Engl. J. Med. 2008;358:580–591. doi: 10.1056/NEJMoa0706245. [DOI] [PubMed] [Google Scholar]
- 152.Prospective UK. Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes. Lancet. 1998;352:854–865. doi: 10.1016/S0140-6736(98)07037-8. [DOI] [PubMed] [Google Scholar]
- 153.Chan JCN, et al. Role of metformin in the initiation of pharmacotherapy for type 2 diabetes: an Asian-Pacific perspective. Diabetes Res. Clin. Pract. 2007;75:255–266. doi: 10.1016/j.diabres.2006.06.023. [DOI] [PubMed] [Google Scholar]
- 154.Bhansali S, Bhansali A, Dutta P, Walia R, Dhawan V. Metformin upregulates mitophagy in patients with T2DM: a randomized placebo-controlled study. J. Cell. Mol. Med. 2020;24:2832–2846. doi: 10.1111/jcmm.14834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bi Y, et al. The beneficial effect of metformin on β-cell function in non-obese Chinese subjects with newly diagnosed type 2 diabetes. Diabetes Metab. Res. Rev. 2013;29:664–672. doi: 10.1002/dmrr.2443. [DOI] [PubMed] [Google Scholar]
- 156.Yang W, et al. Acarbose compared with metformin as initial therapy in patients with newly diagnosed type 2 diabetes: an open-label, non-inferiority randomised trial. Lancet Diabetes Endocrinol. 2014;2:46–55. doi: 10.1016/S2213-8587(13)70021-4. [DOI] [PubMed] [Google Scholar]
- 157.Zhang F, Xu S, Tang L, Pan X, Tong N. Acarbose with comparable glucose-lowering but superior weight-loss efficacy to dipeptidyl peptidase-4 inhibitors: a systematic review and network meta-analysis of randomized controlled trials. Front. Endocrinol. 2020;11:288. doi: 10.3389/fendo.2020.00288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Chiasson J-L, et al. Acarbose for prevention of type 2 diabetes mellitus: the STOP-NIDDM randomised trial. Lancet. 2002;359:2072–2077. doi: 10.1016/S0140-6736(02)08905-5. [DOI] [PubMed] [Google Scholar]
- 159.Zhu Q, Tong Y, Wu T, Li J, Tong N. Comparison of the hypoglycemic effect of acarbose monotherapy in patients with type 2 diabetes mellitus consuming an eastern or western diet: a systematic meta-analysis. Clin. Ther. 2013;35:880–899. doi: 10.1016/j.clinthera.2013.03.020. [DOI] [PubMed] [Google Scholar]
- 160.Hara T, et al. An importance of carbohydrate ingestion for the expression of the effect of α-glucosidase inhibitor in NIDDM. Diabetes Care. 1996;19:642–647. doi: 10.2337/diacare.19.6.642. [DOI] [PubMed] [Google Scholar]
- 161.Weng J, et al. Efficacy of acarbose in different geographical regions of the world: analysis of a real-life database. Diabetes Metab. Res. Rev. 2015;31:155–167. doi: 10.1002/dmrr.2576. [DOI] [PubMed] [Google Scholar]
- 162.Cai X, Han X, Luo Y, Ji L. Comparisons of the efficacy of alpha glucosidase inhibitors on type 2 diabetes patients between Asian and Caucasian. PLoS ONE. 2013;8:e79421. doi: 10.1371/journal.pone.0079421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gao X, et al. Meta-analysis and critical review on the efficacy and safety of alpha-glucosidase inhibitors in Asian and non-Asian populations. J. Diabetes Invest. 2018;9:321–331. doi: 10.1111/jdi.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Holman RR, et al. Effects of acarbose on cardiovascular and diabetes outcomes in patients with coronary heart disease and impaired glucose tolerance (ACE): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2017;5:877–886. doi: 10.1016/S2213-8587(17)30309-1. [DOI] [PubMed] [Google Scholar]
- 165.Colagiuri S, et al. The place of gliclazide MR in the evolving type 2 diabetes landscape: a comparison with other sulfonylureas and newer oral antihyperglycemic agents. Diabetes Res. Clin. Pract. 2018;143:1–14. doi: 10.1016/j.diabres.2018.05.028. [DOI] [PubMed] [Google Scholar]
- 166.Kalra S, et al. Place of sulfonylureas in the management of type 2 diabetes mellitus in South Asia: a consensus statement. Indian. J. Endocrinol. Metab. 2015;19:577–596. doi: 10.4103/2230-8210.163171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Kalra S, et al. The position of gliclazide in the evolving landscapes and disease continuum of T2DM: a collaborative delphi survey-based consensus from India. Diabetes Ther. 2021;12:679–695. doi: 10.1007/s13300-021-01002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Singla R, Bindra J, Singla A, Gupta Y, Kalra S. Drug prescription patterns and cost analysis of diabetes therapy in India: audit of an endocrine practice. Indian. J. Endocrinol. Metab. 2019;23:40–45. doi: 10.4103/ijem.IJEM_646_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Ji L, et al. China type 2 diabetes treatment status survey of treatment pattern of oral drugs users. J. Diabetes. 2015;7:166–173. doi: 10.1111/1753-0407.12165. [DOI] [PubMed] [Google Scholar]
- 170.Wang C, et al. Treatment patterns in patients with newly diagnosed type 2 diabetes in china: a retrospective, longitudinal database study. Clin. Ther. 2019;41:1440–1452. doi: 10.1016/j.clinthera.2019.05.003. [DOI] [PubMed] [Google Scholar]
- 171.ADVANCE Collaborative Group. et al. Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2008;358:2560–2572. doi: 10.1056/NEJMoa0802987. [DOI] [PubMed] [Google Scholar]
- 172.Woodward M, et al. Does glycemic control offer similar benefits among patients with diabetes in different regions of the world? Results from the ADVANCE trial. Diabetes Care. 2011;34:2491–2495. doi: 10.2337/dc11-0755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dennis JM. Precision medicine in type 2 diabetes: using individualized prediction models to optimize selection of treatment. Diabetes. 2020;69:2075–2085. doi: 10.2337/dbi20-0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Dennis JM, et al. Sex and BMI alter the benefits and risks of sulfonylureas and thiazolidinediones in type 2 diabetes: a framework for evaluating stratification using routine clinical and individual trial data. Diabetes Care. 2018;41:1844–1853. doi: 10.2337/dc18-0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Mulvihill EE, Drucker DJ. Pharmacology, physiology, and mechanisms of action of dipeptidyl peptidase-4 inhibitors. Endocr. Rev. 2014;35:992–1019. doi: 10.1210/er.2014-1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J. Diabetes Invest. 2016;7:102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Mitrakou A, et al. Role of reduced suppression of glucose production and diminished early insulin release in impaired glucose tolerance. N. Engl. J. Med. 1992;326:22–29. doi: 10.1056/NEJM199201023260104. [DOI] [PubMed] [Google Scholar]
- 178.Yang A, et al. Trends in glucose-lowering drug use, glycemic control, and severe hypoglycemia in adults with diabetes in Hong Kong, 2002–2016. Dia Care. 2020;43:2967–2974. doi: 10.2337/dc20-0260. [DOI] [PubMed] [Google Scholar]
- 179.Cai X, et al. DPP-4 inhibitor treatment in chinese type 2 diabetes patients: a meta-analysis. Diabetes Technol. Ther. 2016;18:784–793. doi: 10.1089/dia.2016.0302. [DOI] [PubMed] [Google Scholar]
- 180.Kubota K, et al. Penetration of new antidiabetic medications in East Asian countries and the United States: a cross-national comparative study. PLoS ONE. 2018;13:e0208796. doi: 10.1371/journal.pone.0208796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Chowdhury S, et al. Indian Expert Review on Use of teneligliptin in patients with diabetes and its safety and efficacy (INTENSE) J. Assoc. Phys. India. 2021;69:61–70. [PubMed] [Google Scholar]
- 182.Davis TME, et al. Effect of race on the glycaemic response to sitagliptin: Insights from the Trial Evaluating Cardiovascular Outcomes with Sitagliptin (TECOS) Diabetes Obes. Metab. 2018;20:1427–1434. doi: 10.1111/dom.13242. [DOI] [PubMed] [Google Scholar]
- 183.Dennis JM, et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short and long-term glycemic response to DPP4-inhibitor therapy. Diabetes Care. 2018;41:705–712. doi: 10.2337/dc17-1827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Singh AK. Incretin response in Asian type 2 diabetes: are Indians different? Indian. J. Endocrinol. Metab. 2015;19:30. doi: 10.4103/2230-8210.146861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim YG, et al. Differences in the glucose-lowering efficacy of dipeptidyl peptidase-4 inhibitors between Asians and non-Asians: a systematic review and meta-analysis. Diabetologia. 2013;56:696–708. doi: 10.1007/s00125-012-2827-3. [DOI] [PubMed] [Google Scholar]
- 186.Gan S, et al. Efficacy of modern diabetes treatments DPP-4i, SGLT-2i, and GLP-1RA in white and asian patients with diabetes: a systematic review and meta-analysis of randomized controlled trials. Diabetes Care. 2020;43:1948–1957. doi: 10.2337/dc19-2419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Cai X, Han X, Luo Y, Ji L. Efficacy of dipeptidyl-peptidase-4 inhibitors and impact on β-cell function in Asian and Caucasian type 2 diabetes mellitus patients: a meta-analysis. J. Diabetes. 2015;7:347–359. doi: 10.1111/1753-0407.12196. [DOI] [PubMed] [Google Scholar]
- 188.Gao W, Wang Q, Yu S. Efficacy, safety and impact on β-cell function of dipeptidyl peptidase-4 inhibitors plus metformin combination therapy in patients with type 2 diabetes and the difference between Asians and Caucasians: a meta-analysis. J. Endocrinol. Invest. 2016;39:1061–1074. doi: 10.1007/s40618-016-0465-1. [DOI] [PubMed] [Google Scholar]
- 189.Louisa M, Takeuchi M, Takeuchi M, Setiabudy R. A meta-analysis on treatment effects of thiazolidinediones for type 2 diabetes mellitus in Asian populations. Acta Med. Indones. 2011;43:14. [PubMed] [Google Scholar]
- 190.Barnett AH, et al. Rosiglitazone in type 2 diabetes mellitus: an evaluation in British Indo-Asian patients. Diabet. Med. 2003;20:387–393. doi: 10.1046/j.1464-5491.2003.00925.x. [DOI] [PubMed] [Google Scholar]
- 191.Qian X, et al. Pioglitazone improved insulin sensitivity and first phase insulin secretion among obese and lean people with diabetes: a multicenter clamp study. Diabetes Ther. 2018;9:815–826. doi: 10.1007/s13300-018-0401-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Boyko EJ, et al. Effects of ethnicity on diabetes incidence and prevention: results of the Diabetes REduction Assessment with ramipril and rosiglitazone Medication (DREAM) trial. Diabet. Med. 2010;27:1226–1232. doi: 10.1111/j.1464-5491.2010.03064.x. [DOI] [PubMed] [Google Scholar]
- 193.Roughead EE, et al. Variation in association between thiazolidinediones and heart failure across ethnic groups: retrospective analysis of large healthcare claims databases in six countries. Drug. Saf. 2015;38:823–831. doi: 10.1007/s40264-015-0318-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Dormandy JA, et al. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 195.Wilcox R, et al. Effects of pioglitazone in patients with type 2 diabetes with or without previous stroke: results from PROactive (PROspective pioglitAzone Clinical Trial In macroVascular Events 04) Stroke. 2007;38:865–873. doi: 10.1161/01.STR.0000257974.06317.49. [DOI] [PubMed] [Google Scholar]
- 196.Vaccaro O, et al. Cardiovascular effects of pioglitazone or sulfonylureas according to pretreatment risk: moving toward personalized care. J. Clin. Endocrinol. Metab. 2019;104:3296–3302. doi: 10.1210/jc.2019-00361. [DOI] [PubMed] [Google Scholar]
- 197.Miao S, et al. Detecting pioglitazone use and risk of cardiovascular events using electronic health record data in a large cohort of Chinese patients with type 2 diabetes. J. Diabetes. 2019;11:684–689. doi: 10.1111/1753-0407.12894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shukla R, Kalra S. Pioglitazone: Indian perspective. Indian. J. Endocrinol. Metab. 2011;15:294–297. doi: 10.4103/2230-8210.85581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Goyal A, et al. Impact of regulatory spin of pioglitazone on prescription of antidiabetic drugs among physicians in India: a multicentre questionnaire-based observational study. Indian. J. Med. Res. 2017;146:468–475. doi: 10.4103/ijmr.IJMR_1416_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Cai X, et al. No disparity of the efficacy and all-cause mortality between Asian and non-Asian type 2 diabetes patients with sodium–glucose cotransporter 2 inhibitors treatment: a meta-analysis. J. Diabetes Investig. 2018;9:850–861. doi: 10.1111/jdi.12760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Yang L, Zhang L, He H, Zhang M, An Z. Efficacy and safety of sodium-glucose cotransporter 2 inhibitors in East Asians with type 2 diabetes: a systematic review and meta-analysis. Diabetes Ther. 2019;10:1921–1934. doi: 10.1007/s13300-019-0674-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44:1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 203.Khoo CM, et al. Use of sodium-glucose co-transporter-2 inhibitors in Asian patients with type 2 diabetes and kidney disease: an Asian perspective and expert recommendations. Diabetes Obes. Metab. 2021;23:299–317. doi: 10.1111/dom.14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Deerochanawong C, et al. Use of sodium-glucose co-transporter-2 inhibitors in patients with type 2 diabetes mellitus and multiple cardiovascular risk factors: an Asian perspective and expert recommendations. Diabetes, Obes. Metab. 2019;21:2354–2367. doi: 10.1111/dom.13819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Hanif W, et al. Pharmacological management of South Asians with type 2 diabetes: consensus recommendations from the South Asian Health Foundation. Diabet. Med. 2021;38:e14497. doi: 10.1111/dme.14497. [DOI] [PubMed] [Google Scholar]
- 206.Mohan V, Mithal A, Joshi SR, Aravind SR, Chowdhury S. Remogliflozin etabonate in the treatment of type 2 diabetes: design, development, and place in therapy. Drug. Des. Dev. Ther. 2020;14:2487–2501. doi: 10.2147/DDDT.S221093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bizino MB, et al. Efficacy of liraglutide on glycemic endpoints in people of Western European and South Asian descent with T2DM using multiple daily insulin injections: results of the MAGNA VICTORIA studies. Acta Diabetol. 2021;58:485–493. doi: 10.1007/s00592-020-01635-0. [DOI] [PubMed] [Google Scholar]
- 208.Zhang F, Tang L, Zhang Y, Lü Q, Tong N. Glucagon-like peptide-1 mimetics, optimal for Asian type 2 diabetes patients with and without overweight/obesity: meta-analysis of randomized controlled trials. Sci. Rep. 2017;7:15997. doi: 10.1038/s41598-017-16018-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.He L, et al. Subpopulation differences in the cardiovascular efficacy of long-acting glucagon-like peptide 1 receptor agonists in type 2 diabetes mellitus: a systematic review and meta-analysis. Diabetes Ther. 2020;11:2121–2143. doi: 10.1007/s13300-020-00882-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Kang YM, et al. Asian subpopulations may exhibit greater cardiovascular benefit from long-acting glucagon-like peptide 1 receptor agonists: a meta-analysis of cardiovascular outcome trials. Diabetes Metab. J. 2019;43:410–421. doi: 10.4093/dmj.2018.0070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Jones AG, et al. Markers of β-cell failure predict poor glycemic response to GLP-1 receptor agonist therapy in type 2 diabetes. Diabetes Care. 2016;39:250–257. doi: 10.2337/dc15-0258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Pozzilli P, et al. Dulaglutide treatment results in effective glycaemic control in latent autoimmune diabetes in adults (LADA): a post-hoc analysis of the AWARD-2, -4 and -5 trials. Diabetes Obes. Metab. 2018;20:1490–1498. doi: 10.1111/dom.13237. [DOI] [PubMed] [Google Scholar]
- 213.Wang T, et al. Predictive factors associated with glycaemic response to exenatide in Chinese patients with type 2 diabetes mellitus. J. Clin. Pharm. Ther. 2020;45:1050–1057. doi: 10.1111/jcpt.13134. [DOI] [PubMed] [Google Scholar]
- 214.Yoo JH, et al. Clinical efficacy and parameters affecting the response to dulaglutide treatment in patients with type 2 diabetes: a retrospective, real-world data study. Diabetes Ther. 2019;10:1453–1463. doi: 10.1007/s13300-019-0658-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Kalra S, et al. Consensus recommendations on GLP-1 RA use in the management of type 2 diabetes mellitus: south asian task force. Diabetes Ther. 2019;10:1645–1717. doi: 10.1007/s13300-019-0669-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Chan JCN, et al. The Lancet Commission on diabetes: using data to transform diabetes care and patient lives. Lancet. 2020;396:2019–2082. doi: 10.1016/S0140-6736(20)32374-6. [DOI] [PubMed] [Google Scholar]
- 217.Indian Council of Medical Research. ICMR Guideline for Management of Type 2 Diabetes 2018. https://main.icmr.nic.in/content/guidelines-0 (2018). A document summarizing the standard approaches to the management of T2DM recommended in India.
- 218.Buse JB, et al. 2019 Update to: Management of Hyperglycemia in Type 2 Diabetes, 2018. A Consensus Report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Ferrannini E. The target of metformin in type 2 diabetes. N. Engl. J. Med. 2014;371:1547–1548. doi: 10.1056/NEJMcibr1409796. [DOI] [PubMed] [Google Scholar]
- 220.Rena G, Hardie DG, Pearson ER. The mechanisms of action of metformin. Diabetologia. 2017;60:1577–1585. doi: 10.1007/s00125-017-4342-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.LaMoia TE, Shulman GI. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021;42:77–96. doi: 10.1210/endrev/bnaa023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Expert Group of Metformin in Clinical Practice. Chinese expert consensus statement on metformin in clinical practice. Chin. Med. J. 2020;133:1445–1447. doi: 10.1097/CM9.0000000000000883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Chiasson J-L, et al. The effect of acarbose on insulin sensitivity in subjects with impaired glucose tolerance. Diabetes Care. 1996;19:1190–1193. doi: 10.2337/diacare.19.11.1190. [DOI] [PubMed] [Google Scholar]
- 224.Rosenstock J, et al. Effect of linagliptin vs placebo on major cardiovascular events in adults with type 2 diabetes and high cardiovascular and renal risk: the CARMELINA randomized clinical trial. JAMA. 2019;321:69–79. doi: 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Rosenstock J, et al. Effect of linagliptin vs glimepiride on major adverse cardiovascular outcomes in patients with type 2 diabetes: the CAROLINA randomized clinical trial. JAMA. 2019;322:1155–1166. doi: 10.1001/jama.2019.13772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Scheen AJ. Cardiovascular effects of new oral glucose-lowering agents. Circ. Res. 2018;122:1439–1459. doi: 10.1161/CIRCRESAHA.117.311588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Bays H, Mandarino L, DeFronzo RA. Role of the adipocyte, free fatty acids, and ectopic fat in pathogenesis of type 2 diabetes mellitus: peroxisomal proliferator-activated receptor agonists provide a rational therapeutic approach. J. Clin. Endocrinol. Metab. 2004;89:463–478. doi: 10.1210/jc.2003-030723. [DOI] [PubMed] [Google Scholar]
- 228.Home PD, et al. Rosiglitazone evaluated for cardiovascular outcomes in oral agent combination therapy for type 2 diabetes (RECORD): a multicentre, randomised, open-label trial. Lancet. 2009;373:2125–2135. doi: 10.1016/S0140-6736(09)60953-3. [DOI] [PubMed] [Google Scholar]
- 229.Cintra RM, et al. Glucose-lowering drugs and hospitalization for heart failure: a systematic review and additive-effects network meta-analysis with more than 500 000 patient-years. J. Clin. Endocrinol. Metab. 2021;106:3060–3067. doi: 10.1210/clinem/dgab428. [DOI] [PubMed] [Google Scholar]
- 230.Lewis JD, et al. Pioglitazone use and risk of bladder cancer and other common cancers in persons with diabetes. JAMA. 2015;314:265. doi: 10.1001/jama.2015.7996. [DOI] [PubMed] [Google Scholar]
- 231.Scheen AJ. Sodium–glucose cotransporter type 2 inhibitors for the treatment of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2020;16:556–577. doi: 10.1038/s41574-020-0392-2. [DOI] [PubMed] [Google Scholar]
- 232.Verma S, McMurray JJV. SGLT2 inhibitors and mechanisms of cardiovascular benefit: a state-of-the-art review. Diabetologia. 2018;61:2108–2117. doi: 10.1007/s00125-018-4670-7. [DOI] [PubMed] [Google Scholar]
- 233.Singh AK, et al. Evidence-based consensus on positioning of SGLT2i in type 2 diabetes mellitus in Indians. Diabetes Ther. 2019;10:393–428. doi: 10.1007/s13300-019-0562-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Zinman B, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N. Engl. J. Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 235.Neal B, et al. Canagliflozin and cardiovascular and renal events in type 2 diabetes. N. Engl. J. Med. 2017;377:644–657. doi: 10.1056/NEJMoa1611925. [DOI] [PubMed] [Google Scholar]
- 236.Wiviott SD, et al. Dapagliflozin and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2019;380:347–357. doi: 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 237.Palmer SC, et al. Sodium-glucose cotransporter protein-2 (SGLT-2) inhibitors and glucagon-like peptide-1 (GLP-1) receptor agonists for type 2 diabetes: systematic review and network meta-analysis of randomised controlled trials. BMJ. 2021;372:m4573. doi: 10.1136/bmj.m4573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Heerspink HJL, Perkins BA, Fitchett DH, Husain M, Cherney DZI. Sodium glucose cotransporter 2 inhibitors in the treatment of diabetes: cardiovascular and kidney effects, potential mechanisms and clinical applications. Circulation. 2016;134:752–772. doi: 10.1161/CIRCULATIONAHA.116.021887. [DOI] [PubMed] [Google Scholar]
- 239.Nauck MA, Meier JJ, Cavender MA, Aziz MAE, Drucker DJ. Cardiovascular actions and clinical outcomes with glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors. Circulation. 2017;136:849–870. doi: 10.1161/CIRCULATIONAHA.117.028136. [DOI] [PubMed] [Google Scholar]
- 240.Perkovic V, et al. Canagliflozin and renal outcomes in type 2 diabetes and nephropathy. N. Engl. J. Med. 2019;380:2295–2306. doi: 10.1056/NEJMoa1811744. [DOI] [PubMed] [Google Scholar]
- 241.Ueda P, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ. 2018;363:k4365. doi: 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Lin DS-H, Lee J-K, Chen W-J. Clinical adverse events associated with sodium-glucose cotransporter 2 inhibitors: a meta-analysis involving 10 randomized clinical trials and 71,553 individuals. J. Clin. Endocrinol. Metab. 2021;106:2133–2145. doi: 10.1210/clinem/dgab274. [DOI] [PubMed] [Google Scholar]
- 243.Chinese Diabetes SocietyChinese Society of Endocrinology. Expert consensus on glucose-lowering pharmacotherapies in Chinese adults with type 2 diabetes and cardiovascular disease or chronic kidney disease. Chin. J. Endocrinol. Metab. 2020;36:458–468. doi: 10.1002/dmrr.3416. [DOI] [PubMed] [Google Scholar]
- 244.Drucker DJ. The cardiovascular biology of glucagon-like peptide-1. Cell Metab. 2016;24:15–30. doi: 10.1016/j.cmet.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 245.Marso SP, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2016;375:311–322. doi: 10.1056/NEJMoa1603827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hernandez AF, et al. Albiglutide and cardiovascular outcomes in patients with type 2 diabetes and cardiovascular disease (Harmony Outcomes): a double-blind, randomised placebo-controlled trial. Lancet. 2018;392:1519–1529. doi: 10.1016/S0140-6736(18)32261-X. [DOI] [PubMed] [Google Scholar]
- 247.Gerstein HC, et al. Dulaglutide and cardiovascular outcomes in type 2 diabetes (REWIND): a double-blind, randomised placebo-controlled trial. Lancet. 2019;394:121–130. doi: 10.1016/S0140-6736(19)31149-3. [DOI] [PubMed] [Google Scholar]
- 248.Holman RR, et al. Effects of once-weekly exenatide on cardiovascular outcomes in type 2 diabetes. N. Engl. J. Med. 2017;377:1228–1239. doi: 10.1056/NEJMoa1612917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Pfeffer MA, et al. Lixisenatide in patients with type 2 diabetes and acute coronary syndrome. N. Engl. J. Med. 2015;373:2247–2257. doi: 10.1056/NEJMoa1509225. [DOI] [PubMed] [Google Scholar]
- 250.Marso SP, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2016;375:1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 251.Husain M, et al. Oral Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N. Engl. J. Med. 2019;381:841–851. doi: 10.1056/NEJMoa1901118. [DOI] [PubMed] [Google Scholar]
- 252.Chinese Society of EndocrinologyChinese Diabetes Society. [Consensus recommendations on utilizing glucagon-like pepetide-1 (GLP-1) receptor agonists in the treatment of type 2 diabetes mellitus] Zhonghua Nei Ke Za Zhi. 2020;59:836–846. doi: 10.3760/cma.j.cn112138-20200704-00646. [DOI] [PubMed] [Google Scholar]
- 253.Food and Agriculture Organization of the United Nations. Suite of food security indicators. FAOSTAThttp://www.fao.org/faostat/en/#home (2021).
- 254.Food and Agriculture Organization of the United Nations. The Future of Food and Agriculture–Alternative Pathways to 2050 (United Nations, 2018).
- 255.Rhodes EC, Gujral UP, Narayan KM. Mysteries of type 2 diabetes: the Indian elephant meets the Chinese dragon. Eur. J. Clin. Nutr. 2017;71:805–811. doi: 10.1038/ejcn.2017.93. [DOI] [PubMed] [Google Scholar]
- 256.Ma RCW, Lin X, Jia W. Causes of type 2 diabetes in China. Lancet Diabetes Endocrinol. 2014;2:980–991. doi: 10.1016/S2213-8587(14)70145-7. [DOI] [PubMed] [Google Scholar]
- 257.Hills AP, et al. Epidemiology and determinants of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6:966–978. doi: 10.1016/S2213-8587(18)30204-3. [DOI] [PubMed] [Google Scholar]
- 258.Bhavadharini B, et al. White rice intake and incident diabetes: a study of 132,373 participants in 21 countries. Diabetes Care. 2020;43:2643–2650. doi: 10.2337/dc19-2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Wei Y, Wang Z, Wang H, Li Y, Jiang Z. Predicting population age structures of China, India, and Vietnam by 2030 based on compositional data. PLoS ONE. 2019;14:e0212772. doi: 10.1371/journal.pone.0212772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.NCD Risk Factor Collaboration. Worldwide trends in diabetes since 1980: a pooled analysis of 751 population-based studies with 4·4 million participants. Lancet. 2016;387:1513–1530. doi: 10.1016/S0140-6736(16)00618-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.United Nations, Department of Economic and Social Affairs, & Population Division. World Urbanization Prospects: The 2018 Revision (United Nations, 2019).
- 262.Tandon N, et al. The increasing burden of diabetes and variations among the states of India: the Global Burden of Disease study 1990–2016. Lancet Glob. Health. 2018;6:e1352–e1362. doi: 10.1016/S2214-109X(18)30387-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Almgren P, et al. Heritability and familiality of type 2 diabetes and related quantitative traits in the Botnia study. Diabetologia. 2011;54:2811. doi: 10.1007/s00125-011-2267-5. [DOI] [PubMed] [Google Scholar]
- 264.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Flannick J, Florez JC. Type 2 diabetes: genetic data sharing to advance complex disease research. Nat. Rev. Genet. 2016;17:535–549. doi: 10.1038/nrg.2016.56. [DOI] [PubMed] [Google Scholar]
- 266.Sreeramareddy CT, Aye SN. Changes in adult smoking behaviours in ten global adult tobacco survey (GATS) countries during 2008–2018 - a test of ‘hardening’ hypothesis’. BMC Public Health. 2021;21:1209. doi: 10.1186/s12889-021-11201-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Mishra S, et al. Trends in bidi and cigarette smoking in India from 1998 to 2015, by age, gender and education. BMJ Glob. Health. 2016;1:e000005. doi: 10.1136/bmjgh-2015-000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Pan A, Wang Y, Talaei M, Hu FB, Wu T. Relation of active, passive, and quitting smoking with incident type 2 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3:958–967. doi: 10.1016/S2213-8587(15)00316-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jha P, Peto R. Global effects of smoking, of quitting, and of taxing tobacco. N. Engl. J. Med. 2014;370:60–68. doi: 10.1056/NEJMra1308383. [DOI] [PubMed] [Google Scholar]
- 270.Zhou M, et al. Geographical variation in diabetes prevalence and detection in china: multilevel spatial analysis of 98,058 adults. Diabetes Care. 2015;38:72–81. doi: 10.2337/dc14-1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Wu H, et al. Sex differences in the association between socioeconomic status and diabetes prevalence and incidence in China: cross-sectional and prospective studies of 0.5 million adults. Diabetologia. 2019;62:1420–1429. doi: 10.1007/s00125-019-4896-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Seiglie JA, et al. Diabetes prevalence and its relationship with education, wealth, and BMI in twenty-nine low- and middle-income countries. Diabetes Care. 2020;43:767–775. doi: 10.2337/dc19-1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.World Health Organization. Global Spending on Health: Weathering the Storm (World Health Organization, 2020).
- 274.Yip W, Mahal A. The health care systems of China and India: performance and future challenges. Health Aff. 2008;27:921–932. doi: 10.1377/hlthaff.27.4.921. [DOI] [PubMed] [Google Scholar]
- 275.Jan S, et al. Catastrophic health expenditure on acute coronary events in Asia: a prospective study. Bull. World Health Organ. 2016;94:193–200. doi: 10.2471/BLT.15.158303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chan JCN, Zhang Y, Ning G. Diabetes in China: a societal solution for a personal challenge. Lancet Diabetes Endocrinol. 2014;2:969–979. doi: 10.1016/S2213-8587(14)70144-5. [DOI] [PubMed] [Google Scholar]
- 277.Hills AP, et al. Public health and health systems: implications for the prevention and management of type 2 diabetes in south Asia. Lancet Diabetes Endocrinol. 2018;6:992–1002. doi: 10.1016/S2213-8587(18)30203-1. [DOI] [PubMed] [Google Scholar]
- 278.Kalra S, Balhara YPS, Mithal A. Cross-cultural variation in symptom perception of hypoglycemia. J. Midlife Health. 2013;4:176–181. doi: 10.4103/0976-7800.118998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Xu Y, Toobert D, Savage C, Pan W, Whitmer K. Factors influencing diabetes self-management in Chinese people with type 2 diabetes. Res. Nurs. Health. 2008;31:613–625. doi: 10.1002/nur.20293. [DOI] [PubMed] [Google Scholar]
- 280.International Diabetes Federation and the DAR International Alliance. Diabetes and Ramadan: Practical Guidelines 2021 (International Diabetes Federation, 2021).
- 281.Gupta L, Khandelwal D, Singla R, Gupta P, Kalra S. Pragmatic dietary advice for diabetes during Navratris. Indian. J. Endocrinol. Metab. 2017;21:231. doi: 10.4103/2230-8210.196009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Ibrahim SRM, Mohamed GA. Litchi chinensis: medicinal uses, phytochemistry, and pharmacology. J. Ethnopharmacol. 2015;174:492–513. doi: 10.1016/j.jep.2015.08.054. [DOI] [PubMed] [Google Scholar]
- 283.Ge S, et al. A unique understanding of traditional medicine of pomegranate, Punica granatum L. and its current research status. J. Ethnopharmacol. 2021;271:113877. doi: 10.1016/j.jep.2021.113877. [DOI] [PubMed] [Google Scholar]
- 284.Zhao L, Wang K, Wang K, Zhu J, Hu Z. Nutrient components, health benefits, and safety of litchi (Litchi chinensis Sonn.): a review. Compr. Rev. Food Sci. Food Saf. 2020;19:2139–2163. doi: 10.1111/1541-4337.12590. [DOI] [PubMed] [Google Scholar]
- 285.Dutta D, Khandelwal D, Kalra S. Litchi-related hypoglycemia: a public health challenge, an endocrine opportunity. Indian. J. Endocrinol. Metab. 2019;23:380–381. doi: 10.4103/ijem.IJEM_235_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Tanase DM, et al. Role of gut microbiota on onset and progression of microvascular complications of type 2 diabetes (T2DM) Nutrients. 2020;12:3719. doi: 10.3390/nu12123719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.DeFronzo RA, Tobin JD, Andres R. Glucose clamp technique: a method for quantifying insulin secretion and resistance. Am. J. Physiol. 1979;237:E214–E223. doi: 10.1152/ajpendo.1979.237.3.E214. [DOI] [PubMed] [Google Scholar]
- 288.Wallace TM, Levy JC, Matthews DR. Use and abuse of HOMA modeling. Diabetes Care. 2004;27:1487–1495. doi: 10.2337/diacare.27.6.1487. [DOI] [PubMed] [Google Scholar]
- 289.Cersosimo E, Solis-Herrera C, Trautmann ME, Malloy J, Triplitt CL. Assessment of pancreatic β-cell function: review of methods and clinical applications. Curr. Diabetes Rev. 2014;10:2–42. doi: 10.2174/1573399810666140214093600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hannon TS, et al. Review of methods for measuring β-cell function: design considerations from the Restoring Insulin Secretion (RISE) Consortium. Diabetes Obes. Metab. 2018;20:14–24. doi: 10.1111/dom.13005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Wallace TM, Matthews DR. Coefficient of failure: a methodology for examining longitudinal β-cell function in type 2 diabetes. Diabet. Med. 2002;19:465–469. doi: 10.1046/j.1464-5491.2002.00718.x. [DOI] [PubMed] [Google Scholar]
- 292.Brown JB, Nichols GA, Perry A. The burden of treatment failure in type 2 diabetes. Diabetes Care. 2004;27:1535–1540. doi: 10.2337/diacare.27.7.1535. [DOI] [PubMed] [Google Scholar]