Abstract
Background:
Magnetic resonance imaging (MRI) is commonly used for evaluation of ankle cartilage repair, yet its association with clinical outcome is controversial. This study analyzes the correlation between MRI and clinical outcome after cartilage repair of the talus including bone marrow stimulation, cell-based techniques, as well as restoration with allo- or autografting.
Methods:
A systematic search was performed in MEDLINE, Embase, and Cochrane Collaboration. Articles were screened for correlation of MRI and clinical outcome. Guidelines of Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) were used. Chi-square test and regression analysis were performed to identify variables that determine correlation between clinical and radiologic outcome.
Results:
Of 2687 articles, a total of 43 studies (total 1212 cases) were included with a mean Coleman score of 57 (range, 33-70). Overall, 93% were case series, and 5% were retrospective and 2% prospective cohort studies. Associations between clinical outcome and ≥1 imaging variable were found in 21 studies (49%). Of 24 studies (56%) using the composite magnetic resonance observation of cartilage repair tissue (MOCART) score, 7 (29%) reported a correlation of the composite score with clinical outcome. Defect fill was associated with clinical outcome in 5 studies (12%), and 5 studies (50%) reported a correlation of T2 mapping and clinical outcome. Advanced age, shorter follow-up, and larger study size were associated with established correlation between clinical and radiographic outcome (P = .021, P = .028, and P = .033).
Conclusion:
Interpreting MRI in prediction of clinical outcome in ankle cartilage repair remains challenging; however, it seems to hold some value in reflecting clinical outcome in patients with advanced age and/or at a shorter follow-up. Yet, further research is warranted to optimize postoperative MRI protocols and assessments allowing for a more comprehensive repair tissue evaluation, which eventually reflect clinical outcome in patients after cartilage repair of the ankle.
Level of Evidence: Level III, systematic review and meta-analysis.
Keywords: cartilage repair, bone marrow stimulation, cell-based repair, articular cartilage restoration, autologous chondrocyte implantation, microfracture, osteochondral autograft transfer system, magnetic resonance imaging, morphological, magnetic resonance observation of cartilage repair tissue (MOCART), T2 mapping, clinical outcome, correlation
Introduction
The necessity of treating symptomatic articular cartilage defects in orthopaedic surgery has been increasingly recognized worldwide, with the development of several cartilage repair techniques in recent years. To assess postoperative repair tissue formation, magnetic resonance imaging (MRI) is frequently used to assess the structural integrity of both cartilage defects and repair tissue.24,45,51,57,60,65,68,73,88
As described by Hayashi et al, 39 the commonly used 2-dimensional and the more advanced isotropic 3-dimensional MRI sequences can be used to evaluate the morphology of cartilage repair. Other MRI sequences like Spoiled Gradient Recoiled Echo are excellent for cartilage segmentation and quantification of cartilage volume but are often of limited utility in the postoperative setting. In many cases, appropriate metal artifact reduction sequences are necessary (eg, after medial malleolar osteotomy), which further reduce the quality of imaging. 38 Compositional MRI acquisitions like T1rho, T2 mapping, and delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) provide a way to detect biochemical and microstructural changes within the cartilage layer. Quantitative MRI sequences have the potential for tissue characterization after reparative and regenerative surgical treatment of osteochondral lesions of the talus (OLTs). 69 Nevertheless, the correlation between radiologic and clinical outcome is still an ongoing debate among the orthopaedic community.24,40,41,57,67,78
In 2017, leading experts in cartilage repair of the ankle gathered in Pittsburgh for the 1st International Consensus Meeting on Cartilage Repair of the Ankle. Among the published manuscripts from this meeting, van Dijk et al 85 reported that routine MRI is not indicated in the follow-up after cartilage repair because evidence of correlation between clinical outcome and posttreatment imaging is lacking. Thus, the consensus recommended that postoperative imaging should be considered in patients with a mechanical cause for symptoms (eg, loose body or chondral flap). Although information about postoperative imaging in the setting of cartilage repair in the knee has been studied in a systematic review, there is a paucity of comprehensive data in cartilage repair of the ankle. 28 Hence, the purpose of this work is to evaluate the correlation between MRI and clinical outcome after articular cartilage repair of the talus and to identify parameters that associate imaging and clinical outcome.
Methods
A systematic literature review was performed on MRI after articular cartilage repair of the talus. Included cartilage repair techniques ranged from bone marrow stimulation procedures (MS) over cell-based cartilage transplantation (CB) to cartilage restoration techniques. Data from individual articles were analyzed to determine the correlation between MRI parameters and clinical outcome. The search was conducted on October 12, 2020, in the electronic databases of MEDLINE, Embase, and the Cochrane Collaboration using the following parameters: (cartilage repair OR cartilage restoration OR autologous chondrocyte implantation OR autologous chondrocyte transplantation OR matrix-assisted autologous chondrocyte transplantation OR matrix-induced autologous chondrocyte implantation OR MACT OR MACI OR characterized chondrocyte implantation OR autologous osteochondral transplantation OR osteochondral autologous transplantation OR OATS OR osteochondral autograft transplantation OR OCT OR mosaicplasty OR osteochondral allograft transplantation OR OCA OR microfracture OR microfracturing OR autologous matrix-induced chondrogenesis OR AMIC OR Chondro-Gide OR Chondrogide OR particulated juvenile cartilage allograft transplantation OR PJCAT) AND (magnetic resonance imaging OR MRI OR delayed gadolinium enhanced OR dGEMRIC OR T2 mapping OR T2 index OR radiologic OR radiological OR radiographic) AND (talus OR ankle OR talar). Two independent reviewers screened all articles by title and abstract and applied the following inclusion criteria: therapeutic or diagnostic studies of cartilage repair, minimal follow-up of 12 months, clinical assessment, postoperative imaging evaluation with MRI, full text available in English or German. Exclusion criteria were case reports, animal and cadaver studies, etiologic studies, osteoarthritis, and unavailable full texts in English or German. All references of systematic reviews were evaluated for inclusion. All included articles were assessed for established correlation analysis between clinical and imaging outcome.
The guidelines for Preferred Reporting Items for Systematic Reviews and Meta-analysis (PRISMA) were used, 61 and the protocol was registered on PROSPERO database (reg. no. CRD42021232791). 16 The quality of each study was assessed regarding selection bias (patient selection and homogeneity), attrition bias (analysis based on the availability of MRI parameters), detection bias (blinding and independence of MRI observer(s)), and reporting bias (selective reporting of correlation results). The Coleman Methodology score 25 modified by Ramponi et al 66 was used to assess the quality of the methodology. Extracted data from the selected studies included patient demographics, sample sizes, surgical procedure(s), MRI techniques and scores, defect sizes, and clinical outcome scores along with correlation statistics. The primary outcome of this study was to assess established correlations between postoperative MRI and clinical outcome in patients after cartilage repair of the ankle with the secondary aim to identify parameters that associate imaging and clinical outcome.
Statistical Analysis
All statistical analyses were performed in SPSS for Mac (version 23.0, SPSS Inc, Chicago, IL). For meta-analysis, all studies were stratified into 2 groups based on either presence or absence of correlation between postoperative imaging parameters and clinical outcome. The chi-square test and point-biserial analysis were applied to identify variables that determine established associations between imaging and clinical outcome. Following variables were included in the analysis: level of evidence, Coleman score, use of the composite magnetic resonance observation of cartilage repair tissue (MOCART) score, use of T2 mapping, subchondral assessment, cartilage repair technique (MS, CB, cartilage restoration), study size, patient age, defect size, and follow-up time. Correlation analyses that were not performed or Pearson coefficients that could not be obtained were classified as not applicable (NA). Significance was set at P < .05.
Results
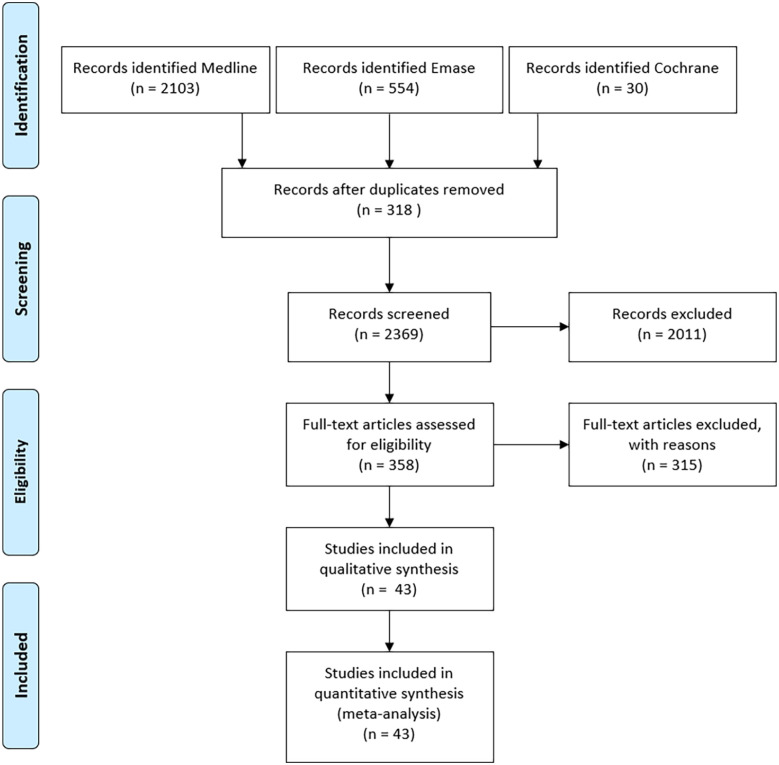
A literature search in MEDLINE resulted in 2103 articles, in Embase in 554 articles and in Cochrane in 30 articles. After removal of 318 duplicates, 2369 articles remained for screening. Following the application of the inclusion and exclusion criteria, 43 articles were finally included in the review and meta-analysis (Figure 1).
Figure 1.
Flowchart of the literature search. 61
Of these, the majority (93%) were case series, 5% retrospective and 2% prospective cohort studies. Besides standardized MRI techniques, T2 mapping was used in 23% and diffusion weight imaging (DWI) in 7% of studies. Procedures were classified in bone marrow stimulation (MS) including microfracture (MF) or autologous matrix induced chondrogenesis (AMIC), cell-based techniques (CB), namely, autologous chondrocyte implantation, and cartilage restoration comprising osteochondral auto- and allograft transplantation. MS was performed in 58% (n = 25), CB in 21% (n = 9), cartilage restoration in 19% (n = 8), and a combination (cohort studies) in 2% (n = 1) of the studies (Table 1).
Table 1.
Study and Patient Characteristics.
| Authors | Principle | Procedure | N | Mean Age, y | Mean Defect Size, cm2 | Follow-up, mo | MRI Protocol | MRI Score | Clinical Score |
|---|---|---|---|---|---|---|---|---|---|
| Battaglia et al 10 | MS | BMDCT | 20 | 28.1 | 0.147 a | 24 | Foot b , DWI | MOCART | AOFAS |
| Becher et al 12 | MS | MF | 45 | 40 | 0.5-2 | 69.6 | Foot | MOCART | HSS |
| Becher et al 13 | MS | MF | 30 | 41 | 0.5-2 | 24 | Foot | Descriptive | HSS |
| Becher et al 14 | MS | MF | 15 | 37 | 0.87 | 94.8 | Foot, T2 mapping | MOCART | AOFAS, Hannover |
| Carlson et al 19 | MS | MF | 22 | 14.4 | n/a | 99.6 | Foot | MOCART | AOFAS |
| Casari et al 20 | MS | AMIC | 35 | 34.4 | 0,9 | 54 | Foot | MOCART | AOFAS, Tegner |
| D’Ambrosi et al 26 | MS | AMIC | 11 | 17.9 | 1.191 | 24 | Foot | Choi et al 23 | AOFAS, SF-12 PCS, SF-12 MCS |
| D’Ambrosi et al 27 | MS | AMIC | 37 | 34 | 1.53 | 24 | Foot | Choi et al 23 | AOFAS |
| Domayer et al 30 | MS | MF | 14 | 41.9 | 1.4 | 55 | Foot | MOCART | AOFAS, CIN |
| Giannini et al 33 | MS | BMDCT | 49 | 18.1 | 1.23 | 48 | Foot, T2 mapping | MOCART | AOFAS |
| Jurina et al 46 | MS | MF | 13 | 15 | n/a | 67 | Foot | MOCART | Berndt & Harty score, AOFAS, SANE question, Martin questionnaire |
| Kanatlı et al 47 | MS | AMIC | 40 | 38 | 2.5 | 33.8 | Foot | MOCART | AOFAS |
| Kim et al 48 | MS | MF & MSC | 50 | 46.1 | n/a | 21.9 | Foot | MOCART | AOFAS, Tegner |
| Kubosch et al 50 | MS | AMIC | 17 | 38.8 | 2.2 | 39.5 | Foot, T2 mapping | MOCART | AOFAS, FFI |
| Kuni et al 51 | MS | MF | 22 | 31 | 3.77 | 24 | Foot | Own criteria | AOFAS |
| Rehnitz et al 68 | MS | MF | 28 | 41.3 | n/a | 42 | Foot, T2 mapping, dGEMRIC | Own criteria | AOFAS |
| Sadlik et al 72 | MS | AMIC | 10 | 37 | n/a | 46.4 | Foot | MOCART | AOFAS |
| Shimozono et al 74 | MS | MF | 42 | 38.4 | 44.1 | 51.7 | Foot, T2 mapping, DWI | SCBH 76 | FAOS |
| Shimozono et al 75 | MS | MF | 43 | 38.4 | 0.46 | 48 | Foot | SCBH 76 | AOFAS |
| Tao et al 79 | MS | MF | 48 | 35.5 | 1.65 | 19.2 | Foot, T2 mapping | Own criteria | AOFAS |
| Usuelli et al 82 | MS | ACIC | 9 | 37.4 | 2.1 | 12 | Foot | MOCART | AOFAS |
| Valderrabano et al 84 | MS | AMIC | 26 | 33 | 1.61 a | 31 | Foot | MOCART | AOFAS |
| Yang et al 91 | MS | MF | 25 | 39.24 | 0.84 | 43.2 | Foot | MOCART | FOAS, AOFAS, SF-36 |
| Ahn et al 3 | MS | MF | 64 | 40.1 | 0.858 | 35.7 | Foot | MOCART | AOFAS |
| Albano et al 4 | MS | AMIC | 16 | 42.6 | >1.5 | 30 | Foot | MOCART | AOFAS |
| Apprich et al 7 | MS& CB | MACI & MF | 20 | 31.7 | 1.09 | 53.8 | Foot, DWI | MOCART | AOFAS |
| Anders et al 5 | CB | MACI | 22 | 23.9 | 1.94 | 63.5 | Foot | MOCART | AOFAS |
| Aurich et al 9 | CB | MACI | 19 | 29.2 | 1.5 | 24.5 | Foot | MOCART | FFI, AOFAS, AAOS |
| Battaglia et al 11 | CB | ACI | 20 | 35 | 0.27 | 60 | Foot, T2 mapping | MOCART | AOFAS |
| Caumo et al 21 | CB | ACI | 41 | 35.2 | n/a | 12 | Foot | Own criteria | Tegner & Lysholm, AOFAS |
| DeSandis et al 29 | CB | JACI & BMAC | 46 | 37.6 | n/a | 24 | Foot | MOCART | FAOS, SF-12v2 |
| Lee et al 53 | CB | ACI | 38 | 35 | 1.94 | 24 | Foot | Anderson et al 6 | AOFAS, HSS |
| Lenz et al 54 | CB | MACI | 15 | 40 | 2.04 | 144 | Foot | MOCART | AOFAS, FAAM |
| Magnan et al 56 | CB | ACI | 30 | 28.9 | 2.36 | 45 | Foot | MOCART | AOFAS |
| Pagliazzi et al 64 | CB | ACI | 20 | 35 | 0.27 | 60 | Foot, T2 mapping, DWI | MOCART | AOFAS |
| Chen et al 22 | R | OAT c | 15 | 40.2 | 2.09 | 44.8 | Foot | MOCART | AOFAS, Ogilvie-Harris scale |
| Fraser et al 31 | R | OAT | 36 | 31 | 1.33 | 70.8 | Foot | Descriptive | AOFAS |
| Haraguchi et al 37 | R | OAT | 9 | 43.8 | n/a | 24 | Foot, T1ρ mapping | Own criteria | AOFAS |
| Hu et al 42 | R | OAT c | 17 | 37.3 | n/a | 32.6 | Foot, T2 mapping | MOCART | AOFAS |
| Imhoff et al 43 | R | OAT | 51 | 33 | 0.15 | 84 | Foot | Own criteria | AOFAS, Tegner |
| Nguyen et al 62 | R | OAT | 38 | 26 | 2.49 | 45 | Foot | MOCART | FAOS, RTS |
| Valderrabano et al 83 | R | OAT | 12 | 43 | 1.35 | 72 | Foot, gadolinium enhanced | Own criteria | AOFAS, Sports activity score, and own criteria |
| Woelfle et al 90 | R | OAT | 32 | 24.5 | n/a | 29 | Foot, T2 mapping | Descriptive | AOFAS, HSS |
Abbreviations: ACI, autologous chondrocyte implantation; ACIC, Autologous collagen induced chondrogenesis; AMIC, autologous matrix–induced chondrogenesis; AOFAS, American Orthopaedic Foot & Ankle Society score; BMAC, bone marrow aspirate concentrate; BMDCT, bone marrow–derived cell transplantation; CB, cell based; CIN, modified Cincinnati rating; DWI, diffusion weight imaging; FAAM, Foot and Ankle Ability Measure; FAOS, Foot and Ankle Outcome Score; FFI, Foot Function Index; HSS, Hannover Scoring System; JACI, juvenile allogenic chondrocyte implantation; MACI, matrix-induced autologous chondrocyte implantation; MCS, mental component summary; MF, microfracturing; MS, bone marrow stimulation; MOCART, magnetic resonance observation of cartilage repair tissue; MSC, mesenchymal stem cells; n/a, not available; R, cartilage restoration; OAT, osteochondral autograft/allograft transplantation; PCS, physical component summary; SANE, Single Assessment Numeric Evaluation; SCBH, subchondral bone health score; SF, Short Form Health Survey.
Defect size in cubic units.
Foot protocol including spin echo (SE), high signal intensity, fast SE sequences, turbo spin-echo, double-echo steady state, short-tau inversion recovery, proton-density fast-spin-echo, true fast imaging with steady state precession, and 3D-gradient echo sequences.
Autologous osteoperiosteal cylinder graft.
The MOCART score 57 was utilized in 28 studies (65%), 10 (23%) used their own defined criteria or only descriptive measures, 2 (5%) the Subchondral Bone Health (SCHB) Score, 74 2 (5%) the Choi classification, 23 and the Anderson’s modified MRI-based classification system 6 was applied once (2%), which also evaluated the Mintz cartilage grading system. 60 Further, 9 studies (21%) assessed postoperative imaging with T2 mapping, 1 (2%) T1ρ mapping, and 1 (2%) dGMERIC.
Regarding clinical outcome, the American Orthopaedic Foot & Ankle Society (AOFAS) score 49 was the most commonly evaluated functional clinical score, with its application in 88% of studies, whereas other scores like the Foot and Ankle Outcome Score (FAOS), 70 Tegner activity scale, 80 Short Form Health Survey (SF-24, SF-36),17,87 Foot Function Index (FFI), 18 and Hannover Scoring System (HSS) 81 were used in a minority of studies.
Methodologic assessment resulted in moderate overall risk of bias with a mean modified Coleman score of 57 (range, 33-70). Although selection bias could not be ruled out in 64% of the included studies, potential detection and attrition bias were found in 22% and 44%, respectively. Risk for reporting bias was low, with only 7% of all studies. Three Level IIb to IIb (Coleman score range, 61-66) and 40 Level IV (Coleman score range, 33-70) studies were included. Detailed study quality assessment can be found in Table 2.
Table 2.
Study Quality.
| Authors | Design | Selection Bias | Attrition Bias | Detection Bias | Reporting Bias | Coleman a | LOE b |
|---|---|---|---|---|---|---|---|
| Ahn et al 3 | CS | Yes | No | No | No | 69 | IV |
| Albano et al 4 | CS | Yes | No | Yes | No | 55 | IV |
| Anders et al 5 | CS | No | No | No | No | 67 | IV |
| Apprich et al 7 | RCS | Yes | No | No | No | 42 | IV |
| Aurich et al 9 | CS | No | No | No | No | 67 | IV |
| Battaglia et al 10 | CS | No | No | No | No | 61 | IV |
| Battaglia et al 11 | CS | No | No | Yes | No | 57 | IV |
| Becher et al 12 | CS | Yes | Yes | Yes | No | 66 | IIb |
| Becher et al 13 | PCS | No | No | Yes | No | 70 | IV |
| Becher et al 14 | CS | Yes | No | No | No | 45 | IV |
| Carlson et al 19 | CS | Yes | Yes | No | No | 69 | IV |
| Casari et al 20 | CS | Yes | No | No | No | 53 | IV |
| Caumo et al 21 | CS | No | No | Yes | No | 33 | IV |
| Chen et al 22 | CS | Yes | No | No | No | 67 | IIb |
| D’Ambrosi et al 26 | CS | Yes | No | No | Yes | 52 | IV |
| D’Ambrosi et al 27 | CS | No | No | No | No | 59 | IV |
| DeSandis et al 29 | CS | Yes | Yes | Yes | Yes | 59 | IV |
| Domayer et al 30 | CS | Yes | No | Yes | No | 48 | IV |
| Fraser et al 31 | CS | No | No | Yes | Yes | 63 | IV |
| Giannini et al 33 | CS | Yes | Yes | Yes | No | 50 | IV |
| Haraguchi et al 37 | CS | Yes | No | Yes | No | 47 | IV |
| Hu et al 42 | CS | No | No | Yes | No | 66 | IV |
| Imhoff et al 43 | CS | Yes | No | No | No | 42 | IV |
| Jurina et al 46 | CS | Yes | Yes | No | Yes | 64 | IV |
| Kanatlı et al 47 | CS | No | No | No | No | 62 | IV |
| Kim et al 48 | RCS | Yes | No | No | No | 61 | IIIb |
| Kubosch et al 50 | CS | No | Yes | Yes | No | 61 | IV |
| Kuni et al 51 | CS | Yes | No | No | Yes | 47 | IV |
| Lee et al 53 | CS | Yes | Yes | Yes | No | 58 | IV |
| Lenz et al 54 | CS | Yes | No | No | No | 48 | IV |
| Magnan et al 56 | CS | Yes | No | Yes | No | 53 | IV |
| Nguyen et al 62 | CS | Yes | No | Yes | No | 63 | IV |
| Pagliazzi et al 64 | CS | Yes | No | Yes | No | 59 | IV |
| Rehnitz et al 68 | CS | Yes | No | No | No | 35 | IV |
| Sadlik et al 72 | CS | No | No | Yes | Yes | 61 | IV |
| Shimozono et al 74 | CS | Yes | Yes | Yes | No | 51 | IV |
| Shimozono et al 75 | CS | Yes | No | No | No | 70 | IV |
| Tao et al 79 | CS | Yes | Yes | Yes | No | 59 | IV |
| Usuelli et al 82 | CS | No | No | No | No | 65 | IV |
| Valderrabano et al 83 | CS | Yes | No | No | Yes | 46 | IV |
| Valderrabano et al 84 | CS | No | No | No | No | 60 | IV |
| Woelfle et al 90 | CS | Yes | Yes | No | No | 58 | IV |
| Yang et al 91 | CS | Yes | No | No | No | 69 | IV |
To evaluate the correlation between clinical and radiological outcome, the majority (n = 28; 65%) used the Spearman rank coefficient or the Pearson correlation coefficient. Magnan et al, 56 Woelfle et al, 90 and Imhoff et al 43 performed parametric or nonparametric statistical hypothesis tests, and D’Ambrosi et al 26 used a multivariant correlation analysis. Rhenitz et al 68 and Nguyen et al 62 both utilized a receiver operating characteristic analysis. There was no clear specification of the statistical analysis in 12% of the included studies. Correlation coefficients could be calculated in n = 5 studies (12%), because detailed case descriptions were available of all included patients.
Of the included 43 studies, a correlation between 1 or more imaging variables and clinical outcome was found in 21 (49%) articles (Table 3). Of the 24 studies (56%) utilizing the composite MOCART score, 7 (29%) reported a correlation with clinical outcome. Five of 13 studies (39%) evaluating the correlation of defect fill and clinical outcome showed an association.9,21,47,64,79 Of all 10 studies evaluating the correlation between T mapping and clinical outcome, 5 (50%, 4 × T2, 1 × T1ρ) showed a correlation.8,11,26,37.51,55,64,68,79 The correlation of diffusion-weighted imaging (DWI) and clinical outcome was shown in none of the 3 (0%) included studies.
Table3.
Correlations Between the MRI and Clinical Outcome Scores. a
| Author Classification | Composite | Filling | Integration | Surface | Structure | Signal Intensity | Subchondral Lamina | Subchondral Bone | Adhesions | Effusion | T2 Mapping | DWI | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOCART | |||||||||||||
| Ahn et al 3 | No | No | No | No | No | No | No | No | No | No | – | – | Yes b |
| Albano et al 4 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Anders et al 5 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Apprich et al 7 | No | – | – | – | – | – | – | – | – | – | – | No | – |
| Aurich et al 9 | No | Yes c | No | No | No | No | No | No | No | No | – | – | – |
| Battaglia et al 11 | No | No | No | No | No | No | No | No | No | No | No | – | – |
| Battaglia et al 10 | No | No | No | No | No | Yesd | No | No | No | No | Yesc | – | – |
| Carlson et al 19 | No | No | No | No | No | No | No | No | No | No | – | – | – |
| Casari et al 20 | No | No | No | No | No | No | No | No | No | No | – | – | – |
| Chen et al 22 | Yes d | – | – | – | – | – | – | – | – | – | – | – | – |
| DeSandis et al 29 | Yes b | – | – | – | – | – | – | – | – | – | – | – | – |
| Hu et al 42 | Yes d | – | – | – | – | – | – | – | – | – | – | – | – |
| Jurina et al 46 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Kanatli et al 47 | No | Yes d | No | No | No | No | No | No | No | No | – | – | – |
| Kim et al 48 | Yes c | – | – | – | – | – | – | – | – | – | – | – | – |
| Kubosch et al 50 | Yes c | – | – | – | – | – | – | – | – | – | No | – | – |
| Lenz et al 54 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Magnan et al 56 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Nguyen et al 62 | Yes d | – | – | – | – | – | – | – | – | – | – | – | – |
| Pagliazzi et al 64 | No | Yes c | No | No | No | No | No | No | No | No | Yes c | – | – |
| Sadlik et al 72 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Usuelli et al 82 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Valderrabano et al 84 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Yang et al 91 | Yes c | – | – | – | – | – | – | – | – | – | – | – | – |
| Choi | |||||||||||||
| D’Ambrosi et al 27 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| D’Ambrosi et al 26 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| Own criteria | |||||||||||||
| Becher et al 12 | – | No | Yes c | – | – | – | – | – | – | Yesd | – | – | – |
| Becher et al 13 | – | No | – | – | – | – | – | No | – | – | – | – | – |
| Becher et al 14 | No | – | – | – | – | – | – | – | – | – | No | – | – |
| Caumo et al 21 | – | Yes b | Yes b | – | – | Yes b | Yes b | Yes b | – | – | – | – | – |
| Fraser et al 31 | – | – | No | No | – | – | – | No | – | – | – | – | – |
| Giannini et al 33 | – | – | – | – | – | – | – | – | – | – | No | – | – |
| Imhoff et al 43 | – | – | – | Yes c | – | – | – | – | – | – | – | – | – |
| Kuni et al 51 | – | No | No | – | – | – | – | Yes d | – | No | – | – | – |
| Rehnitz et al 68 | – | – | – | – | – | – | – | – | – | – | Yes b | No | – |
| Tao et al 79 | – | Yes b | – | – | – | – | – | Yes b | – | – | Yes d | No | – |
| Valderrabano et al 83 | Yes b | – | – | – | – | – | – | – | – | – | – | – | – |
| Woelfle et al 90 | No | – | – | No | No | No | – | No | – | No | – | – | – |
| Anderson | |||||||||||||
| Lee et al 53 | No | – | – | – | – | – | – | – | – | – | – | – | – |
| SCBH Score | |||||||||||||
| Shimozono et al 74 | Yesd | – | – | – | – | – | – | – | – | – | – | – | – |
| Shimozono et al 75 | Yesd | – | – | – | – | – | – | – | – | – | – | – | – |
| T mapping | |||||||||||||
| Domayer et al 30 | – | – | – | – | – | – | – | – | – | – | No | – | – |
| Haraguchi et al 37 | – | – | – | – | – | – | – | – | – | – | Yes b | – | – |
Abbreviations: LOE, level of evidence; MOCART, magnetic resonance observation of cartilage repair tissue; SCBH, subchondral bone health score.
Numbers provided represent exact correlation coefficients; if no coefficients were given, “yes” or “no” was used to indicate that articles found or did not find a correlation between radiologic and clinical outcome, respectively. –, not applicable.
P value unavailable.
P < .05.
P < .01.
Analyzing the metadata, advanced age, shorter follow-up, and study sample size were associated with established correlation between clinical and radiographic outcome (r = 0.367, P = .021; r = −0.335, P = .028; and r = 0.326, P = .033, respectively). None of the other assessed variables showed significant influence on the relationship of MRI and clinical outcome.
Bone Marrow Stimulation
Most studies assessing MS (n=26) evaluated correlation of imaging and clinical outcome after MF (54%; n=14) or a combination of MF with spongiosa, cell-free scaffold, or mesenchymal stem cells (15%; n=4). Other techniques used were AMIC (19%; n=5), bone marrow–derived cell transplantation (BMDCT) (8%; n=2) or autologous collagen-induced chondrogenesis (ACIC) (4%; n=1). Patients were treated at a mean age of 34 (range 14-46) years of age for cartilage defect and evaluated after a follow-up of 43 (range 12-100) months after the cartilage repair. Fifteen of 26 studies (58%) reported 1 or more significant correlations between clinical outcome and imaging parameters. Three studies found a correlation of the composite MOCART score and clinical outcome.48,50,91 Four studies reported a correlation of the subgroups of the MOCART score. Kanatli et al 47 noted that filling of the defect is significantly correlated with outcome, Battaglia et al 10 reported a correlation of the signal of the repair and Ahn et al 3 of the subchondral bone marrow edema (BME) volume with clinical outcomes. Apart from the studies evaluating the MOCART score, BME was associated with inferior outcome in 3 studies,51,75,79 and Shimozono et al 74 reported a correlation of the subchondral bone health score (SCBH) and clinical outcome. Although Becher et al 12 found a correlation between clinical outcome and effusion, D’Ambrosi et al 26 stated that the Choi score on CT was correlated with clinical outcome, yet reported nonsignificance for MRI scores. A positive correlation of clinical outcome and T2 mapping was found in 3 studies.10,68,79
Cell-Based Techniques
Of the 10 studies evaluating CB, 9 studies (90%) investigated the correlation of ACI and 1 (10%) of juvenile articular cartilage allograft with clinical outcome. Patients were treated at a mean age of 33 years (range 24-40) for OLT and evaluated after a follow-up of 51 months (range 12-144). Four of 10 studies (40%) reported 1 or more significant correlations. DeSandis et al 29 reported a correlation of the composite MOCART score and clinical outcome and Aurich et al 9 as well as Pagliazzi et al 64 found a correlation with the subscore defect filling. Caumo et al 21 reported in a descriptive classification that filling, integration, and subchondral signals were correlated with clinical outcome. Additionally, Pagliazzi et al 64 noted a significant association between T2 mapping and clinical outcome.
Cartilage Restoration Techniques
Eight studies evaluated the correlation after cartilage restoration, of which 6 (75%) used OAT and 2 (25%) osteoperiosteal cylinder. Patients were treated at a mean age of 35 years (range 25-44) and evaluated after a follow-up of 50 months (range 24-84). Six of 8 studies (75%) reported 1 or more significant correlations. All 3 studies assessing postoperative imaging using the MOCART score found a significant correlation of the composite score with the clinical outcome.22,42,62 Although Valderrabano et al 83 reported a correlation but did not use a scoring system, Imhoff et al 43 found a correlation of the repair surface with clinical outcome. Haraguchi et al 37 reported a negative correlation of T1ρ mapping and clinical outcome.
Discussion
The main finding of this study is that MRI parameters do not correlate well with the clinical outcome at a minimum follow-up of 12 months after cartilage repair of the talus. However, there is some evidence that postoperative MRI mirrors clinical outcome in patients with advanced age at a short- to midterm follow-up. Nevertheless, there is a paucity of high-quality research regarding the clinical value of postoperative imaging, especially its predictive value for long-term clinical outcome remains uncertain.
Of the included studies, most (n=26) analyzed the correlation of imaging and outcome after MS procedures, and the results were controversial with a small majority of 15 studies finding a correlation (58%). The current study did not identify a radiologic parameter that was predominantly correlated with clinical outcome in these studies. The most consistent reported parameter after MF was the composite MOCART score, which was associated with better clinical outcome in 5 studies,48,50,74,75,91 as well as changes in the subchondral bone like the presence or changes of BME.3,51,74,75,79 In fact, the role of the subchondral bone in cartilage repair has received increasing interest over recent years.35,59,77 Large BME has been shown to negatively affect cartilage repair outcome in the knee, especially in patients undergoing cell-based procedures. 59 Recently, Jung et al emphasized the importance of subchondral parameters in the evaluation of cartilage repair in the knee. 44 They found that subchondral bone defects and bone marrow edema were correlated with cartilage repair tissue quality and clinical symptoms after matrix-associated ACI with concomitant autologous bone grafting. However, the current meta-analysis could not translate these finding to cartilage repair in the ankle with only 6 of 15 studies (40%), which have investigated this association, finding a significant association with clinical outcome. Of these, 5 studies reported a significant correlation of BME with clinical outcome after MS, and only 1 study identified BME as a correlating parameter after CB.3,21,51,74,75,79 Interestingly, Caumo et al 21 reported that the absence of edema was found to correlate with worse clinical outcomes after ACI. The authors described this finding as a sign of insufficient subchondral remodeling after ACI leading to a deficiency in the maturation process, which ultimately results in poor clinical outcome. Conversely, all studies reporting a relationship between BME and clinical outcome after MS stated that the absence or reduction of subchondral BME correlated with superior clinical outcome.3,21,51,74,75,79
Regarding the correlation of MRI and clinical outcome after CB for OLT, evidence is scarce in the current literature, with only 4 of 10 studies (40%) reporting a statistically significant association.9,21,29,64 The most consistent parameter reported was “defect filling” with 3 studies stating statistical significance,29,64 whereas 1 study each identified T2 mapping signal and the composite MOCART as being related to clinical outcome.9,21 Because these studies were heterogenous in follow-up time and assessment of radiologic parameters, the interpretation of its clinical value is challenging. In fact, potential correlations of imaging and clinical outcome after CB have been more intensively studied in the knee joint. In a meta-analysis in 2013, 10 of 19 studies reported a significant correlation between graft hypertrophy and repair tissue signal (as defined by the Henderson score), 40 with a moderate to good correlation of the overall Henderson score with clinical outcomes. 15 Similar to McCarthy et al, 58 who reported a significant association of defect fill, overall signal intensity, and surface of repair tissue with clinical outcome at 12 months after ACI, the mentioned meta-analysis from 2013 had a shorter follow-up period, with studies demonstrating a correlation at 6 months postoperatively and a maximal follow-up of 60 months when compared to the current study. As seen in the results of this study, shorter follow-up was significantly associated with the finding of correlation between clinical and radiographic outcome. Prior studies have shown that complete graft maturation is found 13.5 months after MS and 12-24 months after CB treatment,34,63 yet graft deterioration might start as early as 30 months after cartilage repair with declining imaging scores over time.20,71 Consequently, although optimal timing for MRI evaluation after cartilage repair still remains controversial, some authors suggest a time period between 12 and 30 months as potentially ideal for postoperative imaging in asymptomatic patients to mirror clinical outcome, which is supported by the current findings. 1
Interestingly, the greatest percentage of correlation was found after cartilage restoration techniques. Six of 8 studies (75%) evaluating restoration techniques found a positive correlation to clinical outcome.22,37,42,43,62,83 Similar to the included MS studies, most studies evaluated the composite morphologic appearance on MRI with only limited information about specific subscale parameters. The largest cohort in the current review with 38 patients after OAT, Nguyen et al 62 found a significant correlation between the MOCART score and the ability to return to one’s previous level of activity. In contrast to the ankle joint, studies investigating OAT in the knee were able to identify specific MRI parameters like cystic subchondral change, missing of trabeculae crossing the defect site, abnormal articular cartilage signal and signs of decreased osteointegration that were associated with worse clinical outcome.86,89 Hence, it would be interesting to see if similar findings can be reported after cartilage restoration in the ankle.
Generally, the lack of association between conventional MRI and clinical outcome in cartilage repair of the knee 28 and ankle, as seen in the current study, may stem also from the still rather unspecific nature of current MRI scores such as the MOCART, Osteochondral Allograft MRI Scoring System (OCAMRISS). As Ackermann et al 2 pointed out in a study investigating the effect of the augmentation of bone marrow aspirate on clinical and imaging outcomes after OCA to the knee, the majority of OCAMRISS subscales (same applies to the MOCART) are dichotomous and score solely the absence or presence of the respective MRI feature. Thus, this may result in missing small but potentially clinically relevant differences in graft maturation and integration. This may have also led to the current finding that the correlation of postoperative MRI and clinical outcome is more pronounced in patients with advanced age as increased interindividual differences in cartilage regeneration potentially exist in these patients, thus generating a large enough effect size to be detected by current MRI scores.
In contrast to morphologic MRI techniques, compositional MRI sequences like T2 mapping are able to provide compositional information about tissue formation after cartilage repair. 76
However, this technique is largely used for research settings and is generally not clinically employed. Compared with morphologic MRI sequences, T2 mapping has been able to demonstrate changes in water content and collagen orientation, which is known to play an important role in degeneration of cartilage.8,55 Water content increases in pathologic cartilage and destruction of collagen fiber network increases T2 relaxation, which is an early sign of cartilage degeneration. T2 relaxation in repair tissue differs for each repair technique, which might be helpful in identifying hyaline-like tissue as found in cell transplantation repair techniques compared to fibrocartilage that can be found after MS. 52 Although T2 mapping has been more profoundly studied after cartilage repair of the knee with inconsistent correlation to clinical outcome, 69 there is still insufficient evidence for any association with clinical outcome in cartilage repair of the ankle. The current systematic review identified 5 studies across all groups of cartilage repair (MS, CB, and cartilage restoration) that reported significant correlations with clinical outcome.10,50,64,68,79 Further improvement of current qualitative MRI and more advanced MRI techniques may provide more insight into detailed cartilage repair tissue morphology and maturation, thus generating qualitative data ultimately helping to predict outcome after cartilage repair. 32
This systematic review and meta-analysis has inherit limitations, which have to be acknowledged. First, the included studies show variation in methodology, cartilage repair techniques, and MRI sequences. Whereas cartilage repair with MS is the most investigated technique, studies assessing postoperative imaging in CB and cartilage restoration techniques are scarce making it challenging to draw meaningful conclusions. Because of heterogenous MRI sequences used it is difficult to draw comprehensive conclusions about a specific MRI technique in the evaluation of cartilage restorative procedures. Second, the small sample sizes and different follow-up times of the included studies increase the risk of bias, especially as the quality of cartilage repair tissue varies over time, resulting in the comparison of groups with variable tissue maturation stages. Notably, small sample sizes allow only for the detection of large effect sizes, thus introducing the risk of type II error. Consequently, larger study sizes are needed to identify smaller but potentially clinically relevant effects after cartilage repair. This is highlighted by the findings in the current meta-analysis, where studies with larger sample sizes were more likely to detect a correlation of MRI and clinical outcome.
Conclusion
Interpreting MRI in prediction of clinical outcome in ankle cartilage repair remains challenging; however, it seems to hold some value in reflecting clinical outcome in patients with advanced age and/or at a shorter follow-up. Yet, further research is warranted to optimize postoperative MRI protocols and assessments allowing for a more comprehensive repair tissue evaluation, which eventually reflect clinical outcome in patients after cartilage repair of the ankle.
Footnotes
Ethical Approval: Ethical approval was not sought for the present study because the current study is a systematic review and synthesis of published data. The study uses data from previous published studies which are available in the public domain. No new (personal) data was collected for current study.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article. ICMJE forms for all authors are available online.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Manuel Waltenspül, MD,  https://orcid.org/0000-0002-8192-0233
https://orcid.org/0000-0002-8192-0233
References
- 1. Ackermann J, Merkely G, Mestriner AB, Shah N, Gomoll AH. Increased chondrocytic gene expression is associated with improved repair tissue quality and graft survival in patients after autologous chondrocyte implantation. Am J Sports Med. 2019;47(12):2919-2926. doi: 10.1177/0363546519868213 [DOI] [PubMed] [Google Scholar]
- 2. Ackermann J, Mestriner AB, Shah N, Gomoll AH. Effect of autogenous bone marrow aspirate treatment on magnetic resonance imaging integration of osteochondral allografts in the knee: a matched comparative imaging analysis. Arthroscopy. 2019;35(8):2436-2444. doi: 10.1016/j.arthro.2019.03.033 [DOI] [PubMed] [Google Scholar]
- 3. Ahn J, Choi JG, Jeong BO. Clinical outcomes after arthroscopic microfracture for osteochondral lesions of the talus are better in patients with decreased postoperative subchondral bone marrow edema. Knee Surg Sports Traumatol Arthrosc. 2021;29(5):1570-1576. doi: 10.1007/s00167-020-06303-y [DOI] [PubMed] [Google Scholar]
- 4. Albano D, Martinelli N, Bianchi A, Messina C, Malerba F, Sconfienza LM. Clinical and imaging outcome of osteochondral lesions of the talus treated using autologous matrix-induced chondrogenesis technique with a biomimetic scaffold. BMC Musculoskelet Disord 2017;18(1):306. doi: 10.1186/s12891-017-1679-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Anders S, Goetz J, Schubert T, Grifka J, Schaumburger J. Treatment of deep articular talus lesions by matrix associated autologous chondrocyte implantation–results at five years. Int Orthop 2012;36(11):2279-2285. doi: 10.1007/s00264-012-1635-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Anderson IF, Crichton KJ, Grattan-Smith T, Cooper RA, Brazier D. Osteochondral fractures of the dome of the talus. J Bone Joint Surg Am. 1989;71(8):1143-1152. [PubMed] [Google Scholar]
- 7. Apprich S, Trattnig S, Welsch GH, et al. Assessment of articular cartilage repair tissue after matrix-associated autologous chondrocyte transplantation or the microfracture technique in the ankle joint using diffusion-weighted imaging at 3 Tesla. Osteoarthritis Cartilage 2012;20(7):703-711. doi: 10.1016/j.joca.2012.03.008 [DOI] [PubMed] [Google Scholar]
- 8. Arokoski JP, Jurvelin JS, Väätäinen U, Helminen HJ. Normal and pathological adaptations of articular cartilage to joint loading. Scand J Med Sci Sports. 2000;10(4):186-198. doi: 10.1034/j.1600-0838.2000.010004186.x [DOI] [PubMed] [Google Scholar]
- 9. Aurich M, Bedi HS, Smith PJ, et al. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39(2):311-319. doi: 10.1177/0363546510381575 [DOI] [PubMed] [Google Scholar]
- 10. Battaglia M, Rimondi E, Monti C, et al. Validity of T2 mapping in characterization of the regeneration tissue by bone marrow derived cell transplantation in osteochondral lesions of the ankle. Eur J Radiol. 2011;80(2):e132-e139. doi: 10.1016/j.ejrad.2010.08.008 [DOI] [PubMed] [Google Scholar]
- 11. Battaglia M, Vannini F, Buda R, et al. Arthroscopic autologous chondrocyte implantation in osteochondral lesions of the talus: mid-term T2-mapping MRI evaluation. Knee Surg Sports Traumatol Arthrosc. 2011;19(8):1376-1384. doi: 10.1007/s00167-011-1509-x [DOI] [PubMed] [Google Scholar]
- 12. Becher C, Driessen A, Hess T, Longo UG, Maffulli N, Thermann H. Microfracture for chondral defects of the talus: maintenance of early results at midterm follow-up. Knee Surg Sports Traumatol Arthrosc. 2010;18(5):656-663. doi: 10.1007/s00167-009-1036-1 [DOI] [PubMed] [Google Scholar]
- 13. Becher C, Thermann H. Results of microfracture in the treatment of articular cartilage defects of the talus. Foot Ankle Int. 2005;26(8):583-589. [DOI] [PubMed] [Google Scholar]
- 14. Becher C, Zühlke D, Plaas C, et al. T2-mapping at 3 T after microfracture in the treatment of osteochondral defects of the talus at an average follow-up of 8 years. Knee Surg Sports Traumatol Arthrosc. 2015;23(8):2406-2412 [DOI] [PubMed] [Google Scholar]
- 15. Blackman AJ, Smith MV, Flanigan DC, Matava MJ, Wright RW, Brophy RH. Correlation between magnetic resonance imaging and clinical outcomes after cartilage repair surgery in the knee: a systematic review and meta-analysis. Am J Sports Med. 2013;41(6):1426-1434. doi: 10.1177/0363546513485931 [DOI] [PubMed] [Google Scholar]
- 16. Booth A, Clarke M, Dooley G, et al. The nuts and bolts of PROSPERO: an international prospective register of systematic reviews. Syst Rev. 2012;1(1):1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Brazier JE, Harper R, Jones NM, et al. Validating the SF-36 Health Survey questionnaire: new outcome measure for primary care. BMJ. 1992;305(6846):160-164. doi: 10.1136/bmj.305.6846.160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Budiman-Mak E, Conrad KJ, Roach KE. The Foot Function Index: a measure of foot pain and disability. J Clin Epidemiol 1991;44(6):561-570. doi: 10.1016/0895-4356(91)90220-4 [DOI] [PubMed] [Google Scholar]
- 19. Carlson MJ, Antkowiak TT, Larsen NJ, Applegate GR, Ferkel RD. Arthroscopic treatment of osteochondral lesions of the talus in a pediatric population: A minimum 2-Year follow-up. Am J Sports Med 2020;48(8):1989-1998. [DOI] [PubMed] [Google Scholar]
- 20. Casari FA, Germann C, Weigelt L, Wirth S, Viehöfer A, Ackermann J. The role of magnetic resonance imaging in autologous matrix-induced chondrogenesis for osteochondral lesions of the talus: analyzing MOCART 1 and 2.0. Cartilage. 2020:1947603520946382. doi: 10.1177/1947603520946382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Caumo F, Russo A, Faccioli N, et al. Autologous chondrocyte implantation: prospective MRI evaluation with clinical correlation. Radiol Med. 2007;112(5):722-731. doi: 10.1007/s11547-007-0175-z [DOI] [PubMed] [Google Scholar]
- 22. Chen W, Tang K, Yuan C, Zhou Y, Tao X. Intermediate results of large cystic medial osteochondral lesions of the talus treated with osteoperiosteal cylinder autografts from the medial tibia. Arthroscopy. 2015;31(8):1557-1564. doi:10.1016/j.arthro.2015.02.027 [DOI] [PubMed] [Google Scholar]
- 23. Choi WJ, Park KK, Kim BS, Lee JW. Osteochondral lesion of the talus: is there a critical defect size for poor outcome? Am J Sports Med. 2009;37(10):1974-1980. doi: 10.1177/0363546509335765 [DOI] [PubMed] [Google Scholar]
- 24. Choi YS, Potter HG, Chun TJ. MR imaging of cartilage repair in the knee and ankle. Radiographics. 2008;28(4):1043-1059. doi: 10.1148/rg.284075111 [DOI] [PubMed] [Google Scholar]
- 25. Coleman BD, Khan KM, Maffulli N, Cook JL, Wark JD. Studies of surgical outcome after patellar tendinopathy: clinical significance of methodological deficiencies and guidelines for future studies. Victorian Institute of Sport Tendon Study Group. Scand J Med Sci Sports. 2000;10(1):2-11. doi: 10.1034/j.1600-0838.2000.010001002.x [DOI] [PubMed] [Google Scholar]
- 26. D’Ambrosi R, Maccario C, Ursino C, Serra N, Usuelli FG. Combining microfractures, autologous bone graft, and autologous matrix-induced chondrogenesis for the treatment of juvenile osteochondral talar lesions. Foot Ankle Int. 2017;38(5):485-495. doi: 10.1177/1071100716687367 [DOI] [PubMed] [Google Scholar]
- 27. D’Ambrosi R, Maccario C, Ursino C, Serra N, Usuelli FG. The role of bone marrow edema on osteochondral lesions of the talus. Foot and Ankle Surgery 2018;24(3):229-235. doi: 10.1016/j.fas.2017.02.010 [DOI] [PubMed] [Google Scholar]
- 28. de Windt TS, Welsch GH, Brittberg M, et al. Is magnetic resonance imaging reliable in predicting clinical outcome after articular cartilage repair of the knee? A systematic review and meta-analysis. Am J Sports Med. 2013;41(7):1695-1702. doi: 10.1177/0363546512473258 [DOI] [PubMed] [Google Scholar]
- 29. DeSandis BA, Haleem AM, Sofka CM, O’Malley MJ, Drakos MC. Arthroscopic treatment of osteochondral lesions of the talus using juvenile articular cartilage allograft and autologous bone marrow aspirate concentration. J Foot Ankle Surg. 2018;57(2):273-280. doi: 10.1053/j.jfas.2017.09.009 [DOI] [PubMed] [Google Scholar]
- 30. Domayer SE, Welsch GH, Stelzeneder D, et al. Microfracture in the ankle: Clinical results and MRI with T2-mapping at 3.0 T after 1 to 8 Years. Cartilage 2011;2(1):73-80. doi: 10.1177/1947603510380901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fraser EJ, Harris MC, Prado MP, Kennedy JG. Autologous osteochondral transplantation for osteochondral lesions of the talus in an athletic population. Knee Surg Sports Traumatol Arthrosc 2016;24(4):1272-1279. doi: 10.1007/s00167-015-3606-8 [DOI] [PubMed] [Google Scholar]
- 32. Germann C, Galley J, Falkowski AL, et al. Ultra-high resolution 3D MRI for chondrocalcinosis detection in the knee—a prospective diagnostic accuracy study comparing 7-tesla and 3-tesla MRI with CT. Eur Radiol. 2021;31(12):9436-9445. doi: 10.1007/s00330-021-08062-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Giannini S, Buda R, Battaglia M, et al. One-step repair in talar osteochondral lesions: 4-year clinical results and t2-mapping capability in outcome prediction. Am J Sports Med 2013;41(3):511-518. doi: 10.1177/0363546512467622 [DOI] [PubMed] [Google Scholar]
- 34. Gobbi A, Karnatzikos G, Scotti C, Mahajan V, Mazzucco L, Grigolo B. One-step cartilage repair with bone marrow aspirate concentrated cells and collagen matrix in full-thickness knee cartilage lesions: results at 2-year follow-up. Cartilage. 2011;2(3):286-299. doi: 10.1177/1947603510392023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Gomoll AH, Madry H, Knutsen G, et al. The subchondral bone in articular cartilage repair: current problems in the surgical management. Knee Surg Sports Traumatol Arthrosc. 2010;18(4):434-447. doi: 10.1007/s00167-010-1072-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Group OLoEW. “The Oxford 2011 Levels of Evidence.” Oxford Centre for Evidence-Based Medicine. http://wwwcebmnet/indexaspx?o=5653 2011
- 37. Haraguchi N, Ota K, Nishida N, Ozeki T, Yoshida T, Tsutaya A. T1ρ mapping of articular cartilage grafts after autologous osteochondral transplantation for osteochondral lesions of the talus: a longitudinal evaluation. J Magn Reson Imaging. 2018;48(2):398-403. doi: 10.1002/jmri.25962 [DOI] [PubMed] [Google Scholar]
- 38. Hargreaves BA, Worters PW, Pauly KB, Pauly JM, Koch KM, Gold GE. Metal-induced artifacts in MRI. AJR Am J Roentgenol. 2011;197(3):547-555. doi: 10.2214/ajr.11.7364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hayashi D, Li X, Murakami AM, Roemer FW, Trattnig S, Guermazi A. Understanding magnetic resonance imaging of knee cartilage repair: a focus on clinical relevance. Cartilage. 2018;9(3):223-236. doi: 10.1177/1947603517710309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Henderson I, Tuy B, Connell D, Oakes B, Hettwer W. Prospective clinical study of autologous chondrocyte implantation and correlation with MRI at three and 12 months. J Bone Joint Surg Br. 2003;85(7):1060-1066. doi: 10.1302/0301-620x.85b7.13782 [DOI] [PubMed] [Google Scholar]
- 41. Ho YY, Stanley AJ, Hui JH, Wang SC. Postoperative evaluation of the knee after autologous chondrocyte implantation: what radiologists need to know. Radiographics. 2007;27(1):207-220; discussion 221-222. doi: 10.1148/rg.271065064 [DOI] [PubMed] [Google Scholar]
- 42. Hu Y, Guo Q, Jiao C, et al. Treatment of large cystic medial osteochondral lesions of the talus with autologous osteoperiosteal cylinder grafts. Arthroscopy. 2013;29(8):1372-1379. doi: 10.1016/j.arthro.2013.05.014 [DOI] [PubMed] [Google Scholar]
- 43. Imhoff AB, Paul J, Ottinger B, et al. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39(7):1487-1493. doi: 10.1177/0363546510397726 [DOI] [PubMed] [Google Scholar]
- 44. Jung M, Karampinos DC, Holwein C, et al. Quantitative 3-T magnetic resonance imaging after matrix-associated autologous chondrocyte implantation with autologous bone grafting of the knee: the importance of subchondral bone parameters. Am J Sports Med. 2021;49(2):476-486. doi: 10.1177/0363546520980134 [DOI] [PubMed] [Google Scholar]
- 45. Jungmann PM, Baum T, Bauer JS, et al. Cartilage repair surgery: outcome evaluation by using noninvasive cartilage biomarkers based on quantitative MRI techniques? Biomed Res Int. 2014;2014:840170. doi: 10.1155/2014/840170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jurina A, Dimnjaković D, Mustapić M, Smoljanović T, Bojanić I. Clinical and MRI outcomes after surgical treatment of osteochondral lesions of the talus in skeletally immature children. J Pediatr Orthop 2018;38(2):122-127. doi: 10.1097/bpo.0000000000000745 [DOI] [PubMed] [Google Scholar]
- 47. Kanatlı U, Eren A, Eren TK, Vural A, Geylan DE, Öner AY. Single-step arthroscopic repair with cell-free polymer-based scaffold in osteochondral lesions of the talus: clinical and radiological results. Arthroscopy. 2017;33(9):1718-1726. doi: 10.1016/j.arthro.2017.06.011 [DOI] [PubMed] [Google Scholar]
- 48. Kim YS, Lee HJ, Choi YJ, Kim YI, Koh YG. Does an injection of a stromal vascular fraction containing adipose-derived mesenchymal stem cells influence the outcomes of marrow stimulation in osteochondral lesions of the talus? A clinical and magnetic resonance imaging study. Am J Sports Med. 2014;42(10):2424-2434. doi: 10.1177/0363546514541778 [DOI] [PubMed] [Google Scholar]
- 49. Kitaoka HB, Alexander IJ, Adelaar RS, Nunley JA, Myerson MS, Sanders M. Clinical rating systems for the ankle-hindfoot, midfoot, hallux, and lesser toes. Foot Ankle Int. 1994;15(7):349-353. doi: 10.1177/107110079401500701 [DOI] [PubMed] [Google Scholar]
- 50. Kubosch EJ, Erdle B, Izadpanah K, et al. Clinical outcome and T2 assessment following autologous matrix-induced chondrogenesis in osteochondral lesions of the talus. Int Orthop. 2016;40(1):65-71. doi: 10.1007/s00264-015-2988-z [DOI] [PubMed] [Google Scholar]
- 51. Kuni B, Schmitt H, Chloridis D, Ludwig K. Clinical and MRI results after microfracture of osteochondral lesions of the talus. Arch Orthop Trauma Surg. 2012;132(12):1765-1771. doi: 10.1007/s00402-012-1595-3 [DOI] [PubMed] [Google Scholar]
- 52. Lansdown DA, Wang K, Cotter E, Davey A, Cole BJ. Relationship between quantitative MRI biomarkers and patient-reported outcome measures after cartilage repair surgery: a systematic review. Orthop J Sports Med. 2018;6(4):2325967118765448. doi: 10.1177/2325967118765448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lee KT, Kim JS, Young KW, et al. The use of fibrin matrix-mixed gel-type autologous chondrocyte implantation in the treatment for osteochondral lesions of the talus. Knee Surg Sports Traumatol Arthrosc 2013;21(6):1251-1260. doi: 10.1007/s00167-012-2096-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Lenz CG, Tan S, Carey AL, Ang K, Schneider T. Matrix-induced autologous chondrocyte implantation (MACI) grafting for osteochondral lesions of the talus. Foot Ankle Int 2020;41(9):1099-1105. doi: 10.1177/1071100720935110 [DOI] [PubMed] [Google Scholar]
- 55. Liess C, Lüsse S, Karger N, Heller M, Glüer CC. Detection of changes in cartilage water content using MRI T2-mapping in vivo. Osteoarthritis Cartilage. 2002;10(12):907-913. doi:10.1053/joca.2002.0847 [DOI] [PubMed] [Google Scholar]
- 56. Magnan B, Samaila E, Bondi M, Vecchini E, Micheloni GM, Bartolozzi P. Three-dimensional matrix-induced autologous chondrocytes implantation for osteochondral lesions of the talus: midterm results. Adv Orthop. 2012;2012:942174. doi: 10.1155/2012/942174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57(1):16-23. doi: 10.1016/j.ejrad.2005.08.007 [DOI] [PubMed] [Google Scholar]
- 58. McCarthy HS, McCall IW, Williams JM, et al. Magnetic resonance imaging parameters at 1 year correlate with clinical outcomes up to 17 years after autologous chondrocyte implantation. Orthop J Sports Med. 2018;6(8):2325967118788280. doi: 10.1177/2325967118788280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Merkely G, Ogura T, Bryant T, Minas T. Severe bone marrow edema among patients who underwent prior marrow stimulation technique is a significant predictor of graft failure after autologous chondrocyte implantation. Am J Sports Med. 2019;47(8):1874-1884. doi: 10.1177/0363546519853584 [DOI] [PubMed] [Google Scholar]
- 60. Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19(4):353-359. doi: 10.1053/jars.2003.50041 [DOI] [PubMed] [Google Scholar]
- 61. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. doi: 10.1371/journal.pmed.1000097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Nguyen A, Ramasamy A, Walsh M, McMenemy L, Calder JDF. Autologous osteochondral transplantation for large osteochondral lesions of the talus is a viable option in an athletic population. Am J Sports Med. 2019;47(14):3429-3435. doi: 10.1177/0363546519881420 [DOI] [PubMed] [Google Scholar]
- 63. Niethammer TR, Safi E, Ficklscherer A, et al. Graft maturation of autologous chondrocyte implantation: magnetic resonance investigation with T2 mapping. Am J Sports Med. 2014;42(9):2199-2204. doi: 10.1177/0363546514538756 [DOI] [PubMed] [Google Scholar]
- 64. Pagliazzi G, Vannini F, Battaglia M, Ramponi L, Buda R. Autologous chondrocyte implantation for talar osteochondral lesions: comparison between 5-year follow-up magnetic resonance imaging findings and 7-year follow-up clinical results. J Foot Ankle Surg. 2018;57(2):221-225. doi: 10.1053/j.jfas.2017.05.013 [DOI] [PubMed] [Google Scholar]
- 65. Potter HG, Linklater JM, Allen AA, Hannafin JA, Haas SB. Magnetic resonance imaging of articular cartilage in the knee. An evaluation with use of fast-spin-echo imaging. J Bone Joint Surg Am. 1998;80(9):1276-1284. doi: 10.2106/00004623-199809000-00005 [DOI] [PubMed] [Google Scholar]
- 66. Ramponi L, Yasui Y, Murawski CD, et al. Lesion size is a predictor of clinical outcomes after bone marrow stimulation for osteochondral lesions of the talus: a systematic review. Am J Sports Med. 2017;45(7):1698-1705. doi: 10.1177/0363546516668292 [DOI] [PubMed] [Google Scholar]
- 67. Recht MP, Goodwin DW, Winalski CS, White LM. MRI of articular cartilage: revisiting current status and future directions. AJR Am J Roentgenol. 2005;185(4):899-914. doi: 10.2214/ajr.05.0099 [DOI] [PubMed] [Google Scholar]
- 68. Rehnitz C, Kuni B, Wuennemann F, et al. Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T(2) mapping of talar osteochondral lesions: indicators of clinical outcomes. J Magn Reson Imaging. 2017;46(6):1601-1610. doi: 10.1002/jmri.25731 [DOI] [PubMed] [Google Scholar]
- 69. Rizzo G, Cristoforetti A, Marinetti A, et al. Quantitative MRI T2 mapping is able to assess tissue quality after reparative and regenerative treatments of osteochondral lesions of the talus. J Magn Reson Imaging. 2021;54(5):1572-1582. doi: 10.1002/jmri.27754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Roos EM, Brandsson S, Karlsson J. Validation of the foot and ankle outcome score for ankle ligament reconstruction. Foot Ankle Int. 2001;22(10):788-794. doi: 10.1177/107110070102201004 [DOI] [PubMed] [Google Scholar]
- 71. Rosa D, Balato G, Ciaramella G, Soscia E, Improta G, Triassi M. Long-term clinical results and MRI changes after autologous chondrocyte implantation in the knee of young and active middle aged patients. J Orthop Traumatol. 2016;17(1):55-62. doi: 10.1007/s10195-015-0383-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Sadlik B, Kolodziej L, Blasiak A, Szymczak M, Warchal B. Biological reconstruction of large osteochondral lesions of the talar dome with a modified “sandwich” technique-Midterm results. Foot Ankle Surg 2017;23(4):290-295. doi: 10.1016/j.fas.2016.09.001 [DOI] [PubMed] [Google Scholar]
- 73. Schreiner MM, Mlynarik V, Zbýň Š, et al. New technology in imaging cartilage of the ankle. Cartilage. 2017;8(1):31-41. doi: 10.1177/1947603516632848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Shimozono Y, Coale M, Yasui Y, O’Halloran A, Deyer TW, Kennedy JG. Subchondral bone degradation after microfracture for osteochondral lesions of the talus: an MRI analysis. Am J Sports Med. 2018;46(3):642-648. doi: 10.1177/0363546517739606 [DOI] [PubMed] [Google Scholar]
- 75. Shimozono Y, Hurley ET, Yasui Y, Deyer TW, Kennedy JG. The presence and degree of bone marrow edema influence midterm clinical outcomes after microfracture for osteochondral lesions of the talus. Am J Sports Med. 2018;46(10):2503-2508. doi: 10.1177/0363546518782701 [DOI] [PubMed] [Google Scholar]
- 76. Surowiec RK, Lucas EP, Ho CP. Quantitative MRI in the evaluation of articular cartilage health: reproducibility and variability with a focus on T2 mapping. Knee Surg Sports Traumatol Arthrosc. 2014;22(6):1385-1395. doi: 10.1007/s00167-013-2714-6 [DOI] [PubMed] [Google Scholar]
- 77. Taheri S, Böker KO, Lehmann W, Schilling AF. Knorpel-Knochenmark-Mikro-Konnektoren im subchondralen Knochen [Cartilage-Bone Marrow Micro-Connectors in Subchondral Bone]. Osteologie. 2021;30(01):13-20. [Google Scholar]
- 78. Takahashi T, Tins B, McCall IW, Richardson JB, Takagi K, Ashton K. MR appearance of autologous chondrocyte implantation in the knee: correlation with the knee features and clinical outcome. Skeletal Radiol. 2006;35(1):16-26. doi: 10.1007/s00256-005-0002-3 [DOI] [PubMed] [Google Scholar]
- 79. Tao H, Shang X, Lu R, et al. Quantitative magnetic resonance imaging (MRI) evaluation of cartilage repair after microfracture (MF) treatment for adult unstable osteochondritis dissecans (OCD) in the ankle: correlations with clinical outcome. Eur Radiol. 2014;24(8):1758-1767. doi: 10.1007/s00330-014-3196-8 [DOI] [PubMed] [Google Scholar]
- 80. Tegner Y, Lysholm J. Rating systems in the evaluation of knee ligament injuries. Clin Orthop Relat Res. 1985;(198):43-49. [PubMed] [Google Scholar]
- 81. Thermann H. Treatment of osteochondritis dissecans of the talus: a long-term follow-up. Sports Med Arthrosc Rev. 1994;2:284-288. [Google Scholar]
- 82. Usuelli FG, Grassi M, Manzi L, Guarrella V, Boga M, DEG L. Treatment of osteochondral lesions of the talus with autologous collagen-induced chondrogenesis: clinical and magnetic resonance evaluation at one-year follow-up. Joints 2016;4(2):80-86. doi: 10.11138/jts/2016.4.2.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Valderrabano V, Leumann A, Rasch H, Egelhof T, Hintermann B, Pagenstert G. Knee-to-ankle mosaicplasty for the treatment of osteochondral lesions of the ankle joint. Am J Sports Med. 2009;37(Suppl 1):105S-111S. doi: 10.1177/0363546509351481 [DOI] [PubMed] [Google Scholar]
- 84. Valderrabano V, Miska M, Leumann A, Wiewiorski M. Reconstruction of osteochondral lesions of the talus with autologous spongiosa grafts and autologous matrix-induced chondrogenesis. Am J Sports Med 2013;41(3):519-27. doi: 10.1177/0363546513476671 [DOI] [PubMed] [Google Scholar]
- 85. van Dijk PAD, Murawski CD, Hunt KJ, et al. Post-treatment follow-up, imaging, and outcome scores: proceedings of the international consensus meeting on cartilage repair of the ankle. Foot Ankle Int. 2018;39(1 Suppl):68S-73S. doi: 10.1177/1071100718781861 [DOI] [PubMed] [Google Scholar]
- 86. Wang T, Wang D, Burge AJ, et al. Clinical and MRI outcomes of fresh osteochondral allograft transplantation after failed cartilage repair surgery in the knee. J Bone Joint Surg Am. 2018;100(22):1949-1959. doi: 10.2106/JBJS.17.01418 [DOI] [PubMed] [Google Scholar]
- 87. Ware J, Jr, Kosinski M, Keller SD. A 12-item Short-Form Health Survey: construction of scales and preliminary tests of reliability and validity. Med Care. 1996;34(3):220-233. doi: 10.1097/00005650-199603000-00003 [DOI] [PubMed] [Google Scholar]
- 88. Weber M, Wünnemann F, Jungmann P, Kuni B, Rehnitz C. Modern Cartilage Imaging of the Ankle Moderne Knorpelbildgebung des Sprunggelenks. 2017. [DOI] [PubMed] [Google Scholar]
- 89. Williams RJ, 3rd, Ranawat AS, Potter HG, Carter T, Warren RF. Fresh stored allografts for the treatment of osteochondral defects of the knee. J Bone Joint Surg Am. 2007;89(4):718-726. doi: 10.2106/jbjs.F.00625 [DOI] [PubMed] [Google Scholar]
- 90. Woelfle JV, Reichel H, Javaheripour-Otto K, Nelitz M. Clinical outcome and magnetic resonance imaging after osteochondral autologous transplantation in osteochondritis dissecans of the talus. Foot Ankle Int. 2013;34(2):173-179. doi: 10.1177/1071100712467433 [DOI] [PubMed] [Google Scholar]
- 91. Yang HY, Lee KB. Arthroscopic microfracture for osteochondral lesions of the talus: second-look arthroscopic and magnetic resonance analysis of cartilage repair tissue outcomes. J Bone Joint Surg Am. 2020;102(1):10-20. doi: 10.2106/jbjs.19.00208 [DOI] [PubMed] [Google Scholar]