Abstract
Human epidermal growth factor receptor 2–positive breast cancer (HER2+BC) is a common malignancy that is prone to recurrence and metastasis in the early stages, resulting in a poor prognosis for patients. Many studies have suggested that targeted therapy promotes clinical outcomes in HER2+BC. With the introduction of trastuzumab in 1998, the prognosis of patients with early HER2+BC has improved significantly. However, owing to obstinate drug resistance and adverse events, the addition of new agents in standardized treatment has become a research hotspot. These promising agents include antibodies, antibody-drug conjugates, tyrosine kinase inhibitors, and anti-HER2 combined therapies. This article provides a brief description of the biology of BC and the expression of HER2, with the aim to provide an overview of the therapeutic landscape of HER2+BC by reviewing research results and introducing the latest evidence to provide a reference for clinical treatment.
Keywords: breast cancer, human epidermal growth factor receptor 2, antibody-drug conjugate, tyrosine kinase inhibitor, targeted therapy
Introduction
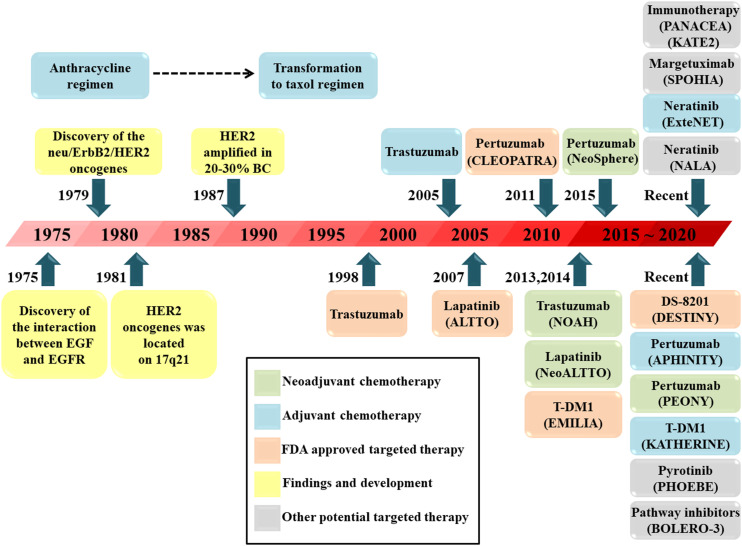
Breast cancer remains the primary disease burden in women worldwide. 1 Breast cancer can be divided into several subtypes, with different subtypes leading to different therapeutic sensitivities and prognoses. 2 HER2 is overexpressed in 15%–20% of all breast cancers plays an indispensable role in the progression of breast cancer. 3 As a result, HER2 is regarded as an effective target for the genomic therapy of various tumors. 4 By forming homodimers or heterodimers, HER2 can drive tumor growth and activate downstream signaling pathways, which promote cell proliferation, survival, and angiogenesis. 5 HER2-targeted therapy has been authorized by the Food and Drug Administration (FDA) because it greatly improves the prognosis of HER2-positive breast cancer (HER2+BC). Chemotherapy plus 1 year of adjuvant HER2-targeted therapy is the standard regimen for HER2+BC. Trastuzumab is the most typical HER2-targeted agent, and the use of trastuzumab in 1998 inspired patients with HER2+BC. For metastatic breast cancer (MBC) with HER2 overexpression, trastuzumab enhances the clinical benefits of chemotherapy. 6 Moreover, the addition of trastuzumab has been shown to halve the recurrence rate in patients with HER2+BC. 7 Although the treatment is valid, some obstinate drug resistance and AEs associated with trastuzumab affect the quality of life of patients during the course of treatment. Therefore, several targeted agents have been explored and approved in recent decades (Figure 1). Further enhancement of the effect of targeted therapy has become the focus of research into HER2+BC. Therefore, there is an urgent need to develop novel and acceptable targeted agents for patients. 8
Figure 1.
Timeline of the findings of HER2 and the development of HER2-positive breast cancer regimens. HER: Human epidermal growth factor receptor, EGF: Epidermal growth factor, EGFR: Epidermal growth factor receptor, TDM-1: Ado-trastuzumab emtansine, BC: Breast cancer, FDA: Food and Drug Administration, DS-8201: Trastuzumab deruxtecan.
This article provides a brief description of the biology of BC and the expression of HER2, with the aim to provide an overview of the therapeutic landscape of HER2+BC by reviewing research results and introducing the latest evidence to provide a reference for clinical treatment.
Breast Cancer Biology and Expression of HER2
According to routine immunohistochemical (IHC) parameters, breast cancers can be classified into four molecular subtypes (Table 1). 9 Different subtypes have different gene expression patterns, which are closely associated with therapeutic responses. Patients with hormone receptor-positive tumors receive endocrine therapy, while a few receive chemotherapy. Patients with HER2-positive tumors receive HER2-targeted therapy in combination with chemotherapy, while those with triple-negative breast cancer usually receive chemotherapy only. 10
Table 1.
Four Molecular Subtypes of Breast Cancer.
| Subtype | ER,PR | HER2 | Ki67 |
|---|---|---|---|
| Luminal A | ER- and/or PR-positive | Negative | Low (<15%) |
| HER2-negative Luminal B | ER- and/or PR-positive | Negative | High (>30%) |
| HER2-positive Luminal B | ER- and/or PR-positive | Positive | Any |
| Triple negative | Both negative | Negative | Any |
| HER2-positive | Both negative | Positive | Any |
Abbreviation: ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; PR, progesterone receptor.
HER2 oncogenes are located on chromosome-17 11 and are responsible for encoding transmembrane receptor tyrosine kinases. 12 The HER2 oncogene is a member of the epidermal growth factor receptor (EGFR) family (also known as ErbB), together with HER1, HER3, and HER4 (Figure 2). By forming homodimers (with HER2) or heterodimers (with HER1, HER3, or HER4), tyrosine residues in the cytoplasmic domain undergo autophosphorylation, which activates downstream signaling pathways (mainly the PI3K/AKT/mTOR and Ras/Raf/MEK/MAPK pathways) and causes adverse biological reactions (Figure 3). HER2 amplification is defined as a vital independent adverse prognostic factor, 13 and HER2+BC is more invasive and more likely to relapse and metastasize in the early stage. 14
Figure 2.
Liner structures and corresponding ligands of the epidermal growth factor receptor family. EC: Extracellular domain, TM: Transmembrane domain, D: Domain. IC: Intracellular domain, TK: Tyrosine kinase domain, APR: Amphiregulin, NRG: Neuregulin, BTC: Betacellulin, HER: Human epidermal growth factor receptor, EPR: Epiregulin.
Figure 3.
Biological mechanism of HER2 and summary of targeted therapies for HER2-positive breast cancer. HER: Human epidermal growth factor receptor, ADCs: Antibody-drug conjugates, TKI: Tyrosine kinase inhibitor, CAR: Chimeric antigen receptor, CD3: Cluster of differentiation 3, TCR: T-cell receptor, PD-1: Programmed death-1, PD-L1: Programmed death-ligand 1, TDM-1: Ado-trastuzumab emtansine, DS-8201: Trastuzumab deruxtecan.
Most patients with HER2+BC are insensitive to endocrine therapy. 15 The emergence of HER2-targeted agents has significantly improved the quality of life of patients, and trastuzumab has significantly improved the survival of patients with early HER2+BC. However, drug resistance and adverse events (AEs) reduce the therapeutic effect. Hence, novel agents have been explored by scientists and are currently undergoing clinical trials (Table 2).
Table 2.
Summary of Major Phase III trials in HER2+BC.
| Trial | Numbers of patients | Race | Line of therapy | Regimen | ORR (%) | PFS (months) | OS (months) |
|---|---|---|---|---|---|---|---|
| CLEOPATRA (NCT00567190) 18 | T:808 E:402 C:406 |
White, Black, Asian, Other | First line | E: Pertuzumab + Trastuzumab + Docetaxel C: Placebo + Trastuzumab + Docetaxel |
E:80.2 C:69.3 |
E:18.7 C:12.4 |
E:56.5 C:40.8 |
| PEONY (NCT02586025) 20 | T:329 E:219 C:110 |
Asian | First line | E: Pertuzumab + Trastuzumab + Docetaxel C: Placebo + Trastuzumab + Docetaxel |
E:88.6 C:78.2 |
E:NA C:NA |
E:NA C:NA |
| SOPHIA (NCT02492711) 26 | T:536 E:266 C:270 |
White, Black, Asian, Other | ≥Second line | E: Margetuximab + Chemotherapy C: Pertuzumab + Chemotherapy |
E:25.2 C:13.7 |
E:5.8 C:4.9 |
E:21.6 C:19.8 |
| EMILIA (NCT00829166) 41 | T:978 E:490 C:488 |
White, Black, Asian, Other | Second line | E: T-DM1 C: Lapatinib + Capecitabine |
E:46.3 C:30.8 |
E:9.6 C:6.4 |
E:30.9 C:25.1 |
| TH3RESA (NCT01419197) 42 | T:602 E:404 C:198 |
White, Asian, Other | ≥Second line | E: T-DM1 C: Physician’s choice |
E:31.0 C:9.0 |
E:6.2 C:3.3 |
E:22.7 C:15.8 |
| NALA (NCT01808573) 57 | T:621 E:307 C:314 |
White, Black, Asian, Other | ≥Second line | E: Neratinib + Capecitabine C: Lapatinib + Capecitabine |
E:32.8 C:26.7 |
E:5.6 C:5.6 |
E:21.0 C:18.7 |
| ExteNET (NCT00878709) 58 | T:2840: E:1420 C:1420 |
White, Black, Asian, Other | ≥Second line | E: Neratinib C: Placebo |
E:NA C:NA |
E:NA C:NA |
E:NA C:NA |
| PHENIX (NCT02973737) 61 | T:279 E:185 C:94 |
Asian | ≥Second line | E: Pyrotinib + Capecitabine C: Placebo + Capecitabine |
E:68.6 C:16.0 |
E:11.1 C:4.1 |
E:NA C:NA |
| PHOEBE (NCT03080805) 61 | T:267 E:134 C:133 |
Asian | ≥Second line | E: Pyrotinib + Capecitabine C: Lapatinib + Capecitabine |
E:67.2 C:51.5 |
E:12.5 C:6.8 |
E:NA C:NA |
| HER2CLIMB (NCT02614794) 64 | T:612 E:410 C:202 |
White, Black, Asian, Other | ≥Second line | E: Tucatinib + Trastuzumab + Capecitabine C: Placebo + Trastuzumab + Capecitabine |
E:40.6 C:22.8 |
E:7.8 C:5.6 |
E:21.9 C:17.4 |
| BOLERO-3 (NCT01007942) 71 | T:569 E:284 C:285 |
White, Black, Asian, Other | ≥Second line | E: Everolimus + Trastuzumab + vinorelbine C: Placebo + Trastuzumab + vinorelbine |
E:40.8 C:37.2 |
E:7.0 C:5.8 |
E:23.5 C:24.1 |
Abbreviation: C, group of control; E, group of experiment; NA, not available; NCT, National Clinical Trial; ORR, objective response rate; OS, overall survival; PFS, progression-free survival; T, total numbers; TDM-1, ado-trastuzumab emtansine.
Therapeutic Antibodies
Several novel antibodies have been identified. Compared to trastuzumab, they either have greater specificity to combine with the HER2 receptor, or they can bind to extra epitopes to enhance activity and generate greater immunologic responses.
Pertuzumab
Pertuzumab is a humanized recombinant monoclonal antibody. In addition to preventing the formation of homodimers and triggering antibody-dependent cell-mediated cytotoxicity (ADCC), which is also triggered by trastuzumab, pertuzumab can combine with HER2 in other regions, restrain the heterodimer (HER2/HER3), and block downstream signaling pathways (Figure 3).16,17
Pertuzumab was first found to be useful in MBC with a combination of trastuzumab and docetaxel in the CLEOPATRA study. The study showed that the regimen extended the median progression-free survival (PFS) to 18.5 months compared to 12.4 months in the placebo group. 18 Later, to determine whether pertuzumab played a role in neoadjuvant therapy of HER2+BC, a phase II trial, NeoSphere, was conducted. 19 The results showed a significantly higher pathologic complete response (pCR) in the treatment group than that in the placebo group (45.8% vs 29.0%; P = .014). It is worth mentioning that the PEONY study, which was conducted in Asians, also confirmed that the application of pertuzumab in neoadjuvant therapy was beneficial in patients with HER2+BC. 20
APHINITY, a phase III clinical trial, showed that pertuzumab could lower the risk of invasive disease-free survival (iDFS) compared to placebo (hazard ratio [HR] = .81; 95% confidence interval [CI], .66–1.00; P = .045). Therefore, pertuzumab has been approved as an adjuvant treatment for HER2+BC. 21 In addition, the regimen (trastuzumab plus pertuzumab and docetaxel) could extend the overall survival (OS) to 56.5 months in patients with HER2+MBC. 22 It is worth noting that this regimen is relatively safe and does not cause additional cardiotoxicity.
According to the latest National Comprehensive Cancer Network (NCCN) clinical practice guidelines in oncology (Version 2.2022), trastuzumab and pertuzumab combined with chemotherapy are the first-line regimens for neoadjuvant chemotherapy. 23 However, there is no precise evidence to support whether the regimen is useful for patients with tumors <2 cm in diameter and without axillary lymph node metastasis.
Margetuximab
Margetuximab (MGAH22) is a chimeric murine monoclonal antibody. Unlike trastuzumab, MGAH22 is designed with an engineered Fc portion to increase affinity for the activating Fcγ receptor and to decrease affinity for the inhibitory Fcγ receptor, thereby increasing the ADCC response. 24 In a phase I trial, MGAH22 exhibited antitumor activity and no cardiotoxicity in patients treated with anti-HER2 therapy. 25 A crucial phase III trial, SOPHIA, showed that the regimen of MGAH22 plus chemotherapy improved PFS compared to the trastuzumab group (HR = .76; 95% CI, .59–.98, P = .03). 26 As a result, MGAH22 combined with chemotherapy has been approved by the FDA for the treatment of HER2+MBC. Further phase II trials, such as MARGOT and NCT04262804, are currently recruiting volunteers. 27
ZW25
ZW25 is a bispecific antibody; in contrast to trastuzumab, ZW25 can combine two HER2 epitopes simultaneously to increase specificity and improve efficacy (Figure 3). In most HER2-overexpressed cancers, ZW25 shows high activity in vivo and in vitro. 28 In a phase I clinical trial, ZW25 was effective in patients with HER2+BC, with a 33% objective response rate (ORR). 29 Recently, a phase I/II trial (NCT02892123) has been conducted. Part 1 was completed with no dose-limiting toxicity, but two common AEs were reported - diarrhea and infusion-related reaction (all grade 2). In part 2, 13 patients with MBC were included in the study, seven of whom improved and six failed. In part 3, which is ongoing, scientists will assess the safety, tolerance, and efficacy of the combination with specific chemotherapy. 30
MM-111
Unlike the HER2/HER2 homodimer regulated by trastuzumab, MM-111 is a fusion antibody consisting of fully human anti-HER2/HER3 single chains linked by modified human serum albumin. It precisely obstructs the combination of heregulin (HRG) and HER3, and blocks ligand-induced signaling and tumor growth. A series of preliminary studies have evaluated the curative effect of MM-111 in the regimen of patients with HER2-positive solid tumors, such as MM-111 monotherapy (NCT00911898), MM-111 plus trastuzumab and chemotherapy regimen (NCT01304784), and MM-111 plus trastuzumab and lapatinib regimen (NCT01097460). 31
MCLA-128
MCLA-128 is a humanized bispecific antibody that contains two arms (“dock” HER2 arm and “block” HER3 arm) and targets extracellular domains, which prevents the phosphorylation of HER3 and downstream oncogenic signaling. 32 In a phase I trial, large doses of MCLA-128 were administered to ten patients with breast cancer, and the results revealed a considerable clinical benefit rate of 70%. Another phase II trial (NCT03321981) is proceeding. 33
Ertumaxomab
The trifunctional immunoglobulin ertumaxomab is an integrated bispecific antibody. 34 Traditional treatment with trastuzumab only affects the EGFR family; however, ertumaxomab can simultaneously target HER2 and cluster of differentiation 3 (CD3) presented on T cells, and activate Fcgamma receptors of innate effector cells. According to these characteristics, ertumaxomab acts as a bridge between tumor cells and immune cells. ADCC can be enhanced by activating the adjunctive cells. In addition, they can relate immune effector cells and tumor cells in a short time and display powerful antineoplastic activity. 35 A phase I trial confirmed that ertumaxomab resulted in one complete response (CR) case and several partial response (PR) cases. A maximum tolerated dose of 100 μg is recommended, and cytokine release is the most related AE. 36 Phase II trials concerned with ertumaxomab (NCT00522457, NCT00452140) were terminated because of changes in the development plan or focus on other projects. Although there is currently no clear conclusion, ertumaxomab could still be an appropriate adjuvant for anti-HER2 therapy.
Antibody-Drug Conjugates (ADCs)
ADCs transmit different cytotoxic drugs to tumors using mAbs. These drugs not only decrease toxicity in normal tissues, but also have increased targeting ability and efficiency. Ado-trastuzumab emtansine (T-DM1) is a representative drug approved by the FDA for the treatment of HER2+MBC, and is composed of cytotoxic agents such as trastuzumab and emtansine. 37 Because T-DM1 combines with HER2, the conjugate can be transported into tumor cells. Subsequently, emtansine is released, which causes the inhibition of tubulin and finally leads to apoptosis. 38 In addition, T-DM1 can block downstream signaling pathways (Figure 3). 39 Phase I/II trials have demonstrated that the addition of TDM-1 results in an excellent response, with better ORR and PFS. 40 EMILIA was a critical phase III trial, which showed that T-DM1 plus capecitabine significantly lengthened the median PFS and OS (HR = .53; 95% CI, .37-.76; P < .05). Based on these results, T-DM1 gained acceptance and was used as a second-line treatment for HER2+MBC patients. 41
Despite the obvious clinical efficacy of T-DM1, its inherent characteristics hamper its potency. Therefore, scientists have focused on the development of new HER2-targeted ADCs. Different ADCs represent various combinations of affiliative antibodies, linker drugs, and payloads. The novel ADCs are summarized in Table 3. 42 A novel ADC named trastuzumab deruxtecan (DS-8201) appeared recently, and quickly gained FDA approval. DS-8201 consists of trastuzumab, a cleavable linker, and a topoisomerase I inhibitor. DS-8201 can affect both antigen+ tumor cells and neighboring antigen-tumor cells in vivo, and can overcome T-DM1 resistance, which might be an appropriate regimen for patients with T-DM1 relapsed or refractory. 43 A preliminary phase I trial demonstrated the safety, pharmacokinetics, and antitumor activity of DS-8201 in patients with HER2+BC. 44 A phase II trial, DESTINY-Breast01, confirmed that DS-8201 has persistent antitumor activity in patients with HER2+BC. 45 However, interstitial pulmonary disease was reported as one of the major AEs in this trial, which became the focus of subsequent phase III trials. 46 The early results of a randomized phase III trial, DESTINY-Breast03, were published at the latest ESMO meeting. The 12 month-PFS rate of patients was 75.8% with DS-8201 and 34.1% with T-DM1 (HR = .28; 95% CI, .22-.37; P < .001). 47 According to the NCCN guidelines, DS-8201 has replaced TDM-1 as the second-line regimen of choice for the systemic treatment of patients with advanced HER2+BC. 23
Table 3.
Summary of Novel ADCs Applied in HER2-Positive Breast Cancer.
| ADCs | Antibody | Payload | Linker drug | DAR | Developmental phase |
|---|---|---|---|---|---|
| DS-8201 | Trastuzumab | Exatecan derivative (topoisomerase I inhibitor) | Maleimide glycynglycynphenylalanyn-glycyn peptide (cleavable) | 7-8 | Phase II, phase III ongoing |
| PF-06804103 | Engineered anti-HER2 antibody | Aur0101 (tubulin inhibitor) | Valine-citrulline (cleavable) | 4 | Phase I ongoing |
| PT-DM1 | Trastuzumab | DM1 maytansinoid | Sulfo-SMCC (cleavable) | 3.5-4.2 | Phase I ongoing |
| HER2-vc0101 | Trastuzumab | Aur0101 (tubulin inhibitor) | Valine-citrulline (cleavable) | 4 | Phase I ongoing |
| SYD985 | Trastuzumab | Duocarmycin (DNA targeting) | Valine-citrulline (cleavable) | 2.8 | Phase I/II, phase III ongoing |
| ARX788 | Engineered anti-HER2 antibody | Monomethyl auristatin F (tubulin inhibitor) | Para-acetylphenylalanine (not cleavable) | 1.9 | Phase I ongoing |
| MEDI4276 | Biparatopic anti-HER2 antibody | Tubulysin (tubulin inhibitor) | Maleimidocaproyl (cleavable) | 4 | Phase I |
| ZW49 | ZW25 | Monomethyl auristatin E (tubulin inhibitor) | Unknown cleavable linker | Unknown | Phase I ongoing |
| RC48 | Hertuzumab | Monomethyl auristatin E (tubulin inhibitor) | MC-Val-Cit-PAB (cleavable) | 4 | Phase I, phase II ongoing |
Abbreviation: ADCs, antibody-drug conjugates; DAR, drug-to-antibody ratio.
Tyrosine Kinase Inhibitors (TKIs)
TKIs are suitable candidates for the treatment of various malignancies. They bind to tyrosine kinases competing with adenosine triphosphate (ATP), inhibit phosphorylation of residues, and block downstream signaling pathways, all of which suppress tumor cell proliferation and metastasis (Figure 3). Therefore, TKIs may be beneficial in patients with early HER2+BC. To date, two HER2-targeted TKIs have been approved by the FDA: lapatinib and neratinib, while several novel TKIs are currently undergoing clinical research. 48 While it is difficult for monoclonal antibodies to traverse the blood–brain barrier (BBB), some TKIs can cross easily, which may represent an effective solution for the metastasis of the central nervous system (CNS) in HER2+BC. 42
Lapatinib
The FDA-approved lapatinib is an effective oral TKI. It can reversibly inhibit HER1 and HER2 receptors, block the downstream pathways (mainly PI3K/AKT/mTOR and Ras/Raf/MEK/MAPK pathways), and ultimately inhibit the proliferation and development of tumor cells. 49 According to the FDA, the regimen of lapatinib plus capecitabine is effective in patients with MBC. As lapatinib can cross the CNS, it has the potential to improve the prognosis of CNS MBC. 50 Studies have shown that short-term treatment with lapatinib (1-7 days) induces cell cycle arrest, apoptosis, and autophagy. However, long-term treatment (3-6 months) may result in acquired drug resistance. 51 Unfortunately, its well-known AE, diarrhea, limits its clinic use. 52
Neratinib
Neratinib is an FDA-approved oral TKI, which irreversibly inhibits members of the EGFR family (except HER3). Compared to lapatinib, neratinib is more valid and consistent. 53 By lowering the phosphorylation of each protein kinase domain, it blocks downstream pathways, which eventually decreases both cyclin D1 expression and RB phosphorylation. 54 As neratinib and trastuzumab have different mechanisms, their combination may be promising. 55
A phase II trial, NEFERT-T, showed that the administration of neratinib led to fewer CNS recurrences. The most frequent AE was diarrhea (20%, grade 3-4). 56 A phase III trial named NALA confirmed that a regimen of neratinib plus capecitabine could lower the cumulative incidence of CNS metastasis. 57 Another phase III trial, ExteNET, explored patients with 1-year neratinib treatment followed by 1-year trastuzumab treatment. In the 5-year follow-up study, the neratinib group showed a survival rate of 90.2%, while the placebo group showed a survival rate of 87.7%, confirming its clinical efficacy. In addition, subgroup analysis suggested that the sequential use of neratinib was more beneficial for hormone receptor (HR)-positive patients. 58
Pyrotinib
Pyrotinib was developed exclusively in China and has similar biological mechanisms to neratinib. 59 Phase I trials showed that a single application of pyrotinib was efficient and safe for patients with advanced HER2+BC. 60 A recent randomized phase II trial (NCT02422199) assessed the clinical efficacy of pyrotinib plus capecitabine, and the results showed that the regimen had a higher ORR (79%) and a longer median PFS (18.1 months). Moreover, phase III trials, including PHENIX and PHOEBE, confirmed that pyrotinib plus capecitabine could achieve longer median PFS (11.1 months, 12.5 months). Based on the above conclusions, this regimen has won regional recognition in China. 61
Tucatinib
Tucatinib is a new oral TKI, and in an in vitro model with HER2-overexpressed cells, tucatinib blocked the phosphorylation of HER2 and protein kinase B (PKB). 62 A phase Ib trial showed that the combination of tucatinib and TDM-1 had expectant outcomes in HER2+MBC. 63 Tucatinib is a promising drug because it does not bind to EGFR, which reflects lower toxicity and indicates the possibility of traversing the BBB. HER2CLIMB is a phase III trial that assessed the clinical efficacy of tucatinib plus trastuzumab and capecitabine in patients with HER2+MBC. The results showed that this regimen increased PFS compared to the placebo group (25% vs 0%, HR = .48; P < .01). Hence, the FDA recently authorized this regimen for adjuvant therapy in patients with advanced HER2+MBC. 64 Other phase III trials, such as HER2CLIMB02 and HER2CLIMB04, have attempted to further assess the availability of tucatinib in MBC. 65
Afatinib
Afatinib is a TKI that irreversibly inhibits HER1, HER2, and HER4 receptors. 66 A phase II trial (NCT01325428) assessed the possibility of applying afatinib in patients with inflammation or MBC. 67 A phase III trial, LUX-Breast 1, attempted to add afatinib to the regimen for patients treated with chemotherapy plus trastuzumab. 68 However, the result showed lower OS than the placebo group, and common AEs such as diarrhea and rash were inevitable. Consequently, the potency of afatinib needs to be investigated in further research.
Anti-HER2 Combined Therapies
PI3K/AKT/mTOR Inhibitors
In a previous statement, we learned that the PI3K/AKT/mTOR signaling pathway plays a vital role in HER2+BC. 69 By activating this pathway, cell proliferation, survival, and angiogenesis can be promoted, suggesting that we can attempt to delay the progression of HER2+BC by inhibiting this pathway. 5
Everolimus is a type of mTOR inhibitor. Several trials have confirmed that the application of everolimus is beneficial in patients with HER2+BC. 70 BOLERO-3 is a phase III trial, scientists have found that the combination regimen of everolimus plus trastuzumab and vinorelbine improved the median PFS compared to the placebo group (7.0 months vs 5.78 months). 71
Buparlisib, an oral inhibitor of both PI3K and mTOR, has been hailed as an exciting discovery in the field. NeoPHOEBE, a phase II trial, proved that the regimen of buparlisib plus trastuzumab and paclitaxel resulted in a higher RR (69% vs 33%, P = .053) and apparent decline of Ki67 (75% vs 27%) compared to the placebo group. 72
PD-1/PD-L1 Inhibitors
Programmed death-1(PD-1) and programmed death-ligand 1 (PD-L1) combine to trigger immune escape and are crucial to tumor growth. 73 As a result, PD-1/PD-L1 inhibitors have attracted considerable attention, and existing trials have shown that PD-1/PD-L1 inhibitors are helpful in the treatment of triple-negative breast cancer. Although there are few studies on HER2+BC, molecular mechanisms and in vitro trials have indicated that PD-1/PD-L1 inhibitors have modest clinical efficacy in HER2+BC. 74
Pembrolizumab is a commonly used PD-1 inhibitor, which can reverse trastuzumab resistance by restoring T cells to tumor cells. A phase Ib and II trial, PANACEA, indicated that pembrolizumab plus trastuzumab is safe and effective, with lasting clinical efficacy. 75
Another inhibitor, atezolizumab (ATEZO), can combine with PD-L1 to block the interaction between PD-L1 and B7.1. In a phase II trial, KATE2, the regimen of ATEZO plus T-DM1 acquired longer PFS than the placebo group (8.2 months vs 6.8 months, HR = .82; 95% CI, .55-1.23; P = .33). 76 Phase III trials are ongoing to determine the advantages of ATEZO. HER2-targeted agents have fast action and high safety, while PD-1/PD-L1 inhibitors have slow action and lasting efficacy, and their combination is expected to play a better role in further research.
HER2-Targeted Vaccines
Tumor vaccines have been explored in recent years, and HER2-targeted vaccines are considered a potential treatment for HER2+BC. 77 Based on the characterized peptide, virus, and tumor cells, HER2-targeted vaccines can be designed in various forms which aim at different substance. Prior clinical trials have shown that HER2-targeted vaccines can generate active immunity. 78 Future research should focus on the development of multi-epitope vaccines.
NeuVax, a peptide-based vaccine, has been widely investigated. Stimulation of CD8+ T cells by NeuVax promotes cytolysis and destroys tumor cells. NeuVax is the only HER2-targeted vaccine that has completed a phase III clinical trial (the PRESENT trial); however, the results indicate that the application of NeuVax might result in a high risk of recurrence. 79
AVX901 is a virus-based vaccine, which can jointly express tumor antigen and virus genes, activating helper T cells in vivo to trigger specific cellular and humoral immune responses. Patients will not become sources of infection because of the low toxicity of AVX901. A completed phase I trial (NCT01526473) has certified its feasibility. 80
ETBX-021 is a virus-based vaccine, which contains a modified adenovirus 5 that is inserted into the HER2 gene. A phase I trial (NCT02751528) has evaluated its safety and preclinical indices.
Chimeric Antigen Receptor (CAR) T-Cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy is a novel approach for treating tumors. The mechanism involves editing tumor-specific T cells, which may be more efficient in killing tumor cells. It has been proven beneficial for several solid tumors, including ovarian carcinoma and prostate cancer.81,82 Scientists have attempted to explore whether therapy is helpful for HER2+ tumors. The phase I/II trial CAR-T-HER2 (NCT01935843) investigated the efficacy, feasibility, and activity of CAR T-cell therapy for HER2+ advanced solid tumors. Eleven patients were enrolled, among whom one obtained PR and five achieved stable disease without severe AEs. 83 The safety and persistence of T-cells, as well as the possibility of the application of CAR T-cell therapy in HER2+BC, need to be further verified.
Conclusions and Future Perspectives
Breast cancer is a major disease burden on women worldwide. Breast cancer can be classified into four subtypes based on IHC parameters, with HER2+BC occupying 15%–20% of all cases. The overexpression of HER2 can drive tumor growth and activate downstream signaling pathways, and, as a result, is extremely aggressive and has poor prognosis in terms of recurrence and metastasis. HER2-targeted therapy is a powerful strategy for the treatment of HER2+BC. The emergence of trastuzumab has improved this dilemma; however, some obstinate drug resistance and AEs associated with trastuzumab have hindered its use. Treatment options for patients with HER2+BC have changed dramatically due to the encouraging results of numerous clinical trials. Pertuzumab has shown good benefit, and patients who were considered likely to experience recurrence could benefit from the combination of trastuzumab and pertuzumab. Trastuzumab plus pertuzumab and taxanes remain the first-line regimen for HER2+BC. Other antibodies, such as ZW25, margetuximab, MM-111, MCLA-128, and ertumaxomab, have shown greater specificity in combination with the HER2 receptor or could bind to extra epitopes to enhance activity and generate greater immunologic responses. T-DM1 is an ADC authorized by the FDA as a second-line agent after failure of a standard chemotherapy regimen. The success of TDM-1 has led scientists to concentrate on the production of new HER2-targeted ADCs such as DS-8201, PF-06804103, PT-DM1, and HER2-vc0101. These ADCs differ in affiliative antibodies, linker drugs, or payloads. It is well accepted that ADCs are taking the central stage as second-or third-line regimens for HER2+BC. Adding small-molecule TKIs to the standard chemotherapy regimen, a third-line regimen, significantly improved the prognosis of patients.
DESTINY-Breast03 was the first trial to compare two ADCs in malignancy to change the standard regimen. The linker is specifically split by tumor cells, which triggers the release of membrane-permeable drug molecules. This “bystander effect” is considered to be advantageous given the heterogeneity of HER2 expression in HER2+ tumors. 47 In addition to the remarkable outcome, the safety of DS-8201 is acceptable and the AEs are within controllable ranges, which can be well managed by monitoring. With rapid development, the future of HER2+BC treatment will likely focus heavily on ADCs.
Although the abovementioned agents are of great value, some patients may be insensitive to them or develop resistance. Therefore, it is necessary to develop novel targeted agents. Preclinical and clinical trials of PI3K/AKT/mTOR inhibitors, PD-1/PD-L1 inhibitors, HER2-targeted vaccines, and CAR T-cell therapy have provided possible anti-HER2 combined strategies. However, the AEs and higher treatment costs need to be weighed by both clinicians and patients. To improve the quality of life and maximally reduce drug toxicity, one of the greatest challenges in the future is to discuss the best combined sequence of these agents. The progress of HER2-targeted therapy is challenging, but with deeper research, the regimens for HER2+BC will gradually become more individualized and precise.
Footnotes
Abbreviations: ADCs, antibody-drug conjugates; ADCC, antibody-dependent cell-mediated cytotoxicity; AEs, adverse events; ATEZO, atezolizumab; ATP, adenosine triphosphate; BBB, blood–brain barrier; CAR, chimeric antigen receptors; CD3, Cluster of Differentiation 3; CD8, Cluster of Differentiation 8; CI, confidence interval; CNS, central nervous system; CR, complete response; DS-8201, trastuzumab deruxtecan; EGFR, epidermal growth factor receptor; FDA, food and drug administration; HER1, human epidermal growth factor receptor 1; HER2, human epidermal growth factor receptor 2; HER2+BC, human epidermal growth factor receptor 2–positive breast cancer; HER3, human epidermal growth factor receptor 3; HER4, human epidermal growth factor receptor 4; HR, hazard ratio; HRG, heregulin; iDFS, invasive disease-free survival; IHC, immunohistochemical; MAGH22, margetuximab; MBC, metastatic breast cancer; NCCN, National Comprehensive Cancer Network; ORR, objective response rate; OS, overall survival; pCR, pathologic complete response; PD-1, programmed death-1; PD-L1, programmed death-ligand 1; PFS, progression-free survival; PKB, protein kinase B; PR, partial response; TDM-1, ado-trastuzumab emtansine; TKIs, tyrosine kinase inhibitors
Authors’ contributions: Jindong Xie: Writing—original draft. Yutian Zou: Writing—original draft. Ting Gao: Writing—original draft. Liming Xie: Writing—review and editing. Duxun Tan: Writing—review and editing. Xiaoming Xie: Writing—review and editing.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was funded by the National Natural Science Foundation of China (81872152, Xiaoming Xie).
ORCID iD
Yutian Zou https://orcid.org/0000-0002-5205-9923
References
- 1.Britt KL, Cuzick J, Phillips KA. Key steps for effective breast cancer prevention. Nat Rev Cancer. 2020;20(8):417-436. [DOI] [PubMed] [Google Scholar]
- 2.Goutsouliak K, Veeraraghavan J, Sethunath V, et al. Towards personalized treatment for early stage HER2-positive breast cancer. Nat Rev Clin Oncol. 2020;17(4):233-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sharma P. Major strides in HER2 blockade for metastatic breast Cancer. N Engl J Med. 2020;382(7):669-671. [DOI] [PubMed] [Google Scholar]
- 4.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2(2):127-137. [DOI] [PubMed] [Google Scholar]
- 5.Moasser MM. The oncogene HER2: its signaling and transforming functions and its role in human cancer pathogenesis. Oncogene. 2007;26(45):6469-6487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Slamon DJ, Leyland-Jones B, Shak S, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783-792. [DOI] [PubMed] [Google Scholar]
- 7.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673-1684. [DOI] [PubMed] [Google Scholar]
- 8.Lambertini M, Del Mastro L, Pescio MC, et al. Cancer and fertility preservation: international recommendations from an expert meeting. BMC Med. 2016;14:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sorlie T, Perou CM, Tibshirani R, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869-10874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288-300. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Xu J, Choi HH, et al. Targeting 17q23 amplicon to overcome the resistance to anti-HER2 therapy in HER2+ breast cancer. Nat Commun. 2018;9(1):4718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Takada K, Kashiwagi S, Goto W, et al. Analysis of HER Family (HER1-4) expression as a biomarker in combination therapy with pertuzumab, trastuzumab and docetaxel for advanced HER2-positive breast cancer. Anticancer Res. 2018;38(4):2285-2294. [DOI] [PubMed] [Google Scholar]
- 13.Luo C, Zhong X, Wang Z, et al. Prognostic nomogram for patients with non-metastatic HER2 positive breast cancer in a prospective cohort. Int J Biol Markers. 2019;34(1):41-46. [DOI] [PubMed] [Google Scholar]
- 14.Turke AB, Song Y, Costa C, et al. MEK inhibition leads to PI3K/AKT activation by relieving a negative feedback on ERBB receptors. Cancer Res. 2012;72(13):3228-3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Montemurro F, Rossi V, Cossu Rocca M, et al. Hormone-receptor expression and activity of trastuzumab with chemotherapy in HER2-positive advanced breast cancer patients. Cancer. 2012;118(1):17-26. [DOI] [PubMed] [Google Scholar]
- 16.Capelan M, Pugliano L, De Azambuja E, et al. Pertuzumab: new hope for patients with HER2-positive breast cancer. Ann Oncol. 2013;24(2):273-282. [DOI] [PubMed] [Google Scholar]
- 17.von Minckwitz G, Procter M, de Azambuja E, et al. Adjuvant pertuzumab and trastuzumab in early HER2-positive breast cancer. N Engl J Med. 2017;377(2):122-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Baselga J, Cortes J, Kim SB, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gianni L, Pienkowski T, Im YH, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25-32. [DOI] [PubMed] [Google Scholar]
- 20.Shao Z, Pang D, Yang H, et al. Efficacy, safety, and tolerability of pertuzumab, trastuzumab, and docetaxel for patients with early or locally advanced ERBB2-positive breast cancer in Asia: the PEONY Phase 3 randomized clinical trial. JAMA Oncol. 2020;6(3):e193692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Irabor OC, Kerry VB, Matton J, Ngwa W. Leveraging the Global health service partnership model for workforce development in global radiation oncology. J Glob Oncol. 2018;4:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Swain SM, Baselga J, Kim SB, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724-734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.NCCN Clinical Practice Guidelines in Oncology-Breast Cancer (2022 Version II) [Internet]. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1419
- 24.Nordstrom JL, Gorlatov S, Zhang W, et al. Anti-tumor activity and toxicokinetics analysis of MGAH22, an anti-HER2 monoclonal antibody with enhanced Fcgamma receptor binding properties. Breast Cancer Res. 2011;13(6):R123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hidalgo M, Martinez-Garcia M, Le Tourneau C, et al. First-in-human phase I study of single-agent vanucizumab, a first-in-class bispecific anti-angiopoietin-2/Anti-VEGF-a antibody, in adult patients with advanced solid tumors. Clin Cancer Res. 2018;24(7):1536-1545. [DOI] [PubMed] [Google Scholar]
- 26.Rugo HS, Im SA, Cardoso F, et al. Efficacy of margetuximab vs trastuzumab in patients with pretreated ERBB2-positive advanced breast cancer: a phase 3 randomized clinical trial. JAMA Oncol. 2021;7(4):573-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markham A. Margetuximab: first approval. Drugs. 2021;81(5):599-604. [DOI] [PubMed] [Google Scholar]
- 28.Weisser N, Wickman G, Davies R, Rowse G. Abstract 31: preclinical development of a novel biparatopic HER2 antibody with activity in low to high HER2 expressing cancers. Cancer Res. 2017;77(suppl 13):31. [Google Scholar]
- 29.Meric-Bernstam F, Johnson AM, Dumbrava EEI, et al. Advances in HER2-targeted therapy: novel agents and opportunities beyond breast and gastric cancer. Clin Cancer Res. 2019;25(7):2033-2041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mitani S, Kawakami H. Emerging targeted therapies for HER2 positive gastric cancer that can overcome trastuzumab resistance. Cancers (Basel). 2020;12(2):400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonagh CF, Huhalov A, Harms BD, et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol Cancer Ther. 2012;11(3):582-593. [DOI] [PubMed] [Google Scholar]
- 32.Schram AM, Odintsov I, Espinosa-Cotton M, et al. Zenocutuzumab, a HER2xHER3 bispecific antibody, is effective therapy for tumors driven by NRG1 gene rearrangements. Cancer Discov. 2022. doi: 10.1158/2159-8290.CD-21-1119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mishra R, Patel H, Alanazi S, Yuan L, Garrett JT. HER3 signaling and targeted therapy in cancer. Oncol Rev. 2018;12(1):355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kiewe P, Thiel E. Ertumaxomab: a trifunctional antibody for breast cancer treatment. Expert Opin Investig Drugs. 2008;17(10):1553-1558. [DOI] [PubMed] [Google Scholar]
- 35.Yu S, Liu Q, Han X, et al. Development and clinical application of anti-HER2 monoclonal and bispecific antibodies for cancer treatment. Exp Hematol Oncol. 2017;6:31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haense N, Atmaca A, Pauligk C, et al. A phase I trial of the trifunctional anti Her2 x anti CD3 antibody ertumaxomab in patients with advanced solid tumors. BMC Cancer. 2016;16:420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wahler J, Suh N. Targeting HER2 positive breast cancer with chemopreventive agents. Curr Pharmacol Rep. 2015;1(5):324-335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oostra DR, Macrae ER. Role of trastuzumab emtansine in the treatment of HER2-positive breast cancer. Breast Cancer. 2014;6:103-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Verma S, Miles D, Gianni L, et al. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783-1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Krop IE, Modi S, LoRusso PM, et al. Phase 1b/2a study of trastuzumab emtansine (T-DM1), paclitaxel, and pertuzumab in HER2-positive metastatic breast cancer. Breast Cancer Res. 2016;18(1):34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Boyraz B, Sendur MA, Aksoy S, et al. Trastuzumab emtansine (T-DM1) for HER2-positive breast cancer. Curr Med Res Opin. 2013;29(4):405-414. [DOI] [PubMed] [Google Scholar]
- 42.Kunte S, Abraham J, Montero AJ. Novel HER2-targeted therapies for HER2-positive metastatic breast cancer. Cancer. 2020;126(19):4278-4288. [DOI] [PubMed] [Google Scholar]
- 43.Rinnerthaler G, Gampenrieder SP, Greil R. HER2 directed antibody-drug-conjugates beyond T-DM1 in breast cancer. Int J Mol Sci. 2019;20(5):1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Doi T, Shitara K, Naito Y, et al. Safety, pharmacokinetics, and antitumour activity of trastuzumab deruxtecan (DS-8201), a HER2-targeting antibody-drug conjugate, in patients with advanced breast and gastric or gastro-oesophageal tumours: a phase 1 dose-escalation study. Lancet Oncol. 2017;18(11):1512-1522. [DOI] [PubMed] [Google Scholar]
- 45.Modi S, Saura C, Yamashita T, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narayan P, Osgood CL, Singh H, et al. FDA approval summary: fam-trastuzumab deruxtecan-Nxki for the treatment of unresectable or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27(16):4478-4485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trastuzumab deruxtecan data impresses at ESMO. Cancer Discov. 2021;11(11):2664-2665. https://aacrjournals.org/cancerdiscovery/article-abstract/11/11/2664/666404/Trastuzumab-Deruxtecan-Data-Impresses-at?redirectedFrom=fulltext. [DOI] [PubMed] [Google Scholar]
- 48.Hudelist G, Kostler WJ, Attems J, et al. Her-2/neu-triggered intracellular tyrosine kinase activation: in vivo relevance of ligand-independent activation mechanisms and impact upon the efficacy of trastuzumab-based treatment. Br J Cancer. 2003;89(6):983-991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhang X, Munster PN. New protein kinase inhibitors in breast cancer: afatinib and neratinib. Expert Opin Pharmacother. 2014;15(9):1277-1288. [DOI] [PubMed] [Google Scholar]
- 50.Saleem A, Searle GE, Kenny LM, et al. Lapatinib access into normal brain and brain metastases in patients with Her-2 overexpressing breast cancer. EJNMMI Res. 2015;5:30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhu X, Wu L, Qiao H, et al. Autophagy stimulates apoptosis in HER2-overexpressing breast cancers treated by lapatinib. J Cell Biochem. 2013;114(12):2643-2653. [DOI] [PubMed] [Google Scholar]
- 52.Oakman C, Pestrin M, Zafarana E, Cantisani E, Di Leo A. Role of lapatinib in the first-line treatment of patients with metastatic breast cancer. Cancer Manag Res. 2010;2:13-25. [PMC free article] [PubMed] [Google Scholar]
- 53.Burstein HJ, Sun Y, Dirix LY, et al. Neratinib, an irreversible ErbB receptor tyrosine kinase inhibitor, in patients with advanced ErbB2-positive breast cancer. J Clin Oncol. 2010;28(8):1301-1307. [DOI] [PubMed] [Google Scholar]
- 54.Segovia-Mendoza M, Gonzalez-Gonzalez ME, Barrera D, Diaz L, Garcia-Becerra R. Efficacy and mechanism of action of the tyrosine kinase inhibitors gefitinib, lapatinib and neratinib in the treatment of HER2-positive breast cancer: preclinical and clinical evidence. Am J Cancer Res. 2015;5(9):2531-2561. [PMC free article] [PubMed] [Google Scholar]
- 55.Blackwell KL, Zaman K, Qin S, et al. Neratinib in combination with trastuzumab for the treatment of patients with advanced HER2-positive breast cancer: a phase I/II study. Clin Breast Cancer. 2019;19(2):97-104.e4. [DOI] [PubMed] [Google Scholar]
- 56.Freedman RA, Gelman RS, Anders CK, et al. TBCRC 022: a phase II trial of neratinib and capecitabine for patients with human epidermal growth factor receptor 2-positive breast cancer and brain metastases. J Clin Oncol. 2019;37(13):1081-1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol. 2020;38(27):3138-3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martin M, Holmes FA, Ejlertsen B, et al. Neratinib after trastuzumab-based adjuvant therapy in HER2-positive breast cancer (ExteNET): 5-year analysis of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2017;18(12):1688-1700. [DOI] [PubMed] [Google Scholar]
- 59.Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: A novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci. 2017;110:51-61. [DOI] [PubMed] [Google Scholar]
- 60.Ma F, Ouyang Q, Li W, et al. Pyrotinib or lapatinib combined with capecitabine in HER2-positive metastatic breast cancer with prior taxanes, anthracyclines, and/or trastuzumab: a randomized, phase II study. J Clin Oncol. 2019;37(29):2610-2619. [DOI] [PubMed] [Google Scholar]
- 61.Blair HA. Pyrotinib: first global approval. Drugs. 2018;78(16):1751-1755. [DOI] [PubMed] [Google Scholar]
- 62.Lee A. Tucatinib: first approval. Drugs. 2020;80(10):1033-1038. [DOI] [PubMed] [Google Scholar]
- 63.Borges VF, Ferrario C, Aucoin N, et al. Tucatinib combined with ado-trastuzumab emtansine in advanced ERBB2/HER2-positive metastatic breast cancer: a phase 1b clinical trial. JAMA Oncol. 2018;4(9):1214-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shah M, Wedam S, Cheng J, et al. FDA approval summary: tucatinib for the treatment of patients with advanced or metastatic HER2-positive breast cancer. Clin Cancer Res. 2021;27(5):1220-1226. [DOI] [PubMed] [Google Scholar]
- 65.Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer. 2021;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Solca F, Dahl G, Zoephel A, et al. Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther. 2012;343(2):342-350. [DOI] [PubMed] [Google Scholar]
- 67.Goh G, Schmid R, Guiver K, et al. Clonal evolutionary analysis during HER2 blockade in HER2-positive inflammatory breast cancer: a phase II open-label clinical trial of Afatinib +/- Vinorelbine. PLoS Med. 2016;13(12):e1002136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Harbeck N, Huang CS, Hurvitz S, et al. Afatinib plus vinorelbine versus trastuzumab plus vinorelbine in patients with HER2-overexpressing metastatic breast cancer who had progressed on one previous trastuzumab treatment (LUX-Breast 1): an open-label, randomised, phase 3 trial. Lancet Oncol. 2016;17(3):357-366. [DOI] [PubMed] [Google Scholar]
- 69.Lee JJ, Loh K, Yap YS. PI3K/Akt/mTOR inhibitors in breast cancer. Cancer Biol Med. 2015;12(4):342-354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Guerrero-Zotano A, Mayer IA, Arteaga CL. PI3K/AKT/mTOR: role in breast cancer progression, drug resistance, and treatment. Cancer Metastasis Rev. 2016;35(4):515-524. [DOI] [PubMed] [Google Scholar]
- 71.Andre F, O’Regan R, Ozguroglu M, et al. Everolimus for women with trastuzumab-resistant, HER2-positive, advanced breast cancer (BOLERO-3): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet Oncol. 2014;15(6):580-591. [DOI] [PubMed] [Google Scholar]
- 72.Loibl S, de la Pena L, Nekljudova V, et al. Neoadjuvant buparlisib plus trastuzumab and paclitaxel for women with HER2+ primary breast cancer: a randomised, double-blind, placebo-controlled phase II trial (NeoPHOEBE). Eur J Cancer. 2017;85:133-145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252-264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou Y, Zou X, Zheng S, et al. Efficacy and predictive factors of immune checkpoint inhibitors in metastatic breast cancer: a systematic review and meta-analysis. Ther Adv Med Oncol. 2020;12:1758835920940928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.De La Cruz LM, Czerniecki BJ. Immunotherapy for breast cancer is finally at the doorstep: immunotherapy in breast cancer. Ann Surg Oncol. 2018;25(10):2852-2857. [DOI] [PubMed] [Google Scholar]
- 76.Nathan MR, Schmid P. The emerging world of breast cancer immunotherapy. Breast. 2018;37:200-206. [DOI] [PubMed] [Google Scholar]
- 77.Curigliano G, Spitaleri G, Pietri E, et al. Breast cancer vaccines: a clinical reality or fairy tale? Ann Oncol. 2006;17(5):750-762. [DOI] [PubMed] [Google Scholar]
- 78.Ladjemi MZ, Jacot W, Chardes T, Pelegrin A, Navarro-Teulon I. Anti-HER2 vaccines: new prospects for breast cancer therapy. Cancer Immunol Immunother. 2010;59(9):1295-1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Dillon PM, Brenin CM, Slingluff CL, Jr. Evaluating nelipepimut-S in the treatment of breast cancer: a short report on the emerging data. Breast Cancer. 2020;12:69-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benedetti R, Dell’Aversana C, Giorgio C, Astorri R, Altucci L. Breast cancer vaccines: new insights. Front Endocrinol (Lausanne). 2017;8:270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kloss CC, Condomines M, Cartellieri M, Bachmann M, Sadelain M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nat Biotechnol. 2013;31(1):71-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lamers CH, Sleijfer S, van Steenbergen S, et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered T cells: clinical evaluation and management of on-target toxicity. Mol Ther. 2013;21(4):904-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang J, Zhou P. New Approaches in CAR-T cell immunotherapy for breast cancer. Adv Exp Med Biol. 2017;1026:371-381. [DOI] [PubMed] [Google Scholar]