Abstract
Background:
The overall incidence and mortality of gastric cancer have steadily declined in the United States over the past few decades, but it is still a serious disease burden for patients. Therefore, it is of great significance to evaluate the latest survival rate of gastric cancer.
Methods:
Based on the Surveillance, Epidemiology, and End Results database, this study analyzed the age-standardized relative survival rates and survival trends of gastric cancer cases in 2007–2011 and 2012–2016 using period analysis, and the survival rate 2017–2021 was predicted using a generalized linear model based on the period analysis.
Results:
During 2007–2016, the 5-year relative survival rate of patients with gastric cancer continued to rise, and the same trend was observed in 2017–2021. The 5-year overall age-standardized relative survival rates in 2007–2011, 2012–2016, and 2017–2021 were 38.3%, 40.6%, and 42.9%, respectively. However, despite these favorable trends, the overall relative survival of patients with gastric cancer remains at a low level. There were significant differences in the relative survival rates of patients with gastric cancer in terms of age, sex, race, primary site, stage, and socioeconomic status. Notably, the survival rate of patients with distant-stage gastric cancer remains very low (10%).
Conclusion:
We found that the survival rate of patients with gastric cancer showed different degrees of improvement in each subgroup. However, the overall relative survival rate of patients with gastric cancer remains low. Analyzing the changes of patients with gastric cancer in the last 10 years will be helpful in predicting the changing trend of cancer in the future. It also provides a scientific basis for relevant departments to formulate effective tumor prevention and control measures.
Keywords: gastric cancer; surveillance, epidemiology, and end results; period analysis; survival rate; prediction
Introduction
Gastric cancer (GC) is the fifth most common cancer worldwide and the fourth leading cause of cancer death, with an estimated 700,000 deaths in 2020. 1 So far, although the incidence and mortality of GC have been steadily declining in many high-income countries, such as the United States, it remains a health issue for which there is widespread global concern.1,2 The incidence of GC is still high in East Asia and Europe. Since GC is often asymptomatic before it progresses to the advanced stage, it has a low early diagnosis rate. Most cases of GC are often diagnosed at an advanced stage. 3 The low survival rate indicates that the prognosis of patients with GC is still poor.
The 5-year survival rate of patients with cancer is an important indicator for evaluating the effects of cancer treatment and monitoring the prognosis of patients with cancer. In order to provide accurate and timely information, estimates of the survival for patients with cancer should be as up-to-date as possible. More importantly, such estimates would accurately indicate the overall status and dynamic trends of the long-term survival rate of patients with cancer in a country or region, and allows determination of the underlying factors, which can motivate relevant departments to perform intervention measures to improve the survival rates of patients.
Due to the general delay in data registration and release of tumor registry systems, it is difficult to obtain the latest data reflecting the current survival situation of patients with cancer. 4 Model-based period analysis can use existing data to accurately estimate survival rates, analyze change trends, and predict future survival rates. 5 The present study used tumor registry follow-up data up to 2016 based on data from 2007 to 2011 and 2012 to 2016 in model-based periodic analysis to estimate survival rates and analyze trends during these two periods. Five-year survival was also predicted for patients during 2017–2021. This method has been widely used worldwide to estimate the long-term survival of patients with cancer.4,6-8
The purpose of this study was to determine the relative survival rates and trends of patients with GC from 2007 to 2016, and to predict the survival rate during2017–2021 using a model-based period analysis method.
Materials and Methods
Data Source
The data analyzed in this study was obtained from the Surveillance, Epidemiology, and End Results (SEER) database, which is a large, population-based data set that collects cancer data from 18 registries across the country and currently covers approximately 35% of the U.S. population. 9 SEER*Stat software (version 8.3.9) was used to extract the data of patients diagnosed with GC from 2002 to 2016. The period of interest in this study was 2007–2016. The flow chart of patient selection was shown in Figure 1.
Figure 1.
Flowchart of sample selection.
All the steps in this study follow the principles outlined in the 1964 Helsinki Declaration and its subsequent amendments. This study was exempted the informed consent and review approval by the Institutional Research Committee of School of Basic Medicine and Public Health of Jinan University, because the SEER data were public and all patient information was de-identified.
Variable Selection
The variables included in this study were sex, age at diagnosis, race, socioeconomic status, stage, primary site, year and month of diagnosis, year and month of last follow-up, time of follow-up and survival status. The year and month of diagnosis were extracted from the SEER database, and the year and month of last follow-up are calculated by the year and month of diagnosis, and the time of follow-up (month). Age was divided into three groups: <40, 40–69, and ≥70 years old. Race was divided into white, black, American Indian/Alaska Native, Asian and Pacific Islander. Socioeconomic status is classified into four groups according to the poverty rate of the patient’s area: rich (<4.9%), low poverty (≥5.0% and <9.9%), and middle poverty (≥10.0% and <19.9%) and high poverty (≥20.0%). 10 The classification criteria for primary site were cardia, non-cardiac, and NOS, and for stage were localized, regional, and distant.
Statistical Analysis
Descriptive statistics were used to summarize the sociodemographic and clinical characteristics of each observation period. Relative survival rate was calculated as the ratio of the observed survival to the expected survival of the general population with the same periods, sexes, and ages
where S𝑘 and represent the observed survival rate and expected survival rate. When calculating the relative survival rate at 5 years, k is 5. The expected survival rate was derived from the life table stratified by age and calendar year released by the Centers for Disease Control and Prevention of the United States. 11 The Ederer II method was used to calculate the expected survival rates. 12 In order to compare with estimates from different periods, the relative survival rates were age-standardized (standardized to the 2000 US population).
Period analysis was used to estimate the relative survival rates during 2007–2011and 2012–2016. In addition, data from 2002 to 2006 were used to estimate survival rates for 2007–2011.A generalized linear model 13 was established to predict the 5-year relative survival rate of patients diagnosed in 2017–2021. The above analysis process was performed using the PeriodR package of R software.
Results
The distribution of GC cases in the three periods is listed in Table 1. From the SEER database, 85,742 GC cases were included in the study from 2002 to 2016, of which 58,669 were in 2007–2016. Most patients were older than 40 years, male, white, and had a primary site of gastric cardia. The distribution of patients between the three stages was relatively balanced, and most patients were from areas with medium poverty and higher poverty. The number of cases in each observation period was relatively stable in terms of age, sex, race, stage, primary site, and socioeconomic status.
Table 1.
Distribution of Gastric Cancer Cases from 2002 to 2016.
| Variables | 2002–2006 n (%) | 2007–2011 n (%) | 2012–2016 n (%) | P-value |
|---|---|---|---|---|
| All | 27,073 | 28,655 | 30,014 | |
| Age | <.001 | |||
| <40 | 1012 (3.7) | 1071 (3.7) | 1107 (3.7) | |
| 40–69 | 12,930 (47.8) | 14,629 (51.1) | 15,880 (52.9) | |
| ≥70 | 13,131 (48.5) | 12,955 (45.2) | 13,027 (43.4) | |
| Sex | .456 | |||
| Male | 16,387 (60.5) | 17,395 (60.6) | 18,318 (61.0) | |
| Female | 10,686 (39.5) | 11,260 (39.3) | 11,696 (39.0) | |
| Race | .069 | |||
| White | 19,686 (72.7) | 20,675 (72.2) | 21,547 (71.8) | |
| Black | 3496 (12.9) | 3889 (13.6) | 4032 (13.4) | |
| American Indian/Alaska Native | 228 (.8) | 220 (.8) | 271 (.9) | |
| Asian and Pacific Islander | 3663 (13.5) | 3871 (13.5) | 4164 (13.9) | |
| Socioeconomic status | .170 | |||
| Rich | 1154 (4.3) | 1251 (4.4) | 1318 (4.4) | |
| Low poverty | 8647 (31.9) | 9102 (31.8) | 9452 (31.5) | |
| Medium poverty | 15,101 (55.8) | 15,852 (55.3) | 16,660 (55.5) | |
| High poverty | 2171 (8.0) | 2450 (8.5) | 2584 (8.6) | |
| Stage | <.001 | |||
| Localized | 9075 (33.5) | 10,152 (35.4) | 10,970 (36.5) | |
| Regional | 9168 (33.9) | 8768 (30.6) | 8465 (28.2) | |
| Distant | 8830 (32.6) | 9735 (34.0) | 10,579 (35.2) | |
| Primary site | <.001 | |||
| Cardia | 6745 (24.9) | 7734 (27.0) | 8682 (28.9) | |
| Non-cardia | 15,407 (56.9) | 15,871 (55.4) | 16,148 (53.8) | |
| NOS | 4921 (18.2) | 5050 (17.6) | 5184 (17.3) | |
Note. Not all columns round to 100% due to rounding.
Abbreviation: NOS, not otherwise specified.
The age-standardized 5-year relative survival rates of GC in terms of sex, race, primary site, stage, and socioeconomic status are shown in Supplementary Material Table S1. The change trends of all gastric cancer patients and between different sexes are shown in Figure 2. According to the results estimated by the period analysis, from 2007 to 2016, the survival rate of patients with GC showed an upward trend. The survival rates of male and female patients differed significantly, with the relative survival rate being much higher in females than males. The generalized linear model predicted that the 5-year overall relative survival of patients with GC during 2017–2021 was 42.9%. The 5-year relative survival for male and female patients was 38.3% and 50.4%, respectively.
Figure 2.
Trends of 5-year relative survival rate of all gastric cancer patients and between different sexes.
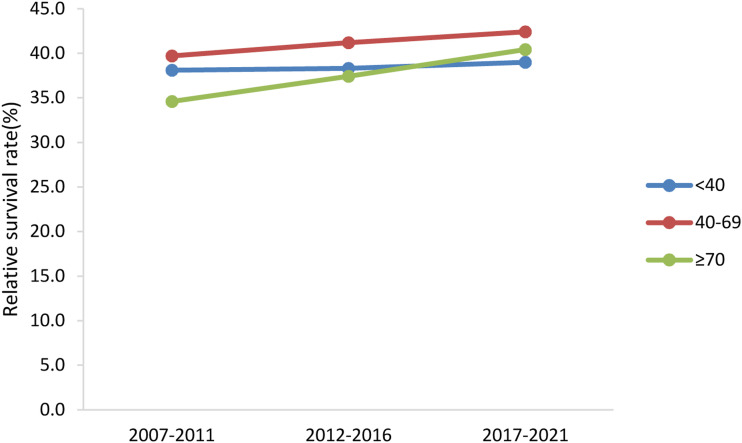
The change trends of age-specific relative survival rate of GC patients were shown in Figure 3. The survival rates of patients of all ages increased over the last 10 years. The survival rate of patients older than 70 and younger than 40 years old was low, and that of those aged 40–69 years was relatively high. The generalized linear model predicted that during 2017–2021, the 5-year relative survival rate for patients younger than 40 years was 39.0%, and that of patients aged 40–69 years was 42.4%. The 5-year relative survival rate for patients ≥70 years was 40.4%.
Figure 3.
Trends of age-specific 5-year relative survival rate in patients with gastric cancer.
The change trends of 5-year relative survival rate of GC patients with different races are shown in Figure 4. Asian and Pacific Islander patients had the highest 5-year relative survival rate among races, reaching 46.5% in 2012–2016. Compared with 2012–2016, the survival rate of all races is expected to increase in 2017–2021. The generalized linear model predicted that during 2017–2021, the 5-year relative survival rates of white, black, American Indian, and Asian patients were 42.3%, 44.3%, 33.0%, and 46.7%, respectively.
Figure 4.
Trends of 5-year relative survival rate in patients with gastric cancer of different races.
Figure 5 displays the change trends of 5-year relative survival rate of GC patients with different socioeconomic status. The 5-year relative survival rate of patients with GC in 2012–2016 was the highest among those from rich areas, and lowest among patients from high-poverty areas. With the decline of socioeconomic status, the survival rate of patients with GC also had been declining. Patients in highly poor areas showed a slight upward trend. The generalized linear model predicted that from 2017 and 2021, the 5-year relative survival rate of patients with GC from rich, low-poor, middle-poor, and high-poor areas were 48.9%, 46.0%, 40.9%, and 35.1%, respectively.
Figure 5.
Trends of 5-year relative survival rate in patients with gastric cancer of different races.
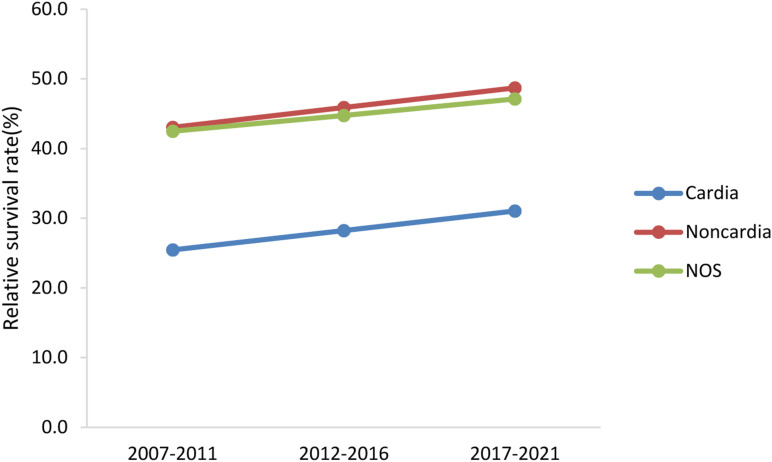
The change trends of 5-year relative survival rate of GC patients with primary site are shown in Figure 6. There was a significant difference in the survival rate between cardia and non-cardiac sites, the survival rate of patients with cardia cancer was much lower than that of non-cardia cancer. The 5-year relative survival rate of patients at each primary site continually increased over the last 10 years, and was predicted to have the same trend during 2017–2021. The generalized linear model predicted that the relative survival rates of patients with cardia and non-cardiac cancer was 31.0% and 48.7%, respectively.
Figure 6.
Trends of 5-year relative survival rate in patients with gastric cancer of different primary sites.
The change trends of 5 - year relative survival rate of GC patients with stage are shown in Figure 7. Compared with distant-stage and regional-stage patients with GC, localized-stage patients had the highest survival rate of more than 70% in 2007–2016, which was also forecasted for 2017–2021. However, the survival rate of distant patients was very low, reaching just 10% in 2012–2016. The generalized linear model predicted that the 5-year survival rates of localized, regional, and distant patients in 2017–2021 were 77.7%, 37.4%, and 10.2%, respectively.
Figure 7.
Trends of 5-year relative survival rate in patients with gastric cancer of different stages.
Discussion
The current study estimated the latest GC age-standardized survival rates and changing trends and the results of the study indicated that the survival rate of GC has only improved moderately in recent years. Overall relative survival for GC remains low.
Our research found that compared with 2007–2011, the 5-year overall relative survival rate of patients with GC increased during 2012–2016, and this trend was predicted to continue during 2017–2021. This increasing tendency was observed in all subgroups (including sex, race, age, stage, primary site, and socioeconomic status). The main reasons might be the improvement of the living conditions and health awareness, the continuously deepening understanding of pathogenesis, and the development of medical technologies such as surgical methods and targeted therapy.
From 2002 to 2016, the proportion of male to female GC cases registered in the SEER database was approximately 1.5:1, which was similar to the results reported in the 2016 American Cancer Statistics. 14 The results also indicated that male patients had a much lower 5-year survival rate than female patients. This might be attributed to differences in lifestyle and diet habits between sexes.15,16 Men are more inclined to high-calorie eating habits and bad habits such as smoking and drinking than women. Some studies have shown that greater exposure to estrogen may be related to a reduced GC risk. 17 GC incidence increases after menopause, and estrogen replacement therapy can significantly reduce the incidence and mortality of GC in postmenopausal women.18-20
This study found that age was an important influencing factor of GC. Elderly patients were comprised a larger proportion of affected subjects. Interestingly, patients aged 40–69 had a higher survival rate than younger patients (<40 years old). This may be because experts recommend regular GC endoscopic screening for people older than 40 years with high-risks of GC, so that they can detect lesions early and perform timely effective treatment. 21 Young people are less likely to suffer from GC, and they do not give it enough attention; once they are diagnosed with stomach cancer, the prognosis is usually poor.
Unlike in other cancers, we observed a large difference in survival rates between white and Asian patients with GC, the survival rate of Asian patients was higher than that of patients from other races. This result was consistent with previous studies. 22 Due to the relatively high prevalence and common screening activities of GC in Asian countries, some immigrants from Asia may have completed screening before immigrating to the United States. 23 At the same time, some Asian Americans may also be more aware of the risk of GC, so they are more likely to be proactive in GC screening, early detection, and aggressive treatment.
The present study showed that the 5-year survival rate of cardia cancer and non-cardia cancer has improved continuously in the past 10 years, and cardia cancer has a poor survival rate compared with non-cardia cancer cardia cancer. Many existing studies around the world have found that the 5-year survival rate of cardiac cancer is significantly lower than that of gastric non-cardiac cancer.24-26 A Chinese-registered study 26 showed that the poor prognosis of GC was associated with a significant increase in the proportion of male patients, older age, advanced pathological stages and poor clinicopathological features at diagnosis compared with non-GC. A study 27 in Sweden from 1990 to 2013 showed that the postoperative survival rates of cardiac cancer and non-cardiac cancer in the two sub-sites were continuously improved in 23 years. The prognosis of these patients has improved significantly, possibly due to better selection of surgical patients, widespread introduction of multidisciplinary team meetings and wider use of neoadjuvant chemotherapy.28,29 A study 30 in Florida showed that non-cardia cancer risk was highest among Asians and Pacific Islanders, and lowest among whites. This is also an important reason why the survival rate of white patients is lower than that of Asian patients.
In addition, the survival rate of patients with different socioeconomic statuses improved in 2002–2016 and 2017–2021. And with the deepening of poverty, the survival rate of GC is gradually decreasing. People with disadvantaged socioeconomic status may have poor survival outcomes due to obstacles in medical and health care services, lack of social assistance, inability to understand or a lack of medical information, and delayed medical treatment.31,32
GC survival is closely related to the stage of diagnosis. In this study, the 5-year relative survival rate of the localize-stage patients was 77.7%, while the 5-year relative survival rate of the advanced (distant-stage) patients was 10.2%. Unfortunately, most patients with GC are diagnosed at the advanced stage. Early detection and treatment is the key to prolonging the survival time of patients with GC. Gastroscopy screening can detect precancerous lesions early and allow timely treatment, which can effectively reduce the mortality of patients with GC.33-35 Since the implementation of population-based GC screening in Japan and South Korea, more patients have been diagnosed early, improving the survival rate.36,37 Due to the relatively low incidence in the United States, large-scale screening is not cost-effective and population-based GC screening is not currently recommended. 23 However, screening for groups with a high-risk of GC should be considered. Screening for GC in appropriate populations may increase likelihood of early GC detection and a decreased death rate from it. There is large amount of evidence that HP infection, family history of GC, and first-generation immigrants from high-incidence areas are associated with an increased GC risk, and so individuals with these risk factors can be regarded as being at high-risk.21,38 It is generally recommended that high-risk groups undergo endoscopic monitoring every 2–3 years. Since endoscopic screening of high-risk groups can further stratify patients, it is necessary to establish a screening and monitoring program for high-risk individuals.
Limitations
This study has some limitations: (1) the inherent limitations of retrospective studies; (2) because the tumor staging changes over time in the SEER database, the results of the analysis must be viewed with caution; (3) the SEER database lacks some potential important factors such as treatment method, certain biological indicators, and behavioral habits and thus it is difficult to evaluate their impacts on survival.
Conclusions
In summary, we found that the relative survival rate of patients with GC continually improved during 2007–2016 and 2017–2021, in various subgroups (age, sex, race, stage, primary site, and socioeconomic status) have increased to varying degrees. However, the relative survival rate of patients with GC remains low. The changing trends of survival rates indicated that the difference between socioeconomic statuses is gradually increasing over time. Notably, the 5-year survival rate of GC in the distant stage is very low. The prognoses of GC with different sites is significantly different primary sites, with the survival rate being much lower at the cardia site than at the non-cardia site, which is worthy of further investigation. Understanding the survival rate of GC over the past 10 years not only helps to predict future trends, but also helps to better design clinical programs and develop appropriate health policies to improve the prognosis of gastric cancer.
Supplemental Material
Supplemental Material for Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis by Yunmei Li, Aozi Feng, Shuai Zheng, Chong Chen, and Jun Lyu in Cancer Control
Acknowledgments
We acknowledge the efforts of the Surveillance, Epidemiology, and End Results (SEER) Program registries for creating the SEER database.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Aozi Feng https://orcid.org/0000-0002-8991-0961
References
- 1.Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality Worldwide for 36 cancers in 185 Countries. CA Cancer J Clin. 2021;71(3):209-249. [DOI] [PubMed] [Google Scholar]
- 2.Allemani C, Matsuda T, Di Carlo V, et al. Global surveillance of trends in cancer survival 2000–14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries. Lancet. 2018;391(10125):1023-1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jim MA, Pinheiro PS, Carreira H, Espey DK, Wiggins CL, Weir HK. Stomach cancer survival in the United States by race and stage (2001-2009): findings from the CONCORD-2 study. Cancer. 2017;123(suppl 24):4994-5013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jiang X, Wang L, Cheng Y, Tang H, Chen T. Assessment of long-term survival of cancer patients using cancer registry data from eastern China: period analysis is superior to traditional methods. Int J Cancer. 2020;147(4):996-1005. [DOI] [PubMed] [Google Scholar]
- 5.Brenner H, Hakulinen T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol. 2006;164(7):689-696. [DOI] [PubMed] [Google Scholar]
- 6.Pulte D, Gondos A, Brenner H. Trends in survival after diagnosis with hematologic malignancy in adolescence or young adulthood in the United States, 1981-2005. Cancer. 2009;115(21):4973-4979. [DOI] [PubMed] [Google Scholar]
- 7.Brenner H. Long-term survival rates of cancer patients achieved by the end of the 20th century: a period analysis. Lancet. 2002;360(9340):1131-1135. [DOI] [PubMed] [Google Scholar]
- 8.Pulte D, Gondos A, Brenner H. Improvement in survival of older adults with multiple myeloma: results of an updated period analysis of SEER data. Oncologist. 2011;16(11):1600-1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yang J, Li Y, Liu Q, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Based Med. 2020;13(1):57-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures—the public health disparities geocoding project. Am J Publ Health. 2003;93(10):1655-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Center for Disease Control and Prevention . Life tables[DB/OL]; 2021:7–20. Available from: https://www.cdc.gov/nchs/products/life_tables.htm. Accessed June 11, 2021.
- 12.Holleczek B, Gondos A, Brenner H. periodR - an R package to calculate long-term cancer survival estimates using period analysis. Methods Inf Med. 2009;48(2):123-128. [DOI] [PubMed] [Google Scholar]
- 13.Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. 2021;8(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7-30. [DOI] [PubMed] [Google Scholar]
- 15.Marques-Lespier JM, Gonzalez-Pons M, Cruz-Correa M. Current perspectives on gastric cancer. Gastroenterol Clin North Am. 2016;45(3):413-428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Abnet CC, Corley DA, Freedman ND, Kamangar F. Diet and upper gastrointestinal malignancies. Gastroenterology. 2015;148(6):1234-1243.e1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Camargo MC, Goto Y, Zabaleta J, Morgan DR, Correa P, Rabkin CS. Sex hormones, hormonal interventions, and gastric cancer risk: a meta-analysis. Cancer Epidemiol Biomarkers Prev. 2012;21(1):20-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frise S, Kreiger N, Gallinger S, Tomlinson G, Cotterchio M. Menstrual and reproductive risk factors and risk for gastric adenocarcinoma in women: findings from the canadian national enhanced cancer surveillance system. Ann Epidemiol. 2006;16(12):908-916. [DOI] [PubMed] [Google Scholar]
- 19.Lindblad M, Ye W, Lagergren CR, Lagergren J. Estrogen and risk of gastric cancer: a protective effect in a nationwide cohort study of patients with prostate Cancer in Sweden. Cancer Epidemiol Biomarkers Prev. 2004;13(12):2203-2207. [PubMed] [Google Scholar]
- 20.Chen C, Gong X, Yang X, et al. The roles of estrogen and estrogen receptors in gastrointestinal disease. Oncol Lett. 2019;18(6):5673-5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim GH, Liang PS, Bang SJ, Hwang JH. Screening and surveillance for gastric cancer in the United States: is it needed? Gastrointest Endosc. 2016;84(1):18-28. [DOI] [PubMed] [Google Scholar]
- 22.Klapheke AK, Carvajal-Carmona LG, Cress RD. Racial/ethnic differences in survival among gastric cancer patients in california. Cancer Causes Control. 2019;30(7):687-696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Taylor VM, Ko LK, Hwang JH, Sin MK, Inadomi JM. Gastric cancer in asian american populations: a neglected health disparity. Asian Pac J Cancer Prev. 2014;15(24):10565-10571. [DOI] [PubMed] [Google Scholar]
- 24.Anderson LA, Tavilla A, Brenner H, et al. Survival for oesophageal, stomach and small intestine cancers in Europe 1999–2007: results from EUROCARE-5. Eur J Cancer. 2015;51(15):2144-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng L, Wu C, Xi P, et al. The survival and the long-term trends of patients with gastric cancer in Shanghai, China. BMC Cancer. 2014;14:300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhao J, Zhao J, Du F, et al. Cardia and non-cardia gastric cancer have similar stage-for-stage prognoses after R0 resection: a large-scale, multicenter study in China. J Gastrointest Surg. 2016;20(4):700-707. [DOI] [PubMed] [Google Scholar]
- 27.Asplund J, Kauppila JH, Mattsson F, Lagergren J. Survival trends in gastric adenocarcinoma: a population-based study in Sweden. Ann Surg Oncol. 2018;25(9):2693-2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nelen SD, Heuthorst L, Verhoeven RHA, et al. Impact of centralizing gastric cancer surgery on treatment, morbidity, and mortality. J Gastrointest Surg. 2017;21:2000-2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Young JA, Shimi SM, Kerr L, McPhillips G, Thompson AM. Reduction in gastric cancer surgical mortality over 10 years: an adverse events analysis. Ann Med Surg. 2014;3(2):26-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu J, Medina H, Reis IM, Sussman DA, Pinheiro PS. Disadvantages for non-Hispanic whites in gastric carcinoma survival in Florida. Cancer Causes Control. 2020;31(9):815-826. [DOI] [PubMed] [Google Scholar]
- 31.Huang RJ, Shah SC, Camargo MC, Palaniappan L, Hwang JH. County rurality and socioeconomic deprivation is associated with reduced survival from gastric cancer in the United States. Gastroenterology. 2020;159(4):1555-1557.e1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Qurayshi Z, Randolph GW, Srivastav S, Kandil E. Outcomes in endocrine cancer surgery are affected by racial, economic, and healthcare system demographics. Laryngoscope. 2016;126(3):775-781. [DOI] [PubMed] [Google Scholar]
- 33.Hamashima C, Shibuya D, Yamazaki H, et al. The Japanese guidelines for gastric cancer screening. Jpn J Clin Oncol. 2008;38(4):259-267. [DOI] [PubMed] [Google Scholar]
- 34.Pasechnikov V, Chukov S, Fedorov E, Kikuste I, Leja M. Gastric cancer: prevention, screening and early diagnosis. World J Gastroenterol. 2014;20(38):13842-13862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hamashima C. Current issues and future perspectives of gastric cancer screening. World J Gastroenterol. 2014;20(38):13767-13774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Isobe Y, Nashimoto A, Akazawa K, et al. Gastric cancer treatment in Japan: 2008 annual report of the JGCA nationwide registry. Gastric Cancer. 2011;14(4):301-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hamashima C, Ogoshi K, Narisawa R, et al. Impact of endoscopic screening on mortality reduction from gastric cancer. World J Gastroenterol. 2015;21(8):2460-2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marcos-Pinto R, Carneiro F, Dinis-Ribeiro M, et al. First-degree relatives of patients with early-onset gastric carcinoma show even at young ages a high prevalence of advanced OLGA/OLGIM stages and dysplasia. Aliment Pharmacol Ther. 2012;35(12):1451-1459. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis by Yunmei Li, Aozi Feng, Shuai Zheng, Chong Chen, and Jun Lyu in Cancer Control